Key Points
Question
Do long-acting insulin analogs reduce the risk of emergency department visits or hospitalization for hypoglycemia compared with neutral protamine Hagedorn (NPH) insulin in older patients with type 2 diabetes?
Findings
In this cohort study of 575 008 patients 65 years or older initiating use of insulin, long-acting insulin analogs were associated with a lower risk of emergency department visits or hospitalizations for hypoglycemia compared with NPH insulin. This finding was not seen with concomitant use of prandial insulin.
Meaning
Long-acting insulin analogs may reduce the risk of severe hypoglycemia compared with NPH insulin in older patients.
Abstract
Importance
Previous studies have found that the risk of severe hypoglycemia does not differ between long-acting insulin analogs and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes. However, these studies did not focus on patients 65 years or older, who are at an increased risk for hypoglycemia, or did not include patients with concomitant prandial insulin use.
Objective
To examine the risk of emergency department (ED) visits or hospitalizations for hypoglycemia among older community-residing patients with type 2 diabetes who initiated long-acting insulin or NPH insulin in real-world settings.
Design, Setting, and Participants
This retrospective, new-user cohort study assessed Medicare beneficiaries 65 years or older who initiated insulin glargine (n = 407 018), insulin detemir (n = 141 588), or NPH insulin (n = 26 402) from January 1, 2007, to July 31, 2019.
Exposures
Insulin glargine, insulin detemir, and NPH insulin.
Main Outcomes and Measures
The primary outcome was time to first ED visit or hospitalization for hypoglycemia, defined using a modified validated algorithm. Propensity score–weighted Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs. The risk of recurring hypoglycemia events was estimated using the Andersen-Gill model. Post hoc analyses were conducted investigating possible effect modification by age.
Results
Of the 575 008 patients initiating use of insulin (mean [SD] age 74.9 [6.7] years; 53% female), 407 018 used glargine, 141 588 used detemir, and 26 402 used NPH insulin. The study included 7347 ED visits or hospitalizations for hypoglycemia (5194 for glargine, 1693 for detemir, and 460 for NPH insulin, with a median follow-up across the 3 cohorts of 0.37 years (interquartile range, 0.20-0.76 years). Initiation of glargine and detemir use was associated with a reduced risk of hypoglycemia compared with NPH insulin use (HR for glargine vs NPH insulin, 0.71; 95% CI, 0.63-0.80; HR, detemir vs NPH insulin, 0.72; 95% CI, 0.63-0.82). The HRs were similar for the recurrent event analysis. The protective association of long-acting insulin analogs varied by age and was not seen with concomitant prandial insulin use.
Conclusions and Relevance
In this cohort study, initiation of long-acting analogs was associated with a lower risk of ED visits or hospitalizations for hypoglycemia compared with NPH insulin in older patients with type 2 diabetes in Medicare. However, this association was not seen with concomitant prandial insulin use.
This cohort study compares the associations of long-acting insulin analogs and NPH insulin with the risk of severe hypoglycemia in older patients with type 2 diabetes.
Introduction
Long-acting insulin analogs, insulin glargine and insulin detemir, are structurally altered human insulins that mimic the pharmacokinetic properties of endogenous insulin more closely than neutral protamine Hagedorn (NPH) insulin and are increasingly used in the management of type 2 diabetes. A recent report1 estimating Medicare Part D spending on insulin in 2017 found that long-acting insulin analogs accounted for 50% of total dispensed insulin (27 513 of 53 102 million units) and almost 62% of insulin analog use was insulin glargine. The price of long-acting insulin analogs has increased substantially,2 and in 2017 alone Medicare was estimated to have spent $7 billion on long-acting insulin analogs, of which glargine accounted for $4.7 billion. Higher costs for these insulin analogs may limit patient access.1
Despite suggested theoretical benefits of long-acting analogs, such as lower risk of hypoglycemia (especially nocturnal) because of the longer duration of action and the less pronounced insulin peak, a Cochrane review of randomized clinical trials (RCTs)3 that compared long-acting insulin analogs with NPH insulin reported no statistically significant difference for severe hypoglycemia rates in any of the RCTs4,5,6,7,8,9 or in a meta-analysis of RCT results.3 Similar findings were reported by Lipska et al10 in an observational study that examined rates of emergency department (ED) visits or hospital admissions for hypoglycemia and changes in levels of glycemic control after initiating use of long-acting insulin analogs compared with NPH insulin among patients with type 2 diabetes in Kaiser Permanente of Northern California (KPNC).10 They reported that initiation of long-acting insulin analog use compared with NPH insulin was not associated with reduced risk of ED visits or hospital admissions for hypoglycemia or with improved glycemic control and suggested that “use of basal insulin analogs in usual practice settings may not be associated with clinical advantages for these outcomes.”10(pE1) However, another meta-analysis of RCTs11 reported a lower risk of severe hypoglycemia among long-acting analog users compared with NPH insulin users across 7 studies. The study by Lipska et al10 was limited in that NPH insulin was used preferentially within KPNC; therefore, 23 561 NPH insulin users but only 1928 long-acting insulin analog users were included in the study. In addition, this study10 did not examine concomitant use of prandial insulin, which is common in real-world settings. The mean age of patients in previous RCTs4,5,8,9 and the study by Lipska et al10 ranged from 55 to 60 years, indicating that these studies were likely underpowered to detect or could not test the impact of older age on their findings. People older than 65 years may be at increased risk for hypoglycemia, and the consequences may be more severe.12,13 In addition, severe hypoglycemia was patient reported in the previous RCTs, whereas in the study by Lipska et al10 it was based on health care utilization data. Given the limitations of the previous observational study10 and the need for real-world evidence on the safety of long-acting insulin analogs compared with NPH insulin in older patients, we used Medicare data to assess the risk of ED visits or hospitalizations for hypoglycemia among older patients with type 2 diabetes who initiated glargine or detemir compared with NPH insulin in real-world settings.
