Abstract
Acanthopanax senticosus (AS) is a well-known, highly effective traditional Chinese herbal medicine. Polysaccharides extracted from AS (ASPS) have multiple pharmacologic and biological activities with potential use as additives in broiler chicken feed. This trial evaluated the effects of dietary ASPS on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. A total of 240 1-day-old Arbor Acres male broiler chicks were randomly divided into 4 groups, with 10 replicates of 6 chicks and fed a corn- and soybean-based diet supplemented with 0, 1, 2, or 4 g/kg ASPS. Compared with the control group, supplementation with 1 g/kg ASPS increased ADG and ADFI in the finisher and overall periods and decreased the feed conversion ratio in the finisher period (both P < 0.05). Serum IgA and IgM were significantly increased by supplementation with 1 and 2 g/kg of ASPS (P < 0.05). Superoxide dismutase and glutathione peroxidase activities were increased and malondialdehyde concentration was decreased in birds fed ASPS-supplemented diets compared with those in the control group (P < 0.05). Polysaccharides extracted from AS supplementation increased Lactobacillus and decreased Escherichia coli and Salmonella counts in the ileal contents compared with the control diet (both P < 0.05). The results show that dietary ASPS improved growth performance, immune status, and antioxidant capacity and stimulated the growth of beneficial gut bacteria in broiler chickens. In conclusion, ASPS was effective as a natural additive in broiler chicken feed; 1 g/kg can be considered as the optimum dosage.
Key words: Acanthopanax senticosus polysaccharide, immune function, antioxidation, microflora, broiler chicken
Introduction
Modern intensive poultry production has achieved recent, phenomenal gains in efficiency. Antibiotic growth promoters are added to poultry feed to improve productivity, product quality, and prevent disease (Brenes and Roura, 2010; Lin et al., 2013). However, their abuse has had serious negative effects, such as drug residue, bacterial resistance, and other problems that challenge food safety and human health. The ban on the use of antibiotic growth promoters in animal diets in the European Union has prompted evaluation of potential alternatives (Landers et al., 2012). Effective, environmentally and consumer-friendly feed additives are needed to support animal production. Recent studies have described the benefits of plant-derived bioactive compounds including polysaccharides, to improve growth performance, immunity, and intestinal health in poultry production (Wu, 2018; Long et al., 2020). Plant extracts of natural polysaccharides have shown great potential as an alternative to antibiotic additives.
Acanthopanax senticosus (AS), also known as Siberian ginseng, is widely distributed throughout northern Asia. It is a well-known traditional Chinese herb that used to treat and prevent inflammation, diabetes mellitus, hypertension, rheumatoid diseases, chronic bronchitis, and ischemic heart diseases, among others (Yi et al., 2001; Li and Zhou, 2007). Polysaccharides extracted from AS (ASPS) have immunomodulatory (Chen et al., 2011), antioxidant (Zhao et al., 2013), anti-inflammatory (Han et al., 2016), and antitumor activities (Meng et al., 2018). As a dietary additive in piglets, ASPS was reported to enhance cellular and humoral immune responses by modulating the production of immunocytes, cytokines, and antibodies (Kong et al., 2007). They were also found to enhance digestion and absorption (Kong et al., 2009), regulate the composition of the gut microbiota, and maintain the normal morphology of the gut mucosa of weaned piglets (Yin et al., 2008). To the best of our knowledge, the use of ASPS in poultry production has not been investigated. This study investigated the effects of dietary supplementation with ASPS on growth performance, immune function, antioxidative activity, and ileal microbial populations in broiler chickens.
