Abstract
The study investigated the benefit of a Bacillus subtilis probiotic (Bs 29,784) in necrotic enteritis (NE)–challenged broilers. Four treatments were performed with 312 male day-old Ross 308 reared in floor pens from day 0 to day 35: 2 groups fed control diet without or with NE challenge (CtrlNC and CtrlNE); 2 groups fed probiotic and antibiotic supplements in the control diet with NE challenge (ProNE and AntNE). Necrotic enteritis challenge procedures commenced with inoculation of Eimeria spp 1 mL/bird per os at day 9 and Clostridium perfringens EHE-NE18 (approximately 108 cfu/mL) 1 mL/bird per os at day 14 and day 15. Performance parameters were measured on day 16 and day 35. Lesion, cecal microbiota, and jejunal gene expression were analyzed on day 16. Necrotic enteritis challenge significantly suppressed the performance parameters compared with CtrlNC: 27% weight gain reduction, 11 points feed conversion ratio (FCR) increase at day 16, and 12% weight gain reduction, 5-point FCR increase at day 35. By day 35, ProNE and AntNE treatments enabled significantly higher weight gain (4 and 9%, respectively) than CtrlNE. Compared with CtlrNE and contrary to AntNE, ProNE treatment exhibited upregulation of genes coding for tight junctions proteins (CLDN1, JAM2, TJP1), cytokines (IL12, interferon gamma, TGFβ), and Toll-like receptors (TLR5, TLR21) suggesting enhanced immunity and intestinal integrity. 16S NGS analysis of cecal microbiota at day 16 showed a decreased alpha diversity in challenged groups. Principal component analysis of operational taxonomic unit (OTU) abundance revealed that ProNE and AntNE grouped closely while both distantly from CtrlNC and CtrlNE, which were separately grouped, indicating the similar effects of ProNE and AntNE on the OTU diversity that were however different from both CtrlNC and CtrlNE. Microbiota analysis revealed an increase of genera Faecalibacterium, Oscillospira, and Butyricicoccus; and a decrease of genera Ruminococcus, Lactobacillus, and Bacteroides; and an increase of the Firmicutes-to-Bacteroidetes ratio in ProNE and AntNE groups compared with the CtlrNE group. It is concluded that Bs 29,784 may enable improved health of broiler chickens under NE conditions thus performance implications.
Key words: probiotic, Bacillus, necrotic enteritis, microbiota, broiler
Introduction
A recent estimate has speculated US$6 billion per annum profitability loss in the poultry industry globally by necrotic enteritis (NE) related to productivity degradation and measures for containment (Wade and Keyburn, 2015). The etiologic agent of the disease were identified in virulent Clostridium perfringens type G through a highly opportunistic pathology with cooperative factors that initialize degradation on intestinal health until an excavation of immune status and drastic distortion to the intestinal microbiome happens (Van Immerseel et al., 2009; Moore, 2016). It was reported that coccidiosis, high level of fish meal in the diet, viscosity increased by nonstarch polysaccharides, and other predisposing factors could compromise intestinal health and trigger proliferation of C. perfringens, encouraging the bacterium to attach to and intrude the compromised intestinal epithelium of the affected chickens (Annett et al., 2002; Collier et al., 2008). It is essential that pathogenic C. perfringens are capable of producing NetB toxins to initiate NE in the poultry flock (Keyburn et al., 2008). Reports have shown that most of NE-affected flocks had isolated C. perfringens carrying NetB in the genome. Furthermore, the threat of NE exists when birds are succumbed to it by predisposing factors because all healthy birds carry various strains of C. perfringens in the gastrointestinal tract as commensal bacteria (Van Immerseel et al., 2009).
Because the nature of the disease is highly relevant to bacterial infection in the intestine, NE can be effectively controlled with the preventive supplementation of antibiotics in feed in the current control measure. However, the implicit withdrawal of antibiotic growth promoters from animal production in the European Union in 2006 (Regulation [EC] N°1831/2003) and voluntary discontinuation of subtherapeutic use of antibiotics in major poultry producer countries exploit alternative methods to counter bacterial borne disease in poultry (Dahiya et al., 2006). Over the decades, genetic selection, continuing improvement in nutrition, and refinement in management practices have demonstrated a significant improvement in modern broiler production (Tsiouris, 2016). Modern broiler production can achieve more efficient use of feed that is translated to weight gain and shorter rearing period compared with older production settings. It is therefore important to maintain a healthy status of the intestine in the animal to ensure this high efficiency is not compromised (Raidal, 2000). Thus, subclinical NE in the flocks with suboptimal health status of intestine may lead to compromised performance and loss of profit for growers. The costs of subclinical NE can quickly escalate owing to extra feed consumption and reduction of the market weight of the animals (Skinner et al., 2010). It is speculated that there is a strong association between intestinal microbiota shift and disease outbreak (Gabriel et al., 2006).
Nutritional immunity of the host can restrain and alter the intestinal ion and protein availability in the gut. This potentially creates a competitive environment among gut commensals whereas the inflamed intestine will have compromised capability to prevent the elevation of enteric pathogens (Liu et al., 2012; Faber and Bäumler, 2014). Probiotics are the broad term of direct-fed microbial which may consist of one or multiple strains of microorganisms that can beneficially affect the intestinal ecosystem and the intestinal health of the host animal (Edens, 2003; Dhama et al., 2008). Various microorganisms have shown beneficial potent in animal production, such as Enterococcus spp., Saccharomyces yeast, spores of Bacillus spp., Bifidobacterium, and to the lesser extent, Lactobacillus spp. (Fan et al., 2006). Probiotics derived from spore-forming organisms, such as Bacillus spores, have shown examples as a promising probiotic candidate in countering Escherichia coli, Salmonella enterica, serotype Enteritidis, and C. perfringens in the poultry settings (La Ragione et al., 2001; La Ragione and Woodward, 2003; Rhayat et al., 2017). However, the protective effect of the direct-fed microbial against disease alleviation may not equally translate to improvements on the performance parameters such as the weight gain (Awad et al., 2009; Waititu et al., 2014).
Bacillus subtilis strain DSM 29784 (Bs 29,784) is a relatively new probiotic (Rhayat et al., 2017). It has been reported that the probiotic can improve feed conversion ratio (FCR) and small intestine growth and villus height in broilers (Mohammadigheisar et al., 2019). Furthermore, the supplementation of Bs 29,784 in the chicken's diet may selectively enrich beneficial bacterial communities, which in turn promote the growth and performance of hens (Neijat et al., 2019). The present study was the continuing research of the capability of Bs 29,784 to improve intestinal health status and the growth performance in broiler chickens under experimentally induced NE condition.
Materials and methods
Experiment Design and Bird Husbandry
The Animal Ethics Committee of the University of New England approved the study. A total of 312 male Ross 308 broiler chickens were procured as day-old from a local hatchery (Biada, Tamworth, Australia) and raised in a floor pen facility for 35 d. Birds were randomly allocated to a group of 13 birds each into a 24 individual floor pen partitions on arrival. Each pen featured 120 × 76 cm2 bedded dry wood shavings providing water and feed ad libitum. Four treatment groups were randomly allocated to 24 pen partitions, including 2 control diet treatments without (CtrlNC) or with (CtrlNE) challenge and 2 NE challenged with additive either with probiotic (ProNE) or antibiotic (AntNE), respectively. Temperature settings followed Ross 308 recommendations with a starting temperature of 34°C–35°C on arrival up to day 3 of age, then decreasing cumulatively 2°C in every 3 d until the temperature of 22°C–23°C was reached by day 21. Birds were inspected at least twice daily for environmental conditions and bird health, welfare, and mortality.
Dietary Treatments
A standard three-phase feeding regime was practised with a starter diet (day 0–10), a grower diet (day 10–24), and a finisher diet (day 24–35), in crumbled form (⌀ ≤ 2 mm) during day 0–10 and pellet form (⌀ 3–3.5 mm) during day 10–35. A cold pelleted (50°C–70°C) wheat–soybean meal was used as the basal diet (Table 1). The control diet was provided to the CtrlNC and the CtrlNE and the addition of either probiotic or antibiotic supplement to ProNE and AntNE treatments, respectively. The probiotic diet was made up of addition of 500 g/ton supplement of B. subtilis DSM29784 (Alterion NE50, Adisseo, France S. A. S) on top of the basal formula to obtain a final concentration of 108 cfu/kg of feed. The antibiotic diet was supplemented with Zn bacitracin 50 mg/kg and salinomycin 75 mg/kg on top of the base formula.
