Abstract
Astrocytes play various important roles such as maintaining brain homeostasis, supporting neurons, and secreting inflammatory mediators to protect the brain cells. In aged subjects, astrocytes show diversely changed phenotypes and dysfunctions. But, the study of aged astrocytes or astrocytes from aged subjects is not yet sufficient to provide a comprehensive understanding of their important processes in the regulation of brain function. In this study, we induced an in vitro aged astrocyte model through late passage cultivation of rat primary cultured astrocytes. Astrocytes were cultured until passage 7 (P7) as late passage astrocytes and compared with passage 1 (P1) astrocytes as early passage astrocytes to confirm the differences in phenotypes and the effects of serial passage. In this study, we confirmed the morphological, molecular, and functional changes of late passage astrocytes showing aging phenotypes through SA-β-gal staining and measurement of nuclear size. We also observed a reduced expression of inflammatory mediators including IL-1β, IL-6, TNFα, iNOS, and COX2, as well as dysregulation of wound-healing, phagocytosis, and mitochondrial functions such as mitochondrial membrane potential and mitochondrial oxygen consumption rate. Culture-conditioned media obtained from P1 astrocytes promoted neurite outgrowth in immature primary cultures of rat cortices, which is significantly reduced when we treated the immature neurons with the culture media obtained from P7 astrocytes. These results suggest that late passage astrocytes show senescent astrocyte phenotypes with functional defects, which makes it a suitable model for the study of the role of astrocyte senescence on the modulation of normal and pathological brain aging.
Keywords: Astrocytes, Late passage cultivation, Cellular senescence, Neuro-inflammatory response, Phagocytosis, Wound healing
INTRODUCTION
Astrocytes are known to be the most abundant cells in the brain that play an important role in maintaining the brain environment. Astrocytes maintain the physical structure of the brain, the ion homeostasis, and the secretion of extracellular matrix proteins to support the maintenance of neuronal cells and the brain environment (Simard and Nedergaard, 2004; Burda et al., 2016). Astrocytes also secrete inflammatory mediators such as cytokines and chemokines to protect brain cells (Abbott, 2002). With these roles, any dysfunction of astrocytes directly or indirectly affects various neuronal cells and the brain environment (Seifert et al., 2006; Sidoryk-Wegrzynowicz et al., 2011; Scuderi et al., 2013; Phatnani and Maniatis, 2015; Acosta et al., 2017; Dossi et al., 2018).
Astrocytes in aged subjects show diverse dysfunction and altered phenotypes. In the brains of the elderly with Alzheimer’s disease, there are increased expressions of aged astrocytes and changes in astrocyte morphology (Bhat et al., 2012). Aged astrocytes show flattened morphology and increase the number of senescence-associated β-galactosidase-positive cells (SA-β-gal-positive cells) and senescence-associated secretory phenotypes (SASPs) (Enokido et al., 2008; Campuzano et al., 2009; Campisi et al., 2011; Salminen et al., 2011; Bhat et al., 2012; Yoon et al., 2016; Yu et al., 2017; Hou et al., 2018). The roles of astrocytes in the aging process of the brain are important, but studies relating to these roles are a few and are only simple observations.
The concept of serial passage cultivation was first described in a study back in 1961 using human diploid cells (Hayflick and Moorhead, 1961). Primary cells as well as stem cells can undergo successive passages. Interestingly, aging-related phenotypes have been observed during late passages of primary cells. For instance, late passage-cultivated mesenchymal stem cells (MSCs) displayed up-regulated SA-β-gal activity and reduced cell migration ability at (Hong et al., 2019) and further induced p21 and c-Myc expressions (Lian et al., 2016) as well as an increased number of S phase cells at passage 20 (Izadpanah et al., 2008). Human fibroblasts also show cellular senescence phenotypes at more than 45-fold population doublings (Gerland et al., 2003).
The aging of astrocytes or brain cells can be caused by various factors such as oxidative stress, shortened or dysfunctional telomeres, DNA damage, mitochondrial dysfunction, cellular senescence, and oncogenic mutations which could lead to diverse results (Munoz-Najar and Sedivy, 2011; Zhu et al., 2011). However, any suitable model for each cause of aging in astrocytes is not yet established. In this study, we aimed to set up an aged astrocyte model and compare the phenotypes of this model in line with known markers or effects of aging in astrocytes. Accordingly, we induced an in vitro senescent cell model through late passage cultivation. The changed phenotypes of astrocytes by late passage cultivation may provide essential clues for brain aging study.
MATERIALS AND METHODS
Materials
The materials used in this study are the following: Dulbecco’s modified Eagle medium (DMEM)/F12, Penicillin-Streptomycin (P/S), 0.25% trypsin-EDTA, and 10% Fetal Bovine Serum (FBS) from Gibco BRL (Grand Island, NY, USA); Tween® 20 and ECLTM Western blotting detection reagent from Amersham Life Science (Arlington Heights, IL, USA); anti-β Actin from Sigma (St. Louis, MO, USA); anti-iNOS and senescence detection kit from Abcam (Cambridge, UK); Agilent Seahorse XF Cell Mito Test Kit from Agilent Technologies (CA, USA); Alexa Fluor® 594 conjugated Escherichia coli (K-12 strain) BioParticles® from Thermo Fisher Scientific (MA, USA); Tetramethylrhodamine Methyl Ester (TMRM) from Thermo Fisher Scientific.
Rat primary cortical astrocyte culture
Animal care and experimental procedures were executed following the protocols and approved by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University (Seoul, Korea) (KU18050). Sprague-Dawley (SD) rats were obtained from Samtako, Inc (Gyeonggi, Korea). Astrocytes were cultured in the brain cortex of postnatal day 2 (P2) SD rats as described previously (Bang et al., 2019b). Briefly, brain cortices were isolated and mechanically triturated to make single cells. triturated single cells were seeded on the poly-D-lysine-coated plate (20 μg/mL) and incubated in DMEM/F12 with 100 U/mL of penicillin, 100 mg/mL of streptomycin, and 10% heat-inactivated FBS in a 95% CO2 incubator at 37°C. The culture medium was changed every 4 days. After 2 weeks, the incubated astrocytes were rinsed twice with serum-free medium and separated from the poly-D-lysine-coated plate by 0.25% trypsin-EDTA. The isolated cells were seeded in the poly-D-lysine-coated well plates. The sub-cultured astrocyte purity was more than 95% based on a positive glial fibrillary acidic protein (GFAP) marker. The first sub-cultured astrocytes were used as passage 1 (P1) and were incubated until confluent growth. After then, P1 astrocytes were sub-cultured into 2.5×105 cells/mL to make the second passage (P2). These processes were repeated until passage 7 (P7) to make the late passage astrocytes.
Rat primary cortical neuron culture
Sprague-Dawley (SD) rats were obtained from ORIENT (Gyeonggi, Korea). The neurons were isolated from the cerebral cortex of embryonic day 18 (E18) SD rats. The isolated primary cortical neurons were seeded on the poly-D-lysine-coated plate (50 μg/mL) and maintained in NBM with B27 and L-glutamine in a 95% CO2 incubator at 37°C for 10 days and the media were half-changed with fresh ones every 3 days.
