Abstract
The Liver Imaging Reporting and Data System (LI-RADS) is a comprehensive system for standardizing liver imaging in patients at risk of developing hepatocellular carcinoma (HCC). We aimed to determine the diagnostic performance of LI-RADS category 5 (LR5) for diagnosing HCC and LI-RADS category M (LRM) for characterizing other non-HCC malignancies (OM) using contrast-enhanced ultrasound (CEUS) and computed tomography (CT)/magnetic resonance imaging (MRI). Multiple databases were searched for articles evaluating the diagnostic accuracy of CEUS LI-RADS and/or CT/MRI LI-RADS. A random-effects model was adopted to synthesize the summary estimates of the diagnostic accuracy of LR5 for diagnosing HCC and LRM for characterizing OM using CEUS and CT/MRI. The pooled sensitivity and specificity of CEUS LR5 for the diagnosis of HCC were 69% and 93%, respectively. The pooled sensitivity was 67% and the specificity, 93% of CT/MRI LR5 for HCC diagnosis. There was no significant difference between the overall diagnostic accuracy for HCC diagnosis of CEUS LR5 and that of CT/MRI LR5 in terms of diagnostic odds ratio (DOR) (p = 0.55). The sensitivity was 84% with a specificity of 90% in the CEUS LRM for characterizing OM, while the sensitivity and specificity of CT/MRI LRM for characterizing OM was 63% and 95%. The DOR of CEUS LRM for characterizing OM was higher than that of CT/MRI LRM without significant difference (50.59 vs. 36.06, p = 0.34). This meta-analysis indicated that CEUS LI-RADS is qualified to characterize HCC and OM and may provide complementary information on liver nodules to CT/MRI LI-RADS.
Keywords: hepatocellular carcinoma, liver neoplasms, contrast media, ultrasonography, routine diagnostic tests
1. Introduction
Hepatocellular carcinoma (HCC) is a major cause of morbidity and mortality of cancer worldwide [1]. Unlike other malignancies, HCC can be non-invasively diagnosed in the absence of histological assessment. Imaging has been recommended as a vital diagnostic tool for HCC diagnosis in patients at high risk for developing HCC [2]. Hence, dependable imaging is critical.
CT and MRI are recommended as the first-line diagnosis methods for HCC diagnosis because of their powerful differential diagnosis of liver neoplasms [2]. In order to standardize reporting and data collection of CT and MRI for HCC diagnosis, the American College of Radiology (ACR) released the CT/MRI Liver Imaging Reporting and Data System (LI-RADS) in 2011 [3]. The three latest versions of CT/MRI LI-RADS were released in 2014, 2017, and 2018. An updated version in 2018 was finally adopted by the American Association for the Study of Liver Disease (AASLD) [4].
Along with the development of sonicated microbubbles, contrast-enhanced ultrasound (CEUS) is able to characterize focal liver lesions commendably. However, several studies have suggested that HCC could be hardly distinguished from some other non-HCC malignancies (OM), such as intrahepatic cholangiocarcinoma (ICC) and mixed hepatocellular and cholangiocarcinoma (HCC-CCA) in CEUS, thus leading to inappropriate clinical strategy [5,6]. That might be the main reason why the AASLD does not accept CEUS as a reliable diagnostic technique. However, other studies have found that when considering the arterial phase hyper-enhancement pattern and the onset and intensity of washout, CEUS did well on distinguishing HCC from OM [7,8]. In recognition of the usefulness of CEUS, several guidelines recommended it as the first- or second-line tool [9,10,11,12]. The CEUS LI-RADS was first introduced in 2016 for trial implementation and it was officially issued in 2017 [13]. Similar to CT/MRI LI-RADS algorithm, liver lesions are assigned into several categories ranked from LI-RADS category 1 (LR1, definitely benign) to LI-RADS category 5 (LR5, definitely HCC) with added categories LI-RADS category M (LRM, probably or definitely malignant but not HCC specific), LI-RADS category TIV (LRTIV, definite tumor in vein) and LI-RADS category NC (LRNC, cannot be categorized due to image degradation) in CEUS LI-RADS. Among them, LR5 lesions can be treated as HCC without biopsy, while LRM lesions need further confirmation through pathological assessment.
A recent systematic review explored the pooled proportion of HCC and overall malignancy in each LR based on CT and MRI [14]. Another two meta-analyses determined the sensitivity and specificity of LR5 for diagnosing HCC using CT/MRI LI-RADS and CEUS LI-RADS, respectively [15,16]. Kim et al. focused on the probability of HCC and OM in the LRM based on MRI [17]. Nevertheless, no systematic review or meta-analysis has compared the diagnostic performance of CEUS LI-RADS and CT/MRI LI-RADS for characterizing HCC and OM. Hence, we performed a meta-analysis to evaluate the diagnostic accuracy of CEUS LR5 for diagnosing HCC and CEUS LRM for characterizing OM in patients at risk of HCC in comparison to CT/MRI ones.
2. Materials and Methods
This meta-analysis was in line with the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement [18]. The study protocol was registered in the INPLASY international platform of registered systematic review and meta-analysis protocols. The registration number was INPLASY202060056.
2.1. Literature Search
We systematically searched Ovid MEDLINE, Ovid Cochrane Central Register of Controlled Trials, EMBASE, and Scopus databases for studies published from 1 January 2014 to 7 April 2020 regardless of languages. The search strategy is shown thoroughly in Supplementary Table S1. The reference lists of the eligibility studies and meta-analyses related to the topic were also manually searched.
2.2. Eligibility Criteria
The original studies that evaluated the diagnostic performance of CEUS LI-RADS or CT/MRI LI-RADS for characterizing HCC and OM in patients at high risk of HCC were included. Here, according to LI-RADS, adults who had cirrhosis, chronic HBV infection, and current or prior HCC were defined as patients at high risk of HCC. The exclusion criteria were as follows: (a) studies that did not enroll either HCC or OM, (b) studies in which the index test did not meet a minimum requirement of LI-RADS technical recommendations, (c) studies without an available reference standard, (d) studies with duplicated patients and (e) reviews, meta-analyses, case reports, letters, and comments.
2.3. Study Selection and Data Extraction
After removing duplicate records, two reviewers independently screened the titles and abstracts of the retrieved studies to remove the irrelevant ones. Full texts of the included abstracts were subsequently reviewed. Data of the eligibility studies were extracted by two reviewers independently using a pre-designed data form, shown in Supplementary Table S2. In cases of discordance, a third reviewer would discuss with the two reviewers until an agreement was reached.
2.4. Quality Assessment
Two reviewers performed methodological quality assessments independently using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool [19] that contains 4 domains including patient and lesion selection, index test, reference standard, and flow and timing. A third reviewer would ask the two reviewers to state their reasons and made final decisions once inconsistency occurred.
2.5. Statistical Analysis
The pooled sensitivity, specificity with their 95% CI of LR5 for diagnosing HCC and LRM for diagnosing OM were calculated using a bivariate random-effects model. Diagnostic odds ratios (DORs) with their 95% CI were also calculated, which represented the overall diagnostic accuracy. It was worth mentioning that when more than one dataset were available in a study (e.g., >1 LI-RADS version, >1 index test >1 reviewer), the data were averaged. The Cochran Q test and I2 statistic were used to assess the heterogeneity. p < 0.05 of Q test and I2 > 50% may suggest apparent heterogeneity. Meta-regression analysis was performed to explore the potential source of the heterogeneity. Publication bias was investigated by Deek’s funnel plot and the statistical significance was tested using Egger’s regression test. All statistical analyses were performed with Stata version 13.0 (StataCorp, College Station, TX, USA) and Review Manager version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark).
