Abstract
The application of probiotics in broiler feed, to alleviate performance deficiencies due to mild infections by coccidia and Clostridium perfringens, is of increasing interest for the poultry industry. Therefore, our objective was to evaluate the capacity of 3 Bacillus strains and their combination as probiotics in vitro and in vivo. Thus, protein and carbohydrate degradation and C. perfringens growth inhibition capabilities were assessed by colometry measurement and an agar diffusion bioassay, respectively. A total of 2,250 1-day-old male broiler chicks were assigned to 5 dietary treatments: 1) non–probiotic-supplemented control (control); 2) control + DSM 32324 at 0.8 × 106 cfu/g of feed; 3) control + DSM 32325 at 0.5 × 106 cfu/g of feed; 4) control + DSM 25840 at 0.3 × 106 cfu/g of feed; and 5) control + DSM 32324 + DSM 32325 + DSM 25840 at 1.6 × 106 cfu/g of feed. A pathogenic field strain of C. perfringens was used to induce the necrotic enteritis challenge on day 19, 20, and 21. All birds and remaining feed were weighed on pen basis on day 0, 21, 35, and 42, to calculate BW gain and mortality-adjusted feed conversion. Mortality and mortality due to necrotic enteritis were recorded daily. On day 21, 45 birds per treatment were evaluated for macroscopic intestinal necrotic enteritis lesions. Performance data were statistically analyzed using an ANOVA and subjected to a least significant difference comparison. Necrotic enteritis lesion scores were statistically analyzed using nonparametric Kruskal-Wallis test. Dunn's test was used for treatment comparison. The tested strains showed different abilities of degrading protein and carbohydrates and inhibiting C. perfringens growth in vitro. The birds fed the multi-train combination presented significantly better performance and lower necrotic enteritis lesion score than those in the control group. Dietary supplementation with probiotics resulted in significantly lower necrotic enteritis mortality. The results demonstrate the suitability of the evaluated Bacillus multistrain combination as an effective probiotic in C. perfringens–challenged chickens.
Key words: DFM, probiotic, Bacillus, Clostridium perfringens, necrotic enteritis
Introduction
Clostridium perfringens (CP) is frequently found as a commensal bacterial species of the poultry intestinal tract that, in general, does not cause any damage to the host (Van Immerseel et al., 2004). Nevertheless, because of the high growth rate of CP, any issue that disrupts the intestinal homeostasis, particularly those that cause cellular damage and gut leakage, will trigger outgrowth of CP (Shojadoost et al., 2012; Moore, 2016). In this sense, when pathogenic CP strains that produce toxins, such as the alpha toxin and/or the NetB toxin, are settled at noteworthy levels in the intestine, necrosis in the epithelium, increased intestinal permeability, hemorrhage, diarrhea, and subsequently performance losses may take place. Thus, the outgrowth of such pathogenic CP strains in the intestine of chickens is the primary etiology of the globally widespread necrotic enteritis (NE) in modern poultry production (Sakurai et al., 2004; Van Immerseel et al., 2009; Cooper et al., 2013; Smyth, 2016).
Until not many year ago, NE stayed controlled in broiler chickens by the addition of antibiotics in feed as growth promoters. However, increasing restrictions on the use of antibiotics in feed as growth promoters and even therapeutic antibiotics in poultry production in many parts of the world have contributed to the resurgence of clinical and subclinical NE, increasing its prevalence in broilers. Subclinical NE may have a greater economic impact for poultry producers than the clinical disease (Hofacre et al., 2018). In response to this phenomenon, increasing attention has been focused by the feed additive industry on research and application of effective products to prevent, control, or palliate NE, the most widespread being direct-fed microbials (DFM) or probiotics, prebiotics, organic acids, phytochemicals, and enzymes (Dahiya et al., 2006). Among all these products, DFM stand out for being safe and natural additives. As accredited by world authorities, probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (Food and Agricultural Organization of the United Nations and World Health Organization, 2001).
At present, the spore-forming genus Bacillus is certainly one of the most important sources of poultry DFM (Zhang et al., 2013; Park and Kim, 2014; Harrington et al., 2016; Haque et al., 2017; Reis et al., 2017; Hong et al., 2019). Bacillus spp. have been considered to be promising DFM, owing to the high stability of spores, which are unaffected by high temperature during feed processing and resistant to harsh gastrointestinal conditions (Mazanko et al., 2017). Another benefit of Bacillus spp., which has made them widely used DFM, is their explicit mechanisms of action in the intestine of the chicken, once they have overcome feed manufacturing and gastric conditions challenges. In this sense, 2 relevant mechanisms of action representative of Bacillus spp. are their pronounced capacity to produce a number of digestive enzymes (e.g., carbohydrases and proteases), increasing nutrient digestibility, as well as their ability to deliver several sturdy antimicrobial peptides, inhibiting the growth of pathogenic bacteria such as CP and subsequently improving performance and alleviating NE in broilers (Sharma et al., 2010; Sen et al., 2011; Seo et al., 2013; Sumi et al., 2015; Wang et al., 2015; Elshaghabee et al., 2017; Whelan et al., 2019). Nevertheless, different Bacillus spp. or even different subspecies or strains from the same species produce different amounts and sets of enzymes and antimicrobial peptides (Burkard et al., 2007; Fuchs et al., 2011; Larsen et al., 2014; Grant et al., 2018), which puts forward that different Bacillus strains may interact in a different way with the digesta and the intestinal microbiota in the gut. Therefore, the development of multistrain DFM requires an unblemished assessment of the ability of each single probiotic strain to produce enzymes and its capability to inhibit the growth of pathogenic bacteria, as well as the synergies between these abilities among different strains.
The objective of this study was to evaluate the capacity of 3 Bacillus strains (DSM 32324, DSM 32325, and DSM 25840) to inhibit the growth of pathogenic CP and their ability to digest protein and carbohydrates in vitro, as bases for their suitability as probiotics, as well as the effects of their dietary supplementation, independently or combined, on the performance of broiler chickens under CP challenge as a model to reproduce subclinical NE.
Materials and methods
As per the described objective, the present study comprised 2 distinct parts. First, in vitro qualitative evaluations were carried out to determine the capacity of the test strains to degrade proteins and carbohydrates of soybean meal, as well as their CP growth inhibitory capacity. In addition, an in vivo trial was carried out with broiler chickens to evaluate the effect of the test strains and their combination on the performance of the birds under a mild challenge by CP.
