INTRODUCTION
Women have consistently been found to have higher levels of total body adiposity than men.1 Women preferentially deposit fat subcutaneously with greater accumulation in the gluteofemoral region. This distribution of fat may provide a buffer for fat storage during periods of positive energy balance and improve glucose metabolism, partially protecting against the development of type 2 diabetes in premenopausal women.2 In contrast, men tend to accumulate fat in the abdominal region, in the visceral compartment, where it contributes to an increased risk for metabolic disease.3 These differences in total lipid storage may have evolved to favor the energy needs of reproduction and lactation in women and suggest fundamental differences in the handling of metabolic fuels by the two sexes. Although these sex-based differences in fat distribution could be related to genetics, the fact that these differences first appear at the onset of puberty and become less pronounced after the menopause in women or in association with declining testosterone levels in men suggest that sex steroids play a central role.4 Studies done in human participants with sex steroid insufficiency occurring naturally or by pharmacologic suppression with our without hormone replacement support this idea. Specifically, although the increase in total fat mass seen in women with the menopausal transition is due in part to advancing age, fat redistribution away from peripheral subcutaneous depots to visceral depots seems to be specifically related to estrogen deficiency.5 Women who receive post-menopausal hormone therapy see an age-adjusted decrease in visceral fat.6 Men with Klinefelter syndrome not on testosterone replacement therapy have increased levels of total body fat that decreases with testosterone replacement, although hormone replacement therapy is associated with an increase in intra-abdominal fat content.7 Studies done in transsexual individuals using gender-affirming hormone therapy show that estrogen use by male-to-female transsexuals is associated with in an increase in total body fat with relatively less fat accumulating in the visceral depot. In contrast, administration of testosterone to female-to-male transsexual individuals results in a reduction in subcutaneous fat area on MRI with a modest increase in visceral fat area.8,9 The importance of sex steroids in adipose tissue metabolism and regional adiposity has been studied in vitro using isolated adipocytes, in studies of animals after gonadectomy with or without hormone replacement, and in studies of mice with genetic manipulations of relevant hormone signaling systems.10 Although energy intake and energy expenditure, including energy expended in habitual physical activity, play critical roles in determining differences in total body adiposity between men and women, even these variables seem to be subject to regulation by sex steroids.11,12 Taken together, these studies strongly support a central role of sex steroids in modifying regional adipose tissue biology, although the details of how these hormones regulate fat mass and distribution are complex.
HOW DOES ONE FAT DEPOT EXPAND RELATIVE TO OTHERS?
The mechanisms determining body fat patterning are not completely understood. Fig. 1 outlines some of the mechanisms that could be involved in the relative expansion of one adipose tissue depot relative to another. The generation of new fat cells (adipogenesis) is necessary across the lifespan to both accommodate expansion of the adipose tissue organ owing to energy surplus, and to maintain the regular turnover of the adipocyte pool, which occurs at a rate of approximately 10% per year.13 When responding to energy surplus, adipose tissue can expand by hypertrophy (increase in cell size) or hyperplasia (increase in cell number). The role of each of these processes in adipose tissue expansion seems to differ by depot (hypertrophy being characteristic of the abdominal depot and hyperplasia of femoral14) and sex (hypertrophy characteristic of adipose tissue in men and hyperplasia in women15), although baseline adipocyte size when weight gain occurs is also of importance.14 Rates of apoptosis of mature adipocytes also affect the total number of adipocytes in a particular depot. Limitations of current methods for measuring adipocyte production and death in vivo in humans has impeded research in this area, although the use of the stable isotope deuterium (2H) labeling technique has made in vivo studies possible.16,17
Fig. 1.
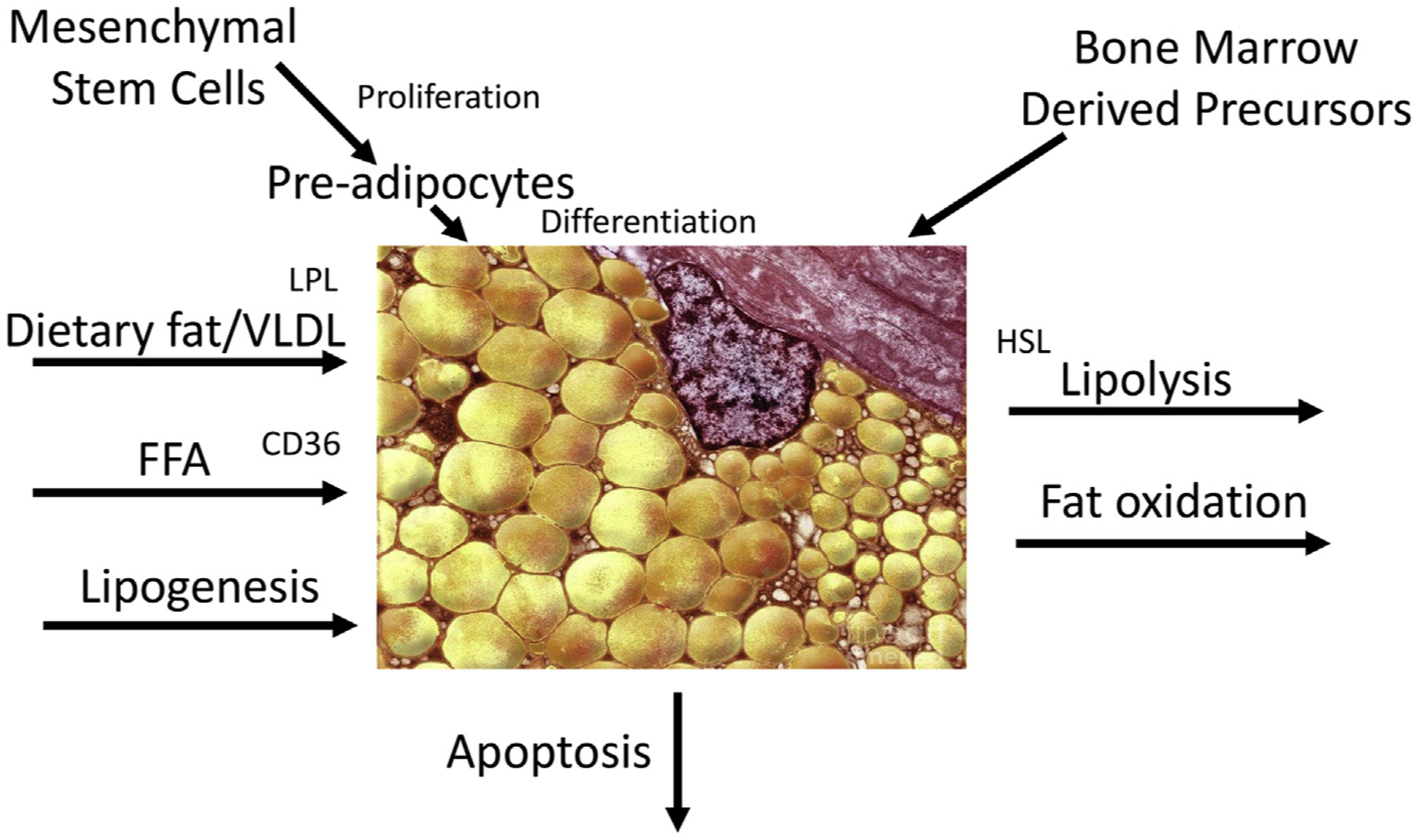
Pathways the influence the accumulation of fat in an adipose tissue depot. CD36, cluster of differentiation 36; HSL, hormone-sensitive lipase.
