Key Points
Question
What is the effect of intermittent vs continuous pulse oximetry in infants hospitalized with stabilized bronchiolitis?
Findings
In this multicenter randomized clinical trial of 229 infants hospitalized with stabilized bronchiolitis with and without supplemental oxygen and with care managed using an oxygen saturation target of 90% or higher, length of hospital stay, medical interventions, safety, and parent-reported outcomes were similar. Nursing satisfaction was greater with intermittent monitoring.
Meaning
Given that other important considerations for clinical practice favor less intense monitoring, these findings support the standard use of intermittent pulse oximetry in hospitalized infants with stabilized bronchiolitis.
Abstract
Importance
There is low level of evidence and substantial practice variation regarding the use of intermittent or continuous monitoring in infants hospitalized with bronchiolitis.
Objective
To compare the effect of intermittent vs continuous pulse oximetry on clinical outcomes.
Design, Setting, and Participants
This multicenter, pragmatic randomized clinical trial included infants 4 weeks to 24 months of age who were hospitalized with bronchiolitis from November 1, 2016, to May 31, 2019, with or without supplemental oxygen after stabilization at community and children’s hospitals in Ontario, Canada.
Interventions
Intermittent (every 4 hours, n = 114) or continuous (n = 115) pulse oximetry, using an oxygen saturation target of 90% or higher.
Main Outcomes and Measures
The primary outcome was length of hospital stay from randomization to discharge. Secondary outcomes included length of stay from inpatient unit admission to discharge and outcomes measured from randomization: medical interventions, safety (intensive care unit transfer and revisits), parent anxiety and workdays missed, and nursing satisfaction.
Results
Among 229 infants enrolled (median [IQR] age, 4.0 [2.2-8.5] months; 136 [59.4%] male; 101 [44.1%] from community hospital sites), the median length of hospital stay from randomization to discharge was 27.6 hours (interquartile range [IQR], 18.8-49.6 hours) in the intermittent group and 25.4 hours (IQR, 18.3-47.6 hours) in the continuous group (difference of medians, 2.2 hours; 95% CI, −1.9 to 6.3 hours; P = .17). No significant differences were observed between the intermittent and continuous groups in the median length of stay from inpatient unit admission to discharge: 49.1 (IQR, 37.2-87.0) hours vs 46.0 (IQR, 32.5-73.8) hours (P = .13) or in frequencies or durations of hospital interventions, such as oxygen supplementation initiation: 4 of 114 (3.5%) vs. 9 of 115 (7.8%) (P = .16) and median duration of oxygen supplementation: 20.6 (IQR, 7.6-46.1) hours vs. 21.4 (11.6-52.9) hours (P = .66). Similarly, there were no significant differences in frequencies of intensive care unit transfer: 1 of 114 (0.9%) vs 2 of 115 (2.7%) (P = .76); readmission to hospital: 3 of 114 (2.6%) in the intermittent group vs 4 of 115 (3.5%) in the continuous group (P > .99); parent anxiety: mean (SD) parent anxiety score, 2.9 (0.9) in the intermittent group vs 2.8 (0.9) in the continuous group (P = .40); or parent workdays missed: median workdays missed, 1.5 (IQR, 0.5-3.0) vs 1.5 (IQR, 0.5-2.5) (P = .36). Mean (SD) nursing satisfaction with monitoring was significantly greater in the intermittent group: 8.6 (1.7) vs 7.1 (2.8) of 10 workdays; the mean difference was 1.5 (95% CI, 0.9-2.2; P < .001).
Conclusions and Relevance
In this randomized clinical trial, among infants hospitalized with stabilized bronchiolitis with and without hypoxia and managed using an oxygen saturation target of 90% or higher, clinical outcomes, including length of hospital stay and safety, were similar with intermittent vs continuous pulse oximetry. Nursing satisfaction was greater with intermittent monitoring. Given that other important clinical practice considerations favor less intense monitoring, these findings support the standard use of intermittent pulse oximetry in stable infants hospitalized with bronchiolitis.
Trial Registration
ClinicalTrials.gov Identifier: NCT02947204
This randomized clinical trial compares the effect of intermittent vs continuous pulse oximetry on clinical outcomes in infants hospitalized with bronchiolitis with or without supplemental oxygen after stabilization.
Introduction
Bronchiolitis is the most common acute lower respiratory tract infection in infants. In the US, it accounts for more than 100 000 hospitalizations and more than $1.7 billion in hospitalization costs annually.1 The focus of bronchiolitis inpatient management is supportive care because drug therapies are ineffective.2,3 Clinical and physiologic monitoring guides fluid and oxygen supplementation, respiratory support, and discharge decisions.
In hospital practice, pulse oximetry for identifying hypoxia is considered the fifth vital sign.4,5 Practitioners choose a pulse oximetry oxygen saturation target to assist with admission and discharge decisions and supplemental oxygen therapy initiation and discontinuation. The American Academy of Pediatrics (AAP) guidelines recommend using an oxygen saturation target of 90% or higher in hospitalized infants with bronchiolitis. This practice is supported by a multicenter trial6 that found that infants randomized to a target of 90% or higher, compared with a target of 94% or higher, were discharged 10 hours earlier and experienced a similarly low rate of adverse events and revisits after discharge.
