Abstract
The calculation of the vapour pressure of organic molecules at 298.15 K is presented using a commonly applicable computer algorithm based on the group-additivity method. The basic principle of this method rests on the complete breakdown of the molecules into their constituting atoms, further characterized by their immediate neighbour atoms. The group contributions are calculated by means of a fast Gauss–Seidel fitting algorithm using the experimental data of 2036 molecules from literature. A ten-fold cross-validation procedure has been carried out to test the applicability of this method, which confirmed excellent quality for the prediction of the vapour pressure, expressed in log(pa), with a cross-validated correlation coefficient Q2 of 0.9938 and a standard deviation σ of 0.26. Based on these data, the molecules’ standard Gibbs free energy ΔG°vap has been calculated. Furthermore, using their enthalpies of vaporization, predicted by an analogous group-additivity approach published earlier, the standard entropy of vaporization ΔS°vap has been determined and compared with experimental data of 1129 molecules, exhibiting excellent conformance with a correlation coefficient R2 of 0.9598, a standard error σ of 8.14 J/mol/K and a medium absolute deviation of 4.68%.
Keywords: group-additivity method, vapour pressure, Gibbs free energy of vaporization, entropy of vaporization
1. Introduction
In recent years, knowledge of the vapour pressure of organic molecules has gained increasing interest in view of the environmental, in particular radiation absorption, effects in the context of global warming, but also in view of their toxicology [1,2], as well as their quality as refrigerants [3]. At the same time, new and highly sophisticated experimental methods, e.g., using a Knudsen effusion apparatus, coupled with a quartz crystal microbalance [4], have been developed for the measurement of molecules exhibiting extremely low vapour pressures. In most cases, these measurements involved the temperature dependence of the vapour pressure over a certain temperature range, the corresponding sequence then being approximated by one of various non-linear functions, mostly by the Antoine equation [5]. In order to enable a comparison of the vapour pressures between molecules at identical conditions, the non-linear functions have been used to interpolate the vapour pressures to a standard temperature, usually 298.15 K. This interpolation method produces reliable results on condition that the experimental temperature range encompassed the standard temperature. However, the comprehensive handbook of Mackay et al. [6], collecting the experimental data from various authors for more than 1000 compounds, revealed that in many cases the standard vapour-pressure data varied by a wide range, depending on the experimental methods. Therefore, many attempts, critically reviewed by O’Meara et al. [7] and Dearden [8], have been undertaken to calculate the vapour pressure based on quantitative structure-property relationships. Some of these attempts require the knowledge of an experimentally determined descriptor, which limits the scope of applicability for vapour-pressure prediction. For example, A. Vetere [9] suggested a non-linear equation relating the vapour pressure to the temperature, based on the reduced temperature Tf (i.e., the critical temperature), which was tested successfully on less than 50 liquids. A neural network approach was presented by R. Kühne et al. [10], whereby the network was trained by 1200 and tested by 638 hydrocarbons and halogenated hydrocarbons, requiring—among further molecular structure data—the melting point of the compounds, achieving an overall error of 0.08 and 0.13 log(Pa) for the training and test set. The authors stressed that neural networks cannot extrapolate reliably outside the given descriptor and target values of the training set. A group-contribution method with the inclusion of group interactions was presented by B. Moller et al. [11], which produced the group parameters, but required the knowledge of one experimental vapour pressure point. The relative error for a training set of 2332 compounds was given as 5%. Several other prediction methods are based on a set of purely structural descriptors and/or on atom groups of the molecules. For the calculation the vapour pressure of a large scope of molecules, these latter methods are entirely dependent on the number and structural variability of molecules with known vapour pressure. In 1994, Ch.-H. Tu [12] presented a group-contribution method based on 5359 experimental vapour pressure data of 342 compounds over a varying temperature range between 90 and 643K, whereby each atom group was defined by four constants, enabling the prediction at various temperatures using a second order equation derived from the Clausius–Clapeyron equation. The medium absolute percentage deviation between experimental and predicted values for 336 compounds was reported as 5%. The reliability of these predictions, however, is said to be limited to molecules carrying at most one functional group. A neural network model was used by E. S. Goll and P. C. Jurs [13] for the vapour-pressure prediction of hydrocarbons and halohydrocarbons, having been trained by the experimental vapour pressures as log(VP) at 25 °C of 352 compounds. The molecules were presented to the neural network by topological, geometric, electronic and hybrid descriptors. The root-mean-square (rms) deviations for the training, cross-validation and prediction sets were given as 0.163, 0.163 and 0.209 log units, respectively. An analogous model, this time based on 420 diverse molecules, was presented by H. E. McClelland and P. C. Jurs [14], yielded an rms error of 0.33 log units. Cohesive energies and solubility parameters derived from molecular dynamics simulations based on forcefield calculations of 22 molecules have been used by P. K. C. Paul [15]. He demonstrated that a single cross term consisting of either the molecular volume or molecular weight and the square of the compound’s solubility parameter—which latter is the square root of the cohesive energy—determined to more than 90% the equation for the vapour pressure, expressed as log(VP). The Abraham descriptors method has been used for the vapour-pressure prediction as log(VP) of liquid and solid organic and organometallic molecules by M. H. Abraham and W. E. Acree [16], the six descriptors being E the excess molar refraction, S the solute polarity/polarizability, A the solute H-bond acidity, B the solute H-bond basicity, V the McGowan’s characteristic molecular volume and L the logarithm of the hexadecane partition coefficient at 298.15 K, all of which, except for L, either being available from commercial databases for more than 8000 compounds or obtainable by calculation procedures. The best standard deviation value has been calculated to 0.28 units for 1016 compounds. These few examples (except for the last one) demonstrate that the studies on the prediction of the vapour pressure published up to now usually either deal with a specific set or a limited number of molecules forestalling an extension beyond them.
The present paper provides a way to predict the vapour pressure at 298.15 K of a very large scope of organic molecules, applying the same basic computer algorithm based on the atom-group additivity method outlined in [17], which has already proven its versatility in the reliable prediction of the 16 molecular descriptors enthalpy of combustion, formation, vaporization, sublimation and solvation, entropy of fusion, logPo/w, logS, logγinf, refractivity, polarizability, toxicity (against the protozoan Tetrahymena pyriformis), viscosity and surface tension of liquids, and heat capacity of solids and liquids [17,18,19,20,21], and which only required a few further peripheral control lines of code to meet the present purpose. In the next section, a brief outline of the calculation procedure is given. In addition, by the inclusion of the experimental and calculated enthalpy of vaporization of the molecules under consideration (their calculated enthalpy having been received by the same method as the present one but published earlier [18]), their experimental and predicted entropies of vaporization have been made accessible and will thus be compared.
2. Method
The present study rests on a regularly updated object-oriented knowledge database of currently 32,697 compounds encompassing the entire spectrum of organic molecules, including pharmaceuticals, herbicides, pesticides, fungicides, textile dyes, ionic liquids, liquid crystals, metal-organics, lab intermediates, and more, each of them stored in a separate datafile containing the 3D-geometry-optimized structure and—as far as available—their experimental and routinely calculated descriptors, including their vapour pressures. The latter is defined in this work as the logarithm to the basis 10 in Pascal, termed as logVP.
The atom-group additivity principle and its translation into a computer algorithm for the calculation of their parameters has been outlined in detail in [17]. Accordingly, the definitions and naming of the atom and special groups are identical to the ones given in Table 1 and Table 2 of [17]. The first preliminary logVP calculations, however, with tentative replacement of certain atom groups by more detailed ones and addition or omission of certain special groups, revealed a significant improvement of the statistical data upon the addition of the groups explained in Table 1.
Table 1.
Refined atom and special groups and their meaning.
| Atom Type | Neighbours | Meaning |
|---|---|---|
| O(prim) | HC | Primary alcohol |
| O(sec) | HC | Secondary alcohol |
| O(tert) | HC | Tertiary alcohol |
| (COH)n | n > 1 | Molecule contains more than 1 saturated OH group |
| (COOH)n | n > 1 | Molecule contains more than 1 carboxylic acid group |
| Endocyclic bonds | No of single bonds | Number of single bonds in cyclic ring |
| Bridgehead atoms | No of bonds | Number of bridgehead C or N (e.g., camphor, DABCO) |
Table 2.
Example calculation of the logVP of 2-Methylcyclohexanol.
| Atom Type/Neighours | C sp3/H3C | C sp3/HC3 | C sp3/HC2O | C sp3/H2C2 | O(sec)/HC | Endocyclic Single bds | Const | Checksum |
|---|---|---|---|---|---|---|---|---|
| Contribution | 0.60 | −1.28 | −2.65 | −0.47 | 0.72 | 0.31 | 4.71 | |
| n | 1 | 1 | 1 | 4 | 1 | 6 | 1 | |
| n x Contrib. | 0.6 | −1.28 | −2.65 | −1.88 | 0.72 | 1.86 | 4.71 | 2.08 |
2.08 is the horizontal checksum.
The separation of the hydroxy group on saturated carbon into primary, secondary and tertiary OH groups as defined in Table 1 (henceforth called “saturated hydroxy group”) has successfully been introduced into the present atom-group additivity approach for the calculation of the heat capacities of molecules [21]. This modification required an additional procedure in the group-additivity algorithm described in [17]. In contrast to this, the group definition of hydroxy groups attached to unsaturated carbon atoms remained unaltered. The tentative first calculations also confirmed an assumption which had already been proven in the calculation of the surface tension of liquids [20]: additional saturated hydroxy groups in a molecule exhibit more than just a linearly additive effect on the descriptor. This non-linear effect has been considered by the special group “(COH)n” for molecules carrying more than 1 saturated hydroxy group (the term “Neighbours” at the header of the second column does not apply to the special groups). Similarly, the first attempts for the prediction of the logVP for dicarboxylic acids indicated that a second carboxylic acid function also showed a nonlinearly increasing impact on the result. Therefore, the special group “(COOH)n” was added for compounds with n>1 to take account of this apparent nonlinearity. A further strong deviation between predicted and experimental data, observed with compounds containing cyclic saturated segments, was remedied by adding a correction value for each single bond that is a part of the ring moiety, defined by the special group “Endocyclic bonds”, yielding a drastic improvement of the prediction statistics data (summed up at the bottom of Table 3). This special group has already found successful use in the prediction of the entropy of fusion [18] and the heat capacities [21] of molecules. Despite this additional special group, the predicted vapour pressures for various bicyclic compounds such as adamantane or camphor have shown to be systematically much lower than their experimental data. Therefore, the special group “Bridgehead atoms” has been introduced. Further details about these special groups will be discussed in the results section.
