Abstract
The 5-hydroxymethylcytosine (5hmC) epigenetic modification is highly enriched in the CNS and a critical modulator of neuronal function and development. We found that cortical 5hmC was enhanced from 5 min to three days of reperfusion following focal ischemia in adult mice. Blockade of the 5hmC-producing enzyme ten-eleven translocase 3 (TET3) increased edema, infarct volume, and motor function impairments. To determine the mechanism by which TET3 provides ischemic neuroprotection, we assessed the genomic regions where TET3 modulates 5hmC. Genome-wide sequencing analysis of differentially hydroxymethylated regions (DhMRs) revealed that focal ischemia robustly increased 5hmC at the promoters of thousands of genes in a TET3-dependent manner. TET3 inhibition reduced 5hmC at the promoters of neuroprotective genes involved in cell survival, angiogenesis, neurogenesis, antioxidant defense, DNA repair, and metabolism demonstrating a role for TET3 in endogenous protection against stroke. The mRNA expression of several genes with known involvement in ischemic neuroprotection were also reduced with TET3 knockdown in both male and female mice, establishing a correlation between decreased promoter 5hmC levels and decreased gene expression. Collectively, our results indicate that TET3 globally increases 5hmC at regulatory regions and overwhelmingly modulates 5hmC in several neuroprotective pathways that may improve outcome after ischemic injury.
Keywords: Epigenetics, gene expression, neuroprotection, cerebral ischemia, angiogenesis
Introduction
Epigenetic modifications, such as DNA methylation and histone acetylation, significantly impact functional outcome after stroke.1 Recently, it has been shown that the mammalian ten-eleven translocases (TET1–3) convert 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), producing a stable epigenetic mark associated with increased gene expression.2–5 The 5hmC modification is highly expressed in the brain where it has been shown to play critical roles in neurodevelopment and neuronal function.4,6,7 Alterations in 5hmC have been associated with a number of neurological disorders, although the specific role of 5hmC in these diseases needs to be elucidated.8,9
Emerging evidence points to a role for TETs and 5hmC in neuroprotection. Neurons from TET1 knockout mice were more susceptible to oxidative stress and TET1 activity protected neurons against reactive oxygen species and caspase-3-dependent neurotoxicity.10,11 Loss of TET2 and 5hmC is associated with decreased neuronal survival in vitro and impaired hippocampal neurogenesis and cognition in adult mice.12,13 Restoration of TET2 was also shown to rescue aged brains from cognitive decline.13 TET3 plays a critical role in DNA damage repair pathways and has been shown to promote axon regeneration after injury.7,14,15 TET3 also promotes synaptic plasticity and behavioral adaptations in learning and memory paradigms.7,16 TET3 has further been implicated as a major protective mechanism against neuronal death in the brain through its regulation of lysosomal and autophagy pathway genes linked to neurodegenerative diseases.17
Several studies have demonstrated that TET activity and global 5hmC levels are rapidly induced following hypoxic conditions.18–20 Our laboratory and others have shown that 5hmC is robustly increased in the cortex following focal ischemia from early to late time points of reperfusion.21,22 In vitro, 5hmC has been shown to promote tolerance to hypoxic conditions through modulation of canonical hypoxia response genes such as, hypoxia-inducible factor.18,20 We have previously shown that TET3 modulates the expression of dozens of antioxidant and DNA repair genes and promotes functional recovery after focal ischemia.21 Since TET3 is a global regulator of 5hmC within the cortex, we presently evaluated specific genomic regions modulated by TET3 after focal ischemia and analyzed if these genes are involved in neuroprotection. We currently focused on the role of TET3-mediated changes in 5hmC and gene expression in the therapeutically relevant peri-infarct region of the mouse cortex.
Materials and methods
Mice
The Research Animal Resources and Care Committee of the University of Wisconsin-Madison approved all experimental mouse protocols. The mice were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services Publication 86-23, revised). Three-month-old male (25–28 g) and female (19–21 g) C57Bl/6J mice (Jackson Laboratory) were used for experiments. Mice were housed with free access to water in a standard pathogen-free environment. This study is in compliance with the ARRIVE guidelines.
Focal ischemia
One hour of transient middle cerebral artery occlusion (MCAO) was induced under isoflurane anesthesia by intraluminal suture method using a 6-0 silicon-coated nylon monofilament (Doccol; catalog# 602345 for male mice and catalog# 602145 for female mice) as described previously.23 Regional cerebral blood flow and physiological parameters were monitored (pH, PaO2, PaCO2, hemoglobin, and blood glucose) and rectal temperature was maintained at 37°C. Mice with unsuccessful occlusion, no evidence of neurological deficits, or that showed hemorrhage after euthanasia were the criteria used for exclusion from the study. Two mice were excluded from this study; one due to hemorrhage and the other due to absence of neurological deficits. Cohorts of mice were euthanized at 5 min, 6 h, 12 h, 24 h, three days or seven days of reperfusion as indicated. Tissue for analyses were obtained from the cortex for sham and 5 min of reperfusion groups or from the peri-infarct regions for 6 h, 12 h, 24 h, three days and seven days of reperfusion groups. The peri-infarct samples were obtained from the region directly adjacent to the necrotic ischemic core as shown in Supplementary Figure 1.
Knockdown of TET3
A cocktail of three in vivo grade siRNAs (ThermoFisher Scientific) targeting non-overlapping regions of TET3 was used to knockdown TET3. The siRNA cocktail (8 nmol) was injected intracerebrally into the cortex (from bregma –0.2 mm posterior, 1.5 mm dorsoventral, and 3.0 mm lateral) with a Hamilton syringe at 0.5 µl/min 48 h prior to MCAO as described previously.24,25 A non-targeting negative control siRNA was used as a control using the same parameters. Mice were randomly assigned to the negative control siRNA or TET3 siRNA group. A small cohort of mice were injected with cyanin 3 (Cy3)-tagged siRNA (8 nmol; ThermoFisher Scientific) to determine the anatomical distribution of siRNA following injection.
DNA dot blotting
An AllPrep DNA/RNA mini kit (Qiagen) was used to isolate DNA from the peri-infarct cortex. The DNA was blotted onto nitrocellulose membrane and then subsequently exposed to 10 min ultraviolet light and baked at 80°C for 2 h. The membranes were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween-20 and probed with an antibody against 5hmC (1: 2000; Active Motif USA), followed by horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies (1:5000; Cell Signaling USA). Chemiluminescence (Life Technologies USA) was used to develop blots on the LI-COR Odyssey Fc platform and quantified with Image Studio software (LI-COR Biotechnology).
Immunohistochemistry
Mice were euthanized at one day and seven days of reperfusion by transcardiac 4% paraformaldehyde (PFA) perfusion fixation. Brains were post-fixed in 4% PFA, cryoprotected in 30% sucrose, and sectioned (coronal: 30 µm). Brain sections were immunostained with antibodies against 5hmC (1:500; Active Motif) as described previously.23 Brain sections were scanned and images were produced by Keyence BZX fluorescence microscope (Keyence, USA).
Western blotting
Radioimmunoprecipitation assay buffer (Sigma Aldrich) was used to obtain protein lysates from the hippocampus, striatum, and cortex at 48 h following siRNA treatment. The protein samples (30 µg) were electrophoresed and transferred to nitrocellulose membranes. Membranes were blocked with 5% BSA in Tris buffered saline with Tween 20 and probed with antibodies against TET3 (1:500; Millipore) followed by secondary horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (1:3000; Cell Signaling Technology). Blots were reprobed with glyceraldehyde-3-phosphate dehydrogenase antibody (1:1000; Cell Signaling Technology) followed by secondary HRP-conjugated anti-mouse secondary antibody (1:3000; Cell Signaling Technology). Chemiluminescence (Life Technologies) was used to visualize blots on the LI-COR Odyssey Fc platform and quantified with Image Studio software (LI-COR Biotechnology).