Table 1.
Feed formulation and nutrient composition.
| Diet | Starter day 0–10 | Grower day 10–24 | Finisher day 24–35 | Unit |
|---|---|---|---|---|
| Ingredient name | ||||
| Wheat | 30.0 | 44.7 | 34.8 | % |
| Sorghum | 31.1 | 20.0 | 30.0 | % |
| Soybean meal | 27.1 | 19.1 | 18.9 | % |
| Solvent-extracted canola meal | 2.0 | 5.0 | 4.5 | % |
| Meat and bone meal | 4.6 | 5.0 | 5.0 | % |
| Canola oil | 2.4 | 3.9 | 4.8 | % |
| Limestone | 0.679 | 0.585 | 0.525 | % |
| Dicalcium phosphorus 18P/21Ca | 0.654 | 0.436 | 0.339 | % |
| Salt | 0.109 | 0.124 | 0.134 | % |
| Sodium bicarb | 0.168 | 0.128 | 0.125 | % |
| UNE vitamin premix1 | 0.090 | 0.090 | 0.090 | % |
| UNE trace mineral premix2 | 0.100 | 0.100 | 0.100 | % |
| Choline chloride 70% | 0.039 | 0.038 | 0.029 | % |
| L-lysine | 0.409 | 0.352 | 0.324 | % |
| DL-methionine | 0.313 | 0.244 | 0.254 | % |
| L-threonine | 0.187 | 0.152 | 0.152 | % |
| Nutrient specification | ||||
| ME poultry | 3,025 | 3,150 | 3,200 | kcal/kg |
| CP | 23.3 | 21.2 | 20.9 | % |
| Crude fat | 4.6 | 6.1 | 7.0 | % |
| Crude fiber | 3.0 | 3.0 | 2.9 | % |
| Isoleucine | 0.983 | 0.867 | 0.852 | % |
| Digestible arginine | 1.321 | 1.153 | 1.125 | % |
| Digestible lysine | 1.270 | 1.100 | 1.060 | % |
| Digestible methionine | 0.608 | 0.521 | 0.526 | % |
| Digestible methionine and creatinine | 0.940 | 0.840 | 0.830 | % |
| Digestible tryptophan | 0.222 | 0.205 | 0.193 | % |
| Digestible threonine | 0.830 | 0.730 | 0.720 | % |
| Digestible valine | 0.940 | 0.840 | 0.830 | % |
| Insoluble NSP | 14.6 | 14.6 | 17.7 | g/kg |
| Calcium | 0.900 | 0.850 | 0.800 | % |
| Available phosphorus | 0.450 | 0.425 | 0.400 | % |
| Sodium | 0.160 | 0.160 | 0.160 | % |
| Potassium | 0.916 | 0.801 | 0.785 | % |
| Chloride | 0.230 | 0.230 | 0.230 | % |
| Choline | 1,600 | 1,500 | 1,400 | mg/kg |
| Linoleic 18:2 | 1.502 | 1.803 | 1.931 | % |
Abbreviation: UNE, University of New England.
Vitamin premix per kg contains the following: vitamin A, 12 MIU; vitamin D, 5 MIU; vitamin E, 75 mg; vitamin K, 3 mg; nicotinic acid, 55 mg; pantothenic acid, 13 mg; folic acid, 2 mg; riboflavin, 8 mg; cyanocobalamin, 0.016 mg; biotin, 0.25 mg; pyridoxine, 5 mg; thiamine, 3 mg; antioxidant, 50 mg.
Mineral premix per kg contains the following: Cu, 16 mg as copper sulfate; Mn, 60.
Necrotic Enteritis Challenge and Postmortem
At day 9, birds in the NE-challenged groups received 1 mL/bird per os Eimeria spp (Eimeria acevulina 5,000 oocytes/mL, Eimeria maxima 5,000 oocytes/mL, Eimeria brunetti 2,500 oocytes/mL) (Eimeria Pty Ltd., Victoria, Australia). Each bird of this group was then inoculated 1 mL per os with a viable growth of C. perfringens type A strain of EHE-NE18 isolated from NE-infected chicken in the field (approximately 108 cfu/mL) (Commonwealth Scientific and Industrial Research Organisation, Geelong, Australia) at day 14 and day 15 as previously reported (Wu et al., 2010; Rodgers et al., 2015). Equivalent sham inoculants (PBS and sterile broth) were administered to unchallenged birds at day 9 and day 14 and 15, respectively. On any occurrence of mortality after the induction of NE challenge, a necropsy was carried out to determine the cause of death. Pen BW and feed intake were measured at day 16 and 35, and feed conversion rate was calculated accordingly taken in consideration of mortalities.
Intestinal Lesions
Necrotic enteritis–caused intestinal lesions were determined after an autopsy of sampled birds at day 16. Two birds per pen were euthanized at day 16, and the entire length of the small intestine was removed to examine the NE lesions. The intestinal lesion score was determined by 2 experienced researchers, using a 0 to 4 lesion scoring criteria reported by Prescott (1979). Briefly, score 0 indicates no gross changes in intestine; 1 represents thin-walled or friable mucosal layers; 2 is for focal necrosis or ulcerations; 3 designates obvious necrosis in large patches along the intestine; and 4 means severe necrosis with resemblance to a typical field cases of NE.
RNA Extraction and cDNA Synthesis
Aseptically, 1 cm length of the jejunum and ileum sections were collected from euthanized birds on day 16, rinsed with sterile PBS, and immediately preserved in a 2-mL Eppendorf tube containing 1.5 mL of RNAlater solution (Sigma-Aldrich, Australia). Total RNA was extracted from the jejunum and ileum tissue after homogenization in TRIsureTM (Bioline, Sydney, Australia) as per the manufacturer's instructions. RNA quantity and purity was determined using a NanoDrop ND-8000 spectrophotometer (Thermo Fisher Scientific, Waltham). RNA integrity (RNA integrity number) was analyzed with an RNA 6000 Nano kit on the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Waldbronn, Germany). RNA samples were considered to be of high integrity if the RNA integrity number was higher than 7.5.
cDNA synthesis was conducted by using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. Briefly, 1 μg of total RNA from each sample was incubated in 2 μL of 7 × gDNA Wipeout Buffer at 42°C for 2 min to remove possible contamination of genomic DNA. Then, the solution was mixed with reverse transcription reaction components that contained 1 μL of Quantiscript Reverse Transcriptase, 4 μL of 7 × Quantiscript RT Buffer, and 1 μL of RT Primer Mix. The mix was incubated on the Rotorgene 6000 real-time PCR machine (Corbett, Sydney, Australia) at 42°C for 15 min and at 95°C for 3 min to convert the RNA into cDNA. The cDNA was diluted 10 times with nuclease-free water and stored at −20°C until required for quantitative PCR.
Quantitative PCR
Primers used to target the specific genes of interest were sourced from publications or were designed by the NCBI primer tool (http://www.ncbi.nlm.nih.gov) with specificity check performed on primer pairs. The information about primers is shown in Table 2. The SYBR Green kit SensiFAST SYBR No-ROX (Bioline, Sydney, Australia) was used for the quantification of the cDNA samples in triplicates. A 10-μL reaction size was applied (5 μL of 2 × SensiFAST, 400 mM of each primer, and 2 μL of DNA template) using a Rotor-Gene 6000 real-time PCR machine (Corbett Research, Sydney, Australia). Thermocycling conditions for a 2-step PCR were as follows: first denaturation at 95°C for 2 min, then 40 cycles of denaturation at 95°C for 5 s and annealing and extension at appropriate annealing temperature for 20 s. The fluorescent data were acquired at the end of each annealing/extension step during PCR cycles. A melting step was conducted to assess the specificity of PCR amplification. After the cycle, the amplification cycle values of each gene were analyzed against 2 optimized reference genes (hypoxanthine phosphoribosyl-transferase 1 and TATA-box binding protein) using qBase +, version 3.0 (Biogazelle, Wijnbeke, Belgium). The logarithmic value of amplification cycle was transformed to linear relative quantity in qBase+, and output data were statistically analyzed with SPSS statistics, version 22 (IBM SPSS, UK). Expression levels of the genes were expressed in means of normalized relative quantities genes (Vandesompele et al., 2002; Hellemans et al., 2007) in respective treatments.
