Abstract
Our aim is to investigate whether vascular risk factors are associated with cerebral deep medullary veins (DMVs) and whether DMVs are associated with MRI markers of cerebral small vessel disease (CSVD) or risk of stroke. In a community-based cohort of 1056 participants (mean age 55.7 years), DMVs were identified on susceptibility-weighted imaging (SWI) and counted in periventricular regions. Neuroimaging markers including lacunes, whiter matter hyperintensity (WMH), microbleeds, enlarged perivascular space, and brain atrophy were evaluated. The number of DMVs decreased with age (p = 0.007). After adjusting for age and sex, the number of DMVs was not associated with traditional vascular risk factors. Fewer DMVs was associated with increase of WMH and lacunes, but the association vanished after adjustment for vascular risk factors. However, fewer DMVs were independently associated with brain atrophy (p < 0.001). DMVs were not associated with three-year risk of stroke. Our results suggest that DMV is significantly different from other MRI markers of CSVD regarding risk factors, association with other CSVD markers, and risk of stroke. Nonetheless, the significant association between DMV and brain atrophy suggested the potential role of venules in age-related neurodegenerative process, which deserves further investigation.
Keywords: Cerebral small vessel disease, deep medullary veins, susceptibility weighted imaging, aging, neurodegeneration
Introduction
The pathological mural changes in intracerebral veins were scarcely discussed until 1995 when Moody et al.1 described non-inflammatory degenerative change in the periventricular and subependymal veins, namely periventricular venous collagenosis (VC). Among 22 postmortem patients, periventricular VC was commonly present in the elderly and was strongly associated with leukoaraiosis.1 Very recently, Keith et al.2 duplicated this pathological finding in both Alzheimer’s disease (AD) and non-AD patients, and described that VC was frequently seen in both veins with diameter less than 150 µm and greater than 200 µm, resulting in stenosis or occlusion of the veins. Although studies are limited, the term “venous collagenosis” has been introduced into the lexicon of small vessel disease.3
Over the past 10 years, susceptibility-weighted imaging (SWI), through exploiting the susceptibility effect from deoxyhemoglobin in veins, has been accepted as a sensitive method of imaging small intracranial veins in vivo.4 Deep medullary veins (DMVs) are the small parenchymal veins located in the periventricular white matter, ranging in diameter from tens to hundreds of microns.1,5 Using SWI, DMVs can be visualized not only on ultra-high field 7-T magnetic resonance imaging (MRI) but also on 3-T MRI.6 In addition, they can be easily assessed because of their regular arrangement perpendicular to the ventricles.7 Some studies suggested that discontinuity or decreased number of DMVs seen on SWI could be considered an imaging manifestation of VC in contexts without infection, inflammation, trauma, or tumor invasion.7,8 To date, only a few studies have focused on DMVs, all of which are of small sample size and in specifically selected groups of patients. Due to the lack of studies of DMVs in the general population, little is known about the potential risk factors related to changes in DMVs, or whether the number of DMVs is associated with brain structural changes during the aging process. As a consequence, we do not have strong evidence to support whether pathological changes of venules, such as VC, are also involved in cerebral small vessel disease (CSVD) in an aging population.
To answer these questions, we quantified the number of DMVs on 3-T SWI in a large population-based cohort and investigated its potential risk factors, association with the risk of stroke, and relationship with the imaging markers of CSVD including white matter hyperintensities (WMHs), lacunes, cerebral microbleeds, dilated perivascular spaces, and brain atrophy.
Methods
Study population
The study population was from the Shunyi Study, an ongoing prospective community-based cohort study that is designed to investigate the risk factors of cardiovascular and age-related diseases. All residents older than 35 years old from five villages in Shunyi, a rural district of Beijing, were invited to join this study. From June 2013 to April 2016, a total of 1586 participants underwent baseline assessment including structured questionnaires, physical examination, and blood tests. All participants were invited to undergo baseline brain MRI. Data of new onset cardiovascular disease and death were collected during the annual follow-up. Among the participants, 329 refused or had contradictions for MRI examination. This current study was performed based on the 1257 participants who underwent baseline MRI. We further excluded subjects with SWI artifacts, large periventricular infarcts or hemosiderin deposition, or enlarged lateral ventricles that hindered measuring the DMVs (n = 167), and further excluded individuals with a history of stroke (n = 34). Finally, a total of 1056 participants were included in the present analysis. The included participants were younger, composed of a higher proportion of male participants, and had increased rates of hyperlipidemia, in addition to having a smaller proportion of current smokers or patients with hypertension than subjects that were not included (Supplementary table 1).