Neurobehavioral function and infarct volume
Motor deficits were examined at one day of reperfusion by the following criteria: no neurological deficit observed (0), failure to fully extend right forepaw (1), turning to right (2), circling to right (3), unable to spontaneously walk (4), and death from stroke (5). Motor function was evaluated by the rotarod test (4 min on a cylinder rotating at 8 r/min) and beam-walk test (number of foot faults while crossing a 120-cm-long beam) at days 1 and 3 of reperfusion. On day 3 of reperfusion, mice were euthanized and serial brain sections (coronal: 1 mm) from each mouse were stained with 1.5% triphenyltetrazolium chloride (TTC). NIH Image J software was used to measure the infarct volume as described previously.23 Infarct volume was corrected to account for edema and differential shrinkage during tissue processing using the Swanson formula. Edema volume was calculated by subtracting the contralateral hemisphere volume from the ipsilateral hemisphere volume.
5hmC sequencing
Hydroxymethylation DNA immunoprecipitation sequencing (hMeDIP-seq) was performed using DNA samples from peri-infarct region of the ischemic cortex (Arraystar Inc). Samples were fragmented to ∼200–800bp with a Diagenode Bioruptor. Approximately 1 µg of fragmented DNA was prepared for Illumina HiSeq 4000 sequencing. The completed libraries were quantified by Agilent 2100 Bioanlyzer, denatured with 0.1 M NaOH to generate single-stranded DNA molecules, captured on Illumina flow cell, amplified in situ, and sequenced on the Illumina HiSeq 4000 following the HiSeq 3000/4000 SBS Kit (300 cycles) protocol. Image analysis and base calling were performed using Off-Line Basecaller software (OLB V1.8). Clean reads that passed Solexa CHASTITY quality filter were aligned to Mouse genome (UCSC MM10) using HISAT2 software (V2.1.0) and used for peak calling of associated hMeDIP-enriched regions (peaks) with statistically significant peaks identified in each sample using a q-value threshold of 10−4 by MACS v2. hMeDIP-enriched regions (peaks) were annotated by the nearest gene using the newest UCSC RefSeq database. A Bioconductor package for differential binding analysis of Chip-seq data using sliding windows26 was used to identify differential peaks with cutoff of log2 FC = 1, p < .05. Input DNA backgrounds were used to filter out regions before the differential analysis. Each experimental group had the minimum sample size (n = 3) to establish significance.
Real-time PCR
RNA was extracted from the peri-infarct cortex using an AllPrep DNA/RNA mini kit (Qiagen, USA) and then reverse transcribed into cDNA with the Reverse Transcription System (Promega, USA). mRNA levels were determined by real-time PCR by SYBR Green method using gene-specific primer pairs as listed in Supplementary Table 1 using QuantStudio 3 (ThermoFisher Scientific, USA).
Statistics
Mann–Whitney U test and Kruskal–Wallis with Dunn’s post hoc test were used to compare differences between two groups or multiple groups, respectively. Log-rank (Mantel–Cox) test was used to compare survival between groups. Repeated measures ANOVA (Sidak’s post hoc) was used to compare differences over time between groups. Values shown are mean ± SD and p < 0.05 was used for significance cut-off. Mice were randomly assigned to experimental groups. Male and female mice were studied in separate experiments. Researchers performing sequencing analysis were blind to group allocation.
Data availability
All sequencing data are deposited in the Gene Omnibus Expression (GEO) repository and will be made publicly available under an accession number after our use of the data.
Results
TET3-modulated 5hmC levels after focal ischemia
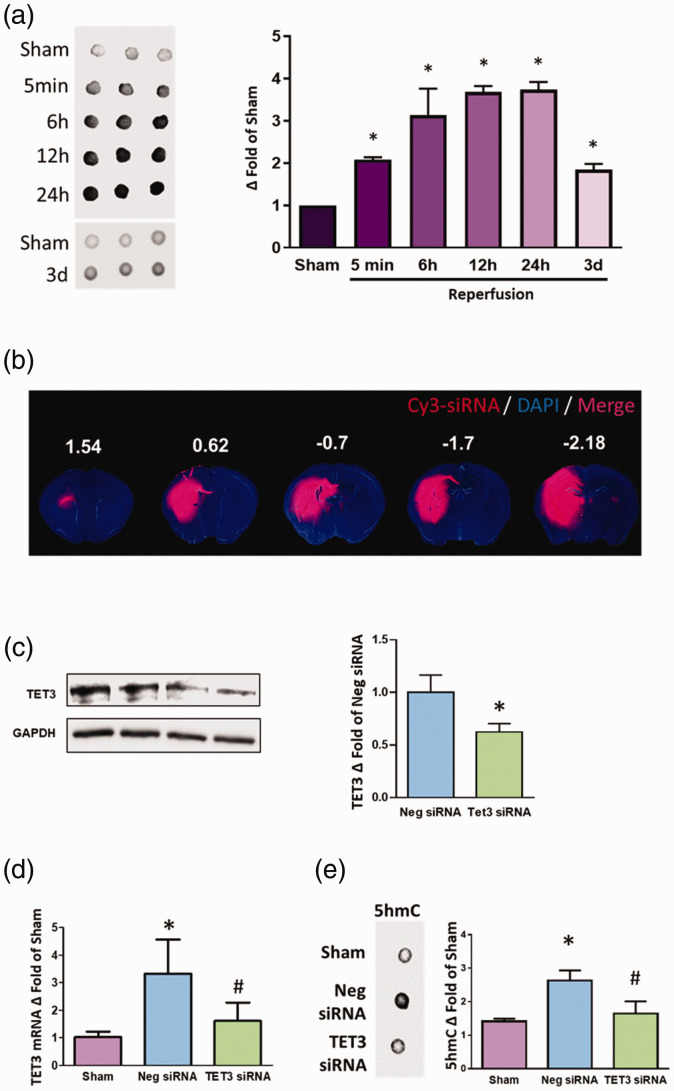
We have previously shown that focal ischemia significantly induces TET activity and preferentially increases the TET3 isoform in the ischemic cortex.21 Following transient MCAO in adult mice, dot blot analysis revealed that cortical 5hmC levels were increased from 5 min to three days of reperfusion (Figure 1(a)). Immunohistochemical staining showed that 5hmC levels were enhanced at one day of reperfusion, but remained unaltered at seven days of reperfusion in the peri-infarct cortex (Supplementary Figure 2). To determine the role of TET3 on 5hmC levels, we injected either TET3 siRNA, negative control siRNA, or Cy3-tagged negative control siRNA into the brains of adult mice. Cy3 fluorescence was observed in the striatum, hippocampus, and cortex at 24 h following injection (Figure 1(b)). Forty-eight hours following injection, western blotting analysis revealed knockdown of TET3 protein levels within the cortex (Figure 1(c)), striatum, and hippocampus in TET3 siRNA-treated cohort compared to control siRNA-treated cohort (Supplementary Figure 3). We subjected mice to transient MCAO 48 h following treatment with either TET3 siRNA or negative control siRNA. TET3 mRNA levels were increased by 3.3-fold in the negative siRNA/MCAO group compared to sham (p < 0.05) which was abrogated with TET3 siRNA treatment at 6 h of reperfusion (Figure 1(d)). Focal ischemia increased 5hmC levels by 2.6-fold (p < 0.05) which was abolished with TET3 knockdown at 6 h of reperfusion (Figure 1(e)). TET3 knockdown reduced 5hmC levels by 45% (p < 0.05) in sham mice (Supplementary Figure 4).
Figure 1.