Table 2.
Sequences of primers used for quantitative real-time PCR.
| Gene | Full name | Sequence | Amplicon size (bp) | Ta oC | References |
|---|---|---|---|---|---|
| IgA | Immunoglobulin A | GTCACCGTCACCTGGACACCA | 192 | 64 | (Lammers et al., 2010) |
| ACCGATGGTCTCCTTCACATC | |||||
| IgG | Immunoglobulin G | ATCACGTCAAGGGATGCCCG | 118 | 60 | (Zhao et al., 2013) |
| ACCAGGCACCTCAGTTTGG | |||||
| IgM | Immunoglobulin M | GCATCAGCGTCACCGAAAGC | 98 | 60 | (Zhao et al., 2013) |
| TCCGCACTCCATCCTCTTGC | |||||
| CLDN1 | Claudin 1 | CTTCATCATTGCAGGTCTGTCAG | 103 | 60 | (Zanu et al., 2020) |
| AAATCTGGTGTTAACGGGTGTG | |||||
| CLDN5 | Claudin 5 | GCAGGTCGCCAGAGATACAG | 162 | 61 | (Zanu et al., 2020) |
| CCACGAAGCCTCTCATAGCC | |||||
| OCLD | Occludin | ACGGCAGCACCTACCTCAA | 123 | 60 | (Du et al., 2016) |
| GGGCGAAGAAGCAGATGAG | |||||
| JAM2 | Junctional adhesion 2 | AGACAGGAACAGGCAGTGCTAG | 135 | 60 | (Zanu et al., 2020) |
| ATCCAATCCCATTTGAGGCTAC | |||||
| TJP1 | Tight junction protein 1 | GGATGTTTATTTGGGCGGC | 187 | 60 | This study |
| GTCACCGTGTGTTGTTCCCAT | |||||
| TLR5 | Toll-like receptor 5 | TGTGGGAGAGAGGTTTATGTTTGG | 169 | 60 | (Yang et al., 2015) |
| CTGAGAGAGAGGTGAGACAATAGG | |||||
| TLR21 | Toll-like receptor 21 | AGTTGTGTCCTGTGCTGAGAG | 130 | 60 | (Wang et al., 2015) |
| AGCAGGTTGTGTTCCACTGTC | |||||
| IL2 | Interleukin 2 | TCTGGGACCACTGTATGCTCT | 256 | 60 | (Liu et al., 2018) |
| ACACCAGTGGGAAACAGTATCA | |||||
| IL6 | Interleukin 6 | CAAGGTGACGGAGGAGGAC | 254 | 60 | (Hong et al., 2012) |
| TGGCGAGGAGGGATTTCT | |||||
| IL7 | Interleukin 7 | GGTTCTGCCACTTCTCCTTG | 160 | 60 | (Khan et al., 2020) |
| CTTGCAGCATCTGTCACGATA | |||||
| IL12-β | Interleukin 12β | TGGGCAAATGATACGGTGAA | 83 | 60 | This study |
| CAGAGTAGTTCTTTGCCTCACATTTT | |||||
| IL18 | Interleukin 18 | TGTGTGTGCAGTACGGCTTAG | 79 | 60 | (Forder et al., 2012) |
| CTTACAAAAGGCATCGCATTC | |||||
| IFN-γ | Interferon gamma | AGCTGACGGTGGTGGACCTATTATT | 259 | 60 | (Liu et al., 2018) |
| R-GGCTTTGCGCTGGATTC | |||||
| TGF-4β | Transforming growth factor-4β | AGGATCTGCAGTGGAAGTGGAT | 137 | 60 | This study |
| CCCCGGGTTGTGTTGGT | |||||
| HPRT1 | Hypoxanthine phosphoribosyl-transferase 1 | ACTGGCTGCTTCTTGTG | 245 | 63 | (Yang et al., 2013) |
| GGTTGGGTTGTGCTGTT | |||||
| TBP | TATA-Box binding protein | TAGCCCGATGATGCCGTAT | 147 | 62 | (Li et al., 2005) |
| GTTCCCTGTGTCGCTTGC |
Microbiota Analysis
On day 16, the same birds autopsied for intestinal lesions were used for cecal digesta collection (1 g) that was aseptically transferred into a 2-mL Eppendorf safe-lock tube, snap-frozen in liquid nitrogen, and stored at −20°C until DNA extraction. DNA extraction was conducted using the QIAGEN Stool Kit (Qiagen, GmbH, Hilden, Germany) as per the manufacturer's instruction. DNA samples were aseptically transferred to an external next-generation sequencing service (BGI Tech Solutions, Hong Kong, Co., Ltd.). DNA samples underwent quality check and library preparation. The jagged ends of the amplicons were performed end-repair into blunt end by using a T4 DNA polymerase, Klenow fragment and T4 polynucleotide kinase. Then, amplicons were added “A” base to each 3′ end, and adapter ligation was performed. Short fragments were removed by AMPure beads. Fusion primers targeting V3–V4 regions of the bacterial 16S rRNA gene (3′- AGATCGGAAGAGCACACGTCTGAACTCCAGTCAC; 5′- AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTA) with dual index and adapters were used for PCR. The qualified library was chosen for sequencing (MiSeq PE300 Dual Index).
The raw 16S sequence data were cleaned by removing adapter sequences and reads of low quality. The consensus sequence was generated by Fast Length Adjustment of SHort reads (v1.2.11) (Magoc and Salzberg, 2011). Paired-end reads without overlaps were removed. Then, a further step of removal of primer sequences was performed, and a final total of 1,622,966 tags without primer were obtained with an average of 67,623 tags per sample and an average tag length of 411 bp. These tags were clustered to operational taxonomic unit (OTU) at 97% sequence similarity using the software USEARCH (v7.0.1090) (Edgar et al., 2011). Chimeras were filtered out by UCHIME (v4.2.40). All tags were mapped to each OTU-representative sequences using USEARCH GLOBAL, then the number of tags of each OTU in each sample was summarized to the OTU abundance table. The relative abundance of each OTU in every sample was calculated from the OTU abundance information, based on relative abundance value; principal component analysis was performed by ade4 package of R (v3.1.1). Taxonomic ranks were then assigned to OTU-representative sequences using the Ribosomal Database Project (Release9 201203) (Cole et al., 2014) Naive Bayesian Classifier v.2.2. The classifier was trained on the Greengenes database (v201305) (DeSantis et al., 2006). Unassigned OTU and OTU not assigned to the target species were removed. Alpha diversity is applied for analyzing complexity of species (Schloss et al., 2009) diversity for each sample through the indices, observed species, Chao1, Shannon, and Simpson, calculated using mothur (Schloss et al., 2009) (v1.31.2).
Statistics
The performance data were analyzed using a 1-way ANOVA (SPSS Statistics 25, IBM). The significance was declared at P < 0.05, and comparisons were separated by Tukey's test. ANOVA procedure of XLSTAT was used for data analysis of gene expression and alpha diversity (followed by a multiple comparison procedure using Dunett's test). Chi-square test was used for data analysis of microbiome relative abundance. Similarly, significance was declared at P < 0.05 among groups.
Results
Growth Performance
Necrotic enteritis–related mortality was less than 2% of the flock, suggesting a mild pathogenic challenge, thus reflecting subclinical NE for the majority of the birds. Parameters of growth performance are shown in Table 3. During day 0–16, birds in the CtrlNE treatment showed 27% reduction (P < 0.001) of BW gain (BWG), suppressed (P < 0.001) feed intake by about 19%, leading to a 11% increased FCR compared with the birds in CtrlNC. In the challenged birds, antibiotic supplementation in the feed (AntNE) alleviated NE challenge effect by 16% BWG increase and 4% FCR decrease over the CtrlNE during day 0–16. Despite a 5% numerical increase of BWG with the use of probiotic-supplemented feed, ProNE birds did not show BWG difference from the CtrlNE birds statistically. However, ProNE birds showed significantly higher feed intake in the treatment than CtrlNE birds during day 0–16, and no was difference shown for FCR between these 2 groups.
Table 3.