3. Results
3.1. Literature Search
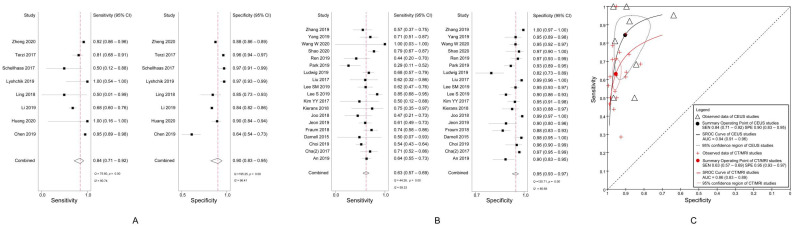
Five hundred and forty-eight studies were screened, of which 39 records were eventually included. The flow diagram of the study selection is shown in Figure 1 and the details of excluded studies are given in Supplementary Table S3. Among 39 studies, nine records [20,21,22,23,24,25,26,27,28] demonstrated the accuracy for HCC diagnosis of CEUS LR5, and 31 [20,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58] evaluated that of CT/MRI LR5, while eight records [21,22,23,24,25,26,27,28] evaluated the performance of CEUS LRM for characterizing OM and 19 reported [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] that of CT/MRI LRM.
Figure 1.
The flow diagram of study selection. CENTRAL, cochrane central register of controlled trials. CEUS, contrast-enhanced ultrasound.
3.2. Study Characteristics
The baseline characteristics of the included studies are shown in Table 1. Eventually, we included 5179 lesions (3841 HCCs, 456 OMs, and 884 benign lesions) for CEUS and 7908 lesions (5443 HCC, 762 OM, and 1713 benign lesions) for CT/MRI (Table 2).
Table 1.
Main characteristics of the included studies.
| Reference | Background | Patients | Index Test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Country | Centre | Study Type | Study Design | No. of Patients | Average Age (y) | Male, % | Imaging Modality | LI-RADS Version | |
| Chen et al., 2019 [21] | China | Single | Retrospective | Case-control | 210 | 54.5 | 77.6 | CEUS | 2017 |
| Huang et al., 2020 [22] | China | Single | Retrospective | Cohort | 172 | 51.8 | 79.1 | CEUS | 2017 |
| Li et al., 2019 [23] | China | Single | Retrospective | Cohort | 1366 | 52.3 | 80.3 | CEUS | 2017 |
| Ling et al., 2018 [24] | China | Single | Retrospective | NR | 56 | 52.5 | 82.1 | CEUS | 2017 |
| Lyshchik et al., 2019 [28] | USA | Multiple | Prospective | Cohort | NR | NR | NR | CEUS | 2017 |
| Schellhaas et al., 2017 [25] | Germany | Single | Prospective | Cohort | 100 | 66.1 | 85.0 | CEUS | 2016 |
| Terzi et al., 2017 [26] | Italy | Multiple | Retrospective | Cohort | 848 | 70 | 53.9 | CEUS | 2016 |
| Zheng et al., 2020 [27] | China | Single | Retrospective | Cohort | 1826 | 54 | 89.9 | CEUS | 2017 |
| Wang et al., 2020 [20] | China | Single | Retrospective | Cohort | 63 | 56 | 74.6 | CEUS | 2017 |
| ECA-MRI | 2018 | ||||||||
| Alhasan et al., 2018 [48] | Canada | Single | Retrospective | Cohort | 59 | 63.2 | 76.3 | CT | 2017 |
| An et al., 2019 [29] | Korea | Multiple | Retrospective | Case-control | 217 | 59 | 76.5 | CT, HBA-MRI | 2014 |
| Basha et al., 2018 [49] | Egypt | Multiple | Prospective | Cohort | 240 | 61.5 | 55.8 | CT, ECA-MRI | 2014 |
| Cha(1) et al., 2020 [50] | Korea | Single | Prospective | Cohort | 122 | 55 | 82.8 | ECA-MRI, HBA-MRI | 2018 |
| Cha(2) et al., 2017 [30] | Korea | Single | Retrospective | Cohort | 421 | 57 | 72.0 | CT, HBA-MRI a | 2014 |
| Choi et al., 2019 [31] | Korea | Single | Retrospective | Case-control | 194 | 57 | 79.9 | HBA-MRI | 2017 |
| Darnell et al., 2015 [32] | Spain | Single | Retrospective | Cohort | 133 | 64 | 50.9 | ECA-MRI | 2014 |
| Forner et al., 2019 [51] | Spain | Single | Retrospective | NR | 262 | NR | NR | MRI | 2018 |
| Fraum et al., 2018 [33] | USA | Single | Prospective | Cohort | 220 | 60.3 | NR | CT, ECA-MRI, HBA-MRI a | 2014 |
| Hwang et al., 2019 [52] | Korea | Single | Retrospective | Cohort | 177 | 58 | 89.3 | HBA-MRI | 2018 |
| Jeon et al., 2019 [34] | Korea | Single | Retrospective | Case-control | 140 | 56 | 71.7 | HBA-MRI | 2017 |
| Joo et al., 2018 [35] | Korea | Single | Retrospective | NR | 288 | NR | NR | HBA-MRI | 2017 |
| Kang et al., 2020 [53] | Korea | Single | Retrospective | Cohort | 266 | 61.3 | 81.2 | HBA-MRI | 2018 |
| Kierans et al., 2018 [36] | USA | Multiple | Retrospective | Cohort | 114 | 57 | 65.8 | ECA-MRI, HBA-MRI | 2017 |
| Kim DH et al., 2019 [54] | Korea | Single | Prospective | Cohort | 258 | 61 | 81.4 | HBA-MRI | 2018 |
| Kim YY et al., 2017 [37] | Korea | Single | Retrospective | Cohort | 143 | 58 | 83.9 | HBA-MRI | 2014 |
| Lee S et al., 2019 [39] | Korea | Single | Retrospective | Cohort | 298 | 57.4 | 72.1 | ECA-MRI, HBA-MRI | 2018 |
| Lee SE et al., 2017 [55] | Korea | Single | Retrospective | Cohort | 103 | 61 | 74.8 | ECA-MRI | 2017 |
| Lee SM 2019 [38] | Korea | Single | Retrospective | Cohort | 387 | 59 | 78.8 | HBA-MRI | 2017, 2018 |
| Liu et al., 2017 [40] | China | Single | Retrospective | NR | 249 | 51 | 85.5 | CT, MRI a | 2014 |
| Ludwig et al., 2019 [41] | USA | Multiple | Retrospective | Cohort | 178 | 61.9 | 77.5 | CT, ECA-MRI, HBA-MRI a | 2018 |
| Min et al., 2019 [56] | Korea | Single | Prospective | Cohort | 125 | 55.3 | 81.6 | CT, ECA-MRI, HBA-MRI | 2018 |
| Park et al., 2019 [42] | Korea | Multiple | Retrospective | Cohort | 267 | 56.2 | 77.9 | HBA-MRI | 2018 |
| Ren et al., 2019 [43] | China | Single | Retrospective | Cohort | 181 | 56.4 | 77.9 | ECA-MRI | 2017, 2018 |
| Renzulli et al., 2018 [57] | Italy | Single | Retrospective | Cohort | 228 | 63.7 | 79.4 | HBA-MRI | 2017 |
| Ronot et al., 2018 [58] | France | Multiple | Prospective | Cohort | 442 | 61.9 | 77.6 | CT, ECA-MRI | 2014 |
| Shao et al., 2020 [44] | China | Single | Retrospective | Case-control | 140 | 48 | 85.7 | ECA-MRI, HBA-MRI | 2018 |
| Wang W et al., 2020 [45] | China | Single | Retrospective | Cohort | 204 | 55 | 89.2 | HBA-MRI | 2018 |
| Yang et al., 2019 [46] | China | Single | Retrospective | NR | 130 | 51.5 | 76.2 | HBA-MRI | 2018 |
| Zhang et al., 2019 [47] | China | Single | Retrospective | Cohort | 203 | 50.3 | 77.3 | HBA-MRI | 2017 |
Abbreviations: LI-RADS, Liver Imaging Reporting and Data System. CEUS, contrast-enhanced ultrasound. CT, computed tomography. MRI, magnetic resonance imaging. ECA, extracellular contrast agents. HBA, hepatobiliary agents. NR, not reported. a, these studies did not evaluate the diagnostic accuracy at CT and MRI separately.
