Summary
Two-photon (2-P) all-optical approaches combine in vivo 2-P calcium imaging and 2-P optogenetic modulations. Here, firstly, we combined in vivo juxtacellular recordings and GCaMP6f-based 2-P calcium imaging in mouse visual cortex to tune our detection algorithm towards a 100% specific identification of action potential-related calcium transients. Secondly, we minimized photostimulation artifacts by using extended-wavelength-spectrum laser sources for optogenetic stimulation. We achieved artifact-free all-optical experiments performing optogenetic stimulation from 1100 nm to 1300 nm. Thirdly, we determined the spectral range for maximizing efficacy until 1300 nm. The rate of evoked transients in GCaMP6f/C1V1-co-expressing cortical neurons peaked already at 1100 nm. By refining spike detection and defining 1100 nm as the optimal wavelength for artifact-free and effective GCaMP6f/C1V1-based all-optical physiology, we increased the translational value of these approaches, e.g., for the development of network-based therapies.
Subject areas: Optical Imaging, Molecular Physiology, Cellular Neuroscience
Graphical abstract

Highlights
-
•
We developed an algorithm for 100 %-specific identification of AP-related calcium transients
-
•
Artifact-free all-optical experiments can be achieved from 1100 nm to 1300 nm
-
•
Efficacy of C1V1 excitation does not increase beyond 1100 nm until 1300 nm
Optical Imaging; Molecular Physiology; Cellular Neuroscience
Introduction
In vivo two-photon (2-P) all-optical approaches are based on simultaneous 2-P optogenetic modulations and 2-P calcium imaging (Emiliani et al., 2015). They allow for causal interrogations of neuronal networks on single-cell level in a minimal-invasive fashion by using spatially confined light for readout and manipulation. In recent years, 2-P-based methods have been successfully employed for all-optical control of neural microcircuits in vivo (Rickgauer et al., 2014; Packer et al., 2015; Carrillo-Reid et al., 2016; Forli et al., 2018; Mardinly et al., 2018; Zhang et al., 2018; Carrillo-Reid et al., 2019; Chen et al., 2019; Marshel et al., 2019). Using parallel (Rickgauer et al., 2014; Mardinly et al., 2018; Chen et al., 2019) or hybrids of parallel and scanning methods (Packer et al., 2012; Yang et al., 2018a; Zhang et al., 2018; Carrillo-Reid et al., 2019; Marshel et al., 2019), mainly involving different variants of computer generated holography (CGH), volumetric imaging of hundreds of neurons across cortical layers and simultaneous manipulation of neuronal ensembles with dozens of neurons became feasible (Carrillo-Reid et al., 2016; Mardinly et al., 2018; Yang et al., 2018a; Marshel et al., 2019). Lately, the revolutionizing concept of all-optical physiology has been successfully applied to modulate complex behavior in mammals (Carrillo-Reid et al., 2019; Marshel et al., 2019) and was extended to closed-loop designs (Zhang et al., 2018). However, although these approaches are advancing rapidly, important aspects concerning specificity and efficacy have not been sufficiently addressed, yet.
Utmost specificity and sensitivity are of crucial importance for applications of all-optical approaches in the context of preclinical research in animal models of neurological disorders. Employing all-optical approaches in this field is not farfetched as the combination of 2-P calcium imaging and 2-P optogenetic stimulation would (a) allow us to detect individual dysregulated neurons (Busche et al., 2012; Arnoux et al., 2018; Ellwardt et al. 2018) and would (b) allow us to modulate their activity in a highly specific manner, e.g., by silencing hyperactive neurons, while leaving intact network components untouched. However, in in vivo imaging experiments optical signals are inherently noisy and only indirectly mirroring neuronal activity. Deflections in the fluorescent trace are not only caused by the underlying neurophysiological signal of interest but can be due to movement or non-physiological artifacts, e.g., stimulation light can cause a deflection in the calcium trace when stimulation and imaging channels are not spectrally well separated, as well as ambient light when the preparation is not well shielded from light sources in its surrounding.
On the detection, ultimately, 2-P calcium imaging does not provide a direct readout of neuronal activity, unlike electrophysiology (Stosiek et al. 2003). It reports—at the level of microcircuits—the fast elevation of intracellular calcium levels upon action potential (AP) firing (Grienberger and Konnerth 2012). Consequently, the first task toward a specific detection of the optical correlate of APs represents the specific identification of these stereotypical calcium transients. The second task towards a specific all-optical physiology represents the avoidance of optical non-AP-related artifacts and the cross talk between imaging and stimulation. While, e.g., movement artifacts strongly depend on the quality of preparation, stimulation artifacts are experimenter independent, as they are caused by cross talk between imaging and stimulation channels. A further important issue, which has not been comprehensively addressed, yet, concerns the spectral efficacy of optogenetic control. The spectral range for effective optogenetic control has still not been thoroughly defined. Studies in slice preparations suggest an increase in photocurrents with increasing wavelength; however, the maximum wavelength was limited to 1080 nm (Prakash et al. 2012; Marshel et al., 2019). Until now, no actuator has been tested using wavelengths beyond this range, mainly due to technical limitations.
Here, we propose a step-by-step approach tackling these aforementioned limitations: (I) we provide data on the sensitivity of 2-P calcium imaging in terms of specific, false-positive-free identification of AP-related calcium transients, (II) we determine the spectral window for artifact-free stimulation, and, lastly, (III) we assess the efficacy of opsin stimulation beyond 1100 nm.
