Abstract
Zinc oxide (ZnO) nano/microparticles (NPs/MPs) have been studied as antibiotics to enhance antimicrobial activity against pathogenic bacteria and viruses with or without antibiotic resistance. They have unique physicochemical characteristics that can affect biological and toxicological responses in microorganisms. Metal ion release, particle adsorption, and reactive oxygen species generation are the main mechanisms underlying their antimicrobial action. In this review, we describe the physicochemical characteristics of ZnO NPs/MPs related to biological and toxicological effects and discuss the recent findings of the antimicrobial activity of ZnO NPs/MPs and their combinations with other materials against pathogenic microorganisms. Current biomedical applications of ZnO NPs/MPs and combinations with other materials are also presented. This review will provide the better understanding of ZnO NPs/MPs as antibiotic alternatives and aid in further development of antibiotic agents for industrial and clinical applications.
Keywords: zinc oxide nano/microparticles, antimicrobial activity, nanoantibiotics, physicochemical characteristics, biomedical application
1. Introduction
Zinc oxide (ZnO) nanoparticles (NPs) have been studied for the development of next-generation nanoantibiotics against pathogenic microorganisms to combat multi-drug resistance [1,2]. These nanoparticles show unique physicochemical properties including morphology, particle size, crystallinity, and porosity [3]. Based on these characteristics, ZnO NPs have a wide spectrum of antimicrobial activity against microorganisms including Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, and the M13 bacteriophage [4,5,6,7]. They can be combined with antibiotic and anti-inflammatory drugs to enhance antimicrobial activity against pathogenic microorganisms without antibiotic resistance in non-clinical and clinical conditions [7,8]. Use of ZnO NPs as well as microparticles (MPs) has been extended to biomedical applications including antibiotic drugs or medical devices, theranostics, implants, and cosmetics for conventional uses in clinics [9,10,11,12].
The physicochemical characterization of ZnO NPs/MPs offers advantageous information regarding biological or biochemical responses to pathogenic microorganisms, enabling the prediction of antimicrobial and toxicological effects [13,14]. Generally, their properties, including morphology, particle size or particle size distribution, porosity, and specific surface area, can affect antimicrobial responses [14,15]. ZnO NPs/MPs of various shapes such as spheres, rods, needles, and platelets, and their average aspect ratios (defined as ratio of length to width) can influence the antimicrobial actions against microorganisms. Their particle size and particle size distribution levels are the key parameters that determine NP uptake into biomembranes and thereby influence antimicrobial activity against pathogenic microorganisms [16,17]. Intra- and interparticle pores of ZnO NPs/MPs also enhance photocatalytic antimicrobial interactions under ultraviolet (UV) and visible light irradiation based on reactive oxygen species (ROS) [5,18]. Furthermore, large specific surface area levels of ZnO NPs/MPs facilitate membrane adsorption for antimicrobial actions [19].
Furthermore, combinations of ZnO NPs/MPs with other antibiotic drugs, metal oxide NPs/MPs, and devices have been used to enhance antimicrobial activity against pathogenic microorganisms [20,21,22,23,24]. They have synergistic or improved antimicrobial activity against E. coli, S. aureus, Aeromonas veronii, P. aeruginosa, B. subtilis, and Klebsiella pneumoniae. ZnO NP/MP combinations with other materials are applied as dosage forms other than particles, including membranes, films, and plates, according to the administration route or usage for enhanced antimicrobial performance [25,26,27,28]. These dosage forms can deliver the drug to the specific site of infection in human diseases including endocarditis, cystic fibrosis, pneumonia, and otitis [29,30].
In this review, we introduce the physicochemical characteristics of ZnO NPs/MPs to explain their antimicrobial activity against pathogenic microorganisms. The antimicrobial activity of ZnO NPs/MPs and their combinations with other antimicrobial materials such as antibiotic and anti-inflammatory drugs, metal oxide NPs/MPs, and polymers is also described. Moreover, current biomedical applications of ZnO NPs/MPs are presented.
2. Characteristics of ZnO Materials Based on Synthesis Techniques
ZnO NPs (81.38 g/mol, <100 nm) are white odorless solid powders of hexagonal wurtzite crystals with various shapes including spheres for zero-dimensional (0D) structures, dumbbells, nanorods, nanotubes, and needles for one-dimensional (1D) structures, disks and platelets for two-dimensional (2D) structures, and flowers, stars and flakes for three-dimensional (3D) structures [6]. They have a wide band gap energy (~3.3 eV) which is similar to that of titanium dioxide (TiO2) NPs. Compared to ZnO NPs, ZnO MPs are generally in the submicron range regarding their size.
The physicochemical properties of ZnO materials mainly affect their antimicrobial activity against pathogenic microorganisms and are critical parameters linked to pharmacological and toxicological responses [31,32]. Their morphology, particle size, and porosity are determined to confer the superior antimicrobial activity in pathogenic microorganisms [3]. These physicochemical properties of ZnO NPs/MPs are influenced by the synthesis techniques used for their preparation [5,6,33].
2.1. Synthesis Techniques of ZnO Materials
The synthesis techniques used for ZnO NPs/MPs are generally categorized by physical, chemical, biological, and microfluidic methods [5,34]. Physical methods of arc plasma, thermal evaporation, physical vapor deposition, ultrasonic irradiation, and laser ablation simply produce chemically pure ZnO NPs/MPs. Wet chemical methods for the synthesis of ZnO NPs/MPs such as microemulsion, sol–gel, precipitation, hydrothermal and solvothermal methods are extensively used to generate the specific physicochemical characteristics, based on their simple and scalable bottom-up approaches [35,36]. Biological methods, so called “green synthesis”, are promoted as ecofriendly synthesis techniques including microorganisms (bacteria, fungi, yeasts, algae, and phages), plant extracts, DNA, and proteins [5,37]. The biosynthesized ZnO NPs/MPs have comparable physicochemical characteristics to those of physically or chemically synthesized ZnO NPs/MPs. Moreover, microfluidic methods can offer high-value ZnO NP/MP products using modular architecture integration for sophisticated reactions [5,38], which enable ZnO NPs/MPs to have targeted physicochemical properties.
Meanwhile, various electrochemical processes for synthesis of ZnO NPs/MPs have been reported including electrodeposition [39,40], sacrificial anode electrolysis [41], and electrochemical deposition under oxidizing conditions (EDOC) [42,43]. Hybrid techniques of electrochemical-thermal processes were also reported in aqueous environment [44,45]. In electrodeposition, ZnO thin films can be produced controlling current density, applied potential, time, and electrolytic bath concentration as major operating parameters using zinc nitrate solution or zinc chloride solution as precursors [40]. This technique is advantageous to achieve simple and cost-effective outcomes in large-surfaced substrate, which produces various structures of nanoneedle-like, prism-like, porous, and continuous shapes with appropriate thickness [39]. On silicon, ZnO deposition occurred from nonaqueous solution of dimethyl sulfoxide (DMSO) containing zinc chloride and potassium chloride, which films of 1–3 μm thick had pore channels (20 nm). Next, sacrificial anode electrolysis from two-electrode cells to thee-electrode cells along with potentiostatic control improves reproducibility of morphological dimension in ZnO NPs/MPs [41]. In the electrode-cell, metal ions are produced in anodic dissolution or added into electrochemical medium and cathodic reduction induces particle growth with tunable morphology. Near-spherical ZnO NPs (<25 nm) in an amorphous phase were produced using sacrificial anodic dissolution of pure zinc metal strip (2 × 6 × 1 cm3) controlling against platinum mesh as the cathode in electrolyte of 0.1 M tetrabutlyammonium bromide solution and acetonitrile (4:1) [46]. In EDOC, sacrificial anodic oxidation, cathodic reduction, and oxidation processes were involved for the synthesis of spherical ZnO NPs (9 nm) [42]. Hybridizing electrochemical and thermal approaches, ZnO NPs/MPs were synthesized in aqueous sodium bicarbonate solution, generating zinc hydroxide species and further oxidized to ZnO in calcination process at >300 °C [44,45]. Specifically, anionic or cationic stabilizers can be used to tune physicochemical properties of ZnO NPs/MPs including morphology and particle size.
2.2. Physicochemical Characteristics of ZnO Materials
We introduce the physicochemical characteristics of ZnO NPs/MPs which antimicrobial functions have already been reported. Table 1 displays morphology, particle size, porosity, surface area, and synthesis technique of ZnO NPs/MPs with antimicrobial functions against pathogenic microorganisms. In general, high aspect ratio, small particle size, multilevel porosity, and large surface area of ZnO NPs/MPs can enhance antimicrobial performance, irrespective of synthesis techniques [3,5,14]. Chemically or biologically synthesized ZnO NPs/MPs have been extensively used to determine their antimicrobial activity against microorganisms, rather than microfluidically or electrochemically synthesized ZnO NPs/MPs, due to simplicity, reproducibility, and production scale, yield or rate for obtaining high-value ZnO NPs/MPs with required characteristics [5,6,8].
Table 1.
Physicochemical characteristics of zinc oxide (ZnO) materials.
| Morphology | Particle Size | Porosity | Surface Area (m2/g) |
Synthesis Method (Precursor) |
Refs. |
|---|---|---|---|---|---|
| Spheres | 12 nm, 25 nm | Slit-like pores | 90.4, 42.8 |
|
[16] |
| 62–94 nm | Not available | Not available |
|
[47] | |
| 15–20 nm | Not available | Not available |
|
[48] | |
| 25–30 nm (TEM); 65 ± 4 nm (dynamic light scattering) | Not available | Not available |
|
[49] | |
| 12–46 nm | Not available | Not available |
|
[50] | |
| Spheres in self-assembled network | 48.3 nm | Mesopores, 5–6.25 nm; macropores, 2–6 μm | Not available |
|
[18] |
| Spheres in NP cluster | NPs-63 nm; cluster-590 nm | 34 nm | 20.72 |
|
[19] |
| Dumbbells | Length 190–250 nm; breadth 50–60 nm | Not available | Not available |
|
[50] |
| Nanorods | Length 523 nm; diameter 47 nm | Not available | 2.3 |
|
[52] |
| Length ~2 μm; diameter ~50 nm | Not available | Not available |
|
[53] | |
| Hollow nanotubes | Length ~500 nm | 53 nm (900 °C annealing), 114.6 nm (700 °C annealing), 255.4 nm (500 °C annealing), 278.6 nm (as-synthesized) | 2.4 (900 °C annealing), 7.2 (700 °C annealing), 16.7 (500 °C annealing), 17.8 (as-synthesized) |
|
[54] |
| Length ~5 μm; thickness 59.5 nm | Internal diameter 178.2 nm | Not available |
|
[55] | |
| Needles | Length ~2 μm; diameter 20–40 nm | Not available | Not available |
|
[56] |
| Disks | 41 nm | Not available | Not available |
|
[57] |
| Platelets | 14.7 nm, 17.5 nm; hydrodynamic environment- 6.4 μm, 5.0 μm | 17.3 nm, 11.6 nm | 39.0, 46.9 |
|
[58] |
| Flowers | Length 200–500 nm; length 1–2 μm | Not available | Not available |
|
[45] |
| 23.7–88.8 nm | Not available | Not available |
|
[59] | |
| 700 nm–2.2 μm | Not available | Not available |
|
[60] | |
| 45 nm | Not available | 28.8 |
|
[61] | |
| Stars | 31 nm | Not available | Not available |
|
[62] |
| Flakes | 32 nm | Not available | Not available |
|
[63] |
First, in the 0D structure of ZnO NPs/MPs, Raghupathi et al. [16] reported the manufacture of spherical ZnO NPs (12–307 nm) with slit-like pores (3.49–90.4 m2/g surface area) using solvothermal and room temperature syntheses. Using the solvothermal technique for ZnO NP synthesis, smaller ZnO NPs (12–25 nm) with larger surface area (42.8–90.4 m2/g) were obtained, compared to ZnO NP synthesis at room temperature (30–307 nm; 3.49–35.6 m2/g surface area). Green synthesized spherical ZnO NPs were reported, using Catharanthus roseus leaf extract and zinc acetate dehydrate [47]. In spherical ZnO NP synthesis, controllable reaction factors were optimized for pH 12, 30 °C, 0.01 M precursor metal ion, and 2 h of reaction time to achieve high-value ZnO NPs for enhanced antimicrobial performance. Using green synthesis and co-precipitation, sphere-shaped ZnO NPs (15–20 nm) were obtained reacting with Bambusa Vulgaris plant extract and zinc nitrate aqueous solution (0.5 mol/50 mL) [48]. Compared to Bambusa Vularis plant extract, Artabotrys Hexapetalu plant extract was used to synthesize mixed spherical and rod-like ZnO NPs (20–30 nm). In green synthesis using Sambucus ebulus leaf extract and 1 M zinc acetate dihydrate, spherical ZnO NPs were also prepared at 25–30 nm for transmission electron microscopy (TEM) and 65 ± 4 nm for dynamic light scattering [49]. Moreover, a self-assembled 3D network structure of spherical ZnO NPs (48.3 nm) on a solid plate was also analyzed, in which NPs were synthesized via sol–gel technique using an annealing process at 150 °C [18]. In the self-assembled ZnO NP network, mesopores at 5–6.25 nm and macropores at 2–6 μm were detected, conferring bimodal porosity for enhanced performance. Meanwhile, spherical nanostructured ZnO (63 nm) manufactured by soft chemical synthesis using micrometric ZnO and urea in glycerol, generated NP clusters (590 nm) with mesopores of 34 nm and a 20.72 m2/g surface area [19].