Senescence-associated-β-galactosidase (SA-β-gal) staining
SA-β-gal staining was performed using Senescence Detection Kit (Abcam) to detect SA-β-gal positive cells in vitro. The experimental procedures were conducted following the manufacturer’s instructions (Dimri et al., 1995). Astrocytes were seeded on 12-well plates at 2.5×105 cells. After 4 days, the SA-β-gal-positive cells were observed with bright-field microscopy. To quantify the senescent cells, the number of SA-β-gal-positive cells were counted in the captured images.
Nuclear staining
The grown cells on a poly-D-lysine-coated coverslip were fixed by 4% paraformaldehyde (PFA) for 10 min at 37°C. After then, the cells were permeabilized with 0.1% Triton X-100 for 20 min and washed 3 times with PBS at room temperature. The fixed samples were stained for 10 min at room temperature with DAPI (4′,6-diamidino-2-phenylindole). After then, the samples are mounted and visualized by a digital microscope (CELENA, Logos Biosystems, Gyeonggi, Korea). To measure the size of the nucleus, the indicating blue color pixels in the captured images by the digital microscope were measured using the Image-J software (NIH, MD, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
The expressions of mRNAs in astrocytes such as IL-1β, IL-6, TNFα, iNOS, COX2, and GAPDH were measured using RT-PCR. The RNA was isolated with TRIzol reagent (Invitrogen) and the RNA concentration was measured using a spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). cDNA was synthesized using RNA and RT reaction mixture with RevertAid Reverse transcriptase reaction buffer (Thermo Fisher Scientific) and dNTP (Promega, WI, USA). A total of 0.5 µg of cDNA was used for PCR amplification under the following cycle parameters: [94°C, 30 s; 60°C, 1 min; 72°C, 30 s]×30 cycles, then 72°C for 10 min to detect IL-1β, IL-6, TNFα, iNOS and COX2; and [94°C, 30 s; 60°C, 1 min; 72°C, 30 s]×23 cycles, then 72°C for 10 min to detect GAPDH. The primers were designed as the following: IL-1β (sense: 5’-AAA ATG CCT CGT GCT GTC TG-3’/ antisense: 5’-CTA TGT CCC GAC CAT TGC TG-3’), IL-6 (sense: 5’-TTG TGC AAT GGC AAT TCT GA-3’/ antisense: 5’-TGG AAG TTG GGG TAG GAA GG-3’), TNFα (sense: 5’-TAG CCC ACG TCG TAG CAA AC-3’/ antisense: 5’- GGA GGC TGA CTT TCT CCT GG-3’), iNOS (sense: 5’-CTG GCT GCC TTG TTC AGC TA-3’/ antisense: 5’-AGT GTA GCG TTT CGG GAT CT-3’), COX2 (sense: 5’-TGC ATG TGG CTG TGGATG TCA TCA A-3’/ antisense: 5’-CAC TAA GAC AGA CCC GTC ATC TCC A-3’), and GAPDH (sense: 5’-GTG AAG GTC GGT GTG AAC GGA TTT-3’/ antisense: 5’-CAC AGT CTT CTG AGT GGC AGT GAT-3’). The PCR products were electrophoresed with 1.2% agarose gel and imaged with ethidium bromide (Sigma). The bands were measured using the Image-J software (NIH). Each band intensity was normalized by GAPDH mRNA.
Western blot analysis
Western blot was analyzed following a previously published protocol (Bang et al., 2019b). Briefly, the cells were harvested with radioimmunoprecipitation assay (RIPA) buffer consisting of 2 mM EDTA, 0.1% (w/v) SDS, 50 mM Tris-HCl, 150 mM sodium chloride, 1% Triton X-100, and 1% (w/v) sodium deoxycholate. The separated proteins were quantified by BCA assay kit (Thermo Fisher Scientific) and boiled for 5 min at 100°C. The SDS-PAGE was performed for 120 min at 100 V. The electrophoresed proteins were transferred to nitrocellulose membranes for 90 min and were blocked with 1 μg/mL polyvinyl alcohol for 5 min at room temperature. After then, samples were washed with Tris-buffered saline and 0.1% Tween 20 (TBS-T). The prepared proteins were incubated with primary antibodies for 16 h at 4°C. The samples were washed and maintained with horseradish peroxidase-conjugated secondary antibody (Life Technologies, Carlsbad, CA, USA) at room temperature for 60 min. The protein blots were detected by a chemiluminescence detection system (Amersham Life Science) and quantified using Image-J software (NIH). β-Actin was used as the loading control.
Wound closure assay
The cells were seeded on poly-D-lysine-coated 96-well plates at a density of 2.5×105 cells/mL and maintained for 4 days. After then, a 700 nm-wide scratch was made in each well using a certified Essen Bioscience automated 96-wound-makerTM (Essen Bioscience, MI, USA). Wound width was detected using the IncuCyte ZOOM system (Essen Bioscience) by imaging each well every 3 h for 72 h and were analyzed using the IncuCyte ZOOM microscope software 2015A (Essen Bioscience).
NO assay
NO was detected as described previously by measuring the nitrite, a stable oxidation product of NO (Green et al., 1990). In brief, Griess reagent was prepared by mixing equal volumes of 0.1% napthylethylenediamine dihydrochloride and 1% sulfanilamide in 5% phosphoric acid. This reagent was added to the supernatant of cell grown medium for 10 min and was measured the absorbance at 550 nm using a UV spectrophotometer (DU-650, Beckman Coulter, CA, USA). Sodium nitrite was used to generate a standard curve.
Mitochondrial oxygen consumption rate (OCR)
The mitochondrial OCR of cells was measured following the Agilent Seahorse XF Cell Mito Test Kit user guide (Agilent Technologies). Briefly, the cartridges were hydrated overnight with a calibrant buffer in a non-CO2 incubator at 37°C. Either of the 1.0 μM oligomycin, 1.0 μM FCCP, or 0.5 μM rotenone/0.5 μM antimycin A were put in the cartridge of each port A to C, respectively. The cell medium was changed to Seahorse XF Base Medium containing 1 mM pyruvate, 2 mM glutamine, and 10 mM glucose and then maintained for 45 min to 1 h. The assay was performed using Agilent Seahorse XFe96 Analyzer (Agilent Technologies). The results were obtained using Wave Desktop 2.6 software (Agilent Technologies) and calculated using the Agilent Seahorse XF Cell Mito Test Kit user guide (Agilent Technologies).
Phagocytosis
Alexa FluorTM 594 conjugated Escherichia coli (K-12 strain) BioParticlesTM were used for detecting phagocytosis activity in astrocytes. The cells were maintained with E. coli BioParticles for 12 h in a 95% CO2 incubator at 37°C. We detected the red fluorescence using the IncuCyte ZOOM system (Essen Bioscience) by imaging each well every 30 min for 12 h and analyzed the red particles using the IncuCyte ZOOM microscope software 2015A (Essen Bioscience).
