Abstract
Traditional Chinese medicine (TCM) was the primary source of medical treatment for the people inhabiting East Asia for thousands of years. These ancient practices have incorporated a wide variety of materia medica including plants, animals and minerals. As modern sciences, including natural products chemistry, emerged, there became increasing efforts to explore the chemistry of this materia medica to find molecules responsible for their traditional use. Insects, including beetles have played an important role in TCM. In our survey of texts and review articles on TCM materia medica, we found 48 species of beetles from 34 genera in 14 different families that are used in TCM. This review covers the chemistry known from the beetles used in TCM, or in cases where a species used in these practices has not been chemically studied, we discuss the chemistry of closely related beetles. We also found several documented uses of beetles in Traditional Korean Medicine (TKM), and included them where appropriate. There are 129 chemical constituents of beetles discussed.
Keywords: Beetle, Traditional Chinese Medicine, Traditional Korean Medicine, Coleoptera, Chemical defense, Secondary metabolites
INTRODUCTION
Traditional Chinese Medicine (TCM) is widely used both inside China and beyond its borders. One survey indicated that approximately 20% of Chinese people over 45 years old used TCM, and use of TCM for chronic conditions was even higher (Liu et al., 2015). In the United States, it was estimated that over 1 million people used TCM, and that approximately 12% of Americans and 9% of Australians used at least one form of herbal medicine in the prior year (Ernst, 2000). Although traditional Korean medicine (TKM) shares a common origin as TCM, and was heavily influenced by TCM, it started to develop into a separate practice around the early 1600’s upon publication of the Dongeuibogam (sometimes spelled Dongui Bogam) (Cha et al., 2007). The use of TKM is still an integral part of the healthcare of the Korean people with over 30,000 practitioners as of 2007, which was an increase from the ten years prior (Cha et al., 2007). These traditional medicines have existed for over 2000 years, constantly evolving to be ever more effective. TCM differs from Western medicine by focusing on addressing the underlying issues rather than treating symptoms. There are several guiding philosophies and treatment modalities including acupuncture, moxibustion, and qi gong (Liu and Liu, 2009; Fung and Linn, 2015; National Center for Complementary, 2019). However, the most relevant form of TCM to the discovery of new chemical entities for drug discovery is the use of materia medica.
In the search for effective ways to treat illness, TCM has accumulated a vast wealth of information on effective materia medica. These components, which are often combined to make treatments, are sometimes referred to in general as herbal medicines even though they can be derived from mineral or animal sources as well as from plants (Yang, 1998; National Administration of Traditional Chinese Medicine, 1999; Liu and Liu, 2009). Animal sources of materia medica are incredibly diverse, ranging from the antlers of deer, to fossilized bones, to snake skin, to scorpions, to human hair (Yang, 1998). The chemical study of arthropods, especially insects, is most compelling for drug discovery when one considers the animal-based medicinal materials. This is due to the fact that they are most likely to be available in large quantities without having major negative implications on their native populations, there are less ethical concerns over the treatment of insects than with vertebrates, and insects are widely known to produce a wide variety of chemical compounds. This, combined with the fact that they are historically under studied when compared to plants, makes insects an ideal group of taxa for chemical investigations (Dossey, 2010; Dettner, 2011; Seabrooks and Hu, 2017).
Beetles are the most diverse group of insects, of the ~1 million described species of insects, over 400,000 are beetles, and some estimates put the total number of beetle species on the planet at over 5 million (Berenbaum and Eisner, 2008; Yuan et al., 2016). They occupy almost every conceivable ecological niche other than that of primary producer. This extreme ecological and behavioral diversity means that beetles are exposed to a wide-range of challenges to which they must overcome, some of which cannot be overcome via physical methods and therefore require chemical or biological approaches, e.g., bacterial infection (Hunt et al., 2007; Yuan et al., 2016). Beetles can sequester or biosynthesize small molecules to increase fitness, and therefore make excellent candidates to search for novel bioactive chemistry (Eisner, 2003; Eisner et al., 2005; Dettner, 2011). Therefore, we decided to explore the known chemistry of beetles used in TCM as a way to demonstrate the value of these resources, as well as to highlight gaps in the current knowledge that warrant additional studies.
To build this article, we first had to make a list of all the beetles known to be used in TCM. To build this list, we relied primarily on six sources (Namba et al., 1988; Ding et al., 1997; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999; Pemberton, 1999; Zhang et al., 2019). We then searched the literature for information on the chemistry of each genus and species of beetle. There were several review articles and books that were particularly helpful in describing the chemistry of beetles that we cite repeatedly throughout the article (Schildknect, 1970; Dettner, 1985, 1987, 2015; Eisner et al., 2005; Vuts et al., 2014), but mostly we relied upon primary research articles for this aspect of the paper. We organized the paper by taxonomic families of beetles, alphabetically, with one exception. The family Geotrupidae was included with Scarabaeidae because the larva of these groups were used interchangeably and Scarabaeidae is the larger and better known taxonomic group. The scientific names of some of the beetles used in TCM have changed since the publication of the article cited, and in these cases we searched for both the name provided and the currently used nomenclature. Where there were uncertainties about which scientific name for a beetle was currently accepted, we frequently used the Global Biodiversity Information Facility as a guide (GBIF Secretariat, 2020).
The goal of this article is to link the knowledge of medicinal use of these beetles with what is known of their chemistry (Table 1). While there are great resources available on which beetles have been used most prominently in TCM and there are similarly impressive reviews on the chemistry of beetles, we could not find a reference that systematically connected the two pools of information. This article is intended to not only show us what we know of the chemistry of beetles used in TCM, but also highlight the fact that there is so much additional work to be done in this field. There are many beetles covered in this article that have never been studied chemically, and many of those that have been chemically investigated have not been done at the same life-stage of the organism that is used medicinally. Furthermore, work on the chemical changes that occur during the preparation of the insects for consumption, e.g., drying, decoction, etc., would provide even greater insights into potentially bioactive compounds. We hope that this article can act as a call to action to chemical ecologists and natural products chemists to fill in the gaps in the knowledge exposed herein.
Table 1.
Beetles used in Traditional Chinese Medicine (TCM) along with their common name, TCM name, traditional use, and chemical class
| Beetle used | Common name | TCM name | Traditional use | Chemical classa |
|---|---|---|---|---|
| Bostrichidae | Detoxification, removal of pus/cystsb | Aliphatic esters | ||
| Lyctus brunneus | Brown powderpost beetle | 竹蠹虫 | ||
| Buprestidae | Insecticidal, eczema, itching,b aphrodisiacc | Buprestins | ||
| Chalcophora japonica | Flat-headed wood-borer | 吉丁虫 | ||
| Chrysochroa elegans | Japanese Jewel beetle | 吉丁虫 | ||
| Carabidae | Stomachache, feverish chills, amenorrheac | 1,4-benzoquinones | ||
| Pheropsophus jessoensis | Bombardier beetle | 行夜 | ||
| Cerambycidae | Improve blood circulation,b,d pain relief, bruises, inflammation, menstrual painb (Bruises, poison, pain, bleeding,b angina pectoris,b,c knife woundsc) | Long-chain ethers, gomadalactones, juvenile hormome III | ||
| Anoplophora chinensis | Longhorn beetle (larva) | 天牛 (桑蠹虫) | ||
| Apriona germari | Longhorn beetle (larva) | 天牛 (桑蠹虫) | ||
| Batocera horsfieldi | White striped longhorn (larva) | 天牛 (桑蠹虫) | ||
| Curculionidae | Treat paralysis pain, arthritisb | Phenol and aliphatic esters, ketones, and aldehydes | ||
| Cyrtotrachelus longimanus | Snout beetle | 竹象鼻虫 | ||
| Dytiscidae | Improve blood circulation,d polyuria, enuresise | Benzoic acid derivatives, steroids | ||
| Cybister japonicus | Diving beetle | 龙虱, 물방개g | ||
| Cybister tripunctatus | Diving beetle | 龙虱, 물방개g | ||
| Elateridae | Increase muscular strength, malariab | Aliphatic acids | ||
| Pleonomus canaliculatus | Click beetle | 叩头虫 | ||
| Geotrupidae | Reduce bruising, constipation, congestion, remove pus or dead skin, indigestion, nausea, pain, swelling,b covulsions, fevers, insanityc | Unknown | ||
| Phelotrupes laevistriatus | Earth-Boring Dung Beetle | 蜣螂 | ||
| Gyrinidae | Remove toxins, treat warts,b nasal polyps,c infected boilsb,c | Norsesquiterpenoids, aliphatic acids | ||
| Gyrinus curtus | Whirligig beetle | 豉虫 | ||
| Lampyridae | Treat burns,b clarify eyesight, cure night blindnessc | Monoterpenoids | ||
| Aquatica lateralis | Firefly | 萤火 | ||
| Meloidae | Cancer, poison, bruises, constipation, amenorrhea, vitiligo, dog bites, scrofula, as a diuretic, nasal polyps, fungal skin infections, menstrual pain,b boils, facial paralysis, STIs,e abortions, urinary obstructionc | Cantharidin and analogues | ||
| Epicauta chinensis | Blister beetle | 葛上亭长 | ||
| Epicauta gorhami | Blister beetle | 葛上亭长 | ||
| Lytta caraganae | Blister beetle | 芫青 | ||
| Lytta chinensis | Blister beetle | 芫青 | ||
| Lytta suturella | Blister beetle | 芫青 | ||
| Meloe coarctatus | Oil beetle | 地胆 | ||
| Mylabris calida | Blister beetle | 斑蝥, 반묘g | ||
| Mylabris cichorii | Blister beetle | 斑蝥, 반묘g | ||
| Mylabris phalerata | Blister beetle | 斑蝥, 반묘g | ||
| Mylabris sidao | Blister beetle | 斑蝥, 반묘g | ||
| Mylabris speciosa | Blister beetle | 斑蝥, 반묘g | ||
| Scarabaeidae | Reduce bruising, constipation, congestion, remove pus or dead skin, indigestion, nausea, pain, swelling,b covulsions, fevers, insanityc (Remove bruises, constipation, relieve pain, remove toxins, reduce menstrual bleeding, gout, tetanus, carbuncle, acute skin infections,b feverish chills,c liver cirrhosise) | Long-chain alcohols, aldehydes, esters, ketones, and lactones, alkaloids, benzoic acid derivatives, branched carboxylic acids, flavonoids, diketopiperazines, β-carbolines, N-acetyl dopamine dimers, N-acetyl dopamine dimer analogues | ||
| Alissonotum impressicolle | Scarab beetle (larva) | 蛴螬 | ||
| Allomyrina dichotoma | Horned beetle | 蜣螂 | ||
| Anomala corpulenta | Leaf chafer (larva) | 蛴螬 | ||
| Anomala cupripes | Leaf chafer (larva) | 蛴螬 | ||
| Anomala exoleta | Leaf chafer (larva) | 蛴螬 | ||
| Catharsius molossus | Dung beetle | 蜣螂 | ||
| Catharsius pithecius | Dung beetle | 蜣螂 | ||
| Gymnopleurus mopsus | Scarab beetle | 蜣螂 | ||
| Heliocopris bucephalus | Northeast block chafer | 蜣螂 | ||
| Holotrichia diomphalia | Chafer (larva) | 蛴螬, 굼벵이g | ||
| Holotrichia morosa | Chafer (larva) | 蛴螬, 굼벵이g | ||
| Holotrichia sauteri | Brown chafer (larva) | 蛴螬, 굼벵이g | ||
| Holotrichia titanis | Chafer (larva) | 蛴螬, 굼벵이g | ||
| Onitis subopacus | Dung beetle | 蜣螂 | ||
| Oxycetonia jucunda | Mulberry chafer (larva) | 蛴螬 | ||
| Pentodon quadridens | Rhinoceros beetle (larva) | 蛴螬 | ||
| Protaetia brevitarsis | Flower chafer (larva) | 蛴螬, 제조g, 굼벵이g | ||
| Protaetia orientalis | Flower chafer (larva) | 蛴螬 | ||
| Scarabaeus sacer | Sacred scarab beetle | 蜣螂 | ||
| Trematodes tenebrioides | Scarab (larva) | 蛴螬 | ||
| Xylotrupes dichotomus | Rhinoceros Beetle (larva) | 蜣螂 (蛴螬) | ||
| Staphylinidae | Treat tooth pain, itching,b skin infections and ailments,b,c remove tattoos,c vitiligo | Pederin and analogues | ||
| Paederus fuscipes | Rove beetle | 青腰虫/花蚁虫 | ||
| Tenebrionidae | Cancer,b coughs, bone problems, stomach illness, stroke,f | 1,4-benzoquinones, limone, long-chain alkenes | ||
| Ulomoides dermestoides | Darkling beetle | 洋虫 | ||
aClass of chemicals identified from the beetle or from closely related beetles. See the text for more detail. b(National Administration of Traditional Chinese Medicine 1999). c(Namba et al. 1988). d(Ding et al. 1997). e(Pemberton 1999). f(Zhang et al. 2019). gTKM name.
BEETLES USED IN TCM BY FAMILY
Bostrichidae
Lyctus: Bostrichids are small, wood-boring beetles often called powderpost beetles. The only record of use of a Bostrichid in TCM is that of Lyctus brunneus (National Administration of Traditional Chinese Medicine, 1999). Lyctus brunneus is one of the most economically destructive powderpost beetles, causing damage to wood products into which they bore, leaving small holes and piles of powdery frass in their efforts to consume the starch within the wood (Parkin, 1940; Martin, 1979; Ide et al., 2016). In TCM, it is typically the larval stage of L. brunneus that is used as a way to detoxify the body and for the removal of pus and cysts, and goes by the name 竹蠹虫 (Zhú Dù Chónɡ) (National Administration of Traditional Chinese Medicine, 1999).
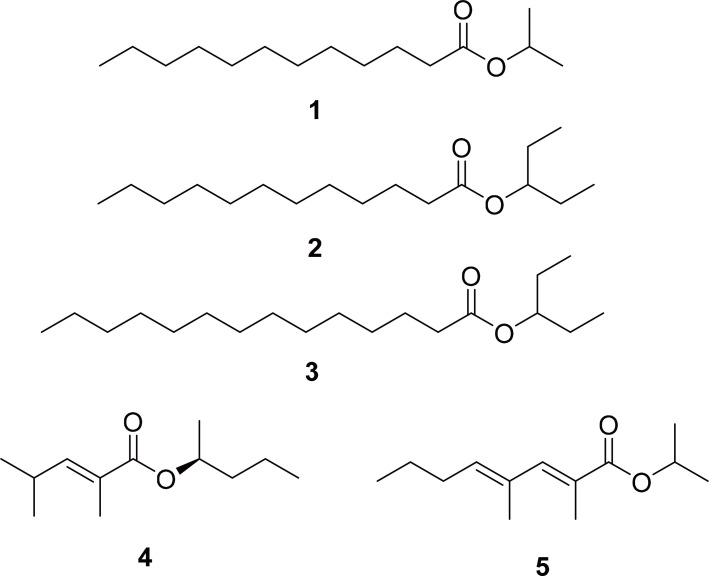
We could not find any chemistry known from L. brunneus, but three of constituents of the aggregation pheromone of the closely related species L. africanus have been determined (1-3 in Fig. 1) (Kartika et al., 2015). Two other species of Bostrichids, Rhizopertha dominica and Prostephanus truncatus, have also have been found to use esters as aggregation pheromones, but those contained shorter, unsaturated chains (4-5 in Fig. 1) (Francke and Dettner, 2005). It therefore seems likely that L. brunneus also uses esters as aggregation pheromones. It should also be mentioned that L. brunneus has pygidial glands that, in other species of beetle, produce defensive compounds, but the products of these glands have not been studied in L. brunneus (Altson, 1924).
Fig. 1.
Pheromones identified from Bostrichid beetles. Compounds 1-3 were identified from Lyctus africanus. Compounds 4 and 5 are examples of pheromones identified from other genera of Bostrichids.
Buprestidae
There are over 15,000 species of beetles in the family Buprestidae, which are often called jewel beetles or metallic wood-boring beetles. They have a world-wide range and many are prized by collectors for their bright metallic colors (Hong et al., 2009). Some Buprestids are economically important pests, including the emerald ash borer (Agrilus planipennis) which is devastating large populations of ash trees in the United States and Canada (Silk and Ryall, 2015). Two genera of Buprestids, Chalcophora and Chrysochroa, have been reported to be used in TCM.
Chalcophora: Within the family Buprestidae, the genus Chalcophora consists of around 15 species of somewhat large wood-boring beetles, some of which are economically important as forestry pests (Maier and Ivie, 2013). Chalcophora japonica, the flat-headed wood-borer, has been used in TCM under the name 吉丁虫 (Jí Dīng Chóng) as an insecticide as well as to treat eczema and itching (National Administration of Traditional Chinese Medicine, 1999).
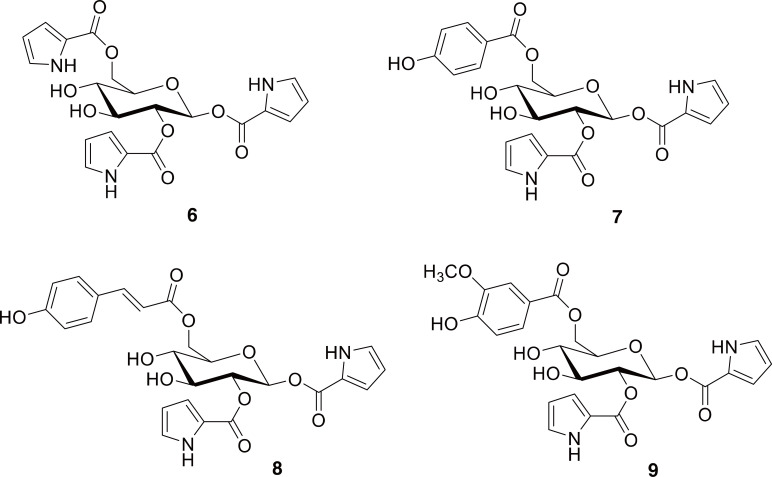
Although we did not find any evidence of chemical studies on Chalcophora japonica in our search, other Buprestids have been investigated. Excellent work by Brown, Moore, and colleagues (Brown et al., 1985; Moore and Brown, 1985) not only isolated and described the main defensive chemicals of Buprestids, but did so by surveying a wide variety of jewel beetles from Australia along with a few from Europe and Asia. They elucidated that these “bitter principles” were based upon β-D-glucose with three aromatic acyl groups attached, and called them buprestins A and B (6-7 in Fig. 2). Later work expanded the number of buprestins known and also confirmed their presence in a species of Chalcophora (C. mariana) (e.g., 8-9 in Fig. 2) (Ryczek et al., 2009; Dettner, 2015). The use of C. japonica as an insecticide corresponds well with the fact that buprestins were found to be repellant to ants (Moore and Brown, 1985; National Administration of Traditional Chinese Medicine, 1999; Ryczek et al., 2009). It would be interesting to test these compounds for anti-inflammatory activity, considering the other uses to which this beetle has been put in TCM.
Fig. 2.
Representative compounds described from jewel beetles (Coleoptera: Buprestidae). Compounds 6 and 7 are buprestins A and B. Compounds 8 and 9 are buprestins D and G.