TET3 knockdown reduced 5hmC after transient MCAO. Transient MCAO in adult mice significantly increased 5hmC between 5 min and three days of reperfusion compared to sham (n = 3/group) *p < 0.05 by Kruskal–Wallis (Dunn’s post hoc) test (a). Visualization of Cy3-tagged siRNA in rostral to caudal brain sections (1.54 to –2.18 from bregma) 24 h following intracerebral injection in adult mice (b). Intracerebral injection of TET3 siRNA significantly reduced TET3 protein levels in the cortex two days following treatment compared with negative control siRNA (Neg siRNA)-treated cohort (n = 6/group). *p < 0.05 by Mann–Whitney U test (c). Levels of TET3 mRNA from the peri-infarct cortex were increased significantly in mice subjected to transient MCAO at 6 h of reperfusion compared to sham. TET3 siRNA treatment significantly reduced TET3 mRNA levels compared with Neg siRNA–treated cohort (n = 3/group; (d)). Transient MCAO significantly increased 5hmC in the peri-infarct cortex at 6 h of reperfusion which was abrogated with TET3 siRNA treatment (n = 5–6/group; (e)). *p < 0.05 compared with sham and #p < 0.05 compared with Neg siRNA by Kruskal–Wallis (Dunn’s post hoc) test ((d) and (e)). Values in the histograms are mean ± SD.
TET: ten-eleven translocase.
TET3 knockdown aggravated secondary brain damage and impaired motor function
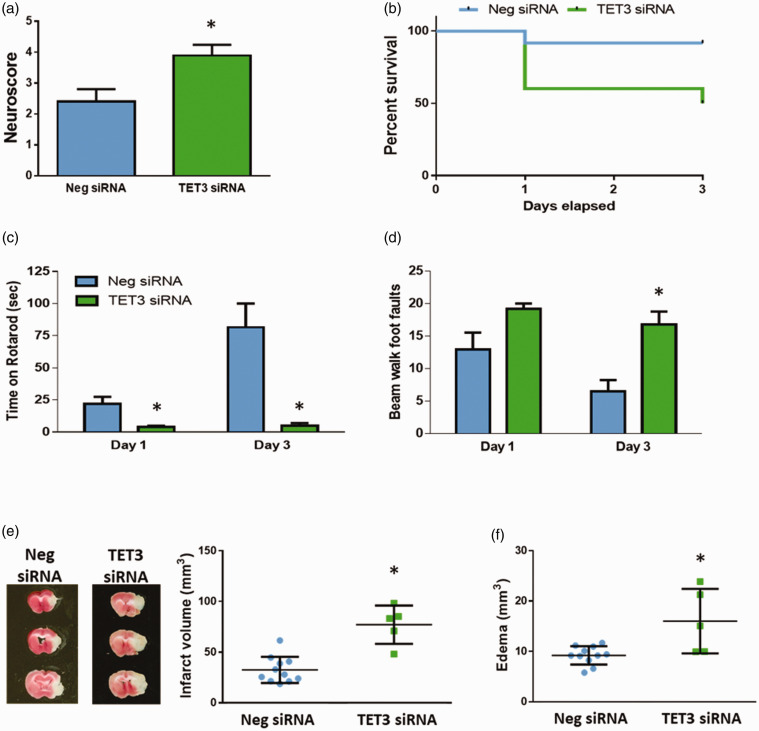
We previously showed that TET3 knockdown exacerbates infarct volume at one day of reperfusion following focal ischemia.21 To assess whether TET3 inhibition also impacts neurobehavioral function, we evaluated neurological decline at one day of reperfusion, and motor function and survival up to three days of reperfusion. TET3 knockdown led to increased neuroscores (Figure 2(a)) and post-ischemic survival at three days of reperfusion was significantly lower (p < 0.05) in the TET3 siRNA-treated cohort (50% survival) compared to control siRNA-treated cohort (92% survival) (Figure 2(b)). TET3 inhibition also led to impairments on rotarod and beam-walk tests (Figure 2(c) and (d)) compared to the negative control group (p < 0.05). Infarct volume was increased by 2.4-fold (p < 0.05) and edema volume was enhanced by >70% (p < 0.05) with TET3 siRNA treatment compared to control siRNA treatment (Figure 2(e) and (f)).
Figure 2.
TET3 knockdown exacerbated motor function impairments and secondary brain damage after transient MCAO. Neurological scores at one day of reperfusion were increased with TET3 knockdown. *p < 0.05 compared to Neg siRNA group by Mann–Whitney U test (a). TET3 knockdown significantly reduced survival compared to the Neg siRNA-treated cohort. At day 3 of reperfusion, 11 of 12 mice survived in the Neg siRNA group, while 5 of 10 mice survived in the TET3 siRNA group. Kaplan–Meier curves show significant difference by Log-rank (Mantel–Cox) test (b). Motor function assessed by time on rotarod and foot faults while walking across a beam at one day and three days of reperfusion was significantly impaired with TET3 knockdown compared to control (negative) siRNA treated cohort. *p < 0.05 compared to Neg siRNA group by repeated measures 2-way ANOVA (Sidak’s post hoc) test ((c) and (d)). TET3 siRNA treatment increased infarct volume (e) and edema volume (f) at three days of reperfusion. TTC-stained serial brain sections are from representative mice of each group. *p < 0.05 compared to Neg siRNA group by Mann–Whitney U test ((e) and (f)). Values in the histograms and scatterplots are mean ± SD (n = 5–11/group).
Neg siRNA: negative control siRNA; TET: ten-eleven translocase.
TET3 inhibition led to loss of 5hmC at gene promoters
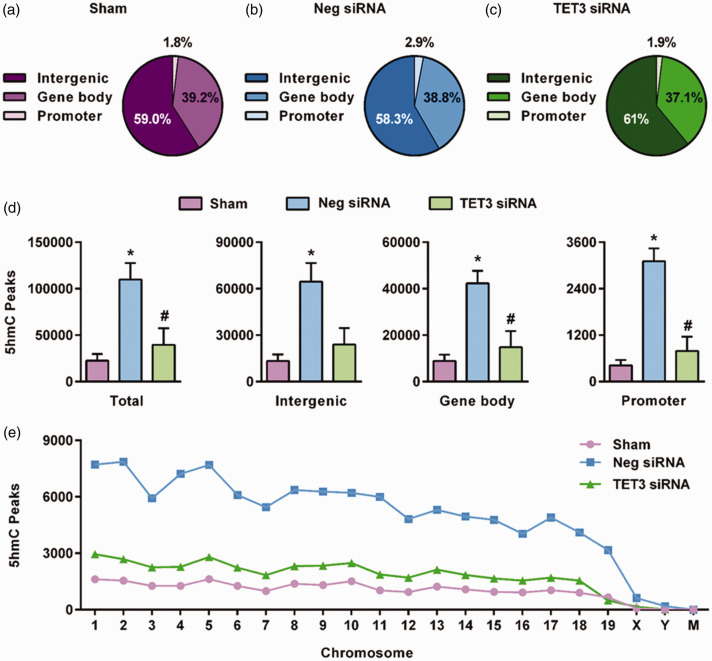
We have previously shown that TET3 inhibition decreases 5hmC levels and the expression of dozens of genes involved in ischemic neuroprotection.21 To analyze the specific genomic regions modulated by TET3 after stroke, we knocked down TET3 and then performed hMeDIP-seq at 6 h of reperfusion following transient MCAO using cortical tissue. The 6 h of reperfusion time-point was chosen since a significant portion of the TET3 siRNA-treated mice do not survive past 24 h of reperfusion, indicating TET3-mediated endogenous protective pathways may be activated at early timepoints following focal ischemia. Mapped reads were used for peak detection of hMeDIP-enriched regions across the genome. Sham, negative siRNA/MCAO control, and TET3 siRNA/MCAO groups all contained similar overall distribution of 5hmC peaks in promoter, gene body, and intergenic regions of the genome (Figure 3(a) to (c)). However, the negative siRNA/MCAO group showed significantly higher promoter 5hmC peaks at 2.9% (p < 0.05) than the sham or TET3 siRNA/MCAO group, at 1.8% and 1.9%, respectively (Figure 3(a) to (c)). Focal ischemia significantly enhanced the number of 5hmC peaks in all genomic regions in the negative control siRNA/MCAO group compared to sham (Figure 3(d)). The TET3 siRNA/MCAO group showed significantly reduced number of 5hmC peaks in the gene body and promoter regions compared to the negative control siRNA/MCAO group (Figure 3(d)). Reduced post-ischemic 5hmC peaks in the TET3 siRNA/MCAO group compared to the negative control siRNA/MCAO group were observed in all chromosomes (except mitochondrial chromosome) (Figure 3(e)).