Ethics statement
All participants provided signed informed consent. The study was approved by the Ethical Committee at Peking Union Medical College Hospital (reference number: B-160). All clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki.
Risk factor assessment
Demographic and clinical information including age, sex, smoking status, body mass index, blood pressure, history of hypertension, diabetes mellitus, hyperlipidemia, and current medication were collected using a structured questionnaire and physical examination. Overnight fasting venous blood samples were drawn and analyzed for plasma total cholesterol, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, and glucose. Hypertension was defined as self-reported hypertension, or treatment with antihypertensive agents, or systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg. Hyperlipidemia was defined as self-reported hyperlipidemia, or treatment with anti-dyslipidemia medication, or fasting total cholesterol >5.2 mmol/l, or low-density lipoprotein >3.36 mmol/l. Diabetes mellitus was defined as self-reported diabetes, or treatment with anti-diabetes drugs or insulin, or fasting glucose ≥7.0 mmol/l. Smoking status was divided into current smoker or non-current smoker.
Assessment of classical imaging markers of CSVD
Participants were scanned using a 3-T Skyra scanner (Siemens, Erlangen, Germany). We performed 3D T1-weighted, T2-weighted, fluid-attenuated inversion recovery, and SWI, as described in detail previously.9
All imaging markers of CSVD were defined according to the Standards for Reporting Vascular Changes on Neuroimaging.10 Lacunes were defined as focal lesions ranging from 3 to 15 mm with the same signal characteristics as cerebrospinal fluid (CSF) on all sequences. CMBs were defined as small, round, or ovoid hypointense lesions on SWI. The severity of dilated PVS in the BG and WM was rated using a previously established four-level severity score.11
The gray matter (GM), WM, and CSF were automatically segmented based on structural T1-weighted images using SPM12. Total intracranial volume was calculated as the sum of the total GM, total WM, and CSF volumes. The brain parenchymal fraction (BPF) was defined as the ratio of brain tissue volume (GM and WM volume) to total intracranial volume. WMH volume was automatically generated using the lesion growth algorithm as implemented in the Lesion Segmentation Tool (https://www.appliedstatistics.de/lst.html) for SPM. The FSL/FIRST (FMRIB Software Library, v5.0) software was used to segment the cerebral cortex and subcortical nucleus, and the average of left and right hippocampal volumes was taken as the hippocampal volume.
Measurement of DMVs
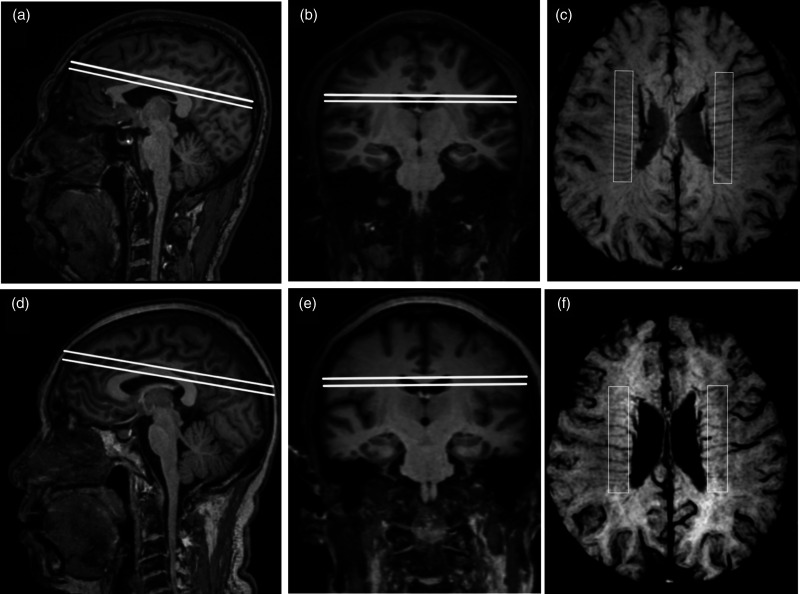
A region of interest (ROI) of 60 mm× 10 mm was placed in the periventricular white matter between the frontal and occipital horn in each cerebral hemisphere. The minimum intensity projection was derived from four consecutive slices of SWI in this region, contoured from the ventricle floor to the superior roof of the corpus callosum, to produce a single 2D image. Veins that traversed perpendicularly to the lateral ventricular and crossed the ROI were quantified by visual inspection. The number of DMVs was defined as the average count of both hemispheres (Figure 1). Intra-rater reliability was assessed using a random sample of 50 individuals with an interval of more than one month between the first and second readings. Two trained and blinded investigators (YCZ and DHA) rated 50 individuals independently to assess the inter-rater reliability. Kappa values for the intra-rater and inter-rater reliability were 0.76 and 0.79, respectively, and inter class correlations for the intra-rater and inter-rater were 0.68 and 0.75, respectively.
