Abstract
Allergic rhinitis (AR) and allergic asthma (AA) exhibit similar inflammatory response in the airways. However, the remodelling is more extensive in the lower airways, suggesting that the inflammation itself is not sufficient for allergic phenotype. We aimed to analyse whether the expression of selected 27 inflammatory and fibrosis-related proteins may be altered in AR and AA in the paediatric population and whether the expression pattern is either similar (due to the inflammation) or disease-specific (due to the remodelling). We analysed 80 paediatric subjects: 39 with AA, 21 with AR and 20 healthy children. The diagnosis of AR and AA was based on clinical manifestation, lung function, positive skin prick tests and increased immunoglobulin E levels. Serum levels of selected inflammatory proteins were measured with custom Magnetic Luminex Assay. Statistical analysis was performed in Statistica v.13. CCL2/MCP1, GM-CSF, gp130 and periostin concentrations were significantly lower, whereas IL-5 levels were higher in AA compared to the control group. CD-40L, CHI3L1/YKL-40, EGF, GM-CSF and periostin levels were significantly decreased in patients with AR than in the control group. Comparison of AA and AR patients revealed significant changes in CHI3L1/YKL-40 (P = 0.021), IL-5 (P = 0.036), periostin (P = 0.013) and VEGFα (P = 0.046). Significantly altered proteins were good predictors to distinguish between AA and AR (P < 0.001, OR 46.00, accuracy 88.57%). Our results suggest that the expression of four fibrotic proteins was significantly altered between AA and AR, suggesting possible differences in airway remodelling between upper and lower airways.
Keywords: allergy, inflammation, paediatric patients, remodelling
Introduction
Allergic rhinitis (AR) and allergic asthma (AA) are the most common chronic disorders in children concerning the respiratory tract: the upper airways in AR and the lower airways in AA.1 Both upper and lower airways have a common respiratory epithelium with the same mucociliary clearance mechanisms.
The link between AA and AR is well-known and many patients have both allergic diseases. Rowe-Jones suggested they may be presented as one disease, taking into account similar epidemiology, pathophysiology and response to treatment, they are, in fact, one disorder, expressed in the upper or lower airways to a greater or lesser extent.2,3 The link between these two disorders has been acknowledged and highlighted in the recent update of the Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines.4
Nasal and bronchial mucosa inflammation plays a crucial role in the pathogenesis of AA and AR. Upper and lower airways are infiltrated by similar inflammatory cells: Th2 lymphocytes, eosinophils, basophils and mast cells.5 Therefore, a similar set of cytokines (e.g. IL-4, IL-5, IL-13, GM-CSF), chemical mediators of inflammation and adhesion molecules is present in both disorders.6,7 Increased expression of inflammatory proteins further enhances inflammation and disease severity.
While both AA and AR have similar Th2 cells-driven inflammation of the mucosa, the remodelling (disease-caused tissue structural changes) is much more extensive in the lower airway suggesting that the inflammation alone cannot be responsible for the development of either disorder.8 Studies in asthmatic children seem to confirm this hypothesis, as the bronchial biopsies show signs of structural changes, abnormal cell activation and reticular basement membrane (RBM) thickening, indicating the remodelling to be an early feature of the disease.9,10 The epithelial-mesenchymal trophic unit signalling pathway might explain the dissociation between airway remodelling and inflammation: the injured epithelium secretes growth factors, that activate the mesenchymal cell unit and subsequently lead to RBM thickening, subepithelial fibrosis and airway smooth muscle (ASM) hyperplasia.11 Hypertrophic ASM cells produce high levels of inflammatory cytokines and growth factors (GM-CSF, IL-1β, IL-5, IL-6, IL-8, FGF, eotaxin and VEGF), promoting cell proliferation and growth, vascularisation and increased inflammatory response.12,13 In AR the data on remodelling are limited and the studies on nasal biopsies report inconsistent findings: some discovered the increased thickness of basement membrane, others did not show any changes compared to healthy controls. These data suggested that in nasal epithelium either the inflammation is not as strong as in the lower airways or that the nasal mucosa is more adapted to environmental injury.8,14
Therefore, we hypothesised that the expression of selected inflammatory and fibrosis-related proteins may be altered in AA and AR and that the expression pattern would be either disease-specific (due to more extensive lower airway remodelling) or similar (as a result of the inflammation) between those two disorders.
Patients and methods
Participants
The study group of this cross-sectional study consisted of 80 children of Caucasian origin: 20 healthy controls, 39 patients with AA and 21 patients with AR. Children were aged from 6 to 18. The study was approved by the Local Bioethics Committee at the Poznan University of Medial Sciences, Poznan, Poland (aprroval no. 954/15). All the parents/legal guardians and children above 16 years gave written informed consent. Patients either visited an outpatient clinic or were hospitalised at the Department of Pulmonology, Pediatric Allergy and Immunology, Poznan University of Medical Sciences between 2012 and 2015. The control group was recruited from healthy volunteers in 2012 and 2015.