Protein and Carbohydrate Degradation
Protein and carbohydrate degradation capacity in vitro was examined on 2 custom-made substrates, S1 and S2, based on dyed soy bean meal (GlycoSpot, Soeborg, Denmark). Soy bean meal protein and polysaccharides were selectively labeled with a nonspecific chlorotriazine dye, Reactive Blue 49. When degraded, a blue color is released into the medium. The intensity of the color in the supernatant corresponds to the degree of degradation and can be measured at an absorbance of 595 nm (FLUOstar Omega microplate reader; BMG Labtech, Ortenberg, Germany). In substrate 1 (S1), the combined effect of carbohydrases and proteases was measured, whereas in substrate 2 (S2), the carbohydrase effect was specifically assessed. By subtracting the absorbances of S2 from S1, the effect of protease was obtained. The Bacillus strains were grown from glycerol stocks overnight at 37 C with shaking in VIB (Veal infusion Broth, BD Difco 234420; Becton, Dickinson and Company, Franklin Lakes, NJ) before inoculating 1% into the 2 soy substrates. The Bacillus strains were grown in the soy substrates for 24 h at 37 C, with shaking, then spun down, and the supernatants used for assaying both the overall degradation of soy bean (S1) and more specifically the degradation of carbohydrate (S2) and protein (S2-S1). The results obtained with the 3 strains were compared with the average of results obtained with the same method for 270 Bacillus subtilis strains and 154 Bacillus amyloliquefaciens strains from the Bacillus collection of Chr Hansen A/S (Hörsholm, Denmark), as a qualitative control to determine the relative efficacy of the tested strains.
C. perfringens Inhibition
Inhibitory activity of the single Bacillus strains was tested towards both CP type A and CP type C, as described in the following.
All bacterial strains were maintained in Brain Heart Infusion (BHI) broth (Chr. Hansen A/S) with 20% glycerol at −80°C. A nonquantified loopful of BS DSM 32324, BS DSM 32325, and BA DSM 25840 from the cryotube was aerobically grown overnight on tryptic soy agar with sheep blood (Oxoid A/S, Denmark) at 37°C. After purity check, Bacillus colonies from the plates were inoculated in BHI broth and incubated aerobically overnight at 37°C and 175 rpm.
Bacillus overnight cultures were diluted 100 times in BHI broth and 10 μL were added to Luria-Bertani agar plates (Chr. Hansen A/S) as spots (106 cfu/spot). As a positive control, ciprofloxacin (0.2 μg/mL) was added as a spot. The plates were incubated aerobically at 37°C for 3 h. After purity check, CP colonies from the plates were suspended in peptone saline buffer (Chr. Hansen A/S) until McFarland 2 was obtained. The suspended CP (CP DSM 756 3.5 mL, CP NCTC 3180 5.7 mL) were added to and mixed with 100 mL liquid BHI agar and the inoculated agar poured onto the surface of the Luria-Bertani agar plates with Bacillus spp. spots as a top layer. The plates were incubated anaerobically at 30°C for 20 h. Radii of the inhibition zones around the spots were recorded. The experiment was repeated twice with duplicates.
Animals and Diets
The study was conducted at Southern Poultry Feed and Research, Inc. (Athens, GA), during the winter months, beginning from December to February. The experiment was conducted in accordance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching. All procedures were supervised by an attending veterinarian. A total of 2,250 1-day-old Cobb 500 male broiler chicks were randomly assigned to 5 dietary treatments consisting of 9 floor pen replicates per treatment and 50 birds per replicate, with pen as an experimental unit. Each pen had an area of 4 × 10 = 40 ft2. All pens had approximately 4 inches of builtup litter with a coating of fresh pine shavings. This study was designed to mimic commercial conditions in the United States as close as possible, so builtup litter was used. This builtup litter was also a possible source of coccidial oocysts. All birds were reared under the same house conditions. All birds were sprayed with commercial coccidia vaccine (Coccivac-B52; Intervet Inc., Omaha, NE) on day 1. Only healthy appearing chicks were used in the study. No birds were replaced during the course of the study. A bird was culled only to relieve suffering. When a bird was culled or found dead, the pen, date, and removal weight (kg) were recorded. A gross necropsy was performed on all dead or culled birds to determine the probable cause of death.
Three nonmedicated (no antibiotic or anticoccidial drug), corn–soybean meal–based diets were used as the basal diets for all treatments during starter (crumbles, day 1–21), grower (pellets, day 22–35), and finisher (pellets, day 36–42) periods, as per the National Research Council (1994) recommendations. All broilers were allowed ad libitum access to water and feed. The feed was provided in 1 tube-type feeder per pen. From day 1 until day 7, feed was also supplied on a tray placed on the litter of each pen. The ingredient and chemical compositions of the experimental diets used in this study are shown in Table 1. Dietary treatments were then produced by supplementing the basal diets with test probiotics. The treatment groups were as follows: 1) infected and nonsupplemented control (control); 2) control + B. subtilis DSM 32324 at 0.8 × 106 cfu/g of feed (BS DSM 32324); 3) control + B. subtilis DSM 32325 at 0.5 × 106 cfu/g of feed (BS DSM 32325); 4) control + B. amyloliquefaciens DSM 25840 at 0.3 × 106 cfu/g of feed (BA DSM 25840;) and 5) control + BS DSM 32324 + BS DSM 32325 + BA DSM 25840 (multistrain combination).
Table 1.
Composition of basal diets.
| Item | Starter | Grower | Finisher |
|---|---|---|---|
| Ingredient (g/kg) | |||
| Corn | 596.98 | 645.28 | 663.64 |
| Soybean meal | 354.87 | 302.73 | 277.46 |
| Vegetable fat | 17.30 | 22.72 | 32.42 |
| Deflourinated phosphate | 21.03 | 19.50 | 17.12 |
| Calcium carbonate | 3.33 | 3.34 | 3.43 |
| DL-Methionine | 2,61 | 2.29 | 1.93 |
| L-Lysine | 1.13 | 1.17 | 0.74 |
| Sodium chloride | 1.50 | 1.70 | 1.99 |
| Trace mineral premix1 | 0.75 | 0.75 | 0.75 |
| Vitamin mineral premix2 | 0.50 | 0.50 | 0.50 |
| Calculated nutrient value | |||
| CP (%) | 21.8 | 19.6 | 18.5 |
| ME (kcal/kg) | 3,008 | 3,086 | 3,167 |
| Crude fiber (%) | 2.20 | 2.13 | 2.09 |
| Lysine (%) | 1.30 | 1.16 | 1.06 |
| Methionine (%) | 0.61 | 0.55 | 0.50 |
| Phosphorus (%) | 0.76 | 0.71 | 0.65 |
| Calcium (%) | 0.90 | 0.84 | 0.76 |
The trace mineral mix provided the following (per kg of diet): manganese (MnSO4∙H2O), 60 mg; iron (FeSO4∙7H2O), 30 mg; zinc (ZnO), 50 mg; copper (CuSO4∙5H2O), 5 mg; iodine (ethylene diamine dihydroiodide), 0.15 mg; selenium (NaSeO3), 0.3 mg.
Vitamin mix provided the following (per kg of diet): vitamin A, 8,818 IU; vitamin D3, 2,480 IU; 25-hydroxyvitamin D3, 69 μg; vitamin E, 35 IU; vitamin B12 (cobalamin),15.5 μg; biotin, 0.17 mg; menadione, 1.98 mg; thiamine, 1.87 mg; riboflavin, 7.7 mg; d-panthothenic acid, 13.23 mg; vitamin B6, 3.3 mg; niacin, 44.1 mg; folic acid, 1.1 mg.
