Abstract
Objectives
The objective of this review was to compare the effectiveness of Colistin monotherapy and combination therapy for the treatment of multidrug-resistant gram-negative bacterial infections.
Data sources
PubMed, Cochrane Library.
Study eligibility, interventions, and exclusions
In this systematic review, we included all retrospective and prospective studies and randomized controlled trials (RCTs) that compared intravenous polymyxin monotherapy and combination therapy with any other antibiotic for treating multidrug-resistant infections. Studies using inhaled polymyxins with 5 or less than 5 patients were excluded. The primary outcome was 30-day all-cause mortality and if not reported at day 30 we extracted and documented the closest time point. Both crude outcome rates and adjusted effect estimates were extracted for mortality.
Study appraisal, data extraction and synthesis
Search string used was “(Colistin OR polymyxin) AND (Enterobacteriaceae OR Klebsiella OR Acinetobacter OR Escherichia coli OR Pseudomonas) AND (random OR prospective OR retrospective OR cohort OR observational OR blind).” Thirty-nine studies were included in our analysis; out of which 6 RCTs were included and 9 studies used carbapenem as the adjunctive antibiotic. Each study was screened and reviewed for eligibility independently by two authors and data extrapolated on an Excel sheet.
Results
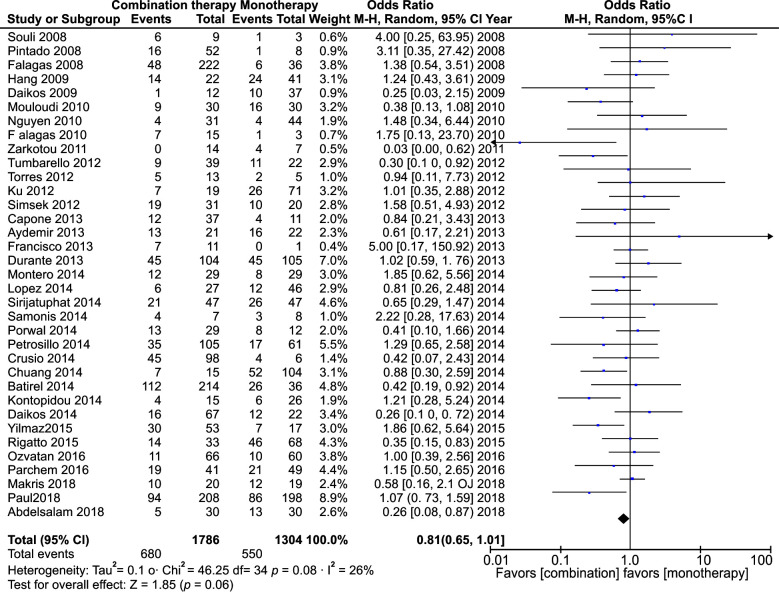
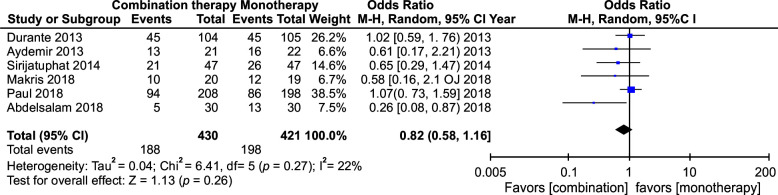
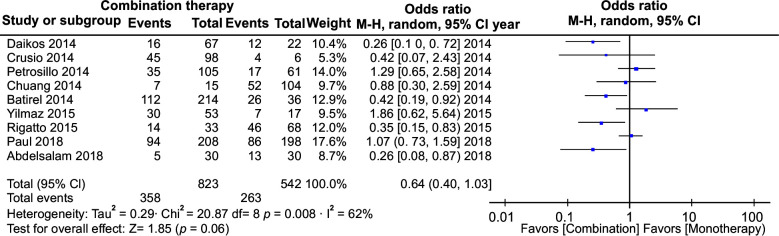
The meta-analysis of polymyxin monotherapy vs. combination therapy in multidrug-resistant infections yielded an odds ratio (OR) of 0.81 (95% confidence interval [CI]: 0.65–1.01) with minimal heterogeneity (I2 = 40%), whereas pooled analysis of this comparison in studies that included carbapenem as combination therapy yielded an OR of 0.64 (CI: 0.40–1.03; I2 = 62%). Likewise, the pooled analysis of the RCTs yielded an OR of 0.82 (95% CI: 0.58–1.16, I2 = 22%). All these showed no statistical significance. However, it was seen that polymyxin combination therapy was more effective in multidrug-resistant infections compared to polymyxin monotherapy. The effectiveness was more glaring when carbapenems were used as the combination drug instead of any other antibiotic and more so in many in vitro studies that used polymyxin combination therapy.