Table 2.
Lesions and their reference standards.
| Reference | Patients | Lesions and Reference Standards | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Cirrhosis, % | n | Pathology, % | Average Size (Range, mm) |
HCC, n | OM, n | Benign Lesion, n | Interval from Index Test to Pathology | Interval from Index Test to Follow-Up | |
| Chen et al., 2019 [21] | 210 | NR £ | 210 | 100.0 | NR | 105 † | 105 † | 0 | NR | N/A |
| Huang et al., 2020 [22] | 172 | NR | 175 | 70.9 | 16.1 (8–20) | 105 †,‡,§ | 2 † | 68 †,‡,§ | (13 ± 7) d | NR |
| Li et al., 2019 [23] | 1366 | 37.5 | 1366 | 100.0 | 47 (5–200) | 985 † | 139 † | 242 † | NR | N/A |
| Ling et al., 2018 [24] | 56 | 8.9 | 56 | 100.0 | 17.1 (14.3–20) | 44 † | 2 † | 10 † | NR | N/A |
| Lyshchik et al., 2019 [28] | NR | NR | 162 | NR | 24 (NR) | 136 †,‡,§ | 6 NR | 20 NR | NR | NR |
| Schellhaas et al., 2017 [25] | 100 | 4.0 | 100 | 63.0 | 52.2 (10–290) | 87 †,‡ | 6 † | 7 †,§ | ≤3 m | ≥6 m |
| Terzi et al., 2017 [26] | 848 | 100.0 | 1066 | 46.9 | 20 (5–150) | 820 †,‡ | 53 † | 133 †,‡ | NR | N/A |
| Wang J et al., 2020 [20] | 63 | 96.8 | 84 | 100.0 | 35 (9–183) | 45 † | 4 † | 35 † | NR | N/A |
| Zheng et al., 2020 [27] | 1826 | 86.3 | 2020 | 35.5 | NR | 1514 †,‡,§ | 138 †,‡ | 368 †,‡,§ | NR | ≥12 m |
| Alhasan et al., 2018 [48] | 59 | 93.2 | 104 | 41.3 | 30.1 (6–110) | 72 †,‡,§ | 4 † | 38 †,‡,§ | HCC:(137 ± 206) d Benign lesions:(251 ± 441) d |
≥6 m |
| An et al., 2019 [29] | 217 | NR | 231 | 100.0 | 21 (3−133) | 114 † | 58 † | 59 † | ≤2 m | N/A |
| Basha et al., 2018 [49] | 240 | NR | 296 | 63.2 | NR (≤50) | 192 †,§ | 9 † | 95 †,§ | NR | NR |
| Cha(1) et al., 2020 [50] | 122 | 59.8 | 147 | 95.9 | 21 (6–50) | 122 † | 10 † | 15 †,§ | ≤1 m | NR |
| Cha(2) et al., 2017 [30] | 421 | NR | 445 | 100.0 | 25 (8–49) | 397 † | 31 † | 17 † | ≤2 m | N/A |
| Choi et al., 2019 [31] | 194 | 100.0 | 194 | 100.0 | 32 (NR) | 97 † | 97 † | 0 | ≤3 m | N/A |
| Darnell et al., 2015 [32] | 133 | 100.0 | 133 | NR | NR (5–20) | 102 † | 4 † | 27 †,‡,§ | NR | NR |
| Forner et al., 2019 [51] | 262 | 100.0 | 262 | NR | NR (≤20) | 197 †,‡,§ | 7 † | 58 †,‡,§ | NR | NR |
| Fraum et al., 2018 [33] | 220 | 66.4 | 220 | 100.0 | NR | 136 † | 42 † | 42 † | NR | N/A |
| Hwang et al., 2019 [52] | 177 | NR | 241 | 71.0 | 19.7 (2.3–48.2) | 149 † | 6 † | 86 †,§ | ≤6 m | ≥24 m |
| Jeon et al., 2019 [34] | 140 | 12.1 | 140 | 100.0 | 39.4 (NR) | 70 † | 70 † | 0 | ≤2 m | N/A |
| Joo et al., 2018 [35] | 288 | 57.6 Ψ | 387 | 39.3 | NR | 292 †,‡ | 15 † | 80 †,‡,§ | ≤2 m | ≥6 m |
| Kang et al., 2020 [53] | 266 | NR | 385 | 62.9 | NR (5–30) | 283 †,§ | 18 † | 84 †,§ | NR | ≥24 m |
| Kierans et al., 2018 [36] | 114 | NR ₴ | 144 | 71.5 | NR | 82 † | 8 † | 54 †,§ | ≤4 m | ≥24 m |
| Kim DH et al., 2019 [54] | 258 | NR | 372 | 63.4 | NR (10–30) | 273 †,§ | 18 † | 81 †,§ | NR | ≥24 m |
| Kim YY et al., 2017 [37] | 143 | NR | 202 | 61.9 | NR | 129 †,§ | 6 † | 67 †,§ | 35 (1–461) d | ≥18 m |
| Lee S et al., 2019 [39] | 298 | 63.1 | 382 | 85.6 | 24 (15–34) | 286 † | 33 † | 63 †,‡,§ | ≤3 m | ≥24 m |
| Lee SE et al., 2017 [55] | 103 | NR | 133 | 75.2 | 22.2 (4.9–192) | 107 †,§ | 3 † | 23 †,§ | NR | ≥18 m |
| Lee SM et al., 2019 [38] | 387 | 74.2 Ψ | 422 | 70.4 | 24 (NR) | 234 † | 45 † | 143 †,§ | ≤2 m | ≥24 m |
| Liu et al., 2017 [40] | 249 | NR | 297 | 64.3 | 22 (3–146) | 178 † | 13 † | 106 †,§ | ≤1 m | ≥24 m |
| Ludwig et al., 2019 [41] | 178 | 97.8 Ψ | 178 | 100.0 | 35 (14–190) | 105 † | 73 † | 0 | HCC:189d; OM:89d | N/A |
| Min et al., 2019 [56] | 125 | 52.8 £ | 163 | 89.6 | 20.7 (9–40) | 124 † | 13 † | 26 †,‡,§ | ≤1 m | NR |
| Park et al., 2019 [42] | 267 | 100.0 | 306 | 100.0 | 19 (5–30) | 280 † | 21 † | 5 † | NR | N/A |
| Ren et al., 2019 [43] | 181 | 32.6 | 217 | 59.0 | 30.5 (NR) | 146 †,§ | 16 † | 55 †,‡,§ | NR | ≥24 m |
| Renzulli et al., 2018 [57] | 228 | NR | 420 | 38.3 | 16.7 (11–150) | 342 †,‡,§ | 8 † | 69 †,§ | NR | ≥24 m |
| Ronot et al., 2018 [58] | 442 | 100.0 Ψ | 595 | NR | 18 (10–30) | 341 †,‡,§ | 8 ¶ | 246 ¶ | ≤6 m | ≥6 m |
| Shao et al., 2020 [44] | 140 | NR | 140 | 100.0 | 48 (10–120) | 70 † | 70 † | 0 | ≤6 m | N/A |
| Wang W et al., 2020 [45] | 204 | 100.0 | 373 | NR | 14 (5.5–19) | 256 †,‡ | 1 † | 116 †,§ | NR | ≥24 m |
| Yang et al., 2019 [46] | 130 | NR | 134 | 100.0 | 49.2 (NR) | 97 † | 29 † | 8 † | ≤1 m | N/A |
| Zhang et al., 2019 [47] | 203 | NR | 245 | 81.6 | 53 (11–128) | 165 † | 30 † | 50 †,§ | NR | ≥24 m |
Abbreviations: HCC, hepatocellular carcinoma. OM, other non-HCC malignancies. £, cirrhosis of any origin confirmed by pathological examination. Ψ, cirrhosis confirmed by pathological assessment or clinical diagnostic criteria. ₴, clinically diagnosed cirrhosis. †, pathological analysis. ‡, contrast-enhanced CT or MRI. §, follow-up. ¶, did not meet the criteria for HCC. d, days. m, months. n, number. NR, not reported. N/A, not applicable.
