Abstract
Coagulase-negative staphylococci (CoNS) are increasingly associated with nosocomial infections, especially among the immunocompromised and those with invasive medical devices, posing a significant concern. We report on clinical multidrug-resistant CoNS from the uMgungundlovu District, KwaZulu-Natal Province, South Africa, as emerging pathogens. One hundred and thirty presumptive CoNS were obtained from blood cultures. Culture, biochemical tests, and the Staphaurex™ Latex Agglutination Test were used for the initial identification of CoNS isolates; confirmation and speciation were undertaken by the VITEK 2 system. Susceptibilities of isolates against a panel of 20 antibiotics were determined using the Kirby-Bauer disk diffusion method, and the multiple antibiotic resistance (MAR) indices of the isolates were determined. The polymerase chain reaction (PCR) was used to amplify the mecA gene to confirm methicillin resistance. Overall, 89/130 presumptive CoNS isolates were confirmed as CoNS by the VITEK 2 system. Of these, 68 (76.4%) isolates were putatively methicillin-resistant by the phenotypic cefoxitin screen test and 63 (92.6%) were mecA positive. Staphylococcus epidermidis (19.1%), S. hominis ssp. hominis (15.7%), and S. haemolyticus (16.9%) were the most common CoNS species. Isolates showed high percentage resistance against penicillin (100.0%), erythromycin (74.2%), and azithromycin (74.2%) while displaying high susceptibilities to linezolid (95.5%), gentamicin (95.5%), and tigecycline (94.4%). Multidrug resistance (MDR) was observed in 76.4% of isolates. MAR index calculation revealed 71.9% of isolates with MAR index >0.2 and 20.2% >0.5. Isolates with the highest MAR indices (0.7 and 0.8) were recovered from the neonatal intensive care unit. Fifty-one MDR antibiograms were observed. The high prevalence of methicillin resistance and multidrug resistance in several species of CoNS necessitates surveillance of this emerging pathogen, currently considered a contaminant of microbial cultures.
Keywords: coagulase-negative staphylococci, antibiotic resistance, multidrug resistance, infections, multiple antibiotic resistance index, public health
1. Introduction
Staphylococci are classified as either coagulase-positive or coagulase-negative, depending on their ability to clot plasma that is facilitated by the enzyme coagulase [1]. Coagulase-negative staphylococci (CoNS) are the most frequent colonizers of the skin and mucous membranes and the most frequently isolated organisms in microbiology laboratories [2]. Although CoNS are mostly considered contaminants in clinical specimens, they have been implicated in clinically relevant infections, including urinary tract infections, endocarditis, bloodstream infections (including neonatal sepsis), and foreign body-related infections [2]. The skin and mucous membranes of the host, which are home to an abundance of CoNS species, are significant sources of endogenous CoNS infections, facilitated by transmission through medical procedures [3].
Pathogenic CoNS are usually associated with clinical environments and found in immunocompromised patients and patients with indwelling metallic or polymer devices, such as orthopedic prostheses, peripheral venous catheters, and artificial pacemakers; however, they are less commonly involved in community-associated diseases [3,4,5,6]. CoNS are the most common pathogens implicated in nosocomial bloodstream infections, responsible for 30–40% of these infections [4]. A study found a CoNS prevalence of 6–68% in suspected human infections in Africa within the last decade [2]. Of the CoNS, Staphylococcus epidermidis is the most common cause of human infection, culpable for about 24–80% of these infections [3].
CoNS are noted for their ability to develop antibiotic resistance against commonly used antibiotic classes such as β-lactams, aminoglycosides, and macrolides, with exceptionally high reported methicillin resistance rates [2] as well as resistance to antibiotics of last resort such as the glycopeptides [7]. Antibiotic resistance genes conferring resistance to these antibiotic classes can be transferred between staphylococcal species such as S. aureus and S. intermedius, limiting the therapeutic options available [1,8].
This study describes the incidence of MDR CoNS from three hospitals in the uMgungundlovu District in the KwaZulu-Natal Province in South Africa.