Determination of mitochondrial membrane potential
TMRM is a dye that penetrates the cells and accumulates in the mitochondria which have active membrane potentials. The cells were maintained in light protection conditions with 100 nM TMRM for 30 min in a 95% CO2 incubator at 37°C. After then, the cells were washed with phosphate-buffered saline (PBS) and visualized by a digital microscope (CELENA, Logos Biosystems). The red particles of the captured images were analyzed using the IncuCyte ZOOM microscope software 2015A (Essen Bioscience).
Measurement of neurite outgrowth
For the neurite outgrowth measurement, neurons were seeded on a PDL-coated well plate at 7×105 cells/mL in the culture media. The plated neurons were maintained for 2 days and were added with astrocyte cultured medium with 1/3 medium. Astrocyte cultured medium was obtained from astrocytes incubated for 24 h in serum-free conditions. We captured and measured the neurite outgrowth image using the IncuCyte® Live-Cell Analysis system by the NeuroTrack Scan Type (Essen Bioscience). We obtained the images every 12 h for 72 h.
Statistical analysis
All the experimental data were expressed as the mean ± SEM and the statistical analyses were conducted using GraphPad Prism version 5 software (GraphPad Software Inc., CA, USA). The group comparisons were performed using two-way ANOVA followed by Bonferroni’s post-test. Two-sample analyses were conducted with unpaired t-test. A p-value of <0.05 was considered significant.
RESULTS
Late passage astrocytes show phenotypes of morphologically aged cells
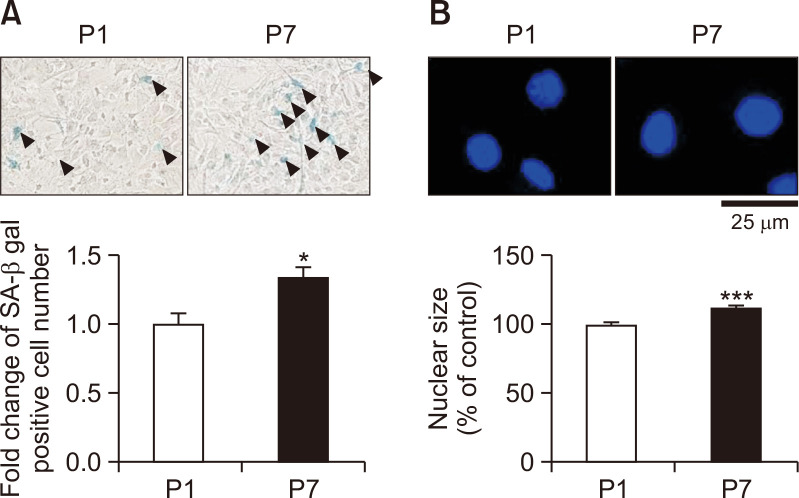
To investigate whether serial passage cultivation induces astrocyte senescence, passage 7 (P7) considered as late passage astrocytes were compared with passage 1 (P1) as early passage astrocytes. We performed SA-β-gal staining in P1 and P7 astrocytes to confirm the differences in phenotypes and the effects of serial passage. Late passage astrocytes showed increased SA-β-gal positive cell number about 1.3 times more than early passage astrocytes (Fig. 1A). The black arrows represent SA-β-gal-positive cells. The late passage astrocytes (P7) showed a significantly increased nuclear size of about 12% more than P1 (Fig. 1B). These results indicate that the P7 serial passage cultivated astrocytes exhibits the phenotypes of aged cells.
Fig. 1.
Late passage astrocytes induced SA-β-gal positive cell number and nuclear size. Astrocytes were sub-cultured either once (passage 1; P1) or 7 times (passage 7; P7). (A) Astrocytes were measured by the SA-β-gal staining. Stained astrocytes were indicated by black triangles as SA-β-gal-positive cells. The graph represents the fold change of SA-β-gal-positive cell number in astrocytes. (B) Astrocytes were stained to determine the nuclear size. The graph shows the nuclear size of P7 compared to P1. The bars indicate the mean ± SEM (n=3). *indicates p<0.05 and, ***indicates p<0.001 vs. P1 astrocytes.
Late passage astrocytes show decreased immune responses with LPS treatment
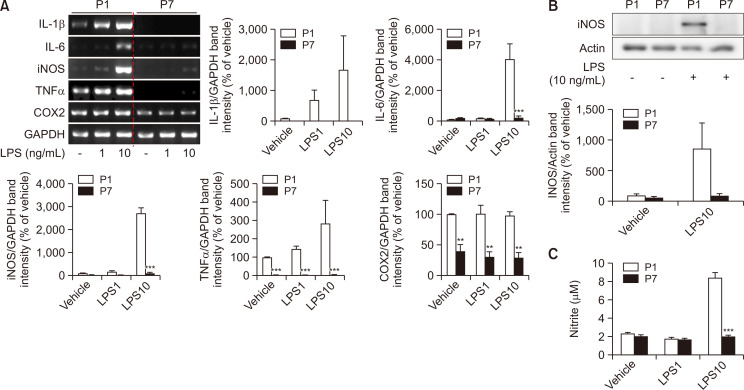
To evaluate whether the expressions of immune-related genes are changed in the late passage astrocytes, the mRNA expressions of IL-1β, IL-6, iNOS, TNFα, and COX2 using RT-PCR were measured with or without LPS. Astrocytes were treated with 0 (vehicle), 1, and 10 ng/mL of LPS for 24 h. The higher dose of LPS increased the mRNA expression of immune-related genes at least three times more in P1 astrocytes than the vehicle group including IL-1β, IL-6, iNOS and TNFα, but not COX2. Late passage astrocytes (P7 astrocytes) were detected with down-regulated mRNA expression of immune-related genes including IL-1β, IL-6, iNOS, TNFα, and COX2. Immune stimulation through LPS treatment did not have any change in the mRNA expression of the immune-related mediators in late passage astrocytes (Fig. 2A). The protein level of iNOS was also not affected by LPS treatment in late passage astrocytes (Fig. 2B). Additionally, the determination of nitrite in late passage astrocytes shows no difference in the non-stimulus group and LPS-stimulated group (Fig. 2C). These data demonstrate that late passage astrocytes may have reduced immune responses, even with LPS stimulation.
Fig. 2.
Late passage astrocytes show decreased immune-related genes and proteins, with no effect on nitrite secretion. (A) The expression level of immune-related genes was indicated by RT-PCR. The graphs show the mRNA expression. Values were normalized based on GAPDH. (B) The expression level of immune-related proteins was represented through Western blot. Densitometry analysis of the protein expression. The graphs show the protein expression levels and the values were normalized to the actin. (C) Production of nitrate was determined using ELISA in astrocytes. The bars display the mean ± SEM (n=3). **indicates p<0.01, and ***indicates p<0.001 vs. vehicle group.
