Abstract
Statement of the Problem:
Zataria multiflora (ZM) is a thyme-like plant that belongs to the Lamiaceae family. It is native to the center and south of Iran, Pakistan, and Afghanistan. Evidence shows that ZM contains thymol and carvacrol and is therefore, effective for the treatment of many conditions especially fungal infections. Oral candidiasis is the most common opportunistic infection of the oral mucosa that plays a role in the development of denture stomatitis.
Purpose:
This study aimed to compare the antifungal efficacy of ZM and nystatin suspension for the treatment of denture stomatitis.
Materials and Method:
This single-blind clinical trial evaluated 28 patients (> 18 years old) suffering from type II or III denture stomatitis. Patients were divided into two groups. The control group used nystatin suspension while the case group used ZM drop. The number of Candida albicans (C. albicans) colony-forming units (CFUs) and erythema of the palate were evaluated at baseline and at 14 days after treatment. Data were analyzed using SPSS version 11 via Student’s t test and repeated measure ANOVA.
Results:
The results showed similar efficacy of nystatin and ZM in the reduction of C.albicans CFUs compared to the baseline value (p= 0.593). Both medications significantly decreased the colony count (p< 0.001). Nystatin and ZM had similar efficacy for the reduction of erythema as well (p= 0.256) and both caused a significant reduction in erythema of the palate (p<0.001).
Conclusion:
ZM drop was as effective as the nystatin drop in the resolution of erythema of the palate and reduction of C. albicans colony count.
Keywords: Candida albicans, Denture stomatitis, Nystatin, Zataria multiflora
Introduction
Oral candidiasis is the most common opportunistic infection affecting the oral mucosa. In most cases, the infection is caused by the Candida albicans (C. albicans) yeast. It has a prevalence of 35% as a member of the normal flora. Under certain circumstances, C. albicans may become invasive. A significant association has been noted between oral candidiasis and presence of local predisposing factors (such as wearing a denture, smoking, and use of inhaled or topical steroids) and some systemic conditions (immune system and endocrine status), causing transformation of C. albicans to its pathogenic variant [ 1 ]. Biofilms containing C. albicans play a role in the development of denture stomatitis. Denture stomatitis is the most common and the most important clinical condition occurring in denture wearers [ 2 ]. It has a multifactorial etiology. Long-term use of denture is the most important risk factor for the colonization of candida species on the mucosal surface of denture and development of oral candidiasis, which is affected by exogenous and endogenous factors [ 3 ]. At present, antifungal medications play a primary role in the treatment of denture stomatitis. However, they have side effects and are associated with a high risk of recurrence. Antifungal agents mainly belong to the family of azoles or polyenes. Polyenes such as nystatin are the primary choice for the treatment of primary oral candidiasis [ 2 ]. However, they have some side effects such as bitter taste, poor acceptance by patients, mucosal irritation, and nausea [ 4 ].
There is a growing trend towards the use of herbal medications worldwide. Affordability, availability, fewer side effects, not causing microbial resistance, and significant therapeutic effects are among the most important properties of herbal medications [ 5 - 7 ]. Zataria multiflora (ZM) is a thyme-like medicinal herb, and the contemporary pharmacological studies have confirmed its anti-inflammatory, analgesic, antispasmodic, and antimicrobial properties. ZM contains thymol and carvacrol and it is used for the treatment of many conditions particularly fungal, bacterial, and parasitic infections. Some studies have reported the antifungal effects of ZM oil against candida [ 5 , 8 ]. In 2018, de Oliveira Santos et al. [ 9 ] showed in a brief review that ZM has the best antifungal effect among various herbal essential oils. Nazzaro et al. [ 10 ] also noted similar results in another study. Considering the scarcity of investigations on the antifungal efficacy of ZM and its comparison with the commonly prescribed synthetic antifungal agents, this study aimed to assess and compare the antifungal effects of ZM and nystatin.
