Abstract
Over 30 million prescriptions of NSAIDs (non-steroidal anti-inflammatory drugs) are issued every year. Considering that these drugs are available without a prescription as over the counter (OTC) drugs, their use will be astronomical. With the increasing use of NSAIDs, their adverse effects are drawing attention. Especially, stomach bleeding, kidney toxicity, liver toxicity, and neurological toxicity are reported as common. Ibuprofen, one of the extensively used NSAIDs along with aspirin, can also induce liver toxicity, but few studies are addressing this point. Here we examined the liver toxicity of ibuprofen and investigated whether co-exposure to ethanol can manifest synergistic effects. We employed 2D and 3D cultured human hepatoma cells, HepG2 to examine the synergistic hepatotoxicity of ibuprofen and alcohol concerning cell viability, morphology, and histology of 3D spheroids. As a result, ibuprofen and alcohol provoked synergistic hepatotoxicity against hepatocytes, and their toxicity increased prominently in 3D culture upon extended exposure. Oxidative stress appeared to be the mechanisms underlying the synergistic toxicity of ibuprofen and alcohol as evidenced by increased production of ROS and expression of the endogenous antioxidant system. Collectively, this study has demonstrated that ibuprofen and EtOH can induce synergistic hepatotoxicity, providing a line of evidence for caution against the use of ibuprofen in combination with alcohol.
Keywords: Ibuprofen, Alcohol, Hepatotoxicity, HepG2, 3D spheroid, Oxidative stress
INTRODUCTION
Ibuprofen is one of the most popular non-steroidal anti-inflammatory drugs (NSAIDs) and is the main ingredients of major over-the-counter (OTC) drugs, such as Advil® and Motrin®. The number of prescription for ibuprofen is over 1.5 million in the US in 2015, with the number steadily increasing (Agency for Healthcare Research and Quality (AHRQ), 2016). Not only is ibuprofen consumed by prescription, but it is also commonly purchased over the counter. Therefore, consumption of ibuprofen is expected to be larger than the figures aforementioned. Although ibuprofen is commonly known as a “safe” OTC drug, it can cause several drug-induced adverse effects, such as liver toxicity, kidney toxicity, and stomach bleeding.
In fact, NSAIDs are one of the most notable causes of drug-induced liver injuries, with about 3 to 23 per 100,000 patient years (Aithal and Day, 2007), and as a result, three different NSAIDS, bromfenac, ibufenac, and benoxaprofen, had been removed from the UK and/or US markets because of their hepatotoxic side effects (Goldkind and Laine, 2006). These adverse effects of NSAIDs have been witnessed in children (Cardile et al., 2016) and elderly (Freytag et al., 2014) alike and can be caused or exacerbated by multiple factors, including overconsumption caused by duplicate prescriptions and/or medication overdose, significant drug interactions, and individual patient susceptibilities (Tolman, 1998).
Meanwhile, alcohol is one most important factor which aggravates the liver toxicity of NSAIDs. It has been reported that acetaminophen, another NSAID, is contra-indicated for the patients drank alcohol due to the increased liver toxicity for the patients with alcoholism (Slattery et al., 1996). Since ibuprofen is frequently used for the patients who drank alcohol and suffer from hangover including headache, attention should be paid to the ibuprofen-alcohol interaction. Yet few studies have examined whether the concomitant administration of alcohol and ibuprofen increases liver toxicity.
Our study sought to examine the impact of liver toxicity of ibuprofen when administered together with alcohol. More specifically, we will use 2D and 3D cultured hepatoma cell line, HepG2. The 3D cell cultures, because of their distinct shape, demonstrate behaviors that strongly resemble in vivo human livers, allowing for more accurate information to be obtained as compared to using just 2D cell cultures, which have been known to demonstrate certain discrepancies from in vivo (Edmondson et al., 2014). HepG2 were grown in both 2D and 3D cell cultures and were exposed to ibuprofen and ethanol. The hepatotoxicity and synergy between the two substances were analyzed using quantitative data (cell viability), as well as qualitative data (cell morphology and histologic analysis). Furthermore, to understand the mechanism underlying, oxidative stress was evaluated by measuring hydrogen peroxide generation with a fluorescent dye, DCF-DA under a fluorescence microscope. Gene expression for antioxidant enzymes was evaluated with quantitative reverse transcription-PCR (qPCR).
MATERIALS AND METHODS
Chemicals
Ethanol and ibuprofen were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were of best quality available.
Cell culture
HepG2 cells (human hepatocellular carcinoma) were purchased from ATCC (Manassas, VA, USA). As previously described (Sooklert et al., 2019), cells were cultured in 75T culture plates and were maintained in standard culture conditions, Dulbecco’s modified eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and incubated in a humidified atmosphere of 5% CO2 in air at 37°C. Culture medium was changed twice a week. At 70 to 80% cell confluence, adherent cells were released with a solution of trypsin (Hyclone, South Logan, UT, USA). HepG2 cells were seeded at 1×106 cell/mL for the experiments.
