Abstract
One avian leukosis virus of subgroup J (ALV-J) strain GX14YYA1 was isolated from a commercial bivalent Newcastle disease (ND)–infectious bronchitis (IB) vaccine in our previous study. To evaluate the pathogenicity of the ALV-J-contaminated vaccine on commercial chickens, day-old Three-Yellow chicks in group I were vaccinated with ALV-J-contaminated bivalent ND-IB live vaccine by intranasal and eye drop at 1-day-old for the primary vaccination and at 7-day-old for the secondary vaccination. Groups II and III were kept as the normal vaccination group with the noncontaminated ND-IB vaccine and blank control groups, respectively. The birds of different groups were maintained separately in isolators for 175 d. The first viremia was detected at 4 wk of age and 20% (2/10) of the birds maintained viremia during 11 to 25 wk of age. At the same time, the birds in group I experienced a significant suppression of body weight gain when compared with those of groups II and III (P < 0.05). In addition, the birds in group I showed obvious ALV-J hemangioma-type anatomical lesions in the liver and tumors were observed in the abdominal cavity. The results demonstrated that the ALV-J contaminated commercial live vaccines can induce pathogenicity in commercial Three-Yellow chickens and indicate that ALV-J-contaminated commercial live vaccines could be one of the transmission routes of ALV-J to commercial chickens.
Key words: avian leukosis virus subgroup J, live vaccine, pathogenicity, hemangioma, transmission
Introduction
The avian leukosis virus of subgroup J (ALV-J) was first isolated from meat-type breeder chickens in 1988 in the United Kingdom, and by the early 2000s, the virus had spread to China (Du et al., 2000; Payne and Nair, 2012). It has caused severe economic losses in the poultry industry worldwide due to its great pathogenicity and transmission ability (Zhou et al., 2018; Li et al., 2019). The ALV-J can be transmitted horizontally and vertically (Yang et al., 2017). Until now, no effective commercial vaccines or drugs have been available (Wang et al., 2019; Dai et al., 2020). Thus, ALV eradication becomes a major measure for the prevention and control of ALV-J (Dai et al., 2020). Owing to the initiation of a Nationwide Eradication Program in 2008 in China, the outbreak of ALV in the commercial white-feather broilers and layers has been reduced (Wei and Cui, 2015). However, the infection and associated clinical problems of ALV-J are still common in local commercial chickens.
In our previous study, an ALV-J strain GX14YYA1 was detected and isolated from a commercial live vaccine (Wang et al., 2018). To evaluate the pathogenicity of the ALV-J-contaminated live vaccine on commercial chickens, day-old Three-Yellow chicks were vaccinated with the ALV-J-contaminated Newcastle disease (ND)–infectious bronchitis (IB) live vaccine. This research explores the possibility of the ALV-J-contaminated commercial live vaccines as one of the transmission routes of ALV to commercial chickens.
Materials and methods
A total of 30 day-old commercial Three-Yellow chicks were purchased from a local commercial farm that was accredited by authorities for the elimination of specific avian diseases including AL. In group I, 10 chicks were vaccinated with the ALV-J-contaminated ND-IB vaccine by intranasal and eye drop, as per the instructions of the manufacturer, at 1-day-old for the primary vaccination and at 7-day-old for the secondary vaccination. In group II, 10 chicks were vaccinated with the noncontaminated ND-IB vaccine (proved by virus isolation by cell culture as the routine method) in the same way and at the same days. In group III, 10 chicks were inoculated with 200 μL phosphate-buffered saline in the same way and at the same days. Birds of different groups were maintained separately in isolators for 175 d with ad lib access to commercial feed and drinking water.
To determine the viremia at 7, 14, 21, 28, 35, 49, 56, 77, 147, and 175 d of age, 10 birds from each group were picked and samples of peripheral blood were obtained and the separated plasma was used for the virus isolation. Briefly, the plasma was inoculated into the DF-1 cells grown in a 24-well plate and then the cultures were grown for 9 d before they were used for ALV-P27 antigen detection with an ELISA kit (Biochek, Holland). The ALV p27 antigen-positive samples were genotyped after virus isolation. DNA from the cell samples was prepared using a commercial DNA extraction kit (Tiangen, Beijing, China) and further used for the PCR detection of ALV, respectively. To understand virus resemblance to former virus (Wang et al., 2018), the ALV-J gp85 genes from the birds with tumor were sequenced as described previously (Wang et al., 2020). The primers for the PCR detections and gp85 gene sequence are listed in Table 1, and the PCR procedure was performed as described previously (Wang et al., 2020).