Practitioners also choose to use pulse oximetry measured continuously or intermittently, such as performing a spot measurement every 4 hours. Retrospective studies7,8 suggest that stable infants with bronchiolitis stay in the hospital longer than needed because of practitioner reliance on the pulse oximeter to make management decisions. Experts and practitioners postulate that continuous measurement of oxygen saturation results in greater detection of transient and false-positive desaturations, leading to unnecessary additional supplemental oxygen use, more interventions, prolonged hospital stays, and higher costs.2,9,10 Increase in desaturation-related alarms may also increase nursing workload and alarm fatigue.2,10 In contrast, some practitioners and parents fear that intermittent monitoring will delay the detection of hypoxia and compromise patient safety.2
According to the AAP guidelines, practitioners may choose not to use continuous pulse oximetry based on lower level of evidence.2 The Choosing Wisely campaign recommends not using continuous monitoring in infants with bronchiolitis but without hypoxia.11 A cross-sectional study12 across 56 North American hospitals found that continuous pulse oximetry is used in 46% of infants without hypoxia hospitalized with bronchiolitis, and hospital use of continuous pulse oximetry varies substantially from 2% to 92%. Experts conclude that the evidence supporting the use of continuous vs intermittent pulse oximetry monitoring is “not equivocal, but rather non-existent.”13(p 1449)
We conducted a multicenter, pragmatic randomized clinical trial in hospitalized infants with stabilized bronchiolitis with and without supplemental oxygen to compare the effect of intermittent vs continuous pulse oximetry. We hypothesized that intermittent pulse oximetry would reduce length of hospital stay compared with continuous pulse oximetry. We also compared the effect of the monitoring strategies on secondary outcomes, including the need for medical interventions, safety, parent anxiety and missed workdays, and nursing satisfaction.
Methods
Trial Design and Oversight
This 6-center, pragmatic, parallel group, superiority randomized clinical trial compared intermittent (every 4 hours) and continuous pulse oximetry monitoring in infants with stabilized bronchiolitis hospitalized from November 1, 2016, to May 31, 2019. The study was conducted by the Canadian Paediatric Inpatient Research Network and included general pediatric inpatient units at 3 children’s and 3 community hospitals. Parents or guardians provided written informed consent to participate. Data were deidentified, and unique study identification numbers were used. (A master code list, stored separately from the deidentified data, linked the study identification numbers to participants identifying information.) Research ethics boards at all hospital sites approved the trial protocol (Supplement 1), which has been published previously.14
Trial Participants
After admission to the general pediatric inpatient unit, infants 4 weeks to 24 months of age were eligible for inclusion if they had a clinical diagnosis of first-episode bronchiolitis, were generally healthy, and had a stable clinical status (full eligibility criteria are provided in the trial protocol in Supplement 1). Bronchiolitis was defined as signs and symptoms of respiratory distress associated with a viral respiratory tract infection in accordance with the AAP guidelines.2 Only infants with a stable clinical status were included because during the stabilization period infants would not be considered for discharge because of being too unwell (ie, significant hypoxia) and/or having an uncertain illness trajectory. Stable clinical status criteria were defined by the trial investigators based on clinical consensus, institutional practices, and a pilot trial. Specifically, hospitalized infants who required supplemental oxygen had to be stable for at least 6 hours defined by the following: stable or decreasing requirement for supplemental oxygen, stable or decreasing respiratory rate by at least 2 measurements, respiratory rate less than 70 breaths/min, heart rate less than 180 beats/per min, or oxygen supplementation less than 40% fractional inspired oxygen or 2 L/min by nasal prongs. In infants who did not require supplemental oxygen, the clinical status needed to be stable (as defined above) for at least 6 hours from presentation to the emergency department. Infants who required intensive care unit (ICU) admission for mechanical or noninvasive ventilatory support were excluded. Infants treated with heated high-flow oxygen only became eligible after use of the heated high-flow oxygen was discontinued. Infants whose parents could not complete questionnaires because of limited English-language proficiency were excluded.
Randomization
We randomized (1:1) infants to intermittent or continuous oxygen saturation monitoring. A computer-generated randomization sequence, stratified by center, with random permuted block sizes of 4 or 6 was used. A central, internet-based randomization system used the Research Electronic Data Capture software (REDCap) application.15 Research assistants at the sites enrolled infants and then used REDCap to obtain the participant group allocation. Masking of the assigned treatment was not possible, given the obvious differences between the 2 interventions.
Trial Interventions
Bronchiolitis management followed institutional practices and the trial protocol (Supplement 1 and eTable 1 in Supplement 2).14 Hospitals used 1 of 2 oxygen saturation targets for clinical management (ie, oxygen supplementation and discharge decision-making) in keeping with their usual practice: (1) 90% or greater in room air while the infant was awake or asleep (2 children’s hospitals and 1 community hospital) and (2) 90% or greater in room air while awake and 88% or greater in room air while asleep (1 children’s hospital and 2 community hospitals). Infants received continuous monitoring in the emergency department and inpatient unit until stabilization as per institutional practices.
For the intermittent monitoring group, nurses measured oxygen saturation levels every 4 hours until discharge. If the infant had a clinical deterioration as assessed by the clinical team, the infant could be switched to continuous monitoring and intermittent monitoring restarted when the status stabilized. In the continuous monitoring group, nurses measured oxygen saturation continuously until discharge. For both groups, vital signs were measured every 4 hours, and weaning of supplemental oxygen was managed by the clinical team (Supplement 1).
Trial Outcomes
The primary outcome was length of hospital stay from randomization on the inpatient unit to discharge from the hospital, measured in hours. Prespecified secondary outcomes included length of hospital stay from randomization to meeting discharge criteria, defined as absence of fever (temperature <38 °C), no supplemental oxygen, normal respiratory rate (using World Health Organization age-specific criteria16), and parent-reported adequate feeding (defined as a score of ≥7 on a 10-cm visual analog feeding scale); length of hospital stay from inpatient unit admission to discharge; the need for medical interventions after randomization (blood testing, blood culture testing, chest radiography, oxygen supplementation [duration and initiation], bronchodilator treatment, systemic corticosteroid therapy, nasopharyngeal virus testing, nasopharyngeal suctioning, and intravenous fluids nasogastric feeds); parent anxiety level on a 4-point Likert scale after randomization (1 indicating not at all at ease; 2, somewhat at ease; 3, moderately at ease; and 4, very much at ease)17; number of parent workdays missed from randomization to 15 days after discharge; nursing satisfaction with the monitoring strategy assigned to their patient, measured on a 10-mm visual analog scale (0 indicating not at all satisfied and 10 indicating completely satisfied); unscheduled bronchiolitis-related ambulatory physician visits within 15 days of discharge; ICU admission and consultation after randomization; revisits to the emergency department or admission to the inpatient unit at the participating hospital within 15 days of discharge; and mortality. Research staff also assessed participants for crossover from the assigned to the alternate monitoring group by direct observation twice daily and review of electronic records.
