Abstract
This study compares titers of binding and neutralizing antibodies after a single mRNA coronavirus vaccine dose in health care workers previously infected with SARS-CoV-2.
Current shortages in COVID-19 vaccine production and distribution have led some experts to suggest untested regimens.1 Persons who have had COVID-19 are thought to have protective immunity and memory responses2 for at least 6 months; however, neither recall responses nor ideal vaccine dosing regimens have been studied in those previously infected with SARS-CoV-2. We assessed whether health care workers with previous COVID-19 infection could mount recall responses to a single dose of an mRNA-based COVID-19 vaccine.
Methods
Health care workers who had previously enrolled in a hospital-wide serosurvey study,3 conducted from July to August 2020 at the University of Maryland Medical Center, were randomly contacted based on stratification into 3 groups: SARS-CoV-2 IgG antibody–negative (Ab-negative); IgG-positive asymptomatic COVID-19 (asymptomatic); and IgG-positive with history of symptomatic COVID-19 (symptomatic). Participants were vaccinated with either the Pfizer-BioNTech or Moderna vaccine, depending on personal preference and availability. Blood was drawn at days 0 (baseline), 7, and 14 postvaccination in December 2020 and January 2021 (draws could be within 1 day from assigned day). Plasma was tested using enzyme-linked immunosorbent assay (ELISA) for IgG to spike trimer, which was modified from an assay4 to give a readout of half-maximal binding titers. The reciprocal half-maximal binding titers represent the dilution of plasma that achieves 50% of maximal binding of a known control that reaches saturation. Day 0 and 14 samples from vaccinees were also tested for ID99 (the 99% inhibitory dose, the highest dilution at which 99% of cells were protected) by live virus neutralization (presented as reciprocals).5 Samples from each day were compared between each prior Ab-positive (asymptomatic or symptomatic) group to the Ab-negative group.
All health care workers provided written informed consent; the study was approved by the University of Maryland institutional review board. Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software). Antibody titers between groups were tested using the 2-tailed Mann-Whitney test, with P < .05 considered significant.
Results
Of 3816 health care workers enrolled in the serosurvey study,3 151 were randomly contacted and 59 volunteers enrolled: 17 in the Ab-negative, 16 in the asymptomatic, and 26 in the symptomatic group (Table). The median age was 38 years for the Ab-negative, 40 years for the asymptomatic, and 38 years for the symptomatic group. The percentage of women was 71% for the Ab-negative, 75% for the asymptomatic, and 88% for the symptomatic group. At 0, 7, and 14 days, median reciprocal half-maximal binding titers were higher in each of the asymptomatic (208, 29 364, and 34 033) and symptomatic (302, 32 301, and 35 460) groups compared with the Ab-negative group (<50, <50, and 924) (P < .001 for each). At 0 and 14 days, median reciprocal ID99 virus neutralization titers of each of the asymptomatic (80 and 40 960) and symptomatic (320 and 40 960) groups were higher than the Ab-negative group (<20 and 80) (P < .001 for each) (Figure).
Table. Study Population Baseline Characteristics.
| No. (%) | |||
|---|---|---|---|
| Ab-negative (n = 17) | Asymptomatic (n = 16) | Symptomatic (n = 26) | |
| Age, median (range), y | 38 (29-55) | 40 (25-72) | 38 (23-59) |
| Sex | |||
| Male | 5 (29) | 4 (25) | 3 (12) |
| Female | 12 (71) | 12 (75) | 23 (88) |
| Race/ethnicitya | |||
| Black or African American | 2 (12) | 5 (31) | 5 (19) |
| White | 12 (71) | 9 (56) | 18 (69) |
| Asian | 3 (18) | 2 (13) | 3 (14) |
| Vaccine (by manufacturer) | |||
| Pfizer-BioNTech | 10 (59) | 6 (37) | 13 (50) |
| Moderna | 7 (41) | 10 (63) | 13 (50) |
| Days symptomatic, median (range) | 0 (0-2) | 10 (3-34) | |
| Hospitalization | 0 | 4 (15) | |
| Months from COVID-19 PCR+ to vaccination, median (% PCR tested) [range] | 9.0 (44) [6.5-9.7] | 8.0 (73) [6.0-10.3] | |
| Months from COVID-19 IgG+ to vaccination, median (range) | 6.2 (4.8-7.1) | 6.1 (3.8-7.2) | |
Abbreviation: PCR, polymerase chain reaction.
Race/ethnicity data derived from self-report. This variable was of research interest because individuals of different race/ethnicity may react differently to vaccine.
Figure. Anti–SARS-CoV-2 Antibody Responses After a Single Dose of Vaccine in Health Care Workers.