Materials and Method
This study is a randomized clinical trial and single-blinded design. After obtaining ethical approval (IR.IAU, DENTAL.REC.1395, 35) and registration in the Iranian Registry of Clinical Trials (IRCT) (IRCT201706183461 9 N1) (2017-09-24), written informed consent was obtained from all patients before their enrollment. Patients were selected among those presenting to the dental clinic of the Islamic Azad University of Tehran. A total of 28 patients over 18 years of age suffering from type II or III denture stomatitis were selected (Figure 1). Denture stomatitis has three types. Type II presents an extensive erythematous mucosa under the denture. Type III is characterized by the presence of a granular mucosa at the center of the palate in addition to the involved areas mentioned for type II [ 11 ]. The exclusion criteria were patients with hepatic cirrhosis, renal insufficiency, thyroid dysfunction (contraindication for use of ZM), diabetes mellitus, xerostomia, hypoparathyroidism, pregnancy or nursing, immunocompromised patients, chemotherapy, radiotherapy, and patients who had taken antibiotics or antifungal agents in the past 4 weeks [ 12 - 13 ]. Patients were questioned about the duration of using removable denture, its cleaning protocol (water, toothbrush, denture cleansing agents), frequency of cleansing the denture (zero, once a week, twice a week, three times a week, more than three times a week) and that whether they remove it at night. The presence of erythema of the palate was also recorded. For this purpose, the affected area was outlined with a copying pencil and its surface area was measured using a 5mm squared transparent grid and reported in square millimeters. Eventually, the patients were requested to rinse their mouth with drinking water. Then a microbial sample was taken from the erythematous area from hard palate using a sterile swab to count the C. albicans colony forming units (CFUs) before treatment [ 12 ]. The patients were then divided into two groups of 14, using stratified block randomization since patients had type II or III denture stomatitis and we wanted to ensure an equal number of both types in the two groups.
Figure 1.
CONSORT Flow Diagram
In the control group, patients were requested to rinse 40 drops of 100,000-unit nystatin suspension (Emad Pharmaceuticals, Tehran, Iran) for 2 minutes four times a day for two weeks [ 13 ]. In the case group, patients were requested to rinse one teaspoon (5mL) of ZM essential oil 0.05 % (Gastroli; Bridge Essence) containing 5mg/mL thymol and carvacrol for 2minutes four times a day for two weeks [ 12 ]. It should be noted that the medications were given to patients by another person not involved in the study and according to computer-generated random numbers (2 series from 1 to 28 for types II and III denture stomatitis).
Patients were instructed to remove the denture, rinse their mouth with water, and then use the medication. In addition, they were instructed to use their denture only for eating. Cleaning the denture after meals with a soft toothbrush without toothpaste, removal of the denture at night, and its immersion in water overnight were also emphasized [ 14 ]. Since nystatin interferes with chlorhexidine, patients were requested not to immerse their dentures in chlorhexidine during the study period [ 15 ].
Patients were examined at the end of day 14. A microbial sample was taken from the palate using a sterile swab and the presence/absence of erythema was recorded. The C. albicans colony count was recorded again. The C. albicans colonies were counted using the method as follows. Microbial samples taken from the palate were placed in sterile saline and transferred to the microbiology lab within 2 hours. The test tube containing the swab and saline was shaken on a shaker and then 0.1 mL of the homogenous solution was taken by a sampler, cultured on Sabouraud dexterous agar plate, and incubated for 24 hours. Next, the presence of C. albicans colonies was confirmed using the germ tube test and they were counted under a light microscope (SE, Nikon, Japan). In the germ tube test, one colony was chosen and placed in 2 cc of human serum. After 2 hours, a slide was prepared of the serum, and the presence of C. albicans colonies was microscopically confirmed [ 16 ].
Statistics analysis
We used Student’s t test for comparison of quantitative data with normal distribution between two groups and repeated measure ANOVA for comparison more than two groups or to compare a group in more than one position or time, respectively. Data were analyzed with SPSS 11 (IBM Corp.) and Statistical significance was accepted as p< 0.05.
Results
A total of 7 females (25%) and 21 males (75%) participated in this study. The nystatin group included 5 females and 9 males with a mean age of 60.93±13.04 years (range 38 to 82 years) while the ZM group included 2 females and 12 males with a mean age of 55.86± 10.04 years (range 37 to 67 years). Demographic and clinical characteristics in two groups, before and after treatment were shown in Table 1.
Table 1.