Late passage astrocytes show decreased astrocytic functions
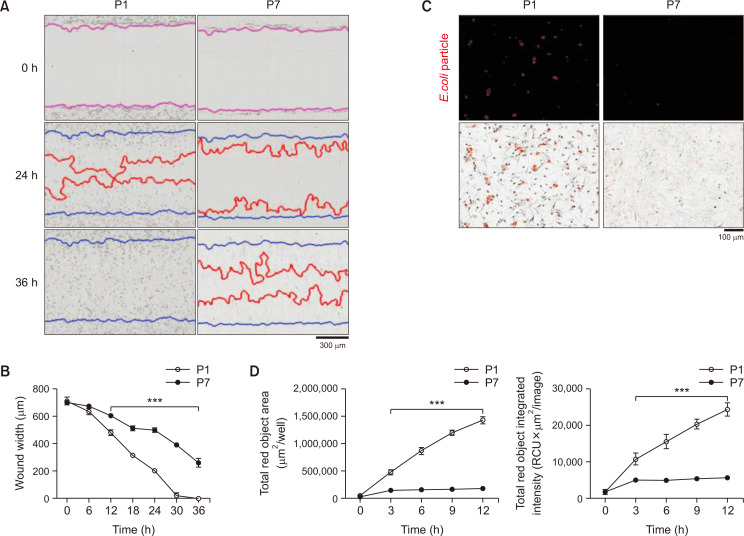
To investigate the change of astrocytic function in late passage astrocytes, we evaluated the wound healing ability and phagocytic capacity. The wound healing ability was measured using a wound-scratch assay in late passage astrocytes (P7) compared with early passage astrocytes (P1). Late passage astrocytes show a wider wound width and lower relative wound density than early passage astrocytes (P1) and 36 h after making the wound scratch, the wound of P1 was closed while the wound width of P7 was still about 260 μm (Fig. 3A, 3B). The wound healing ability was also significantly decreased in the late passage astrocytes. To confirm the phagocytotic capacity in late passage astrocytes, E. coli BioParticles were introduced. Early astrocytes show a brighter red fluorescence than late passage astrocytes in the cells over time. The total fluorescence objective area of P1 was about 8 times wider than P7 (Fig. 3C, 3D). The total fluorescence intensity and total fluorescence objective area were significantly decreased than early passage astrocytes as time goes on. These results indicate that late passage astrocytes have reduced functions such as wound healing ability and phagocytotic capacity.
Fig. 3.
Late passage astrocytes show decreased wound healing and phagocytosis ability. (A) Representative images of astrocytes were obtained through the wound-healing assay. Blue lines represent the initial scratch-wound area. Red lines indicate wound closure at each time-point. Images and data gathered using IncuCyte (Essen Bioscience). (B) Cell migration every 12 h into the wound area, represented by wound width (μm). (C) Astrocytes were examined for its phagocytosis ability using E. coli BioParticles. (D) The graphs represent the total red object area per well (μm2/well) and the total red object integrated intensity (RCU×μm2/image) every 30 min for 12 h. Lines show the mean ± SEM (n=3). ***indicates p<0.001 vs. vehicle group.
Late passage astrocytes show decreased mitochondrial functions
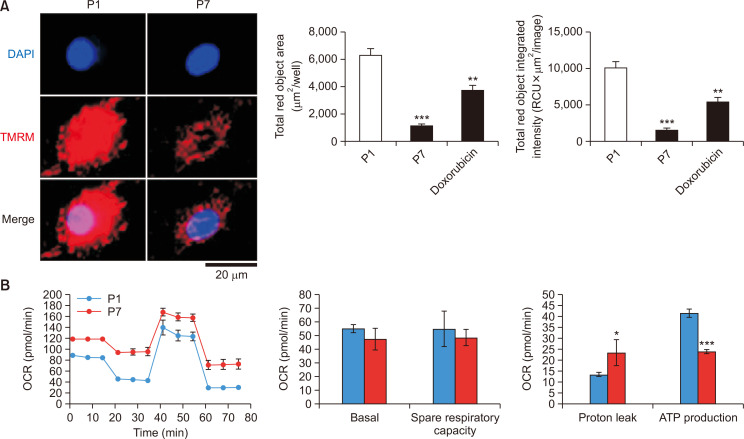
To investigate the mitochondrial function in late passage astrocytes, we measured the mitochondrial membrane potential through TMRM and the mitochondrial oxygen consumption rate (OCR) in the late passage astrocytes (P7) and early passage astrocytes (P1). Late passage astrocytes (P7) showed decreased TMRM objective area and total TMRM intensity about 80% less than early passage astrocytes (P1) (Fig. 4A). Doxorubicin was used as a positive control. Mitochondrial OCR was experimented using Agilent Seahorse XFe96 Analyzer (Agilent Technologies) showing that late passage astrocytes have reduced ATP production (Fig. 4B). These results show that late passage astrocytes have decreased mitochondrial function, which indicates reduced energy metabolism.
Fig. 4.
Late passage astrocytes show reduced mitochondrial membrane potential and ATP production. (A) Astrocytes were conducted for mitochondrial membrane potential by fluorescence staining of TMRM. Representative images were obtained by the IncuCyte ZOOM microscope software 2015A (Essen Bioscience). The data were measured by IncuCyte software (Essen Bioscience). The representative graph shows the total TMRM object integrated intensity (RCU x μm2/image) and the total TMRM object area (μm2/image). Bars represent the mean ± SEM (n=3). **indicates p<0.01, and ***indicates p<0.001 vs. vehicle group. (B) The mitochondrial OCR of astrocytes was experimented using Cell Mito Test Kit and Agilent Seahorse XFe96 Analyzer (Agilent Technologies). The representative graphs were visualized using Wave Desktop 2.6 software (Agilent Technologies). The graphs represent the basal level, spare respiratory capacity, proton leak, and ATP production level. OCRs are mean ± SEM (n=3), normalized to the third measurement point and expressed as % baseline OCR. *indicates p<0.05 and, ***indicates p<0.001 vs. P1 astrocytes.
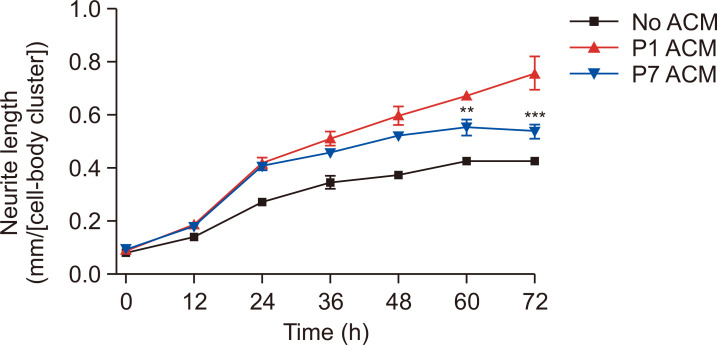
Late passage astrocytes affected the neurite outgrowth
The results above demonstrated that late passage astrocytes have decreased astrocytic functions (Fig. 3, 4). To determine the effects of late passage astrocytes on neurons, astrocyte conditioned media (ACM) was added on neurons with 1/3 medium. The ACM was harvested in serum-free conditions. The primary cortical neurons in DIV2 were treated with ACM, and the neurite outgrowth was measured every 12 h for 72 h. P1 ACM-treated neurons have increased neurite outgrowth compared with vehicle-treated neurons (no ACM). The late passage (P7) astrocyte conditioned media (P7 ACM)-treated neurons presented a down-regulated neurite outgrowth of about 30% shorter neurite in comparison with early passage (P1) ACM-treated neurons (Fig. 5). These results demonstrate that late passage astrocytes have lesser supportive functions on neurons than P1.
