Abstract
This study aimed to investigate whether the antidiabetic drugs dipeptidyl peptidase 4 (DPP4) inhibitors such as evogliptin and sitagliptin affect the membrane DPP4 (mDPP4) enzymatic activity and immune function of T helper1 (Th1) cells in terms of cytokine expression and cell profiles. The mDPP4 enzymatic activity, cytokine expression, and cell profiles, including cell counts, cell viability, DNA synthesis, and apoptosis, were measured in pokeweed mitogen (PWM)-activated CD4+CD26+ H9 Th1 cells with or without the DPP4 inhibitors, evogliptin and sitagliptin. PWM treatment alone strongly stimulated the expression of mDPP4 and cytokines such as interleukin (IL)-2, IL-10, tumor necrosis factor-alpha, interferon-gamma, IL-13, and granulocyte-macrophage colony stimulating factor in the CD4+CD26+ H9 Th1 cells. Evogliptin or sitagliptin treatment potently inhibited mDPP4 activity in a dose-dependent manner but did not affect either the cytokine profile or cell viability in PWM-activated CD4+CD26+ H9 Th1 cells. These results suggest that, following immune stimulation, Th1 cell signaling pathways for cytokine expression function normally after treatment with evogliptin or sitagliptin, which efficiently inhibit mDPP4 enzymatic activity in Th1 cells.
Keywords: DPP4, CD26, Evogliptin, Sitagliptin, Type 2 diabetes, Th1 cell-specific cytokines
INTRODUCTION
Dipeptidyl peptidase 4 (DPP4, also known as CD26) is a homodimer protein existing in the soluble form in the blood or in the membrane form on the surface of various cell types, including T cells and endothelial cells (Ohnuma et al., 2011; Klemann et al., 2016; Shao et al., 2020). DPP4 has multiple functions via either its enzymatic activity through which it can degrade substrate peptides or its non-enzymatic activity through which it interacts with other signaling molecules (Ohnuma et al., 2006; Mulvihill and Drucker, 2014; Mortier et al., 2016). DPP4 catalytically cleaves dipeptides such as X-Pro/Ala at the N-terminus and subsequently regulates the bioactivity of various substrates, including hormones, growth factors, chemokines, cytokines, and incretins (Mulvihill and Drucker, 2014). In particular, soluble DPP4 (sDPP4), originating from the cell surface, cleaves incretin peptides such as gastric inhibitory polypeptide and glucagon-like peptide-1, thereby regulating insulin-mediated glucose metabolism (Ussher and Drucker, 2012; Sheikh, 2013; Mulvihill and Drucker, 2014). Therefore, sDPP4 has become a critical therapeutic target in the treatment of type 2 diabetes (Röhrborn et al., 2015; Zhong et al., 2015). To date, many DPP4 inhibitors, including sitagliptin and evogliptin, have been developed (Cho et al., 2011; Kim et al., 2011; Aso et al., 2012; Kim et al., 2012; Chae et al., 2015; Jung et al., 2015; Hong et al., 2017; Lee et al., 2017; Park et al., 2017; Kim et al., 2018).
DPP4/CD26 has also been reported to play important roles in immune cells, including T helper 1 (Th1) cells (Ohnuma et al., 2007, 2008a, 2008b; Ou et al., 2015). CD26 expression on the cell surface is higher in Th1 cells than in Th2 cells (Boonacker et al., 2002). The interaction of membrane DPP4 (mDPP4) with adenosine deaminase facilitates T cell activation through the regulation of pericellular adenosine levels (Dong et al., 1997). The interaction of mDPP4 with caveolin-1 of antigen-presenting cells stimulates the recruitment of caspase recruitment domain-containing membrane-associated guanylate kinase protein-1 (CARMA-1) to the cytosolic region of DPP4 and activates nuclear factor kappa B (NF-κB), leading to interleukin-2 (IL-2) production and T cell proliferation (Ohnuma et al., 2007, 2008a, 2008b). Recent studies showed that DPP4 catalytic activity leads to the cleavage of chemotactic trafficking chemokines such as C-X-C motif chemokine 10, chemokine (C-C motif) ligand 5, and C-X-C motif chemokine 12, which inhibit T cell migration to inflammatory sites, whereas DPP4 inhibition enhances T cell chemotaxis in the tumor parenchyma (Richter et al., 2014; Barreira da Silva et al., 2015; Ou et al., 2015). As DPP4 plays multifunctional roles in T cell immune responses, it can be a crucial therapeutic target protein in DPP4-mediated T cell diseases as well as in type 2 diabetes (Klemann et al., 2016; Shao et al., 2020). Nevertheless, whether or how the enzymatic and non-enzymatic activities of DPP4 contribute to immune functions in T cells is not yet known.
Although diverse DPP4 inhibitors have been extensively studied as synthetic therapeutic drugs to suppress DPP4 activity in the treatment of type 2 diabetes, little is known about their effects on other DPP4-related diseases including cardiovascular disorders, and autoimmune diseases such as type 1 diabetes, rheumatoid arthritis, Grave’s disease, allograft rejection, inflammatory bowel disease, multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) (Yazbeck et al., 2009; White et al., 2010; Zhao et al., 2014; Waumans et al., 2015; Zhong et al., 2016; Hu et al., 2017). Sitagliptin, a representative antidiabetic drug, has been investigated for other unknown therapeutic effects in DPP4-related T cell diseases (Yazbeck et al., 2009; Zhao et al., 2014; Waumans et al., 2015; Zhong et al., 2016). Some studies have shown that sitagliptin does not affect CD4+ T cell activation in patients with type 2 diabetes, whereas recent reports indicate that it may inhibit NF-κB activation and inflammatory cytokine expression in rat insulinoma cells (White et al., 2010; Hu et al., 2017). Although the manner in which sitagliptin may contribute to T cell immune responses is not yet known, these studies indicate the potential therapeutic effects of sitagliptin in DPP4-related T cell immune diseases. Recent clinical studies revealed a new antidiabetic drug, evogliptin; it is potent and safe for the treatment of type 2 diabetes (Cho et al., 2011; Kim et al., 2011, 2012; Gu et al., 2014; Chae et al., 2015; Jung et al., 2015; Hong et al., 2017; Lee et al., 2017; Park et al., 2017; Kim et al., 2018). For newly developed DPP4 inhibitors, including evogliptin, information regarding their putative effects on T cells is insufficient. Further studies are warranted to carefully determine the therapeutic and/or adverse effects of DPP4 inhibitors, including evogliptin and sitagliptin, on DPP4-related immune diseases.
This study aimed to elucidate whether mDPP4 enzymatic activity is essential for T cell signaling pathways for cytokine expression and whether DPP4 inhibition by therapeutic drugs affects the biologic profiles of T cells. We investigated the effects of the antidiabetic drugs evogliptin and sitagliptin in activated CD4+CD26+ Th1 cells in terms of mDPP4 activity, cytokine expression, cell profiles, and cell surface marker profiles.
MATERIALS AND METHODS
Cell cultures
The human Th1 cell lines CD4+CD26–Jurkat E6 and CD4+CD26+H9 T cells were obtained from the Korean Type Culture Collection (KTCC, Seoul, Korea). The cells were cultured in RPMI 1640 medium (Cat. LM011-01; WELGENE Inc., Gyeongsan, Korea) supplemented with 10% fetal bovine serum (FBS; Ref. 26140-079, GibcoTM, PA, USA) and 1% penicillin–streptomycin (Ref. 15140-0122, GibcoTM) at 37°C under a 5% CO2 atmosphere.