In the 1D structure of ZnO NPs/MPs, Bala et al. [50] reported dumbbell-shaped ZnO NPs (190–250 nm in length and 50–60 nm in breadth), which were prepared via green synthesis by reacting with Hibiscus subdariffa leaf extract and zinc acetate in water. The morphology of ZnO NPs was modified depending on drying temperature. After particle precipitation, dumbbell-shaped ZnO NPs were generated with further heating at 100 °C for 4 h. In contrast to the dumbbell-shaped ZnO NPs, sphere-shaped ZnO NPs (12–46 nm) resulted from drying at 60 °C. The submicron ZnO dumbbell structure was generated using a chemical bath deposition technique at low temperature (80 °C) [51]. The resulting ZnO dumbbells were 1–2 μm long, 200–300 nm wide at one end, and 250–400 nm wide at the other end.
ZnO nanorods (523 nm in length and 47 nm in diameter) were reported as photocatalytic antibacterial agents by Singh et al. [52]. They were synthesized using the sol–gel technique by reacting with 1 M zinc nitrate and 1 M hexamethyl tetraamine (HMTA) at pH 6.5. Reactions carried out at pH 5.0–6.5 in mediated smaller ZnO nanorods. Using the green synthesis technique of reacting with egg white albumen and zinc acetate dihydrate in water (1 mmol/mL), the resulting ZnO nanorods had a highly crystalline structure at ~2 μm in length and ~50 nm in diameter for enhanced antimicrobial performance [53]. Calcined temperature was determined as a controllable parameter for high-value ZnO nanorod synthesis, ranged at 400–650 °C. ZnO nanorods calcined at the highest temperature (650 °C) displayed pure and highly crystalline products.
Hollow ZnO nanotubes in nanopowders, ranging at ~500 nm were manufactured via hydrothermal synthesis using polyvinyl alcohol (PVA) with a further annealing process at 500–900 °C [54]. After annealing, the surface area and pore size of the ZnO nanopowders were reduced from 17.8 m2/g and 278.6 nm to 2.4–16.7 m2/g and 53–255.4 nm, respectively, compared to as-synthesized nanopowders, owing to sintering and growing boundaries to form microscale aggregates. Lopez de Dicastillo et al. [55] described hollow ZnO nanotubes 5 μm in length and 59.5 nm in thickness with an internal diameter of 178.2 nm, prepared via template methods using electrospun PVA nanofibers through atomic layer deposition of ZnO NPs from water and diethyl zinc. Thermal treatment or hydrolysis was additionally performed to remove the PVA template from the hollow ZnO nanotubes. Besides the nanotubes, ZnO needles were also reported as antimicrobial agents for urinary tract pathogens [56]. These needle-shaped ZnO NPs were green synthesized using Berberis aristata leaf extract and 0.1 M zinc acetate dihydrate adjusting pH with 1 M sodium hydroxide. Their size range was ~2 μm in length and 20–40 nm in diameter.
In 2D structures of ZnO NPs/MPs, ZnO nanodisks were prepared using co-precipitation by reacting with 1 mM zinc acetate dihydrate and 2 M sodium hydroxide in water at room temperature [57]. The nanodisks, theoretically calculated at 41 nm, showed a minimum toxicity (LD50 for brine shrimps, >200 μg/mL) compared to those synthesized in other solvents, including acetone (size, 29.1 nm; LD50, 83.2 μg/mL), ethyl acetate (size, 40.1 nm; LD50, 140.3 μg/mL), ethanol (size, 33.7 nm; LD50, 90 μg/mL), and methanol (size, 32.8 nm; LD50, 49.4 μg/mL), with the exception of chloroform (size, 38.5 nm; LD50, >200 μg/mL). Platelet-shape ZnO NPs of different sizes (14.7 nm and 17.5 nm) were also reported by Pasquet et al. [58], in which size distribution was less than 100 nm despite the particle size increasing to 5.0–6.4 μm in a hydrodynamic environment. The particles had mesopores of 11.6–17.3 nm and a specific surface area of 39.0–46.9 m2/g.
In 3D structured ZnO NPs/MPs such as antibiotic agents, flower-like ZnO MPs were synthesized using electrochemical-thermal method with poly-diallyl-(dimethylammonium) chloride (PDDA) and benzyl-dimethylammonium chloride (BAC) as a stabilizer, which were 200–500 nm or 1–2 μm long [45]. Flower-shaped ZnO NPs were also synthesized using the sol–gel method reacting with 5% zinc acetate dihydrate and 0.2 w/v% cetyltrimethyl ammonium bromide (CTAB) at pH 11.7–11.9, and 25–75 °C of near-room temperatures [59]. They showed enhanced antibacterial and antifungal performance via larger zones of growth inhibition when compared to ZnO powders and conventional products. Flower-like ZnO NPs were further reported by Quek et al. [60], which were sized at 700 nm–2.2 μm in diameter for visible light-responsive photocatalytic antimicrobial action. Talebian et al. [61] described higher antibacterial activity of flower-shaped ZnO NPs compared to those of rod- and sphere-shaped ZnO NPs. The flower-shaped ZnO NPs were synthesized using the solvothermal technique reacting with zinc acetate dihydrate in water (5.6 g/50 mL) and 0.5 M sodium hydroxide. They resulted in a smaller particle (45 nm) with larger surface area (28.8 m2/g) than ZnO NPs synthesized in 1-hexanol (rod shape; 76 nm; 15.5 m2/g surface area) and ethylene glycol (sphere shape; 65 nm; 19.2 m2/g surface area), predicting enhanced antimicrobial performance. At this point, it is believed that the solvent in the solvothermal technique is a critical parameter in the synthesis of ZnO NPs, which results in differentiated morphology. Star-shaped ZnO NPs were also synthesized at 31 nm using liquid precipitation for preparing microorganism-protective defense clothing on cotton or polyester fabrics due to their biocompatibility [62]. Using co-precipitation, flake-shaped ZnO NPs were prepared at 32 nm size for enhanced antimicrobial performance [63].
Therefore, irrespective of ZnO NP/MP variety, the physicochemical characteristics of ZnO NPs/MPs affect their antimicrobial activity against pathogenic microorganisms [14,64], and synthesis techniques can be controlled to improve the properties of ZnO NPs/MPs for enhanced antimicrobial performance [13,65].
3. Antimicrobial ctivity against Pathogenic Microorganisms
3.1. Antimicrobial Mechanisms of ZnO Materials
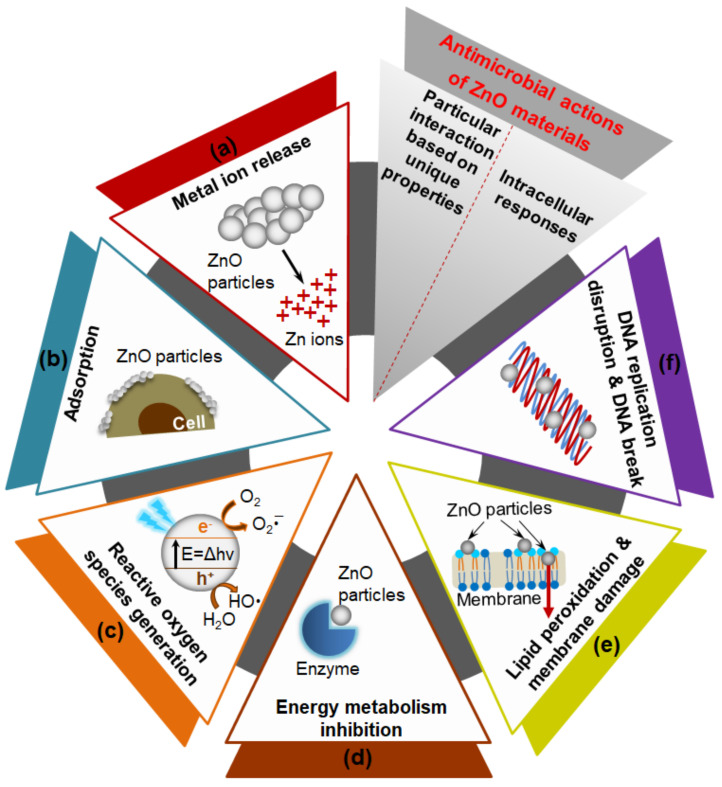
Mechanisms of antimicrobial actions of ZnO materials have been explained in association with particular interaction based on their unique physicochemical properties of (a) Zn2+ ion release, (b) adsorption, and (c) ROS generation [14,66], and the intracellular responses in microorganisms of (d) energy metabolism inhibition; (e) lipid peroxidation, and cell membrane damage; and (f) DNA replication disruption, and DNA break [67,68] (Figure 1). The Zn2+ ions that are released from ZnO NPs/MPs induce an antimicrobial response in microorganisms due to interference in metabolic processes and disturbance in enzymatic systems [69,70]. ZnO NPs/MPs also have functions of particle adsorption to the biomembrane via a charge–charge interaction, and ROS generation as photocatalysts under UV and visible light irradiation [3,12,71,72]. Positively charged surfaces of ZnO NPs/MPs interact with the negatively charged cell wall or biomembrane of microorganisms [73]. They are internalized into the microorganisms after adsorption resulting in loss of cell integrity based on cell wall or membrane rupture, and further mediate oxidative stress owing to lipid peroxidation leading to deoxyribonucleic acid (DNA) damage. Based on the fundamental mechanisms of action, ZnO NPs/MPs have a differential susceptibility against pathogenic microorganisms, affected by their physicochemical characteristics including morphology, particle size, and porosity [3,17,18,73].
Figure 1.
Mechanisms of zinc oxide (ZnO) materials used in antimicrobial applications: Particular interactions of (a) metal ion release, (b) adsorption, and (c) reactive oxygen species generation based on their physicochemical properties; and following intracellular responses of (d) energy metabolism inhibition, (e) lipid peroxidation and membrane damage, and (f) DNA replication disruption and DNA break.
In the photocatalytic activation of ZnO NPs/MPs for ROS generation linked to oxidative stress, electrons (e−) are transmitted from the valence band to the conduction band by the photoexcitation over band gap energy (E = Δhν) leaving positive holes (h+) [14,65]. Holes in the valence band produce hydroxyl radicals (OH·), whereas electrons in the conduction band generate superoxide radical anions (O2·−) in an aqueous environment. Reacting with electrons and hydrogens, hydroxyl peroxide radicals (HO2·) and finally hydrogen peroxide (H2O2) are formed. In addition, surface defects of ZnO NPs can enhance ROS production even in the dark for enhanced antimicrobial performance [74].
In 3D structures of ZnO NPs/MPs followed by aggregate formation or nanostructure generation, multiple scattering can be one of several antimicrobial mechanisms to improve photocatalytic performance based on enhanced mass transfer and exchange [18,75]. The bimodal porous self-assembled network of ZnO NPs, with mesopores and macropores was reported to enhance the photocatalytic antimicrobial action caused by multiple scattering under dual UV irradiation [18]. Hierarchically porous self-forming structured aggregates of polydispersed ZnO NPs showed a submicron size of 100–500 nm in diameter, which enhanced the multiple scattering phenomenon [75]. Thus, the hierarchically porous structure of ZnO NPs/MPs mediating multiple scattering can counteract the undesirable photocatalytic performance, which prevents large particles from retaining a small surface area.
Compared to other metal or metal oxide NPs/MPs, ZnO NPs/MPs have multiple functional mechanisms of antimicrobial performance [4]. In antibiotic metals such as silver (Ag) and gold (Au), NPs have the primary functions of metal ion release and membrane adsorption, respectively. In general, titanium dioxide, cupric oxide (CuO), and magnesium oxide (MgO) NPs display ROS generation, NP internalization, and membrane damage, respectively. Regardless of the susceptibility of pathogenic microorganisms to antimicrobial activity, ZnO NPs/MPs can combat microbial regrowth to overcome multi-drug resistance of conventional antibiotics based on targeting antibiotic-resistant pathways in a non-specific manner, irrespective of NP penetration into microorganisms [7,30,76].
Meanwhile, ZnO NP/MP combinations with other materials displayed comparable antimicrobial activity against pathogenic microorganisms [77,78]. In these studies, the materials were mixed or conjugated with ZnO NPs/MPs, with a view to improving their usability and sustainability in biomedical applications, compared to ZnO NPs/MPs alone. Combinatorial materials used with ZnO NPs/MPs also showed antimicrobial activity using different mechanisms to overcome multi-drug resistance. These included antibiotic or anti-inflammatory drugs [20,47,79], other metal oxide NPs/MPs or metal doping [80,81], polymers (e.g., chitosan and alginate) [28,82,83], carbon-based materials (e.g., graphene [84,85], graphene oxide (GO) [86,87], and reduced graphene oxide (rGO) [88]), and quantum dots (QDs) [89,90]. They enhanced ROS generation or mediated the other antimicrobial pathways of ZnO NPs/MPs in microorganisms to create an additive or synergistic microbial growth inhibition benefit. However, the reduced or antagonistic disadvantages of antimicrobial performance sometimes occurred due to microbial susceptibility [20,81,91].