Fig. 5.
Late passage astrocytes affected the neurite outgrowth through ACM. ACM was harvested and treated on neurons to measure the effects on neuronal functions. The neurite length of ACM-treated neurons was calculated through IncuCyte at each time-point. Values are shown as the mean ± SEM (n=3). **indicates p<0.01, and ***indicates p<0.001 vs. P1 ACM group. Abbreviations: passage1, P1; passage7, P7; astrocyte conditioned medium, ACM.
DISCUSSION
Astrocytes play important physical and molecular roles in the brain. Any disruption of their normal physiological function can lead to the pathology of CNS disorders. Therefore, the aging of astrocytes has the potential to affect the brain environment and function. As an important mechanism of aging, cellular senescence has been considered as an inducing factor of age-related neurodegenerative disorders. However, little is known about the importance or effect of astrocyte aging in the brain. Therefore, we attempted to make an in vitro aged astrocyte model to aid in the study of astrocytes during the aging process. Late passage cultivation is a method that is used in both stem cells and primary cells. Several studies showed that late passage can induce cellular senescence. Cultured stem cells undergo senescence by late passage, whereby the cells expand and eventually stop proliferation. Age-related gene expression in cultured cells of late passages is similar to the gene profiles of aging in human progenitor cells in vivo (Wagner et al., 2009). Evidence from late culture study suggested replicative senescence in astrocyte-like cells from normal CNS tissues but not from gliomas (Ponten and Macintyre, 1968). Similarly, a study using a technique called minicloning found that glial cells from late passages had a reduced capacity for population doublings, which may result in earlier cellular senescence (Blomquist et al., 1980). Subsequent studies suggested that a gradual and constant increase in non-dividing cells, just like the case in fibroblast (Hayflick and Moorhead, 1961), is responsible for the reduced proliferative capacity of late passage cells (Ponten et al., 1983), which is reminiscent of Shall and Stein model of cellular aging (Shall and Stein, 1979). Replicative senescence of astrocytes has also been demonstrated using primary cultures derived from normal or AD postmortem brain tissues (Evans et al., 2003; Blasko et al., 2004).
Astrocytes that underwent serial passage cultivation for several times showed aged astrocyte phenotypes. Various studies have reported nuclear enlargement of aged primary astrocytes (Yoon et al., 2016; Bang et al., 2019a). The increased nuclear or cell size in aged cells could be due to the increased macromolecule contents such as DNA, RNA, and proteins (De Cecco et al., 2011). Senescence-associated β-galactosidase (SA-β-gal) staining is a method most widely used as a biomarker for cellular senescence due to the overexpression and accumulation of the lysosomal β-galactosidase protein specifically in senescent cells (Lee et al., 2006). Late passage astrocytes manifest cellular senescence phenotypes such as increased nuclear size and SA-β-gal positive cell number.
We also examined the molecular and functional changes in late passage astrocytes. Astrocytes along with microglia are known to have immune-related functions in the brain. All neuroinflammatory and regulatory processes in the CNS are generally initiated to prevent the disruption of cellular homeostasis. The acute inflammatory response in the CNS induces the repair of damaged brain regions and is rapidly triggered by activated glial cells. However, sustained secretion of inflammatory mediators by dysregulated astrocytes can induce chronic inflammation which can lead to brain degeneration (Sochocka et al., 2017). All of these processes can contribute to prominent neurodegeneration and cognitive decline (Morales et al., 2014). SASPs are excessively increased by aging. Excessive SASP activity was suppressed by reducing the inflammatory cytokine levels (Bhaumik et al., 2009; Rodier and Campisi, 2011). Further research is needed to determine the mechanism of the reduction of cytokine by late passage cultivation in aged astrocytes. In addition, the decreased immune response of late passage cells by LPS may be induced by immunosenescence.
Immunosenescence, a term first introduced by Walford in 1969, is characterized by a quantitative reduction in the adequate immune responses, a process that decreases responsiveness and increases vulnerability to extrinsic factors such as bacterial, viral, and fungal pathogens (Rosenstiel et al., 2008; Boraschi and Italiani, 2014; Fulop et al., 2014; Poland et al., 2014; Montgomery and Shaw, 2015). Aging is associated with declined innate and adaptive immunity (Lutz and Quinn, 2012; Wong and Goldstein, 2013; Golomb et al., 2015). Several papers reported the down-regulation of toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) during the aging process (Rosenstiel et al., 2008). Taken together, aging can suppress the response to the presence of bacterial invasion which shows a similar response in the induction of LPS. Down-regulated TLRs also affect the reduced susceptibility to inflammation. Most immunosenescence studies are focused on the peripheral nervous system (PNS), therefore, studies on the central nervous system (CNS) are also needed.
In addition to the morphological and molecular features of aged astrocytes, we investigated the role of late passages on brain function including wound healing, phagocytosis, mitochondrial energy metabolism, and neurite outgrowth. Our results showed that late passage cultivated astrocytes exhibited functional changes manifested by decreased wound healing capacity. These results are consistent with our previous reports showing the decreased wound healing capacity in aging-induced astrocytes through tenovin-1 or etoposide treatment (Bang et al., 2019a, 2019b). Old satellite cells and tenocytes show impaired migration in vitro similar to aged bone marrow-derived dendritic cells (Grolleau-Julius et al., 2008; Chang et al., 2012; Collins-Hooper et al., 2012). The regulatory mechanisms of astrocyte migration could be associated with matrix metalloproteinase (MMP) such as MMP2 and MMP9 in brain injury states, although the specific mechanisms are not yet clear (Hsu et al., 2008). For instance, uveal melanoma cell lines such as 92.1 and Mel 270 show reduced wound healing capacity by down-regulated MMP2 and MMP9 (Dai et al., 2016; McHugh and Gil, 2018). Astrocytes move rapidly during injury and form glial scars, thus, astrocytic migration ability is important. The astrocyte migration during aging is attenuated by downregulating the growth factors such as EGF and VEGF which is associated with astrocyte migration (Wittko et al., 2009; Li et al., 2014; Moraga et al., 2015). In addition, the activation of the P2X7 receptor increases the migration of glial cells, while P2X7 receptor deficiency inhibits immune cell migration such as microglia (Murphy and Lynch, 2012; Nadal-Nicolas et al., 2016). Several proteins are regulating the astrocyte migration including Integrin, Syndecan-4 proteoglycan, P2X7, and Pannexin1 (Caldeira et al., 2014). Further studies are needed on the mechanism of astrocyte migration and its functional role.