Chrysochroa: The genus Chrysochroa is renowned for its brilliant iridescent colors, and is a major reason why Buprestids in general are called jewel beetles. Members of this genus were literally used in jeweled ornaments in Asia, including in the Silla Dynasty, Korea (Han et al., 2012). According to Namba et al. (1988), Chrysochroa elegans was also called 吉丁虫 (Jí Dīng Chóng) and kept in peoples clothing as an aphrodisiac. It appears that C. elegans is a synonym of the species C. fulgidissima, however, C. fulgidissima may itself be a species complex (Han et al., 2012; Kim et al., 2014c; GBIF Secretariat, 2019a).
We were unable to find any chemical studies on beetles from the genus Chrysochroa, however based on the wide distribution of buprestins as defensive molecules in the Buprestidae, it seems quite likely that these beetles contain this class of molecule (Fig. 2) (Moore and Brown, 1985). A complete mitogenome of C. fulgidissima has been sequenced, published, and used in taxonomic studies (Hong et al., 2009; Kim et al., 2014c). Unsurprisingly, the majority of the scientific studies on Chrysochroa spp. has been on their bright coloration and the physical structure of the cuticle that is responsible, along with attempts to mimic this beautiful feature (Stavenga et al., 2011; Yoshioka et al., 2012; Tzeng et al., 2015).
Carabidae
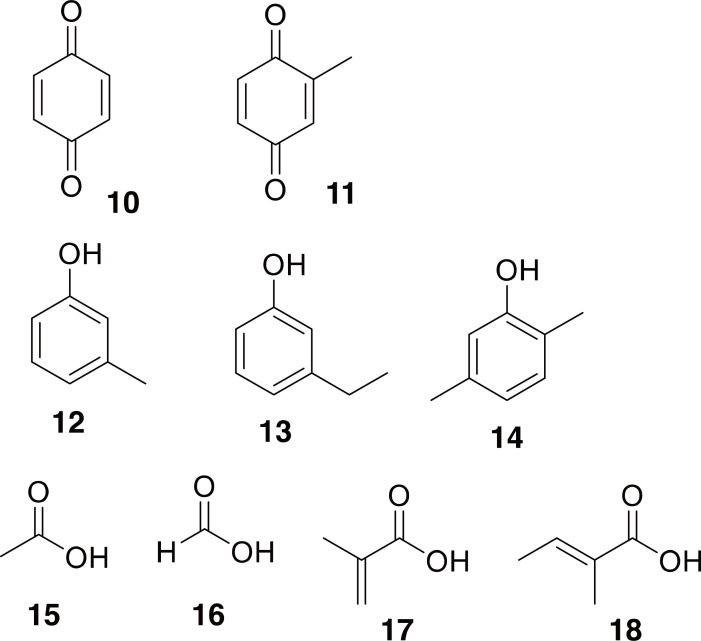
Carabids are often called ground beetles (not to be confused with Eupolyphaga sinensis, a flightless cockroach also sometimes referred to as “ground beetle”, 土鳖虫, in TCM) and are predatory rather than herbivorous (Ding et al., 1997). In Namba et al. (1988), they mention unidentified Carabids called “Tian Ke Chong” being used in order to “stimulate mutual love”. Different subfamilies of Carabids have different defensive chemistry (10-18 in Fig. 3) including 1,4-benzoquinones from the Brachininae, substituted phenols from the Harpalinae, and simple acids from the Carabinae (Schildknect 1970; Eisner et al., 2005; Holliday et al., 2012), so without more details on the identification, it is difficult to predict the chemistry of “Tian Ke Chong”.
Fig. 3.
Chemical defenses identified from Carabid beetles. Compound 10 and 11 are 1,4-benzoquinone and 2-methyl-1,4-benzoquinone identified from members of the subfamily Brachininae. Compounds 12-14 are in substituted phenols identified from members of the subfamily Harpalinae. Compounds 15-18 are examples of some defensive compounds identified from the subfamily Carabinae.
Pheropsophus: We could find two sources indicating that the bombardier beetle (Carabidae: Brachininae) Pheropsophus jessoensis is used in TCM. This beetle is called 行夜 (Xíng Yè) and is used to treat stomachache, feverish chills, and amenorrhea (Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999). Being in the subfamily Brachininae, it most likely contains 1,4-benzoquinones which it uses as a chemical defense (10-11 in Fig. 3) (Schildknect, 1970; Eisner, 2003; Eisner et al., 2005). This is quite interesting, because 1,4-benzoquinones are known to exhibit hepatotoxicity (Moore et al., 1987; Abernethy et al., 2004; Chan et al., 2008). Caution should be taken when handling these beetles, because, like other bombardier beetles, Pheropsophus spp. propel their chemical defenses as hot (nearly 100°C) liquid mixtures, and there are reports of burns that required medical attention (Schildknect, 1970; Eisner et al., 2005; de Oliveira Pardal et al., 2016).
Cerambycidae
The family Cerambycidae, known as longhorn beetles (a.k.a., long-horned beetles or longicorn beetles), is a group of beetles that are well known for having several large, brightly colored species, often with very long antennae. There are over 35,000 species of Cerambycids currently described (Allison et al., 2004). Larvae of these beetles bore into living or dead wood, and several species are serious economic pests, with potential damage caused in the USA by a single introduced species (Anoplophora glabripennis) estimated at $669 billion (Allison et al., 2004; Nowak et al., 2009).
In addition to the specific Cerambycid genera being used in TCM (see descriptions below) we also found reference to unidentified longhorn beetles being used in TCM. According to Namba et al. (1988), the ancient Chinese medicine Fei Shen Chong was most likely the adults of Cerambycids and was used to aid during difficult childbirth, while the larval stage was used as 木蠹虫 (Mù Dù Chóng) to treat weakness, poor blood circulation, amenorrhea, back pain, anemia, and pain in the upper thorax (epigastrium). Several longhorn beetles in the subfamily Cerambycinae have been shown to secrete defensive chemicals from metasternal glands, however, the three specified genera of longhorn beetles still in use in TCM, Anoplophora, Apriona, and Batocera, are all in the subfamily Lamiinae, for which we could not find reports of defensive secretions (Francke and Dettner, 2005).
The genera Anoplophora, Apriona, and Batocera are still used in TCM in both the larval and adult life stages. The larval stages of beetles in these genera are called 桑蠹虫 (Sāng Dù Chóng), and are used to remove poison, remove bruises, reduce pain, decrease bleeding, treat knife wounds, and to ameliorate angina pectoris (Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999). Adults of Anoplophora chinensis, Apriona germarii (=Apriona germari), and Batocera horsfieldi are termed 天牛 (Tiān Niú), and are used to improve blood circulation, relieve pain (including menstrual pain), reduce bruising, and reduce inflammation (Namba et al., 1988; Ding et al., 1997; National Administration of Traditional Chinese Medicine, 1999).
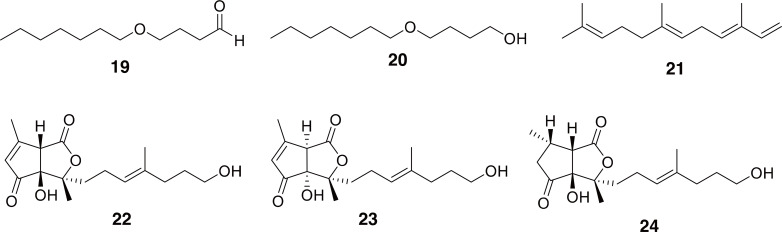
Anoplophora: Because some species of Anoplophora, including A. glabripennis, A. malasiaca, and even A. chinensis (the species used in TCM) are agricultural and forestry pests, there have been several studies on their chemical signaling, especially pheromones (Allison et al., 2004; Nowak et al., 2009; Yasui, 2009). Work has been done to elucidate male sex pheromones (Zhang et al., 2002; Crook et al., 2014; Hansen et al., 2015), female sex pheromones (Yasui et al., 2003, 2007; Zhang et al., 2003; Wickham et al., 2012), and female trail pheromones (Hoover et al., 2014), which provides insights into some of the chemical constituents of adult Anoplophora spp. (19-21 in Fig. 4).
Fig. 4.
Pheromones identified from Anoplophora spp. Male sex pheromones, 4-(n-heptyloxy)-butanal (19), 4-(n-heptyloxy)-butan-1-ol (20), and (3E,6E)-α-farnesene (21). Compounds 22-24 are female sex pheromones gomadalatone A-C (left to right). Several long-chain alkanes, alkenes, and ketones were also identified as female specific sex and trail pheromones, but are not shown here (see text for citations).
The most interesting of the compounds found in Anoplophora are the gomadalactones (22-24 in Fig. 4). These molecules were isolated as part of the female sex pheromone and contain an intriguing oxabicyclo[3.3.0]octane ring-system (Yasui et al., 2007). This ring-system is analogous to the one found in the endogenous vasodilator prostaglandins I2, also known as prostacyclin, and when synthesized as epoprostenol (Sitbon and Vonk Noordegraaf, 2017). Synthetic molecules with oxabicyclo[3.3.0]octane rings have also been shown to be vasodilators and platelet aggregation factor antagonists (Akiba et al., 1986; Peçanha et al., 1998). Therefore, the structures of the gomadalactones intriguingly match some of the functions of the TCM made from A. chinensis, namely improving blood circulation.
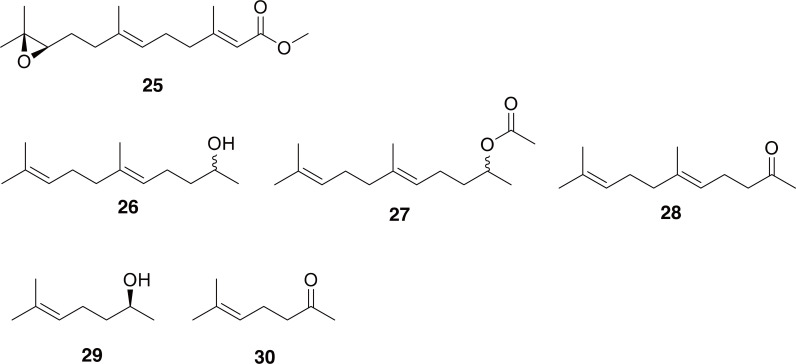
Apriona and Batocera: Both Apriona germarii (=A. germari) and Batocera horsefieldi are in the subfamily Lamiinae, and are used interchangeably with Apolophora chinensis in TCM (described above). However, the chemistry of these genera has been less well studied. It has been found that A. germarii males produce juvenile hormone III (JHIII) in a male accessory gland which they then transfer to females during copulation (Tian et al., 2010). While studies on Apriona and Batocera chemistry are somewhat sparse, the sex pheromones of several other genera in the Lamiinae have been described (25-30 in Fig. 5) (Hughes et al., 2013; Meier et al., 2019, 2020). Interestingly, it seems like the absolute configurations of these pheromones can vary based on species (Hughes et al., 2016).
Fig. 5.
Compound 25 is juvenile hormone III, which is produced by Apriona germari males and transferred to females during copulation. Compounds 26-28 are fuscumol, fuscumol acetate, and geranylacetone, respectively, which are sex pheromones identified from Lamiinae longhorn beetles. Both enantiomers are made by Lamiinae, but they vary by species. (S)-Sulcatol (29) and sulcatone (30) are sex pheromones identified from Lamiinae longhorn beetles.
Curculionidae
Cyrtotrachelus: Curculionids are the familiar group of beetles commonly called weevils or snout beetles. This family is incredibly diverse with over 62,000 described species so far and estimates of 220,000 extant species (Oberprieler et al., 2007). Whole bodies of adult Cyrtotrachelus longimanus are used in TCM under the name 竹象鼻虫 (Zhú Xiànɡ Bí Chónɡ) to treat pain due to paralysis and arthritis, and have also been eaten as a prepared food dish (National Administration of Traditional Chinese Medicine, 1999; Pemberton, 2003). Bamboo borers (C. longimanus and C. buqueti) are serious pests in commercial bamboo and bamboo shoot production (Li et al., 2017; Yang et al., 2018). Due to the close relationship of these two species, along with their similar habitats, it is likely that either one may be used as 竹象鼻虫 (Zhú Xiànɡ Bí Chónɡ).
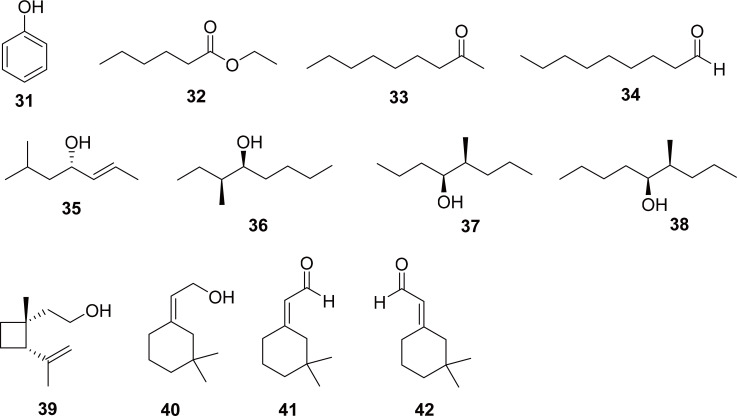
As with many groups of beetles that are primarily known as agricultural pests, the vast majority of chemical studies on Curculionids have been directed towards pheromones, both aggregation and sex pheromones, in an effort to aid in control of these species. Cyrtotrachelus buqueti has been shown to have produce phenol, ethyl hexanoate, 2-nonanone, nonanal, and methyl pentadecanoate as sex pheromones (31-34 in Fig. 6) (Mang et al., 2012). Other compounds have been found from the palm weevils, which are in the same subfamily, Dryophthorinae, but in the genus Rhynchophorus (35-38 in Fig. 6) (Hallett et al., 1993; Francke and Dettner, 2005). Less closely related weevils, in the subfamily Curculioninae, tend to have cyclic structures in their pheromones (39-42 in Fig. 6) (Hedin et al., 1997). There are also indications that some species of weevils sequester chemical defenses from their food plants (Francke and Dettner, 2005). The secretions from the male accessory glands of C. buqueti have been shown to have antibacterial activity against Gram-positive bacteria (Liang et al., 2016). However, the isolation of phenol from C. buqueti, a well-known topical anesthetic, correlates most closely with its use in TCM to treat localized pain due to arthritis or paralysis (Mang et al., 2012; Kumar et al., 2015).
Fig. 6.
Pheromones identified from weevils. Compounds 31-34 are pheromones isolated from Cyrtotrachelus buqueti. Compounds 35-38 are pheromones isolated from Rhynchophorus spp., which are also in the subfamily Dryophthorinae. Compounds 39-42 are pheromones from weevils in the subfamily Curculioninae.
Dytiscidae
Cybister: The genus Cybister consists of diving beetles, many of which are large, that are found worldwide (Miller et al., 2007; Michat et al., 2017). These beetles inhabit fresh-water environments including ponds, lakes, and streams and are predatory in both the larval and adult life-stage (Eisner et al., 2005). Several of our sources cited whole insects of either C. tripunctatus and/or C. japonicus as having been used in TCM and TKM. The TCM name is 龙虱 (Long Shī), the TKM name is 물방개 (Mul Bang Gae), and it has been recorded as being used to improve blood circulation and to treat polyuria and enuresis (Ding et al., 1997; National Administration of Traditional Chinese Medicine, 1999; Pemberton, 1999). Beetles in the genus Cybister have also been substituted, perhaps erroneously, for the more commonly used Eupolyphaga sinensis or Steleophaga plancyi (土鳖虫) (Hu et al., 2004). Cybister japonicus is also eaten as a food in parts of China and work on rearing them in artificial environments has been done (Jäch, 2003; Wang et al., 2017).
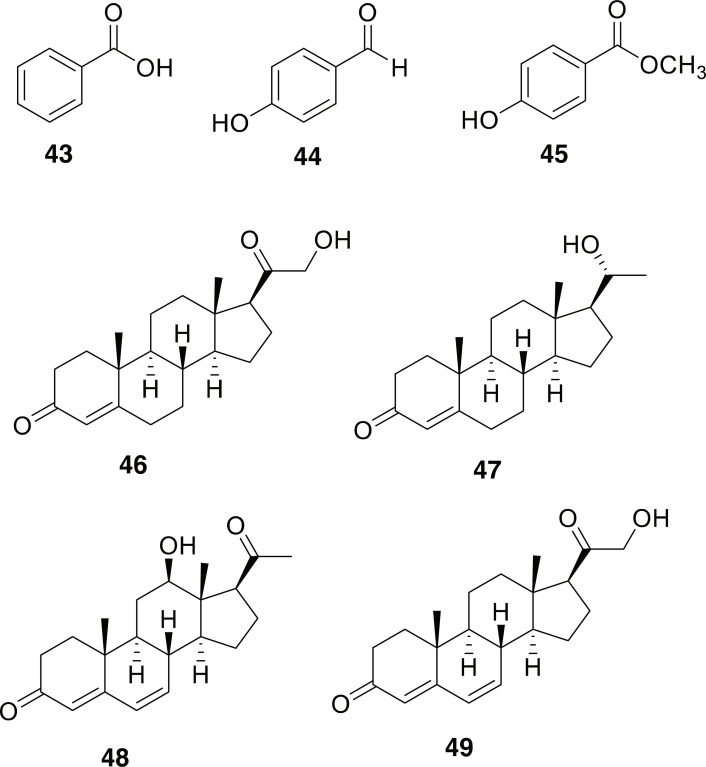
There have been several chemical studies on species within the genus Cybister, including on C. tripunctatus which have been summarized in several excellent reviews on beetle defensive chemistry (Schildknect, 1970; Dettner, 1987, 2014). It does not appear that C. japonicus (=C. chinensis) has been investigated for chemical composition, but it has recently been the subject genetic investigation using RNA seq technology (Hwang et al., 2018). Beetles in this genus contain a series of simple aromatic compounds as well as steroids, both used as glandular secretions (chemical constituents of C. tripunctatus, are shown in 43-49 in Fig. 7) (Schildknect, 1970; Dettner, 1987, 2014). The presence of steroids in these beetles is particularly interesting due to the many extremely potently bioactive steroids used as medicines.
Fig. 7.
Chemical constituents of Cybister tripunctatus. Compounds 43-45 are benzoic acid, 4-hydroxybenzaldehyde, methyl 4-hydroxybenzoate. Compounds 46 and 47 are 11-deoxycorticosterone, and 20β-hydroxypregn-4-ene-3-one. Compounds 48 and 49 are cybisterol, and 21-hydroxypregna-4,6-diene-3,20-dione.
Elateridae
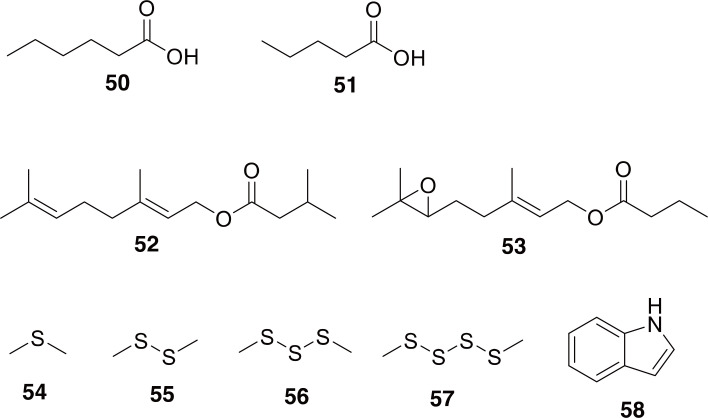
Pleonomus: Members of the family Elateridae are commonly known as click beetles. These beetles are most well-known for their ability to “click” when they rapidly move their prosternum in relation to their mesosternum with the aid of a structural peg and groove system, which allows them to flip themselves with great force relative to their size (Eisner et al., 2005). The most prominent Elaterid used in TCM is Pleonomus canaliculatus, which is called 叩头虫 (Kòu Tóu Chóng) and is used to increase muscular strength, especially in children with underdeveloped limb musculature, and to treat malaria (National Administration of Traditional Chinese Medicine, 1999). However, most work done on P. canaliculatus is in relation to controlling the population since it is a serious pest of Chinese winter wheat, referred to as a “wireworm” (Zhang et al., 2017).