Statistical Analysis
We anticipated a median length of hospital stay from randomization to discharge of 36 hours based on our pilot study and published studies.18,19,20 Using a significance level of P < .05 (2-sided), we calculated that a sample size of 210 (105 per group) would provide 90% power to detect a minimum median difference of 12 hours in length of stay between groups. We choose a 12-hour minimal clinically important difference based on discussions with practitioners, administrators, and parents. It is also aligned with the frequency at which discharge decisions are made on the inpatient unit.
All analyses were specified a priori. The primary and secondary outcomes were analyzed according to the intention-to-treat principle. To control for multiple testing, the level for declaring statistical significance for the secondary outcomes was set at .002 using the Bonferroni correction. Analyses were performed using R software, version 3.6.2.21 All statistical tests were 2-sided.
For the primary outcome of length of hospital stay from randomization to discharge, we estimated the difference in median time between groups and the ratio of the 2 medians along with a 95% CI.22 Because no censoring was necessary, the groups were compared using a Mann-Whitney–type test stratified for site, using the method of Kawaguchi and Koch.23 A priori, we planned a secondary analysis of the primary outcome adjusted for any clinically important imbalances in baseline variables. Because there were between-group sex differences, additional adjustment of the primary outcome for sex was performed using the method of Kawaguchi and Koch.23 In addition, a Cox proportional hazards regression analysis stratified for site and adjusted for sex was performed. For each of the secondary outcomes, we report the ratio of medians (interquartile ranges [IQRs]), odds ratios (ORs) (95% CIs), or mean differences (95% CIs). Statistical testing for continuous, dichotomous, and count secondary outcomes was performed using linear, logistic, and negative binomial models, respectively, with site as a covariate.
Results
Participants
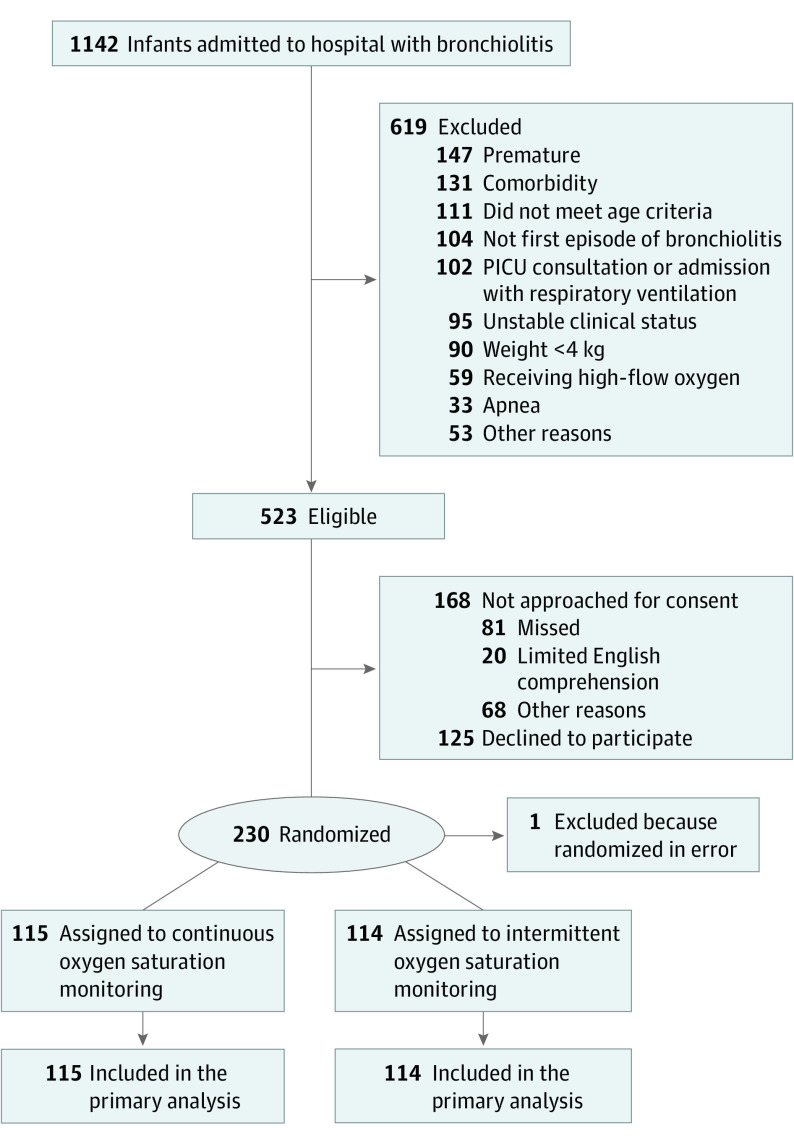
A total of 229 infants (median [IQR] age, 4.0 [2.2-8.5] months; 136 [59.4%] male) were enrolled in the study, with 101 (44.1%) from community hospital sites (Figure 1 and eMethods in Supplement 2). A total of 100 infants (43.7%) received supplemental oxygen before randomization on the inpatient unit, and 34 (14.8%) were receiving supplemental oxygen at randomization. The median time from inpatient unit admission to randomization was 16.4 hours (IQR, 11.1-24.4 hours). Except for sex, the baseline characteristics of the infants were similar between groups, including the proportion receiving supplemental oxygen, feeding adequacy, and time from inpatient unit admission to randomization (Table 1). For 17 participants (7.4%), there was crossover from the assigned monitoring group: 11 of 114 (9.6%) in the intermittent group and 6 of 115 (5.2%) in the continuous group (eTable 2 in the Supplement).
