This cohort study analyzes the neuroanatomic, functional, and other outcomes during the emergent, critical care, and rehabilitation phases after new-onset traumatic brain injury with disrupted consciousness.
Key Points
Question
What are the trajectory of and factors associated with recovery of consciousness in patients with a disorder of consciousness (DOC) after traumatic brain injury (TBI)?
Findings
In this cohort study of 17 470 patients with TBI, 57% of patients experienced initial loss of consciousness, which persisted after acute care treatment in 12% of patients. However, 98% of these patients recovered consciousness by the end of subsequent inpatient rehabilitation, and their trajectory of functional improvement mirrored that of patients with TBI who did not lose consciousness.
Meaning
Results of this study indicated that most individuals who became comatose after moderate or severe TBI recovered consciousness in the short term and almost half of them regained functional independence, suggesting that caution is warranted in early decisions to withdraw or withhold treatment in patients with TBI and a DOC.
Abstract
Importance
Traumatic brain injury (TBI) leads to 2.9 million visits to US emergency departments annually and frequently involves a disorder of consciousness (DOC). Early treatment, including withdrawal of life-sustaining therapies and rehabilitation, is often predicated on the assumed worse outcome of disrupted consciousness.
Objective
To quantify the loss of consciousness, factors associated with recovery, and return to functional independence in a 31-year sample of patients with moderate or severe brain trauma.
Design, Setting, and Participants
This cohort study analyzed patients with TBI who were enrolled in the Traumatic Brain Injury Model Systems National Database, a prospective, multiyear, longitudinal database. Patients were survivors of moderate or severe TBI who were discharged from acute hospitalization and admitted to inpatient rehabilitation from January 4, 1989, to June 19, 2019, at 1 of 23 inpatient rehabilitation centers that participated in the Traumatic Brain Injury Model Systems program. Follow-up for the study was through completion of inpatient rehabilitation.
Exposures
Traumatic brain injury.
Main Outcomes and Measures
Outcome measures were Glasgow Coma Scale in the emergency department, Disability Rating Scale, posttraumatic amnesia, and Functional Independence Measure. Patient-related data included demographic characteristics, injury cause, and brain computed tomography findings.
Results
The 17 470 patients with TBI analyzed in this study had a median (interquartile range [IQR]) age at injury of 39 (25-56) years and included 12 854 male individuals (74%). Of these patients, 7547 (57%) experienced initial loss of consciousness, which persisted to rehabilitation in 2058 patients (12%). Those with persisting DOC were younger; had more high-velocity injuries; had intracranial mass effect, intraventricular hemorrhage, and subcortical contusion; and had longer acute care than patients without DOC. Eighty-two percent (n = 1674) of comatose patients recovered consciousness during inpatient rehabilitation. In a multivariable analysis, the factors associated with consciousness recovery were absence of intraventricular hemorrhage (adjusted odds ratio [OR], 0.678; 95% CI, 0.532-0.863; P = .002) and intracranial mass effect (adjusted OR, 0.759; 95% CI, 0.595-0.968; P = .03). Functional improvement (change in total functional independence score from admission to discharge) was +43 for patients with DOC and +37 for those without DOC (P = .002), and 803 of 2013 patients with DOC (40%) became partially or fully independent. Younger age, male sex, and absence of intraventricular hemorrhage, intracranial mass effect, and subcortical contusion were associated with better functional outcome. Findings were consistent across the 3 decades of the database.
Conclusions and Relevance
This study found that DOC occurred initially in most patients with TBI and persisted in some patients after rehabilitation, but most patients with persisting DOC recovered consciousness during rehabilitation. This recovery trajectory may inform acute and rehabilitation treatment decisions and suggests caution is warranted in consideration of withdrawing or withholding care in patients with TBI and DOC.
Introduction
Protracted loss of consciousness is an ominous sign in patients who have experienced acute traumatic brain injury (TBI) and, if severe or persisting over days, may portend worse outcome, including disability or death. In the US each year, approximately 2.9 million individuals present to emergency departments (EDs) with head trauma and 288 000 are hospitalized, and these rates are increasing.1 Disorder of consciousness (DOC) in TBI begins with coma and, if continuing, may evolve into the vegetative and/or minimally conscious states.2,3 The frequency of persisting DOC in TBI has been estimated in smaller settings4,5 but has not been well quantified in the broader TBI population.
Mortality in moderate and severe TBI is 12% to 44% during acute inpatient care.6,7,8,9 In addition, 45% to 87% of this mortality is associated with withdrawal of life-sustaining therapy.6,7,10,11 Persisting disturbance in consciousness that occurs acutely after TBI is associated with greater short- and longer-term cognitive and functional deficits.12,13,14 When DOC continues in severe brain trauma, early life-sustaining therapy is often withdrawn or withheld, and specialized inpatient rehabilitation is not pursued after completion of acute care.7,8,15,16,17 Thus, it is difficult to ascertain the extent to which current outcomes reflect the natural course in TBI or a self-fulfilling prophesy of assumed poor survival and recovery for these patients.
Previous reports have evaluated outcomes for patients with a protracted DOC in small samples and at 1 to 10 years after TBI.18,19,20,21,22 We sought to quantify the loss of consciousness, factors associated with recovery, and functional ability, including return to functional independence, in a large cohort of patients with TBI during the acute stage of emergent and critical care and in subsequent inpatient rehabilitation. This time frame encompasses the treatment phase in which early decisions are made on withdrawal of life-sustaining therapy, aggressiveness of interventions, and pursuit of follow-up rehabilitation. To address this aim, we evaluated survivors of moderate and severe brain trauma with a DOC in a geographically diverse sample of patients with moderate or severe TBI who were admitted for specialized hospital rehabilitation over a 31-year period.
Methods
This cohort study was a retrospective analysis of patients with acute, moderate, or severe TBI who were enrolled in the Traumatic Brain Injury Model Systems (TBIMS) National Database, a longitudinal, prospective multicenter database.23 The sample included patients who were discharged from acute hospitalization and admitted to inpatient rehabilitation from January 4, 1989, to June 19, 2019, at 1 of 23 US inpatient rehabilitation centers that participated in the TBIMS program (The Virginia Commonwealth TBI Model System, Richmond, Virginia; The Institute for Rehabilitation and Research [Memorial Hermann], Houston, Texas; Southeastern Michigan Traumatic Brain Injury System, Detroit, Michigan; Northern California TBI Model System, San Jose, California; The Ohio Regional TBI Model System, Columbus, Ohio; Moss TBI Model System, Elkins Park, Pennsylvania; University of Alabama at Birmingham TBI Care System, Birmingham, Alabama; Rocky Mountain Regional Brain Injury System, Englewood, Colorado; Georgia Model Brain Injury System, Atlanta, Georgia; Spaulding-Harvard TBI Model System, Boston, Massachusetts; Mayo Clinic TBI Model System, Rochester, Minnesota; TBI Model System of Mississippi, Jackson, Mississippi; Northern New Jersey TBI System, West Orange, New Jersey; Carolinas TBI Rehabilitation and Research System, Charlotte, North Carolina; University of Washington TBI Model System, Seattle, Washington; JFK-Johnson Rehabilitation Institute TBI Model System, Edison, New Jersey; University of Pittsburgh Medical Center TBI Model System, Pittsburgh, Pennsylvania; North Texas TBI Model System, Dallas, Texas; New York TBI Model System [Mount Sinai], New York, New York; Midwest Regional TBI Model System, Chicago, Illinois; Rusk Rehabilitation TBIMS at NY, New York, New York; Indiana University Rehabilitation Hospital of Indiana, Indianapolis, Indiana; and South Florida TBI Model System, Miami, Florida). The study was approved by the institutional review board at each participating center. All patients or their surrogates provided written informed consent to be included in the database. No separate consent was obtained for the present study.