Although a few genera within the family Elateridae have been chemically studied, we could not find any information on the chemical composition of P. canaliculatus or any member of the genus Pleonomus. The genus Pleonomus resides within the subfamily Dendrometrinae, as does the genus Limonius (Etzler and Johnson, 2018). Limonius spp. have been found to use simple carboxylic acids as sex pheromones, including hexanoic acid and pentanoic acid (50-51 in Fig. 8) (Francke and Dettner 2005). Other subfamilies within Elateridae have been found to have isoprenoid-derived pheromones (52-53) (Elaterinae) and glandular defensive secretions (Agrypinae) using nitrogen- and sulfur-containing compounds (54-58 in Fig. 8) (Dettner, 1987; Francke and Dettner, 2005; Kundrata et al., 2018).
Fig. 8.
Compounds identified from Elaterid beetles. Compounds 50 and 51 are hexanoic acid and pentanoic acid, identified from members of the subfamily Dendrometrinae. Compounds 52 and 53 are examples of pheromones identified from members of the subfamily Elaterinae (absolute configuration of 53 is unknown). Compounds 54-58 are examples of some defensive compounds identified from the subfamily Agrypinae.
Gyrinidae
Gyrinus: Whirligig beetles, members of the family Gyrinidae, are aquatic beetles often seen in swarms on freshwater. They tend to move in tight circles or gyrate on the surfaces of water, this behavior is the basis for their common name (Eisner et al., 2005). Beetles in the genus Gyrinus are used in TCM under the name 豉虫 (Chǐ Chóng). References specifically cite Gyrinus curtus as the species primarily used to treat nasal polyps and infected boils (Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999). It also seems likely that G. japonicus and larger species within the genus Dineutus may also be used occasionally in addition to G. curtus (Namba et al., 1988; Meyer-Rochow, 2017).
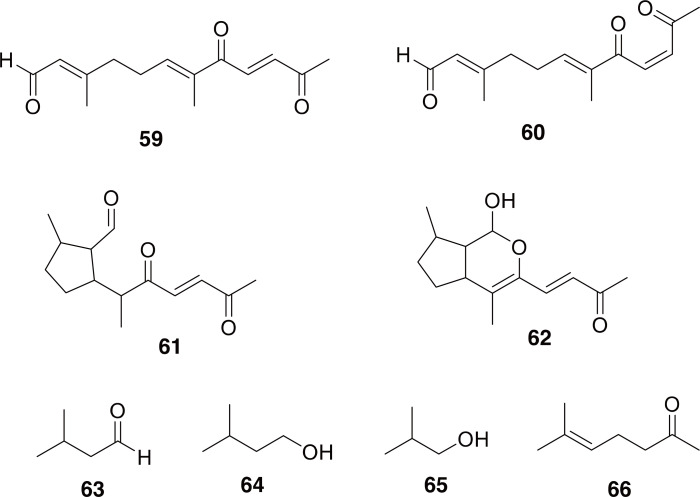
Although we could not find any chemical studies on G. curtus, there have been several investigations into the chemical defenses and chemical signaling of other Gyrinus species. Gyrinus spp. use a series of norsequiterpenoids as their primary chemical defenses, including gyrinidal (59-62 in Fig. 9) (Meinwald et al., 1972; Miller et al., 1975). Based on the widespread presence of the same type of chemical defenses within Gyrinus, and even in the related genus Dineutus, it seems likely that G. curtus also contains this class of molecule (Dettner, 1985, 2019). Additional studies have shown that Gyrinus and Dineutus species release small volatile molecules (63-66 in Fig. 9) as well that play a role in intraspecific communication and chemical defense (Ivarsson et al., 1996; Karlsson et al., 1999; Härlin, 2005). However, most interestingly, it has been shown that the secretion of the pygidial glands (which contain the aforementioned norsesquiterpenoids) have antibacterial activity against E. coli (Kovac and Maschwitz, 1990), which corresponds intriguingly to the use of these beetles to treat infected boils (Namba et al., 1988).
Fig. 9.
Chemical constituents of Gyrinus spp. Compounds 59 and 60 are gyrinidal, and isogyrinidal. Compounds 61 and 62 are gyrinidione, and gyrinidone (relative and absolute configuration not fully described in the literature). Compounds 63-66 are 3-methylbutanal, 3-methyl-1-butanol, 2-methyl-1-propanol, 6-methyl-5-hepten-2-one.
Lampyridae
Aquatica: Beetles in the family Lampyridae are known as fireflies and are famous for their beautiful displays of bioluminescence. There are over 2000 described species and all of them emit light in at least one life-stage (Bessho-Uehara and Oba, 2017; Maeda et al., 2017). Although there are around 100 genera of fireflies, we could only find reference to one species as being used in TCM, which is Luciola vitticollis (Namba et al., 1988; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999). However, it appears that this species has been reclassified to now be called Aquatica lateralis, although it is still often referred to as Luciola lateralis in the literature (Fu et al., 2010). When used in TCM, A. lateralis is called 萤火 (Yíng Huǒ) (or occasionally Ye Guang), and is used to clarify eyesight, cure night blindness, and treat wounds and burns caused by fire (Namba et al., 1988; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999).
Several species of fireflies from different genera have been studied for their chemical defenses and have been found to contain steroidal compounds called lucibufagins that are similar in structure to the bufadienolides found in toads that are used in TCM (69 in Fig. 10) (Eisner et al., 1978, 1997; Tyler et al., 2008; Smedley et al., 2017). However, recent work was unable to show evidence of lucibufagin presence in A. lateralis (Fallon et al., 2018). A study on the related firefly (also in the subfamily Luciolinae) Luciola leii demonstrated that larvae have glands that produce the monoterpenoids δ-terpinene and γ-terpinene (67-68 in Fig. 10) as defensive substances (Fu et al., 2007). Another study showed that some firefly species produce pyrazine compounds (70 in Fig. 10) as an olfactory aposematic signal (Vencl et al., 2016). There was also work on the cuticular hydrocarbons of a Luciola species (Shibue et al., 2004), but most of the chemical work on this genus have related to their bioluminescence, primarily interested in luciferin and luciferase (Oba et al., 2010; Bessho-Uehara and Oba, 2017). Aquatica lateralis is one of the few species of firefly that has been raised in large numbers in the lab (Noh et al., 1990; Kim et al., 2014a). Although their chemistry remains somewhat mysterious, a complete mitochondrial genome and a nuclear genome of A. lateralis have now been published (Maeda et al., 2017; Fallon et al., 2018).
Fig. 10.
Representative defensive compounds from fireflies (Coleoptera: Lampyridae). Compounds 67 and 68 are δ-terpinene, and γ-terpinene. Compounds 69 and 70 are lucibufagin C, and 2-methoxy-3-(1-methylpropyl) pyrazine (absolute configuration of 70 was not described in the literature).
Meloidae
There are over 3,000 described species of beetles in the family Meloidae, which are called blister beetles. Their common name derives from the fact that most beetles in this family produce the powerful vesicant (blistering agent) cantharidin (Eisner et al., 2005). This group of beetles is probably the most widely known and used beetle in traditional medicines, not only being used in traditional East Asian medicine, but it has also been described as a medicine in ancient Greek texts and in Europe in general, where some species were called “Spanish fly” and used as an aphrodisiac (Young, 1984; Wang, 1989). We found evidence that four genera of Meloids are used in TCM, those being Epicauta, Lytta, Meloe, and Mylabris.
Epicauta: Within the genus Epicauta, we were able to find references for the use of two species in TCM. Epicauta gorhami and E. chinensis are both referred to as 葛上亭长 (Gě Shànɡ Tínɡ Zhǎnɡ). These beetles are used to treat poison, bruises, and constipation, as well as to induce abortions and clear obstructed urinary passages (Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999).
Blister beetles in the genus Epicauta, like most beetles in the family Meloidae, contain the potently bioactive and toxic terpenoid cantharidin (71 in Fig. 11) (Walter and Cole, 1967; Carrel et al., 1986). Due to its original use as an aphrodisiac, along with subsequent discovery of anticancer activity, there have been numerous studies on the bioactivity and toxicity of cantharidin and its analogs, including several high-quality review articles and book chapters including Schmitz 1989; Karras et al., 1996; Liu and Chen, 2009; Dettner, 2011; Deng et al., 2013; Ghoneim, 2013; Puerto Galvis et al., 2013. Cantharidin is used in western medicine as a treatment for various skin diseases, including molluscum contagiosum and verruca vulgaris, due to its strong vesicant action (Torbeck et al., 2014). Cantharidin is extremely toxic to humans and livestock when ingested and can cause death due to hemorrhage of the gastrointestinal and urinary tracts, among other dangerous symptoms (Schmitz, 1989; Karras et al., 1996). Recently, there have been several studies investigating the biosynthetic genes and tissues responsible for cantharidin production in Epicauta (Jiang et al., 2017a, 2017b, 2019).
Fig. 11.
Compounds likely to be present in most Meloidae, including Epicauta, Lytta, Meloe, and Mylabris. Compound 71 is cantharidin, compounds 72 and 73 are R-(+)-palasonin, and S-(-)-palasonin.
Based on the wide range of Meloids that have been shown to produce both enantiomers of palasonin (demethylcantharidin, 72-73 in Fig. 11), these molecules are also likely to occur in Epicauta (Fietz et al., 2002; Nikbakhtzadeh and Tirgari, 2002; Nikbakhtzadeh and Ebrahimi, 2007; Mebs et al., 2009). Although it is unusual to find mixtures of enantiomers formed biosynthetically, in this case it seems plausible since it is believed that cantharidin, which is achiral, has one of two methyl groups removed via oxidative decarboxylation to form the chiral product, palasonin (Fietz et al., 2002).
Lytta: Our sources specified three blister beetles from the genus Lytta used in TCM, L. caraganae, L. chinensis, and L. suturella. These three species are used interchangeably under the name 芫青 (Yuán Jīng) as a diuretic and to treat bruises, poison, scrofula, bites from rabid dogs, infections, and to induce abortions (Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999). The genus Lytta is closely related to Epicauta, and it appears that some species are even synonymous (Lytta chinensis=Epicauta sibirica) (Pan and Ren, 2018). Being Meloids, these beetles contain cantharidin. In fact, cantharidin was first isolated from beetles in this genus in 1810 (Young, 1984). It is likely that Lytta spp. also contain palasonin due to its widespread distribution in the Meloidae.
Meloe: Beetles in the genus Meloe, in addition to being generalized as blister beetles because they are in the family Meloidae, are also sometimes called oil beetles. Meloe coarctatus is used in TCM under the name 地胆 (Dì Dǎn), and is used to remove poison, bruises, boils, warts, and necrotic tissue, as well as to increase liver function, treat scrofula, clear bowel obstruction, reduce feverish colds, and induce abortions (Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999). As with Epicauta and Lytta, beetles in the genus Meloe contain cantharidin (Young, 1984), and are likely to contain palasonin.
Mylabris: Of all of the genera of blister beetles (Meloidae) used in TCM, Mylabris has been studied the most intensely. We found references for five species of Mylabris used in TCM. The most common species used is Mylabris phalerata, but M. calida, M. cichorii, M. sidao, and M. speciosa are also used (Namba et al., 1988; Jiang, 1990; Ding et al., 1997; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999; Pemberton, 1999; Zhang et al., 2019). The TKM name for Mylabris is 반묘 (Ban Myo) and it is used to treat skin diseases including boils, fungal infection, and paralysis due to stroke, as well as swelling and lymphangitis, rabies, cancer, gonorrhea, and syphilis (Pemberton, 1999). The TCM name for Mylabris spp. is 斑蝥 (Bān Máo), and it is used to treat a long list of disorders including: infectious fevers, scrofula, boils, necrotic tissue, bladder stones, baldness, bruises, urinary blockage, to induce abortions, and, most famously, to fight several forms of cancer (Namba et al., 1988; Jiang, 1990; Ding et al., 1997; National Administration of Traditional Chinese Medicine, 1999; Zhang et al., 2019). Use of Mylabris spp. in TCM dates back over 2000 years (Wang, 1989). There is some confusion in the literature about the taxonomy of Mylabris and several papers use the genus name Hycleus for some Mylabris species, but it appears that Mylabris is a currently accepted genus (Bologna and Pinto, 2002).
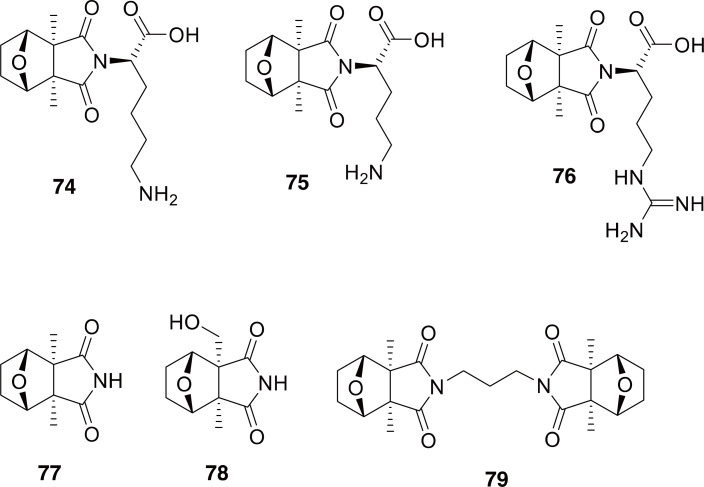
The chemistry of Mylabris spp. has been the subject of several studies. Interestingly, in addition to cantharidin and palasonin, there have been additional cantharidin analogs, known as cantharimides and cantharidinimides found in this beetle (74-79 in Fig. 12) (Nakatani et al., 2004; Nikbakhtzadeh and Ebrahimi, 2007; Dettner, 2011; Zeng et al., 2020). The most recent of these studies used a very sensitive UPLC-MS technique to propose the structures of 34 chemical constituents of M. phalerata, the majority of which were cantharidinimides (Zeng et al., 2020).
Fig. 12.
Compounds 74-79 are examples of cantharidin analogs identified from Mylabris phalerata.
Scarabaeidae and Geotrupidae
The scarab beetles, members of the family Scarabaeidae, are a large and diverse group with over 2,000 genera and 25,000 species. In addition to the beetles commonly known as scarabs, many dung beetles, chafers, and rhinoceros beetles also belong in this group. Although this is a well-known group of beetles, not many chemical defenses have been described from scarabs (Eisner et al., 2005). There have been numerous studies on the pheromones of scarabs, especially on pest species (Leal, 1998; Francke and Dettner, 2005; Vuts et al., 2014, and references therein). The earth-boring dung beetles, family Geotrupidae, are a smaller group, with only around 350 described species (Cunha et al., 2011). We have included the family Geotrupidae in our discussion of the Scarabaeidae because beetles from these families are called the same name in TCM and are used interchangeably as the same medicine to perform the same function (Namba et al., 1988; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999).
Beetles in the family Scarabaeidae along with those in the Geotrupidae are used in both the adult and larval form in TCM, but the larval stage is primarily used in TKM. In TKM, the larval stages of scarabs are called 굼벵이 (Kum Bang Yi). It is used frequently (70% of TKM clinics surveyed had prescribed this medicine) to treat cirrhosis of the liver (Pemberton, 1999). Scarab larvae are called 蛴螬 (Qí Cáo) in TCM and are used to reduce bruising, remove toxins, reduce menstrual bleeding, treat constipation, relieve pain, and to treat gout, tetanus, infected boils, feverish chills and acute skin infections (Namba and Inagaki, 1984; Namba et al., 1988; Ding et al., 1997; Yang, 1998; National Administration of Traditional Chinese Medicine 1999; Zhang et al., 2019). Larvae from several subfamilies, including the Cetoniinae, Dynastinae, Melolonthinae, Rutelinae are still used as Qí Cáo in Hong Kong and China (Namba and Inagaki, 1984). Adult scarab beetles and Geotrupids are called 蜣螂 (Qiānɡ Lánɡ) in TCM and are used to treat convulsions, feverish chills, adult insanity, to reduce bruising, relieve constipation, ameliorate congestion, remove pus, remove dead skin, treat indigestion, alleviate nausea, and to reduce pain and swelling (Namba et al., 1988; Ding et al., 1997; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999; Zhang et al., 2019).
Alissonotum: We could find evidence that the larvae of one species from the genus Alissonotum, A. impressicolle, has been used in TCM as Qí Cáo (National Administration of Traditional Chinese Medicine, 1999). This species is known to be a pest on Chinese sugarcane, yet we still could not find any chemical studies for this genus (Liu et al., 1985). Chemical studies on other members of the subfamily Dynastinae may be informative (e.g., Allomyrina, below).
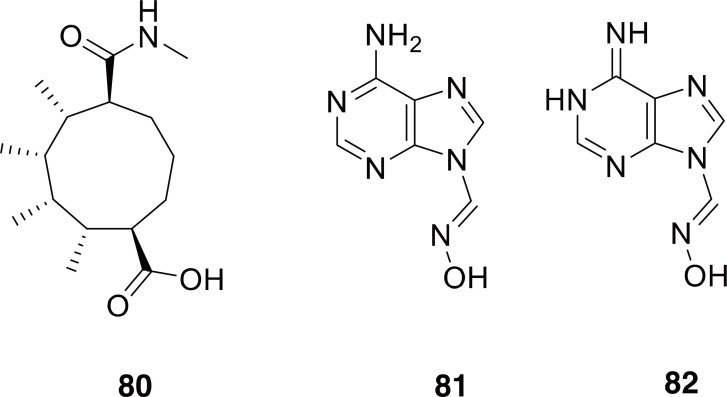
Allomyrina: The genus Allomyrina is in the subfamily Dynastinae, which includes many of the rhinoceros beetles (Bouchard et al., 2011). The larvae of A. dichotoma have been widely used as kumbangi in TKM and Qí Cáo in TCM (see description and references above). Due to its widespread use in TCM and TKM, A. dichotoma extracts have been studied for bioactivity and chemical constituents. Even though A. dichotoma was determined to be non-toxic up to doses of 2.5 g/kg in rats (Noh et al., 2015), various forms and extracts were found to contain antibacterial proteins (Miyanoshita et al., 1996; Sagisaka et al., 2001), to exhibit anti-obesity effects (Yoon et al., 2015), and be antioxidant in nature (Suh et al., 2010). The fatty acid composition of A. dichotoma was described (Youn et al., 2012), however, what most likely sets Allomyrina apart from other scarabs was the discovery of three new alkaloids from adult A. dichotoma with moderate antibacterial activity (80-82 in Fig. 13) (Niu et al., 2016). The finding that A. dichotoma has at least two types of antibacterial substances (proteins and alkaloids) corresponds intriguingly with its use in TCM to treat infections.
