Abstract
The mitogen-activated protein kinase (MAPK) pathway controls intestinal epithelial barrier permeability by regulating tight junctions (TJs) and epithelial cells damage. Heme oxygenase-1 (HO-1) and carbon monoxide (CO) protect the intestinal epithelial barrier function, but the molecular mechanism is not yet clarified. MAPK activation and barrier permeability were studied using monolayers of Caco-2 cells treated with tissue necrosis factor α (TNF-α) transfected with FUGW-HO-1 or pLKO.1-sh-HO-1 plasmid. Intestinal mucosal barrier permeability and MAPK activation were also investigated using carbon tetrachloride (CCl4) administration with CoPP (a HO-1 inducer), ZnPP (a HO-1 inhibitor), CO releasing molecule 2 (CORM-2), or inactived-CORM-2-treated wild-type mice and mice with HO-1 deficiency in intestinal epithelial cells. TNF-α increased epithelial TJ disruption and cleaved caspase-3 expression, induced ERK, p38, and JNK phosphorylation. In addition, HO-1 blocked TNF-α-induced increase in epithelial TJs disruption, cleaved caspase-3 expression, as well as ERK, p38, and JNK phosphorylation in an HO-1-dependent manner. CoPP and CORM-2 directly ameliorated intestinal mucosal injury, attenuated TJ disruption and cleaved caspase-3 expression, and inhibited epithelial ERK, p38, and JNK phosphorylation after chronic CCl4 injection. Conversely, ZnPP completely reversed these effects. Furthermore, mice with intestinal epithelial HO-1 deficient exhibited a robust increase in mucosal TJs disruption, cleaved caspase-3 expression, and MAPKs activation as compared to the control group mice. These data demonstrated that HO-1-dependent MAPK signaling inhibition preserves the intestinal mucosal barrier integrity by abrogating TJ dysregulation and epithelial cell damage. The differential targeting of gut HO-1-MAPK axis leads to improved intestinal disease therapy.
Keywords: HO-1, Intestinal barrier function, Tight junction, MAPK, Inflammatory bowel disease
INTRODUCTION
The intestinal epithelial barrier consists of a monolayer of epithelial cells and intercellular junctions between adjacent cells that seal the paracellular space and regulate the barrier permeability (Jin and Blikslager, 2020; Nighot and Ma, 2020; Slifer and Blikslager, 2020). This barrier separates harmful luminal substances, such as microorganisms, toxins, and antigens from the body, and thus plays a critical role in maintaining intestinal homeostasis. Impaired barrier function can lead to gut hyperpermeability and trigger mucosal inflammation (Tabat et al., 2020). Increased gut permeability usually results from aberrant apoptosis of intestinal epithelial cells and/or dysregulation of tight junctions (TJs) on the barrier (Su et al., 2013; Otani et al., 2020).
The mitogen-activated protein kinases (MAPKs) contain highly conserved serine/threonine protein kinases that play a central role in the regulation of TJ permeability (Xiong et al., 2020), including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK. MAPKs are required for the transcription and production of various pro-inflammatory agents and the regulation of barrier function in vitro (Yang et al., 2019) and in vivo (Meir et al., 2019). Tissue necrosis factor α (TNF-α), a proinflammatory cytokine central to the pathogenesis of inflammatory bowel diseases (IBD) has been shown to promote TJ dysregulation and induce epithelial barrier loss by upregulating MAPK expression and enzymatic activity (Petecchia et al., 2012; Borgonetti et al., 2020).
Heme oxygenase-1 (HO-1) is an enzyme specialized in degrading heme and is assembled with biliverdin, carbon monoxide (CO), and free iron (Vijayan et al., 2018). HO-1 is normally expressed in the mucosal layer of gastrointestinal track (Naito et al., 2011). The upregulation of HO-1 expression and CO level inhibits inflammatory responses and attenuates intestinal barrier disruption (Chi et al., 2018; Stefanson and Bakovic, 2018; Wang et al., 2020). Heme supplementation modifies the microenvironment of the colonic tissue, which plays a protective role in dextran sodium sulfate (DSS)-induced colitis mice via regulation of macrophage polarization in both HO-1-dependent and -independent manner (Wu et al., 2020). However, how HO-1 and/or CO controls TJs and epithelial cells damage remains unclear. Therefore, in this study, we aimed at elucidating the effects and molecular mechanisms of HO-1/CO in regulating TJ function and epithelial damage. The current study demonstrated that HO-1-CO axis suppresses the MAPK pathway activation to maintain intestinal mucosal barrier permeability.
MATERIALS AND METHODS
Cell culture and treatments
The human colonic adenocarcinoma cell line Caco-2 was cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% fetal calf serum (FCS, Gibco, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C in a 5% CO2 atmosphere. Then, TNF-α (100 ng/mL, PeproTech, USA) was added to Caco-2 cells for 24 h. Lentiviral transfection was performed using LipofiterTM (Hanbio Biotechnology, China), according to the manufacturer’s instructions. The FUGW-HO-1 and pLKO.1-sh-HO-1 plasmids were obtained from Hanbio Biotechnology. The sequences used for HO-1 overexpression were as follows: F-CACAGACCGGTATGGAGCGTCCGCAACCCGACAG, R-CACAGGAATTCTCACATGGCATAAAGCCCTACAGC. The sequence used for short hairpin RNA (shRNA) targeting HO-1 was F-CCGGACAGTTGCTGTAGGGCTTTATCTCGAGATAAAGCCCTACAGCAACTGTTTTTTG; R-AATTCAAAAAACAGTTGCTGTAGGGCTTTATCTCGAGATAAAGCCCTACAGCAACTGT.