2. Results
2.1. Isolation, Identification, and Species’ Distribution
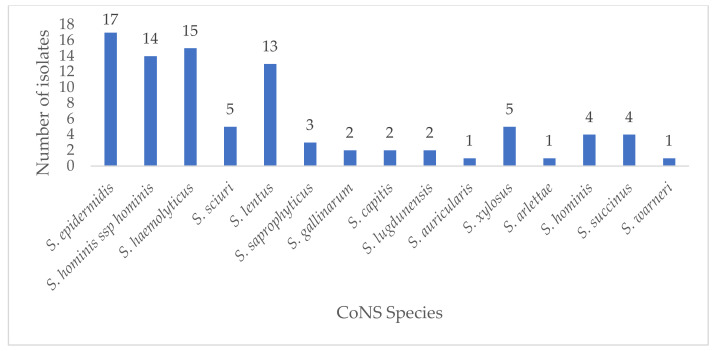
Eighty-nine (89) out of the 130 presumptive isolates were confirmed as CoNS by the automated VITEK 2 system and used for further analysis. Of these, Staphylococcus epidermidis (17, 19.1%) was the most frequent species identified. Identities of other CoNS isolates were S. hominis ssp. hominis (14, 15.7%), S. haemolyticus (15, 16.9%), and S. lentus (13, 14.6%) (Figure 1). In terms of the distribution of CoNS by department, the emergency department (3), pediatric Outpatient Department (OPD) (3), and pediatric and extension wards (2) recorded the highest number of S. epidermidis isolates. S. hominis ssp. hominis had the highest distribution in the pediatric OPD (4) and medical ward (3). In the intensive care unit (ICU), S. haemolyticus (3) was the most frequently isolated, while S. hominis ssp. hominis (1), S. lentus (1), and S. warneri (1) were also found in the ICU. The rest of the species were sparingly distributed across all departments. Of the remaining 41 non-CoNS isolates, Enterococcus faecalis (12), Enterococcus faecium (8), S. aureus (6), and Aerococcus viridans (6) were the most abundant. Others were Leuconostoc mesenteroides ssp. cremoris (5), Enterococcus columbae (1), and Dermacoccus nishinomiyaensis (1).
Figure 1.
Distribution of CoNS isolates in this study.
2.2. Antibiotic Resistance Phenotypic Patterns of CoNS and mecA Detection
Of the 89 isolates, 68 (76.4%) were putatively methicillin-resistant by the phenotypic cefoxitin screen test. All isolates displayed resistance to at least one agent in one antibiotic class. High levels of antibiotic resistance rates were recorded for penicillin (100.0%), erythromycin (74.2%), azithromycin (74.2%), cefoxitin (76.4%), and trimethoprim/sulfamethoxazole (68.5%). Isolates were highly susceptible to linezolid (95.5%), gentamicin (95.5%), tigecycline (94.4%), nitrofurantoin (92.1%), amikacin (89.9%), vancomycin (86.5%), teicoplanin (82.0%), and ceftaroline (76.4%). The detailed phenotypic and genotypic profile of isolates is available in the supplementary Table S1. Table 1 delineates the percentage resistance of all CoNS, and methicillin-resistant coagulase-negative staphylococci (MRCoNS) isolates to the different antibiotics tested. None of the isolates was resistant to all antibiotics tested. Sixty-three (92.6%) of the 68 MRCoNS by cefoxitin phenotypic test were confirmed as MRCoNS by PCR detection of the mecA gene.
Table 1.
Antimicrobial susceptibility pattern of CoNS and MRCoNS isolates from clinical sources.
| Antibiotic | CoNS Isolates (n = 89) | MRCoNS Isolates (n = 68) | ||||
|---|---|---|---|---|---|---|
| No of Susceptible (%) | No of Intermediate (%) | Number Resistant (%) | No of Susceptible (%) | No of Intermediate (%) | Number of Resistant (%) | |
| Cefoxitin | 21 (23.6) | NA | 68 (76.4) | 0 (0.0) | NA | 68 (100.0) |
| Penicillin G | 0 (0) | NA | 89 (100) | 0 (0.0) | NA | 68 (100.0) |
| Ceftaroline | 68 (76.4) | 12 (13.5) | 9 (10.1) | 50 (73.5) | 12 (17.6) | 6 (8.8) |
| Ciprofloxacin | 41 (46.1) | 10 (11.2) | 38 (42.7) | 25 (36.8) | 7 (10.3) | 36 (52.9) |
| Moxifloxacin | 46 (51.7) | 6 (6.7) | 37 (41.6) | 30 (44.1) | 5 (7.4) | 33 (48.5) |
| Azithromycin | 19 (21.3) | 4 (4.5) | 66 (74.2) | 8 (11.8) | 3 (4.4) | 57 (83.8) |
| Erythromycin | 17 (19.1) | 6 (6.7) | 66 (74.2) | 6 (8.8) | 5 (7.4) | 57 (83.8) |
| Gentamicin | 85 (95.5) | 2 (2.2) | 2 (2.2) | 64 (94.1) | 2 (2.9) | 2 (2.9) |
| Amikacin | 80 (89.9) | 5 (5.6) | 4 (4.5) | 61 (89.7) | 3 (4.4) | 4 (5.9) |
| Chloramphenicol | 64 (71.9) | 2 (2.2) | 23 (25.8) | 47 (69.1) | 2 (2.9) | 19 (27.9) |
| Tetracycline | 62 (69.7) | 4 (4.5) | 23 (25.8) | 44 (64.7) | 3 (4.4) | 21 (30.9) |
| Doxycycline | 65 (73.0) | 3 (3.4) | 21 (23.6) | 46 (67.4) | 2 (2.9) | 20 (29.4) |
| Teicoplanin | 73 (82.0) | 10 (11.2) | 6 (6.7) | 59 (86.8) | 4 (5.9) | 5 (7.4) |
| Tigecycline | 84 (94.4) | NA | 5 (5.6) | 64 (94.1) | NA | 4 (5.9) |
| Linezolid | 85 (95.5) | NA | 4 (4.5) | 65 (95.6) | NA | 3 (4.4) |
| Clindamycin | 47 (52.8) | 10 (11.2) | 32 (35.9) | 32 (47.1) | 7 (10.3) | 29 (42.6) |
| Rifampicin | 51 (57.3) | 0 (0.0) | 38 (42.7) | 34 (50.0) | 0 (0.0) | 34 (50.0) |
| Sulfamethoxazole/trimethoprim | 24 (26.9) | 4 (4.5) | 61 (68.5) | 16 (23.5) | 3 (4.4) | 49 (72.1) |
| Nitrofurantoin | 82 (92.1) | 2 (2.2) | 5 (5.6) | 63 (92.6) | 2 (2.9) | 3 (4.4) |
| Vancomycin * | 77 (86.5) | 13 (13.5) | 0 (0.0) | 62 (91.2) | 6 (8.8) | 0 (0.0) |
* Susceptibility against vancomycin was determined using the broth microdilution method.