Fig. 13.
Compounds 80-82 are identified from adult Allomyrina dichotoma.
Anomala: The subfamily Rutelinae are called metallic leaf chafers, and includes the one of the most diverse genera (>1,000 species) in the animal kingdom, Anomala (Jameson, 1997). There are three species in the genus Anomala that have larvae used as Qí Cáo in TCM, A. corpulenta, A. cupripes, and A. exoleta (Namba et al., 1988; Ding et al., 1997; National Administration of Traditional Chinese Medicine, 1999). Although we could not find studies on the chemistry of the species of Anomala used in TCM, there has been a lot of work done revealing the pheromones of other Anomala spp. (83-90 in Fig. 14) (Leal, 1998; Francke and Dettner, 2005; Vuts et al., 2014, and references therein).
Fig. 14.
Pheromones identified from Anomala spp. Compounds 83-85 are (R)-buibuilactone, (R)-japonilure, (S)-japonilure. Compounds 86-88 are (E)-2-nonen-1-ol, (E)-2-nonenal, (Z)-7-tetradecen-2-one. Compounds 89 and 90 are methyl (Z)-5-tetradecenoate and methyl benzoate.
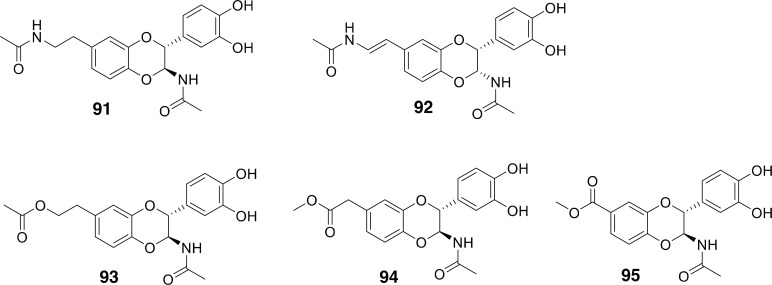
Catharsius: The genus Catharsius is a group of scarabs consisting mostly of dung beetles in the subfamily Scarabaeinae. Two species in this genus, C. molossus and C. pithecius are used in TCM as Qiānɡ Lánɡ (Namba et al., 1988; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999; Zhang et al., 2019). In addition to the uses of Qiānɡ Lánɡ described above, C. molossus has also been used to treat enlarged prostate (Zhao et al., 2006; Jiang et al., 2012).
Due to its use in TCM, there have been some studies on the chemistry of Catharsius molossus. The structure and characteristics of melanin (Xin et al., 2015) and chitosan (Ma et al., 2015) isolated from C. molossus have been investigated. A protein with fibrinolytic activity was also isolated from this beetle (Ahn et al., 2003). A chemical study of C. molossus identified N-acetyldopamine dimers and derivatives (91-95 in Fig. 15), similar to what was found in Protaetia (and other insects) (Lu et al., 2015). The finding of N-acetyldopamine dimers and derivatives in C. molossus, which are similar in structure to compounds with demonstrated anti-inflammatory activity (Xu et al., 2006; Yan et al., 2015), corresponds well with its use to treat enlarged prostates and the use of it as Qiānɡ Lánɡ to reduce pain and swelling.
Fig. 15.
Compounds identified from Catharsius molossus. Compounds 91 and 92 are N-acetyldopamine dimers. Compounds 93-95 are N-acetyldopamine dimer derivatives.
Gymnopleurus: The genus Gymnopleurus consists of dung beetles, including G. mopsus, a dung-rolling beetle found in Mongolia, Northern China, and the Korean peninsula that is used in TCM as Qiānɡ Lánɡ (Namba et al., 1988; Kang et al., 2018). Although we could not find any bioactivity or chemical constituent studies on any species of Gymnopleurus, they are in the subfamily Scarabaeinae, so they may contain similar compounds to those from Catharsisus (91-95 in Fig. 15). Additionally, several pheromones have been identified from dung beetles in the genus Kheper (96-99 in Fig. 16), which is also in the Scarabaeinae, so there could be compounds of this type present in G. mopsus (Francke and Dettner, 2005).
Fig. 16.
Compounds 96-99 are pheromones identified from dung beetles in the genus Kheper, subfamily Scarabaeinae.
Heliocopris: Dung beetles in the genus Heliocopris are also in the subfamily Scarabaeinae. These beetles have been eaten as food in some regions, and H. bucephalus has been used as Qiānɡ Lánɡ in TCM, and to treat diarrhea in Laos (Namba et al., 1988; Ratcliffe, 2006). We could not find any chemical or bioactivity data for H. bucephalus, or any other members of this genus, but they may have similar chemistry to other members of the Scarabaeinae (91-95 in Fig. 15 and 96-99 in Fig. 16).
Holotrichia: There are over 300 species in the genus Holotrichia, which are scarabs in the subfamily Melolonthinae (Pathania et al., 2016). Some members of this genus are referred to by the non-specific term “white grub” and can be serious economic pests on a variety of crops (Wang et al., 2019a). We found records of six species of Holotrichia used as Qí Cáo in TCM, H. diomphalia, H. parallela (=H. morosa), H. oblita (cited as H. obrita), H. sauteri, H. sinensis, and H. titanis (Namba et al., 1988; Ding et al., 1997; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999). Several papers also mention the use of H. diomphalia in TKM, but do not provide a Korean name, and it is likely that these also fall under the term 굼벵이 (Kum Bang Yi) (scarab larvae, see description above under Scarabaeidae and Geotrupidae) (Kang et al., 2002; Oh et al., 2003). A number of studies have shown Holotrichia larvae or their extracts to have bioactivities using in vitro and in vivo assays including immunomodulatory (Kang et al., 2002), hepatoprotective (Oh et al., 2003), antifungal (Dong et al., 2008), antioxidant (Liu et al., 2012), anticancer (Song et al., 2014), anticoagulant (Xu et al., 2016), and anti-asthma (Hong et al., 2019). Additionally, antifungal and antibacterial proteins have been isolated from H. diomphalia (Lee et al., 1994, 1995a, 1995b).
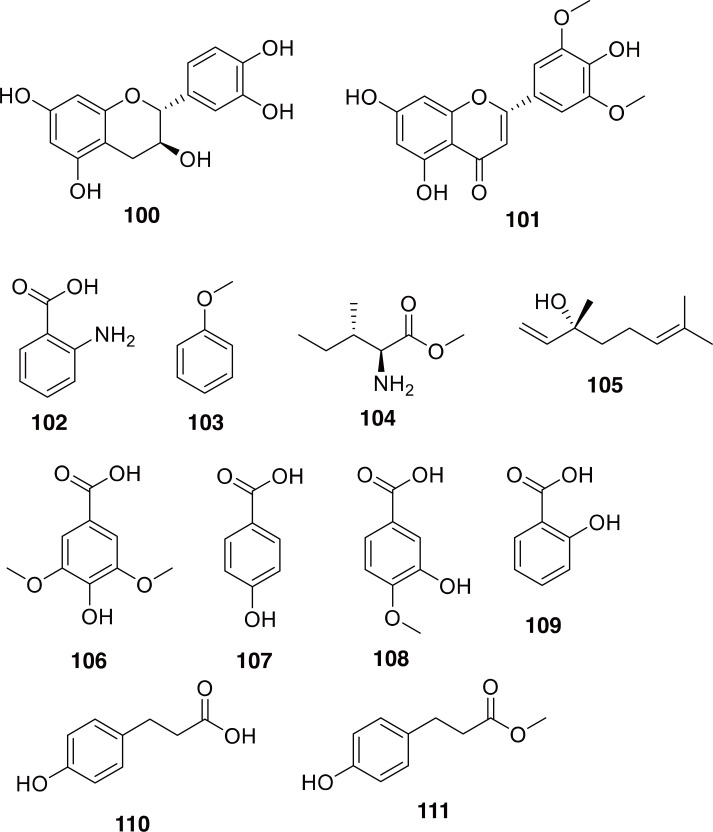
Due to the pest status of some Holotrichia species, along with their usage in TCM and TKM, there have been studies on the chemical constituents of these beetles. The sex pheromones of some species have been elucidated (Leal, 1998; Francke and Dettner, 2005; Vuts et al., 2014, and references therein), as have some chemical constituents of these beetles (100-105 in Fig. 17) (Dong et al., 2011; Liu et al., 2012; Wang et al., 2012). Many of the isolated compounds are phenolic, which corresponds well with the antioxidant findings, although the flavonoids are likely to be obtained from the diet and could change upon feeding on a different substrate (100-105 in Fig. 17) (Liu et al., 2012; Wang et al., 2012). The hepatoprotective effects of the extracts, along with the antioxidant activity corresponds well to the use of these scarab larvae to treat cirrhosis in TKM, while the antibacterial proteins correlate well with the use of these larvae in TCM to treat infections. It should be noted, however, that Ding et al. (1997) mention that beetles in the genus Holotrichia are toxic and caution should be used in their application.
Fig. 17.
Compounds reported from Holotrichia spp. Compounds 100 and 101 are flavonoids catechin and tricin. Compounds 102-105 are pheromones identified from Holotrichia spp. Compounds 106-111 are phenolic compounds from H. diomphalia.
Onitis: The genus Onitis is in the subfamily Scarabaeinae and consists of dung beetles. Onitis subopacus has been used as Qiānɡ Lánɡ in TCM (Namba et al., 1988). It is notable that beetles in this genus have been eaten as a food, so are unlikely to have significant toxicity (Ratcliffe, 2006). Some species of Onitis have exocrine glands that likely produce pheromones, however the structures of these compounds have not been elucidated (Houston, 1986). Other compounds from dung beetles in the Scarabaeinae were discussed earlier (91-95 in Fig. 15 and 96-99 in Fig. 16).
Oxycetonia: The smaller green flower chafer, Oxycetonia jucunda, is the only member of the species Oxycetonia that we could find references for as being used in TCM as Qí Cáo (Namba et al., 1988). This beetle is known to visit citrus flowers, but is not a major pest (Nishino et al., 1970). Little is known about the chemistry of beetles in the genus Oxycetonia. They do seem to be attracted to floral scented lure traps, but not to pheromones of the Japanese beetle, Popillia japonica (Klein and Edwards, 1989; Leal, 1998; Vuts et al., 2014).
Pentodon: The genus Pentodon is a relatively small group of scarabs with only 14 species and is in the subfamily Dynastinae (Jang and Kim, 2019). One species has been cited as being used in TCM as Qí Cáo, Pentodon quadridens (=Pentodon patruelis) (Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999). Even though P. idiota is a pest species on turfgrass, we could not find any chemical studies on members of this genus (Hosseini et al., 2019). There could be some clues of general chemical types based on analysis of the chemistry from other genera in the same subfamily (Dynastinae), e.g., Allomyrina (80-82 in Fig. 13).
Phelotrupes: The genus Phelotrupes is currently regarded to be in the family Geotrupidae, the earth-boring dung beetles (Cunha et al., 2011). Three references indicated that Phelotrupes laevistriatus (cited as Geotrupes laevistriatus) is used in TCM as Qiānɡ Lánɡ (Namba et al., 1988; Yang, 1998; National Administration of Traditional Chinese Medicine, 1999). We could not find any chemical or bioactivity studies on Phelotrupes. It has been noted that other Geotrupids excrete a red fluid when disturbed, so the chemical constituents of the Geotrupidae definitely warrant further examination (Cunha et al., 2011).
Protaetia: The genus Protaetia is in the subfamily Cetoniinae and are often referred to as flower-chafers. We found evidence that the larval stage of two species were used in TCM as Qí Cáo, P. brevitarsis, and P. orientalis (Namba and Inagaki 1984; Namba et al., 1988). Additionally, P. brevitarsis is used in TKM, either called 제조 (Je Jo), or used as 굼벵이 (Kum Bang Yi) to treat liver problems and cancer, and has been approved as a food ingredient by the Korean Ministry of Food and Drug Safety (Yoo et al., 2007; Wang et al., 2019b). Various bioactivities of P. brevitarsis and P. orientalis larvae and extracts have been described, including hepatoprotective (Kang et al., 2001; Hwang et al., 2005; Chon et al., 2012; Lee et al., 2014), anticancer (Yoo et al., 2007; Lee et al., 2014), antioxidant (Park et al., 2012; Suh and Kang, 2012; Lee et al., 2017a), anti-inflammatory (Sung et al., 2016; Lee et al., 2019), and antithrombotic (Lee et al., 2017c). Antibacterial and antifungal peptides have also been isolated from P. brevitarsis (Park et al., 1994; Yoon et al., 2003; Hwang et al., 2008). A complete mitochondrial genome and nuclear genome of P. brevitarsis have been published (Kim et al., 2014b; Wang et al., 2019b).
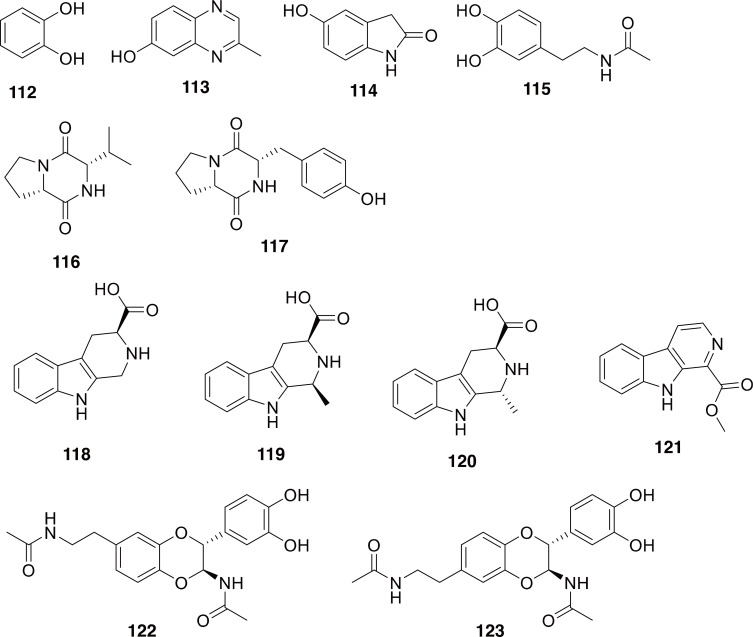
There have been several chemical studies on P. brevitarsis, primarily due to its use in traditional medicines. The fatty-acid and volatile constituents of P. brevitarsis have been published, as has the purported anticancer activity of some of the fatty acids (namely, palmitic acid, oleic acid, and stearic acid) (Yoo et al., 2007; Yeo et al., 2013). A detailed investigation of the chemical constituents of P. brevitarsis identified 16 compounds (112-123 in Fig. 18) (Lee et al., 2017b). Several of the classes of compounds identified from this scarab have shown potent bioactivity, perhaps the most exciting of which are the β-carboline-type structures, which are very similar to antidepressant drug candidates (Ferraz et al., 2019) and the N-acetyldopamine dimers which are similar to some reported to have anti-inflammatory activity (including COX-2 inhibition), which matches the use of Qí Cáo for pain relief (Xu et al., 2006; Yan et al., 2015).
Fig. 18.
Representative compounds reported from Protaetia brevitarsis. Compounds 112-115 are simple aromatic compounds. Compounds 116 and 117 are diketopiperazines. Compounds 118-121 are β-carbolines. Compounds 122 and 123 are N-acetyldopamine dimers.
Scarabaeus: Dung beetles in the genus Scarabaeus may be the famous scarabs depicted in ancient Egyptian artwork. Beetles in this genus have been eaten by people throughout their range, as well as used in traditional medicines (Ratcliffe, 2006). Most relevant to this work, however, is that S. sacer was noted as being used as Qiānɡ Lánɡ in TCM (National Administration of Traditional Chinese Medicine, 1999). We could not find any in-depth studies of the chemical properties of S. sacer, but chitosan isolated from this beetle has recently been tested for and displayed anticancer activity (Wahid et al., 2018). There has also been work on the cuticular hydrocarbon profile of S. sacer, as well as indication that they have sternal glands that likely produce organic compounds, but no specific compounds have been elucidated (Niogret et al., 2006, 2018).
Trematodes: The genus Trematodes is in the subfamily Melolonthinae, similar to Holotrichia. It was reported in Namba et al. (1988) that T. tenebrioides is used as Qí Cáo in TCM. Although T. tenebrioides is one of the most common scarabs in Inner Mongolia (Liu and Wu, 2004), we could find no information on the chemistry of this genus. They may have similar compounds as Holotrichia since they are in the same subfamily (100-111 in Fig. 17).
Xylotrupes: The genus Xylotrupes is in the subfamily Dynastinae, and are therefore rhinoceros beetles. Even though we found sources indicating that X. dichotomus was used in TCM as both Qí Cáo (in the larval stage) and Qiānɡ Lánɡ (in the adult stage), we could not find any studies on the chemistry of this beetle (Namba and Inagaki, 1984; Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999). Considering that it is in the same subfamily as Allomyrina, it would be interesting to see if it had similar chemistry (80-82 in Fig. 13).
Staphylinidae
Paederus: The Staphylinidae, or rove beetles, are a large group (~1,500 genera and 25,000 species) of predacious beetles that have long, narrow bodies that when combined with their short elytra often leave large portions of their abdomen exposed (Eisner et al., 2005). The fact that so much of the abdomen is physically unprotected has led to the assumption, and later support of this hypothesis, that most species have some sort of chemical defense (Dettner, 1987, 2015; Eisner et al., 2005). We have found one species of Staphylinid used in traditional Chinese and traditional Korean medicine, Paederus fuscipes, although it is sometimes listed as the synonymous names P. densipennis or P. idae (GBIF Secretariat, 2019b). In TCM it is called 青腰虫, Qīng Yāo Chónɡ (although one source called it 花蚁虫, Huā Yǐ Chónɡ) and is used to remove tattoos, and treat skin ailments including infected boils, nasal polyps, and ringworm (Frank and Kanamitsu, 1987; Namba et al., 1988; National Administration of Traditional Chinese Medicine, 1999). We were unable to find the Korean name for this insect, but it has been traditionally used to treat vitiligo (You et al., 2003).