Figure 3.
TET3 modulates increased genomic levels of 5hmC after transient MCAO. Composition of 5hmC peaks in sham (a), negative siRNA/transient MCAO (b), and TET3 siRNA/transient MCAO (c) groups at 6 h of reperfusion in the peri-infarct cortex. TET3 knockdown significantly decreased total 5hmC peaks in promoters and gene bodies and regions (d). Significantly altered 5hmC peaks between sham, negative siRNA, and TET3 siRNA by chromosome following transient MCAO (e). Values in the histograms are mean ± SD (n = 3/group). *p < 0.05 compared with sham and #p < 0.05 compared with the negative siRNA group by Kruskal–Wallis (Dunn’s post hoc) test. Significant 5hmC peaks identified using q value threshold of 10−4.
Neg siRNA: negative control siRNA; TET: ten-eleven translocase.
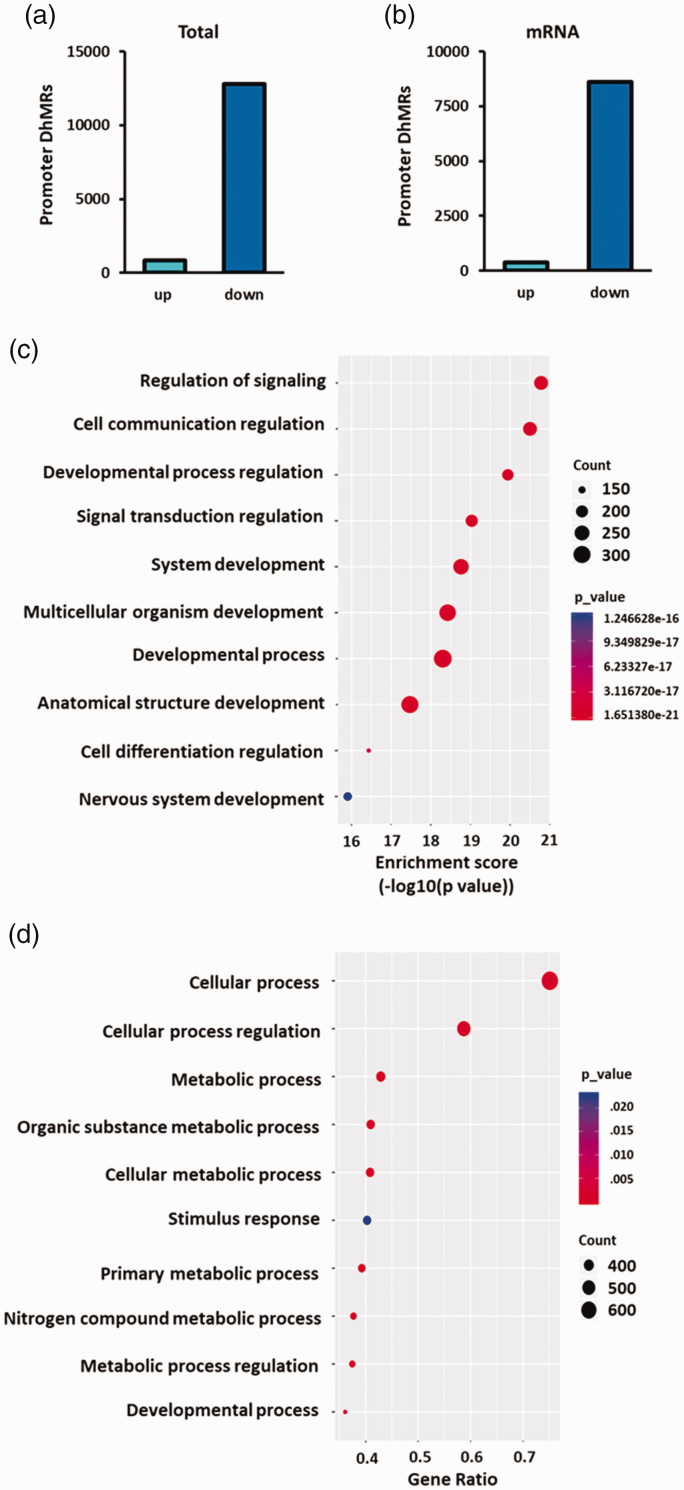
We further analyzed the post-ischemic differentially hydroxymethylated regions (DhMRs) within promoters between TET3 siRNA/transient MCAO group compared to negative control siRNA/transient MCAO groups. There were 12,797 DhMRs significantly downregulated and 863 DhMRs significantly upregulated with TET3 knockdown within promoter regions (Figure 4(a)). Of these, there were 8601 DhMRs significantly downregulated and 374 DhMRs significantly upregulated with TET3 knockdown in the promoter regions of genes encoding mRNAs (Figure 4(b)). Significantly downregulated DhMRs were identified in all chromosomes, except the Y and mitochondrial chromosomes (Supplementary Figure 5).
Figure 4.
TET3 knockdown reduced post-ischemic 5hmC at the promoters of neuroprotective genes. DhMRs significantly altered in the promoters between the TET3 siRNA and Neg siRNA groups at 6 h of reperfusion following transient MCAO (n = 3/group) for total promoter regions (a) and mRNA promoters (b) in the peri-infarct cortex. Gene ontological analysis of biological pathways of reduced DhMRs at gene promoters following TET3 knockdown compared to negative siRNA treatment at 6 h of reperfusion following transient MCAO ((c) and (d)). Top 10 biological pathways ranked by enrichment score (c) and by gene ratio (genes/pathway) (d). Statistically significant DhMRs were identified by differential analysis with a cut-off of log2 FC = 1.0 and p < 0.05.
DhMR: differentially hydroxymethylated region.
Gene ontology (GO) analysis of TET3-mediated downregulated DhMRs identified hundreds of genes involved in various cellular components (Supplementary Figure 6), molecular functions (Supplementary Figure 7), and biological processes (Supplementary Figure 8). Further analysis of the biological processes revealed that the most significantly downregulated DhMRs were in genes related to cell signaling and development (Figure 4(c)), while the most abundant downregulated DhMRs were involved in cellular and metabolic processes (Figure 4(d)).
