Abstract
How to cite this article: Kumar N, Kumar A, Pradhan S, Kumar A, Singh K. Painful Blisters of Left Hand Following Extravasation of Remdesivir Infusion in COVID-19. Indian J Crit Care Med 2021;25(2):240–241.
Keywords: COVID pneumonia, Extravasation, Intensive care, Remdesivir, Skin lesion
Extravasation is the unintentional leakage of medications from the veins into the perivascular space and should be treated as a medical emergency. Any patient receiving intravenous (IV) medication is at risk of extravasation. Drugs that tend to result in an inflammatory reaction at the site of administration are considered irritants.1 The severity of extravasation and the amount of tissue injury depends on several factors like drug dose, drug concentration, drug administration site, and duration of drug exposure.2 Every infusion site must be regularly checked for assessment of free flow. These extravasations may cause ulceration and tissue necrosis at the injection site.3
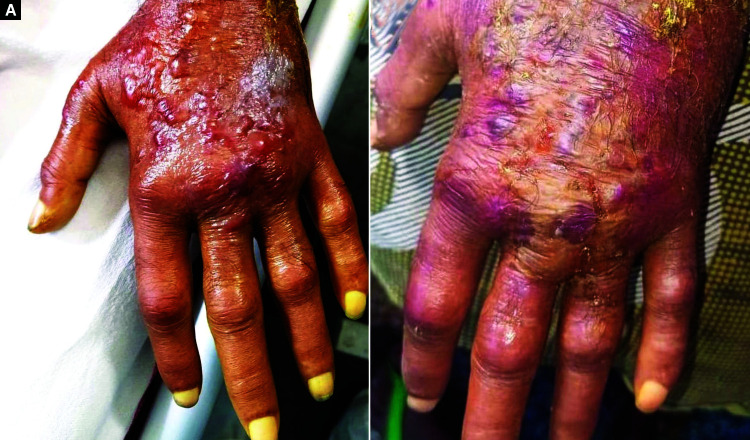
As per literature search, this is the first-reported case of extravasation of intravenously infused remdesivir in an elderly male, which resulted in an infusion site adverse reaction in the form of blisters, hemorrhage, and localized edema over the dorsum of the left hand (Fig. 1A).
Fig. 1.
(A) Remdesivir-induced infusion site reaction over the left dorsum of the hand; (B) After 72 hours of conservative treatment
A 64-year-old male patient was admitted to our hospital with moderate COVID-19 pneumonia. Remdesivir was infused into the patient's left hand after diluting with 0.9% saline over 60 minutes using 20 g IV cannula (Vygonüle V, Vygon, Haryana, India). The infusion site was free of any signs of inflammation or infection prior to the infusion. On the second day, 25 minutes after remdesivir administration, the patient complained of mild pain and a burning sensation at the catheter insertion site. The IV line was checked and, due to the lack of patency, the infusion was stopped and the cannula was removed. No other drug was concomitantly administered along with remdesivir infusion through the cannula. After 6 to 7 hours, at the same site the patient developed localized erythema and edema over the dorsum of the hand, which subsequently within 24 hours progressed in severity with a formation of blisters on a hemorrhagic background along with the extension of edema and induration of the fingers. There was an associated severe burning pain. A clinical diagnosis of infusion site reaction to remdesivir due to the drug extravasation was made after consultation with dermatology.
Although no guidelines apply to the management of all drug extravasations, however some general recommendations do exist. Local injection of dexamethasone or hydrocortisone in the treatment of extravasation is controversial. However, it is suggested that steroids would suppress the local inflammation caused by a tissue trauma that occurs during the treatment process.4 So, the patient was started on oral prednisolone 1 mg/kg body weight because of the progressive erythema, edema, and blisters over the left hand along with other conservative approaches (limb elevation, immobilization, analgesics, and nonocclusive dressing). After 72 hours, the edema subsided by 50–60% with the disappearance of all the blisters (Fig. 1B). The remaining doses of remdesivir were infused through the venous access in the right hand after obtaining written informed consent from the patient. The drug did not cause any adverse events while infused for the second time into the right hand.
Discussion
There are various chemical properties of medications, such as high drug concentration, high osmolarity, and high or low pH, and selection of a small-sized vein and rapid infusion rate may lead to an increased risk of soft tissue damage with extravasation. Remdesivir for injection contains inactive ingredients, such as sulfobutylether-β-cyclodextrin sodium salt (SBECD), water, hydrochloric acid, and sodium hydroxide. Hydrochloric acid and sodium hydroxide are used to adjust the formulation to a pH of 3.0–4.0. One of the most common adverse events with remdesivir use is skin rashes. The chemotherapeutic drugs are commonly known for their vesicant effect causing infusion site reactions. The severe type of reaction occurs due to drug extravasation which can occur immediately or may be delayed up to 6–12 hours. It is characterized by pain at the site of injection, blister formation, and severe skin tissue damage. As remdesivir is highly acidic (pH of 3.0–4.0, osmolality of 278 mOsm/kg), it may be the cause of significant tissue damage in our case. Remdesivir is being used widely all over the globe these days as an emergency drug for the treatment of COVID-19. Proper instruction is to be followed during administration. So, always routinely check and document the rate of drug administration, location and condition of the site, verification of patency, and patient's responses to prevent extravasation. So, for early recognition and prompt reporting of IV-induced extravasation, proper patient education and better communication is of paramount importance.
Orcid
Neeraj Kumar https://orcid.org/0000-0002-9161-7000
Abhyuday Kumar https://orcid.org/0000-0002-9247-6713
Swetalina Pradhan https://orcid.org/0000-0003-2832-1538
Amarjeet Kumar https://orcid.org/0000-0002-4272-5750
Kunal Singh https://orcid.org/0000-0003-4485-8757
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.DeDea L. How should extravasation injuries be treated? JAAPA. 2011;24(12):17. [PubMed] [Google Scholar]
- 2.Pentam (Pentamindine isethionate) [package insert]. Schamburg, IL: APP Pharmaceuticals, LLC; 2008. p. 7. [Google Scholar]
- 3.Grein J, Ohmagari N, Shin D, et al. Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–36. doi: 10.1056/NEJMoa2007016. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemotherapy extravasation in practice guideline. WOSCAN. Sep, 2009. p. 12. Available from: https://www.beatson.scot.nhs.uk/content/mediaassets/doc/Extravasation%20guidance.pdf .