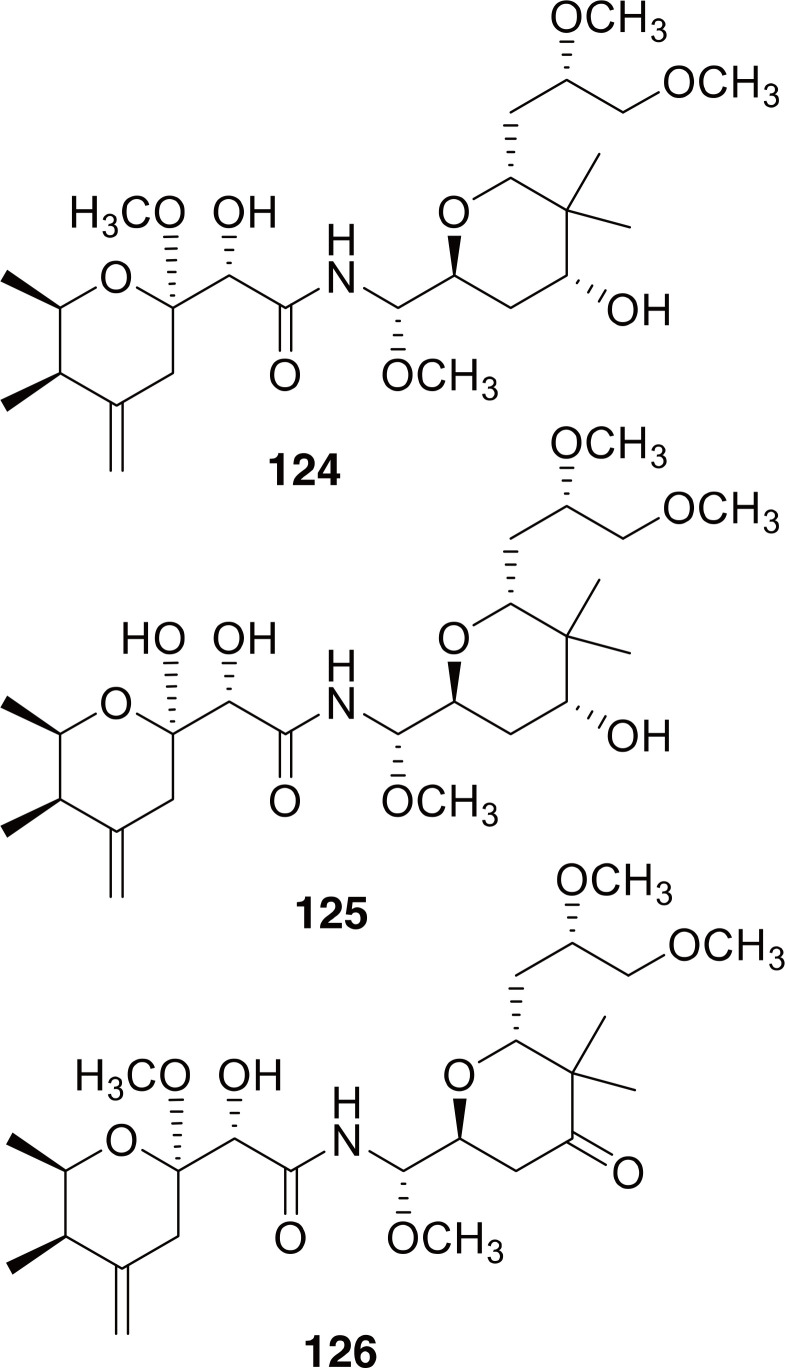
Beetles in the genus Paederus have been studied intensively due to their role in causing outbreaks of severe dermatitis called Paederus dermatitis or dermatitis linearis. Swarms of Paederus spp. occasionally occur, especially when they are attracted to bright lights at night near agricultural areas, and can cause many cases of acute skin and eye damage (Huang et al., 2009; Bong et al., 2015; Prasher et al., 2017). There have been several excellent reviews relating to Paederus spp. including on the natural history and medical importance (Frank and Kanamitsu, 1987), outbreaks of Paederus dermatitis (Bong et al., 2015), chemical defenses (Dettner, 2011, 2015), and chemistry and biological activity (Narquizian and Kocienski, 2000; Mosey and Floreancig, 2012). Three compounds have been identified from P. fuscipes: pederin, pseudopederin, and pederone (Fig. 19) (Dettner, 2011, 2015).
Fig. 19.
Compounds identified from Paederus fuscipes. Compounds 124-126 are pederin, pseudopederin, pederone.
Pederin (124 in Fig. 19) was found to be the causative agent in Paederus dermatitis, and displayed extraordinary cytotoxicity, being one of the most potent cytotoxic natural compounds. It has been said that it is over 15 times more toxic than cobra venom (Frank and Kanamitsu, 1987). When applied to skin it causes painful burn-like wounds and blistering which can take one to three weeks to heal. After healing, the skin sometimes shows hyperpigmentation, which along with the complete renewal of the underlying skin, would explain the traditional use to remove tattoos, treat skin infections, and vitiligo (Frank and Kanamitsu, 1987; You et al., 2003; Huang et al., 2009). It has been found that female Paederus spp. contain up to ten times more pederin than males (Kellner and Dettner, 1995), and that this compound is biosynthesized by symbiotic bacteria, probably Pseudomonas (Dettner, 2011; Mosey and Floreancig, 2012, and references therein). The mode of action of pederin has been determined to be the inhibition of protein synthesis by binding to the ribosome (Narquizian and Kocienski, 2000, and references therein). Pederin and analogs (124-126) have been investigated for possible use as anticancer agents, but unfortunately they tend to kill cells indiscriminately and are not specific to cancer cells (Narquizian and Kocienski, 2000; Dettner, 2011; Mosey and Floreancig, 2012; Schleissner et al., 2017).
Tenebrionidae
Ulomoides: The family Tenebrionidae, commonly called darkling beetles, include approximately 1,700 genera and 18,000 species. They are notable for the fact that they are predominantly protected by chemicals produced in large glands (Eisner et al., 2005). We could only find mention of one species of Tenebrionid used in TCM, Martianus dermestoides, which is called 洋虫 (Yánɡ Chónɡ) (Ding et al., 1997; Zhang et al., 2019). However, upon further investigation, it became apparent that this species is now classified as Ulomoides dermestoides (Gustavo et al., 2002). In TCM, U. dermestoides is used as a tonic, to treat coughing, stomach illness, bone problems, stroke, and, most notably, cancer (Ding et al., 1997; National Administration of Traditional Chinese Medicine, 1999; Zhang et al., 2019).
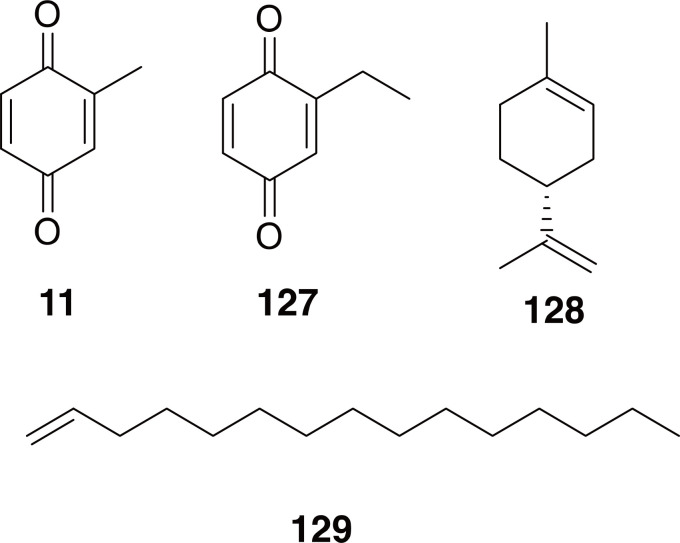
Consistent with earlier studies of other Tenebrionids, the chemical constituents of the defensive secretions of U. dermestoides were determined to contain 1,4-benzoquinones, and long-chain 1-alkenes (11, 127-129 in Fig. 20) along with other, minor constituents, including limonene (Eisner et al., 2005; Francke and Dettner, 2005; Villaverde et al., 2009; Martins et al., 2010). Tests of extracts from U. dermestoides and closely related species, along with some of the individual compounds that make up the defensive secretions, have shown a wide-range of bioactivities, including cytotoxicity (Crespo et al., 2011), anti-diabetes (Jasso-Villagomez et al., 2018), anti-senility (Yan et al., 2009), anticoagulatory (Wu et al., 1996), and anti-asthma/anti-inflammatory (Wahrendorf and Wink, 2006; Santos et al., 2010). These results should be tempered, however, by the reminder that 1,4-benzoquinones are known to be hepatotoxic (Moore et al., 1987; Abernethy et al., 2004; Chan et al., 2008).
Fig. 20.
Compounds 11 and 127-129 are major compounds identified from the defensive secretions of Ulomoides dermestoides.
The use of U. dermestoides as a folk medicine has exploded worldwide in recent years, especially in Latin America, where this species does not occur naturally, but has been introduced (Gustavo et al., 2002). This beetle has many common names in its usage outside of the structure of TCM, including “peanut beetle”, “Chinese beetle”, “Chinese weevil” (even though it has no protruding rostrum and is therefore easily identifiable as not being a weevil), “asthma beetle”, and most notoriously, “cancer beetle”. It should be noted that the widespread use of U. dermestoides without the care, knowledge, and experience of a TCM practitioner can cause severe side effects including eosinophilic pneumonia, palpable purpura, and colitis (Natt et al., 2014; Martínez-Rodríguez et al., 2015; Saldarriaga Rivera et al., 2017).
DISCUSSION AND CONCLUSIONS
We were able to find evidence of 48 species of beetles from 34 genera in 14 different families that are used in TCM. The fact that so many species of beetles are used in TCM would likely surprise most TCM researchers and practitioners, even though this pales in comparison to the number of plant species used in TCM (>2,400) (Dai et al., 2016). Beetles are used for treatment of such critical diseases as cancer, stroke, heart disease, and bacterial infection. These are heavily researched areas that are still in dire need of additional therapeutics, and the beetles used in TCM may be a good resource to study for new lead compounds.
It should be noted that the beetles used in TCM were carefully selected over hundreds to thousands of years based, at least in part, by outcomes of patients. This selection is made clear when one compares and contrasts the taxa used by practitioners versus those not used. Several families of beetles bearing compounds with potent bioactivity (and even toxicity) are used including the Meloidae, the Tenebrionidae, and the Staphylinidae. However, other families that are known to have potent and/or high concentrations of bioactive molecules are not represented in TCM use, including the Chrysomelidae, Coccinellidae, Lycidae, and Silphidae (Dettner, 1987; Eisner et al., 2005). It seems fair to assume that these unrepresented families of beetles were the subjects of experimentation at least once over the >2,000-year history of TCM, but were discarded for lack of efficacy, presence of side effects, difficulty accumulating significant sources, or some other problem. This does not mean that these taxa are chemically uninteresting. However, it does emphasize that when searching for new potential drug leads, one should start with taxa that have been selected for through the long traditions of TCM.
The depth to which the beetles used in TCM have been chemically investigated varies widely. For instance, blister beetles, especially those in the genus Mylabris which are used to treat cancer, have been studied in detail yielding primarily cantharidin, palasonin, and cantharidinimides (Nakatani et al., 2004; Nikbakhtzadeh and Ebrahimi, 2007; Zeng et al., 2020). These structures and related analogs have been tested and showed potent anti-cancer activity (although toxicity remains problematic) (Liu and Chen, 2009; Dettner, 2011; Puerto Galvis et al., 2013). However, the in-depth studies on Mylabris and its chemical constituents serves as “the exception that proves the rule”, highlighting how few of these medicinal beetles have been intensively investigated.
There is a spectrum of how well beetles used in TCM have been studied, ranging from the aforementioned Mylabris example to no chemical studies at all. There are beetles that have known chemistry, and the bioactivities of those molecules matches the TCM use, yet these molecules have not been subjected to rigorous medicinal chemistry work to determine feasibility of progressing to preclinical trials. A good example of this is the genus Gyrinus, which produces norsesquiterpenoids including gyrinidal, which have been shown to have antibacterial activity and are used in TCM to treat infected boils. There are also beetles that have known chemistry, yet these molecules have not been tested to see if they demonstrate the bioactivity which has been attributed to the beetle. Good examples of these are the buprestins from Chalcophora, the gomadalactones from Anoplophora, steroids from Cybister, β-carbolines from Protaetia, and N-acetyldopamine dimers from Catharsius. Some beetles, like Lyctus brunneus, are known to have glands that likely produce organic molecules, but the constituents of these glands have not been elucidated. Finally, there are some groups for which we could find no chemical studies even though they are used in TCM, including Pleonomus and Phelotrupes. Therefore, there is plenty of work to be done on beetles used in TCM for scientists ranging from natural products chemists to medicinal chemists to molecular biologists and pharmaceutical scientists.
Many of the beetles that have been chemically studied have only been investigated for pheromone content, which is incredibly valuable, especially for biocontrol work, but tends to focus heavily on volatile and/or cuticular compounds and could overlook non-volatile and more polar compounds. Further work on these taxa should use methodologies such as LC-MS and 2D NMR spectroscopy, in addition to GC-MS, to elucidate the presence of non-volatile molecules. Additionally, studies on the chemical constituents of bacterial and fungal endosymbionts of medicinal beetles could also be extremely valuable, and are underrepresented in the literature when compared to work on endophytic bacteria and fungi (Dettner, 2011, 2015).
Beetles are the most diverse group of insects, and insects are the most numerous group of macroscopic organisms (Berenbaum and Eisner, 2008; Yuan et al., 2016). We know that beetles are capable of biosynthesis and/or sequestration of potently bioactive compounds from a number of biosynthetic origins. The chemical prospecting of beetles is therefore likely to yield multitudes of new compounds with exciting biological activities (Dossey, 2010; Dettner, 2011; Seabrooks and Hu, 2017). What is more, the use of beetles in TCM gives us an obvious path towards new chemical entities with potential as lead compounds for therapeutic agents. Study of these insects not only provides the possibility of finding new drugs, but also has the additional benefit of providing insights into millennia-old cultural and medical practices. It is the goal of this paper to not only act as a valuable resource in connecting the ethnopharmacological data on which beetles are used in TCM with the chemical data of what compounds these beetles contain, but also to incite the scientific community into action to fill in the gaps in the knowledge herein exposed.
ACKNOWLEDGMENTS
The authors thank Dr. Clark (Kanglun) Liu for his help with translations of selected text from Chinese. A Fulbright US Scholar Award to study TCM at Hong Kong Baptist University was invaluable for this work, and we especially thank Dr. Hongjie Zhang for hosting STD in the School of Chinese Medicine at HKBU. Help from the staff at Standish Library at Siena College was greatly appreciated, as were several enlightening conversations with Dr. Mark Deyrup on beetles and their taxonomy. Thanks to an anonymous reviewer for providing us with the Hangul and pronunciations for the beetles used in TKM. Funding for this work was provided by Siena College.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interest.