TET3 inhibition reduced genomic 5hmC levels in the promoters of neuroprotective genes
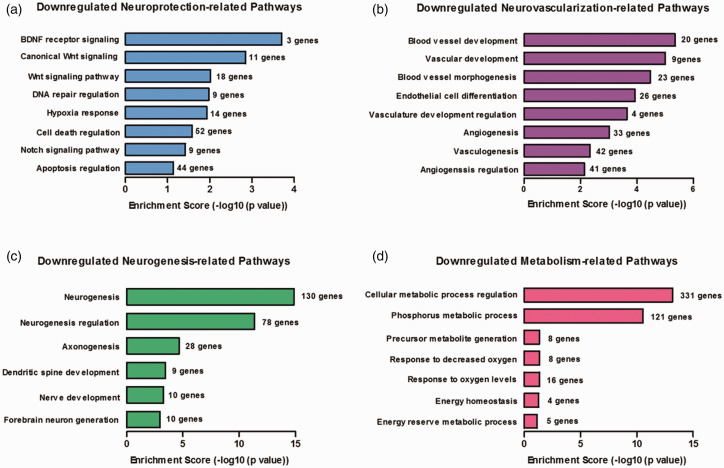
We further screened the downregulated GO biological processes for pathways related to protection against secondary brain damage after stroke. Several neuroprotective pathways involved in recovery after ischemic injury, such as hypoxia response, DNA repair, brain-derived neurotrophic factor (BDNF), and Wnt signaling,27,28 were downregulated with TET3 knockdown (Figure 5(a)). The large majority of these neuroprotective genes were related to negative regulation of cell death (52 genes) and negative regulation of apoptosis (44 genes) (Figure 5(a)). Since downregulated developmental processes were among the top pathways with the highest enrichment scores and gene ratios (Figure 4), we assessed the involvement of pathways related to neovascularization and neurogenesis, both of which have been shown to promote survival and recovery after stroke.29 We identified several pathways involved in angiogenesis and vasculogenesis (Figure 5(b)) as well as neurogenesis (Figure 5(c)) that were significantly downregulated with TET3 knockdown. Metabolic processes important for maintaining energy homeostasis and responding to altered oxygen levels were also reduced (Figure 5(d)). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of downregulated DhMRs revealed dozens of genes involved in various disease-associated pathways, several of which are associated with neuroprotection against ischemic injury (Supplementary Figure 9). For example, vascular endothelial growth factor (VEGF) signaling is an important mediator of angiogenesis and forkhead box class O transpition factor (FOXO) signaling plays a central role in hypoxia response.29 While the vast majority of pathways inhibited are involved in neuroprotection, a few pathways involved in cellular injury after stroke were also downregulated. For example, the mTOR signaling pathway that has been shown to exacerbate ischemic injury30 and the mitogen-activated protein kinase and AMP-activated protein kinase pathways that have controversial roles in stroke outcome,31,32 were also decreased with TET3 knockdown (Supplementary Figure 9(a), (b), and (e)). However, overall TET3 modulated 5hmC in the promoters of neuroprotective genes.
Figure 5.
TET3 knockdown downregulated genes involved in neuroprotective pathways following transient MCAO. The specific gene ontological biological pathways related to neuroprotection (a), neovascularization (b), neurogenesis (c), and metabolism (d) downregulated in the TET3 siRNA group compared to negative control siRNA group at 6 h of reperfusion following transient MCAO in the peri-infarct cortex.
BDNF: brain-derived neurotrophic factor.
TET3-mediated reduction in 5hmC decreased the expression of neuroprotective genes
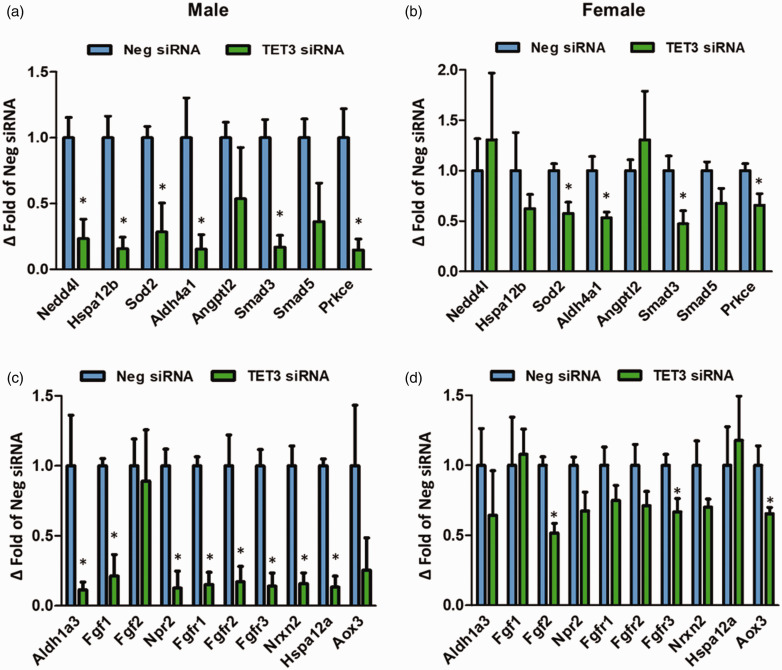
In order to determine whether decreased promoter 5hmC levels correlate with decreased gene expression, we selected 18 genes with a range of DhMR Log2 fold changes that have known involvement in ischemic neuroprotection (Table 1) and tested their mRNA expression by real-time PCR. TET3 knockdown significantly decreased the post-ischemic expression of almost a third of these genes at 6 h reperfusion (Supplementary Figure 10) and three-fourths of these genes by 24 h reperfusion (Figure 6(a) and (c)) compared to the negative control siRNA group at 6 h or 24 h reperfusion, respectively. We also selected the top three DhMR-associated genes that showed increased Log2 fold change following TET3 knockdown and confirmed that increased promoter 5hmC also correlates with increased gene expression at 6 h of reperfusion following transient MCAO (Supplementary Figure 11).
Table 1.
DhMRs in the promoters of genes downregulated after stroke that are putatively involved in ischemic outcome.
| Log2 FC (down) | Gene | Putative function after focal ischemia |
|---|---|---|
| 17 | NEDD4l | Promotes survival, modulates PTEN/Akt pathway |
| 13 | HSPa12b | Promotes angiogenesis |
| 12 | SOD2 | Antioxidant |
| 11 | ALDH4a1 | Antioxidant |
| 10 | ANGPTL2 | Accelerates angiogenesis |
| 10 | SMAD3 & SMAD5 | Suppress apoptosis, reduce inflammation, stabilize vasculature |
| 10 | PRKCE | Protects mitochondria, modulates CBF |
| 9 | ALDH1a3 | Antioxidant |
| 8, 3 | FGF1, FGF2 | Promote neurogenesis and angiogenesis |
| 7 | NPR2 | Suppress edema |
| 7, 3, 6 | FGFR1, 2, 3 | Angiogenesis |
| 6 | NRXN2 | Synaptic protein associated with enhanced recovery |
| 5 | HSPa12a | Pro-survival chaperone |
| 2 | AOX3 | Antioxidant |
NEDD4l: neural precursor cell expressed, developmentally downregulated 4-like, E3 ubiquitin protein ligase; HSP: heat shock protein; SOD2: superoxide dismutase 2; ALDH4a1: aldehyde dehydrogenase 4a1; ANGPTL2: angiopoietin-related protein 2 precursor; SMAD: mothers against DPP homolog; PRKCE: protein kinase C, epsilon; ALDH1a3: aldehyde dehydrogenase 1A3; FGF: fibroblast growth factor; NPR2: atrial natriuretic peptide receptor 2 precursor; FGFR: FGF receptor; NRNX2: neurexin 2; AOX3: aldehyde oxidase 3.
Figure 6.
TET3 knockdown decreased gene expression of neuroprotective genes in adult male and female mice. Expression of selected neuroprotective genes displaying downregulated DhMRs with TET3 inhibition at 24 h of reperfusion was assessed by real-time PCR analysis in the peri-infarct cortex. Expression of genes with Log2 fold change value of 10 or above (a) and (b)). Expression of genes with Log2 fold change values of less than 10 (c) and (d)). Values in the histograms are mean ± SD (n = 3/group). *p < 0.05 compared to negative siRNA by Mann–Whitney U test.
Nedd4l: neural precursor cell expressed developmentally downregulated gene 4-like; Hspa12: heat shock 70 kDa protein 12; Sod2: superoxide dismutase 2; Prkce: protein kinase C epsilon; Aox3: aldehyde oxidase 3; Aldh: aldehyde dehydrogenase; Angptl2: angiopoietin like 2; Npr2: natriuretic peptide receptor 2; Smad: mothers against decapentaplegic; Fgf: fibroblast growth factor; Nrxn: neurexin; Neg siRNA: negative control siRNA; TET: ten-eleven translocase.
The data presented thus far was from adult male mice. We have previously shown that TET3 inhibition exacerbates ischemic injury, while TET3 activation decreases the infarct volume and improves functional recovery in both male and female mice subjected to transient MCAO.21 Since there are sex-specific differences in the pathogenic mechanisms governing stroke,33 we assessed whether TET3 knockdown in female mice modulates the expression of similar neuroprotective genes. TET3 siRNA treatment reduced TET3 expression by 53% at 24 h of reperfusion compared to the negative control siRNA group, while TET1 and TET2 remained unchanged (Supplementary Figure 12). TET3 knockdown significantly decreased the expression of 39% of the selected genes at 24 h reperfusion following transient MCAO compared with control siRNA group (Figure 6(b) and (d)), indicating that a large portion of the genes are modulated by TET3 in female mice as well.