2.3. Multidrug Resistance and Multiple Antibiotic Resistance Index(MARI)
The MAR index ranged from 0.05 to 0.80, with an overall mean of 0.34 (Table 2 and Table 3). Multidrug resistance was observed in 68 (76.4%) of the isolates. Fifty-one antibiograms were observed (Table 4).
Table 2.
Multiple antibiotic resistance index (MARI) of CoNS isolates.
| MAR Index | Number of Isolates |
|---|---|
| 0.05 | 5 (5.6%) |
| 0.10 | 5 (5.6%) |
| 0.15 | 10 (11.2%) |
| 0.20 | 5 (5.6%) |
| 0.25 | 10 (11.2%) |
| 0.30 | 6 (6.7%) |
| 0.35 | 8 (8.9%) |
| 0.40 | 12 (13.5%) |
| 0.45 | 10 (11.2%) |
| 0.50 | 8 (8.9%) |
| 0.55 | 2 (2.2%) |
| 0.60 | 6 (6.7%) |
| 0.70 | 1 (1.1%) |
| 0.80 | 1 (1.1%) |
Table 3.
Distribution of CoNS isolates depending on MARI value >0.2 in various departments.
| Department | Number of Isolates with MAR Index > 0.2 (n = 64) | Percentage |
|---|---|---|
| Emergency | 5 | 7.8% |
| ICU | 11 | 17.2% |
| Medical Ward | 10 | 15.6% |
| Obstetrics/gynecology | 1 | 1.6% |
| OPD | 7 | 10.9% |
| Surgical Ward | 5 | 7.8% |
| Extension Ward | 7 | 10.9% |
| Pediatric Ward | 9 | 14.1% |
Table 4.
Resistance pattern observed in MDR CoNS (n = 68).
| Resistance Pattern 1 | Number |
|---|---|
| FOX-PEN-CIP-MXF-AZM-ERY-GEN-CLI-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CHL-CLI-RIF-SXT | 3 |
| PEN- AZM-ERY-CHL-CLI-SXT | 1 |
| PEN-CPT-CHL-RIF-NIT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CLI-RIF | 4 |
| FOX-PEN-CIP-MXF-AZM-ERY-GEN-DOX-RIF-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-AMK-CLI-RIF | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CHL-TET-DOX-TGC-TEC-LZD-CLI-RIF-SXT-NIT | 1 |
| FOX-PEN-MXF-AZM-ERY-CHL-CLI | 1 |
| PEN-AZM-ERY-SXT | 3 |
| FOX-PEN-AZM-ERY-SXT | 6 |
| PEN-CPT-MXF- CHL-TET-DOX-TGC-TEC-LZD-RIF-SXT-NIT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CLI-RIF-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-TET-DOX-RIF-SXT | 2 |
| FOX-PEN-AMK-CHL-TET-DOX-TGE-TGC-LZD-RIF-SXT-NIT | 1 |
| FOX-PEN-CPT-CIP-MXF-AZM-ERY-CHL-TET-DOX-CLI-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CHL-TET-DOX-TGC-TEC-LZD-RIF-SXT-NIT | 1 |
| PEN- CIP-MXF-AZM-ERY-CHL-CLI-RIF-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CHL-TET-DOX-CLI-RIF-SXT | 2 |
| FOX-PEN-MXF-AZM-ERY-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-AMK-CHL-CLI-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-TET-CLI-RIF | 1 |
| FOX-PEN-AZM-ERY-CLI-RIF-SXT | 1 |
| FOX-PEN-CPT-MXF-AZM-ERY-TET-DOX-CLI-RIF-SXT | 1 |
| FOX-PEN-CPT-CIP-MXF-AZM-ERY-AMK-CHL-CLI-RIF-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-RIF-SXT | 1 |
| FOX-PEN-AZM-ERY-CHL-TET-DOX-SXT | 2 |