Figure 1. Flow of Participants in the Trial.
Reasons for meeting exclusion criteria and reasons for not being approached for consent are not mutually exclusive. A total of 101 of 229 infants (44.1%) were recruited from community hospital sites. See the trial protocol in Supplement 1 for further details. PICU indicates pediatric intensive care unit.
Table 1. Baseline Characteristics of the Enrolled Participantsa.
| Characteristic | Monitoring group | |
|---|---|---|
| Intermittent (n = 114) | Continuous (n = 115) | |
| Age, median (IQR), mo | 4.0 (2.2-8.4) | 4.0 (2.2-9.2) |
| Female sexb | 40 (35.1) | 53 (46.1) |
| Weight, median (IQR), kg | 7.2 (5.6-9.0) | 7.0 (5.2-9.0) |
| History of atopyc | 59 (52.2) | 68 (59.6) |
| Parental cigarette smoking | 19 (16.7) | 18 (15.7) |
| Emergency department or inpatient unit management before randomization | ||
| Antibiotic treatment | 36 (31.6) | 34 (29.6) |
| Salbutamol treatment | 45 (39.5) | 48 (41.7) |
| Nebulized epinephrine | 43 (37.7) | 46 (40.0) |
| Corticosteroid treatment | 17 (14.9) | 19 (16.5) |
| Supplemental oxygen | 53 (46.5) | 47 (40.9) |
| High-flow oxygen therapyd | 11 (9.6) | 11 (9.6) |
| Continuous oxygen monitoring | 66 (57.9) | 72 (62.6) |
| Intravenous fluids | 60 (52.6) | 63 (54.8) |
| Nasogastric feeds | 2 (1.8) | 3 (2.6) |
| Time from inpatient unit admission to randomization, median (IQR), h | 16.6 (11.7-31.5) | 16.1 (10.0-21.4) |
| Clinical status at randomization on inpatient unit | ||
| Respiratory rate, median (IQR), breaths/min | 42 (36-48) | 42 (37-50) |
| Heart rate, median (IQR), beats/min | 142 (128-152) | 140 (132-148) |
| Oxygen saturation, median (IQR), % | 97 (96-99) | 97 (95-98) |
| Oxygen supplementation | 16 (14.0) | 18 (15.7) |
| Feeding adequacy scale score, mean (SD)e | 5.9 (2.7) | 5.8 (2.8) |
| Hospital sitef | ||
| A | 24 (21.1) | 24 (20.9) |
| B | 23 (20.2) | 23 (20.0) |
| C | 21 (18.4) | 20 (17.4) |
| D | 19 (16.7) | 20 (17.4) |
| E | 14 (12.3) | 15 (13.0) |
| F | 13 (11.4) | 13 (11.3) |
Abbreviation: IQR, interquartile range.
Data are presented as number (percentage) of patients unless otherwise indicated.
There was an important difference in sex between the groups.
Refers to family history of asthma or personal history of atopy. One participant in the intermittent and continuous group was missing this information.
High-flow oxygen therapy discontinued before randomization.
Feeding adequacy scale scores range from 0 to 10, with 0 indicating not feeding and 10 indicating feeding as when healthy. One participant in the continuous group was missing this score.
Hospitals A, C, and D were children’s hospitals and hospitals B, E, and F were community hospitals.
Primary Outcome
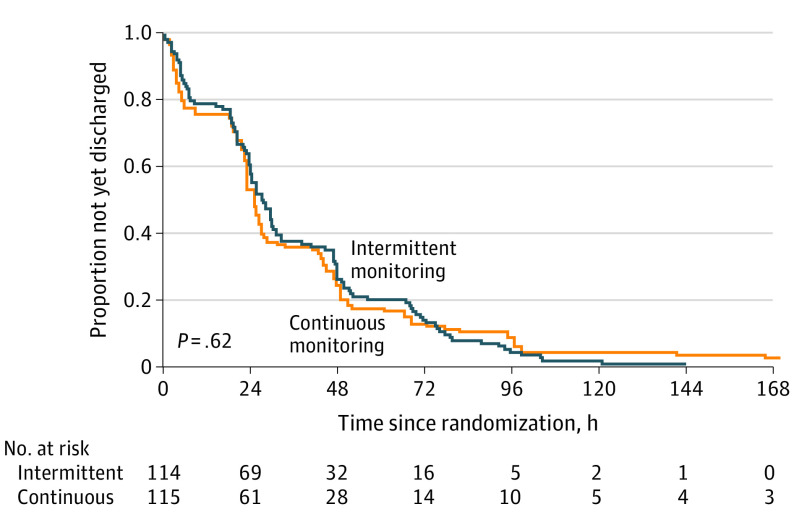
The median length of hospital stay from randomization to discharge was 27.6 hours (IQR, 18.8-49.6 hours) in the intermittent group and 25.4 hours (IQR, 18.3-47.6 hours) in the continuous group (difference of medians, 2.2 hours; 95% CI, −1.9 to 6.3 hours); ratio of medians, 1.09 (95% CI, 0.93-1.27; P = .17) (Table 2).23 A Kaplan-Meier plot shows that the length of stay between groups is similar (Figure 2). Sex-adjusted analysis that included hospital site as a stratification variable using Cox proportional hazards models did not show a statistically significant difference in the hazard of discharge between groups (adjusted hazard ratio, 0.93; 95% CI, 0.71-1.23; P = .62).