Material and methods
Animals, Experimental Design, and Management
All procedures were approved by the Animal Care and Use Committee of Hunan Agricultural University, China. A total of 240 1-day-old male Arbor Acres broiler chicks with similar BW were obtained from a local hatchery. They were randomly assigned to 4 groups with 10 replicates per group and 6 broilers per replicate. All birds were offered the same basal diet with the addition of ASPS at 0 (control), 1, 2, and 4 g/kg. Powdered ASPS was purchased from Shaanxi Sihai Biotechnology Co. Ltd. (Xian, China). As per the manufacturer, it had been extracted from A. senticosus using an ethanol/salt aqueous 2-phase system. The polysaccharide content, measured by the phenol-sulfuric acid method, was ≥50% (Zhao et al., 2013). The animals were fed for 42 d, including starter (Day 1–Day 21) and finisher (Day 21–Day 42) phases. The diets met the 1994 NRC nutrient requirements (Table 1). The chicks were raised in wire cages (110 cm × 60 cm × 50 cm) with 6 birds per cage and housed in an environmentally controlled room with continuous lighting. All chicks were given ad libitum access to feed and water. The room temperature was maintained at 32°C to 34°C for the first 3 d and decreased by 2°C to 3°C per week to a final temperature of 22°C. Light was provided for 24 h during the first 3 d and then reduced to 22 h in the subsequent 4 to 7 d. All birds were weighed individually after arrival from the hatchery. The study lasted 42 d. Final BW and feed consumption of the birds per cage were determined on day 21 and 42. ADG, ADFI, and feed conversion ratio (FCR) (feed consumed [g]:weight gain [g]) were calculated.
Table 1.
Composition and nutrient content of experimental diets.
| Items | Age (d) |
|
|---|---|---|
| Starter |
Grower |
|
| (0–21 d) | (21–42 d) | |
| Ingredient (%) | ||
| Corn | 57.20 | 62.70 |
| Soybean meal (43% CP) | 34.70 | 29.30 |
| Soy oil | 2.70 | 2.80 |
| Fish meal (60.2% CP) | 1.50 | 1.50 |
| Dicalcium phosphate | 1.65 | 1.41 |
| Limestone | 1.30 | 1.30 |
| Salt | 0.25 | 0.21 |
| DL-Methionine | 0.20 | 0.20 |
| HCl- Lysine | - | 0.08 |
| Vitamin–mineral premix1 | 0.50 | 0.50 |
| Nutrient content | ||
| ME (MJ/kg) | 12.92 | 13.43 |
| CP(g/kg) | 21.50 | 19.60 |
| Ca (g/kg) | 0.95 | 0.88 |
| Total P (%) | 0.69 | 0.64 |
| Available P (g/kg) | 0.46 | 0.35 |
| Lys (%) | 1.21 | 1.09 |
| Met (%) | 0.50 | 0.44 |
Supplied per kilogram of diet: vitamin A (trans-retinyl acetate), 10,050 IU; vitamin D3, 2,800 IU; vitamin E (DL-α-tocopheryl acetate), 50 mg; vitamin K3, 3.5 mg; thiamine, 2.5 mg; riboflavin, 7.5 mg; pantothenic acid, 15.3 mg; pyridoxine, 4.3 mg; vitamin B12 (cyanocobalamin), 0.02 mg; niacin, 35 mg; choline chloride, 1,000 mg; biotin, 0.20 mg; folic acid, 1.2 mg; Mn, 100 mg; Fe, 85 mg; Zn, 60 mg; Cu, 9.6 mg; I, 0.30 mg; Co, 0.20 mg; and Se, 0.20 mg.
Sample Collection
At 42 d of age, 10 birds were randomly selected from each treatment group. After 12 h of feed withdrawal (water was offered ad libitum), blood samples were collected before slaughter from the wing vein using 5-mL vacuum tubes and allowed to clot at 37°C for 2 h before centrifuging at 3,000 g for 10 min at 4°C. The serum supernatant was stored at −20°C until assay of IgG, IgM, IgA, and antioxidant enzyme activities.
Serum Ig
Serum Ig (IgM, IgG and IgA) were assayed with ELISA kits purchased from Bogoo Biotechnology Co. Ltd. (Shanghai, China). All measurements were performed at least in triplicate following the manufacturer's instructions.
Serum Antioxidant Indices
Serum superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity and malondialdehyde (MDA) concentration were assayed with commercial reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's instructions. All assays were conducted at least in triplicate.