Table 3.
Atom groups and their contributions for logVP calculations.
| Entry | Atom Type | Neighbours | Contribution | Occurrences | Molecules |
|---|---|---|---|---|---|
| 1 | Const | 4.71 | 2036 | 2036 | |
| 2 | B | HN2 | −1.17 | 6 | 2 |
| 3 | B | BN2 | −1.6 | 2 | 1 |
| 4 | B | BO2 | −1.71 | 4 | 2 |
| 5 | B | C2N | −0.35 | 1 | 1 |
| 6 | B | C2O | −0.44 | 1 | 1 |
| 7 | B | C2S | −0.44 | 1 | 1 |
| 8 | B | CO2 | −1.56 | 1 | 1 |
| 9 | B | O3 | −1.57 | 6 | 6 |
| 10 | B | S3 | −3.17 | 1 | 1 |
| 11 | C sp3 | H3B | 0 | 7 | 4 |
| 12 | C sp3 | H3C | 0.6 | 2211 | 1077 |
| 13 | C sp3 | H3N | −1.07 | 113 | 62 |
| 14 | C sp3 | H3N(+) | −1.64 | 1 | 1 |
| 15 | C sp3 | H3O | −0.95 | 152 | 116 |
| 16 | C sp3 | H3S | −0.52 | 23 | 17 |
| 17 | C sp3 | H3P | −1.22 | 8 | 7 |
| 18 | C sp3 | H3Si | −0.42 | 87 | 16 |
| 19 | C sp3 | H2C2 | −0.47 | 4196 | 831 |
| 20 | C sp3 | H2CN | −2.07 | 240 | 138 |
| 21 | C sp3 | H2CN(+) | −2.01 | 5 | 5 |
| 22 | C sp3 | H2CO | −1.82 | 460 | 314 |
| 23 | C sp3 | H2CP | −2.3 | 5 | 3 |
| 24 | C sp3 | H2CS | −1.6 | 79 | 54 |
| 25 | C sp3 | H2CF | 0.39 | 15 | 15 |
| 26 | C sp3 | H2CCl | −0.48 | 59 | 48 |
| 27 | C sp3 | H2CBr | −0.76 | 22 | 20 |
| 28 | C sp3 | H2CJ | −1.23 | 11 | 11 |
| 29 | C sp3 | H2CSi | −1.58 | 11 | 6 |
| 30 | C sp3 | H2N2 | −11.73 | 1 | 1 |
| 31 | C sp3 | H2NO | −3.82 | 2 | 2 |
| 32 | C sp3 | H2NS | −1.24 | 3 | 3 |
| 33 | C sp3 | H2O2 | −3.81 | 6 | 6 |
| 34 | C sp3 | H2OF | −1.22 | 3 | 3 |
| 35 | C sp3 | H2OCl | −2.1 | 2 | 2 |
| 36 | C sp3 | H2S2 | −2.59 | 3 | 3 |
| 37 | C sp3 | HC3 | −1.28 | 342 | 231 |
| 38 | C sp3 | HC2N | −2.87 | 35 | 28 |
| 39 | C sp3 | HC2N(+) | −2.87 | 3 | 3 |
| 40 | C sp3 | HC2O | −2.65 | 115 | 95 |
| 41 | C sp3 | HC2S | −2.36 | 11 | 8 |
| 42 | C sp3 | HC2F | −0.6 | 10 | 9 |
| 43 | C sp3 | HC2Cl | −1.22 | 31 | 15 |
| 44 | C sp3 | HC2Br | −1.59 | 16 | 12 |
| 45 | C sp3 | HC2J | −1.96 | 1 | 1 |
| 46 | C sp3 | HCN2 | −2.07 | 2 | 1 |
| 47 | C sp3 | HCNO | −5.99 | 1 | 1 |
| 48 | C sp3 | HCNS | −2.48 | 1 | 1 |
| 49 | C sp3 | HCO2 | −3.65 | 7 | 7 |
| 50 | C sp3 | HCOBr | −4.78 | 1 | 1 |
| 51 | C sp3 | HCF2 | 0.37 | 31 | 27 |
| 52 | C sp3 | HCFCl | −0.01 | 7 | 7 |
| 53 | C sp3 | HCCl2 | −0.94 | 12 | 11 |
| 54 | C sp3 | HCClBr | −0.76 | 1 | 1 |
| 55 | C sp3 | HCBr2 | −1.93 | 3 | 2 |
| 56 | C sp3 | HOF2 | −1.09 | 6 | 6 |
| 57 | C sp3 | C4 | −2.19 | 98 | 87 |
| 58 | C sp3 | C3N | −3.6 | 11 | 11 |
| 59 | C sp3 | C3N(+) | −3.57 | 2 | 2 |
| 60 | C sp3 | C3O | −3.46 | 36 | 35 |
| 61 | C sp3 | C3S | −3.21 | 6 | 6 |
| 62 | C sp3 | C3Si | −3.37 | 3 | 2 |
| 63 | C sp3 | C3Cl | −2.84 | 6 | 3 |
| 64 | C sp3 | C3Br | −2.2 | 2 | 2 |
| 65 | C sp3 | C3F | −1.39 | 13 | 10 |
| 66 | C sp3 | C2O2 | −5.46 | 4 | 2 |
| 67 | C sp3 | C2OF | −2.8 | 5 | 5 |
| 68 | C sp3 | C2F2 | −0.37 | 184 | 71 |
| 69 | C sp3 | C2FCl | −0.8 | 1 | 1 |
| 70 | C sp3 | C2Cl2 | 0 | 3 | 3 |
| 71 | C sp3 | CNF2 | −2.03 | 12 | 5 |
| 72 | C sp3 | CNF2(+) | −0.37 | 1 | 1 |
| 73 | C sp3 | CNCl2 | −0.4 | 1 | 1 |
| 74 | C sp3 | COF2 | −1.69 | 49 | 39 |
| 75 | C sp3 | CSF2 | −1.15 | 24 | 12 |
| 76 | C sp3 | CF3 | 0.67 | 152 | 107 |
| 77 | C sp3 | CF2Cl | 0.3 | 8 | 7 |
| 78 | C sp3 | CF2Br | −0.07 | 5 | 4 |
| 79 | C sp3 | CFCl2 | −0.37 | 5 | 4 |
| 80 | C sp3 | CFClBr | −0.73 | 1 | 1 |
| 81 | C sp3 | CCl3 | −0.98 | 15 | 14 |
| 82 | C sp3 | CCl2Br | 0 | 1 | 1 |
| 83 | C sp3 | NF3 | −1.09 | 5 | 3 |
| 84 | C sp3 | OF3 | −0.36 | 13 | 10 |
| 85 | C sp3 | O2F2 | −2.67 | 1 | 1 |
| 86 | C sp3 | S2F2 | −1.83 | 4 | 2 |
| 87 | C sp3 | SF3 | −0.01 | 10 | 7 |
| 88 | C sp3 | SCl3 | −7.92 | 1 | 1 |
| 89 | C sp3 | PF3 | −0.08 | 20 | 8 |
| 90 | C sp2 | H2=C | 0.67 | 127 | 113 |
| 91 | C sp2 | HC=C | −0.38 | 272 | 175 |
| 92 | C sp2 | HC=N | −1.49 | 7 | 7 |
| 93 | C sp2 | HC=O | −0.47 | 27 | 27 |
| 94 | C sp2 | H=CN | −1.84 | 19 | 12 |
| 95 | C sp2 | H=CO | −0.79 | 5 | 5 |
| 96 | C sp2 | H=CS | −0.79 | 8 | 6 |
| 97 | C sp2 | H=CP | −1.03 | 3 | 1 |
| 98 | C sp2 | H=CF | 0.68 | 3 | 3 |
| 99 | C sp2 | H=CCl | −0.15 | 13 | 11 |
| 100 | C sp2 | H=CBr | −0.56 | 5 | 3 |
| 101 | C sp2 | H=CJ | −1.2 | 2 | 1 |
| 102 | C sp2 | HN=N | −1.89 | 11 | 9 |
| 103 | C sp2 | HN=O | −2.47 | 9 | 8 |
| 104 | C sp2 | HO=O | −1.25 | 8 | 8 |
| 105 | C sp2 | C2=C | −1.25 | 79 | 67 |
| 106 | C sp2 | C2=N | −3.09 | 2 | 2 |
| 107 | C sp2 | C=CN | −2.26 | 2 | 2 |
| 108 | C sp2 | C2=O | −1.27 | 56 | 53 |
| 109 | C sp2 | C=CO | −1.5 | 6 | 6 |
| 110 | C sp2 | C=CP | −3.09 | 1 | 1 |
| 111 | C sp2 | C=CS | −1.78 | 6 | 5 |
| 112 | C sp2 | C=CF | −0.25 | 3 | 3 |
| 113 | C sp2 | C=CCl | −1.24 | 18 | 13 |
| 114 | C sp2 | CN=N | −4.13 | 2 | 2 |
| 115 | C sp2 | CN=O | −3.17 | 35 | 32 |
| 116 | C sp2 | C=NS | −1.47 | 2 | 1 |
| 117 | C sp2 | CO=O | −2.33 | 222 | 184 |
| 118 | C sp2 | C=OCl | −0.54 | 4 | 4 |
| 119 | C sp2 | C=OBr | −1.1 | 1 | 1 |
| 120 | C sp2 | C=OJ | −1.67 | 1 | 1 |
| 121 | C sp2 | =CF2 | 0.95 | 7 | 6 |
| 122 | C sp2 | =CFCl | 0.14 | 1 | 1 |
| 123 | C sp2 | =CFBr | −0.25 | 1 | 1 |
| 124 | C sp2 | =CCl2 | −0.53 | 10 | 8 |
| 125 | C sp2 | =CBr2 | 0.65 | 1 | 1 |
| 126 | C sp2 | N2=N | −4.7 | 1 | 1 |
| 127 | C sp2 | N2=O | −5.19 | 5 | 5 |
| 128 | C sp2 | N=NS | −1.58 | 1 | 1 |
| 129 | C sp2 | N2=S | 0.14 | 2 | 1 |
| 130 | C sp2 | NO=O | −4.55 | 15 | 13 |
| 131 | C sp2 | N=OS | −0.64 | 7 | 7 |
| 132 | C sp2 | =NOS | −0.26 | 1 | 1 |
| 133 | C sp2 | NS=S | 1.24 | 1 | 1 |
| 134 | C sp2 | O2=O | −3.59 | 4 | 4 |
| 135 | C aromatic | H:C2 | −0.2 | 3662 | 751 |
| 136 | C aromatic | H:C:N | −0.41 | 34 | 21 |
| 137 | C aromatic | H:N2 | 0.48 | 2 | 2 |
| 138 | C aromatic | :C3 | −1.06 | 260 | 85 |
| 139 | C aromatic | C:C2 | −1.06 | 929 | 508 |
| 140 | C aromatic | C:C:N | −1.21 | 15 | 13 |
| 141 | C aromatic | :C2N | −2.3 | 40 | 38 |
| 142 | C aromatic | :C2N(+) | −2.59 | 33 | 29 |
| 143 | C aromatic | :C2:N | −1.42 | 4 | 3 |
| 144 | C aromatic | :C2O | −2 | 381 | 195 |
| 145 | C aromatic | :C2P | −4.07 | 1 | 1 |
| 146 | C aromatic | :C2S | −1.69 | 8 | 6 |
| 147 | C aromatic | :C2F | −0.1 | 63 | 26 |
| 148 | C aromatic | :C2Cl | −0.84 | 1630 | 386 |
| 149 | C aromatic | :C2Br | −1.13 | 166 | 58 |
| 150 | C aromatic | :C2J | −1.57 | 10 | 9 |
| 151 | C aromatic | :C2Si | 0.89 | 1 | 1 |
| 152 | C aromatic | C:N2 | −1.39 | 2 | 2 |
| 153 | C aromatic | :C:NO | −1.85 | 6 | 6 |
| 154 | C aromatic | :C:NCl | −1.33 | 5 | 5 |
| 155 | C aromatic | N:N2 | −2.72 | 17 | 10 |
| 156 | C aromatic | :N2O | −0.96 | 2 | 2 |
| 157 | C aromatic | :N2S | 1.89 | 3 | 3 |
| 158 | C aromatic | :N2Cl | −1.38 | 3 | 3 |
| 159 | C sp | H#C | 0.81 | 14 | 13 |
| 160 | C sp | C#C | −0.49 | 22 | 17 |
| 161 | C sp | =C2 | −0.51 | 3 | 3 |
| 162 | C sp | C#N | −0.61 | 34 | 27 |
| 163 | C sp | =N=O | 0.75 | 3 | 3 |
| 164 | C sp | =N=S | 1.19 | 1 | 1 |
| 165 | N sp3 | HB2 | 0.45 | 3 | 2 |
| 166 | N sp3 | H2C | 1.45 | 62 | 47 |
| 167 | N sp3 | H2C(pi) | 0.15 | 18 | 18 |
| 168 | N sp3 | H2N | −0.52 | 3 | 3 |
| 169 | N sp3 | HC2 | 2.36 | 26 | 26 |
| 170 | N sp3 | HC2(pi) | 0.56 | 35 | 26 |
| 171 | N sp3 | HC2(2pi) | 0.44 | 14 | 10 |
| 172 | N sp3 | HCN | 0.7 | 3 | 2 |
| 173 | N sp3 | HCN(pi) | −0.36 | 1 | 1 |
| 174 | N sp3 | HCN(2pi) | 0.38 | 1 | 1 |
| 175 | N sp3 | HCP(pi) | −4.25 | 1 | 1 |
| 176 | N sp3 | HCS(pi) | 5.56 | 1 | 1 |
| 177 | N sp3 | B2C | 1.1 | 3 | 2 |
| 178 | N sp3 | BC2 | 2.13 | 5 | 2 |
| 179 | N sp3 | C3 | 3.52 | 49 | 45 |
| 180 | N sp3 | C3(pi) | 2.95 | 27 | 26 |
| 181 | N sp3 | C3(2pi) | 3.52 | 11 | 11 |
| 182 | N sp3 | C3(3pi) | 3.4 | 3 | 3 |
| 183 | N sp3 | C2N(pi) | 0.11 | 4 | 4 |
| 184 | N sp3 | C2N(2pi) | 3.37 | 8 | 8 |
| 185 | N sp3 | C2N(3pi) | 2.89 | 1 | 1 |
| 186 | N sp3 | C2O | 3.47 | 1 | 1 |
| 187 | N sp3 | C2S | 2.57 | 3 | 3 |
| 188 | N sp3 | C2S(pi) | 3.96 | 3 | 2 |
| 189 | N sp3 | C2S(2pi) | 7.1 | 1 | 1 |
| 190 | N sp3 | C2P | 2.07 | 7 | 4 |
| 191 | N sp3 | C2F(pi) | 4.38 | 1 | 1 |
| 192 | N sp3 | CF2 | 0.61 | 1 | 1 |
| 193 | N sp3 | CSi2 | 1.18 | 2 | 2 |
| 194 | N sp3 | SF2 | 0.07 | 1 | 1 |
| 195 | N sp2 | C=C | 0.39 | 16 | 15 |
| 196 | N sp2 | C=N | −3.19 | 1 | 1 |
| 197 | N sp2 | C=N(+) | 0.96 | 7 | 7 |
| 198 | N sp2 | =CN | −0.04 | 10 | 9 |
| 199 | N sp2 | =CO | 0.68 | 4 | 3 |
| 200 | N sp2 | =CS | −0.39 | 1 | 1 |
| 201 | N sp2 | N=N | 0 | 1 | 1 |
| 202 | N sp2 | N=O | 0 | 4 | 4 |
| 203 | N sp2 | =NP(+) | −0.39 | 1 | 1 |
| 204 | N sp2 | O=O | 1.58 | 6 | 6 |
| 205 | N aromatic | :C2 | −0.06 | 61 | 39 |
| 206 | N(+) sp2 | CO=O(−) | 0.34 | 45 | 41 |
| 207 | N(+) sp2 | O2=O(−) | 0.54 | 50 | 26 |
| 208 | N(+) sp | =N2(−) | 0 | 8 | 8 |
| 209 | O(prim) | HC | 0.44 | 95 | 78 |
| 210 | O(sec) | HC | 0.72 | 48 | 47 |
| 211 | O(tert) | HC | 0.74 | 11 | 11 |
| 212 | O | HC(pi) | 0.04 | 102 | 90 |
| 213 | O | HN(pi) | −1.29 | 1 | 1 |
| 214 | O | HO | −1.16 | 4 | 3 |
| 215 | O | BC | 1.39 | 26 | 8 |
| 216 | O | BP | 0.16 | 3 | 2 |
| 217 | O | C2 | 2.38 | 150 | 132 |
| 218 | O | C2(pi) | 2.3 | 228 | 191 |
| 219 | O | C2(2pi) | 1.49 | 151 | 130 |
| 220 | O | CN | 0 | 1 | 1 |
| 221 | O | CN(pi) | 0 | 6 | 6 |
| 222 | O | CN(2pi) | 0.26 | 3 | 2 |
| 223 | O | CN(+)(pi) | 0 | 50 | 26 |
| 224 | O | CO | 1.03 | 8 | 3 |
| 225 | O | CO(pi) | 1.59 | 3 | 2 |
| 226 | O | CS | 1.25 | 6 | 4 |
| 227 | O | CS(pi) | 1.44 | 2 | 2 |
| 228 | O | CP | 0.06 | 95 | 44 |
| 229 | O | CP(pi) | −0.29 | 14 | 12 |
| 230 | O | CSi | 0.65 | 7 | 2 |
| 231 | O | OS | −0.67 | 3 | 2 |
| 232 | O | S2 | −1.14 | 5 | 3 |
| 233 | O | Si2 | −0.3 | 22 | 7 |
| 234 | P3 | C3 | 0 | 1 | 1 |
| 235 | P3 | HC2 | 2.57 | 1 | 1 |
| 236 | P3 | C2N | 1.59 | 2 | 2 |
| 237 | P3 | C2O | 0 | 3 | 2 |
| 238 | P3 | C2S | −0.09 | 5 | 4 |
| 239 | P3 | CN2 | −0.35 | 1 | 1 |
| 240 | P3 | CS2 | −0.94 | 1 | 1 |
| 241 | P4 | HO2=O | −0.55 | 1 | 1 |
| 242 | P4 | C3=S | 0.19 | 1 | 1 |
| 243 | P4 | CO2=O | 0.62 | 4 | 4 |
| 244 | P4 | CO2=S | 3.03 | 1 | 1 |
| 245 | P4 | CO=OS | 0.38 | 2 | 2 |
| 246 | P4 | COS=S | −0.5 | 1 | 1 |
| 247 | P4 | N3=O | −0.83 | 1 | 1 |
| 248 | P4 | NO=OS | −0.06 | 1 | 1 |
| 249 | P4 | N=OF2 | 0 | 1 | 1 |
| 250 | P4 | O3=O | 0.23 | 9 | 9 |
| 251 | P4 | O3=S | 0.22 | 13 | 13 |
| 252 | P4 | O2=OS | −0.36 | 1 | 1 |
| 253 | P4 | O=OS2 | −1.76 | 1 | 1 |
| 254 | P4 | O2S=S | −0.58 | 12 | 11 |
| 255 | S2 | HC | 0.83 | 29 | 23 |
| 256 | S2 | HC(pi) | 0.28 | 1 | 1 |
| 257 | S2 | HS | −0.26 | 2 | 1 |
| 258 | S2 | HP | 0.06 | 1 | 1 |
| 259 | S2 | BC | 0.52 | 4 | 2 |
| 260 | S2 | C2 | 1.07 | 30 | 28 |
| 261 | S2 | C2(pi) | −1.97 | 14 | 13 |
| 262 | S2 | C2(2pi) | 1.52 | 9 | 9 |
| 263 | S2 | CN | 0 | 1 | 1 |
| 264 | S2 | CN(2pi) | −2.3 | 1 | 1 |
| 265 | S2 | CS | 0.05 | 8 | 4 |
| 266 | S2 | CP | −0.07 | 22 | 19 |
| 267 | S2 | CP(pi) | 0 | 1 | 1 |
| 268 | S2 | N2 | −1.45 | 2 | 2 |
| 269 | S2 | NCl | −0.43 | 1 | 1 |
| 270 | S2 | P2 | −0.7 | 1 | 1 |
| 271 | S2 | Si2 | 0.33 | 3 | 3 |
| 272 | S4 | C2=O | −0.96 | 4 | 4 |
| 273 | S4 | C2=O2 | 1.6 | 2 | 2 |
| 274 | S4 | C2O2 | −2.15 | 1 | 1 |
| 275 | S4 | C2F2 | 0.41 | 5 | 5 |
| 276 | S4 | CO=O2 | 2.16 | 1 | 1 |
| 277 | S4 | CN=O2 | −2.13 | 1 | 1 |
| 278 | S4 | NO=O2 | −2.51 | 1 | 1 |
| 279 | S4 | N=O2Cl | 0 | 1 | 1 |
| 280 | S4 | O2=O | −0.56 | 1 | 1 |
| 281 | S4 | O2=O2 | −0.94 | 1 | 1 |
| 282 | S4 | O=O2F | 0.12 | 4 | 4 |
| 283 | S6 | C2F4 | 0.72 | 5 | 3 |
| 284 | S6 | O2F4 | −0.78 | 1 | 1 |
| 285 | S6 | OF5 | 1.08 | 7 | 5 |
| 286 | Si | H3C | 1.72 | 4 | 4 |
| 287 | Si | H3N | 0 | 4 | 2 |
| 288 | Si | H3S | −0.3 | 2 | 1 |
| 289 | Si | H3Si | −0.53 | 2 | 1 |
| 290 | Si | H2C2 | 1.78 | 2 | 2 |
| 291 | Si | H2Si2 | 0 | 2 | 1 |
| 292 | Si | HC2O | 0.78 | 2 | 1 |
| 293 | Si | HC2S | 0.11 | 2 | 1 |
| 294 | Si | HC2J | 0.23 | 1 | 1 |
| 295 | Si | HCCl2 | 0.47 | 1 | 1 |
| 296 | Si | HO3 | 0.21 | 1 | 1 |
| 297 | Si | C4 | 1.97 | 2 | 2 |
| 298 | Si | C3O | 1.08 | 6 | 3 |
| 299 | Si | C3S | 0.19 | 2 | 1 |
| 300 | Si | C3Cl | 1.05 | 1 | 1 |
| 301 | Si | C3Si | −0.79 | 2 | 1 |
| 302 | Si | C2O2 | −0.18 | 18 | 5 |
| 303 | Si | C2F2 | 1.69 | 1 | 1 |
| 304 | Si | C2Cl2 | 0.41 | 1 | 1 |
| 305 | Si | CF3 | 0 | 1 | 1 |
| 306 | Si | CCl3 | 0.06 | 1 | 1 |
| 307 | Si | O4 | −0.16 | 1 | 1 |
| 308 | (COH)n | n>1 | −0.74 | 23 | 22 |
| 309 | (COOH)n | n>1 | −1.73 | 12 | 12 |
| 310 | Endocyclic bonds | No of single bds | 0.31 | 1072 | 193 |
| 311 | Bridgehead atoms | No of atoms | 0.23 | 80 | 27 |
| 312 | Angle60 | 0.19 | 42 | 14 | |
| 313 | Angle90 | 0.17 | 72 | 21 | |
| 314 | Angle102 | 0.11 | 323 | 110 | |
| A | Based on | Valid groups | 171 | 2036 | |
| B | Goodness of fit | R2 | 0.9946 | 1908 | |
| C | Deviation | Average | 0.18 | 1908 | |
| D | Deviation | Standard | 0.24 | 1908 | |
| E | K-fold cv | K | 10 | 1842 | |
| F | Goodness of fit | Q2 | 0.9938 | 1842 | |
| G | Deviation | Average (cv) | 0.2 | 1842 | |
| H | Deviation | Standard (cv) | 0.26 | 1842 |
Lines A to H are the statistics data of the table.