Spheroid culture
HepG2 spheroids were cultivated on 96-well ultra-low-attachment plates (Corning Co., Corning, NY, USA). Briefly, 1×104 cells/well were seeded in 100 μL of media and cultured for 14 days with media changes every 2-3 days. The formation of spheroids was examined under a microscope. After culturing, ibuprofen was added to a concentration indicated from a stock solution in dimethyl sulfoxide (final concentration 0.1%) with or without ethanol, and incubation was continued for 72 h. For histology, spheroids were washed in Dulbecco’s phosphate-buffered saline (DPBS) and collected in a tube. After removing DBPS, 100 μL of 2% agarose was added, and the samples were centrifuged (2 s, 150×g) to pellet and immobilize spheroids. The agarose-immobilized spheroids were separated from the tube, fixed in 4% formalin for more than 3 h, and then underwent processing for hematoxylin and eosin (H&E) staining.
Cell viability assay
WST-1 (4-[3-(iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) (Roche, Indianapolis, IN, USA) solution was used to evaluate cell viability (Joo et al., 2019). For the WST-1 assay, 1×104 cells were seeded into 96-well plates. Ibuprofen or ethanol-treated HepG2 cells were incubated with 200 μL of WST-1 solution for 2.5 h at 37°C, 5% CO2 in the dark. 100 μL of supernatant was transferred into each well of 96 well plates, and absorbance was measured at 450 nm. All measurements were performed in triplicate.
Visual examination and photographing
After the treatment, the media was removed and cells were washed twice with phosphate-buffered saline (PBS). HepG2 cells were observed and photographed under a phase-contrast optical microscope with the magnification of 200 (×200, ECLIPSE TS100, Nikon, Tokyo, Japan). For individual cell tracking, cells were culture on a microgrid slide chamber (μ-slide 8 well Grid-500, Ibidi Co., Fitchburg, WI, USA) and observed under the microscope.
Intracellular reactive oxygen species (ROS) level
Production of ROS was measured by 2′,7′-dichlorofluorescein diacetate (DCF-DA, Eugene, OR, USA)-enhanced fluorescence assay as described previously (Kim et al., 2019). HepG2 cells were pretreated with the indicated concentrations of ibuprofen with or without ethanol for 24 h, washed with PBS and stained with 5 mM DCF-DA for 10 min at 37°C. For positive control, cells were treated with 100 mM H2O2 for 10 min before staining. The resulting cells were pictured using NIS-Elements BR 4.6 program and Ts2R FLPH microscope. Cellular fluorescence was measured using the ImageJ program (NIH, Bethesda, MD, USA).
RNA sample preparation
HepG2 cells treated for 24 h were collected and washed once with PBS, and the total ribonucleic acid (RNA) was extracted with TRIzol reagent (Invitrogen, CARLSBAD, CA, USA) according to the manufacturer’s protocol. RNA precipitates were dissolved in RNase free DEPC treated water (usb, USA). The concentration of RNA was determined with NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA).
Reverse transcription-PCR
Relative expression levels of mRNAs were measured by quantitative real-time PCR. Total RNA, extracted from HepG2 cells, was used to synthesize cDNA using a pre-master mix with oligo dT (Bioepis, Seoul, Korea). Each reaction was performed using Power SYBR Green PCR master mix in a StepOnePlusTM Real-time PCR machine (Applied Biosystems, Warrington, UK). The sequence of primers were as follows: forward superoxide dismutase (SOD), 5’- ATGGACCAGTGAAGGTGTGG-3’, reverse SOD 5’-GCCCACCGTGTTTTCTGGAT-3’; forward catalase (CAT), 5’-CTCCGGAACAACAGCCTTCT-3’, reverse CAT5’-ATAGAATGCCCGCACCTGAG-3’; forward glutathione peroxidase (GPX3), 5’-AGAAGTCGAAGATGGACTGCC-3’, reverse GPX3 5’-CAAAGAGGACGTATTTGCCAGC-3’; forward cytochrome P450 family 2 subfamily E member 1 (CYP2E1), 5’-TTGAAGCCTCTCGTTGACCC, reverse CYP2E1 5’-TCATGAGCGGGGAATGACAC-3’; forward glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5’-GCATCCTGGGCTACACTGAG-3’, reverse GAPDH 5’- AAGTGGTCGTTGAGGGCAAT-3’. Cycling parameters were 52°C for 2 min, 95°C for 10 min, 40 cycles of 95°C 15 s and 52°C 1 min. Semi-quantitative RT-PCR was performed using electrophoresis through a 1.5% agarose gel with eco dye (EcoDye DNA staining solution, Biofact, Daejeon, Korea).