Methods
Study Cohort
Medicare beneficiaries 65 years or older and enrolled in Medicare fee-for-service Parts A, B, and D were eligible for study inclusion if they initiated a study insulin (glargine, detemir, or NPH insulin) between January 1, 2007, and July 31, 2019. Beneficiaries were also required to have continuous enrollment in Medicare, a type 2 diabetes diagnosis, and no prescriptions for a study insulin or a prandial insulin in the 365 days before the date of the qualifying prescription (index date). Patients were excluded if they were in a skilled nursing facility or nursing home, received their index prescription from a long-term care pharmacy, or were receiving hospice care on the index date. Kidney transplant recipients, patients undergoing dialysis in the year before index date, and anyone who entered Medicare for reasons other than age were also excluded (eFigure 1 in the Supplement). This study was classified as public health surveillance by the US Food and Drug Administration and was exempted from review by the agency’s institutional review board in accordance with the updated Common Rule. In addition, patient consent was not required for this study because it was observational and used deidentified health care claims data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Exposure
The primary exposure definition was new use14 of any formulation of glargine, detemir, or NPH insulin alone (eTable 1 in the Supplement); NPH insulin combination products with prandial insulin were excluded from the primary analysis. Patients were considered exposed to a study insulin from their index date (first insulin dispensing based on Part D dispensing claims) until a censoring or outcome event occurred.
Outcomes
Emergency department visits and hospitalizations for hypoglycemia were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), diagnosis codes. We used a modified version of the algorithm developed by Ginde et al,15 which was validated for use in claims data based on International Classification of Diseases, Ninth Revision (ICD-9) coding. For hypoglycemic events identified after the October 1, 2015, International Classification of Diseases, Tenth Revision (ICD-10) transition date, we used the General Equivalence Mappings to map ICD-9-CM codes from the algorithm of Ginde et al15 to ICD-10-CM codes. The algorithm was modified to include inpatient and outpatient ED claims with hypoglycemia in the primary position only, similar to the algorithm used by Lipska et al10 (eFigure 2 and eTable 2 in the Supplement).
Follow-up
An as-treated approach to follow-up time was applied in our primary analyses. Follow-up time started on the first day after the index date and continued until the earliest of the following: switch to another study insulin, initiation of prandial insulin use, death, disenrollment from Medicare Parts A, B, or D or enrollment in Medicare Part C, end of study (July 31, 2019), treatment cessation (defined as no observed refill within 45 days of the last day of the previous refill’s supply), a hypoglycemia outcome, or an invalid prescription (ie, prescriptions with implausible dose values) (eTable 3 in the Supplement).
Covariates
Demographic characteristics, preexisting medical conditions, medication use, an adapted Diabetes Complications Severity Index,16 and health care utilization (including inpatient, outpatient, ED, and physician visits; number of glycated hemoglobin tests; and physician specialty) were identified in the 12-month baseline period before cohort entry. Time-varying concomitant use of noninsulin antidiabetic drugs, including sulfonylureas, metformin, incretins (dipeptidyl peptidase-4 inhibitors and glucagon-like peptide 1 agonists), and other antidiabetic drugs (thiazolidinediones and other) were also evaluated during follow-up.
Statistical Analysis
Logistic regression was used to estimate the probability of receiving glargine vs NPH insulin and detemir vs NPH insulin conditional on baseline covariates (ie, propensity score) and to calculate inverse probability of treatment weights (IPTWs) for each comparison (glargine vs NPH insulin and detemir vs NPH insulin). Because the target population for this study was the glargine-detemir cohort for each comparison, IPTWs for average treatment effects among treated patients, considering glargine-detemir as the reference treatment, were applied. Distributions of propensity scores and IPTWs were inspected for outliers. Weight truncation at the 99th percentile was conducted to account for extreme weights in the NPH insulin cohort. Covariate balance between weighted cohorts was assessed using standardized mean differences, with a value of 0.1 or less indicating a negligible difference between groups.17
All analyses were based on IPTW-adjusted cohorts to account for potential confounding at baseline and additionally adjusted for time-varying concomitant use of noninsulin antidiabetic drugs, including sulfonylureas, metformin, incretins (dipeptidyl peptidase-4 inhibitors and glucagon-like peptide 1 agonists), and other antidiabetic drugs (thiazolidinediones and other) during follow-up. Weighted Cox proportional hazards regression with robust SEs was used to estimate hazard ratios (HRs) and 95% CIs for time to first ED visit or hospitalization for hypoglycemia among glargine users compared with users of NPH insulin and separately for detemir users compared with NPH insulin. Secondary analyses used the Andersen-Gill model, an extended Cox proportional hazards regression model,18 to examine the association between use of long-acting insulins and recurrent hypoglycemia events, additionally adjusting for number of prior events. For recurrent events to be counted as separate outcomes, we required at least 1 day between the end of prior outcome and start of the next outcome.
Several prespecified sensitivity analyses were undertaken that modified the primary analysis by (1) increasing the gap allowance between successive insulin prescriptions from 45 to 90 days; (2) restricting the analysis to the ICD-9 coding era (January 2007 to September 2015); (3) combining long-acting insulin analog users (glargine and detemir) into 1 cohort; (4) using the original algorithm of Ginde et al,15 which includes hypoglycemia recorded in any position; and (5) not truncating IPTW at the 99th percentile.
We also conducted prespecified exploratory analyses to evaluate the risk of hypoglycemia among concomitant prandial insulin users, defined as initiation of prandial insulin use on index date or at any time during follow-up. For these analyses, the primary analysis study population was expanded to include patients in the NPH insulin cohort who initiated an NPH insulin combination with prandial insulin. The primary analysis was repeated in this expanded cohort with the following modifications: (1) removal of censoring for initiation of prandial insulin use during follow-up, (2) an additional adjustment for time-varying concomitant prandial insulin use in the weighted model, and (3) inclusion of interaction terms for concurrent prandial insulin use for each of the study drugs.
Post hoc analyses were conducted to investigate possible effect modification by age given that the findings varied from the previous study by Lipska et al,10 which involved a younger population. We first examined effect modification by age category (65-74, 75-84, and ≥85 years) and then used a penalized spline19 to explore effect modification in a continuous age scale. Post hoc analyses also investigated the effect of socioeconomic status and year of insulin initiation. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) and R software, version 3.4.3 (R Foundation for Statistical Computing). A 2-sided P < .05 was considered statistically significant.
Results
Of the 575 008 patients initiating use of insulin (mean [SD] age 74.9 [6.7] years; 53% female), 407 018 used glargine, 141 588 used detemir, and 26 402 used NPH insulin. Glargine initiators contributed 299 098 person-years of follow-up, detemir initiators contributed 101 426 person-years of follow-up, and NPH insulin initiators contributed 14 994 person- years of follow-up.
Before IPTW adjustment, no substantial differences were found in demographic or clinical characteristics between the glargine and detemir cohorts. However, when the NPH insulin cohort was compared with each long-acting analog cohort, slight differences were noted, including a higher proportion of NPH insulin users who were Black (13% for NPH insulin vs 9% for analogs), fewer physician visits and glycated hemoglobin tests before the index date in NPH insulin users than analog users, and a smaller proportion of NPH insulin users who used oral diabetes medications and statins compared with analog users (Table 1). After IPTW adjustment, cohorts for each analysis were closely balanced for all baseline covariates (eTable 4 in the Supplement).