Study Population and TBIMS Procedures
Patients were survivors of new-onset moderate or severe TBI. For the TBIMS database, TBI was defined as brain tissue damage from an external mechanical force. Inclusion criteria were age of 16 years or older at injury onset; presentation to a TBIMS-designated acute care hospital within 72 hours of injury; and at least 1 of the following: loss of consciousness for more than 30 minutes, a Glasgow Coma Scale24 (GCS) total score lower than 13 on initial presentation to a health care facility, posttraumatic amnesia (PTA) for more than 24 hours, or trauma-related intracranial abnormalities identified on neuroimaging. Selection of study participants followed the TBIMS database procedures.25
Demographic and TBI characteristics were recorded for all patients in the TBIMS database. Acute care hospital and rehabilitation treatment variables included intracranial pressure monitoring, surgical procedures such as craniotomy and craniectomy, and lengths of stay at acute and rehabilitation hospitals. For these patients, outcome was assessed during acute care and at the time of inpatient rehabilitation admission and discharge. Outcome measures were GCS in the ED, Disability Rating Scale,26 PTA, and Functional Independence Measure (FIM).27 For the TBIMS program, study investigators are trained to perform all assessments, and data are collected per quality control guidelines maintained by the TBIMS National Data and Statistical Center.28
Consciousness and Functional Status Assessment
Consciousness was assessed by the GCS motor score (score range: 1-6, with the highest score indicating obeying commands) at acute care hospital presentation and by the Disability Rating Scale motor component (score range: 0-5, with the lowest score indicating obeying commands) at inpatient rehabilitation admission and discharge. For the purposes of this study, DOC was defined as not following commands or having an ED GCS motor score lower than 6 or a Disability Rating Scale motor score greater than 0. Orientation was evaluated by assessing PTA. For the acute care course, PTA was abstracted from the hospitalization medical record. During inpatient rehabilitation, PTA was assessed by trained investigators through the prospective administration of the Galveston Orientation and Amnesia Test29 or the Orientation Log.30 Posttraumatic amnesia as an outcome measure was recorded as a dichotomous variable (emerged vs no emergence).
Functional status was measured with FIM total, motor, and cognitive scores. For the FIM total measure, the range of scores is 13 to 126, with the lowest indicating complete dependence and the highest indicating complete independence. The FIM was assessed by trained raters at the time of inpatient rehabilitation admission and discharge.
Radiologic Neuroanatomic Evaluation
Neuroanatomic data for study patients were derived from radiologic reports of brain computed tomography (CT) obtained clinically during acute care. The most abnormal CT findings in the initial 7 days after injury onset were recorded in the TBIMS database. Computed tomography findings were categorized according to the modified Marshall Scale criteria31 used in the TBIMS database method.23 For evidence of intracranial mass effect, the TBIMS database records presence of shift and shift larger than 5 mm of midline cerebral hemispheric structures and compression of basal cisterns but no measurement of lesion volume. Computed tomography evidence of intraventricular hemorrhage, subarachnoid hemorrhage, epidural and subdural fluid collections, and cortical and subcortical injury was recorded.
To evaluate possible rehabilitation referral bias, we performed a systematic comparison of the TBIMS database sample to patients with moderate or severe TBI included in published studies that used 3 other large databases.32,33,34,35 This comparison included patient and clinical characteristics and outcome measures.
Statistical Analysis
For the main analyses, patients were dichotomized into those with DOC vs without DOC at acute care initial presentation and at inpatient rehabilitation admission and discharge. Injury type was categorized as high velocity (eg, motor vehicle, motorcycle, or bicycle crashes) or lower velocity (eg, falls, pedestrian injuries). Clinical characteristics and outcome measures were treated as continuous or categorical variables, as appropriate, and were dichotomized or categorized into logical or clinically relevant groupings. Functional status was treated as a continuous FIM score, and change in functional status was calculated as the difference in FIM total scores between rehabilitation admission and discharge. Functional status at discharge was further categorized for level of independence based on FIM total scores using previously designated caregiver burden cutoffs: dependent (18-79), semidependent (80-99), and fully independent (100-126).36 Key portions of the analysis were repeated in a subset of patients, which included only those with an obtainable ED GCS total score of 12 or lower (a traditional GCS cutoff for moderate or severe TBI).
Dichotomous variables were analyzed with the Pearson χ2 test or Fisher exact test, where appropriate. Continuous variables were analyzed with the independent samples t test (unpaired, 2-tailed) when normally distributed. For ordinal variables and those not normally distributed, nonparametric tests (Mann-Whitney test and Kruskal-Wallis test) were used. Independent associations with DOC, orientation, and functional status were identified with backward stepwise logistic regression for dichotomous outcome variables and with multivariable linear regression for continuous outcome variables. Factors with clinical relevance found to be associated with DOC, orientation, and functional status in univariate analyses were incorporated in multivariable models.
Statistical significance was set at a 2-sided P < .05. Data were analyzed with SPSS, version 26.0 (IBM Corporation).
Results
A total of 17 470 patients with TBI were included in the study. These patients had a median (interquartile range [IQR]) age at injury of 39 (25-56) years, 12 854 were male (74%), and 11 565 were White (66%) individuals. Cause of TBI was motor vehicle collision or other high-velocity impact for 8651 patients (50%) and falls or other lower-velocity incidents for 4866 patients (28%). At the time of acute hospital presentation, the GCS score was available for 13 458 patients in the ED, of whom 7624 (57%) had a DOC. Neuroanatomic injuries included subarachnoid hemorrhage or subdural fluid collection (13 128 [75%]), cortical contusion (10 857 [62%]), and subcortical white matter damage (3265 [19%]), and 6731 patients (39%) had intracranial mass effect with midline shift or compression of basal cisterns. Median (IQR) duration of acute hospital care for all patients was 16 (9-26) days (Table 1).