Western blot analysis
Hepg2 cells were washed after 24-h exposure to Ibuprofen 0.8 mM and EtOH 200 mM. Then, cells were homogenized in RIPA buffer (Sigma-Aldrich) containing 1% protease inhibitor cocktail and a phosphatase inhibitor cocktail. The homogenate was centrifuged (12,000 rpm, 10 min) and the supernatant was collected. The protein concentration was measured and an aliquot (15.6 μg protein) was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the fractionated proteins were then transferred to a nitrocellulose membrane. For immunoblotting, the following primary antibodies were used: rabbit anti- SOD antibody (1:300; Bioss, MA, USA), rabbit anti- Catalase (1:200; Bioss), goat anti- Glutathione Peroxidase 3/GPx-3 antibody (1:500 dilution; abcam, Cambridge, UK) and rabbit anti- Cytochrome P450 2E1 antibody (1:200 dilution; abcam) after incubation with HRP-conjugated secondary antibodies (KPL, Gaithersburg, MD, USA), the immunoreactive bands were visualized using ECL Western blotting detection reagents (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) and an LAS. Band intensity was measured using the ImageJ program (NIH). Rabbit anti- GAPDH Polyclonal Antibody (1:150 dilution; Bioss), was used as a control for immunoblotting.
Statistics
Data are presented as the mean ± SE unless indicated otherwise. Difference between groups was examined using student t-test with p<0.05 as the criteria for statistical significance. Excel 2013 (Microsoft, Washington, WA, USA) was used.
RESULTS
To evaluate whether ibuprofen can potentiate the hepatotoxicity of ethanol (EtOH), HepG2, human hepatocellular carcinoma cell system was cultured in and seeded to 96 well plates. Then, HepG2 cells were exposed to ibuprofen (0, 0.4, 0.8 and 2 mM) with or without ethanol (EtOH 200 mM or 700 mM) for 24 h and measured for cell viability using WST-1 assay.
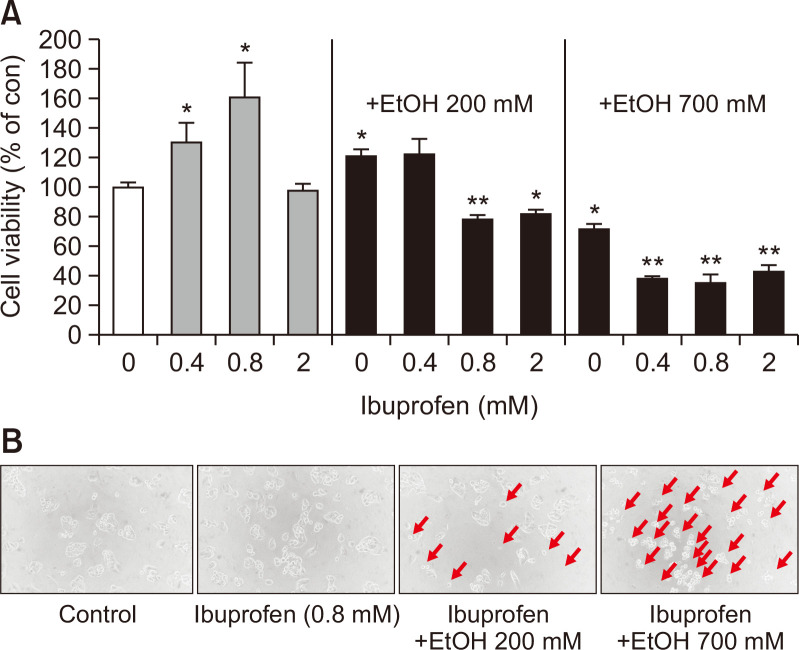
Therapeutic blood concentration of ibuprofen is ~0.25 mM (Janssen and Venema, 1985) and EtOH reaches up to >20 mM when extremely drunken (Grant et al., 2000). As shown in Fig. 1, combined treatment of ibuprofen increased the cytotoxicity of EtOH. When EtOH was 200 mM and ibuprofen was ≥0.8 mM or when EtOH was 700 mM and ibuprofen ≥0.4 mM, significant cytotoxicity was observed, indicating the potentiation of EtOH-induced hepatotoxicity by ibuprofen (Fig. 1).
Fig. 1.
Cell viability of 2D HepG2 evaluated with WST-1 assay. 24 h after exposure to ibuprofen +/- EtOH. N=3, *p<0.05, **p<0.01. Morphological changes in 2D cultured hepatocytes after exposure to ibuprofen with or without EtOH (200×).