Discussion
Epigenetic factors involved in gene repression, such as DNA methylation and histone deacetylation, have previously been shown to worsen outcome after focal ischemia.34–37 We recently provided evidence that DNA hydroxymethylation, which stimulates gene expression, is beneficial for promoting recovery after stroke.21 In the current study, we show that TET3 controls global levels of 5hmC, but in particular modulates 5hmC at the promoters of hundreds of neuroprotective genes involved in cell survival and recovery after stroke. These results demonstrate that TET3 may provide ischemic protection through a multifaceted approach of several neuroprotective pathways.
The 5hmC modification is present in all tissue and cell types, but its abundance is highest in neuronal cells.38 Immunohistochemical analysis revealed that focal ischemia overwhelmingly increases 5hmC within neurons of the peri-infarct cortex, the ischemic region which can survive after a stroke.21 5hmC is associated with euchromatin and enhances gene expression by increasing accessibility of transcriptional machinery to chromatin.39 The TET enzymes produce 5hmC through oxidation of methylated DNA (5mC) and further TET-mediated oxidation of 5hmC leads to removal of the oxidized methylated cytosine through a mechanism of active demethylation.6,40,41 However, since the TET enzymes show preference for 5mC versus other oxidized products, 5hmC promotes transcription both as a stable epigenetic modification and via demethylation of DNA.42
The TET enzymes are known to play critical roles in neuronal maintenance and response to stress. The TET3 isoform has previously been associated with pathways involved in synaptic plasticity, DNA repair, and autophagy.7,14,16,17,43 Following focal ischemia, TET3 was shown to modulate several oxidative stress and DNA repair genes, but the specific genomic regions regulated by TET3 were not identified.21 The current study widens the scope of TET3-associated protective pathways to include neurotrophin signaling (such as BDNF), neurogenesis, angiogenesis, antioxidant defense, and longevity/cell survival mechanisms following experimental stroke. Interestingly, although global levels of 5hmC are increased after focal ischemia, TET3 overwhelmingly modulated pathways involved in neuroprotection. This parallels the role of TET3 during neuronal differentiation where increased TET3 and 5hmC affect specific gene targets rather than the genome in an indiscriminate manner.49 The mechanisms underlying TET-mediated specificity are still emerging, but there is evidence that TET-targeting kinases, transcriptional regulators such as REST, and noncoding RNAs are involved in mediating context-specific changes in the hydroxymethylome.50–52
In the cerebral cortex, expression levels of the TET3 isoform is higher than TET1 and TET2 isoforms, and overexpression of TET3 has been shown to increase 5hmC levels and positively modulate transcription.43,44 Recent evidence indicates that in addition to producing 5hmC, TET3 can modulate the epigenome through non-enzymatic activity. For example, in neuronal stem cells, TET3 was shown to bind to the small nuclear ribonucleoprotein-associated protein N promoter and thereby repress the gene in a manner independent of TET catalytic activity.45 In HEK 293 T cells, TET3 was shown to bind to O-linked β-N-acetylglucosamine transferase, an enzyme which catalyzes serine and threonine glycosylation that interacts with other epigenetic regulators.46 In neural progenitor cell lines, TET3 facilitates the recruitment and stabilization of thyroid hormone receptor on chromatin.47 Furthermore, catalytically-inactive TET3 was shown to partially rescue developmental defects caused by TET3 knockout in Xenopus eye development.48 However, whether TET3 displays non-catalytic epigenetic activity in the adult mammalian brain has not been investigated. In our study, we specifically focus on the TET3-modulated changes to the hydroxymethylome.
TET-mediated 5hmC has been established as a major regulator of neurogenesis in both the embryonic and adult brain.53 The TET3 isoform is important for the conversion of stem cells to the neuronal phenotype and has been shown to be a critical regulator of neurogenic pathways.52,54 Furthermore, TET3 was shown to be critical for axonal regeneration after peripheral nerve injury.15,55 Following focal ischemia, markers for neuronal precursors have been detected in the ischemic penumbra as early as 24 h of reperfusion,56 and migrating neuroblasts have been observed in the cortex as early as one week of reperfusion.57 Cortical neurogenesis has been shown to persist up to two months within the ischemic boundary zone following focal ischemic injury.58 However, whether TET3 or 5hmC play a role in post-ischemic neurogenesis in the cortex is not known at this time. Although, our study indicates that 5hmC levels were decreased by seven days following stroke, introduction of TET3 activators could potentially enhance neurogenesis as a therapeutic modulator of long-term recovery. For example, ascorbate, a known activator of TET and thus a modulator of 5hmC, has been shown to enhance neurogenesis in the subventricular zone and cerebellum.59,60 We previously showed that ascorbate leads to activation of TET3 in the post-stroke cortex. However, whether ascorbate modulates neurogenesis via TET3 after stroke needs to be explored in future studies.
Previous studies indicated that 5hmC modulates the development and maintenance of the vascular system.61 Following vascular injury, 5hmC levels are reduced, but restoration of 5hmC diminished several pathogenic mechanisms including autophagy and inflammation.61,62 In the present study, GO analysis identified dozens of genes modulated by TET3 involved in the development of blood vessels and vasculature. Neovascularization is considered a key restorative mechanism that promotes brain plasticity and functional recovery after ischemic stroke.29 Angiogenesis following focal ischemia peaks at 2–3 days of reperfusion, although the expression of dozens of pro-angiogenic factors have been shown to increase as early as one hour of reperfusion.63 Since 5hmC was induced from 5 min to three days of reperfusion, our data suggest that increased 5hmC following focal ischemia could promote angiogenesis through modulation of pro-angiogenic genes. A previous study showed that decreased 5hmC levels were associated with decreased VEGF expression in the mouse brain.64 Our current study and previous work by other laboratories have shown an association between TET3/5hmC and NOTCH genes in the brain, which regulate both angiogenesis and neurogenesis.54,65 Thus, TET3 activation may be important for long-term cell survival and recovery in addition to its role in acute neuroprotection.
Sexual dimorphism plays a major role in the pathogenic mechanisms that govern ischemic injury.33 We have previously shown that TET3 decreases lesion size and improves motor function recovery following focal ischemia in both young and aged male and female mice.21 In the current study, we observed that TET3 modulates a significant portion of genes involved in neuroprotection in adult mice of both sexes. However, while TET3 inhibition reduced the expression of about 75% of the selected 5hmC-associated neuroprotective genes in the male peri-infarct cortex at 24 h reperfusion, only about 40% of the same 5hmC-associated neuroprotective genes were reduced in the female peri-infarct cortex. These differences may be due to the fact that the 5hmC sequencing analysis was performed with male tissue, and thus gene expression changes were better correlated in the male mice. Additionally, there may be temporal differences between sexes that govern the timing of 5hmC-associated gene expression in the post-stroke cortex. Notably, in female mice, the expression of the pro-angiogenic/pro-neurogenic fibroblast growth factor 2 and the antioxidant aldehyde oxidase 3 were significantly reduced with TET3 inhibition, while there was no change in male mice. Estradiol treatment has previously been shown to promote both angiogenesis and neurogenesis in ovariectomized female mice.66,67 Additionally, female neurons display more resistance to oxidative stress than male neurons.68 Therefore, although TET3 modulates ischemic protection in both male and female mice, there may be divergent pathways governing this protection between sexes.