Table 1. Baseline Patient and Clinical Characteristics at Time of Injury.
| Characteristic | No. (%) |
|---|---|
| Patient characteristics | |
| All patients | 17 470a |
| Demographics | |
| Age at injury onset, median (IQR), yb | 39 (25-56) |
| Male sex | 12 854 (74) |
| Race/ethnicity | |
| White | 11 565 (66) |
| Black | 3208 (18) |
| Asian/Pacific Islander | 472 (3) |
| Native American | 92 (1) |
| Hispanic | 1903 (11) |
| Otherc | 212 (1) |
| Acute clinical characteristics | |
| ED GCS motor scored | |
| 6: Follows commands | 5755 (33) |
| 5: Localizes | 2406 (14) |
| 4: Withdraws | 1664 (10) |
| 3: Flexion | 533 (3) |
| 2: Extension | 393 (2) |
| 1: No response | 2551 (15) |
| Sedated, paralyzed | 3796 (22) |
| Unknown/missing data | 337 (2) |
| ED GCS motor score <6 | 7547 (57) |
| TBI cause | |
| MVC or other high velocity | 8651 (50) |
| Fall or other lower velocity | 4866 (28) |
| Acute hospital course characteristics | |
| Duration of acute care, median (IQR), d | 16 (9-26) |
| Craniotomy or craniectomy | 3617 (21) |
| Radiologic characteristics | |
| Intracranial mass effect | |
| Compression or midline shift | 6731 (39) |
| Midline shift >5 mm | 2284 (13) |
| IPH or IVH | |
| Punctate/petechial hemorrhage | 3684 (21) |
| IVH | 4185 (24) |
| Extra-axial fluid collection | |
| SAH or subdural | 13 128 (75) |
| Epidural | 1810 (10) |
| Cortical contusion | 10 857 (62) |
| Subcortical contusion | 3265 (19) |
Abbreviations: ED, emergency department; GCS, Glasgow Coma Scale; IPH, intraparenchymal hemorrhage; IQR, interquartile range; IVH, intraventricular hemorrhage; MVC, motor vehicle collision; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury.
Total excluded 213 patients with no Disability Rating Scale motor score at inpatient admission.
Age ≥89 years was set at 89 (n = 157).
Other is among the race/ethnicity categories included in the Traumatic Brain Injury Model Systems database.
ED GCS scores were available for 13 458 patients (76%).
Outcomes
A total of 7547 patients (57%) experienced initial loss of consciousness. Of the patients subsequently admitted for inpatient rehabilitation, 2058 (12%) had a DOC after completion of acute care treatment. Patients with DOC vs 15 412 without DOC at this stage were younger (median [IQR] age at injury onset, 35 [23-53] years vs 40 [25-57] years; P < .001) and were more likely to have a high-velocity TBI (55% [n = 1124] vs 49% [n = 7527]; odds ratio [OR], 1.266 [95% CI, 1.154-1.389]; P < .001), intracranial mass effect (52% [n = 984] vs 39% [n = 5747]; OR, 1.690 [95% CI, 1.535-1.861]; P < .001), intraventricular hemorrhage (40% [n = 770] vs 23% [n = 3415]; OR, 2.245 [95% CI, 2.033-2.479]; P < .001), subcortical contusion (32% [n = 603] vs 18% [n = 2662]; OR, 2.100 [95% CI, 1.890-2.333]; P < .001), and longer acute care (median [IQR] duration, 25 [17-36] days vs 15 [9-25] days; P < .001) (Table 2).
Table 2. Association of Patient and Clinical Characteristics With Consciousness Level During Inpatient Rehabilitation.
| Characteristics | Rehabilitation admission | Rehabilitation discharge | ||||||
|---|---|---|---|---|---|---|---|---|
| Consciousness level, No. (%)a | Consciousness level, No. (%)a | |||||||
| With DOC (n = 2058)b | Without DOC (n = 15 412)c | P value | OR (95% CI) | With DOC (n = 414)b,d | Without DOC (n = 16 994)c,e | P value | OR (95% CI) | |
| Patient characteristics | ||||||||
| Age at injury onset, median (IQR), yf | 35 (23-53) | 40 (25-57) | <.001 | NA | 35 (22-58) | 39 (25-56) | .06 | NA |
| Male sex | 1555/2053 (76) | 11 299/15 408 (73) | .02 | 1.136 (1.020-1.264) | 305/414 (74) | 12 501/16 991 (74) | .97 | 1.005 (0.805-1.254) |
| Race/ethnicity | ||||||||
| White | 1393/2057 (68) | 10 172/15 395 (66) | .69 | NA | 266/412 (65) | 11 260/16 978 (66) | .07 | NA |
| Black | 358/2057 (17) | 2850/15 395 (18) | NA | 66/412 (16) | 3127/16 978 (18) | NA | ||
| Asian/Pacific Islander | 51/2057 (2) | 421/15 395 (3) | NA | 20/412 (5) | 452/16 978 (3) | NA | ||
| Native American | 9/2057 (1) | 83/15 395 (1) | NA | 2/412 (1) | 89/16 978 (1) | NA | ||
| Hispanic | 219/2057 (11) | 1684/15 395 (11) | NA | 51/412 (12) | 1846/16 978 (11) | NA | ||
| Otherg | 27/2057 (1) | 185/15 395 (1) | NA | 7/412 (2) | 204/16 978 (1) | NA | ||
| Acute clinical characteristics | ||||||||
| ED GCS motor score, No./total No. (%)h | ||||||||
| 6: Follows commands | 249/1454 (17) | 5506/11 848 (46) | <.001 | NA | 53/311 (17) | 5686/12 946 (44) | <.001 | NA |
| 5: Localizes | 247/1454 (17) | 2159/11 848 (18) | NA | 43/311 (14) | 2354/12 946 (18) | NA | ||
| 4: Withdraws | 240/1454 (17) | 1424/11 848 (12) | NA | 39/311 (12) | 1622/12 946 (13) | NA | ||
| 3: Flexion | 100/1454 (7) | 433/11 848 (4) | NA | 19/311 (6) | 512/12 946 (4) | NA | ||
| 2: Extension | 109/1454 (7) | 284/11 848 (2) | NA | 26/311 (8) | 362/12 946 (3) | NA | ||
| 1: No response | 509/1454 (35) | 2042/11 848 (17) | NA | 131/311 (42) | 2410/12 946 (19) | NA | ||
| Sedated, paralyzed | 530/1984 (27) | 3266/15 114 (22) | NA | 92/403 (23) | 3699/16 645 (22) | NA | ||
| Unknown/missing data | 74/2058 (4) | 298/15 412 (2) | NA | 11/414 (3) | 349/16 994 (2) | NA | ||
| ED GCS motor score <6 | 1205/1454 (83) | 6342/11 848 (54) | <.001 | 4.201 (3.648-4.838) | 258/311 (83) | 7260/12 946 (56) | <.001 | 3.813 (2.831-5.134) |
| TBI cause | ||||||||
| MVC or other high velocity | 1124/2050 (55) | 7527/15 379 (49) | <.001 | 1.266 (1.154-1.389) | 207/413 (50) | 8422/16 961 (50) | .85 | 1.019 (0.838-1.238) |