Figure 1.
Demonstration of DMVs assessment. (a) to (c): a 45-year-old male. (d) to (f): a 70-year-old male. (a), (d): Sagittal view of a 6 mm block parallel to ACPC plane. (b), (e): Coronal view of the selected block. (c), (f): Minimum intensity projection in 3T SWI and vessel counting.
Statistical analyses
Continuous variables are described as mean ± standard deviation (SD) or median with range value, and categorical variables are described as frequencies and proportions. General linear regression was used to assess the potential risk factors for the number of DMVs. The associations between the number of DMVs and MRI markers of cerebral SVD (BPF was used to depict brain atrophy) were tested using general linear regression for continuous variables and logistic regression for binominal variables. We applied Cox proportional hazard models to investigate the association of the number of DMVs with incident stroke. All regression models were initially adjusted for age and sex, and then additionally adjusted for other vascular risk factors (hypertension, smoking, and diabetes). WMH volume and lacunes were included in models testing the association of the number of DMVs with BPF. Covariates in the multivariable regression models were selected based on prior knowledge and univariable analyses. WMH volumes were natural log-transformed to normalize skewness. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA), and two-sided P-values of <0.05 were considered statistically significant.
Results
Baseline demographic and clinical characteristics, imaging markers of SVD, and number of DMVs are listed in Table 1. Among the 1056 participants, the mean (SD) age was 55.7 (9.1) years and 378 (35.8%) were male. Among these, lacunes, cerebral microbleed, and severe PVS in the basal ganglia and white matter were present in 153 (14.5%), 111 (10.5%), 147 (14.0%), and 154 (14.6%), respectively. The median (range value) WMH volume of the subjects was 0.80 ml (0.24–49.6), and the median (range value) BPF was 76.5% (65.0–85.7). In total, the median (range value) number of DMVs was 19.0 (12.5–24).
Table 1.
Baseline characteristics of the 1056 participants.
| Baseline clinical characteristic | Mean ± SD or N (%) |
|---|---|
| Age (year) | 55.7 ± 9.1 |
| Male | 378 (35.8%) |
| Current smoker | 229 (22.5%) |
| Alcohol user | 272 (26.7%) |
| BMIa (kg/m2) | 26.3 ± 3.7 |
| Hypertension | 517 (49.5%) |
| Systolic blood pressure (mmHg) | 133 ± 19 |
| Diastolic blood pressure (mmHg) | 79 ± 11 |
| Hyperlipidemia | 506 (48.4%) |
| Diabetes mellitus | 169 (16.1%) |
| Imaging characteristics | |
| WMH volume (mm3)b | 0.80 (0.24, 49.6) |
| Lacunes | 153 (14.5%) |
| Cerebral microbleeds | 111 (10.5%) |
| Basal ganglia PVS (degree 3 and 4) | 147 (14.0%) |
| White matter PVS (degree 3 and 4) | 154 (14.6%) |
| Brain parenchymal fraction (%)b | 76.5 (65.0, 85.7) |
| Number of DMVsb | 19.0 (12.5, 24) |
BMI: body mass index; DMV: deep medullary vein; PVS: perivascular space; SD: standard deviation; WMH: white matter hyperintensity.
aBody mass index is calculated as weight in kilograms divided by height in meters squared.
bVariable is described as median (range value).