Table 2. Primary and Secondary Outcomes of Length of Hospital Stay.
| Outcome | Monitoring group, median (IQR) | Difference in medians (95% CI) | Ratio of medians (95% CI) | P valuea | |
|---|---|---|---|---|---|
| Intermittent (n = 114) | Continuous (n = 115) | ||||
| Time from randomization to discharge, h (primary outcome) | 27.6 (18.8 to 49.6) | 25.4 (18.3 to 47.6) | 2.2 (−1.9 to 6.3) | 1.09 (0.93 to 1.27) | .17b |
| Other length of hospital stay outcomes (secondary outcomes) | |||||
| Time from randomization to meeting discharge criteria, h | 18.8 (2.7 to 32.6) | 19.4 (2.4 to 30.1) | −0.6 (−9.6 to 8.4) | 0.97 (0.48 to 1.95) | .97 |
| Time from inpatient unit admission to discharge, h | 49.1 (37.2 to 87.0) | 46.0 (32.5 to 73.8) | 3.1 (−10.9 to 17.0) | 1.07 (0.81 to 1.40) | .13 |
Abbreviation: IQR, interquartile range.
P values calculated using a Mann-Whitney–type test stratified for site, using the method of Kawaguchi and Koch.23
In a secondary analysis of the primary outcome, using a Mann-Whitney–type test stratified for site and adjusted for sex using the method of Kawaguchi and Koch,23 P = .22.
Figure 2. Kaplan-Meier Plot of the Proportion of Infants With Bronchiolitis in Hospital.
Cox proportional hazards regression of the primary outcome of length of stay from randomization to discharge, stratified by site and adjusted for sex, resulted in a hazard ratio of 0.93 (95% CI, 0.71-1.23; P = .62).
Secondary Outcomes
Other Length of Hospital Stay Outcomes
No significant difference between groups was found for median length of stay from randomization to meeting discharge criteria (18.8 hours [IQR, 2.7-32.6 hours] in the intermittent group and 19.4 hours [IQR, 2.4-30.1 hours] in the continuous group; P = .97) or in the median length of stay from inpatient unit admission to discharge (49.1 hours [IQR, 37.2-87.0 hours] in the intermittent group and 46.0 hours [IQR, 32.5-73.8 hours] in the continuous group; P = .13) (Table 2).23
Interventions
Fewer infants in the intermittent group had oxygen supplementation initiated after randomization than in the continuous group (4 of 114 [3.5%] vs 9 of 115 [7.8%]; OR, 0.43; 95% CI, 0.13-1.43; P = .16]), although this finding was not statistically significant. No other significant differences were found between groups in medical interventions, including median duration of oxygen supplementation: 20.6 (IQR, 7.6-46.1) hours vs 21.4 (IQR, 11.6-52.9) hours (ratio of medians, 0.96; 95% CI, 0.37-2.52; P = .66); chest radiography: 8 of 114 (7.0%) vs 11 of 115 (9.6%) (OR, 0.71; 95% CI, 0.28-1.85; P = .49); blood tests: 14 of 114 (12.3%) vs 18 of 115 (15.7%) (OR, 0.75; 95% CI, 0.36-1.60; P = .49); nasopharyngeal virus: 14 of 114 (12.3%) vs 19 of 115 (16.5%) (OR, 0.71; 95% CI, 0.34-1.49; P = .33); blood culture testing: 5 of 114 (4.4%) vs 5 of 115 (4.3%) (OR, 1.01; 95% CI, 0.28-3.58; P = .96); bronchodilator treatment: 21 of 114 (18.4%) vs 20 of 115 (17.4%) (OR, 1.07; 95% CI, 0.55-2.11; P = .88); systemic corticosteroid treatment: 7 of 114 (6.1%) vs 9 of 115 (7.8%) (OR, 0.77; 95% CI, 0.28-2.14; P = .59); or nasal suctioning: 63 of 114 (55.3%) vs 63 of 114 (54.8%) (OR, 1.02; 95% CI, 0.61-1.72; P = .85). Likewise, no statistically significant differences were found in frequency of initiation of intravenous fluid therapy: 9 of 114 (7.9%) vs 8 of 115 (7.0%) (OR, 1.15; 95% CI, 0.43-3.08; P = .76); median duration of intravenous fluid therapy: 26.9 (IQR, 17.0-48.3) hours vs 24.0 (IQR, 17.7-49.4) hours (ratio of medians, 1.12; 95% CI, 0.71-1.76; P = .86); frequency of initiation of nasogastric fluid therapy: 1 of 114 (0.9%) vs 3 of 115 (2.6%) (OR, 0.33; 95% CI, 0.03-3.22; P = .33) or median duration of nasogastric fluid therapy: 34.1 (IQR, 18.1-50.2) hours vs 18.4 (IQR, 10.5-47.2) hours (ratio of medians, 1.86; 95% CI, 0.01-471.44; P = .37) (Table 3).