REFERENCES
- Abernethy D. J., Kleymenova E. V., Rose J., Faiola B. Human CD34 hematopoietic progenitor cells are sensitive targets for toxicity induced by 1,4-benzoquinone. Toxicol. Sci. 2004;79:82–89. doi: 10.1093/toxsci/kfh095. [DOI] [PubMed] [Google Scholar]
- Ahn M. Y., Hahn B. S., Ryu K. S., Kim J. W., Kim I., Kim Y. S. Purification and characterization of a serine protease with fibrinolytic activity from the dung beetles, Catharsius molossus. Thromb. Res. 2003;112:339–347. doi: 10.1016/j.thromres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Akiba T., Miyazaki M., Toda N. Vasodilator actions of TRK-100, a new prostaglandin I2 analogue. Br. J. Pharmacol. 1986;89:703–711. doi: 10.1111/j.1476-5381.1986.tb11174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J. D., Borden J. H., Seybold S. J. A review of the chemical ecology of the Cerambycidae (Coleoptera) Chemoecology. 2004;14:123–150. doi: 10.1007/s00049-004-0277-1. [DOI] [Google Scholar]
- Altson A. M. On the genital system of Lyctus brunneus Steph., with a note on Lyctus linearis Goeze (Coleoptera) Zool. J. Linn. Soc. 1924;35:581–597. doi: 10.1111/j.1096-3642.1924.tb00056.x. [DOI] [Google Scholar]
- Berenbaum M. R., Eisner T. Bugs' bugs. Science. 2008;322:52–53. doi: 10.1126/science.1164873. [DOI] [PubMed] [Google Scholar]
- Bessho-Uehara M., Oba Y. Identification and characterization of the Luc2-type luciferase in the Japanese firefly, Luciola parvula, involved in a dim luminescence in immobile stages. Luminescence. 2017;32:924–931. doi: 10.1002/bio.3273. [DOI] [PubMed] [Google Scholar]
- Bologna M. A., Pinto J. D. The Old World genera of Meloidae (Coleoptera): a key and synopsis. J. Nat. Hist. 2002;36:2013–2102. doi: 10.1080/00222930110062318. [DOI] [Google Scholar]
- Bong L. J., Neoh K. B., Jaal Z., Lee C. Y. Paederus outbreaks in human settings: a review of current knowledge. J. Med. Entomol. 2015;52:517–526. doi: 10.1093/jme/tjv041. [DOI] [PubMed] [Google Scholar]
- Bouchard P., Bousquet Y., Davies A. E., Alonso-Zarazaga M. A., Lawrence J. F., Lyal C. H. C., Newton A. F., Reid C. A. M., Schmitt M., Ślipiński S. A., Smith A. B. T. Family-group names in Coleoptera (Insecta) ZooKeys. 2011;88:1–972. doi: 10.3897/zookeys.88.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W., Jones A., Lacey M., Moore B. Chemistry of buprestins A and B. Bitter principles of jewel beetles (Coleoptera: Buprestidae) Aust. J. Chem. 1985;38:197–206. doi: 10.1071/CH9850197. [DOI] [Google Scholar]
- Carrel J. E., Doom J. P., McCormick J. P. Cantharidin biosynthesis in a blister beetle: Inhibition by 6-fluoromevalonate causes chemical disarmament. Experientia. 1986;42:853–854. doi: 10.1007/BF01941552. [DOI] [PubMed] [Google Scholar]
- Cha W. S., Oh J. H., Park H. J., Ahn S. W., Hong S. Y., Kim N. I. Historical difference between traditional Korean medicine and traditional Chinese medicine. Neurol. Res. 2007;29 Suppl 1:S5–S9. doi: 10.1179/016164107X172293. [DOI] [PubMed] [Google Scholar]
- Chan K., Jensen N., O'Brien P. J. Structure-activity relationships for thiol reactivity and rat or human hepatocyte toxicity induced by substituted p-benzoquinone compounds. J. Appl. Toxicol. 2008;28:608–620. doi: 10.1002/jat.1312. [DOI] [PubMed] [Google Scholar]
- Chon J. W., Kweon H., Jo Y. Y., Yeo J. H., Lee H. S. Protective effects of extracts of Protaetia brevitarsis on carbon tetrachloride-induced hepatotoxicity in the mice. Korean J. Sericult. Sci. 2012;50:93–100. doi: 10.7852/jses.2012.50.2.93. [DOI] [Google Scholar]
- Crespo R., Villaverde M. L., Girotti J. R., Güerci A., Juárez M. P., De Bravo M. G. Cytotoxic and genotoxic effects of defence secretion of Ulomoides dermestoides on A549 cells. J. Ethnopharmacol. 2011;136:204–209. doi: 10.1016/j.jep.2011.04.056. [DOI] [PubMed] [Google Scholar]
- Crook D. J., Lance D. R., Mastro V. C. Identification of a potential third component of the male-produced pheromone of Anoplophora glabripennis and its effect on behavior. J. Chem. Ecol. 2014;40:1241–1250. doi: 10.1007/s10886-014-0520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha R. L., Verdú J. R., Lobo J. M., Zardoya R. Ancient origin of endemic Iberian earth-boring dung beetles (Geotrupidae) Mol. Phylogenet. Evol. 2011;59:578–586. doi: 10.1016/j.ympev.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Dai S. X., Li W. X., Han F. F., Guo Y. C., Zheng J. J., Liu J. Q., Wang Q., Gao Y. D., Li G. H., Huang J. F. In silico identification of anti-cancer compounds and plants from traditional Chinese medicine database. Sci. Rep. 2016;6:25462. doi: 10.1038/srep25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Pardal P. P., da Silva C. T. C., Monteiro W. M., da Costa Gadelha M. A. Dermatitis after contact with Pheropsophus sp (Coleoptera, Carabidae, Brachininae) in the Pará State, Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2016;49:799–801. doi: 10.1590/0037-8682-0218-2016. [DOI] [PubMed] [Google Scholar]
- Deng L. P., Dong J., Cai H., Wang W. Cantharidin as an antitumor agent: a retrospective review. Curr. Med. Chem. 2013;20:159–166. doi: 10.2174/092986713804806711. [DOI] [PubMed] [Google Scholar]
- Dettner K. Ecological and phylogenetic significance of defensive compounds from pygidial glands of Hydradephaga (Coleoptera) Proc. Acad. Nat. Sci. Philadelphia. 1985;137:156–171. [Google Scholar]
- Dettner K. Chemosystematics and evolution of beetle chemical defenses. Annu. Rev. Entomol. 1987;32:17–48. doi: 10.1146/annurev.en.32.010187.000313. [DOI] [Google Scholar]
- Dettner K. In: Potential pharmaceuticals from insects and their co-occurring microorganisms. In Insect Biotechnology. Vilcinskas A., editor. Springer Science+Business Media; 2011. pp. 95–119. [DOI] [Google Scholar]
- Dettner K. In: Chemical ecology and biochemistry of Dytiscidae. In Ecology, Systematics, and the Natural History of Predaceous Diving Beetles (Coleoptera: Dytiscidae) Yee D. A., editor. Springer Science+Business Media; 2014. pp. 235–306. [DOI] [Google Scholar]
- Dettner K. In: Toxins, defensive compounds and drugs from insects. In Insect Molecular Biology and Ecology. Hoffmann K. H., editor. Taylor & Francis; 2015. pp. 39–93. [Google Scholar]
- Dettner K. In: Defenses of water insects. In Aquatic Insects. Del-Claro K., Guillermo R., editors. Springer Nature; 2019. pp. 191–262. [DOI] [Google Scholar]
- Ding Z., Zhao Y., Gao X. Medicinal insects in China. Ecol. Food Nutr. 1997;36:209–220. [Google Scholar]
- Dong Q. F., Wang Z., Liu H. J., Zhang C. F., He D. X., Wu G., Zhang L. Flavonoid and other compounds from Holotrichia diomphalia larvae. Chem. Nat. Compd. 2011;47:114–115. doi: 10.1007/s10600-011-9848-x. [DOI] [Google Scholar]
- Dong Q. F., Wang J. L., Zhang S. F., Wang Z., Zhang C. X., Gao H., Zhang H. M., Zhang L. Antifungal activity of crude extracts and fat-soluble constituents of Holotrichia diomphalia larvae. Bioresour. Technol. 2008;99:8521–8523. doi: 10.1016/j.biortech.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Dossey A. T. Insects and their chemical weaponry: new potential for drug discovery. Nat. Prod. Rep. 2010;27:1737–1757. doi: 10.1039/c005319h. [DOI] [PubMed] [Google Scholar]
- Eisner T., Goetz M. A., Hill D. E., Smedley S. R., Meinwald J. Firefly "femmes fatales" acquire defensive steroids (lucibufagins) from their firefly prey. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9723–9728. doi: 10.1073/pnas.94.18.9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner T., Wiemer D. F., Haynes L. W., Meinwald J. Lucibufagins: defensive steroids from the fireflies Photinus ignitus and P. marginellus (Coleoptera: Lampyridae) Proc. Natl. Acad. Sci. U.S.A. 1978;75:905–908. doi: 10.1073/pnas.75.2.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner T. For Love of Insects. The Belknap Press of Harvard University Press; 2003. [Google Scholar]
- Eisner T., Eisner M., Siegler M. Secret Weapons: Defenses of Insects, Spiders, Scorpions, and Other Many-Legged Creatures. The Belknap Press of Harvard University Press; 2005. [Google Scholar]
- Ernst E. Prevalence of use of complementary/alternative medicine: a systematic review. Bull. World Health Organ. 2000;78:252–257. [PMC free article] [PubMed] [Google Scholar]
- Etzler F. E., Johnson P. J. Athoplastus Johnson and Etzler (Coleoptera: Elateridae: Dendrometrinae), a new genus of click beetle from the northwestern continental USA. Coleopt. Bull. 2018;72:503–521. doi: 10.1649/0010-065X-72.3.503. [DOI] [Google Scholar]
- Fallon T. R., Lower S. E., Chang C. H., Bessho-Uehara M., Martin G. J., Bewick A. J., Behringer M., Debat H. J., Wong I., Day J. C., Suvorov A., Silva C. J., Stanger-Hall K. F., Hall D. W., Schmitz R. J., Nelson D. R., Lewis S. M., Shigenobu S., Bybee S. M., Larracuente A. M., Oba Y., Weng J. K. Firefly genomes illuminate parallel origins of bioluminescence in beetles. ELife. 2018;7:e36495. doi: 10.7554/eLife.36495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz C. A. A., de Oliveira Júnior R. G., Picot L., da Silva Almeida J. R. G., Nunes X. P. Pre-clinical investigations of β-carboline alkaloids as antidepressant agents: a systematic review. Fitoterapia. 2019;137:104196. doi: 10.1016/j.fitote.2019.104196. [DOI] [PubMed] [Google Scholar]
- Fietz O., Dettner K., Görls H., Klemm K., Boland W. (R)-(+)-palasonin, a cantharidin-related plant toxin, also occurs in insect hemolymph and tissues. J. Chem. Ecol. 2002;28:1315–1327. doi: 10.1023/A:1019561517040. [DOI] [PubMed] [Google Scholar]
- Francke W., Dettner K. In: Chemical signalling in beetles. In The Chemistry of Pheromones and Other Semiochemicals II. Schultz S., editor. 2005. pp. 85–166. [DOI] [Google Scholar]
- Frank J. H., Kanamitsu K. Paederus, sensu lato (Coleoptera: Staphylinidae): natural history and medical importance. J. Med. Entomol. 1987;24:155–191. doi: 10.1093/jmedent/24.2.155. [DOI] [PubMed] [Google Scholar]
- Fu X., Ballantyne L. A., Lambkin C. L. Aquatica gen. nov. from mainland China with a description of Aquatica wuhana sp. nov. (Coleoptera: Lampyridae: Luciolinae) Zootaxa, 2010;2530:1–18. doi: 10.11646/zootaxa.2530.1.1. [DOI] [Google Scholar]
- Fu X., Vencl F. V., Nobuyoshi O., Meyer-Rochow V. B., Lei C., Zhang Z. Structure and function of the eversible glands of the aquatic firefly Luciola leii (Coleoptera: Lampyridae) Chemoecology. 2007;17:117–124. doi: 10.1007/s00049-007-0370-3. [DOI] [Google Scholar]
- Fung F. Y., Linn Y. C. Developing traditional Chinese medicine in the era of evidence-based medicine: current evidences and challenges. Evid. Based Complement. Alternat. Med. 2015;2015:425037. doi: 10.1155/2015/425037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBIF Secretariat Chrysochroa fulgidissima (Schönherr, 1817) GBIF Backbone Taxonomy. 2019a Available from: https://doi.org/https://doi.org/10.15468/39omei/
- GBIF Secretariat Paederus fuscipes subsp. fuscipes Curtis, 1826. GBIF Backbone Taxonomy. 2019b Available from: https://doi.org/https://doi.org/10.15468/39omei//
- GBIF Secretariat [retrieved 2020 Jun 3];GBIF. 2020 Available from: https://www.gbif.org/
- Ghoneim K. Cantharidin toxicosis to animal and human in the world: A review. Stand. Res. J. Toxicol. Environ. Health Sci. 2013;1:1–16. [Google Scholar]
- Gustavo E., Padín S. B., Stetson R. E. First records of the Oriental species Ulomoides dermestoides (Coleoptera: Tenebrionidae) in Argentina. Rev. Soc. Entomol. Argent. 2002;61:48–50. [Google Scholar]
- Hallett R. H., Gries G., Gries R., Borden J. H., Czyzewska E., Oehlschlager A. C., Pierce H. D., Angerilli N. P. D., Rauf A. Aggregation pheromones of two asian palm Weevils, Rhynchophorus ferrugineus and R. vulneratus. Naturwissenschaften. 1993;80:328–331. doi: 10.1007/BF01141908. [DOI] [Google Scholar]
- Han T., Kang T., Jeong J., Lee Y., Chung H., Park S., Lee S., Kim K., Park H. Pseudocryptic speciation of Chrysochroa fulgidissima (Coleoptera: Buprestidae) with two new species from Korea, China and Vietnam. Zool. J. Linn. Soc. 2012;164:71–98. doi: 10.1111/j.1096-3642.2011.00763.x. [DOI] [Google Scholar]
- Hansen L., Xu T., Wickham J., Chen Y., Hao D., Hanks L. M., Millar J. G., Teale S. A. Identification of a male-produced pheromone component of the citrus longhorned beetle, Anoplophora chinensis. PLoS ONE. 2015;10:e0134358. doi: 10.1371/journal.pone.0134358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härlin C. To have and have not: volatile secretions make a difference in gyrinid beetle predator defence. Anim. Behav. 2005;69:579–585. doi: 10.1016/j.anbehav.2004.06.014. [DOI] [Google Scholar]
- Hedin P. A., Dollar D. A., Collins J. K., Dubois J. G., Mulder P. G., Hedger G. H., Smith M. W., Eikenbary R. D. Identification of male pecan weevil pheromone. J. Chem. Ecol. 1997;23:965–977. doi: 10.1023/B:JOEC.0000006382.70034.66. [DOI] [Google Scholar]
- Holliday A. E., Holliday N. J., Mattingly T. M., Naccarato K. M. Defensive secretions of the Carabid beetle Chlaenius cordicollis: chemical components and their geographic patterns of variation. J. Chem. Ecol. 2012;38:278–286. doi: 10.1007/s10886-012-0078-x. [DOI] [PubMed] [Google Scholar]
- Hong J. H., Kim S. H., Lee Y. C. The ethanol extract of Holotrichia diomphalia larvae, containing fatty acids and amino acids, exerts anti-asthmatic effects through inhibition of the GATA-3/Th2 signaling pathway in asthmatic mice. Molecules. 2019;24:852. doi: 10.3390/molecules24050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M. Y., Jeong H. C., Kim M. J., Jeong H. U., Lee S. H., Kim I. Complete mitogenome sequence of the jewel beetle, Chrysochroa fulgidissima (Coleoptera: Buprestidae) Mitochondrial DNA. 2009;20:46–60. doi: 10.1080/19401730802644978. [DOI] [PubMed] [Google Scholar]
- Hoover K., Keena M., Nehme M., Wang S., Meng P., Zhang A. Sex-specific trail pheromone mediates complex mate finding behavior in Anoplophora glabripennis. J. Chem. Ecol. 2014;40:169–180. doi: 10.1007/s10886-014-0385-5. [DOI] [PubMed] [Google Scholar]
- Hosseini H. R., Salehi H., Alichi M. Acquirement of CRY8DB transgenic tall fescue (Festuca arundinacea Schreb.) by Agrobacterium tumefaciens to develop resistance against Pentodon idiota Herbest. Mol. Biotechnol. 2019;61:528–540. doi: 10.1007/s12033-019-00183-5. [DOI] [PubMed] [Google Scholar]
- Houston W. W. K. Exocrine glands in the forelegs of dung beetles in the genus Onitis F. (Coleoptera: Scarabidae) J. Austral. Ent. Soc. 1986;25:161–169. doi: 10.1111/j.1440-6055.1986.tb01096.x. [DOI] [Google Scholar]
- Hu Y., Kang T., Zhao Z. Studies on microscopic identification of animal drugs' remnant hair (2) Identification of ground beetle and its counterfeits. Natural Medicines. 2004;58:185–192. [Google Scholar]
- Huang C., Liu Y., Yang J., Tian J., Yang L., Zhang J., Li Y., Li J., Wang C., Tu Y., Tao J. An outbreak of 268 cases of Paederus dermatitis in a toy-building factory in central China. Int. J. Dermatol. 2009;48:128–131. doi: 10.1111/j.1365-4632.2009.03876.x. [DOI] [PubMed] [Google Scholar]
- Hughes G. P., Meier L. R., Zou Y., Millar J. G., Hanks L. M., Ginzel M. D. Stereochemistry of fuscumol and fuscumol acetate influences attraction of longhorned beetles (Coleoptera: Cerambycidae) of the subfamily Lamiinae. Environ. Entomol. 2016;45:1271–1275. doi: 10.1093/ee/nvw101. [DOI] [PubMed] [Google Scholar]
- Hughes G. P., Zou Y., Millar J. G., Ginzel M. D. (S)-fuscumol and (S)-fuscumol acetate produced by a male Astyleiopus variegatus (Coleoptera: Cerambycidae) Can. Entomol. 2013;145:327–332. doi: 10.4039/tce.2013.3. [DOI] [Google Scholar]
- Hunt T., Bergsten J., Levkanicova Z., Papadopoulou A., St. John O., Wild R., Hammond P. M., Ahrens D., Balke M., Caterino M. S., Gómez-Zurita J., Ribera I., Barraclough T. G., Bocakova M., Bocak L., Vogler A. P. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science. 2007;318:1913–1916. doi: 10.1126/science.1146954. [DOI] [PubMed] [Google Scholar]
- Hwang H., Patnaik B. B., Kang S. W., Park S. Y., Chung J. M., Sang M. K., Park J. E., Min H. R., Seong J., Jo Y. H., Noh M. Y., Lee J. D., Jung K. Y., Park H. S., Jeong H. C., Lee Y. S. RNA Sequencing, de novo assembly, functional annotation and SSR analysis of the endangered diving beetle Cybister chinensis (= Cybister japonicus) using the Illumina platform. Entomol. Res. 2018;48:60–72. doi: 10.1111/1748-5967.12292. [DOI] [Google Scholar]
- Hwang J. S., Kang B. R., Kim S. R., Yun E. Y., Park K. H., Jeon J. P., Nam S. H., Suh H. J., Hong M. Y., Kim I. S. Molecular characterization of a defensin-like peptide from larvae of a beetle, Protaetia brevitarsis. Int. J. Ind. Entomol. 2008;17:131–135. [Google Scholar]
- Hwang S. Y., Kim Y. B., Lee S. H., Yun C. Y. Preventive effect of a chafer, Protaetia brevitarsis extract on carbon tetrachloride-induced liver injuries in rats. Korean J. Orient. Physiol. Pathol. 2005;19:1337–1343. [Google Scholar]
- Ide T., Kanzaki N., Ohmura W., Okabe K. Molecular identification of an invasive wood-boring insect Lyctus brunneus (Coleoptera: Bostrichidae: Lyctinae) using frass by loop-mediated isothermal amplification and nested PCR assays. J. Econ. Entomol. 2016;109:1410–1414. doi: 10.1093/jee/tow030. [DOI] [PubMed] [Google Scholar]
- Ivarsson P., Henrikson B. I., Stenson J. A. E. Volatile substances in the pygidial secretion of gyrinid beetles (Coleoptera: Gyrinidae) Chemoecology. 1996;7:191–193. doi: 10.1007/BF01266313. [DOI] [Google Scholar]
- Jäch M. A. Fried water beetles cantonese style. Am. Entomol. 2003;49:34–37. doi: 10.1093/ae/49.1.34. [DOI] [Google Scholar]
- Jameson M. L. Phylogenetic analysis of the subtribe Rutelina and revision of the Rutela generic groups (Coleoptera: Scarabaeidae: Rutelinae: Rutelini) Bulletin of the University of Nebraska State Museum. 1997;14:1–184. [Google Scholar]