3.3. Quality Assessment
The overall risk of bias of the 39 studies was low to moderate, shown in Supplementary Figure S1. Patient selection was the most significant source of bias. Thirteen studies were identified as being at high risk of bias in the domain of patient selection because they did not avoid a case-control design and/ or enroll lesions diagnosed by composite clinical reference standards. Studies that employed LR5 of another imaging modality without follow-up as a reference standard were considered at high risk of bias in the domain of reference standard.
3.4. Diagnostic Performance of LR5 for Diagnosing HCC
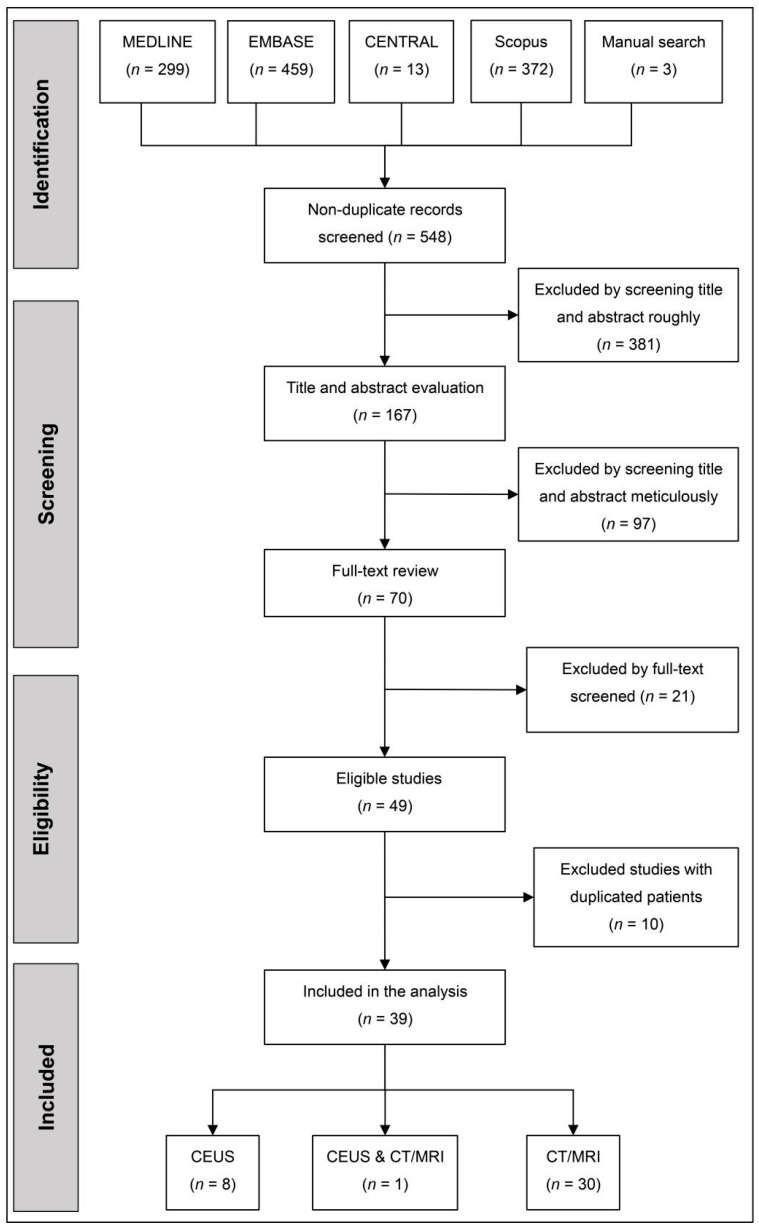
The pooled sensitivity and specificity of CEUS LR5 for diagnosing HCC were, respectively, 69% (95% CI, 64%–74%; I2 = 89.45%; p < 0.01) and 93% (95% CI, 87%–97%; I2 = 88.85%; p < 0.01) (Figure 2A).
Figure 2.
(A) Sensitivity and specificity of CEUS LR5 for diagnosing HCC; (B) Sensitivity and specificity of CT/MRI LR5 for diagnosing HCC; (C) Summary receiver operating characteristic curves of CEUS LR5 and CT/MRI LR5 for diagnosing HCC. CEUS, contrast-enhanced ultrasound; LR5, Liver Imaging Reporting and Data System category 5; HCC, hepatocellular carcinoma.
The pooled sensitivity was 67% (95% CI, 62–71%; I2 = 91.01%; p < 0.01) and the specificity, 93% (95% CI, 91–95%; I2 = 78.86%; p < 0.01) of CT/MRI LR5 for diagnosing HCC (Figure 2B).
There was no significant difference in the DOR of the LR5 for HCC diagnosis between CEUS (31.83, 95% CI, 14.04–72.15) and CT/MRI (26.55, 95% CI, 19.81–35.56) (p = 0.55). The area under the summary receiver operating characteristic (SROC) curve of CEUS LR5 for HCC and that of CT/MRI LR5 were 0.82 (95% CI, 0.79–0.85) and 0.88 (95% CI, 0.85–0.91), respectively (Figure 2C).
3.5. Diagnostic Performance of LRM for Characterizing OM
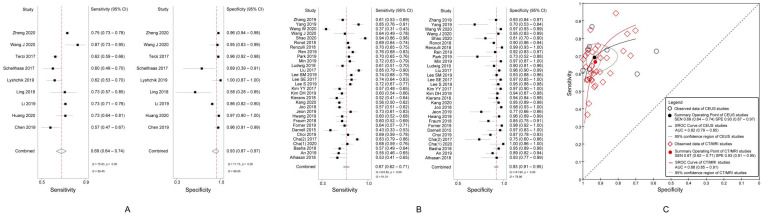
A pooled analysis showed a sensitivity of 84% (95% CI, 71–92%; I2 = 90.74%; p < 0.01) and a specificity of 90% (95% CI, 83–95%; I2 = 96.41%; p < 0.01) for characterizing OM in respect of CEUS LRM (Figure 3A).
Figure 3.
(A) Sensitivity and specificity of CEUS LRM for characterizing OM; (B) Sensitivity and specificity of CT/MRI LRM for characterizing OM; (C) Summary receiver operating characteristic curves of CEUS LRM and CT/MRI LRM for characterizing OM. CEUS, contrast-enhanced ultrasound; LRM, Liver Imaging Reporting and Data System category M; OM, other non-HCC malignancies.
The pooled sensitivity was 63% (95% CI, 57–69%; I2 = 59.33%; p < 0.01) and the specificity, 95% (95% CI, 93–97%; I2 = 85.68%; p < 0.01) of CT/MRI LRM for characterizing OM (Figure 3B).
The DOR of CEUS LRM for characterizing OM was 50.59 (95% CI, 22.16–115.52), and that of CT/MRI LRM was 36.06 (95% CI, 23.70–54.87) (p = 0.34). Analysis of the area under the SROC curves showed that the accuracy of CEUS LRM for characterizing OM and that of CT/MRI was 0.94 (95% CI, 0.91–0.96) and 0.86 (95% CI, 0.83–0.89), respectively (Figure 3C).