Potential risk factors of reduction in number of DMVs
The associations of risk factors with DMVs are shown in Table 2. The decreased number of DMV was significantly associated with the increase of age (β coefficient, −0.16 per 10 years; 95% CI, −0.27 to −0.05; P = 0.005), current smoking status (β coefficient, −0.32; 95% CI, −0.57 to −0.08; P = 0.010), history of hypertension (β coefficient, −0.24; 95% CI, −0.44 to −0.03; P = 0.024); particularly higher systolic blood pressure (β coefficient, −0.10 per 10 mmHg; 95% CI, −0.15 to −0.04; P = 0.001), and diabetes (β coefficient,−0.30; 95% CI, −0.58 to −0.03; P = 0.032). When adjusting for sex, the reduction in the number of DMVs remained significantly associated with increasing age (β coefficient, −0.15 per 10 years; 95% CI, −0.26 to −0.04; P = 0.007), while the association of DMV with traditional vascular risk factors including hypertension, smoking, and diabetes disappeared after adjusting for age and sex.
Table 2.
Associations between potential risk factors and number of deep medullary veins.a
| Variable | Model 1b |
Model 2c |
||
|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | |
| Age (per 10-year increase) | −0.16 (−0.27, −0.05) | 0.005 | −0.15 (−0.26, −0.04) | 0.007 |
| Male sex (reference = female) | −0.22 (−0.43, −0.01) | 0.041 | −0.21 (−0.42, 0.01) | 0.057 |
| BMI (per 1-unit increase) | −0.02 (−0.05, 0.00) | 0.084 | −0.03 (−0.06, 0.00) | 0.044 |
| Current smoker (reference = no) | −0.32 (−0.57, −0.08) | 0.010 | −0.31 (−0.63, 0.02) | 0.064 |
| Alcohol use (reference = no) | −0.14 (−0.38, 0.09) | 0.222 | 0.03 (−0.29, 0.35) | 0.848 |
| Hypertension (reference = no) | −0.24 (−0.44, −0.03) | 0.024 | −0.16 (−0.37, 0.05) | 0.137 |
| SBP (per 10-mmHg increase) | −0.10 (−0.15, −0.04) | 0.001 | −0.08 (−0.13, −0.02) | 0.007 |
| DBP (per 10-mmHg increase) | −0.08 (−0.18, 0.02) | 0.099 | −0.08 (−0.17, 0.02) | 0.121 |
| Hyperlipidemia (reference = no) | −0.03 (−0.24, 0.17) | 0.768 | −0.01 (−0.22, 0.20) | 0.898 |
| Diabetes mellitus (reference = no) | −0.30 (−0.58, −0.03) | 0.032 | −0.24 (−0.52, 0.04) | 0.091 |
BMI: body mass index; CI: confidence interval; DBP: diastolic blood pressure; SBP: systolic blood pressure.
aIn the general linear regression models, the number of deep medullary veins was treated as dependent variable, and the potential risk factors were treated as independent variables.
bModel 1 is univariate.
cModel 2 is adjusted for age and sex.
Associations between number of DMVs and conventional imaging markers of CSVD
Table 3 summarizes the associations between the number of DMVs and conventional MRI markers of CSVD. In the crude models, a lower number of DMVs was associated with the presence of lacunes (OR, 0.85; 95% CI, 0.77–0.94, 0.94; P = 0.001) and larger WMH volume (β coefficient, −0.10 per 1 DMV; 95% CI, −0.16 to −0.04; P = 0.003), but not with the presence of CMBs, or severe basal ganglia or white matter PVS. When adjusting for age, sex, and other vascular risk factors, the association of reduced DMVs with WMH volume or presence of lacunes vanished.
Table 3.