| Fall or other lower velocity | 487/2052 (24) | 4379/15 404 (28) | <.001 | 0.783 (0.704-0.872) | 102/413 (25) | 4748/16 988 (28) | .15 | 0.845 (0.674-1.060) |
| Acute hospital course characteristics | ||||||||
| Duration of acute care, median (IQR), d | 25 (17-36) | 15 (9-25) | <.001 | NA | 33 (19-47) | 16 (9-26) | <.001 | NA |
| Craniotomy or craniectomy, No./total No. (%) | 559/1577 (35) | 3058/12 299 (25) | <.001 | 1.659 (1.485-1.854) | 132/325 (41) | 3470/13 520 (26) | <.001 | 1.981 (1.582-2.480) |
| Radiologic characteristics, No./total No. (%) | ||||||||
| Intracranial mass effect | ||||||||
| Compression or midline shift | 984/1884 (52) | 5747/14 630 (39) | <.001 | 1.690 (1.535-1.861) | 222/380 (58) | 6488/16 088 (40) | <.001 | 2.079 (1.691-2556) |
| Midline shift >5 mm | 362/1884 (19) | 1922/14 630 (13) | <.001 | 1.572 (1.389-1.781) | 90/380 (24) | 2191/16 088 (14) | <.001 | 1.968 (1.547-2.504) |
| IPH or IVH | ||||||||
| Punctate/petechial hemorrhage | 557/1903 (29) | 3127/14 693 (21) | <.001 | 1.531 (1.376-1.702) | 102/384 (27) | 3565/16 166 (22) | .04 | 1.278 (1.016-1.608) |
| IVH | 770/1903 (40) | 3415 (23) | <.001 | 2.245 (2.033-2.479) | 172 (45) | 4002 (25) | <.001 | 2.467 (2.011-3.026) |
| Extra-axial fluid collection | ||||||||
| SAH or subdural | 1630/1904 (86) | 11 498/14 706 (78) | <.001 | 1.660 (1.452-1.897) | 336/384 (87) | 12 758/16 180 (79) | <.001 | 1.878 (1.384-2.547) |
| Epidural | 220/1900 (12) | 1590/14 701 (11) | .32 | 1.080 (0.930-1.254) | 50/384 (13) | 1757/16 171 (11) | .18 | 1.228 (0.909-1.660) |
| Cortical contusion | 1448/1903 (76) | 9409/14 700 (64) | <.001 | 1.790 (1.602-1.999) | 292/384 (76) | 10 529/16 173 (65) | <.001 | 1.701 (1.343-2.155) |
| Subcortical contusion | 603/1901 (32) | 2662/14 695 (18) | <.001 | 2.100 (1.890-2.333) | 131/384 (34) | 3118/16 166 (19) | <.001 | 2.167 (1.748-2.685) |
Abbreviations: DOC, disorder of consciousness; DRS, Disability Rating Scale; ED, emergency department; GCS, Glasgow Coma Scale; IPH, intraparenchymal hemorrhage; IVH, intraventricular hemorrhage; MVC, motor vehicle collision; NA, not applicable; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury.
Total excluded 213 patients with no DRS motor score at rehabilitation admission.
With DOC defined as not following commands, or a DRS motor score >0.
DRS motor score of 0.
Included 51 patients (12%) who did not follow commands at rehabilitation admission.
Included 1674 patients (10%) who did not follow commands at rehabilitation admission.
Age ≥89 years was set at 89 (n = 157).
Other is among the race/ethnicity categories included in the Traumatic Brain Injury Model Systems database.
ED GCS scores were available for 13 458 patients (76%).
By completion of inpatient rehabilitation, 414 of 17 470 patients (2%) had a DOC. Patients with DOC were more likely than those without DOC to have had a DOC on initial presentation after injury (83% [258 of 311] vs 56% [7260 of 12 946]; OR, 3.813 [95% CI, 2.831-5.134]; P < .001) and had longer acute care (median 33 days vs 16 days; P < .001) and longer rehabilitation (median [IQR] duration, 37 [22-65] days vs 19 [12-30] days; P < .001). Neuroanatomically, patients with DOC at the end of rehabilitation were more likely than those without DOC to have intracranial mass effect (58% [n = 222] vs 40% [n = 6488]; OR, 2.079 [95% CI, 1.691-2.556]; P < .001), intraventricular hemorrhage (45% [n = 172] vs 25% [n = 4002]; OR, 2.467 [95% CI, 2.011-3.026]; P < .001), subcortical contusion (34% [n = 131] vs 19% [n = 3118]; OR, 2.167 [95% CI, 1.748- 2.685]; P < .001), or cortical contusion (76% [n = 292] vs 65% [n = 10 529]; OR, 1.701 [95% CI, 1.343-2.155]; P < .001) (Table 2). Fourteen patients died during rehabilitation, of whom 1 (7%) had a DOC at rehabilitation admission.
Trajectory of Recovery of Consciousness
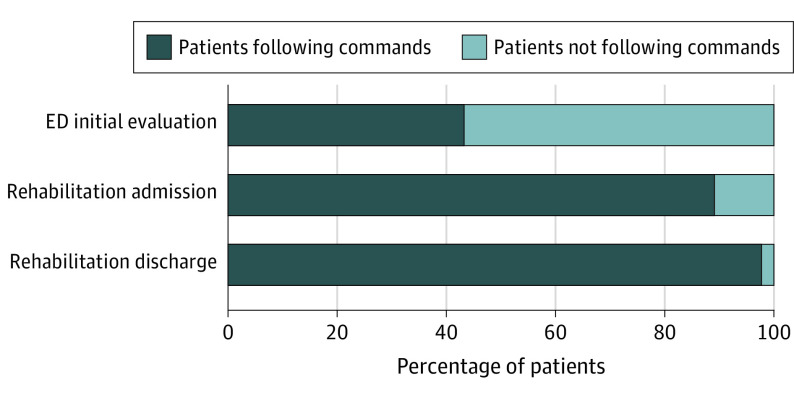
Of all patients, 13 458 (76%) were successfully evaluated for consciousness level at the time of acute hospital presentation after injury, and 7624 (57%) did not follow commands (with DOC). Of this subset of patients, 11 848 (89%) were following commands (without DOC) at the time of subsequent inpatient rehabilitation admission and 12 946 (98%) were following commands by the time of inpatient rehabilitation discharge. Of those who were following commands at initial presentation, 249 patients (4%) regressed and had a DOC at the time of admission to rehabilitation, and 51 (1%) of those following commands at rehabilitation start had a DOC when discharged (eTable 1 in the Supplement) (Figure 1). Eighty-two percent (n = 1674) of patients in a coma recovered consciousness during inpatient rehabilitation.
Figure 1. Progression in Percentage of Patients With Disorder of Consciousness During Treatment.
ED indicates emergency department.
Key components of the analysis were repeated in a subset of 4643 patients with an obtainable admission GCS total score of 12 or lower, a conventional definition of moderate and severe brain injury used in previous reports. The main findings for recovery of consciousness identified in the full TBIMS database population were confirmed in this subset.
In a multivariable analysis, controlling for age, sex, and TBI cause, the independent factors associated with recovery of consciousness by the end of rehabilitation were absence of intraventricular hemorrhage (adjusted OR, 0.678; 95% CI, 0.532-0.863; P = .002) and absence of intracranial mass effect (adjusted OR, 0.759; 95% CI, 0.595-0.968; P = .03) (eTable 2 in the Supplement).