The calculation of the parameter values of the atom and special groups of Table 3 is carried out in a step-by-step process as explained in [17]: in a first step, a temporary list of compounds for which the experimental vapour pressure is known, is extracted from the database. In a second step, for each of the “backbone” atoms (i.e., atoms bound to at least two other direct neighbour atoms) in the molecules the atom type and its neighbourhood is defined by two character strings according to the rules defined in [17], corresponding to the atom type and neighbours terms listed in Table 3, and then its occurrence in the molecule is counted. Thirdly, an M × (N + 1) matrix is generated where M is the number of molecules and N + 1 the molecules’ number of atom and special groups plus their experimental value and where each matrix element (i,j) receives the number of occurrences of the jth atomic or special group in the ith molecule. In the final steps, normalization of this matrix into an Ax = B matrix and its subsequent balancing using a fast Gauss–Seidel calculus, as e.g., described by E. Hardtwig [22], yield the group contributions, which are then stored in Table 3.
Following the philosophy of the group-additivity approach, these group contributions can now be used to calculate the descriptor, in this case the vapour pressure as logVP at 298.15 K, by simply summing up the contributions for each of the molecule’s atom and special group, according to Equation (1), wherein ai and bj are the respective atom and special group contributions, Ai is the number of occurrences of the ith atom group, Bj is the number of occurrences of the jth special group and C is a constant. However, an important restriction has to be observed when using Table 3 in connection with Equation (1): the group contributions are only reliable enough for use—i.e., “valid”—if they are supported by at least three independent molecules, i.e., if the number in the rightmost column of Table 3 exceeds 2.
| (1) |
The plausibility of the descriptor results is immediately tested in the present method by means of a 10-fold cross-validation algorithm wherein in each of the 10 recalculations another 10% of the complete set of compounds is used as a test set, ensuring that each compound has been entered alternatively as a training and a test sample. The respective statistics data of the training and accumulated test calculations are finally collected at the bottom of Table 3. Due to the restriction mentioned above, the number of molecules for the evaluation of the training correlation coefficient, average and standard deviations (lines B, C and D) and for the corresponding test data from cross-validation (lines F, G and H) are smaller than the number of compounds shown on line A, upon which the complete list of atom-group parameters is based. The number of “valid” groups (line A) is significantly lower than the total of atom and special groups listed in Table 3, leaving a substantial number of “invalid” groups. Although not applicable for vapour-pressure predictions at present, they have deliberately been left in Table 3 for future use in this continuous project (and may motivate interested scientists to focus on measuring the vapour pressure of molecules carrying the under-represented atom groups). At present, the elements list for vapour-pressure predictions is limited to H, B, C, N, O, P, S, Si, and/or halogen.
A simple example may help to understand the application of the data of Table 3 in Equation (1): 2-methylcyclohexanol consists of the atom and special groups listed in Table 2. Accordingly, the sum of all the contributions is 2.08. The experimental logVP was published in [16] as 2.216.
3. Sources of Vapor-Pressure Data
An overview of the literature concerning the vapour-pressure data of molecules revealed that generally their measurements were either carried out at 298.15 K or over a certain temperature range encompassing this standard temperature. In the latter case, the authors mostly provided a set of constants to be used in a non-linear equation, usually the Antoine-equation [6], allowing to calculate the vapour pressure at the standard temperature by interpolation. However, in several publications the authors chose a temperature range which remained above this standard, particularly with compounds having a high melting point. On condition that the lowest experimental temperature was not higher than 5 K above the standard, the extrapolated vapour-pressure data have been included in the present study, well aware of the uncertainty of these values. Some authors overcame the problem of the high melting point by supercooling the melt prior to measuring the vapour pressure, examples of which are cited in [6]. While past publications usually expressed the vapour pressure in older units such as Torr, mm(Hg) or atm, newer ones generally used Pa, kPa, MPa or mPa, often converted to their natural logarithm. In the present paper, these various units have been translated throughout to the logarithm to the basis 10 of the unit Pa, expressed as logVP.
Several comprehensive papers provided the majority of the vapour-pressure data: D. Mackay’s Handbook [6] is a compilation of over 1000 compounds, collecting—among several further physico-chemical data—the results of the vapour-pressure measurements of various authors for each compound, revealing the general extent of the experimental uncertainty, depending on the method of measurement. Another rich source was the comprehensive collection of M. H. Abraham and W. E. Acree Jr [16], contributing the vapour-pressure data of additional more than 1000 compounds. Further collective sources have been used to complement—and compare—the experimental data [8,14,15,23,24,25,26,27,28,29,30]. In addition, vapour-pressure data have been published specifically for various saturated and unsaturated hydrocarbons [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], alcohols [53,54,55,56,57,58], phenols [59,60], alkyl- and arylethers [61,62,63,64], acetals [65,66], carboxylic acids [67,68,69,70,71], carboxylic halides [72], carboxylic esters and lactones [73,74,75,76,77,78,79,80,81,82,83,84,85,86], carbonates [87,88,89,90,91], ketones [92,93,94], peroxides [95,96], amines [97,98,99,100,101,102,103,104,105,106], amides [107,108,109,110], azides [111,112], hydrazines [113,114,115], isocyanates and isothiocyanates [116,117,118,119], nitriles [120,121], nitro-substituted compounds [122,123,124,125,126], nitrites [127], nitrates [128], nitrosamines [129], ureas [130,131], alkyl- and arylsulfides [132], sulfoxides [133,134,135,136], thiophenes [137,138,139], phosphines and phosphoranes [140,141,142,143,144], phosphonates, phosphates and thiophosphates [145,146,147,148,149,150,151,152], boranes and borates [153,154,155,156,157,158,159,160], silanes, siloxanes and silthianes [161,162,163,164,165,166,167,168,169,170,171,172,173,174,175] and hetarenes [176,177,178,179]. A particularly large number of publications studied the vapour-pressure data of halogen-substituted compounds, which in many cases belonged to one of the aforementioned groups, hereinafter subdivided in haloalkanes [180,181,182,183,184,185,186,187,188,189,190], haloalkenes [191,192,193,194,195,196,197,198,199,200,201,202], haloaromatics [203,204,205,206,207,208,209,210,211], haloalkyl-amines, -aminoxides and -alkoxyamines [212,213,214,215,216,217], haloalkylethers [218,219,220,221,222,223], haloarylethers [224], haloalkylsulfides, -sulfoxides, -sulfones and -sulfates [225,226,227,228,229,230,231], haloalkylsilanes [232,233], haloalkylcarboxylic acids, -esters, and peresters [234,235]. Finally, a number of authors published the vapour pressures of some individual compounds, as there were the terpenes and their derivatives carvone, 2-hydroxy-3-pinanone, iso-pinocampheol, myrtanol, pinocarveol, eugenol, camphor, menthone, damascenone and (-)-methyl jasmonate [236,237,238], several hydroxycarbonyl- and formyl-derivatives of naphthalene, fluorene, anthracene and pyrene with exceptionally low vapour pressure [239], 2-aminoethanol and its N-methyl derivatives [240], 2-dialkylaminoethanethiols [241], derivatives of 2- and 3-amino-1-propanols [242], phthalan [243], the aroma compounds d-linalool, 2-nonanone, d-limonene and isoamyl acetate [244], hydroxyacetaldehyde and hydroxyacetone [245], l-deprenyl, benzphetamine and alverine [246], 2-adamantanone and 1-acetyl-adamantane [247], fenpropidin and phencyclidine [248], ambroxide and galaxolide [249], the lignin fragments trans-anethole, estragole, eugenol as well as hydroxy- and methoxy-substituted benzaldehydes [250,251,252,253], benzocaine [254] and bicifadine [255].
4. Results
4.1. Vapour Pressure
The contributions of the atom groups in Table 3 for the prediction of the vapour pressure as logVP in Pascal are the final result of a series of direct and cross-validation calculations according to the method outlined in [17], whereby in preceding step-by-step calculations any outliers, defined by the deviation of their experimental from their predicted value exceeding three times the current cross-validated standard error Q2, have been removed from further calculations. At the end, they made up ca. 11% of the total number of molecules for which experimental vapour pressures were given, which have been collected in an outliers list accessible in the Supplemental Material. The statistical data are collected at the bottom of Table 3 in rows A to H. As is shown in row A, of the 314 atom and special groups required to cover all remaining 2036 compounds, only 171 groups have been found to be valid for predictions. Accordingly, the number of compounds, for which a prediction was possible, has been reduced to 1908 in the complete training set and to 1842 in the cross-validation test sets. The high compliance of the direct and the cross-validation correlation coefficients R2 and Q2 (lines B and F), also evident in the low scatter of both the respective data points about the correlation line in the corresponding diagram (Figure 1) and confirmed by the narrow symmetrical Gaussian bell curve of the histogram (Figure 2), confirms the excellent reliability of the present atom-groups approach for vapour-pressure predictions. Its low cross-validation standard deviation of only 0.26 units compares very favourably with the best values of other prediction methods such as that of McClelland and Jurs [14] or that of Abraham and Acree [16], although it is based on a much larger number of molecular structures. The large range of experimental vapour pressures of between ca. 107 Pa for tetrafluoroethylene and 10−19 Pa for hexapentacontane and the broad structural variety of molecules, upon which these calculations are based, enabled the trustworthy prediction of the vapour pressure of more than 57% of the compounds listed in the database which can well be considered as representative for the entire realm of chemical structures. A list of the molecules with their experimental and predicted vapour pressure data is accessible in the Supplemental Material.
Figure 1.
Correlation Diagram of the logVP data at 298.15 K. Cross-validation data are added as red circles. (N = 1907; R2 = 0.9945; Q2 = 0.9938; regression line: intercept = −0.0001; slope = 0.9924).
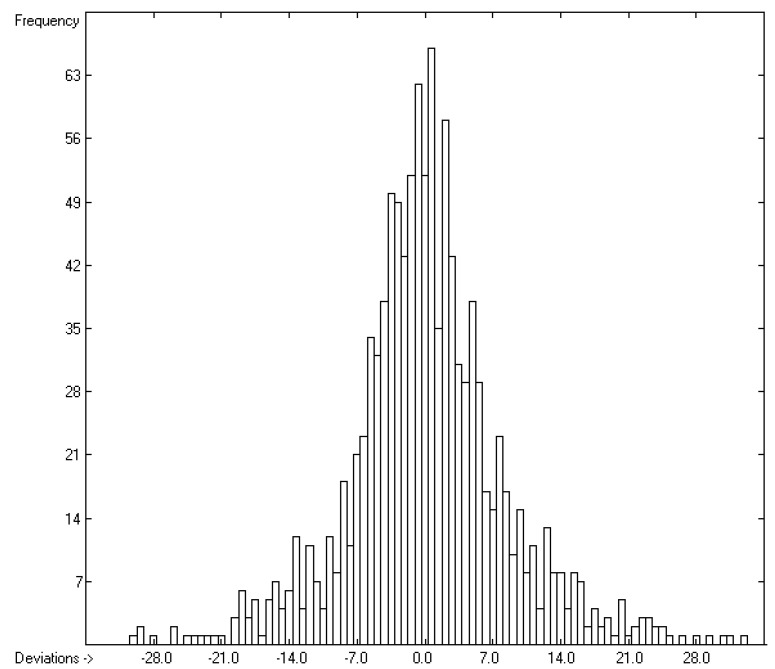
Figure 2.
Histogram of the logVP data at 298.15 K. Cross-validation data are superpositioned as red bars. (σ = 0.24; S = 0.26; experimental values range: −19.36–+6.591).
A few observations concerning certain atom and special groups are worth being highlighted: separation of the hydroxy group at a saturated carbon atom into primary, secondary and tertiary OH groups (group numbers 209–211 in Table 3) led to an appreciable improvement of the statistics data. A comparison of their individual parameter values reveals that primary hydroxy groups generally cause noticeably lower vapour pressures than secondary or tertiary analogues. This pattern matches with the observation made in their influence on the heat capacities of molecules [21,256]. It may be explained by an effect which was discussed in studies by Huelsekopf and Ludwig [257] which, based on the quantum cluster equilibrium theory (QCE), demonstrated that primary alcohols principally form cyclic tetramers and pentamers in the liquid phase, whereas tertiary alcohols only form mono- and dimers. (Secondary alcohols have not been considered.) This clustering of primary alcohols could also prevail in the gas phase, consequently leading to a lower saturated vapour pressure.
Another peculiarity was found with di- and trihydroxyalkyl compounds in that the negative impact of any additional OH group on the vapour pressure was clearly larger than just cumulative. This effect was taken account of by the special group “(COH)n” which was invoked for n > 1. Similarly, and even more drastically, the second carboxylic function in dicarboxylic acids lowered the vapour pressure in more than a cumulative way, which required the additional special group “(COOH)n”, again called up for n > 1. Both these nonlinearities have already been reported by Compernolle et al. [258] and have been considered by additional parameters in their development of EVAPORATION, a group-additivity model for vapour-pressure prediction especially designed for secondary organic aerosols (SOA) comprising alkyl and alkenyl compounds optionally carrying various functional groups but ignoring aromatic systems. They did not provide a reason for the nonlinearities of the additional OH and COOH groups on the vapour pressure; the seemingly obvious argument pointing to intermolecular hydrogen-bridge effects is questionable in view of their observation of a similar nonlinearity with polynitrates. It should be mentioned however, that the present method did not require an additional parameter for the vapour-pressure prediction of polynitrates.
Linear and cyclic, unbranched alkanes having the same number of carbon atoms exhibit comparable experimental vapour pressures (in logVP), e.g., butane vs. cyclobutane: 5.38 vs. 5.195, pentane vs. cyclopentane: 4.84 vs. 4.62, hexane vs. cyclohexane: 4.3 vs. 4.11, heptane vs. cycloheptane: 3.78 vs. 3.45, or octane vs. cyclooctane: 3.27 vs. 2.876 (cited from [25]). For the prediction of logVP of the linear alkanes, the present method simply sums up the contributions of the n-2 methylene groups (i.e., n-2 x -0.47) and adds twice the contribution of the end methyl groups (i.e., 2 x +0.6) to the constant (4.71). In cyclic alkanes however, the two end methyl groups with their large positive contributions are replaced by two methylene groups contributing with large negative values. Therefore, in order to still achieve the goal of vapour pressures comparable to their linear counterparts, the methyl-methylene replacement effect had to be compensated. This was achieved by the introduction of special group “Endocyclic bonds”, whereby its parameter value of +0.31 represents the additional contribution of each single bond of the cyclic moieties of the molecule. For 3-, 4- and 5-membered saturated rings the special groups “Angle60”, “Angle90” and “Angle102” (successfully used for the calculation of the heats of combustion [17]) have been added to Table 3 to take account of their further increasing effect on the vapour pressure. Yet, it turned out that for bicyclic molecules, e.g., camphor or adamantane and its derivatives, the combined contributions of these special groups are still too small to compensate for the even larger negative atom-group contributions of the three- or four-bonded atoms at their bridge heads, (defined by e.g., “C sp3 / HC3”: −1.28, “C sp3 / C4”: −2.19, or “C sp3 / C3O”: −3.46). As a consequence, the special group “Bridgehead atoms” had to be introduced, successfully lifting this deficiency by the additional parameter value of +0.23 units for each bridgehead atom.
4.2. Gibbs Free Energy of Vaporization
Some authors [28,29] derived the experimental vapour pressure of molecules from the experimental data of their enthalpy and entropy of vaporization or sublimation at standard conditions, applying Equation (2), wherein ΔG° is the Gibbs free energy, ΔH° the enthalpy and ΔS° the entropy of vaporization/sublimation, and Θ the reference temperature of 298.15 K. By insertion of ΔG° into the integrated Clausius–Clapeyron Equation (3), wherein p° is the standard pressure of 101,325 Pascal and R the gas constant, and assuming ideal gas-phase conditions and neglecting the volume of the condensed phases, they received the vapour pressure p at 298.15 K in Pascal.
| ΔG°vap,sub(Θ) = ΔH°vap,sub(Θ) − ΘΔS°vap,sub(Θ) | (2) |
| p(Θ) = p°exp[−ΔG°vap,sub(Θ)/(RΘ)] | (3) |
In a logical inversion of the mathematical approach, the vapour pressures, calculated by the present group-additivity method, have been used to predict the Gibbs free energy in kJ/mol by simply using the rearranged form of Equation (3), i.e., Equation (4), and focusing on vaporization.
| ΔG°vap(Θ) = −RΘln(p(Θ)/p°) = −RΘln(10logVP/101325) | (4) |
Applying Equation (4) on the experimental and predicted vapour pressures yielded the correlation diagram of the Gibbs free energies in Figure 3. Evidently, since both ΔG°vap are simple translations according to Equation (4), their correlation coefficient is identical with that in Figure 1 for the vapour pressures. A list of the molecules with their experimental and predicted free energies is available in the Supplemental Material.
Figure 3.
Correlation Diagram of the ΔG°vap data in kJ/mol at 298.15 K. (N = 1907; R2 = 0.9945; σ = 1.38 kJ/mol; MAPD = 14.2%; regression line: intercept = 0.2167; slope = 0.9924).