Ileal Microbial Populations
At the end of the trial, ileal microbial populations were determined as previously described by Yang et al. (2012), with some modifications. Briefly, 10 broilers per treatment group were euthanized by CO2 inhalation before extracting the ileal contents. The ileum was ligated at both ends and removed from the gastrointestinal tract. One gram of ileal contents per sample was weighed out, suspended in 99 mL of sterile 0.9% saline solution, and homogenized for 5 min in a stomacher. Each homogenate was diluted 10 times (10% wt/vol) with sterile ice-cold normal saline. Diluted samples (0.1 mL) were inoculated into selective agar for bacterial enumeration. Escherichia coli was incubated on MacConkey agar, and Salmonella was incubated on Salmonella Shigella agar at 37°C for 24 h. Lactobacillus and Bifidobacterium were incubated on Luria-Bertani agar and Briggs liver agar, respectively, in an anaerobic incubator at 37°C for 48 h. Agars were purchased from Beijing Ruizekang Technology Co., Ltd. (Beijing, China).
Statistical Analysis
The statistical analysis was performed by SPSS 20.0 software (SPSS Inc., Chicago, IL). Data were analyzed by 1-way ANOVA as a completely randomized design using the GLM procedure. Cages was used as an experimental unit to analyze the performance data. For the analysis of other data, each bird per replicate was treated as an experimental unit. Differences between dietary treatment means were compared using Duncan's multiple-range tests. A significance level of P < 0.05 was used.
Results
Growth Performance
The effects of dietary ASPS on broiler performance are shown in Table 2. In the starter period, differences in ADG, ADFI, and FCR among the study groups were not significant (P > 0.05). Supplementation with 1 g/kg ASPS resulted in significant increases in ADG and ADFI in the finisher period and the entire 42 d and a significant decrease in FCR in the finisher period compared with the control birds (P < 0.05). The overall difference in FCR from day 0 to day 42 was not significant.
Table 2.
Effects of polysaccharide-enriched extract from Acanthopanax senticosus (ASPS) on the performance of broilers.1
| Items | ASPS level (g/kg)3 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0.0 | 1.0 | 2.0 | 4.0 | |||
| Initial BW (g) | 44.5 | 44.5 | 45.3 | 44.8 | 0.15 | 0.165 |
| Final BW (g) | 2219.7 | 2427.6 | 2307.9 | 2242.8 | 19.8 | 0.341 |
| Starter period, day 1 to 21 of age | ||||||
| ADG (g) | 30.3 | 32.4 | 31.4 | 30.7 | 30.3 | 0.472 |
| ADFI (g) | 47.5 | 49.9 | 48.2 | 48.3 | 47.5 | 0.518 |
| FCR (feed/gain, g/g)2 | 1.57 | 1.54 | 1.54 | 1.57 | 0.02 | 0.196 |
| Finisher period, day 22 to 42 of age | ||||||
| ADG (g) | 75.4b | 83.2a | 78.5a,b | 76.1b | 0.12 | 0.026 |
| ADFI (g) | 126.6b | 134.4a | 132.5a,b | 130.1a,b | 6.65 | 0.038 |
| FCR (feed/gain, g/g) | 1.68a | 1.62b | 1.69a | 1.71a | 0.01 | 0.047 |
| Overall, day 1 to 42 of age | ||||||
| ADG (g) | 51.79b | 56.74a | 53.87a,b | 52.60a,b | 1.79 | 0.029 |
| ADFI (g) | 92.20b | 98.50a | 96.40a,b | 94.10a,b | 2.20 | <0.001 |
| FCR (feed/gain, g/g) | 1.78 | 1.74 | 1.79 | 1.79 | 0.03 | 0.132 |
a,bMeans within a row lacking a common superscript differ (P < 0.05).
Data represent the means of 10 replicate cages (n = 10).
FCR, feed conversion ratio.
1.0, 2.0, 4.0 represented the data from broilers fed with 1 g, 2 g, and 4 g ASPS per kilogram of the diet, respectively.
Serum Ig
As shown in Table 3, dietary supplementation had no effect on serum IgG, but on day 42, broilers fed diets supplemented with 1or 2 g/kg ASPS had higher serum IgA and IgM levels (P < 0.05) than those fed the control diets.