Functional Outcome
Patients with DOC vs those without DOC after acute care had lower functional status at the start of inpatient rehabilitation (median [IQR] FIM total score, 19 [18-28] vs 54 [36-70]; P < .001) and at rehabilitation discharge (median [IQR] FIM total score, 71 [37-90] vs 96 [82-108]; P < .001). At the start of rehabilitation, patients with DOC vs those without DOC were less likely to be partially or fully functionally independent by rehabilitation discharge (40% [n = 803] vs 79% [n = 11 941]; P < .001) (Table 3; eTable 3 and eFigure 1 in the Supplement).
Table 3. Association of Consciousness Level With Functional Outcome During Inpatient Rehabilitation.
| Outcome characteristic | All patients (n = 17 470), median (IQR) | Rehabilitation admission consciousness level, median (IQR)a | P value | |
|---|---|---|---|---|
| With DOC (n = 2058)b | Without DOC (n = 15 412)c | |||
| Rehabilitation admission | ||||
| FIM motor score | 34 (19 to 49) | 13 (13 to 19) | 38 (23 to 51) | <.001 |
| FIM cognitive score | 15 (8 to 21) | 5 (5 to 8) | 16 (10 to 22) | <.001 |
| FIM total score | 51 (30 to 69) | 19 (18 to 28) | 54 (36 to 70) | <.001 |
| Rehabilitation discharge | ||||
| FIM motor score | 69 (57 to 80) | 53 (25 to 68) | 70 (60 to 81) | <.001 |
| FIM cognitive score | 25 (20 to 29) | 17 (10 to 23) | 25 (21 to 29) | <.001 |
| FIM total score | 94 (79 to 107) | 71 (37 to 90) | 96 (82 to 108) | <.001 |
| Dependent, No./total No. (%)d | 4354/17 098 (25) | 1210/2013 (60) | 3144/15 085 (21) | <.001 |
| Semi-independent, No./total No. (%)e | 6058/17 098 (35) | 494/2013 (25) | 5564/15 085 (37) | |
| Independent, No./total No. (%)f | 6686/17 098 (39) | 309/2013 (15) | 6377/15 085 (42) | |
| Progression during rehabilitation | ||||
| Δ FIM total score, admission to discharge | +38 (+26 to +51) | +43 (+15 to +61) | +37 (+26 to +50) | .002 |
| PTA emergence, No./total No. (%) | 13 673/16 809 (81) | 882/1976 (45) | 12 791/14 833 (86) | <.001 |
Abbreviations: DOC, disorder of consciousness; DRS, Disability Rating Scale; FIM, Functional Independence Measure; IQR, interquartile range; PTA, posttraumatic amnesia.
Excluded 213 patients with no DRS motor score at inpatient admission.
With DOC defined as not following commands, or a DRS motor score >0 at inpatient admission.
DRS motor score of 0 at inpatient admission.
FIM total score of 18-79 indicated requiring professional assistance.
FIM total score of 80-99 indicated compatibility with home care by family members.
FIM total score of 100-126 indicated full independence.
Among patients with DOC at rehabilitation admission, those who achieved higher functional independence vs those who were dependent during the rehabilitation course were more likely to be male (83% [n = 256 of 309] vs 74% [n = 1269 of 1704]; OR, 1.656 [95% CI, 1.208-2.269]; P = .002), less likely to have had a high-velocity TBI (47% [n = 144 of 307] vs 57% [n = 964 of 1703]; OR, 0.677 [95% CI, 0.531-0.864]; P = .002), less likely to have had craniotomy or craniectomy (26% [n = 54 of 204] vs 36% [n = 491 of 1349]; OR, 0.629 [95% CI, 0.452-0.875]; P = .006), and had a shorter acute care stay (median [IQR] duration, 18 [12-25] days vs 26 [18-38] days; P < .001). Patients with DOC who regained higher functional independence also had less severe neuroanatomic injuries. They were less likely than those who were dependent to have intracranial mass effect (44% [n = 114 of 261] vs 53% [n = 851 of 1593]; OR, 0.676 [95% CI, 0.519-0.880]; P = .003), intraventricular hemorrhage (25% [n = 66 of 264] vs 44% [n = 701 of 1609]; OR, 0.432 [95% CI, 0.321-0.580]; P < .001), or subcortical white matter injury (17% [n = 45 of 264] vs 34% [n = 552 of 1607]; OR, 0.393 [95% CI, 0.280-0.550]; P < .001) (eTable 4 in the Supplement).
Independent factors associated with functional independence among patients with DOC at the time of rehabilitation admission were younger age; male sex; lower-velocity TBI cause; and absence of intraventricular hemorrhage, subcortical white matter injury, or intracranial mass effect (eTable 5 in the Supplement).
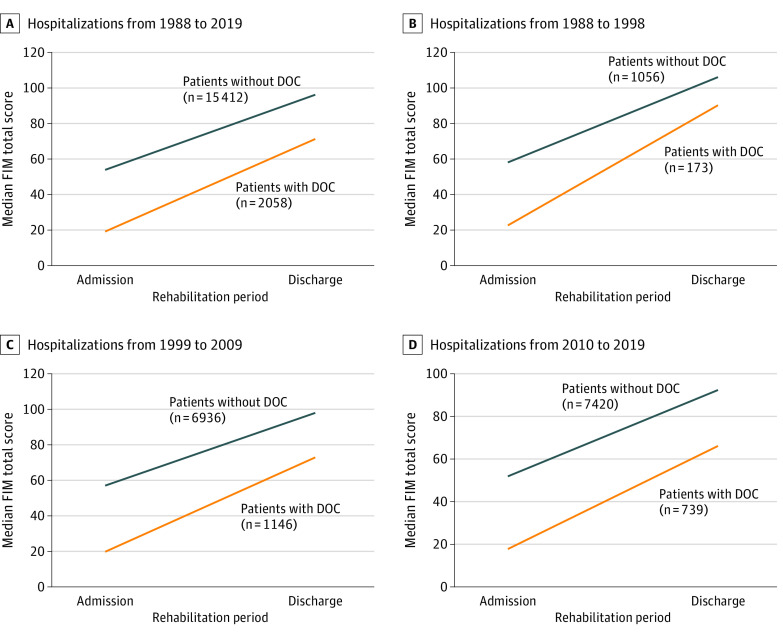
Patients with or without DOC at rehabilitation admission experienced improvement in functional status during rehabilitation. For patients with DOC, the median FIM total score increased from 19 to 71 during the rehabilitation course compared with an increase from 54 to 96 in those without DOC (change in FIM total score, +43 vs +37; P = .002) (Figure 2 and eFigure 2 in the Supplement).
Figure 2. Comparison of Trajectory of Recovery in Functional Status During Inpatient Rehabilitation by Hospitalization Year.
DOC indicates disorder of consciousness; FIM, Functional Independence Measure.