| PEN-MXF-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CLI-RIF-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-TET-DOX-CLI-RIF-SXT | 1 |
| FOX-PEN-AZM-ERY-TET-CLI | 1 |
| PEN-CIP-MXF-AZM-ERY-TET-CLI-RIF-SXT | 1 |
| FOX-PEN-CIP-AZM-ERY-TET-DOX-RIF-SXT | 1 |
| FOX-PEN-TET-DOX-TGC-RIF | 1 |
| FOX-PEN-AZM-ERY-RIF | 1 |
| FOX-PEN-CPT-AZM-ERY-TET-DOX-SXT | 2 |
| FOX-PEN-AZM-ERY-CHL-SXT | 1 |
| FOX-PEN-AZM-ERY-CHL-CLI-RIF-SXT | 2 |
| FOX-PEN-CIP-MXF-AZM-ERY-CLI-SXT | 1 |
| FOX-PEN-CIP-AZM-ERY-TET-DOX-SXT | 1 |
| FOX-PEN-CPT-CIP-AZM-ERY-CLI-RIF-SXT | 1 |
| FOX-PEN-CIP-AZM-ERY-TET-DOX | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CHL-TET-DOX-SXT | 1 |
| FOX-PEN-CIP-AZM-ERY-CLI-SXT | 1 |
| FOX-PEN-CIP-MXF-ERY-RIF | 1 |
| FOX-PEN-AZM-ERY-TEC-RIF-SXT | 1 |
| FOX-PEN-AZM-ERY-TEC | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-CHL-CLI-SXT | 1 |
| FOX-PEN-CIP-MXF-AZM-ERY-RIF | 1 |
| FOX-PEN-CIP-MXF-RIF | 1 |
1 FOX = cefoxitin; PEN = penicillin G; CPT = ceftaroline; CIP = ciprofloxacin; MXF = moxifloxacin; AZM = azithromycin; ERY = erythromycin; GEN = gentamicin; AMK = amikacin; CHL = chloramphenicol; TET = tetracycline; DOX = doxycycline; TEC = teicoplanin; TGC = tigecycline; LZD = linezolid; CLI = clindamycin; RIF = rifampicin; SXT = sulfamethoxazole/trimethoprim; NIT = nitrofurantoin.
2.4. Demographic Characteristics of Patients and Statistical Analysis
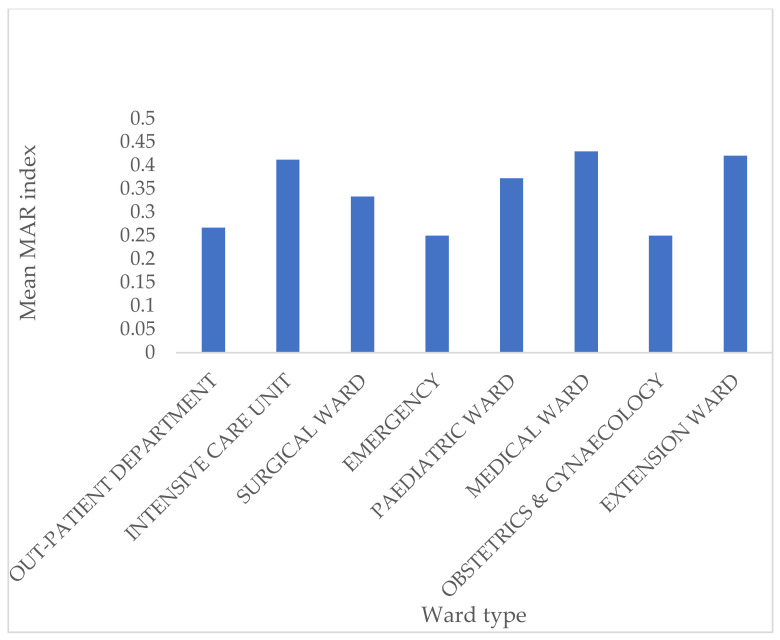
Patients’ ages ranged from 0 to 77. More than half (50.6%) of isolates were obtained from patients who were less than 1 year old. Of the remaining 49.4%, ages ranged from 1 to 77, with a mean age of 37.03 ± 23.22. Isolates were obtained from 46 (51.7%) males and 33 (37.1%) females, while seven (7.9%) were unknown/unspecified. Outpatients and inpatients made up 19.1% and 80.9% of samples, respectively. The distribution of isolates by wards was as follows: pediatric ward (17.9%), nursery (6.7%), emergency unit (5.7%), ICU (13.5%), medical ward (11.2%), surgical ward (6.7%), extension ward (7.9%), obstetrics and gynecology ward (1.1%), and maternity ward (1.1%). The Pearson Chi-Square test showed no significant association between the ward type and MAR index: X2(104, n = 89) =116.05, p = 0.197), even though the isolates with the highest MAR indices were from the ICU (Figure 2). Also, there was no statistically significant association between CoNS species and MAR index, X2(182, n = 89) = 203.07, p = 0.136). Furthermore, even though one-way ANOVA showed that S. saprophyticus isolates had a higher MAR mean (p = 0.012), the effect size was small (0.201).
Figure 2.
Distribution of MAR index against various ward types.