Chemicals and reagents
The T lymphocytes were stimulated with various agents, including pokeweed mitogen (PWM; Cat. L9379, Sigma-Aldrich Co., MO, USA) as a primary activator, phorbol 12-myristate 13-acetate (PMA; Cat. P1585, Sigma-Aldrich Co.), and Dynabeads human T-activator CD3/CD28 (Cat. 111.31D, Invitrogen, MA, USA) used as the positive control to activate the T lymphocytes. Evogliptin (Suganon; Dong-A ST, Seoul, Korea) and sitagliptin (Januvia; Merck & Co., Inc., NJ, USA) were used as the synthetic therapeutic DPP-4 inhibitors. Diprotin A (Cat. 416200, Calbiochem, MO, USA) as a peptidyl peptide inhibitor and berberine (Cat. 141433-60-5, Sigma-Aldrich Co.), a natural compound extracted from Berberis vulgaris, were used as DPP4 inhibitors. Recombinant human CD26/DPP4 protein (Cat. Ab79138, Abcam, Cambridge, UK) was used as a positive control to analyze the DPP4 enzymatic activity. Dulbecco’s phosphate-buffered saline (DPBS) and dimethyl sulfoxide (DMSO), the solutions used for dissolving the DPP4 inhibitors, were co-administered with PWM. DPBS did not influence mDPP4 enzymatic activity on the cell surface and Th1 cell-specific cytokine expression, whereas DMSO slightly affected mDPP4 enzymatic activity and most Th1 cell-specific cytokines (Supplementary Fig. 1). Negative matrix effects were minimized by using DPBS at a volume of 1/100, and DMSO was added to the culture at 1/250.
Cell counts and viability analysis
The cell pellets were washed and suspended in ice-cold DPBS (Cat. 14190-144, GibcoTM); subsequently, the cell count and viability were immediately measured. The cells were stained using an Accustain 4X kit (Cat. AD4K-200, NanoEnTek Inc., Seoul, Korea) in a 1:1 ratio for counting total cells and non-viable cells, and 20 µL of the sample mixture was loaded onto the Accuchip. The cell number and viability were measured using an Adam MC automatic cell counter (NanoEnTek Inc.).
Cytokine quantification using a fluorescent multiplex bead assay
At the end of each incubation, the cell supernatant was harvested and stored at –20°C until cytokine quantification. The cell supernatant was analyzed for 18 cytokines, including IL-1β (Cat. 171B5001M), IL-2 (Cat. 171B5003M), IL-4 (Cat. 171B5004M), IL-5 (Cat. 171B5005M), IL-6 (Cat. 171B5006M), IL-7 (Cat. 171B5007M), IL-8 (Cat. 171B5008M), IL-10 (Cat. 171B5010M), IL-12p70 (Cat. 171B5011M), IL-13 (Cat. 171B5012M), IL-15 (Cat. 171B5013M), and IL-17 (Cat. 171B5014M); granulocyte-macrophage colony-stimulating factor (GM-CSF; Cat. 171B5018M); interferon-gamma (IFN-γ; Cat. 171B5019M); tumor necrosis factor-alpha (TNF-α; Cat. 171B5026M); basic fibroblast growth factor (bFGF; Cat. 171B5016M); vascular endothelial growth factor (VEGF; Cat. 171B5027M); and platelet-derived growth factor (PDGF-BB; Cat. 171B5024M) using a Bio-Plex Pro assay (BIO-RAD Laboratories, Inc., CA, USA), and for 6 matrix metalloproteinases (MMPs), including MMP-1 (Cat. LMP901B), MMP-2 (Cat. LMP902C), MMP-3 (Cat. LMP513B), MMP-8 (Cat. LMP908B), MMP-9 (Cat. LMP911B), and MMP-12 (Cat. LMP919B) using a Luminex® performance assay (R&D Systems, Inc., MN, USA). The Luminex® performance assay is a multiplex immunoassay based on xMAP technology developed by LuminexTM that uses analyte-specific capture antibodies coupled to different magnetic microspheres with a distinct color code. After a series of reactions, the microspheres react with the biotinylated detection antibody and streptavidin–phycoerythrin as a fluorescent reporter. The microspheres were read using the flow-based dual laser system (635 and 532 nm) of the Bio-Plex 200 system (BIO-RAD Laboratories, Inc.) to determine the fluorescence intensity (FI) of each cytokine. Standard curves were fitted to determine the concentration of each cytokine in every batch. The measurement was performed in three different batches to confirm that the results were reproducible and the coefficient of variation (CV) was under 25%.
DPP4 enzyme kinetics
After the cell pellets were washed with DPBS, the DPP4 enzymatic activity of the cells was measured kinetically using substrate buffers containing 100 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (HEPES; Cat. 11344-041, GibcoTM) pH 7.6; 20 µM H-Gly-Pro-7-amino-4-methyl coumarin (AMC) substrate (Cat. I-1225, Bachem); and 100 µg/mL BSA (Cat. 0332, Amresco, OH, USA) in the dark at 25°C for 5 min. After the enzymatic reaction by mDPP4 on the surface of the cells, the FI of the released AMC was measured using a spectrofluorometer (SpectraMax M5; Molecular Devices. Co., CA, USA) at Ex 360 nm/Em 465 nm. The DPP4 enzymatic activity (mU/min×105 cells) is shown as the initial velocity (V0) of the reaction during which AMC (μM) is generated in 500,000 cultured cells for 5 min. The IC50 values of these drugs were determined using a series of dose-response curve fits for mDPP4 enzymatic activity using SoftMax4.1 software (Molecular Devices, Co.). All samples were analyzed in triplicate with a CV under 20% for FI and an R-value over 0.99 for V0; the analysis was repeated at least three times.
Cell surface markers and cell cycle analysis via flow cytometry
The cell surface markers were analyzed by harvesting a million cultured cells and washing with phosphate-buffered saline (PBS). The washed cells were resuspended with 100 µL of DPBS and stained with 5 µL of fluorescently labeled monoclonal antibodies in the dark for 20 min at room temperature. The antibodies used were mouse anti-human CD3-eFluor 450 (Cat. 48-0037-41, clone OKT3, eBioscienceTM, CA, USA), mouse anti-human CD4-PerCP-Cyanine5.5 (Cat. 45-0049-41, clone RPA-T4, eBioscienceTM), mouse anti-human CD8-FITC 5 (Cat. 11-0087-41, clone SK1, eBioscienceTM), mouse anti-human CD26-PE (Cat. 12-0269-41, clone 2A6, eBioscienceTM), and mouse anti-human CD28-APC (Cat. 17-0289-41, clone CD28.2, eBioscienceTM). The labeled cells were washed twice with DPBS and suspended in 200 µL of DPBS. CD3 was analyzed at Ex 405 nm/Em 445 nm; CD4, Ex 488 nm/Em 695 nm; CD8a, Ex 488 nm/Em 520 nm; CD26, Ex 488 nm/Em 578 nm; and CD28, Ex 633 nm/Em 660 nm using the BD FACSCantoTM system (BD Biosciences, CA, USA) and FACSDiva software (BD Biosciences).
Cell cycle progression, including DNA synthesis, was detected by harvesting the cultured cells and washing them twice with PBS. The cells were fixed with ice-cold 70% ethanol at –20°C for 2 h. After the cells were washed with PBS, they were stained with a DNA-staining solution containing propidium iodide (PI; Cat. P4170, Sigma-Aldrich Co.), RNase (Cat. R6513, Sigma-Aldrich Co.), and triton X-100 (Cat. 108603, Merck & Co., Inc.) in the dark for 20 min at room temperature. Cell cycle progression was analyzed at an Ex 488 nm/Em 585 nm using the BD FACSCaliburTM system (BD Biosciences) and Cell Quest Pro program; next, DNA synthesis (S phase) was determined using the ModFit LT 3.0 DNA analysis program (Verity Software House, ME, USA). For the apoptosis analysis, a commercially available Annexin V Apoptosis Detection Kit FITC (Cat. 88-8005, eBioscienceTM) was used, which contains recombinant annexin V conjugated to fluorescein (FITC annexin V) and a solution of the red-fluorescent PI, a nucleic acid-binding dye. The cultured cells were washed once with cold PBS and then with annexin-binding buffer. The cells were resuspended in 100 µL of annexin-binding buffer, following which 5 µL of annexin V-FITC was added; then, the mixture was incubated for 10 min in the dark at room temperature. After incubation, the cells were washed with annexin-binding buffer, and 200 µL of the same buffer and 5 µL of PI staining solution were added. Apoptosis was measured at Ex 530 nm/Em 695 nm using the BD FACSCantoTM system (BD Biosciences) and FACSDiva software (BD Biosciences). All analyses were performed in triplicate.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 21 (NY, USA) and Prism 5 (GraphPad Software, CA, USA). A paired sample t-test was used to compare each group with a control group, either an activator- or inhibitor-treated group. The differences in the t-test results were considered significant at p<0.05, p<0.005, or p<0.001.