The 5hmC modification has previously been implicated in pathways involved in several neurodegenerative disorders, but the specific role of 5hmC in these diseases is not yet clear.8,39 Preliminary evidence indicated that Huntington’s disease is associated with loss of the 5hmC with concomitant increase in 5mC.69 Whereas in Alzheimer’s disease (AD) patients, conflicting studies showed both increased and decreased global 5hmC levels in the hippocampus.8 In the prefrontal cortex of AD patients, differentially hydroxymethylated loci in genes that maintain neuronal morphology, synaptic function, and tau neurotoxicity have been observed, indicating that 5hmC may specifically modulate pathogenic genes.70 Significant loss of 5hmC is a hallmark of various cancers, including gliomas that cause neurodegeneration,71 and pharmacologic doses of the TET-activator ascorbate has been shown to prevent proliferation of glioma cells.72 Selectively inducing TET3 expression was also shown to inhibit both glioblastoma tumor growth and stem cell renewal.73 Interestingly, three of the most statistically significant pathways modulated by 5hmC induction identified by KEGG analysis in the present study are involved in cancer. Furthermore, several additional TET3-regulated pathways related to neurotrophin signaling, antioxidant defense, and cell survival are important targets of neuroprotection in neurodegenerative diseases.74–76 Therefore, in addition to stroke, TET3 and 5hmC may be neuroprotective over a spectrum of disorders.
Together with our previous studies, the present analysis indicates that TET3 and 5hmC are robust mediators of protection in the ischemic brain. Furthermore, the current study revealed the genomic regions targeted by TET3 and the cellular pathways by which TET3/5hmC may provide endogenous protection against cerebral ischemia. This indicates that post-stroke TET3 activation may be therapeutically beneficial by preventing secondary brain damage after ischemic insults. Ascorbate has also recently been identified as an epigenetic modulator through its ability to function as a cofactor and inducer of TET activity.77,78 Ascorbate has previously been shown to modulate a number of pathways involved in neuroprotection such as angiogenesis79 and neurogenesis80 and is associated with decreased impairments and lesion size following acute brain injuries.81 However, the role of 5hmC or TET activity was not explored in these studies. We previously showed that TET3 activation by ascorbate leads to robust protection against focal ischemia by modulating lesion size and motor function recovery.21 Thus, additional molecular approaches that modulate TET3 and 5hmC may be useful as potential stroke therapies and should be explored in future studies.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20912965 for TET3 regulates DNA hydroxymethylation of neuroprotective genes following focal ischemia by Kahlilia C Morris-Blanco, Anil K Chokkalla, Mario J Bertogliat and Raghu Vemuganti in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in part by National Institute of Health (R01 NS109459) and Department of Neurological Surgery, University of Wisconsin.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Morris-Blanco KC and Vemuganti R designed the experiments. Morris-Blanco KC performed MCAO surgeries, PCR, animal behavior/survival studies, and infarct analyses. Chokkalla AK performed IHC and western blotting. Morris-Blanco KC and Chokkalla AK performed intracerebral siRNA injections. Morris-Blanco KC and Bertogliat MJ performed dot blots and post-sequencing analyses. Morris-Blanco KC and Vemuganti R wrote the manuscript.
ORCID iD: Raghu Vemuganti https://orcid.org/0000-0002-7915-2810
Supplemental material: Supplemental material for this article is available online.
References
- 1.Qureshi IA, Mehler MF.Emerging role of epigenetics in stroke: part 1: DNA methylation and chromatin modifications. Arch Neurol 2010; 67: 1316–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriaucionis S, Heintz N.The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009; 324: 929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruzov A, Tsenkina Y, Serio A, et al. Lineage-specific distribution of high levels of genomic 5-hydroxymethylcytosine in mammalian development. Cell Res 2011; 21: 1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen L, Li X, Yan L, et al. Whole-genome analysis of 5-hydroxymethylcytosine and 5-methylcytosine at base resolution in the human brain. Genome Biol 2014; 15: R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellen M, Ayata P, Dewell S, et al. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell 2012; 151: 1417–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009; 324: 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Su Y, Shin J, et al. Tet3 regulates synaptic transmission and homeostatic plasticity via DNA oxidation and repair. Nat Neurosci 2015; 18: 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mahdawi S, Virmouni SA, Pook MA.The emerging role of 5-hydroxymethylcytosine in neurodegenerative diseases. Front Neurosci 2014; 8: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherwani SI, Khan HA.Role of 5-hydroxymethylcytosine in neurodegeneration. Gene 2015; 570: 17–24. [DOI] [PubMed] [Google Scholar]
- 10.Xin YJ, Yuan B, Yu B, et al. Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Sci Rep 2015; 5: 7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gou P, Qi X, Yuan R, et al. Tet1-mediated DNA demethylation involves in neuron damage induced by bilirubin in vitro. Toxicol Mech Methods 2018; 28: 55–61. [DOI] [PubMed] [Google Scholar]
- 12.Mi Y, Gao X, Dai J, et al. A novel function of TET2 in CNS: sustaining neuronal survival. Int J Mol Sci 2015; 16: 21846–21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gontier G, Iyer M, Shea JM, et al. Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep 2018; 22: 1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang D, Wei S, Chen F, et al. TET3-mediated DNA oxidation promotes ATR-dependent DNA damage response. EMBO Rep 2017; 18: 781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng YL, An R, Cassin J, et al. An intrinsic epigenetic barrier for functional axon regeneration. Neuron 2017; 94: 337–346.e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Wei W, Zhao QY, et al. Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc Natl Acad Sci U S A 2014; 111: 7120–7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin SG, Zhang ZM, Dunwell TL, et al. Tet3 reads 5-carboxylcytosine through its CXXC domain and is a potential guardian against neurodegeneration. Cell Rep 2016; 14: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariani CJ, Vasanthakumar A, Madzo J, et al. TET1-mediated hydroxymethylation facilitates hypoxic gene induction in neuroblastoma. Cell Rep 2014; 7: 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laukka T, Mariani CJ, Ihantola T, et al. Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J Biol Chem 2016; 291: 4256–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Zhang D, Du J, et al. Tet1 facilitates hypoxia tolerance by stabilizing the HIF-alpha proteins independent of its methylcytosine dioxygenase activity. Nucleic Acids Res 2017; 45: 12700–12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris-Blanco KC, Kim T, Lopez MS, et al. Induction of DNA hydroxymethylation protects the brain after stroke. Stroke 2019; 50: 2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao Z, He Y, Xin N, et al. Altering 5-hydroxymethylcytosine modification impacts ischemic brain injury. Hum Mol Genet 2015; 24: 5855–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakka VP, Lang BT, Lenschow DJ, et al. Increased cerebral protein ISGylation after focal ischemia is neuroprotective. J Cereb Blood Flow Metab 2011; 31: 2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta SL, Kim T, Vemuganti R.Long noncoding RNA FosDT promotes ischemic brain injury by interacting with REST-associated chromatin-modifying proteins. J Neurosci 2015; 35: 16443–16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris-Blanco KC, Kim T, Bertogliat MJ, et al. Inhibition of the epigenetic regulator REST ameliorates ischemic brain injury. Mol Neurobiol 2019; 56: 2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lun AT, Smyth GK.csaw: a Bioconductor package for differential binding analysis of ChIP-seq data using sliding windows. Nucleic Acids Res 2016; 44: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen A, Xiong LJ, Tong Y, et al. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep 2013; 1: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shruster A, Ben-Zur T, Melamed E, et al. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS One 2012; 7: e40843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Font MA, Arboix A, Krupinski J.Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev 2010; 6: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Hei C, Liu P, et al. Inhibition of mTOR pathway by rapamycin reduces brain damage in rats subjected to transient forebrain ischemia. Int J Biol Sci 2015; 11: 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nozaki K, Nishimura M, Hashimoto N.Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol 2001; 23: 1–19. [DOI] [PubMed] [Google Scholar]
