Abstract
Histone acetylation is a well-characterized epigenetic modification controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs). Imbalanced histone acetylation has been observed in many primary cancers. Therefore, efforts have been made to find drugs or small molecules such as HDAC inhibitors that can revert acetylation levels to normal in cancer cells. We observed dose-dependent reduction in the endogenous and exogenous protein expression levels of KAT8 (also known as human MOF), a member of the MYST family of HATs, and its corresponding histone acetylation at H4K5, H4K8, and H4K16 in chemotherapy drug gemcitabine (GEM)-exposed T24 bladder cancer (BLCA) cells. Interestingly, the reduction in MOF and histone H4 acetylation was inversely proportional to GEM-induced γH2AX, an indicator of chemotherapy drug effectiveness. Furthermore, pGL4-MOF-Luc reporter activities were significantly inhibited by GEM, thereby suggesting that GEM utilizes an MOF-mediated anti-BLCA mechanism of action. In the CCK-8, wound healing assays and Transwell® experiments, the additive effects on cell proliferation and migration were observed in the presence of exogenous MOF and GEM. In addition, the promoted cell sensitivity to GEM by exogenous MOF in BLCA cells was confirmed using an Annexin V-FITC/PI assay. Taken together, our results provide the theoretical basis for elucidating the anti-BLCA mechanism of GEM.
Keywords: KAT8/MOF, Bladder cancer, Chemotherapy, Gemcitabine
INTRODUCTION
Bladder cancer (BLCA) is one of the most common cancers worldwide and is also the main cause of cancer morbidity and mortality involving the urinary system (Yu et al., 2018). Based on the depth of infiltration of the tumor, BLCA is clinically divided into muscle-invasive BLCA (MIBC) and non-muscle-invasive BLCA (NMIBC) (Sanchez-Carbayo et al., 2006). About 75% of patients with BLCA have an initial diagnosis of NMIBC (Nieder et al., 2008); thus, cystectomy remains the main treatment for NMIBC and localized MIBC. However, about 50% of patients develop metastases within 2 years after cystectomy (Buttiglier et al., 2017). For this reason, intravesical immunotherapy or chemotherapy drugs have also been employed for the treatment of this disease (Shelley et al., 2012). The chemotherapy drug GEM is often clinically used for the treatment of BLCA by intravesical chemotherapy (Shelley et al., 2012; Takeuchi et al., 2015). For example, in a phase I/II study in patients with NMIBC, GEM effectively inhibited cancer progression and delayed the postoperative recurrence of tumors (Gontero et al., 2004). In particular, the combination of intravesical GEM and mitomycin C in patients with BLCA who are not candidates for (or refuse) cystectomy reduced BLCA recurrence and prolonged survival (Cockerill et al., 2016). However, the exact mechanisms underlying the effects of GEM on BLCA remain unclear. Studies in the past decade have shown that epigenetics is closely related to tumor pathogenesis, placing it at the forefront of cancer research (Jeronimo and Henrique, 2014). Epigenetic-based biomarkers have provided new insights into the clinical treatment of BLCA.
Epigenetic-based biomarkers have been studied in an attempt to better understand the molecular mechanisms involved in BLCA. Histone acetylation, as a fully characterized epigenetic modification, is conducted by HATs and HDACs. Human KAT8/MOF is a highly conserved member of the MYST (Moz-Ybf2/Sas3-Sas2-Tip60) family of HATs that was originally discovered in a study of X-chromosome dosage compensation in Drosophila (Hilfiker et al., 1997; Akhtar and Becker, 2000). MOF is a catalytic subunit that can form 2 distinct protein complexes. One is the male-specific lethal (MSL) complex, which plays a key role in dosage compensation in the fly and is mainly responsible for histone H4 on lysine 16 acetylation (H4K16ac) in cells (Mellert and McMahon, 2009). The other complex is the non-specific lethal (NSL)-associated MOF complex, which is capable of catalyzing substantial acetylation of histone H4K5, H4K8, and H4K16 (Cai et al., 2010).
Numerous studies have shown that MOF plays pivotal roles in various cellular functions, including genome stability, gene transcription, DNA damage repair, cell cycle regulation, and early embryonic development (Rea et al., 2007; Gupta et al., 2008; Kind et al., 2008; Sharma et al., 2010). The expression patterns of human MOF vary among different primary cancers. For example, it is frequently downregulated in breast cancer (Kapoor-Vazirani, et al., 2011), ovarian cancer (Liu et al., 2013), medulloblastoma (Pfister et al., 2008), renal cancer (Wang et al., 2013), colorectal carcinoma (Cao et al., 2014), and gastric cancer (Zhu et al., 2015), but is upregulated in non-small cell lung cancer (Zhao et al., 2013). The expression levels of MOF proteins are tightly associated with H4K16ac, strongly suggesting the involvement of MOF and its corresponding H4K16ac in certain tumorigenic pathways. However, the role of MOF in BLCA remains largely unknown. It is thought that MOF and its corresponding acetylation activities may be related to the sensitivity of cells to external stimuli, as overexpression of MOF has been shown to inhibit the sensitivity of 293T cells to arsenic trioxide (As2O3), but MOF-knockdown promotes the sensitivity of HeLa cells to As2O3 (Liu et al., 2015). However, the impact of MOF on the therapeutic effects of chemotherapy agents remains unclear.
In this study, T24 human urinary BLCA cells were employed as an experimental model to investigate the effects of GEM on BLCA. Our results showed a decrease in the MOF protein expression and its corresponding histone H4K16ac in T24 cells exposed to GEM, suggesting the potential interaction between MOF and GEM. To explore the potential MOF-mediated molecular mechanisms of GEM in T24 BLCA cells, we conducted a series of biochemical and molecular biological experiments such as the cell viability assay, flow cytometry, colony formation assay, and wound healing assay.
MATERIALS AND METHODS
Materials
Anti-mouse IgG-HRP (IH-0031) and anti-rabbit IgG-HRP (IH-0011) were obtained from Beijing Dingguo Changsheng Biotechnology Co. Ltd (Beijing, China). Anti-Flag (M2) (F3165) monoclonal antibody and anti-H4K16ac (H9164) polyclonal antibody were from Sigma-Aldrich (St. Louis, MO, USA). Anti-MOF (A02757) monoclonal antibody got from BosterBio (Wuhan, China). Antibodies for H4K5ac (07-327), H4K8ac (07-328), H3K4me1 (07-436), and H3K4me2 (07-030) were purchased from Merck Millipore (Darmstadt, Germany). Antibodies for H4 (16047-1-AP) and HDAC2 (12922-3-AP) were purchased from Proteintech Group (Wuhan, China). Antibodies for H2AX (CBS-PA15429A0R6) was from Cusabio Technology (Wuhan, China). Anti-γH2AX (07-164) monoclonal antibody was purchased from Merck Millipore. Antibodies for H3K4me3 (RLM3104), H3K9me2 (RLM3108), Parp1 (RLM3145), HDAC1 (RLT2145) and E-cadherin (RLT1453) were obtained from Ruiying (Suzhou, China). Antibodies for N-cadherin (ab18203) and Vimentin (ab45939) were from Abcam (Cambridge, UK). While anti-GAPDH (NM_002046, full length) rabbit polyclonal antibody was raised against bacterially expressed proteins (Jilin University, Changchun, China). The chemotherapy drug GEM was kindly gifted by Dr. Yong Wang (Urology Department, Jilin province People’s Hospital, Changchun, China).