- Jang Y., Kim S. Description of larva and pupa of Pentodon quadridens bidentulus (Fairmaire, 1887) (Coleoptera, Scarabaeidae, Dynastinae) and notes on its biology. Korean J. Appl. Entomol. 2019;58:165–174. [Google Scholar]
- Jasso-Villagomez E. I., Garcia-Lorenzana M., Almanza-Perez J. C., Fortis-Barrera M. A., Blancas-Flores G., Roman-Ramos R., Prado-Barragan L. A., Alarcon-Aguilar F. J. Beetle (Ulomoides dermestoides) fat improves diabetes: effect on liver and pancreatic architecture and on PPARγ expression. Braz. J. Med. Biol. Res. 2018;51:e7238. doi: 10.1590/1414-431x20187238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Lü S. M., Qi Z. Y., Zhang Y. L. Characterized cantharidin distribution and related gene expression patterns in tissues of blister beetles, Epicauta chinensis. Insect Sci. 2019;26:240–250. doi: 10.1111/1744-7917.12512. [DOI] [PubMed] [Google Scholar]
- Jiang M., Lü S., Zhang Y. Characterization of juvenile hormone related genes regulating cantharidin biosynthesis in Epicauta chinensis. Sci. Rep. 2017a;7:2308. doi: 10.1038/s41598-017-02393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Lü S., Zhang Y. The potential organ involved in cantharidin biosynthesis in Epicauta chinensis Laporte (Coleoptera: Meloidae) J. Insect Sci. 2017b;17:52. doi: 10.1093/jisesa/iex021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Tan C., Ma J., Yang M. Screening of active fraction of anti-benign prostatic hyperplasia from Catharsius molossus (I) Pharmacol. Clin. Chin. Mater. Med. 2012;6:35. [Google Scholar]
- Jiang S. J. Anti-cancer insect medicinal materials in China. Zhong Yao Cai. 1990;13:11–14. [Google Scholar]
- Kang I. J., Chung C. K., Kim S. J., Nam S. M., Oh S. H. Effects of Protaetia orientalis (Gory et Perchlon) larva on the lipid metabolism in carbon tetrachloride administered rats. Kor. Jour. Electron Microscopy. 2001;31:9–18. [Google Scholar]
- Kang J. H., Lim C. S., Park S. H., Seok S. W., Yoon T. J., Bayartogtokh B., Bae Y. J. Historical domestication-driven population expansion of the dung beetle Gymnopleurus mopsus (Coleoptera: Scarabaeidae) from its last refuge in Mongolia. Sci. Rep. 2018;8:3963. doi: 10.1038/s41598-018-22182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N. S., Park S. Y., Lee K. R., Lee S. M., Lee B. G., Shin D. H., Pyo S. Modulation of macrophage function activity by ethanolic extract of larvae of Holotrichia diomphalia. J. Ethnopharmacol. 2002;79:89–94. doi: 10.1016/S0378-8741(01)00369-5. [DOI] [PubMed] [Google Scholar]
- Karlsson A. B., Henrikson B., Härlin C., Ivarsson P., Stenson J. A. E., Svensson B. W. The possible role of volatile secretions as intra- and interspecific alarm signals in Gyrinus species. OIKOS. 1999;87:220–227. doi: 10.2307/3546737. [DOI] [Google Scholar]
- Karras D. J., Farrell S. E., Harrigan R. A., Henretig F. M., Gealt L. Poisoning from "spanish fly" (cantharidin) Am. J. Emerg. Med. 1996;14:478–483. doi: 10.1016/S0735-6757(96)90158-8. [DOI] [PubMed] [Google Scholar]
- Kartika T., Shimizu N., Yoshimura T. Identification of esters as novel aggregation pheromone components produced by the male powder-post beetle, Lyctus africanus Lesne (Coleoptera: Lyctinae) PLoS ONE. 2015;10:e0141799. doi: 10.1371/journal.pone.0141799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner R. L. L., Dettner K. Allocation of pederin during lifetime of Paederus rove beetles (Coleoptera: Staphylinidae): evidence for polymorphism of hemolymph toxin. J. Chem. Ecol. 1995;21:1719–1733. doi: 10.1007/BF02033672. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Kim H. G., Jeong J. H. Seasonal characteristics of eggs and adults of Luciola lateralis (Coleoptera: Lampyridae) reared in the laboratory. Korean J. Appl. Entomol. 2014a;53:225–229. doi: 10.5656/KSAE.2014.03.1.082. [DOI] [Google Scholar]
- Kim M. J., Im H. H., Lee K. Y., Han Y. S., Kim I. Complete mitochondrial genome of the whiter-spotted flower chafer, Protaetia brevitarsis (Coleoptera: Scarabaeidae) Mitochondrial DNA. 2014b;25:177–178. doi: 10.3109/19401736.2013.792064. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Hwang U. W., Kwon O. Three different genetic lineages of the jewel beetle Chrysochroa fulgidissima (Buprestidae; Chrysochroinae) inferred from mitochondrial COI gene. J. Ecol. Environ. 2014c;37:35–39. doi: 10.5141/ecoenv.2014.005. [DOI] [Google Scholar]
- Klein M. G., Edwards D. C. Captures of Popillia lewisi (Coleoptera: Scarabaeidae) and other scarabs on Okinawa with Japanese beetle lures. J. Econ. Entomol. 1989;82:101–103. doi: 10.1093/jee/82.1.101. [DOI] [Google Scholar]
- Kovac D., Maschwitz U. Secretion-grooming in aquatic beetles (Hydradephaga): a chemical protection against contamination of the hydrofuge respiratory region. Chemoecology. 1990;1:131–138. doi: 10.1007/BF01241654. [DOI] [Google Scholar]
- Kumar M., Chawla R., Goyal M. Topical anesthesia. J. Anaesthesiol. Clin. Pharmacol. 2015;31:450–456. doi: 10.4103/0970-9185.169049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundrata R., Gunter N. L., Janosikova D., Bocak L. Molecular evidence for the subfamilial status of Tetralobinae (Coleoptera: Elateridae), with comments on parallel evolution of some phenotypic characters. Arthropod Syst. Phylo. 2018;76:137–145. [Google Scholar]
- Leal W. S. Chemical ecology of phytophagous scarab beetles. Annu. Rev. Entomol. 1998;43:39–61. doi: 10.1146/annurev.ento.43.1.39. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Ryu H. J., Song H. J., Lee S. O. Enzymatic preparation and antioxidant activities of protein hydrolysates from Protaetia brevitarsis larvae. J. Korean Soc. Food Sci. Nutr. 2017a;46:1164–1170. [Google Scholar]
- Lee H. J., Seo M., Lee J. H., Kim I. W., Kim S. Y., Hwang J. S., Kim M. A. Inhibitory effect of Protaetia brevitarsis seulensis ethanol extract on neuroinflammation in LPS-stimulated BV-2 microglia. J. Life Sci. 2019;29:1096–1103. [Google Scholar]
- Lee J. E., Jo D. E., Lee A. J., Park H. K., Youn K., Yun E. Y., Hwang J. S., Jun M., Kang B. H. Hepatoprotective and antineoplastic properties of Protaetia brevitarsis larvae. Entomol. Res. 2014;44:244–253. doi: 10.1111/1748-5967.12075. [DOI] [Google Scholar]
- Lee J. I., Hwang I. H., Kim J. H., Kim M. A., Hwang J. S., Kim Y. H., Na M. K. Quinoxaline-, dopamine-, and amino acid-derived metabolites from the edible insect Protaetia brevitarsis seulensis. Arch. Pharm. Res. 2017b;40:1064–1070. doi: 10.1007/s12272-017-0942-x. [DOI] [PubMed] [Google Scholar]
- Lee J. I., Lee W., Kim M. A., Hwang J. S., Na M. K., Bae J. S. Inhibition of platelet aggregation and thrombosis by indole alkaloids isolated from the edible insect Protaetia brevitarsis seulensis (Kolbe) J. Cell. Mol. Med. 2017c;21:1217–1227. doi: 10.1111/jcmm.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Moon H. J., Kawabata S., Kurata S., Natori S., Lee B. L. A sapecin homologue of Holotrichia diomphalia: Purification, sequencing, and determination of disulfide pairs. Biol. Pharm. Bull. 1995a;18:457–459. doi: 10.1248/bpb.18.457. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Moon H. J., Kurata S., Kurama T., Natori S., Lee B. L. Purification antibacterial diomphalial and molecular cloning of cDNA for an inducible protein of larvae of a Coleopteran insect, Holotrichia diomphalia. J. Biochem. 1994;115:82–86. doi: 10.1093/oxfordjournals.jbchem.a124309. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Moon H. J., Kurata S., Natori S., Lee B. L. Purification and cDNA clonging of an antifungal protein from the hemolymph of Holotrichia diomphalia larvae. Biol. Pharm. Bull. 1995b;18:1049–1052. doi: 10.1248/bpb.18.1049. [DOI] [PubMed] [Google Scholar]
- Li L., Guo C., Li X., Xu S., Han C. Microstructure and mechanical properties of rostrum in Cyrtotrachelus longimanus (Coleoptera: Curculionidae) Anim. Cells Syst. 2017;21:199–206. doi: 10.1080/19768354.2017.1330764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Du C., Yang Y., Nong X., Liao H., Yan S. Study on antibacterial activity of male accessory-gland extracts from Cyrtotrachelus buqueti. Sichuan J. Zool. 2016;35:66–69. [Google Scholar]
- Liu D., Chen Z. The effects of cantharidin and cantharidin derivates on tumour cells. Anti-Cancer Agents Med. Chem. 2009;9:392–396. doi: 10.2174/1871520610909040392. [DOI] [PubMed] [Google Scholar]
- Liu S., Sun J., Yu L., Zhang C., Bi J., Zhu F., Qu M., Yang Q. Antioxidant activity and phenolic compounds of Holotrichia parallela Motschulsky extracts. Food Chem. 2012;134:1885–1891. doi: 10.1016/j.foodchem.2012.03.091. [DOI] [PubMed] [Google Scholar]
- Liu T., Li X., Zou Z. Y., Li C. The prevalence and determinants of using Traditional Chinese Medicine among middle-aged and older Chinese adults: Results from the China health and retirement longitudinal study. J. Am. Med. Dir. Assoc. 2015;16:1002.e1–1002.e5. doi: 10.1016/j.jamda.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Liu X., Wu N. Community features of Scarabaeoidea larvae in Stipa Grandis Steppe. Ying Yong Sheng Tai Xue Bao. 2004;15:1607–1610. [PubMed] [Google Scholar]
- Liu Z. C., Sun Y. R., Wang Z. Y., Liu G. F. The role of biological control in integrated management of sugarcane insect pests. Natural Enemies of Insects. 1985;7:216–222. [Google Scholar]
- Liu Z., Liu L. In: Essentials of Chinese Medicine: Volume 1. Liu Z., Liu L., editors. Springer-Verlag London; 2009. [DOI] [Google Scholar]
- Lu J., Sun Q., Tu Z. C., Lv Q., Shui P. X., Cheng Y. X. Identification of N-acetyldopamine dimers from the dung beetle Catharsius molossus and their COX-1 and COX-2 inhibitory activities. Molecules. 2015;20:15589–15596. doi: 10.3390/molecules200915589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Xin C., Tan C. Preparation, physicochemical and pharmaceutical characterization of chitosan from Catharsius molossus residue. Int. J. Biol. Macromol. 2015;80:547–556. doi: 10.1016/j.ijbiomac.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Maeda J., Kato D. I., Arima K., Ito Y., Toyoda A., Noguchi H. 2017. [DOI] [Google Scholar]
- Maier C. A., Ivie M. A. Reevaluation of Chalcophora angulicollis (LeConte) and Chalcophora virginiensis (Drury) with a review and key to the North American species of Chalcophora Dejean (Coleoptera: Buprestidae) Coleopt. Bull. 2013;67:457–469. doi: 10.1649/0010-065X-67.4.457. [DOI] [Google Scholar]
- Mang D. Z., Luo Q. H., Shu M., Wei W. Extraction and identification of cuticular semiochemical components of Cyrtotrachelus buqueti Guerin-Meneville (Coleoptera: Curculionidae) Acta Entomol. Sin. 2012;55:291–302. [Google Scholar]
- Martin M. M. Biochemical implications of insect mycophagy. Biol. Rev. 1979;54:1–21. doi: 10.1111/j.1469-185X.1979.tb00865.x. [DOI] [Google Scholar]
- Martínez-Rodríguez L. A., Bernal-Méndez A. R., Valdovinos-Andraca F., Martínez-Lozano J. A., Grajales-Figueroa G., Téllez-Ávila F. I. Chinese weevils (Ulomoides dermestoides) found incidentally during colonoscopy. Endoscopy. 2015;47:E114. doi: 10.1055/s-0034-1391128. [DOI] [PubMed] [Google Scholar]
- Martins C. B. C., Zarbin P. H. G., Almeida L. M. Evidence for sex-specific pheromones in Ulomoides dermestoides (Coleoptera, Tenebrionidae) Fla. Entomol. 2010;93:639–641. doi: 10.1653/024.093.0424. [DOI] [Google Scholar]
- Mebs D., Pogoda W., Schneider M., Kauert G. Cantharidin and demethylcantharidin (palasonin) content of blister beetles (Coleoptera: Meloidae) from southern Africa. Toxicon. 2009;53:466–468. doi: 10.1016/j.toxicon.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Meier L. R., Millar J. G., Mongold-Diers J. A., Hanks L. M. (S)-Sulcatol is a pheromone component for two species of Cerambycid beetles in the subfamily Lamiinae. J. Chem. Ecol. 2019;45:447–454. doi: 10.1007/s10886-019-01071-7. [DOI] [PubMed] [Google Scholar]
- Meier L. R., Zou Y., Mongold-Diers J. A., Millar J. G., Hanks L. M. Pheromone composition and chemical ecology of six species of Cerambycid beetles in the subfamily Lamiinae. J. Chem. Ecol. 2020;46:30–39. doi: 10.1007/s10886-019-01128-7. [DOI] [PubMed] [Google Scholar]
- Meinwald J., Opheim K., Eisner T. Gyrinidal: a sesquiterpenoid aldehyde from the defensive glands of Gyrinid beetles. Proc. Natl. Acad. Sci. U.S.A. 1972;69:1208–1210. doi: 10.1073/pnas.69.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Rochow V. B. Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: a comparative survey and review. J. Ethnobiol. Ethnomed. 2017;13:9. doi: 10.1186/s13002-017-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michat M. C., Alarie Y., Miller K. B. Higher-level phylogeny of diving beetles (Coleoptera: Dytiscidae) based on larval characters. Syst. Entomol. 2017;42:734–767. doi: 10.1111/syen.12243. [DOI] [Google Scholar]
- Miller J. R., Hendry L. B., Mumma R. O. Norsesquiterpenes as defensive toxins of whirligig beetles (Coleoptera: Gyrinidae) J. Chem. Ecol. 1975;1:59–82. doi: 10.1007/BF00987720. [DOI] [Google Scholar]
- Miller K. B., Bergsten J., Whiting M. F. Phylogeny and classification of diving beetles in the tribe Cybistrini (Coleoptera, Dytiscidae, Dytiscinae) Zool. Scr. 2007;36:41–59. doi: 10.1111/j.1463-6409.2006.00254.x. [DOI] [Google Scholar]
- Miyanoshita A., Hara S., Sugiyama M., Asaoka A., Taniai K., Yukuhiro F., Yamakawa M. Isolation and characterization of a new member of the insect defensin family from a beetle, Allomyrina dichotoma. Biochem. Biophys. Res. Comm. 1996;220:526–531. doi: 10.1006/bbrc.1996.0438. [DOI] [PubMed] [Google Scholar]
- Moore B. P., Brown W. V. The buprestins: bitter principles of jewel beetles (Coleoptera: Buprestidae) Aust. J. Entomol. 1985;24:81–85. doi: 10.1111/j.1440-6055.1985.tb00191.x. [DOI] [Google Scholar]
- Moore G. A., Rossi L., Nicotera P., Orrenius S., O'Brien P. J. Quinone toxicity in hepatocytes: studies on mitochondrial Ca2+ release induced by benzoquinone derivatives. Arch. Biochem. Biophys. 1987;259:283–295. doi: 10.1016/0003-9861(87)90495-4. [DOI] [PubMed] [Google Scholar]
- Mosey R. A., Floreancig P. E. Isolation, biological activity, synthesis, and medicinal chemistry of the pederin/mycalamide family of natural products. Nat. Prod. Rep. 2012;29:980–995. doi: 10.1039/c2np20052j. [DOI] [PubMed] [Google Scholar]
- Nakatani T., Konishi T., Miyahara K., Noda N. Three novel cantharidin-related compounds from the Chinese blister beetle, Mylabris phalerata PALL. Chem. Pharm. Bull. 2004;52:807–809. doi: 10.1248/cpb.52.807. [DOI] [PubMed] [Google Scholar]
- Namba T., Inagaki K. Pharmacognostical studies on the Chinese crude drugs derived from insects (VII): on the original insects of Qicao. Shoyakugaku Zasshi. 1984;38:118–126. [Google Scholar]
- Namba T., Ma Y. H., Inagaki K. Insect-derived crude drugs in the Chinese Song dynasty. J. Ethnopharmacol. 1988;24:247–285. doi: 10.1016/0378-8741(88)90157-2. [DOI] [PubMed] [Google Scholar]
- Narquizian R., Kocienski P. J. The pederin family of antitumor agents : structures, synthesis and biological activity. In: Mulzer J., Bohlmann R., editors. The Role of Natural Products in Drug Discovery. Springer Berlin Heidelberg; 2000. pp. 25–56. [DOI] [PubMed] [Google Scholar]
- National Administration, of Traditional Chinese Medicine Chinese Herbal Medicine Editorial Committee, editor. Chinese Herbal Medicine, Volume IX. Shanghai Scientific & Technical Publishers; 1999. [Google Scholar]
- National Center for Complementary and Integrative Health, author. [accessed 2020 Jul 1];Traditional Chinese Medicine. 2019 Available from: https://www.nccih.nih.gov/health/traditional-chinese-medicine-what-you-need-to-know/
- Natt B. S., Campion J. M., Knox K. S. Acute eosinophilic pneumonia associated with ingestion of Ulomoides dermestoides larvae ("Chinese beetles") Ann. Am. Thorac. Soc. 2014;11:1667–1668. doi: 10.1513/AnnalsATS.201410-483LE. [DOI] [PubMed] [Google Scholar]
- Nikbakhtzadeh M. R., Tirgari S. Cantharidin component of Iranian blister beetles (Col: Meloidae) and their differences between Iranian and exotic species. Iranian J. Public Health. 2002;31:113–117. [Google Scholar]
- Nikbakhtzadeh M. R., Ebrahimi B. Detection of cantharidin-related compounds in Mylabris impressa (Coleoptera: Meloidae) J. Venom. Anim. Toxins Inc. Trop. Dis. 2007;13:686–693. doi: 10.1590/S1678-91992007000300011. [DOI] [Google Scholar]
- Niogret J., Lumaret J., Bertrand M. Comparison of the cuticular profiles of several dung beetles used as host carriers by the phoretic mite Macrocheles saceri (Acari: Mesostigmata) Nat. Volatiles Essent. Oils. 2018;4:8–13. [Google Scholar]
- Niogret J., Lumaret J. P., Bertrand M. Semiochemicals mediating host-finding behaviour in the phoretic association between Macrocheles saceri (Acari: Mesostigmata) and Scarabaeus species (Coleoptera: Scarabaeidae) Chemoecology. 2006;16:129–134. doi: 10.1007/s00049-006-0338-8. [DOI] [Google Scholar]
- Nishino T., Ohgushi R., Ono K. Observations on the daily fluctuation in flower visiting activity of smaller green flower chafer, Oxycetonia jucunda Falderman on citrus flowers. Japanese J. Appl. Entomol. Zool. 1970;14:39–44. doi: 10.1303/jjaez.14.39. [DOI] [Google Scholar]
- Niu L., Gao J., Li H., Liu J., Yin W. Novel skeleton compound allomyrinanoid A and two purine alkaloids from the adult of Allomyrina dichotoma L. Bioorg. Med. Chem. Lett. 2016;26:366–369. doi: 10.1016/j.bmcl.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Noh J. H., Yun E. Y., Park H., Jung K. J., Hwang J. S., Jeong E. J., Moon K. S. Subchronic oral dose toxicity of freeze-dried powder of Allomyrina dichotoma larvae. Toxicol. Res. 2015;31:69–75. doi: 10.5487/TR.2015.31.1.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh Y. T., Baek K. M., Shin I. C., Moon I. H. Propagation of Korean fireflies, Luciola lateralis Motschulsky. Korean J. Entomol. 1990;20:1–9. [Google Scholar]