4.3. Standard Entropy of Vaporization
The standard entropy of vaporization ΔS°vap(Θ) of a molecule can be calculated from the Gibbs free energy ΔG°vap(Θ) using Equation (5), which is the rearranged form of Equation (2), provided that the standard enthalpy of vaporization ΔH°vap(Θ) is known. The present database of currently 32697 molecules has the advantage of encompassing—besides the experimental vapour pressures for 2036 samples—the experimental heat-of-vaporization data for 4029 compounds and, based on these, the predicted heats of vaporization for 24309 compounds, calculated by means of the same group-additivity method as the present one, described in [18]. Hence, it was exciting to compare the results of Equation (5), if in the first case both input data have been experimental values and in the second case both originate from predicted data.
| ΔS°vap(Θ) = [ΔH°vap(Θ) − ΔG°vap(Θ)]/Θ | (5) |
Accordingly, the correlation diagram in Figure 4 compares the entropies of vaporization received from both the experimental enthalpies and energies of vaporization with those calculated from both the predicted enthalpies and energies, revealing a surprisingly small medium absolute percentage deviation (MAPD) of less than 5%. The corresponding histogram in Figure 5 confirms the narrow scatter about the correlation line. The limited number of only 1129 samples in this diagram is owed to the fact that only for these both experimental enthalpies and free energies (or more precisely: vapour pressures) have been available, whereas the large number of calculated enthalpies and free energies (>20800) in the database enabled the reliable prediction of the entropies of vaporization for 20232 (i.e., ca. 62%) compounds of the database. A list of the compounds with their experimental and predicted entropies of vaporization as well as their experimental and predicted enthalpies of vaporization have been added to the Supplementary Material.
Figure 4.
Correlation Diagram of the ΔS°vap data in J/mol/ K at 298.15 K. (N = 1129; R2 = 0.9598; MAPD = 4.68%; regression line: intercept = 4.0448; slope = 0.9660).
Figure 5.
Histogram of the ΔS°vap data in J/mol/ K at 298.15 K. (σ = 8.14 J/mol/K; values range: 64.26–463.16 J/mol/K).
5. Conclusions
The present paper is the result of an extension of a common group-additivity approach applied in an ongoing software project enabling the direct and indirect calculation of 16 physical, thermodynamic, solubility-, optics-, charge- and environment-related descriptors, which led to a series of earlier publications [17,18,19,20,21]. The present project extension, enabling the trustworthy prediction of the vapour pressure and subsequent Gibbs free energy of vaporization at 295.15 K of molecules, also immediately allowed the reliable calculation of the molecules’ standard entropy of vaporization due to the project’s direct access to their predicted heat of vaporization as outlined in [18]. The big advantage of the present group-additivity approach, encoded in the common computer algorithm outlined in [17], not only rests on its simple extensibility by the addition of just a few further lines of control code to fulfil the present task, but also in its simple applicability, basically even allowing accurate prediction of any of the mentioned molecular descriptors by means of paper and pencil and usage of their corresponding group-parameters table. A further advantage is the easy extensibility of the group-parameters lists (if required) to take account of molecules with known descriptor values newly added to the database, usually followed by a recalculation of the group parameters. The disadvantage of the large number of group parameters due to the radical breakdown of the molecules and its subsequent limitation of the calculations to molecules only, for which all the group parameters are found in the respective tables, is well compensated by the high accuracy of the predicted values.
The mentioned software project is called ChemBrain IXL, available from Neuronix Software (www.neuronix.ch (accessed on 17 February 2021), Rudolf Naef, Lupsingen, Switzerland).
Acknowledgments
R. Naef is indebted to the library of the University of Basel for allowing him.
Supplementary Materials
The list of compounds used in the present studies, their experimental and calculated data and 3D structures is available online as standard SDF files, accessible for external chemistry software, under the name of “S1. Compounds List for logVP Calculations.sdf” In addition, compounds lists used for the correlation diagrams containing the compound names and their experimental and calculated values are available under the names of “Table S2. Experimental vs. calculated logVP Data Table.doc”, “Table S3. Experimental vs. calculated deltaG°(vap) Data Table.doc” and “Table S4. Experimental vs. calculated deltaS°(vap) Data Table.doc”. Furthermore, a compounds list of the logVP calculation outliers has been added under the name of “Table S5. Outliers of logVP.xls”. Finally, the experimental and calculated enthalpies of vaporization ΔH°(vap) used in this study, extracted from [18], have been collected in “Table S6. Exp. and calc. deltaH°(vap) Data Table.xls”. The figures are available as tif files and the tables as doc files under the names given in the text.
Author Contributions
R.N. developed project ChemBrain and its software upon which this paper is based, and also fed the database, calculated and analysed the results and wrote the paper. W.E.A.J. suggested the extension of ChemBrain’s tools to include the presented descriptors and contributed experimental data and the majority of the literature references. Beyond this, R.N. is indebted to W.E.A.J. for the many valuable discussions. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples Wagner, of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pope III C.A., Dockery D.W. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 2.Heal M.R., Kumar P., Harrison R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012;41:6606–6630. doi: 10.1039/c2cs35076a. [DOI] [PubMed] [Google Scholar]
- 3.Hundy G.F., Trott A.R., Welch T.C. Refrigeration, Air Conditioning and Heat Pumps (Fifth ed.) Elsevier; Amsterdam, The Netherland: 2016. Chapter 16—Food Refrigeration—Product by Product; pp. 253–271. [DOI] [Google Scholar]
- 4.Santos L.M.N.B.F., Lobo Ferreira A.I.M.C., Stejfa V., Rodrigues A.S.M.C., Rocha M.A.A., Torres M.C., Tavares F.M.S., Carpinteiro F.S. Development of the Knudsen effusion methodology for vapour pressure measurements of low volatile liquids and solids based on a quartz crystal microbalance. J. Chem. Thermodynamics. 2018;126:171–186. doi: 10.1016/j.jct.2018.07.004. [DOI] [Google Scholar]
- 5.Antoine C. Tension des vapeurs: Nouvelle relation entre les tension et les temperatures. Comptes Rendus. 1888;107:681–684. [Google Scholar]
- 6.Mackay D., Shiu W.Y., Ma K.-C., Lee S.C. Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals. 2nd ed. CRC Press; Boca Raton, FL, USA: 2006. [Google Scholar]
- 7.O’Meara S., Booth A.M., Barley M.H., Topping D., McFiggans G. An assessment of vapour pressure estimation methods. Phys. Chem. Chem. Phys. 2014;16:19453. doi: 10.1039/C4CP00857J. [DOI] [PubMed] [Google Scholar]
- 8.Dearden J.C. Quantitative structure-property relationships for prediction of boiling point, vapor pressure and melting point. Toxicol. Chem. 2003;22:1696–1709. doi: 10.1897/01-363. [DOI] [PubMed] [Google Scholar]
- 9.Vetere, A; Methods for Predicting and Correlating the Vapour Pressures of Pure Compounds. Fluid Phase Equil. 1988;43:191–203. doi: 10.1016/0378-3812(88)87006-7. [DOI] [Google Scholar]
- 10.Kühne R., Ebert R.-U., Schüürmann G. Estimation of Vapour Pressures for Hydrocarbons and Halogenated Hydrocarbons from Chemical Structure by a Neural Network. Chemosphere. 1997;34:671–686. doi: 10.1016/S0045-6535(97)00460-8. [DOI] [Google Scholar]
- 11.Moller B., Rarey J., Ramjugernath D. Estimation of the vapour pressure of non-electrolyte organic compounds via group contributions and group interactions. J. Mol. Liquids. 2008;143:52–63. doi: 10.1016/j.molliq.2008.04.020. [DOI] [Google Scholar]
- 12.Tu C.-H. Group-contribution method for the estimation of vapor pressures. Fluid Phase Equil. 1994;99:105–120. doi: 10.1016/0378-3812(94)80025-1. [DOI] [Google Scholar]
- 13.Goll E.S., Jurs P.C. Prediction of Vapor Pressures of Hydrocarbons and Halohydrocarbons from Molecular Structure with a Computational Neural Network Model. J. Chem. Inf. Comput. Sci. 1999;39:1081–1089. doi: 10.1021/ci9900433. [DOI] [Google Scholar]
- 14.McClelland H.E., Jurs P.C. Quantitative Structure-Property Relationships for the Prediction of Vapor Pressures of Organic Compounds from Molecular Structures. J. Chem. Inf. Comput. Sci. 2000;40:967–975. doi: 10.1021/ci990137c. [DOI] [PubMed] [Google Scholar]
- 15.Paul P.K.C. Prediction of vapour pressure using descriptors derived from molecular dynamics. Org. Biomol. Chem. 2005;3:1176–1179. doi: 10.1039/b418834a. [DOI] [PubMed] [Google Scholar]
- 16.Abraham M.H., Acree W.E., Jr. Estimation of vapor pressure of liquid and solid organic and organometallic compounds at 298.15K. Fluid Phase Equil. 2020;519:112595. doi: 10.1016/j.fluid.2020.112595. [DOI] [Google Scholar]
- 17.Naef R. A generally applicable computer algorithm based on the group additivity method for the calculation of seven molecular descriptors: Heat of combustion, LogPO/W, LogS, refractivity, polarizability, toxicity and LogBB of organic compounds; scope and limits of applicability. Molecules. 2015;20:18279–18351. doi: 10.3390/molecules201018279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naef R., Acree W.E. Calculation of five thermodynamic molecular descriptors by means of a general computer algorithm based on the group-additivity method: Standard enthalpies of vaporization, sublimation and solvation, and entropy of fusion of ordinary organic molecules and total phase-change entropy of liquid crystals. Molecules. 2017;22:1059. doi: 10.3390/molecules22071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naef R., Acree W.E. Application of a general computer algorithm based on the group-additivity method for the calculation of two molecular descriptors at both ends of dilution: Liquid viscosity and activity coefficient in water at infinite dilution. Molecules. 2018;23:5. doi: 10.3390/molecules23010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naef R., Acree W.E., Jr. Calculation of the surface tension of ordinary organic and ionic liquids by means of a generally applicable computer algorithm based on the group-additivity method. Molecules. 2018;23:1224. doi: 10.3390/molecules23051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naef R. Calculation of the Isobaric Heat Capacities of the Liquid and Solid Phase of Organic Compounds at 298.15 K by Means of the Group-Additivity Method. Molecules. 2020;25:1147. doi: 10.3390/molecules25051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardtwig E. Fehler- und Ausgleichsrechnung. Hochschultaschenbücher 262/262a. Bibliographisches Institut AG; Mannheim, Germany: 1968. [Google Scholar]
- 23.McDonald R.A., Shrader S.A., Stull D.R. Vapor Pressures and Freezing Points of 30 Organics. J. Chem. Eng. Data. 1959;4:311–313. doi: 10.1021/je60004a009. [DOI] [Google Scholar]
- 24.Quina F.H., Carroll F.A., Cheuy D.M. A Linear Solvation Energy Relationship to Predict Vapor Pressure from Molecular Structure. J. Braz. Chem. Soc. 2005;16:1010–1016. doi: 10.1590/S0103-50532005000600019. [DOI] [Google Scholar]
- 25.Katritzky A.R., Slavov S.H., Dobchev D.A., Karelson M. Rapid QSPR model development technique for prediction of vapor pressure of organic compounds. Comp. Chem. Eng. 2007;31:1123–1130. doi: 10.1016/j.compchemeng.2006.10.001. [DOI] [Google Scholar]
- 26.Poling B.E., Thomson G.H., Friend D.G., Rowley R.L., Wilding W.V. Perry’s Chemical Engineers’ Handbook. 8th ed. The McGraw-Hill Companies; New York, NY, USA: 2008. Physical and Chemical Data. [DOI] [Google Scholar]
- 27.Lepori L., Matteoli E., Gianni P. Vapor Pressure and Its Temperature Dependence of 28 Organic Compounds: Cyclic Amines, Cyclic Ethers, and Cyclic and Open Chain Secondary Alcohols. J. Chem. Eng. Data. 2017;62:194–203. doi: 10.1021/acs.jced.6b00576. [DOI] [Google Scholar]
- 28.Costa J.C.S., Mendes A., Santos L.M.B.F. Chain Length Dependence of the Thermodynamic Properties of n-Alkanes and their Monosubstituted Derivatives. J. Chem. Eng. Data. 2018;63:1–20. doi: 10.1021/acs.jced.7b00837. [DOI] [Google Scholar]
- 29.Costa J.C.S., Santos L.M.B.F. Chain-Length Dependence of the Thermodynamic Behavior of Homologous α,ω-Disubstituted Alkanes. J. Chem. Eng. Data. 2019;64:2229–2246. doi: 10.1021/acs.jced.9b00125. [DOI] [Google Scholar]
- 30.Dang C., Bannan T., Shelley P., Priestley M., Worrall S.D., Waters J., Coe H., Percival C.J., Topping D. The effect of structure and isomerism on the vapor pressures of organic molecules and its potential atmospheric relevance. Aerosol Sci. Tech. 2019;53:1040–1055. doi: 10.1080/02786826.2019.1628177. [DOI] [Google Scholar]
- 31.Noyes R.M., Noyes W.A., Steinmetz H. Vapor Pressures of cis and trans Disubstituted Ethylenes. J. Am. Chem. Soc. 1950;72:33–34. doi: 10.1021/ja01157a008. [DOI] [Google Scholar]
- 32.Nass K., Lenoir D., Kettrup A. Calculation of the Thermodynamic Properties of Polycyclic Aromatic Hydrocarbons by an Incremental Procedure. Angew. Chem Int. Ed. Engl. 1995;34:1735–1736. doi: 10.1002/anie.199517351. [DOI] [Google Scholar]
- 33.Verevkin S.P. Thermochemical Properties of Diphenylalkanes. J. Chem. Eng. Data. 1999;44:175–179. doi: 10.1021/je980200e. [DOI] [Google Scholar]
- 34.Mokbel I., Ruzicka K., Majer V., Ruzicka V., Ribeiro M., Jose J., Zabransky M. Vapor pressures and thermal data for three high-boiling compounds of petroleum interest: 1-phenyldodecane, (5α)-cholestane, adamantane. Fluid Phase Equil. 2000;169:191–207. doi: 10.1016/S0378-3812(99)00342-8. [DOI] [Google Scholar]