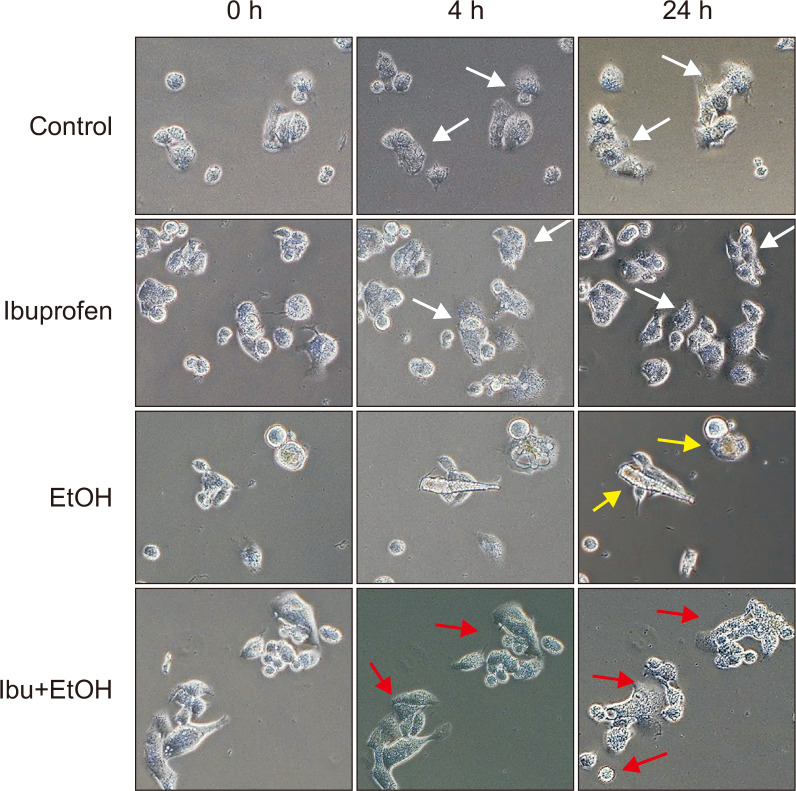
Cells under cytotoxic conditions show damaged pattern such as floating from the culture dish or rounding by loss of attachment. We examined the morphology of HepG2 cells after exposure to ibuprofen with or without ethanol under a microscope. In accordance with the increased hepatotoxicity of combined treatment of ibuprofen and EtOH as measured with WST-1 assay, cell morphology observed under a light microscope, showed floating and rounding appearance, characteristic features of cell death, when exposed to both ibuprofen and EtOH (Fig. 1). This pattern appeared more evident when the exposed individual cells were tracked under a microscope with a microgrid slide chamber as shown in Fig. 2. While non-treated cells showed normal cell division and attachment onto the dish surface, cells treated with EtOH alone or combination of ibuprofen and EtOH showed rounding and deformation. Cells exposed to both ibuprofen and EtOH showed the severest changes.
Fig. 2.
Time lapsed imaging of HepG2 cells (200×). White arrow, normal cells; Yellow arrow, rounding; Red arrow, deformed cells.
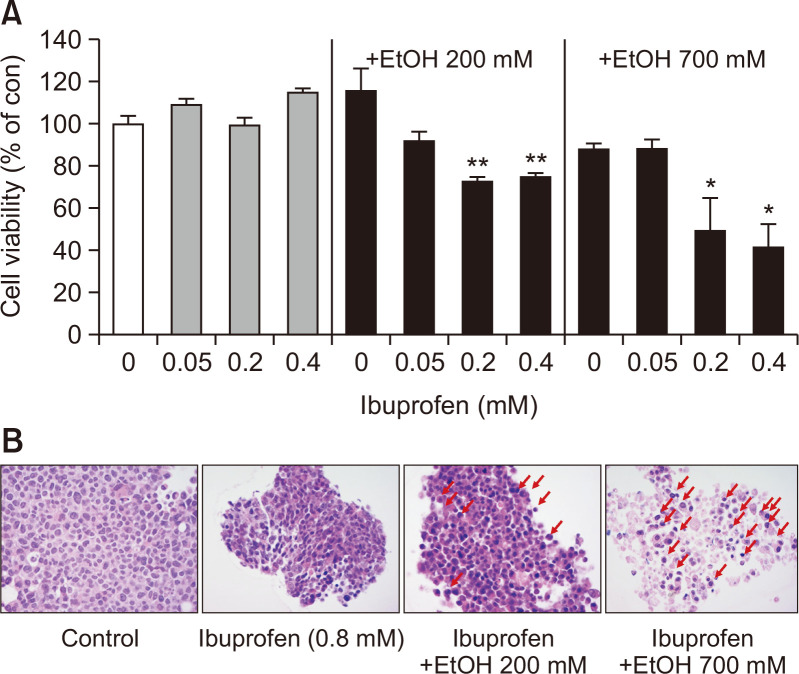
2D cultured cells have limited value in predicting organ responses to chemicals since the cells are growing in stretched appearance over plastic culture ware. To overcome this, 3D spheroid-cultured HepG2 cells were employed. 3D spheroid has a multi-cell layered structure and exhibits natural cell shapes which is in spheroid or aggregated forms. 3D spheroids were also advantageous in examining longer time-frame of exposure since they can be cultured for a longer time than 2D culture. To examine the synergy of combined exposure to ibuprofen and EtOH in hepatotoxicity, HepG2 spheroids were exposed for 72 h. As shown in Fig. 3A, synergistic hepatotoxicity of ibuprofen and EtOH could be observed as evidenced by decreased cell viability. Notably, much lower concentrations of ibuprofen were determined to be hepatotoxic in 3D spheroids than observed in 2D culture (0.2 mM in spheroid vs 0.8 mM in 2D).