AA was diagnosed according to GINA recommendations based on clinical asthma symptoms and lung function tests. Spirometry was performed on LungTest 1000 (MES) in accordance with ERS/ATS guidelines.15 All asthmatic children received inhaled steroids in the dose ranges between 200 and 400 µg equivalent to budesonide and SABA (salbutamol) on demand. About 35% of asthmatic patients received additionally LABA (salmeterol). Patients receiving biological drugs or undergoing immunotherapy were excluded from the study.
AR diagnosis was based on clinical manifestation (rhinorrhoea, sneezing, nasal itching and obstruction) in agreement with ARIA guidelines.4 The atopic background was confirmed by total immunoglobulin E level (IgE) higher than the normal upper limits for age and the positive skin prick test to at least one aero-allergen (Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat, dog, feathers, Alternaria alternata, Cladosporium herbarum; pollen: grass mix, rye, birch pollen, alder, hazel – Allergopharma, Reinbek, Germany). Children with autoimmune diseases, immunodeficiency, neurological diseases and parasitic infections were excluded from the study. All allergic rhinitis patients received oral antihistamine drugs and nasal steroids to control the symptoms. For the control group, any allergic diseases or asthma were excluded based on medical history and clinical examination, spirometry and total IgE level. Individuals with chronic diseases other than allergy and asthma were also excluded.
Inflammatory proteins measurement
Blood samples were collected into tubes without anticoagulant. After 1 h, samples were centrifuged and the obtained serum was frozen at −80°C for further analysis. Proteins concentration in undiluted serum were measured in duplicates using custom Magnetic Luminex Assay: Human Premixed Multi-Analyte Kit (catalog no. LXSAHM R&D Systems, Minneapolis, USA), according to the manufacturer’s protocol. The proteins analysed in this study were selected based on their documented Th2-dependent inflammatory cascade or fibrotic and airway remodelling processes and the previous association with allergic rhinitis or asthma. This included chemokines (CCL7/MCP-3, CX3CL1, CXCL10/IP-10), interleukins (CXCL8/IL-8, IL-10, IL-17α, IL-1Rα, IL-2, IL-33, IL-4, IL-5, IL-6), chemotactic proteins (CCL11/eotaxin, CCL2/MCP1), transmembrane proteins (CD-40L, TNFβ), glycoproteins (CHI3L1/YKL-40, gp130), proteoglycans (SYND4), growth factors (EGF, FGF basic, GM-CSF, VEGF-α), signalling proteins (IFN-β), interferons (IFN-γ ) and ligands (LGALS9, periostin). The detailed list of analysed proteins is presented in the Supplemental File (Supplemental Table S1).
Statistical analysis
Statistical analysis was performed in Statistica v.13 (Statsoft, Cracow, Poland). Data were not normally distributed after using the Shapiro-Wilk test. Therefore, we used non-parametric test (U-Mann-Whitney test) to compare differences in cytokine levels between groups. Power analysis of U-Mann-Whitney test assuming 80% power and 95% confidence level was evaluated using online calculator available at: https://www.benchmarksixsigma.com/calculators/sample-size-calculator-for-mann-whitney-test/. Stepwise logistic regression analysis with the Wald test was performed using disease status (disease–control) as a dichotomous-dependent variable with the cytokine expression as predictors. The statistical significance level was defined below 0.05.
Results
The study consisted of patients diagnosed with AA (n = 39) or AR (n = 21) and healthy children (n = 20) from the Polish population (Caucasian origin). Clinical characteristics are presented in Table 1. The analysed groups were similar in gender and age. The allergic patients showed significantly higher total IgE levels than the control group. Total IgE levels also differed significantly between AA and AR group.
Table 1.
Clinical characteristic of the group.
| Variable | Controls | Allergic asthma | Allergic rhinitis |
|---|---|---|---|
| Number of subjects | 20 | 39 | 21 |
| Female | 15 | 17 | 6 |
| Age (mean ± SD) | 11.25 ± 2.79 | 10.61 ± 3.53 | 12.47 ± 3.57 |
| Total IgE (mean ± SD) (IU/mL) | 7.37 | 242.35 ± 206.86*† | 27.78 ± 20.25* |
| Positive SPT (%) | 0.0 | 75%*† | 81.3%* |
| exNO (mean ± SD) (ppb) | <20 | 32.05 ± 49.17 | – |
| Blood eosinophils (mean ± SD) (%) | – | 6.2 ± 3.85 | – |
| FEV1/FVC pred (mean ± SD) | 99.87 ± 5.82 | 91.94 ± 15.59 | 93.00 ± 8.66 |
| FEV1% pred (mean ± SD) | 99.93 ± 9.88 | 94.19 ± 20.38 | 101.60 ± 13.94 |
| PEF% pred (mean ± SD) | 97.73 ± 13.31 | 89.80 ± 22.48 | 91.60 ± 15.09 |
SD: standard deviation.