Animals experiments
C57BL/6 male wild type (WT) mice (6-8-week-old and weighing 20-25 g) were obtained from the Laboratory Animal Center of Dalian Medical University (Liaoning, China). Briefly, the mice were administered 2 mL/kg carbon tetrachloride (CCl4, Aladdin Biochemical Technology Co., Ltd, Shanghai, China) via intraperitoneal injection (CCl4:olive oil=1:3) twice a week for 12 weeks to induce barrier loss. The control group was administered olive oil. In the last 2 weeks, according to the groups, all surviving mice were administered cobalt protoporphyrin (CoPP, 5 mg/kg, Sigma-Aldrich, Saint Louis, MO, USA) (Bakhautdin et al., 2014), zinc protoporphyrin (ZnPP, 5 mg/kg, Sigma-Aldrich) (Liu et al., 2018), carbon monoxide releasing molecule-2 (CORM-2, 8 mg/kg, Sigma-Aldrich) (Xue and Habtezion, 2014), inactivated-CORM-2 (iCORM-2, 8 mg/kg), or normal saline via intraperitoneal injection twice a week. iCORM-2 was generated as described previously by addition to cell culture medium overnight (18 h) at 37°C and bubbling with air (N2) to remove the residual CO (Xue and Habtezion, 2014). The WT C57BL/6 mice were randomized into six groups: Control (n=6), CCl4 (n=10), CCl4+CoPP (n=10), CCl4+ZnPP (n=10), CCl4+CORM-2 (n=10), and CCl4+iCORM-2 (n=10). VillinCreHmox1floxp/floxp mice with knockout HO-1, specifically on the intestinal epithelial cells, were constructed using C57BL/6 mice from Beijing Viewsolid Biotechnology Co. (Beijing, China) by crossing Villin-Cre transgenic mice with Hmox1floxp/floxp mice that contain LoxP sites flanking exon 2 of the Hmox-1 gene. WT and Hmox1floxp/floxp mice were bred in the same room of our vivarium and used as controls for experiments involving VillinCreHmox1floxp/floxp mice. WT, Hmox1floxp/floxp, and VillinCreHmox1floxp/floxp C57BL/6 mice were also induced by CCl4. The mice were randomized to six groups: WT-Control (n=5), Hmox1floxp/floxp-Control (n=5), VillinCreHmox1floxp/floxp-Control (n=6), WT-CCl4 (n=6), Hmox1floxp/floxp-CCl4 (n=5), and VillinCre Hmox1floxp/floxp-CCl4 (n=6). Then, intestine samples were collected. The excised ileum tissues in each group were fixed with 4% paraformaldehyde for histopathological staining, and the remnants of intestine tissues were stored at –80°C for subsequent use. All animals and experiments were carried out in strict accordance with the recommendations of the Animal Care and Use Committee of Dalian Medical University, and the protocols were approved by the institutional Animal Experimental Ethics Committee (Approval No. AEE18006).
Histopathological examination
Freshly dissected ileum biopsies were fixed with 4% formaldehyde in phosphate-buffered saline (PBS) (pH 7.2-7.4), embedded in paraffin, and stained with hematoxylin and eosin (H&E), according to the standard protocol. The slides were examined by an experienced histopathologist who was blinded to the study design. Intestinal mucosal injuries were graded according to the Chiu’s scoring system (Li et al., 2019): 0=normal mucosa; 1=mucosal degeneration with extended subepithelial space; 2=intestinal villus epithelium raised and more extended subepithelial space; 3=intestinal villus epithelium deciduation; 4=intestinal villus epithelium shedding, only lamina propria; 5=severe degeneration and mucosal digestion with disintegration of lamina propria, bleeding, and ulcers.
Western blot analysis
Western blot analysis was performed, as described previously (Mishra et al., 2017). The primary antibodies used in our experiments were as follows: anti-ZO-1 (1:500, Proteintech, Shanghai, China), anti-occludin (1:1,000, Abcam, Cambridge, UK), anti-caspase-3 (1:500, Cell Signaling Technology, Boston, MA, USA), anti-cleaved caspase-3 (1:500, Cell Signaling Technology), anti-ERK (1:1,000, Abcam), anti-phospho-ERK (1:1,000, Abcam), anti-p38 (1:500, Abbkine, Wuhan, China), anti-phospho-p38 (1:500, Abbkine), anti-JNK (1:1,000, Abcam), anti-phospho-JNK (1:1,000, Abcam), anti-HO-1 (1:500, Proteintech), anti-β-actin (1:2,000, Proteintech), or anti-GAPDH (1:2,000, Proteintech). Also, the secondary antibodies, including anti-rabbit IgG (H+L) and anti-mouse IgG (H+L), were purchased from Proteintech (1:2,000). The intensity of the immunoreactive bands was quantitated using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The data were representative of three or more independent experiments, each with similar results. The continuous data are shown as mean ± standard deviation. The comparison between two groups were performed using Mann-Whitney U test. The comparisons among multiple groups were made using Kruskal-Wallis H test. p-value ≤0.05 was considered statistically significant.