Table 1. Baseline Demographic and Clinical Characteristics of Glargine, Detemir, and Neutral Protamine Hagedorn (NPH) Insulin Users Before Inverse Probability of Treatment Weighting.
| Characteristic | No. (%) of users | Standardized mean difference | ||||
|---|---|---|---|---|---|---|
| Glargine | Detemir | NPH insulin | Glargine vs detemir | Glargine vs NPH | Detemir vs NPH | |
| Base population total | 407 018 (100) | 141 588 (100) | 26 402 (100) | NA | NA | NA |
| Age, mean (SD), y | 75.00 (6.76) | 74.72 (6.65) | 75.11 (6.84) | 0.04 | 0.02 | 0.06 |
| Age categorical, y | ||||||
| 65-74 | 218 367 (54) | 78 402 (55) | 13 936 (53) | 0.03 | 0.02 | 0.05 |
| 75-84 | 145 613 (36) | 49 494 (35) | 9595 (36) | 0.02 | 0.01 | 0.03 |
| >85 | 43 038 (11) | 13 692 (10) | 2871 (11) | 0.03 | 0.01 | 0.04 |
| Race/ethnicity | ||||||
| White | 324 641 (80) | 112 528 (79) | 19 833 (75) | 0.01 | 0.11 | 0.10 |
| Black | 38 547 (9) | 13 291 (9) | 3395 (13) | 0.00 | 0.11 | 0.11 |
| Other | 43 830 (11) | 15 769 (11) | 3174 (12) | 0.01 | 0.04 | 0.03 |
| Sex | ||||||
| Male | 190 240 (47) | 65 675 (46) | 11 898 (45) | 0.01 | 0.03 | 0.03 |
| Female | 216 778 (53) | 75 913 (54) | 14 504 (55) | 0.01 | 0.03 | 0.03 |
| Low-income status | ||||||
| Yes | 126 173 (31) | 43 770 (31) | 8815 (33) | 0.00 | 0.05 | 0.05 |
| No | 280 845 (69) | 97 818 (69) | 17 587 (67) | 0.00 | 0.05 | 0.05 |
| Zip code–level annual income, $ | ||||||
| <30 000 | 68 260 (17) | 26 653 (19) | 5320 (20) | 0.05 | 0.09 | 0.03 |
| 30 000-60 000 | 267 954 (66) | 92 342 (65) | 17 054 (65) | 0.01 | 0.03 | 0.01 |
| >60 000 | 55 478 (14) | 17 192 (12) | 2965 (11) | 0.04 | 0.07 | 0.03 |
| Unknown | 15 326 (4) | 5401 (4) | 1063 (4) | 0.00 | 0.01 | 0.01 |
| Metropolitan statistical area | ||||||
| Nonrural | 302 571 (74) | 102 537 (72) | 19 376 (73) | 0.04 | 0.02 | 0.02 |
| Rural | 104 447 (26) | 39 051 (28) | 7026 (27) | 0.04 | 0.02 | 0.02 |
| Area Deprivation Index score | ||||||
| Missing | 15 323 (4) | 5449 (4) | 1152 (4) | 0.00 | 0.03 | 0.03 |
| 0-19 | 65 940 (16) | 19 877 (14) | 3863 (15) | 0.06 | 0.04 | 0.02 |
| 20-39 | 85 255 (21) | 28 027 (20) | 5171 (20) | 0.03 | 0.03 | 0.01 |
| 40-59 | 87 795 (22) | 30 595 (22) | 5572 (21) | 0.00 | 0.01 | 0.01 |
| 60-79 | 83 774 (21) | 31 129 (22) | 5493 (21) | 0.03 | 0.01 | 0.03 |
| 80-100 | 68 931 (17) | 26 511 (19) | 5151 (20) | 0.05 | 0.07 | 0.02 |
| No. of IP visits | ||||||
| 0 | 255 489 (63) | 91 781 (65) | 15 923 (60) | 0.04 | 0.05 | 0.09 |
| 1 | 85 608 (21) | 28 093 (20) | 5450 (21) | 0.03 | 0.01 | 0.02 |
| 2 | 36 119 (9) | 11 937 (8) | 2570 (10) | 0.02 | 0.03 | 0.05 |
| ≥3 | 29 802 (7) | 9777 (7) | 2459 (9) | 0.02 | 0.07 | 0.09 |
| No. of OP/ED visits | ||||||
| 0 | 274 892 (68) | 96 295 (68) | 17 824 (68) | 0.01 | 0.00 | 0.01 |
| 1 | 82 488 (20) | 28 429 (20) | 5244 (20) | 0.00 | 0.01 | 0.01 |
| 2 | 28 588 (7) | 9747 (7) | 1938 (7) | 0.01 | 0.01 | 0.02 |
| ≥3 | 21 050 (5) | 7117 (5) | 1396 (5) | 0.01 | 0.01 | 0.01 |
| No. of physician visitsa | ||||||
| 0 | 17 506 (4) | 4277 (3) | 2225 (8) | 0.07 | 0.17 | 0.23 |
| 1-4 | 57 216 (14) | 17 174 (12) | 4383 (17) | 0.06 | 0.07 | 0.13 |
| 5-10 | 101 467 (25) | 35 346 (25) | 6324 (24) | 0.00 | 0.02 | 0.02 |
| 11-20 | 122 618 (30) | 44 911 (32) | 7182 (27) | 0.03 | 0.06 | 0.10 |
| 21-30 | 58 705 (14) | 21 752 (15) | 3333 (13) | 0.03 | 0.05 | 0.08 |
| ≥31 | 49 506 (12) | 18 128 (13) | 2955 (11) | 0.02 | 0.03 | 0.05 |
| No. of glycated hemoglobin tests | ||||||
| 0 | 36 798 (9) | 11 850 (8) | 4214 (16) | 0.02 | 0.21 | 0.23 |
| 1 | 66 303 (16) | 22 166 (16) | 4774 (18) | 0.02 | 0.05 | 0.06 |
| 2-3 | 201 273 (49) | 70 921 (50) | 11 760 (45) | 0.01 | 0.10 | 0.11 |
| 4-5 | 90 720 (22) | 32 361 (23) | 4975 (19) | 0.01 | 0.09 | 0.10 |
| ≥6 | 11 924 (3) | 4290 (3) | 679 (3) | 0.01 | 0.02 | 0.03 |
| Physician specialtya | ||||||
| Endocrinology | 43 696 (11) | 14 747 (10) | 3570 (14) | 0.01 | 0.09 | 0.10 |
| Primary care | 240 379 (59) | 87 982 (62) | 12 871 (49) | 0.06 | 0.21 | 0.27 |
| Other | 122 943 (30) | 38 859 (27) | 9961 (38) | 0.06 | 0.16 | 0.22 |
| Other diabetes medications | ||||||
| Metformin | 263 194 (65) | 93 095 (66) | 13 532 (51) | 0.02 | 0.27 | 0.30 |
| Sulfonylureas | 269 206 (66) | 93 664 (66) | 13 806 (52) | 0.00 | 0.28 | 0.28 |
| Thiazolidinediones | 79 231 (19) | 26 167 (18) | 4202 (16) | 0.03 | 0.09 | 0.07 |
| DPP-4 | 120 166 (30) | 47 431 (33) | 3417 (13) | 0.09 | 0.41 | 0.50 |