3.2. ZnO Materials and Combinations Based on Antimicrobial Functions
ZnO NPs/MPs have been studied to enhance their antimicrobial activity against pathogenic microorganisms [33,92]. In addition to improving their physicochemical characteristics, antimicrobial activities of combinations of ZnO NPs/MPs with other biomaterials have been explored for enhanced antimicrobial performance [93,94]. Table 2 presents the antimicrobial activity of (i) ZnO NPs/MPs, (ii) ZnO NPs/MPs with antibiotic or anti-inflammatory drugs, (iii) ZnO NPs/MPs with other metal oxide NPs/MPs or metal doping, (iv) ZnO NPs/MPs with other polymeric biomaterials or films, and (v) ZnO QDs.
Table 2.
Antimicrobial activity of zinc oxide (ZnO) materials against pathogenic microorganisms.
| Materials | Synthesis Techniques (Precursor) |
Material Description (Morphology/Particle Size) | Antimicrobial Activity Test Methods | Antimicrobial Activity against Pathogenic Microorganisms | Refs. |
|---|---|---|---|---|---|
| ZnO materials | |||||
| 0D structured ZnO NPs | Hydrothermal method or room temperature synthesis (zinc nitrate hexahydrate; zinc sulfate; zinc acetate dihydrate) |
Spheres/12 nm, 25 nm; 30 nm, 88 nm, 142 nm, 212 nm, 307 nm | Turbidity | 6 mM, [Staphylococcus aureus] 30 nm: 10.11% growth; 88 nm: 54.34% growth; 142 nm: 78.12% growth; 212 nm: 79.79% growth; 307 nm: 96.67% growth, [Proteus vulgaris] 12 nm: 8.71% growth, [Salmonella typhimurium] 12 nm: 46.96% growth, [Shigella flexneri] 12 nm: 5.95% growth, [Bacillus cereus] 12 nm: 7.62% growth |
[16] |
| Green method using Aloe vera leaf extract (zinc acetate dihydrate) |
|
Well diffusion, broth microdilution; MIC | MIC, [E. coli] ZnO-1, ZnO-2, and ZnO-3: 1562 μg/mL; [S. aureus] ZnO-1: 781 μg/mL, ZnO-2 and ZnO-3: 391 μg/mL; [B. subtilis] ZnO-1 and ZnO-2: 391 μg/mL; ZnO-3: 195 μg/mL |
[17] | |
| Precipitation method (zinc acetate dihydrate) | Nanopyramids/~15.4 nm in segments | Colony counting, serial dilution; MIC | [Methicillin-resistant S. aureus (MRSA)] MIC, 333 μg/mL (3 log (CFU/mL) inhibition at 800 μg/mL) | [96] | |
| Soft chemical synthesis (micrometric ZnO) | Spherical NP cluster/cluster-590 nm; NPs-63 nm | Colony counting | Antimicrobial activity value of log (control/sample), [E. coli] > 3.5; [S. aureus] > 3.5 |
[19] | |
| 1D structured ZnO NPs | Hydrothermal method (zinc acetate dihydrate) |
Hollow nanotubes/particle size ~500 nm in length; surface area 17.8 m2/g; average pore size, 278.6 nm | Disk diffusion; MIC | As-synthesized ZnO nanopowders, MIC, [Escherichia coli] 0.0585 mg/mL, [S. aureus] 0.234 mg/mL, [Pseudomonas aeruginosa] 0.234 mg/mL, [Bacillus subtilis] 0.938 mg/mL |
[54] |
| Atomic layer deposition process over polymer template and template removal | Hollow nanotubes/length, ~5 μm; thickness, 59.5 nm; internal diameter, 178.2 nm | Colony counting | ZnO nanotubes at 1 wt% with acrylic polymer/extruded 32 μm-polyethylene (PE) substrate film coating [E. coli] 4.67 log reduction (cells/cm2), [S. aureus] 2.46 log reduction (cells/cm2) |
[55] | |
| Precipitation method (zinc nitrate) | Nanorods/88.7 nm using 2-mercaptoethanol as a capping agent | Disk diffusion method, serial dilution; MIC | [Klebsiella pneumoniae] MIC, 40 μg/mL | [99] | |
| Green method using Chlorella vulgaris culture supernatant (zinc acetate dihydrate) | Nanorods/crystal size, 3.4 nm; length-150 nm; width-21 nm (aspect ratio of length to width 7.14) | Microdilution; MIC | [E. coli, P. aeruginosa, S. aureus] MIC, 250 μg/mL (excluding Enterococcus faecalis: resistant to ZnO nanorods) |
[100] | |
| Sol–gel method (zinc nitrate hexahydrate) | Nanorods/500 nm–1 μm | Disk diffusion | [Capping agent: ethylenediamine] E. coli-inhibition, Micrococcus luteus-no inhibition, [Capping agent: citric acid monohydrate] E. coli-no inhibition, B. subtilis-inhibition |
[101] | |
| 2D structured ZnO NPs | Not available (conventional products) |
|
Disk diffusion, broth dilution; MIC | ZnO-1, ZnO-2, ZnO -3, MIC (wt%), [E. coli] 0.12, 0.18, 2.30; [S. aureus] 0.25, 0.30, 3.40; [P. aeruginosa] 1.28, 4.68, 5.70; [Candida albicans] >8; >8; >8; [Aspergillus brasiliensis] nil, nil, nil |
[58] |
| 3D structured ZnO NP network | Sol–gel method (zinc acetylacetonate hydrate) | Spherical NP aggregate network/aggregates: ~3 μm; NPs: 48.3 nm | Colony counting; photocatalytic incubation | [E. coli] {Dual UV for 30 s} no particles-~approx. 2.8–3.0 log (CFU/mL), 0.05 mg/mL-no colonies detected | [18] |
| ZnO NP mixtures | Green method using Butea monsoperma seed extract (zinc nitrate hexahydrate) | Mixtures/25 nm | Broth dilution; MIC | [P. aeruginosa (resistant to amikacin (30 μg), cefepime (30 μg), sparfloxacin (5 μg), piperacillin (100 μg), levofloxacin (5 μg), piperacilin-tazobactum (100/10 μg), imipenem (10 μg), tobramycin (10 μg), nitrofurantoin (300 μg), and ceftazidime (30 μg))] MIC, 1600 μg/mL; 1600–3200 μg/mL for isolates from patients |
[102] |
| ZnO MPs | Flame transport synthesis (zinc microparticles) | Tetrapods/~30 μm | Plaque assay | [Herpes simplex virus type-2 (HSV-2)] ZnO tetrapod NP and HSV-2 cocktail for live virus vaccine | [95,103] |
| Green method using starch (zinc acetate) | Self-assembled hollow microdonuts/1–2 μm | Disk diffusion | [Enterobacter aerogenes and Staphylococcus epidermidis] 0.5–5 mM | [104] | |
| Combinations of ZnO materials with drugs | |||||
| ZnO NPs with azithromycin, gentamicin, oxacillin, cefotaxime, cefuroxime, fosfomycin, chloramphenicol, and oxytetracycline | Green method using Ulva fasciata alga extract (zinc acetate dehydrate) |
Flakes/length, ~200 nm | Disk diffusion; MIC |
|
[20] |
| ZnO with cephalexin nanohybrids | Ion exchange via sol–gel method (ZnO, and 2,4-chlorophenoxyacetic acid) | Squeezed ZnO crystals/not available | Disk diffusion; MIC | ZnO with cephalexin nanohybrids: MIC, [Aeromonas spp.] 1 mg/mL | [21] |
| ZnO NPs with β-lactam antibiotics (ciprofloxacin and imipenem) | Not available (conventional product) | Not available | Disk diffusion; agar dilution; MIC | MIC
|
[79] |
| ZnO NPs with ceftriaxone (CFX-ZnO NPs), ceftazidime (CFZ-ZnO NPs), and gentamicin (GTM-ZnO NPs) | Green method using Enterococcus faecalis culture supernatant (zinc sulfate) | Spheres/15.3–37 nm | Broth dilution; MIC |
|
[105] |
| ZnO NPs with ampicillin/sulbactam | Milling method (zinc chloride) | Not available/25 nm | Disk diffusion; broth dilution; MIC | MIC,
|
[106] |
| Combinations of ZnO materials with other metal oxide NPs / MPs, or metal doping | |||||
| ZnO NPs with TiO2 NPs in 4A zeolite | Ion exchange process (zinc acetate dehydrate) |
Cubes/400–600 nm (NPs: spheres, ~50 nm) | Disk diffusion; MIC | MIC, [E. coli O157:H7] 1 mg/mL, [S. aureus] 2 mg/mL, [Pseudomonas fluorescens] 1 mg/mL, [Listeria Monocytogenes] 2 mg/mL |
[80] |
| Ag-ZnO·mSiO2 composites | Precipitation of sodium water glass in zinc acetate solution (zinc acetate dihydrate) | Lamellar porous nanostructure with silver spots/not available (specific surface area, 250 m2/g) | Microdilution; MIC | MIC, [E. coli] 2.9 mg/cm3, [P. aeruginosa] 3.9 mg/cm3, [Streptococcus salivarius] 5.9 mg/cm3, [S. aureus] 5.9 mg/cm3, [C. albicans] 23.5 mg/cm3—synergistic | [81] |
| ZnO-SiO2 composites | Solid state mixing (zinc acetate dihydrate) |
|
Broth microdilution; MIC | MIC, [S. aureus] 2 mg/mL (except for ZnO/S-S-20, C-A-10, or C-A-20, >2) as 0.228–0.632 mg/mL of ZnO NPs, [C. albicans] 1 or 2 mg/mL (except for ZnO/S-A-10 or S-S-10 >2) as 0.187–0.632 mg/mL of ZnO NPs | [115] |
| CuZnO NPs on mesoporous silica SBA-3 | Co-condensation method (zinc nitrate) |
|
Colony counting; MIC | MIC, [E. coli] 25 mg/mL (CuZnO, 0.558 mg/mL), [S. aureus] 6.25 mg/mL (CuZnO, 0.139 mg/mL) | [117] |
| ZnO MPs with TiO2 MPs | Sol–gel method (zinc chloride) |
|
Broth dilution; MIC; photocatalytic incubation under visible light irradiation | ZnO MPs (0.25%) with TiO2 MPs (1%): [E. coli] 76.8% growth inhibition, [Streptococcus pyogenes] 70.2% growth inhibition, [K. pneumoniae] 80.8% growth inhibition | [118] |
| Combinations of ZnO materials with other biomaterials | |||||
| Chitosan | |||||
| Chitosan-ZnO NP-loaded gallic acid (C-ZnO@gal) films |
Hydrothermal method (zinc sulfate) |
|
Agar well diffusion | 0.5 mg/mL, [E. coli] 28 mm zone of inhibition, [B. subtilis] 25 mm zone of inhibition |
[28] |
| ZnO NP-containing chitosan coating | Not available (conventional product: 2% w/v solution, 10–30 nm) |
|
Broth dilution; MIC | [E. coli O157:H7] ▪ Chitosan (2.5%, w/v): 2.5 log (CFU/g) reduction at 4 °C ▪ ZnO NP (1%, w/v)-containing chitosan (2.5%, w/v): 2.8 log (CFU/g) reduction at 4 °C |
[82] |
| ZnO NP-containing chitosan/gelatin hybrid nanocomposite (nZnO-chitosan/gelatin) films | Green method using Cassia fistula fruit extract (zinc nitrate hexahydrate) |
|
Disk diffusion | [E. coli] ZnO NPs in films: 1%-10.5 mm zone of inhibition, 2%—10.5 mm zone of inhibition, 4%—10.7 mm zone of inhibition [S. aureus] not prominent compared to E. coli |
[91] |
| 3D porous ZnO NP-chitosan/silk/sericin scaffolds for wound dressing | Not available (conventional product: solution, ~35 nm) | Porous microstructures/not available (porosity ~86%; pore size 4–200 μm) |
Disk diffusion | 1.5 × 1.5 cm2, 2% (w/v) chitosan, 100 μL and 250 μL of ZnO NPs (40 wt% dispersion), [E. coli] 2–4.5 mm zone of growth inhibition, [S. aureus] 2.5–5.5 mm zone of growth inhibition |
[136] |
| Chitosan-based ZnO nanocomposites | Co-precipitation (zinc acetate dihydrate) |
|
Disc diffusion | Zone of inhibition (mm), [K. pneumoniae] 13 mm (the highest) > [E. coli] > [P. aeruginosa] > [B. subtillis], [S. aureus], and [MRSA] 6 mm (the lowest) |
[137] |
| Hydroxyapatite & alginate | |||||
| Hydroxyapatite-biphasic ZnO NP/MP-embedded alginate beads | Precipitation (zinc nitrate) |
|
Agar diffusion; colony counting; MIC | 0.1 mg/mL, [E. coli] 56% inhibition, [P. aeruginosa] 65% inhibition, [S. aureus and S. epidermidis] 100% inhibition |
[83] |
| Polyethylene glycol | |||||
| Tungsten-doped polyethylene glycol-capped ZnO (W-PEG-ZnO) NPs | Electrochemical method (Zn electrodes) |