Cell culture and maintenance
T24 and 5637 human urinary BLCA cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium (Gibco Life TechnologiesTM, Waltham, MA, USA) containing 10% fetal bovine serum (KY-01003, Kang Yuan Biology, Beijing, China) and 1% penicillin-streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in the presence of 5% CO2. The cells were treated with GEM according to the experimental design.
shRNA knockdown
The pLVX-shRNA system was used to knockdown endogenous MOF. Two shRNA sequences (shMOF-1, GTGATCCAGTCTCGAGTGA; shMOF-2, CGAAATTGATGCCTGGTAT), which targeted the coding DNA sequence of MOF, were introduced into the pLVX vector. The pLVX-shRNA plasmids were transiently transfected using polyethylenimine (PEI, 23966) (Polysciences, Shenzhen, China) according to the manufacturer’s recommendation.
Cell viability and growth assay
Cells were cultured at a density of 3×103/well in 96-well plates and treated with GEM. Then, cells were incubated with CCK-8 reagent (017319, Promega Corporation, Madison, WI, USA) for 1 h. The absorbance at a wavelength of 450 nm was measured using a microplate reader (Infinite F200 Pro, TECAN, Shanghai, China).
Wound healing assay
T24 cells were cultured in 6-well plates as ~90% confluent monolayers, and wounds were introduced by scraping the cells with the tip of a 10-μL pipette. Floating cells were removed and medium without serum was added. To analyze scratch wound closure, optical images were captured at 0 and 24 h time points using a microscope (Olympus IX73 microscope; Olympus Corporation, Tokyo, Japan). The wound area was analyzed with ImageJ (bundled with 64-bit Java 1.8.0_112; National Institutes of Health, MD, USA).
Colony formation assay
Cells (2×103) were seeded into a 12-well plate. After seven days of culture, formed colonies were stained with 0.1% crystal violet. Colonies were photographed by Gel Imaging System (Liuyi Instrument Plant, Beijing, China).
Transwell® migration assay
Twenty-four-well Transwell® chambers (Corning Inc., Corning, NY, USA) were used in the migration assay. Filters (8-μm pore size) were used for estimating cell migration. Cells in 0.2 mL of serum-free medium were placed into the upper chamber (2×104 cells). Medium containing 10% FBS was then added into the lower chamber. After incubation for 24 h, cells were fixed in methanol for 20 min, stained with 0.1% crystal violet for 10 min, and counted under a microscopy (Olympus Corporation, Miyazaki, Japan).
Luciferase reporter assay
The MOF promoter region (1470 bp, –1464 to +6 bp) was introduced into the pGL4-Luc vector. The luciferase reporter assay was conducted as described (Su et al., 2016a). Briefly, T24 cells were co-transfected with 0.4 μg pGL4, which encodes firefly luciferase; 1 ng control plasmid renilla luciferase vector, which encodes renilla luciferase; and effector plasmid expressing pGL4-MOF using PEI (PolyScience, Warrington, PA, USA). Total effector plasmid in each transfection was adjusted to 0.4 μg with empty vector. After 48 h, pGL4-MOF transactivation activity was determined by measuring firefly and renilla luciferase activities using a Dual-Luciferase Reporter Assay Kit (Promega Corporation) and by normalizing firefly to Renilla luciferase.
Plasmid construction and transient transfection
Full-length cDNA encoding the hMOF (BC037773) protein was subcloned with Flag-tag into pcDNA3.1(-). Then, the T24 cells were transiently transfected with FLAG-MOF (Fl:MOF) plasmid using PEI according to the manufacturer’s instructions (PolyScience).
Flow cytometry analysis
Cultured T24 cells were harvested by trypsinization. ~1×106 cells were suspended as single cell dispersions in 70% ethanol at –20°C for at least 4 h. After centrifugation at 300×g for 5 min, cells were washed twice with PBS and then re-suspended in 300 μl PBS containing 0.1% (v/v) Triton X-100, 0.3 mg/mL DNase-free, RNase A, 50 μg/mL propidium iodide, and then were incubated at 37°C for 1 h. Data collection was performed using the EPICS XLTM flow cytometer (Beckman Coulter, Brea, CA, USA). Acquired data were analyzed using ModFit LT software (Verity Software House, Topsham, ME, USA). The experiment was repeated three times under the same conditions.
Analysis of apoptosis
T24 cells were cultured in RPMI-1640 medium with or without GEM (0.15 μM or 0.75 μM). After transfection for 48 h, cells were harvested and stained with an Annexin V-FITC/PI kit (KeyGEN Biotech, Nanjing, China). Propidium iodide (PI) was used to discriminate between apoptotic cells with membrane integrity (Annexin V+/PI–) and necrotic cells that lost membrane integrity (Annexin V+/PI+).
Statistical analysis
Statistical analysis was conducted using data from at least three independent experiments. All results are presented as mean ± standard deviation (SD). SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Independent samples t-test was used to compare means between two groups. One-way ANOVA with Bonferroni’s post-test was used to measure the overall effect of drug treatment. For the drug treatment and multiple time-points groups, two-way ANOVA analysis of variance and the Tukey honest significant difference post hoc test were used. Significance was defined at p<0.05.