Fig. 3.
Cell viability of 3D HepG2 spheroids evaluated with WST-1 assay. 72 h after exposure to ibuprofen +/- EtOH. N=3, *p<0.05, **p<0.01. Histological changes in 3D cultured HepG2 after exposure to ibuprofen with or without EtOH. Red arrows indicate cell nucleus accumulation.
3D spheroid can be further observed for cellular morphology through histology. Spheroids were sectioned, stained with hematoxylin and eosin and observed under a light microscope. As shown in Fig. 3B, spheroids exposed to ibuprofen and EtOH showed loss of cell number and cytosol, and the accumulation of nucleus (pyknosis), indicative of necrosis and apoptosis.
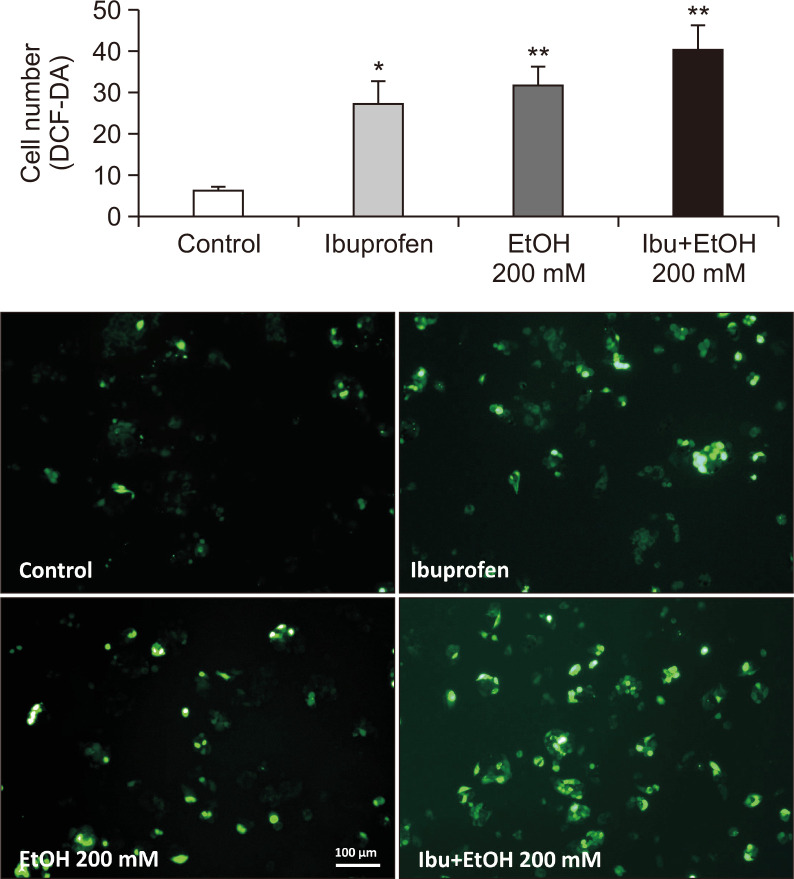
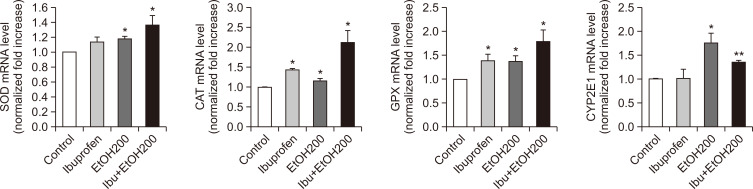
To examine the mechanism underlying the synergistic hepatotoxicity of ibuprofen and EtOH, the generation of hydrogen peroxide, as an indicator of oxidative stress, was measured with a fluorescent dye, DCF-DA. As shown in Fig. 4, combined exposure to ibuprofen and EtOH resulted in the increased generation of hydrogen peroxide suggestive of potentiation of oxidative stress. Increased oxidative stress induces augmentation of antioxidant system in cells. Indeed, antioxidant systems such as glutathione peroxidase (GPX) and cytochrome P450 family 2 subfamily E member 1 (CYP2E1) were augmented as evidenced by increased mRNA expression of these enzymes in qPCR measurement and Western blot analysis (Fig. 5, 6).
Fig. 4.
Measurement of hydrogen peroxide generation in 2D HepG2 culture after exposure to ibuprofen with or without EtOH. H2O2 was used as a positive control for hydrogen peroxide generation (100×). N=3, *p<0.05, **p<0.01.
Fig. 5.
Augmentation of expression of anti-oxidant enzymes and CYP2E1 after exposure to ibuprofen and/ or EtOH. N=3, *p<0.05, **p<0.01.
Fig. 6.
Effect of ibuprofen with or without EtOH on the protein level of SOD, CAT, GPX and CYP2E1 in HepG2 cells. Protein levels were determined by Western blotting. The cells were treated with ibuprofen 0.8 mM +/- EtOH 200 mM for 24 h.