In multivariate analyses, younger age and male sex were associated with both better absolute median FIM total score at discharge and improvement in functional status during rehabilitation. All neuroanatomic injuries considered except cortical contusion were associated with worse functional outcomes. For the FIM total score at rehabilitation discharge, intraventricular hemorrhage accounted for a 10-point decrease (95% CI, –12.706 to –7.051; P < .001), intracranial mass effect accounted for a 9-point decrease (95% CI, –11.458 to –5.987; P < .001), and subcortical injury accounted for a 9-point decrease (95% CI, –11.658 to –5.657; P < .001). For the change in FIM total score during rehabilitation, intraventricular hemorrhage accounted for a 5-point decrease (95% CI, –7.591 to –2.492; P < .001), intracranial mass effect accounted for a 7-point decrease (95% CI, –9.063 to –4.146; P < .001), and subcortical injury accounted for a 6-point decrease (95% CI, –8.932 to –3.527; P < .001) (eTable 6 in the Supplement).
Comparison of Outcome and DOC Over 3 Decades
Findings were compared for patients with DOC for each of the 3 decades in the TBIMS database (1988-1998, 1999-2009, and 2010-2019). Male patients consistently composed three-quarters of the sample in all decades. Age of patients increased from decade 1 to decade 3 (median [IQR] age, 34 [23-43] years to 38 (25-58) years), and falls represented a greater proportion of TBI causes over time (10% in decade 1 to 31% in decade 3).
Outcome for all measures decreased slightly from decade 1 to decade 3. Median (IQR) FIM total scores at the time of rehabilitation discharge were 90 (59-105) in decade 1, 73 (39-91) in decade 2, and 66 (32-85) in decade 3. Median (IQR) changes in FIM total score during rehabilitation were +46 (+24 to +68) in decade 1, +44 (+16 to +62) in decade 2, and +39 (+13 to +58) in decade 3. The proportion of patients with DOC who achieved functional independence by the end of rehabilitation was 36% in decade 1, 16% in decade 2, and 10% in decade 3. Emergence from PTA by rehabilitation discharge occurred in 79 of 158 patients with DOC (50%) in decade 1, 501 of 1102 patients with DOC (45%) in decade 2, and 302 of 716 patients with DOC (42%) in decade 3 (eTable 7 in the Supplement and Figure 2).
Discussion
In this cohort study of 17 470 patients with moderate or severe TBI over a 31-year period who went on to receive inpatient rehabilitation, at least 57% experienced initial loss of consciousness, 12% of those admitted to rehabilitation had a DOC at the time of rehabilitation admission, and of those patients, 82% recovered consciousness (ie, followed commands) by the end of rehabilitation. Among patients with DOC after acute care, 40% achieved semi- or full-functional independence during this rehabilitation, less than in patients without DOC, of whom 80% recovered functional independence. However, the study demonstrated that although patients with DOC completed emergent and critical hospital care with lower functional status, they experienced greater absolute improvement during rehabilitation than patients without DOC. This change occurred over a longer period for those with DOC than for those without DOC (median inpatient rehabilitation duration, 37 vs 18 days).
Acute care had a median (IQR) duration of 25 (17-36) days for patients with DOC at the time of rehabilitation admission, 10 days longer than for patients without DOC. For patients with DOC at rehabilitation discharge, the median (IQR) duration of rehabilitation was 37 (22-65) days. Updated guidelines for care of patients with DOC persisting 28 days or more recommend that these patients receive treatment for confounding conditions, optimal arousal stimulation, and serial standardized assessment to improve diagnostic accuracy.37
This study found that structural neuroanatomic injuries were associated with initial loss of consciousness after injury and independently estimated all of the outcome measures assessed. These neuroanatomic pathologies included intraventricular hemorrhage, subcortical white matter damage, and shift of midline cerebral structures attributed to intracranial mass effect. These findings are consistent with those of previous research on the association of coma with intracranial mass and shift of midline cerebral structures,38 intracranial hemorrhage,39 and white matter damage.40 This study defined DOC as not following commands or having an ED GCS motor score lower than 6. This criterion was selected partly because of the inherent limitation of the verbal component of the GCS for assessing intubated patients in the intensive care unit and the interrater variability in calculating the total score. The GCS motor score also has the advantage of simplicity and ease of bedside administration.
Potential changes over time were evaluated by separately analyzing each of the 3 decades of the TBIMS database. This analysis found an aging of the TBI population and an increasing proportion of falls as opposed to high-velocity injuries as the cause of TBI. Recovery of consciousness and improvement of functional outcome during inpatient rehabilitation remained largely steady across the 3 decades. The proportion of patients who achieved functional independence decreased steadily from decade 1 to decade 3. We believe that this finding may reflect that patients in the most recent decade arrived at inpatient rehabilitation facilities after shorter acute care stays and earlier in the process of recovery than in previous decades, but a thorough analysis of this pattern is beyond the scope of this study. Nonetheless, we observed a consistent parallel in trajectory of functional recovery between patients with DOC and those who were fully conscious in each of the 3 decades and during the overall period.
We considered the potential of referral bias favoring patients with better expected outcomes for inpatient rehabilitation. Although a thorough comparison was beyond the scope of this study, evidence suggests that social and demographic factors, such as younger age, review by a physiatrist, and proximity of acute hospitalization to inpatient rehabilitation care, played a similar or greater role in the decision-making for inpatient rehabilitation referral than patient clinical status.41,42,43,44 In addition, given that no validated tools exist for estimating the recovery of consciousness in patients with DOC, it seemed unlikely that referral bias was a substantial factor in the outcomes of the present study. To evaluate potential referral bias, we compared these results with those of reports that examined 3 large databases of patients with moderate or severe TBI and mixed referrals (rehabilitation and no rehabilitation).
Longitudinal studies suggest continued functional improvement for at least several years after rehabilitation discharge.18,20 Adverse outcomes of neuroanatomic injuries may provide guidance for prognosis and early clinical management. Consistency in patterns of recovery of consciousness and functional improvement across each of the 3 decades suggests that the trajectory of recovery is a natural process in moderate and severe TBI. Although the findings specifically concerned patients who received inpatient rehabilitation care after initial hospitalization, caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DOC. Furthermore, the factors associated with DOC recovery that we identified could be used as a sample enrichment strategy in future TBI clinical trials. This strategy aligns with the move toward precision medicine to reduce sample heterogeneity, improving the probability of detecting treatment signal.
Limitations
This study has some limitations. First, the cohort included only TBI survivors who were admitted to inpatient rehabilitation. The generalizability of the findings, therefore, is limited, given that they do not account for outcome in patients whose care was withdrawn or withheld or who did not proceed to inpatient rehabilitation because of perceived poor prognosis, lack of insurance, family choice, or other reasons. Second, a referral bias possibly exists in inpatient rehabilitation for patients with TBI and a perceived better prognosis. The study findings, therefore, may not be directly applicable to the overall population of patients with moderate or severe TBI. Data do not exist to quantify this potential referral bias, and thus the issue warrants further research. Third, the TBIMS database does not include initial consciousness assessment for patients who were chemically sedated or paralyzed at initial presentation, precluding the evaluation of the full progression of consciousness from injury through rehabilitation for this TBI subset. Neuroanatomic injury findings were derived from radiologic reports of head CT obtained clinically. Thus, incomplete information was provided on the disruption of white matter connectivity, which is believed to be a major cause of protracted unconsciousness. Fourth, this study did not account for variations in treatment regimens over the 3 decades, which may have affected outcome.