Table 1.
Primers for PCR amplifications to detect ALV.
| Name | Sequence (5′-3′) | Annealing temperature (°C) | Expected size (bp) |
|---|---|---|---|
| ALV-A-F | GGATGAGGTGACTAAGAAAG | 50 | 692 |
| ALV-A-R | AGAGAAAGAGGGGTGTCTAAGGAG | ||
| ALV-B-F | GGATGAGGTGACTAAGAAAG | 50 | 847 |
| ALV-B-R | ATGGACCAATTCTGACTCATT | ||
| ALV-C-F | GGATGAGGTGACTAAGAAAG | 50 | 860 |
| ALV-C-R | GAGGCCAGTACCTCCCACG | ||
| ALV-D-F | GGATGAGGTGACTAAGAAAG | 50 | 797 |
| ALV-D-R | ATCCATACGCACCACAGTATTCG | ||
| ALV-J-F | GGATGAGGTGACTAAGAAAG | 56 | 545 |
| ALV-J-R | CGAACCAAAGGTAACACACG |
Abbreviation: ALV, avian leukosis virus.
To determine the effect of infection of the ALV-J-contaminated ND-IB vaccine on the live body weights of the birds at 1, 7, 21, 28, 35, 56, 77, 105, 147, and 175 d of age, all birds from each group were picked to weigh. All birds that died during the experiment or were sacrificed humanely at the end point of the experiment at 175 d were examined postmortem. The liver and other organs with gross tumors were collected and soaked in 10% neutral buffered formalin for fixation overnight. After embedding in paraffin wax, the tissues were sliced into 5-μm sections, which were routinely stained by means of hematoxylin and eosin. The processed sections were mounted on glass slides and examined under a light microscope (ECLIPSE 80i Nikon, Japan)
All the experimental animals of this study were cared for and maintained throughout the experiments strictly following the ethics and biosecurity guidelines approved by the Institutional Animal Care and Use Committee of Guangxi University.
Results and discussion
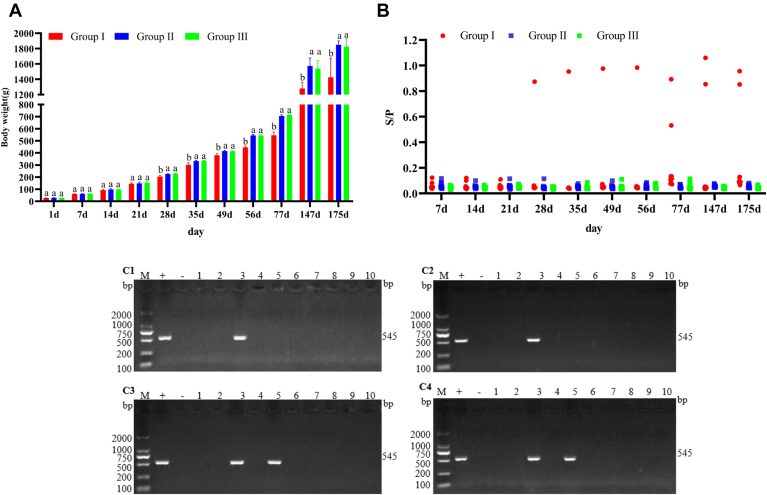
As shown in Figure 1A, at 28 d of age and then afterward, the body weight gains of birds in group I were dramatically suppressed when compared with the birds in groups II and III (P < 0.05). As indicated in Figure 1B, one bird (No.3) in group I developed viremia at 28 d of age, and the positive rate of viremia increased to 20% (2/10) (No.3 and No.5) at 77 d of age and was maintained to the end of the experiment at 175 d of age. The PCR results (Figure 1C) showed that the ALV P27 antigen-positive samples (C1, 28 d, No.3; C2, 56 d, No.3; C3, 77 d, No.3 and 5; C4, 175 d, No.3 and 5) were all ALV-J. These results indicated that the ALV-J-contaminated commercial live vaccines could cause viremia and inhibit body weight gain in vaccinated birds.
Figure 1.
Viremia, PCR results, and the influence of ALV-J infection on the body weights of birds (X±SE). (A) Influence of the viral infection on the body weights of birds. Each mean body weight, followed by a different lower-case letter, was significantly different (P < 0.05) based on Duncan's multiple range test. (B) ALV-positive identification by ALV P27 antigen ELISA; an S/P value greater than or equal to 0.2 was regarded as positive. (C) PCR was used to identify the ALV-J in the samples of 10 birds. The results showed that all ALV-positive samples were ALV-J; C1-C4, PCR results of 28, 56, 77, and 175 d samples. M, DL2000 DNA marker; +, DF-1 cells infected with GX14YYA1 used as positive control; -, uninfected DF-1 cells used as a negative control; 1-10, samples. Abbreviation: ALV-J, avian leukosis virus of subgroup J.