RESULTS
Anti-cancer effects of GEM were verified in T24 BLCA cells
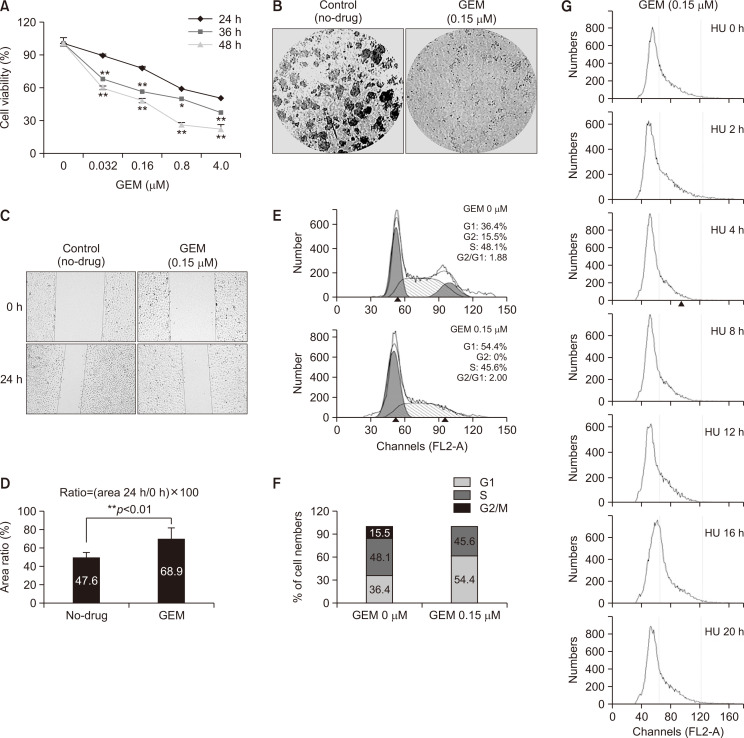
Our initial research results suggest that the protein level of MOF in bladder cancer T24 and 5637 cells was suppressed in a dose-dependent manner after treatment with chemotherapeutic drugs, including GEM, mitomycin C, and camptothecin (data not shown). Thus, GEM was selected for the subsequent study on MOF-related mechanisms. To determine the appropriate concentrations of GEM for our experiments, the CCK-8 cell viability assay was first performed to verify their cytotoxicity in T24 cells. Fig. 1A shows that dose-dependent cytotoxicity of GEM in T24 cells was observed after treatment with gradient concentrations of GEM for 24 h, 36 h, and 48 h. Cytotoxicity increased with prolonged drug exposure time (48 h>36 h>24 h). Next, long-term clonogenic cell survival and cell migration ability were evaluated by colony formation and wound healing assays. Based on the CCK-8 cell viability assay, we selected 0.15 µM GEM for subsequent experiments because this drug concentration did not induce any cell death. Compared to the control group (No-drug group), the ability of cells to form colonies was significantly suppressed by GEM (Fig. 1B), and the progression of wound closure was much slower in GEM-exposed cells than in control cells (Fig. 1C). The normalized percentage of the scratch wound is shown in Fig. 1D. Next, FACS analysis was performed to verify the effects of GEM on T24 cell cycle progression. Fig. 1E shows that compared to the control group, GEM-exposed cells appeared to be arrested in G1/S phase (lower panel). The percentage of cells in the G1, S, and G2/M phases is shown in Fig. 1F. To further confirm this observation, T24 cells were treated with 1 mM hydroxyurea (HU) to block cells in the G1/S phase so that no new G2/M phase cells could be generated, and then released the cells at different time points (0, 2, 4, 8, 12, 16, and 20 h). As expected, 0.15 µM GEM-treated cells were completely arrested at the G1/S phase throughout the experiment (Fig. 1G).
Fig. 1.
The inhibitory effects of GEM on T24 BLCA cells are confirmed by a set of experiments. (A) Cell viability (n=3). Cells were treated with 0-4.0 µM GEM for 24 h, 36 h, and 48 h. The cell viability was determined by CCK-8 assay (one-way ANOVA with Bonferroni’s post-test, *p<0.05, **p<0.01). (B) Colony formation assay (n=3). The no-drug group was used as the control. (C) Wound healing assay. Scratch wound healing assay was performed in T24 cells with or without GEM (0.15 µM). The no-drug group was used as the control. Representative images were captured at 0 h and 24 h after the initiation of the scratch wound. (D) Cell migration was quantified as a percentage of the wound closure. **p<0.01 compared to the no-drug group (student’s t-test). (E) Fluorescence-activated cell sorting (FACS) analysis. FACS analysis of propidium iodide-stained cells was performed in cells with or without GEM (0.15 µM) for 24 h. (F) Quantified cell cycle distribution in GEM 0 and 0.15 µM exposed cells. (G) Delayed progression of G1/S to G2/M phases in 0.15 µM GEM-treated cells. 0.15 µM GEM exposed T24 cells were synchronized by treatment with 1 mM HU for 24 h. Cells were harvested by trypsinization 0, 2, 4, 6, 8, 12, 16 and 20 h after removal of HU. Acquired data was analyzed using ModFit LT software (Verity Software House, Topsham, ME, USA).
MOF-mediated global histone H4K5ac/K8ac/K16ac in T24 BLCA cells are targeted and regulated by GEM
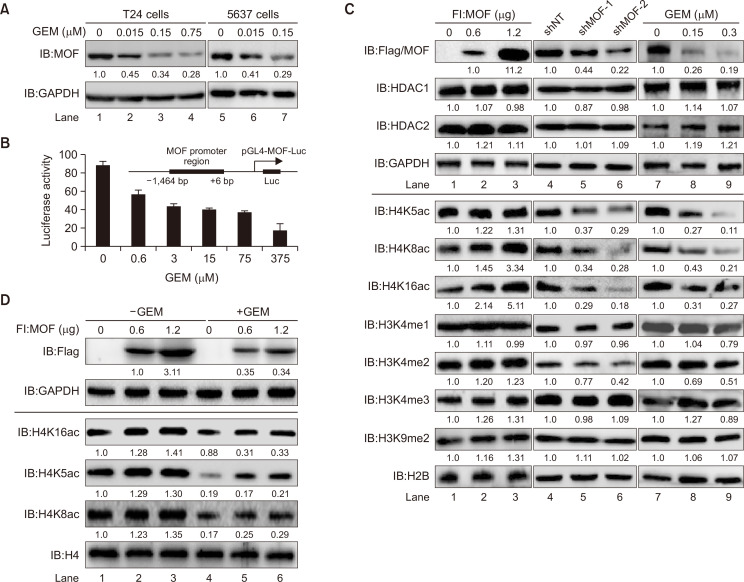
Both BLCA T24 and 5637 cells treated with GEM showed downregulated endogenous levels of the HAT MOF protein, and this reduction was dose-dependent (Fig. 2A). Thus, we selected T24 cells for a series of subsequent experiments. At first, to explore whether GEM affects the transactivation of MOF, the MOF promoter region (–1,464 to +6 bp) was subcloned into the pGL4 luciferase vector (Fig. 2B, right upper). Then, the impact of GEM on MOF transactivation was estimated by measuring luciferase activity of pGL4-MOF in GEM- (0-375 nM) exposed cells. In contrast to basal level luciferase activity, a dose-dependent decrease in luciferase activity was observed in all concentrations of GEM treated cells (Fig. 2B), indicating the regulatory role of GEM on MOF transactivation. Considering that MOF acts as a catalytic subunit in cells, it can form 2 different protein complexes (NSL and MSL complexes) that are responsible for catalyzing the acetylation of histones H4K5, H4K8, and H4K16 (Cai et al., 2010). Therefore, to further confirm that GEM targets MOF, Fl:MOF and pLVX-shMOF plasmids were constructed. We then compared the changes in MOF protein expression and global histone H4 acetylation, H3 methylation, and HDAC1/2 levels in MOF-overexpression, MOF-knockdown, or GEM-exposed T24 cells. Fig. 2C shows that similar to the results of MOF-knockdown (lanes 4-6), GEM dose-dependently reduced the expression of the MOF protein and its corresponding histone H4K5ac/H4K8ac/H4K16ac (lanes 7-9), suggesting that GEM can target endogenous MOF. Conversely, in cells overexpressing Fl:MOF, in addition to the highly expressed MOF, its corresponding global histone acetylation level also increased (lane 1-3). However, the histone H3K4me1/me2/me3 and H3K9me2 and the levels of HDAC1/2 did not significantly change in cells that exhibited MOF overexpression, knockdown, or were treated with GEM, suggesting that GEM specifically targets MOF, thereby affecting its corresponding histone acetylation level at lysine K5, K8, and K16. The following experiment confirmed the synergy between MOF and GEM. Fig. 2D shows that the cells were transiently transfected with increasing amounts of Fl:MOF plasmids in the presence or absence of GEM. Compared to the Fl:MOF plasmid-transfected group (lanes 1-3), GEM remarkably decreased the level of exogenous MOF and the acetylation of histone H4 (lanes 4-6).