RESULTS
Elevation of HO-1 increases the level of TJ proteins and reduces cell apoptosis
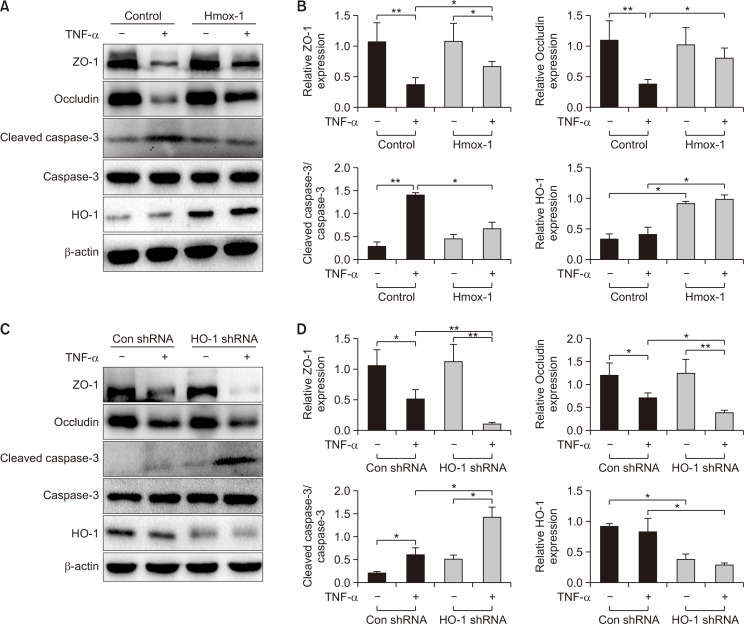
In order to examine the effects of HO-1 on the intestinal epithelial barrier, gain- or loss-of-HO-1-function experiments were conducted using Caco-2 cells transfected with FUGW-HO-1 or pLKO.1-sh-HO-1 plasmid. The cells transfected with empty plasmids served as the control groups. For these in-vitro studies, TNF-α was used to stimulate the Caco-2 epithelial cells as it is one of the most frequently used cell models for mimicking the intestinal epithelial barrier injury, and TNF-α stimulation does not affect the expression of HO-1 (Fig. 1A-1D, all p>0.05). Subsequently, the levels of TJ proteins (ZO-1 and occludin) and cell apoptosis-related factors (caspase-3 and cleaved caspase-3) were determined by Western blot analysis. As shown in Fig. 1A and 1B, the expression of ZO-1 and occludin was significantly increased, and the ratio of cleaved caspase-3/caspase-3 was remarkedly decreased in cells transfected with FUGW-HO-1 after TNF-α treatment as compared to the control group (all p<0.05). However, relative to the control group, HO-1 shRNA significantly decreased the expression of ZO-1 (p<0.01) and occludin (p<0.05) and distinctly increased the ratio of cleaved caspase-3/caspase-3 (p<0.05) as a response to TNF-α treatment (Fig. 1C, 1D). Together, these data indicated that the elevation of HO-1 maintained the integrity of the intestinal epithelial barrier by upregulating the level of TJ proteins and reducing the apoptosis of intestinal epithelial cells.
Fig. 1.
Overexpressed HO-1 elevates the expression of TJ proteins while reducing the ratio of cleaved caspase-3/caspase-3 in Caco-2 cells. Caco-2 cells were transfected with FUGW-HO-1 or pLKO. 1-sh-HO-1 plasmid and cells transfected with empty plasmid as the control groups. (A, B) The representative protein bands and the level of ZO-1, occludin, cleaved caspase-3/caspase-3, and HO-1 in Caco-2 cells transfected with FUGW-HO-1 or empty plasmid, as measured by Western blot analysis. (C, D) The representative protein bands and the expression of ZO-1, occludin, cleaved caspase-3/caspase-3, and HO-1 in Caco-2 cells transfected with the pLKO.1-sh-HO-1 or empty plasmid, as measured by Western blot analysis. Measurement data are expressed as mean ± SD. All data presented were representative of three or more independent experiments, each with similar results. Bar *p<0.05 and **p<0.01.
Elevation of HO-1 blocks the MAPK signaling pathway
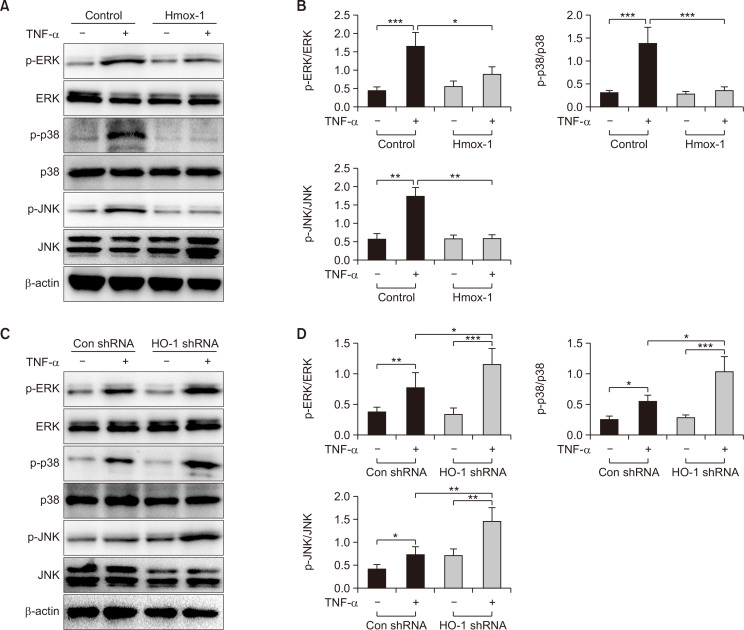
We used RNA-seq to explore the putative molecular mechanism of HO-1 in barrier disruption, and the results demonstrated that HO-1 regulates the expression of MAPK signaling pathway-related genes in TNF-α-induced Caco-2 cells treated with CoPP or ZnPP (Supplementary Fig. 1A-1D). We also examined the expression of MAPK signaling pathway-related proteins, such as ERK, phosphorylated-ERK (p-ERK), p38, phosphorylated-p38 (p-p38), JNK, and phosphorylated-JNK (p-JNK), by Western blot analysis and found that the ratios of p-ERK/ERK (p<0.05), p-p38/p38 (p<0.001), and p-JNK/JNK (p<0.01) were significantly decreased in cells transfected with FUGW-HO-1 after treatment with TNF-α as compared to that in the control group (Fig. 2A, 2B). However, compared to the control group, HO-1 shRNA significantly increased the ratios of p-ERK/ERK (p<0.05), p-p38/p38 (p<0.05), and p-JNK/JNK (p<0.01) in cells treated with TNF-α (Fig. 2C, 2D). These findings indicated that elevated HO-1 might suppress the phosphorylation of the MAPK signaling pathway in Caco-2 cells.