3.6. Meta-Regression Analysis
Meta-regression analyses of the sensitivity and specificity of CEUS LR5 for diagnosing HCC and CEUS LRM for characterizing OM are demonstrated in Supplementary Table S4. Among six covariates, country, average lesion size and proportion of OM were the factors that contributed to the heterogeneity of the sensitivity of CEUS LR5 for diagnosing HCC. Regarding the reference standard, the specificity of CEUS LR5 for diagnosing HCC was higher for studies using a mixed reference standard as a reference standard than for those that employed only pathology (96% vs. 89%; p = 0.06). Country, study design, proportion of OM, and reference standard were the factors that significantly influenced the heterogeneity of the specificity of the CEUS LRM category for characterizing OM.
Meta-regression analyses of the sensitivity and specificity of the CT/MRI LR5 category for diagnosing HCC and the CT/MRI LRM category for characterizing OM were also performed (Supplementary Table S5). We noted that the pooled sensitivity was 67% (95% CI, 62–72%) and the specificity, 93% (95% CI, 91–96%) of LR5 for diagnosing HCC with CT/MRI LI-RAD v2017/2018, superior to those with v2014 (sensitivity, 65%, 95% CI, 56–74%; specificity, 92%, 95% CI, 88–96%; p = 0.05). As for average size, the sensitivity of CT/MRI LI-RADS for diagnosing HCC and characterizing OM was reduced when small lesions were enrolled (Sensitivity of LR5 for diagnosing HCC, 61% vs. 68%; Sensitivity of LRM for characterizing OM, 57% vs. 64%), similar to the findings observed in CEUS studies. Besides, the index test was one of the factors that significantly influenced the heterogeneity of the sensitivity and specificity for both HCC and non-HCC malignancies with CT/MRI LI-RADS.
3.7. Publication Bias
No obvious publication bias was identified through the funnel plot and Egger’s test (p > 0.05), shown in Supplementary Figure S2.
4. Discussion
The meta-analysis included more than 5000 lesions in each LI-RADS. A pooled analysis revealed a sensitivity of 69% and a specificity of 93% of CEUS LR5 and a sensitivity of 67% and a specificity of 93% of CT/MRI LR5 for diagnosing HCC. This study also indicated that the pooled sensitivity and specificity for characterizing OM with respect to CEUS LRM were 84% and 90%, respectively, and those of CT/MRI LRM were 63% and 95%.
Our meta-analysis found that the pooled sensitivity and specificity for diagnosing HCC of CEUS LR5 was 69% and 93%, respectively, which was quite similar to those summary estimates of CT/MRI LR5 (sensitivity 67% and specificity 93%). A study conducted by Shin et al. [16] suggested that the diagnostic performances for diagnosing HCC of LI-RADS with CEUS and CT/MRI can be compared. In our study, there was no statistical difference between CEUS LR5 and CT/MRI LR5 in the overall diagnostic accuracy for diagnosing HCC (p = 0.55), but a specificity of 93% for HCC diagnosis of LR5 was lower than that preconceived in the LI-RADS algorithm (definitely HCC).
Patients with ICC and HCC-CCA have a poorer prognosis and fewer treatment options than those patients with HCC. Therefore, distinguishing OM from HCC will be of great significance for making clinical decisions. In our meta-analysis, CEUS LRM showed high sensitivity (84%) and high specificity (90%) for characterizing OM, while moderate sensitivity (63%) and high specificity (95%) were observed in CT/MRI LRM. The overall diagnostic accuracy of CEUS LRM was higher than that of CT/MRI LRM without significant difference (p = 0.34). In other words, compared to CT/MRI, CEUS did not increase the risk of misdiagnosing OM as HCC. This finding is not in agreement with the results of some previous studies which indicated CEUS was associated with a high risk of misdiagnosis [7].
CEUS is different from CT/MRI in the following aspects [59]: (a) physicochemical properties of contrast agent used, (b) method to obtain images (for CEUS, dynamic real-time imaging; for CT/MRI, static imaging) and (c) criteria adopted to assign the LI-RADS category to a liver nodule. Based on the above, CEUS may provide additional information of focal liver nodules to CT/MRI. We suggested that CEUS is useful in assisting to provide an accurate diagnosis for patients at risk of HCC and a multimodality approach might be needed to improve the diagnostic performance of characterizing HCC and OM.
Regarding the baseline characteristics of the included studies, we noted that most were from Asian countries, followed by European and North American countries. Although the epidemiological features and imaging characteristics of HCC in non-Asian countries were different from that in Asian countries, CEUS LI-RADS and CT/MRI LI-RADS introduced by the American association were also suitable to be applied in Asian countries where HBV is endemic. Patients with HBV infection are at risk for HCC even without cirrhosis. In a cirrhotic liver, we need to differentiate HCC from regenerating nodules, and for patients with HBV infection in the absence of cirrhosis, we do not need to do so. This might lead to superior diagnostic performance for HCC. However, the prevalence of cirrhosis in the whole sample was lower than that in western countries, so the generalizability of these results should be further assessed. There are only a limited number of studies evaluating the diagnostic performance of CEUS LI-RADS.The main reasons are as follows: (a) The CEUS LI-RADS was officially issued in 2017, while CT/MRI LI-RADS was first released in 2011 and multiple revisions were updated after that. In other words, CEUS LI-RADS has a short time of application worldwide in comparison to CT/MRI LI-RADS. (b) Some centers adopted other CEUS-based standardized algorithms (such as ESCULAP, EFSUMB) in clinical practice instead of LI-RADS. Among 39 included records, only eight were prospective studies, eight were multicenter studies and twenty-nine employed a cohort study design. More prospective, multicenter cohort researches that focus on the LI-RADS algorithm are expected to be published in the future.
QUADAS-2 was used to assess the methodological quality of the included studies. More than half of the studies had at least one domain at high risk of bias. In our study, case-control studies were viewed to be inferior to those cohort studies since the percentage of HCC and OM depended on the researchers. Using pathology as a reference standard exclusively might introduce verification bias because lesions receiving pathological assessment are more inclined to be malignant and be assigned to higher LI-RADS categories. There is likely to be incorporation bias for studies employing LR5 of another imaging modality without follow-up as a reference standard [14].
To a certain extent, there were clinical and methodological similarities between studies because of cautious study selection and methodological quality assessment, but there was still statistical heterogeneity between the included studies. The meta-regression analysis showed that the specificity of the CEUS LR5 for diagnosing HCC in the studies employing merely pathology as a reference standard was significantly lower than those employing a mixed reference standard (89% vs. 96%, p = 0.03). A similar finding was observed in the CT/MRI studies. HCC with typical imaging features can be easily diagnosed by composite imaging, whereas atypical HCC was difficult to be definitely diagnosed by imaging and should be biopsied. Studies using only a pathological reference standard might contain more atypical HCC than those using a mixed reference standard, which may explain their lower specificity for characterizing HCC. We also noted that when small lesions were enrolled, the sensitivity for diagnosing HCC and characterizing OM was reduced both at CEUS and CT/MRI. Small liver nodules usually possess atypical imaging features, so that it remains a challenge to distinguish small HCC and OM from other focal liver lesions, especially under the background of cirrhosis.
Comparing CT/MRI LI-RADS v2014, v2017 and v2018 made major revisions to applicable patients, diagnostic categories, threshold growth definition, LRM criteria, and ancillary features. The pooled sensitivity was 67% and the specificity was 93% of LR5 for diagnosing HCC with CT/MRI LI-RAD. v2017/2018 was superior to those with v2014 (sensitivity, 65%; specificity, 92%; p = 0.05). It seemed that the revisions significantly improved the diagnostic performance for diagnosing HCC. The index test significantly influenced the heterogeneity of the sensitivity and specificity for both HCC and non-HCC malignancies with CT/MRI LI-RADS. So we performed three meta-analyses that compared the different techniques (CEUS, CT, MRI) two by two after removing four studies [30,33,40,41] which didn’t evaluate the diagnostic accuracy at CT and MRI imaging separately (Supplementary Table S6).