Table 3.
Effects of polysaccharide-enriched extract from Acanthopanax senticosus (ASPS) on serum Ig in broilers.1
| ASPS levels (g/kg)2 | IgA (μg/ml) | IgG (mg/mL) | IgM (mg/mL) |
|---|---|---|---|
| Control (0) | 53.3b | 3.38 | 1.15b |
| 1.0 | 72.8a | 4.06 | 2.27a |
| 2.0 | 70.6a | 4.19 | 1.68a |
| 4.0 | 61.5a,b | 3.66 | 1.35b |
| SEM | 1.02 | 0.16 | 0.11 |
| P-value | <0.01 | 0.118 | 0.019 |
a,bMeans within column with different superscript letters differed significantly (P < 0.05).
Data represent the means of 10 birds for 1 bird per replicate (n = 10).
1.0, 2.0, 4.0 represented the data from broilers fed with 1 g, 2 g, and 4 g ASPS per kilogram of the diet, respectively.
Antioxidant Capacity
Table 4 shows the effect of ASPS supplementation on serum SOD and GSH-Px activity and MDA concentration in 42-day-old broiler chickens. The birds fed with ASPS-supplemented diets had higher SOD and GSH-Px activities and lower MDA concentration than those fed the control diet (P < 0.05).
Table 4.
Effects of polysaccharide-enriched extract from Acanthopanax senticosus (ASPS) on superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities and malondialdehyde (MDA) concentration in serum of broilers.1
| ASPS levels (g/kg)2 | SOD (U/mL) | GSH-Px (U/mL) | MDA (nmol/mL) |
|---|---|---|---|
| Control (0) | 145.3c | 613.9b | 7.26a |
| 1.0 | 170.7a | 755.3a | 6.17b |
| 2.0 | 163.6a,b | 783.2a | 5.08c |
| 4.0 | 155.5b | 721.8a | 5.13c |
| SEM | 3.67 | 15.9 | 0.56 |
| P-value | 0.018 | <0.01 | 0.027 |
a–cMeans within column with different superscript letters differed significantly (P < 0.05).
Data represent the means of 10 birds for 1 bird per replicate (n = 10).
1.0, 2.0, 4.0 represented the data from broilers fed with 1 g, 2 g, and 4 g ASPS per kilogram of the diet, respectively.
Ileal Microflora Populations
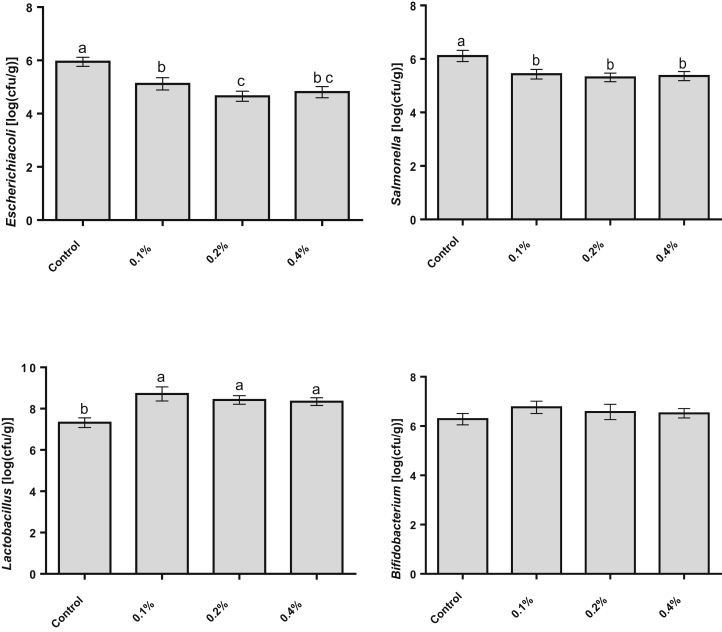
As shown in Figure 1, dietary supplementation had no effect on the Bifidobacterium population (P > 0.05), but ASPS supplementation increased Lactobacillus and decreased the E. coli and Salmonella populations in the ileal contents compared with the control diet.