Table 3. Secondary Outcomes Measured From Time of Randomization.
| Outcome | Monitoring groupa | Mean difference (95% CI) | Odds ratio (95% CI)b | P valuec | |
|---|---|---|---|---|---|
| Intermittent (n = 114) | Continuous (n = 115) | ||||
| Medical interventions | |||||
| Nasopharyngeal testing for viruses | 14 (12.3) | 19 (16.5) | NA | 0.71 (0.34 to 1.49) | .33 |
| Blood testing | 14 (12.3) | 18 (15.7) | NA | 0.75 (0.36 to 1.60) | .49 |
| No. of blood tests, median (IQR) | 1.0 (1.0 to 2.0) | 1.5 (1.0 to 2.0) | NA | 0.67 (0.41 to 1.07) | .13 |
| Blood culture testing | 5 (4.4) | 5 (4.3) | NA | 1.01 (0.28 to 3.58) | .96 |
| Chest radiography | 8 (7.0) | 11 (9.6) | NA | 0.71 (0.28 to 1.85) | .49 |
| Oxygen supplementation initiatedd | 4 (3.5) | 9 (7.8) | NA | 0.43 (0.13 to 1.43) | .16 |
| Duration of oxygen supplementation, median (IQR), he | 20.6 (7.6 to 46.1) | 21.4 (11.6 to 52.9) | NA | 0.96 (0.37 to 2.52) | .66 |
| Bronchodilator treatment | 21 (18.4) | 20 (17.4) | NA | 1.07 (0.55 to 2.11) | .88 |
| No. of bronchodilator treatments, median (IQR) | 3.0 (1.0 to 12.0) | 3.0 (1.0 to 7.5) | NA | 1.00 (0.24 to 4.23) | .52 |
| Systemic corticosteroid treatment | 7 (6.1) | 9 (7.8) | NA | 0.77 (0.28 to 2.14) | .59 |
| Nasopharyngeal suctioning | 63 (55.3) | 63 (54.8) | NA | 1.02 (0.61 to 1.72) | .85 |
| No. of times the nasopharynx was suctioned, median (IQR) | 3.0 (2.0 to 6.5) | 3.0 (1.5 to 6.0) | NA | 1.00 (0.63 to 1.59) | .71 |
| Intravenous fluids initiatedf | 9 (7.9) | 8 (7.0) | NA | 1.15 (0.43 to 3.08) | .76 |
| Duration of intravenous fluids, median (IQR), hg | 26.9 (17.0 to 48.3) | 24.0 (17.7 to 49.4) | NA | 1.12 (0.71 to 1.76) | .86 |
| Nasogastric feeds initiatedh | 1 (0.9) | 3 (2.6) | NA | 0.33 (0.03 to 3.22) | .33 |
| Duration of nasogastric feeds, median (IQR), hi | 34.1 (18.1 to 50.2) | 18.4 (10.5 to 47.2) | NA | 1.86 (0.01 to 471.44) | .37 |
| Safety | |||||
| ICU consultation | 1 (0.9) | 2 (1.7) | NA | 0.50 (0.04 to 5.59) | .59 |
| ICU transfer | 1 (0.9) | 2 (1.7) | NA | 0.50 (0.04 to 5.59) | .76 |
| Emergency department revisit | 7 (6.1) | 10 (8.7) | NA | 0.69 (0.25 to 1.87) | .71 |
| Readmission to hospital | 3 (2.6) | 4 (3.5) | NA | 0.75 (0.16 to 3.43) | >.99 |
| Mortality | 0 | 0 | NA | NA | |
| Other secondary outcomes | |||||
| Nursing satisfaction score with monitoring, 0 to 10, mean (SD)j | 8.6 (1.7) | 7.1 (2.8) | 1.5 (0.9 to 2.2) | NA | <.001 |
| Parent anxiety score, mean (SD)k | 2.9 (0.9) | 2.8 (0.9) | 0.1 (−0.1 to 0.4) | NA | .40 |
| Parent workdays missed up to 15 d after discharge, median (IQR), dl | 1.5 (0.5 to 3.0) | 1.5 (0.5 to 2.5) | NA | 1.00 (0.67 to 1.50) | .36 |
| Unscheduled visits to a physician | 15 (13.2) | 10 (8.7) | NA | 1.59 (0.68 to 3.71) | .28 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; NA, not applicable.
Data are presented as number (percentage) of patients unless otherwise indicated.
Reported as the ratio of medians for outcomes that report median values.
P values for continuous, dichotomous, and continuous secondary outcomes were calculated using linear, logistic, and negative binomial regression models, respectively, with site as a covariate.
Represents the number of infants who had oxygen supplementation initiated after randomization (ie, were not receiving oxygen supplementation at randomization but had it initiated after randomization).
Among the 47 infants who received supplemental oxygen at randomization and/or after randomization (20 infants in the intermittent monitoring group and 27 in the continuous monitoring group).
Represents the number of infants who had intravenous feeds initiated after randomization.
Among the 132 infants who received intravenous fluids (64 infants in the intermittent monitoring group and 68 in the continuous monitoring group).
Represents the number of infants who had nasogastric feeds initiated after randomization.
Among the 9 infants who received nasogastric feeds (3 infants in the intermittent monitoring group and 6 in the continuous monitoring group).
Measured on a 10-mm visual analog scale, with 0 indicating not at all satisfied and 10 indicating completely satisfied. Four patients in the intermittent group and 10 patients in the continuous group had missing data.
Measured on a 4-point Likert scale, with 1 indicating not at all at ease and 4 indicating very much at ease. Five patients in the intermittent group and 9 patients in the continuous group had missing data.
Among the 184 infants whose parent(s) reported working in paid or unpaid employment. One patient in the intermittent group and 1 patient in the continuous group had missing data.
Safety
The frequency of the need for an ICU transfer was similar between groups: 1 of 114 (0.9%) in the intermittent group and 2 of 115 (1.7%) in the continuous group (OR, 0.50; 95% CI, 0.04-5.59; P = .76). Similarly, the frequency of an emergency department revisit within 15 days of discharge was not significantly different between groups: 7 of 114 (6.1%) in the intermittent group and 10 of 115 (8.7%) in the continuous group (OR, 0.69; 95% CI, 0.25-1.87; P = .71), and no statistically significant difference was found in the frequency of readmissions to hospital within 15 days of discharge: 3 of 114 (2.6%) in the intermittent group and 4 of 115 (3.5%) in the continuous group (OR, 0.75; 95% CI, 0.25-3.43; P > .99). No deaths occurred in either group (Table 3).