DISCUSSION
Drug-induced liver toxicity is affected by several factors including the ingested amount, alcohol consumption, co-treated medications, diets, and health conditions. Accordingly, individual variation is considerable in the outcome of drug-induced liver toxicity. Frequently, chronic alcoholics are known to be susceptible to drug-induced liver toxicity (Prescott, 2000; Riordan and Williams, 2002). NSAIDs are a major culprit for drug-induced liver toxicity, consisting almost 10% of whole cases (Bessone, 2010) and showing stronger associations with liver injury than other drug classes (Garcia Rodriguez et al., 1994).
Ibuprofen is known to be relatively safer than other NSAIDs. Despite the heavy use of ibuprofen around the world, low incidence of adverse events indicates an excellent safety. Indeed, there are no reports demonstrating the association of ibuprofen with liver diseases (Boureau et al., 2004). This may be attributable to a short biological half-life and absence of toxic metabolites. However, co-existing risk factors like liver diseases and co-consumption of liver injuring agents may potentiate the liver toxicity of ibuprofen. Indeed, in chronic hepatitis type C patients, the prominent elevation of transaminases (>5x) has been reported following the intake of ibuprofen, which was confirmed by a re-challenge test (Riley and Smith, 1998). We could also confirm that ibuprofen treatment alone does not induce cell death. Paradoxically in 2D HepG2 culture, ibuprofen alone increased the cell viability while when combined with EtOH it decreased CV. Previous studies reported that NSAIDs induce apoptosis through activating NF-κB pathway (Stark et al., 2007; Jana, 2008). Of note, NF-κB signaling is associated with cell survival as well as cell death (Mattson and Camandola, 2001), reflecting that NF-κB may act as an arbitrator determining the fate of cell upon the exposure to toxic stimuli. We speculate that at low extent of insult from ibuprofen alone, NF-κB pathway might have been geared toward cell survival and proliferation while at higher extent of insults from ibuprofen and EtOH, the cell survival pathway was overwhelmed and cell death was induced although further studies are necessary to convince it.
Alcohol is one of most frequent causes for liver diseases, including fatty liver, liver fibrosis and liver cancer. Mechanism underlying alcohol-induced hepatotoxicity is mostly ascribable to the generation of reactive oxygen species (ROS) during alcohol metabolism (Lieber, 1990). Oxidative stress is generated from ROS produced intrinsically and extrinsically, which ultimately disrupts cellular antioxidant capacity (Toyokuni et al., 1995) Aberrant production of ROS which cannot be mitigated by cellular antioxidant system, can damage cellular organelles, and prime inflammation, contributing to progression into ischemia, apoptosis, or necrosis (Sanchez-Valle et al., 2012). Especially, mitochondria are vulnerable to alcohol-induced oxidative stress and disruption of mitochondrial respiratory chain, ATP production and mitochondrial membrane potential ensue after heavy alcohol consumption (Zhong et al., 2014). This further potentiates ROS generation, aggravating alcohol-induced hepatotoxicity further.
Indeed, ROS appears to mediate the toxicity of ibuprofen and EtOH as evidenced by increased ROS generation and expression of endogenous antioxidant systems, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). SOD is one of the most effective antioxidant enzymes, which catalyzes the transformation of O2− (superoxide anion) to O (molecular oxygen) or H2O2 (hydrogen peroxide) (Flora, 2009). CAT and GPx remove H2O2 produced by SOD using NADP+ and glutathione, respectively (Ha et al., 2010). Since hydroperoxides can generate highly toxic and reactive hydroxyl radicals, effective clearance of H2O2 is important to maintain cellular integrity (Mates et al., 1999). Excessive ROS generation and failure of endogenous antioxidant systems in its disposition lead to damages in macromolecules like lipid peroxidation, protein sulfhydryl depletion, and DNA adduct formations (Zhen et al., 2019). Increased ROS also promotes cell death through activating cell death signaling, contributing to pathophysiological development (Giordano, 2005). Indeed, the involvement of ROS in the hepatotoxicity of NSAIDs has been reported for diclofenac, ketoprofen, and piroxicam (Jurima-Romet et al., 1994; Ghosh et al., 2015).
Moreover, CYP2E1, which is accountable for microsomal oxidation of alcohol can metabolize xenobiotics into toxic metabolites, contributing to the synergistic hepatotoxicity of alcohol and xenobiotics like industrial solvents and prescription drugs, over-the-counter analgesics and chemical carcinogens. CYP2E1 produces a large amount of ROS during the metabolism of ethanol, which plays a pivotal role in alcohol-induced oxidative stress in liver (Bang et al., 2016). In addition, NSAIDs also can be substrates for CYP2E1. Overdose of acetaminophen will lead to the saturation of conjugation metabolism, and surplus acetaminophen can undergo oxidation by CYP2E1, resulting in the formation of a highly reactive N-acetyl-p-benzoquinone imine (NAPQI). Under normal condition, NAPQI is deactivated through the conjugation with GSH but depletion of antioxidant capacity can lead to the damages on cellular organelles including mitochondria and endoplasmic reticulum (McGill and Jaeschke, 2013). Actually, CYP2E1 can also metabolize ibuprofen (Chang et al., 2008), which may explain the oxidative stress and synergistic hepatotoxicity of ibuprofen and alcohol at least in part. Indeed, we demonstrated that the co-treatment of ibuprofen and alcohol led to increased expression of CYP2E1, which may support this hypothesis, although further studies are needed to confirm it.