Figure 1.
Effects of polysaccharide-enriched extract from Acanthopanax senticosus (ASPS) on ileal microbial populations in broilers. 0.1, 0.2, 0.4% represented the data from broilers fed with 1 g, 2 g, and 4 g ASPS per kilogram of the diet, respectively. Bacterial number is expressed as log10 cfu/g wet digesta. Each bar represents the mean for 10 birds per treatment ± SE. a–cWithin the same day, bars with different letters differ significantly (P < 0.05).
Discussion
The use of botanic polysaccharides, such as those from Achyranthes bidentate, Camellia oleifera, and Lycium barbarum, as feed additives for improving growth performance, immunity, or antioxidant activity in broilers has previously been reported (Long et al., 2020; Wang et al., 2020). The objectives of this study were to determine whether ASPS added to broiler feed would improve growth performance and immune and antioxidant status and to determine the optimal supplementation level. The study results demonstrated that dietary supplementation with 1 g/kg ASPS increased ADG and ADFI in the finisher period and overall reduced the FCR in the finisher period. Kong et al. (2009) reported that dietary supplementation with an AS extract enhanced the digestion and absorption of amino acids in weaned piglets. Another recent study showed that dietary ASPS supplementation in weaned piglets alleviated detrimental responses to a lipopolysaccharide challenge, which may have been achieved by improving gut health (Han et al., 2014). Oral pretreatment with ASPS has also been shown to inhibit activation of nuclear factor kappa B and changes in intestinal mucosal integrity in response to inflammation (Han et al., 2016; 2017). However, understanding how ASPS benefits the gut health and growth of broilers requires further study.
Plant polysaccharides have been shown to benefit immune function and immune system diseases in animal models (Qiao et al., 2013; Zhang et al., 2013; Fu et al., 2017). Serum Ig have key roles in immune functions in poultry (Liao et al., 2015). In this study, dietary supplementation with ASPS promoted the humoral immune response by increasing serum IgA and IgM concentrations. Similar results were reported by Kong et al. (2007), who demonstrated that dietary supplementation with an AS extract in weaned piglets increased serum IgG and IgM concentrations compared with piglets fed nonsupplemented diets. Ig is produced by lymphocytes in response to foreign substances (Rezaei et al., 2015). It has been shown that ASPS can stimulate B cell proliferation and Ig production (Han et al., 2003), which might account for the beneficial effects of ASPS on the health status of the broiler chickens in this study.
Reactive oxygen species play an important role in maintaining health (Estévez, 2015). The lipid content of broiler chickens is relatively high and may induce the production of reactive oxygen species that have detrimental effects on the body (Bai et al., 2017). The removal of lipid peroxides involves antioxidant enzymes including SOD, GSH-Px, and catalase (Pisoschi and Pop, 2015). Superoxide dismutase and GSH-Px are the first line of enzymatic antioxidant defense and convert free radicals into water and oxygen to maintain the intracellular redox balance (Chueh et al., 2019). Malondialdehyde is an end product of lipid peroxidation. It can be assayed to determine the extent of lipid peroxidation, and indirectly indicate the extent of cell damage (Pirinccioglu et al., 2010). Many studies have shown that Chinese herbal polysaccharides can increase the activity of antioxidant enzymes, scavenge free radicals, and inhibit lipid oxidation (Jing et al., 2009; Shi et al., 2017). Oral ASPS administration has been shown to increase total antioxidant competence, catalase, SOD, and GSH-Px and to decrease the MDA concentration in the serum, liver, and kidney of rats (Wang et al., 2010; Fu et al., 2012). In vitro, ASPS has been shown to scavenge superoxide and hydroxyl radicals (Fu et al., 2012). In this study, dietary ASPS supplementation improve the antioxidant capacity in broilers by increasing both SOD and GSH-Px activity and decreasing serum MDA concentration. The results indicated that dietary ASPS supplementation protected broiler chicks against damage from reactive oxygen species and free radicals.