3. Discussion
This study describes the species distribution, antibiotic resistance profiles, MDR, and the MAR indices of clinical CoNS isolates from hospitals in the uMgungundlovu District of KwaZulu-Natal Province in South Africa. There was a diversity of CoNS species isolated, with S. epidermidis as the most abundant species, in agreement with previous studies conducted in ICUs investigating vancomycin heteroresistance and reduced glycopeptide susceptibility among bloodstream CoNS in Egypt and Italy [9,10]. Generally, the CoNS species distribution in this study agrees with detection rates of studies in Africa. A review paper [2] based on 35 studies in Africa, describing epidemiologically relevant CoNS found the most abundant CoNS species to be S. epidermidis and S. haemolyticus but found low detection rates of S. capitis (three studies), S. lugdunensis (three studies), S. caprae (one study), and S. gallinarum (one study). The MRCoNS prevalence rate (76.4%) was higher than that found in previous studies from clinical samples in healthcare settings in Nigeria (46.3%) [11] and Egypt (75.9%) [9]. However, higher MRCoNS prevalence figures of 86% [12] and 100% [13] were detected for CoNS implicated in infections in South Africa. The S. epidermidis group (S. epidermidis and S. haemolyticus) is an important cause of nosocomial infections [1]. S. epidermidis is the most frequently isolated staphylococcal species in humans and considered the most important CoNS species [3]. In this study, the S. epidermidis group was part of the three most abundant CoNS species.
There was a 92.6% agreement between phenotypic and genotypic confirmation of methicillin resistance in this study. Methicillin resistance in isolates that lack the mecA gene may be mediated by other mechanisms of methicillin resistance, such as possession of mecC [14], mecB genes [15], or the overproduction of β-lactamases [2]. The development of methicillin resistance has been observed in about 80% of CoNS species, contributing to increased morbidity and mortality in hospitals due to their prominence in healthcare-associated infections (HAIs) [11].
The majority (76.4%) of the isolates in this study showed a multidrug resistance phenotype, with isolates displaying high resistance to commonly used antibiotics such as penicillin (100.0%), macrolides (74.2% each for erythromycin and azithromycin), and sulfamethoxazole/trimethoprim (68.5%). Similar high-resistance patterns have been observed against these antibiotics in other studies elsewhere [16,17]. However, the isolates in this study displayed high susceptibility against reserve antibiotics such as linezolid (95.5%) and the anti-MRSA (methicillin-resistant Staphylococcus aureus) β-lactam antibiotic ceftaroline (76.4%). Similarly, complete susceptibility of CoNS isolates was observed against vancomycin, tigecycline, teicoplanin, and linezolid in another South Africa study [13]. The high susceptibilities recorded against these antibiotics could be due to the reserved use of those antibiotics, mainly for resistant staphylococcal infections. Thus, the last resort antibiotics still retain high activity against CoNS and can be used for empirical treatment of conditions such as suspected CoNS sepsis, even though resistance against these antibiotics is gradually increasing [2]. The 51 antibiogram types seen in MDR isolates indicate a wide diversity of resistance phenotypes. Resistance to FOX, PEN, AZM, ERY, and SXT (R7) was the most frequently observed pattern in six (8.8%) MDR isolates. The next most common pattern was resistance to FOX, PEN, CIP, MXF, AZM, ERY, CLI, and RIF, observed in four (5.9%) MDR isolates. Due to its ability to penetrate biofilm, rifampicin is one of the preferred antibiotics for treating bone and joint infections [18]. However, the development of resistance due to continued use of the antibiotic has necessitated its use in combination with other antibiotics to treat bone and joint infections [18]. The resistance to rifampicin observed in this study (42.7%) means that the drug may not be relied upon alone in treating infections caused by CoNS.
Considering their susceptibility profiles, vancomycin, nitrofurantoin, linezolid, tigecycline, teicoplanin, gentamicin, amikacin, and the anti-MRSA cephalosporin ceftaroline retained high activities against CoNS in the study setting and may be relied upon in the treatment of CoNS infections. Decreasing vancomycin susceptibility is reported with increasing frequency in the clinically relevant CoNS literature and may be associated with increased vancomycin exposure [19]. The current study recorded 13.5% CoNS isolates with intermediate susceptibility to vancomycin. It is imperative to mitigate the development of vancomycin non-susceptibility, considering the vital role of glycopeptides in treating multidrug-resistant infections.
Multidrug resistance (MDR) in CoNS is problematic in low/middle-income countries due to the limited access to newer antibiotics and the high cost of alternative treatment [2]. The study showed that most isolates were multidrug-resistant, with 71.9% of isolates having MAR indices of >0.20 and 18 (20.2%) had MAR values of ≥0.50. Other studies have, as well, recorded high MDR rates of CoNS [11]. MAR values higher than 0.2 indicate isolates, possibly originating from environments where antibiotics are frequently used, and may also hint at possible nosocomial transmission within the hospital setting [20]. There was no statistically significant association between the type of ward and the MAR index (p = 0.197), even though some isolates with high MAR indices were recovered in the neonatal ICU.
It is important to note that more than half (50.6%) of the patients in this study were less than 1 year old. This is important considering that neonates have underdeveloped immune systems, making them prone to HAIs, especially in ICUs [2]. CoNS are also a leading cause of bacteremia in neonatal ICUs [2], and neonatal sepsis is quite common in neonates with poor perinatal events. Considering that CoNS are recognized neonatal pathogens in upper- and high-income countries, they should be given equal attention in low- and middle-income countries [21].