Near-spheres/~40.46 nm | Agar well diffusion | ▪ Ampicillin: 100 μg/μL [E. coli] 35 mm zone of inhibition, [B. cereus] 20 mm zone of inhibition ▪ W-PEG-ZnO NPs: [E. coli] 400–600 μg/μL, 5–6 mm zone of inhibition, [B. cereus] 300–600 μg/μL, 6–8 mm zone of inhibition |
[120] |
| K-carrageenan, bacterial cellulose, propolis extract& curcumin | |||||
| Biopolymer K-carrageenan wrapped ZnO (KC-ZnO) NPs | Precipitation (zinc acetate dehydrate) |
KC-ZnO NPs: ovals/97 nm | Disk diffusion; MIC | [MRSA] MIC, 7.50 μg/mL | [27] |
| Bacterial cellulose-ZnO NP-propolis extract (BC-ZnO-propolis) films | Ultrasound (zinc acetate) |
|
Disk diffusion; MIC; photocatalytic incubation (254 nm, 30 min) | MIC, [E. coli] >1.89 mg/mL, [B. subtilis] 0.44 or <0.44 mg/mL, [C. albicans] >0.8, 1.3, 1.89, >1.89 mg/mL |
[121] |
| Curcumin-loaded ZnO (C-ZnO) NPs | Sol–gel method (zinc nitrate hexahydrate) |
|
Well diffusion; colony counting | Zone of inhibition (mm), [E. coli] C-sZnO, 8.4; C-rZnO, 10.1; C-jZnO, 11.1; C-spZnO, 8.1; C-lpZnO, 9.8; C, 7.4 [S. epidermis] C-sZnO, 19.1; C-rZnO, 17.2; C-jZnO, 19.4; C-spZnO, 16.4; C-lpZnO, 20.1; C, 8.2 [S. aureus] C-sZnO, 15.4; C-rZnO, 16.6; C-jZnO, 13.4; C-spZnO, 15.2; C-lpZnO, 17.0; C, 8.1 [B. cereus] C-sZnO, 17.4; C-rZnO, 18.7; C-jZnO, 18.7; C-spZnO, 14.2; C-lpZnO, 14.2; C, 8.4 |
[124] |
| Graphene, graphene oxide, & reduced graphene oxide | |||||
| Graphene/ZnO nanocomposite films | Ion exchange process (zinc acetate) |
|
Microdilution; MIC | [Streptococcus mutans] MIC, 125 μg/mL | [84] |
| Graphene/ZnO nanocomposite with curcumin (C-ZnO) | Ion exchange process (zinc acetate dihydrate) |
|
Agar diffusion; colony counting; microdilution; MIC | MIC,
|
[85] |
| Graphene oxide (GO)/ZnO nanocomposite for wound care | Co-precipitation (zinc nitrate) |
▪ GO: smooth and wrinkled surface layers/not available▪ GO/ZnO nanocomposite: well incorporated and distributed ZnO NPs (0.1–0.4 M) on GO sheets forming agglomerates/not available | Disk diffusion, colony counting; dark (D) and visible light-irradiated (L) conditions | Zone of growth inhibition (mm), ▪ ZnO NPs (0.4 M) on GO sheets, 100 μg/mL [E. coli] GO-D, 11 mm; L, 11.5 mm; GO/ZnO (0.4 M)-D, 11 mm; L, 13 mm [P. aeruginosa] GO-D, 10 mm; L, 10.5 mm; GO/ZnO (0.4 M)-D, 10 mm; L, 13 mm [S. typhi] GO-D, 10.5 mm; L, 9 mm; GO/ZnO (0.4 M)-D, 11 mm; L, 11.5 mm [S. flexneri] GO-D, 8 mm; L, 10.6 mm; GO/ZnO (0.4 M)-D, 12 mm; L, 12.5 mm |
[86] |
| GO/ZnO composites | Ion exchange process (zinc acetate dihydrate) |
|
Agar disk diffusion; MIC | [E. coli] MIC, 2 μg | [87] |
| Reduced graphene oxide (rGO)/ZnO films | Sol–gel synthesis (zinc acetate dihydrate) |
|
Serial dilution; colony counting; MIC; photocatalytic incubation (UV at 365 nm) | [S. aureus] 1 wt% rGO >99% (>2-log) reduction |
[88] |
| Cotton fabric | |||||
| ZnO MPs-loaded chitosan-coated cotton fabrics | Precipitation (zinc chloride) |
Uniformly distributed dense microstructure of rods/not available | Disk diffusion | [E. coli] 2.5 cm zone of growth inhibition (ZnCl2 4%, chitosan 1–2%/1 g cotton fabric) | [126] |
| Cotton-ZnO NP composites (C-nZnO) | Precipitation (zinc chloride) |
|
Disk diffusion; colony counting | nZnO amounts in C-nZnO: 2.2, 1.7, 4.9, 4.3, 11.1, 7.8, 22.2, and 16.7 wt% 9 mm in diameter, [E. coli] 97–100% growth reduction, [S. aureus] 96–98% growth reduction |
[127] |
| ZnO NP-coated fabric | Green method using starch (zinc nitrate) |
|
Colony counting | 4.8 cm in diameter of fabric, [E. coli] 80% reduction [S. aureus] 99.99% reduction |
[129] |
| ZnO quantum dots (QDs) | |||||
| Different nanostructure-based ZnO QDs (ZnO QD-1–ZnO QD-14) | Sol–gel method (zinc acetate dihydrate) |
Nanorods, nanotubes, nanospheres, nanowhiskers, nanoflowers/not available | Agar well diffusion; agar dilution; MIC | MIC, [E. coli] ZnO QD-1: 25 mg/mL, [Enterobacter aerogenes] ZnO QD-4 and ZnO QD-6: 25 mg/mL, [K. pneumonia] ZnO QD-3 and ZnO QD-5: 12.5 mg/mL, [P. aeruginosa] ZnO QD-3 and ZnO QD-7: 12.5 mg/mL, [Bacillus anthracis] ZnO QD-2, ZnO QD-3 and ZnO QD-8: 6.25 mg/mL, [S. aureus] ZnO QD-8: 6.25 mg/mL, [L. monocytogenes] ZnO QD-6 and ZnO QD-7: 50 mg/mL, [E. faecalis] ZnO QD-2 and ZnO QD-7: 25 mg/mL, [B. cereus] ZnO QD-3 and ZnO QD-5: 12.5 mg/mL, [S. epidermidis] ZnO QD-8: 1.5 mg/mL |
[89] |
| ZnO QDs | Green method using Eclipta alba leaf extract (zinc acetate dihydrate) |
Spheres/~6 nm | Agar diffusion | [E. coli] 15.69 mm zone of inhibition (1.6-fold increase compared to bulk zinc acetate at 5 mM) | [90] |
| Antimicrobial peptide-based ZnO QDs containing vancomycin and methicillin (Van@ZnO-BSA-PEP-MPA; Met@ZnO-BSA-PEP-MPA) | Precipitation (zinc acetate) |
ZnO@BSA-PEP-MPA: spheres/104 nm | Broth dilution; MIC; in vivo diagnostics-4 × 108 CFU S. aureus for infection and Van@ZnO-BSA-PEP-MPA at 5.0 mg/kg for theranostics | MIC,
|
[141] |
| Polyvinylpyrrolidone-capped ZnO (PVP-ZnO) QDs | Precipitation (zinc acetate hydrate) |
|
Agar diffusion; colony counting |
|
[142] |
3.2.1. ZnO Materials
The antimicrobial activity of various-sized or -shaped ZnO NPs/MPs has been investigated against pathogenic bacteria as well as viruses [3,69,95]. ZnO NPs/MPs show various forms as spheres, cubes, pyramids, rods, tubes, donuts, platelets, mixtures, and 3D architectures of clusters or networks, ranging from 3.4 nm to 30 μm in size. Size-dependent antimicrobial activity of spherical ZnO NPs of 12–307 nm was described against S. aureus, Proteus vulgaris, Salmonella typhimurium, Shigella flexneri, and Bacillus cereus [16]. Smaller NPs (12–30 nm) showed a superior antimicrobial growth inhibition (~94.05%) than larger NPs at the same concentration (6 mM). In addition, three types of ZnO NPs shaped like hexagons, spheres, and cubes or rods showed antimicrobial activity against E. coli, S. aureus, and B. subtilis [17]. The minimum inhibitory concentration (MIC) levels of ZnO NPs at ~63 nm, ~65 nm and 60–180 nm, and 40–45 nm were determined as 1562 μg/mL against E. coil, 391–781 μg/mL against S. aureus, and 195–391 μg/mL against B. subtilis, respectively. ZnO nanopyramids at ~15.4 nm in a segment showed an MIC of 333 μg/mL against MRSA [96]. DeLucas-Gil et al. [19] illustrated that nanostructured ZnO of spherical NP clusters at 590 nm in diameter, had superior antimicrobial activity against E. coli and S. aureus at >3.5 log (control/sample) value than the ZnO NPs at 63 nm in diameter.
ZnO nanorods, at <100 nm or ranging from 500 nm to 1 μm, also showed an antimicrobial activity against pathogenic microorganisms [97,98]. Reddy et al. [99] reported antimicrobial activity of ZnO nanorods at 88.7 nm against K. pneumoniae, for which the MIC was 40 μg/mL. These NPs were synthesized by the precipitation method using 2-mercaptoethanol as a capping agent. Tahizdeh et al. [100] reported that ZnO nanorods at an MIC of 250 μg/mL had antimicrobial activity against E. coli, P. aeruginosa, S. aureus, and Enterococcus faecalis, excluding E. faecalis which was resistant to ZnO nanorods. These nanorods were small crystals 3.4 nm in size and the NPs were 150 nm in length and 21 nm in width (aspect ratio, 7.14). Nanometric ZnO were synthesized using ethylenediamine and citric acid monohydrate as capping agents; the antimicrobial activity of the resulting nanorod-shaped particles (500 nm to 1.0 μm) was affected by the synthesis technique [101]. ZnO nanorods synthesized with ethylenediamine showed antimicrobial activity against E. coli but not against Micrococcus luteus. Conversely, ZnO nanorods synthesized using citric acid monohydrate showed growth inhibition for M. luteus but not for E. coli.
Elkady et al. [54] reported antimicrobial activity against E. coli, S. aureus, P. aeruginosa, and B. subtilis using as-synthesized ZnO nanopowders of hexagonal hollow tube shapes ~500 nm in length with 17.8 m2/g surface area and 278.6 nm average pore size. Their MIC values were found to be 0.0585 mg/mL for E. coli (zone of inhibition, 13 mm), 0.234 mg/mL for S. aureus (zone of inhibition, 14 mm), 0.234 mg/mL for P. aeruginosa (zone of inhibition, 18 mm), and 0.938 mg/mL for B. subtilis (zone of inhibition, 15 mm) using disk diffusion. Hollow nanotubes of ZnOs were also reported by López de Dicastillo et al. [55]. They were coated on acrylic polymer/extruded 32 μm-polyethylene (PE) substrate at 1% (wt) and were ~5 μm in length, 59.5 nm in thickness, and 178.2 nm in internal diameter. The hollow nanotubes reduced microbial growth against E. coli by 4.67 log (cells/cm2) and S. aureus by 2.46 log (cells/cm2).
Pasquet et al. [58] reported antimicrobial activity by three-different ZnO NPs (ZnO-1, ZnO-2, and ZnO-3) based on differences in the physicochemical characteristics including NP size and morphology of platelets and rods against E. coli, S. aureus, P. aeruginosa, Candida albicans, and Aspergillus brasiliensis. Platelet-like ZnO NPs (ZnO-1 and ZnO-2) and rod-like ZnO NPs (ZnO-3) formed crystal sizes of 14.7 nm, 17.5 nm, and 76.2 nm, with corresponding surface areas and mesopore sizes of 39.0 m2/g and 17.3 nm, 46.9 m2/g and 11.6 nm, and 8.25 m2/g and 10.6 nm, respectively. The MIC levels of ZnO-1, ZnO-2, and ZnO-3 were 0.12%, 0.18%, and 2.30% (w/w) for E. coli; 0.25%, 0.30%, and 3.40% (w/w) for S. aureus; 1.28%, 4.68%, and 5.70% (w/w) for P. aeruginosa; and >8%, >8%, and >8% (w/w) for C. albicans, respectively. No growth inhibition was detected against A. brasiliensis. Lower MIC levels against microorganisms were detected when using smaller ZnO NPs (ZnO-1 < ZnO-2 < ZnO-3).
Three-dimensional structured ZnO NPs also displayed antimicrobial activity against pathogenic microorganisms [14,18]. Jin et al. [18] presented the antimicrobial activity of self-assembled networks of spherical ZnO NPs at ~3 μm against E. coli under dual UV irradiation for 30 s. No colonies were detected at 0.05 mg/mL of self-assembled ZnO networks, whereas colonies at 2.8–3.0 log (CFU/mL) were grown after no particle treatment.
ZnO NP mixtures at 25 nm (1600–3200 μg/mL) particularly had an antimicrobial activity against P. aeruginosa isolates from patients which were resistant to amikacin (30 μg), cefepime (30 μg), sparfloxacin (5 μg), piperacillin (100 μg), levofloxacin (5 μg), piperacilin-tazobactum (100/10 μg), imipenem (10 μg), tobramycin (10 μg), nitrofurantoin (300 μg), and ceftazidime (30 μg) [102].