Collectively, this study has demonstrated that ibuprofen and EtOH can induce synergistic hepatotoxicity, providing an important line of evidence for caution against the use of ibuprofen in alcoholic patients. By introducing 3D spheroids, this study has shown that prolonged exposure to ibuprofen and EtOH at realistic condition can induce hepatotoxicity at much lower concentrations, which would be important to predict their toxicity in chronic alcoholics. Oxidative stress appeared to be key in mediating the synergistic hepatotoxicity of ibuprofen and EtOH, which suggests the utility of antioxidant dietary supplement in preventing liver toxicity from them, although the further confirmatory study is necessary.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (2018R1D1A1B07042919 and NRF-2020R1I1A1A01067636) and the Korea government (MSIT) (2018R1A5A2025286). Authors are grateful to Eunice Bae Lee for her support in the preparation of manuscript.
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest regarding this work.
REFERENCES
- Agency for Healthcare Research and Quality (AHRQ), author Ibuprofen Drug Usage Statistics, United States, 2005-2015 2016
- Aithal G. P., Day C. P. Nonsteroidal anti-inflammatory drug-induced hepatotoxicity. Clin. Liver Dis. 2007;11:563–575. doi: 10.1016/j.cld.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Bang C. Y., Byun J. H., Choi H. K., Choi J. S., Choung S. Y. Protective effects of Ecklonia stolonifera extract on ethanol-induced fatty liver in rats. Biomol. Ther. (Seoul) 2016;24:650–658. doi: 10.4062/biomolther.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessone F. Non-steroidal anti-inflammatory drugs: what is the actual risk of liver damage? World J. Gastroenterol. 2010;16:5651–5661. doi: 10.3748/wjg.v16.i45.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boureau F., Schneid H., Zeghari N., Wall R., Bourgeois P. The IPSO study: ibuprofen, paracetamol study in osteoarthritis. A randomised comparative clinical study comparing the efficacy and safety of ibuprofen and paracetamol analgesic treatment of osteoarthritis of the knee or hip. Ann. Rheum. Dis. 2004;63:1028–1034. doi: 10.1136/ard.2003.011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardile S., Martinelli M., Barabino A., Gandullia P., Oliva S., Di Nardo G., Dall'Oglio L., Rea F., de'Angelis G. L., Bizzarri B., Guariso G., Masci E., Staiano A., Miele E., Romano C. Italian survey on non-steroidal anti-inflammatory drugs and gastrointestinal bleeding in children. World J. Gastroenterol. 2016;22:1877–1883. doi: 10.3748/wjg.v22.i5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. Y., Li W., Traeger S. C., Wang B., Cui D., Zhang H., Wen B., Rodrigues A. D. Confirmation that cytochrome P450 2C8 (CYP2C8) plays a minor role in (S)-(+)- and (R)-(-)-ibuprofen hydroxylation in vitro. Drug Metab. Dispos. 2008;36:2513–2522. doi: 10.1124/dmd.108.022970. [DOI] [PubMed] [Google Scholar]
- Edmondson R., Broglie J. J., Adcock A. F., Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora S. J. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell. Longev. 2009;2:191–206. doi: 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag A., Quinzler R., Freitag M., Bickel H., Fuchs A., Hansen H., Hoefels S., Konig H. H., Mergenthal K., Riedel-Heller S. G., Schon G., Weyerer S., Wegscheider K., Scherer M., van den Bussche H., Haefeli W. E., Gensichen J. Use and potential risks of over-the-counter analgesics. Schmerz. 2014;28:175–182. doi: 10.1007/s00482-014-1415-5. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez L. A., Williams R., Derby L. E., Dean A. D., Jick H. Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch. Intern. Med. 1994;154:311–316. doi: 10.1001/archinte.1994.00420030117012. [DOI] [PubMed] [Google Scholar]
- Ghosh R., Alajbegovic A., Gomes A. V. NSAIDs and cardiovascular diseases: role of reactive oxygen species. Oxid. Med. Cell. Longev. 2015;2015:536962. doi: 10.1155/2015/536962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F. J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkind L., Laine L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: lessons learned from the bromfenac experience. Pharmacoepidemiol. Drug Saf. 2006;15:213–220. doi: 10.1002/pds.1207. [DOI] [PubMed] [Google Scholar]