- 35.Lei Y.D., Chankalal R., Chan A., Wania F. Supercooled Liquid Vapor Pressures of the Polycyclic Aromatic Hydrocarbon. J. Chem. Eng. Data. 2002;47:801–806. doi: 10.1021/je0155148. [DOI] [Google Scholar]
- 36.Chickos J.S., Hanshaw W. Vapor Pressures and Vaporization Enthalpies of the n-Alkanes from C21 to C30 at T = 298.15 K by Correlation Gas Chromatography. J. Chem. Eng. Data. 2004;49:77–85. doi: 10.1021/je0301747. [DOI] [Google Scholar]
- 37.Haftka J.J.H., Parsons J.R., Govers H.A.J. Supercooled liquid vapour pressures and related thermodynamic properties of polycyclic aromatic hydrocarbons determined by gas chromatography. J. Chromatogr. A. 2006;1135:91–100. doi: 10.1016/j.chroma.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 38.Chickos J., Wang T., Sharma E. Hypothetical Thermodynamic Properties: Vapor Pressures and Vaporization Enthalpies of the Even n-Alkanes from C40 to C76 at T = 298.15 K by Correlation-Gas Chromatography. Are the Vaporization Enthalpies a Linear Function of Carbon Number? J. Chem. Eng. Data. 2008;53:481–491. doi: 10.1021/je7005852. [DOI] [Google Scholar]
- 39.Chickos J., Lipkind D. Hypothetical Thermodynamic Properties: Vapor Pressures and Vaporization Enthalpies of the Even n-Alkanes from C78 to C92 at T = 298.15 K by Correlation-Gas Chromatography. J. Chem. Eng. Data. 2008;53:2432–2440. doi: 10.1021/je800442s. [DOI] [Google Scholar]
- 40.Hanshaw W., Nutt M., Chickos J.S. Hypothetical Thermodynamic Properties. Subcooled Vaporization Enthalpies and Vapor Pressures of Polyaromatic Hydrocarbons. J. Chem. Eng. Data. 2008;53:1903–1913. doi: 10.1021/je800300x. [DOI] [Google Scholar]
- 41.Stejfa V., Fulem M., Ruzicka K., Cervinka C., Rocha M.A.A., Santos L.M.N.B.F., Schröder B. Thermodynamic study of selected monoterpenes. J. Chem. Thermodyn. 2013;60:117–125. doi: 10.1016/j.jct.2013.01.009. [DOI] [Google Scholar]
- 42.Stejfa V., Fulem M., Ruzicka K., Cervinka C. Thermodynamic study of selected monoterpenes II. J. Chem. Thermodyn. 2014;79:272–279. doi: 10.1016/j.jct.2013.12.012. [DOI] [Google Scholar]
- 43.Stejfa V., Fulem M., Ruzicka K., Cervinka C. Thermodynamic study of selected monoterpenes III. J. Chem. Thermodyn. 2014;79:280–289. doi: 10.1016/j.jct.2014.04.022. [DOI] [Google Scholar]
- 44.Zaitsaua D.H., Emel’yanenko V.N., Pimerzin A.A., Verevkin S.P. Benchmark properties of biphenyl as a liquid organic hydrogen carrier: Evaluation of thermochemical data with complementary experimental and computational methods. J. Chem. Thermodyn. 2018;122:1–12. doi: 10.1016/j.jct.2018.02.025. [DOI] [Google Scholar]
- 45.Orf M., Kurian M., Espinosa L., Nelson C., Simmons D., Chickos J. Thermochemical properties of sesquiterpenes in natural products by correlation gas chromatography: Application to bergamotene oil. J. Chem. Thermodyn. 2018;126:128–136. doi: 10.1016/j.jct.2018.06.017. [DOI] [Google Scholar]
- 46.Zafar A., Chickos J. The vapor pressure and vaporization enthalpy of squalene and squalene by correlation gas chromatography. J. Chem. Thermodyn. 2019;135:192–197. doi: 10.1016/j.jct.2019.03.032. [DOI] [Google Scholar]
- 47.Siripoltangman N., Chickos J. Vapor pressure and vaporization enthalpy studies of the major components of ginger, α-zingiberene, β-sesquiphellandrene and (-) ar curcumene by correlation chromatography. J. Chem. Thermodyn. 2019;138:107–115. doi: 10.1016/j.jct.2019.06.011. [DOI] [Google Scholar]
- 48.Fischer-Lodike C., Zafar A., Chickos J. The vapor pressure and vaporization enthalpy of pristane and phytane by correlation gas chromatography. J. Chem. Thermodyn. 2020;141:105931. doi: 10.1016/j.jct.2019.105931. [DOI] [Google Scholar]
- 49.Stejfa V., Fulem M., Ruzicka K. Thermodynamic study of selected monoterpenes IV. Chem. Thermodyn. 2020;144:106013. doi: 10.1016/j.jct.2019.106013. [DOI] [Google Scholar]
- 50.Barton D., Chickos J. The vapor pressure and vaporization enthalpy of (-) β-Elemene and (-) β-Bisabolene By correlation gas chromatography. J. Chem. Thermodyn. 2020;148:106139. doi: 10.1016/j.jct.2020.106139. [DOI] [Google Scholar]
- 51.Stejfa V., Mahnel T., Skorepova E., Rohli cek J., Eigner V., Schröder B., Ruzicka K., Fulem M. A combined thermodynamic and crystallographic study of 1,3-diisopropylnaphthalene. J. Chem. Thermodyn. 2020;150:106193. doi: 10.1016/j.jct.2020.106193. [DOI] [Google Scholar]
- 52.Pimenova S.M., Lukyanova V.A., Druzhinina A.I., Dorofeeva O.V. Thermodynamic properties of 1,3,3-trimethylcyclopropene. J. Chem. Thermodyn. 2020;151:106240. doi: 10.1016/j.jct.2020.106240. [DOI] [Google Scholar]
- 53.N’Guimbi J., Berro C., Mokbel I., Rauzy E., Jose J. Experimental vapour pressures of 13 secondary and tertiary alcohols—correlation and prediction by a group contribution method. Fluid Phase Equil. 1999;162:143–158. doi: 10.1016/S0378-3812(99)00168-5. [DOI] [Google Scholar]
- 54.Verevkin S.P. Determination of vapor pressures and enthalpies of vaporization of 1,2-alkanediols. Fluid Phase Equil. 2004;224:23–29. doi: 10.1016/j.fluid.2004.05.010. [DOI] [Google Scholar]
- 55.Umnahanant P., Kweskin S., Nichols G., Dunn M.J., Smart-Ebinne H., Chickos J.S. Vaporization Enthalpies of the α,ω-Alkanediols by Correlation Gas Chromatography. J. Chem. Eng. Data. 2006;51:2246–2254. doi: 10.1021/je060333x. [DOI] [Google Scholar]
- 56.Stejfa V., Bazyleva A., Fulem M., Rohli cek J., Skorepova E., Ruzicka K., Blokhin A.V. Polymorphism and thermophysical properties of l- and dl-menthol. J. Chem. Thermodyn. 2018;131:524–543. doi: 10.1016/j.jct.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albinsaad M., Scott N.I., Chickos J. Vapor Pressures and Vaporization Enthalpies of 1-Nonadecanol. Isophytol, 2 Z- and 2 E-Phytol, and (2E,7R,11R) phytol by Correlation Gas Chromatography. J. Chem. Thermodyn. 2020;153:106307. doi: 10.1016/j.jct.2020.106307. [DOI] [Google Scholar]
- 58.Pokorny V., Stejfa V., Klajmon M., Fulem M., Ruzicka K. Vapor Pressures and Thermophysical Properties of 1-Heptanol, 1-Octanol, 1-Nonanol, and 1-Decanol: Data Reconciliation and PC-SAFT Modeling. J. Chem. Eng. Data. 2020;66:805–821. doi: 10.1021/acs.jced.0c00878. [DOI] [Google Scholar]
- 59.Verevkin S.P. Thermochemistry of phenols: Quantification of the ortho-, para-, and meta-interactions in tert-alkyl substituted phenols. J. Chem. Thermodyn. 1999;31:559–585. doi: 10.1006/jcht.1998.0459. [DOI] [Google Scholar]
- 60.Freitas V.L.S., Lima A.C.M.O., Sapei E., Ribeiro da Silva M.D.M.C. Comprehensive thermophysical and thermochemical studies of vanillyl alcohol. J. Chem. Thermodyn. 2016;102:287–292. doi: 10.1016/j.jct.2016.07.015. [DOI] [Google Scholar]
- 61.del Rio A., Coto B., Pando C., Renuncio J.A.R. Vapor–liquid equilibria and excess properties of octane + 1,1-dimethylpropyl methyl ether (TAME) mixtures. Fluid Phase Equil. 2002;200:41–51. doi: 10.1016/S0378-3812(02)00011-0. [DOI] [Google Scholar]
- 62.Verevkin S.P., Krasnykh E.L., Vasiltsova T.V., Heintz A. Determination of Ambient Temperature Vapor Pressures and Vaporization Enthalpies of Branched Ethers. J. Chem. Eng. Data. 2003;48:591–599. doi: 10.1021/je0255980. [DOI] [Google Scholar]
- 63.Efimova A.A., Druzhinina A.I., Varushchenko R.M., Dorofeeva O.V., Krasnykh E.L. Phase Equilibria and Thermodynamic Properties of Some Branched Alkyl Ethers. J. Chem. Eng. Data. 2009;54:2457–2469. doi: 10.1021/je800998u. [DOI] [Google Scholar]
- 64.Verevkin S.P., Siewert R., Emel’yanenko V.N., Bara J.E., Cao H., Pimerzin A.A. Diphenyl Ether Derivatives as Potential Liquid Organic Hydrogen Carriers: Thermochemical and Computational Study. J. Chem. Eng. Data. 2020;65:1108–1116. doi: 10.1021/acs.jced.9b00502. [DOI] [Google Scholar]
- 65.Jiang H., Li H., Wang C., Tan T., Han S. (Vapour + liquid) equilibria for (2,2-dimethoxypropane + methanol) and (2,2-dimethoxypropane + acetone) J. Chem. Thermodyn. 2003;35:1567–1572. doi: 10.1016/S0021-9614(03)00146-0. [DOI] [Google Scholar]
- 66.Verevkin S.P., Konnova M.E., Zherikova K.V., Pimerzin A.A. Thermodynamics of glycerol and diols valorisation via reactive systems of acetals synthesis. Fluid Phase Equil. 2020;510:112503. doi: 10.1016/j.fluid.2020.112503. [DOI] [Google Scholar]
- 67.de Kruif C.G., Oonk H.A.J. Enthalpies of vaporization and vapour pressures of seven aliphatic carboxylic acids. J. Chem. Tlermodyn. 1979;11:287–290. doi: 10.1016/0021-9614(79)90153-8. [DOI] [Google Scholar]
- 68.Ribeiro da Silva M.A.V., Monte M.J.S., Ribeiro J.R. Vapour pressures and the enthalpies and entropies of sublimation of five dicarboxylic acids. J. Chem. Thermodyn. 1999;31:1093–1107. doi: 10.1006/jcht.1999.0522. [DOI] [Google Scholar]
- 69.Lagoa A.L.C., Diogo H.P., Minas da Piedade M.E., Amaral L.M.P.F., Guedes R.C., Costa Cabral B.J., Kulikov D.V., Verevkin S.P., Siedler M., Epple M. Energetics of the C-Cl Bond in CH3CH(Cl)COOH. Enthalpy of Formation of (S)-(-)-2-Chloropropionic Acid and of the 1-Carboxyethyl Radical. J. Phys. Chem. A. 2002;106:9855–9861. doi: 10.1021/jp020412p. [DOI] [Google Scholar]
- 70.Bruns E.A., Greaves J., Finlayson-Pitts B.J. Measurement of Vapor Pressures and Heats of Sublimation of Dicarboxylic Acids Using Atmospheric Solids Analysis Probe Mass Spectrometry. J. Phys. Chem. A. 2012;116:5900–5909. doi: 10.1021/jp210021f. [DOI] [PubMed] [Google Scholar]
- 71.Huisman A.J., Krieger U.K., Zuend A., Marcolli C., Peter T. Vapor pressures of substituted polycarboxylic acids are much lower than previously reported. Atmos. Chem. Phys. 2013;13:6647–6662. doi: 10.5194/acp-13-6647-2013. [DOI] [Google Scholar]
- 72.Devore J.A., O’Neal H.E. Heats of Formation of the Acetyl Halides and of the Acetyl Radical. J. Phys. Chem. 1969;73:2644–2648. doi: 10.1021/j100842a031. [DOI] [Google Scholar]
- 73.Bureau N., Mokbel J.J.I., de Hemptinne J.-C. Vapour pressure measurements and prediction for heavy esters. J. Chem. Thermodyn. 2001;33:1485–1498. doi: 10.1006/jcht.2001.0859. [DOI] [Google Scholar]
- 74.Chickos J.S., Zhao H., Nichols G. The vaporization enthalpies and vapor pressures of fatty acid methyl esters C18, C21 to C23, and C25 to C29 by correlation – gas chromatography. Thermochim. Acta. 2004;424:111–121. doi: 10.1016/j.tca.2004.05.020. [DOI] [Google Scholar]
- 75.Rohac V., Ruzicka K., Ruzicka V., Zaitsau D.H., Kabo G.J., Diky V., Aim K. Vapour pressure of diethyl phthalate. J. Chem. Thermodyn. 2004;36:929–937. doi: 10.1016/j.jct.2004.07.025. [DOI] [Google Scholar]
- 76.Vasiltsova T.V., Verevkin S.P., Bich E., Heintz A., Bogel-Lukasik R., Domanska U. Thermodynamic Properties of Mixtures Containing Ionic Liquids. 7. Activity Coefficients of Aliphatic and Aromatic Esters and Benzylamine in 1-Methyl-3-ethylimidazolium Bis(trifluoromethylsulfonyl) Imide Using the Transpiration Method. J. Chem. Eng. Data. 2006;51:213–218. doi: 10.1021/je050334+. [DOI] [Google Scholar]
- 77.Lipp S.V., Krasnykh E.L. Vapor Pressures and Enthalpies of Vaporization of a Series of the Symmetric Linear n-Alkyl Esters of Dicarboxylic Acids. J. Chem. Eng. Data. 2011;56:800–810. doi: 10.1021/je100231g. [DOI] [Google Scholar]
- 78.Gobble C., Chickos J. Vapor Pressures and Vaporization Enthalpies of a Series of Dialkyl Phthalates by Correlation Gas Chromatography. J. Chem. Eng. Data. 2014;59:1353–1365. doi: 10.1021/je500110d. [DOI] [Google Scholar]
- 79.Kozlovskiy M., Gobble C., Chickos J. Vapor pressures and vaporization enthalpies of a series of esters used in flavors by correlation gas chromatography. J. Chem. Thermodyn. 2015;86:65–74. doi: 10.1016/j.jct.2015.02.015. [DOI] [Google Scholar]
- 80.Ishak H., Stephan J., Karam R., Goutaudier C., Mokbel I., Saliba C., Saab J. Aqueous solubility, vapor pressure and octanol-water partition coefficient of two phthalate isomers dibutyl phthalate and di-isobutyl phthalate contaminants of recycled food packages. Fluid Phase Equil. 2016;427:362–370. doi: 10.1016/j.fluid.2016.07.018. [DOI] [Google Scholar]
- 81.Simmons D., Chickos J. Enthalpy of vaporization and vapor pressure of whiskey lactone and menthalactone by correlation gas chromatography. J. Chem. Thermodyn. 2017;110:65–70. doi: 10.1016/j.jct.2017.02.010. [DOI] [Google Scholar]
- 82.Emel’yanenko V.N., Altuntepe E., Held C., Pimerzin A.A., Verevkin S.P. Renewable platform chemicals: Thermochemical study of levulinic acid esters. Thermochim. Acta. 2018;659:213–221. doi: 10.1016/j.tca.2017.12.006. [DOI] [Google Scholar]
- 83.Verevkin S.P., Emel’yanenko V.N., Pimerzin A.A., Yermalayeu A.V. How much different are thermochemical properties of enantiomers and their racemates? Thermochemical properties of enantiopure and racemate of methyl- and butyl lactates. J. Chem. Phys. 2018;149:054506. doi: 10.1063/1.5029433. [DOI] [PubMed] [Google Scholar]
- 84.Emel’yanenko V.N., Yermalayeu A.V., Portnova S.V., Pimerzin A.A., Verevkin S.P. Renewable platform chemicals: Evaluation of thermochemical data of alkyl lactates with complementary experimental and computational methods. J. Chem. Thermodyn. 2019;128:55–67. doi: 10.1016/j.jct.2018.07.029. [DOI] [Google Scholar]
- 85.Portnova S.V., Yamshchikova Y.F., Krasnykh E.L., Nikitin E.D., Popov A.P., Faizullin M.Z. Vapor Pressure, Vaporization Enthalpies, Critical Parameters, and Heat Capacities of Alkyl Glycolates. J. Chem. Eng. Data. 2020;65:2566–2577. doi: 10.1021/acs.jced.9b01195. [DOI] [Google Scholar]
- 86.Siewert R., Zaitsau D.H., Emel’yanenko V.N., Verevkin S.P. Biomass Valorization: Thermodynamics of the Guerbet Condensation Reaction. J. Chem. Eng. Data. 2019;64:4904–4914. doi: 10.1021/acs.jced.9b00419. [DOI] [Google Scholar]
- 87.Francesconi R., Comelli F. Vapor-Liquid Equilibria, Excess Molar Enthalpies, and Excess Molar Volumes of Dialkyl Carbonates + Methyl tert-Butyl Ether at 298.15 K. J. Chem. Eng. Data. 1997;42:697–701. doi: 10.1021/je960342d. [DOI] [Google Scholar]