Intestinal microbiota contribute to the health status of host animals and are the first barrier against pathogens from food. The health effects can be both beneficial (e.g., Lactobacillus and Bifidobacterium) and harmful (e.g., E. coli and Salmonella) (Li et al., 2009). Plant polysaccharides can increase the number of intestinal probiotics (e.g., Lactobacillus) and inhibit populations of pathogenic bacteria (e.g., E. coli) in broiler chickens and pigs (Li et al., 2009; Li et al., 2011). Selective fermentation of nutrients by probiotics may increase the number of lactic acid bacteria (Zhu et al., 2015). Fang et al. (2009) reported that dietary supplementation with an AS extract significantly increased Lactobacillus and Bacillus subtilis and decreased the ileal and cecal populations of E. coli and Salmonella in piglets (Fang et al., 2009). In this study, dietary ASPS supplementation reduced the E. coli and Salmonella populations and increased that of Lactobacillus in the ileal contents of broilers. The result is consistent with a report by Yin et al. (2008) that dietary ASPS increased the Lactobacillus and decreased the E. coli populations in the ileal and cecal contents of weaned piglets. Dietary ASPS was also found to regulate the immune responses of weaned piglets by modulating the production of immunocytes, cytokines, and antibodies that inhibited a wide range of pathogenic bacteria in an in vitro model (Kong et al., 2007). The antibacterial activity associated with ASPS supplementation needs additional study.
In conclusion, this study found that dietary ASPS supplementation improved BW gain and feed efficiency in poultry. It also resulted in improved immune status, antioxidant capacity, and balance of the intestinal microflora, which may have beneficial effects on the health and performance of broiler chickens. The findings support a recommendation of supplementation with 1 g/kg ASPS in a poultry diet.
Acknowledgments
This research was supported by the Innovation Team Construction Project of the Poultry Industry Technology System of Modern Agriculture in Guangdong Province, China (2019KJ28), the Scientific Research Foundation in the Higher Education Institutions of Educational Commission of Guangdong Province, China (2017GCZX006), the Project in Key Areas of Serving Rural Revitalization of Department of Education of Guangdong Province, China (2019KZDZX2006), Special Fund for Science and Technology of Guangdong Province (DZX20192520309) and the Open Foundation of CAS Key Laboratory of Agro-ecological Processes in Subtropical Region, Institute of Subtropical Agriculture (ISA2020101). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Disclosures
The authors declare no conflicts of interest.
References
- Bai K., Huang Q., Zhang J., He J., Zhang L., Wang T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017;96:74–82. doi: 10.3382/ps/pew246. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010;158:1–14. [Google Scholar]
- Chen R., Liu Z., Zhao J., Chen R., Meng F., Zhang M., Ge W. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011;127:434–440. doi: 10.1016/j.foodchem.2010.12.143. [DOI] [PubMed] [Google Scholar]
- Chueh C.C., Lin L., Lin W.C., Huang S.H., Jan M.S., Chang S.C., Chung W.S., Lee T.T. Antioxidant capacity of banana peel and its modulation of Nrf2-ARE associated gene expression in broiler chickens. Ital. J. Anim. Sci. 2019;18:1394–1403. [Google Scholar]
- Estévez E. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Fang J., Yan F.Y., Kong X.F., Ruan Z., Liu Z.Q., Huang R.L., Li T.J., Geng M.M., Yang F., Zhang Y.Z., Li P., Gong J., Wu G.Y., Fan M.Z., Liu Y.L., Hou Y.Q., Yin Y.L. Dietary supplementation with Acanthopanax senticosus extract enhances gut health in weanling piglets. Livest. Sci. 2009;123:268–275. [Google Scholar]