Results
Implementing a highly specific optical detection of neural spiking activity in cortical circuits
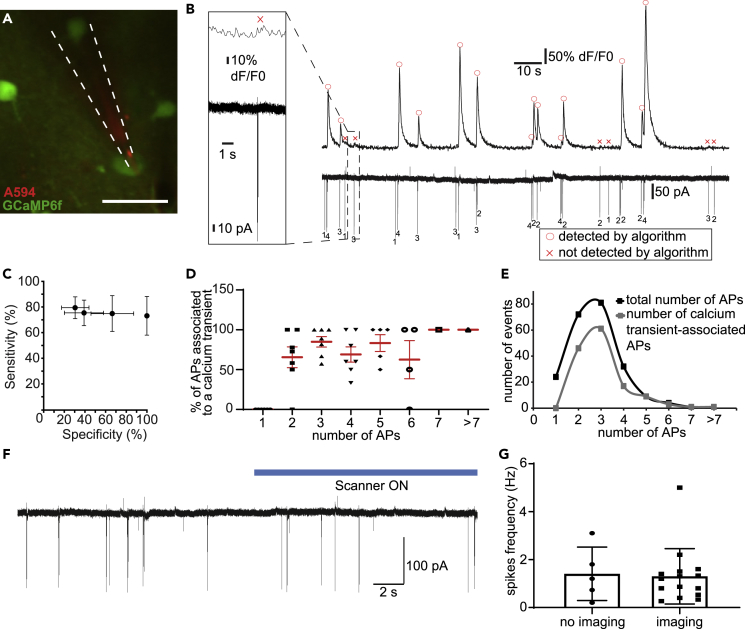
For the application of optical imaging of cortical microcircuit dynamics, i.e., the optical correlate of APs, high specificity combined with high sensitivity is paramount. We have developed a program suite comprising different analysis tools (Arnoux et al., 2018), which take into account the rather stereotypical dynamics of an AP-related calcium transient such as the dynamics of its rise, its duration, and its decay (see Transparent methods Arnoux et al., 2018). This is of particular importance, as a mere deflection of the calcium traces from the baseline could very well have non-AP-related origins, such as motion or light artifacts. To test for the specificity and sensitivity of its built-in peak detection algorithm, here, we conducted simultaneous juxtacellular electrophysiological recordings and 2-P calcium imaging in the visual cortex of the lightly anesthetized mouse. For that, we performed viral-based gene transfer of the genetically encoded calcium indicator GCaMP6f in cortical neurons. In these bimodal recordings, a stable calcium trace with large-scale deflections could be observed (Figures 1A and 1B). This calcium trace was synchronized with the juxtacellular recordings in voltage clamp mode, displaying typical sparse spontaneous AP firing (Rochefort et al. 2009). We set the peak detection criteria in a way that no false positives, i.e., detection of calcium peaks in the absence of temporally locked APs, were identified (Figure 1B). It has to be noted that, indeed, simply correlating relative fluorescence changes with, e.g., the behavioral context, without taking into account the typical temporal dynamics of AP-related calcium transients would have led to the false identification of calcium transients being related to APs. Keeping the specificity at 100%, the average sensitivity of our transient detection was 73 ± 15% (Figure 1C). Of note, a detection of the optical correlate of single APs was not achieved, employing this rigorous analysis aimed for maximizing specificity, even though we used state-of-the-art optics and indicators with high expression levels. For achieving single-action potential resolution requires optimal imaging conditions, with a low baseline noise, low movement particularly in z-direction, and high specific expression of the indicator. By comparing the automated transient identification used in our study with current state-of-art techniques, such as OASIS (Online Active Set method to Infer Spikes), we obtained a higher sensitivity employing the OASIS deconvolution approach in comparison to our algorithm (Figure S1A). But, and this is critical, this comes at a cost of lower specificity, i.e., a significant portion of false positives. The likelihood to identify an AP-related calcium transient increased with the number of APs exhibited by the GCaMP6f-positive neuron (Figure 1D) resulting in converging curves for AP and transient detection at higher firing rates (Figure 1E). To test whether line scanning at 920 nm for imaging of GCaMP6f impacts neuronal physiology, e.g., by heating the brain tissue, we compared firing rates between periods of scanning and periods when the scanner was off (Figures 1F and 1G). Notably, using typical light intensities of 10–30 mW in resonant imaging mode with a pixel dwell time of 1 μs, we did not observe a significant change in the spiking rate between periods of scanning and non-scanning. In conclusion, while achieving high specificity comes at a cost, we could still capture above 70% of all electrophysiologically detected APs.
Figure 1.
Implementing a highly specific optical detection of neural spiking activity in cortical circuits
(A) Simultaneous 2-P imaging and juxtacellular recordings of GCaMP6f-expressing neuron (green). Patch pipette filled with AlexaFluo594 (red), scale bar: 20 μm.
(B) Spontaneous activity of a GCaMP6f-expressing neuron. Calcium trace acquired with 2-P imaging (upper) and current trace (lower) obtained by juxtacellular recording of the same neuron. AP-associated calcium transients as detected by the algorithm are marked by a red circle. APs not accompanied by detected calcium transients are marked by a red cross in the calcium trace. Numbers of APs are indicated below the trace.
(C) Sensitivity and specificity for calcium transient detection based on the chosen standard deviation threshold. Applying a threshold of 0.05 standard deviation (SD) results in a mean sensitivity of 80 ± 9%, reaching a specificity of 30 ± 13%. An increase of the threshold to 0.2, 0.5 SD, and 1 SD is reducing the sensitivity to 75 ± 10%, 75 ± 14%, and 73 ± 15% respectively. The corresponding specificity for an SD threshold of 0.2 and 0.5 increases to 40 ± 19% and 66 ± 21%, respectively; a threshold of 1 SD reaches 100% specificity (n = 7 neurons, 3 mice).
(D) Relation of APs associated to a detected calcium transient to all detected APs, n = 7 neurons, 3 mice.
(E) Histogram of APs (black) and detected calcium transient-associated APs (gray), n = 7 neurons, 3 mice.
(F) Representative current trace without and with (indicated by blue line above) line scanning in resonant mode at 920 nm.
(G) Analysis of spikes frequency w/o 2-P resonant scanning. n = 7 neurons, 3 mice (p = 0.84, unpaired t test with Welch's correction).
Data are represented as mean ± SD. Significance levels were ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Functional calcium transients can only be detected in a rather narrow spectral window
For an all-optical experiment with minimal cross talk, it is essential to spectrally separate imaging and stimulation. Therefore, we needed to define the spectral window allowing for the reliable detection of functional calcium transients as done before (Akerboom et al. 2012). We performed in vivo imaging of GCaMP6f-expressing neurons at different wavelengths of the same field of view, again in layer II/III of visual cortex in lightly anesthetized mice (Figure S1B). First, we investigated the shortest wavelength allowing for the identification of individual GCaMP6f-expressing neurons. The number of visually detectable neurons was similar across wavelengths ranging from 860 to 920 nm (Figure S1B). Therefore, 860 nm was still in the pumping spectrum of the optical parametric oscillator (OPO, see below) and therefore might allow a system based on one laser source. However, while the neurons could be clearly identified, below 880 nm hardly any functional transient was detectable (18.3 ± 3.6%, Figure S1D). At 880 nm, a minor fraction (27.6 ± 10.0%) and at 900 nm, less than half of the transients could be recorded (48.8 ± 10.6%) compared to imaging at 920 nm, where the number of detected calcium transients increased substantially (Figures S1C and S1E). Likewise, the number of detectable active cells increased in similar fashion when increasing the imaging wavelength from 860 to 920 nm (18.3 ± 3.6% at 860 nm, 27.6 ± 9.9% at 880 nm, 49 ± 11% at 900 nm, 100 ± 0% at 920 nm, Figure S1D). Also, spontaneous transient frequencies increased significantly from 860 to 920 nm (0.33 ± 0.1 transients/min at 860 nm, 1.17 ± 0.29 transients/min at 880 nm, 1.33 ± 0.23 transients/min at 900 nm, 1.69 ± 0.22 transients/min at 920 nm, Figure S1F). Consequently, we could replicate that the spectral window for the detection of functional calcium transients is significantly smaller than the window for the morphological identification of GCaMP6f-expressing neurons (Akerboom et al. 2012).