Conclusions
Most patients with moderate or severe TBI and early-onset DOC who received inpatient rehabilitation recovered consciousness during acute care, and 82% of patients with persisting DOC regained consciousness (ie, followed commands) by the end of inpatient rehabilitation. Those with persisting DOC made greater absolute improvement, albeit from a lower baseline, in functional status during the rehabilitation phase. This result points to the potential for delayed, but better, outcomes than might otherwise be expected in TBI with DOC. Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DOC.
eTable 1. Progression of Consciousness in Acute Care and Inpatient Rehabilitation Following TBI
eTable 2. Predictors of Recovery of Consciousness During Inpatient Rehabilitation Following TBI
eTable 3. Comparison of Patient Characteristics and Rehabilitation Admission Disordered Consciousness With Functional Independence at Discharge
eTable 4. Association of Patient and Clinical Characteristics With Functional Independence at Inpatient Rehabilitation Discharge in Patients With Disordered Consciousness at Start of Inpatient Rehabilitation
eTable 5. Predictors of Functional Independence at Rehabilitation Discharge in Patients With Disordered Consciousness at Start of Inpatient Rehabilitation
eTable 6. Predictors of Inpatient Rehabilitation Functional Outcome for Patients With Disordered Consciousness Following TBI
eTable 7. Comparison of Patient, Injury and Outcome Characteristics by Decade in Disordered Consciousness Following TBI
eFigure 1. Comparison of Disordered Consciousness at Inpatient Rehabilitation Admission and Functional Independence at Inpatient Rehabilitation Discharge
eFigure 2. Comparison of Change in Functional Status During Inpatient Rehabilitation
References
- 1.Centers for Disease Control and Prevention. Traumatic brain injury & concussion. Accessed May 20, 2020. https://www.cdc.gov/traumaticbraininjury/get_the_facts.html
- 2.Giacino J, Whyte J. The vegetative and minimally conscious states: current knowledge and remaining questions. J Head Trauma Rehabil. 2005;20(1):30-50. doi: 10.1097/00001199-200501000-00005 [DOI] [PubMed] [Google Scholar]
- 3.Hirschberg R, Giacino JT. The vegetative and minimally conscious states: diagnosis, prognosis and treatment. Neurol Clin. 2011;29(4):773-786. doi: 10.1016/j.ncl.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 4.Godbolt AK, Deboussard CN, Stenberg M, Lindgren M, Ulfarsson T, Borg J. Disorders of consciousness after severe traumatic brain injury: a Swedish-Icelandic study of incidence, outcomes and implications for optimizing care pathways. J Rehabil Med. 2013;45(8):741-748. doi: 10.2340/16501977-1167 [DOI] [PubMed] [Google Scholar]
- 5.Løvstad M, Andelic N, Knoph R, et al. Rate of disorders of consciousness in a prospective population-based study of adults with traumatic brain injury. J Head Trauma Rehabil. 2014;29(5):E31-E43. doi: 10.1097/HTR.0000000000000017 [DOI] [PubMed] [Google Scholar]
- 6.Jochems D, van Wessem KJP, Houwert RM, et al. Outcome in patients with isolated moderate to severe traumatic brain injury. Crit Care Res Pract. 2018;2018:3769418. doi: 10.1155/2018/3769418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: do they exist in traumatic brain injury, too? Neurocrit Care. 2013;19(3):347-363. doi: 10.1007/s12028-013-9925-z [DOI] [PubMed] [Google Scholar]
- 8.Turgeon AF, Lauzier F, Simard JF, et al. ; Canadian Critical Care Trials Group . Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581-1588. doi: 10.1503/cmaj.101786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertsen A, Førde R, Skaga NO, Helseth E. Treatment-limiting decisions in patients with severe traumatic brain injury in a Norwegian regional trauma center. Scand J Trauma Resusc Emerg Med. 2017;25(1):44. doi: 10.1186/s13049-017-0385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verkade MA, Epker JL, Nieuwenhoff MD, Bakker J, Kompanje EJ. Withdrawal of life-sustaining treatment in a mixed intensive care unit: most common in patients with catastrophic brain injury. Neurocrit Care. 2012;16(1):130-135. doi: 10.1007/s12028-011-9567-y [DOI] [PubMed] [Google Scholar]
- 11.Sise MJ, Sise CB, Thorndike JF, Kahl JE, Calvo RY, Shackford SR. Withdrawal of care: a 10-year perspective at a level I trauma center. J Trauma Acute Care Surg. 2012;72(5):1186-1193. doi: 10.1097/TA.0b013e31824d0e57 [DOI] [PubMed] [Google Scholar]
- 12.Malone C, Erler KS, Giacino JT, et al. Participation following inpatient rehabilitation for traumatic disorders of consciousness: a TBI model systems study. Front Neurol. 2019;10:1314. doi: 10.3389/fneur.2019.01314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakase-Richardson R, McNamee S, Howe LL, et al. Descriptive characteristics and rehabilitation outcomes in active duty military personnel and veterans with disorders of consciousness with combat- and noncombat-related brain injury. Arch Phys Med Rehabil. 2013;94(10):1861-1869. doi: 10.1016/j.apmr.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 14.Lucca LF, Lofaro D, Pignolo L, et al. Outcome prediction in disorders of consciousness: the role of coma recovery scale revised. BMC Neurol. 2019;19(1):68. doi: 10.1186/s12883-019-1293-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCredie VA, Alali AS, Xiong W, et al. Timing of withdrawal of life-sustaining therapies in severe traumatic brain injury: impact on overall mortality. J Trauma Acute Care Surg. 2016;80(3):484-491. doi: 10.1097/TA.0000000000000922 [DOI] [PubMed] [Google Scholar]
- 16.Pratt AK, Chang JJ, Sederstrom NO. A fate worse than death: prognostication of devastating brain injury. Crit Care Med. 2019;47(4):591-598. doi: 10.1097/CCM.0000000000003647 [DOI] [PubMed] [Google Scholar]
- 17.Côte N, Turgeon AF, Lauzier F, et al. Factors associated with the withdrawal of life-sustaining therapies in patients with severe traumatic brain injury: a multicenter cohort study. Neurocrit Care. 2013;18(1):154-160. doi: 10.1007/s12028-012-9787-9 [DOI] [PubMed] [Google Scholar]
- 18.Whyte J, Nakase-Richardson R, Hammond FM, et al. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch Phys Med Rehabil. 2013;94(10):1855-1860. doi: 10.1016/j.apmr.2012.10.041 [DOI] [PubMed] [Google Scholar]
- 19.Hammond FM, Giacino JT, Nakase Richardson R, et al. Disorders of consciousness due to traumatic brain injury: functional status ten years post-injury. J Neurotrauma. 2019;36(7):1136-1146. doi: 10.1089/neu.2018.5954 [DOI] [PubMed] [Google Scholar]
- 20.Katz DI, Polyak M, Coughlan D, Nichols M, Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1-4 year follow-up. Prog Brain Res. 2009;177:73-88. doi: 10.1016/S0079-6123(09)17707-5 [DOI] [PubMed] [Google Scholar]