We tried to answer the question of whether CEUS is qualified to characterize HCC and OM accurately, which were closely relevant to the management of liver nodules. There were some limitations. First, apparent heterogeneity was identified in our meta-analysis. To seek the covariates that contributed to the heterogeneity, a meta-regression analysis was performed, but the heterogeneity still cannot be ignored. Second, an adjusted indirect comparison was used instead of direct comparison, mainly due to insufficient evidence comparing the diagnostic accuracy of CEUS LI-RADS and CT/MRI LI-RADS head-to-head. A study reported that adjusted indirect comparison might be less biased than direct comparison [60]. Finally, we did not perform subgroup analyses based on major and ancillary features of the LI-RADS because of insufficient data. Thus, our results should be confirmed by more evidence. Considering the limitations, more high-quality direct comparisons between CEUS LI-RADS and CT/MRI LI-RADS are required to update this meta-analysis. At present, we have been working on a multicenter study comparing the diagnostic accuracy of CEUS LI-RADS and CT/MRI LI-RADS for characterizing HCC and OM directly with a large sample size. We also keep a concurring opinion with the study [14] that an individual participant meta-analysis is needed.
In conclusion, both CEUS LR5 and CT/MRI LR5 showed moderate sensitivity and high specificity for diagnosing HCC. CEUS LRM showed high sensitivity and high specificity for characterizing OM, while CT/MRI LRM showed moderate sensitivity and high specificity.
Abbreviations
| AASLD | American Association for the Study of Liver Disease |
| ACR | American College of Radiology |
| CEUS | Contrast-enhanced ultrasound |
| DOR | Diagnostic odds ratio |
| HCC | Hepatocellular carcinoma |
| HCC-CCA | Mixed hepatocellular and cholangiocarcinoma |
| ICC | Intrahepatic cholangiocarcinoma |
| LI-RADS | Liver Imaging Reporting and Data System |
| LR5 | LI-RADS category 5 |
| LRM | LI-RADS category M |
| OM | Other non-HCC malignancies |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies 2 |
| SROC | Summary receiver operating characteristic |
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/11/2/323/s1, Figure S1. Methodological quality assessment using the Quality Assessment of Diagnostic Accuracy Studies 2 tool; Figure S2. Deek’s funnel plot and Egger’s test to evaluate the publication bias of (A) studies evaluating the diagnostic performance of CEUS LR5 for HCC diagnosis, (B) studies evaluating the diagnostic performance of CT/MRI LR5 for HCC diagnosis, (C) studies evaluating the diagnostic performance of CEUS LRM for characterizing OM, and (D) studies evaluating the diagnostic performance of CT/MRI LRM for characterizing OM. CEUS, contrast-enhanced ultrasound; HCC, hepatocellular carcinoma; OM, other non-HCC malignancies; LR5, Liver Imaging Reporting and Data System category 5; LRM, Liver Imaging Reporting and Data System category M; ESS, effective sample sizes; p > 0.05, no obvious publication bias was identified; Table S1. Details of search strategy; Table S2. Data form; Table S3. Details of excluded studies; Table S4. The results of meta-regression of the sensitivity and specificity of the CEUS LR5 for diagnosing HCC and the CEUS LRM for characterizing OM; Table S5. The results of meta-regression of the sensitivity and specificity of the CT/MRI LR5 for diagnosing HCC and the CT/MRI LRM for characterizing OM; Table S6. The diagnostic performance of LR5 for diagnosing HCC and LRM for characterizing OM with CEUS, CT and MRI separately.
Author Contributions
Study design: all authors; study selection: J.H., Q.L. and J.Z.; data collection: L.L., C.P., and J.Z.; quality assessment: C.P., Q.L. and J.H.; data analysis and interpretation: L.L., Y.H., and J.H.; manuscript writing: L.L.; final approval of manuscript: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent was waived for this study because all analyses were based on previously published studies.
Ethical Approval
Institutional Review Board approval was waived because all analyses were based on previously published studies.
Data Availability Statement
All analyses were based on previously published studies. No new data were created in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., Roberts L.R., Heimbach J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 3.American College of Radiology CT/MRI Liver Imaging Reporting and Data System Version 2018. [(accessed on 3 April 2020)]; Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018.
- 4.Kim T.-H., Kim S.Y., Tang A., Yoon J.H. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin. Mol. Hepatol. 2019;25:245–263. doi: 10.3350/cmh.2018.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilana R., Forner A., Bianchi L., García-Criado Á., Rimola J., De Lope C.R., Reig M., Ayuso C., Brú C., Bruix J. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatolology. 2010;51:2020–2029. doi: 10.1002/hep.23600. [DOI] [PubMed] [Google Scholar]
- 6.Galassi M., Iavarone M., Rossi S., Bota S., Vavassori S., Rosa L., Leoni S., Venerandi L., Marinelli S., SanGiovanni A., et al. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013;33:771–779. doi: 10.1111/liv.12124. [DOI] [PubMed] [Google Scholar]
- 7.Wildner D., Bernatik T., Greis C., Seitz K., Neurath M.F., Strobel D. CEUS in Hepatocellular Carcinoma and Intrahepatic Cholangiocellular Carcinoma in 320 Patients—Early or Late Washout Matters: A Subanalysis of the DEGUM Multicenter Trial. Ultraschall der Med.-Eur. J. Ultrasound. 2015;36:132–139. doi: 10.1055/s-0034-1399147. [DOI] [PubMed] [Google Scholar]
- 8.Li F., Li Q., Liu Y., Han J., Zheng W., Huang Y., Zheng X., Cao L., Zhou J.-H. Distinguishing intrahepatic cholangiocarcinoma from hepatocellular carcinoma in patients with and without risks: The evaluation of the LR-M criteria of contrast-enhanced ultrasound liver imaging reporting and data system version 2017. Eur. Radiol. 2019;30:461–470. doi: 10.1007/s00330-019-06317-2. [DOI] [PubMed] [Google Scholar]
- 9.Omata M., Cheng A.-L., Kokudo N., Kudo M., Lee J.M., Jia J., Tateishi R., Han K.-H., Chawla Y.K., Shiina S., et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 11.2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J. Radiol. 2019;20:1042–1113. doi: 10.3348/kjr.2019.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokudo N., Takemura N., Hasegawa K., Takayama T., Kubo S., Shimada M., Nagano H., Hatano E., Izumi N., Kaneko S., et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 13.American College of Radiology Contrast-Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS) Version 2016. [(accessed on 3 April 2020)]; Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CEUS-LI-RADS-v2016.