Indicates significance (P < 0.05) as compared to the control group.
Indicates significance (P < 0.05) as compared to the AR group.
Median concentrations of 27 proteins measured in the serum of the study population are presented in Table 2.
Table 2.
Serum mean concentrations of 27 proteins in the studied population.
| Protein | Full name | Allergic asthma [pg/ml] | Allergic rhinitis [pg/ml] | Control [pg/ml] |
|---|---|---|---|---|
| CCL11/eotaxin | C-C Motif Chemokine Ligand 11/Eotaxin | 44.35 ± 24.47 | 43.61 ± 16.23 | 43.28 ± 14.94 |
| CCL2/MCP1 | C-C Motif Chemokine Ligand 2/Monocyte Chemotactic Protein 1 | 105.83 ± 38.22* | 124.67 ± 37.97 | 134.28 ± 41.54 |
| CCL7/MCP-3 | C-C Motif Chemokine Ligand 7/Monocyte Chemotactic Protein 3 | 15.22 ± 1.53 | 15.05 ± 2.21 | 14.65 ± 1.42 |
| CD-40L | CD40 Ligand/CD154 | 2923.08 ± 1763.76 | 2013.49 ± ± 1483.53* | 3741.32 ± 1348.98 |
| CHI3L1/YKL-40 | Chitinase 3 Like 1 | 10944.36 ± 6370.54† | 7203.79 ± 2867.24* | 8750.37 ± 2584.40 |
| CX3CL1 | C-X3-C Motif Chemokine Receptor 1/Fractalkine | 737.06 ± 267.21 | 664.38 ± 325.89 | 640.02 ± 215.75 |
| CXCL10/IP-10 | C-X-C Motif Chemokine Ligand 10/Interferon-Inducible Cytokine IP-10 | 8.92 ± 13.84 | 11.01 ± 13.99 | 7.45 ± 4.65 |
| CXCL8/IL-8 | C-X-C Motif Chemokine Ligand 8/Interleukin-8 | 4.99 ± 5.95 | 6.60 ± 11.67 | 5.40 ± 4.50 |
| EGF | Epidermal Growth Factor | 92.12 ± 96.06 | 46.39 ± 41.94* | 76.29 ± 48.96 |
| FGFbasic | Fibroblast Growth Factor 2 (Basic) | 2.32 ± 2.59 | 1.60 ± 0.89 | 1.67 ± 1.49 |
| GM-CSF | Granulocyte Macrophage-Colony Stimulating Factor/Colony Stimulating Factor 2 | 4.15 ± 2.22* | 3.81 ± 1.40* | 5.34 ± 1.24 |
| gp130 | Interleukin 6 Signal Transducer | 39580.48 ± 18467.90* | 40678.83 ± 11932.25 | 48284.53 ± 12547.13 |
| IFN-β | Interferon Beta | 0.73 ± 1.03 | 1.18 ± 1.20 | 2.77 ± 3.16 |
| IFN-γ | Interferon Gamma | 40.04 ± 42.86 | 26.60 ± 21.79 | 32.94 ± 31.85 |
| IL-10 | Interleukin-10 | 0.95 ± 0.81 | 1.55 ± 3.28 | 0.90 ± 0.80 |
| IL-17α | Interleukin-17α | 2.97 ± 1.77 | 2.76 ± 1.73 | 2.15 ± 1.47 |
| IL-1Rα | Interleukin-1 Receptor α | 475.39 ± 440.19 | 396.65 ± 233.14 | 386.60 ± 111.89 |
| IL-2 | Interleukin-2 | 20.34 ± 11.15 | 14.53 ± 8.75 | 28.12 ± 20.55 |
| IL-33 | Interleukin-33 | 4.96 ± 4.39 | 3.18 ± 2.03 | 4.42 ± 4.96 |
| IL-4 | Interleukin-4 | 17.29 ± 4.63 | 17.07 ± 3.53 | 17.61 ± 5.56 |
| IL-5 | Interleukin-5 | 1.39 ± 1.95*† | 0.58 ± 0.28 | 0.49 ± 0.20 |
| IL-6 | Interleukin-6 | 2.72 ± 10.83 | 1.34 ± 2.12 | 0.72 ± 0.26 |
| LGALS9 | Galectin-9 | 3662.42 ± 1976.34 | 3444.93 ± 1699.02 | 4210.22 ± 1633.96 |
| LT-α/TNF-β | Lymphotoxin-α/Tumour Necrosis Factor β | 1.01 ± 0.77 | 0.97 ± 0.82 | 0.83 ± 0.69 |
| Periostin | Periostin | 56961.67 ± 18760.38*† | 74228.37 ± 23669.18* | 93418.73 ± 22278.76 |
| SYND4 | Syndecan-4 | 939.59 ± 330.59 | 902.05 ± 324.06 | 1075.27 ± 219.26 |
| VEGFα | Vascular Endothelial Growth Factor α | 52.27 ± 22.06† | 41.57 ± 24.37 | 41.60 ± 16.49 |
Indicates significance (P < 0.05) as compared to the control group.