The birds BW gain (BWG), feed intake (FI), and feed conversion ratio (FCR) in response to the treatments.
| Treatment | CtrlNC | CtrlNE | ProNE | AntNE | P value | SEM |
|---|---|---|---|---|---|---|
| Day 0–16 | ||||||
| BWG | 624a | 454c | 476c | 525b | 0.001 | 9.8 |
| FI | 732a | 591c | 626b | 660b | 0.001 | 11.7 |
| FCR | 1.172c | 1.302a | 1.317a | 1.256b | 0.001 | 0.012 |
| Day 0–35 | ||||||
| BWG | 2,529a | 2,231d | 2,315c | 2,425b | 0.001 | 26.1 |
| FI | 3,498a | 3,232c | 3,365b | 3,453a,b | 0.001 | 39.0 |
| FCR | 1.384c | 1.449a | 1.454a | 1.424b | 0.001 | 0.008 |
| Day 16–35 | ||||||
| BWG | 1,905a | 1,777b | 1,839a,b | 1,899a | 0.003 | 33.5 |
| FI | 2,766a | 2,642b | 2,738a,b | 2,793a | 0.038 | 50.5 |
| FCR | 1.453 | 1.487 | 1.489 | 1.471 | 0.105 | 0.016 |
a–cMeans within a row lacking a common superscript differ as per the corresponding P value (column P).
During day 0–35, NE challenge–induced performance suppression remained in all parameters, as shown by poorer performance in CtrlNE compared with those in CtrlNC. Necrotic enteritis challenge with antibiotics enabled a 9% increase of BWG (P < 0.001) and lower (P < 0.001) FCR compared with CtrlNE. The ProNE treatment enabled a significantly higher (P < 0.001) BWG (4% improvement) than the CtrlNE birds.
Necrotic Enteritis Lesions
Overall, low levels of lesion scores were observed in the all the birds in the challenged groups indicating a mild challenge and subclinical disease. At the duodenum level, there was almost no lesion, and this was true for all treatments. At the jejunum level, NE-related lesions were recorded higher in challenged animal, compared with non-challenged animals, and were significantly (P < 0.05) higher in CtrlNE treatment than that of the CtrlNC treatment (Table 4). However, the magnitude of NE-related lesions was not relieved with the antibiotic (AntNE) or the probiotic treatment (ProNE) where the lesion did not differ from nonchallenged birds (CtrlNC) either. Surprisingly, no difference was recorded between the control groups in the ileal necropsy. However, the ProNE-treated birds showed a significantly higher lesion score than the rest of the groups, despite the overall low level of lesion for all the groups.
Table 4.
Measurement of macro lesion related to necrotic enteritis in the small intestine.1
| Treatment | Duodenum | Jejunum | Ileum |
|---|---|---|---|
| CtrlNC | 0.0 | 0.0b | 0.0b |
| CtrlNE | 0.1 | 1.0a | 0.1b |
| ProNE | 0.0 | 0.7a,b | 0.7a |
| AntNE | 0.1 | 0.5a,b | 0.3b |
| SEM | 0.1 | 0.3 | 0.2 |
| P value | 0.582 | 0.032 | 0.006 |
a–cMeans within a column lacking a common superscript differ as per the corresponding P value indicated in the P value row.
Abbreviations: AntNE, necrotic enteritis challenge with antibiotic supplementation; CtrlNC, control diet without necrotic enteritis challenge; CtrlNE, control diet with necrotic enteritis challenge;ProNE, necrotic enteritis challenge with probiotic supplementation.
Intestinal lesion score was determined using a 0 to 4 lesion scoring criteria reported by Prescott (1979). Score 0, no gross changes in intestine; 1, thin-walled or friable mucosal layers; 2, for focal necrosis or ulcerations; 3, obvious necrosis in large patches along the intestine; 4, severe necrosis with resemblance to a typical field cases of necrotic enteritis.
Expression of Genes Coding Proteins Related to Gut Integrity and Immunity in the Jejunum
As the intestine is the first defense system of the animals, the examination of intestinal responses of the host to enteric diseases such as NE provides vital information about the severity of infection. The jejunum represents a major part of the small intestine, and the nutrient absorption such as the absorption of carbohydrates and proteins occurs largely in the jejunum. Therefore, the jejunum was chosen for the analysis of gene expression. The localized immune response in the jejunum was stimulated by NE challenge at day 16 as shown by the change in the expression of a cytokine-coding gene (Table 5). The genes IL6 and IL7 were downregulated by NE challenge relative to nonchallenged birds (CtrlNC), whereas the gene interferon gamma was upregulated. The ProNE treatment upregulated the expression of IL-12B, interferon gamma, TGF-4β genes when compared with the CtrlNE treatment. Addition of antibiotic (AntNE) did not alter the expression of the cytokine-coding gene, except for IL18 that was upregulated by the antibiotic supplementation compared with that of the CtrlNE group.
Table 5.
Expression of cytokine genes in the jejunum.1
| Treatments | IL2 | IL6 | IL7 | IL12-b | IL18 | Interferon gamma | TGF-4β |
|---|---|---|---|---|---|---|---|
| CtrlNC | 1.720a | 2.395a | 2.466a | 0.260b | 1.158a,b | 0.264c | 0.852b |
| CtrlNE | 1.236a,b | 0.692b | 0.870b | 1.207b | 0.823b | 1.348b | 0.828b |
| ProNE | 0.836b | 0.735b | 0.721b | 3.873a | 0.925b | 2.211a | 1.959a |
| AntNE | 0.762b | 0.964b | 0.799b | 1.227b | 1.389a | 1.450b | 0.846b |
| P value | 0.007 | <0.001 | <0.001 | <0.001 | 0.049 | <0.001 | 0.004 |
a–cMeans within a column lacking a common superscript differ as per the corresponding P value indicated in the P value row.
Abbreviations: AntNE, necrotic enteritis challenge with antibiotic supplementation; CtrlNC, control diet without necrotic enteritis challenge; CtrlNE, control diet with necrotic enteritis challenge;ProNE, necrotic enteritis challenge with probiotic supplementation.
Expression levels of the genes were expressed in means of normalized relative quantities genes.
The expression of Ig gene IgM in the jejunum decreased (P < 0.001) when animals were challenged (CtrlNE) compared with the nonchallenged group (CtrlNC) (Table 6). Both probiotic and antibiotic supplementation did not show effect for IgM gene expression compared with the CtrlNE (P > 0.05). IgA and IgG did not show changes in response to the treatments statistically.
Table 6.
Expression of Ig genes in the jejunum.1
| Treatments | IgA | IgG | IgM |
|---|---|---|---|
| CtrlNC | 2.78 | 1.976 | 1.910a |
| CtrlNE | 1.605 | 1.915 | 0.692b |
| ProNE | 2.277 | 1.277 | 0.897b |
| AntNE | 0.48 | 0.885 | 1.169b |
| P value | 0.056 | 0.077 | <0.001 |
a–cMeans within a row lacking a common superscript differ as per the corresponding P value.
Abbreviations: AntNE, necrotic enteritis challenge with antibiotic supplementation; CtrlNC, control diet without necrotic enteritis challenge; CtrlNE, control diet with necrotic enteritis challenge;ProNE, necrotic enteritis challenge with probiotic supplementation.
Expression levels of the genes were expressed in means of normalized relative quantities genes.
The expression of tight junction genes, namely CLDN5, OCLD and TJP1, was downregulated (P < 0.001, 0.001 and 0.05, respectively) by NE challenge compared with CtrlNC (Table 7). On the other hand, probiotic treatments showed upregulated CLDN5 gene compared with antibiotic treatment, while no difference was observed between probiotic treatments (ProNE) and challenged control (CtrNE). For OCLD and TJP1 genes, no differences were observed between antibiotic and probiotic treatments of the birds (P > 0.05). Interestingly, there was no effect of the challenge on the expression of CLDN1 and JAM2 (P > 0.05), but probiotic treatment significantly upregulated the expression of these 2 tight junction genes compared with the other 3 groups (P < 0.05).
Table 7.
Expression of tight junction gene in the jejunum.1
| Treatments | CLDN1 | CLDN5 | OCLD | JAM2 | TJP1 |
|---|---|---|---|---|---|
| CtrlNC | 0.059b | 1.270a | 1.571a | 0.837b | 1.212a |
| CtrlNE | 0.906b | 1.033b | 1.035b | 0.894b | 0.782b |
| ProNE | 2.423a | 1.037b | 0.974b | 1.446a | 1.102a,b |
| AntNE | 0.962b | 0.639c | 0.715b | 0.998b | 0.939a,b |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.027 |
a–cMeans within a column lacking a common superscript differ as per the corresponding P value indicated in the P value row.