In ZnO MPs, tetrapod-type ZnO NPs which were ~30 μm in size had antimicrobial activity against Herpes simplex virus type-2 (HSV-2) as ZnO tetrapod NPs and HSV-2 cocktail for live virus vaccine [95,103]. ZnO microdonuts were reported by Jeyabharathi et al. [104]. They were nano-to microscale sized donuts at 1–2 μm, and showed growth inhibition against Enterobacter aerogenes and Staphylococcus epidermidis at 0.5–5 mM.
3.2.2. ZnO Materials with Drugs
Combinations of ZnO NPs/MPs with drugs including antibiotic agents and anti-inflammatory agents have been studied to enhance antimicrobial activity against pathogenic microorganisms [20,21,79,105,106]. Pharmaceutical formulations of ZnO NPs/MPs with antibiotic drugs, anti-inflammatory drugs, or natural products were conventionally used as endodontic dressing (clindamycin and triamcinolone) [107]; dental cements for temporary implants (eugenol) [108,109]; and medical devices or health care products for skin irritation, minor burns, or wounds (aloe vera) [110,111]. In recent approaches to ZnO NP/MP combinations with drugs, various antibiotic drugs were used including azithromycin, gentamicin, oxacillin, cefotaxime, cefuroxime, fosfomycin, chloramphenicol, oxytetracycline [20], cephalexin [21], ciprofloxacin, imipenem [79], ceftriaxone, ceftazidime, gentamicin [105], and ampicillin/sulbactam [106].
The antimicrobial activity of flake ZnO NPs (~200 nm in length) with azithromycin, gentamicin, oxacillin, cefotaxime, cefuroxime, fosfomycin, chloramphenicol, and oxytetracycline, was demonstrated against E. coli, S. aureus, Salmonella enterica subsp. Bukuru, and C. albicans [20]. The combination effects of ZnO NPs with these antibiotics were categorized by synergistic and antagonistic responses compared to ZnO NPs alone at MIC levels of 1.25 mg against E. coli, S. aureus, and S. enterica subsp. Bukuru, excluding C. albicans, which had no growth inhibition. Synergistic antimicrobial responses were presented in ZnO NPs (10 mg) with specific antibiotics against microorganisms: azithromycin, oxacillin, cefotaxime, cefuroxime, fosfomycin, and oxytetracycline against E. coli; azithromycin, cefotaxime, cefuroxime, fosfomycin, chloramphenicol, and oxytetracycline against S. aureus; and oxacillin, cefuroxime, and fosfomycin against Sallmonella species. However, ZnO NPs (10 mg) with azithromycin, gentamicin, cefotaxime, neomycin, ampicillin/sulbactam, chloramphenicol, and oxytetracycline had antagonistic antimicrobial responses against Sallmonella species. Meanwhile, using ZnO nanohybrids of squeezed ZnO crystals with cephalexin, an MIC level was reported at 1 mg/mL even against Aeromonas species [21].
Farzana et al. [79] reported MIC levels of ZnO NPs with ciprofloxacin against E. coli and K. pneumonia at 0.2–1 mg/mL compared to 0.08 and 0.05 mg/mL for ZnO NPs alone, respectively. However, ZnO NPs with imipenem had no growth inhibition with decreasing antimicrobial effect except for one K. pneumonia strain.
Spherical ZnO NPs (15.3–37 nm) with ceftriaxone, ceftazidime, and gentamicin showed growth inhibition against E. coli, K. pneumonia, S. aureus, E. faecalis, P. aeruginosa, and Shigella flexneri [105]. Compared to ZnO NPs (MIC, 4–16 μg/mL; except P. aeruginosa, >64 μg/mL) and antibiotics (MIC, 9–13 μg/mL), after 24 h incubation, ZnO NPs with ceftriaxone, ceftazidime, and gentamicin showed 89–90%, 96–99%, and 95–98% growth inhibition levels against E. coli, K. pneumonia, and S. aureus, respectively. Growth inhibition levels of 95%, 3.8%, and 96% were also observed against E. faecalis, P. aeruginosa, and S. flexneri, respectively, for ZnO NPs with ceftriaxone, ceftazidime, and gentamicin.
Sharma et al. [106] described antimicrobial activity of ZnO NPs (25 nm) with ampicillin/sulbactam against E. coli, K. pneumoniae, P. aeruginosa, S. typhi, and S. aureus. MIC levels of ampicillin/sulbactam were 50 μg/mL against E. coli, S. typhi, and S. aureus; and 100 μg/mL against K. pneumoniae and P. aeruginosa, whereas MIC levels of ZnO NPs alone were 25–200 μg. Although the MIC level of ZnO NPs was 25 μg and that of ampicillin/sulbactam was 100 μg/mL against K. pneumoniae, the MIC level of the ZnO NP-ampicillin/sulbactam conjugated form against K. pneumoniae was 6.25 μg/mL, suggesting antimicrobial activity enhancement.
3.2.3. ZnO Materials with Other Metal Oxide NPs/MPs or Metal Doping
ZnO NPs/MPs have also been studied as combinatorial composites or surface coating agents for enhanced antimicrobial activity against pathogenic microorganisms. Titanium dioxide (TiO2) NPs/MPs, 4A zeolite (Na12[(AlO2)12(SiO2)12]·27H2O), and silica (SiO2) were included in combinations of ZnO NPs/MPs [112,113,114]. In addition, doping of antibiotic metals (e.g., Ag and Cu) to ZnO NPs/MPs has been reported for enhanced antimicrobial performance [81,105,115,116,117].
Azizi-Lalabadi et al. [80] described growth inhibition of ZnO NPs with TiO2 NPs in 4A zeolite against E. coli O157:H7, S. aureus, Pseudomonas fluorescens, and Listeria monocytogenes at MIC levels of 1, 2, 1, and 2 mg/mL, respectively. The ZnO NPs/TiO2 NPs/4A zeolites in combinations were cube-shaped at 400–600 nm, in which spherical ZnO NPs with ~50 nm were included. In addition, Ag-ZnO·mSiO2 composites reported by Bednář et al. [81], were lamellar porous nanostructures with Ag spots having 250 m2/g of specific surface area, and had antimicrobial activity against E. coli, P. aeruginosa, Streptococcus salivarius, S. aureus, and C. albicans at MIC levels of 2.9 mg/cm3, 3.9, 5.9, 5.9, and 23.5 mg/cm3, respectively. The Ag-ZnO·mSiO2 composites also showed synergistic antimicrobial activity based on Ag and ZnO interaction, compared to ZnO·mSiO2 alone. However, ZnO-SiO2 composites displayed by Donnadio et al. [115] had antimicrobial activity against S. aureus and C. albicans at MIC levels of 2 mg/mL (except for ZnO/S-S-20, C-A-10, or C-A-20, >2) as 0.228–0.632 mg/mL of ZnO NPs and 1 or 2 mg/mL (except for ZnO/S-A-10 or S-S-10 >2) as 0.187–0.632 mg/mL of ZnO NPs, respectively. These ZnO-SiO2 composites were structured ZnO nanorods of 30–50 nm on the silica surface with specific surface areas of 20–70 m2/g.
In 3D porous architectures, CuZnO NPs on mesoporous silica SBA-3 had highly ordered mesoporous structures of near-spherical NPs ~2 μm in size, 3.6274 nm in pore size, and 829 m2/g in specific surface area with a relatively rough surface [117]. They showed growth inhibition against E. coli and S. aureus at MIC levels of 25 mg/mL (0.558 mg/mL as CuZnO NPs) and 6.25 mg/mL (0.139 mg/mL as CuZnO NPs), respectively.
Moreover, ZnO MPs with TiO2 MPs showed antimicrobial activity against E. coli, Streptococcus pyogenes, and K. pneumoniae under UV and visible light irradiation [118]. Using ZnO MPs at 0.25% and TiO2 MPs at 1%, growth inhibition levels were 76.8% against E. coli, 70.2% against S. pyrogenes, and 80.8% against K. pneumoniae. ZnO MPs synergistically enhanced antimicrobial activity against pathogenic microorganisms compared to UV and visible light-irradiated TiO2 MPs alone. The ZnO MPs in combinations had near-spherical shape, with sizes of 3.17–10.3 μm compared to 2.15–37.1 μm of the TiO2 MPs.
3.2.4. ZnO Materials with Other Biomaterials
Antimicrobial polymeric biomaterials or films are used as additive composites in ZnO NP/MP combinations for enhanced antimicrobial activity against pathogenic microorganisms. Recently studied antibiotic biomaterials or films [26,28] in ZnO NPs/MPs combinations included chitosan [28,82,84], silk sericin [84], gelatin [91], gallic acid [28], hydroxyapatite [83,119], alginate [83], polyethyleneglycol (PEG) [120], biopolymer K [27], carrageenan [27], bacterial cellulose [121,122], propolis extract [121], curcumin [84,123,124], graphene [84,85], graphene oxide (GO) [86,87,125], reduced graphene oxide (rGO), and cotton [88,126,127,128,129]. In general, they enhanced the antimicrobial activity of ZnO NPs/MPs and overcame formulation challenges of ZnO NPs/MPs in manufacturing beads or films and covering surfaces for coating.
Among natural products, chitosan has been extensively studied as a pharmaceutical excipient for drug delivery carriers in biomedical applications owing to their biocompatibility and physicochemical characteristics [113,114]. It is beneficial to have inherent therapeutic functions including antimicrobial activity and anti-inflammatory activity for accelerated wound healing, hemostasis, and reduced fat absorption to decrease plasma and liver lipids and increase fecal excretion [114,130]. Regarding the biomedical functions of chitosan, the antimicrobial mechanisms are explained by cell binding via charge–charge interaction owing to the polycationic nature of chitosan, leading to disruption of the membrane and inhibition of nucleic acid processes, thereby causing cell death. As a chelating agent, chitosan also interacts with trace metal elements, which are toxic to cells, inducing microbial growth inhibition. It is applied for wound dressing/healing, cotton fabric, daily food packaging, and paper packaging as an antimicrobial agent, mostly in films [113,131,132,133,134]. Therefore, chitosan can be extended to the combinations with ZnO NPs/MPs [135].
Chitosan-ZnO NP-loaded gallic acid (C-ZnO@gal) films showed antimicrobial activity against E. coli and B. subtilis [28]. ZnO@gal NPs were near-rod-shape at ~19.2 nm, and C-ZnO@gal films included homogeneously distributed ZnO@gal NPs on chitosan matrixes (2 g chitosan/70 mg ZnO@gal) at a thickness of 0.10 mm. Using C-ZnO@gal films at 0.5 mg/mL, growth inhibition zone levels were reported as 28 mm against E. coli and 25 mm against B. subtilis. Al-Nabulsi et al. [82] described the antimicrobial activity of ZnO NP-containing chitosan against E. coli O157:H7, which was used as a biocompatible coating agent for smart storage. The ZnO NPs had a near-spherical shape at ~65 nm and were uniformly distributed in chitosan matrixes. The growth reduction levels against E. coli O157:H7 at 4 °C were 2.5 log (CFU/g) for chitosan (2.5%, w/v) and 2.8 log (CFU/g) for ZnO NP (1%, w/v)-containing chitosan (2.5%, w/v). Although combinations of ZnO NPs in a chitosan matrix did not show a significant improvement of antimicrobial activity against E. coli O157:H7 (p > 0.05) compared to ZnO NPs or chitosan alone, the ZnO NP combination in the chitosan matrix presented better antimicrobial results.
Kumar et al. [91] reported growth inhibition levels of chitosan/gelatin hybrid nanocomposite films containing ZnO NPs against E. coli and S. aureus along with their improved structural integrity. In these films, ZnO NPs had various shapes such as polyhedrons, quasi-spheres, or rods of 20–40 nm, 500–1000 nm, and 200–400 nm, respectively, which were evenly distributed on smooth, compact, and heterogeneous surfaces with 86–92 μm in thickness. Depending on the ZnO NP contents in the films, their antimicrobial activity against E. coli presented a 10.5 mm zone of growth inhibition for ZnO NPs at 1% and 2% in the films and a 10.7 mm zone of growth inhibition for ZnO NPs at 4% in the films. The films showed comparable growth inhibition results based on inherent antimicrobial activity of chitosan. Unlike the effects against E. coli, no prominent results were obtained for S. aureus.
Three-dimensional porous ZnO NP scaffolds with chitosan/silk/sericin for wound dressing/healing were described as antimicrobial agents against E. coli and S. aureus [136]. Their porous microstructures (1.5 × 1.5 cm2) comprising 2% (w/v) chitosan, and 100 μL or 250 μL of ZnO NPs (40% dispersion, wt), showed ~86% porosity with 4–200 μm in pore size. Based on the zone of growth inhibition via the disk diffusion method for evaluation, these particles displayed 2–4.5 mm growth inhibition against E. coli and 2.5–5.5 mm growth inhibition against S. aureus.
In particular, Javed et al. [137] reported a biodental materials for orthodontics, composed of star-shaped, chitosan-based ZnO NPs (20–25 nm), which showed enhanced antimicrobial activity against K. pneumoniae (13 mm zone of inhibition, the highest); E. coli, P. aeruginosa; and B. subtillis, S. aureus, and MRSA (6 mm zone of inhibition, the lowest), compared to those composed of ZnO NPs or chitosan alone. In the synthesis of chitosan-based ZnO NPs via co-precipitation, zinc acetate dihydrate (0.06 M) and chitosan were used as a precursor and a capping agent, respectively. Conventionally used adhesive was coated using chitosan-based ZnO NPs at 2–10%. The pure ZnO NPs had a spherical shape with a diameter of 25–30 nm.