- 32.Li J, McCullough LD.Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab 2010; 30: 480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim T, Chelluboina B, Chokkalla AK, et al. Age and sex differences in the pathophysiology of acute CNS injury. Neurochem Int 2019; 127: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endres M, Meisel A, Biniszkiewicz D, et al. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci 2000; 20: 3175–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langley B, Brochier C, Rivieccio MA.Targeting histone deacetylases as a multifaceted approach to treat the diverse outcomes of stroke. Stroke 2009; 40: 2899–2905. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Myers SJ, Dingledine R.Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat Neurosci 1999; 2: 867–872. [DOI] [PubMed] [Google Scholar]
- 37.Morris-Blanco KC, Kim T, Bertogliat MJ, et al. Inhibition of the epigenetic regulator REST ameliorates ischemic brain injury. Mol Neurobiol 2019; 56: 2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Globisch D, Munzel M, Muller M, et al. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One 2010; 5: e15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song CX, Szulwach KE, Fu Y, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol 2011; 29: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011; 333: 1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He YF, Li BZ, Li Z, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011; 333: 1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu L, Lu J, Cheng J, et al. Structural insight into substrate preference for TET-mediated oxidation. Nature 2015; 527: 118–122. [DOI] [PubMed] [Google Scholar]
- 43.Szwagierczak A, Bultmann S, Schmidt CS, et al. Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res 2010; 38: e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colquitt BM, Allen WE, Barnea G, et al. Alteration of genic 5-hydroxymethylcytosine patterning in olfactory neurons correlates with changes in gene expression and cell identity. Proc Natl Acad Sci U S A 2013; 110: 14682–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montalban-Loro R, Lozano-Urena A, Ito M, et al. TET3 prevents terminal differentiation of adult NSCs by a non-catalytic action at Snrpn. Nat Commun 2019; 10: 1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Q, Chen Y, Bian C, et al. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature 2013; 493: 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan W, Guyot R, Samarut J, et al. Methylcytosine dioxygenase TET3 interacts with thyroid hormone nuclear receptors and stabilizes their association to chromatin. Proc Natl Acad Sci U S A 2017; 114: 8229–8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Xu C, Kato A, et al. Tet3 CXXC domain and dioxygenase activity cooperatively regulate key genes for xenopus eye and neural development. Cell 2012; 151: 1200–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn MA, Qiu R, Wu X, et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in mammalian neurogenesis. Cell Rep 2013; 3: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perera A, Eisen D, Wagner M, et al. TET3 is recruited by REST for context-specific hydroxymethylation and induction of gene expression. Cell Rep 2015; 11: 283–294. [DOI] [PubMed] [Google Scholar]
- 51.Zhou L, Ren M, Zeng T, et al. TET2-interacting long noncoding RNA promotes active DNA demethylation of the MMP-9 promoter in diabetic wound healing. Cell Death Dis 2019; 10: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao VK, Swarnaseetha A, Tham GH, et al. Phosphorylation of Tet3 by cdk5 is critical for robust activation of BRN2 during neuronal differentiation. Nucleic Acids Res 2020; 48: 1225–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Tang B, He Y, et al. DNA methylation dynamics in neurogenesis. Epigenomics 2016; 8: 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seritrakul P, Gross JM.Tet-mediated DNA hydroxymethylation regulates retinal neurogenesis by modulating cell-extrinsic signaling pathways. PLoS Genet 2017; 13: e1006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loh YE, Koemeter-Cox A, Finelli MJ, et al. Comprehensive mapping of 5-hydroxymethylcytosine epigenetic dynamics in axon regeneration. Epigenetics 2017; 12: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin K, Sun Y, Xie L, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 2003; 24: 171–189. [DOI] [PubMed] [Google Scholar]
- 57.Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006; 26: 13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang W, Gu W, Brannstrom T, et al. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke 2001; 32: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 59.Corbett AM, Sieber S, Wyatt N, et al. Increasing neurogenesis with fluoxetine, simvastatin and ascorbic acid leads to functional recovery in ischemic stroke. Recent Pat Drug Deliv Formul 2015; 9: 158–166. [DOI] [PubMed] [Google Scholar]
- 60.Oyarce K, Silva-Alvarez C, Ferrada L, et al. SVCT2 is expressed by cerebellar precursor cells, which differentiate into neurons in response to ascorbic acid. Mol Neurobiol 2018; 55: 1136–1149. [DOI] [PubMed] [Google Scholar]
- 61.Liu R, Jin Y, Tang WH, et al. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 2013; 128: 2047–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng J, Yang Q, Li AF, et al. Tet methylcytosine dioxygenase 2 inhibits atherosclerosis via upregulation of autophagy in ApoE –/– mice. Oncotarget 2016; 7: 76423–76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayashi T, Noshita N, Sugawara T, et al. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 2003; 23: 166–180. [DOI] [PubMed] [Google Scholar]
- 64.Tang Y, Han S, Asakawa T, et al. Effects of intracerebral hemorrhage on 5-hydroxymethylcytosine modification in mouse brains. Neuropsychiatr Dis Treat 2016; 12: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terragni J, Zhang G, Sun Z, et al. Notch signaling genes: myogenic DNA hypomethylation and 5-hydroxymethylcytosine. Epigenetics 2014; 9: 842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki S, Gerhold LM, Bottner M, et al. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol 2007; 500: 1064–1075. [DOI] [PubMed] [Google Scholar]
- 67.Jesmin S, Hattori Y, Sakuma I, et al. Estrogen deprivation and replacement modulate cerebral capillary density with vascular expression of angiogenic molecules in middle-aged female rats. J Cereb Blood Flow Metab 2003; 23: 181–189. [DOI] [PubMed] [Google Scholar]
- 68.Du L, Bayir H, Lai Y, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem 2004; 279: 38563–38570. [DOI] [PubMed] [Google Scholar]
- 69.Villar-Menendez I, Blanch M, Tyebji S, et al. Increased 5-methylcytosine and decreased 5-hydroxymethylcytosine levels are associated with reduced striatal A2AR levels in Huntington’s disease. Neuromolecular Med 2013; 15: 295–309. [DOI] [PubMed] [Google Scholar]
- 70.Bernstein AI, Lin Y, Street RC, et al. 5-Hydroxymethylation-associated epigenetic modifiers of Alzheimer’s disease modulate Tau-induced neurotoxicity. Hum Mol Genet 2016; 25: 2437–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin SG, Jiang Y, Qiu R, et al. 5-Hydroxymethylcytosine is strongly depleted in human cancers but its levels do not correlate with IDH1 mutations. Cancer Res 2011; 71: 7360–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castro ML, Carson GM, McConnell MJ, et al. High dose ascorbate causes both genotoxic and metabolic stress in glioma cells. Antioxidants (Basel) 2017; 6: pii: E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui Q, Yang S, Ye P, et al. Downregulation of TLX induces TET3 expression and inhibits glioblastoma stem cell self-renewal and tumorigenesis. Nat Commun 2016; 7: 10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miranda M, Morici JF, Zanoni MB, et al. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 2019; 13: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trist BG, Hare DJ, Double KL.Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019: e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radi E, Formichi P, Battisti C, et al. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis 2014; 42Suppl 3: S125–S152. [DOI] [PubMed] [Google Scholar]
- 77.Minor EA, Court BL, Young JI, et al. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem 2013; 288: 13669–13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yin R, Mao SQ, Zhao B, et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc 2013; 135: 10396–10403. [DOI] [PubMed] [Google Scholar]
- 79.May JM, Harrison FE.Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal 2013; 19: 2068–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tveden-Nyborg P, Johansen LK, Raida Z, et al. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am J Clin Nutr 2009; 90: 540–546. [DOI] [PubMed] [Google Scholar]
- 81.Polidori MC, Mecocci P, Frei B.Plasma vitamin C levels are decreased and correlated with brain damage in patients with intracranial hemorrhage or head trauma. Stroke 2001; 32: 898–902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20912965 for TET3 regulates DNA hydroxymethylation of neuroprotective genes following focal ischemia by Kahlilia C Morris-Blanco, Anil K Chokkalla, Mario J Bertogliat and Raghu Vemuganti in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
All sequencing data are deposited in the Gene Omnibus Expression (GEO) repository and will be made publicly available under an accession number after our use of the data.