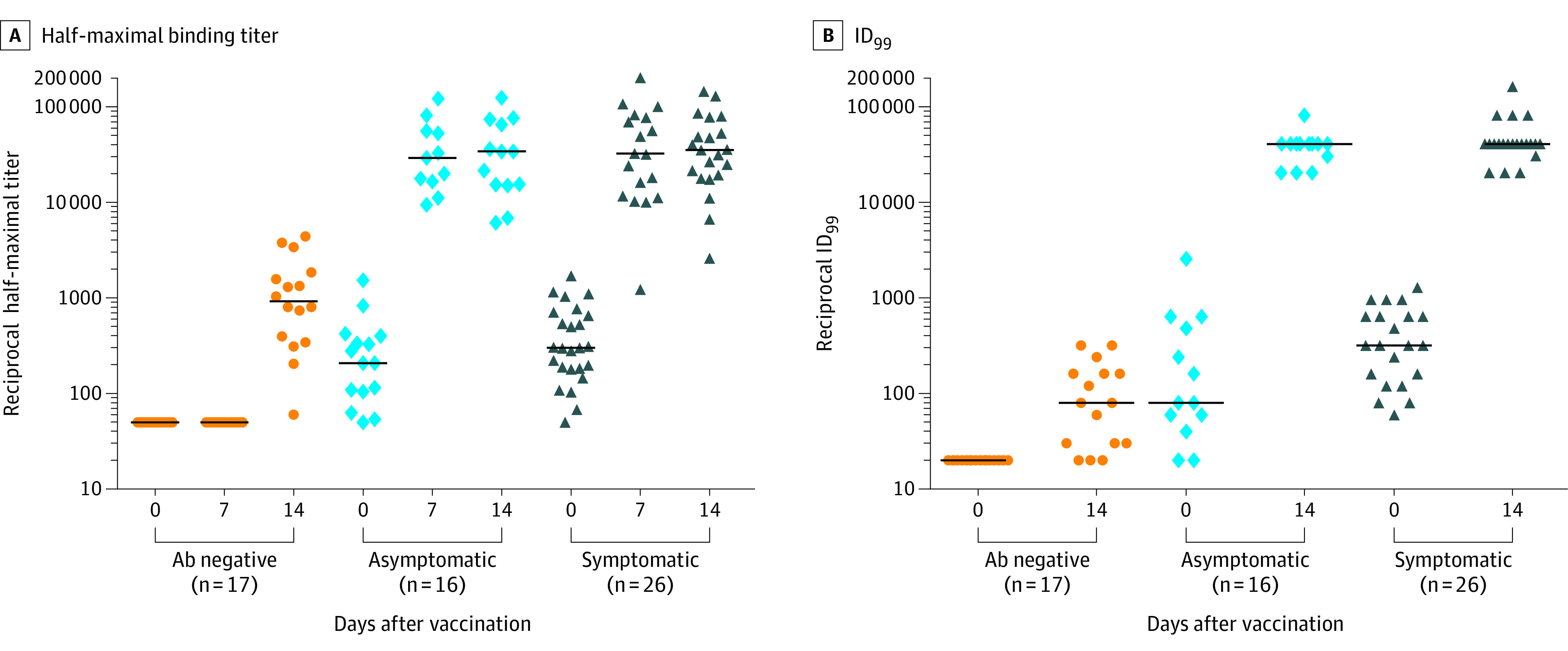
After COVID-19 vaccination, plasma was drawn at 0, 7, and 14 days; IgG binding titers against spike trimer were measured by enzyme-linked immunosorbent assay and live virus neutralization was assessed at days 0 and 14. A, IgG spike trimer half-maximal titers. By 7 days and continuing through 14 days following vaccination, both groups of health care workers with prior infection (asymptomatic and symptomatic) who received a single vaccine dose developed higher peak IgG titers than the antibody (Ab)-negative group. B, Live virus neutralization ID99 (the 99% inhibitory dose, the dilution at which 99% of cells were protected). At 14 days, both groups of health care workers with prior infection (asymptomatic and symptomatic) who received a single vaccine dose developed higher neutralization titers than the Ab-negative group. Horizontal black lines represent median values.
Discussion
Health care workers with previous COVID-19 infection, based on laboratory-confirmed serology testing, had higher antibody titer responses to a single dose of mRNA vaccine than those who were not previously infected. Antibody titers started peaking at 7 days and achieved higher titers and neutralization in 14 days compared with Ab-negative volunteers. Limitations of the study are the small sample size, lack of demonstration of vaccine efficacy, and potential bias introduced by those enrolling not being representative of the larger original population. Given the ongoing worldwide vaccine shortages, the results inform suggestions for a single-dose vaccination strategy for those with prior COVID-19 or placing them lower on the vaccination priority list.6
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Winter L. US officials debate efficacy of half doses of COVID-19 vaccine. The Scientist. Published January 5, 2021. Accessed February 13, 2021. https://www.the-scientist.com/news-opinion/us-officials-debate-efficacy-of-half-doses-of-covid-19-vaccine-68316
- 2.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullins KE, Merrill V, Ward M, et al. Validation of COVID-19 serologic tests and large scale screening of asymptomatic healthcare workers. Clin Biochem. 2021;S0009-9120(21)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rikhtegaran Tehrani Z, Saadat S, Saleh E, et al. Performance of nucleocapsid and spike-based SARS-CoV-2 serologic assays. PLoS One. 2020;15(11):e0237828. doi: 10.1371/journal.pone.0237828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320-2332. doi: 10.1056/NEJMoa2026920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haute Autorité de Santé (HAS) . Stratégie de vaccination contre le SARS-CoV-2: vaccination des personnes ayant un antécédent de Covid-19 [SARS-CoV-2 vaccination strategy: vaccination of people with a history of Covid-19]. Published February 11, 2021. Accessed February 13, 2021. https://www.has-sante.fr/upload/docs/application/pdf/2021-02/strategie_de_vaccination_contre_le_sars-cov-2___vaccination_des_personnes_ayant_un_antecedent_de_covid-19_-_synthese.pdf