Demographic information of patients in the two groups
| Parameter | Groups* | |
|---|---|---|
| Nystatin | ZM | |
| Age (yrs.) | ||
| mean± SD | 60.93±13.04 | 55.86±10.04 |
| Median (range) | 38-82 | 37-67 |
| Gender | ||
| Female | 5 (35.7%) | 2 (14.3%) |
| Male | 9 (64.3%) | 12 (85.7%) |
| Denture stomatitis type | ||
| II | 7 (50 %) | 7 (50 %) |
| III | 7 (50 %) | 7 (50 %) |
| Duration of wearing a denture | ||
| <5(yrs) | 2(14.3%) | 2(14.3%) |
| >5(yrs) | 12(85.7%) | 12(85.7%) |
| Cleaning method | ||
| Water | 4(28.6%) | 2(14.3%) |
| Tooth brush | 10(71.4%) | 12(85.7%) |
| Cleaning agent | 0(0%) | 0(0%) |
| Frequency of cleaning | ||
| None | 0(0%) | 0(0%) |
| 1 qw | 3(21.4%) | 0(0%) |
| 2 qw | 1(7.1%) | 3(21.4%) |
| 3 qw | 0(0%) | 3(21.4%) |
| >3 qw | 10(71.4%) | 8(57.1%) |
| Nocturnal denture use | ||
| No | 2(14.3%) | 1(7.1%) |
| Yes | 12(85.7%) | 13(92.9%) |
Statistical analysis was revealed no significant difference (p> 0.05)
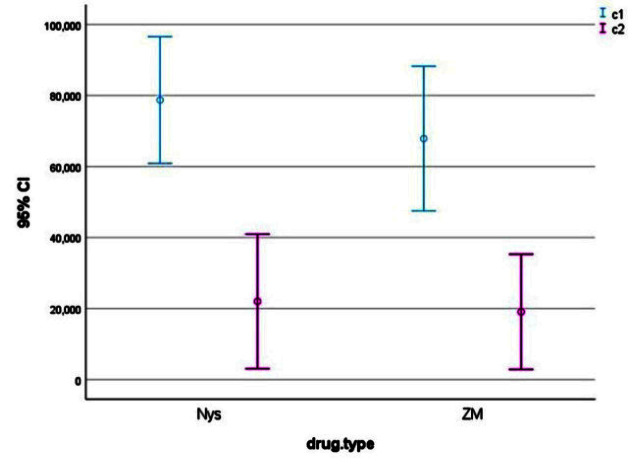
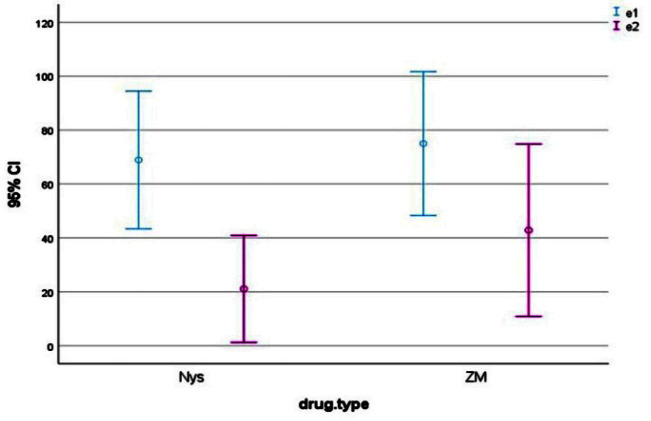
Patients in the case and control groups were compared in terms of C. albicans colony count at baseline and 14 days after treatment. The results showed that ZM and nystatin had no significant difference with each other in this regard (p= 0.593) and both caused a significant reduction of C. albicans colony count (p< 0.001, Table 2, Figures 2-4). Comparison of erythema before and at 14 days after treatment revealed that both nystatin and ZM significantly decreased the erythema (p< 0.001) and both were equally effective in this regard with no significant difference with each other (p= 0.256; Table 3, Figures 5-9).
Table 2.
Colony count (CFUs) before and after treatment in the two groups
| Groups | p Value | ||
|---|---|---|---|
| Nystatin | ZM | ||
| CFU (before) | p= 0.593 | ||
| Min | 15000 | 20000 | |
| Max | 100000 | 100000 | |
| mean±SD | 78714.29± | 30897.91 | |
| 67857.14± | 35285.33 | ||
| CFU (after) | |||
| Min | 0 | 0 | |
| Max | 80000 | 100000 | |
| mean±SD | 22000± | 32825.88 | |
| 19071.43± | 28089.04 | ||
| p value | p< 0.001 | ||
SD: Standard deviation
Figure 2.
Colony count (CFUs) before and after treatment in the two groups
Figure 3.
Candida albicans colony forming units before treatment with ZM
Figure 4.