Changes in the size of an adipose tissue depot through hypertrophy also depends on the net delivery of lipid (uptake of free fatty acids [FFA], dietary fat, or very low-density lipoprotein [VLDL]) and lipogenesis as balanced against the loss of lipid through lipolysis (basal or hormone stimulated) and fat oxidation. Extensive studies of the effects of sex steroids on these processes have been performed in an effort to understand the sexual dimorphism in regional adiposity.
ADIPOCYTE PRODUCTION AND TURNOVER
Sex Differences in Adipogenesis
Adipose tissue in males is characterized by more adipocyte hypertrophy, whereas females demonstrate more hyperplasia.14,15 Tchoukalova and coworkers14 found that women had a greater fraction of stromal vascular cells that were early differentiated adipocytes compared with men, particularly in the femoral depot.18 They additionally found a tendency for preadipocytes from the femoral region of women to be less susceptible to apoptosis compared with subcutaneous abdominal preadipocytes. Additionally, in vivo studies in overweight and obese premenopausal women suggest that subcutaneous femoral adipose tissue has a higher capacity for adipogenesis compared with abdominal adipose tissue.19
Effects of Estrogen and Testosterone on Adipocyte Development
Estrogen
Human adipocyte precursors exposed to estradiol (E2) in culture consistently demonstrate increased replication and proliferation.20 This effect is mediated by estrogen receptors and varies with the estrogen receptor content of the adipose tissue being studied. In vivo data also support the notion that estrogen promotes adipocyte precursor proliferation in both visceral and gluteofemoral regions.18 This finding is consistent with the overall effects of estrogen to promote adipose tissue accumulation. However, the effects are complex, with evidence also showing E2 to be a negative regulator of adipogenesis.21,22 The effect of E2 seems to depend on the stage of adipocyte development considered. Estrogens seem to be more effective in promoting the proliferation of adipocyte precursors in women compared with men, although this sex difference is not observed in vitro suggesting an important role for features of the local adipose tissue environment that have yet to be defined.23,24
Testosterone
Androgens seem to have a more consistent effect on adipocyte development than estrogens. In vivo and in vitro studies demonstrate decreased preadipocyte proliferation in testosterone replete conditions and testosterone incubation seems to inhibit commitment and differentiation of adipocyte precursors from men.25–27 These results are consistent with the overall effect of testosterone to reduce adipose tissue proliferation. These effects are mediated through androgen receptors and downstream effects on IGF-1 and PPAR-γ2. Recent data suggest that the suppressive effects of testosterone on preadipocyte differentiation may depend on intermediate effects on macrophage polarization.28
Although androgens have been implicated as having an important role altering adipocyte development, increases in fat mass in male murine and human models of disruption of estrogen signaling and estrogen deficiency implicate estrogens in mediating some effects of androgens on fat after their aromatization to E2.29–31
Bone Marrow-Derived Adipocytes
Recent evidence suggests that some adipocyte precursors come from the bone marrow in both rodents and humans.32 These precursor cells seem to be preferentially directed toward visceral adipose tissue depots. In rodents, ovariectomy results in an increase in bone marrow-derived adipocytes in gonadal (visceral) fat as compared with control animals and this increase was prevented with estrogen replacement.33
Brown Adipose Tissue
Although controversial, there is increasing interest in the possible role of brown adipose tissue (BAT) in weight regulation in humans. There is evidence that sex steroids play a role in the function of BAT.12 In both rodents and humans, females have more BAT activity than males.34,35 Experimental studies support the notion that estrogens promote UCP1 expression, whereas testosterone decreases it.36 In female rats, ovariectomy result in a reduction in UCP1 expression that is restored with estrogen treatment.37
Potential Effects of Follicle-Stimulating Hormone
As women transition through menopause, E2 levels decrease and follicle-stimulating hormone increases. A recent study by Liu and colleagues38 found that administration of an antibody to follicle-stimulating hormone that blocked the interaction of the hormone with its cognate receptor protected both male and female mice against high fat diet induced obesity. A similar effect was observed in mice that were genetically modified to not express the cognate receptor of follicle-stimulating hormone. In these studies, there was an increase total energy expenditure and UCP1 expression and evidence for “beiging” of white adipose tissue. It is not clear why food intake did not increase in response to increased energy expenditure, but these results raise the question as to whether some of the increase in visceral adipose tissue observed in postmenopausal women could be due in part to increases in follicle-stimulating hormone and not solely due to decreases in E2.39