| GLP-1 receptor agonist | 31 686 (8) | 13 154 (9) | 1254 (5) | 0.05 | 0.13 | 0.18 |
| Other antidiabetic drugs | 38 557 (9) | 13 428 (9) | 1455 (6) | 0.00 | 0.15 | 0.15 |
| No antidiabetic drug | 45 160 (11) | 14 140 (10) | 7316 (28) | 0.04 | 0.43 | 0.47 |
| Other medications | ||||||
| ACE inhibitors and ARBs | 295 620 (73) | 103 035 (73) | 18 422 (70) | 0.00 | 0.06 | 0.07 |
| Antiarrhythmics | 20 077 (5) | 6961 (5) | 1238 (5) | 0.00 | 0.01 | 0.01 |
| Anticoagulants, oral | 60 079 (15) | 19 762 (14) | 3499 (13) | 0.02 | 0.04 | 0.02 |
| Antiplatelets | 69 693 (17) | 24 590 (17) | 4391 (17) | 0.01 | 0.01 | 0.02 |
| β-Blockers | 221 524 (54) | 76 437 (54) | 13 574 (51) | 0.01 | 0.06 | 0.05 |
| Calcium channel blockers | 154 515 (38) | 54 145 (38) | 9993 (38) | 0.01 | 0.00 | 0.01 |
| Digoxin | 26 603 (7) | 8780 (6) | 1560 (6) | 0.01 | 0.03 | 0.01 |
| Diuretics | ||||||
| Loop | 131 010 (32) | 44 328 (31) | 8721 (33) | 0.02 | 0.02 | 0.04 |
| Potassium | 39 516 (10) | 13 494 (10) | 2535 (10) | 0.01 | 0.00 | 0.00 |
| Thiazides | 142 886 (35) | 50 287 (36) | 8694 (33) | 0.01 | 0.05 | 0.05 |
| Fibrates | 38 987 (10) | 14 977 (11) | 2050 (8) | 0.03 | 0.06 | 0.10 |
| Nitrates | 34 065 (8) | 11 361 (8) | 2465 (9) | 0.01 | 0.03 | 0.05 |
| Statins | 291 861 (72) | 102 197 (72) | 17 438 (66) | 0.01 | 0.12 | 0.13 |
| Medical conditions | ||||||
| Atrial fibrillation | 80 256 (20) | 26 726 (19) | 4895 (19) | 0.02 | 0.03 | 0.01 |
| COPD | 83 476 (21) | 28 957 (20) | 5617 (21) | 0.00 | 0.02 | 0.02 |
| Chronic kidney disease stage | ||||||
| 1 | 3000 (1) | 1105 (1) | 168 (1) | 0.00 | 0.01 | 0.02 |
| 2 | 10 026 (2) | 3819 (3) | 590 (2) | 0.01 | 0.02 | 0.03 |
| 3 | 64 961 (16) | 23 408 (17) | 3583 (14) | 0.02 | 0.07 | 0.08 |
| 4 | 13 555 (3) | 4578 (3) | 846 (3) | 0.01 | 0.01 | 0.00 |
| 5 | 1062 (0) | 318 (0) | 103 (0) | 0.01 | 0.02 | 0.03 |
| ESRD | 1886 (0) | 596 (0) | 185 (1) | 0.01 | 0.03 | 0.04 |
| Unspecified stage | 22 570 (6) | 7408 (5) | 1553 (6) | 0.01 | 0.01 | 0.03 |
| Unspecified kidney failure | 9822 (2) | 3147 (2) | 680 (3) | 0.01 | 0.01 | 0.02 |
| Kidney sclerosis | 456 (0) | 154 (0) | 33 (0) | 0.00 | 0.00 | 0.00 |
| None | 279 680 (69) | 97 055 (69) | 18 661 (71) | 0.00 | 0.04 | 0.05 |
| Coronary revascularization | 67 657 (17) | 22 980 (16) | 4208 (16) | 0.01 | 0.02 | 0.01 |
| Dementia | 28 390 (7) | 9509 (7) | 1666 (6) | 0.01 | 0.03 | 0.02 |
| Heart failure | 104 161 (26) | 34 038 (24) | 7056 (27) | 0.04 | 0.03 | 0.06 |
| Hospitalized myocardial infarction | 21 233 (5) | 6722 (5) | 1455 (6) | 0.02 | 0.01 | 0.03 |
| Hospitalized stroke | 34 226 (8) | 11 531 (8) | 2353 (9) | 0.01 | 0.02 | 0.03 |
| Hypercholesterolemia | 201 288 (49) | 72 001 (51) | 12 098 (46) | 0.03 | 0.07 | 0.10 |
| Hypertension | 378 959 (93) | 132 865 (94) | 24 095 (91) | 0.03 | 0.07 | 0.10 |
| Liver disease | 23 614 (6) | 8441 (6) | 1395 (5) | 0.01 | 0.02 | 0.03 |
| Malignant tumor | 108 562 (27) | 37 634 (27) | 6982 (26) | 0.00 | 0.01 | 0.00 |
| Obesity | 98 237 (24) | 34 788 (25) | 5815 (22) | 0.01 | 0.05 | 0.06 |
| Prior hypoglycemia | 4140 (1) | 1311 (1) | 386 (1) | 0.01 | 0.04 | 0.05 |
| Smoking | 78 957 (19) | 27 926 (20) | 4655 (18) | 0.01 | 0.05 | 0.05 |
| Transient ischemic attack | 19 081 (5) | 6726 (5) | 1224 (5) | 0.00 | 0.00 | 0.01 |
| Diabetes systemic complications | ||||||
| Diabetic ketoacidosis | 6304 (2) | 2152 (2) | 419 (2) | 0.00 | 0.00 | 0.01 |
| Nephropathy | 158 742 (39) | 55 230 (39) | 9856 (37) | 0.00 | 0.03 | 0.03 |
| Neuropathy | 129 831 (32) | 45 635 (32) | 8511 (32) | 0.01 | 0.01 | 0.00 |
| Peripheral arterial disease | 125 712 (31) | 43 651 (31) | 8294 (31) | 0.00 | 0.01 | 0.01 |
| Prior amputation | 4902 (1) | 1647 (1) | 421 (2) | 0.00 | 0.03 | 0.04 |
| Retinopathy | 86 712 (21) | 29 922 (21) | 6255 (24) | 0.00 | 0.06 | 0.06 |
| Adapted Diabetes Complications Severity Index score (categorical) | ||||||
| 0 | 66 792 (16) | 23 622 (17) | 4460 (17) | 0.01 | 0.01 | 0.01 |
| 1-2 | 124 402 (31) | 43 782 (31) | 7926 (30) | 0.01 | 0.01 | 0.02 |
| 3-4 | 110 884 (27) | 38 132 (27) | 7101 (27) | 0.01 | 0.01 | 0.00 |
| ≥5 | 104 940 (26) |
36 052 (25) | 6915 (26) | 0.01 | 0.01 | 0.02 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; DPP-4, dipeptidyl peptidase-4 inhibitors; ED, emergency department; ESRD, end-stage renal disease; GLP-1, glucagon-like peptide 1; IP, inpatient; NA, not applicable; OP, outpatient.