- Fu J., Yuan J., Tu Y., Fu J., Zhang N., Gao B., Fu G., Zhang Y. A polysaccharide from Acanthopanax senticosus improves the antioxidant status in alloxan-induced diabetic mice. Carbohyd. Polym. 2012;88:517–521. [Google Scholar]
- Fu L., Gang Z., Pan J., Li Y., Li X. Effects of Astragalus polysaccharides on antioxidant abilities and non-specific immune responses of Chinese mitten crab, Eriocheir sinensis. Aquacult. Int. 2017;25:1333–1343. [Google Scholar]
- Han J., Bian L., Liu X., Zhang F., Zhang Y., Yu N. Effects of Acanthopanax senticosuspolysaccharide supplementation on growth performance, immunity, blood parameters and expression of pro-inflammatory cytokines genes in challenged weaned piglets. Asian Australas. J. Anim. Sci. 2014;27:1035–1043. doi: 10.5713/ajas.2013.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Li J.H., Bai G., Shen G.S., Chen J., Liu J.N., Wang S., Liu X.J. Acanthopanax senticosus polysaccharides-induced intestinal tight junction injury alleviation via inhibition of NF-κB/MLCK pathway in a mouse endotoxemia model. World J. Gastroenterol. 2017;23:2175–2184. doi: 10.3748/wjg.v23.i12.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Liu L., Yu N., Chen J., Liu B., Yang D., Shen G. Polysaccharides from Acanthopanax senticosus enhances intestinal integrity through inhibiting TLR4/NF-κB signaling pathways in lipopolysaccharide-challenged mice. Anim. Sci. J. 2016;87:1011–1018. doi: 10.1111/asj.12528. [DOI] [PubMed] [Google Scholar]
- Han S.B., Yoon Y.D., Ahn H.J., Lee H.S., Lee C.W., Yoon W.K., Park S.K., Kim H.M. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int. Immunopharmacol. 2003;3:1301–1312. doi: 10.1016/S1567-5769(03)00118-8. [DOI] [PubMed] [Google Scholar]
- Jing X., Liu W., Yao W., Pang X., Yin D., Gao X. Carboxymethylation of a polysaccharide extracted from Ganoderma lucidum enhances its antioxidant activities in vitro. Carbohyd. Polym. 2009;78:227–234. [Google Scholar]
- Kong X.F., Yin Y.L., Wu G.Y., Liu H.J., Yin F.G., Li T.J., Huang R.L., Ruan Z., Xiong H., Deng Z.Y. Dietary supplementation with Acanthopanax senticosusextract modulates cellular and humoral immunity in weaned piglets. Asian Australas. J. Anim. Sci. 2007;20:1453–1461. [Google Scholar]
- Kong X.F., Yin F.G., He Q.H., Liu H.J., Li T.J., Huang R.L., Fan M.Z., Liu Y.L., Hou Y.Q., Li P., Ruan Z., Deng Z.Y., Xie M.Y., Xiong H., Yin Y.L. Acanthopanax senticosus extract as a dietary additive enhances the apparent ileal digestibility of amino acids in weaned piglets. Livest. Sci. 2009;123:261–267. [Google Scholar]
- Landers T.F., Cohen B., Wittum T.E., Larson E.L. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127:4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.L., Yin F.G., Zhang B., Peng H.Z., Li F.N., Zhu N.S., Hou D.X., Yin Y.L., Luo J.J., Tang Z.R., Liu G. Dietary supplementation with Atractylodes Macrophala Koidz polysaccharides ameliorate metabolic status and improve immune function in early-weaned pigs. Livest. Sci. 2011;142:33–41. [Google Scholar]
- Li S.P., Zhao X.J., Wang J.Y. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009;88:519–525. doi: 10.3382/ps.2008-00365. [DOI] [PubMed] [Google Scholar]
- Li X.L., Zhou A.G. Preparation of polysaccharides from Acanthopanax senticosus and its inhibition against irradiation-induced injury of rat. Carbohydr. Polym. 2007;67:219–226. [Google Scholar]
- Liao X.D., Ma G., Cai J., Fu Y., Yan X.Y., Wei X.B., Zhang R.J. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 2015;94:662–667. doi: 10.3382/ps/pev038. [DOI] [PubMed] [Google Scholar]