Associations between mean number of DMVs and MRI markers of cerebral small vessel disease.a
| WMH volumeb (mm3) |
Lacunes |
CMBs |
Severe PVS-BGc |
Severe PVS-WMc |
BPFd (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | β (95% CI) | P |
| Model 1e | −0.10 (−0.16, −0.04) | 0.003 | 0.85 (0.77, 0.94) | 0.001 | 0.97 (0.86, 1.08) | 0.560 | 1.07 (0.96, 1.19) | 0.195 | 1.04 (0.94, 1.15) | 0.461 | 0.32 (0.20, 0.44) | <0.001 |
| Model 2f | −0.04 (−0.10, 0.02) | 0.167 | 0.90 (0.82, 1.00) | 0.047 | 1.02 (0.91, 1.15) | 0.697 | 1.11 (1.00, 1.23) | 0.057 | 1.07 (0.96, 1.18) | 0.212 | 0.23 (0.13, 0.33) | <0.001 |
| Model 3g | −0.03 (−0.09, 0.03) | 0.359 | 0.92 (0.82, 1.03) | 0.159 | 1.02 (0.89, 1.16) | 0.814 | 1.13 (1.00, 1.27) | 0.057 | 1.08 (0.97, 1.22) | 0.171 | 0.21 (0.13, 0.29) | <0.001 |
BPF: brain parenchymal fraction; CI: confidence interval; CMB: cerebral microbleed; DMV: deep medullary vein; MRI: magnetic resonance imaging; OR: odds ratio; PVS-BG: perivascular space in basal ganglia; PVS-WM: perivascular space in white matter; WMH: white matter hyperintensity.
aIn both the general linear and logistic regression models, the markers of cerebral vessel disease were treated as dependent variables, and the number of deep medullary veins was treated as independent variable. The OR and β were calculated based on per one deep medullary vein increase.
bWMH volume was natural log transformed and had 132 missing data.
cPerivascular space had three missing data.
dBPF had 64 missing data.
eModel 1 is univariate.
fModel 2 is adjusted for age and sex.
gModel 3 is adjusted for age, sex, hypertension, current smoker status, diabetes mellitus status.
A significant association was showed between the number of DMVs and BPF (β coefficient, 0.32 per 1 DMV; 95% CI, 0.20–0.44; P < 0.001), this association remained significant after adjustment for age and gender (β coefficient, 0.23 per 1 DMV; 95% CI, 0.13–0.33; P < 0.001). Even further adjustment for other cardiovascular risk factors and WMH volume and lacunes did not change the magnitude of this association (β coefficient, 0.21 per 1 DMV; 95% CI, 0.13–0.29; P < 0.001). In addition, a similar significant association was found between reduced number of DMVs and hippocampal volume (β coefficient, 0.002 per 1 DMV; 95% CI, 0.001–0.003; P = 0.008).
Additionally, we assessed the associations of the number of veins with all CSVD markers in one general linear model using the method of stepwise selection after adjusting for conventional risk factors. In the model, we treated the number of veins as dependent variable and treated all CSVD markers as independent variables. At last, only the BPF retained in the model and remained statistically significant (β coefficient, 0.11 per 1%; 95% CI, 0.06–0.18; P < 0.001), which was consistent with the results of separate analyses.
Association of number of DMVs with stroke incidence
During the follow-up of 3034 person-years (median of 3.0 years), 28 cases experienced stroke. Of these, 24 had ischemic stroke, three had hemorrhagic stroke, and one had both ischemic and hemorrhagic stroke. The number of DMVs was not associated with the risk of stroke (HR: 1.01; 95% CI: 0.79–1.29; P = 0.944), neither with ischemic nor hemorrhagic stroke.
Discussion
In this population-based cohort study, we observed a significant reduction in the number of DMVs with increased age, whereas no association between the number of DMVs and traditional vascular risk factors was found. We also found that number of DMVs was not associated with any MRI marker of CSVD, including WMH, lacunes, CMB, or PVS after adjusted for age, sex, and vascular risk factors. Furthermore, fewer DMVs were strongly related to brain atrophy independent of vascular risk factors and vascular parenchymal lesions, which may imply the potential relationship between DMVs and neurodegenerative process during aging. Finally, we found no association between the number of DMVs at baseline and three-year stroke risk.
Because of the limited number and sample size of previous studies on DMVs, there was no reliable candidate concerning potential risk factors for the number of DMVs except age. The significant reduction of the number of DMVs with increasing age found in our study could be well explained by a previous pathological study that found collagenous thickening of the venous wall increases with age and may result in stenosis or occlusion of the veins. Moody et al.1 reported no association between hypertension and VC, yet the small sample size limited the reliability of this conclusion. Although the number of DMVs is significantly associated with SBP, it is not associated with hypertension and DBP; this inconsistent result suggested that the relationship between blood pressure and number of DMVs is not robust. We further proved the absence of association between other vascular risk factors and DMV number. It seems that the factors which relate to atherosclerosis or arteriolosclerosis might not drive the reduction in the number of DMVs.