RESULTS
PWM is a potent stimulator that induces Th1 cell-specific cytokine expression in human CD26+ Th1 cells
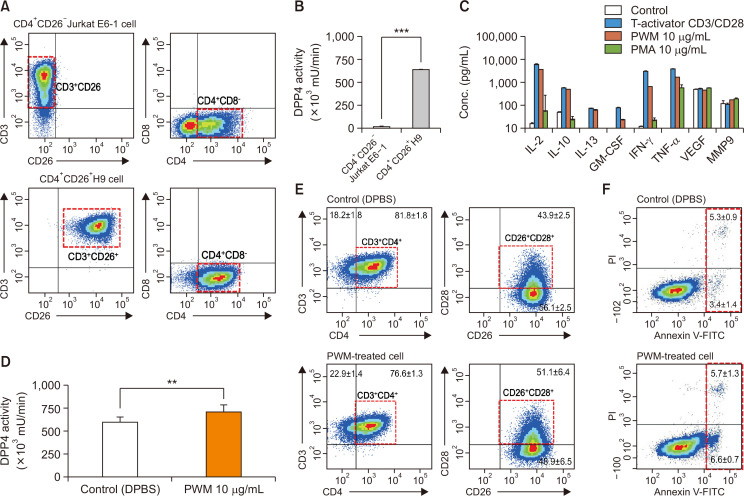
The expression of CD26 on the cell surface of Th1 cells, as shown in previous studies (Dong et al., 1997; Boonacker et al., 2002; Ohnuma et al., 2007, 2008) was determined using human Th1 cell lines, including Jurkat E6 and H9 Th1 cells, and the mDPP4 enzymatic activity in Th1 cells was measured using a fluorescent enzymatic kinetic assay (Fan et al., 2004; Villhauer et al., 2003; Kirino et al., 2009; Lee et al., 2009; Asakura et al., 2015). CD4+CD26+ H9 Th1 cells, but not CD4+CD26– Jurkat E6, exhibited the high level of basal mDPP4 enzymatic activity (Fig. 1A, 1B). After H9 Th1 cells were treated for 3 h with various stimulators, including the T-activator CD3/CD28 as an activation control or PWM (10 µg/mL) and PMA (10 µg/mL) as representative T cell activators, the concentrations of expressed cytokines were quantified in the cell culture supernatants using fluorescent multiplex bead assays containing 18 different cytokines (Fig. 1C). Th1 cell-specific cytokines such as IL-2, IL-10, IL-13, GM-CSF, IFN-γ, and TNF-α were highly induced in the stimulated H9 Th1 cells, whereas angiogenetic factors VEGF and MMP9 were consistently expressed regardless of the treatment. Moreover, PWM was as potent as T-activator CD3/CD28 and more potent than PMA in inducing Th1 cell-specific cytokine expression. PWM induced Th1 cell-specific cytokine expression in H9 Th1 cells in a dose-dependent manner (0-100 µg/mL), and the 10 µg/mL concentration was sufficient to induce the maximal expression of these cytokines (Supplementary Fig. 1). PWM (10 µg/mL) treatment only marginally increased the mDPP4 activity of H9 Th1 cells as well as CD28 surface expression, as shown in the analysis of the CD3+CD4+/CD26+CD28+ subsets of H9 Th1 cells (Fig. 1D, 1E, Supplementary Fig. 2A-2C). The annexin V-staining results showed that early apoptotic cells were slightly increased in the PWM-activated H9 Th1 cells (Fig. 1F, Supplementary Fig. 2D, 3D).
Fig. 1.
Characterization of CD26 expression, mDPP4 enzymatic activity, and cytokine expression in T helper cell lines. (A, B) Jurkat E6 and H9 Th1 cells were harvested and analyzed for the expression of T cell-specific surface markers, including CD3, CD4, and CD26 (A), and for their mDPP4 enzymatic activity (B). (C) H9 Th1 cells were treated with various T cell activators, including T-Activator CD3/CD28, PWM (10 µg/mL), and PMA (10 µg/mL) for 3 h. The expressions of 18 cytokines and 6 MMPs were measured in the culture supernatants of treated cells using two different panels of fluorescent multiplex bead assays. Activated T helper cell-specific cytokines, including IL-2, IL-10, IL-13, GM-CSF, IFN-γ, and TNF-α, are shown. VEGF and MMP 9 are also included as detected high in all the samples. (D-F) H9 Th1 cells were treated with 10 µg/mL PWM for 12 h and subjected to mDPP4 enzymatic assays (D), FACS analysis for T helper cell-specific surface markers such as CD3, CD4, CD26, and CD28 (E), and analysis for apoptosis using an annexin V kit and flow cytometry (F). All analyses were repeated in three independent batches, and the data are shown as the means and standard deviation. **p<0.005; ***p<0.001 (paired t-test).
Evogliptin and sitagliptin efficiently inhibit mDPP4 enzymatic activity, but do not affect Th1 cell-specific cytokine expression in activated H9 Th1 cells
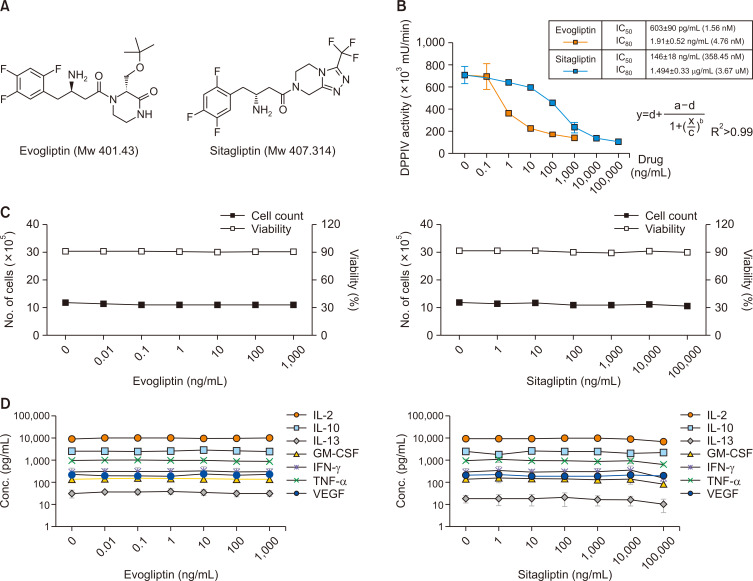
Next, we tested the inhibitory effects of the antidiabetic drugs evogliptin and sitagliptin on the mDPP4 enzymatic activity of CD26 in the PWM-activated Th1 cells. Evogliptin and sitagliptin are antihyperglycemic agents that bind directly at a DPP4 catalytic region and are used to treat type 2 diabetes (Fig. 2A; White et al., 2010; Chae et al., 2015; Hong et al., 2017; Hu et al., 2017; Lee et al., 2017; Park et al., 2017; Kim et al., 2018). H9 Th1 cells stimulated with PWM (10 µg/mL) were treated with various doses of evogliptin (0-1 µg/mL) or sitagliptin (0-100 µg/mL) for 12 h and assayed for mDPP4 activity (Fig. 2B). First, a series of dose-response curve fits of mDPP4 enzymatic activity with each drug was used to calculate the IC50 and IC80—IC50=1.56 nM (603 pg/mL) and IC80=4.76 nM (1.91 ng/mL) for evogliptin; IC50=358.45 nM (146 ng/mL) and IC80=3.67 M (1.494 µg/mL) for sitagliptin—indicating that evogliptin was a stronger DPP4 inhibitor than sitagliptin in these cells. Based on the results, we determined the doses of evogliptin (2 ng/mL) and sitagliptin (2 µg/mL) that inhibit 80% or higher mDPP4 enzymatic activity. Second, whether mDPP4 inhibition by evogliptin and sitagliptin influences the cell profiles of H9 Th1 cells was investigated by measuring the cell count and viability using cells from the same batch used for the analysis (Fig. 2C). Although evogliptin and sitagliptin inhibited mDPP4 enzymatic activity, they did not alter the pattern of cell viability in the PWM-activated H9 Th1 cells (Supplementary Fig. 2A, Fig. 2C). Furthermore, to assess whether mDPP4 inhibition by the antidiabetic drugs affects cytokine expression in H9 Th1 cells, we analyzed the expression of Th1 cell-specific cytokines in supernatant obtained from the batch mentioned above (Fig. 2B). Co-treatment with the antidiabetic drugs did not alter the expression patterns of most Th1 cell-specific cytokines in the PWM-activated H9 Th1 cells (Fig. 2D). Although PWM slightly decreased the CD3+CD4+ subsets, but increased the CD26+CD28+ subsets, of the activated H9 Th1 cells, the co-treatment did not affect the expression of Th1 cell-specific cytokines (Supplementary Fig. 2B, 2C). Taken together, the results indicate that evogliptin inhibited the mDPP4 activity more potently than sitagliptin; moreover, mDPP4 inhibition by these antidiabetic drugs did not affect the cell profiles and Th1 cell-specific cytokine expression in the PWM-activated H9 Th1 cells.