- Nowak D. J., Pasek J. E., Sequeira R. A., Crane D. E., Mastro V. C. Potential effect of Anoplophora glabripennis (Coleoptera: Cerambycidae) on urban trees in the United States. J. Econ. Entomol. 2009;94:116–122. doi: 10.1603/0022-0493-94.1.116. [DOI] [PubMed] [Google Scholar]
- Oba Y., Mori N., Yoshida M., Inouye S. Identification and characterization of a luciferase isotype in the Japanese firefly, Luciola cruciata, involving in the dim glow of firefly eggs. Biochemistry. 2010;49:10788–10795. doi: 10.1021/bi1016342. [DOI] [PubMed] [Google Scholar]
- Oberprieler R. G., Marvaldi A. E., Anderson R. S. Weevils, weevils, weevils everywhere. Zootaxa. 2007;1668:491–520. doi: 10.11646/zootaxa.1668.1.24. [DOI] [Google Scholar]
- Oh W. Y., Pyo S., Lee K. R., Lee B. K., Shin D. H., Cho S. I., Lee S. M. Effect of Holotrichia diomphalia larvae on liver fibrosis and hepatotoxicity in rats. J. Ethnopharmacol. 2003;87:175–180. doi: 10.1016/S0378-8741(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Pan Z., Ren G. Taxonomic revision of the subfamily Meloinae (Coleoptera: Meloidae) from Xizang, China, with description of a new species. Zoological Systematics. 2018;43:66–88. [Google Scholar]
- Park H. Y., Park D. S., Park S. S., Oh H. W., Shin S. W., Lee H. K., Joo C. K., Hong S. D. Bacteria-induced antibiotic peptide, protaecin from the white-spotted flower chafer, Protaetia brevitarsis. Korean J. Appl. Microbiol. Biotechnol. 1994;22:52–58. [Google Scholar]
- Park J. H., Kim S. Y., Kang M., Yoon M., Lee Y. I., Park E. Antioxidant activity and safety evaluation of juice containing Protaetia brevitarsis. J. Korean Soc. Food Sci. Nutr. 2012;41:41–48. doi: 10.3746/jkfn.2012.41.1.041. [DOI] [Google Scholar]
- Parkin E. A. The digestive enzymes of some wood-boring beetle larvae. J. Exp. Biol. 1940;17:364–377. [Google Scholar]
- Pathania M., Chandel R. S., Verma K. S., Mehta P. K. Seasonal life cycle of Holotrichia longipennis (Blanchard) (Coleoptera: Scarabaeidae: Melolonthinae): a serious foliage and root feeding pest in India. Phytoparasitica. 2016;44:615–629. doi: 10.1007/s12600-016-0557-7. [DOI] [Google Scholar]
- Peçanha E. P., Fraga C. A. M., De Sant' Anna C. M. R., De Miranda A. L. P., Barreiro E. J. Synthesis and pharmacological evaluation of a new class of bicyclic phospholipids, designed as platelet activating factor antagonists. Farmaco. 1998;53:327–336. doi: 10.1016/S0014-827X(98)00027-5. [DOI] [PubMed] [Google Scholar]
- Pemberton R. W. Persistence and change in traditional use of insects in contemporary East Asian cultures. In: Motte-FLorac E., Thomas J. M. C., editors. Les Insects Dans La Tradition Orale - Insects in Oral Literature and Tradition. Peeters; 2003. pp. 139–154. [Google Scholar]
- Pemberton R. W. Insects and other arthropods used as drugs in Korean traditional medicine. J. Ethnopharmacol. 1999;65:207–216. doi: 10.1016/S0378-8741(98)00209-8. [DOI] [PubMed] [Google Scholar]
- Prasher P., Kaur M., Singh S., Kaur H., Bala M., Sachdeva S. Ophthalmic manifestations of Paederus dermatitis. Int. Ophthalmol. 2017;37:885–891. doi: 10.1007/s10792-016-0352-y. [DOI] [PubMed] [Google Scholar]
- Puerto Galvis C. E., Vargas Méndez L. Y., Kouznetsov V. V. Cantharidin-based small molecules as potential therapeutic agents. Chem. Biol. Drug Des. 2013;82:477–499. doi: 10.1111/cbdd.12180. [DOI] [PubMed] [Google Scholar]
- Ratcliffe B. C. Scarab beetles in human culture. Coleopt. Soc. Monographs. 2006;5:85–101. doi: 10.1649/0010-065X(2006)60[85:SBIHC]2.0.CO;2. [DOI] [Google Scholar]
- Ryczek S., Dettner K., Unverzagt C. Synthesis of buprestins D, E, F, G and H; structural confirmation and biological testing of acyl glucoses from jewel beetles (Coleoptera: Buprestidae) Bioorg. Med. Chem. 2009;17:1187–1192. doi: 10.1016/j.bmc.2008.12.038. [DOI] [PubMed] [Google Scholar]
- Sagisaka A., Miyanoshita A., Ishibashi J., Yamakawa M. Purification, characterization and gene expression of a glycine and proline-rich antibacterial protein family from larvae of a beetle, Allomyrina dichotoma. Insect Mol. Biol. 2001;10:293–302. doi: 10.1046/j.0962-1075.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- Saldarriaga Rivera L., Lopez Villegas V., Rivera Toquica F. Association of Ulomoides Dermestoides "beetle-peanut" as cause of palpable purpura. Revista Cubana de Reumatología. 2017;19:224–227. [Google Scholar]
- Santos R. C. V., Lunardelli A., Caberlon E., Bastos C. M. A., Nunes F. B., Pires M. G. S., Biolchi V., Paul E. L., Vieira F. B. C., Do Carmo Aquino A. R., Corseuil E., De Oliveira J. R. Anti-inflammatory and immunomodulatory effects of Ulomoides dermestoides on induced pleurisy in rats and lymphoproliferation in vitro. Inflammation. 2010;33:173–179. doi: 10.1007/s10753-009-9171-x. [DOI] [PubMed] [Google Scholar]
- Schildknect H. The defensive chemistry of land and water beetles. Angew. Chem. Int. Ed. 1970;9:1–9. doi: 10.1002/anie.197000011. [DOI] [Google Scholar]
- Schleissner C., Cañedo L. M., Rodríguez P., Crespo C., Zúñiga P., Peñalver A., De La Calle F., Cuevas C. Bacterial production of a pederin analogue by a free-living marine alphaproteobacterium. J. Nat. Prod. 2017;80:2170–2173. doi: 10.1021/acs.jnatprod.7b00408. [DOI] [PubMed] [Google Scholar]
- Schmitz D. G. Cantharidin toxicosis in horses. J. Vet. Intern. Med. 1989;3:208–215. doi: 10.1111/j.1939-1676.1989.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Seabrooks L., Hu L. Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharmaceutica Sinica B. 2017;7:409–426. doi: 10.1016/j.apsb.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue K., Goto Y., Kawashima I., Shibue T. Chemical analysis of surface hydrocarbons in fireflies by direct contact extraction and gas chromatography-mass spectrometry. Anal. Sci. 2004;20:1729–1731. doi: 10.2116/analsci.20.1729. [DOI] [PubMed] [Google Scholar]
- Silk P., Ryall K. Semiochemistry and chemical ecology of the emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae) Can. Entomol. 2015;147:277–289. doi: 10.4039/tce.2014.58. [DOI] [Google Scholar]
- Sitbon O., Vonk Noordegraaf A. Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. Eur. Respir. Rev. 2017;26:160055. doi: 10.1183/16000617.0055-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley S. R., Risteen R. G., Tonyai K. K., Pitino J. C., Hu Y., Ahmed Z. B., Christofel B. T., Gaber M., Howells N. R., Mosey C. F., Rahim F. U., Deyrup S. T. Bufadienolides (lucibufagins) from an ecologically aberrant firefly (Ellychnia corrusca) Chemoecology. 2017;27:141–153. doi: 10.1007/s00049-017-0240-6. [DOI] [Google Scholar]
- Song L., Sun S., Jin L., Xue L., Fu Y. The extracts of Holotrichia diomphalia larvae inhibit proliferation and induce apoptosis of cancer cells in vitro and in vivo. Mol. Cell. Toxicol. 2014;10:251–259. doi: 10.1007/s13273-014-0028-5. [DOI] [Google Scholar]
- Stavenga D. G., Wilts B. D., Leertouwer H. L., Hariyama T. Polarized iridescence of the multilayered elytra of the Japanese jewel beetle, Chrysochroa fulgidissima. Philos. Trans. R. Soc. B. 2011;366:709–723. doi: 10.1098/rstb.2010.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H. J., Kang S. C. Antioxidant activity of aqueous methanol extracts of Protaetia brevitarsis Lewis (Coleoptera: Scarabaedia) at different growth stages. Nat. Prod. Res. 2012;26:510–517. doi: 10.1080/14786419.2010.530267. [DOI] [PubMed] [Google Scholar]
- Suh H. J., Kim S. R., Lee K. S., Park S., Kang S. C. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: Insecta) larvae. J. Photochem. Photobiol. B. 2010;99:67–73. doi: 10.1016/j.jphotobiol.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Sung G. A., Kim M. H., Park S. N. Anti-inflammatory and whitening effects of Protaetia brevitarsis seulensis extracts by oriental conversion methods. J. Soc. Cosmet. Sci. Korea. 2016;42:421–432. doi: 10.15230/SCSK.2016.42.4.421. [DOI] [Google Scholar]
- Tian L., Ji B. Z., Liu S. W., Jin F., Gao J., Li S. Juvenile hormone III produced in male accessory glands of the longhorned beetle, Apriona germari, is transferred to female ovaries during copulation. Arch. Insect Biochem. Physiol. 2010;75:57–67. doi: 10.1002/arch.20385. [DOI] [PubMed] [Google Scholar]
- Torbeck R., Pan M., de Moll E., Levitt J. Cantharidin: a comprehensive review of the clinical literature. Dermatol. Online J. 2014;20:13030. [PubMed] [Google Scholar]
- Tyler J., McKinnon W., Lord G. A., Hilton P. J. A defensive steroidal pyrone in the Glow-worm Lampyris noctiluca L. (Coleoptera: Lampyridae) Physiol. Entomol. 2008;33:167–170. doi: 10.1111/j.1365-3032.2007.00610.x. [DOI] [Google Scholar]
- Tzeng P., Hewson D. J., Vukusic P., Eichhorn S. J., Grunlan J. C. Bio-inspired iridescent layer-by-layer-assembled cellulose nanocrystal Bragg stacks. J. Mater. Chem. C. 2015;3:4260–4264. doi: 10.1039/C5TC00590F. [DOI] [Google Scholar]
- Vencl F. V., Ottens K., Dixon M. M., Candler S., Bernal X. E., Estrada C., Page R. A. Pyrazine emission by a tropical firefly: an example of chemical aposematism? Biotropica. 2016;48:645–655. doi: 10.1111/btp.12336. [DOI] [Google Scholar]
- Villaverde M. L., Girotti J. R., Mijailovsky S. J., Pedrini N., Juárez M. P. Volatile secretions and epicuticular hydrocarbons of the beetle Ulomoides dermestoides. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009;154:381–386. doi: 10.1016/j.cbpb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Vuts J., Imrei Z., Birkett M. A., Pickett J. A., Woodcock C. M., Tóth M. Semiochemistry of the Scarabaeoidea. J. Chem. Ecol. 2014;40:190–210. doi: 10.1007/s10886-014-0377-5. [DOI] [PubMed] [Google Scholar]
- Wahid A. A. A., Maged M., Mohamed A. F. Evaluation of Scarabaeus sacer derived-chitosan, anti-cancer potentials and related changes: in vitro study. J. Egypt. Soc. Parasitol. 2018;48:443–448. [Google Scholar]
- Wahrendorf M. S., Wink M. Pharmacologically active natural products in the defence secretion of Palembus ocularis (Tenebrionidae, Coleoptera) J. Ethnopharmacol. 2006;106:51–56. doi: 10.1016/j.jep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Walter W. G., Cole J. F. Isolation of cantharidin from Epicauta pestifera. J. Pharm. Sci. 1967;56:174–176. doi: 10.1002/jps.2600560204. [DOI] [PubMed] [Google Scholar]
- Wang C. Q., Li J. Q., Li E. T., Nyamwasa I., Li K., Bin , Zhang S., Peng Y., Wei Z. J., Yin J. Molecular and functional characterization of odorant-binding protein genes in Holotrichia oblita Faldermann. Int. J. Biol. Macromol. 2019a;136:359–367. doi: 10.1016/j.ijbiomac.2019.06.013. [DOI] [PubMed] [Google Scholar]
- Wang G. S. Medical uses of Mylabris in ancient China and recent studies. J. Ethnopharmacol. 1989;26:147–162. doi: 10.1016/0378-8741(89)90062-7. [DOI] [PubMed] [Google Scholar]
- Wang J., Yan Y., Tan R., Cheng Y. Phenolic compounds from Holotrichia diomphalia Bates. Nat. Prod. Res. Dev. 2012;24:622–626. [Google Scholar]
- Wang K., Li P., Gao Y., Liu C., Wang Q., Yin J., Zhang J., Geng L., Shu C. De novo genome assembly of the white-spotted flower chafer (Protaetia brevitarsis) GigaScience. 2019b;8:giz019. doi: 10.1093/gigascience/giz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang T., Sun Y., Wang S. Preliminary construction of the behavior spectrum of Cybister Japonicus Sharp under artificial breeding conditions. Ad. Eng. Res. 2017;141:1507–1510. [Google Scholar]
- Wickham J. D., Xu Z., Teale S. A. Evidence for a female-produced, long range pheromone of Anoplophora glabripennis (Coleoptera: Cerambycidae) Insect Sci. 2012;19:355–371. doi: 10.1111/j.1744-7917.2012.01504.x. [DOI] [Google Scholar]
- Wu Z., Hong C., Jin C., Zhang T., Yin J., Wang J., Wu M. Effect of Martianus dermestoides on coagulation and bleeding time in mice. Zhong Yao Cai. 1996;7:18. [Google Scholar]
- Xin C., Ma J. H., Tan C. J., Yang Z., Ye F., Long C., Ye S., Hou D. B. Preparation of melanin from Catharsius molossus L. and preliminary study on its chemical structure. J.Biosci. Bioeng. 2015;119:446–454. doi: 10.1016/j.jbiosc.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Xu M. Z., Lee W. S., Han J. M., Oh H. W., Park D. S., Tian G. R., Jeong T. S., Park H. Y. Antioxidant and anti-inflammatory activities of N-acetyldopamine dimers from Periostracum Cicadae. Bioorg. Med. Chem. 2006;14:7826–7834. doi: 10.1016/j.bmc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- Xu X., Liu W., Li W., Liu S. Anticoagulant activity of crude extract of Holotrichia diomphalia larvae. J. Ethnopharmacol. 2016;177:28–34. doi: 10.1016/j.jep.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Yan S. C., Wang L., Li Q., Fu Y. Anti-senile effects of water extraction of Martianus dermestoides (Coleoptera: Tenebrionidae) feeding different foods on aging mice. Acta Entomologica Sinica. 2009;52:820–824. [Google Scholar]
- Yan Y. M., Li L. J., Qin X. C., Lu Q., Tu Z. C., Cheng Y. X. Compounds from the insect Blaps japanensis with COX-1 and COX-2 inhibitory activities. Bioorg. Med. Chem. Lett. 2015;25:2469–2472. doi: 10.1016/j.bmcl.2015.04.085. [DOI] [PubMed] [Google Scholar]
- Yang S. The Divine Farmer's Materia Medica A: Translation of the Shen Nong Ben Cao Jing. 7th ed. Blue Poppy Press; 1998. [Google Scholar]
- Yang W. J., Yang D. X., Xu K. K., Cao Y., Meng Y. L., Wu Y., Li G. Y., Zhang G. Z., Wang Y. W., Li C. Complete mitochondrial genome of the bamboo snout beetle, Cyrotrachelus buqueti (Coleoptera: Curculionidae) Mitochondrial DNA Part B. 2018;3:88–89. doi: 10.1080/23802359.2017.1422411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui H. Chemical communication in mate location and recognition in the white-spotted longicorn beetle, Anoplophora malasiaca (Coleoptera: Cerambycidae) Appl. Entomol. Zool. 2009;44:183–194. doi: 10.1303/aez.2009.183. [DOI] [Google Scholar]
- Yasui H., Akino T., Yasuda T., Fukaya M., Ono H., Wakamura S. Ketone components in the contact sex pheromone of the white-spotted longicorn beetle, Anoplophora malasiaca, and pheromonal activity of synthetic ketones. Entomol. Exp. Appl. 2003;107:167–176. doi: 10.1046/j.1570-7458.2003.00053.x. [DOI] [Google Scholar]
- Yasui H., Akino T., Yasuda T., Fukaya M., Wakamura S., Ono H. Gomadalactones A, B, and C: novel 3-oxabicyclo[3.3.0]octane compounds in the contact sex pheromone of the white-spotted longicorn beetle, Anoplophora malasiaca. Tetrahedron Lett. 2007;48:2395–2400. doi: 10.1016/j.tetlet.2007.01.101. [DOI] [Google Scholar]
- Yeo H., Youn K., Kim M., Yun E. Y., Hwang J. S., Jeong W. S., Jun M. Fatty acid composition and volatile constituents of Protaetia brevitarsis larvae. Prev. Nutr. Food Sci. 2013;18:150–156. doi: 10.3746/pnf.2013.18.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo Y. C., Shin B. H., Hong J. H., Lee J., Chee H. Y., Song K. S., Lee K. B. Isolation of fatty acids with anticancer activity from Protaetia brevitarsis larva. Arch. Pharm. Res. 2007;30:361–365. doi: 10.1007/BF02977619. [DOI] [PubMed] [Google Scholar]
- Yoon H. S., Lee C. S., Lee S. Y., Choi C. S., Lee I. H., Yeo S. M., Kim H. R. Purification and cDNA cloning of inducible antibacterial peptides from Protaetia brevitarsis (Coleoptera) Arch. Insect Biochem. Physiol. 2003;52:92–103. doi: 10.1002/arch.10072. [DOI] [PubMed] [Google Scholar]
- Yoon Y., Il , Chung M. Y., Hwang J. S., Han M. S., Goo T. W., Yun E. Y. Allomyrina dichotoma (arthropoda: Insecta) larvae confer resistance to obesity in mice fed a high-fat diet. Nutrients. 2015;7:1978–1991. doi: 10.3390/nu7031978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S., Kinoshita S., Iida H., Hariyama T. Phase-adjusting layers in the multilayer reflector of a jewel beetle. J. Phys. Soc. Jpn. 2012;81:054801. doi: 10.1143/JPSJ.81.054801. [DOI] [Google Scholar]
- You D. O., Kang J. D., Youn N. H., Park S. D. Bullous contact dermatitis caused by self-applied crushed Paederus fuscipes for the treatment of vitiligo. Cutis. 2003;72:385–388. [PubMed] [Google Scholar]
- Youn K., Kim J. Y., Yeo H., Yun E. Y., Hwang J. S., Jun M. Fatty acid and volatile oil compositions of Allomyrina dichotoma larvae. Prev. Nutr. Food Sci. 2012;17:310–314. doi: 10.3746/pnf.2012.17.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. K. Cantharidin and insects: an historical review. Great Lakes Entomol. 1984;17:187–194. [Google Scholar]
- Yuan M. L., Zhang Q. L., Zhang L., Guo Z. L., Liu Y. J., Shen Y. Y., Shao R. High-level phylogeny of the Coleoptera inferred with mitochondrial genome sequences. Mol. Phylogenet. Evol. 2016;104:99–111. doi: 10.1016/j.ympev.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Guo Y., Zhang Y., Wang X., Jiang Y., Yang D. Rapid profiling of cantharidin analogs in Mylabris phalerata Pallas by ultra-performance liquid chromatography-quadrupole time-of-flight-tandem mass spectrometry. Biomed. Chromatogr. 2020;34:e4801. doi: 10.1002/bmc.4801. [DOI] [PubMed] [Google Scholar]
- Zhang A., Oliver J. E., Aldrich J. R., Wang B., Mastro V. C. Stimulatory beetle volatiles for the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky) Z. Naturforsch. C. 2002;57:553–558. doi: 10.1515/znc-2002-5-626. [DOI] [PubMed] [Google Scholar]
- Zhang A., Oliver J. E., Chauhan K., Zhao B., Xia L., Xu Z. Evidence for contact sex recognition pheromone of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae) Naturwissenschaften. 2003;90:410–413. doi: 10.1007/s00114-003-0452-1. [DOI] [PubMed] [Google Scholar]
- Zhang X., Ruan J., Ma Z. Research on history and present situation of medicinal insect resources in China. Chinese Journal of Bioprocess Engineering. 2019;17:615–622. [Google Scholar]
- Zhang Z., Zhang X., Zhao Y., Mu W., Liu F. Efficacy of insecticidal seed treatments against the wireworm Pleonomus canaliculatus (Coleoptera: Elateridae) in China. Crop Prot. 2017;92:134–142. doi: 10.1016/j.cropro.2016.11.004. [DOI] [Google Scholar]
- Zhao X., Zhu M., Yang M., Tao K., Wang J. The study of Catharsius molossus L. on experimental prostatic hyperplasia. Pharmacol. Clin. Chin. Mater. Med. 2006;22:37. [Google Scholar]