Fig. 2.
HO-1 overexpression suppresses the MAPK signaling pathway in Caco-2 cells. Caco-2 cells were transfected with FUGW-HO-1 or pLKO.1-sh-HO-1 plasmid, and cells transfected with empty plasmid as the control groups. (A, B) The representative protein bands and the ratios of p-ERK/ERK, p-p38/p38, and p-JNK/JNK in Caco-2 cells transfected with FUGW-HO-1 or empty plasmid, as measured by Western blot analysis. (C, D) The representative protein bands and the ratios of p-ERK/ERK, p-p38/p38, and p-JNK/JNK in Caco-2 cells transfected with pLKO.1-sh-HO-1, as measured by Western blot analysis. Measurement data are expressed as mean ± SD. All data presented were representative of three or more independent experiments, each with similar results. Bar *p<0.05, **p<0.01, and ***p<0.001.
Effect of HO-1 and CORM-2 on intestinal epithelial barrier injury and MAPK signaling pathway in CCl4-induced mice
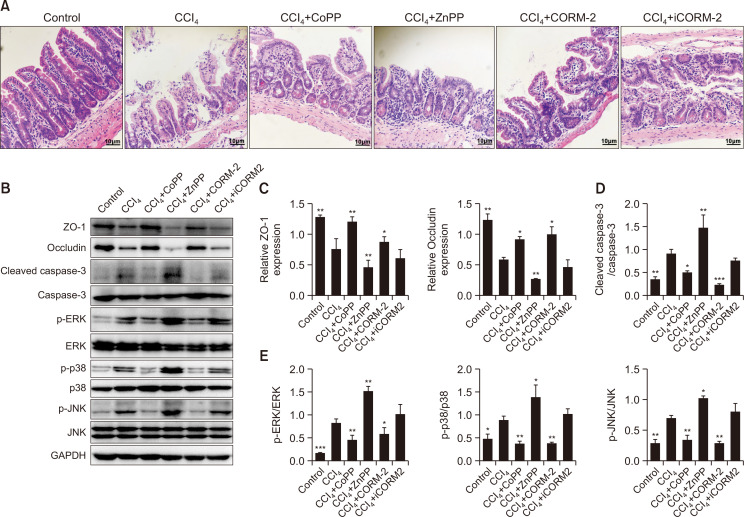
To determine the therapeutic effects of CoPP, ZnPP, CORM-2, or iCORM-2, we used CCl4 to induce intestinal epithelial barrier damage. Mice were administered CoPP, ZnPP, CORM-2, iCORM-2, or normal saline after CCl4 administration, and then, serum was collected and intestinal tissues harvested. The disrupted architecture and the disarranged ileal epithelial mucosal villi were observed by H&E staining in the CCl4-treated group (Fig. 3A). CoPP significantly upregulated the level of HO-1 protein in the intestine of the mice model (Supplementary Fig. 2, p<0.05). H&E staining revealed that CoPP and CORM-2 administration attenuated the CCl4-induced pathological changes in the ileum mucosa (Fig. 3A); however, groups treated with ZnPP showed severe pathological changes than the CCl4-treated group. Western blot analysis revealed that CoPP and CORM-2 administration significantly increased the expression of ZO-1 (p<0.01 and p<0.05, respectively) and occludin (both p<0.05) proteins, and reduced the ratios of cleaved caspase-3/caspase-3 (p<0.01 and p<0.001, respectively), p-ERK/ERK (p<0.01 and p<0.05, respectively), p-p38/p38 (both p<0.01), and p-JNK/JNK (both p<0.01) as compared to the CCl4-treated group (Fig. 3B-3E). However, ZnPP treatment reversed these effects (Fig. 3B-3E), and iCORM-2 had no effects on the barrier loss and the level of MAPK pathway-related proteins after CCl4 challenge (all p>0.05). These findings indicated that CoPP and CORM-2 repaired the intestinal epithelial barrier loss, which was characterized by the alleviated intestinal mucosal lesions, the upregulated expression of TJ proteins, and the reduced cell apoptosis. Moreover, CoPP and CORM-2 inhibited the phosphorylation of the MAPK signaling pathway.
Fig. 3.
Upregulation of HO-1 and exogenous supply of CO prevent mucosal lesion, TJ protein disruption, and intestinal epithelial cell apoptosis and block the activation of the MAPK signaling pathway in CCl4-induced mice. Mice were treated with CoPP (an HO-1 inducer), ZnPP (an HO-1 inhibitor), CORM-2 (exogenous supply of CO), iCORM-2, or normal saline after CCl4 administration. (A) Representative H&E staining images of ileal mucosa in mice (400×, scale bar=10 μm) (n=3). (B) The representative protein bands of ZO-1, occludin, cleaved caspase-3, caspase-3, p-ERK, ERK, p-p38, p38, p-JNK, and JNK in mice, as measured by Western blot analysis. (C) The level of ZO-1 and occludin relative to GAPDH in mice. (D, E) The ratios of cleaved caspase-3/caspase-3, p-ERK/ERK, p-p38/p38, and p-JNK/JNK in mice. Measurement data are expressed as mean ± SD. All data presented were representative of three or more independent experiments, each with similar results. *p<0.05, **p<0.01, and ***p<0.001 vs. the CCl4 group.