Fig. 2.
Both endogenous and exogenous HAT MOF and its corresponding histone H4 acetylation levels in T24 cells are suppressed by GEM. (A) Reduction in endogenous MOF protein levels in GEM-exposed BLCA cells. T24 and 5637 cells were treated with the indicated amount of GEM for 24 h. MOF protein expression was determined by western blotting with the anti-MOF antibody. The MOF proteins in different GEM-treated groups were compared with the untreated group (all MOF proteins were quantified with GAPDH). The quantified results are marked below the MOF signals. (B) Luciferase reporter assays. GEM-induced MOF transactivation was detected by dual luciferase reporter assays. Schematic of the promoter region of MOF gene (–1,464 to +6 bp) used for luciferase reporter assay. Cells were treated with indicated concentration of GEM. Then, dual luciferase activities were detected after transfection of pGL4-MOF-Luc for 48 h. (C) Western blot analysis. Cells were transfected with Fl:MOF plasmid (0.6 µg and 1.2 µg) or shMOF plasmid (1.5 µg) or treated with GEM (0.15 µM or 0.3 µM). Approximately 48 h later, the WCL protein expression was measured by western blotting with specific antibodies. MOF, HDAC1, and HDAC2 expression levels were normalized to that of GAPDH, and different histone modifications were quantified using H2B. Proteins signals were compared to the untreated group. The data below the protein signals represents the quantified results. (D) Reduction of exogenous MOF in GEM-treated cells. 0.6 µg and 1.2 µg Fl:MOF plasmids were transiently transfected into T24 cells. After 24 h, the cells were treated with 0.15 µM GEM for 24 h. MOF protein and histone H4 acetylation levels were detected by western blot with specific antibodies. MOF protein was normalized by GAPDH, and histone H4 modifications were quantified by H4. Signals in GEM-treated groups were compared to the corresponding untreated groups.
GEM and MOF coordinatively induces DNA damage in T24 BLCA cells
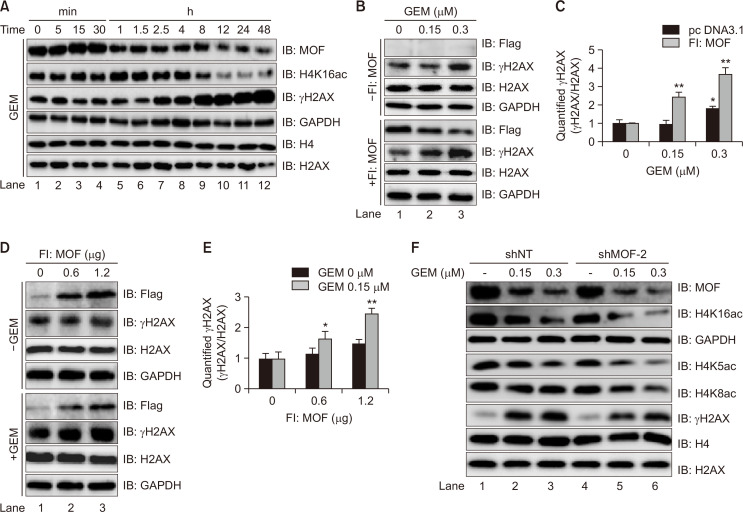
Double-strand DNA breaks (DSBs) often initiate phosphorylation of histone H2AX at serine 139, which is referred to as γH2AX, and the detection of γH2AX is a rapid method for assessing the effectiveness of chemotherapy drugs that induce DSBs (Wang et al., 2010). Fig. 3A shows that using γH2AX as an indicator, we observed the correlation between MOF and γH2AX in GEM-exposed cells. As a result, the levels of γH2AX in GEM-treated cells gradually increased during the observation period, indicating the therapeutic effects of GEM on BLCA cells (IB: γH2AX). Contrary to the observed changes in γH2AX, MOF and histone H4K16ac levels gradually decreased (IB:MOF and H4K16ac), suggesting the potential relevance of MOF in the anti-cancer mechanism of GEM. To further confirm this hypothesis, γH2AX was measured in cells with or without Fl:MOF plasmid transfection after GEM treatment (Fig. 3B). As expected, a highly significantly increase in γH2AX levels was observed in cells co-treated with GEM and MOF (**p<0.01, compared to cells treated with GEM alone) (Fig. 3C). The γH2AX levels in Fl:MOF-transfected cells with or without GEM were also tested. Similarly, a remarkable enhancement of γH2AX was revealed in the presence of both GEM and MOF (*p<0.05 and **p<0.01, compared to cells treated with MOF alone) (Fig. 3D, 3E). However, the addition of GEM to MOF-knockdown cells reduced γH2AX levels compared to the shNT group, which may have been due to the decrease in endogenous MOF and its corresponding acetylation of histone H4K5/ H4K8/ H4K16, leading to a decrease in cell sensitivity to GEM (Fig. 3F).