Meanwhile, hydroxyapatite-biphasic ZnO NP/MP-embedded alginate (HA-ZnO-Alg) beads showed antimicrobial activity against E. coli, P. aeruginosa, S. aureus, and S. epidermidis [83]. In alginate beads, ZnO NPs/MPs had a snowflake shape at <1 μm, which were evenly distributed with hydroxyapatite in alginate bead matrixes. Antimicrobial growth inhibition levels of HA-ZnO-Alg beads at 0.1 mg/mL were 56% against E. coli, 65% against P. aeruginosa, and 100% against S. aureus and S. epidermidis.
Jose et al. [120] also reported growth inhibition of tungsten-doped polyethylene glycol (PEG)-capped ZnO (W-PEG-ZnO) NPs against E. coli and B. cereus, which were near-spherical at ~40.46 nm. Compared to ampicillin at 100 μg/μL inhibiting growth at 35 mm against E. coli and 20 mm against B. cereus, W-PEG-ZnO NPs showed a 5–6 mm zone of growth inhibition against E. coli at 400–600 μg/μL and a 6–8 mm zone of growth inhibition against B. cereus at 300–600 μg/μL, suggesting B. cereus was more susceptible to W-PEG-ZnO NPs than E. coli.
Antimicrobial activity of biopolymer K-carrageenan-wrapped ZnO (KC-ZnO) NPs, oval-shaped at 97 nm, was reported against MRSA [27]; the MIC level of KC-ZnO NPs was 7.5 μg/mL. ZnO NP films manufactured with bacterial cellulose and propolis extract resulted in quasi-spherical ZnO NPs (70–90 nm), homogeneously distributed on the substrate surface in bacterial cellulose-ZnO NP-propolis extract (BC-ZnO-propolis) films [121]. The BC-ZnO-propolis films had antimicrobial activity against E. coli at an MIC level of >1.89 mg/mL, B. subtilis at 0.44 or <0.44 mg/mL, and C. albicans at >0.8, 1.3, 1.89, and >1.89 mg/mL, depending on ZnO NP types according to ultrasound frequency (40 kHz and 100 kHz) during the synthesis, and propolis extract contents (3.5%, 7%, 11%, and 15%, wt).
Curcumin in turmeric is known to have beneficial anti-inflammatory activity, and is used as a multipurpose nutraceutical in various dosage forms of tablets, capsules, and ointments [123]. After loading curcumin on ZnO (C-ZnO) NPs, the antimicrobial activity of C-ZnO NPs against E. coli, S. epidermis, S. aureus, and B. cereus was determined [124]. Among the C-ZnO NPs, spheres (C-sZnO), rods (C-rZnO), javelin (C-jZnO), short petal nanoflowers (C-spZnO), and long petal nanoflowers (C-lpZnO) were detected with sizes of 40–100 nm, 600–900 nm (<300 nm of width), 300–600 nm (<300 nm of width), 2–4 μm, and ~500 nm, respectively. Their microbial growth inhibition levels were confirmed via measurements of well diffusion zones of inhibition as follows: C-sZnO, 8.4 mm; C-rZnO, 10.1 mm; C-jZnO, 11.1 mm (the highest); C-spZnO, 8.1 mm; C-lpZnO, 9.8 mm; and curcumin, 7.4 mm (the lowest) against E. coli; C-sZnO, 19.1 mm; C-rZnO, 17.2 mm; C-jZnO, 19.4 mm; C-spZnO, 16.4 mm; C-lpZnO, 20.1 mm (the highest); and curcumin, 8.2 mm (the lowest) against S. epidermis; C-sZnO, 15.4 mm; C-rZnO, 16.6 mm; C-jZnO, 13.4 mm; C-spZnO, 15.2 mm; C-lpZnO, 17.0 mm (the highest); curcumin, 8.1 mm (the lowest) against S. aureus; and C-sZnO, 17.4 mm; C-rZnO, C-jZnO, 18.7 mm (the highest); C-spZnO, 14.2 mm; C-lpZnO, 14.2 mm; and curcumin, 8.4 mm (the lowest) against B. cereus.
Graphene, graphene oxide (GO), and reduced GO (rGO) have also been studied as antibiotic agents in combinations of ZnO NPs with other biomaterials. Antimicrobial activity of graphene/ZnO nanocomposite films against Streptococcus mutans was reported by Kulshrestha et al. [84]. The ZnO NPs distributed in the graphene sheet films were near-spherical at 20–40 nm. The MIC level of graphene/ZnO nanocomposite films was 125 μg/mL. Oves et al. [85] described the antimicrobial activity of graphene/ZnO nanocomposites with curcumin against MRSA strains (ATCC 43300 and ATCC BAA-1708). In the nanocomposites, spherical ZnO NPs at 35 nm were homogenously distributed on graphene sheets. Compared to curcumin with an MIC of 125 μg/mL, the graphene/ZnO nanocomposite induced growth inhibition at 125 μg/mL against MRSA ATCC 43300 and at 250 μg/mL against MRSA ATCC BAA-1708. Use of curcumin together with the graphene/ZnO nanocomposites achieved growth inhibition against MRSA ATCC 43300 and MRSA ATCC BAA-1708 at 31.25 and 62.5 μg/mL, respectively. Against in vivo topical dermatitis infection, the growth of MRSA was inhibited by up to 64%.
GO/ZnO nanocomposites for wound care showed growth inhibition against E. coli, S. typhi, P. aeruginosa, and S. flexneri [86]. GO with smooth and wrinkled surface layers was used to prepare GO/ZnO nanocomposites. The ZnO NPs were well incorporated and distributed on the GO sheets generating agglomerates. Analysis of the zone of growth inhibition with GO/ZnO nanocomposites at levels of 25–100 μg/mL under dark or visible light irradiation, showed that the composites were more susceptible to E. coli, P. aeruginosa, S. typhi, and S. flexneri than with GO alone. In addition, Wang et al. [87] reported antimicrobial activity against E. coli of GO/ZnO composites, including rod-like ZnO NPs at 4 nm, homogenously attached on GO sheets. Using 1–4 μg GO/ZnO composites, growth inhibition zones were detected at 2 and 4 μg (MIC, 2 μg).
In the case of rGO, combination of ZnO NPs with rGO had antimicrobial activity against microorganisms [88]. Spherical ZnO NPs at 100–300 nm were attached to rGO sheets for manufacturing rGO/ZnO films with roughness at 159 nm, containing homogenously distributed ZnO NPs on rGO sheets. They displayed >99% (2-log) growth reduction against S. aureus at 1% (wt) rGO.
In cotton fabrics, ZnO NPs have been used as protective agents against textile degradation, unpleasant odor, and potential health risks caused by microbial growth after contact with the human body [128,130,138]. As an alternative to chemical biocides, ZnO NPs provide enhanced antimicrobial activity against pathogenic microorganisms in surface-modified textiles during the manufacturing process. Noman, M.T. and M. Petrů [127] reported eight types of cotton-ZnO NP (C-nZnO) composites and their antimicrobial actions against E. coli and S. aureus. The ZnO NPs were spherical at 27 nm, and formed thick and dense layers on cotton surfaces in C-nZnO composites at 2.2%, 1.7%, 4.9%, 4.3%, 11.1%, 7.8%, 22.2%, and 16.7% (wt). They showed microbial growth reduction of 97–100% against E. coli and 96–98% against S. aureus. Subash et al. [129] also demonstrated microbial growth reduction by ZnO NP-coated fabric against E. coli and S. aureus. The ZnO-coated fabric comprised evenly distributed spherical ZnO NPs (200 nm) on the fabric surface. In a 4.8 cm-diameter circular cut of the fabric, E. coli and S. aureus growth levels were reduced by 80% and 99.99%, respectively. In the case of ZnO MPs, they were coated on cotton fabrics together with chitosan and formed a uniformly distributed dense microstructure of rods showing antimicrobial growth inhibition against E. coli [126]. Immersion of ZnCl2 (4%) for ZnO MP synthesis on chitosan-loaded cotton fabrics (1–2%/1 g cotton fabric) resulted in a growth inhibition zone of 2.5 cm in disk diffusion against E. coli.
3.2.5. ZnO QDs
ZnO QDs have been studied for imaging and therapy as theranostic agents having photoluminescence properties with antimicrobial activity against pathogenic microorganisms [139,140]. They have superior characteristics including high quantum yield, high stability, and a narrow emission range.
Based on different nanostructures of nanorods/nanotubes (ZnO QD-1, ZnO QD-2, and ZnO QD-12), nanospheres (ZnO QD-3, ZnO QD-4, ZnO QD-6, ZnO QD-7, ZnO QD-8, ZnO QD-10, ZnO QD-11, and ZnO QD-14), nanowhiskers (ZnO QD-5), nanoflowers, and mixtures of nanorods and nanoflowers (ZnO QD-13) (ZnO QD-9: not mentioned), the growth inhibition levels of 14 ZnO QDs were evaluated against E. coli, K. pneumonia, P. aeruginosa, S. aureus, L. monocytogenes, E. faecalis, E. aerogenes, B. anthracis, B. cereus, and S. epidermidis [89]. The lowest MIC levels among the ZnO QDs from ZnO-1 to ZnO-10 were as follows: ZnO QD-1, 25 mg/mL against E. coli; ZnO QD-4 and ZnO QD-6, 25 mg/mL against E. aerogenes; ZnO QD-3, and ZnO QD-5, 12.5 mg/mL against K. pneumonia; ZnO QD-3 and ZnO QD-7, 12.5 mg/mL against P. aeruginosa; ZnO QD-2, ZnO QD-3, and ZnO QD-8, 6.25 mg/mL against B. anthracis; ZnO QD-8, 6.25 mg/mL against S. aureus; ZnO QD-6 and ZnO QD-7, 50 mg/mL against L. monocytogenes; ZnO QD-2 and ZnO QD-7, 25 mg/mL against E. faecalis; ZnO QD-3 and ZnO QD-5, 12.5 mg/mL against B. cereus; and ZnO QD-8, 1.5 mg/mL against S. epidermidis. Singh et al. [90] reported spherical ZnO QDs of ~6 nm that showed an inhibition zone of 15.69 mm against E. coli. Compared to the growth inhibition level at 5 mM of bulk zinc acetate, that of ZnO QDs against E. coli increased by 1.6-fold.
ZnO QDs are also functionalized with polymers, peptides, and antibiotic drugs [140,141,142]. Antimicrobial peptide (BSA-PEP-MPA) was attached to ZnO QDs (spheres, 104 nm) containing vancomycin (Van@ZnO-BSA-PEP-MPA) and methicillin (Met@ZnO-BSA-PEP-MPA) for enhanced antimicrobial activity against S. aureus, B. subtilis, and MRSA [141]. Analysis of MIC levels showed that ZnO@BSA-PEP-MPA had no growth inhibition potential, used as a nanoprobe promoting permeability of antibiotics through microbial membrane. In the case of Van@ZnO-BSA-PEP-MPA, 2.0 μg/mL against S. aureus and 1.0 μg/mL against B. subtilis were detected as MIC levels. Growth inhibition at 6 × 104 log (CFU/g) was induced after administration of 4 × 108 CFU of S. aureus for infection and 5.0 mg/kg of Van@ZnO-BSA-PEP-MPA as in vivo diagnostic agent with no changes in activity and body weight for theranostics. However, Met@ZnO-BSA-PEP-MPA showed 64 μg/mL of MIC against MRSA. Polyvinylprolidone-capped ZnO (PVP-ZnO) QDs also showed antimicrobial activity against E. coli O157:H7, S. enteritidis, and L. monocytogenes. Spherical ZnO QDs of ~5 nm were used to prepare PVP-ZnO QDs, which were highly crystalline spheres at 4 nm (<ZnO QDs) [142]. Compared to ZnO QDs at 1.12 mg/mL mediating growth inhibition of 63.9% against S. enteritidis and 80.6% against L. monocytogenes, PVP-ZnO QDs at 40 mg/mL showed growth inhibition of 66.7% against E. coli O157:H7 and 58.9% against L. monocytogenes.