Conditional knockout of HO-1 on intestinal epithelial cells loses the protective effect against CCl4-induced barrier loss
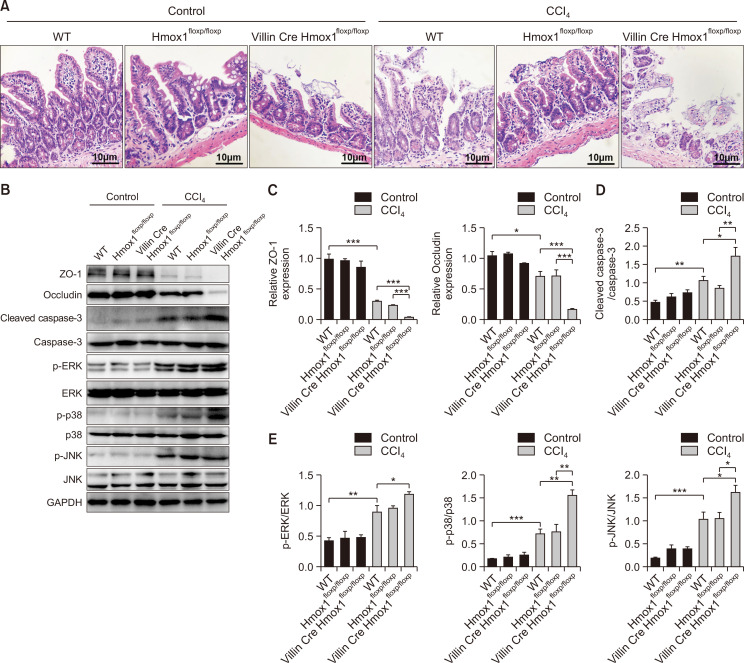
To confirm the intestinal epithelial cell-specific function of HO-1 in mediating the barrier loss, we specifically deleted HO-1 from intestinal epithelial cells (VillinCreHmox1floxp/floxp) (Supplementary Fig. 3) by crossing Villin-Cre mice with Hmox1floxp/floxp mice. WT and Hmox1floxp/floxp mice displayed relatively mild mucosal lesion, TJ disruption, and cell apoptosis after CCl4 challenge (Fig. 4A-4D), while VillinCreHmox1floxp/floxp mice showed a significant aggravation of mucosal lesions (Fig. 4A), disruption of TJ proteins (Fig. 4B, 4C), and increase in cell apoptosis (Fig. 4B, 4D). Moreover, the animals presented a significantly elevated MAPK phosphorylation as compared to the WT and Hmox1floxp/floxp mice (Fig. 4B, 4E). In summary, HO-1 on intestinal epithelial cells contributed to the protection of the paracellular leakage pathway, effectuating the repair of barrier disruption. These processes were accompanied by the inhibition of MAPK phosphorylation in a HO-1-dependent manner.
Fig. 4.
HO-1-/- mice fail to prevent mucosal lesion, TJ disruption, and intestinal epithelial cells apoptosis and promote the MAPK signaling pathway phosphorylation after CCl4 administration. C57BL/6 WT, Hmox1floxp/floxp, and VillinCreHmox1floxp/floxp mice were studied using CCl4. (A) Representative H&E staining images of ileal mucosa in mice (400×, scale bar=10 μm) (n=3). (B) The representative protein bands of ZO-1, occludin, cleaved caspase-3, caspase-3, p-ERK, ERK, p-p38, p38, p-JNK, and JNK in mice, as measured by Western blot analysis. (C) The expression of ZO-1 and occludin relative to GAPDH in mice. (D, E) The ratios of cleaved caspase-3/caspase-3, p-ERK/ERK, p-p38/p38, and p-JNK/JNK in mice. Measurement data are expressed as mean ± SD. The data presented were representative of three or more independent experiments, each with similar results. Bar *p<0.05, **p<0.01, and ***p<0.001.
DISCUSSION
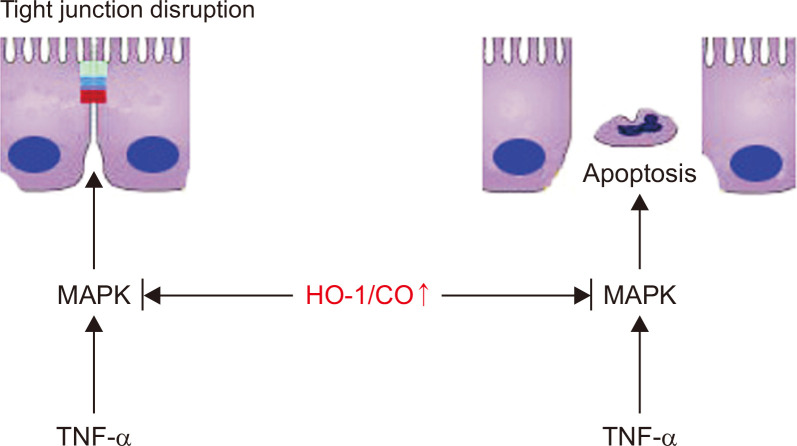
Gut leakiness is a well-characterized phenomenon in patients with many diseases (Shao et al., 2018; Albillos et al., 2020; Hall et al., 2020; Wang et al., 2020). In this study, we demonstrated that the dysregulation of TJs and the apoptosis of intestinal epithelial cells induced intestinal barrier loss in vitro and in vivo. The elevated expression of HO-1 repaired the intestinal epithelial barrier dysfunction by increasing the expression of TJ proteins, regulating the epithelial structure and reducing the apoptotic frequency of cells. Furthermore, the loss of HO-1 enhanced the disruption of TJs. In addition, HO-1 was shown to interact with the MAPK signaling pathway, and the overexpression of HO-1 suppressed the upregulation of phosphorylated ERK, phosphorylated p38, and phosphorylated JNK, while the loss of HO-1 induced an increase in the phosphorylation of ERK, p38, and JNK. Herein, we provided novel evidence for the relative contributions of HO-1-mediated MAPK pathway involved in repairing intestinal barrier function (Fig. 5).