Implementations of spectrally independent 2-P light sources
As the lower edge of the spectral range for our all-optical experiments was now set to 920 nm due to the functional limitations of GCaMP6f discussed above, we had to devise a light source, which flexibly delivered longer wavelengths for cross talk-free optogenetic modulations. Previous work on all-optical physiology used fixed-wavelength ytterbium lasers typically between 1040 and 1080 nm for the 2-P excitation of opsins. We chose to probe whether longer wavelength might both reduce cross talk and improve efficacy.
For achieving this goal, several technical solutions are amenable: One conceivable approach is to use one tunable 2-P Ti:Sa laser source and splitting the beam power for pumping an OPO and for imaging GCaMP6f (Figure S2A). However, 920 nm, the wavelength for functional GCaMP6f imaging, is well beyond the pumping spectrum of most OPO models. In this configuration, the system could therefore only be used in combination with indicators effectively excitable with lower wavelengths, such as Oregon-Green-BAPTA 1, excitable at 800 nm. For all-optical experiments reported in this study, based on GCaMP6f, we probed two different configurations both based on two primary laser sources: The first configuration integrates a second 2-P tunable Ti:Sa laser. One of the two independently tunable beams is dedicated for imaging, allowing full Ti:Sa-spectrum (680–1060 nm), and the other Ti:Sa is set at the optimal wavelength for pumping the subsequent OPO, resulting in full OPO spectrum (1100–1400 nm) for optogenetic stimulation (Figure S2B). The second configuration comprises a single femtosecond laser with two independently tunable output channels simultaneously emitting two beams for imaging (680–960 nm) and stimulation (950–1300 nm) (Figure S2C). Each channel's maximum output power exceeds 1 W. In both configurations, we temporally uncoupled dwell times of the excitation and imaging beams using separate lasers and scanners for imaging and stimulation. This is important, as the pixel dwell time achieved in resonant scanning mode is in the nanosecond range and therefore not sufficient for effective excitation of an opsin, mandating more than 4 μs (Prakash et al. 2012). Consequently, only the 2-P beam used for imaging was coupled to the fast resonant scanner, and the second longer wavelength was coupled to an additional galvanometric scanner. To achieve a high spatial specificity in modulating activity of single cells, it is crucial that the excitation volume is confined and does not exceed the spatial extent of the neuron supposed to be stimulated. Only then, specific scanning patterns can be applied to, e.g., specifically target neurons showing aberrant activity without interfering the activity of well-functioning neighboring network components. The excitation volume of individual microscope systems is defined by their point spread function (PSF), and 2-P illumination theoretically allows excitation of femtoliter volumes. At 920 nm, the PSF of our system had an acceptable full width half maximum (FWHM) of 0.20 ± 0.03 μm in x and 0.26 ± 0.01 μm in y-direction, while the FWHM in z-direction was approximately 1.60 ± 0.05 μm (Figure S2F). These measurements were stable using different excitation wavelengths (Figures S2D–S2F) and allowed to concentrate the excitation on a 0.08 μm3 volume (Figure S2E). In these configurations, our 2-P system was therefore capable of performing spatially specific and spectrally independent optogenetic modulations temporally uncoupled from high-speed GCaMP6f imaging.
Probing functional co-expression of indicator and actuator applying 1- and 2-P optogenetic stimulation
For highly specific all-optical interrogations combining precise optogenetic control and optical readout from individual neurons, the indicator and the opsin protein need to be co-expressed in the neuronal population of interest. We therefore co-injected two Adeno-associated virus (AAV)-based viral vectors encoding for the 2-P excitable opsin C1V1T/T and the calcium indicator GCaMP6f. We titrated both viruses to achieve strong co-expression of both sensor and actuator protein by examining different dilutions of each virus. When increasing the titer of either AAV, we could observe a predominant expression of either GCaMP6f or C1V1T/T-mCherry due to the limited protein synthesis capacity of single neurons (Figures S3A–S3F). Hence, viral vector-based schemes for transduction of postmitotic neurons with opsin and indicator require careful titration. If a given titer is effective for strong expression of the indicator, the second virus encoding the opsin cannot be simply added, as this might lead to overexpression-mediated apoptosis (Aschauer et al. 2013; Yang et al., 2018b).
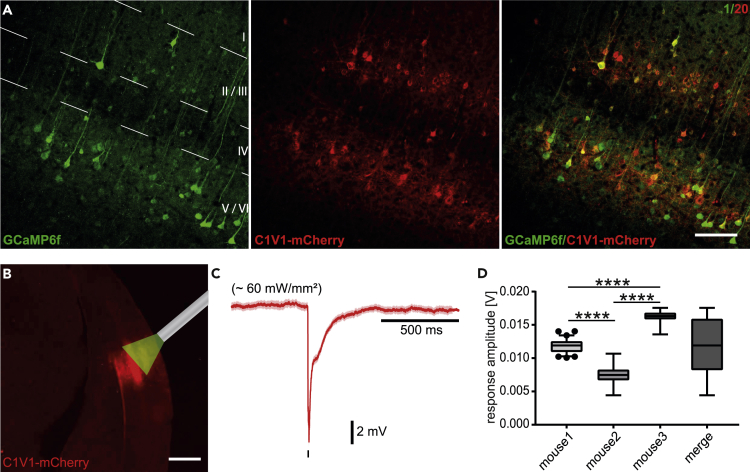
Stable co-expression of C1V1T/T and GCaMP6f could be observed in confocal analysis of the tissue sections employing a final titer of 20:1 for C1V1T/T (3.33 ∗ 1011/mL) and GCaMP6f (1.84 ∗ 1010/mL) (Figures 2A and S4). Using this titer, the density of C1V1T/T-mCherry-positive cells was 1392 ± 97 cells/mm3 in layer II/III and 4336 ± 313 cells/mm3 in layer V/VI. The density of cells co-expressing C1V1T/T-mCherry and GCaMP6f was 735 ± 99 cells/mm3 in layer II/III and 1226 ± 139 cells/mm3 in layer V/VI resulting in a fraction of 52.5 ± 4.4% and 28.3 ± 2.3% of C1V1T/T/GCaMP6f co-expressing cells in layer II/III and in layer V/VI, respectively. Hardly any co-expressing neurons were found in layer IV. We found strong and dense co-expression across targeted cortical layers II/III and V/VI. Note that layer IV seems to be difficult to target with AAVs of serotype I/II, as reported previously (Schmid et al. 2016; Yang et al. 2017).
Figure 2.
Achieving strong co-expression of actuator and indicator and probing functional expression
(A) Confocal micrographs of co-expression pattern in the primary visual cortex. Left: green channel at 488 nm. Middle: red channel at 584 nm. Right: overlay. Scale bar: 75 μm.
(B) Epifluorescence micrograph of typical C1V1T/T-mCherry expression and schematic inset of the optic fiber for 1-P stimulation at 552 nm. Scale bar: 500 μm.
(C) Average LFP response upon optogenetic stimulation at 60 mW/mm2, n = 31 responses.