We found the inverse correlation between the number of DMVs and volume of WMH vanished after adjustment for age, suggesting this association was probably because of the synchronous change of DMV and WMH with increasing age. Moody et al.12 found advanced leukoaraiosis in 10/13 patients with more severe VC. Keith et al.2 reported stenosis of large caliber DMVs (>200 µm) which was a predictor for WMH. However, previous studies failed to prove whether this association is independent because of their small sample size. Moreover, we found no association between DMV and other MRI markers of CSVD including lacunes, CMB, and the perivascular space after adjustment for traditional vascular risk factors. Thus, our results suggest that DMV was significantly different from all other MRI markers of CSVD. First, traditional vascular risk factors, including hypertension, smoking, diabetes, hyperlipidemia, and history of coronary heart disease, showed no association with decreased venous number after adjustment for age. Second, small venous visibility was not associated with traditional MRI markers of CSVD including WMH, lacunes, MB, or the perivascular space. Finally, no association was detected between the number of DMVs and risk of stroke.
Our finding of the strong association between the decreased number of DMVs and brain atrophy is striking. We found that fewer DMVs are significantly related to not only lower total whole brain volume, but also lower GM, white matter, and hippocampal volume. This association is independent of age, other vascular risk factors, and vascular parenchymal lesions, such as WMH or lacunes. Using the susceptibility effect, deoxyhemoglobin appears as an intrinsic contrast agent in the veins; in this way, cerebral veins can be noninvasively assessed using the SWI sequence.4 Therefore, one potential explanation of this association might be that brain atrophy results in reduced cerebral metabolism and lowers deoxyhemoglobin levels in the vessel. However, the association between DMV and the hippocampus is difficult to be explained by a significant change in the level of metabolism as they are quite widely spatially separated. In addition, both imaging- and pathology-based studies found structural changes, such as tortuosity, stenosis, and occlusion, of the venules according to age.13 From our experience with MRI reading, the presence of fewer DMVs usually occurs simultaneously with discontinuity or tortuosity in shape. Therefore, it is more likely that structural changes, such as stenosis or occlusion of DMVs, may also be associated with brain atrophy rather than just merely a consequence of change in metabolism. Bouvy et al.8 also reported tortuosity of venules in early AD patients. To date, studies on brain venules are still very limited, while the results highlight the importance of further study on the relationship between venules and neurodegenerative process during aging.
The strengths of our study include the population-based design and large sample size of SWI sequences obtained using a 3-T MRI scanner. However, there are several limitations that should be considered. First, we used a 3T brain MRI to assess DMVs as this lower field strength may reduce the quality of detecting DMVs as compared with 7T brain MRI; beyond that, as the conspicuity of venules on SWI images depends on the effect of deoxygenated hemoglobin, decreased venular visibility could be a result of venous occlusion or decreased concentration of deoxyhemoglobin. Thus, people previously speculate that the concentration of hemoglobin might be associated with the visibility of DMV. However, we did not find the number of DMVs was associated with the concentration of hemoglobin in our general population (rpearson = −0.025, P = 0.305). What is more, large artery stenosis might also relate to higher visibility of veins by causing hypoperfusion state of the downstream small vessels. However, exclusion of individuals with stenosis of carotid artery or intracranial artery (N = 37) do not change the present results. Second, one might query the reliability of visual assessment used in this present study. Visual assessment and several automatic segmentation methods have been applied in previous studies on brain veins; however, none has been accepted as a unified method because of the very limited studies in this field. An automatic segmentation technique is more likely to have an advantage when whole brain veins are evaluated, whereas visual counting is feasible and reliable when DMVs are assessed due to their regular arrangement perpendicular to the ventricles with little anatomic variation. To our experience, since DMVs are only counted in a ROI of 60 mm × 10 mm in the periventricular white matter of each cerebral hemisphere, the evaluation process of one case takes only a couple of minutes. This simple and speedy method also guarantees the feasibility of DMV evaluation. Moreover, the intra- and inter-rater agreement in our study was found to be 0.76 and 0.79, which suggested a good reproducibility of visual assessment. We need to note that this is the first population-based study on risk factors and potential significance of brain venules and was performed in a Chinese population; hence, further studies are needed to confirm this finding in other samples.