Abbreviations: AntNE, necrotic enteritis challenge with antibiotic supplementation; CtrlNC, control diet without necrotic enteritis challenge; CtrlNE, control diet with necrotic enteritis challenge;ProNE, necrotic enteritis challenge with probiotic supplementation.
Expression levels of the genes were expressed in means of normalized relative quantities genes.
For Toll-like receptor (TLR) genes, the NE challenge (CtrlNE) did not affect TLR5 gene expression compared with the nonchallenge group (CtrlNC) (P > 0.05), but probiotic supplementation upregulated this gene compared with other groups (P < 0.05). The expression of TLR21 gene was downregulated by NE challenge regardless of additive supplementations, while probiotic supplementation (ProNE) upregulated its expression compared with NE challenge control (CtrlNE) and antibiotic (AntNE) groups (P < 0.001), (Table 8).
Table 8.
Expression of Toll-like receptor genes in the jejunum.1
| Treatments | TLR5 | TLR21 |
|---|---|---|
| CtrlNC | 0.865b | 1.543a |
| CtrlNE | 0.794b | 0.747c |
| ProNE | 1.285a | 1.113b |
| AntNE | 0.961b | 0.773c |
| P value | 0.013 | <0.001 |
a–cMeans within a column lacking a common superscript differ as per the corresponding P value indicated in the P value row.
Abbreviations: AntNE, necrotic enteritis challenge with antibiotic supplementation; CtrlNC, control diet without necrotic enteritis challenge; CtrlNE, control diet with necrotic enteritis challenge;ProNE, necrotic enteritis challenge with probiotic supplementation.
Expression levels of the genes were expressed in means of normalized relative quantities genes.
Microbiota Dynamics
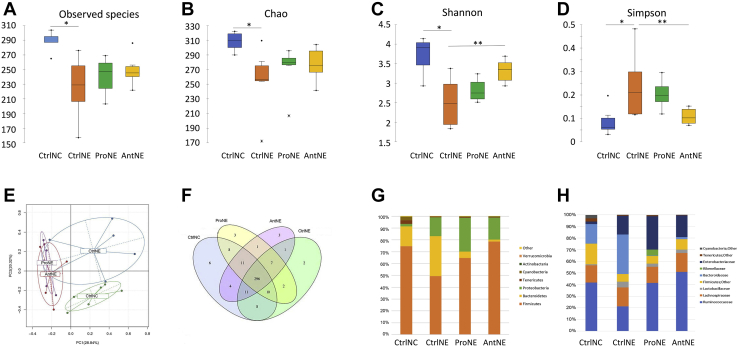
Microbiota of the cecum content of day 16 was analyzed by 16S sequencing. Alpha diversity quantification by richness (total number of observed species and Chao1 index) and by calculation of Shannon and Simpson indexes, which relates both species richness and evenness, is shown in Figure 1A-D. Analysis of the total number of observed species and Chao1 index revealed that CtrlNC had the highest richness of all groups (P < 0.05). Similarly, CtrlNC also presented by analyses of Shannon and Simpson indexes the highest diversity of all groups (P < 0.05). Richness was the same in all the challenged groups (CtrlNE, ProNE, and AntNE), as shown by the Chao1 index (Figure 1B). In terms of microbial diversity, antibiotic treatment increased significantly the Shannon index (P < 0.05) and reduced the Simpson index (P < 0.05) when compared with CrtlNE. Even if the increase was not significant P > 0.05), ProNE showed also a numerical higher diversity, estimated by Shannon and Simpson indexes, than the CtrlNE group. Principal component analysis of OTU abundance revealed that ProNE and AntNE grouped closely while distinctly from the CtrlNC and CtrlNE which were grouped separately, indicating the similarity of OTU between the ProNE and AntNE birds that were however different from both CtrlNC and CtrlNE birds (Figure 1E). The Venn diagram was used to show the number of common/unique OTU in the groups (Figure 1F). The core microbiomes of different environments could be obtained if combined with the OTU representing species level. As a result, there were eight OTU, each shared between CtrlNC and ProNE and between CtrlNC and CtrlNE, four between CtrlNC and AntNE, while only 2 between ProNE and CtrlNE, and 1 each between AntNE and CtrlNE, and between AntNE and ProNE.
Figure 1.
Microbiota diversity in samples from cecal content of 16-day-old broilers from CtrlNC, CtrlNE, ProNE, and AntNE groups. (A–D)., alpha diversity: CtrlNC (blue), CtrlNE (orange), ProNE (green), and AntNE (yellow) box plots showing alpha diversity in samples using the Observed Species index, Chao1, Shannon, and the Simpson indexes. ∗ and ∗∗ mean significant difference (P < 0.05 and P < 0.01, respectively) between the groups. (E, F) Principal Component Analysis (PCA): OTU abundance and Venn diagram, respectively; PCA of OTU abundance indicates that ProNE and AntNE are grouped closely, while both distinctly from the CtrlNC and CtrlNE. CtrlNC and CtrlNE were grouped separately; Venn diagram analysis shows the number of common/unique OTU in the groups. (G, H) Microbiota in the relative abundance at phylum and family levels, respectively. Abbreviations: AntNE, necrotic enteritis challenge with antibiotic supplementation; CtrlNC, control diet without necrotic enteritis challenge; CtrlNE, control diet with necrotic enteritis challenge;ProNE, necrotic enteritis challenge with probiotic supplementation.
Microbiota of the cecal content were also analyzed in term of relative abundance at the genus level (Figure 1G). Necrotic enteritis challenge exhibited an increase of Proteobacteria and Bacteroidetes in addition to a decrease of Firmicutes leading to a decrease of Firmicutes-to-Bacteroidetes ratio (4.5 and 1.5 for CtrlNC and CtrlNE, respectively). The Firmicutes-to-Bacteroidetes ratio increased considerably to reach 12.0 and 43.8 in ProNE and AntNE, respectively. This difference with the challenged animals (CtrlNE) was driven by a very important decrease of the Bacteroidetes population. The main bacterial known genera increased by the challenge, arbitrarily determined as significant difference higher than 2 points between CtrlNC and CtrlNE, were as follows: Ruminococcus (from 2.4 to 6.4%, P < 0.001), Lactobacillus (from 0.9 to 4.9%, P < 0.001), Bacteroides (from 16.6 to 33.7%, P < 0.001), Escherichia (from 2.0 to 14.4%, P < 0.001) (Figure 1H, Table 9). Other genera also increased but the extent of the change was not very high (less than 2 points between CtrlNC and CtrlNE). Faecalibacterium and Oscillospira were the 2 genera that were the most decreased (from 16.2 to 10.2% and 14.5 to 6.0%, respectively).
Table 9.
Microflora of cecal content in the relative abundance at the genus level.
| Phylum |
Clostridiales; other |
Clostridiaceae |
Firmicutes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family |
Ruminococcaceae |
Lachnospiraceae |
Lactobacillaceae |
|||||||||
| Genus | Clostridiales; Other | Butyricicoccus | Faecalibacterium | Oscillospira | Ruminococcus | Ruminococcus 2 | Other | Coprococcus | Dorea | Blautia | Other | Lactobacillus |
| CtrlNC | 17.3%d | 0.8%b | 16.2%b | 14.5%c | 2.4%a | 1.9%c | 7.6%d | 0.6%a | 0.7%a | 0.4%a | 9.8%d | 0.9%a |
| CtrlNE | 6.6%b | 0.5%a | 10.2%a | 6.0%a | 6.4%d | 0.9%a | 3.0%a | 1.2%b | 0.9%b | 0.3%a | 7.3%b | 4.9%d |
| ProNE | 6.3%a | 1.3%c | 23.6%c | 9.8%b | 4.6%c | 1.1%b | 4.5%b | 2.3%c | 1.0%c | 0.7%c | 5.1%a | 2.6%b |
| AntNE | 8.7%c | 1.7%d | 23.3%c | 17.1%d | 3.1%b | 2.1%d | 5.8%c | 2.3%c | 0.7%a | 0.6%b | 9.3%c | 2.9%c |
| P value (Khi2) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Phylum |
Bacteroidetes |
Proteobacteria |
Cyanobacteria |
Tenericutes |
|||
|---|---|---|---|---|---|---|---|
| Family |
Bacteroidaceae |
Rikenellaceae |
Enterobacteriaceae |
Cyanobacteria; other |
Mollicutes; RF39; other |
||
| Genus | Bacteroides | Other | Escherichia | Proteus | Other | Other | Mollicutes; RF39; Other; |
| CtrlNC | 16.6%c | 0.1%b | 2.0%a | 0.0%a | 0.1%a | 3.0%c | 2.7%d |
| CtrlNE | 33.7%d | 0.0%a | 14.4%b | 0.1%c | 1.3%c | 0.1%a | 0.8%c |
| ProNE | 0.3%a | 5.2%c | 25.9%d | 0.6%d | 1.8%d | 0.5%b | 0.6%b |
| AntNE | 1.8%b | 0.0%a | 17.5%c | 0.0%b | 0.8%b | 0.1%a | 0.4%a |
| P value (Khi2) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
a–cMeans within a column lacking a common superscript differ as per the corresponding P value indicated in the P value row.