Three feed samples were collected, 1 each from the beginning, middle, and end of the batch of each diet and mixed to form a composite sample. One sample was then obtained from the composite for each treatment and was used for Bacillus spore count analysis. The total spore count analysis was performed twice by the dilution plate series culture method. In Table 2, spore counts are reported as the log10 transformation of cfu per gram of feed (Log10 cfu/g feed; VDLUFA, 2012).
Table 2.
Bacillus cfu counts (cfu/g) in experimental feed.1
| Experimental treatments | Starter feed | Grower feed | Finisher feed | Expected |
|---|---|---|---|---|
| Control | 1.2 × 105 | 1.1 × 105 | 3.1 × 104 | |
| BS DSM 32324 | 4.4 × 105 | 4.2 × 105 | 4.0 × 105 | 8.0 × 105 |
| BS DSM 32325 | 5.0 × 105 | 3.0 × 105 | 3.9 × 105 | 5.0 × 105 |
| BA DSM 25840 | 3.3 × 105 | 3.2 × 105 | 3.0 × 105 | 3.0 × 105 |
| Multistrain combination | 2.2 × 106 | 2.0 × 106 | 1.9 × 106 | 1.6 × 106 |
cfu counts are average values of five technical replicates of 2 biological replicate per sample.
Necrotic Enteritis Challenge
On day 0, birds in each pen were exposed to approximately 2 kg of used litter that was sourced from healthy chickens that were not exposed to dietary probiotics. A pathogenic field strain of CP, alpha toxin and NetB positive, that was isolated from a commercial poultry operation diagnosed with NE was used to induce the NE challenge. Fresh challenge inoculant was prepared before each challenge day; the CP isolate was incubated in cooked meat broth for approximately 24 h and then transferred into sterile nutrient broth and incubated for approximately 18 h. On 3 consecutive day (day 19, 20, and 21), all birds were administered a fresh broth culture of CP (approximately 1 × 109 cfu/mL) by mixing the inoculum into the feed in the base of the tube feeders. Each pen received the same amount of inoculum and fresh inoculum was used each day.
Performance Evaluation and NE Lesion Score
All birds and feeds were weighed on day 0, 21, 35, and 42. Feed consumption and BW were recorded on pen basis for each treatment group. BW gain and mortality-adjusted feed conversion ratio (aFCR) were reported as average per bird. Mortality and mortality due to NE (NE mortality) were recorded daily, calculated as a percentage for each pen replicate, and reported as average of all replicate pens in each treatment. The diagnosis of NE mortality was based on macroscopical examination of intestinal necrosis found in the jejunum and ileum—mucosa lined by a loosely-to-tightly adherent yellow-to-green pseudomembrane, often described as having a “Turkish towel” appearance—as well as dark, swollen, and firm liver (Opengart, 2008).
On day 21, 5 birds per pen (45 birds per treatment) were randomly selected, euthanized by cervical dislocation, and evaluated for macroscopic intestinal NE lesions (Hofacre et al., 1998). Lesion scores were based on a 0 to 3 scoring system, with 0 being normal and 3 being the most severe (Opengart, 2008).
Statistical Analysis
A randomized complete block design was used. BW, aFCR, and mortality data were analyzed using ANOVA (Statistix 10.0, Tallahassee, Florida) and subjected to a least significant difference comparison. Differences were considered significant when P < 0.05. Percentage mortality was arcsine transformed before ANOVA analysis. Necrotic enteritis lesion scores were statistically analyzed using nonparametric Kruskal-Wallis test, with Dunn's test nonparametric pairwise multiple comparisons as a post hoc test for treatment comparison.
Results
In Vitro Results
The 3 strains used in the present study were selected from the Bacillus collection of Chr. Hansen A/S (Hörsholm, Denmark). Therefore, protein and carbohydrate degradation data were obtained from single experiment screens of more than 1,300 Bacillus isolates; of which, 270 strains were B. subtilis and 154 B. amyloliquefaciens. Table 3 shows the protein and carbohydrate degradation results of the 2 B. subtilis strains selected (DSM 32324 and DSM 32325), compared with all the B. subtilis strains (N = 270) from the referred collection, as well as the results of the B. amyloliquefaciens strain selected (DSM 25840), compared with all the B. amyloliquefaciens strains (N = 154) from the same collection.
Table 3.
Degree of carbohydrate and protein degradation by Bacillus subtilis DSM 32324 and DSM 32325 strains, compared with 270 B. subtilis strains, and Bacillus amyloliquefaciens DSM 25840 strain, compared with 154 B. amyloliquefaciens strains. Results are expressed as the absorbance measured at 595 nm.
| Class of degradation |
Bacillus subtilis |
Bacillus amyloliquefaciens |
|||||
|---|---|---|---|---|---|---|---|
| DSM 32324 | DSM 32325 | Control1 | STD | DSM 25840 | Control2 | STD | |
| S1: Carbohydrate and protein 3 | 2.2 | 2.2 | 1.6 | 0.85 | 2.9 | 2.6 | 0.66 |
| S2: Carbohydrate4 | 2.1 | 2.9 | 2.0 | 1.08 | 0.9 | 1.4 | 0.38 |
| S1–S2: Protein 5 | 0.1 | 0.0 | 0.0 | 0.90 | 1.9 | 1.2 | 0.70 |
Average of 270 Bacillus subtilis strains.
Average of 154 Bacillus amyloliquefaciens strains.
Degree of carbohydrate and protein degradation.
Degree of carbohydrate degradation.
Degree of protein degradation, by subtracting the absorbance of S2 from absorbance of S1.
All 3 strains showed a high overall degradation of soy bean meal (S1), whereas the fiber degradation/carbohydrase activity (S2) was most pronounced for B. subtilis strains (DSM 32324 and DSM 32325), both having high activity. B. amyloliquefaciens DSM 25840 showed medium activity. In contrast, there was no activity of both B. subtilis strains (DSM 32324 and BS DSM 32325) on protein degradation (S2-S1), whereas B. amyloliquefaciens DSM 25840 had high activity. Comparing with all the B. subtilis strains tested, both DSM 32324 and DSM 32325 were in the high-end on overall degradation of soy bean meal (S1) and in the medium and high-end, respectively, for fiber degradation (S2). All B. subtilis scored low on protein degradation (S2-S1). On the contrary, DSM 25840 was in the high end for both the overall degradation of soybean meal (S1) and the protein degradation (S2-S1), whereas the fiber degradation activity was in the low end, compared with all B. amyloliquefaciens strains tested.
The Bacillus spp. strains exhibited inhibitory activity against the CP strains though in varying extent. DSM 32324 was strongly inhibitory (r = 5–10 mm) against both tested CP strains, whereas DSM 32325 and DSM 25840 exhibited moderate inhibition (r = 1–5 mm) against both tested CP strains.