LIPID METABOLISM
Upper body (subcutaneous and visceral) and lower body fat depots show distinct properties in the uptake of fatty acids derived from circulating triglycerides (Tg) and FFA as well as the rates and net amounts of the release of FFA through lipolysis. Investigators have hypothesized that differences in the rate of lipid uptake and release may be responsible for the depot-specific characteristics of adipose tissue. A large number of studies over the last 30 years have examined these pathways. The results of these studies have not always been consistent nor have they supported the importance of a single pathway in explaining the observed differences in body fat distribution. Studies in this area face a number of design challenges. The processes under study are dynamic and differences in the pathways studied may be most relevant under specific circumstances (eg, during puberty, during exercise, or after overfeeding). Direct studies of visceral fat metabolism in humans are quite difficult and obtaining samples of adipose tissue from this depot in humans is rare. Data can be expressed per gram of fat, which is relevant for understanding the cellular mechanisms, or at a whole depot level, which may be more relevant for whole body metabolism. In vitro studies can provide control of experimental conditions, but may remove relevant local effectors present in vivo (e.g., local levels of estrogen or cortisol,40,41 local sympathetic nerve activity,42 or local adenosine levels). A number of reviews have highlighted the results of these studies.11,23,43 What emerges is a picture of great complexity, but one where sex and sex steroids clearly play important roles.
The Uptake and Storage of Triglyceride-Derived Fatty Acids
Dietary fat is an important source of the lipid stored in adipose tissue. The rate-limiting step in the uptake of dietary fat carried in chylomicron particles (and VLDL) is thought to be lipoprotein lipase (LPL) that is made by and acts locally in adipose tissue (ATLPL). ATLPL content is increased by insulin and so, ideally, measures would be taken in both fasted and fed states. The uptake of dietary fat by adipose tissue has been examined using test meals that contain a dietary fat tracer. Tissues can then be sampled at some interval after ingestion. The specific results may depend on what time point is selected because lipid within adipose tissue is constantly turning over. Sex hormones may have a role in modulating LPL expression and activity as well as direct Tg-derived fatty acid uptake.
Sex differences in the uptake and storage of triglyceride-derived fatty acids
Meal fat studies in rats44 and humans45 have not shown marked sex based differences in the uptake of dietary fat by adipose tissue. Several of these studies find more dietary fat being stored in the upper body as compared with the lower body fat in both sexes.46,47 However, when participants were overfed a high-fat meal, women stored more fat in lower body adipose tissue as compared with men.48 Furthermore, women with lower body obesity stored more dietary fat per gram of adipose tissue in the gluteal as compared with the abdominal region, whereas men with upper body obesity stored less dietary fat in subcutaneous depots as compared with women.49
Effects of estrogens and testosterone on the uptake and storage of triglyceride-derived fatty acids
Estrogen
Santosa and Jensen50 found that dietary fat uptake was greater in the femoral depot in premenopausal women as compared with postmenopausal, although no group differences in ATLPL were evident. In a different study, estrogen decreased LPL and Tg accumulation in cultured adipocytes.51 These investigators were unable to find an estrogen response element in the LPL gene and thus concluded that the effect was indirect. Yamaguchi and coworkers52 found that ATLPL varied systematically across the estrous cycle in female rats. This variation was attributed to differences in plasma insulin concentrations during some phases of the estrous cycle and estrogen concentration in others.52 Eckel53 reported that ATLPL is higher in gluteofemoral fat as compared with abdominal subcutaneous fat in premenopausal women. Rebuffe-Scrive and associates54 found that femoral ATLPL increased markedly in postmenopausal women after treatment with E2 and progesterone. The results of these studies generally support the idea that estrogen favors the storage of dietary fat in lower body adipose tissue depots.