Fig. 5.
HO-1/CO signaling protects the mucosal barrier. HO-1/CO signaling preserves tight junction barrier and epithelial apoptosis by blocking MAPK activation.
HO-1 is the rate-limiting enzyme in the degradation of heme, converting heme to free iron, biliverdin, and CO, which in turn, plays a major role in conferring protection against the different types of damage, such as intestinal diseases (Onyiah et al., 2013; Wu et al., 2020), liver injury (Chen et al., 2019), sepsis (Zhan et al., 2018), and pulmonary inflammation (Cho et al., 2018). However, a majority of the studies used HO-1 as the damage stimuli, while no data are available on the protective role of HO-1 in the intestinal barrier disruption after CCl4 via direct regulation of HO-1. TNF-α increases the intestinal TJ permeability (Feng and Teitelbaum, 2013; Su et al., 2013; Du et al., 2015), which contributes to the increase in the intestinal permeability (Hartmann et al., 2012; Chen et al., 2015). Our previous study has demonstrated that TNF-α levels are increased in BDL-induced cholestatic liver injury (Zhang et al., 2017). The present study demonstrated that TNF-α treatment increased the permeability of epithelial barrier, reduced the intestinal TJs, and induced the expression of cell apoptosis-related proteins, such as cleaved caspase-3 in vitro. In addition, TNF-α stimulation did not exert a significant effect on the expression of HO-1. The overexpression of HO-1 and exogenous administration of the HO-1 inducer CoPP repaired the intestinal epithelial barrier dysfunction in TNF-α-induced Caco-2 cells and CCl4-induced mice. Intriguingly, CORM-2 mimicked the protective role of HO-1 via exogenous administration of CO. Conversely, HO-1 shRNA and exogenous administration of the HO-1 inhibitor ZnPP reversed these effects. Our findings indicated that HO-1/CO repaired the intestinal TJ permeability and reduced the cell apoptosis, and therefore, restored the intestinal epithelial barrier defects. However, the potential mechanisms of HO-1/CO protecting the barrier loss are currently under intensive focus.
The MAPK signaling pathway (including p38, ERK, and JNK) harbor highly conserved serine/threonine protein kinases that function in various fundamental cellular processes, such as growth/proliferation, differentiation, motility, apoptosis/survival, inflammation, and innate immunity (Hua et al., 2017). The MAPK signaling pathway has been shown to play a major role in the disruption of the intestinal barrier (Song et al., 2010; Wang et al., 2017; Yang et al., 2019). In addition, colonic inflammation and downregulation of occludin and claudin-1 was mediated by p38, JNK, and ERK phosphorylation (Sun et al., 2015; Ran et al., 2018). In the present study, RNA-seq analysis data revealed that the differentially expressed genes were mainly clustered to the MAPK signaling pathway in TNF-α-induced Caco-2 cells. Based on these data, we found an obvious increase in the phosphorylation of p38, ERK, and JNK as a response to TNF-α stimulation. Furthermore, dysregulation of TJ, apoptosis of intestinal epithelial cells, and disarrangement of ileum villus structure were accompanied by the activation of the MAPK signaling pathway after CCl4. These data showed that the MAPK signaling pathway might participate in intestinal epithelial barrier injury. Nuclear factor E2 related factor 2 (Nrf2)-mediated HO-1 elevation significantly reduced the phosphorylation levels p38-MAPK in the small intestine (Zhuang et al., 2019). Hirsutenone reverses the disordered intestinal permeability by activating the EGFR/Akt and ERK pathways, which are involved in the regulation of HO-1 expression (Seo et al., 2014). Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signaling and reduction of oxidative stress (De Backer et al., 2009). In this study, HO-1 overexpression and CoPP and CORM-2 administration blocked the phosphorylation of ERK, p38, and JNK, while HO-1 shRNA and ZnPP treatment enhanced the phosphorylation. Our findings suggested that HO-1/CO protects the barrier loss by inhibiting the phosphorylation of the MAPK signaling pathway. However, further studies are needed to delineate the upstream (e.g., p-MAPKKs and their total) and downstream (p-c-Jun/p-c-Fos and their total) effects of MAPK activation or inhibition in the intestinal barrier damage.
In summary, this study indicated that intestinal HO-1 contributes to maintaining the integrity of intestinal epithelial barrier, increasing the intestinal TJs and reducing the apoptosis of intestinal epithelial cells through inhibiting the phosphorylation of the MAPK signaling pathway. Targeting the gut HO-1-MAPK crosstalk would track the clinical management of patients with barrier loss.