- Lin J., Hunkapiller A.A., Layton A.C., Chang Y.J., Robbins K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne. Pathog. Dis. 2013;10:331–337. doi: 10.1089/fpd.2012.1348. [DOI] [PubMed] [Google Scholar]
- Long L.N., Kang B.J., Jiang Q., Chen J.S. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020;99:744–751. doi: 10.1016/j.psj.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q., Pan J., Liu Y., Chen L., Ren Y. Anti-tumour effects of polysaccharide extracted from Acanthopanax senticosus and cell-mediated immunity. Exp. Ther. Med. 2018;15:1694–1701. doi: 10.3892/etm.2017.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirinccioglu A.G., Gökalp D., Pirinccioglu M., Kizil G., Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010;43:1220–1224. doi: 10.1016/j.clinbiochem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Qiao J., Li H.H., Zheng C.J., Feng Z.Y., Wang W. Dietary supplementation with Aloe vera polysaccharide enhances the growth performance and immune function of weaned piglets. J. Anim. Feed. Sci. 2013;22:329–334. [Google Scholar]
- Rezaei S., Faseleh Jahromi M., Liang J.B., Zulkifli I., Farjam A.S., Laudadio V., Tufarelli V. Effect of oligosaccharides extract from palm kernel expeller on growth performance, gut microbiota and immune response in broiler chickens. Poult. Sci. 2015;94:2414–2420. doi: 10.3382/ps/pev216. [DOI] [PubMed] [Google Scholar]
- Shi M.J., Wei X., Xu J., Chen B.-J., Zhao D.-Y., Cui S., Zhou T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem. 2017;215:76–83. doi: 10.1016/j.foodchem.2016.07.151. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang M., Gou Z., Jiang S., Zhang Y., Wang M., Tang X., Xu B. The effect of camellia oleifera cakepolysaccharides on growth performance, carcass traits, meat quality, blood profile, and caecum microorganisms in yellow broilers. Animals. 2020;10:266. doi: 10.3390/ani10020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hai C.X., Liang X., Yu S.X., Zhang W., Li Y.L. The protective effects of Acanthopanax senticosus Harms aqueous extracts against oxidative stress: role of Nrf2 and antioxidant enzymes. J. Ethnopharmacol. 2010;127:424–432. doi: 10.1016/j.jep.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Yang C.M., Cao G.T., Ferket P.R., Liu T.T., Zhou L., Zhang L., Xiao Y.P., Chen A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012;91:2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- Yi J.M., Kim M.S., Seo S.W., Lee K.N., Yook C.S., Kim H.M. Acanthopanax senticosus root inhibits mast cell-dependent anaphylaxis. Clin. Chim. Acta. 2001;312:163–168. doi: 10.1016/s0009-8981(01)00613-1. [DOI] [PubMed] [Google Scholar]
- Yin F.G., Yin Y.L., Kong X.F., Liu Y.L., He Q.H., Li T.J., Huang R.L., Hou Y.Q., Shu X.G., Tan L.G., Chen L.X., Gong J.H., Kim S.W., Wu G.Y. Dietary supplementation with Acanthopanax senticosus extract modulates gut microflora in weaned piglets. Asian Australas. J. Anim. Sci. 2008;21:1330–1338. [Google Scholar]
- Zhang X., Cao F., Sun Z., Yu W., Zhao L., Wang T. Sulfation of Agrocybe chaxingu polysaccharides can enhance the immune response in broiler chicks. J. Appl. Poult. Res. 2013;22:778–791. [Google Scholar]
- Zhao Z., Xu X., Ye Q., Dong L. Ultrasound extraction optimization of Acanthopanax senticosus polysaccharides and its antioxidant activity. Int. J. Biol. Macromol. 2013;59:290–294. doi: 10.1016/j.ijbiomac.2013.04.067. [DOI] [PubMed] [Google Scholar]
- Zhu W., Li D., Wang J., Wu H., Xia X., Bi W., Guan H., Zhang L. Effects of polymannuronate on performance, antioxidant capacity, immune status, cecal microflora, and volatile fatty acids in broiler chickens. Poult. Sci. 2015;94:345–352. doi: 10.3382/ps/pev006. [DOI] [PubMed] [Google Scholar]