Candida albicans colony forming units after treatment with ZM
Table 3.
Erythema before and after treatment in the two groups
| Groups | p Value | ||
|---|---|---|---|
| Nystatin | ZM | ||
| Erythema (before)(mm2) | p= 0.256 | ||
| Min. | 10 | 20 | |
| Max. | 150 | 170 | |
| Mean± SD | 68.93±44.25 | 75±46.24 | |
| Erythema (after) (mm2) | |||
| Min | 0 | 0 | |
| Max | 100 | 170 | |
| Mean± SD | 21.07±34.42 | 42.86±55.39 | |
| p Value | p< 0.001 | ||
SD: Standard deviation
Figure 5.
Erythema before and after treatment in the two groups
Figure 6.
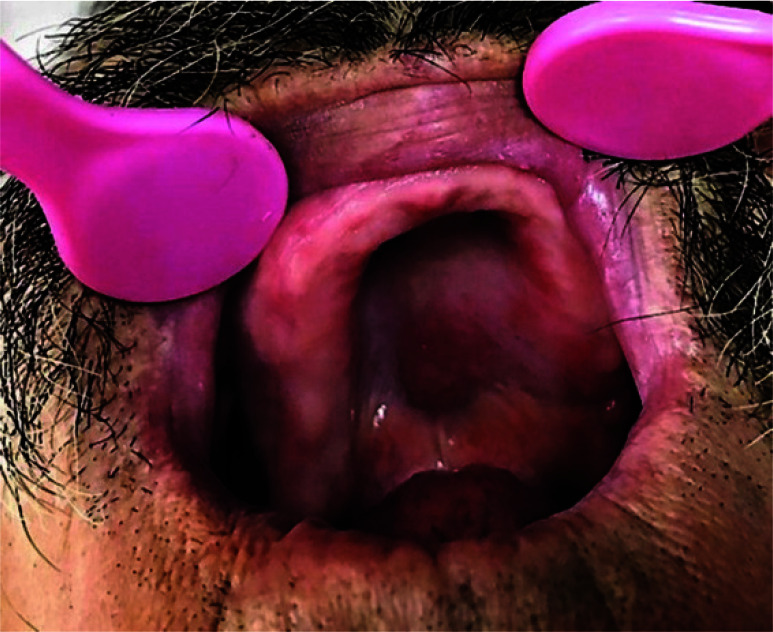
Erythema before treatment with nystatin
Figure 7.
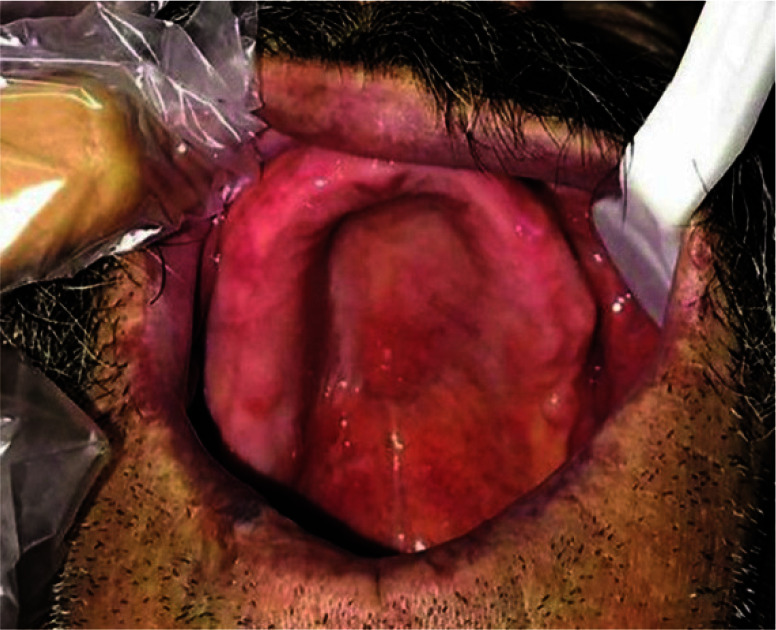
Erythema after treatment with nystatin
Figure 8.