Testosterone
Rebuffé-Scrive and coworkers55 administered androgens to normal young men and found increases in abdominal ATLPL. Subsequently, this group measured the uptake of a dietary fat tracer by abdominal and femoral fat in men receiving androgens. They found supplemental androgens did not alter ATLPL or fat uptake in femoral fat, but reduced both in abdominal fat.46 Santosa and coworkers acutely suppressed testosterone production in normal men with the gonadotropin-releasing hormone agonist Lupron. Participants were then studied before and after testosterone replacement. They found that testosterone deficiency was associated with increases in LPL in both the fasting and fed states, as well as increased uptake of dietary fat.7 Rynders and colleagues56 studied healthy young men once after suppression of testosterone and E2 levels with a gonadotropin-releasing hormone antagonist and an aromatase inhibitor and again after testosterone add back. They found the low testosterone/estrogen condition was associated with greater lower body up-take of a dietary fat tracer. Blouin and colleagues26 found that LPL production by adipose tissue explants declined after exposure to testosterone. Taken together, these findings are consistent with the idea that testosterone decreases the expansion of subcutaneous fat but promotes abdominal obesity by decreasing the delivery of Tg-derived fatty acid to lower body fat depots.
Lipolysis and Free Fatty Acid Release
Lipid is lost from adipose tissue through oxidation but, more important, through lipolysis resulting in the liberation of FFAs to fuel tissues and organs. Rates of lipolysis are decreased by insulin after feeding and increased by catecholamines in the fasted state and during exercise. It has long been thought that the products of visceral adipose tissue lipolysis preferentially go to the liver,57 where they may promote VLDL synthesis and perhaps insulin resistance. It is important to note that although this finding is true in well-fed individuals, FFAs provide energy for hepatic gluconeogenesis and substrate for ketogenesis in malnourished individuals. This finding may be relevant given that the metabolic regulatory systems seem to prioritize fat storage in the visceral depot in men (why not women?).
Sex differences in lipolysis and free fatty acid release
If differences in the rate of lipolysis were the cause of differences in total body adiposity and regional fat distribution in men and women, one might think that lipolysis would be lower in women than in men and lower in regions that accumulate fat in both sexes. This is not what studies have shown. Whole body rates of lipolysis are similar in men and women and women suppress lipolysis in response to insulin to a greater extent than men.58 In addition, women have higher rates of nonoxidative FFA disposal as compared with men.59 Lipolysis in upper body fat is suppressed less by insulin in men than women, a result that is the opposite of what one would predict. On a whole body level, women with upper body obesity have higher rates of basal lipolysis than women with lower body obesity or lean controls, but are less responsive to the lipolytic stimulatory effects of catecholamines.60 Lower body fat is also less responsive to stimulation by catecholamines compared with upper body fat in women.61 Women have higher rates of lipolysis during exercise, which is associated with a greater reliance on fat oxidation as compared with men when exercising at an equivalent workload.62 This finding seems to be due to sex-based differences in the sensitivity to α2-adrenergic antilipolytic activation.63
Effects of estrogen and testosterone on lipolysis and free fatty acid release
Estrogen
The effects of estrogens on lipolysis are complicated. Most in vivo studies in postmenopausal women demonstrate a suppressive effect of E2 treatment on basal lipolysis.64–66 This antilipolytic action of estrogen could be mediated by an increase in α2-adrenergic receptors67 or improved insulin-mediated suppression of lipolysis.68 Acute E2 treatment also seems to inhibit catecholamine stimulated lipolysis in femoral subcutaneous adipose tissue,65 whereas chronic E2 treatment decreases norepinephrine stimulated lipolysis in the abdominal subcutaneous adipose tissue.69 However, studies in premenopausal women find lipolysis does not vary with alterations in E2 over the menstrual cycle70,71 and that chronic oral contraceptive use actually increases submaximal exercise stimulated lipolysis.70 Local perfusion of E2 into subcutaneous adipose tissue of premenopausal women does not alter basal lipolysis, but results in a depot-specific effect on maximally stimulated lipolysis, blunting lipolysis in the gluteal region, but potentiating it in the abdominal region.72 Thus, the role of estrogens in regulating lipolysis varies between studies of premenopausal and postmenopausal women, adipose depots, basal or stimulated conditions, and chronic (genomic) or acute (nongenomic) exposures.
Testosterone
Studies of male rodents in the late 1980s and early 1990s demonstrated a role for testosterone in the regulation of lipolysis. A study of castrated hamsters before and after testosterone supplementation found that basal and catecholamine-stimulated rates of lipolysis were decreased in the testosterone-deficient state and were restored by testosterone treatment.73 A similar study in rats found that only stimulated, but not basal, lipolysis was altered by testosterone status.74,75 In contrast, in studies of preadipocytes isolated from a mix of men and women of varying age and body mass index, in vitro testosterone exposure decreased both the lipolytic response to catecholamines and the expression of hormone-sensitive lipase in cells from the subcutaneous but not visceral depot.76 In another set of studies in elderly men, testosterone supplementation did not alter systemic rates of basal lipolysis,77 postprandial lipolysis, or responses of lipolysis to insulin.78
In summary, studies on the sex-based differences in lipolysis and the effects of sex steroids on the regulation of lipolysis are conflicting and suggest that differences in lipolysis are likely involved in, but do not play the primary role in determining adipose tissue distribution in women and men.