Live Performance Results
The performance of broilers in each feeding phase is summarized in Table 4. At the end of the starter phase (0–21 d), the control group showed significantly lower BW gain than any of the DFM-supplemented groups. With regard to feed efficiency during the starter phase, the control group showed the highest aFCR, whereas the 2 B. subtilis single strain (BS DSM 32324 and BS DSM 32325), as well as the multistrain combination group resulted in the lowest aFCR. The B. amyloliquefaciens single-strain-supplemented group (BA DSM 25840) showed an intermedium value.
Table 4.
The effect of 3 Bacillus probiotic strains and their combination on performance of Clostridium perfringens–challenged broilers in different feeding phases.1
| Feeding phase and probiotic inclusion | BW gain (g) | aFCR |
|---|---|---|
| Starter phase (0–21 d) | ||
| Control | 430.40b ± 10.24 | 1.90a ± 0.03 |
| BS DSM 32324 | 475.81a ± 14.76 | 1.69c ± 0.02 |
| BS DSM 32325 | 509.17a ± 13.31 | 1.65c ± 0.03 |
| BA DSM 25840 | 436.36b ± 9.65 | 1.79b ± 0.02 |
| Multi-strain combination | 488.65a ± 15.28 | 1.65c ± 0.02 |
| Grower phase (22–35 d) | ||
| Control | 1,175.32 ± 14.13 | 1.91a ± 0.03 |
| BS DSM 32324 | 1,190.97 ± 12.92 | 1.86a,b,c ± 0.01 |
| BS DSM 32325 | 1,185.48 ± 14.99 | 1.90a,b ± 0.03 |
| BA DSM 25840 | 1,204.71 ± 21.14 | 1.81c ± 0.01 |
| Multistrain combination | 1,201.26 ± 8.08 | 1.84b,c ± 0.02 |
| Finisher phase (36–42 d) | ||
| Control | 631a,b ± 13.31 | 2.10a,b ± 0.04 |
| BS DSM 32324 | 624a,b ± 9.10 | 2.08a,b ± 0.04 |
| BS DSM 32325 | 627a,b ± 16.20 | 2.14a,b ± 0.05 |
| BA DSM 25840 | 603b ± 14.56 | 2.20a ± 0.04 |
| Multistrain combination | 654a ± 20.15 | 2.04b ± 0.03 |
a–cGroups that are significantly different from each other (P < 0.05),within a feeding phase in the same column, are indicated by different superscripts (n = 9).
Abbreviations: aFCR, mortality-adjusted feed conversion ratio; BWG, BW gain.
The results are reported as mean ± SE.
In the grower phase (22–35 d), once the challenge with C. perfringens had already been completed, no significant differences in BW gain were observed between the different experimental treatments. However, the birds fed with BA DSM 25840 had the lowest aFCR, whereas the control birds had the highest aFCR. The 2 groups that received the diets supplemented with B. subtilis (BS DSM 32324 and BS DSM 32325) did not show significant differences compared with the control group. However, the BS DSM 32324 group did not differ from the BA DSM 25840 one either. The multi–train combination group presented an intermediate aFCR between the 2 groups supplemented with B. subtilis strains (BS DSM 32324 and BS DSM 32325) and the one supplemented with B. amyloliquefaciens (BA DSM 25840).
In the finisher phase (36–42 d), the chickens that received the diet supplemented with the multistrain combination obtained the best performance, both in BW gain and in aFCR. In the short finisher period, the birds that were fed the BA DSM 25840 diet had the poorest performance. The other 3 groups showed intermediate values for both parameters.
In the overall experimental period (Table 5), the group fed the multistrain combination was the only one that presented significantly better performance than the control group in both BW gain and aFCR. The birds fed the diets containing B. subtilis (BS DSM 32324 and BS DSM 32325) showed similar BW gain to that of the multistrain combination group, whereas the BS DSM 32324 group and BA DSM 25840 group showed no significant differences in aFCR compared with the multistrain group. No significant differences in mortality were observed between experimental treatments. However, dietary supplementation with DFM, regardless of the DFM, resulted in significantly lower NE mortality (control: 4.2%; BS DSM 32324: 0.4%; BS DSM 32325: 0.9%; BA DSM 25840: 1.6%; multi-strain group: 0.4%; P < 0.05).
Table 5.
The effect of 3 Bacillus probiotic strains and their combination on performance of Clostridium perfringens–challenged broilers in the entire experimental period (1–42 d).1
| Probiotic inclusion | BW gain (g) | aFCR |
|---|---|---|
| Control | 2,236.80b ± 19.97 | 1.96a ± 0.02 |
| BS DSM 32324 | 2,290.94a,b ± 14.27 | 1.87b,c ± 0.01 |
| BS DSM 32325 | 2,321.93a ± 30.98 | 1.90b ± 0.02 |
| BA DSM 25840 | 2,243,63b ± 31.05 | 1.90b ± 0.01 |
| Multistrain combination | 2,342.43a ± 32.40 | 1.84c ± 0.01 |
a–cGroups that are significantly different from each other within a column at P < 0.05 are indicated by different superscripts (n = 9).
The results are reported as mean ± SE.
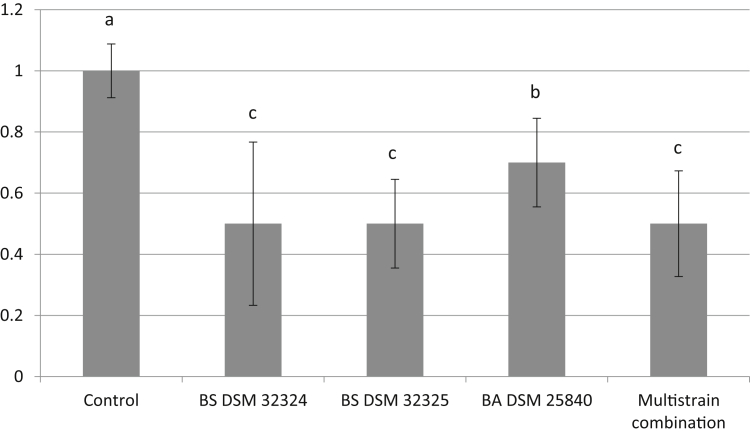
Figure 1 shows the lesion score of the different experimental groups. In general, all groups showed a fairly low score. The multistrain combination and the 2 strains of B. subtilis (BS DSM 32324 and BS DSM 32325) resulted in the lowest score, whereas the control group showed the highest one. The BA DSM 25840 group showed intermediate score. Furthermore, the multistrain combination resulted in the highest percentage of birds with lesion score of zero (Table 6).
Figure 1.
Intestinal lesion scores of the control group and groups supplemented with Bacillus strains and their combination on day 21 of trial. Groups that are significantly different from each other at P < 0.05 are indicated by different letters (a–c).
Table 6.