Fig. 3.
GEM-induced γH2AX levels are regulated by MOF. (A) Dynamic changes of γH2AX in GEM (0.15 µM)-treated T24 cells. (B, C) Impact of GEM (0, 0.15 and 0.3 µM) on γH2AX levels in T24 cells with or without 1.2 µg Fl:MOF transfection. (D, E) Effects of MOF (0, 0.6 and 1.2 µg) on γH2AX in the presence or absence of 0.15 µM GEM in T24 cells. (F) Effects of shMOF on histone H4K16ac, H4K5ac, H4K8ac, and γH2AX in GEM (0, 0.15 and 0.3 µM)-exposed T24 cells. GAPDH, H4, and H2AX were used as internal controls (two-way ANOVA with Tukey’s post-hoc analysis for C and E, *p<0.05, **p<0.01).
MOF enhances the inhibitory effect of GEM on T24 BLCA cell proliferation
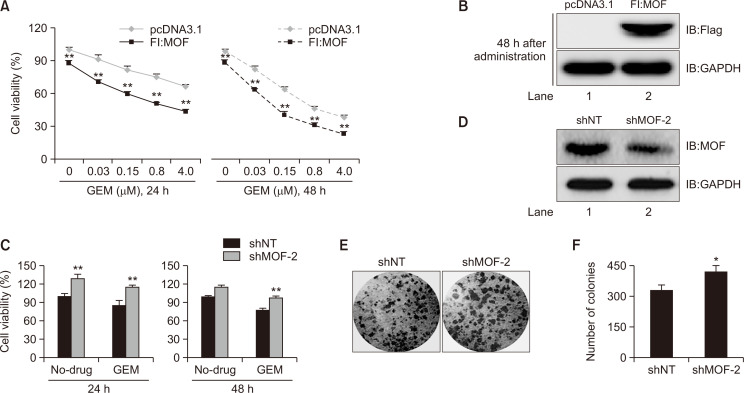
Our previous results confirmed that GEM targets and regulates intracellular MOF and its corresponding histone H4K5/K8/K16 acetylation levels, prompting us to further explore whether the regulatory effect of GEM on MOF is involved in its anti-cancer effect. To address this question, the viability of T24 cells treated with 0, 0.03, 0.15, 0.8 or 4.0 µM GEM for 24 h and 48 h in the presence or absence of exogenous MOF was assessed using the CCK-8 assay kit (017319, Promega Corporation). Compared with the pcDNA3.1-transfected control group, GEM dose-dependently inhibited cell viability at both 24 h and 48 h time points (Fig. 4A), suggesting that MOF facilitated the sensitivity of T24 cells to GEM. The expression level of Fl:MOF at 48 h after transfection is shown in Fig. 4B (lane 2). On the contrary, by knocking down the MOF with specific shRNA, cell viability increased regardless of whether the cells were treated with GEM for 24 h or 48 h (Fig. 4C). The protein level of endogenous MOF at 48 h after shMOF transfection was shown in Fig. 4D (lane 2). The results of the colony formation assay further support the previous results of cell viability. Fig. 4E shows that knocking down MOF with shRNA in T24 cells promotes colony formation. The number of colonies in the control and MOF-knockdown groups is shown in Fig. 4F. The above experimental results show the synergistic effects of GEM and MOF on BLCA tumorigenesis.
Fig. 4.
Confirmation of the additive effects of MOF and GEM on proliferation of T24 cells. (A) Cell viability of GEM-exposed cells with or without Fl:MOF (n=3). Cells cultured in a 3.5-cm dish were transiently transfected with pcDNA3.1 and Fl:MOF plasmids separately (two-way ANOVA with Tukey’s post-test, **p<0.01). Approximately 1.2 µg pcDNA3.1 or Fl:MOF transfected (48 h) cells were treated with different concentrations of GEM (0-4.0 µM) for 24 h and 48 h. Cell viability was determined by CCK-8 assay. Fl:MOF protein levels after 48 h transient transfection are shown in (B). (C) Cell viability of MOF-knockdown T24 cells. shNT- and shMOF-transfected cells were treated with GEM (0.15 µM) for 24 h and 48 h, then, CCK-8 assay was performed (n=3) (two-way ANOVA with Tukey’s post-hoc analysis, **p<0.01). (D) MOF-knockdown efficiency was analyzed by western blotting. (E) Increase of colony formation in shMOF-transfected cells. Colony formation assay was performed in shNT- and shMOF-transfected T24 cells. (F) Quantified number of colony in control and MOF-knockdown groups (*p=0.0177, student’s t-test).
MOF and GEM engage in cooperative inhibition of cell motility
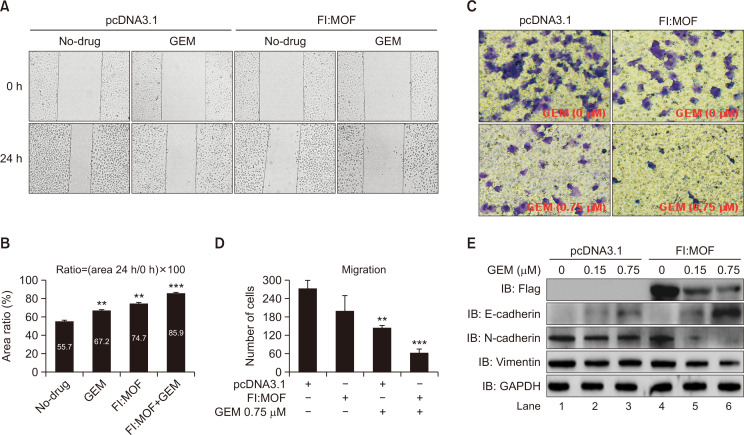
Cancer cells move within tissues during invasion and metastasis by their own motility (Yamazaki et al., 2005). To observe the synergistic effect of MOF and GEM on cell motility, the wound healing assay and Transwell® experiments were performed. Fig. 5A shows the images 24 h after making an initial scratch wound. The progression of wound closure was slower in the GEM, MOF, and MOF+GEM cells than in the no-drug cells. Importantly, when cells were co-treated with MOF and GEM, the progression of wound closure was much slower than in cells treated with MOF or GEM alone. The percentages of the scratch wound at 24 h were 55.7%, 67.2%, 74.7%, and 85.9% in the control, GEM, MOF, and MOF+GEM groups, respectively (Fig. 5B). Next, the Transwell® migration assay was performed to examine the function of MOF and GEM in metastasis. Similarly, cells co-treated with MOF and GEM had lower migrant ability than cells treated with MOF or GEM alone (Fig. 5C). The number of migrant cells is shown in Fig. 5D. Epithelial-mesenchymal transition (EMT) plays a key role in tumor metastasis. Using western blotting, we examined the protein levels of several key EMT-related molecules. Fig. 5E shows that compared to the GEM alone-treated cells (left panel), when GEM-exposed cells were transfected with Fl:MOF plasmids, the epithelial cell marker E-cadherin was strongly upregulated, whereas mesenchymal markers N-cadherin and vimentin were distinctly downregulated (right panel). The above results show the synergistic inhibitory effect of MOF and GEM on the metastasis of BLCA T24 cells.