The primary care specialty included 3 specialties (internal medicine, family practice, and general practice), whereas the other specialty included all other prescribing specialties, except for endocrinology.
A total of 7347 ED visits or hospitalizations for hypoglycemia occurred during the study period: 5194 for glargine, 1693 for detemir, and 460 for NPH insulin, with a median follow-up across the 3 cohorts of 0.37 years (interquartile range [IQR], 0.20-0.76 years). The main reason for censoring in each exposure cohort was treatment discontinuation (responsible for the censoring of approximately 25% in each cohort within 3 months and approximately 60% within a year [eTable 3 in the Supplement]). For the glargine and NPH insulin comparison, the weighted incidence rates for ED visits or hospitalizations for hypoglycemia per 1000 person-years were 17.37 (95% CI, 16.89-17.84) for glargine and 26.64 (95% CI, 26.01-27.3) for NPH insulin. For the detemir and NPH insulin comparison, the incidence rates were 16.69 (95% CI, 15.92-17.51) for detemir and 25.04 (95% CI, 24.01-26.11) for NPH insulin (Table 2).
Table 2. Incidence Rates and Hazard Ratios for ED Visits or Hospitalizations for Hypoglycemia Among New Users of Glargine and Detemir Compared With Neutral Protamine Hagedorn (NPH) Insulin.
| Agent | No. of patients | Follow-up time, median (IQR), y | Total No. of person-years | No. of events | Unweighted | Weighted incidence rates per 1000 person-years | Adjusted hazard ratio (95% CI)a | |
|---|---|---|---|---|---|---|---|---|
| Incidence rates per 1000 person-years | Hazard ratio (95% CI) | |||||||
| Glargine vs NPH insulin | ||||||||
| Glargine | 407 018 | 0.37 (0.20-0.73) | 299 098 | 5194 | 17.37 (16.89-17.84) | 0.61 (0.55-0.67) | 17.37 (16.89-17.84) | 0.71 (0.63-0.80) |
| NPH insulin | 26 402 | 0.27 (0.2-0.55) | 14 994 | 460 | 30.68 (27.88-33.48) | 1 [Reference] | 26.64 (26.01-27.30) | 1 [Reference] |
| Detemir vs NPH insulin | ||||||||
| Detemir | 141 588 | 0.37 (0.20-0.76) | 101 426 | 1693 | 16.69 (15.92-17.51) | 0.58 (0.52-0.64) | 16.69 (15.92-17.51) | 0.72 (0.63-0.82) |
| NPH insulin | 26 402 | 0.27 (0.20-0.55) | 14 994 | 460 | 30.68 (27.88-33.48) | 1 [Reference] | 25.04 (24.01-26.11) | 1 [Reference] |
Abbreviations: ED, emergency department; IQR, interquartile range.
Adjusted for inverse probability of treatment weights and time-varying concomitant use of noninsulin antidiabetic drugs, including sulfonylureas, metformin, incretins (dipeptidyl peptidase 4 inhibitors and glucagon-like peptide 1 agonists), and other antidiabetic drugs (thiazolidinediones and others).
Compared with patients who initiated NPH insulin, those taking glargine and detemir had a reduced risk of ED visits or hospitalizations for hypoglycemia; the adjusted HRs were 0.71 (95% CI, 0.63-0.80; P < .001) for glargine and 0.72 (95% CI, 0.63-0.82; P < .001) for detemir (Table 2; eFigures 3 and 4 in the Supplement).
In the analysis that included concomitant prandial insulin as a time-varying covariate, the HR for hypoglycemia comparing glargine vs NPH insulin was 0.99 (95% CI, 0.90-1.09; P = .85) during time taking concomitant prandial insulin and 0.78 (95% CI, 0.67-0.87; P < .001) during time not taking concomitant prandial insulin (P = .001 for the interaction between study drug and concomitant prandial insulin use). For detemir vs NPH insulin, the corresponding risk estimates were 0.96 (95% CI, 0.86-1.08; P = .53) during time taking concomitant prandial insulin and 0.78 (95% CI, 0.68-0.89; P < .001) during time not taking concomitant prandial insulin (P = .02 for the interaction between study drug and concomitant prandial insulin use) (Table 3). These findings indicate that the association between each long-acting insulin analog (vs NPH insulin) and severe hypoglycemia during periods of receiving and not receiving treatment with prandial insulin was significantly different (effect modification).