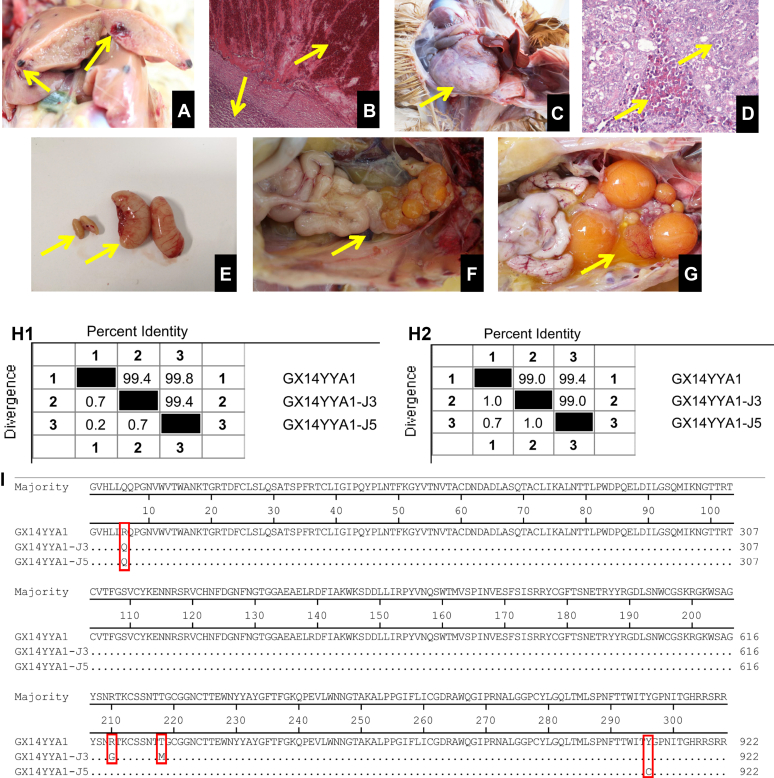
During the whole experiment, no death occurred in the birds of the 3 groups. At 175 d of age, one bird (No.5) in group I had hemangioma in the liver and microscopically, masses containing blood cells and heterophilic lymphoid cells were found in the hemangioma regions (Figures 2A and 2B). A gross tumor was found in the abdomen in one of the birds (No.3) in group I (Figure 2C) and microscopically, numerous infiltrations of myelocytes were found and that were characterized by acidophilic granules in the cytoplasm (Figure 2D). The testes, ovaries, and fallopian tubes in group I birds were poorly developed (Figures 2E and 2F), whereas those in the control groups developed normally (Figures 2E and 2G). These results indicated that the ALV-J-contaminated commercial live vaccines could cause tumor.
Figure 2.
Anatomical, histopathological lesions and sequence analysis results. (A) Hemangioma in the liver; (B) Blood cells and heterophilic lymphoid cells in the hemangioma region; (C) Tumor in the abdomen; (D) The infiltrated myelocytes that were characterized by acidophilic granules in the cytoplasm; (E) Comparison of normal (without arrow point) and abnormal testicles (with arrow point); (F) Ovaries poorly developed at 175-day-old; (G) Ovaries normally developed at 175-day-old. Similarity of nucleotide (H1) and amino acids (H2) sequences of gp85 between GX14YYA1-J3, GX14YYA1-J5 and GX14YYA1; (I) Comparison of the amino acid sequences of gp85 between GX14YYA1-J3, GX14YYA1-J5, and GX14YYA1.
To understand virus resemblance to former virus (GX14YYA1), the ALV-J gp85 genes from the birds of No.3 and No.5 with tumors at 175 d were sequenced and analyzed. The similarities of nucleotide and amino acids sequence of gp85 gene between GX14YYA1-J3 (isolated from No.3 chicken, MW477383), GX14YYA1-J5 (isolated from No.5 chicken, MW477383) and GX14YYA1 were 99.4, 99.8% and 99.0, 99.4%, respectively (Figures H1, H2). Further analysis shows that there were only 3 (R6Q, R210G, and T218M) and 2 (R6Q and Y296G) amino acid mutation sites between GX14YYA1-J3, GX14YYA1-J5, and GX14YYA1, respectively (Figure 1). These results confirmed that the virus causing tumors in the chickens is the same strain as virus reported previously (Wang et al., 2018).
