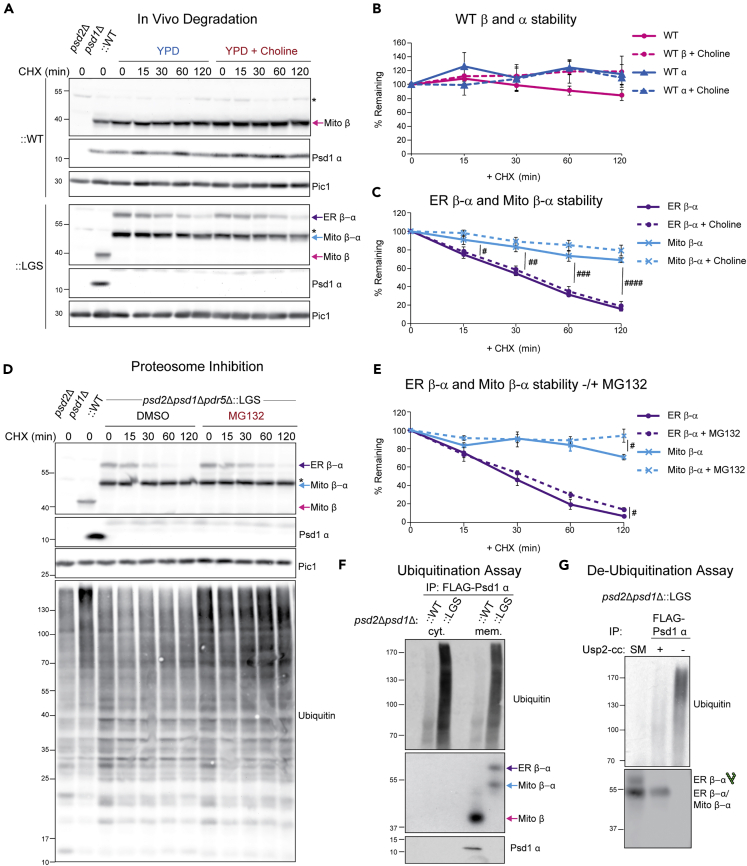
Figure 6.
Glycosylated non-functional mutant Psd1 is short lived and ubiquitinated
(A) In vivo degradation assay. Cell extracts from designated strains were isolated at the indicated times following growth in YPD containing cycloheximide (CHX) only or CHX and choline. Samples were resolved by SDS-PAGE and immunoblotted as indicated (n = 5). ∗, nonspecific bands.
(B) The percentages of WT α and β subunits remaining at each time point were quantified (mean ± SEM for n = 5).
(C) The percentages of nonfunctional glycosylated ER β-α and mitochondrial β-α remaining at each time point were quantified (mean ± SEM for n = 5). Statistical differences (1 symbol p ≤ 0.05; 2 symbols p ≤ 0.01; 3 symbols p ≤ 0.001; 4 symbols p ≤ 0.0001) between ER β-α and Mito β-α were determined at each time point by unpaired student t-test.
(D) An overnight YPD culture of the psd2Δpsd1Δpdr5Δ:LGS strain was resuspended in SCD with 2mM choline and further spiked with vehicle (DMSO) or the proteosomal inhibitor MG132. After a 1hr incubation at 30°C, CHX was added and cell extracts harvested following growth at 30°C for the indicated times. Samples were resolved by SDS-PAGE and immunoblotted as indicated (n = 5).
(E) The percentages of nonfunctional glycosylated ER β-α and mitochondrial β-α remaining at each time point were quantified (mean ± SEM for n = 5). Statistical differences (1 symbol p ≤ 0.05) between vehicle and MG132 treated samples for ER β-α and Mito β-α were determined at each time point by unpaired Student's t-test.
(F) In vivo ubiquitination assay. Crude lysate was prepared from the indicated strains and lysates were ultracentrifuged into soluble, cytosolic (cyt.) and membrane (mem.) fractions, the latter of which was solubilized with digitonin. FLAG-tagged WT and mutant Psd1 were immunoprecipitated from the soluble and digitonin-extracted membrane fractions and recovered Mito β, Mito α, ER β-α, and Mito β-α detected by immunoblot using 4,077.4 antisera; ubiquitin antibody detected ubiquitination (n = 3).
(G) In vivo re-translocation assay. Ubiquitin removal with Usp2Core, quenched with SUME, immunoprecipitated with anti-FLAG resin, and immunoblotted for Psd1 β and ubiquitin. SM, starting material before anti-FLAG immunoprecipitation (n = 3).