- 88.Chernyak Y., Clements J.H. Vapor Pressure and Liquid Heat Capacity of Alkylene Carbonates. J. Chem. Eng. Data. 2004;49:1180–1184. doi: 10.1021/je034173q. [DOI] [Google Scholar]
- 89.Kozlova S.A., Emel’yanenko V.N., Georgieva M., Verevkin S.P., Chernyak Y., Schäffner B., Börner A. Vapour pressure and enthalpy of vaporization of aliphatic dialkyl carbonates. J. Chem. Thermodyn. 2008;40:1136–1140. doi: 10.1016/j.jct.2008.02.012. [DOI] [Google Scholar]
- 90.Verevkin S.P., Toktonov A.V., Chernyak Y., Schäffner B., Börner A. Vapour pressure and enthalpy of vaporization of cyclic alkylene carbonates. Fluid Phase Equil. 2008;268:1–6. doi: 10.1016/j.fluid.2008.03.013. [DOI] [Google Scholar]
- 91.Pokorny V., Stejfa V., Fulem M., Cervinka C., Ruzicka K. Vapor Pressures and Thermophysical Properties of Ethylene Carbonate, Propylene Carbonate, γ-Valerolactone, and γ-Butyrolactone. J. Chem. Eng. Data. 2017;62:4174–4186. doi: 10.1021/acs.jced.7b00578. [DOI] [Google Scholar]
- 92.Jimenez P., Roux M.V., Davalos J.Z., Martin-Luengo M.A., Abboud J.-L. Structural effects on the thermochemical properties of cycloalkanones II. Enthalpy of combustion, vapour pressures, enthalpy of sublimation, and standard molar enthalpy of formation in the gaseous phase of cyclopentadecanone. J. Chem. Thermodyn. 1997;29:1281–1288. doi: 10.1006/jcht.1997.0244. [DOI] [Google Scholar]
- 93.Zaitsau D.H., Verevkin S.P., Sazonova A.Y. Vapor pressures and vaporization enthalpies of 5-nonanone, linalool and 6-methyl-5-hepten-2-one. Data evaluation. Fluid Phase Equil. 2015;386:140–148. doi: 10.1016/j.fluid.2014.11.026. [DOI] [Google Scholar]
- 94.Almeida A.R.R.P., Monte M.J.S. Vapour pressures and phase transition properties of four substituted acetophenones. J. Chem. Thermodyn. 2017;107:42–50. doi: 10.1016/j.jct.2016.12.012. [DOI] [Google Scholar]
- 95.Indritz D., Stone J., Williams F. Vapor Pressure of Di-tert-butyl Peroxide. J. Chem. Eng. Data. 1978;23:6–7. doi: 10.1021/je60076a002. [DOI] [Google Scholar]
- 96.Oxley J.C., Smith J.L., Luo W., Brady J. Determining the Vapor Pressures of Diacetone Diperoxide (DADP), and Hexamethylene Triperoxide Diamine (HMTD) Propellants Explos. Pyrotech. 2009;34:539–543. doi: 10.1002/prep.200800073. [DOI] [Google Scholar]
- 97.Messerly J.F., Finke H.L., Osborn A.G., Douslin D.D. Low-temperature calorimetric and vaporpressure studies on alkanediamines. J. Chem. Thermodyn. 1975;7:1029–1046. doi: 10.1016/0021-9614(75)90237-2. [DOI] [Google Scholar]
- 98.Verevkin S.P., Beckhaus H.-D., Schüle U., Rüchardt C. Experimental Enthalpies of Formation and Strain of the Methylated l-Amino-2-phenylethanes. Struct. Chem. 1998;9:1–7. doi: 10.1023/A:1022475329407. [DOI] [Google Scholar]
- 99.Verevkin S.P., Georgieva M., Melkhanova S.V. Vapor Pressures and Phase Transitions of a Series of the Aminonaphthalenes. J. Chem. Eng. Data. 2007;52:286–290. doi: 10.1021/je060394v. [DOI] [Google Scholar]
- 100.Razzouk A., Hajiaji A., Mokbel I., Mougin P., Jose J. Experimental vapor pressures of 1,2-bis(dimethylamino)ethane, 1-methylmorpholine, 1,2-bis(2-aminoethoxy)ethane and N-benzylethanolamine between 273.18 and 364.97 K. Fluid Phase Equil. 2009;282:11–13. doi: 10.1016/j.fluid.2009.04.006. [DOI] [Google Scholar]
- 101.Efimova A.A., Emel’yanenko V.N., Verevkin S.P., Chernyak Y. Vapour pressure and enthalpy of vaporization of aliphatic poly-amines. J. Chem. Thermodyn. 2010;42:330–336. doi: 10.1016/j.jct.2009.09.003. [DOI] [Google Scholar]
- 102.Gobble C., Rath N., Chickos J. The Vaporization Enthalpies and Vapor Pressures of Some Primary Amines of Pharmaceutical Importance by Correlation Gas Chromatography. J. Chem. Eng. Data. 2013;58:2600–2609. doi: 10.1021/je400498a. [DOI] [Google Scholar]
- 103.Fulem M., Ruzicka K., Cervinka C., Bazyleva A., Della Gatta G. Thermodynamic study of alkane-α,ω-diamines – Evidence of odd–even pattern of sublimation properties. Fluid Phase Equil. 2014;371:93–105. doi: 10.1016/j.fluid.2014.03.013. [DOI] [Google Scholar]
- 104.Bouzina Z., Negadi A., Mokbel I., Jose J., Negadi L. Phase equilibrium properties of binary mixtures containing 1,3-pentanediamine (or 1,5-diamino-2-methylpentane) and water at several temperatures. J. Chem. Thermodyn. 2015;84:81–86. doi: 10.1016/j.jct.2014.12.008. [DOI] [Google Scholar]
- 105.Bouzina Z., Dergal F., Mokbel I., Negadi A., Saab J. Liquid-vapor equilibria of pure and aqueous solutions of diethylenetriamine or dipropylenetriamine. Fluid Phase Equil. 2016;414:164e169. doi: 10.1016/j.fluid.2016.01.022. [DOI] [Google Scholar]
- 106.Emel’yanenko V.N., Turovtsev V.V., Orlov Y.D., Fedina Y.A. Vapour pressure and enthalpy of vaporization of cyclic imines. Thermochim. Acta. 2019;682:178049. doi: 10.1016/j.tca.2018.07.016. [DOI] [Google Scholar]
- 107.Zaitseva K.V., Zaitsau D.H., Varfolomeev M.A., Verevkin S.P. Vapour pressures and enthalpies of vaporisation of alkyl formamides. Fluid Phase Equil. 2019;494:228–238. doi: 10.1016/j.fluid.2019.04.036. [DOI] [Google Scholar]
- 108.Zaitseva K.V., Zaitsau D.H., Varfolomeev M.A., Verevkin S.P. Vapour pressures and enthalpies of vaporisation of N alkyl acetamides. J. Mol. Liquids. 2019;293:111453. doi: 10.1016/j.molliq.2019.111453. [DOI] [Google Scholar]
- 109.Zaitseva K.V., Zaitsau D.H., Varfolomeev M.A., Verevkin S.P. Vapour pressures and enthalpies of vaporisation of N,N-di-alkyl-acetamides. Fluid Phase Equil. 2019;499:112241. doi: 10.1016/j.fluid.2019.112241. [DOI] [Google Scholar]
- 110.Stejfa V., Chun S., Pokorny V., Fulem M., Ruzicka K. Thermodynamic study of acetamides. J. Mol. Liquids. 2020;319:114019. doi: 10.1016/j.molliq.2020.114019. [DOI] [Google Scholar]
- 111.Verevkin S.P., Emel’yanenko V.N., Algarra M., Lopez-Romero J.M., Aguiar F., Rodriguez-Borges J.E. Esteves da Silva, Vapor pressures and enthalpies of vaporization of azides. J. Chem. Thermodyn. 2011;43:1652–1659. doi: 10.1016/j.jct.2011.05.028. [DOI] [Google Scholar]
- 112.Emel’yanenko V.N., Algarra M., Esteves da Silva J.C.G., Hierrezuelo J., Lopez-Romero J.M., Verevkin S.P. Thermochemistry of organic azides revisited. Thermochim. Acta. 2014;597:78–84. doi: 10.1016/j.tca.2014.10.015. [DOI] [Google Scholar]
- 113.Scott D.W., Oliver G.D., Gross M.E., Hubbard W.N., Huffman H.M. Hydrazine: Heat Capacity, Heats of Fusion and Vaporization, Vapor Pressure, Entropy and Thermodynamic Functions. J. Am. Chem. Soc. 1949;71:2293–2297. doi: 10.1021/ja01175a007. [DOI] [Google Scholar]
- 114.Aston J.G., Fink H.L., Janz G.J., Russell K.E. The Heat Capacity, Heats of Fusion and Vaporization, Vapor Pressures, Entropy and Thermodynamic Functions of Methylhydrazine. J. Am. Chem. Soc. 1951;73:1939–1943. doi: 10.1021/ja01149a010. [DOI] [Google Scholar]
- 115.Aston J.G., Janz G.J., Russell K.E. The Heat Capacity, Heats of Fusion and Vaporization, Vapor Pressures and Entropy of Symmetrical Dimethylhydrazine. J. Am. Chem. Soc. 1951;73:1943–1945. doi: 10.1021/ja01149a011. [DOI] [Google Scholar]
- 116.Ahmad S., Giesen R., Lucas K. Vapor-Liquid Equilibrium Studies for Systems Containing n-Butylisocyanate at Temperatures between 323.15 K and 371.15 K. J. Chem. Eng. Data. 2004;49:826–831. doi: 10.1021/je030222n. [DOI] [Google Scholar]
- 117.Ramos L.A., Ulic S.E., Romano R.M., Vishnevskiy Y.V., Mitzel N.W., Beckers H., Willner H., Tong S., Ge M., Della Vedova C.O. Chlorodifluoroacetyl Isothiocyanate, ClF2CC(O)NCS: Preparation and Structural and Spectroscopic Studies. J. Phys. Chem. A. 2013;117:5597–5606. doi: 10.1021/jp403549f. [DOI] [PubMed] [Google Scholar]
- 118.Davis P.S., Kilpatrick J.E. Entropy, related thermodynamic properties, and structure of methylisocyanate. J. Chem. Thermodyn. 2013;58:134–141. doi: 10.1016/j.jct.2012.10.011. [DOI] [Google Scholar]
- 119.Xu M., Wu L., Zhang J. Measurement of vapor-liquid equilibrium data and azeotropic analysis for 2,4-toluene diisocyanate+entrainer. CIESC. 2016 doi: 10.11949/j.issn.0438-1157.20150433. [DOI] [Google Scholar]
- 120.Woodman A.L., Murbach W.J., Kaufman M.H. Vapor Pressure and Viscosity Relationships for a Homologous Series of α,ι-Dinitriles. J. Phys. Chem. 1960;64:658–660. doi: 10.1021/j100834a032. [DOI] [Google Scholar]
- 121.Cao Y., Dai X., Song H., Yao S., Lan X. Vapor Pressure Measurement and Isobaric Vapor−Liquid Equilibria for Binary Mixtures of ZE-2-Methyl-2-butenenitrile and 2-Methyl-3-butenenitrile. J. Chem. Eng. Data. 2016;61:1573–1577. doi: 10.1021/acs.jced.5b00943. [DOI] [Google Scholar]
- 122.Toops Jr. E.E. Physical Properties of eight High-Purity Nitroparaffins. J. Phys. Chem. 1956;60:304–306. doi: 10.1021/j150537a012. [DOI] [Google Scholar]
- 123.Verevkin S.P. Thermochemistry of nitro compounds. Experimental standard enthalpies of formation and improved group-additivity values. Thermochim. Acta. 1997;307:17–25. doi: 10.1016/S0040-6031(97)00359-6. [DOI] [Google Scholar]
- 124.Verevkin S.P., Heintz A. Thermochemistry of substituted benzenes: Quantification of ortho-, para-, meta-, and buttress interactions in alkyl-substituted nitrobenzenes. J. Chem. Thermodyn. 2000;32:1169–1182. doi: 10.1006/jcht.2000.0589. [DOI] [Google Scholar]
- 125.Ribeiro da Silva M.A.V., Lima L.M.S.S., Amaral L.M.P.F., Ferreira A.I.M.C.L., Gomes J.R.B. Standard molar enthalpies of formation, vapour pressures, and enthalpies of sublimation of 2-chloro-4-nitroaniline and 2-chloro-5-nitroaniline. J. Chem. Thermodyn. 2003;35:1343–1359. doi: 10.1016/S0021-9614(03)00109-5. [DOI] [Google Scholar]
- 126.Ribeiro da Silva M.A.V., Monte M.J.S., Ferreira A.I.M.C.L., Oliveira J.A.S.A., Cimas A. Experimental and Computational Thermodynamic Study of Three Monofluoronitrobenzene Isomers. J. Phys. Chem. B. 2010;114:7909–7919. doi: 10.1021/jp102024y. [DOI] [PubMed] [Google Scholar]
- 127.Thompson H.W., Dainton F.S. The Photochemistry of Alkyl Nitrites. Trans. Faraday Soc. 1937;33:1546–1555. doi: 10.1039/tf9373301546. [DOI] [Google Scholar]
- 128.Fischer R.G., Ballschmiter K. Prediction of the environmental distribution of alkyl dinitrates—Chromatographic determination of vapor pressure p0, water solubility SH2O, gas-water partition coefficient KGW (Henry’s law constant) and octanol-water partition coefficient KOW. Fresenius, J. Anal. Chem. 1998;360:769–776. doi: 10.1007/s002160050803. [DOI] [Google Scholar]
- 129.Klein R.G. Calculations and Measurements on the Volatility of Nitrosamines and their Aqueous Solutions. Toxicology. 1982;23:135–147. doi: 10.1016/0300-483X(82)90093-2. [DOI] [PubMed] [Google Scholar]
- 130.Jakli G., Van Hook W.A. Excess Thermodynamic Properties of H2O and D2O Solutions of Tetramethylurea, an Azeotropic System. Vapor Pressures, Excess Vapor Pressures, and Vapor Pressure Isotope Effects. J. Chem. Eng. Data. 2001;46:777–781. doi: 10.1021/je000362m. [DOI] [Google Scholar]
- 131.Ribeiro da Silva M.M.C., Ribeiro da Silva M.A.V., Freitas V.L.S., Roux M.V., Jimenez P., Temprado M., Davalos J.Z., Cabildo P., Claramunt R.M., Elguero J. Structural studies of cyclic ureas: 1. Enthalpies of formation of imidazolidin-2-one and N,N’-trimethyleneurea. J. Chem. Thermodyn. 2008;40:386–393. doi: 10.1016/j.jct.2007.08.004. [DOI] [Google Scholar]
- 132.Sawaya T., Mokbel I., Rauzy E., Saab J., Berro C., Jose J. Experimental vapor pressures of alkyl and aryl sulfides Prediction by a group contribution method. Fluid Phase Equil. 2004;226:283–288. doi: 10.1016/j.fluid.2004.10.007. [DOI] [Google Scholar]
- 133.Sassa Y., Konishi R., Katayama T. Isothermal Vapor-Liquid Equilibrium Data of DMSO Solutions by Total Pressure Method. DMSO-Acetone, DMSO-Tetrahydrofuran, and DMSO-Ethyl Acetate Systems. J. Chem. Eng. Data. 1974;19:44–48. doi: 10.1021/je60060a004. [DOI] [Google Scholar]
- 134.Markarian S.A., Zatikyan A.L., Grigoryan V.V., Grigoryan G.S. Vapor Pressures of Pure Diethyl Sulfoxide from (298.15 to 318.15) K and Vapor-Liquid Equilibria of Binary Mixtures of Diethyl Sulfoxide with Water. J. Chem. Eng. Data. 2005;50:23–25. doi: 10.1021/je034278t. [DOI] [Google Scholar]
- 135.Grigoryan G.S., Markarian S.A. Thermodynamics of Liquid–Gas Phase Equilibria in the Dipropylsulfoxide–Water System in the Range of 303.15 to 323.15 K. Russ. J. Phys. Chem. A. 2013;87:191–193. doi: 10.1134/S0036024413020027. [DOI] [Google Scholar]
- 136.Grigoryan G.S., Markarian S.A. Thermodynamics of Liquid–Gas Phase Equilibria of Diisopropyl and Dibutyl Sulfoxides. Russ. J. Phys. Chem. A. 2014;88:1071–1072. doi: 10.1134/S0036024414060132. [DOI] [Google Scholar]
- 137.Edwards D.R., Prausnitz J.M. Vapor Pressures of Some Sulfur-Containing, Coal-Related Compounds. J. Chem. Eng. Data. 1981;26:121–124. doi: 10.1021/je00024a005. [DOI] [Google Scholar]
- 138.Anton V., Artigas H., Lomba L., Giner B., Lafuente C. Thermophysical properties of the thiophene family. J. Therm. Anal. Calorim. 2016;125:509–518. doi: 10.1007/s10973-016-5448-0. [DOI] [Google Scholar]
- 139.Anton V., Giner B., Artigas H., Gascon I., Lafuente C. Comparative Study of the Thermophysical Properties of 2-Ethylthiophene and 2-Ethylfuran. J. Chem. Eng. Data. 2018;63:3274–3284. doi: 10.1021/acs.jced.8b00153. [DOI] [Google Scholar]
- 140.Burg A.B., Slota Jr. P.J. Dimethylaminodimethylphosphine. J. Am. Chem. Soc. 1958;80:1107–1109. doi: 10.1021/ja01538a023. [DOI] [Google Scholar]
- 141.Burg A.B., Slota Jr. P.J. Chemistry of the C4H8P Ring: The Aminophosphine (CH3)2NPC4H8, the Cyclophosphine C4H8PH and the Tetracyclic Trimer (C4H8PBH2) J. Am. Chem. Soc. 1960;82:2148–2151. doi: 10.1021/ja01494a015. [DOI] [Google Scholar]
- 142.Burg A.B., Griffiths J.E. Trifluoromethyl-bromo-phosphines and Phosphoranes. J. Am. Chem. Soc. 1960;82:3514–3517. doi: 10.1021/ja01499a009. [DOI] [Google Scholar]
- 143.Holmes R.R., Wagner R.P. Phosphorus Nitrogen Chemistry. IV. The Reactions of Dimethylaminophosphines with Boron Trihalides and Trialkyls. J. Am. Chem. Soc. 1962;84:357–361. doi: 10.1021/ja00862a008. [DOI] [Google Scholar]