- 14.Van der Pol C.B., Lim C.S., Sirlin C.B., McGrath T.A., Salameh J., Bashir M.R., Tang A., Singal A.G., Costa A.F., Fowler K., et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology. 2019;156:976–986. doi: 10.1053/j.gastro.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Lee S., Kim S., Roh Y.H., Choi J., Park M., Kim M. Diagnostic Performance of CT/MRI Liver Imaging Reporting and Data System v2017 for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Int. 2020;40:1488–1497. doi: 10.1111/liv.14424. [DOI] [PubMed] [Google Scholar]
- 16.Shin J., Lee S., Bae H., Chung Y.E., Choi J., Huh Y., Park M. Contrast-enhanced ultrasound liver imaging reporting and data system for diagnosing hepatocellular carcinoma: A meta-analysis. Liver Int. 2020;40:2345–2352. doi: 10.1111/liv.14617. [DOI] [PubMed] [Google Scholar]
- 17.Kim D.H., Choi S.H., Park S.H., Kim K.W., Byun J.H., Kim S.Y., Lee S.S., Shin Y.M., Won H.J., Kim P. Liver imaging reporting and data system category M: A systematic review and meta-analysis. Liver Int. 2020;40:1477–1487. doi: 10.1111/liv.14420. [DOI] [PubMed] [Google Scholar]
- 18.McInnes M., Moher D., Thombs B.D., McGrath T.A., Bossuyt P.M., Clifford T., Cohen J.F., Deeks J.J., Gatsonis C., Hooft L., et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 19.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M.M., The QUADAS-2 Group QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Wang J.-Y., Feng S.-Y., Yi A.-J., Zhu D., Xu J.-W., Li J., Cui X.-W., Dietrich C.F. Comparison of Contrast-Enhanced Ultrasound versus Contrast-Enhanced Magnetic Resonance Imaging for the Diagnosis of Focal Liver Lesions Using the Liver Imaging Reporting and Data System. Ultrasound Med. Biol. 2020;46:1216–1223. doi: 10.1016/j.ultrasmedbio.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Chen L.-D., Ruan S.-M., Lin Y., Liang J.-Y., Shen S.-L., Hu H.-T., Huang Y., Li W., Wang Z., Xie X.-Y., et al. Comparison between M-score and LR-M in the reporting system of contrast-enhanced ultrasound LI-RADS. Eur. Radiol. 2019;29:4249–4257. doi: 10.1007/s00330-018-5927-8. [DOI] [PubMed] [Google Scholar]
- 22.Huang J.-Y., Li J.-W., Lu Q., Luo Y., Lin L., Shi Y.-J., Li T., Liu J.-B., Lyshchik A. Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology. 2020;294:329–339. doi: 10.1148/radiol.2019191086. [DOI] [PubMed] [Google Scholar]
- 23.Li J., Ling W., Chen S., Ma L., Yang L., Lu Q., Luo Y. The interreader agreement and validation of contrast-enhanced ultrasound liver imaging reporting and data system. Eur. J. Radiol. 2019;120:108685. doi: 10.1016/j.ejrad.2019.108685. [DOI] [PubMed] [Google Scholar]
- 24.Ling W., Wang M., Ma X., Qiu T., Li J., Lu Q., Luo Y. The preliminary application of liver imaging reporting and data system (LI-RADS) with contrast-enhanced ultrasound (CEUS) on small hepatic nodules (≤2 cm) J. Cancer. 2018;9:2946–2952. doi: 10.7150/jca.25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schellhaas B., Gortz R.S., Pfeifer L., Kielisch C., Neurath M.F., Strobel D. Diagnostic accuracy of contrast-enhanced ultrasound for the differential diagnosis of hepatocellular carcinoma: ESCULAP versus CEUS-LI-RADS. Eur. J. Gastroen. Hepat. 2017;29:1036–1044. doi: 10.1097/MEG.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 26.Terzi E., Iavarone M., Pompili M., Veronese L., Cabibbo G., Fraquelli M., Riccardi L., De Bonis L., SanGiovanni A., Leoni S., et al. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J. Hepatol. 2018;68:485–492. doi: 10.1016/j.jhep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Zheng W., Li Q., Zou X.-B., Wang J.-W., Han F., Li F., Huang L.-S., Li A.-H., Zhou J.-H. Evaluation of Contrast-enhanced US LI-RADS version 2017: Application on 2020 Liver Nodules in Patients with Hepatitis B Infection. Radiology. 2020;294:299–307. doi: 10.1148/radiol.2019190878. [DOI] [PubMed] [Google Scholar]
- 28.Lyshchik A KYPF Clinical Validation of CEUS LI-RADS in Prospective Multi-Center Study: Preliminary Results. [(accessed on 3 April 2020)]; Radiological Society of North America, 2019 Scientific Assembly and Annual Meeting 2019. Available online: http://archive.rsna.org/2019/19014914.html.
- 29.An C., Lee C.H., Byun J.H., Lee M.H., Jeong W.K., Choi S.H., Kim D.Y., Lim Y.-S., Kim Y.S., Kim J.H., et al. Intraindividual Comparison between Gadoxetate-Enhanced Magnetic Resonance Imaging and Dynamic Computed Tomography for Characterizing Focal Hepatic Lesions: A Multicenter, Multireader Study. Korean J. Radiol. 2019;20:1616–1626. doi: 10.3348/kjr.2019.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha D.I., Jang K.M., Kang T.W., Kim S.H., Song K.D. Liver Imaging Reporting and Data System on CT and gadoxetic acid-enhanced MRI with diffusion-weighted imaging. Eur. Radiol. 2017;61:1056–4405. doi: 10.1007/s00330-017-4804-1. [DOI] [PubMed] [Google Scholar]
- 31.Choi S.H., Lee S.S., Park S.H., Kim K.M., Yu E., Park Y., Shin Y.M., Lee M.-G. LI-RADS Classification and Prognosis of Primary Liver Cancers at Gadoxetic Acid–enhanced MRI. Radiology. 2019;290:388–397. doi: 10.1148/radiol.2018181290. [DOI] [PubMed] [Google Scholar]
- 32.Darnell A., Forner A., Rimola J., Reig M., García-Criado Á., Ayuso C., Bruix J. Liver Imaging Reporting and Data System with MR Imaging: Evaluation in Nodules 20 mm or Smaller Detected in Cirrhosis at Screening US. Radiology. 2015;275:698–707. doi: 10.1148/radiol.15141132. [DOI] [PubMed] [Google Scholar]
- 33.Fraum T.J., Tsai R., Rohe E., Ludwig D.R., Salter A., Nalbantoglu I., Heiken J.P., Fowler K.J. Differentiation of Hepatocellular Carcinoma from Other Hepatic Malignancies in Patients at Risk: Diagnostic Performance of the Liver Imaging Reporting and Data System Version 2014. Radiology. 2018;286:158–172. doi: 10.1148/radiol.2017170114. [DOI] [PubMed] [Google Scholar]
- 34.Jeon S.K., Joo I., Lee D.H., Lee S.M., Kang H.-J., Lee K.-B., Lee J.M. Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur. Radiol. 2019;29:373–382. doi: 10.1007/s00330-018-5605-x. [DOI] [PubMed] [Google Scholar]
- 35.Joo I., Lee J.M., Lee D.H., Jeon J.H., Han J.K. Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: Can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur. Radiol. 2019;29:1724–1732. doi: 10.1007/s00330-018-5727-1. [DOI] [PubMed] [Google Scholar]
- 36.Kierans A.S., Makkar J., Guniganti P., Cornman-Homonoffm J., Lee M.J., Pittman M., Askin G., Hecht E.M. Validation of Liver Imaging Reporting and Data System 2017 (LI-RADS) Criteria for Imaging Diagnosis of Hepatocellular Carcinoma. J. Magn. Reson. Imaging. 2019;49:e205–e215. doi: 10.1002/jmri.26329. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y., An C., Kim S., Kim M. Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. Eur. Radiol. 2018;28:2038–2046. doi: 10.1007/s00330-017-5188-y. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.M., Lee J.M., Ahn S.J., Kang H.-J., Yang H.K., Yoon J.H. LI-RADS Version 2017 versus Version 2018: Diagnosis of Hepatocellular Carcinoma on Gadoxetate Disodium–enhanced MRI. Radiology. 2019;292:655–663. doi: 10.1148/radiol.2019182867. [DOI] [PubMed] [Google Scholar]