It has been more than 30 yr since ALV-J was first isolated in the UK (Payne et al., 1991). Since then, scientists have invested a lot of labor, financial and material resources in the research of the virus, and have achieved a number of remarkable achievements (Ye et al., 2005; Guan et al., 2017; Li et al., 2020). Unfortunately, up to the present time, there is still no effective commercial vaccine or drug available to treat and control the ALV-J infection (Dai et al., 2020). Eradication schemes against exogenous ALV have been the only effective routine measures for the prevention and control of ALV-J. However, in some companies that have carried out strict ALV eradication program for years, there still is a rebound in the positive rate of ALV infection in flocks. One of the reasons easily overlooked might be that the live vaccines used for the chickens were contaminated with ALV.
In 2006, Fadly et al reported the commercial vaccine of Marek's disease virus was confirmed to be ALV-positive (Fadly et al., 2006), and in 2014, Zhao et al reported an ALV-A contamination in a live vaccine in China (Zhao et al., 2014). Our team reported a live ALV-J-contaminated commercial live vaccine in 2018 (Wang et al., 2018), and in 2019, another team in Nigeria reported 9% (4/44) of the live commercially poultry vaccines were contaminated with ALV-J (Shittu et al., 2019). Although these reports speculated that the injection of the vaccines contaminated with ALV might be the transmission route of ALV to the commercial chickens, no direct evidence was provided.
In this study, we established an infection model in which 1-day-old Three-Yellow chickens were vaccinated with the ALV-J-contaminated ND-IB live vaccine in the same route and at the same time as that of the routine commercial vaccine, and then the viremia, body weight gain, tumor incidence, and ALV-J gp85 gene were detected and sequenced, respectively. The birds vaccinated with the ALV-J-contaminated ND-IB live vaccine not only showed viremia and significantly inhibited weight gain but also showed typical pathological changes, including tumors, of an ALV-J infection. The evidences of a sudden rebound of the positive rate of ALV infection and clinical tumor incidence in flocks that had been vaccinated with the ALV-J-contaminated vaccine were also observed on the farm (Wang et al., 2018).
These results demonstrated that ALV-J-contaminated commercial live vaccines could be one of the transmission routes of ALV to commercial chickens.
The present experimental results raise the alarm that exogenous viruses must be tested before the commercial live vaccines were used, and any of the contaminated vaccine must be eliminated. That is one of the important measures to ensure the success of eradication programs in the poultry industry.
Acknowledgments
This work was supported by the Guangxi Special Funding on Science and Technology Research [AA17204057], the Guangxi Program for Modern Agricultural Industry Technical System Construction-Chicken Industry [nycytxgxcxtd-19-03], the Shandong Provincial Natural Science Foundation, China [ZR2019BC047]. The article was kindly reviewed by Dr. Richard Roberts, Aurora, CO 80014, USA.
Disclosures
The authors declare that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Dai M.M., Li S.B., Shi K.Y., Liao J.Y., Hui S., Liao M. Systematic identification of host Immune Key Factors influencing viral infection in PBL of ALV-J infected SPF chicken. Viruses. 2020;12:114. doi: 10.3390/v12010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Cui Z.Z., Qin A.J., Silva R.F., Lee L.F. Isoaltion of Subgroup J avian leukosis viruses and their partial sequence comparison. Chin. J. Virol. 2000;6:341–346. [Google Scholar]
- Fadly A., Pandiri R. Silva.A., Davis C. Isolation and characterization of an Adventitious avian leukosis virus isolated from commercial Marek's disease vaccines. Avian Dis. 2006;50:380–385. doi: 10.1637/7497-122905R.1. [DOI] [PubMed] [Google Scholar]