Table 3. Hazard Ratios for ED Visits or Hospitalizations for Hypoglycemia Among New Users of Glargine and Detemir Compared With Neutral Protamine Hagedorn (NPH) Insulin According to Prandial Use During Follow-up.
| Concomitant prandial insulin use during follow-up | Hazard ratio (95% CI) |
|---|---|
| Insulin glargine vs NPH insulin (expanded cohort) | |
| Time using prandial insulin | |
| Glargine | 0.99 (0.90-1.09) |
| NPH insulin | 1 [Reference] |
| Time not using prandial insulin | |
| Glargine | 0.78 (0.69-0.87) |
| NPH insulin | 1 [Reference] |
| P value for interaction (glargine × time using prandial insulin during follow-up) | .001 |
| Insulin detemir vs NPH insulin (expanded cohort) | |
| Time using prandial insulin | |
| Detemir | 0.96 (0.86-1.08) |
| NPH insulin | 1 [Reference] |
| Time not using prandial insulin | |
| Detemir | 0.78 (0.68-0.89) |
| NPH insulin | 1 [Reference] |
| P value for interaction (detemir × time using prandial insulin during follow-up) | .02 |
Abbreviation: ED, emergency department.
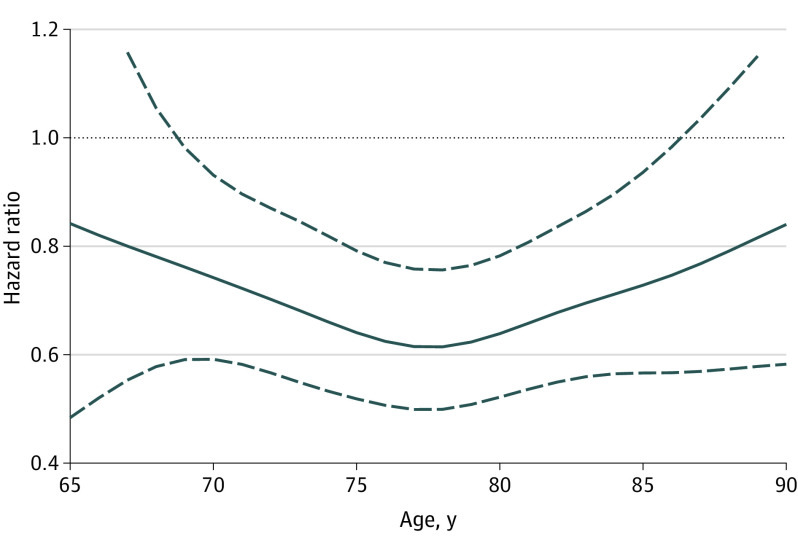
There was also evidence that the association of hypoglycemia with glargine and detemir may vary by age. In a post hoc analysis, exploring effect modification in a continuous age scale, results from penalized spline models illustrated in Figure 1 and Figure 2 suggested that the observed protective association of long-acting analogs compared with NPH insulin was stronger at 69 to 87 years of age than at other ages.
Figure 1. Hazard Ratios of Hypoglycemia Risk by Age for Glargine vs Neutral Protamine Hagedorn (NPH) Insulin Users.
The hazard ratios are indicated by the solid line and the corresponding upper, and lower bounds of the 95% CIs are indicated by the dashed lines.
Figure 2. Hazard Ratios of Hypoglycemia Risk by Age for Detemir vs Neutral Protamine Hagedorn (NPH) Insulin Users.
The hazard ratios are indicated by the solid line, and the corresponding upper and lower bounds of the 95% CIs are indicated by the dashed lines.
The analysis allowing for recurrent hypoglycemia events found similar results for glargine (HR, 0.70; 95% CI, 0.63-0.79; P < .001) and detemir (HR, 0.74; 95% CI, 0.65-0.84; P < .001). In addition, this analysis identified that the risk of recurrent ED visits or hospitalizations for hypoglycemia across all comparisons increased with the number of prior events (eTable 5 and eFigure 4 in the Supplement). Prespecified sensitivity analyses had similar patterns and trends of associations as those for the primary analysis (eFigure 4 and eTable 6 in the Supplement).
In a post hoc subgroup analysis by low-income subsidy, a proxy for low-income status, the HR for the glargine vs NPH insulin comparison was 0.67 (95% CI, 0.56-0.80) for beneficiaries who received the subsidy, whereas in those who did not receive the subsidy it was 0.73 (95% CI, 0.62-0.85), and the corresponding estimates for the detemir vs NPH insulin comparison were 0.76 (95% CI, 0.60-0.95) for beneficiaries who received the subsidy vs 0.71 (95% CI, 0.60-0.85) for those who did not (P > .40 for interaction) (eTable 7 in the Supplement). Including year of initiation of insulin use in the model did not substantially change the estimates from the primary analysis (glargine vs NPH insulin: HR, 0.73; 95% CI, 0.65-0.83; detemir vs NPH insulin: HR, 0.77; 95% CI, 0.67-0.90).
Discussion
In this new-user, active comparator cohort study, which included more than 575 000 insulin users, initiation of glargine and detemir use was associated with a nearly 30% lower risk of ED visits or hospitalizations for hypoglycemia compared with initiation of NPH insulin use, which translated to 9.3 fewer hypoglycemia events with glargine and 8.4 fewer with detemir per 1000 patient-years of treatment. In terms of number needed to harm,20 one would need to treat 154 patients with glargine or 167 with detemir rather than with NPH insulin for a year to prevent 1 excess case of severe hypoglycemia. However, this protective association was no longer seen when insulin analogs were used concomitantly with prandial insulin.
These results contrast with findings of a recent observational study3 examining the same question in KPNC. Lipska et al10 reported no difference in risk of ED visits or hospital admissions for hypoglycemia among long-acting analog users compared with NPH insulin users. The mean age of participants in that study10 was 60 years compared with 75 years in the current study, suggesting that age may have contributed to the disparity in study results. In post hoc analyses, we observed potential effect modification by age. It appeared that in those 65 to 68 years of age, the use of glargine or detemir compared with NPH insulin may not be associated with ED visits or hospitalizations for hypoglycemia. These findings aligned more closely with those from the study by Lipska et al,10 whereas in middle to old age (69-87 years of age), use of long-acting analogs was associated with a reduced risk of hypoglycemia compared with NPH insulin. Differences in the populations between the 2 studies included age, race/ethnicity, and type of insurance. The KPNC population was substantially younger, likely had health insurance based on employment, and had a lower proportion of White people than the Medicare population in the current study. It is possible that adherence to insulin therapy may be better in younger, actively employed individuals, which might explain the longer follow-up in the study by Lipska et al.10 In addition, the practice of medicine within KPNC, with system-wide, evidence-based practice guidelines and physician performance monitoring, may contribute to improved patient adherence compared with the more heterogeneous guidelines and monitoring seen in the fee-for-service environment. However, despite a shorter median follow-up, we still had large cohorts of glargine and detemir users with follow-up beyond 1 year, indicating that our estimates of hypoglycemia risk during longer periods are still well powered. Differences in race/ethnicity between KPNC and Medicare reflect the race/ethnicity distribution in Northern California compared with the racial/ethnic distribution of older people in the entire US.