Conclusion
Although statistically insignificant, it would be prudent to use polymyxin combination therapy to treat multidrug-resistant gram-negative bacilli (GNB) infection over monotherapy with preference to use carbapenem as the adjunct alongside polymyxins.
How to cite this article
Samal S, Mishra SB, Patra SK, Rath A, Dash A, Nayak B, et al. Polymyxin Monotherapy vs. Combination Therapy for the Treatment of Multidrug-resistant Infections: A Systematic Review and Meta-analysis. Indian J Crit Care Med 2021;25(2):199–206.
Keywords: Carbapenems, Colistin, Combination therapy, Gram-negative bacilli infections, Monotherapy, Multidrug resistant (MDR), Polymyxin B
Introduction
Polymyxin E (Colistin) was originally isolated in 1947 from soil bacterium Paenibacillus polymyxa subsp. colistinus.1 Polymyxin B and colistin belong to the class of polymyxins, which is a polypeptide group of antibiotics. Polymyxins share their structure with cationic antimicrobial peptides (CAMPs) like defensins and gramicidins.2 CAMPs represent the first line of defense against bacterial infections. Polymyxins are cationic polypeptides that consist of a cyclic heptapeptide possessing a tripeptide side chain acylated at the N-terminus by a fatty acid tail.3,4 N-terminal fatty acyl segment is hydrophobic and is associated with both toxicity and the antimicrobial property.5,6 Colistin and polymyxin B differ by only a single amino acid in the peptide ring with phenylalanine in polymyxin B and leucine in Colistin.7 Polymyxin B is administered directly as an active antibiotic, whereas Colistin is administered as an inactive prodrug, colistin methanesulfonate (also known as colistimethate [CMS]).7 Colistimethate is formed by the reaction of colistin with formaldehyde and sodium bisulfite.8 This prodrug is transformed in aqueous media and also in vivo in biological fluids and is converted into colistin and several inactive methanesulfonated compounds.9,10
Mechanism of Action
The outer membrane of gram-negative bacteria is the target for polymyxins. Because of an electrostatic interaction occurring between the -diaminobutyric acid (Dab) residue of the positively charged polymyxin on one side and the phosphate groups of the negatively charged lipid A membrane on the other side, divalent cations (Ca2+ and Mg2+) are displaced from the negatively charged phosphate groups of membrane lipids.11 The lipopolysaccharide (LPS) is therefore destabilized consequently increasing the permeability of the bacterial membrane leading to leakage of the cytoplasmic content and ultimately causing cell death.4,12 The endotoxin of gram-negative pathogens corresponds to the lipid A portion of the LPS; polymyxins have the ability to bind to and neutralize this LPS molecule released during cell lysis.13 Inhibition of vital respiratory enzymes (inhibition of type II nicotinamide adenine dinucleotide–quinone oxidoreductases [NDH-2]) in the bacterial inner membrane is another proposed mechanism of action.14 Even though the LPS is the initial target, the exact mode of action of polymyxins still remains uncertain.
Spectrum of Activity
Polymyxins have a narrow antibacterial spectrum including most members of the Enterobacteriaceae and common nonfermentative gram-negative bacteria including Acinetobacter baumannii, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia.12
Proteus spp., Morganella morganii, Providencia spp., Serratia marcescens, Pseudomonas mallei, Burkholderia cepacia, Chromobacterium spp., Edwardsiella spp., Brucella, Legionella, Campylobacter, and Vibrio cholerae are intrinsically resistant to the drug. Gram-negative cocci (Neisseria spp.), gram-positive bacteria, and anaerobic bacteria are not covered by polymyxins.12
Rationale
Zusman et al. conducted a meta-analysis to look into the in vitro studies of synergism between polymyxin and other antibiotics.15 High synergism (up to 75%) is seen between polymyxins and carbapenems against A. baumannii strains. The combination therapy increased the bactericidal property of A. baumannii from 24 to 75%. Synergy was higher with meropenem and doripenem compared to imipenem for A. baumannii. Carbapenem-resistant, Colistin-susceptible A. baumannii showed a synergy rate of 71%. For Klebsiella pneumoniae, 44% synergism was seen. Doripenem showed higher synergism in comparison with imipenem and meropenem. Carbapenem-resistant, Colistin-susceptible K. pneumoniae showed a synergy rate of 55%. P. aeruginosa showed a synergy rate of 50%. So, overall, in vitro studies showed synergy between carbapenems and polymyxins.
We planned to do a systematic meta-analysis of all the clinical trials to date.
Objective
We sought to examine the effectiveness of polymyxin-based combination vs. monotherapy by antibiotic types and bacterial species.