Fig. 2.
Effects of antidiabetic drugs on mDPP4 enzymatic activity, cell profiles, and Th1-specific cytokine expression in H9 Th1 cells. (A) The chemical structures of antidiabetic drugs, evogliptin and sitagliptin, are shown. (B-D) Cell pellets and culture supernatants were harvested 12 h after treatment of H9 Th1 cells with evogliptin (dose range, 0-1 µg/mL) or sitagliptin (dose range, 0-100 µg/mL) in the presence of 10 µg/mL PWM. The mDPP4 enzymatic activity of the cell pellets was measured using a spectrofluorometer, and the IC50 values of these drugs were determined using a series of dose-response curve fits for mDPP4 enzymatic activity (B). The cell profiles, including cell number and viability, of the PWM-treated cells with or without antidiabetic drugs were assessed using an automatic cell counter (C). The values of Th1-specific cytokines, including IL-2, IL-10, IL-13, IFN-γ, TNF-α, and GM-CSF, and VEGF in the culture supernatants were quantified using a fluorescent multiplex bead assay (D). These analyses were performed in over three independent batches, and the data are shown as means and standard deviation.
Diprotin A and berberine do not inhibit mDPP4 enzymatic activity in activated H9 Th1 cells
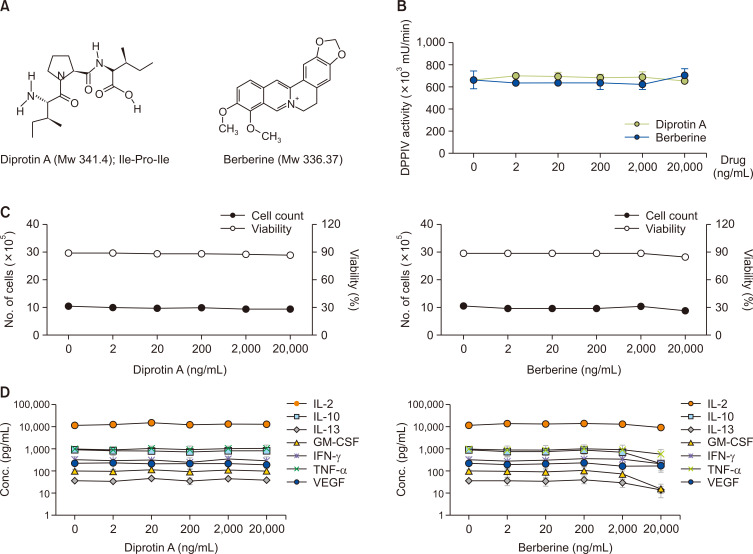
In addition to these antidiabetic DPP4 inhibitors, we also tested other known DPP4 inhibitors with antihyperglycemic effects, such as diprotin A and berberine (Fig. 3A). Diprotin A (Ile-Pro-Ile) is a peptidyl DPP IV inhibitor that binds to the DPP4 enzyme as a substrate. Berberine is a natural compound that inhibits the DPP4 enzyme, albeit its mechanism of action is still unclear (Lee et al., 2009; Asakura et al., 2015; Krupina and Khlebnikova, 2016). Although diprotin A and berberine are known to be active in recombinant DPP4 in vitro assays with IC50 values of 3.5 µM and 25 µM, respectively (Barzegar et al., 2015; Mohanty et al., 2017), they did not reduce mDPP4 enzyme activity in the PWM-activated H9 Th1 cells even at a concentration of 20 µg/mL (approximately 60 µM; Fig. 3B). While diprotin A did not affect the cell count and viability and Th1 cell-specific cytokine expression at the tested doses in the PWM-activated H9 Th1 cells, berberine inhibited IL-10, IL-13, and GM-CSF among the Th1 cell-specific cytokines the highest dose of 20 µg/mL, albeit statistically insignificant (paired t-test) (Fig. 3C, 3D). Like the results with evogliptin and sitagliptin, diprotin A treatment did not alter the population of the CD3+CD4+ or CD26+CD28+ subset in the PWM-activated H9 Th1 cells (Supplementary Fig. 3B, 3C). The effect of berberine could not be determined because of the interference of berberine with the fluorescent dye during cell surface marker staining.
Fig. 3.
Effects of diprotin A and berberine on mDPP4 enzymatic activity, cell profiles, and T helper-specific cytokine expression in H9 Th1 cells. (A) The chemical structures of diprotin A and berberine, representative DPP4 inhibitors, but not antidiabetic drugs, are shown. (B-D) Cell pellets and culture supernatants were harvested 12 h after treatment of H9 Th1 cells with diprotin A and berberine (dose range, 0-20 µg/mL) in the presence of 10 µg/mL PWM. The mDPP4 enzymatic activity of the cell pellets was determined (B). The cell profiles, including cell counts and viability, of the PWM-treated cells with or without DPP4 inhibitors were determined (C). The values of Th1-specific cytokines, including IL-2, IL-10, IL-13, GM-CSF, IFN-γ, and TNF-α, and VEGF in the culture supernatants were measured simultaneously (D). All analyses were the same as those described in Fig. 2.