- 144.Burg A.B., Gosling K. Stable Thiotrifluoromethyl phosphines. J. Am. Chem. Soc. 1965;87:2113–2116. doi: 10.1021/ja01088a007. [DOI] [Google Scholar]
- 145.Michou-Saucet M.-A., Jose J., Michou-Saucet C., Merlin J.C. Pressions de Vapeur et Enthalpies Libre d’Exces de Systèmes Binaires: Hexamethylphosphorotriamide (HMPT) + n-Hexane; n-Heptane; n-Octane; A 288.15 K; 303.15 K; 313.15 K; 323.15 K; 333.15 K. Thermochim. Acta. 1984;75:85–106. doi: 10.1016/0040-6031(84)85009-1. [DOI] [Google Scholar]
- 146.Skene W.G., Krzymien M.E. Vapor Pressure of Tri-n-butyl Phosphate. J. Chem. Eng. Data. 1995;40:394–397. doi: 10.1021/je00018a006. [DOI] [Google Scholar]
- 147.Tsuzuki M. Thermodynamic Estimation of Vapor Pressure for Organophosphorous Pesticides. Environ. Tox. Chem. 2000;19:1717–1726. doi: 10.1002/etc.5620190704. [DOI] [Google Scholar]
- 148.Butrow A.B., Buchanan J.H., Tevault D.E. Vapor Pressure of Organophosphorus Nerve Agent Simulant Compounds. J. Chem. Eng. Data. 2009;54:1876–1883. doi: 10.1021/je8010024. [DOI] [Google Scholar]
- 149.Tevault D.E., Brozena A., Buchanan J.H., Abercrombie-Thomas P.L., Buettner L.C. Thermophysical Properties of VX and RVX. J. Chem. Eng. Data. 2012;57:1970–1977. doi: 10.1021/je200891j. [DOI] [Google Scholar]
- 150.Brozena A., Buchanan J.H., Miles Jr. R.W. Vapor Pressure of Triethyl and Tri-n-Propyl Phosphates and Diethyl Malonate. J. Chem. Eng. Data. 2014;59:2649–2659. doi: 10.1021/je500428d. [DOI] [Google Scholar]
- 151.Althoff M.A., Grieger K., Härtel M.A.C., Karaghiosoff K.L., Klapötke T.M., Metzulat M. Application of the Transpiration Method To Determine the Vapor Pressure and Related Physico-Chemical Data of Low Volatile, Thermolabile, and Toxic Organo(thio)phosphates. J Phys Chem. A. 2017;121:2603-–2609. doi: 10.1021/acs.jpca.7b01177. [DOI] [PubMed] [Google Scholar]
- 152.Bikelyté G., Härtel M.A.C., Klapötke T.M., Krumm B., Sadaunykas A. Experimental thermochemical data of CWA simulants: Triethyl phosphate, diethyl ethylphosphonate, malathion and methyl salicylate. J. Chem. Thermodyn. 2020;143:106043. doi: 10.1016/j.jct.2019.106043. [DOI] [Google Scholar]
- 153.Schlesinger H.I., Ritter D.M., Burg A.B. Hydrides of Boron. IX. The Preparation of Some Methyl Triborine Triamines. J. Am. Chem. Soc. 1938;60:1296–1300. doi: 10.1021/ja01273a006. [DOI] [Google Scholar]
- 154.Burg A.B., Wagner R.I. Chemistry of S-B Bonding: Polymeric Thioborines, Methanethiodiborane and Related Substances. J. Am. Chem. Soc. 1954;76:3307–3310. doi: 10.1021/ja01641a069. [DOI] [Google Scholar]
- 155.Burg A.B., Good C.D. Nitrogen bond strain effects in the chemistry of ring-amino boron hydrides. J. Inorg. Nucl. Chem. 1956;2:237–245. doi: 10.1016/0022-1902(56)80050-X. [DOI] [Google Scholar]
- 156.Parsons T.D., Silverman M.B., Ritter D.M. Alkenylboranes. I. Preparation and Properties of Some Vinyl- and Propenylboranes. J. Am. Chem. Soc. 1957;79:5091–5098. doi: 10.1021/ja01576a005. [DOI] [Google Scholar]
- 157.Brotherton R.J., McCloskey A.L. Tetra-(amino)-diborons. J. Am. Chem. Soc. 1960;82:6242–6245. doi: 10.1021/ja01509a009. [DOI] [Google Scholar]
- 158.Brotherton R.J., McCloskey A.L., Boone J.L., Manasevit H.M. The Preparation and Properties of Some Tetraalkoxydiborons. J. Am. Chem. Soc. 1960;82:6245–6248. doi: 10.1021/ja01509a010. [DOI] [Google Scholar]
- 159.Christopher P.M., Shilman A. Vapor pressures of trialkyl borates. J. Chem. Eng. Data. 1967;12:333–335. doi: 10.1021/je60034a012. [DOI] [Google Scholar]
- 160.Burg A.B., Basi J.S. Boron bis(trifluoromethyl)phosphinites. J. Am. Chem. Soc. 1969;91:1937–1940. doi: 10.1021/ja01036a012. [DOI] [Google Scholar]
- 161.Emeléus H.J., Miller N. 175. Derivatives of monosilane. Part, I. The reactions of chlorosilane with aliphatic amines. J. Chem. Soc. 1939:819–823. doi: 10.1039/JR9390000819. [DOI] [Google Scholar]
- 162.Aston J.G., Kennedy R.M., Messerly G.H. The Heat Capacity and Entropy, Heats of Fusion and Vaporization and the Vapor Pressure of Silicon Tetramethyl. J. Am. Chem. Soc. 1941;63:2343–2348. doi: 10.1021/ja01854a012. [DOI] [Google Scholar]
- 163.Emeléus H.J., Maddock A.G. 251. The chemistry of the higher silanes. Part I. Tetrasilane. J. Chem. Soc. 1946:1131–1134. doi: 10.1039/JR9460001131. [DOI] [PubMed] [Google Scholar]
- 164.Tannenbaum S., Kaye S., Lewenz G.F. Synthesis and Properties of Some Alkylsilanes. J. Am. Chem. Soc. 1953;75:3753–3757. doi: 10.1021/ja01111a043. [DOI] [Google Scholar]
- 165.Opitz H.E., Peake J.S., Nebergall W.H. The Preparation of Monobromosilane and Organic Silyl Derivatives. J. Am. Chem. Soc. 1956;78:292–294. doi: 10.1021/ja01583a012. [DOI] [Google Scholar]
- 166.Emeléus H.J., Smythe L.E. 118. Preparation and properties of dimethyliodosilane, 1:1′:2:2′-tetramethyl-disiloxane and -disilthiane. J. Chem. Soc. 1958:609–611. doi: 10.1039/JR9580000609. [DOI] [Google Scholar]
- 167.Takagi S., Ishikawa M., Kumada M., Kimura T., Fujishiro R. Vapour Pressure and Thermodynamic Properties of Hexamethyldisilane at 305–387 K. Thermochim. Acta. 1986;109:55–61. doi: 10.1016/0040-6031(86)85007-9. [DOI] [Google Scholar]
- 168.Fulem M., Ruzicka K., Ruzicka V., Simecek T., Hulicius E., Pangrac J., Becker J., Koch J., Salzmann A. Vapor Pressure of Di-tert-butylsilane. J. Chem. Eng. Data. 2005;50:1613–1615. doi: 10.1021/je050084x. [DOI] [Google Scholar]
- 169.Sternbach B., MacDiarmid A.G. The Preparation of Methoxysilanes by the Interaction of Monosilane and Methanol. J. Am. Chem. Soc. 1959;81:5109–5110. doi: 10.1021/ja01528a023. [DOI] [Google Scholar]
- 170.Marsh K.N. Thermodynamics of octamethylcyclotetrasiloxane mixtures. Trans. Faraday Soc. 1968;64:883–893. doi: 10.1039/tf9686400883. [DOI] [Google Scholar]
- 171.van der Vis M.G.M., Cordfunke E.H.P. Tetraethoxysilane, Si(OC2H5)4: Vapour pressure measurements at temperatures from 323 to 442 K by means of a Bourdon spoon gauge. Thermochim. Acta. 1995;265:129–134. doi: 10.1016/0040-6031(95)02403-O. [DOI] [Google Scholar]
- 172.Kochetkov A., Smith J.S., Ravikrishna R., Valsaraj K.T., Thibodeaux L.J. Air-Water Partition Constants for Volatile Methyl Siloxanes. Environ. Tox. Chem. 2009;20:2184–2188. doi: 10.1002/etc.5620201008. [DOI] [PubMed] [Google Scholar]
- 173.Abbas R., Schedemann A., Ihmels C., Enders S., Gmehling J. Measurement of Thermophysical Pure Component Properties for a Few Siloxanes Used as Working Fluids for Organic Rankine Cycles. Ind. Eng. Chem. Res. 2011;50:9748–9757. doi: 10.1021/ie200256f. [DOI] [Google Scholar]
- 174.Emeléus H.J., MacDiarmid A.G., Maddock A.G. Sulphur and Selenium Derivatives of Monosilanes. J. Inorg. Nucl. Chem. 1955;1:194–201. doi: 10.1016/0022-1902(55)80056-5. [DOI] [Google Scholar]
- 175.Louis E., Urry G. Preparation of hexamethyldisilthiane. Inorg. Chem. 1968;7:1253–1254. doi: 10.1021/ic50064a051. [DOI] [Google Scholar]
- 176.Wisniewska B., Lencka M., Rogalski M. Vapour pressures of 2,4-, 2,6-, and 3,5-dimethylpyridine at temperatures from 267 to 360 K. J. Chem. Thermodyn. 1986;18:703–708. doi: 10.1016/0021-9614(86)90102-3. [DOI] [Google Scholar]
- 177.Verevkin S.P., Zaitseva K.V., Stanton A.D., Hindman M.S., Irvin A.C., Bara J.E. Building Blocks for Ionic Liquids: Vapor Pressures and Vaporization Enthalpies of N-Functionalized Imidazoles with Branched and Cycloalkyl Substituents. Ind. Eng. Chem. Res. 2015;54:9850–9856. doi: 10.1021/acs.iecr.5b01599. [DOI] [Google Scholar]
- 178.Emel’yanenko V.N., Kaliner M., Strassner T., Verevkin S.P. Thermochemical properties of different 1-(R-phenyl)-1H-imidazoles. Fluid Phase Equil. 2017;433:40–49. doi: 10.1016/j.fluid.2016.11.010. [DOI] [Google Scholar]
- 179.Safronov S.P., Nagrimanov R.N., Samatov A.A., Emel’yanenko V.N., Zaitsau D.H., Pimerzin A.A., Skrzypczak A., Verevkin S.P. Benchmark properties of pyrazole derivatives as a potential liquid organic hydrogen carrier: Evaluation of thermochemical data with complementary experimental and computational methods. J. Chem. Thermodyn. 2019;128:173–186. doi: 10.1016/j.jct.2018.07.020. [DOI] [Google Scholar]
- 180.Stiles V.E., Cady G.H. Physical Properties of Perfluoro-n-hexane and Perfluoro-2-methylpentane. J. Am. Chem. Soc. 1952;74:3771–3773. doi: 10.1021/ja01135a017. [DOI] [Google Scholar]
- 181.Barber J.B., Cady G.H. Vapor Pressures of Perfluoropentanes. J. Phys. Chem. 1956;60:504–505. doi: 10.1021/j150538a030. [DOI] [Google Scholar]
- 182.Rowlinson J.S., Thacker R. The physical properties of some fluorine compounds and their solutions. Part 3.—Perfluorocyclohexane and perfluoromethylcyclohexane. Trans. Faraday Soc. 1957;53:1–8. doi: 10.1039/TF9575300001. [DOI] [Google Scholar]
- 183.Good W.D., Douslin D.R., Scott D.W., George A., Lacina J.L., dawson J.P., Waddington G. Thermochemistry and Vapor Pressure of Aliphatic Fluorocarbons. A Comparison of the C–F and C–H Thermochemical Bond Energies. J. Phys. Chem. 1959;63:1133–1138. doi: 10.1021/j150577a024. [DOI] [Google Scholar]
- 184.Crowder G.A., Taylor Z.L., Reed III T.M., Young J.A. Vapor pressures and triple point temperatures for several pure fluorocarbons. J. Chem. Eng. Data. 1967;12:481–485. doi: 10.1021/je60035a005. [DOI] [Google Scholar]
- 185.Majer V., Svoboda V., Posta A., Pick J. Determination of heats of vaporization and some other thermodynamic quantities for three fluorinated halogen ethanes. Collect. Czech. Chem. Commun. 1981;46:817–822. doi: 10.1135/cccc19810817. [DOI] [Google Scholar]
- 186.Marrucho I.M., Oliveira N.S., Dohrn R. Vapor-Phase Thermal Conductivity, Vapor Pressure, and Liquid Density of R365mfc. J. Chem. Eng. Data. 2002;47:554–558. doi: 10.1021/je015534+. [DOI] [Google Scholar]
- 187.Dias A.M.A., Goncalves C.M.B., Caco A.I., Santos L.M.N.B.F., Pineiro M.M., Vega L.F., Coutinho J.A.P., Marrucho I.M. Densities and Vapor Pressures of Highly Fluorinated Compounds. J. Chem. Eng. Data. 2005;50:1328–1333. doi: 10.1021/je050056e. [DOI] [Google Scholar]
- 188.Sarraute S., Mokbel I., Costa Gomes M.F., Majer V., Jose J. Atmosphere/water partition of halocyclohexanes from vapour pressure and solubility data. Atmos. Env. 2008;42:4724–4734. doi: 10.1016/j.atmosenv.2008.01.041. [DOI] [Google Scholar]
- 189.Morgado P., Das G., McCabe C., Filipe E.J.M. Vapor Pressure of Perfluoroalkylalkanes: The Role of the Dipole. J. Phys. Chem. B. 2015;119:4–1623. doi: 10.1021/jp5109448. [DOI] [PubMed] [Google Scholar]
- 190.Nagrimanov R.N., Solomonov B.N., Emel’yanenko V.N., Verevkin S.P. Six-membered ring aliphatic compounds: A search for regularities in phase transitions. Thermochim. Acta. 2016;638:80–88. doi: 10.1016/j.tca.2016.06.014. [DOI] [Google Scholar]
- 191.Lister M.W. Heats of Organic Reactions. X. Heats of Bromination of Cyclic Olefins. J. Am. Chem. Soc. 1941;63:143–149. doi: 10.1021/ja01846a035. [DOI] [Google Scholar]
- 192.Bobbo S., Fedele L., Scattolini M., Camporese M. Compressed Liquid Densities, Saturated Liquid Densities, and Vapor Pressures of Hexafluoro-1,3-butadiene (C4F6) J. Chem. Eng. Data. 2002;47:179–182. doi: 10.1021/je010194d. [DOI] [Google Scholar]
- 193.Subramoney S.C., Wayne M.N., Valtz A., Coquelet C., Richon D., Naidoo P., Ramjugernath D. Pure Component and Binary Vapor-Liquid Equilibrium + Modeling for Hexafluoropropylene and Hexafluoropropylene Oxide with Toluene and Hexafluoroethane. J. Chem. Eng. Data. 2010;55:411–418. doi: 10.1021/je900400v. [DOI] [Google Scholar]
- 194.Fedele L., Di Nicola G., Brown J.S., Bobbo S., Zilio C. Measurements and Correlations of cis-1,3,3,3-Tetrafluoroprop-1-ene (R1234ze(Z)) Saturation Pressure. Int. J. Thermophys. 2014;35:1–12. doi: 10.1007/s10765-013-1553-5. [DOI] [Google Scholar]
- 195.Yang Z.-q., Kou L.-g., Mao W., Lu J., Zhang W., Lu J. Isothermal Vapor–Liquid Equilibrium for the Binary System of 2,3,3,3-Tetrafluoropropene and 2-Chloro-1,1,1,2-tetrafluoropropane. J. Chem. Eng. Data. 2015;60:1153–1156. doi: 10.1021/je501130r. [DOI] [Google Scholar]
- 196.Sakoda N., Higashi Y. Measurements of PvT Properties, Vapor Pressures, Saturated Densities, and Critical Parameters for cis-1-Chloro-2,3,3,3-tetrafluoropropene (R1224yd(Z)) J. Chem. Eng. Data. 2019;64:3983–3987. doi: 10.1021/acs.jced.9b00374. [DOI] [Google Scholar]
- 197.Li S., Yang F., Zhang K., Duan Y., Yang Z. Vapor Pressure Measurements and Correlation for trans-1-Chloro-3,3,3-trifluoroprop-1-ene. J. Chem. Eng. Data. 2019;64:2947–2954. doi: 10.1021/acs.jced.9b00001. [DOI] [Google Scholar]
- 198.Sakoda N., Higashi Y., Akasaka R. Measurements of Vapor Pressures for trans-1-Chloro-3,3,3-trifluoropropene (R1233zd(E)) and cis-1,1,1,4,4,4-Hexafluoro-2-butene (R1336mzz(Z)) J. Chem. Eng. Data. 2020;65:4285–4289. doi: 10.1021/acs.jced.0c00239. [DOI] [Google Scholar]
- 199.Bobbo S., Bet A., Scattolini M., Fedele L. Saturated Pressure Measurements of cis-1-Chloro-2,3,3,3- tetrafluoropropene (R1224yd (Z)) Saturation Pressure. J. Chem. Eng. Data. 2020;65:4263–4267. doi: 10.1021/acs.jced.0c00231. [DOI] [Google Scholar]
- 200.Fang Y., Ye G., Ni H., Jiang Q., Bao K., Han X., Chen G. Vapor–Liquid Equilibrium for the Binary Systems 1,1,2,3,3,3-Hexafluoro-1-propene (R1216) + 2,3,3,3-Tetrafluoroprop-1-ene (R1234yf) and 1,1,2,3,3,3-Hexafluoro-1-propene (R1216) + trans-1,3,3,3-Tetrafluoropropene (R1234ze(E)) J. Chem. Eng. Data. 2020;65:4215–4222. doi: 10.1021/acs.jced.0c00004. [DOI] [Google Scholar]
- 201.Yang T., Hu X., Meng X., Wu J. Vapour-liquid equilibria for the binary systems of pentafluoroethane {(R125) + 2,3,3,3-tetrafluoroprop-1-ene (R1234yf)} and {trans-1,3,3,3-tetrafluoropropene R1234ze(E)} J. Chem. Thermodyn. 2020;150:106222. doi: 10.1016/j.jct.2020.106222. [DOI] [Google Scholar]
- 202.Yin J., Ke J., Zhao G., Ma S. Experimental vapor pressures and gaseous pvT properties of trans-1-Chloro-3,3,3-trifluoropropene (R1233zd(E)) Int. J. Refrig. 2021;121:253–257. doi: 10.1016/j.ijrefrig.2020.09.010. [DOI] [Google Scholar]