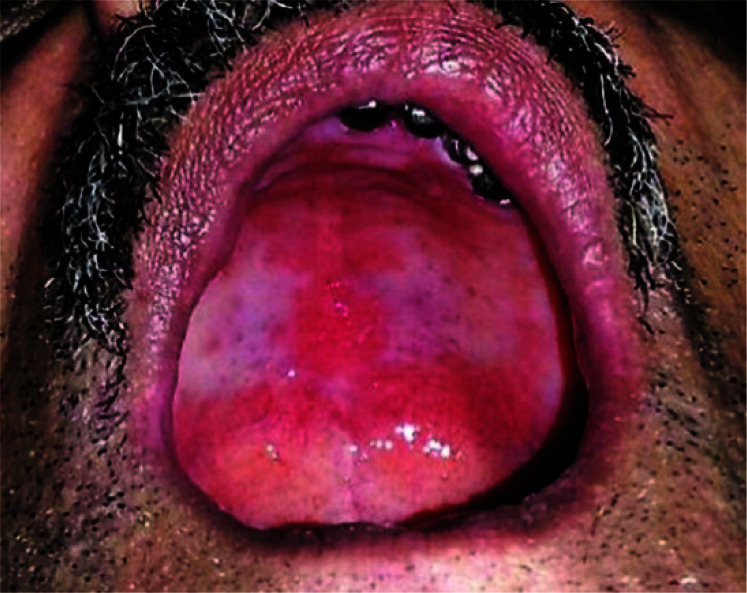
Erythema before treatment with ZM
Figure 9.
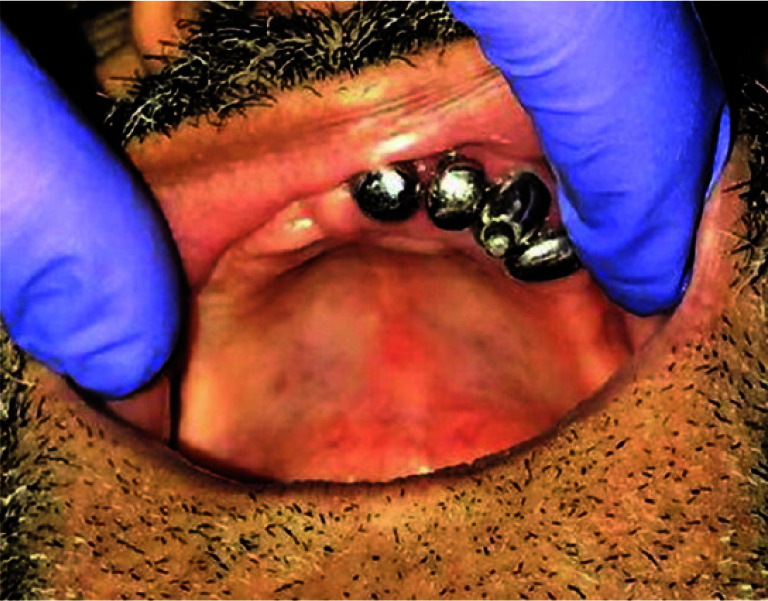
Erythema after treatment with ZM
Discussion
The current findings revealed similar efficacy of nystatin and ZM in the reduction of C. albicans colony count (p= 0.593) and they both caused a significant reduction in C. albicans CFUs (p< 0.001).
The same results were obtained for resolution of erythema and both medications equally decreased erythema (p< 0.001) with no significant difference with each other (p= 0.256).
Amanlou et al. [ 12 ] compared the efficacy of miconazole and ZM gel for the treatment of denture stomatitis in a clinical trial. Similarly, in our study, erythema significantly decreased in both groups with no significant difference between them. A reduction was also noted in C. albicans colony count on day 14 in both groups with no significant difference between nystatin and ZM in this respect. However, Amanlou et al. [ 12 ] showed superior efficacy of miconazole in this respect. This difference in the results can be due to the differences in the efficacy of nystatin and miconazole and different forms of medications used (gel versus drop).
Sajed et al. [ 8 ] performed a systematic review of ethnopharmacology, pharmacology, toxicity, modern pharmaceutical applications, and pharmacochemistry of ZM. They reviewed all relevant articles published until 2012 and concluded that ZM has antimicrobial, antioxidative, anti-inflammatory, antispasmodic, and analgesic properties. ZM oil contains high concentrations of oxygenated monoterpenes, thymol, and carvacrol and possesses favorable antimicrobial properties. Our study also confirmed the anti-candida effects of ZM.