Methods
Protocol
Our protocol was inspired by the meta-analysis and systematic review by Zusman et al. (J Antimicrob Chemother, DOI: 10.1093/jac/dkw377).15
Eligibility Criteria
We included all clinical studies [whether retrospective, prospective or RCTs] comparing intravenous polymyxin (Colistin or polymyxin B) monotherapy vs. any polymyxin-based combination therapy in adult patients with documented infection caused by polymyxin-susceptible, carbapenem-resistant (CR) or carbapenemase-producing GNB provided that the study reported on the outcomes for a specific polymyxin and a specific combination regimen (named antibiotics). If more than one comparison was reported, we included all reported comparisons. No language or year restrictions were applied. We did not include studies using inhaled polymyxins. Only studies reporting on more than 5 patients per treatment group were included.
Information Sources
We searched PubMed, the Cochrane Library, references of all included studies, and narrative or systematic reviews on the topic.
Search
In the databases, we used the following search string: “(Colistin OR polymyxin) AND (Enterobacteriaceae OR Klebsiella OR Acinetobacter OR E. coli OR Pseudomonas) AND (random OR prospective OR retrospective OR cohort OR observational OR blind).” We estimated that this search string will be relatively sensitive. The last search was run on December 31, 2018.
Study Selection
Any study from our search that compared Colistin or polymyxin in combination against being used alone for treating intensive care unit (ICU)-acquired infections were considered provided the number of subjects included were more than 5. Studies that used inhaled Colistin were excluded.
Data Collection Process and Data Items
Each study was screened and reviewed for eligibility independently by two authors. In case of missing data for an eligible study, an attempt was made to contact the study authors for clarification. Data were tabulated in an Excel sheet. The primary outcome was 30-day all-cause mortality and if not reported at day 30 we extracted and documented the closest time point. Both crude outcome rates and adjusted effect estimates were extracted for mortality. Data were extracted independently by two authors using a predefined data extraction form and then compared for verification. In the event of a dispute, a third author acted as a referee. From individual studies, we sought to extract patient demographics, formulation and dosage including loading for polymyxins and the combination antibiotic, clinical data regarding the infection including source, place of acquisition of sepsis presentation and severity, and types and resistance profile of the bacteria.
Synthesis of Results
Data in the Excel sheet were analyzed and evaluated using RevMan 5.3.
Additional Analysis
RCTs were studied separately and a separate analysis was done for them.
Results
Study Selection
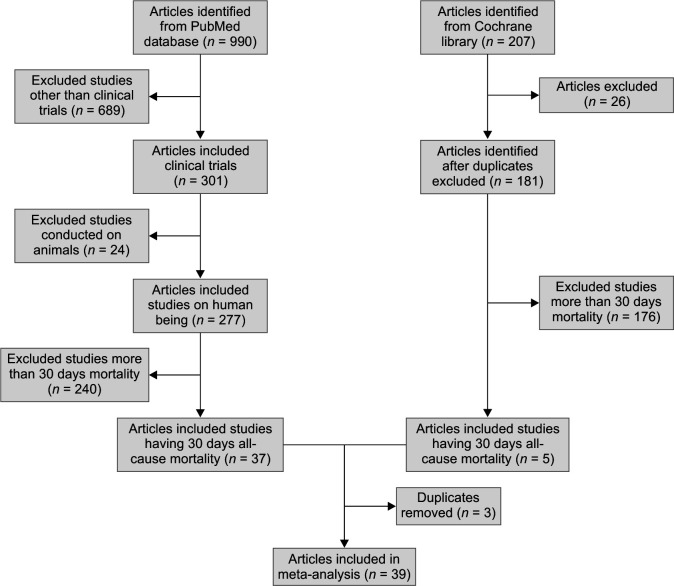
Using the search key mentioned above within the stipulated time period, 990 articles were identified from PubMed database, whereas 207 were identified from the Cochrane Library. After removing duplicates, animal studies, and studies with more than 30-day mortality rate, 39 clinical trials finally qualifying our criteria were considered. Only studies that were clinical trials were considered (Flowchart 1).
Flowchart 1.