In conclusion, our study offers new insights into brain venules. The significant association between DMVs and aging, as well as the association between DMV and brain atrophy are intriguing, and its elucidation may improve our understanding of the complex relationship between vascular alterations and age-related neurodegenerative process. Cerebral venules obviously deserve more attention and further investigation.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20918467 for Brain deep medullary veins on 3-T MRI in a population-based cohort by Dong-Hui Ao, Ding-Ding Zhang, Fei-Fei Zhai, Jiang-Tao Zhang, Fei Han, Ming-Li Li, Jun Ni, Ming Yao, Shu-Yang Zhang, Li-Ying Cui, Zheng-Yu Jin, Li-Xin Zhou Yi-Cheng Zhu in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Key Research and Development Program of China (No. 2016YFB1001402), National Natural Science Foundation of China (No. 81671173), the Strategic Priority Research Program (Pilot study) “Biological basis of aging and therapeutic strategies” of the Chinese Academy of Sciences (Grant XDPB10), 2016 PUMCH science fund for junior faculty (pumch-2016-1.3), the Fundamental Research Funds for the Central Universities(3332018034), and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (No. 2016-12M-1-004).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contribution: DHA and DDZ drafted the manuscript, participated in study design and data collection, conducted the statistical analyses, analyzed, and interpreted the data. YCZ and LXZ participated in study design and data collection, data interpretation, and made a major contribution in revising the manuscript. FFZ, JTZ, and FH participated in the data collection and imaging analysis and made contribution in revising the manuscript. MLL assisted in designing the MRI sequences. JN, MY, SYZ, LYC, and ZYJ made contribution in supervising and coordinating the study.
ORCID iD: Dong-Hui Ao https://orcid.org/0000-0002-7388-3372
Supplemental material: Supplemental material for this article is available online.
References
- 1.Moody DM, Brown WR, Challa VR, et al. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 1995; 194: 469–476. [DOI] [PubMed] [Google Scholar]
- 2.Keith J, Gao F, Noor R, et al. Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropathol Exp Neurol 2018; 76: 299–312. [DOI] [PubMed] [Google Scholar]
- 3.Pantoni L.Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 4.Reichenbach JR, Venkatesan R, Schillinger DJ, et al. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 1997; 204: 272–277. [DOI] [PubMed] [Google Scholar]
- 5.Okudera T, Huang YP, Fukusumi A, et al. Micro-angiographical studies of the medullary venous system of the cerebral hemisphere. Neuropathology 1999; 19: 93–118. [DOI] [PubMed] [Google Scholar]
- 6.Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 2016; 12: 714–722. [DOI] [PubMed] [Google Scholar]
- 7.De Guio F, Vignaud A, Ropele S, et al. Loss of venous integrity in cerebral small vessel disease: a 7-T MRI study in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke 2014; 45: 2124–2126. [DOI] [PubMed] [Google Scholar]
- 8.Bouvy WH, Kuijf HJ, Zwanenburg JJM, et al. Abnormalities of cerebral deep medullary veins on 7 tesla MRI in amnestic mild cognitive impairment and early Alzheimer’s disease: a pilot study. J Alzheimer’s Dis 2017; 57: 705–710. [DOI] [PubMed] [Google Scholar]
- 9.Zhai FF, Yan S, Li ML, et al. Intracranial arterial dolichoectasia and stenosis: risk factors and relation to cerebral small vessel disease. Stroke 2018; 49: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Tzourio C, Soumaré A, et al. Severity of dilated Virchow-Robin spaces is associated. Stroke 2010; 41: 2483–2490. [DOI] [PubMed] [Google Scholar]
- 12.Moody DM, Brown WR, Challa VR, et al. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer’s disease. Ann N Y Acad Sci 1997; 826: 103–116. [DOI] [PubMed] [Google Scholar]
- 13.Brown WR, Thore CR.Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 2011; 37: 56–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20918467 for Brain deep medullary veins on 3-T MRI in a population-based cohort by Dong-Hui Ao, Ding-Ding Zhang, Fei-Fei Zhai, Jiang-Tao Zhang, Fei Han, Ming-Li Li, Jun Ni, Ming Yao, Shu-Yang Zhang, Li-Ying Cui, Zheng-Yu Jin, Li-Xin Zhou Yi-Cheng Zhu in Journal of Cerebral Blood Flow & Metabolism