Sex Differences in the Uptake and Storage of Free Fatty Acid
For many years, the role that the direct uptake of FFA by adipose tissue plays in net lipid uptake was felt to be negligible. However, Jensen and colleagues79,80 demonstrated experimentally that indeed direct uptake of FFA was a significant contributor to the overall lipid supply of adipose tissue. It is less clear whether this pathway is entirely independent of or linked to the pathway that delivers lipid from Tg-rich lipoproteins.79 Direct uptake and storage of circulating FFA is significantly greater in the subcutaneous fat of women as compared with men.80 Furthermore, direct uptake of FFA is greater in abdominal fat as compared with femoral fat in men, but this regional difference was not observed in women. A study of more than 80 participants confirmed the greater uptake of FFA in the subcutaneous fat of women as compared with men and showed alignment of regional FFA uptake with differences in regional adiposity with women having greater uptake of FFA in lower body fat depots and men in upper body fat.81 FFA uptake also correlated with circulating FFA concentrations. In summary, whole body and regional direct uptake of FFA correlates with sex-based differences in whole body and regional fat accumulation.
SEX DIFFERENCES IN PRODUCTS SECRETED BY ADIPOSE TISSUE
Adipose tissue is not only a site for energy storage and liberation, but also serves an important role in the secretion of cytokines and adipokines. These secreted factors act locally and systemically to mediate a range of physiologic functions, including but not limited to insulin sensitivity, energy intake, inflammation, and blood pressure.82 Here we discuss two important adipokines, leptin and adiponectin.
Leptin
Leptin is secreted by adipose tissue with circulating levels generally proportional to total fat mass. Because women have higher levels of adiposity than men, they also have higher circulating levels of leptin. However, many but not all83,84 studies show that the higher level of leptin in women is maintained even after correcting for total body fat. Some of the sex-based differences in leptin concentration may be in part due to the fact that the relationship between percent body fat and leptin concentration is not linear but logarithmic.85 Differences in leptin concentration between males and females are most pronounced during puberty when leptin seems to be important in sexual maturation.86 There is evidence that subcutaneous adipose tissue produces more leptin per gram of fat than intra-abdominal fat.87 Because girls and women have more subcutaneous fat relative to visceral fat than men, this factor could explain the sex-based difference. In one study, correcting for regional fat distribution indeed eliminated sex-based differences in leptin concentrations.88 Other studies have found correlations with estrogen concentration directly. The importance of estrogen is suggested by the fact that leptin levels corrected for the increase in adiposity at puberty89 and decrease with the menopause.90
Adiponectin
Adiponectin is a factor secreted by adipose tissue that is associated with improved insulin sensitivity. It also is associated with a decreased risk of cardiovascular disease. In contrast with leptin, adiponectin levels are inversely related to fat mass. Interestingly, adiponectin levels are actually higher in adult females as compared with males,83 with an inverse correlation with visceral adipose tissue in women only.91 The sex-based difference develops during puberty when adiponectin levels decrease dramatically in boys.92 During this period, fat mass increases in females and decreases in males. These changes in fat mass would predict decreasing adiponectin levels in females and increasing levels in males, but the opposite is observed. In the one study that measured sex steroids, testosterone levels were inversely correlated with adiponectin levels.93 Consistent with these data, androgen receptor-null mice have high levels of adiponectin and are insulin sensitive.94 Low levels of adiponectin secretion from adipose tissue may in part underlie the relatively greater risk of type 2 diabetes in males.
SUMMARY
Differences in the amount and distribution of body fat between men and women are the result of a large number of complex yet coordinated adjustments to basic aspects of adipocyte biology resulting in reduced total fat but relatively more visceral fat in men and greater total fat with more subcutaneous gluteofemoral fat in women. Although we focus on the potential effects of these differences on health, the broad effects of sex steroids on adipose function suggest that lipid metabolism is fundamentally different in men as compared with women. Visceral fat, the relatively preferred site for fat accumulation in men, delivers at least some of its products of hydrolysis to the liver, supporting gluconeogenesis and ketogenesis in states of undernutrition or providing substrate for VLDL Tg synthesis in states of full nutrition. VLDL Tg is available to tissues as a source of fuel based on the hormonal regulation of LPL in that tissue by insulin and catecholamines. Conversely, subcutaneous fat releases its products of hydrolysis into the systemic circulation where these FFA are available to all tissues and their uptake is less subject to regulation by hormones. The fact that sex steroids alter so many processes in adipocyte function suggests that sex differences in lipid metabolism are important for supporting normal sex-specific functions. The implications of these differences in adipose tissue function extend to glucose and protein metabolism as well as nutrient sensing and appetite. Many questions remain, but it may be useful to try to develop a whole body model of sex differences in fuel metabolism that can be a foundation for future studies of both normal metabolism and disease risk and treatment. Understanding sex differences in adipose tissue metabolism and function may be a good place to start.
KEY POINTS.
Males have less total body fat, but accumulate a disproportionate amount in the abdominal visceral compartment; women preferentially store fat in the gluteofemoral depot.
Sex steroids play a central role in these differences by altering developmental and biochemical processes in adipocytes that are, in part, depot specific.
Effects of sex steroids on adipocytes occur directly through hormone receptors or indirectly by modulating tissue responses to other hormones, including catecholamines and insulin.
Emerging areas of research include sex differences in the recruitment of brown adipose tissue, disposition of bone marrow-derived adipocyte precursors, and potential independent effects of follicle-stimulating hormone.
Although these differences have relevance for metabolic disease risk, they may inform us about differences in the priorities of fuel metabolism in men versus women.
ACKNOWLEDGMENTS
The authors are supported by NIH grants R01DK114272, R01DK111622, P50 HD073063, U54 AG062319, K01 DK109053 and P30 DK048520.
Footnotes
DISCLOSURE
Neither author has any commercial or financial conflict of interest to report. Both authors receive funding from the NIH.