That the ICU had the highest number of recovered isolates is significant as CoNS are frequently isolated in bloodstream infections in ICU patients [2]. The use of invasive devices such as catheters, especially in ICUs, increases the risk of infection by CoNS, which is further facilitated by biofilm formation. The study was, however, limited by the lack of clinical data that precluded an analysis of risk factors associated with pathogenicity and/or contamination, warranting detailed epidemiological data.
4. Materials and Methods
4.1. Ethical Considerations
The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (Reference: BREC/00001302/2020). This study was a substudy of the overarching research program on Antibiotic Resistance and One Health (Reference: BCA444/16).
4.2. Study Setting, Sample Collection, and Identification
A total of one hundred thirty (130) suspected staphylococcal isolates were recovered from routine clinical specimens processed by the central microbiology laboratory for the uMgungundlovu District between October 2019 and February 2020. Isolates were obtained from blood cultures from both outpatients and inpatients, the latter from the intensive care unit (ICU), neonatal intensive care unit (NICU), pediatric ward, pediatric outpatient department (OPD), emergency departments, surgical ward, and nursery.
Presumptive identification was undertaken by Gram staining, colony morphology on blood agar, and the catalase test. The Staphaurex™ Latex Agglutination Test (Thermo Scientific, Kent, UK) was used to differentiate staphylococci based on their coagulase activity. Speciation was undertaken using the automated VITEK 2 system (BioMérieux, Marcy-L’Etoile, France). Patients’ demographic data (age, sex, ward type, and specimen source) were obtained from anonymous patient records. Isolates were stored at −86 °C in tryptic soy broth (Oxoid Ltd., Basingstoke, UK) containing 10% glycerol (VWR Lifescience Biotechnology, Missouri, TX, USA) and used for further analyses.
4.3. Antimicrobial Susceptibility Testing and Determination of Methicillin-Resistant Coagulase-Negative Staphylococci (MRCoNS)
Antibacterial susceptibility profile of isolates against a selected antibiotic panel was ascertained by the Kirby–Bauer disk diffusion method and interpreted according to the Clinical and Laboratory Standard Institute (CLSI) [22] guidelines using the following antibiotic discs: penicillin G (10 µg), cefoxitin (30 µg), ceftaroline (30 µg), ciprofloxacin (5 µg), moxifloxacin (5 µg), azithromycin (15 µg), erythromycin (15 µg), gentamicin (120 µg), amikacin (30 µg), chloramphenicol (30 µg), tetracycline (30 µg), doxycycline (30 µg), teicoplanin (30 µg), tigecycline (15 µg), linezolid (30 µg), clindamycin (10 µg), rifampicin (5 µg), sulfamethoxazole/trimethoprim (1.25/23.75 µg), and nitrofurantoin (300 µg). The cefoxitin test (disk diffusion) was used to screen methicillin resistance [22]. All disks were purchased from Oxoid (Oxoid Ltd., Basingstoke, UK). Multidrug resistance was defined as resistance to at least one agent in three or more distinct antibiotic drug classes. Susceptibility testing for vancomycin was done by minimum inhibitory concentration (MIC) (according to the CLSI guidelines) using the broth microdilution method due to the absence of breakpoints for the disc diffusion method [22]. Staphylococcus aureus ATCC 25923 and Staphylococcus epidermidis ATCC 12228 were used as the control strains. The multiple antibiotic resistance (MAR) index was calculated using the formula MAR = x/y, where x is the number of antibiotics an isolate displayed resistance toward and y is the total number of antibiotics tested against the isolate. The MAR index was used as an indicator of health risk assessment to identify if isolates originate from high or low antibiotic use environments.
4.4. DNA Extraction and PCR Amplification of MecA
Genomic DNA was extracted using the GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) according to the manufacturer’s instructions. The purity and concentration of extracted DNA were determined by Nanodrop™ 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and stored at −20 °C for PCR. PCR detection of the mecA gene was done for presumptive methicillin-resistant coagulase-negative staphylococci (MRCoNS) isolates. The mecA gene, conferring resistance to methicillin, was amplified using the T100™ Thermal cycler (Bio-Rad, Hercules, CA, USA), using the primer set F-AACAGGTGAATTATTAGCACTTGTAAG and R-ATTGCTGTTAATATTTTTTGAGTTGAA (Inqaba Biotech, Pretoria, South Africa) [23], generating a 174 base pair fragment [24].
PCR was performed in a 25-µL reaction mixture containing 12.5 µL DreamTaq Green PCR Master Mix (ThermoScientific, Carthage, CA, USA), 0.5 µL each of forward and reverse primers, and 3 µL of template DNA. The PCR protocol was denaturation at 94 °C for 5 min, 35 cycles of 94 °C for 30 s, 55 °C for 45 s, and 72 °C for 45 s, and a final extension step of 10 min at 72 °C. PCR products were run on 1.5% agarose gel at 120 V for 60 min in a 1× Tris-acetate-EDTA (TAE) buffer (BioConcept Ltd., Basel, Switzerland) and visualized by UV transillumination using Bio-Rad ChemiDoc™ MP System (Bio-Rad, Hercules, CA, USA). S. aureus ATCC 43300 was used as a positive control.