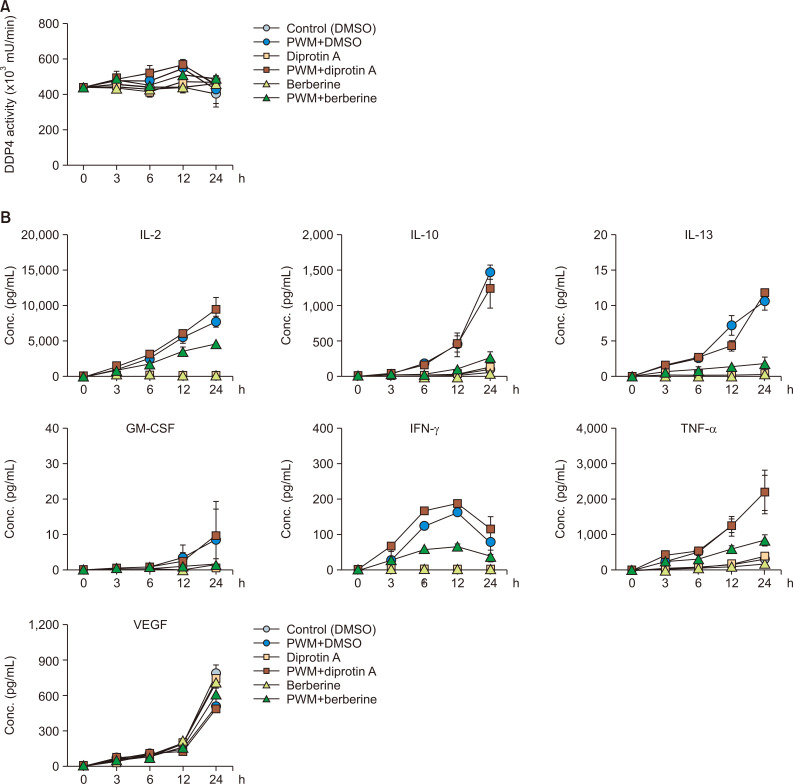
Time-course effect of DPP4 inhibitors on Th1 cell-specific cytokine expression and the cell profiles of PWM-activated H9 Th1 cells
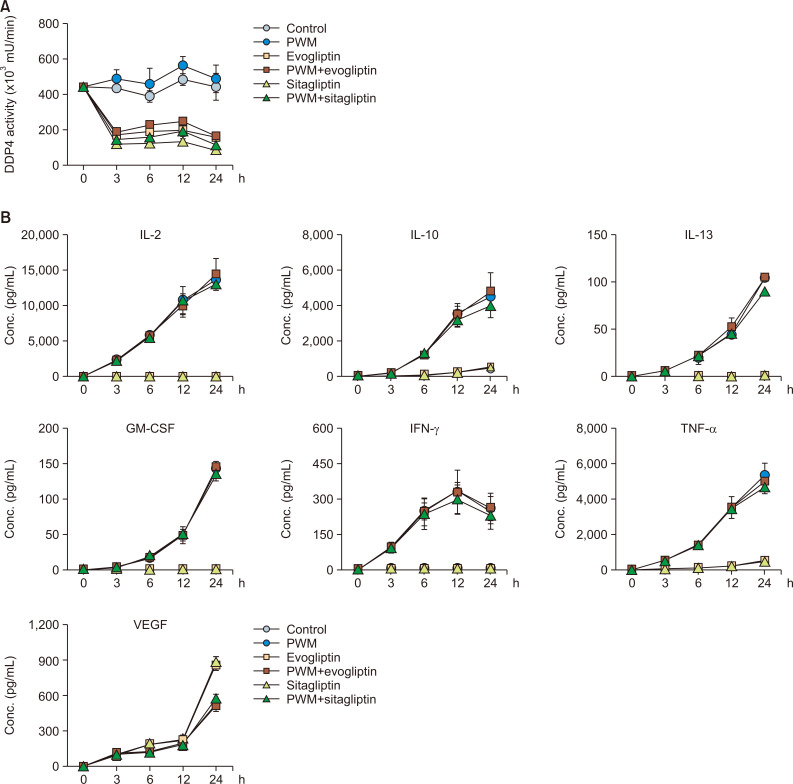
To further determine the time-course effects of the antidiabetic drugs, we treated the PWM-stimulated H9 cells with evogliptin and sitagliptin for 3, 6, 12, and 24 h at concentrations of 2 ng/mL and 2 µg/mL, respectively, and analyzed the mDPP4 activity and Th1 cell-specific cytokines. Although mDPP4 enzymatic activity was the highest at 12 h after PWM treatment, the increase in mDPP4 activity induced by PWM was independent of co-treatment with the antidiabetic drugs (Fig. 4A). Evogliptin and sitagliptin efficiently inhibited mDPP4 activity in the PWM-treated and untreated H9 Th1 cells at all time points. The expression of Th1 cell-specific cytokines, including IL-2, IL-10, IL-13, GM-CSF, IFN-γ, and TNF-α, gradually increased over time in the PWM-activated cells, whereas VEGF exceptionally decreased at 24 h after treatment with PWM (Fig. 4B). However, the expression levels of the cytokines were not affected by co-treatment with the antidiabetic drugs in H9 Th1 cells. When we further investigated the cell profiles, including cell count, cell viability, DNA synthesis, and apoptosis, in cultured cells prepared from the same batches, the results of which are shown in Fig. 4, evogliptin and sitagliptin did not change any of the cell profiles (Supplementary Fig. 4). In summary, our results indicate that evogliptin and sitagliptin are specific inhibitors that control the mDPP4 enzymatic activity of CD26 on the surface of Th1 cells but do not influence the expression of Th1 cell-specific cytokines and cell profiles, including cell count, viability, DNA synthesis, and apoptosis, in the PWM-activated H9 Th1 cells.
Fig. 4.
Time-course effects of evogliptin and sitagliptin on mDPP4 enzymatic activity and Th1-specific cytokine expression in H9 Th1 cells. The cells were stimulated with various combinations of DPBS and PWM (10 µg/mL) and a high dose of the antidiabetic drugs, evogliptin (2 ng/mL) or sitagliptin (2 µg/mL). Cell pellets and culture supernatant were harvested at the indicated time points. The mDPP4 enzyme activity of the collected cells was immediately measured at each time point (A). The values of Th1-specific cytokines, including IL-2, IL-10, IL-13, IFN-γ, TNF-α, and GM-CSF, and VEGF in the culture supernatants were quantified simultaneously using a fluorescent multiplex bead assay (B). All analyses were repeated in three independent batches, and the data are presented as means and standard deviation.
Time-course effect of diprotin A and berberine on Th1 cell-specific cytokine expression and the cell profiles of PWM-activated H9 Th1 cells
Since diprotin A and berberine did not inhibit the mDPP4 activity of H9 Th1 cells after 12-h treatment, we analyzed their effects at various time points at a high concentration of 20 µg/mL. Despite the change in the incubation time from 3 to 24 h, the high dose of diprotin A and berberine did not reduce the mDPP4 activity in H9 Th1 cells (Fig. 5A). In addition, diprotin A did not influence cytokine expression at any time point (Fig. 5B). In contrast, from 6 to 24 h, berberine treatment significantly inhibited the expression of Th1 cell-specific cytokines except VEGF in the PWM-activated H9 Th1 cells. Diprotin A did not affect the overall cell profiles of the PWM-activated H9 cells, but berberine decreased the cell count, viability, and DNA synthesis at 24 h (Supplementary Fig. 5). These results suggest that diprotin A inhibits neither mDPP4 enzymatic activity nor Th1 cell-specific cytokine expression despite the different incubation time and that berberine may exhibit cytotoxic effects even without mDPP4 inhibitory activity, leading to decreased cytokine expression and cellular growth in H9 Th1 cells.
Fig. 5.
Time-course effects of diprotin A and berberine on mDPP4 activity and Th1-specific cytokine expression in H9 Th1 cells. The cells were stimulated with various combinations of DMSO and PWM (10 µg/mL) and the DPP4 inhibitors, diprotin A (20 µg/mL) and berberine (20 µg/mL). The mDPP4 enzyme activity of the harvested cells was measured at the indicated time points (A). The values of Th1-specific cytokines, including IL-2, IL-10, IL-13, IFN-γ, TNF-α, and GM-CSF, and VEGF in the culture supernatants were quantified (B). All the analyses were performed in the same manner as that described in Fig. 4.