REFERENCES
- 1.Jensen MD. Adipose tissue and fatty acid metabolism in humans. J R Soc Med 2002;95(Suppl 42):3–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Mauvais-Jarvis F Gender differences in glucose homeostasis and diabetes. Physiol Behav 2018;187:20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathieu P, Boulanger MC, Despres JP. Ectopic visceral fat: a clinical and molecular perspective on the cardiometabolic risk. Rev Endocr Metab Disord 2014; 15(4):289–98. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan AJ. Does oestrogen allow women to store fat more efficiently? A biological advantage for fertility and gestation. Obes Rev 2009;10(2):168–77. [DOI] [PubMed] [Google Scholar]
- 5.Ambikairajah A, Walsh E, Tabatabaei-Jafari H, et al. Fat mass changes during menopause: a metaanalysis. Am J Obstet Gynecol 2019;221(5):393–409.e50. [DOI] [PubMed] [Google Scholar]
- 6.Papadakis GE, Hans D, Rodriguez EG, et al. Menopausal hormone therapy is associated with reduced total and visceral adiposity: the osteolaus cohort. J Clin Endocrinol Metab 2018;103(5):1948–57. [DOI] [PubMed] [Google Scholar]
- 7.Host C, Bojesen A, Erlandsen M, et al. A placebo-controlled randomized study with testosterone in Klinefelter syndrome - beneficial effects on body composition. Endocr Connect 2019;8(9):1250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbers JM, Asscheman H, Seidell JC, et al. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol 1999;276(2):E317–25. [DOI] [PubMed] [Google Scholar]
- 9.Elbers JM, Giltay EJ, Teerlink T, et al. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 2003; 58(5):562–71. [DOI] [PubMed] [Google Scholar]
- 10.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 2015;402:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank AP, de Souza Santos R, Palmer BF, et al. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res 2019;60(10):1710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavin KM, Kohrt WM, Klemm DJ, et al. Modulation of energy expenditure by estrogens and exercise in women. Exerc Sport Sci Rev 2018;46(4):232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453(7196):783–7. [DOI] [PubMed] [Google Scholar]
- 14.Tchoukalova YD, Votruba SB, Tchkonia T, et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A 2010;107(42):18226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tchoukalova YD, Koutsari C, Karpyak MV, et al. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 2008;87(1):56–63. [DOI] [PubMed] [Google Scholar]
- 16.Strawford A, Antelo F, Christiansen M, et al. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab 2004;286(4):E577–88. [DOI] [PubMed] [Google Scholar]
- 17.Tchoukalova YD, Fitch M, Rogers PM, et al. In vivo adipogenesis in rats measured by cell kinetics in adipocytes and plastic-adherent stroma-vascular cells in response to high-fat diet and thiazolidinedione. Diabetes 2012;61(1):137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchoukalova YD, Koutsari C, Votruba SB, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring) 2010; 18(10):1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White UA, Fitch MD, Beyl RA, et al. Differences in in vivo cellular kinetics in abdominal and femoral subcutaneous adipose tissue in women. Diabetes 2016;65(6):1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatima LA, Campello RS, Barreto-Andrade JN, et al. Estradiol stimulates adipogenesis and Slc2a4/GLUT4 expression via ESR1-mediated activation of CEBPA. Mol Cell Endocrinol 2019;498:110447. [DOI] [PubMed] [Google Scholar]
- 21.Luo F, Huang WY, Guo Y, et al. 17beta-estradiol lowers triglycerides in adipocytes via estrogen receptor alpha and it may be attenuated by inflammation. Lipids Health Dis 2017;16(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell-Fugate AE. The role of sex steroids in white adipose tissue adipocyte function. Reproduction 2017;153(4):R133–49. [DOI] [PubMed] [Google Scholar]
- 23.White UA, Tchoukalova YD. Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta 2014;1842(3):377–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson LA, McTernan PG, Barnett AH, et al. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab 2001;86(10):5045–51. [DOI] [PubMed] [Google Scholar]
- 25.O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol 2014;143:277–84. [DOI] [PubMed] [Google Scholar]
- 26.Blouin K, Nadeau M, Perreault M, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2010;72(2):176–88. [DOI] [PubMed] [Google Scholar]
- 27.Zerradi M, Dereumetz J, Boulet MM, et al. Androgens, body fat distribution and adipogenesis. Curr Obes Rep 2014;3(4):396–403. [DOI] [PubMed] [Google Scholar]
- 28.Ren X, Fu X, Zhang X, et al. Testosterone regulates 3T3-L1 pre-adipocyte differentiation and epididymal fat accumulation in mice through modulating macrophage polarization. Biochem Pharmacol 2017;140:73–88. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013;369(11): 1011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callewaert F, Venken K, Ophoff J, et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J 2009;23(1):232–40. [DOI] [PubMed] [Google Scholar]
- 31.Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol 2001;79(1–5):3–9. [DOI] [PubMed] [Google Scholar]