PRISMA flowchart/study selection
Out of the 39 studies that were selected for this meta-analysis, the majority of the studies were conducted in Europe (Greece, Turkey, Spain, and Italy).16–43 Five of them were conducted in America,44–48 Asia49–53, and 1 in Africa (Egypt).54 Seventeen of the studies were prospective studies,16,18–20,25–29,31,39,41,42,47,54 whereas 22 were retrospective studies. In total, there were 6 RCTs. Sixteen of the 39 studied the effect of Colistin or another drug or in combination on A. baumannii,18,19,24,25,27,30,35,38,40,41,43,48–51,5311 studied the effect of therapy on K. pneumoniae,16,17,20,21,26,28,31,37,39,46,54 2 studies dealt with the role of therapy in P. aeruginosa34,48, and the rest dealt with any kind of sepsis/infection in ICU or polymicrobial infection.22,23,29,32,33,36,42,44,45,47,52 Twenty-seven studies were conducted solely in the ICU setup18–24,26–28,30–32,37,38,41–45,48–54, whereas the rest of the studies included patients from any setup (ward/high dependency unit/ICU) with the infections being treated with Colistin or combination therapy. Nine of these 39 focused on bloodstream infections alone17,20,21,28,29,39,40,46,51, 2 on pneumonia30,45, 6 on ventilator-associated pneumonia18,38,41,43,53,54, 4 on mixed or multiple-site infections27,31–33 and the rest took any site of infection into account. All of these studies except 2 studied the effect of Colistin as compared to combination therapy. Polymyxin B was the antibiotic studied in the other 2.47,48 Carbapenems and tigecycline were the most used antibiotics for combination therapy followed by aminoglycosides, rifampicin, and beta-lactam plus beta-lactamase inhibitor. Fosfomycin and vancomycin were used in 1 study each.
All-cause Mortality in Combination vs. Monotherapy
All 39 studies were analyzed. In total, 4863 patients were included in the analysis. The meta-analysis yielded an OR of 0.81 with CI from 0.65 to 1.01. This had a p-value of 0.06. Heterogeneity measured I2 was 26% (Fig. 2). We did a separate meta-analysis of only the RCTs. Six studies were analyzed and yielded an OR of 0.82 with a CI of 0.58–1.16. This was not statistically significant. The heterogeneity was I2 = 22 (Fig. 3). We also did an analysis including those studies, which had only carbapenems as a combination. Nine studies were analyzed and yielded an OR of 0.64 with a CI of 0.40–1.03. This was not statistically significant. The heterogeneity was I2 = 62 (Fig. 1).
Fig. 2.
All-cause mortality in all included studies
Fig. 3.
All-cause mortality in RCTs
Fig. 1.
All-cause mortality in studies where carbapenems were used in combination therapy
Discussion
Klebsiella and Acinetobacter are the commonest organisms found in ICUs with very high resistance to antibiotics. The newer strains isolated in most of the studies were resistant to carbapenems in most of the cases.54 Targeting these organisms with a multifaceted approach seems to be a way out. Various combinations of antibiotics have been used to treat these multidrug-resistant infections in the hope to achieve results in vivo where in vitro tests have deemed the infections to be untreatable.
We made an attempt to compile the evidence available to date on the use of combination therapy with polymyxins in comparison to monotherapy with polymyxins. These 39 studies were found which had comparative groups of monotherapy in comparison to combination therapy which had mortality as an outcome. We did not assess the clinical clearance of the organisms as there is always a very high risk of bias associated with such outcomes, both in terms of definition as well as data collection.
The in vitro studies have shown that there is a synergism between carbapenems and polymyxins.15 This is seen predominantly in Acinetobacter strains. Klebsiella and Pseudomonas also show synergism in up to 50% of strains. The clinical trials though are very varied. Various combinations have been tried. The most common agent that has been used in combination with Colistin/polymyxin B is the tigecycline. We did a subgroup analysis of the studies that included only carbapenems as combination therapy. It showed a significant improvement in all-cause mortality. Though the largest RCT did not find any benefit, this finding may be a hypothesis for future trials.42
The overall analysis showed lesser mortality with combination therapy, though it was not statistically significant. This was consistent if we included all the studies or if we looked only at the RCT. This finding is consistent with previous meta-analyses and trials. Thirty-three studies were observational in nature. The major risk of bias in these studies is the classification bias which means the authenticity that the patient in the said group actually received that therapy or not. There was a high risk of bias as there were no clear definitions. The other major problem with observational studies is the selection bias. In some studies, some patients were given monotherapy as they were selected because of less severe infection or an easily treatable source of infection, e.g., urinary tract infections. Most of the studies do not report on the minimum inhibitory concentration (MIC) values for carbapenems. Only the study by Navarro–San Francisco has mentioned about the MIC.29
In the 6 RCTs included, almost all showed a trend towards lower mortality in patients receiving combination therapy; however, only the RCT by Abdelsalam et al. found a significant difference in mortality.54 The largest RCT by Paul et al. showed significant results in case of infections with K. pneumoniae, though not adequately powered for it.42 Two of the RCTs had a very small sample size whose results could not be extrapolated to larger populations.18,41 Though nearly all of the studies did not find the benefit of the combination therapy, they, however, did show a better bacteriological clearance in patients receiving combination therapy.19,41,49,54 The higher mortality in patients was attributed to the higher acute physiology and chronic health evaluation (APACHE) II score at admission and higher age. In one of the RCTs, no significant difference in microbiological clearance was found, but the time to the clearance was significantly shorter in patients who received combination therapy.18 Multiple regression analysis in a study found that combination therapy was an independent predictor of good clinical response.41
Our meta-analysis shows that there is a trend towards mortality benefits with combination therapy. This trend is more pronounced when carbapenem is the antibiotic used for combination therapy. This is supported by in vitro trials and meta-analysis which support the synergism of combination between polymyxins and carbapenems. This is also supported theoretically by the fact that polymyxins are bacteriostatic agents and maybe a combination will help in more penetration into the bacterial cells and enhance the tidal properties of the carbapenems.