Comparison of the inhibitory effects of DPP4 inhibitors on recombinant DPP4 and mDPP4 activities
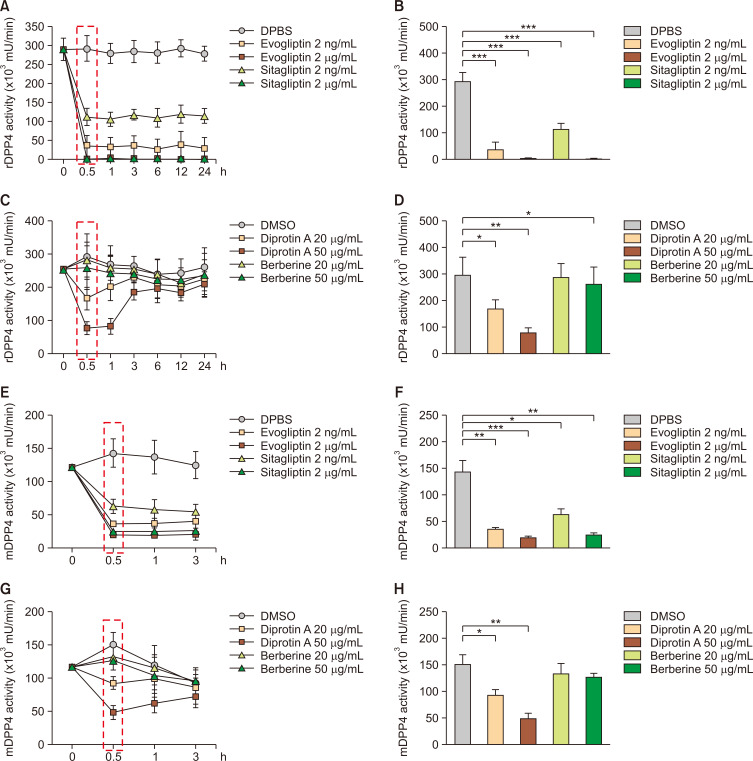
Our results showing the lack of inhibitory effects of diprotin A and berberine on the mDPP4 activity of H9 Th1 cells are contradictory to those of previous studies showing their activities as DPP4 inhibitors, which could be attributed to the different assay systems used: previous DPP4 enzymatic assays were performed using the recombinant DPP4 protein (rDPP4; (Barzegar et al., 2015; Jao et al., 2015; Krupina and Khlebnikova, 2016; Mohanty et al., 2017). We conducted a kinetic analysis of in vitro DPP4 assays using rDPP4 for the DPP4 inhibitors used in this study (Fig. 6A-6D). Evogliptin and sitagliptin were highly efficient in inhibiting rDPP4 activity at concentrations of 2 ng/mL and 2 µg/mL at as early as 30 min until 24 h, albeit evogliptin was more potent than sitagliptin at a concentration of 2 ng/mL (Fig. 6A, 6B). Diprotin A could reduce the rDPP4 activity at concentrations of 20 and 50 µg/mL in a dose-dependent manner after 30 min and 1 h incubation, but its inhibitory effects decreased over time starting from 3 h incubation (Fig. 6C). In contrast, berberine had little or no inhibitory effect even on rDPP4 activity at any time point (Fig. 6D).
Fig. 6.
Comparison of the inhibitory effects of various DPP4 inhibitors on rDPP4 and mDPP4 activities at different time points. The DPP4 inhibitory activities of evogliptin, sitagliptin, diprotin A, and berberine were measured using recombinant DPP4 (rDPP4) protein or cell pellets of H9 Th1 cells. DPBS or DMSO were used as a control. The recombinant DPP4 proteins were incubated with evogliptin and sitagliptin (2 ng/mL and 2 µg/mL) or diprotin A and berberine (20 and 50 µg/mL); harvested after 0, 0.5, 1, 3, 6, 12, and 24 h; and subjected to assays for measuring the DPP4 activity (A, C). The results of DPP4 enzymatic activity after 30 min of incubation are shown (B, D). The H9 Th1 cell pellets were incubated with evogliptin and sitagliptin (2 ng/mL and 2 µg/mL, respectively) or diprotin A and berberine (20 and 50 µg/mL); harvested after 0, 0.5, 1, and 3 h; and subjected to DPP4 assays (E, G). The results of DPP4 activity after 30 min incubation are shown (F, H). All analyses were performed in three independent experiments, and the data are represented as the means and standard deviation. *p<0.05; **p<0.005; and ***p<0.001 (paired t-test).
The highly transient inhibition of DPP4 enzymatic activity by diprotin A could be the reason we did not observe any inhibition of mDPP4 activity in the PWM-activated H9 Th1 cells co-treated with diprotin A. Next, we evaluated the DPP4 inhibitory effects of these inhibitors in H9 Th1 cells for less than 3 h (Fig. 6E-6H). Consistent with the results obtained using rDPP4, evogliptin and sitagliptin were highly potent in inhibiting the mDPP4 activity of H9 Th1 cells even after 30 min incubation (Fig. 6E, 6F). Diprotin A also exhibited dose-dependent inhibition of mDPP4 activity in H9 Th1 cells after 30 min and 1 h incubation, but not after 3 h incubation, whereas berberine had no effect (Fig. 6G, 6H).
Thus, we observed that evogliptin and sitagliptin were potent inhibitors of both the rDPP4 protein and mDPP4 in CD4+CD26+ H9 Th1 cells after incubation for as little as 30 min until 24 h. Conversely, diprotin A transiently inhibited both the rDPP4 protein and mDPP4 in H9 Th1 cells, but this was not noted for more than 1 h. Berberine did not actively inhibit DPP4 activity regardless of the assay systems used. Therefore, these results suggest that evogliptin and sitagliptin are more effective mDPP4 inhibitors without any adverse effects on Th1 cells than the peptidyl DPP4 inhibitor, diprotin A.
DISCUSSION
In this study, we investigated the effects of evogliptin and sitagliptin, which are antidiabetic drugs and DPP4 inhibitors, on the mDPP4 enzymatic activity of CD26 and Th1 cell-specific cytokine expression in PWM-activated CD4+CD26+ H9 Th1 cells. PWM treatment stimulated the substantial expression of Th1 cell-specific cytokines such as IL-2, IL-10, TNF-α, IFN-γ, IL-13, and GM-CSF in the H9 Th1 cells (Fig. 1C). These results were consistent with those of previous studies showing that CD26 expression was closely related to T cell signaling pathways for Th1 cell-specific cytokine expression in PWM-activated Th1 cells (Yan et al., 2003). However, it is also noted that PWM treatment only enhanced CD26 expression and mDPP4 activity to limited levels in H9 Th1 cells (Fig. 1D, 1E, Supplementary Fig. 2A). Given that the basal levels of CD26 expression and mDPP4 activity were already high in H9 Th1 cells, these results suggest that there may be little correlation between Th1-specific cytokine induction and mDPP4 activity in PWM-activated CD4+CD26+ H9 Th1 cells.
Evogliptin and sitagliptin efficiently inhibited mDPP4 activity in a dose-dependent manner but did not alter cytokine expression in PWM-treated H9 Th1 cells. According to the IC50 values of the drugs, evogliptin was more potent than sitagliptin in inhibiting mDPP4 enzymatic activity in the PWM-activated H9 cells as well as rDPP4 activity in vitro at all incubation times tested from 30 min up to 24 h. In contrast, we found that diprotin A, a synthetic peptidyl inhibitor, only transiently inhibited the enzymatic activities of mDPP4 and rDPP4 after less than 30 min incubation with no effect on Th1-specific cytokine expression. Berberine, a natural DPP4 inhibitor, showed minimal effects on the enzymatic activities of mDPP4 and rDPP4 but decreased the expression of cytokines, possibly because of its cytotoxicity.
While the non-enzymatic activities of CD26 are essential for T cell signaling pathways, whether mDPP4 enzymatic activity is also critical for the immune responses of T cells is not yet known. Sitagliptin treatment of patients with type 2 diabetes did not affect CD4+ T cell activation (White et al., 2010). Treatment with both sitagliptin and a matching placebo in human immunodeficiency virus (HIV)-positive men did not alter the CD4+ T cell number, plasma HIV viremia, or immune activation for 24 weeks (Goodwin et al., 2013). In contrast, sitagliptin was reported to affect the subsets of circulating CD4+ T cells, especially Th17 and Treg cells, in vitro as well as in patients with type 2 diabetes, when patients with sitagliptin or a control drug were followed up for 12 weeks (Aso et al., 2015; Pinheiro et al., 2017).
Our results showed that the treatment of PWM-activated H9 Th1 cells with DPP4 inhibitors did not change the cell profiles such as cell viability and CD3+CD4+ or CD26+CD28+ subset populations (Supplementary Fig. 2, 3). In addition, potent DPP4 inhibitors had no or little effect on Th1 cell-specific cytokine expression in the PWM-activated Th1 cells, although they directly inhibited DPP4 enzymatic activity in these cells. Despite our limitation using a Th1 cell line rather than primary cells, these results suggest that mDPP4 enzymatic activity may not be required for Th1 cell-specific cytokine expression. Thus, patients with type 2 diabetes treated with evogliptin or sitagliptin may not exhibit adverse effects on T cell immune responses, though the effects of long-term treatment with these drugs should be evaluated in vitro as well as in vivo. A recent study reported that sitagliptin therapy for 12 months reduces DPP4 activity yet does not increase markers of inflammation or levels of sDPP4 in the patients with type 2 diabetes (Baggio et al., 2020). Since sDPP4 are mainly derived from lymphocytes (Casrouge et al., 2018), these results support our hypothesis that prolonged treatment of DPP4 inhibitors may not alter the degree of inflammation or the levels of lymphocytes-associated DPP4 in humans.