- 32.Gavin KM, Gutman JA, Kohrt WM, et al. De novo generation of adipocytes from circulating progenitor cells in mouse and human adipose tissue. FASEB J 2016; 30(3):1096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavin KM, Sullivan TM, Kohrt WM, et al. Ovarian hormones regulate the production of adipocytes from bone marrow-derived cells. Front Endocrinol (Lausanne) 2018;9:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011;96(1):192–9. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Cuenca S, Pujol E, Justo R, et al. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem 2002;277(45):42958–63. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Cuenca S, Monjo M, Gianotti M, et al. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17beta-estradiol, testosterone, and progesterone. Am J Physiol Endocrinol Metab 2007;292(1):E340–6. [DOI] [PubMed] [Google Scholar]
- 37.Nadal-Casellas A, Proenza AM, Llado I, et al. Effects of ovariectomy and 17-beta estradiol replacement on rat brown adipose tissue mitochondrial function. Steroids 2011;76(10–11):1051–6. [DOI] [PubMed] [Google Scholar]
- 38.Liu P, Ji Y, Yuen T, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017;546(7656):107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohrt WM, Wierman ME. Preventing fat gain by blocking follicle-stimulating hormone. N Engl J Med 2017;377(3):293–5. [DOI] [PubMed] [Google Scholar]
- 40.McInnes KJ, Andersson TC, Simonyte K, et al. Association of 11beta-hydroxysteroid dehydrogenase type I expression and activity with estrogen receptor beta in adipose tissue from postmenopausal women. Menopause 2012;19(12):1347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dieudonne MN, Sammari A, Dos Santos E, et al. Sex steroids and leptin regulate 11beta-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: sex specificities. J Steroid Biochem Mol Biol 2006;99(4–5): 189–96. [DOI] [PubMed] [Google Scholar]
- 42.Lazzarini SJ, Wade GN. Role of sympathetic nerves in effects of estradiol on rat white adipose tissue. Am J Physiol 1991;260(1 Pt 2):R47–51. [DOI] [PubMed] [Google Scholar]
- 43.Santosa S, Jensen MD. The sexual dimorphism of lipid kinetics in humans. Front Endocrinol (Lausanne) 2015;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackman MR, Kramer RE, MacLean PS, et al. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab 2006;291(5): E1083–91. [DOI] [PubMed] [Google Scholar]
- 45.Horton TJ, Commerford SR, Pagliassotti MJ, et al. Postprandial leg uptake of triglyceride is greater in women than in men. Am J Physiol Endocrinol Metab 2002; 283(6):E1192–202. [DOI] [PubMed] [Google Scholar]
- 46.Marin P, Oden B, Bjorntorp P. Assimilation and mobilization of triglycerides in sub-cutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab 1995;80(1):239–43. [DOI] [PubMed] [Google Scholar]
- 47.Votruba SB, Jensen MD. Short-term regional meal fat storage in nonobese humans is not a predictor of long-term regional fat gain. Am J Physiol Endocrinol Metab 2012;302(9):E1078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 2006;291(5):E1115–23. [DOI] [PubMed] [Google Scholar]
- 49.Santosa S, Hensrud DD, Votruba SB, et al. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am J Clin Nutr 2008;88(4):1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santosa S, Jensen MD. Adipocyte fatty acid storage factors enhance subcutaneous fat storage in postmenopausal women. Diabetes 2013;62(3):775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Homma H, Kurachi H, Nishio Y, et al. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J Biol Chem 2000;275(15):11404–11. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi M, Katoh S, Morimoto C, et al. The hormonal responses of lipoprotein lipase activity and lipolysis in adipose tissue differ depending on the stage of the estrous cycle in female rats. Int J Obes Relat Metab Disord 2002;26(5):610–7. [DOI] [PubMed] [Google Scholar]
- 53.Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med 1989;320(16):1060–8. [DOI] [PubMed] [Google Scholar]
- 54.Rebuffe-Scrive M, Lonnroth P, Marin P, et al. Regional adipose tissue metabolism in men and postmenopausal women. Int J Obes 1987;11(4):347–55. [PubMed] [Google Scholar]
- 55.Rebuffe-Scrive M, Marin P, Bjorntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes 1991;15(11):791–5. [PubMed] [Google Scholar]
- 56.Rynders CA, Schmidt SL, Bergouignan A, et al. Effects of short-term sex steroid suppression on dietary fat storage patterns in healthy males. Physiol Rep 2018; 6(2). 10.14814/phy2.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113(11):1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 1995;96(5):2297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koutsari C, Basu R, Rizza RA, et al. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab 2011;96(2):541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen MD, Haymond MW, Rizza RA, et al. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 1989;83(4):1168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo Z, Johnson CM, Jensen MD. Regional lipolytic responses to isoproterenol in women. Am J Physiol 1997;273(1 Pt 1):E108–12. [DOI] [PubMed] [Google Scholar]
- 62.Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc 2008;40(4):648–54. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt SL, Bessesen DH, Stotz S, et al. Adrenergic control of lipolysis in women compared with men. J Appl Physiol (1985) 2014;117(9):1008–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jensen MD, Martin ML, Cryer PE, et al. Effects of estrogen on free fatty acid metabolism in humans. Am J Physiol 1994;266(6 Pt 1):E914–20. [DOI] [PubMed] [Google Scholar]