- Grant S., Millar K., Kenny G. Blood alcohol concentration and psychomotor effects. Br. J. Anaesth. 2000;85:401–406. doi: 10.1093/bja/85.3.401. [DOI] [PubMed] [Google Scholar]
- Ha H. L., Shin H. J., Feitelson M. A., Yu D. Y. Oxidative stress and antioxidants in hepatic pathogenesis. World J. Gastroenterol. 2010;16:6035–6043. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana N. R. NSAIDs and apoptosis. Cell. Mol. Life Sci. 2008;65:1295–1301. doi: 10.1007/s00018-008-7511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen G., Venema J. Ibuprofen: plasma concentrations in man. J. Int. Med. Res. 1985;13:68–73. doi: 10.1177/030006058501300110. [DOI] [PubMed] [Google Scholar]
- Joo K. M., Kim S., Koo Y. J., Lee M., Lee S. H., Choi D., Lim K. M. Development and validation of UPLC method for WST-1 cell viability assay and its application to MCTT HCE™ eye irritation test for colorful substances. Toxicol. In Vitro. 2019;60:412–419. doi: 10.1016/j.tiv.2019.06.017. [DOI] [PubMed] [Google Scholar]
- Jurima-Romet M., Crawford K., Huang H. S. Comparative cytotoxicity of non-steroidal anti-inflammatory drugs in primary cultures of rat hepatocytes. Toxicol. In Vitro. 1994;8:55–66. doi: 10.1016/0887-2333(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Kim M., Lee C. S., Lim K. M. Rhododenol activates melanocytes and induces morphological alteration at sub-cytotoxic levels. Int. J. Mol. Sci. 2019;20:5665. doi: 10.3390/ijms20225665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S. Mechanism of ethanol induced hepatic injury. Pharmacol. Ther. 1990;46:1–41. doi: 10.1016/0163-7258(90)90032-W. [DOI] [PubMed] [Google Scholar]
- Mates J. M., Perez-Gomez C., Nunez, de Castro I. Antioxidant enzymes and human diseases. Clin. Biochem. 1999;32:595–603. doi: 10.1016/S0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Camandola S. NF-κB in neuronal plasticity and neurodegenerative disorders. J. Clin. Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M. R., Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L. F. Paracetamol, alcohol and the liver. Br. J. Clin. Pharmacol. 2000;49:291–301. doi: 10.1046/j.1365-2125.2000.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T. R., Smith J. P. Ibuprofen-induced hepatotoxicity in patients with chronic hepatitis C: a case series. Am. J. Gastroenterol. 1998;93:1563–1565. doi: 10.1111/j.1572-0241.1998.00484.x. [DOI] [PubMed] [Google Scholar]
- Riordan S. M., Williams R. Alcohol exposure and paracetamol-induced hepatotoxicity. Addict. Biol. 2002;7:191–206. doi: 10.1080/13556210220120424. [DOI] [PubMed] [Google Scholar]
- Sanchez-Valle V., Chavez-Tapia N. C., Uribe M., Mendez-Sanchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- Slattery J. T., Nelson S. D., Thummel K. E. The complex interaction between ethanol and acetaminophen. Clin. Pharmacol. Ther. 1996;60:241–246. doi: 10.1016/S0009-9236(96)90050-8. [DOI] [PubMed] [Google Scholar]
- Sooklert K., Wongjarupong A., Cherdchom S., Wongjarupong N., Jindatip D., Phungnoi Y., Rojanathanes R., Sereemaspun A. Molecular and morphological evidence of hepatotoxicity after silver nanoparticle exposure: a systematic review, in silico, and ultrastructure investigation. Toxicol. Res. 2019;35:257–270. doi: 10.5487/TR.2019.35.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark L. A., Reid K., Sansom O. J., Din F. V., Guichard S., Mayer I., Jodrell D. I., Clarke A. R., Dunlop M. G. Aspirin activates the NF-κB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis. 2007;28:968–976. doi: 10.1093/carcin/bgl220. [DOI] [PubMed] [Google Scholar]
- Tolman K. G. Hepatotoxicity of non-narcotic analgesics. Am. J. Med. 1998;105:13S–19S. doi: 10.1016/S0002-9343(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Toyokuni S., Okamoto K., Yodoi J., Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-B. [DOI] [PubMed] [Google Scholar]
- Zhen A. X., Piao M. J., Kang K. A., Fernando P., Kang H. K., Koh Y. S., Yi J. M., Hyun J. W. Niacinamide protects skin cells from oxidative stress induced by particulate matter. Biomol. Ther. (Seoul), 2019;27:562–569. doi: 10.4062/biomolther.2019.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Ramshesh V. K., Rehman H., Liu Q., Theruvath T. P., Krishnasamy Y., Lemasters J. J. Acute ethanol causes hepatic mitochondrial depolarization in mice: role of ethanol metabolism. PLoS ONE. 2014;9:e91308. doi: 10.1371/journal.pone.0091308. [DOI] [PMC free article] [PubMed] [Google Scholar]