Indicates significance (P < 0.05) as compared to the AR group.
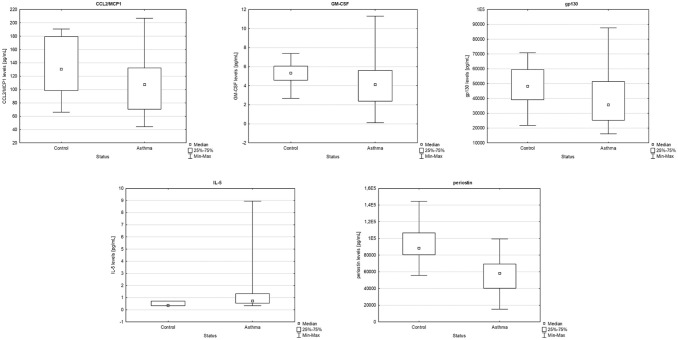
Comparison analysis revealed that CCL2/MCP1, GM-CSF, gp130 and periostin concentrations were significantly lower in patients with AA compared to control (P = 0.027, P = 0.013, P = 0.018, P < 0.001, respectively), whereas IL-5 levels were significantly increased (P = 0.017), as presented in Figure 1.
Figure 1.
Comparison between allergic asthmatic patients and the control group of CCL2/MCP1 (P = 0.027), GM-CSF (P = 0.013), gp130 (P = 0.018), IL-5 (P = 0.017) and periostin (P < 0.001) levels in serum (U-Mann-Whitney, box plot).
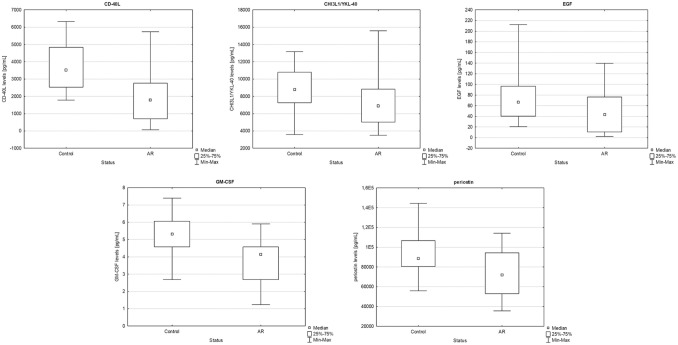
Moreover, we found that that CD-40L, CHI3L1/YKL-40, EGF, GM-CSF and periostin levels were significantly decreased (P < 0.001, P = 0.033, P = 0.043, P = 0.002 and P = 0.024, respectively) in patients with AR when compared to the control group (Figure 2).
Figure 2.
Comparison between allergic rhinitis patients and the control group of CD-40L (P < 0.001), CHI3L1/YKL-40 (P = 0.033), EGF (P = 0.043), GM-CSF (P = 0.002) and periostin (P = 0.024) levels in serum (U-Mann-Whitney, box plot).
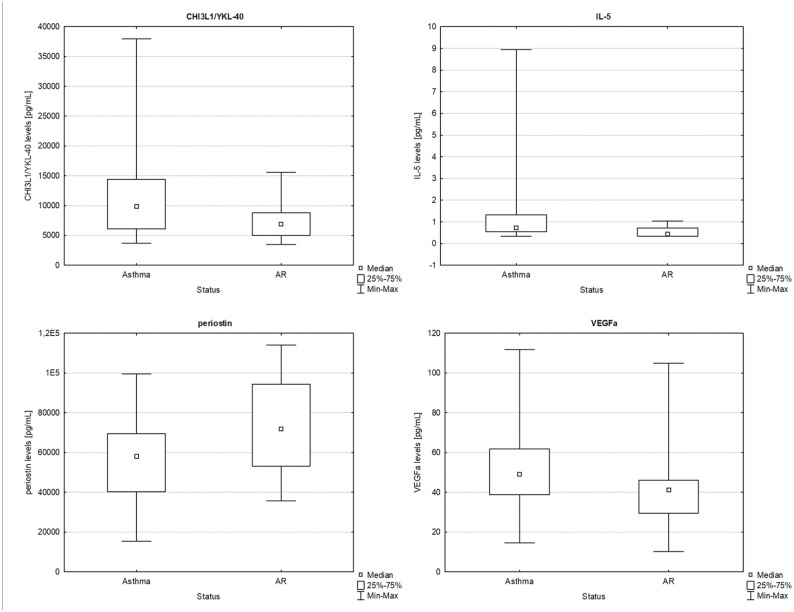
Comparison of AA and AR patients showed significantly higher expression of CHI3L1/YKL-40 (P = 0.021), IL-5 (P = 0.036) and VEGFα (P = 0.046) in AA, as well as significantly decreased expression of periostin (P = 0.013) in AR. The results are presented in Figure 3.
Figure 3.