Astrocytes show a reduced phagocytic capacity during reactive astrogliosis, while the microglia have increased phagocytosis following various stimuli and aging (Jung and Chung, 2018). Our results demonstrated that aged astrocytes through late passage cultivation have decreased phagocytotic capacity. There are some regulatory factors related to phagocytosis in astrocytes. Drosophila glial cells in aged brains have decreased phagocytosis through a decreased translation regulator, draper, a homolog of Meg10 for phagocytosing synapses in astrocytes. Also, A1 reactive astrocytes have decreased phagocytic ability capacity by decreasing the mRNA expression of Mertk and Megf10 (Liddelow et al., 2017). Therefore, these results indicate that late passage cultivation can induce aging in primary cultured astrocytes as manifested by decreased phagocytotic capacity. The regulatory factors of phagocytosis in aged astrocytes by late passage cultivation may be an interesting area for future investigation.
Glial cells including astrocytes and microglia play a central role in Aβ regulation and clearance (Ries and Sastre, 2016). Microglia and astrocytes can generate Aβ degrading proteases such as neprilysin (NEP), endothelin-converting enzyme (ECE), cathepsin B (CAT-B), and matrix metalloproteases (MMPs) (Eckman et al., 2001, 2003; Yan et al., 2006; Yin et al., 2006; Halle et al., 2008). Senescent astrocytes have reduced physiological functions and increased SASPs, which play a role in Aβ accumulation, tau hyperphosphorylation, and the deposition of NFTs (neurofibrillary tangles) in AD. Receptors such as receptor for advanced glycation end products (RAGE), triggering receptor expressed on myeloid cells 2 (TREM-2), scavenger receptors (SR), and formyl peptide receptors (FPR) play roles in the uptake and clearance of Aβ and are located on the surface of glial cells. Astrocytes are also contacted with blood vessels, which support the excretion of Aβ from the brain. Therefore, aged astrocytes lead to blood-brain barrier (BBB) disruption, and have a putative role in the pathologic progress of AD regarding astrocytes senescence. Astrocytes and microglia play an essential role in the phagocytosis of Aβ through numerous receptors on the surface (Ries and Sastre, 2016). Likewise, the dysfunction of phagocytosis in aged glial cells may have increased relevance to neurodegenerative diseases via the accumulation of Aβ, debris, or misfolded proteins. In the therapeutics development for age-related neurodegenerative disorders such as AD, targeting astrocyte senescence by late passage cultivation can be a feasible approach.
Mitochondrial dysfunction of aged astrocytes was confirmed through mitochondrial membrane potential and mitochondrial OCR. Aged astrocytes by replicative senescence showed glutamate uptake and decreased mitochondrial activity (Pertusa et al., 2007). The impaired mitochondrial function of astrocytes may lead to abnormalities in maintaining the extracellular environment, which is expected to affect neurons directly or indirectly. A previous study also reported that senescent astrocytes by in vitro culturing for 90 days showed decreased mitochondrial activity (Pertusa et al., 2007). Astrocytes release proteins such as growth factors, ECM, cytokines, and chemokines, which affect neuroprotection, synapse formation, and neuronal growth. There are several factors related to mitochondrial defects in aged astrocyte. IGF-1 receptor (IGFR) expression is attenuated in aging with altered mitochondrial structure and function (Sadaba et al., 2016; Logan et al., 2018). In addition, HO-1 upregulation in glia can lead to mitochondrial dysfunction in brain aging and age-related neurodegenerative disorders such as MCI (mild cognitive impairment) (Pertusa et al., 2007). Overexpression of HO-1 in rat astrocytes in MCI promotes mitochondrial iron sequestration and free radical-induced MnSOD expression that lead to oxidative damage of mitochondrial protein and lipid, which result in cellular toxicity (Schipper et al., 1999; Frankel et al., 2000). Our data demonstrate that aged astrocytes by late passage cultivation exhibit alterations of mitochondrial activity. The detrimental role of mitochondrial activity and protein oxidation in aged astrocytes will be our further study.
Although there is no direct study on the effects of aged astrocytes on neurons, one study isolated astrocytes and neurons from old rats (24 months) and co-cultured both for 5 days. Astrocytes from old rats supported the neurite outgrowth less than astrocytes from young rats (3 months) (Rozovsky et al., 2005). These data suggest that aged astrocytes can negatively affect neurons. Conditioned media from aged astrocytes reduced the proliferation of neural progenitor cells (NPC) via decreasing the Wnt signal in aged astrocytes and anti-apoptotic protein survival in NPCs (Miranda et al., 2012). These results suggest that astrocyte senescence could affect neuron survival and neurogenesis during aging. Taken together, we suggest that late passage cultivation can be used as an in vitro aged astrocyte model which exhibited morphological, molecular, and functional characteristics of well-known senescent cells in comparison to P1 astrocytes. Also, additional phagocytic and mitochondrial function studies confirmed that this model exhibits mitochondrial dysfunction, and can affect neurons in the neurite outgrowth process. The results could be used for the study of brain aging and age-related neurodegenerative diseases. Aged astrocyte research usually consists of simple observational experiments and further in-depth studies on this area are needed. Our studies could become the cornerstone in the understanding of neuroinflammation by aging.
ACKNOWLEDGMENTS
This paper was supported by Konkuk University in 2020.
Footnotes
CONFLICT OF INTEREST
There are no conflict of interest.