Abbreviations: AntNE, necrotic enteritis challenge with antibiotic supplementation; CtrlNC, control diet without necrotic enteritis challenge; CtrlNE, control diet with necrotic enteritis challenge;ProNE, necrotic enteritis challenge with probiotic supplementation.
Administration of probiotics to challenge animals led to the changes in the microbiota; 3 main genera were increased: Faecalibacterium (from 10.2 to 23.6%, P < 0.001), Oscillospira (from 6.0 to 9.8%, P < 0.001), Escherichia (from 14.4 to 25.9%, P < 0.001), whereas Lactobacillus and Bacteroides were decreased (from 4.9 to 2.6% and from 33.7 to 0.3%, respectively). In the animals receiving antibiotics, there were also changes at the microbiota level, the main ones were the same than those observed with the probiotic treatment. Only differences were that in the case of antibiotic treatment, there was an additional decrease in Ruminococcus.
Discussion
The present study demonstrated that successful subclinical NE challenge was made as shown by the significantly worsened performance and intestinal necrosis but with very low mortality. The poorer performance of the challenged birds shown from day 16 to 35 suggests a lasting impact of the subclinical NE. As has been reported that subclinical NE has been associated with severe economic loss due to high FI and less weight gain, thus low feed efficiency (Skinner et al., 2010). Therefore, the use of feed additives to combat subclinical NE has been explored for year to reduce such economic loss.
It has been reported that NE challenge can shift the microbiota in the small intestine (Stanley et al., 2014; Hernandez-Patlan et al., 2019). Such microbiota shifts may suggest that the intestinal microbiota may modulate immune responses of the host and thus play a critical role in NE infection (Antonissen et al., 2016). In the present study, alpha diversity revealed decreased microbiota diversity after the challenge compared with the nonchallenged treatment, and microbiota of cecal content analysis in term of relative abundance at genus level showed that NE challenge led to distinct bacterial abundance from nonchallenged birds. These results are in agreement with the previous observations that NE shifts the microbiota in the intestine of chickens. Furthermore, it has been shown that the supplementation of probiotics to the chickens modulates the microbiota balance in the intestine, which reduces bacterial groups prone to be inflammatory inducer and increased bacterial groups that may be of those beneficial to the hosts (Hernandez-Patlan et al., 2019). In the present study, both alpha diversity and microbiota relative abundance analyses indicated that probiotic treatment resulted in similar bacterial diversity to that in antibiotic-treated birds, while distinct from both challenged and nonchallenged controls. This may be indicative of a similar effect of probiotic to antibiotics to the birds at least from the microbiota point of view. In this study, it was shown that a decrease in Faecalibacterium abundance was observed in the NE-challenged control group. Interestingly, there was a favorable proliferation of Butyricicoccus and Faecalibacterium genera with the supplementation of probiotics and antibiotics to the birds, which suggests their beneficial effect to reverse the NE challenge effect toward the “nonchallenged” microbiota. As the probiotic treatment had a significant impact on the dynamics of the intestinal microbiota, this might explain weight gain improvements in the ProNE compared with the CtrlNE birds. Eeckhaut et al. (2016) reported that significant growth performance improvements were achieved in broilers receiving dietary supplements of Butyricicoccus pullicaecorum under the NE challenge condition. In addition, butyric acid produced by Butyricicoccus has been reported to inhibit pathogenic bacteria such as C. perfringens and Salmonella in broiler chickens (Van Immerseel et al., 2005; Timbermont et al., 2010). Therefore, the findings in the present study that the increased Butyricicoccus and Faecalibacterium abundance that associates with the performance improvement by the antibiotic and probiotic treatments confirmed the beneficial effects of these butyrate-producing bacteria in the intestine of the chickens.
The Bs 29,784 supplementation affected the expression of genes involved in intestinal immunity and tight junction complexes. In the present study, the probiotic supplementation upregulated the expression of interferon gamma gene, which is known to be the key effector of the cell-mediated immunity. The protein interferon gamma is reported to activate and increase antimicrobial activity of macrophages and induce autophagy which is an antimicrobial response of the host (Singh et al., 2006; Li et al., 2012). The interferon gamma cytokines mainly facilitate cellular immunity against intracellular bacteria by stimulating cellular mechanisms that produce phagocyte-dependent inflammation (del Carmen Rodríguez-Sáinz et al., 2002). B. subtilis has been previously reported to activate immune responses and regulate innate immunity in chickens (Lee et al., 2015). Furthermore, the addition of Bs 29,784 also increased the expression of IL12-β and transforming growth factor-β4 in present study. The IL12 family proteins are known to be proinflammatory cytokines that generate T cells and develop adaptive immunity characterized by the induction of interferon gamma protein (Gee et al., 2009). Twardzik et al. (1990) has previously concluded that interferon gamma can enhance release of mononuclear phagocytes of transforming growth factor-β which are generally known as anti-inflammatory cytokines. Our results are in agreement with these reports, and the higher expression of INF-γ along with IL-12 and transforming growth factor-β may suggest higher stimulated defense mechanisms activated by Bs 29,784.
Furthermore, downregulation of TLR21 in the jejunal tissues during the NE condition was also observed in the present study, while probiotic supplementation upregulated the expression of TLR21 in the post-NE challenge of the chickens. Toll-like receptors are a member of the Pattern Recognition Receptor system family (Akira, 2004) which can detect molecular structures known as pathogen-associated molecular patterns, such as lipopolysaccharides, peptidoglycans, flagellin, and bacterial DNA that are shared by many pathogens (Medzhitov and Janeway, 2000). The TLR play a key role in innate immunity by recognizing the pathogen-associated molecular patterns and inducing inflammatory responses by the production of reactive oxygen and nitrogen intermediates and inflammatory cytokines (Werling and Jungi, 2003). The TLR21 in chicken is reported to act as a homologue to TLR9 in mammals which can recognize microbial DNA and initiate innate and adaptive immune responses (Brownlie et al., 2009). Previous studies have reported that supplementation of Lactobacillus and Bacillus probiotics in diets can elevate the expression of TLR21 gene in laying hen blood (Sheoran et al., 2017). On the other hand, TLR5 is important in host defense against bacteria, and bacterial flagellin is reported to be a natural ligand for TLR5 (Hayashi et al., 2001). Because B. subtilis is a flagellated bacterium, it may result in the activation of TLR5 expression; however, there are studies that disagree on the ability of B. subtillis to activate this gene (Kojima et al., 2008; Im et al., 2009). Further study is warranted to investigate the functions underling the upregulation of TLR5. The upregulated expression of TLR21 and TLR5 with the supplementation of Bs 29,784 may lead to the conclusion that this probiotic can possibly benefit the chickens by the increase resistance against NE infection.
Moreover, the present study shows upregulation of the junctional adhesion 2 (JAM2) expression in birds fed with Bs 29,784. The JAM proteins are expressed by different cell types including epithelium, endothelium, and immune cells (Williams et al., 1999) and along with occludins and claudins contribute to the tight junction complex (Mitic and Anderson, 1998). The JAM proteins are glycoproteins that belong to the Ig superfamily (Williams and Barclay, 1988), and JAM2 is widely expressed in the endothelial and lymphatic cells (Aurrand-Lions et al., 2001). Johnson-Léger et al. (2002) reported that JAM2 is involved in transmigration of lymphocytes which occurs at sites of inflammation. Upregulation of JAM2 has been previously reported by Bacillus supplementation in broiler feed (Gadde et al., 2017), which are in line with the results observed in the present study. It can be concluded that the supplementation of Bs 29,784 may improve intestinal immunity responses, thus, positively affecting the tight junction complex in the current NE infection model.