Comparison between allergic asthmatic and rhinitis patients of CHI3L1/YKL-40 (P = 0.021), IL-5 (P = 0.036), periostin (P = 0.013) and VEGFα (P = 0.046) levels in serum (U-Mann-Whitney, box plot).
Minimum sample size required to verify if the two medians are similar or not for above comparisons assuming 80% power and 95% confidence level, was between 27 and 84 subjects in each group, depending on the inter-individual variability of the protein level.
We have also used stepwise logistic regression analysis to check whether inflammatory protein serum levels could be predictors of the AA or AR risk. All variables in the models were not specific. However, when the variables were reduced to the proteins with significantly changed expression in each of the analyses, the predictive models have revealed them to be good predictors for AA (P = 0.006, odds ratio 9.36, accuracy 75%), AR (P < 0.001, odds ratio 24.08, accuracy 82.93%), or as a good predictors to differentiate between these diseases (P < 0.001, odds ratio 46.00, accuracy 88.57%).
Discussion
The main finding of our study is the significantly changed expression of the inflammatory proteins in the serum of AA and AR paediatric children when compared to healthy controls, as well as the different expression of four fibrotic proteins between those two disorders.
CCL2/MCP1, GM-CSF, gp130 and periostin concentrations were significantly lower, whereas IL-5 levels were higher in patients with AA compared to control. These results are in agreement with previous findings regarding CCL2/MCP1, periostin and IL-5, but contradictory to the existing literature in case of gp130 and GM-CSF .
Previous study showed that MCP-1 levels were decreased in serum from paediatric asthmatic patients when compared to controls.16 Different outcomes were also reported: MCP1 was significantly higher in serum and exhaled breath condensate from asthmatic patients, but the sample size was limited (n = 20 and n = 25).17,18 Interestingly, GM-CSF had higher expression in serum, sputum, BALF and bronchial mucosa, which is not consistent with our results, probably due to the fact that the subjects were either adults or adolescents.19–21 Signal-transducing receptor, gp130, is involved in inflammatory cytokines pathway via JAK/STAT,22 which may explain its decreased levels in our study. However, our observations of its decreased concentrations in serum of asthmatic children compared to control group are in contrast to previous findings by Xu et al.23 The difference in results may result from different source of biological material (plasma) and age group of the subjects (<3 years). Serum IL-5 levels (essential cytokine for eosinophil differentiation and survival) in AA patients were significantly higher than in controls in the previous studies, as confirmed by our research.24 Periostin, an extracellular matrix protein, was deposited in the thickened basement membrane in asthmatic patients, contributing to subepithelial fibrosis.25–27 However, it was also found that periostin decreases allergic airway inflammation in mice.28,29 Previous cohort study showed no difference between asthmatic patients and healthy controls.30 Different research outcomes show that the role of periostin in allergic inflammation is still unclear.
Comparison of AR patients with control group revealed significant decrease in CD-40L, CHI3L1/YKL-40, EGF, GM-CSF and periostin levels in the AR group. Our results differ from the other published works, as almost all of these proteins were found to be up-regulated in AR. This discrepancy may be explained by different age of our population (children in our study vs adults in the previously published papers). Previous studies showed that the concentrations of these proteins in serum was strongly influenced by age in different diseases.26,31–33
CD-40L (a member of the TNF ligand family) expression in serum was significantly increased in AR patients.34 Secreted glycoprotein CHI3L1/YKL-40 expression level was up-regulated in allergic nasal mucosa and serum from moderate/severe AR patients.35,36 EGF receptor was upregulated in seasonal AR, which suggest that this molecule plays a role in the allergic inflammation.37 Previously, GM-CSF expression in nasal secretions from AR patients was significantly increased or normal, when compared to healthy controls.38,39 Periostin mRNA and protein levels were upregulated in AR and were associated with AR and asthma comorbidity.40,41 Interestingly, Korean children with AR did not have significantly altered periostin levels compared to healthy controls.42