A study by Luo et al21 compared severe hypoglycemia rates before and after a managed care organization’s intervention encouraging Medicare beneficiaries (mean age, 72.5 years) with type 2 diabetes to switch from a long-acting analog to human insulin. They found that switching was not associated with changes in rates of serious hypoglycemia. However, as with the study by Lipska et al,10 the findings from a managed care organization often cannot be generalized to other health care settings with less intensive practitioner support for chronic disease management. Indeed, the intervention in that study10 included support from pharmacists, nurse practitioners, physician assistants, and physicians with experience in chronic disease management, which may have included counseling about hypoglycemia not offered to participants in the current study.
A Cochrane review of RCTs3 to assess the effects of long-term treatment with long-acting insulin analogs compared with NPH insulin in patients with type 2 diabetes also reported no difference in severe hypoglycemia among users. The summary odds ratios from the meta-analysis were 0.70 (95% CI, 0.40-1.23) for glargine vs NPH insulin based on 4 RCTs4,5,8,9 and 0.50 (95% CI, 0.18-1.38) for detemir vs NPH insulin based on 2 RCTs.6,7 However, the meta-analytic summary estimates and the individual risk estimates from the RCTs trended in the same direction as the overall estimate in the current study, toward a protective association for long-acting analogs, despite lacking statistical significance. Of note, the RCTs had small sample sizes (ranging from 476 to 756 study participants) and limited numbers of recorded outcome events, and the mean age of participants was also lower than in the current study.
Other studies11,22 have also found results similar to ours. Another meta-analysis of RCTs11 reported a lower risk of severe hypoglycemia across 7 studies among long-acting analog users compared with NPH insulin (glargine: HR, 0.80; 95% CI, 0.67 to 0.96; detemir: HR, 0.74; 95% CI, 0.58-0.96). However, a high level of heterogeneity among studies that reported hypoglycemia outcomes was noted, which may have been related to differences in patient characteristics or treatment strategies. A network meta-analysis22 of 41 studies reported a summary risk ratio for documented symptomatic hypoglycemia in glargine vs NPH insulin users of 0.65 (95% CI, 0.27-1.49).22
In real-world clinical practice, basal insulin and prandial insulin are frequently used together in the management of type 2 diabetes. In the current study, up to 29% of basal insulin users had concomitant use of prandial insulin during follow-up and were censored in the primary analysis. When time-varying use of concomitant prandial insulin was accounted for in exploratory analyses, the protective association conferred by use of long-acting analogs compared with NPH insulin seen in the primary analysis was not observed. This is an important finding and suggests that the advantages of long-acting analogs on hypoglycemia, observed in the main analysis, at least in certain age groups may not be seen if the patient initiates concomitant prandial insulin. The inclusion of prandial insulin in type 2 diabetes treatment regimens may increase complexity, especially for older users,23 thereby increasing the possibility of using too much insulin, which might increase the likelihood of hypoglycemia to such an extent that the benefits from long-acting analogs compared with NPH insulin are lost. Further studies are needed to investigate the potential influence of prandial insulin use. The study by Lipska et al10 did not include prandial insulin users in their analyses.
Strengths and Limitations
This study has several strengths. First, to our knowledge, it is the largest study to date to examine the association between long-acting insulin analog use and ED visits or hospitalizations for hypoglycemia, and it had substantially more long-acting insulin analog users compared with a recent study by Lipska et al10 (548 606 vs 1928 long-acting insulin analog users). The study by Lipska et al10 was conducted in an integrated delivery health care system setting. These settings tend to have formularies that restrict the use of long-acting analogs in patients with type 2 diabetes initiating use of insulin. This means that in the study by Lipska et al,10 those with the most severe diabetes may have been channeled to receive a prescription for a long-acting analog and may have had a higher risk of hypoglycemia. This selection bias may result in an NPH insulin user cohort that has a lower risk of hypoglycemia. The lack of such restrictions in the choice to initiate analog insulin vs NPH insulin in Medicare is a strength of the current study. A new-user design14 was applied to avoid missing potential early effects of drugs and to accurately ascertain baseline confounders. The current study was the first study to date, to our knowledge, that examined the association of long-acting insulin analog vs NPH insulin use on ED visits or hospitalizations for hypoglycemia by age and with concomitant use of prandial insulin. The current study used a propensity score method to balance observed confounders across exposure cohorts. Because NPH insulin use has declined over calendar time, bias may have been introduced by not accounting for the study year in the primary analysis; however, post hoc analyses, including year in the propensity score, found that the results did not change substantially.
The study has limitations. It was observational and therefore may be subject to potential residual confounding by factors not adjusted for in the analysis, such as unmeasured aspects of socioeconomic status, insulin dose, and glycated hemoglobin levels. Lower socioeconomic status could conceivably result in channeling to the cheapest insulin (NPH insulin) and be associated with severe hypoglycemia. However, post hoc subgroup analyses by low-income subsidy, a proxy measure for low-income status, found that the results of the primary analysis were not influenced by confounding related to socioeconomic status as measured by low-income subsidy. The study population was 65 years or older; therefore, the generalizability of findings to those outside that age range may be limited. The Medicare claims data used in this study are ideal for capturing drug exposure but may be limited in identifying hypoglycemia. To address this, the study used a modified validated algorithm15 that has been applied in a previous study10 and a sensitivity analysis was conducted using an unmodified version of the validated algorithm. Hypoglycemia events that did not result in an ED visit or hospitalization were not captured in this study, which may have underestimated the true incidence of hypoglycemia.24 Although information on diabetes duration or baseline glycated hemoglobin levels (an indicator of baseline glycemic control) was not available, the study estimated a modified diabetes severity index16 and included it in the propensity score model to account for any potential confounding by diabetes severity.