Supplementary Materials
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 81670479).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Borgonetti V., Cocetta V., Biagi M., Carnevali I., Governa P., Montopoli M. Anti-inflammatory activity of a fixed combination of probiotics and herbal extract in an in vitro model of intestinal inflammation by stimulating Caco-2 cells with LPS-conditioned THP-1 cells medium. Minerva Pediatr. 2020 doi: 10.23736/S2724-5276.20.05765-5. doi: 10.23736/S0026-4946.20.05765-5 [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Bakhautdin B., Das D., Mandal P., Roychowdhury S., Danner J., Bush K., Pollard K., Kaspar J. W., Li W., Salomon R. G., McMullen M. R., Nagy L. E. Protective role of HO-1 and carbon monoxide in ethanol-induced hepatocyte cell death and liver injury in mice. J. Hepatol. 2014;61:1029–1037. doi: 10.1016/j.jhep.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J. H., Kim Y. H., Sohn D. H., Seo G. S., Lee S. H. Ameliorativeeffect of Alnus japonica ethanol extract on colitis through the inhibition of inflammatory responses and attenuation of intestinal barrier disruption in vivo and in vitro. Biomed. Pharmacother. 2018;108:1767–1774. doi: 10.1016/j.biopha.2018.10.050. [DOI] [PubMed] [Google Scholar]
- Cho R. L., Lin W. N., Wang C. Y., Yang C. C., Hsiao L. D., Lin C. C., Yang C. M. Heme oxygenase-1 induction by rosiglitazone via PKCα/AMPKα/p38MAPKα/SIRT1/PPARγ pathway suppresses lipopolysaccharide-mediated pulmonary inflammation. Biochem. Pharmacol. 2018;148:222–237. doi: 10.1016/j.bcp.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Chen Y., Park H. J., Park J., Song H. C., Ryter S. W., Surh Y. J., Kim U. H., Joe Y., Chung H. T. Carbon monoxide ameliorates acetaminophen-induced liver injury by increasing hepatic HO-1 and parkin expression. FASEB J. 2019;33:13905–13919. doi: 10.1096/fj.201901258RR. [DOI] [PubMed] [Google Scholar]
- Chen P., Stärkel P., Turner J. R., Ho S. B., Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–894. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Chen Y., Shi Y., Liu T., Cao Y., Tang Y., Ge X., Nie H., Zheng C., Li Y. C. 1,25-Dihydroxyvitamin D protects intestinal epithelial barrier by regulating the myosin light chain kinase signaling pathway. Inflamm. Bowel Dis. 2015;21:2495–2506. doi: 10.1097/MIB.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer O., Elinck E., Blanckaert B., Leybaert L., Motterlini R., Lefebvre R. A. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut. 2009;58:347–356. doi: 10.1136/gut.2008.155481. [DOI] [PubMed] [Google Scholar]
- Feng Y., Teitelbaum D. H. Tumour necrosis factor-α-induced loss of intestinal barrier function requires TNFR1 and TNFR2 signalling in a mouse model of total parenteral nutrition. J. Physiol. 2013;591:3709–3723. doi: 10.1113/jphysiol.2013.253518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Chi W., Su L., Li J., Zhang Z., Yuan X. ROS-induced oxidative injury involved in pathogenesis of fungal keratitis via p38MAPK activation. Sci. Rep. 2017;7:10421. doi: 10.1038/s41598-017-09636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann P., Haimerl M., Mazagova M., Brenner D. A., Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330–1340. doi: 10.1053/j.gastro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. H. T., Lee J. S., Murphy E. M., Gerich M. E., Dran R., Glover L. E., Abdulla Z. I., Skelton M. R., Colgan S. P. Creatine transporter, reduced in colon tissues from patients with inflammatory bowel diseases, regulates energy balance in intestinal epithelial cells, epithelial integrity, and barrier function. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.033. doi: 10.1053/j.gastro.2020.05.033 [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Blikslager A. T. The regulation of intestinal mucosal barrier bymyosin light chain kinase/Rho kinases. Int. J. Mol. Sci. 2020;21:E3550. doi: 10.3390/ijms21103550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu M., Gao S., Cai L., Zhang Q., Yan S., Liu G., Ji B. Cold-inducible RNA-binding protein maintains intestinal barrier during deep hypothermic circulatory arrest. Interact. Cardiovasc. Thorac. Surg. 2019;29:583–591. doi: 10.1093/icvts/ivz147. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhu Q., Zhang M., Yin T., Xu R., Xiao W., Wu J., Deng B., Gao X., Gong W., Lu G., Ding Y. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2018;2018:7161592. doi: 10.1155/2018/7161592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir M., Burkard N., Ungewiß H., Diefenbacher M., Flemming S., Kannapin F., Germer C. T., Schweinlin M., Metzger M., Waschke J., Schlegel N. Neurotrophic factor GDNF regulates intestinal barrier function in inflammatory bowel disease. J. Clin. Invest. 2019;129:2824–2840. doi: 10.1172/JCI120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Tiwari S., Gomes A. V. Protein purification and analysis: next generation western blotting techniques. Expert Rev. Proteomics. 2017;14:1037–1053. doi: 10.1080/14789450.2017.1388167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nighot P., Ma T. Endocytosis of intestinal tight junction proteins: in time and space. Inflamm. Bowel Dis. 2020 doi: 10.1093/ibd/izaa141. doi: 10.1093/ibd/izaa141 [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Takagi T., Uchiyama K., Yoshikawa T. Heme oxygenase-1: a novel therapeutic target for gastrointestinal diseases. J. Clin. Biochem. Nutr. 2011;48:126–133. doi: 10.3164/jcbn.10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S., Oami T., Yoseph B. P., Klingensmith N. J., Chen C. W., Liang Z., Coopersmith C. M. Overexpression of BCL-2 in the intestinal epithelium prevents sepsis-induced gut barrier dysfunction via altering tight junction protein expression. Shock. 2020;54:330–336. doi: 10.1097/SHK.0000000000001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyiah J. C., Sheikh S. Z., Maharshak N., Steinbach E. C., Russo S. M., Kobayashi T., Mackey L. C., Hansen J. J., Moeser A. J., Rawls J. F., Borst L. B., Otterbein L. E., Plevy S. E. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology. 2013;144:789–798. doi: 10.1053/j.gastro.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petecchia L., Sabatini F., Usai C., Caci E., Varesio L., Rossi G. A. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab. Invest. 2012;92:1140–1148. doi: 10.1038/labinvest.2012.67. [DOI] [PubMed] [Google Scholar]