Moghim et al. [ 5 ] evaluated the antifungal effects of ZM and Nigella sativa on C. albicans. They measured the minimum inhibitory concentration (MIC), 50% MIC, 90% MIC, and minimum fungicidal concentration (MFC) of ZM and Nigella sativa separately by counting the fungal colonies. The results revealed that both ZM and Nigella sativa were effective against C.albicans (p< 0.005). This finding was in agreement with our results.
Jafari et al. [ 17 ] evaluated the antifungal effects of ZM essence on acrylic resin plates contaminated with C. albicans. In their in vitro experimental study, they compared five different concentrations of ZM essence (3.125 mg/mL to 50mg/mL), 100,000-unit nystatin as the positive control, and saline as the negative control. They concluded that ZM essence in 25mg/mL and 50mg/mL concentrations had a MFC similar to that of nystatin and eliminated 100% of the C. albicans colonies. Similarly, ZM in our study caused a significant reduction in C. albicans colony count.
Sedigh-Shams et al. [ 18 ] compared the antifungal effects of sodium hypochlorite and ZM essence as irrigating solutions for root canals contaminated with C. albicans in vitro. They first calculated the MFC of ZM and sodium hypochlorite. The results showed that sodium hypochlorite in its MFC and ZM in twice its MFC had the highest antifungal effect with no significant difference with each other (p> 0.05). However, their antifungal effects were significantly different from those of ZM in MFC and distilled water (p< 0.05). Their findings confirmed the antifungal effect of ZM and were in line with our results.
Khosravi et al. [ 19 ] compared the effects of ZM essential oil and itraconazole on disseminated C. albicans infection in rats. They administered 30mg/mL, 48mg/ mL, and 64mg/mL of the essential oil of ZM and 200mg/mL itraconazole intraperitoneally. The results showed that injection of 64 mg/mL essential oil of ZM had the highest efficacy for the reduction of C. albicans colony count and itraconazole was less effective for this purpose (p< 0.01 for the brain, p < 0.0005 for the lungs, and p< 0.0005 for the kidneys). However, itraconazole was more effective than 30 mg/mL concentration of ZM for the elimination of C. albicans in the brain (p< 0.02), kidneys (p< 0.02), and spleen (p< 0.04). No significant difference was noted between itraconazole and 48 mg/mL concentration of ZM. The difference between their results and ours regarding the efficacy of ZM can be due to some reasons. First, dissimilarities between human and animal models, second, diversities in types of C. albicans infection, and finally differences between ZM concentrations.
Fouladi et al. [ 20 ] compared the efficacy of ZM cream with vaginal clotrimazole cream for the treatment of vaginal candidiasis in 73 patients. They reported that 1% clotrimazole cream and ZM both caused a significant improvement in patients (p< 0.05). Their findings were in agreement with our results.
Considering all the above and the current results, it may be concluded that ZM is effective for the reduction of C. albicans colony count due to its antifungal and anti-inflammatory properties.
Conclusion
As a potent antifungal drug, ZM may be as effective as nystatin for the reduction of erythema of the palate and
C. albicans colony count.
Footnotes
Conflict of Interest: Authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) in our work and authors do not have any conflict of interest.