- 21.Lammi MH, Smith VH, Tate RL, Taylor CM. The minimally conscious state and recovery potential: a follow-up study 2 to 5 years after traumatic brain injury. Arch Phys Med Rehabil. 2005;86(4):746-754. doi: 10.1016/j.apmr.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 22.Nakase-Richardson R, Whyte J, Giacino JT, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma. 2012;29(1):59-65. doi: 10.1089/neu.2011.1829 [DOI] [PubMed] [Google Scholar]
- 23.Traumatic Brain Injury Model Systems National Data and Statistical Center. Welcome to the NDSC. Accessed November 13, 2020. https://www.tbindsc.org/
- 24.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81-84. doi: 10.1016/S0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 25.Traumatic Brain Injury Model Systems National Data and Statistical Center. Standardized operating procedure 101a: identification of subjects for the TBI Model Systems National Database. Accessed November 13, 2020. https://www.tbindsc.org/StaticFiles/SOP/101a%20-%20Identification%20of%20Subjects.pdf
- 26.Gouvier WD, Blanton PD, LaPorte KK, Nepomuceno C. Reliability and validity of the Disability Rating Scale and the Levels of Cognitive Functioning Scale in monitoring recovery from severe head injury. Arch Phys Med Rehabil. 1987;68(2):94-97. doi: 10.1097/00001199-198712000-00015 [DOI] [PubMed] [Google Scholar]
- 27.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6-18. [PubMed] [Google Scholar]
- 28.Traumatic Brain Injury Model Systems National Data and Statistical Center. Standardized operating procedures. Accessed November 13, 2020. https://www.tbindsc.org/SOP.aspx
- 29.Levin HS, O’Donnell VM, Grossman RG. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J Nerv Ment Dis. 1979;167(11):675-684. doi: 10.1097/00005053-197911000-00004 [DOI] [PubMed] [Google Scholar]
- 30.Jackson WT, Novack TA, Dowler RN. Effective serial measurement of cognitive orientation in rehabilitation: the Orientation Log. Arch Phys Med Rehabil. 1998;79(6):718-720. doi: 10.1016/S0003-9993(98)90051-X [DOI] [PubMed] [Google Scholar]
- 31.Marshall LF, Marshall SB, Klauber MR, et al. A new classification of head injury based on computerised tomography. J Neurosurg. 1991;75(suppl):S14-S20. [Google Scholar]
- 32.Lingsma H, Andriessen TMJC, Haitsema I, et al. Prognosis in moderate and severe traumatic brain injury: external validation of the IMPACT models and the role of extracranial injuries. J Trauma Acute Care Surg. 2013;74(2):639-646. doi: 10.1097/TA.0b013e31827d602e [DOI] [PubMed] [Google Scholar]
- 33.Castaño-Leon AM, Lora D, Munarriz PM, et al. Predicting outcomes after severe and moderate traumatic brain injury: an external validation of impact and crash prognostic models in a large Spanish cohort. J Neurotrauma. 2016;33(17):1598-1606. doi: 10.1089/neu.2015.4182 [DOI] [PubMed] [Google Scholar]
- 34.Roozenbeek B, Lingsma HF, Lecky FE, et al. ; International Mission on Prognosis Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) Study Group; Corticosteroid Randomisation After Significant Head Injury (CRASH) Trial Collaborators; Trauma Audit and Research Network (TARN) . Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid Randomisation After Significant Head injury (CRASH) prognostic models. Crit Care Med. 2012;40(5):1609-1617. doi: 10.1097/CCM.0b013e31824519ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camarano JG, Ratliff HT, Korst GS, Hrushka JM, Jupiter DC. Predicting in-hospital mortality after traumatic brain injury: external validation of CRASH-basic and IMPACT-core in the national trauma data bank. Injury. Published online October 10, 2020. doi: 10.1016/j.injury.2020.10.051 [DOI] [PubMed] [Google Scholar]
- 36.Uniform Data System for Medical Rehabilitation. The FIM Instrument: its background, structure, and usefulness. Published 2012. Accessed May 19, 2020. http://docshare02.docshare.tips/files/23187/231876238.pdf
- 37.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91(10):450-460. doi: 10.1212/WNL.0000000000005926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kowalski RG, Buitrago MM, Duckworth J, et al. Neuroanatomical predictors of awakening in acutely comatose patients. Ann Neurol. 2015;77(5):804-816. doi: 10.1002/ana.24381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Mufti F, Amuluru K, Changa A, et al. Traumatic brain injury and intracranial hemorrhage-induced cerebral vasospasm: a systematic review. Neurosurg Focus. 2017;43(5):E14. doi: 10.3171/2017.8.FOCUS17431 [DOI] [PubMed] [Google Scholar]
- 40.Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(pt 2):449-463. doi: 10.1093/brain/awq347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrigley JM, Yoels WC, Webb CR, Fine PR. Social and physical factors in the referral of people with traumatic brain injuries to rehabilitation. Arch Phys Med Rehabil. 1994;75(2):149-155. doi: 10.1016/0003-9993(94)90387-5 [DOI] [PubMed] [Google Scholar]
- 42.Cnossen MC, Lingsma HF, Tenovuo O, et al. Rehabilitation after traumatic brain injury: a survey in 70 European neurotrauma centres participating in the CENTER-TBI study. J Rehabil Med. 2017;49(5):395-401. doi: 10.2340/16501977-2216 [DOI] [PubMed] [Google Scholar]
- 43.Foster M, Fleming J, Tilse C, Rosenman L. Referral to post-acute care following traumatic brain injury (TBI) in the Australian context. Brain Inj. 2000;14(12):1035-1045. doi: 10.1080/02699050050203531 [DOI] [PubMed] [Google Scholar]
- 44.Swaine B, Cullen N, Messier F, et al. Post-acute care referral and inpatient rehabilitation admission criteria for persons with brain injury across two Canadian provinces. Disabil Rehabil. 2018;40(6):697-704. doi: 10.1080/09638288.2016.1262911 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Progression of Consciousness in Acute Care and Inpatient Rehabilitation Following TBI
eTable 2. Predictors of Recovery of Consciousness During Inpatient Rehabilitation Following TBI
eTable 3. Comparison of Patient Characteristics and Rehabilitation Admission Disordered Consciousness With Functional Independence at Discharge
eTable 4. Association of Patient and Clinical Characteristics With Functional Independence at Inpatient Rehabilitation Discharge in Patients With Disordered Consciousness at Start of Inpatient Rehabilitation
eTable 5. Predictors of Functional Independence at Rehabilitation Discharge in Patients With Disordered Consciousness at Start of Inpatient Rehabilitation
eTable 6. Predictors of Inpatient Rehabilitation Functional Outcome for Patients With Disordered Consciousness Following TBI
eTable 7. Comparison of Patient, Injury and Outcome Characteristics by Decade in Disordered Consciousness Following TBI
eFigure 1. Comparison of Disordered Consciousness at Inpatient Rehabilitation Admission and Functional Independence at Inpatient Rehabilitation Discharge
eFigure 2. Comparison of Change in Functional Status During Inpatient Rehabilitation