Conclusions
Initiation of long-acting insulin analog use was associated with a lower risk of ED visits or hospitalizations for hypoglycemia compared with NPH insulin in older patients with type 2 diabetes in Medicare. However, this protective association may vary by age and was not seen with use of concomitant prandial insulin.
eFigure 1. Cohort Creation Process
eFigure 2. Modified Hypoglycemia Outcome Definition Flowchart
eFigure 3. Kaplan-Meier Plots: Glargine vs NPH During the First Two Years of Follow-up (A) and Detemir vs NPH During the First Two Years of Follow-up (B)
eFigure 4. Forest Plot Displaying All Results for Each Comparison
eTable 1. National Drug Codes (NDCs) Used to Define Insulin Exposure for the Primary Analysis
eTable 2. ICD-9 and ICD-10 Codes Used to Define the Hypoglycemia Outcome, and Step of Algorithm They Correspond With
eTable 3. Summary of Censoring Reasons by Follow-up Time Among Insulin Users in the Primary Analysis (45-Day Gap Rule)
eTable 4. Baseline Demographic and Clinical Characteristics of Insulin Glargine, Insulin Detemir, and NPH Users After Inverse Probability of Treatment Weighting
eTable 5. Adjusted Hazard Ratios for Hypoglycemia-Related ED Visits and Hospitalizations Among Ever Users of Glargine and Detemir Compared to NPH Who Had Recurrent Events
eTable 6. Adjusted Hazard Ratios for Hypoglycemia-Related ED Visits and Hospitalizations Among Ever Users of Glargine and Detemir Compared to NPH According to Sensitivity Analyses
eTable 7. Primary Analysis by Low Income Subsidy Status
References
- 1.Feldman WB, Rome BN, Lehmann LS, Kesselheim AS. Estimation of Medicare Part D spending on insulin for patients with diabetes using negotiated prices and a defined formulary. JAMA Intern Med. 2020;180(4):597-601. doi: 10.1001/jamainternmed.2019.7018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herkert D, Vijayakumar P, Luo J, et al. Cost-related insulin underuse among patients with diabetes. JAMA Intern Med. 2019;179(1):112-114. doi: 10.1001/jamainternmed.2018.5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613. doi: 10.1002/14651858.CD005613.pub3 [DOI] [PubMed] [Google Scholar]
- 4.Eliaschewitz FG, Calvo C, Valbuena H, et al. ; HOE 901/4013 LA Study Group . Therapy in type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Arch Med Res. 2006;37(4):495-501. doi: 10.1016/j.arcmed.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 5.Fritsche A, Schweitzer MA, Häring HU, 4001 Study Group . Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2003;138(12):952-959. doi: 10.7326/0003-4819-138-12-200306170-00006 [DOI] [PubMed] [Google Scholar]
- 6.Haak T, Tiengo A, Draeger E, Suntum M, Waldhäusl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7(1):56-64. doi: 10.1111/j.1463-1326.2004.00373.x [DOI] [PubMed] [Google Scholar]
- 7.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naïve people with type 2 diabetes. Diabetes Care. 2006;29(6):1269-1274. doi: 10.2337/dc05-1365 [DOI] [PubMed] [Google Scholar]
- 8.Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators . The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080-3086. doi: 10.2337/diacare.26.11.3080 [DOI] [PubMed] [Google Scholar]
- 9.Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24(4):631-636. doi: 10.2337/diacare.24.4.631 [DOI] [PubMed] [Google Scholar]
- 10.Lipska KJ, Parker MM, Moffet HH, Huang ES, Karter AJ. Association of initiation of basal insulin analogs vs neutral protamine hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320(1):53-62. doi: 10.1001/jama.2018.7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ. 2009;180(4):385-397. doi: 10.1503/cmaj.081041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong AP, Yang X, Luk A, et al. Severe hypoglycemia identifies vulnerable patients with type 2 diabetes at risk for premature death and all-site cancer: the Hong Kong diabetes registry. Diabetes Care. 2014;37(4):1024-1031. doi: 10.2337/dc13-2507 [DOI] [PubMed] [Google Scholar]
- 13.Yun JS, Ko SH. Risk factors and adverse outcomes of severe hypoglycemia in type 2 diabetes mellitus. Diabetes Metab J. 2016;40(6):423-432. doi: 10.4093/dmj.2016.40.6.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 15.Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. doi: 10.1186/1472-6823-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18(11):721-726. [PubMed] [Google Scholar]
- 17.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44(1):324-333. doi: 10.1093/ije/dyu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roshani D, Ghaderi E. Comparing smoothing techniques for fitting the nonlinear effect of covariate in Cox models. Acta Inform Med. 2016;24(1):38-41. doi: 10.5455/aim.2016.24.38-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495. doi: 10.1136/bmj.319.7223.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, Khan NF, Manetti T, et al. Implementation of a health plan program for switching from analogue to human insulin and glycemic control among Medicare beneficiaries with type 2 diabetes. JAMA. 2019;321(4):374-384. doi: 10.1001/jama.2018.21364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freemantle N, Chou E, Frois C, et al. Safety and efficacy of insulin glargine 300 u/mL compared with other basal insulin therapies in patients with type 2 diabetes mellitus: a network meta-analysis. BMJ Open. 2016;6(2):e009421. doi: 10.1136/bmjopen-2015-009421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association . Older adults: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S152-S162. doi: 10.2337/dc20-S012 [DOI] [PubMed] [Google Scholar]
- 24.Karter AJ, Moffet HH, Liu JY, Lipska KJ. Surveillance of hypoglycemia-limitations of emergency department and hospital utilization data. JAMA Intern Med. 2018;178(7):987-988. doi: 10.1001/jamainternmed.2018.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Cohort Creation Process
eFigure 2. Modified Hypoglycemia Outcome Definition Flowchart
eFigure 3. Kaplan-Meier Plots: Glargine vs NPH During the First Two Years of Follow-up (A) and Detemir vs NPH During the First Two Years of Follow-up (B)
eFigure 4. Forest Plot Displaying All Results for Each Comparison
eTable 1. National Drug Codes (NDCs) Used to Define Insulin Exposure for the Primary Analysis
eTable 2. ICD-9 and ICD-10 Codes Used to Define the Hypoglycemia Outcome, and Step of Algorithm They Correspond With
eTable 3. Summary of Censoring Reasons by Follow-up Time Among Insulin Users in the Primary Analysis (45-Day Gap Rule)
eTable 4. Baseline Demographic and Clinical Characteristics of Insulin Glargine, Insulin Detemir, and NPH Users After Inverse Probability of Treatment Weighting
eTable 5. Adjusted Hazard Ratios for Hypoglycemia-Related ED Visits and Hospitalizations Among Ever Users of Glargine and Detemir Compared to NPH Who Had Recurrent Events
eTable 6. Adjusted Hazard Ratios for Hypoglycemia-Related ED Visits and Hospitalizations Among Ever Users of Glargine and Detemir Compared to NPH According to Sensitivity Analyses
eTable 7. Primary Analysis by Low Income Subsidy Status