- Ran X., Li Y. H., Chen G. X., Fu S., He D., Huang B., Wei L., Lin Y., Guo Y., Hu G. Farrerol ameliorates TNBS-induced colonic inflammation by inhibiting ERK1/2, JNK1/2, and NF-κB signaling pathway. Int. J. Mol. Sci. 2018;19:2037. doi: 10.3390/ijms19072037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifer Z. M., Blikslager A. T. The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int. J. Mol. Sci. 2020;21:972. doi: 10.3390/ijms21030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanson A. L., Bakovic M. Falcarinol is a potent inducer of heme oxygenase-1 and was more effective than sulforaphane in attenuating intestinal inflammation at diet-achievable doses. Oxid. Med. Cell. Longev. 2018;2018:3153527. doi: 10.1155/2018/3153527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo G. S., Jiang W. Y., Park P. H., Sohn D. H., Cheon J. H., Lee S. H. Hirsutenone reduces deterioration of tight junction proteins through EGFR/Akt and ERK1/2 pathway both converging to HO-1 induction. Biochem. Pharmacol. 2014;90:115–125. doi: 10.1016/j.bcp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Su L., Nalle S. C., Shen L., Turner E. S., Singh G., Breskin L. A., Khramtsova E. A., Khramtsova G., Tsai P. Y., Fu Y. X., Abraham C., Turner J. R. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. B., Wang Y. Y., Meng F. S., Zhang Q. H., Zeng J. Y., Xiao L. P., Yu X. P., Peng D. D., Su L., Xiao B., Zhang Z. S. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-κB activation. PLoS ONE. 2010;5:e12969. doi: 10.1371/journal.pone.0012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. H., Xu C., Chen G. Q., Yu M., Yang S., Qiu Y., Peng K., Wang W., Xiao W., Yang H. A novel role of OS-9 in the maintenance of intestinal barrier function from hypoxia-induced injury via p38-dependent pathway. Int. J. Biol. Sci. 2015;11:664–671. doi: 10.7150/ijbs.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao T., Zhao C. Q., Li F. Y., Gu Z. L., Liu L. M., Zhang L. H., Wang Y. H., He L. Q., Liu Y. H., Liu Q., Chen Y. P., Donde H., Wang R., Jala V. R., Barve S., Chen S. Y., Zhang X., Chen Y. P., McClain C. J., Wenke F. Intestinal HIF-1α deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction. J. Hepatol. 2018;69:886–895. doi: 10.1016/j.jhep.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabat M. W., Marques T. M., Markgren M., Löfvendahl L., Brummer R. J., Wall R. Acute effects of butyrate on induced hyperpermeability and tight junction protein expression in human colonic tissues. Biomolecules. 2020;10:766. doi: 10.3390/biom10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan V., Wagener F. A. D. T. G., Immenschuh S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018;153:159–167. doi: 10.1016/j.bcp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Wang H., Liu Y., Shi H., Wang X., Zhu H., Pi D., Leng W., Li S. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-κB and p38 signaling in weaned pigs after LPS challenge. Eur. J. Nutr. 2017;56:1433–1443. doi: 10.1007/s00394-016-1189-x. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang S., Zhao H., Qin H., Zhang J., Dong J., Zhang H., Liu X., Zhao Z., Zhao Y., Shao M., Wu F., Zhang W. Carbon monoxide inhibits the expression of proteins associated with intestinal mucosal pyroptosis in a rat model of sepsis induced by cecal ligation and puncture. Med. Sci. Monit. 2020;26:e920668. doi: 10.12659/MSM.920668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wu B., Zhang Z., Lu H., Fan C., Qi Q., Gao Y., Li H., Feng C., Zuo J., Tang W. Heme protects intestinal mucosal barrier in DSS-induced colitis through regulating macrophage polarization in both HO-1-dependent and HO-1-independent way. FASEB J. 2020;34:8028–8043. doi: 10.1096/fj.202000313RR. [DOI] [PubMed] [Google Scholar]
- Xue J., Habtezion A. Carbon monoxide-based therapy amelioratesacute pancreatitis via TLR4 inhibition. J. Clin. Invest. 2014;124:437–447. doi: 10.1172/JCI71362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Huang J., Li X., Zhang Z., Jin M., Wang J., Xu Y., Wang Z. Icariin and its phosphorylated derivatives alleviate intestinal epithelial barrier disruption caused by enterotoxigenic Escherichia coli through modulate p38 MAPK in vivo and in vitro. FASEB J. 2020;34:1783–1801. doi: 10.1096/fj.201902265R. [DOI] [PubMed] [Google Scholar]
- Yang L., Liu G., Lian K., Qiao Y., Zhang B., Zhu X., Luo Y., Shang Y., Gu X. L. Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the NF-κB/MAPK signaling pathway in broilers. J. Anim. Sci. 2019;97:1679–1692. doi: 10.1093/jas/skz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C. Y., Chen D., Luo J. L., Shi Y. H., Zhang Y. P. Protective role of down-regulated microRNA-31 on intestinal barrier dysfunction through inhibition of NF-κB/HIF-1α pathway by binding to HMOX1 in rats with sepsis. Mol. Med. 2018;24:55. doi: 10.1186/s10020-018-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. J., Zhang Z. L., Liu B. J., Jin Y. L., Tian Y., Xin Y., Duan Z. J. The protective effect of heme oxygenase-1 against intestinal barrier dysfunction in cholestatic liver injury is associated with NF-κB inhibition. Mol. Med. 2017;23:215–224. doi: 10.2119/molmed.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S., Zhong J., Bian Y. F., Fan Y., Chen Q., Liu P., Liu Z. Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury viamodulation of Nrf2 and MAPKs. Life Sci. 2019;216:168–175. doi: 10.1016/j.lfs.2018.11.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.