Fig. 5.
Additive effects of MOF and GEM on wound healing and migration of T24 cells. (A) Wound healing assay. Scratch wound healing assay was performed in pcDNA3.1 vector (as control) and Fl:MOF expressed cells with or without GEM (0.15 µM) treatment. Images were captured at 0 h and 24 h after initiation of the scratch wound. (B) The percentage of scratch area (%). Y-axis represented the percentage of scratch area. The percentage of the wound area expressed as the ratio of 24 h scratch area to 0 h area. **p<0.01, ***p<0.001 compared to the no-drug group (Student t-test). (C) Transwell migration assay. The 24-well transwell chambers were used for migration assay. T24 cells were transfected with pcDNA3.1 vector (as control) or Fl:MOF plasmids. Then, cells were exposed in GEM (0.75 µM) for 24 h. After transfection for 48 h, cells were seeded, stained, and counted. (D) The number of migrant cells was calculated (two-way ANOVA with Tukey’s post-hoc analysis, **p<0.01, ***p<0.001). (E) EMT assay: T24 cells with or without Fl:MOF transfection were treated with GEM (0, 0.15 and 0.75 µM) for 24 h. EMT-related proteins were then analyzed by western blotting with specific antibodies. GAPDH was used as internal control.
MOF enhances GEM-induced cell death
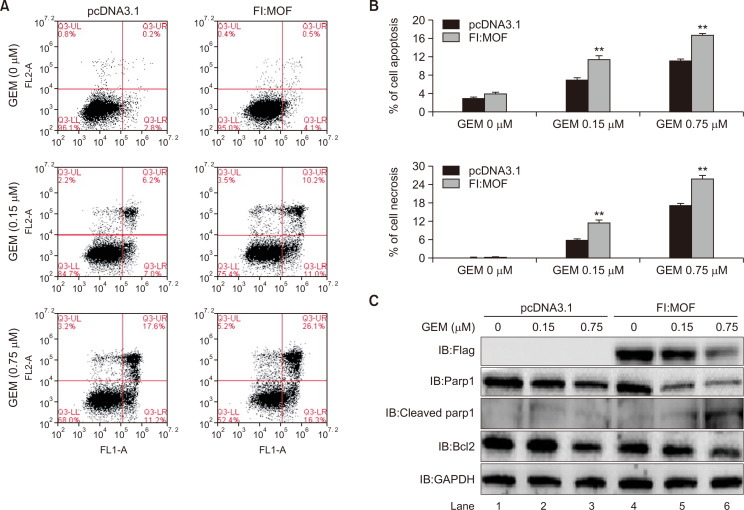
Cell viability experiments suggest that MOF increases cell sensitivity to GEM. To further confirm whether MOF has an influence on GEM-induced cell apoptosis and necrosis, flow cytometry of Annexin V binding/PI uptake was assessed in cells exposed to increasing GEM concentrations with or without Fl:MOF transfection (Fig. 6A). Apoptosis and necrosis are depicted in Fig. 6B. Compared to the GEM-only treated cells, exogenous MOF significantly increased GEM-induced apoptosis (upper panel) and necrosis (lower panel) (**p<0.01 in 0.15 and 0.75 µM GEM group), suggesting that MOF promoted GEM-induced cell death. This result has also been confirmed by detecting the apoptosis-related proteins using western blot analysis. Fig. 6C shows that overexpression of Fl:MOF increased expression of apoptosis-associated protein Cleaved Parp1 and inhibited Bcl2 expression, suggesting that exogenous MOF enhances the T24 BLCA cell sensitivity to GEM.
Fig. 6.
MOF enhances GEM-induced apoptosis and necrosis of T24 cells. (A) Annexin V/PI-FACS analysis in T24 cells. The numbers in the upper left and right and lower left and right quadrants represent the percentage of damaged, necrotic, live and apoptotic cells, respectively. (B) Quantified percentages of apoptotic (upper) and necrotic (lower) cells in pcDNA3.1 (as the control) and Fl:MOF-transfected groups. Two-way ANOVA with Tukey’s post-hoc analysis, **p<0.01. (C) Apoptosis-associated proteins were detected by western blotting with specific antibodies. GAPDH was used as internal control.
DISCUSSION
Different histone post-translational modifications lead to distinct effects on chromatin architecture (Biswas and Rao, 2018). Among these, histone acetylation is controlled by HATs and HDACs. In cells, human HAT MOF is a catalytic subunit that can form at least 2 distinct multiprotein complexes (MSL and NSL). Both complexes can acetylate the histone H4K16 site; however, the H4K5 and H4K8 sites can also be acetylated by the NSL complex, suggesting the complexity of the functions of MOF (Cai et al., 2010; Su et al., 2016b). In cells, MOF participates in many critical biological processes, including gene transcription, cell proliferation, and the DNA repair response (Mendjan et al., 2006; Sharma et al., 2010). Our previous study and other literature reports have suggested that MOF plays a key role in tumorigenesis (Pfister et al., 2008; Kapoor-Vazirani et al., 2011; Liu et al., 2013; Wang et al., 2013; Zhao et al., 2013; Cao et al., 2014; Zhu et al., 2015), demonstrating that it is likely to be a target for cancer treatment. Interestingly, GEM, a chemotherapy drug commonly used in BLCA, inhibited both endogenous and exogenous MOF protein and global histone H4 acetylation at Lys5, Lys8, and Lys16 sites in T24 cells (Fig. 2, 3), suggesting the involvement of MOF-containing NSL complex in the anti-BLCA mechanism of GEM. Further research results suggest that the downregulation of MOF protein and its corresponding histone H4K5ac, H4K8ac, and H4K16ac in GEM-exposed cells may be due to GEM inhibiting the transactivation because pGL4-MOF-Luc reporter activities were suppressed by treating cells with GEM and followed a dose-dependent manner (Fig. 2). In line with our results, the well-known p300/CBP HAT has become the target of several chemotherapy drugs. For instance, 5-fluorouracil (5-FU), a ribonucleic acid and deoxyribonucleic acid synthesis-interfering agent, can result in hypoacetylation of global histones by promoting the degradation of p300/CBP in multiple colorectal cancer cell lines (Du et al., 2017).