Table 1.
Comparison of RCTs
| Sl. No. | Author | Year | Location | Organism | Setting | Infection type | Polymyxin | Combination | No. of Pt. | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aydemir et al.18 | 2013 | Turkey | CR-AB | ICU | VAP | Colistin | Rifampin | 43 | No difference in mortality or clinical, laboratory or microbiological clearance Significant reduction in time to microbiological clearance in combination arm |
| 2 | Durante-Mangoni et al.19 |
2013 | Italy | XDR-AB | ICU | Any | Colistin | Rifampin | 210 | No mortality benefit, but better bacteriological clearance |
| 3 | Sirijatuphat et al.49 | 2014 | Thailand | CR-AB | ICU | Any | Colistin | Fosfomycin | 94 | More favorable microbiological response significantly Trend of lower 28-day all-cause mortality levels |
| 4 | Abdelsalam et al.54 | 2018 | Egypt | MDR KP | ICU | VAP | Colistin | Meropenem | 60 | Combination group showed a significant decrease in mortality |
| 5 | Makris et al.41 | 2018 | Greece | MDRAB | ICU | VAP | Colistin | Ampicillin sulbactam | 39 | Multiple regression analysis—combination therapy was an independent predictor of good clinical response |
| 6 | Paul et al.42 | 2018 | Greece | Any | ICU | Any | Colistin | Meropenem | 406 | No overall mortality benefit. Benefit only against Klebsiella pneumoniae infections, (not adequately powered) |
CR, carbapenem resistant; AB, Acinetobacter baumannii; ICU, intensive care unit; VAP, ventilator-acquired pneumonia; XDR, extremely drug resistant; MDR, multidrug resistant
Limitations
The limitations of our study originate from the studies that were included in this analysis. Since most of the studies are observational, they suffer from the shortcomings associated with these trials as explained before. The study endpoint was not mortality in many of the studies. The methodological flaws in the included trial affect this study as well. The in vitro evidence is there for carbapenem combination therapy, but it has been evaluated in limited trials. Only 2 RCTs have looked into this combination therapy.42,54 Though there were a few other studies who did use carbapenems or meropenem, this was not the only adjunctive antibiotic used in these studies.21–23,26,27,29,38,39,48 Either tigecycline, tetracycline, aminoglycoside or sulbactam was also studied along with carbapenems/meropenem as an adjunct to Colistin. These other antibiotics are not supported by in vitro trials. The largest RCT looked at an invalidated composite outcome in a mixed bacterial population.42 They also reported a reduced renal complication in combination group, which currently we do not have any physiological basis for but something worth looking at in the future.
Risk of Bias
Risk of bias was present in some of the studies, the most prominent being the selection bias as clinicians were not blinded in some.
Conclusion
The use of combination therapy with polymyxin in comparison to monotherapy with polymyxin in a gram-negative infection may show benefits. The combination with a carbapenem especially meropenem is preferred. We need larger RCTs with specific bacterial infection groups to find more conclusive evidence.