Other Secondary Outcomes
The mean (SD) nursing satisfaction score with the assigned oxygen monitoring group was greater in the intermittent (8.6 [1.7] of 10) compared with the continuous group (7.1 [2.8] of 10) (mean difference, 1.5; 95% CI, 0.9-2.2; P < .001). Parent anxiety score during the hospitalization, frequency of unscheduled visits to a physician within 15 days of discharge, and median workdays missed by parents did not differ significantly between groups (Table 3).
Discussion
This pragmatic randomized clinical trial of infants with and without hypoxia hospitalized for bronchiolitis at children’s and community hospitals found that clinical outcomes were similar with intermittent or continuous pulse oximetry when using an oxygen saturation target of 90% or higher. No important differences were found between groups in the primary outcome of median length of hospital stay from randomization. Furthermore, the confidence limits around the difference exclude our prespecified minimal clinically important difference of 12 hours. Although no significant differences were found between groups in medical interventions and safety outcomes as well as parent anxiety or parent workdays missed, nursing satisfaction was greater with intermittent pulse oximetry.
Several possible explanations exist for why a reduction in length of hospital stay was not found with intermittent monitoring. In contrast to the previous nonrandomized and retrospective studies8,9 that suggested that continuous monitoring prolongs hospital stay, this study was a randomized clinical trial. This trial used the current recommended oxygen target saturation of 90% or higher rather than a target of 93% or higher or 94% used in the earlier retrospective studies.8,9 Oxygen saturation target level may be a more important determinant of oxygen supplementation and length of hospital stay than monitoring frequency. For safety reasons, this trial compared oxygen monitoring strategies when patients were clinically improving and met stability criteria. Although initiation of intermittent monitoring early in the stabilization period when a greater proportion of infants had hypoxia might have been effective in reducing length of hospital stay, that strategy may not be acceptable to many practitioners.
One previous bronchiolitis trial (n = 161) that compared intermittent with continuous pulse oximetry found no significant differences in mean length of stay or ICU transfer.24 However, that study24 did not include infants with hypoxia; the time of intermittent monitoring initiation was not reported, limiting the comparison on the time saved to discharge25; and parent, nurse, and postdischarge outcomes were not measured.
This trial and several quality improvement reports found similar percentages of ICU transfers and hospital revisits with intermittent pulse oximetry, supporting the safety of this strategy in infants with stabilized bronchiolitis.26,27,28 Of importance, this study also found that parental anxiety in the hospital was similar between groups, suggesting that less monitoring does not erode parental confidence, as has been postulated.13,29
Current AAP guideline recommendations to limit continuous pulse oximetry in hospitalized infants with bronchiolitis carry “the worst evidence-quality grade (D) and weakest recommendation strength.”2,13(p 1449) Almost half of infants without hypoxia hospitalized with bronchiolitis in North American hospitals are monitored continuously.12 This practice continues because of the weak evidence base; perceived value that nurses, physicians, and parents may place on technology when dealing with uncertainty30,31; and barriers to deimplementing the established practice of continuous monitoring.13
This trial found that clinical outcomes were similar between intermittent and continuous pulse oximetry. Young et al32 suggest application of broader considerations to make a practice recommendation when a randomized trial of 2 established treatments finds nonstatistically and nonclinically significant differences in the primary outcome based on the prespecified criteria. Broader considerations for the use of intermittent vs continuous pulse oximetry argue for the use of less intense monitoring. These considerations include, first, lower nursing workload because of fewer oximeter alarms and enhanced patient safety because of reduced alarm fatigue.2,10 Of importance, this study found that nursing satisfaction was greater with intermittent monitoring. Second, infants recovering from bronchiolitis commonly experience transient desaturations that are of little clinical importance.33 Third, intermittent monitoring better aligns with the parent and child hospital-to-home transition preparation. Fourth, doing less simplifies care, which is a valued principle in hospital care.32 Given these considerations, the findings of this study support the standard use of intermittent pulse oximetry in infants hospitalized with stabilized bronchiolitis and provide strong evidence for quality improvement efforts in deimplementing continuous pulse oximetry.
Limitations
This study has several limitations. First, it was not possible to mask the oxygen monitoring intervention. However, in this pragmatic trial, the interest was in the real-world effect of the intervention, with practitioner and parent knowledge of the monitoring strategy used. Second, this trial does not exclude smaller differences between groups in the primary outcome of length of stay from randomization to discharge, such as 4 hours. Third, data on the frequency of desaturations were not collected, which might have provided a mechanistic understanding between monitoring strategies and clinical outcomes. In addition, future qualitative studies will provide further understanding of nursing monitoring preferences. Fourth, revisits to hospital after discharge did not capture revisits to other hospitals. Fifth, neurocognitive outcomes were not assessed; although there is controversy, some have raised concern that transient hypoxia associated with the acute illness may have neurocognitive impacts.34
Conclusions
In infants hospitalized with stabilized bronchiolitis with and without hypoxia and managed using an oxygen saturation target of 90% or higher, clinical outcomes of intermittent and continuous pulse oximetry monitoring were similar. Given that other important clinical practice considerations favor less intense monitoring, these findings support the standard use of intermittent pulse oximetry in infants hospitalized with stabilized bronchiolitis.