The effect of 3 Bacillus probiotic strains and their combination on the distribution of intestinal lesion scores on day 21 of trial.
| Probiotic inclusion | n | Number of birds with 0, 1, 2, 3 lesion score |
Percentage of birds with 0, 1, 2, 3 lesion score (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Control | 45 | 7 | 33 | 5 | 0 | 15.6 | 73.3 | 11.1 | 0.0 |
| BS DSM 32324 | 45 | 22 | 23 | 0 | 0 | 48.9 | 51.1 | 0.0 | 0.0 |
| BS DSM 32325 | 45 | 23 | 22 | 0 | 0 | 51.1 | 48.9 | 0.00 | 0.0 |
| BA DSM 25840 | 45 | 15 | 29 | 1 | 0 | 33.3 | 64.6 | 2.2 | 0.0 |
| Multistrain combination | 45 | 24 | 21 | 0 | 0 | 53.3 | 46.7 | 0.00 | 0.0 |
Discussion
In recent times, several studies have evaluated the use of Bacillus spp. as DFM for the prevention and palliation of NE effects on the performance in chickens (De Oliveira et al., 2019; Whelan et al., 2019; Bortoluzzi et al., 2019; Hernández-Patlan et al., 2019). However, preceding exhaustive strain selection procedures need to be completed as per the mentioned prophylactic and alleviating purpose. Thus, the 3 strains tested in the present study (DSM32324, DSM 32325, and DSM 25840) were previously selected, among numerous B. subtilis and B. amyloliquefaciens strains, on their efficacy with regard to 2 representative properties of the Bacillus genus, which make it 1 of the genera of choice for the development of poultry DFM: the ability to degrade carbohydrates and proteins and the capacity of inhibiting the growth of CP. It is well-known that Bacillus spp. isolates differ in their capacity to produce and secrete bioactive metabolites, even if they belong to the same species (Larsen et al., 2014). It is therefore essential to discern how effective each strain is, compared with other strains of the same species, to definitively become an effective DFM in practice. The tested strains in this trial showed indeed different abilities among them, in addition to excelling in most of the cases with respect to the studied collection of strains of the same species (Table 3), which enables them as complementary strains to develop an effective DFM for birds. In this sense, the B. subtilis DSM 32324 strain showed both a relatively high carbohydrate degradation capacity (Table 3) and a high ability to inhibit CP growth. In turn, the B. subtilis DSM 32325 strain was also effective in carbohydrate degradation, with a moderate ability to inhibit CP. The B. amyloliquefaciens DSM 25840 strain stood out for its high protein degradation capacity (Table 3), while its CP inhibitory capacity was middle.
In the present study, the abilities of the tested strains as DFM, confirmed in the in vitro tests, were definitely corroborated in the in vivo trial, giving full alignment between the in vitro and the in vivo results. The suitability of B. subtilis and B. amyloliquefaciens strains as poultry DFM has previously been verified by other researchers. Accordingly, Latorre et al. (2016) screened 31 Bacillus spp. from environmental and poultry sources as DFM candidates in view of their enzyme production profile, biofilm synthesis capacity, and pathogen-inhibition activity and found that a B. subtilis strain and 2 B. amyloliquefaciens strains were superior enzyme producers. Furthermore, the majority of the tested Bacillus spp. strains showed antimicrobial activity against Salmonella enterica serovar Enteritidis, Escherichia coli, and Clostridium difficile. Likewise, Penaloza-Vázquez et al. (2019), assessed the suitability as poultry DFM of 200 endospore-forming bacteria isolated from sourdough and the gastrointestinal tract of young broiler chicks based on the production of a series of exoenzymes, survivability under stress conditions, sporulation efficiency, biofilm formation, compatibility among themselves, and antagonistic effects against pathogenic bacteria such as Enterococcus cecorum, S. enterica and E. coli. The authors also concluded that the strains identified as B. amyloliquefaciens and B. subtilis demonstrated remarkable potential as probiotics for poultry. However, in the previously referenced studies, CP growth inhibition capacity was not studied, as it was performed in the present study. Nevertheless, the inhibition of CP growth by B. subtilis and B. amyloliquefaciens has certainly been previously described by several researchers (Teo and Tan, 2005; Klose et al., 2010; Latorre et al., 2015; Geeraerts et al., 2016; Horng et al., 2019).
The observed carbohydrate and protein degradation by the tested strains in the present study is undoubtedly triggered by the capacity of both Bacillus species of producing nonstarch polysaccharide enzymes and proteases (Larsen et al., 2014; Latorre et al. 2015, 2016; Gong et al., 2018). Thus, the production of exogenous enzymes by Bacillus DFM leads to improvements in the use of nutrients when these strains are added to poultry feed. The dietary supplementation with Bacillus spp. can improve energy and CP digestibility, which is highly associated with a subsequent digestive capacity improvement (Sen et al., 2011). It has been observed in several studies that the use of Bacillus DFM in diets for healthy chickens with a lower energy and protein content than control diets (without any added DFM), equals the growth performance of the DFM-fed birds to that of control birds, corroborating the positive effect of Bacillus DFM on the digestive capacity of birds (Harrington et al., 2016; Goodarzi Boroojeni et al., 2018; Upadhaya et al., 2019). Besides the well-defined positive effect on the nutrient utilization, the enzyme production of B. subtilis and B. amyloliquefaciens may have a subsequent positive effect on the gut health of the birds under challenging conditions. In this sense, the improvement of nutrient digestibility has been described as an important beneficial factor in controlling NE in chickens (Williams, 2005; Moore, 2016). The increase of the digestion of dietary carbohydrates—particularly nonstarch polysaccharides—and the subsequent decreased digesta viscosity, as well as the improvement of the protein digestion and the consequent reduction of undigested protein available to feed pathogenic bacteria, provide a nutritional environment that rather hinders the growth of CP in the gastrointestinal tract of chickens (Shojadoost et al., 2012; Latorre et al., 2015). The reported in vitro results in the present study show the capacity of the tested Bacillus strains and their combination to inhibit the growth of CP, probably owing to a combination of both the production of antimicrobial-like compounds—as it appears from the CP inhibition test in agar—and changes in environmental conditions—as it seems from the carbohydrate and protein degradation test (Table 3). Consequently, the 3 strains and their combination tested were considered appropriate to be evaluated as DFM in the succeeding in vivo trial, under CP challenge conditions.
In the in vivo part of the present study, the relative efficacy among each of the tested strains and their combination on the performance of the birds was different depending on the feeding phase (Table 4). Thus, the supplementation of the feed with each 1 of the 2 strains of B. subtilis (DSM 32324 and DSM 32325) and the multistrain combination resulted in superior performance in relation to the control diet and BA DSM 25840 treatment in the starter period (0–21 d) and the multistrain combination also in the finisher period (36–42 d). However, the supplementation with the B. amyloliquefaciens strain (DSM 25840) was prominent in the grower period (22–35 d), just after the challenge with CP. These differences between experimental treatments, depending on the feeding phase, are conceivably because of the different effect of each DFM on the intestinal microbiota of chickens in each of the periods studied. Li et al. (2019) observed variations in the effect of different strains of Bacillus spp. added in feed on the composition and diversity of the intestinal microbiota of chickens, depending on the age of the birds. It has been observed that microbial diversity increases during chicken development, reaching at the peak approximately on day 14 for the foregut and then remaining stable or decreasing slightly thereafter under challenge-free conditions (Huang et al., 2018). However, in the present study, a CP challenge was held as an external factor on day 19, 20 and 21, which obviously destabilized the microbiota balance in the gut, simulating what, to a greater or lesser extent, occurs in practice.