REFERENCES
- Abbott N. J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta C., Anderson H. D., Anderson C. M. Astrocyte dysfunction in Alzheimer disease. J. Neurosci. Res. 2017;95:2430–2447. doi: 10.1002/jnr.24075. [DOI] [PubMed] [Google Scholar]
- Bang M., Kim D. G., Gonzales E. L., Kwon K. J., Shin C. Y. Etoposide induces mitochondrial dysfunction and cellular senescence in primary cultured rat astrocytes. Biomol. Ther. (Seoul) 2019a;27:530–539. doi: 10.4062/biomolther.2019.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M., Ryu O., Kim D. G., Mabunga D. F., Cho K. S., Kim Y., Han S. H., Kwon K. J., Shin C. Y. Tenovin-1 induces senescence and decreases wound-healing activity in cultured rat primary astrocytes. Biomol. Ther. (Seoul) 2019b;27:283–289. doi: 10.4062/biomolther.2018.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R., Crowe E. P., Bitto A., Moh M., Katsetos C. D., Garcia F. U., Johnson F. B., Trojanowski J. Q., Sell C., Torres C. Astrocyte senescence as a component of Alzheimer's disease. PLoS ONE. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik D., Scott G. K., Schokrpur S., Patil C. K., Orjalo A. V., Rodier F., Lithgow G. J., Campisi J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany N.Y.) 2009;1:402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I., Stampfer-Kountchev M., Robatscher P., Veerhuis R., Eikelenboom P., Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3:169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Blomquist E., Westermark B., Ponten J. Ageing of human glial cells in culture: increase in the fraction of non-dividers as demonstrated by a minicloning technique. Mech. Ageing Dev. 1980;12:173–182. doi: 10.1016/0047-6374(80)90093-7. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol. Lett. 2014;162:346–353. doi: 10.1016/j.imlet.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Burda J. E., Bernstein A. M., Sofroniew M. V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016;275 Pt 3:305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira C., Oliveira A. F., Cunha C., Vaz A. R., Falcao A. S., Fernandes A., Brites D. Microglia change from a reactive to an age-like phenotype with the time in culture. Front. Cell. Neurosci. 2014;8:152. doi: 10.3389/fncel.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Andersen J. K., Kapahi P., Melov S. Cellular senescence: a link between cancer and age-related degenerative disease? Semin. Cancer Biol. 2011;21:354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano O., Castillo-Ruiz M. M., Acarin L., Castellano B., Gonzalez B. Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J. Neurosci. Res. 2009;87:2484–2497. doi: 10.1002/jnr.22074. [DOI] [PubMed] [Google Scholar]
- Chang H. N., Pang J. H., Chen C. P., Ko P. C., Lin M. S., Tsai W. C., Yang Y. M. The effect of aging on migration, proliferation, and collagen expression of tenocytes in response to ciprofloxacin. J. Orthop. Res. 2012;30:764–768. doi: 10.1002/jor.21576. [DOI] [PubMed] [Google Scholar]
- Collins-Hooper H., Woolley T. E., Dyson L., Patel A., Potter P., Baker R. E., Gaffney E. A., Maini P. K., Dash P. R., Patel K. Age-related changes in speed and mechanism of adult skeletal muscle stem cell migration. Stem Cells. 2012;30:1182–1195. doi: 10.1002/stem.1088. [DOI] [PubMed] [Google Scholar]
- Dai W., Zhou J., Jin B., Pan J. Class III-specific HDAC inhibitor Tenovin-6 induces apoptosis, suppresses migration and eliminates cancer stem cells in uveal melanoma. Sci. Rep. 2016;6:22622. doi: 10.1038/srep22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cecco M., Jeyapalan J., Zhao X., Tamamori-Adachi M., Sedivy J. M. Nuclear protein accumulation in cellular senescence and organismal aging revealed with a novel single-cell resolution fluorescence microscopy assay. Aging (Albany N.Y.) 2011;3:955–967. doi: 10.18632/aging.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G. P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E. E., Linskens M., Rubelj I., Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossi E., Vasile F., Rouach N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018;136:139–156. doi: 10.1016/j.brainresbull.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman E. A., Reed D. K., Eckman C. B. Degradation of the Alzheimer's amyloid beta peptide by endothelin-converting enzyme. J. Biol. Chem. 2001;276:24540–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- Eckman E. A., Watson M., Marlow L., Sambamurti K., Eckman C. B. Alzheimer's disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J. Biol. Chem. 2003;278:2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Enokido Y., Yoshitake A., Ito H., Okazawa H. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem. Biophys. Res. Commun. 2008;376:128–133. doi: 10.1016/j.bbrc.2008.08.108. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Wyllie F. S., Wynford-Thomas D., Kipling D., Jones C. J. A P53-dependent, telomere-independent proliferative life span barrier in human astrocytes consistent with the molecular genetics of glioma development. Cancer Res. 2003;63:4854–4861. [PubMed] [Google Scholar]
- Frankel D., Mehindate K., Schipper H. M. Role of heme oxygenase-1 in the regulation of manganese superoxide dismutase gene expression in oxidatively-challenged astroglia. J. Cell. Physiol. 2000;185:80–86. doi: 10.1002/1097-4652(200010)185:1<80::AID-JCP7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Fulop T., Le Page A., Fortin C., Witkowski J. M., Dupuis G., Larbi A. Cellular signaling in the aging immune system. Curr. Opin. Immunol. 2014;29:105–111. doi: 10.1016/j.coi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Gerland L. M., Peyrol S., Lallemand C., Branche R., Magaud J. P., Ffrench M. Association of increased autophagic inclusions labeled for beta-galactosidase with fibroblastic aging. Exp. Gerontol. 2003;38:887–895. doi: 10.1016/S0531-5565(03)00132-3. [DOI] [PubMed] [Google Scholar]
- Golomb L., Sagiv A., Pateras I. S., Maly A., Krizhanovsky V., Gorgoulis V. G., Oren M., Ben-Yehuda A. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death Differ. 2015;22:1764–1774. doi: 10.1038/cdd.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Mellouk S., Hoffman S. L., Meltzer M. S., Nacy C. A. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol. Lett. 1990;25:15–19. doi: 10.1016/0165-2478(90)90083-3. [DOI] [PubMed] [Google Scholar]
- Grolleau-Julius A., Harning E. K., Abernathy L. M., Yung R. L. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68:6341–6349. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L., Moorhead P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hong S. H., Lee M. H., Koo M. A., Seon G. M., Park Y. J., Kim D., Park J. C. Stem cell passage affects directional migration of stem cells in electrotaxis. Stem Cell Res. 2019;38:101475. doi: 10.1016/j.scr.2019.101475. [DOI] [PubMed] [Google Scholar]
- Hou J., Cui C., Kim S., Sung C., Choi C. Ginsenoside F1 suppresses astrocytic senescence-associated secretory phenotype. Chem. Biol. Interact. 2018;283:75–83. doi: 10.1016/j.cbi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Hsu J. Y., Bourguignon L. Y., Adams C. M., Peyrollier K., Zhang H., Fandel T., Cun C. L., Werb Z., Noble-Haeusslein L. J. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J. Neurosci. 2008;28:13467–13477. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadpanah R., Kaushal D., Kriedt C., Tsien F., Patel B., Dufour J., Bunnell B. A. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–4238. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y. J., Chung W. S. Phagocytic roles of glial cells in healthy and diseased brains. Biomol. Ther. (Seoul) 2018;26:350–357. doi: 10.4062/biomolther.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. Y., Han J. A., Im J. S., Morrone A., Johung K., Goodwin E. C., Kleijer W. J., DiMaio D., Hwang E. S. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Li Y. N., Pan R., Qin X. J., Yang W. L., Qi Z., Liu W., Liu K. J. Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression. J. Neurochem. 2014;129:120–129. doi: 10.1111/jnc.