- 65.Gormsen LC, Host C, Hjerrild BE, et al. Estradiol acutely inhibits whole body lipid oxidation and attenuates lipolysis in subcutaneous adipose tissue: a randomized, placebo-controlled study in postmenopausal women. Eur J Endocrinol 2012; 167(4):543–51. [DOI] [PubMed] [Google Scholar]
- 66.Van Pelt RE, Gozansky WS, Hickner RC, et al. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring) 2006;14(12):2163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedersen SB, Kristensen K, Hermann PA, et al. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab 2004;89(4):1869–78. [DOI] [PubMed] [Google Scholar]
- 68.Pereira RI, Casey BA, Swibas TA, et al. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab 2015;100(12):4456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindberg UB, Crona N, Silfverstolpe G, et al. Regional adipose tissue metabolism in postmenopausal women after treatment with exogenous sex steroids. Horm Metab Res 1990;22(6):345–51. [DOI] [PubMed] [Google Scholar]
- 70.Casazza GA, Jacobs KA, Suh SH, et al. Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. J Appl Physiol (1985) 2004;97(1):302–9. [DOI] [PubMed] [Google Scholar]
- 71.Horton TJ, Miller EK, Bourret K. No effect of menstrual cycle phase on glycerol or palmitate kinetics during 90 min of moderate exercise. J Appl Physiol (1985) 2006;100(3):917–25. [DOI] [PubMed] [Google Scholar]
- 72.Gavin KM, Cooper EE, Raymer DK, et al. Estradiol effects on subcutaneous adipose tissue lipolysis in premenopausal women are adipose tissue depot specific and treatment dependent. Am J Physiol Endocrinol Metab 2013;304(11): E1167–74. [DOI] [PubMed] [Google Scholar]
- 73.Pecquery R, Leneveu MC, Giudicelli Y. Influence of androgenic status on the alpha 2/beta-adrenergic control of lipolysis in white fat cells: predominant alpha 2-antilipolytic response in testosterone-treated-castrated hamsters. Endocrinology 1988;122(6):2590–6. [DOI] [PubMed] [Google Scholar]
- 74.Xu X, De Pergola G, Bjorntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology 1990;126(2):1229–34. [DOI] [PubMed] [Google Scholar]
- 75.Xu XF, De Pergola G, Bjorntorp P. Testosterone increases lipolysis and the number of beta-adrenoceptors in male rat adipocytes. Endocrinology 1991;128(1): 379–82. [DOI] [PubMed] [Google Scholar]
- 76.Dicker A, Ryden M, Naslund E, et al. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 2004;47(3):420–8. [DOI] [PubMed] [Google Scholar]
- 77.Koutsari C, Ali AH, Nair KS, et al. Fatty acid metabolism in the elderly: effects of dehydroepiandrosterone and testosterone replacement in hormonally deficient men and women. J Clin Endocrinol Metab 2009;94(9):3414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Espinosa De Ycaza AE, Rizza RA, Nair KS, et al. Effect of dehydroepiandrosterone and testosterone supplementation on systemic lipolysis. J Clin Endocrinol Metab 2016;101(4):1719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sondergaard E, Gormsen LC, Nellemann B, et al. Body composition determines direct FFA storage pattern in overweight women. Am J Physiol Endocrinol Metab 2012;302(12):E1599–604. [DOI] [PubMed] [Google Scholar]
- 80.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 2007;56(5):1369–75. [DOI] [PubMed] [Google Scholar]
- 81.Koutsari C, Ali AH, Mundi MS, et al. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 2011;60(8):2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci 2015;36(7):461–70. [DOI] [PubMed] [Google Scholar]
- 83.Christen T, Trompet S, Noordam R, et al. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides 2018; 107:25–31. [DOI] [PubMed] [Google Scholar]
- 84.Hunma S, Ramuth H, Miles-Chan JL, et al. Do gender and ethnic differences in fasting leptin in Indians and Creoles of Mauritius persist beyond differences in adiposity? Int J Obes (Lond) 2018;42(2):280–3. [DOI] [PubMed] [Google Scholar]
- 85.Jensen MD, Hensrud D, O’Brien PC, et al. Collection and interpretation of plasma leptin concentration data in humans. Obes Res 1999;7(3):241–5. [DOI] [PubMed] [Google Scholar]
- 86.Horlick MB, Rosenbaum M, Nicolson M, et al. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab 2000; 85(7):2509–18. [DOI] [PubMed] [Google Scholar]
- 87.Montague CT, Prins JB, Sanders L, et al. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 1997;46(3):342–7. [DOI] [PubMed] [Google Scholar]
- 88.Nagy TR, Gower BA, Trowbridge CA, et al. Effects of gender, ethnicity, body composition, and fat distribution on serum leptin concentrations in children. J Clin Endocrinol Metab 1997;82(7):2148–52. [DOI] [PubMed] [Google Scholar]
- 89.Ahmed ML, Ong KK, Morrell DJ, et al. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab 1999;84(3):899–905. [DOI] [PubMed] [Google Scholar]
- 90.Isidori AM, Strollo F, More M, et al. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab 2000;85(5):1954–62. [DOI] [PubMed] [Google Scholar]
- 91.Bidulescu A, Liu J, Hickson DA, et al. Gender differences in the association of visceral and subcutaneous adiposity with adiponectin in African Americans: the Jackson Heart Study. BMC Cardiovasc Disord 2013;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohman-Hanson RA, Cree-Green M, Kelsey MM, et al. Ethnic and sex differences in adiponectin: from childhood to adulthood. J Clin Endocrinol Metab 2016; 101(12):4808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bottner A, Kratzsch J, Muller G, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab 2004;89(8):4053–61. [DOI] [PubMed] [Google Scholar]
- 94.Fan W, Yanase T, Nomura M, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes 2005;54(4):1000–8. [DOI] [PubMed] [Google Scholar]