References
- 1.Di Stasio D, Lauritano D, Minervini G, Paparella RS, Petruzzi M, Romano A, et al. Management of denture stomatitis: a narrative review. J Biol Regul Homeost Agents. 2018; 32: 113–116. [PubMed] [Google Scholar]
- 2.De Souza RF, Khiyani MF, Chaves CA, Feine J, Barbeau J, Fuentes R, et al. Improving practice guidelines for the treatment of denture-related erythematous stomatitis: a study protocol for a randomized controlled trial. Trials. 2017; 18: 211–217. doi: 10.1186/s13063-017-1947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauch LM, Pedrosa SS, Silveira-Gomes F, Esteves RA, Marques-da-Silva SH. Isolation of Candida spp. from denture-related stomatitis in Pará, Brazil. Braz J Microbiol. 2017; 49: 148–151. doi: 10.1016/j.bjm.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhshi M, Taheri JB, Basir Shabestari S, Tanik A, Pahlevan R. Comparison of therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatitis. Gerodontology. 2012; 29: e680–e684. doi: 10.1111/j.1741-2358.2011.00544.x. [DOI] [PubMed] [Google Scholar]
- 5.Moghim H, Taghipoor S, Shahinfard N, Kheiri S, Panahi R. Antifungal effects of Zataria multiflora and Nigella sativa extracts against Candida albicans. JHP. 2015; 4: 138–141. [Google Scholar]
- 6.Zomorodian K, Saharkhiz MJ, Rahimi MJ, Bandegi A, Shekarkhar G, Bandegani A, et al. Chemical composition and antimicrobial activities of the essential oils from three ecotypes of Zataria multiflora. Pharmacogn Mag. 2011; 7: 53. doi: 10.4103/0973-1296.75902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat N, Mitra R, Reddy JJ, Oza S, Vinayak KM. Evaluation of efficacy of chlorhexidine and a herbal mouthwash on dental plaque: An in vitro comparative study. Int J Pharm Bio Sci. 2013; 4: 625–632. [Google Scholar]
- 8.Sajed H, Sahebkar A, Iranshahi M. Zataria multiflora Boiss. (Shirazi thyme)-an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 2013; 145: 686–698. doi: 10.1016/j.jep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 9.De Oliveira Santos GC, Vasconcelos CC, Lopes AJ, de Sousa Cartágenes MD, Allan Filho KD, do Nascimento FR, et al. Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Frontiers in Microbiology. 2018; 9: 1351. doi: 10.3389/fmicb.2018.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazzaro F, Fratianni F, Coppola R, Feo VD. Essential oils and antifungal activity. Pharmaceuticals. 2017; 10: 86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offenbacher S, Barros SP, Bencharit S, Yu N, Preisser J, Moss K, et al. Differential Mucosal Gene Expression Patterns in Candida‐Associated, Chronic Oral Denture Stomatitis. J Prosth. 2019; 28: 202–208. doi: 10.1111/jopr.13007. [DOI] [PubMed] [Google Scholar]
- 12.Amanlou M, Beitollahi JM, Abdollahzadeh S, Tohidast‐Ekrad Z. Miconazole gel compared with Zataria multiflora Boiss, gel in the treatment of denture stomatitis. Phytother Res. 2006; 20: 966–969. doi: 10.1002/ptr.1986. [DOI] [PubMed] [Google Scholar]
- 13.Lyu X, Zhao C, Yan ZM, Hua H. Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Design Development and Therapy. 2016; 10: 1161. doi: 10.2147/DDDT.S100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duyck J, Vandamme K, Krausch-Hofmann S, Boon L, De Keersmaecker K, Jalon E, et al. Impact of denture cleaning method and overnight storage condition on denture biofilm mass and composition: A cross-over randomized clinical trial. PLoS One. 2016; 11: e0145837. doi: 10.1371/journal.pone.0145837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein JB, Vickars L, Spinelli J, Reece D. Efficacy of chlorhexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surgery Oral Medicine Oral Pathology. 1992; 73: 682–689. doi: 10.1016/0030-4220(92)90009-f. [DOI] [PubMed] [Google Scholar]
- 16.Mattei AS, Alves SH, Severo CB, Guazzelli LD, Oliveira FD, Severo LC. Use of Mueller-Hinton broth and agar in the germ tube test. Rev Inst Med Trop Sao Paulo. 2014; 56: 483–485. doi: 10.1590/S0036-46652014000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafari AA, Tafti AF, Hoseiny SM, Kazemi A. Antifungal Effect of Zataria multiflora Essence on Experimentally Contaminated Acryl Resin Plates With Candida albicans. IRCMJ. 2015; 17: 165–170. doi: 10.5812/ircmj.16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedigh-Shams M, Badiee P, Adl A, Sarab MD, Abbaszadegan A, Nabavizadeh M. In vitro comparison of antimicrobial effect of sodium hypochlorite solution and Zataria multiflora essential oil as irrigants in root canals contaminated with Candida albicans. JCD. 2016; 19: 101–105. doi: 10.4103/0972-0707.173212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khosravi A, Shokri H, Tootian Z, Alizadeh M, Yahyaraeyat R. Comparative efficacies of Zataria multiflora essential oil and itraconazole against disseminated Candida albicans infection in BALB/c mice. Braz J Microbiol. 2009; 40: 439–445. doi: 10.1590/S1517-83822009000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouladi Z, Afshari P, Gharibi T, Dabbagh MA. The comparison of Zataria multiflora boiss (Avishan Shirazi) and Clotrimazol vaginal cream in the treatment of candidiasis vaginitis. ISMJ. 2009; 12: 214–224. [Google Scholar]