- 203.Fischer R.C., Wittlinger R., Ballschmiter K. Retention-index based vapor pressure estimation for polychlorobiphenyl (PCB) by gas chromatography. Fresenius, J. Anal. Chem. 1992;342:421–425. doi: 10.1007/BF00322199. [DOI] [Google Scholar]
- 204.Lei Y.D., Wania F., Shiu W.Y. Vapor Pressures of the Polychlorinated Naphthalenes. J. Chem. Eng. Data. 1999;44:577–582. doi: 10.1021/je9802827. [DOI] [Google Scholar]
- 205.Verevkin S.P., Schick C. Determination of vapor pressures, enthalpies of sublimation, enthalpies of vaporization, and enthalpies of fusion of a series of chloro-aminobenzenes and chloro-nitrobenzenes. Fluid Phase Equil. 2003;211:161–177. doi: 10.1016/S0378-3812(03)00181-X. [DOI] [Google Scholar]
- 206.Verevkin S.P., Emel’yanenko V.N., Klamt A. Thermochemistry of Chlorobenzenes and Chlorophenols: Ambient Temperature Vapor Pressures and Enthalpies of Phase Transitions. J. Chem. Eng. Data. 2007;52:499–510. doi: 10.1021/je060429r. [DOI] [Google Scholar]
- 207.Nakajoh K., Grabda M., Oleszek-Kudlak S., Shibata E., Eckert F., Nakamura T. Prediction of vapour pressures of chlorobenzenes and selected polychlorinated biphenyls using the COSMO-RS model. J. Mol. Struct. 2009;895:9–17. doi: 10.1016/j.theochem.2008.10.015. [DOI] [Google Scholar]
- 208.Almeida A.R.R.P., Monte M.J.S. Crystalline and liquid vapour pressures of the four p-monohalophenols: A thermodynamic study of their phase transitions. J. Chem. Thermodyn. 2013;65:150–158. doi: 10.1016/j.jct.2013.05.047. [DOI] [Google Scholar]
- 209.Verevkin S.P., Emel’yanenko V.N., Varfolomeev M.A., Solomonov B.N., Zherikova K.V., Melkhanova S.V. Vaporization enthalpies of a series of the halogen-substituted fluorobenzenes. Fluid Phase Equil. 2015;387:160–168. doi: 10.1016/j.fluid.2014.12.023. [DOI] [Google Scholar]
- 210.Oliveira J.A.S.A., Oliveira T.S.M., Gaspar A., Borges F., Ribeiro da Silva M.D.M.C., Monte M.J.S. Study on the volatility of halogenated fluorenes. Chemosphere. 2016;157:25–32. doi: 10.1016/j.chemosphere.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 211.Almeida A.R.R.P., Pinheiro B.D.A., Lima C.F.R.A.C., Santos A.F.L.O.M., Ferreira A.C.S., Almeida Paz F.A., Monte M.J.S. Thermodynamic Properties of Moldy-Musty Contaminants of Wine. J. Chem. Eng. Data. 2019;64:4741–4753. doi: 10.1021/acs.jced.9b00062. [DOI] [Google Scholar]
- 212.Rotariu G.J., Hanrahan R.J., Fruin R.E. Liquid-Liquid Solubility and Vapor Pressure of Heptacosafluorotributylamine. J. Am. Chem. Soc. 1954;76:3752–3756. doi: 10.1021/ja01643a050. [DOI] [Google Scholar]
- 213.Barr D.A., Haszeldine R.N. 663. Perfluoroalkyl derivatives of nitrogen. Part III. Heptafluoronitrosopropane, perfluoro-2-n-propyl-1: 2-oxazetidine, perfluoro-(methylene-n-propylamine), and related compounds. J. Chem. Soc. 1956:3416–3428. doi: 10.1039/jr9560003416. [DOI] [Google Scholar]
- 214.Good W.D., Todd S.S., Messerly J.F., Lacina J.L., Dawson J.P., Scott D.W., McCullough J.P. Perfluoropiperidine: Entropy, heat of formation, and vapor pressure.; N-F bond energy.; and solid-state transitions. J. Phys. Chem. 1963;67:1306–1311. doi: 10.1021/j100800a036. [DOI] [Google Scholar]
- 215.Nash L.L., Babb D.P., Couville J.J., Shreeve J.M. Some bis(trifluoromethyl)nitroxides. J. Inorg. Nucl. Chem. 1968;30:3373–3375. doi: 10.1016/0022-1902(68)80138-1. [DOI] [Google Scholar]
- 216.Takashima M., Shreeve J.M. Preparation and Reactions of Heptafluoroazazcyclopentan-2-one. Inorg. Chem. 1979;18:3281–3283. doi: 10.1021/ic50201a070. [DOI] [Google Scholar]
- 217.Varouchtchenko R.M., Droujinina A.I. Thermodynamics of vaporization of some perfluorotrialkylamines. J. Chem. Thermodyn. 1995;27:355–368. doi: 10.1006/jcht.1995.0034. [DOI] [Google Scholar]
- 218.Aim K., Svoboda V., Majer V. Vapour pressures, densities, and refractive indices of 2-chloro-1,1,2-trifluoroethyl ethyl ether, 2-chloro-1,1,2-trifluoroethyl propyl ether, 2-chloro-1,1,2-trifluoroethyl chloromethyl ether, and 2-chloro-1,1,2-trifluoroethyl difluoromethyl ether. J. Chem. Thermodyn. 1983;15:531–536. doi: 10.1016/0021-9614(83)90052-6. [DOI] [Google Scholar]
- 219.Salvi-Narkhede M., Adcock J.L., Gakh A., Van Hook W.A. Vapor pressures, liquid molar volumes, vapor non-ideality, and critical properties of CF3OCF2CF2CF3, c-CF2CF2CF2CF2O, CF3OCF2OCF3, and CF3OCF2CF2H. J. Chem. Thermodyn. 1993;25:643–647. doi: 10.1006/jcht.1993.1060. [DOI] [Google Scholar]
- 220.Satoh K., Nishiumi H., Kasatani T. Vapor pressure of CH3OCHF2 synthesized from HCFC22. Fluid Phase Equil. 1998;144:211–216. doi: 10.1016/S0378-3812(97)00259-8. [DOI] [Google Scholar]
- 221.Murata J., Yamashita S., Akiyama M., Katayama S., Hiaki T., Sekiya A. Vapor Pressures of Hydrofluoroethers. J. Chem. Eng. Data. 2002;47:911–915. doi: 10.1021/je010322y. [DOI] [Google Scholar]
- 222.Garate M.P., Bejarano A., de la Fuente J.C. Vapour pressures for 1-(butoxymethoxy)butane (dibutoxymethane) and 1,1,1,2,2,3,3,4,4-nonafluoro-4-methoxybutane (methyl nonafluorobutyl ether) over the pressure range of (15–80) kPa. J. Chem. Thermodyn. 2016;101:351–355. doi: 10.1016/j.jct.2016.06.025. [DOI] [Google Scholar]
- 223.Black J.E., Silva G.M.C., Klein C., Iacovella C.R., Norgado P., Martins L.F.G., Filipe E.J.M., McCabe C. Perfluoropolyethers: Development of an All-Atom Force Field for Molecular Simulations and Validation with New Experimental Vapor Pressures and Liquid Densities. J. Phys. Chem. B. 2017;121:6588–6600. doi: 10.1021/acs.jpcb.7b00891. [DOI] [PubMed] [Google Scholar]
- 224.Wong A., Lei Y.D., Alaee M., Wania F. Vapor Pressures of the Polybrominated Diphenyl Ethers. J. Chem. Eng. Data. 2001;46:239–242. doi: 10.1021/je0002181. [DOI] [Google Scholar]
- 225.Brandt G.A.R., Emeléus H.J., Haszeldine R.N. 410. Organometallic and organometalloidal fluorine compounds. Part III. Trifluoromethyl derivatives of sulphur. J. Chem. Soc. 1952:2198–2205. doi: 10.1039/JR9520002198. [DOI] [Google Scholar]
- 226.Shreeve J.M., Cady G.H. Some Reactions of Peroxydisulfuryl Difluoride. J. Am. Chem. Soc. 1961;83:4521–4525. doi: 10.1021/ja01483a007. [DOI] [Google Scholar]
- 227.Merrill C.E., Cady G.H. Some Reactions of Bis(pentasulfur)peroxide. Defence Tech. Inf. Ctr. 1962:AD0277435. [Google Scholar]
- 228.Delfing J.J., Shreeve J.M. Enneafluoro-2-(fluorosulfato)butane and Trifluoroacetyl Fluorosulfate. Inorg. Chem. 1965;5:308–309. doi: 10.1021/ja00890a017. [DOI] [Google Scholar]
- 229.Sauer D.T., Shreeve J.M. Bis(perfluoroalkyl)sulfur difluorides and bis(perfluoroalkyl) sulfoxides. J. Fluor. Chem. 1971;1:1–11. doi: 10.1016/S0022-1139(00)82528-2. [DOI] [Google Scholar]
- 230.Abe T., Shreeve J.M. Perfluorotetramethylenesulfur difluoride and its derivatives. Perfluoro-1,3-dithietane octafluoride and perfluoro-1,4-dithiane octafluoride. J. Fluor. Chem. 1973;3:17–26. doi: 10.1016/S0022-1139(00)82857-2. [DOI] [Google Scholar]
- 231.Kitazume T., Shreeve J.M. Stable fluorinated sulfuranes and sulfurane oxides. Synthesis and reactions. Inorg. Chem. 1978;17:2173–2176. doi: 10.1021/ic50186a029. [DOI] [Google Scholar]
- 232.Emeléus H.J., Wilkins C.J. 122. Some new ethyl and phenyl silicon fluorides. J. Chem. Soc. 1944:454–456. doi: 10.1039/JR9440000454. [DOI] [Google Scholar]
- 233.Rugina T., Sacarescu L. Isobaric vapor liquid equilibria for the binary systems dichloromethylsilane with chlorotrimethylsilane, dichlorodimethylsilane, trichloromethylsilane, or silicon tetrachloride. J. Chem. Eng. Data. 1992;37:143–145. doi: 10.1021/je00006a003. [DOI] [Google Scholar]
- 234.Kopitzky R., Willner H., Hermann A., Oberhammer H. Bis(trifluoroacetyl) Peroxide, CF3C(O)OOC(O)CF3. Inorg. Chem. 2001;40:2693–2698. doi: 10.1021/ic0014639. [DOI] [PubMed] [Google Scholar]
- 235.Schindler B.J., Buchanan J.H., Mahle J.J., Peterson G.W., Glover T.G. Ambient Temperature Vapor Pressure and Adsorption Capacity for (Perfluorooctyl) Ethylene, 3-(Perfluorobutyl)propanol, Perfluorohexanoic Acid, Ethyl Perfluorooctanoate, and Perfluoro-3,6-dioxaheptanoic Acid. J. Chem. Eng. Data. 2013;58:1806–1812. doi: 10.1021/je400205g. [DOI] [Google Scholar]
- 236.Widegren J.A., Bruno T.J. Vapor Pressure Measurements on Low-Volatility Terpenoid Compounds by the Concatenated Gas Saturation Method. Env. Sci. Tech. 2009;44:388–393. doi: 10.1021/es9026216. [DOI] [PubMed] [Google Scholar]
- 237.Vilas-Boas S.M., Pokorny V., Stejfa V., Ferreira O., Pinho S.P., Ruzicka K., Fulem M. Vapor pressure and thermophysical properties of eugenol and (+)-carvone. Fluid Phase Equil. 2019;499:112248. doi: 10.1016/j.fluid.2019.112248. [DOI] [Google Scholar]
- 238.Fonseca L.A.A.P., Sartoratto A., Cremasco M.A. Experimental determination of thermodynamic properties of terpene and aromatic ketones by gas chromatography. J. Mol. Liquids. 2020:114531. doi: 10.1016/j.molliq.2020.114531. [DOI] [Google Scholar]
- 239.Goldfarb J.L., Suuberg E.M. Vapor pressures and thermodynamics of oxygen-containing polycyclic aromatic hydrocarbons measured using Knudsen effusion. Environ. Tox. Chem. 2008;27:1244–1249. doi: 10.1897/07-486.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Touhara H., Okazaki S., Okino F., Tanaka H., Nakanishi K. Thermodynamic properties of aqueous mixtures of hydrophilic compounds 2. Aminoethanol and its methyl derivatives. J. Chem. Thermodyn. 1982;14:145–156. doi: 10.1016/0021-9614(82)90026-X. [DOI] [Google Scholar]
- 241.Williams B.R., Hulet M.S., Brozena A., Miles Jr. R.W., Tevault D.E. Vapor Pressure of 2-Dialkyl Aminoethanethiols. J. Chem. Eng. Data. 2013;58:1679–1684. doi: 10.1021/je400136y. [DOI] [Google Scholar]
- 242.Belabbaci A., Ahmed N.C.-B., Mokbel I., Negadi L. Investigation of the isothermal (vapour + liquid) equilibria of aqueous 2-amino-2-methyl-1-propanol (AMP), N-benzylethanolamine, or 3-dimethylamino-1-propanol solutions at several temperatures. J. Chem. Thermodyn. 2010;42:1158–1162. doi: 10.1016/j.jct.2010.04.015. [DOI] [Google Scholar]
- 243.Steele W.V., Chirico R.D., Knipmeyer S.E., Nguyen A., Smith N.K., Tasker I.R. Thermodynamic Properties and Ideal-Gas Enthalpies of Formation for Cyclohexene, Phthalan (2,5-Dihydrobenzo-3,4-furan), Isoxazole, Octylamine, Dioctylamine, Trioctylamine, Phenyl Isocyanate, and 1,4,5,6-Tetrahydropyrimidine. J. Chem. Eng. Data. 1996;41:1269–1284. doi: 10.1021/je960093t. [DOI] [Google Scholar]
- 244.Espinosa Diaz M.A., Guetachew T., Landy P., Jose J., Voilley A. Experimental and estimated saturated vapour pressures of aroma compounds. Fluid Phase Equil. 1999;157:257–270. doi: 10.1016/S0378-3812(99)00016-3. [DOI] [Google Scholar]
- 245.Petitjean M., Reyes-Perez E., Perez D., Mirabel P., Le Calvé S. Vapor Pressure Measurements of Hydroxyacetaldehyde and Hydroxyacetone in the Temperature Range (273 to 356) K. J. Chem. Eng. Data. 2010;55:852–855. doi: 10.1021/je9004905. [DOI] [Google Scholar]
- 246.Gobble C., Vikman J., Chickos J.S. Evaluation of the Vaporization Enthalpies and Liquid Vapor Pressures of (R)-Deprenyl, (S)-Benzphetamine, Alverine, and a Series of Aliphatic Tertiary Amines by Correlation Gas Chromatography at T/ K = 298.15. J. Chem. Eng. Data. 2014;59:2551–2562. doi: 10.1021/je500358r. [DOI] [Google Scholar]
- 247.Emel’yanenko V.N., Nagrimanov R.N., Solomonov B.N., Verevkin S.P. Adamantanes: Benchmarking of thermochemical properties. J. Chem. Thermodyn. 2016;101:130–138. doi: 10.1016/j.jct.2016.05.017. [DOI] [Google Scholar]
- 248.Gobble C., Walker B., Chickos J.S. The Vaporization Enthalpy and Vapor Pressure of Fenpropidin and Phencyclidine (PCP) at T/ K = 298.15 by Correlation Gas Chromatography. J. Chem. Eng. Data. 2016;61:896–902. doi: 10.1021/acs.jced.5b00737. [DOI] [Google Scholar]
- 249.Wootitunthipong K., Chickos J. Vaporization enthalpy and vapor pressure of (−) Ambroxide and Galaxolide by correlation gas chromatography. J. Chem. Thermodyn. 2019;129:121–129. doi: 10.1016/j.jct.2018.09.012. [DOI] [Google Scholar]
- 250.Russo A.Y., Konnova M.E., Andreeva I.V., Verevkin S.P. Vaporization thermodynamics of compounds modeling lignin structural units. Fluid Phase Equil. 2019;491:45–55. doi: 10.1016/j.fluid.2019.03.004. [DOI] [Google Scholar]
- 251.Verevkin S.P., Emel’yanenko V.N., Siewert R., Pimerzin A.A. Thermochemistry of the lignin broken bits. Fluid Phase Equil. 2020;522:112751. doi: 10.1016/j.fluid.2020.112751. [DOI] [Google Scholar]
- 252.Verevkin S.P., Konnova M.E., Emel’yanenko V.N., Pimerzin A.A. Weaving a web of reliable thermochemistry around lignin building blocks: Vanillin and its isomers. J. Chem. Thermodyn. 2020:106362. doi: 10.1016/j.jct.2020.106362. [DOI] [Google Scholar]
- 253.Verevkin S.P., Emel’yanenko V.N. Vapor pressures of methoxy substituted benzaldehydes. Fluid Phase Equil. 2020:112912. doi: 10.1016/j.fluid.2020.112912. [DOI] [Google Scholar]
- 254.Ledo J.M., Flores H., Freitas V.L.S., Solano-Altamirano J.M., Hernandez-Perez J.M., Camarillo E.A., Ramos F., Ribeiro da Silva M.D.M.C. Benzocaine: A comprehensive thermochemical study. J. Chem. Thermodyn. 2020;147:106119. doi: 10.1016/j.jct.2020.106119. [DOI] [Google Scholar]
- 255.Umnahanant P. Vaporization enthalpy and liquid vapor pressure of bicifadine using correlation gas chromatography. J. Chem. Thermodyn. 2020;150:106204. doi: 10.1016/j.jct.2020.106204. [DOI] [Google Scholar]
- 256.Naef R. Calculation of the Isobaric Heat Capacities of the Liquid and Solid Phase of Organic Compounds at and around 298.15 K Based on Their “True” Molecular Volume. Molecules. 2019;24:1626. doi: 10.3390/molecules24081626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Huelsekopf M., Ludwig R. Temperature dependence of hydrogen bonding in alcohols. J. Mol. Liq. 2000;85:105–125. doi: 10.1016/S0167-7322(99)00168-3. [DOI] [Google Scholar]
- 258.Compernolle S., Ceulemans K., Müller J.-F. EVAPORATION: A new vapour pressure estimation method for organic molecules including non-additivity and intramolecular interactions. Atmos. Chem. Phys. 2011;11:9431–9450. doi: 10.5194/acp-11-9431-2011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.