4. Current Biomedical Applications
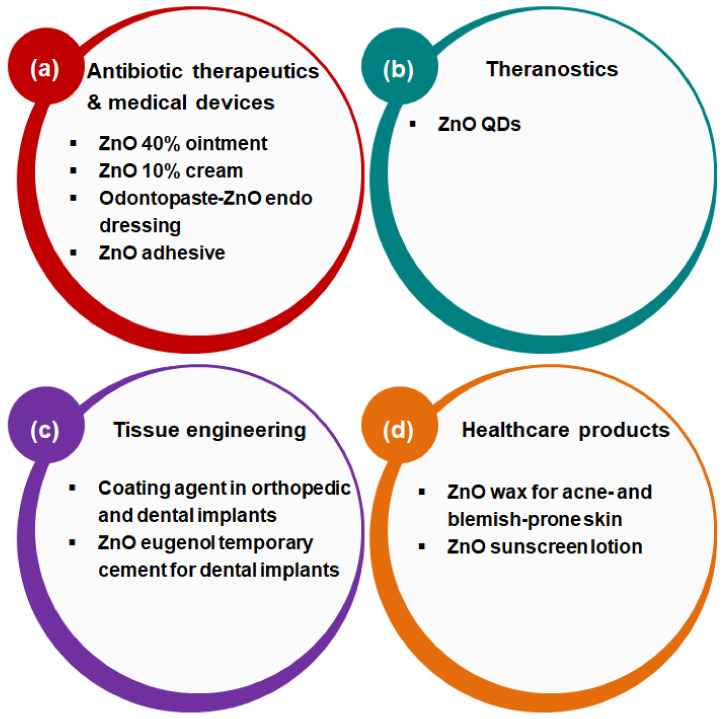
ZnO NPs/MPs are one of Zn-based materials that have been extensively used in various biomedical fields including antibiotic therapy, medical devices, theranostics, tissue engineering, and health care because of their antimicrobial functions against pathogenic microorganisms (Figure 2) [11,143,144,145,146,147,148]. In the human body, Zn plays a pivotal role in life cycle maintenance and Zn deficiency causes cell impairment and malignancy leading to severe diseases related to immune responses such as infection and cancer [69]. As a first aid antibiotic alternative for the protection of skin [143,147], ZnO ointment (40%) or cream (10%) is conventionally used. In addition, ZnO NPs/MPs and antibiotic drugs are applied as endo dressing in paste forms [107]. They are also used as ZnO surgical adhesives in medical devices [144,145]. Moreover, ZnO NPs/MPs have been utilized as postoperative dressing on leg excisions [148]. In theranostics, biocompatible ZnO QDs are explored as photodynamic therapeutics as well as diagnostic agents owing to their photoluminescence properties based on photochemical stability [11]. For dental tissue engineering, ZnO NPs/MPs are used as coating agents in orthopedic and dental implants [146]. ZnO NPs/MPs and eugenol are also extensively used as temporary cement for dental implants [108]. ZnO waxes or sunscreen lotions are used as health care products for skin protection against acne/blemish or UV and visible light [149,150]. Although ZnO NPs/MPs still have exposure risks to health via the inhalation route [151,152], ZnO is considered as “generally recognized as safe” and is approved by the U.S. Food and Drug Administration and the health risks of ZnO NPs/MPs are guided by the Organisation for Economic Co-operation and Development, based on their unique physicochemical characteristics via synthesis techniques [153]. Therefore, ZnO NPs/MPs have a promising potential for use in biomedical applications including medicine, medical devices, and cosmeceuticals connected with antibiotic functions overcoming drug resistance.
Figure 2.
Functional approaches of ZnO materials for biomedical applications: (a) antibiotic therapeutics and medical devices, (b) theranostics, (c) tissue engineering, and (d) healthcare products.
5. Conclusions
The antibiotic properties of ZnO materials have been highlighted for their biomedical applications in combating multi-drug resistance. Compared to currently available antibiotic drugs, they exhibit different mechanisms of antimicrobial actions; these mechanisms are mainly induced by the Zn2+ ion, particle adsorption, ROS generation and photocatalytic responses based on physicochemical characteristics of these materials, which vary substantially according to their synthesis techniques. ZnO NPs, sometimes MPs, show enhanced antimicrobial performance even toward pathogenic viruses. Their combinations with other antibiotic drugs, metal oxide NPs/MPs or metal doping, and other biomaterials also have a wide-spectrum antimicrobial activity that can be customized for multi-therapeutic options and used to improve their applicability as industrial and clinical translation platforms. Moreover, ZnO QDs display enhanced antimicrobial actions as theranostic agents. Therefore, ZnO materials can be a next-generation antibiotic drug against multi-drug resistant pathogenic microorganisms and, in the near future, combinations of these can be developed further with industrial and clinical impacts.
Abbreviations
| BC | Bacterial cellulose |
| BSA | Bovine serum albumin |
| CFU | Colony forming unit |
| CFX | Ceftriaxone |
| CFZ | Ceftazidime |
| C-jZnO | Curcumin-loaded ZnO javelin |
| C-lpZnO | Curcumin-loaded long petal zno nanoflower |
| C-nZnO | Cotton-ZnO nanoparticle nanocomposite |
| C-rZnO | Curcumin-loaded ZnO rod |
| C-spZnO | Curcumin-loaded short petal zno nanoflower |
| C-sZnO | Curcumin-loaded ZnO sphere |
| C-ZnO | Curcumin-loaded ZnO |
| C-ZnO@gal | Chitosan-ZnO nanoparticle-loaded gallic acid |
| D | Dark |
| GO | Graphene oxide |
| GTM | Gentamicin |
| HA-ZnO-Alg | Hydroxyapatite-biphasic ZnO nanoparticle/microparticle-embedded alginate |
| HSV-2 | Herpes simplex virus type-2 |
| KC | K-carrageenan |
| L | Light |
| Met | Methicillin |
| MIC | Minimum inhibitory concentration |
| MPs | Microparticles |
| MRSA | Methicillin-resistant staphylococcus aureus |
| nZnO-chitosan/gelatin | ZnO NP-containing chitosan/gelatin hybrid nanocomposite |
| PE | Polyethylene |
| PVP | Polyvinylpyrrolidone |
| QDs | Quantum dots |
| rGO | Reduced graphene oxide |
| UV | Ultraviolet |
| Van | Vancomycin |
| W-PEG-ZnO | Tungsten-doped polyethylene glycol-capped ZnO |
Author Contributions
Conceptualization, S.-E.J. and H.-E.J.; investigation, S.-E.J. and H.-E.J.; writing—original draft preparation, S.-E.J.; writing—review and editing, S.-E.J. and H.-E.J.; supervision, S.-E.J. and H.-E.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makabenta J.M.V., Nabawy A., Li C.-H., Schmidt-Malan S., Patel R., Rotello V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2020 doi: 10.1038/s41579-020-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muzammil S., Hayat S., Fakhar E.A.M., Aslam B., Siddique M.H., Nisar M.A., Saqalein M., Atif M., Sarwar A., Khurshid A., et al. Nanoantibiotics: Future nanotechnologies to combat antibiotic resistance. Front. Biosci. 2018;10:352–374. doi: 10.2741/e827. [DOI] [PubMed] [Google Scholar]
- 3.da Silva B.L., Abuçafy M.P., Berbel Manaia E., Oshiro Junior J.A., Chiari-Andréo B.G., Pietro R.C.R., Chiavacci L.A. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019;14:9395–9410. doi: 10.2147/IJN.S216204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dizaj S.M., Lotfipour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Jin S.-E., Jin H.-E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics. 2019;11:575. doi: 10.3390/pharmaceutics11110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ul Haq A.N., Nadhman A., Ullah I., Mustafa G., Yasinzai M., Khan I. Synthesis Approaches of Zinc Oxide Nanoparticles: The Dilemma of Ecotoxicity. J. Nanomater. 2017;2017:8510342. doi: 10.1155/2017/8510342. [DOI] [Google Scholar]
- 7.Sánchez-López E., Gomes D., Esteruelas G., Bonilla L., Lopez-Machado A.L., Galindo R., Cano A., Espina M., Ettcheto M., Camins A., et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials. 2020;10:292. doi: 10.3390/nano10020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Giau V., An S.S.A., Hulme J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des. Dev. Ther. 2019;13:327–343. doi: 10.2147/DDDT.S190577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajwa N., Mehra N.K., Jain K., Jain N.K. Pharmaceutical and biomedical applications of quantum dots. Artif. Cells Nanomed. Biotechnol. 2016;44:758–768. doi: 10.3109/21691401.2015.1052468. [DOI] [PubMed] [Google Scholar]
- 10.Khan S.T., Musarrat J., Al-Khedhairy A.A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: Current status. Colloids Surf. B Biointerfaces. 2016;146:70–83. doi: 10.1016/j.colsurfb.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Carmona M., Gun’ko Y., Vallet-Regí M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials. 2018;8:268. doi: 10.3390/nano8040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelgrift R.Y., Friedman A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Czyżowska A., Barbasz A. A review: Zinc oxide nanoparticles—Friends or enemies? Int. J. Environ. Health Res. 2020 doi: 10.1080/09603123.2020.1805415. [DOI] [PubMed] [Google Scholar]
- 14.Sirelkhatim A., Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., Bakhori S.K.M., Hasan H., Mohamad D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-micro Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Z., Li Q., Wang J., Yu Y., Wang Y., Zhou Q., Li P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020;15:115. doi: 10.1186/s11671-020-03344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghupathi K.R., Koodali R.T., Manna A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir. 2011;27:4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S., Kumar K., Thakur N., Chauhan S., Chauhan M.S. The effect of shape and size of ZnO nanoparticles on their antimicrobial and photocatalytic activities: A green approach. Bull. Mater. Sci. 2019;43:20. doi: 10.1007/s12034-019-1986-y. [DOI] [Google Scholar]
- 18.Jin S.E., Jin J.E., Hwang W., Hong S.W. Photocatalytic antibacterial application of zinc oxide nanoparticles and self-assembled networks under dual UV irradiation for enhanced disinfection. Int. J. Nanomed. 2019;14:1737–1751. doi: 10.2147/IJN.S192277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lucas-Gil E., Leret P., Monte-Serrano M., Reinosa J.J., Enríquez E., Del Campo A., Cañete M., Menéndez J., Fernández J.F., Rubio-Marcos F. ZnO Nanoporous Spheres with Broad-Spectrum Antimicrobial Activity by Physicochemical Interactions. ACS Appl. Nano Mater. 2018;1:3214–3225. doi: 10.1021/acsanm.8b00402. [DOI] [Google Scholar]
- 20.Abo-Shama U.H., El-Gendy H., Mousa W.S., Hamouda R.A., Yousuf W.E., Hetta H.F., Abdeen E.E. Synergistic and Antagonistic Effects of Metal Nanoparticles in Combination with Antibiotics Against Some Reference Strains of Pathogenic Microorganisms. Infect. Drug Resist. 2020;13:351–362. doi: 10.2147/IDR.S234425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Hisnawi M.S., Jabor M.A. Preparation of nanohybrid compound from the drugs (naproxen and cephalexin) with zinc oxide and studying biological activities against Aeromonas bacteria. J. Contemp. Med. Sci. 2016;1:16–19. [Google Scholar]
- 22.Bian Z., Tachikawa T., Zhang P., Fujitsuka M., Majima T. A nanocomposite superstructure of metal oxides with effective charge transfer interfaces. Nat. Commun. 2014;5:3038. doi: 10.1038/ncomms4038. [DOI] [PubMed] [Google Scholar]
- 23.Shanmugam N.R., Muthukumar S., Prasad S. A review on ZnO-based electrical biosensors for cardiac biomarker detection. Future Sci. OA. 2017;3:FSO196. doi: 10.4155/fsoa-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stankic S., Suman S., Haque F., Vidic J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016;14:73. doi: 10.1186/s12951-016-0225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannossa L., Longano D., Ditaranto N., Nitti M., Paladini F., Pollini M., Rai M., Sannino A., Valentini A., Cioffi N. Metal nanoantimicrobials for textile applications. Nanotechnol. Rev. 2013;2 doi: 10.1515/ntrev-2013-0004. [DOI] [Google Scholar]
- 26.González-Henríquez C.M., Sarabia-Vallejos M.A., Rodriguez-Hernandez J. Advances in the Fabrication of Antimicrobial Hydrogels for Biomedical Applications. Materials. 2017;10:232. doi: 10.3390/ma10030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vijayakumar S., Saravanakumar K., Malaikozhundan B., Divya M., Vaseeharan B., Durán-Lara E.F., Wang M.H. Biopolymer K-carrageenan wrapped ZnO nanoparticles as drug delivery vehicles for anti MRSA therapy. Int. J. Biol. Macromol. 2020;144:9–18. doi: 10.1016/j.ijbiomac.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Yadav S., Mehrotra G.K., Dutta P.K. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021;334:127605. doi: 10.1016/j.foodchem.2020.127605. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee D., Shivapriya P.M., Gautam P.K., Misra K., Sahoo A.K., Samanta S.K. A Review on Basic Biology of Bacterial Biofilm Infections and Their Treatments by Nanotechnology-Based Approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020;90:243–259. doi: 10.1007/s40011-018-01065-7. [DOI] [Google Scholar]
- 30.Sharma D., Misba L., Khan A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019;8:76. doi: 10.1186/s13756-019-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canta M., Cauda V. The investigation of the parameters affecting the ZnO nanoparticle cytotoxicity behaviour: A tutorial review. Biomater. Sci. 2020;8:6157–6174. doi: 10.1039/D0BM01086C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu Q., David C.A., Galceran J., Rey-Castro C., Krzemiński L., Wallace R., Bamiduro F., Milne S.J., Hondow N.S., Brydson R., et al. Systematic investigation of the physicochemical factors that contribute to the toxicity of ZnO nanoparticles. Chem. Res. Toxicol. 2014;27:558–567. doi: 10.1021/tx4004243. [DOI] [PubMed] [Google Scholar]