(D) Average LFP response amplitudes upon stimulations at 60 mW/mm2 (unpaired t: test mouse 1 vs mouse 2 p < 0.0001, Mann-Whitney test: mouse 1 vs mouse 3 p < 0.0001, mouse 2 vs mouse 3 p < 0.0001, n = 31 responses each, 3 mice).
Data are represented as mean ± SEM (standard error of the mean). Significance levels were ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Electrophysiological recordings provide a direct readout of neuronal activity and are therefore well suited to probe functionality of opsin expression and test 2-P excitation paradigms. To ensure functional expression of our optogenetic actuator, we therefore performed easy to implement optic fiber-based 1-P stimulations at 552 nm in combination with local field potential LFP recordings ((Fois et al. 2014), Figures 2B and 2C). Within single animals, we found a stable response pattern with a low variability of response amplitudes at approximately 60 mW/mm2 (Figure 2D). However, between animals amplitudes varied, with an average amplitude of 11.9 ± 0.4 mV. Next, in a proof-of-principle experiment we tested whether our 2-P light source in combination with a raster scan paradigm was capable of eliciting spikes in individual opsin expressing neurons (Figure S3G). Simultaneous juxtacellular recordings demonstrated stable responses upon every trial of single-cell 2-P optogenetic stimulations with a light intensity of 37 mW at 1100 nm (Figures S3H and S3I). We applied a pixel dwell time of 6 μs with a raster width of 0.5 μm. Like this, specific modulations of single neurons were achievable by applying our stimulation approach with a success rate of 100% using light intensities ≥37 mW in 26 consecutive trials (96% at 18 mW, Figure S3M). Latencies decreased with increasing light intensities (Figure S3J), as reported previously (Stroh et al. 2013), while photocurrents increased (Figure S5L). Interestingly, light intensities ≤37 mW only triggered approximately one event whereas light intensities around 60 mW already triggered two events (Figure S3K). This is important to note as for low AP numbers the sensitivity of our peak detection algorithm is decreasing substantially. Thus, we decided to perform our stimulations in imaging experiments at light intensities ≥40 mW. In summary, these quality control and feasibility experiments provide the needed foundation for subsequent all-optical experiments.
Above noise stimulation artifacts of 2-P excitation are wavelength dependent and absent above 1100 nm
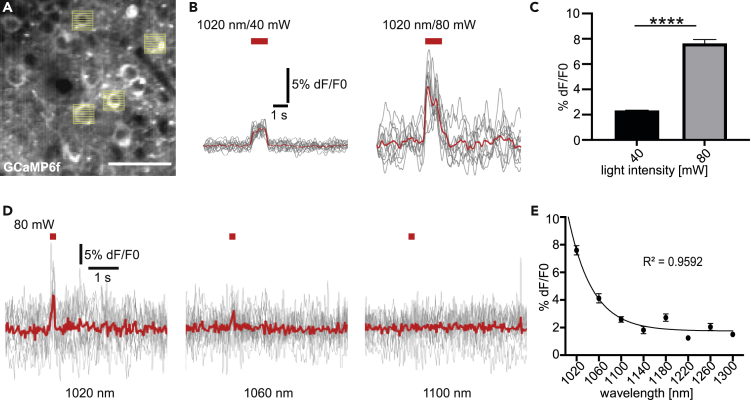
Unlike electrophysiological recordings, optical imaging using genetically encoded calcium indicators does not provide a specific signal per se. Identification of AP-derived calcium transients critically depends on several components of the signal, such as the sharp rise time. An artifact of the stimulation pulse in the GCaMP6f emission channel renders a highly specific identification of AP-related calcium transients difficult. This is of particular concern, if this method is applied in preclinical research, which should provide evidence guiding translation to human studies (Duda et al. 2014; Dirnagl 2016, 2019; Piper et al. 2019). Here, we defined the spectral windows for artifact-free all-optical interrogations by testing different wavelengths for stimulation. To ensure that photostimulation artifacts were in fact no mistaken calcium transients, we performed our stimulation paradigm on mice which were lacking C1V1 expression and solely expressed GCaMP6f (Figure 3A). Notably, applying stimulation wavelengths and power used for all-optical experiments (Packer et al. 2015; Yang et al., 2018a; Carrillo-Reid et al. 2019; Marshel et al., 2019), we observed a significant artifact in the calcium trace when stimulating at 1020 nm, already at 40 mW power (Figure 3B). Increasing power levels to 80 mW which increases the efficacy of stimulation led to even more pronounced artifacts (Figures 3B and 3C). Note that this artifact displayed a sharp rise time and an amplitude well above the noise level so that it might be misinterpreted as an AP-related calcium transient. Also, the amplitude of the artifact varied drastically between trials, rendering automated subtraction of mean artifacts, as conducted in a previous study using single-wavelength all-optical physiology, obsolete (Schmid et al. 2016). To define the spectral range for artifact-free all-optical physiology, we now increased the stimulation wavelength step by step. We found that the amplitude of the averaged and normalized artifact decreased with increasing wavelength (Figure 3D). At 1060 nm, again, often used in ytterbium-laser-based 2-P optogenetics, an artifact was still clearly visible beyond noise level (Figures 3D and 3E). Above 1100 nm, the amplitude of the stimulation artifact was greatly reduced and within the noise level (Figures 3D and 3E). Above 1100 nm, the artifact amplitude stayed approximately constant in the tested wavelength spectrum up to 1300 nm. In summary, it has to be stated that the artifacts induced by photostimulation were highly heterogeneous in shape and amplitude. This might be explained by unintended excitation of GCaMP's fluorescent core eGFP and varies with varying expression levels of the reporter and varying levels of autofluorescence. We suggest using wavelengths of 1100 nm or higher for artifact-free all-optical physiology.
Figure 3.
Stimulation artifact of 2-P excitation is wavelength dependent and absent above 1100 nm
(A) In vivo 2-P calcium imaging of GCaMP6f expressing neurons in layer II/III of the mouse visual cortex and simultaneous raster scan stimulations of selected cells at different wavelengths and light intensities. Please note the photostimulation artifact (white vertical lines). Scale bar: 50 μm.
(B) Average artifacts (red line, n = 10 artifacts, single trials are depicted in gray) in calcium traces of selected neurons upon 40 and 80 mW raster scan stimulations at 1020 nm. The amplitude of the artifact is increasing with increasing stimulation light power.
(C) Quantification of artifact amplitude at 40 and 80 mW. Mann-Whitney test: 40 mW vs 80 mW p < 0.0001. 40 mW n = 1192 trials, 3 mice, 80 mW n = 790 trials, 5 mice.
(D) Averaged artifact (red lines, n = 10 artifacts, single trials are depicted in gray) upon 2-P raster scan stimulations (80 mW) at varying stimulation wavelengths (1020, 1060, and 1100 nm).
(E) Quantification of artifact amplitudes at varying stimulation wavelengths at 80 mW light intensity. Average artifact amplitudes are decreasing with increasing wavelength. Please note that the stimulation artifact ranges within the noise level beyond 1100 nm. Non-linear regression with a monoexponential function (R2 = 0.96) (733–788 artifacts/wavelength, n = 5 mice).