In the present study, the feed supplementation with both B. subtilis strains (DSM 32324 and DSM 32325) and with the multistrain combination resulted in significantly higher BW and lower aFCR than the control group during the starter period (0–21 d), including the challenge d with CP. The group fed B. amyloliquefaciens–supplemented feed (BA DSM25840) showed a similar BW to that of the control group and an intermedium aFCR between the other 3 DFM treatments and the control group (Table 4). The marked preeminence of the 2 groups supplemented with B. subtilis (BS DSM 32324 and BA DSM 32325) and the group supplemented with the multistrain combination is most likely owing to the particularly high carbohydrate-degrading ability of both tested strains of B. subtilis (Table 3), as well as the strong CP-inhibitory power by strain DSM 32324. Dietary carbohydrates are a primary source of energy for young chicks. It has been suggested that the higher the energy and nutrient availability during early ages of chickens, the better the development of their gastrointestinal tract (Adedokun and Olojede, 2019). With regard to CP-inhibitory capacity of B. subtilis, the synthesis of CP growth inhibitory factors by B. subtilis has been known for a long time (Teo and Tan, 2005). In this regard, B. subtilis–derived surfactin is an important antimicrobial peptide with antibacterial activity through disruption of the bacterial membrane. Thus, it has been observed that the mentioned surfactin is able to cause the death of CP in a dose- and time-dependent manner (Horng et al., 2019). The results on NE lesion score at 21 d of age (Figure 1 and Table 6) are consistent with the performance results during the 0- to 21-d onset period (Table 4) and with the in vitro CP inhibition results. All DFM treatments showed a significantly lower NE lesion score than the control treatment, with the 2 B. subtilis treatments (BS DSM32324 and BS DSM 32 325) and the multistrain combination showing the lowest score (Figure 1). Despite the significant differences in NE lesion score between the control group and the DFM groups, the low scores observed, owing to the subclinical induction of NE, make it difficult to draw consistent conclusions in this regard, given that a difference of 0.5 score is most likely not biologically relevant enough. Nevertheless, it does deserve to emphasize that the multistrain combination was the experimental treatment that resulted in the largest number of animals without lesion (Table 6).
In our study, feed supplementation with B. amyloliquefaciens (BA DSM 25840) significantly decreased FCR, compared with the control group, in the grower period, immediately after the CP challenge (day 22–35). As in this study, other researchers (De Oliveira et al., 2019) have recently observed a positive effect of B. amyloliquefaciens as DFM on FCR and lower CP counts in ileal content when added to diets of chickens right after challenging birds with CP (De Oliveira et al., 2019). According to Hong et al. (2019), B. amyloliquefaciens DFM are able to modulate the intestinal microbiota of chickens, increasing the Firmicutes/Bacteroidetes ratio until day 35 of life. The described microbiota modulation by B. amyloliquefaciens could explain the improvement in aFCR in B. amyloliquefaciens–supplemented birds (BA DSM 25840) compared with control birds, after the infection with CP. It has been proven that a higher Firmicutes/Bacteroidetes ratio promotes broiler performance (Singh et al., 2013; Mancabelli et al., 2016). In addition to the intestinal microbiota modulating effect of B. amyloliquefaciens, its great ability to degrade dietary protein may also contribute to a faster recovery of the digestive capacity of the birds and consequently of their intestinal health, after a mild infection with CP, as stated previously. Contrarily, Geeraerts et al. (2016), despite the substantial CP inhibitory activity of B. amyloliquefaciens they observed in vitro, did not detect any beneficial effect of the referred DFM against NE in vivo. The lack of effect in vivo in the aforementioned study could be explained by the fact of evaluating the addition of vegetative cells. On the other hand, in the present study, all the tested DFM were added to the feed in the form of spores. Sporulated DFM survive feed-manufacturing processes and pass through the stomach, reaching the intestine where spores germinate and function through mechanisms which require them to be metabolically active, such as secretion of antimicrobial compounds (Cartman et al., 2008).
In the finisher period, the group supplemented with B. amyloliquefaciens (BA DSM 25840) did not only stand out with respect to the other dietary treatments, but it was the group that showed the worst performance (Table 4). The lack of positive effect of the tested B. amyloliquefaciens strain (DSM 25840) in the last period of the trial (36–42 d) could be owing to the fact that its modulating effect on the intestinal microbiota is diluted after 35 d of life. Hong et al. (2019) indicated that, as time progress, the proportion of Bacteroidetes in the gut increases, and the proportion of Firmicutes decreases, regardless of the dietary supplementations. Furthermore, another reason behind the better performance results with the other 3 experimental treatments (BS DSM 32324, BS DSM 32325, and multistrain combination) than with BA DSM 25840, from 36 to 42 d of life, could be a compensatory growth of those birds that did not receive BA DSM 25840. In general, a compensatory growth can be observed after challenge periods (Arczewska-Wlosek and Światkiewicz, 2013). With regard to the other experimental treatments of the present study, the multistrain combination resulted in a significant growth performance improvement compared with the control group in the finisher period (36–42 d). The positive effect observed with the multistrain combination in our trial in the finisher period most probably is because of a synergistic effect between its 3 component strains. As aforementioned, the performance results observed in the finisher period also show the different behavior of each DFM treatments depending on the life span of the birds.
In the global experimental period (0–42 d), BS DSM 32325 and the multistrain combination significantly improved BW gain with respect to the control group. However, aFCR was also improved by BA DSM 25804 besides BS DSM 32325 and the multistrain combination (Table 5). Definitely, the multistrain combination was the only experimental treatment that significantly improved both BW and aFCR in the overall period. In the same way, Ramlucken et al. (2020) recently observed a significant FCR improvement but a nonsignificantly higher BW in birds fed a multistrain DFM compared with control birds without any DFM in their diet. In addition to using different DFM strains in the present study and in the previously referred study, the difference observed in the significance of the BW improvement when feeding birds with a multistrain DFM could be potentially affected by the virulence of the CP strains used in each study as well as differences in the environments where both trials were performed. In our study, a pathogenic field strain of CP, alpha toxin and NetB positive, that had been isolated from a commercial poultry operation diagnosed with NE was used, trying to reliably simulate conditions that usually occur in the field. These stark trial conditions potentially allowed the multistrain combination to express its full probiotic effect through the whole experimental period. Regarding the positive effect of DFM supplementation on NE mortality observed in our study, it agrees with the findings of other researchers using Bacillus-based DFM in poultry diets (Bortoluzzi et al., 2019; Whelan et al., 2019). On the contrary, in other recent studies (De Oliveira et al., 2019; Hernandez-Patlan et al., 2019; Ramlucken et al., 2020), no significant improvements in mortality are reported when DFM is used in chickens affected by NE. This shows again the probiotic efficacy of the DFM tested in this trial, which, as explained earlier in this article, were selected from a wide collection of Bacillus spp.