Our study investigated Th1 cell immune responses in PWM-activated H9 cells, after treatment with clinical DPP4 inhibitors such as evogliptin and sitagliptin. Our results suggest that the non-enzymatic activity of CD26 is more important than its enzymatic activity for the regulation of Th1 cell immune responses for the treatment of patients with type 2 diabetes.
Supplementary Materials
ACKNOWLEDGMENTS
This work was supported in part by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (No. 2020R1A2C2013827) and by the grants from Korea University and the Biomedical Research Institute, Seoul National University Hospital. Evogliptin and sitagliptin were provided by the Department of Clinical Pharmacology and Therapeutics, Seoul National University Hospital.
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- Asakura M., Fujii H., Atsuda K., Itoh T., Fujiwara R. Dipeptidyl peptidase-4 greatly contributes to the hydrolysis of vildagliptin in human liver. Drug Metab. Dispos. 2015;43:477–484. doi: 10.1124/dmd.114.062331. [DOI] [PubMed] [Google Scholar]
- Aso Y., Fukushima M., Sagara M., Jojima T., Iijima T., Suzuki K., Momobayashi A., Kasai K., Inukai T. Sitagliptin, a DPP-4 inhibitor, alters the subsets of circulating CD4+ T cells in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2015;110:250–256. doi: 10.1016/j.diabres.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Aso Y., Ozeki N., Terasawa T., Naruse R., Hara K., Suetsugu M., Takebayashi K., Shibazaki M., Haruki K., Morita K., Inukai T. Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea. Transl. Res. 2012;159:25–31. doi: 10.1016/j.trsl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Baggio L. L., Varin E. M., Koehler J. A., Cao X., Lokhnygina Y., Stevens S. R., Holman R. R., Drucker D. J. Plasma levels of DPP4 activity and sDPP4 are dissociated from inflammation in mice and humans. Nat. Commun. 2020;11:3766. doi: 10.1038/s41467-020-17556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreira, da Silva R., Laird M. E., Yatim N., Fiette L., Ingersoll M., Albert M. L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015;16:850–858. doi: 10.1038/ni.3201. [DOI] [PubMed] [Google Scholar]
- Barzegar E., Fouladdel S., Komeili M. T., Atashpour S., Ghahremani M. H., Ostad S. N., Azizi E. Effects of berberine on proliferation, cell cycle distribution and apoptosis of human breast cancer T47D and MCF7 cell lines. Iran. J. Basic Med. Sci. 2015;18:334–342. [PMC free article] [PubMed] [Google Scholar]
- Boonacker E. P., Wierenga E. A., Smits H. H., Van Noorden C. J. CD26/DPPIV signal transduction function, but not proteolytic activity, is directly related to its expression level on human Th1 and Th2 cell lines as detected with living cell cytochemistry. J. Histochem. Cytochem. 2002;50:1169–1177. doi: 10.1177/002215540205000903. [DOI] [PubMed] [Google Scholar]
- Casrouge A., Sauer A. V., Barreira, da Silva R., Tejera-Alhambra M., Sánchez-Ramón S., ICAReB , Cancrini C., Ingersoll M. A., Aiuti A., Albert M. L. Lymphocytes are a major source of circulating soluble dipeptidyl peptidase 4. Clin. Exp. Immunol. 2018;194:166–179. doi: 10.1111/cei.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae Y. N., Kim T. H., Kim M. K., Shin C. Y., Jung I. H., Sohn Y. S., Son M. H. Beneficial effects of evogliptin, a novel dipeptidyl peptidase 4 inhibitor, on adiposity with increased Ppargc1a in white adipose tissue in obese mice. PLoS ONE. 2015;10:e0144064. doi: 10.1371/journal.pone.0144064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J. M., Jang H. W., Cheon H., Jeong Y. T., Kim D. H., Lim Y. M., Choi S. H., Yang E. K., Shin C. Y., Son M. H., Kim S. H., Kim H. J., Lee M. S. A novel dipeptidyl peptidase IV inhibitor DA-1229 ameliorates streptozotocin-induced diabetes by increasing β-cell replication and neogenesis. Diabetes Res. Clin. Pract. 2011;91:72–79. doi: 10.1016/j.diabres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Dong R. P., Tachibana K., Hegen M., Munakata Y., Cho D., Schlossman S. F., Morimoto C. Determination of adenosine deaminase binding domain on CD26 and its immunoregulatory effect on T cell activation. J. Immunol. 1997;159:6070–6076. [PubMed] [Google Scholar]
- Fan H., Yan S., Stehling S., Marguet D., Schuppaw D., Reutter W. Dipeptidyl peptidase IV/CD26 in T cell activation, cytokine secretion and immunoglobulin production. In Dipeptidyl Aminopeptidases in Health and Disease. In: Back N., Cohen I. R., Kritchevsky D., Lajtha A., Paoletti R., editors. Advances in Experimental Medicine and Biology, Vol. 524. Springer; Boston, MA: 2004. https://doi.org/10.1007/0-306-47920-6_20/ [DOI] [PubMed] [Google Scholar]
- Goodwin S. R., Reeds D. N., Roya M., Struthers H., Laciny E., Yarasheski K. E. Dipeptidyl peptidase IV inhibition does not adversely affect immune or virological status in HIV infected men and women: a pilot safety study. J. Clin. Endocrinol. Metab. 2013;98:743–751. doi: 10.1210/jc.2012-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N., Park M. K., Kim T. E., Bahng M. Y., Lim K. S., Cho S. H., Yoon S. H., Cho J. Y., Jang I. J., Yu K. S. Multiple-dose pharmacokinetics and pharmacodynamics of evogliptin (DA-1229), a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. Drug Des. Devel. Ther. 2014;8:1709–1721. doi: 10.2147/DDDT.S65678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. M., Park C. Y., Hwang D. M., Han K. A., Lee C. B., Chung C. H., Yoon K. H., Mok J. O., Park K. S., Park S. W. Efficacy and safety of adding evogliptin versus sitagliptin for metformin-treated patients with type 2 diabetes: a 24-week randomized, controlled trial with open label extension. Diabetes Obes. Metab. 2017;19:654–663. doi: 10.1111/dom.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Liu S., Liu X., Zhang J., Liang Y., Li Y. DPP-4 (CD26) inhibitor sitagliptin exerts anti-inflammatory effects on rat insulinoma (RINm) cells via suppressing NF-κB activation. Endocrine. 2017;55:754–763. doi: 10.1007/s12020-016-1073-8. [DOI] [PubMed] [Google Scholar]
- Jao C. L., Hung C. C., Tung Y. S., Lin P. Y., Chen M. C., Hsu K. C. The development of bioactive peptides from dietary proteins as a dipeptidyl peptidase IV inhibitor for the management of type 2 diabetes. BioMedicine. 2015;5:14. doi: 10.7603/s40681-015-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. H., Park C. Y., Ahn K. J., Kim N. H., Jang H. C., Lee M. K., Park J. Y., Chung C. H., Min K. W., Sung Y. A., Park J. H., Kim S. J., Lee H. J., Park S. W. A randomized, double-blind, placebo-controlled, phase II clinical trial to investigate the efficacy and safety of oral DA-1229 in patients with type 2 diabetes mellitus who have inadequate glycaemic control with diet and exercise. Diabetes Metab. Res. Rev. 2015;31:295–306. doi: 10.1002/dmrr.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Kwak W. Y., Min J. P., Lee J. Y., Yoon T. H., Kim H. D., Shin C. Y., Kim M. K., Choi S. H., Kim H. S., Yang E. K., Cheong Y. H., Chae Y. N., Park K. J., Jang J. M., Choi S. J., Son M. H., Kim S. H., Yoo M., Lee B. J. Discovery of DA-1229: a potent, long acting dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Bioorg. Med. Chem. Lett. 2011;21:3809–12. doi: 10.1016/j.bmcl.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Kim M. K., Chae Y. N., Kim H. D., Yang E. K., Cho E. J., Choi S. H., Cheong Y. H., Kim H. S., Kim H. J., Jo Y. W., Son M. H., Kim S. H., Shin C. Y. DA-1229, a novel and potent DPP4 inhibitor, improves insulin resistance and delays the onset of diabetes. Life Sci. 2012;90:21–29. doi: 10.1016/j.lfs.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Kim T. H., Lee J. H., Chae Y. N., Jung I. H., Kim M. K. Additive effects of evogliptin in combination with pioglitazone on fasting glucose control through direct and indirect hepatic effects in diabetic mice. Eur. J. Pharmacol. 2018;830:95–104. doi: 10.1016/j.ejphar.2018.04.033. [DOI] [PubMed] [Google Scholar]