Data are represented as mean ± SEM. Significance levels were ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Optimal wavelength for effective and cross talk-free optogenetic control in C1V1/GCaMP6f-based experimental approaches is 1100 nm
Finally, we conducted in vivo experiments in animals expressing high and functional levels of both opsin and indicator in the lightly anesthetized mouse. First, we performed GCaMP6f imaging only. We assessed simultaneously the full-field activity of the local ensemble of neurons. We set the anesthetic depth in a way that persistent, desynchronized population activity could be observed (Figures S4A and S4B). Deeper anesthesia will result in slow-wave-brain state, which is characterized by large amplitude oscillations (Figures S4A and S4B). This brain state would not be suitable for probing the excitability and connectivity of each neuron, as the excitability of the respective neuron would be governed by the current phase of the population-wide oscillation (Schwalm et al., 2017). In persistent brain state, we next carried out cell-specific interventions on genetically defined C1V1T/T-expressing neurons using 2-P-based optogenetic stimulation. We carried out sequential stimulations of individual cells at the artifact-free wavelength of 1100 nm using aforementioned raster scans while simultaneously imaging GCaMP6f fluorescence at 920 nm (Figure 4A). The performed stimulation paradigm robustly elicited putatively AP-related GCaMP6f transients in stimulated cells, applying the highly specific detection algorithm implemented above at different power (210 mW: Figure 4B; 80 mW: Figure S4C). We now asked whether increasing stimulation wavelengths beyond 1100 nm, not being possible with standard Ti:Sa or ytterbium lasers, will lead to an increased efficacy. As stated above, the early studies in the field revealed a linear increase in photocurrents, until the technical limit of 1040 nm (Prakash et al. 2012). It has to be noted, however, that these studies were conducted in slice preparations, with a drastically different inhibitory tone. As the main goal of this methodological pipeline is testing the limitations and prospects of 2-P all-optical physiology in preclinical in vivo applications, we believe that measuring the capability of evoking detectable and specific AP-associated calcium transients represents the most appropriate avenue. Surprisingly, we found that a further increase of the stimulation wavelength from 1100 up to 1300 nm did not yield a significant increase in the rate of evoked calcium transients (Figure 4C), as well as no significant increase in the fraction of responding cells (Figure 4D). However, increasing stimulation power from rather low values ranging at 40 or 80 mW up to values ranging at 120 or 210 mW significantly increased the rate of evoked transients 2–3 fold (Figure 4E, 26.7 ± 6.7% at 40 mW, 21.6 ± 3.5% at 80 mW, 56.7 ± 13.1% at 120 mW, 76.3 ± 7.3% at 210 mW).
Figure 4.
The optimal wavelength for effective and cross talk-free optogenetic control is 1100 nm
(A) All-optical control of individual GCaMP6f (green)/C1V1T/T (red) co-expressing neurons in layer II/III of mouse visual cortex. Depiction of raster scan patterns as described above. Six individual neurons can be targeted for sequential photostimulation. Scale bar: 50 μm.
(B) 2-P stimulation (indicated by red marker) of GCaMP6f/C1V1T/T co-expressing neurons at 1100 nm. GCaMP6f calcium transients of four co-expressing neurons of ten stimulation trials.
(C) Average fraction of responding cells at varying wavelengths at 80 mW.
(D) Average rate of evoked calcium transients at varying wavelengths at 80 mW (n = 27 neurons [10 trials each], 3 mice). One-way ANOVA nonparametric test, p = 0.68 (C), p = 0.74 (D).
(E) Average rate of evoked transients at stimulation wavelength of 1100 nm, at varying light intensities (40, 80, 120, and 210 mW). n = 26 neurons each, 3 mice. Unpaired t test, 40 mW vs 80 mW (p = 0.46), 40 mW vs 120 mW (p = 0.012), 40 mW vs 210 mW (p = 0.0039), 80 mW vs 120 mW (p = 0.012), 80 mW vs 210 mW (p < 0.0001), 120 mW vs 210 mW (p = 0.19).
Data are represented as mean ± SEM. Significance levels were ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Discussion
In this study, we provide a methodological and conceptional framework for a highly specific all-optical physiology approach. For that, challenges had to be overcome in devising a light source for spectrally independent imaging and optogenetics. Here, we used a dual-scanner approach, which is well suited for establishing this framework. In our view, holographic or combined holographic and scanning approaches based on spatial light modulators (Packer et al. 2015; Carrillo-Reid et al. 2016; Yang et al., 2018a; Carrillo-Reid et al. 2019; Marshel et al., 2019), temporal focusing (Rickgauer et al. 2014; Chen et al. 2019), or new techniques such as 3D-SHOT (Mardinly et al. 2018) are superior to scanning approaches as used in this study as they allow temporally uncoupled, parallel manipulation and imaging of a multitude of cells in three dimensions. However, holography-based systems are typically set at defined stimulation wavelengths as they are often used with fixed-wavelength ytterbium lasers, which do not allow for flexible exploration of spectral windows for effective and artifact-free optogenetic control. Therefore, a 2-P scanner-based solution as applied here might still be advantageous at least for methodological ground work in the field of all-optical physiology. It has to be noted that, in terms of light sources, novel designs providing two independently tunable femtosecond-pulsed laser beams with extended wavelength spectrum could be introduced in a CGH-based setups as well, thereby increasing flexibility in contrast to fixed-wavelength lasers.