In conclusion, our results show the suitability of the evaluated Bacillus multistrain combination as a fully effective and reliable DFM to be considered within strategies for coping with CP-associated disorders in broiler chickens. Its positive effect on the performance may be attributable to complementary effects of its constituent single strains on nutrient availability, pathogen inhibition and, in the end, on intestinal health and function. Future considerations include elucidating further the modes of action of this novel Bacillus multistrain DFM.
Acknowledgments
The authors would like to acknowledge the excellent technical assistance of Rikke Dollerup Bech (Chr. Hansen A/S, R&D Discovery, Microbial Screening), Christel Galschioet and Nina Milora (Chr. Hansen A/S, Animal Health Innovation). This work was supported by Chr. Hansen A/S, Animal Health Innovation, Hoersholm, Denmark. This work is financially supported by Chr. Hansen A/S. Authors are employed by Chr. Hansen or have worked in consultancy conditions and the experiment has been financial supported by Chr. Hansen.
Disclosures
The authors declare no conflicts of interest.
References
- Adedokun S.A., Olojede O.C. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front. Vet. Sci. 2019;5:348. doi: 10.3389/fvets.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arczewska-Wlosek A., Światkiewicz S. Improved performance due to dietary supplementation with selected herbal extracts of broiler chickens infected with Eimeria spp. J. Anim. Feed Sci. 2013;22:257–263. [Google Scholar]
- Bortoluzzi C., Vieira B.S., De Paula Dorigam J.C., Menconi A., Sokale A., Doranalli K., Applegate T.J. Bacillus subtilis DSM 32315 supplementation attenuates the effects of Clostridium perfringens challenge on the growth performance and intestinal microbiota of broiler chickens. Microorganisms. 2019;7:71. doi: 10.3390/microorganisms7030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard M., D: Entian K., Stein T. Development and application of a microtiter plate-based autoinduction bioassay for detection of the lantibiotic subtilin. J. Microbiol. Methods. 2007;70:179–185. doi: 10.1016/j.mimet.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Cartman S.T., La Ragione R.M., Woodward M.J. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl. Environ. Microbiol. 2008;74:5254–5258. doi: 10.1128/AEM.00580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G., Uzal F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 2013;25:314–327. doi: 10.1177/1040638713483468. [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Wilkie D.C., Van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:60–88. [Google Scholar]
- De Oliveira M.J.K., Sakomura N.K., de Paula Dorigam J.C., Doranalli K., Soares L., Viana G.D.S. Bacillus amyloliquefaciens CECT 5940 alone or in combination with antibiotic growth promoters improves performance in broilers under enteric pathogen challenge. Poult. Sci. 2019;98:4391–4400. doi: 10.3382/ps/pez223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaghabee F.M.F., Rokana N., Gulhane R.D., Sharma C., Panwar H. Bacillus as potential probiotics: Status, concerns and future perspectives. Front. Microbiol. 2017;8:1490. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agricultural Organization of the United Nations and World Health Organization . Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. American Córdoba Park Hotel; Córdoba, Argentina: 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria; pp. 1–34. [Google Scholar]
- Fuchs S.W., Jaskolla T.W., Bochmann S., Kötter P., Wichelhaus T., Karas M., Stein T., Entian K.D. Entianin, a novel subtilin-like lantibiotic from Bacillus subtilis subsp. spizizenii DSM 15029 T with high antimicrobial activity. Appl. Environ. Microbiol. 2011;77:1698–1707. doi: 10.1128/AEM.01962-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraerts S., Delezie E., Ducatelle R., Haesebrouck F., Devreese B., Van Immerseel F. Vegetative Bacillus amyloliquefaciens cells do not confer protection against necrotic enteritis in broilers despite high antibacterial activity of its supernatant against Clostridium perfringens in vitro- Br. Poult. Sci. 2016;57:324–329. doi: 10.1080/00071668.2016.1169246. [DOI] [PubMed] [Google Scholar]
- Gong L., Wang B., Mei X., Xu H., Qin Y., Li W., Zhou Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018;89:1561–1571. doi: 10.1111/asj.13089. [DOI] [PubMed] [Google Scholar]
- Goodarzi Boroojeni F., Vahjen W., Männer K., Blanch A., Sandvang .D., Zentek J. Bacillus subtilis in broiler diets with different levels of energy and protein. Poult. Sci. 2018;97:3967–3976. doi: 10.3382/ps/pey265. [DOI] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Haque M.I., Ahmad N., Miah A.M. Comparative analysis of body weight and serum biochemistry in broilers supplemented with some selected probiotics and antibiotic growth promoters. Adv. J. Vet. Anim. Res. 2017;4:288–294. [Google Scholar]
- Harrington D., Sims M., Kehlet A.B. Effect of Bacillus subtilis supplementation in low energy diets on broiler performance. J. Appl. Poult. Res. 2016;25:29–39. [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Pontin K.P., Hernandez-Velasco X., Merino-Guzman R., Adhikari B., López-Arellano R., Kwon Y.M., Hargis B.M., Arreguin-Nava M.A., Tellez-Isaias G., Latorre J.D. Impact of a Bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions, and ileal microbiota in broiler chickens using a laboratory challenge model. Front. Vet. Sci. 2019;6:108. doi: 10.3389/fvets.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacre C.L., Froyman R., Gautrais B., George B., Goodwin M.A., Brown J. Use of Aviguard, Virginiamycin, or Bacitracin MD in experimental Clostridium perfringens-associated necrotizing enteritis. J. Appl. Poult. Res. 1998;7:412–418. [PubMed] [Google Scholar]
- Hofacre C.L., Smith J.A., Mathis G.F. An optimist’s view on limiting necrotic enteritis and maintaining broiler gut health and performance in today’s marketing, food safety and regulatory climate. Poult. Sci. 2018;97:1929–1933. doi: 10.3382/ps/pey082. [DOI] [PubMed] [Google Scholar]