- Kirino Y., Sato Y., Kamimoto T., Kawazoe K., Minakuchi K., Nakahori Y. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: A streptozotocin-induced model using wild-type and DPP4-deficient rats. J. Endocrinol. 2009;200:53–61. doi: 10.1677/JOE-08-0424. [DOI] [PubMed] [Google Scholar]
- Klemann C., Wagner L., Stephan M., von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016;185:1–21. doi: 10.1111/cei.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupina N. A., Khlebnikova N. N. Neonatal exposure to the dipeptidyl peptidase-IV inhibitors diprotin A and sitagliptin induces depression-like behavior, anxiety, and latent aggression in adolescent and adult rats. J. Behav. Brain Sci. 2016;6:167–183. doi: 10.4236/jbbs.2016.64018. [DOI] [Google Scholar]
- Lee H. K., Kim M. K., Kim H. D., Kim H. J., Kim J. W., Lee J. O., Kim C. W., Kim E. E. Unique binding mode of evogliptin with human dipeptidyl peptidase IV. Biochem. Biophys. Res. Commun. 2017;494:452–459. doi: 10.1016/j.bbrc.2017.10.101. [DOI] [PubMed] [Google Scholar]
- Lee K. N., Jackson K. W., Terzyan S., Christiansen V. J., McKee P. A. Using substrate specificity of antiplasmin-cleaving enzyme for fibroblast activation protein inhibitor design. Biochemistry. 2009;48:5149–5158. doi: 10.1021/bi900257m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty I. R., Kumar S., Suman R. Dipeptidyl peptidase IV inhibitory activity of berberine and mangiferin: an in silico approach. Int. J. Clin. Endocrinol. Metab. 2017;3:18–22. doi: 10.17352/ijcem.000024. [DOI] [Google Scholar]
- Mortier A., Gouwy M., Van Damme J., Proost P., Struyf S. CD26/dipeptidylpeptidase IV-chemokine interactions: double-edged regulation of inflammation and tumor biology. J. Leukoc. Biol. 2016;99:955–969. doi: 10.1189/jlb.3MR0915-401R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E. E., Drucker D. J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma K., Dang N. H., Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008a;29:295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Ohnuma K., Hosono O., Dang N. H., Morimoto C. Dipeptidyl peptidase in autoimmune pathophysiology. Adv. Clin. Chem. 2011;53:51–84. doi: 10.1016/B978-0-12-385855-9.00003-5. [DOI] [PubMed] [Google Scholar]
- Ohnuma K., Inoue H., Uchiyama M., Yamochi T., Hosono O., Dang N. H., Morimoto C. T-cell activation via CD26 and caveolin-1 in rheumatoid synovium. Mod. Rheumatol. 2006;16:3–13. doi: 10.3109/s10165-005-0452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma K., Takahashi N., Yamochi T., Hosono O., Dang N. H., Morimoto C. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front. Biosci. 2008b;13:2299–2310. doi: 10.2741/2844. [DOI] [PubMed] [Google Scholar]
- Ohnuma K., Uchiyama M., Yamochi T., Nishibashi K., Hosono O., Takahashi N., Kina S., Tanaka H., Lin X., Dang N. H., Morimoto C. Caveolin-1 triggers T-cell activation via CD26 in association with CARMA1. J. Biol. Chem. 2007;282:10117–10131. doi: 10.1074/jbc.M609157200. [DOI] [PubMed] [Google Scholar]
- Ou X., O'Leary H. A., Broxmeyer H. E. Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood. 2015;122:161–169. doi: 10.1182/blood-2013-02-487470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Park S. W., Yoon K. H., Kim S. R., Ahn K. J., Lee J. H., Mok J. O., Chung C. H., Han K. A., Koh G. P., Kang J. G., Lee C. B., Kim S. H., Kwon N. Y., Kim D. M. Efficacy and safety of evogliptin monotherapy in patients with type 2 diabetes and moderately elevated glycated haemoglobin levels after diet and exercise. Diabetes Obes. Metab. 2017;19:1681–1687. doi: 10.1111/dom.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro M. M., Stoppa C. L., Valduga C. J., Okuyama C. E., Gorjão R., Pereira R. M. S., Diniz S. N. Sitagliptin inhibit human lymphocytes proliferation and Th1/Th17 differentiation in vitro. Eur. J. Pharm. Sci. 2017;100:17–24. doi: 10.1016/j.ejps.2016.12.040. [DOI] [PubMed] [Google Scholar]
- Richter R., Jochheim-Richter A., Ciuculescu F., Kollar K., Seifried E., Forssmann U., Verzijl D., Smit M. J., Blanchet X., von Hundelshausen P., Weber C., Forssmann W., Henschler R. Identification and characterization of circulating variants of CXCL12 from human plasma: effects on chemotaxis and mobilization of hematopoietic stem and progenitor cells. Stem Cells Dev. 2014;23:1959–1974. doi: 10.1089/scd.2013.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrborn D., Wronkowitz N., Eckel J. DPP4 in diabetes. Front. Immunol. 2015;6:386. doi: 10.3389/fimmu.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S., Xu Q. Q., Yu X., Pan R., Chen Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol. Ther. 2020;209:107503. doi: 10.1016/j.pharmthera.2020.107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A. Direct cardiovascular effects of glucagon like peptide-1. Diabetol. Metab. Syndr. 2013;5:47. doi: 10.1186/1758-5996-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher J. R., Drucker D. J. Cardiovascular biology of the incretin system. Endocr. Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villhauer E. B., Brinkman J. A., Naderi G. B., Burkey B. F., Dunning B. E., Prasad K., Mangold B. L., Russell M. E., Hughes T. E. 1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 2003;46:2774–2789. doi: 10.1021/jm030091l. [DOI] [PubMed] [Google Scholar]
- Waumans Y., Baerts L., Kehoe K., Lambeir A. M., De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front. Immunol. 2015;6:387. doi: 10.3389/fimmu.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., Chamberlain-Shea H., de la Morena M. T. Sitagliptin treatment of patients with type 2 diabetes does not affect CD4+ T-cell activation. J. Diabetes Complications. 2010;24:209–213. doi: 10.1016/j.jdiacomp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Yan S., Marguet D., Dobers J., Reutter W., Fan H. Deficiency of CD26 results in a change of cytokine and immunoglobulin secretion after stimulation by pokeweed mitogen. Eur. J. Immunol. 2003;33:1519–1527. doi: 10.1002/eji.200323469. [DOI] [PubMed] [Google Scholar]
- Yazbeck R., Howarth G. S., Abbott C. A. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol. Sci. 2009;30:600–607. doi: 10.1016/j.tips.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yang L., Wang X., Zhou Z. The new insights from DPP-4 inhibitors: their potential immune modulatory function in autoimmune diabetes. Diabetes Metab. Res. Rev. 2014;30:646–653. doi: 10.1002/dmrr.2530. [DOI] [PubMed] [Google Scholar]
- Zhong J., Kankanala S., Rajagopalan S. Division DPP4 inhibition: insights from the bench and recent clinical studies. Curr. Opin. Lipidol. 2016;27:484–492. doi: 10.1097/MOL.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Maiseyeu A., Davis S. N., Rajagopalan S. DPP4 in cardiometabolic disease: Recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ. Res. 2015;116:1491–1504. doi: 10.1161/CIRCRESAHA.116.305665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.