Orcid
Samir Samal https://orcid.org/0000-0002-2496-1434
Shakti B Mishra https://orcid.org/0000-0001-6634-1877
Shantanu K Patra https://orcid.org/0000-0003-2707-6825
Arun Rath https://orcid.org/0000-0002-0382-4342
Abhilash Dash https://orcid.org/0000-0001-8287-5975
Biswajit Nayak https://orcid.org/0000-0001-6314-7550
Diganta Mohanty https://orcid.org/0000-0003-0924-4303
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Benedict RG, Langlykke AF. Antibiotic activity of Bacillus polymyxa. J Bacteriol. 1947;54(1):24. [PubMed] [Google Scholar]
- 2.Hancock RE. Peptide antibiotics. Lancet. 1997;349(9049):418–422. doi: 10.1016/S0140-6736(97)80051-7. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist Updat. 2010;13(4–5):132–138. doi: 10.1016/j.drup.2010.05.002. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gramnegative bacterial infections. Lancet Infect Dis. 2006;6(9):589–601. doi: 10.1016/S1473-3099(06)70580-1. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Brink AJ, Richards GA, Colombo G, Bortolotti F, Colombo P, Jehl F. Multicomponent antibiotic substances produced by fermentation: implications for regulatory authorities, critically ill patients and generics. Int J Antimicrob Agents. 2014;43(1):1–6. doi: 10.1016/j.ijantimicag.2013.06.013. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Gallardo-Godoy A, Muldoon C, Becker B, Elliott AG, Lash LH, Huang JX, et al. Activity and predicted nephrotoxicity of synthetic antibiotics based on polymyxin B. J Med Chem. 2016;59(3):1068–1077. doi: 10.1021/acs.jmedchem.5b01593. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis. 2014;59(1):88–94. doi: 10.1093/cid/ciu213. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett M, Bushby SR, Wilkinson S. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol Chemother. 1964;23(3):552–574. doi: 10.1111/j.1476-5381.1964.tb01610.x. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. Stability of colistin and colistin methanesulfonate in aqueous media and plasma asdetermined by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003;47(4):1364–1370. doi: 10.1128/aac.47.4.1364-1370.2003. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53(5):837–840. doi: 10.1093/jac/dkh167. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Dixon RA, Chopra I. Leakage of periplasmic proteins from Escherichia coli mediated by polymyxin B nonapeptide. Antimicrob Agents Chemother. 1986;29(5):781–788. doi: 10.1128/aac.29.5.781. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis. 2005;40(9) doi: 10.1086/429323. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25(1):11–25. doi: 10.1016/j.ijantimicag.2004.10.001. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Deris ZZ, Akter J, Sivanesan S, Roberts KD, Thompson PE, Nation RL, et al. A secondary mode of action of polymyxins against Gramnegative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. J Antibiot (Tokyo) 2013;67(2):147–151. doi: 10.1038/ja.2013.111. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zusman O, Altunin S, Koppel F, Benattar YD, Gedik H, Paul M. Polymyxin monotherapy or in combination against carbapenemresistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72(1):29–39. doi: 10.1093/jac/dkw377. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19(1):E23–E30. doi: 10.1111/1469-0691.12070. DOI: [DOI] [PubMed] [Google Scholar]
- 17.Tumbarello M, Viale V, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase.producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943–950. doi: 10.1093/cid/cis588. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Aydemir H, Akduman D, Piskin N, Comert F, Horuz E, Terzi A, et al. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilatorassociated pneumonia. Epidemiol Infect. 2013;141(6):1214–1222. doi: 10.1017/S095026881200194X. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57(3):349–358. doi: 10.1093/cid/cit253. DOI: [DOI] [PubMed] [Google Scholar]
- 20.Daikos GL, Petrikkos P, Psichogiou M, Kosmidis C, Vryonis E, Skoutelis A, et al. Prospective observational study of the impact of VIM-1 metallo-β-lactamase on the outcome of patients with Kebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2009;53(5):1868–1873. doi: 10.1128/AAC.00782-08. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322–2328. doi: 10.1128/AAC.02166-13. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falagas ME, Rafailidis PI, Matthaiou DK, Virtzili S, Nikita D, Michalopoulos A. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents. 2008;32(5):450–454. doi: 10.1016/j.ijantimicag.2008.05.016. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35(2):194–199. doi: 10.1016/j.ijantimicag.2009.10.005. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Garnacho-Montero J, Amaya-Villar R, Gutiérrez-Pizarraya A, Espejo-Gutiérrez De Tena E, Artero-González ML, Corcia-Palomo Y, et al. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy. 2014;59(3):225–231. doi: 10.1159/000356004. DOI: [DOI] [PubMed] [Google Scholar]