Subsequent research studies have shown that inhibiting the degradation of p300/CBP can enhance the cytotoxicity of 5-FU in colorectal cancer cells, whereas reducing the protein expression levels of p300/CBP by siRNA improves cellular resistance to 5-FU. Moreover, the downregulation of p300/CBP in colorectal carcinoma tissue is closely associated with poor clinical response to 5-FU-based chemotherapy (Du et al., 2017), demonstrating the involvement of HAT p300/CBP in the anti-cancer mechanism of 5-FU. Furthermore, in A549 cells (adenocarcinomic human alveolar basal epithelial cells), the significant increase in cell sensitivity to cis-diamminedichloroplatinum (cisplatin), 5-FU, and bleomycin was observed after knocking down MOF with siRNA (Chen et al., 2014), strongly suggesting that MOF is the target of those anti-cancer drugs. GEM only affects global acetylation of histone H4K5, H4K8, and H4K16, but it has little or no effect on histone H3K4me1/me2/me3, H3K9me2, HDAC1, and HDAC2 levels, suggesting that at least part of the anti-cancer mechanism of GEM in T24 BLCA cells may be caused by selective inhibition of MOF. This is also consistent with the view that overexpressing MOF decreases cell viability, and knocking down MOF increases cell viability in GEM-exposed T24 BLCA cells.
In view of the research results described above, the role of MOF-mediated anti-cancer effects of GEM can be deduced. However, it is worth noting that the modulation of MOF expression by GEM not only directly affects the global intracellular acetylation of histone H4, but also may indirectly participate in the pathogenesis of cancer through acetylation of non-histone proteins. For instance, MOF is required for directly acetylating histone demethylase LSD1 in T47D, MDA-MB-468, BT474, and MCF7 cells, and increasing MOF expression can suppress the LSD1’s accessibility to chromatin, EMT, and invasion in A549 cells. In contrast, depletion of MOF promoted EMT and cell invasion, suggesting the function of acetylation of LSD1 by MOF in tumor malignant progression (Luo et al., 2016). In this study, the overexpression of MOF in GEM-exposed T24 BLCA cells led to an additive inhibition in cell motility, which was confirmed by wound healing and Transwell® assays (Fig. 5), suggesting the cooperative function of MOF and GEM in T24 BLCA cell migration. In line with this, upregulation of E-cadherin and downregulation of N-cadherin/vimentin were observed in the MOF/GEM co-treated group. Furthermore, this additive effect between MOF and GEM is also reflected in flow cytometry of Annexin V binding/PI uptake assay. The percentage of cell apoptosis and necrosis significantly increased in the MOF and GEM co-treated group compared those to GEM-only treated group. At the same time, lower levels of the apoptosis-related protein Parp1 and Bcl2 and higher levels of cleaved Parp1 were detected by western blotting (Fig. 6).
In summary, our present findings uncovered novel mechanisms by which the anti-bladder cancer effect of GEM is at least partially achieved by inhibiting the expression of MOF and its corresponding histone H4 acetylation. Thus, our results suggest that human MOF or MOF-containing NSL complex may be potentially utilized as a target for the development of new drugs for the treatment of BLCA.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (31401086 and 31771421). In addition, we thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- Akhtar A., Becker P. B. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell. 2000;5:367–375. doi: 10.1016/S1097-2765(00)80431-1. [DOI] [PubMed] [Google Scholar]
- Biswas S., Rao C. M. Epigenetic tools (The Writers, The Readers and The Erasers) and their implications in cancer therapy. Eur. J. Pharmacol. 2018;837:8–24. doi: 10.1016/j.ejphar.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Buttiglier T. M., Tucci M., Vignani F., Scagliotti G. V., Di Maio M. Molecular biomarkers to predict response to neoadjuvant chemotherapy for bladder cancer. Cancer Treat. Rev. 2017;54:1–9. doi: 10.1016/j.ctrv.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Cai Y., Jin J., Swanson S. K., Cole M. D., Choi S. H., Florens L., Washburn M. P., Conaway J. W., Conaway R. C. Subunit composition and substrate specificity of a MOF-containing histone acetyltransferase distinct from the male-specific lethal (MSL) complex. J. Biol. Chem. 2010;285:4268–4272. doi: 10.1074/jbc.C109.087981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Zhu L., Yang J., Su J., Ni J., Du Y., Liu D., Wang Y., Wang F., Jin J., Cai Y. Correlation of low expression of hMOF with clinicopathological features of colorectal carcinoma, gastric cancer and renal cell carcinoma. Int. J. Oncol. 2014;44:1207–1214. doi: 10.3892/ijo.2014.2266. [DOI] [PubMed] [Google Scholar]
- Chen Z., Ye X., Tang N., Shen S., Li Z., Niu X., Lu S., Xu L. The histone acetylranseferase hMOF acetylates Nrf2 and regulates anti-drug responses in human non-small cell lung cancer. Br. J. Pharmacol. 2014;171:3196–3211. doi: 10.1111/bph.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill P. A., Knoedler J. J., Frank I., Tarrell R., Karnes R. J. Intravesical gemcitabine in combination with mitomycin C as salvage treatment in recurrent non-muscle-invasive bladder cancer. BJU Int. 2016;117:456–462. doi: 10.1111/bju.13088. [DOI] [PubMed] [Google Scholar]
- Du C., Huang D., Peng Y., Yao Y., Zhao Y., Yang Y., Wang H., Cao L., Zhu W. G., Gu J. 5-Fluorouracil targets histone acetyltransferases p300/CBP in the treatment of colorectal cancer. Cancer Lett. 2017;400:183–193. doi: 10.1016/j.canlet.2017.04.033. [DOI] [PubMed] [Google Scholar]