- Guan X., Zhang Y., Yu M., Ren C., Gao Y., Yun B., Liu Y., Wang Y., Qi X., Liu C., Cui H., Zhang Y., Gao L., Li K., Pan Q., Zhang B., Wang X., Gao Y. Residues 28 to 39 of the Extracellular Loop 1 of chicken Na+/H+ Exchanger type I Mediate cell Binding and Entry of subgroup J avian leukosis virus. J. Virol. 2017;92 doi: 10.1128/JVI.01627-17. e01627-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.J., Wang P.K., Lin L.L., Shi M.Y., Gu Z.M., Huang T., Mo M.L., Wei T.C., Zhang H.M., Wei P. The emergence of the infection of subgroup J avian leucosis virus escalated the tumour incidence in commercial Yellow chickens in Southern China in recent years. Transbound. Emerg. Dis. 2019;66:312–316. doi: 10.1111/tbed.13023. [DOI] [PubMed] [Google Scholar]
- Li T.F., Yao X.H., Li C.P., Zhang J., Xie Q., Wang W.K., Lu H., Fu H., Li L.Y., Xie J., Shao H.X., Gao W., Qin A.J., Ye J.Q. Gp37 regulates the pathogenesis of avian leukosis virus subgroup J via its C-terminus. J. Virol. 2020;94 doi: 10.1128/JVI.02180-19. e02180-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L.N., Brown S.R., Umstead N.B., Howes K., Frazier J.A., Thouless M.E. A novel subgroup of exogenous avian leukosis virus in chickens. J. Gen. Virol. 1991;72:801–807. doi: 10.1099/0022-1317-72-4-801. [DOI] [PubMed] [Google Scholar]
- Payne L.N., Nair V. The long view: 40 years of avian leukosis research. Avian Pathol. 2012;41:11–19. doi: 10.1080/03079457.2011.646237. [DOI] [PubMed] [Google Scholar]
- Shittu I., Adedeji A.J., Luka P.D., Asala O.O., Sati N.M., Nwagbo I.O., Chinyere C.N., Arowolo O.O., Adole J.A., Emennaa P., Abdu P.A., Joannis M. Avian leukosis virus subgroup - J as a contaminant in live commercially available poultry vaccines distributed in Nigeria. Biologicals. 2019;57:29–33. doi: 10.1016/j.biologicals.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Wang P.K., Lin L.L., Li H.J., Shi M.Y., Gu Z.M., Wei P. Full-length genome sequence analysis of an avian leukosis virus subgroup J (ALV-J) as contaminant in live poultry vaccine: the commercial live vaccines might be a potential route for ALV-J transmission. Transbound. Emerg. Dis. 2018;65:1103–1106. doi: 10.1111/tbed.12841. [DOI] [PubMed] [Google Scholar]
- Wang P.K., Li M., Li H.J., Lin L.L., Shi M.Y., Gu Z.M., Gao Y.L., Huang T., Mo M.L., Wei T.C., Wei P. Full-length cDNA sequence analysis of 85 avian leukosis virus subgroup J strains isolated from chickens in China during the years 1988–2018: coexistence of 2 extremely different clusters that are highly dependent upon either the host genetic background or the geographic location. Poult. Sci. 2020;99:3469–3480. doi: 10.1016/j.psj.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Miao Y., Xu Y., Meng X., Cui W., Wang Y., Zhu L., Sha Z., Wei K., Zhu R. Taishan Pinus Massoniana pollen polysaccharide inhibits the replication of acute tumorigenic ALV-J and its associated tumor growth. Vet. Microbiol. 2019;236:108376. doi: 10.1016/j.vetmic.2019.07.028. [DOI] [PubMed] [Google Scholar]
- Wei P., Cui Z.Z. Avian leukosis and pullorum disease: threats to Chinese native breeder chickens and preventivemeasures for elimination. China Poult. 2015;37:1–4. [Google Scholar]
- Yang L., Cui S., Li W.H., Wang Y.X., Cui Z.Z., Zhao P., Chang S. Vertical transmission of avian leukosis virus subgroup J (ALV-J) from hens infected through artificial insemination with ALV-J infected semen. Bmc Vet. Res. 2017;13:204. doi: 10.1186/s12917-017-1122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.Q., Qin A.J., Shao H., Liu H., Jin W., Liu Y. Development of chicken embryo fibroblast cell line resistant to J subgroup avian leukosis virus(ALV-J) infection. Chin. J. Virol. 2005;21:456–460. [Google Scholar]
- Zhao P., Dong X., Cui Z.Z. Isolation, identification, and gp85 characterization of a subgroup A avian leukosis virus from a contaminated live Newcastle Disease virus vaccine, first report in China. Poult. Sci. 2014;93:2168–2174. doi: 10.3382/ps.2014-03963. [DOI] [PubMed] [Google Scholar]
- Zhou D., Xue J., Zhang Y., Wang G., Feng Y., Hu L., Shang Y., Cheng Z. Outbreak of myelocytomatosis caused by mutational avian leukosis virus subgroup J in China, 2018. Transbound. Emerg. Dis. 2018;66:622–626. doi: 10.1111/tbed.13096. [DOI] [PubMed] [Google Scholar]