- 25.Hernández-Torres A, García-Vázquez E, Gómez J, Canteras M, Ruiz J, Yagüe G. Multidrug and carbapenem-resistant Acinetobacter baumannii infections: factors associated with mortality. Med Clin (Barc) 2012;138(15):650–655. doi: 10.1016/j.medcli.2011.06.024. DOI: [DOI] [PubMed] [Google Scholar]
- 26.Kontopidou F, Giamarellou H, Katerelos P, Maragos A, Kioumis I, Trikka-Graphakos E, et al. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect [Internet] 2014;20(2):O117.O123. doi: 10.1111/1469-0691.12341. DOI: [DOI] [PubMed] [Google Scholar]
- 27.López-Cortés LE, Cisneros JM, Fernández-Cuenca F, Bou G, Tomás M, Garnacho-Montero J, et al. Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii: analysis of a multicentre prospective cohort. J Antimicrob Chemother. 2014;69(11):3119–3126. doi: 10.1093/jac/dku233. DOI: [DOI] [PubMed] [Google Scholar]
- 28.Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, Giasnetsova T, et al. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol. 2010;31(12):1250–1256. doi: 10.1086/657135. DOI: [DOI] [PubMed] [Google Scholar]
- 29.Navarro-San Francisco C, Mora-Rillo M, Romero-Gómez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, et al. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect. 2013;19(2):E72–E79. doi: 10.1111/1469-0691.12091. DOI: [DOI] [PubMed] [Google Scholar]
- 30.Özvatan T, Akalin H, Sinirtaş M, Ocako.lu G, Yilmaz E, Heper Y, et al. Nosocomial Acinetobacter pneumonia: treatment and prognostic factors in 356 cases. Respirology. 2016;21(2):363–369. doi: 10.1111/resp.12698. DOI: [DOI] [PubMed] [Google Scholar]
- 31.Papadimitriou-Olivgeris M, Marangos M, Christofidou M, Fligou F, Bartzavali C, Panteli ES, et al. Risk factors for infection and predictors of mortality among patients with KPC-producing Klebsiella pneumoniae bloodstream infections in the intensive care unit. Scand J Infect Dis. 2014;46(9):642–648. doi: 10.3109/00365548.2014.923106. DOI: [DOI] [PubMed] [Google Scholar]
- 32.Petrosillo N, Giannella M, Antonelli M, Antonini M, Barsic B, Belanci L, et al. Clinical experience of colistin-glycopeptide combination in critically ill patients infected with gram-negative bacteria. Antimicrob Agents Chemother. 2014;58(2):851–858. doi: 10.1128/AAC.00871-13. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pintado V, San Miguel LG, Grill F, Mejía B, Cobo J, Fortún J, et al. Intravenous colistin sulphomethate sodium for therapy of infections due to multidrug-resistant gram-negative bacteria. J Infect. 2008 Mar;56(3):185–190. doi: 10.1016/j.jinf.2008.01.003. DOI: Epub 2008 Feb 15. PubMed PMID: 18280570. [DOI] [PubMed] [Google Scholar]
- 34.Samonis G, Korbila IP, Maraki S, Michailidou I, Vardakas KZ, Kofteridis D, et al. Trends of isolation of intrinsically resistant to colistin Enterobacteriaceae and association with colistin use in a tertiary hospital. Eur J Clin Microbiol Infect Dis. 2014 Sep;33(9):1505–1510. doi: 10.1007/s10096-014-2097-8. DOI: [DOI] [PubMed] [Google Scholar]
- 35.Şimsek F, Gedik H, Yildirmak MT, Iris NE, Türkmen A, Ersoy A, et al. Colistin against colistin-only-susceptible Acinetobacter baumannii-related infections: monotherapy or combination therapy? Indian J Med Microbiol. 2012;30(4):448–452. doi: 10.4103/0255-0857.103767. DOI: [DOI] [PubMed] [Google Scholar]
- 36.Souli M, Kontopidou FV, Papadomichelakis E, Galani I, Armaganidis A, Giamarellou H. Clinical experience of serious infections caused by Enterobacteriaceae producing VIM-1 metallo-beta-lactamase in a Greek University Hospital. Clin Infect Dis. 2008;46(6):847–854. doi: 10.1086/528719. DOI: [DOI] [PubMed] [Google Scholar]
- 37.Vardakas KZ, Mavroudis AD, Georgiou M, Falagas ME. Intravenous colistin combination antimicrobial treatment vs. monotherapy: a systematic review and meta-analysis. Int J Antimicrob Agents [Internet] 2018;51(4):535–547. doi: 10.1016/j.ijantimicag.2017.12.020. DOI: [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz GR, Guven T, Guner R, Tufan ZK, Izdes S, Tasyaran MA, et al. Colistin alone or combined with sulbactam or carbapenem against A. baumannii in ventilator-associated pneumonia. J Infect Dev Ctries. 2015;9(5):476–485. doi: 10.3855/jidc.6195. DOI: [DOI] [PubMed] [Google Scholar]
- 39.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect [Internet] 2011;17(12):1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. DOI: [DOI] [PubMed] [Google Scholar]