Studies in asthmatic children showed that the structural changes of the lung tissue originated from injury and often preceded the inflammation and that lower airways remodelling is much more extensive compared to that of the upper airways.8 Therefore, we have also compared the AA and AR patients to see whether the expression pattern of analysed proteins would be disease-specific. CHI3L1/YKL-40, IL-5 and VEGFα showed significantly higher expression in AA, whereas the expression of periostin was significantly lower in AA than AR. Hoppenot et al. reported that IL-5 serum levels were significantly increased for AR and asthma compared to healthy controls; IL-5 concentrations were also significantly higher in asthma than AR.43 There is a lack of literature comparing CHI3L1/YKL-40, periostin and VEGF in AA and AR. Interestingly, all of these inflammatory proteins contribute to the airway fibrosis and remodelling. CHI3L1/YKL-40 both inhibits injury and increases fibroproliferative repair, leading to tissue scarring.44 IL-5-deficient mice had less peribronchial fibrosis and peribronchial smooth muscle layer, whereas anti-IL-5 treatment in human subjects decreased the thickness of reticular basement membrane by regulating the composition of the matrix.45,46 VEGF regulates surfactant production and bronchial angiogenesis,47 whereas periostin controls the matrix composition via interaction with other extracellular matrix proteins.48 Therefore, we hypothesise that the distinct expression of these proteins in AA and AR might be caused by different mechanisms of the remodelling: in the lower airways, the epithelium activates the mesenchymal cell unit, responsible for reticular basement membrane thickening, subepithelial fibrosis and airway smooth muscle hyperplasia, subsequently increasing inflammatory response, whereas, in the upper airways, the reaction to the injury and following inflammation is not as strong.8,11
Interestingly, periostin has been found to have decreased expression in both diseases compared to control and differential expression between both of these diseases. Since periostin was found to cause subepithelial fibrosis in upper and lower airways (although the nasal mucosa is more affected), it may explain the significantly higher expression in AR. Moreover, GM-CSF expression significantly differed in both AA and AR when compared to controls, indicating the common background of these disorders, as GM-CSF plays a role in the allergic sensitisation.49
Our analysis has revealed that proteins with significantly changed expression in each of the analyses were good predictor variables for AA or AR, as well as for the differentiation between these two diseases. Periostin, significantly changed in all of the comparisons, has already been proposed as a biomarker of asthma.25
Despite this is the first comprehensive analysis of inflammatory and fibrosis-related proteins in allergic children, we are aware of the limitations of this study. The main limitation is the limited size of the analysed groups, in particular for proteins showing high variability within the group, that resulted in low power of the comparisons and might affected the interpretation of the results. However, the strength of this study is analysis in a paediatric population, as the most of the previous studies was carried out in adult patients. Moreover, previous studies were performed in the sample sizes comparable to our sample.
Conclusion
In conclusion, our results suggest that the expression of several inflammatory proteins significantly differs between AA and AR compared to controls, suggesting their involvement in the allergic inflammation. Moreover, the different levels of four fibrotic proteins (CHI3L1/YKL, IL-5, VEGFα and periostin) between AA and AR, implicate distinct patomechanism in the lower and upper airway remodelling and could become a diagnostic aid for better differentiation between these diseases. These proteins could also serve as targets to develop novel therapies focused on differences in airway remodelling between allergic rhinitis and allergic asthma. However, further studies on larger populations are necessary to confirm our observations.
Supplemental Material
Supplemental material, sj-pdf-1-iji-10.1177_2058738421990493 for Expression of proteins associated with airway fibrosis differs between children with allergic asthma and allergic rhinitis by Paulina Sobkowiak, Beata Narożna, Irena Wojsyk-Banaszak, Anna Bręborowicz and Aleksandra Szczepankiewicz in International Journal of Immunopathology and Pharmacology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by the Department of Pediatric Pulmonology, Allergy and Clinical Immunology, Poznan University of Medical Sciences and the National Science Centre in Poland, grant no. 2011/01/D/NZ5/02771 (A.S.) and 2019/35/B/NZ5/02906 (A.S.). The funding body had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Ethical approval: Ethical approval for this study was obtained from Local Bioethics Committee at the Poznan University of Medial Sciences, Poznan, Poland (approval no. 954/15).
Informed consent: Written informed consent was obtained from legally authorised representatives before the study.
ORCID iDs: Irena Wojsyk-Banaszak  https://orcid.org/0000-0001-6724-1499
https://orcid.org/0000-0001-6724-1499
Aleksandra Szczepankiewicz  https://orcid.org/0000-0003-3379-9106
https://orcid.org/0000-0003-3379-9106
Data availability: The dataset supporting the conclusions of this article is available upon the written request from the corresponding author.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Kim H, Bouchard J, Renzi PM. (2008) The link between allergic rhinitis and asthma: A role for antileukotrienes? Canadian Respiratory Journal 15(2): 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simons FE. (1999) Allergic rhinobronchitis: The asthma-allergic rhinitis link. Journal of Allergy and Clinical Immunology 104(3 Pt 1): 534–540. [DOI] [PubMed] [Google Scholar]
- 3. Rowe-Jones JM. (1997) The link between the nose and lung, perennial rhinitis and asthma–Is it the same disease? Allergy 52(36 Suppl): 20–28. [DOI] [PubMed] [Google Scholar]