4.5. Statistical Analysis
Statistical analysis was carried out using Microsoft Excel 2016 (Microsoft Corperation, Redmond, DC, USA) and Statistical Package for the Social Sciences v. 26 (IBM Corperation, Armonk, NY, USA). Possible relationships between variables were investigated using the Pearson Chi-Square test and one-way analysis of variance (ANOVA). A p-value of <0.05 was considered statistically significant.
5. Conclusions
The study reports relatively high percentages of methicillin and multidrug resistance among CoNS isolates with a wide range of MAR indices. Considering that CoNS naturally inhabit the skin and mucous membranes, they may only be contaminants of clinical specimens. However, they are increasingly emerging pathogens, necessitating due diligence when recovered from clinical specimens.
Acknowledgments
Authors are grateful to Sumayya Haffejee of the National Health Laboratory Service for her assistance during sample collection.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/10/2/198/s1. Table S1: Detailed phenotypic and mecA profile of isolates.
Author Contributions
Co-conceptualization, S.Y.E. and J.A.; methodology, J.A., B.A.H., and L.A.B.; validation, S.Y.E., A.L.K.A., D.G.A., and L.A.B.; formal analysis, J.A. and B.A.H.; investigation, J.A.; resources, S.Y.E.; writing—original draft preparation, J.A.; writing—review and editing, all.; supervision, D.G.A., A.L.K.A., and S.Y.E.; project administration, S.Y.E.; funding acquisition, S.Y.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No. 98342), the South African Medical Research Council (SAMRC) under a Self-Initiated Research Grant, and the College of Health Sciences, University of Kwa-Zulu Natal, South Africa.
Institutional Review Board Statement
The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (Reference: BREC/00001302/2020). This study was a substudy of the overarching research program on Antibiotic Resistance and One Health (Reference: BCA444/16).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
S.Y.E. is the chairperson of the Global Respiratory Infection Partnership and a member of the Global Hygiene Council, both sponsored by unrestricted educational grants from Reckitt and Benckiser (Pty.), UK. For all other authors, no competing financial interests exist. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gómez-Sanz E., Ceballos S., Ruiz-Ripa L., Zarazaga M., Torres C. Clonally Diverse Methicillin and Multidrug Resistant Coagulase Negative Staphylococci Are Ubiquitous and Pose Transfer Ability Between Pets and Their Owners. Front. Microbiol. 2019;10:485. doi: 10.3389/fmicb.2019.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asante J., Amoako D.G., King A.A.L., Somboro A.M., Govinden U., Bester L.A., Essack S.Y. Review of Clinically and Epidemiologically Relevant Coagulase-Negative Staphylococci in Africa. Microb. Drug Resist. 2020;26:951–970. doi: 10.1089/mdr.2019.0381. [DOI] [PubMed] [Google Scholar]
- 3.Becker K., Heilmann C., Peters G. Coagulase-Negative Staphylococci. Clin. Microbiol. Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David M.D., Elliott P.T. Coagulase-negative staphylococci. Br. J. Hosp. Med. 2015;76:C126–C128. doi: 10.12968/hmed.2015.76.8.C126. [DOI] [PubMed] [Google Scholar]
- 5.García P., Benítez R., Lam M., Salinas A.M., Wirth H., Espinoza C.F., Garay T., Depix M.S., Labarca J., Guzmán A.M. Coagulase-negative staphylococci: Clinical, microbiological and molecular features to predict true bacteraemia. J. Med Microbiol. 2004;53:67–72. doi: 10.1099/jmm.0.04994-0. [DOI] [PubMed] [Google Scholar]
- 6.Iweriebor C., Ramalivhana N.J., Hattori T., Okoh A.I., Obi C.L. Vancomycin resistant coagulase-negative Staphylococcal isolates from HIV positive patients in the Limpopo Province, South Africa. J. Microbiol. Antimicrob. 2013;5:18–24. doi: 10.5897/JMA11.090. [DOI] [Google Scholar]
- 7.May L., Klein E.Y., Rothman R.E., Laxminarayan R. Trends in Antibiotic Resistance in Coagulase-Negative Staphylococci in the United States, 1999 to 2012. Antimicrob. Agents Chemother. 2013;58:1404–1409. doi: 10.1128/AAC.01908-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amoako D.G., Somboro A.M., King A.A.L., Molechan C., Perrett K., Bester L.A., Essack S.Y. Antibiotic Resistance in Staphylococcus aureus from Poultry and Poultry Products in uMgungundlovu District, South Africa, Using the “Farm to Fork” Approach. Microb. Drug Resist. 2020;26:402–411. doi: 10.1089/mdr.2019.0201. [DOI] [PubMed] [Google Scholar]