- Hong Y., Cheng Y., Li Y., Li X., Zhou Z., Shi D., Li Z., Xiao Y. Preliminary study on the effect of Bacillus amyloliquefaciens TL on cecal bacterial community ctructure of broiler chickens. Hindawi Biomed. Res. Int. 2019;2019:1–11. doi: 10.1155/2019/5431354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng Y., Yu Y., Dybus A., Hsiao F.S., Cheng Y. Antibacterial activity of Bacillus species-derived surfactin on Brachyspira hyodysenteriae and Clostridium perfringens. AMB Expr. 2019;9:188. doi: 10.1186/s13568-019-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.Y., Zhang K.P., Xiao F., Jiang H.C., Wang D.Z., Tang D., Liu B., Liu Y., Liu X., He H., Liu X., Liu Z., Qing C., Liu J., Huang Y., Ren L., Yun L., Yin Q., Lin C., Zeng X., Su J., Yuan L., Lin N., Hu H., Cao S., Huang Y., Guo W., Fan, Zeng J. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 2018;6:211. doi: 10.1186/s40168-018-0590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose V., Bayer K., Bruckbeck R., Schatzmayr G., Loibner A.P. In vitro antagonistic activities of animal intestinal strains against swine-associated pathogens. Vet. Microbiol. 2010;144:515–521. doi: 10.1016/j.vetmic.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Larsen N., Thorsen L., Kpikpi E.N., Stuer-Lauridsen B., Cantor M.D., Nielsen B., Brockmann E., Derkx P.M.F., Jespersen L. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl. Microbiol. Biotechnol. 2014;98:1105–1118. doi: 10.1007/s00253-013-5343-6. [DOI] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Kuttappan V.A., Wolfenden R.E., Vicente J.L., Wolfenden A.D., Bielke L.R., Prado-Rebolledo O.F., Morales E., Hargis B.M., Tellez G. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Front. Vet. Sci. 2015;2:25. doi: 10.3389/fvets.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Wolfenden R.E., Vicente J.L., Wolfenden A.D., Menconi A., Bielke L.R., Hargis B.M., Tellez G. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 2016;3:95. doi: 10.3389/fvets.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang J., Zhang H., Wu S., Hui Q., Yang C., Fang R., Qi G. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 2019;9:1968. doi: 10.3389/fphys.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancabelli L., Ferrario C., Milani C., Mangifesta M., Turroni F., Duranti S., Lugli G.A., Viappiani A., Ossiprandi M.C., Van Sinderen D., Ventura M. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016;18:4727–4738. doi: 10.1111/1462-2920.13363. [DOI] [PubMed] [Google Scholar]
- Mazanko M.S., Gorlov I.F., Prazdnova E.V., Makarenko M.S., Usatov A.V., Bren A.B. Bacillus probiotic supplementations improve laying performance, egg quality, hatching of laying hens, and sperm quality of roosters. Probiot. Antimicrobiol. Proteins. 2017;10:367–373. doi: 10.1007/s12602-017-9369-4. [DOI] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- 53.National Research Council. 1994. Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. Press, Washington, DC.
- Opengart K. Necrotic enteritis. In: Saif Y.M., editor. Diseases of Poultry. 12th ed. BlackWell Publishing; Ames, IA: 2008. pp. 872–879. [Google Scholar]
- Park J.H., Kim I.H. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult. Sci. 2014;93:2054–2059. doi: 10.3382/ps.2013-03818. [DOI] [PubMed] [Google Scholar]
- Penaloza Vázquez A., Ma L.M., Rayas-Duarte P. Isolation and characterization of Bacillus spp. strains as potential probiotics for poultry. Can. J. Microbiol. 2019;65:762–774. doi: 10.1139/cjm-2019-0019. [DOI] [PubMed] [Google Scholar]
- Ramlucken U., Ramchuran S.O., Moonsamy G., Lalloo R., Thantsha M.S., Jansen van Rensburg C. A novel Bacillus based multi-strain probiotic improves growth performance and intestinal properties of Clostridium perfringens challenged broilers. Poult. Sci. 2020;99:331–341. doi: 10.3382/ps/pez496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M.P., Fassani E.J., García Junior A.A.P., Rodrigues B.P., Bertechini A.G., Barrett N., Persia M.E., Schmid C.J. Effect of Bacillus subtilis (DSM 17299) on performance, digestibility, intestine morphology, and pH in broiler chickens. J. Appl. Poult. Res. 2017;26:573–583. [Google Scholar]
- Sakurai J., Nagahama M., Oda M. Clostridium perfringens alpha-toxin: characterization and mode of action. J. Biochem. 2004;136:569–574. doi: 10.1093/jb/mvh161. [DOI] [PubMed] [Google Scholar]
- Sen S., Ingale S.L., Kim Y.W., Kim J.S., Kim K.H., Lohakare J.D., Kim E.K., Ryu M.H., Kwon I.K., Chae B.J. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2011;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Seo J.K., Park T.S., Kwon I.H., Piao M.Y., Lee C.H., Ha J.K. Characterization of cellulolytic and xylanolytic enzymes of Bacillus licheniformis JK7 isolated from the rumen of a native Korean goat. Asian-Aust. J. Anim. Sci. 2013;26:50–58. doi: 10.5713/ajas.2012.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Singh R.L., Kakkar P. Bacillus licheniformis IITRHR2: a novel source of antimicrobial proteinaceous food substance. J. Microbiol. Antimicrob. 2010;2:127–133. [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Karimi A., Devendra K., Waldroup P.W., Cho K.K., Kwon Y.M. Inuence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poult. Sci. 2013;92:272–276. doi: 10.3382/ps.2012-02603. [DOI] [PubMed] [Google Scholar]
- Smyth J.A. Pathology and diagnosis of necrotic enteritis: is it clear-cut? Avian Pathol. 2016;45:282–287. doi: 10.1080/03079457.2016.1158780. [DOI] [PubMed] [Google Scholar]
- Sumi C.D., Yang B.W., Yeo I.-C., Hahm Y.T. Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can. J. Microbiol. 2015;61:93–103. doi: 10.1139/cjm-2014-0613. [DOI] [PubMed] [Google Scholar]
- Teo A.Y., Tan H.M. Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl. Environ. Microbiol. 2005;71:4185–4190. doi: 10.1128/AEM.71.8.4185-4190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya S.D., Rudeaux F., Kim I.H. Effects of inclusion of Bacillus subtilis (Gallipro) to energy- and protein-reduced diet on growth performance, nutrient digestibility, and meat quality and gas emission in broilers. Poult. Sci. 2019;98:2169–2178. doi: 10.3382/ps/pey573. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Buck J.D., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Wang S., Zeng X.F., Wang Q.W., Zhu J.L., Peng Q., Hou C.L., Thacker P., Qiao S.Y. The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by Clostridium perfringens in broilers. J. Anim. Sci. 2015;93:4750–4760. doi: 10.2527/jas.2015-9284. [DOI] [PubMed] [Google Scholar]
- Whelan R.A., Doranalli K., Rinttilä T., Vienola K., Jurgens G., Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult. Sci. 2019;98:3450–3463. doi: 10.3382/ps/pey500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Zhang Z.F., Cho J.H., Kim I.H. Effects of Bacillus subtilis UBT-MO2 on growth performance, immune organ relative weight, fecal gas concentration and intestinal microbial shedding in broiler chickens. Livest Sci. 2013;155:343–347. [Google Scholar]