- 4. Brozek JL, Bousquet J, Agache I, et al. (2017) Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. Journal of Allergy and Clinical Immunology 140(4): 950–958. [DOI] [PubMed] [Google Scholar]
- 5. American Thoracic Society Workshop (1999) Immunobiology of asthma and rhinitis. Pathogenic factors and therapeutic options. American Journal of Respiratory and Critical Care Medicine 160(5 Pt 1): 1778–1787. [DOI] [PubMed] [Google Scholar]
- 6. Scadding G. (2014) Cytokine profiles in allergic rhinitis. Current Allergy and Asthma Reports 14(5): 435. [DOI] [PubMed] [Google Scholar]
- 7. Kips JC. (2001) Cytokines in asthma. European Respiratory Journal Supplement 34: 24s–33s. [DOI] [PubMed] [Google Scholar]
- 8. Samitas K, Carter A, Kariyawasam HH, et al. (2018) Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited. Allergy 73(5): 993–1002. [DOI] [PubMed] [Google Scholar]
- 9. Payne DN, Rogers AV, Adelroth E, et al. (2003) Early thickening of the reticular basement membrane in children with difficult asthma. American Journal of Respiratory and Critical Care Medicine 167(1): 78–82. [DOI] [PubMed] [Google Scholar]
- 10. Pohunek P, Warner JO, Turzikova J, et al. (2005) Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatric Allergy and Immunology 16(1): 43–51. [DOI] [PubMed] [Google Scholar]
- 11. Holgate ST, Davies DE, Lackie PM, et al. (2000) Epithelial-mesenchymal interactions in the pathogenesis of asthma. Journal of Allergy and Clinical Immunology 105(2 Pt 1): 193–204. [DOI] [PubMed] [Google Scholar]
- 12. Hirst SJ. (2003) Regulation of airway smooth muscle cell immunomodulatory function: Role in asthma. Respiratory Physiology & Neurobiology 137(2–3): 309–326. [DOI] [PubMed] [Google Scholar]
- 13. Alexandrova E, Nassa G, Corleone G, et al. (2016) Large-scale profiling of signalling pathways reveals an asthma specific signature in bronchial smooth muscle cells. Oncotarget 7(18): 25150–25161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim MC, Taylor RM, Naclerio RM. (1995) The histology of allergic rhinitis and its comparison to cellular changes in nasal lavage. American Journal of Respiratory and Critical Care Medicine 151(1): 136–144. [DOI] [PubMed] [Google Scholar]
- 15. Graham BL, Steenbruggen I, Miller MR, et al. (2019) Standardization of spirometry 2019 update. An official american thoracic society and european respiratory society technical statement. American Journal of Respiratory and Critical Care Medicine 200(8): e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keszei M, Nagy A, Kozma GT, et al. (2006) Pediatric asthmatic patients have low serum levels of monocyte chemoattractant protein-1. Journal of Asthma 43(5): 399–404. [DOI] [PubMed] [Google Scholar]
- 17. Jahnz-Royk K, Plusa T, Mierzejewska J. (2000) Eotaxin in serum of patients with asthma or chronic obstructive pulmonary disease: Relationship with eosinophil cationic protein and lung function. Mediators of Inflammation 9(3–4): 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Majak P, Jerzynska J, Bojo M, et al. (2016) Cytokine profiling in exhaled breath condensate after exercise challenge in asthmatic children with post-exercise symptoms. Archives of Medical Science 12(4): 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saha S, Doe C, Mistry V, et al. (2009) Granulocyte-macrophage colony-stimulating factor expression in induced sputum and bronchial mucosa in asthma and COPD. Thorax 64(8): 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dente FL, Carnevali S, Bartoli ML, et al. (2006) Profiles of proinflammatory cytokines in sputum from different groups of severe asthmatic patients. Annals of Allergy, Asthma & Immunology 97(3): 312–320. [DOI] [PubMed] [Google Scholar]
- 21. Woolley KL, Adelroth E, Woolley MJ, et al. (1994) Granulocyte-macrophage colony-stimulating factor, eosinophils and eosinophil cationic protein in subjects with and without mild, stable, atopic asthma. European Respiratory Journal 7(9): 1576–1584. [DOI] [PubMed] [Google Scholar]
- 22. Silver JS, Hunter CA. (2010) gp130 at the nexus of inflammation, autoimmunity, and cancer. Journal of Leukocyte Biology 88(6): 1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu H, Radabaugh T, Lu Z, et al. (2016) Exploration of early-life candidate biomarkers for childhood asthma using antibody arrays. Pediatric Allergy and Immunology 27(7): 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joseph J, Benedict S, Safa W, et al. (2004) Serum interleukin-5 levels are elevated in mild and moderate persistent asthma irrespective of regular inhaled glucocorticoid therapy. BMC Pulmonary Medicine 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woodruff PG, Boushey HA, Dolganov GM, et al. (2007) Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proceedings of the National Academy of Sciences of the United States of America 104(40): 15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Izuhara K, Ohta S, Ono J. (2016) Using periostin as a biomarker in the treatment of asthma. Allergy, Asthma & Immunology Research 8(6): 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masuoka M, Shiraishi H, Ohta S, et al. (2012) Periostin promotes chronic allergic inflammation in response to Th2 cytokines. Journal of Clinical Investigation 122(7): 2590–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sehra S, Yao W, Nguyen ET, et al. (2011) Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. Journal of Immunology 186(8): 4959–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gordon ED, Sidhu SS, Wang ZE, et al. (2012) A protective role for periostin and TGF-beta in IgE-mediated allergy and airway hyperresponsiveness. Clinical & Experimental Allergy 42(1): 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. James A, Janson C, Malinovschi A, et al. (2017) Serum periostin relates to type-2 inflammation and lung function in asthma: Data from the large population-based cohort Swedish GA(2)LEN. Allergy 72(11): 1753–1760. [DOI] [PubMed] [Google Scholar]