Trial Protocol
eTable 1. Pulse Oximetry Monitoring Characteristics at Trial Hospital Sites
eTable 2. Distribution of Participants Across Trial Hospital Sites
eMethods. Supplementary Methods
Data Sharing Statement
References
- 1.Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132(1):28-36. doi: 10.1542/peds.2012-3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralston SL, Lieberthal AS, Meissner HC, et al. ; American Academy of Pediatrics . Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. doi: 10.1542/peds.2014-2742 [DOI] [PubMed] [Google Scholar]
- 3.Hartling L, Fernandes RM, Bialy L, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ. 2011;342:d1714. doi: 10.1136/bmj.d1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mower WR, Sachs C, Nicklin EL, Baraff LJ. Pulse oximetry as a fifth pediatric vital sign. Pediatrics. 1997;99(5):681-686. doi: 10.1542/peds.99.5.681 [DOI] [PubMed] [Google Scholar]
- 5.Enoch AJ, English M, Shepperd S. Does pulse oximeter use impact health outcomes? a systematic review. Arch Dis Child. 2016;101(8):694-700. doi: 10.1136/archdischild-2015-309638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham S, Rodriguez A, Adams T, et al. ; Bronchiolitis of Infancy Discharge Study (BIDS) group . Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386(9998):1041-1048. doi: 10.1016/S0140-6736(15)00163-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527-530. doi: 10.1001/archpedi.158.6.527 [DOI] [PubMed] [Google Scholar]
- 8.Unger S, Cunningham S. Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121(3):470-475. doi: 10.1542/peds.2007-1135 [DOI] [PubMed] [Google Scholar]
- 9.Bergman AB. Pulse oximetry: good technology misapplied. Arch Pediatr Adolesc Med. 2004;158(6):594-595. doi: 10.1001/archpedi.158.6.594 [DOI] [PubMed] [Google Scholar]
- 10.Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. doi: 10.1136/bmj.j3850 [DOI] [PubMed] [Google Scholar]
- 11.Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. doi: 10.1002/jhm.2064 [DOI] [PubMed] [Google Scholar]
- 12.Bonafide CP, Xiao R, Brady PW, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network . Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. doi: 10.1001/jama.2020.2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheston CC, Vinci RJ. Overuse of continuous pulse oximetry for bronchiolitis: the need for deimplementation science. JAMA. 2020;323(15):1449-1450. doi: 10.1001/jama.2020.4359 [DOI] [PubMed] [Google Scholar]
- 14.Mahant S, Wahi G, Giglia L, et al. Intermittent versus continuous oxygen saturation monitoring for infants hospitalised with bronchiolitis: study protocol for a pragmatic randomised controlled trial. BMJ Open. 2018;8(4):e022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Handbook: Integrated Management of Childhood Illnesses. 2005. Accessed March 15, 2020. https://apps.who.int/iris/handle/10665/42939
- 17.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, & Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press Inc; 1983. [Google Scholar]
- 18.Mansbach JM, Piedra PA, Teach SJ, et al. ; MARC-30 Investigators . Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700-706. doi: 10.1001/archpediatrics.2011.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013;6(6):CD004878. doi: 10.1002/14651858.CD004878.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2008;7(4):CD006458. doi: 10.1002/14651858.CD006458.pub2 [DOI] [PubMed] [Google Scholar]
- 21.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020.
- 22.Price RM, Bonett DG. Distribution-free confidence intervals for difference and ratio of medians. J Stat Comput Simul. 2002;72(2):119-124. doi: 10.1080/00949650212140 [DOI] [Google Scholar]
- 23.Kawaguchi A, Koch GG. sanon: an R package for stratified analysis with nonparametric covariable adjustment. J Stat Softw. 2015; 67(9):1-37. doi: 10.18637/jss.v067.i09 [DOI] [Google Scholar]
- 24.McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs. continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. doi: 10.1001/jamapediatrics.2015.1746 [DOI] [PubMed] [Google Scholar]
- 25.Cunningham S. Intermittent monitoring of oxygen saturation in infants and children with acute bronchiolitis: peekaboo paediatrics or good clinical care? JAMA Pediatr. 2015;169(10):891-892. doi: 10.1001/jamapediatrics.2015.1971 [DOI] [PubMed] [Google Scholar]
- 26.Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. doi: 10.1542/hpeds.2018-0023 [DOI] [PubMed] [Google Scholar]
- 27.Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. doi: 10.1542/peds.2014-2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heneghan M, Hart J, Dewan M, et al. No cause for alarm: decreasing inappropriate pulse oximetry use in bronchiolitis. Hosp Pediatr. 2018;8(2):109-111. doi: 10.1542/hpeds.2017-0126 [DOI] [PubMed] [Google Scholar]
- 29.Hendaus MA, Nassar S, Leghrouz BA, Alhammadi AH, Alamri M. Parental preference and perspectives on continuous pulse oximetry in infants and children with bronchiolitis. Patient Prefer Adherence. 2018;12:483-487. doi: 10.2147/PPA.S152880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. doi: 10.1001/jama.2014.8637 [DOI] [PubMed] [Google Scholar]
- 31.Vinci R, Bauchner H. Bronchiolitis, deception in research, and clinical decision making. JAMA. 2014;312(7):699-700. doi: 10.1001/jama.2014.8638 [DOI] [PubMed] [Google Scholar]
- 32.Young PJ, Nickson CP, Perner A. When should clinicians act on non-statistically significant results from clinical trials? JAMA. 2020;323(22):2256-2257. doi: 10.1001/jama.2020.3508 [DOI] [PubMed] [Google Scholar]
- 33.Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. doi: 10.1001/jamapediatrics.2016.0114 [DOI] [PubMed] [Google Scholar]
- 34.Wainwright CE, Kapur N. Oxygen saturation targets in infants with bronchiolitis. Lancet. 2015;386(9998):1016-1018. doi: 10.1016/S0140-6736(15)00155-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Pulse Oximetry Monitoring Characteristics at Trial Hospital Sites
eTable 2. Distribution of Participants Across Trial Hospital Sites
eMethods. Supplementary Methods
Data Sharing Statement