Lactobacillus has been considered beneficial bacteria (Penders et al., 2006; Inoguci et al., 2012), thus higher abundance of Lactobacillus may indicate a more healthy gut. However, it may not always be the case. For example, NE-challenged chickens showed increased abundance of Lactobacillus (M'Sadeq et al., 2015; Gharib-Naseri et al., 2019). Therefore, the higher Lactobacillus does not necessarily always present in a healthy gut. Likewise, the beneficial additives might not always increase the abundance of Lactobacillus as shown in the present study. As Lactobacillus is composed of many different species and strains/genotypes, the abundance of the genus may not be critical as an indication of the health status of the gut. On the other hand, its individual members as species or even strains of the species may be more important in gut health. Similarly, the principle applies to other bacterial groups such as Escherichia and Enterobacteriaceae. Nonetheless, bacterial diversity and balance are more important for gut health than the abundance of some bacterial groups. Therefore, without in-depth understanding of the functionality of particular species and/or strains of the bacteria, it is not like to consider any bacterial population as a reliable biomarker for healthy gut. Further studies are warranted to elucidate the microbiota balance and changes in response to the gut health status.
In summary, experimentally induced NE in the meat chicken had suppressed the growth performance and disrupted the balance in the intestinal microbiota. Necrotic enteritis–affected flocks even with a moderate-mortality display could still sustain significant performance loss consistently carried up to the market age. Direct feeding of Bs 29,784 yielded a significant BWG to the chicken under the NE condition. An abundance of butyric-producing cecal commensals increased in the Bs 29,784–supplemented birds; however, the treatment also encouraged abundance of Escherichia and Enterobacteriaceae. The current analysis however suggests that clustering of principal component analysis and abundance of identified bacterial genus in the B. subtilis treatment shared similarity with antibiotic treatment. The benefit of Bs 29,784 may also be categorized in the regulatory effect on the tight junction gene expression and immune responses in a positive manner. Direct feeding of B. subtilis could be beneficial to NE-affected flocks as the current findings, but further investigation might be needed to deeper explain its mode of action.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
ADISSEO France SAS funded this study. Centre for Animal Research and Teaching (CART) at the University of New England for assistance in raising and management of the animal facilities. Petrina Young of Eimeria Pty Ltd. for providing Eimeria species and Prof Robert Moore for providing Clostridium perfringens.
References
- Akira S. Toll receptor families: structure and function. Semin. Immunol. 2004;16:1–2. doi: 10.1016/j.smim.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Annett C., Viste J., Chirino-Trejo M., Classen H., Middleton D., Simko E. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- Antonissen G., Eeckhaut V., Van Driessche K., Onrust L., Haesebrouck F., Ducatelle R., Moore R.J., Van Immerseel F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016;45:308–312. doi: 10.1080/03079457.2016.1152625. [DOI] [PubMed] [Google Scholar]
- Aurrand-Lions M., Duncan L., Ballestrem C., Imhof B.A. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J. Biol. Chem. 2001;276:2733–2741. doi: 10.1074/jbc.M005458200. [DOI] [PubMed] [Google Scholar]
- Awad W., Ghareeb K., Abdel-Raheem S., Bohm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Brownlie R., Zhu J., Allan B., Mutwiri G.K., Babiuk L.A., Potter A., Griebel P. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol. Immunol. 2009;46:3163–3170. doi: 10.1016/j.molimm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Cole J.R., Wang Q., Fish J.A., Chai B., McGarrell D.M., Sun Y., Brown C.T., Porras-Alfaro A., Kuske C.R., Tiedje J.M. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier C., Hofacre C., Payne A., Anderson D., Kaiser P., Mackie R., Gaskins H. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Dahiya J., Wilkie D., Van Kessel A., Drew M. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:60–88. [Google Scholar]
- del Carmen Rodríguez-Sáinz M., Sánchez-Ramón S., de Andrés C., Rodríguez-Mahou M., Muñoz-Fernández M.A. Th1/Th2 cytokine balance and nitric oxide in cerebrospinal fluid and serum from patients with multiple sclerosis. Eur. Cytokine Netw. 2002;13:110–114. [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Mahendran M., Tomar S., Chauhan R. Beneficial effects of probiotics and prebiotics in livestock and poultry: the current perspectives. Intas Polivet. 2008;9:1–12. [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:19. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens F. An alternative for antibiotic use in poultry: probiotics. Rev. Bras. Cienc. Avic. 2003;5:75–97. [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics (Oxf.) 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhaut V., Wang J., Van Parys A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., Van Immerseel F. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front. Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber F., Bäumler A.J. The impact of intestinal inflammation on the nutritional environment of the gut microbiota. Immunol. Lett. 2014;162:48–53. doi: 10.1016/j.imlet.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.-j., Chen S.-j., Yu Y.-c., Si J.-m., Liu B. A probiotic treatment containing Lactobacillus, Bifidobacterium and Enterococcus improves IBS symptoms in an open label trial. J. Zhejiang Univ. Sci. B. 2006;7:987–991. doi: 10.1631/jzus.2006.B0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forder R.E., Nattrass G.S., Geier M.S., Hughes R.J., Hynd P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- Gabriel I., Lessire M., Mallet S., Guillot J.F. Microflora of the digestive tract: critical factors and consequences for poultry. World's Poult. Sci. J. 2006;62:499–512. [Google Scholar]
- Gadde U.D., Oh S., Lee Y., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res. Vet. Sci. 2017;114:236–243. doi: 10.1016/j.rvsc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Gee K., Guzzo C., Mat C., Nor F., Ma W., Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy Drug Targets. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- Gharib-Naseri K., Kheravii S.K., Keerqin C., Morgan N., Swick R.A., Choct M., Wu S.B. Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poult. Sci. 2019;98:6422–6432. doi: 10.3382/ps/pez480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F., Smith K.D., Ozinsky A., Hawn T.R., Eugene C.Y., Goodlett D.R., Eng J.K., Akira S., Underhill D.M., Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Pontin K.P., Hernandez X., Merino-Guzman R., Adhikari B., López-Arellano R., Kwon Y.M., Hargis B.M., Arreguin-Nava M. Impact of a Bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions and ileal microbiota in broiler chickens using a laboratory challenge model. Front. Vet. Sci. 2019;6:108. doi: 10.3389/fvets.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.H., Song W., Lee S.H., Lillehoj H.S. Differential gene expression profiles of beta-defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poult. Sci. 2012;91:1081–1088. doi: 10.3382/ps.2011-01948. [DOI] [PubMed] [Google Scholar]
- Im J., Jeon J.H., Cho M.K., Woo S.S., Kang S.-S., Yun C.-H., Lee K., Chung D.K., Han S.H. Induction of IL-8 expression by bacterial flagellin is mediated through lipid raft formation and intracellular TLR5 activation in A549 cells. Mol. Immunol. 2009;47:614–622. doi: 10.1016/j.molimm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Inoguchi S., Ohashi Y., Narai-Kanayama A., Aso K., Nakagaki T., Fujisawa T. Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. Int. J. Food Sci. Nutr. 2012;63:402–410. doi: 10.3109/09637486.2011.630992. [DOI] [PubMed] [Google Scholar]
- Johnson-Léger C.A., Aurrand-Lions M., Beltraminelli N., Fasel N., Imhof B.A. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–2486. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Di Rubbo A., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Roberts J., Wu S.B. Regulation of immunity-related genes by infectious Bronchitis virus challenge in spleen of laying chickens. Viral Immunol. 2020;33:413–420. doi: 10.1089/vim.2019.0139. [DOI] [PubMed] [Google Scholar]