- 31. Meybosch S, De Monie A, Anne C, et al. (2019) Epidermal growth factor and its influencing variables in healthy children and adults. PLoS One 14(1): e0211212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santos CB, Davidson J, Covar RA, et al. (2014) The chitinase-like protein YKL-40 is not a useful biomarker for severe persistent asthma in children. Annals of Allergy, Asthma & Immunology 113(3): 263–266. [DOI] [PubMed] [Google Scholar]
- 33. Lee KY, Suh BG, Kim JW, et al. (2000) Varying expression levels of colony stimulating factor receptors in disease states and different leukocytes. Experimental & Molecular Medicine 32(4): 210–215. [DOI] [PubMed] [Google Scholar]
- 34. Zhu R, Liu G, Li W, et al. (2008) A study of costimulatory molecules in allergic allergic rhinitis patients. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 22(17): 780–782, 784. [PubMed] [Google Scholar]
- 35. Park SJ, Jun YJ, Kim TH, et al. (2013) Increased expression of YKL-40 in mild and moderate/severe persistent allergic rhinitis and its possible contribution to remodeling of nasal mucosa. American Journal of Rhinology & Allergy 27(5): 372–380. [DOI] [PubMed] [Google Scholar]
- 36. Pirayesh A, Shahsavan S, Zargari Samani O, et al. (2020) Local expression of mucosal YKL-40; correlation of YKL-40 with clinical manifestations and immunopathogenesis of moderate/severe persistent allergic rhinitis patients. Immunological Investigations 49(1–2): 46–57. [DOI] [PubMed] [Google Scholar]
- 37. Matovinovic E, Solberg O, Shusterman D. (2003) Epidermal growth factor receptor - but not histamine receptor - is upregulated in seasonal allergic rhinitis. Allergy 58(6): 472–475. [DOI] [PubMed] [Google Scholar]
- 38. Peric A, Spadijer-Mirkovic C, Matkovic-Jozin S, et al. (2016) Granulocyte-macrophage colony-stimulating factor production and tissue eosinophilia in chronic rhinitis. International Archives of Otorhinolaryngology 20(4): 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Konig K, Klemens C, Eder K, et al. (2015) Cytokine profiles in nasal fluid of patients with seasonal or persistent allergic rhinitis. Allergy, Asthma & Clinical Immunology 11(1): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimura H, Konno S, Makita H, et al. (2018) Serum periostin is associated with body mass index and allergic rhinitis in healthy and asthmatic subjects. Allergology International 67(3): 357–363. [DOI] [PubMed] [Google Scholar]
- 41. Ishida A, Ohta N, Suzuki Y, et al. (2012) Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergology International 61(4): 589–595. [DOI] [PubMed] [Google Scholar]
- 42. Kim DY, Kim JH, Lee KH, et al. (2017) Serum periostin level is not associated with allergic rhinitis or allergic sensitization in Korean children. International Journal of Pediatric Otorhinolaryngology 93: 24–29. [DOI] [PubMed] [Google Scholar]
- 43. Hoppenot D, Malakauskas K, Lavinskiene S, et al. (2015) Peripheral blood Th9 cells and eosinophil apoptosis in asthma patients. Medicina (Kaunas) 51(1): 10–17. [DOI] [PubMed] [Google Scholar]
- 44. Zhou Y, Peng H, Sun H, et al. (2014) Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in Mammalian lung fibrosis. Science Translational Medicine 6(240): 240–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cho JY, Miller M, Baek KJ, et al. (2004) Inhibition of airway remodeling in IL-5-deficient mice. Journal of Clinical Investigation 113(4): 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flood-Page P, Menzies-Gow A, Phipps S, et al. (2003) Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. Journal of Clinical Investigation 112(7): 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barratt SL, Flower VA, Pauling JD, et al. (2018) VEGF (vascular endothelial growth factor) and fibrotic lung disease. International Journal of Molecular Sciences 19(5): 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O’Dwyer DN, Moore BB. (2017) The role of periostin in lung fibrosis and airway remodeling. Cellular and Molecular Life Sciences 74(23): 4305–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gajewska BU, Wiley RE, Jordana M. (2003) GM-CSF and dendritic cells in allergic airway inflammation: Basic mechanisms and prospects for therapeutic intervention. Current Drug Targets – Inflammation & Allergy 2(4): 279–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-iji-10.1177_2058738421990493 for Expression of proteins associated with airway fibrosis differs between children with allergic asthma and allergic rhinitis by Paulina Sobkowiak, Beata Narożna, Irena Wojsyk-Banaszak, Anna Bręborowicz and Aleksandra Szczepankiewicz in International Journal of Immunopathology and Pharmacology