- Gontero P., Casetta G., Maso G., Sogni F., Pretti G., Zitella A., Frea B., Tizzani A. Phase II study to investigate the ablative efficacy of intravesical administration of gemcitabine in intermediate-risk superficial bladder cancer (SBC) Eur. Urol. 2004;46:339–343. doi: 10.1016/j.eururo.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Gupta A., Guerin-Peyrou T. G., Sharma G. G., Park C., Agarwal M., Ganju R. K., Pandita S., Choi K., Sukumar S., Pandita R. K., Ludwig T., Pandita T. K. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol. Cell. Biol. 2008;28:397–409. doi: 10.1128/MCB.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker A., Hilfiker-Kleiner D., Pannuti A., Lucchesi J. C. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997;16:2054–2060. doi: 10.1093/emboj/16.8.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo C., Henrique R. Epigenetic biomarkers in urological tumors: a systematic review. Cancer Lett. 2014;342:264–274. doi: 10.1016/j.canlet.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Kapoor-Vazirani P., Kagey J. D., Vertino M. P. SUV420H2-mediated H4K20 trimethylation enforces RNA polymerase II promoter-proximal pausing by blocking hMOF-dependent H4K16 acetylation. Mol. Cell. Biol. 2011;31:1594–1609. doi: 10.1128/MCB.00524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind J., Vaquerizas J. M., Gebhardt P., Gentzel M., Luscombe N. M., Bertone P., Akhtar A. Genome-wide analysis reveals MOF as a key regulator of dosage compensation and gene expression in Drosophila. Cell. 2008;133:813–828. doi: 10.1016/j.cell.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Liu D., Wu D., Zhao L., Yang Y., Ding J., Dong L., Hu L., Wang F., Zhao X., Cai Y., Jin J. Arsenic trioxide reduces global histone H4 acetylation at lysine 16 through direct binding to histone acetyltransferase hMOF in human cells. PLoS ONE. 2015;10:e0141014. doi: 10.1371/journal.pone.0141014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Zhang R., Zhao X., Su J., Bian X., Ni J., Yu Y., Cai Y., Jin J. A potential diagnostic marker for ovarian cancer: involvement of the histone acetyltransferase, human males absent on the first. Oncol. Lett. 2013;6:393–400. doi: 10.3892/ol.2013.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Shenoy A. K., Li X., Jin Y., Jin L., Cai Q., Tang M., Liu Y., Chen H., Reisman D., Wu L., Seto E., Qiu Y., Dou Y., Casero R. A., Jr, Lu J. MOF acetylates the histone demethylase LSD1 to suppress epithelial-to-mesenchymal transition. Cell Rep. 2016;15:2665–2678. doi: 10.1016/j.celrep.2016.05.050. [DOI] [PubMed] [Google Scholar]
- Mellert H. S., McMahon S. B. hMOF, a KAT(8) with many lives. Mol. Cell. 2009;36:174–175. doi: 10.1016/j.molcel.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Mendjan S., Taipale M., Kind J., Holz H., Gebhardt P., Schelder M., Vermeulen M., Buscaino A., Duncan K., Mueller J., Wilm M., Stunnenberg H. G., Saumweber H., Akhtar A. Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol. Cell. 2006;21:811–823. doi: 10.1016/j.molcel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Nieder A. M., Mackinnon J. A., Huang Y., Fleming L. E., Koniaris L. G., Lee D. J. Florida bladder cancer trends 1981 to 2004: minimal progress in decreasing advanced disease. J. Urol. 2008;179:491–495. doi: 10.1016/j.juro.2007.09.082. [DOI] [PubMed] [Google Scholar]
- Pfister S., Rea S., Taipale M., Mendrzyk F., Straub B., Ittrich C., Thuerigen O., Sinn H. P., Akhtar A., Lichter P. The histone acetyltransferase hMOF is frequently downregulated in primary breast carcinoma and medulloblastoma and constitutes a biomarker for clinical outcome in medulloblastoma. Int. J. Cancer. 2008;122:1207–1213. doi: 10.1002/ijc.23283. [DOI] [PubMed] [Google Scholar]
- Rea S., Xouri G., Akhtar A. Males absent on the first (MOF): from flies to humans. Oncogene. 2007;26:5385–5394. doi: 10.1038/sj.onc.1210607. [DOI] [PubMed] [Google Scholar]
- Sanchez-Carbayo M., Socci N. D., Lozano J., Saint F., Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- Sharma G. G., So S., Gupta A., Kumar R., Cayrou C., Avvakumov N., Bhadra U., Pandita R. K., Porteus M. H., Chen D. J., Cote J., Pandita T. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol. Cell. Biol. 2010;30:3582–3595. doi: 10.1128/MCB.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley M. D., Jones G., Cleves A., Wilt T. J., Mason M. D., Kynaston H. G. Intravesical gemcitabine therapy for non-muscle invasive bladder cancer (NMIBC): a systematic review. BJU Int. 2012;109:496–505. doi: 10.1111/j.1464-410X.2011.10880.x. [DOI] [PubMed] [Google Scholar]
- Su J., Sui Y., Ding J., Li F., Shen S., Yang Y., Lu Z., Wang F., Cao L., Liu X., Jin J., Cai Y. Human INO80/YY1 chromatin remodeling complex transcriptionally regulates the BRCA2- and CDKN1A-interacting protein (BCCIP) in cells. Protein Cell. 2016a;7:749–760. doi: 10.1007/s13238-016-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Wang F., Cai Y., Jin J. The functional analysis of histone acetyltransferase MOF in tumorigenesis. Int. J. Mol. Sci. 2016b;17:99. doi: 10.3390/ijms17010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Mmeje C. O., Jinesh G. G., Taoka R., Kamat A. M. Sequential gemcitabine and tamoxifen treatment enhances apoptosis and blocks transformation in bladder cancer cells. Oncol. Rep. 2015;34:2738–2744. doi: 10.3892/or.2015.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Pfister T. D., Parchment R. E., Kummar S., Rubinstein L., Evrard Y. A., Gutierrez M. E., Murgo A. J., Tomaszewski J. E., Doroshow J. H., Kinders R. J. Monitoring drug-induced γH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clin. Cancer Res. 2010;16:1073–1084. doi: 10.1158/1078-0432.CCR-09-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang R., Wu D., Lu Z., Sun W., Cai Y., Wang C., Jin J. Epigenetic change in kidney tumor: downregulation of histone acetyltransferase MYST1 in human renal cell carcinoma. J. Exp. Clin. Cancer Res. 2013;32:8. doi: 10.1186/1756-9966-32-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D., Kurisu S., Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–386. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Cao H., Zhang M., Shi F., Wang R., Liu X. Prognostic value of DNA methylation for bladder cancer. Clin. Chim. Acta. 2018;484:207–212. doi: 10.1016/j.cca.2018.05.056. [DOI] [PubMed] [Google Scholar]
- Zhao L., Wang D. L., Liu Y., Chen S., Sun F. L. Histone acetyltransferase hMOF promotes S phase entry and tumorigenesis in lung cancer. Cell. Signal. 2013;25:1689–1698. doi: 10.1016/j.cellsig.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Zhu L., Yang J., Zhao L., Yu X., Wang L., Wang F., Cai Y., Jin J. Expression of hMOF, but not HDAC4, is responsible for the global histone H4K16 acetylation in gastric carcinoma. Int. J. Oncol. 2015;46:2535–2545. doi: 10.3892/ijo.2015.2956. [DOI] [PubMed] [Google Scholar]