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J., Lv S., Liu C., Liu Y., Wang S., Guo X., Nan F., Yu H., He X., Sun G., Ma X. Effects of serial passage on the characteristics and cardiac and neural differentiation of human umbilical cord Wharton's jelly-derived mesenchymal stem cells. Stem Cells Int. 2016;2016:9291013. doi: 10.1155/2016/9291013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., Bennett M. L., Munch A. E., Chung W. S., Peterson T. C., Wilton D. K., Frouin A., Napier B. A., Panicker N., Kumar M., Buckwalter M. S., Rowitch D. H., Dawson V. L., Dawson T. M., Stevens B., Barres B. A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S., Pharaoh G. A., Marlin M. C., Masser D. R., Matsuzaki S., Wronowski B., Yeganeh A., Parks E. E., Premkumar P., Farley J. A., Owen D. B., Humphries K. M., Kinter M., Freeman W. M., Szweda L. I., Van Remmen H., Sonntag W. E. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol. Metab. 2018;9:141–155. doi: 10.1016/j.molmet.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C. T., Quinn L. S. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany N.Y.) 2012;4:535–546. doi: 10.18632/aging.100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D., Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 2018;217:65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda C. J., Braun L., Jiang Y., Hester M. E., Zhang L., Riolo M., Wang H., Rao M., Altura R. A., Kaspar B. K. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell. 2012;11:542–552. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R. R., Shaw A. C. Paradoxical changes in innate immunity in aging: recent progress and new directions. J. Leukoc. Biol. 2015;98:937–943. doi: 10.1189/jlb.5MR0315-104R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga A., Pradillo J. M., Garcia-Culebras A., Palma-Tortosa S., Ballesteros I., Hernandez-Jimenez M., Moro M. A., Lizasoain I. Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia. J. Neuroinflammation. 2015;12:87. doi: 10.1186/s12974-015-0314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales I., Guzman-Martinez L., Cerda-Troncoso C., Farias G. A., Maccioni R. B. Neuroinflammation in the pathogenesis of Alzheimer's disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Najar U., Sedivy J. M. Epigenetic control of aging. Antioxid. Redox Signal. 2011;14:241–259. doi: 10.1089/ars.2010.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N., Lynch M. A. Activation of the P2X(7) receptor induces migration of glial cells by inducing cathepsin B degradation of tissue inhibitor of metalloproteinase 1. J. Neurochem. 2012;123:761–770. doi: 10.1111/jnc.12031. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolas F. M., Galindo-Romero C., Valiente-Soriano F. J., Barbera-Cremades M., deTorre-Minguela C., Salinas-Navarro M., Pelegrin P., Agudo-Barriuso M. Involvement of P2X7 receptor in neuronal degeneration triggered by traumatic injury. Sci. Rep. 2016;6:38499. doi: 10.1038/srep38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertusa M., Garcia-Matas S., Rodriguez-Farre E., Sanfeliu C., Cristofol R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J. Neurochem. 2007;101:794–805. doi: 10.1111/j.1471-4159.2006.04369.x. [DOI] [PubMed] [Google Scholar]
- Phatnani H., Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2015;7:a020628. doi: 10.1101/cshperspect.a020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland G. A., Ovsyannikova I. G., Kennedy R. B., Lambert N. D., Kirkland J. L. A systems biology approach to the effect of aging, immunosenescence and vaccine response. Curr. Opin. Immunol. 2014;29:62–68. doi: 10.1016/j.coi.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten J., Macintyre E. H. Long term culture of normal and neoplastic human glia. Acta Pathol. Microbiol. Scand. 1968;74:465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Ponten J., Stein W. D., Shall S. A quantitative analysis of the aging of human glial cells in culture. J. Cell. Physiol. 1983;117:342–352. doi: 10.1002/jcp.1041170309. [DOI] [PubMed] [Google Scholar]
- Ries M., Sastre M. Mechanisms of Abeta clearance and degradation by glial cells. Front. Aging Neurosci. 2016;8:160. doi: 10.3389/fnagi.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F., Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel P., Derer S., Till A., Hasler R., Eberstein H., Bewig B., Nikolaus S., Nebel A., Schreiber S. Systematic expression profiling of innate immune genes defines a complex pattern of immunosenescence in peripheral and intestinal leukocytes. Genes Immun. 2008;9:103–114. doi: 10.1038/sj.gene.6364454. [DOI] [PubMed] [Google Scholar]
- Rozovsky I., Wei M., Morgan T. E., Finch C. E. Reversible age impairments in neurite outgrowth by manipulations of astrocytic GFAP. Neurobiol. Aging. 2005;26:705–715. doi: 10.1016/j.neurobiolaging.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Sadaba M. C., Martin-Estal I., Puche J. E., Castilla-Cortazar I. Insulin-like growth factor 1 (IGF-1) therapy: mitochondrial dysfunction and diseases. Biochim. Biophys. Acta. 2016;1862:1267–1278. doi: 10.1016/j.bbadis.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Salminen A., Ojala J., Kaarniranta K., Haapasalo A., Hiltunen M., Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur. J. Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- Schipper H. M., Bernier L., Mehindate K., Frankel D. Mitochondrial iron sequestration in dopamine-challenged astroglia: role of heme oxygenase-1 and the permeability transition pore. J. Neurochem. 1999;72:1802–1811. doi: 10.1046/j.1471-4159.1999.0721802.x. [DOI] [PubMed] [Google Scholar]
- Scuderi C., Stecca C., Iacomino A., Steardo L. Role of astrocytes in major neurological disorders: the evidence and implications. IUBMB Life. 2013;65:957–961. doi: 10.1002/iub.1223. [DOI] [PubMed] [Google Scholar]
- Seifert G., Schilling K., Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat. Rev. Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Shall S., Stein W. D. A mortalization theory for the control of the cell proliferation and for the origin of immortal cell lines. J. Theor. Biol. 1979;76:219–231. doi: 10.1016/0022-5193(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M., Wegrzynowicz M., Lee E., Bowman A. B., Aschner M. Role of astrocytes in brain function and disease. Toxicol. Pathol. 2011;39:115–123. doi: 10.1177/0192623310385254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M., Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Sochocka M., Sobczynski M., Sender-Janeczek A., Zwolinska K., Blachowicz O., Tomczyk T., Zietek M., Leszek J. Association between periodontal health status and cognitive abilities. The role of cytokine profile and systemic inflammation. Curr. Alzheimer Res. 2017;14:978–990. doi: 10.2174/1567205014666170316163340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Bork S., Horn P., Krunic D., Walenda T., Diehlmann A., Benes V., Blake J., Huber F. X., Eckstein V., Boukamp P., Ho A. D. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittko I. M., Schanzer A., Kuzmichev A., Schneider F. T., Shibuya M., Raab S., Plate K. H. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J. Neurosci. 2009;29:8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Goldstein D. R. Impact of aging on antigen presentation cell function of dendritic cells. Curr. Opin. Immunol. 2013;25:535–541. doi: 10.1016/j.coi.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P., Hu X., Song H., Yin K., Bateman R. J., Cirrito J. R., Xiao Q., Hsu F. F., Turk J. W., Xu J., Hsu C. Y., Holtzman D. M., Lee J. M. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J. Biol. Chem. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- Yin K. J., Cirrito J. R., Yan P., Hu X., Xiao Q., Pan X., Bateman R., Song H., Hsu F. F., Turk J., Xu J., Hsu C. Y., Mills J. C., Holtzman D. M., Lee J. M. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. B., Park K. R., Kim S. Y., Han S. Y. Induction of nuclear enlargement and senescence by sirtuin inhibitors in glioblastoma cells. Immune. Netw. 2016;16:183–188. doi: 10.4110/in.2016.16.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Yi M., Wei T., Gao X., Chen H. KCa3.1 inhibition switches the astrocyte phenotype during astrogliosis associated with ischemic stroke via endoplasmic reticulum stress and MAPK signaling pathways. Front. Cell. Neurosci. 2017;11:319. doi: 10.3389/fncel.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Yu J., Shi Q., Lu W., Liu B., Xu S., Wang L., Han J., Wang X. Strain- and age-related alteration of proteins in the brain of SAMP8 and SAMR1 mice. J. Alzheimers Dis. 2011;23:641–654. doi: 10.3233/JAD-2010-101389. [DOI] [PubMed] [Google Scholar]