- Kojima K., Ueta M., Hamuro J., Hozono Y., Kawasaki S., Yokoi N., Kinoshita S. Human conjunctival epithelial cells express functional Toll-like receptor 5. Br. J. Ophthalmol. 2008;92:411–416. doi: 10.1136/bjo.2007.128322. [DOI] [PubMed] [Google Scholar]
- La Ragione R.M., Casula G., Cutting S.M., Woodward M.J. Bacillus subtilis spores competitively exclude Escherichia coli O78: K80 in poultry. Vet. Microbiol. 2001;79:133–142. doi: 10.1016/s0378-1135(00)00350-3. [DOI] [PubMed] [Google Scholar]
- La Ragione R.M., Woodward M.J. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 2003;94:245–256. doi: 10.1016/s0378-1135(03)00077-4. [DOI] [PubMed] [Google Scholar]
- Lammers A., Wieland W.H., Kruijt L., Jansma A., Straetemans T., Schots A., den Hartog G., Parmentier H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010;34:1254–1262. doi: 10.1016/j.dci.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Lee K.-W., Kim D.K., Lillehoj H.S., Jang S.I., Lee S.-H. Immune modulation by Bacillus subtilis-based direct-fed microbials in commercial broiler chickens. Anim. Feed Sci. Technol. 2015;200:76–85. [Google Scholar]
- Li P., Du Q., Cao Z., Guo Z., Evankovich J., Yan W., Chang Y., Shao L., Stolz D.B., Tsung A. Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1) Cancer Lett. 2012;314:213–222. doi: 10.1016/j.canlet.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.P., Bang D.D., Handberg K.J., Jorgensen P.H., Zhang M.F. Evaluation of the suitability of six host genes as internal control in real-time RT-PCR assays in chicken embryo cell cultures infected with infectious bursal disease virus. Vet. Microbiol. 2005;110:155–165. doi: 10.1016/j.vetmic.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu L., Li L., Tian D., Li W., Xu L., Yan R., Li X., Song X. Protective immunity induced by Eimeria common antigen 14-3-3 against Eimeria tenella, Eimeria acervulina and Eimeria maxima. BMC Vet. Res. 2018;14:337. doi: 10.1186/s12917-018-1665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.Z., Jellbauer S., Poe A.J., Ton V., Pesciaroli M., Kehl-Fie T.E., Restrepo N.A., Hosking M.P., Edwards R.A., Battistoni A. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. 2012;11:227–239. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoc T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics (Oxf.) 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. How does the immune system distinguish self from nonself? Semin. Immunol. 2000;12:185–188. doi: 10.1006/smim.2000.0230. [DOI] [PubMed] [Google Scholar]
- Mitic L., Anderson J. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Mohammadigheisar M., Shirley R., Barton J., Welsher A., Thiery P., Kiarie E. Growth performance and gastrointestinal responses in heavy Tom turkeys fed antibiotic free corn− soybean meal diets supplemented with multiple doses of a single strain Bacillus subtilis probiotic (DSM29784) Poult. Sci. 2019;98:5541–5550. doi: 10.3382/ps/pez305. [DOI] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- M’Sadeq S.A., Wu S.-B., Swick R.A., Choct M. Dietaryacylated starch improves performance and gut health in necrotic enteritis challenged broilers. Poult. Sci. 2015;94:2434–2444. doi: 10.3382/ps/pev219. [DOI] [PubMed] [Google Scholar]
- Neijat M., Habtewold J., Shirley R.B., Welsher A., Barton J., Thiery P., Kiarie E. Bacillus subtilis strain DSM 29784 modulates the cecal microbiome, concentration of short-chain fatty acids, and apparent retention of dietary components in Shaver White chickens during grower, developer, and laying phases. Appl. Environ. Microbiol. 2019;85:e00402–e00419. doi: 10.1128/AEM.00402-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., van den Brandt P.A., Stobberingh E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- Prescott J.F. The prevention of experimentally induced necrotic enteritis in chickens by avoparcin. Avian Dis. 1979;23:1072–1074. [PubMed] [Google Scholar]
- Raidal S. Poultry health and management. Aust. Vet. J. 2000;78:830. [Google Scholar]
- Rhayat L., Jacquier V., Brinch K.S., Nielsen P., Nelson A., Geraert P.A., Devillard E. Bacillus subtilis strain specificity affects performance improvement in broilers. Poult. Sci. 2017;96:2274–2280. doi: 10.3382/ps/pex018. [DOI] [PubMed] [Google Scholar]
- Rodgers N.J., Swick R.A., Geier M.S., Moore R.J., Choct M., Wu S.-B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015;59:38–45. doi: 10.1637/10774-011614-reg.1. [DOI] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran N., Sihag S., Maan N. Expression analysis of immunity related genes in White Leghorn layers supplemented with probiotics and prebiotics. Pharma Innovation. 2017;6:14. [Google Scholar]
- Singh S.B., Davis A.S., Taylor G.A., Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- Skinner J.T., Bauer S., Young V., Pauling G., Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010;54:1237–1240. doi: 10.1637/9399-052110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S., Rodgers N., Swick R., Moore R. Differential responses of caecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9:1–10. doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Dewulf J., Nollet N., Schwarzer K., Haesebrouck F., Ducatelle R., Van Immerseel F. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010;39:117–121. doi: 10.1080/03079451003610586. [DOI] [PubMed] [Google Scholar]
- Tsiouris V.V. Poultry management: a useful tool for the control of necrotic enteritis in poultry. Avian Pathol. 2016;45:323–325. doi: 10.1080/03079457.2016.1154502. [DOI] [PubMed] [Google Scholar]
- Twardzik D.R., Mikovits J.A., Ranchalis J.E., Purchio A., Ellingsworth L., Ruscetti F.W. γ-interferon-induced activation of latent transforming growth factor-β by human monocytes. Ann. Phytopatholn. Y. Acad. Sci. 1990;593:276–284. doi: 10.1111/j.1749-6632.1990.tb16119.x. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Boyen F., Gantois I., Timbermont L., Bohez L., Pasmans F., Haesebrouck F., Ducatelle R. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 2005;84:1851–1856. doi: 10.1093/ps/84.12.1851. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Waititu S.M., Yitbarek A., Matini E., Echeverry H., Kiarie E., Rodriguez-Lecompte J.C., Nyachoti C.M. Effect of supplementing direct-fed microbials on broiler performance, nutrient digestibilities, and immune responses. Poult. Sci. 2014;93:625–635. doi: 10.3382/ps.2013-03575. [DOI] [PubMed] [Google Scholar]
- Wang J., Tang C., Wang Q., Li R., Chen Z., Han X., Wang J., Xu X. Apoptosis induction and release of inflammatory cytokines in the oviduct of egg-laying hens experimentally infected with H9N2 avian influenza virus. Vet. Microbiol. 2015;177:302–314. doi: 10.1016/j.vetmic.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Werling D., Jungi T.W. TOLL-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunopathol. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- Williams A.F., Barclay A.N. The immunoglobulin superfamily—domains for cell surface recognition. Annu. Rev. Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Williams L., Martin-Padura I., Dejana E., Hogg N., Simmons D. Identification and characterisation of human junctional adhesion molecule (JAM) Mol. Immunol. 1999;36:1175–1188. doi: 10.1016/s0161-5890(99)00122-4. [DOI] [PubMed] [Google Scholar]
- Wu S.-B., Rodgers N., Choct M. Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Dis. 2010;54:1058–1065. doi: 10.1637/9338-032910-Reg.1. [DOI] [PubMed] [Google Scholar]
- Yang F., Lei X., Rodriguez-Palacios A., Tang C., Yue H. Selection of reference genes for quantitative real-time PCR analysis in chicken embryo fibroblasts infected with avian leukosis virus subgroup. J. BMC Res. Notes. 2013;6:402. doi: 10.1186/1756-0500-6-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu L., Sheikhahmadi A., Wang Y., Li C., Jiao H., Lin H., Song Z. Effects of corticosterone and dietary energy on immune function of broiler chickens. PLoS One. 2015;10:e0119750. doi: 10.1371/journal.pone.0119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanu H.K., Keerqin C., Kheravii S.K., Morgan N., Wu S.B., Bedford M.R., Swick R.A. Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 2. intestinal permeability, organ weights, hematology, intestinal morphology, and jejunal gene expression. Poult. Sci. 2020;99:2581–2594. doi: 10.1016/j.psj.2019.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.Q., Zhang Z.W., Yao H.D., Wang L.L., Liu T., Yu X.Y., Li S., Xu S.W. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013;95:146–155. doi: 10.1016/j.rvsc.2013.01.021. [DOI] [PubMed] [Google Scholar]