We commenced our framework with setting the specificity to detect underlying APs of our detection algorithm to 100% by correlating our optical data to the actual electrical activity of a neuron. This allowed us to completely avoid type I errors, i.e., mistaking movement- or stimulation light-induced artifacts with calcium transients devoid of an electrophysiological correlate and therefore contributing to false-positive detection of pathological activity patterns (e.g. early cortical hyperactivity) in a healthy brain. In our recent studies, we already applied a rigorous analysis routine to 2-P imaging data (Figure S4D). And indeed, this provided an improved platform for testing the efficacy of drug treatments (Arnoux et al., 2018; Ellwardt et al. 2018). Here, we extend this greatly conservative approach to all-optical physiology. Of course, this comes at cost of sensitivity of activity detection, especially of low numbers of APs. It has to be noted that the initial proof-of-principle publications on new indicators, e.g., on GCaMP6f (Chen et al. 2013) do provide sensitivity but no specificity data. Certainly, also in our setup, we did identify calcium deflections upon single APs, but these could not be unambiguously separated from calcium deflections of non-physiological sources. Deconvolution-based algorithms, such as OASIS, may be superior in terms of sensitivity, and indeed, using this approach, the identification of single-AP-related calcium transients is feasible. Yet, it comes at a price of overall reduced specificity, particularly in noisy data (please see also (Stringer et al. 2019)). Conventional threshold-based algorithms, such as ours, might be advantageous particularly in cases, in which no ground truth detection exists. In addition, the specificity of these approaches is compromised by systematic artifacts induced by photostimulation. We here demonstrate that the amplitude of these artifacts can be in the range of the amplitude of calcium transients and define the spectral range for artifact-free all-optical experiments. On the basis of this finding, we cannot recommend automated analysis algorithms, which merely correlate normalized fluorescence values with other physiological parameters or the behavioral context and strongly suggest that a detection algorithm should be based on an event detection, i.e., the optical correlate of a neuronal action potential. Indeed, several elegant approaches take these characteristic dynamics into account, such as the peeling algorithm (Greenberg et al. 2014). But, ultimately, given the high variability of technical setups, expression levels and brain areas investigated, we still suggest employing single-cell electrophysiology to titrate each detection algorithm, as exemplified in this study. Of course, in 2-P all-optical experiments, these artifacts are less dramatic than in 1-P excitation (Schmid et al. 2016). However, also in 2-P excitation, these photostimulation artifacts still cause significant problems (Rickgauer et al. 2014; Carrillo-Reid et al. 2016; Forli et al. 2018; Mardinly et al. 2018; Chen et al. 2019; Marshel et al., 2019) and might resemble AP-related calcium transients (Carrillo-Reid et al. 2019), especially when high light intensities are applied. Up to now, this problem was addressed using three main approaches: (1) sacrificing pixels containing the artifact resulting in data loss (Chen et al. 2019; Marshel et al., 2019), (2) post hoc identification based on the temporal dynamics of the artifact in contrast to the AP-related transients (Rickgauer et al. 2014; Carrillo-Reid et al. 2016), which in our view is amenable only in data with highest signal-to-noise ratio, not reflecting the reality particularly in awake measurements, and (3) gating stimulation and recording lasers, reducing effective field of view (Mardinly et al. 2018). In contrast to the aforementioned studies, one study used a different configuration combining a blue-shifted opsin with a red-shifted indicator (Forli et al. 2018). However, also here, a photostimulation artifact was apparent, most likely due to the fact that the indicator was excited on his blue shoulder by the strong stimulation light and was dealt with by subtracting background or blanking a short period in the calcium trace (Forli et al. 2018). One further, major problem which cannot be addressed with any of these methods and is independent of the artifact amplitude is the following one: The photostimulation artifact will inevitably make it impossible to determine latencies of responses and will therefore hinder precise timing of evoked spikes as the exact onset of a transient will be hidden within the artifact.
Here, we provide evidence that at stimulation wavelengths of 1100 nm and above, stimulation artifacts range below noise level. This frees the researcher from the need for any post hoc and specificity-reducing methods of artifact minimization and opens up the use of higher stimulation light intensities, e.g., to overcome strong inhibitory tones, as reducing light intensities to minimize artifacts will necessarily reduce photocurrents (Rickgauer et al. 2014; Mardinly et al. 2018; Chen et al. 2019; Marshel et al., 2019). Indeed, increasing stimulation light intensities at the optimal stimulation wavelength 1100 nm significantly increased cellular response rates. And above all, stimulation at 1100 nm will still allow the exact determination of response latencies. On the aspect of spurious activation of the opsin by the light used for exciting the indicator: This type of cross talk is actually a far more significant problem for 1-P stimulation of opsins and indicators, i.e., 1-P all-optical physiology. We conducted a study on this earlier, finding a two-order-of magnitude safety margin when using low yet efficient light intensities (Adelsberger et al. 2014). Efficient 2-P excitation of opsin is typically only effective at pixel dwell times of 6 μs and beyond, at least for the currently used opsins (Prakash et al. 2012). The current state of the art in 2-P calcium imaging uses resonant mirrors, yielding temporal resolutions of 30 Hz, and pixel dwell times of 1 μs or less, or inertia-free AOM (Acousto-optic modulator)-based systems with even higher frequencies and shorter pixel dwell times. Also, the wavelength used for excitation of GCaMP6f is well outside of the efficient excitation peak. And indeed, three recent studies did not find any significant spurious excitation of the opsin by the illumination light used for imaging, at least for light intensities of 40 mW and below. Mardinly and colleagues (Mardinly et al. 2018) explored the spurious activation of the newly designed soma-targeted opsin ST-ChroME while Ronzitti and colleagues (Ronzitti et al. 2017) used Chronos. We would like to emphasize that, Rickgauer et al. (Rickgauer et al. 2014) employed C1V1 as in this study and found that with an imaging power of 30–40 mW, this type of cross talk was negligible. Here, in our study we used even lower light intensities for imaging (10–30 mW).
All-optical physiology approaches using 2-P light sources for stimulation and imaging do not only face obstacles such as cross talk between indicator and opsin or limited efficacy of optogenetic stimulation, but the field needs to address putative heat-related side effects, which might impact neuronal physiology. This is particularly relevant for high stimulation laser powers, as used both in holographic and line-scanning-based 2-P optogenetic stimulation schemes. Chen et al. (Chen et al. 2019) and Mardinly et al. (Mardinly et al. 2018) conducted an assessment of heating-related artifacts in a CGH approach. In terms of line-scanning-based approaches, as conducted here, putative heat effects critically depend on the pixel dwell time, light power and wavelength. Earlier studies using wavelength of 920 nm, as employed for the excitation of the indicator, did not reveal effects on neuronal firing rate, confirmed by our own results here (Stujenske et al. 2015; Podgorski and Ranganathan 2016). Longer wavelengths as used for the stimulation of opsins should even reduce heat-related effects, at least only considering the effect of the wavelength (Stujenske et al. 2015; Podgorski and Ranganathan 2016). However, pixel dwell times in imaging schemes with resonant scanners range around 1 μs, while for optogenetics, pixel dwell times typically exceed 6 μs (Packer et al. 2015). Yet a designated study addressing heat-related effects using longer wavelength line scanning optogenetic schemes is clearly desirable.
Finally, we addressed an open question of the field in terms of the spectral efficacy of opsin activation beyond 1100 nm. As aforementioned, in vitro whole-cell electrophysiology measurements found an increase in photocurrents with increasing wavelengths up to 1040 nm (Prakash et al. 2012). Here, albeit only using C1V1 in in vivo 2-P calcium imaging experiments, we did not find a further significant increase in spectral efficacy beyond 1100 nm. However, this might be different for other 2-P excitable opsins.
The still new and evolving field of 2-P all-optical physiology opens up tremendous prospects (Iaccarino et al. 2018; Martorell et al. 2019), but we suggest a careful and holistic approach including a pipeline for further exploratory research as devised here in order to maximize translational power. In sum, for minimizing the artifact, stimulation wavelengths of 1100 nm and above are advisable, albeit beyond 1100 nm no further reduction became apparent. Efficacy for opsin stimulation did not exceed above 1100 nm, albeit a larger spectral separation could be beneficial, depending on the opsin-indicator pair used. For GCaMP6f, imaging wavelength of 920 nm seems to be optimal, combined with C1V1-based optogenetics at wavelength of 1100 nm, an efficacy beyond 70% is well achievable for light intensities between 150 and 200 mW. Note that all these parameters will critically depend on the imaging setup and also require tailored expression strategies, optimizing the entire pipeline (Stroh 2018).