- 9.Mashaly G.E.-S., El-Mahdy R. Vancomycin heteroresistance in coagulase negative Staphylococcus blood stream infections from patients of intensive care units in Mansoura University Hospitals, Egypt. Ann. Clin. Microbiol. Antimicrob. 2017;16:63. doi: 10.1186/s12941-017-0238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natoli S., Fontana C., Favaro M., Bergamini A., Testore G.P., Minelli S., Bossa M.C., Casapulla M., Broglio G., Beltrame A., et al. Characterization of coagulase-negative staphylococcal isolates from blood with reduced susceptibility to glycopeptides and therapeutic options. BMC Infect. Dis. 2009;9:83. doi: 10.1186/1471-2334-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibadin E., Enabulele I.O., Muinah F. Prevalence of mecA gene among staphylococci from clinical samples of a tertiary hospital in Benin City, Nigeria. Afr. Heal. Sci. 2017;17:1000–1010. doi: 10.4314/ahs.v17i4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballot D.E., Nana T., Sriruttan C., Cooper P.A. Bacterial Bloodstream Infections in Neonates in a Developing Country. ISRN Pediatr. 2012;2012:508512. doi: 10.5402/2012/508512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers M.M., Strasheim W., Lowe M., Ueckermann V., Kock M.M. Molecular Epidemiology of Staphylococcus epidermidis Implicated in Catheter-Related Bloodstream Infections at an Academic Hospital in Pretoria, South Africa. Front. Microbiol. 2018;9:417. doi: 10.3389/fmicb.2018.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhaouadi S., Soufi L., Campanile F., Dhaouadi F., Sociale M., Lazzaro L., Cherif A., Stefani S., Elandoulsi R.B. Prevalence of meticillin-resistant and -susceptible coagulase-negative staphylococci with the first detection of the mecC gene among cows, humans and manure in Tunisia. Int. J. Antimicrob. Agents. 2020;55:105826. doi: 10.1016/j.ijantimicag.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Becker K., van Alen S., Idelevich E.A., Schleimer N., Seggewiß J., Mellmann A., Kaspar U., Peters G. Plasmid-Encoded Transferable mecB-Mediated Methicillin Resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018;24:242–248. doi: 10.3201/eid2402.171074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adeyemi A.I., Sulaiman A.A., Solomon B.B., Chinedu O.A., Victor I.A. Bacterial Bloodstream Infections in HIV-infected Adults Attending a Lagos Teaching Hospital. J. Heal. Popul. Nutr. 2010;28:318–326. doi: 10.3329/jhpn.v28i4.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klingenberg C., Rønnestad A., Anderson A., Abrahamsen T., Zorman J., Villaruz A., Flægstad T., Otto M., Sollid J.E. Persistent strains of coagulase-negative staphylococci in a neonatal intensive care unit: Virulence factors and invasiveness. Clin. Microbiol. Infect. 2007;13:1100–1111. doi: 10.1111/j.1469-0691.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 18.Nicolosi D., Cinà D., Di Naso C., D’Angeli F., Salmeri M., Genovese C. Antimicrobial Resistance Profiling of Coagulase-Negative Staphylococci in a Referral Center in South Italy: A Surveillance Study. Open Microbiol. J. 2020;14:91–97. doi: 10.2174/1874285802014010091. [DOI] [Google Scholar]
- 19.Center K.J., Reboli A.C., Hubler R., Rodgers G.L., Long S.S. Decreased Vancomycin Susceptibility of Coagulase-Negative Staphylococci in a Neonatal Intensive Care Unit: Evidence of Spread of Staphylococcus warneri. J. Clin. Microbiol. 2003;41:4660–4665. doi: 10.1128/JCM.41.10.4660-4665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul S., Bezbaruah R.L., Roy M.K., Ghosh A.C. Multiple antibiotic resistance (MAR) index and its reversion in Pseudomonas aeruginosa. Lett. Appl. Microbiol. 1997;24:169–171. doi: 10.1046/j.1472-765X.1997.00364.x. [DOI] [PubMed] [Google Scholar]
- 21.Patel G., Perez F., A Bonomo R. Carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii: Assessing their impact on organ transplantation. Curr. Opin. Organ Transplant. 2010;15:676–682. doi: 10.1097/MOT.0b013e3283404373. [DOI] [PubMed] [Google Scholar]
- 22.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 6th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. pp. 74–80. Informational Supplement M100-S27. [Google Scholar]
- 23.Martineau F., Picard F.J., Grenier L., Roy P.H., Ouellette M., Bergeron M.G. Multiplex PCR assays for the detection of clinically relevant antibiotic resistance genes in staphylococci isolated from patients infected after cardiac surgery. J. Antimicrob. Chemother. 2000;46:527–534. doi: 10.1093/jac/46.4.527. [DOI] [PubMed] [Google Scholar]
- 24.Asante J., Govinden U., Owusu-Ofori A., Bester L., Essack S. Molecular characterization of methicillin-resistant Staphylococcus aureus isolates from a hospital in Ghana. Afr. J. Clin. Exp. Microbiol. 2019;20:164. doi: 10.4314/ajcem.v20i3.1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.