- 39.Lee S., Kim M.-J., Kim S.-S., Shin H., Kim D.Y., Choi J.-Y., Park M.-S., Mitchell D.G. Retrospective comparison of EASL 2018 and LI-RADS 2018 for the noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Hepatol. Int. 2020;14:70–79. doi: 10.1007/s12072-019-10002-3. [DOI] [PubMed] [Google Scholar]
- 40.Liu W., Qin J., Guo R., Xie S., Jiang H., Wang X., Kang Z., Wang J., Shan H. Accuracy of the diagnostic evaluation of hepatocellular carcinoma with LI-RADS. Acta Radiol. 2018;59:140–146. doi: 10.1177/0284185117716700. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig D.R., Fraum T.J., Cannella R., Ballard D.H., Tsai R., Naeem M., Leblanc M., Salter A., Tsung A., Shetty A.S., et al. Hepatocellular carcinoma (HCC) versus non-HCC: Accuracy and reliability of Liver Imaging Reporting and Data System v2018. Abdom. Radiol. 2019;44:2116–2132. doi: 10.1007/s00261-019-01948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park S.H., Kim B., Kim S.Y., Shim Y.S., Kim J.H., Huh J., Kim H.J., Kim K.W., Lee S.S. Abbreviated MRI with optional multiphasic CT as an alternative to full-sequence MRI: LI-RADS validation in a HCC-screening cohort. Eur. Radiol. 2019;30:2302–2311. doi: 10.1007/s00330-019-06546-5. [DOI] [PubMed] [Google Scholar]
- 43.Ren A.-H., Zhao P.-F., Yang D.-W., Du Bs J., Wang Z.-C., Yang Z.-H. Diagnostic performance of MR for hepatocellular carcinoma based on LI-RADS v2018, compared with v2017. J. Magn. Reson. Imaging. 2019;50:746–755. doi: 10.1002/jmri.26640. [DOI] [PubMed] [Google Scholar]
- 44.Shao S., Liang Y., Kuang S., Chen J., Shan Q., Yang H., Zhang Y., Wang B., Fowler K.J., Wang J., et al. Diagnostic performance of LI-RADS version 2018 in differentiating hepatocellular carcinoma from other hepatic malignancies in patients with hepatitis B virus infection. Bosn. J. Basic Med Sci. 2020;20:401–410. doi: 10.17305/bjbms.2019.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W., Yang C., Zhu K., Yang L., Ding Y., Luo R., Zhu S., Chen C., Sun W., Zeng M., et al. Recurrence after Curative Resection of HBV-related Hepatocellular Carcinoma: Diagnostic Algorithms on Gadoxetic Acid-enhanced Magnetic Resonance Imaging. Liver. Transpl. 2020;26:751–763. doi: 10.1002/lt.25713. [DOI] [PubMed] [Google Scholar]
- 46.Yang C., Jiang H., Song B., Chen J. The diagnostic performance of version 2018 LI-RADS for hepatocellular carcinoma on Gd-EOB-DTPA enhanced MRI. Chin. J. Radiol. 2019;53:1060–1064. [Google Scholar]
- 47.Zhang T., Huang Z.-X., Wei Y., Jiang H.-Y., Chen J., Liu X.-J., Cao L.-K., Duan T., He X.-P., Xia C.-C., et al. Hepatocellular carcinoma: Can LI-RADS v2017 with gadoxetic-acid enhancement magnetic resonance and diffusion-weighted imaging improve diagnostic accuracy? World J. Gastroenterol. 2019;25:622–631. doi: 10.3748/wjg.v25.i5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alhasan A., Cerny M., Olivié D., Billiard J.-S., Bergeron C., Brown K., Bodson-Clermont P., Castel H., Turcotte S., Perreault P., et al. LI-RADS for CT diagnosis of hepatocellular carcinoma: Performance of major and ancillary features. Abdom. Radiol. 2018;44:517–528. doi: 10.1007/s00261-018-1762-2. [DOI] [PubMed] [Google Scholar]
- 49.Basha M.A.A., AlAzzazy M.Z., Ahmed A.F., Yousef H.Y., Shehata S.M., Sammak D.A.E.A.E., Fathy T., Obaya A.A., Abdelbary E.H. Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. Eur. Radiol. 2018;28:2592–2603. doi: 10.1007/s00330-017-5232-y. [DOI] [PubMed] [Google Scholar]
- 50.Cha D.I., Choi G.S., Kim Y.K., Kim J.M., Kang T.W., Song K.D., Ahn S.H. Extracellular contrast-enhanced MRI with diffusion-weighted imaging for HCC diagnosis: Prospective comparison with gadoxetic acid using LI-RADS. Eur. Radiol. 2020;30:3723–3734. doi: 10.1007/s00330-020-06753-5. [DOI] [PubMed] [Google Scholar]
- 51.Forner A., Darnell A., Caparroz C., Rimola J., García-Criado M.Á., Belmonte E., Reig M., Ayuso C., Bruix J. PS-115-Evaluation of LI-RADS v2018 by magnetic resonance in US-detected nodules <2 cm in cirrhotics. J. Hepatol. 2019;70:e73. doi: 10.1016/s0618-827830127-6. [DOI] [PubMed] [Google Scholar]
- 52.Hwang S.H., Park S., Han K., Choi J.-Y., Park Y.-N., Park M.-S. Optimal lexicon of gadoxetic acid-enhanced magnetic resonance imaging for the diagnosis of hepatocellular carcinoma modified from LI-RADS. Abdom. Radiol. 2019;44:3078–3088. doi: 10.1007/s00261-019-02077-1. [DOI] [PubMed] [Google Scholar]
- 53.Kang J.H., Choi S.H., Byun J.H., Kim D.H., Lee S.J., Kim S.Y., Won H.J., Shin Y.M., Kim P.-N. Ancillary features in the Liver Imaging Reporting and Data System: How to improve diagnosis of hepatocellular carcinoma ≤ 3 cm on magnetic resonance imaging. Eur. Radiol. 2020;30:2881–2889. doi: 10.1007/s00330-019-06645-3. [DOI] [PubMed] [Google Scholar]
- 54.Kim D.H., Choi S.H., Kim S.Y., Kim M.-J., Lee S.S., Byun J.H. Gadoxetic Acid–enhanced MRI of Hepatocellular Carcinoma: Value of Washout in Transitional and Hepatobiliary Phases. Radiology. 2019;291:651–657. doi: 10.1148/radiol.2019182587. [DOI] [PubMed] [Google Scholar]
- 55.Lee S.E., An C., Hwang S.H., Choi J.-Y., Han K., Kim M.-J. Extracellular contrast agent-enhanced MRI: 15-min delayed phase may improve the diagnostic performance for hepatocellular carcinoma in patients with chronic liver disease. Eur. Radiol. 2017;28:1551–1559. doi: 10.1007/s00330-017-5119-y. [DOI] [PubMed] [Google Scholar]
- 56.Min J.H., Kim J.M., Kim Y.K., Cha D.I., Kang T.W., Kim H., Choi G.S., Choi S.-Y., Ahn S. Magnetic Resonance Imaging With Extracellular Contrast Detects Hepatocellular Carcinoma With Greater Accuracy Than With Gadoxetic Acid or Computed Tomography. Clin. Gastroenterol. Hepatol. 2020;18:2091–2100.e7. doi: 10.1016/j.cgh.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Renzulli M., Biselli M., Brocchi S., Granito A., Vasuri F., Tovoli F., Sessagesimi E., Piscaglia F., D’Errico A., Bolondi L., et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: A new diagnostic algorithm. Gut. 2018;67:1674–1682. doi: 10.1136/gutjnl-2017-315384. [DOI] [PubMed] [Google Scholar]
- 58.Ronot M., Fouque O., Esvan M., Lebigot J., Aubé C., Vilgrain V. Comparison of the accuracy of AASLD and LI-RADS criteria for the non-invasive diagnosis of HCC smaller than 3 cm. J. Hepatol. 2018;68:715–723. doi: 10.1016/j.jhep.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 59.Kim T.K., Noh S.Y., Wilson S.R., Kono Y., Piscaglia F., Jang H., Lyshchik A., Dietrich C.F., Willmann J.K., Vezeridis A., et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin. Mol. Hepatol. 2017;23:280–289. doi: 10.3350/cmh.2017.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song F., Harvey I., Lilford R. Adjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. J. Clin. Epidemiol. 2008;61:455–463. doi: 10.1016/j.jclinepi.2007.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analyses were based on previously published studies. No new data were created in this study. Data sharing is not applicable to this article.