Limitations of the study
In this study, we probed the indicator-opsin pair GCaMP6f/C1V1, as this combination seemed to be mostly used in the field (Rickgauer et al. 2014; Packer et al. 2015; Carrillo-Reid et al. 2016; Yang et al., 2018a; Zhang et al. 2018; Carrillo-Reid et al. 2019). However, the development of new opsin and indicator variants has advanced (Ronzitti et al. 2016; Mardinly et al. 2018; Chen et al. 2019; Marshel et al., 2019), and for newer opsin-indicator couples, different stimulation or imaging parameters might be optimal. Recent developments of new members of the family such as GCaMP7 or 8 might display even higher sensitivity toward single action potentials (Dana et al., 2019; Zhang et al., 2020), albeit as they are also based on the same or similar fluorophore, the same behavior concerning the wavelength dependency of the artifact can be expected. What is more, also somatic targeting of the opsin (Mardinly et al., 2018) might increase the efficacy of 2-P optogenetic stimulation, yet it will not change the wavelength dependency, provided the same opsin is used. Furthermore, as mentioned above, our approach has important technical limitations compared to CGH-based all-optical experiments and is currently limited to one plane for imaging and stimulation.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be responded to by the lead contact, Prof Albrecht Stroh (albrecht.stroh@unimedizin-mainz.de)
Material availability
This study did not generate new unique reagents.
Data and code availability
All data associated with study will be made available upon request. Original 2P cacium imaging data analysis have been deposited to Mendeley Data: https://doi.org/10.17632/v36fvgzzn2.1. The code for analysis of calcium imaging is available at: https://github.com/Strohlab.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Nicolas Ruffini for support in analyzing the photostimulation artifact, Nico Bürger for viral injections, and Marcel Kegel for technical consulting. This study was supported by the German Research Council (DFG CRC 1193) and the German Federal Ministry of Education and Research (BMBF Eurostars E! 10324 FEMSCOPY), and the Böhringer-Ingelheim Foundation.
Author contributions
T.F., I.A., and J.D. are equally contributing first authors. A.S. designed the study. T.F. performed calcium imaging, all-optical experiments, and virus titration. I.A. and H.W. performed cell-attached recordings combined with calcium imaging and optogenetic stimulation. J.D. performed LFP experiments combined with optic fiber stimulations. T.F., I.A., J.D., H.B., and H.W. performed the imaging analysis. I.A. and J.D. performed the analysis of in vivo electrophysiology. J.D. performed confocal analysis. I.S. performed PSF measurements. A.S., J.D., T.F., I.A., H.W., and I. S. wrote the manuscript.
Declaration of interests
I.S. is an employee of Light Conversion Ltd., the producer of one of the lasers used in this study.
Published: March 19, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2021.102184.
Supplemental information
References
- Adelsberger H., Grienberger C., Stroh A., Konnerth A. In vivo calcium recordings and channelrhodopsin-2 activation through an optical fiber. Cold Spring Harb Protoc. 2014;2014 doi: 10.1101/pdb.prot084145. pdb prot084145. [DOI] [PubMed] [Google Scholar]
- Akerboom J., Chen T.W., Wardill T.J., Tian L., Marvin J.S., Mutlu S., Calderon N.C., Esposti F., Borghuis B.G., Sun X.R. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoux I., Willam M., Griesche N., Krummeich J., Watari H., Offermann N., Weber S., Narayan Dey P., Chen C., Monteiro O. Metformin reverses early cortical network dysfunction and behavior changes in Huntington’s disease. Elife. 2018;7:e38744. doi: 10.7554/eLife.38744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer D.F., Kreuz S., Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche M.A., Chen X., Henning H.A., Reichwald J., Staufenbiel M., Sakmann B., Konnerth A. Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reid L., Han S., Yang W., Akrouh A., Yuste R. Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell. 2019;178:447–457.e5. doi: 10.1016/j.cell.2019.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reid L., Yang W., Bando Y., Peterka D.S., Yuste R. Imprinting and recalling cortical ensembles. Science. 2016;353:691–694. doi: 10.1126/science.aaf7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.W., Ronzitti E., Lee B.R., Daigle T.L., Dalkara D., Zeng H., Emiliani V., Papagiakoumou E. In vivo submillisecond two-photon optogenetics with temporally focused patterned light. J. Neurosci. 2019;39:3484–3497. doi: 10.1523/JNEUROSCI.1785-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H., Sun Y., Mohar B., Hulse B.K., Kerlin A.M., Hasseman J.P., Tsegaye G., Tsang A., Wong A., Patel R. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods. 2019;16:649–657. doi: 10.1038/s41592-019-0435-6. [DOI] [PubMed] [Google Scholar]
- Dirnagl U. Thomas Willis lecture: is translational stroke research broken, and if so, how can we fix it? Stroke. 2016;47:2148–2153. doi: 10.1161/STROKEAHA.116.013244. [DOI] [PubMed] [Google Scholar]
- Dirnagl U. Rethinking research reproducibility. EMBO J. 2019;38:e101117. doi: 10.15252/embj.2018101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda G.N., Grainger D.W., Frisk M.L., Bruckner-Tuderman L., Carr A., Dirnagl U., Einhaupl K.M., Gottschalk S., Gruskin E., Huber C. Changing the mindset in life sciences toward translation: a consensus. Sci. Transl. Med. 2014;6:264cm212. doi: 10.1126/scitranslmed.aaa0599. [DOI] [PubMed] [Google Scholar]
- Ellwardt E., Pramanik G., Luchtman D., Novkovic T., Jubal E.R., Vogt J., Arnoux I., Vogelaar C.F., Mandal S., Schmalz M. Maladaptive cortical hyperactivity upon recovery from experimental autoimmune encephalomyelitis. Nat. Neurosci. 2018;21:1392–1403. doi: 10.1038/s41593-018-0193-2. [DOI] [PubMed] [Google Scholar]
- Emiliani V., Cohen A.E., Deisseroth K., Hausser M. All-optical interrogation of neural circuits. J. Neurosci. 2015;35:13917–13926. doi: 10.1523/JNEUROSCI.2916-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fois C., Prouvot P.H., Stroh A. A roadmap to applying optogenetics in neuroscience. Methods Mol. Biol. 2014;1148:129–147. doi: 10.1007/978-1-4939-0470-9_9. [DOI] [PubMed] [Google Scholar]
- Forli A., Vecchia D., Binini N., Succol F., Bovetti S., Moretti C., Nespoli F., Mahn M., Baker C.A., Bolton M.M. Two-photon bidirectional control and imaging of neuronal excitability with high spatial resolution in vivo. Cell Rep. 2018;22:3087–3098. doi: 10.1016/j.celrep.2018.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D.S., Wallace D.J., Kerr J.N. Imaging neuronal population activity in awake and anesthetized rodents. Cold Spring Harb. Protoc. 2014;2014:912–922. doi: 10.1101/pdb.top083535. [DOI] [PubMed] [Google Scholar]