- 40.Batirel A, Balkan II, Karabay O, Agalar C, Akalin S, Alici O, et al. Comparison of colistin-carbapenem, colistin-sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drugresistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol Infect Dis. 2014;33(8):1311–1322. doi: 10.1007/s10096-014-2070-6. DOI: [DOI] [PubMed] [Google Scholar]
- 41.Makris D, Petinaki E, Tsolaki V, Manoulakas E, Mantzarlis K, Apostolopoulou O, et al. Colistin versus colistin combined with ampicillin-sulbactam for multiresistant Acinetobacter baumannii ventilator-associated pneumonia treatment: an open-label prospective study. Indian J Crit Care Med. 2018 Feb;22(2):67–77. doi: 10.4103/ijccm.IJCCM_302_17. DOI: PubMed PMID: 29531445; PubMed Central PMCID PMC5842460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis [Internet]. 2018;18(4):391–400. doi: 10.1016/S1473-3099(18)30099-9. DOI: [DOI] [PubMed] [Google Scholar]
- 43.Kalin G, Alp E, Akin A, Coskun R, Doganay M. Comparison of colistin and colistin/sulbactam for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Infection. 2014;42(1):37–42. doi: 10.1007/s15010-013-0495-y. DOI: [DOI] [PubMed] [Google Scholar]
- 44.Ku K, Pogue JM, Moshos J, Bheemreddy S, Wang Y, Bhargava A, et al. Retrospective evaluation of colistin versus tigecycline for the treatment of Acinetobacter baumannii and/or carbapenem-resistant Enterobacteriaceae infections. Am J Infect Control [Internet] 2012;40(10):983–987. doi: 10.1016/j.ajic.2011.12.014. DOI: [DOI] [PubMed] [Google Scholar]
- 45.Parchem NL, Bauer KA, Cook CH, Mangino JE, Jones CD, Porter K, et al. Colistin combination therapy improves microbiologic cure in critically ill patients with multi-drug resistant gram-negative pneumonia. Eur J Clin Microbiol Infect Dis. 2016 Sep;35(9):1433–1439. doi: 10.1007/s10096-016-2681-1. DOI: Epub 2016 May 26. PubMed PMID: 27230510. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen M, Eschenauer GA, Bryan M, O'Neil K, Furuya EY, Della-Latta P, et al. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis [Internet] 2010;67(2):180–184. doi: 10.1016/j.diagmicrobio.2010.02.001. DOI: [DOI] [PubMed] [Google Scholar]
- 47.Crusio R, Rao S, Changawala N, Paul V, Tiu C, van Ginkel J, et al. Epidemiology and outcome of infections with carbapenemresistant Gram-negative bacteria treated with polymyxin B-based combination therapy. Scand J Infect Dis. 2014;46(1):1–8. doi: 10.3109/00365548.2013.844350. DOI: [DOI] [PubMed] [Google Scholar]
- 48.Rigatto MH, Vieira FJ, Antochevis LC, Behle TF, Lopes NT, Zavasckic AP. Polymyxin B in combination with antimicrobials lacking in vitro activity versus polymyxin B in monotherapy in critically ill patients with Acinetobacter baumannii or Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 2015;59(10):6575–6580. doi: 10.1128/AAC.00494-15. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2014 Sep;58(9):5598–5601. doi: 10.1128/AAC.02435-13. DOI: Epub 2014 Jun 30. PubMed PMID: 24982065; PubMed CentralPMCID: PMC4135862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuang YC, Cheng CY, Sheng WH, Sun HY, Wang JT, Chen YC, et al. Effectiveness of tigecycline-based versus colistin- based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumannii in a critical setting: a matched cohort analysis. BMC Infect Dis. 2014;14(1):1–8. doi: 10.1186/1471-2334-14-102. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim SK, Lee SO, Choi SH, Choi JP, Kim SH, Jeong JY, et al. The outcomes of using colistin for treating multidrug resistant Acinetobacter species bloodstream infections. J Korean Med Sci. 2011;26(3):325–331. doi: 10.3346/jkms.2011.26.3.325. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porwal R, Gopalakrishnan R, Rajesh NJ, Ramasubramanian V. Carbapenem resistant Gram-negative bacteremia in an Indian intensive care unit: a review of the clinical profile and treatment outcome of 50 patients. Indian J Crit Care Med. 2014 Nov;18(11):750–753. doi: 10.4103/0972-5229.144021. DOI: PubMed PMID: 25425843; PubMed Central PMCID: PMC4238093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang HJ, Kim MN, Lee K, Hong SB, Lim CM, Koh Y. The comparative efficacy of colistin monotherapy and combination therapy based on in vitro antimicrobial synergy in ventilator-associated pneumonia caused by multi-drug resistant Acinetobacter baumannii. Tuberc Respir Dis. 2009;67(3):212–220. doi: 10.4046/trd.2009.67.3.212. DOI: [DOI] [Google Scholar]
- 54.Abdelsalam MFA, Abdalla MS, El-Abhar HSED. Prospective, comparative clinical study between high-dose colistin monotherapy and colistin–meropenem combination therapy for treatment of hospital-acquired pneumonia and ventilator-associated pneumonia caused by multidrug-resistant Klebsiella pneumoniae. J Glob Antimicrob Resist [Internet] 2018;15:127–135. doi: 10.1016/j.jgar.2018.07.003. DOI: [DOI] [PubMed] [Google Scholar]