- Grienberger C., Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Iaccarino H.F., Singer A.C., Martorell A.J., Rudenko A., Gao F., Gillingham T.Z., Mathys H., Seo J., Kritskiy O., Abdurrob F. Author Correction: gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2018;562:E1. doi: 10.1038/s41586-018-0351-4. [DOI] [PubMed] [Google Scholar]
- Mardinly A.R., Oldenburg I.A., Pegard N.C., Sridharan S., Lyall E.H., Chesnov K., Brohawn S.G., Waller L., Adesnik H. Precise multimodal optical control of neural ensemble activity. Nat. Neurosci. 2018;21:881–893. doi: 10.1038/s41593-018-0139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel J.H., Kim Y.S., Machado T.A., Quirin S., Benson B., Kadmon J., Raja C., Chibukhchyan A., Ramakrishnan C., Inoue M. Cortical layer-specific critical dynamics triggering perception. Science. 2019;365:eaaw5202. doi: 10.1126/science.aaw5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell A.J., Paulson A.L., Suk H.J., Abdurrob F., Drummond G.T., Guan W., Young J.Z., Kim D.N., Kritskiy O., Barker S.J. Multi-sensory gamma stimulation ameliorates Alzheimer's-associated pathology and improves cognition. Cell. 2019;177:256–271 e222. doi: 10.1016/j.cell.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer A.M., Peterka D.S., Hirtz J.J., Prakash R., Deisseroth K., Yuste R. Two-photon optogenetics of dendritic spines and neural circuits. Nat. Methods. 2012;9:1202–1205. doi: 10.1038/nmeth.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer A.M., Russell L.E., Dalgleish H.W., Hausser M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat. Methods. 2015;12:140–146. doi: 10.1038/nmeth.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper S.K., Grittner U., Rex A., Riedel N., Fischer F., Nadon R., Siegerink B., Dirnagl U. Exact replication: foundation of science or game of chance? PLoS Biol. 2019;17:e3000188. doi: 10.1371/journal.pbio.3000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski K., Ranganathan G. Brain heating induced by near-infrared lasers during multiphoton microscopy. J. Neurophysiol. 2016;116:1012–1023. doi: 10.1152/jn.00275.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R., Yizhar O., Grewe B., Ramakrishnan C., Wang N., Goshen I., Packer A.M., Peterka D.S., Yuste R., Schnitzer M.J., Deisseroth K. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickgauer J.P., Deisseroth K., Tank D.W. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 2014;17:1816–1824. doi: 10.1038/nn.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort N.L., Garaschuk O., Milos R.I., Narushima M., Marandi N., Pichler B., Kovalchuk Y., Konnerth A. Sparsification of neuronal activity in the visual cortex at eye-opening. Proc. Natl. Acad. Sci. U S A. 2009;106:15049–15054. doi: 10.1073/pnas.0907660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzitti E., Conti R., Papagiakoumou E., Tanese D., Zampini V., Chaigneau E., Foust A.J., Klapoetke N.C., Boyden E.S., Emiliani V. Sub-millisecond optogenetic control of neuronal firing with two-photon holographic photoactivation of Chronos. bioRvix. 2016;37:10679–10689. doi: 10.1523/JNEUROSCI.1246-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzitti E., Conti R., Zampini V., Tanese D., Foust A.J., Klapoetke N., Boyden E.S., Papagiakoumou E., Emiliani V. Submillisecond optogenetic control of neuronal firing with two-photon holographic photoactivation of chronos. J. Neurosci. 2017;37:10679–10689. doi: 10.1523/JNEUROSCI.1246-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid F., Wachsmuth L., Schwalm M., Prouvot P.H., Jubal E.R., Fois C., Pramanik G., Zimmer C., Faber C., Stroh A. Assessing sensory versus optogenetic network activation by combining (o)fMRI with optical Ca2+ recordings. J. Cereb. Blood Flow Metab. 2016;36:1885–1900. doi: 10.1177/0271678X15619428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm M., Schmid F., Wachsmuth L., Backhaus H., Kronfeld A., Aedo Jury F., Prouvot P.H., Fois C., Albers F., van Alst T. Cortex-wide BOLD fMRI activity reflects locally-recorded slow oscillation-associated calcium waves. Elife. 2017;6:e27602. doi: 10.7554/eLife.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stosiek C., Garaschuk O., Holthoff K., Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C., Pachitariu M., Steinmetz N., Reddy C.B., Carandini M., Harris K.D. Spontaneous behaviors drive multidimensional, brainwide activity. Science. 2019;364:255. doi: 10.1126/science.aav7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh A. Humana Press; 2018. Optogenetics: A Roadmap. [Google Scholar]
- Stroh A., Adelsberger H., Groh A., Ruhlmann C., Fischer S., Schierloh A., Deisseroth K., Konnerth A. Making waves: initiation and propagation of corticothalamic Ca2+ waves in vivo. Neuron. 2013;77:1136–1150. doi: 10.1016/j.neuron.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Stujenske J.M., Spellman T., Gordon J.A. Modeling the spatiotemporal dynamics of light and heat propagation for in vivo optogenetics. Cell Rep. 2015;12:525–534. doi: 10.1016/j.celrep.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.W., Prouvot P.H., Reyes-Puerta V., Stuttgen M.C., Stroh A., Luhmann H.J. Optogenetic modulation of a minor fraction of parvalbumin-positive interneurons specifically affects spatiotemporal dynamics of spontaneous and sensory-evoked activity in mouse somatosensory cortex in vivo. Cereb. Cortex. 2017;27:5784–5803. doi: 10.1093/cercor/bhx261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Carrillo-Reid L., Bando Y., Peterka D.S., Yuste R. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. Elife. 2018;7 doi: 10.7554/eLife.32671. e32671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-W., Prouvot P.-H., Stroh A., Luhmann H.J. Combining optogenetics with MEA, depth-resolved LFPs and assessing the scope of optogenetic network modulation. Neuromethods. 2018;133:133–152. SpringerNature (Optogenetics: A Roadmap) [Google Scholar]
- Zhang, Y., M. Rózsa, D. Bushey, J. Zheng, D. Reep, G.J. Broussard, A. Tsang, G. Tsegaye, R. Patel, S. Narayan, et al (2020). jGCaMP8 fast genetically encoded calcium indicators. https://doi.org/10.25378/janelia.13148243.
- Zhang Z., Russell L.E., Packer A.M., Gauld O.M., Hausser M. Closed-loop all-optical interrogation of neural circuits in vivo. Nat. Methods. 2018;15:1037–1040. doi: 10.1038/s41592-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with study will be made available upon request. Original 2P cacium imaging data analysis have been deposited to Mendeley Data: https://doi.org/10.17632/v36fvgzzn2.1. The code for analysis of calcium imaging is available at: https://github.com/Strohlab.