- 33.Pomastowski P., Król-Górniak A., Railean-Plugaru V., Buszewski B. Zinc Oxide Nanocomposites—Extracellular Synthesis, Physicochemical Characterization and Antibacterial Potential. Materials. 2020;13:4347. doi: 10.3390/ma13194347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao N., Zhang M., Zhang J.X.J. Microfluidics for ZnO micro-/nanomaterials development: Rational design, controllable synthesis, and on-chip bioapplications. Biomater. Sci. 2020;8:1783–1801. doi: 10.1039/C9BM01787A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikam A.V., Prasad B.L.V., Kulkarni A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. CrystEngComm. 2018;20:5091–5107. doi: 10.1039/C8CE00487K. [DOI] [Google Scholar]
- 36.Thakur V., Verma U.P., Rajaram P. Wet chemical synthesis of ZnO nanocrystals: Dependence of growth and morphology on the solvent composition. J. Mater. Sci. Mater. Electron. 2014;25:3242–3250. doi: 10.1007/s10854-014-2009-9. [DOI] [Google Scholar]
- 37.Bandeira M., Giovanela M., Roesch-Ely M., Devine D.M., da Silva Crespo J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020;15:100223. doi: 10.1016/j.scp.2020.100223. [DOI] [Google Scholar]
- 38.Liu Y., Jiang X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip. 2017;17:3960–3978. doi: 10.1039/C7LC00627F. [DOI] [PubMed] [Google Scholar]
- 39.Balucani M., Nenzi P., Chubenko E., Klyshko A., Bondarenko V. Electrochemical and hydrothermal deposition of ZnO on silicon: From continuous films to nanocrystals. J. Nanopart. Res. 2011;13:5985–5997. doi: 10.1007/s11051-011-0346-7. [DOI] [Google Scholar]
- 40.Kumar M., Sasikumar C. Electrodeposition of Nanostructured ZnO Thin Film: A Review. Am. J. Mater. Sci. Eng. 2014;2:18–23. doi: 10.12691/ajmse-2-2-2. [DOI] [Google Scholar]
- 41.Izzi M., Sportelli M.C., Ditaranto N., Picca R.A., Innocenti M., Sabbatini L., Cioffi N. Pros and Cons of Sacrificial Anode Electrolysis for the Preparation of Transition Metal Colloids: A Review. ChemElectroChem. 2020;7:386–394. doi: 10.1002/celc.201901837. [DOI] [Google Scholar]
- 42.Dierstein A., Natter H., Meyer F., Stephan H.O., Kropf C., Hempelmann R. Electrochemical deposition under oxidizing conditions (EDOC): A new synthesis for nanocrystalline metal oxides. Scr. Mater. 2001;44:2209–2212. doi: 10.1016/S1359-6462(01)00906-X. [DOI] [Google Scholar]
- 43.Natter H., Hempelmann R. Tailor-made nanomaterials designed by electrochemical methods. Electrochim. Acta. 2003;49:51–61. doi: 10.1016/j.electacta.2003.04.004. [DOI] [Google Scholar]
- 44.Picca R.A., Sportelli M.C., Hötger D., Manoli K., Kranz C., Mizaikoff B., Torsi L., Cioffi N. Electrosynthesis and characterization of ZnO nanoparticles as inorganic component in organic thin-film transistor active layers. Electrochim. Acta. 2015;178:45–54. doi: 10.1016/j.electacta.2015.07.122. [DOI] [Google Scholar]
- 45.Sportelli M.C., Picca R.A., Izzi M., Palazzo G., Gristina R., Innocenti M., Torsi L., Cioffi N. ZnO Nanostructures with Antibacterial Properties Prepared by a Green Electrochemical-Thermal Approach. Nanomaterials. 2020;10:473. doi: 10.3390/nano10030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jabeera B., Anirudhan T., Shibli S.M.A. Nano zinc oxide for efficient activation of aluminium zinc alloy sacrificial anode. J. New Mater. Electrochem. Syst. 2005;8:291. [Google Scholar]
- 47.Gupta M., Tomar R.S., Kaushik S., Mishra R.K., Sharma D. Effective Antimicrobial Activity of Green ZnO Nano Particles of Catharanthus roseus. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shanavas S., Duraimurugan J., Kumar G.S., Ramesh R., Acevedo R., Anbarasan P.M., Maadeswaran P. Ecofriendly green synthesis of ZnO nanostructures using Artabotrys Hexapetalu and Bambusa Vulgaris plant extract and investigation on their photocatalytic and antibacterial activity. Mater. Res. Express. 2019;6:105098. doi: 10.1088/2053-1591/ab3efe. [DOI] [Google Scholar]
- 49.Alamdari S., Sasani Ghamsari M., Lee C., Han W., Park H.-H., Tafreshi M.J., Afarideh H., Ara M.H.M. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus ebulus. Appl. Sci. 2020;10:3620. doi: 10.3390/app10103620. [DOI] [Google Scholar]
- 50.Bala N., Saha S., Chakraborty M., Maiti M., Das S., Basu R., Nandy P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015;5:4993–5003. doi: 10.1039/C4RA12784F. [DOI] [Google Scholar]
- 51.Borade P., Joshi K.U., Gokarna A., Lerondel G., Walke P., Late D., Jejurikar S.M. Synthesis and self-assembly of dumbbell shaped ZnO sub-micron structures using low temperature chemical bath deposition technique. Mater. Chem. Phys. 2016;169:152–157. doi: 10.1016/j.matchemphys.2015.11.042. [DOI] [Google Scholar]
- 52.Singh J., Juneja S., Palsaniya S., Manna A.K., Soni R.K., Bhattacharya J. Evidence of oxygen defects mediated enhanced photocatalytic and antibacterial performance of ZnO nanorods. Colloids Surf. B Biointerfaces. 2019;184:110541. doi: 10.1016/j.colsurfb.2019.110541. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed F., Arshi N., Jeong Y.S., Anwar M.S., Dwivedi S., Alsharaeh E., Koo B.H. Novel Biomimatic Synthesis of ZnO Nanorods Using Egg White (Albumen) and Their Antibacterial Studies. J. Nanosci. Nanotechnol. 2016;16:5959–5965. doi: 10.1166/jnn.2016.12127. [DOI] [PubMed] [Google Scholar]
- 54.Elkady M.F., Shokry Hassan H., Hafez E.E., Fouad A. Construction of Zinc Oxide into Different Morphological Structures to Be Utilized as Antimicrobial Agent against Multidrug Resistant Bacteria. Bioinorg. Chem. Appl. 2015;2015:536854. doi: 10.1155/2015/536854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Dicastillo C.L., Vidal C.P., Falcó I., Sánchez G., Márquez P., Escrig J. Antimicrobial Bilayer Nanocomposites Based on the Incorporation of As-Synthetized Hollow Zinc Oxide Nanotubes. Nanomaterials. 2020;10:503. doi: 10.3390/nano10030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandra H., Patel D., Kumari P., Jangwan J.S., Yadav S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C. 2019;102:212–220. doi: 10.1016/j.msec.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 57.Ali A., Ambreen S., Javed R., Tabassum S., ul Haq I., Zia M. ZnO nanostructure fabrication in different solvents transforms physio-chemical, biological and photodegradable properties. Mater. Sci. Eng. C. 2017;74:137–145. doi: 10.1016/j.msec.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Pasquet J., Chevalier Y., Couval E., Bouvier D., Noizet G., Morlière C., Bolzinger M.-A. Antimicrobial activity of zinc oxide particles on five micro-organisms of the Challenge Tests related to their physicochemical properties. Int. J. Pharm. 2014;460:92–100. doi: 10.1016/j.ijpharm.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 59.Khan M.F., Hameedullah M., Ansari A.H., Ahmad E., Lohani M.B., Khan R.H., Alam M.M., Khan W., Husain F.M., Ahmad I. Flower-shaped ZnO nanoparticles synthesized by a novel approach at near-room temperatures with antibacterial and antifungal properties. Int. J. Nanomed. 2014;9:853–864. doi: 10.2147/IJN.S47351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quek J.A., Lam S.M., Sin J.C., Mohamed A.R. Visible light responsive flower-like ZnO in photocatalytic antibacterial mechanism towards Enterococcus faecalis and Micrococcus luteus. J. Photochem. Photobiol. B Biol. 2018;187:66–75. doi: 10.1016/j.jphotobiol.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 61.Talebian N., Amininezhad S.M., Doudi M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013;120:66–73. doi: 10.1016/j.jphotobiol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Mousa M., Khairy M. Synthesis of nano-zinc oxide with different morphologies and its application on fabrics for UV protection and microbe-resistant defense clothing. Text. Res. J. 2020;90:2492–2503. doi: 10.1177/0040517520920952. [DOI] [Google Scholar]
- 63.Mahalakshmi S., Hema N., Vijaya P.P. In Vitro Biocompatibility and Antimicrobial activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route—A Comparative Study. BioNanoScience. 2020;10:112–121. doi: 10.1007/s12668-019-00698-w. [DOI] [Google Scholar]
- 64.Auría-Soro C., Nesma T., Juanes-Velasco P., Landeira-Viñuela A., Fidalgo-Gomez H., Acebes-Fernandez V., Gongora R., Almendral Parra M.J., Manzano-Roman R., Fuentes M., et al. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials. 2019;9:1365. doi: 10.3390/nano9101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gharpure S., Ankamwar B. Synthesis and Antimicrobial Properties of Zinc Oxide Nanoparticles. J. Nanosci. Nanotechnol. 2020;20:5977–5996. doi: 10.1166/jnn.2020.18707. [DOI] [PubMed] [Google Scholar]
- 66.Espitia P.J.P., Soares N.d.F.F., Coimbra J.S.d.R., de Andrade N.J., Cruz R.S., Medeiros E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012;5:1447–1464. doi: 10.1007/s11947-012-0797-6. [DOI] [Google Scholar]
- 67.Cheeseman S., Christofferson A.J., Kariuki R., Cozzolino D., Daeneke T., Crawford R.J., Truong V.K., Chapman J., Elbourne A. Antimicrobial Metal Nanomaterials: From Passive to Stimuli-Activated Applications. Adv. Sci. 2020;7:1902913. doi: 10.1002/advs.201902913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 69.Ali A., Phull A.-R., Zia M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol. Rev. 2018;7:413. doi: 10.1515/ntrev-2018-0067. [DOI] [Google Scholar]
- 70.Pasquet J., Chevalier Y., Pelletier J., Couval E., Bouvier D., Bolzinger M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014;457:263–274. doi: 10.1016/j.colsurfa.2014.05.057. [DOI] [Google Scholar]
- 71.Adhikari S., Gupta R., Surin A., Kumar T.S., Chakraborty S., Sarkar D., Madras G. Visible light assisted improved photocatalytic activity of combustion synthesized spongy-ZnO towards dye degradation and bacterial inactivation. RSC Adv. 2016;6:80086–80098. doi: 10.1039/C6RA10472J. [DOI] [Google Scholar]
- 72.Kaliraj L., Ahn J.C., Rupa E.J., Abid S., Lu J., Yang D.C. Synthesis of panos extract mediated ZnO nano-flowers as photocatalyst for industrial dye degradation by UV illumination. J. Photochem. Photobiol. B Biol. 2019;199:111588. doi: 10.1016/j.jphotobiol.2019.111588. [DOI] [PubMed] [Google Scholar]
- 73.Hajipour M.J., Fromm K.M., Akbar Ashkarran A., de Aberasturi D.J., Larramendi I.R.d., Rojo T., Serpooshan V., Parak W.J., Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Lakshmi Prasanna V., Vijayaraghavan R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir. 2015;31:9155–9162. doi: 10.1021/acs.langmuir.5b02266. [DOI] [PubMed] [Google Scholar]
- 75.Li Y., Fu Z.-Y., Su B.-L. Hierarchically Structured Porous Materials for Energy Conversion and Storage. Adv. Funct. Mater. 2012;22:4634–4667. doi: 10.1002/adfm.201200591. [DOI] [Google Scholar]
- 76.Picone A., Riva M., Brambilla A., Calloni A., Bussetti G., Finazzi M., Ciccacci F., Duò L. Reactive metal–oxide interfaces: A microscopic view. Surf. Sci. Rep. 2016;71:32–76. doi: 10.1016/j.surfrep.2016.01.003. [DOI] [Google Scholar]
- 77.Eleraky N.E., Allam A., Hassan S.B., Omar M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics. 2020;12:142. doi: 10.3390/pharmaceutics12020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wahid F., Zhong C., Wang H.S., Hu X.H., Chu L.Q. Recent Advances in Antimicrobial Hydrogels Containing Metal Ions and Metals/Metal Oxide Nanoparticles. Polymers. 2017;9 doi: 10.3390/polym9120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farzana R., Iqra P., Shafaq F., Sumaira S., Zkia K., Hunaiza T., Husna M. Antimicrobial Behavior of Zinc Oxide Nanoparticles and β-Lactam Antibiotics against Pathogenic Bacteria. Arch. Clin. Microbiol. 2017;8:57. [Google Scholar]
- 80.Azizi-Lalabadi M., Ehsani A., Divband B., Alizadeh-Sani M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019;9:17439. doi: 10.1038/s41598-019-54025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bednář J., Svoboda L., Rybková Z., Dvorský R., Malachová K., Stachurová T., Matýsek D., Foldyna V. Antimicrobial Synergistic Effect Between Ag and Zn in Ag-ZnO·mSiO2 Silicate Composite with High Specific Surface Area. Nanomaterials. 2019;9:1265. doi: 10.3390/nano9091265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Nabulsi A., Osaili T., Sawalha A., Olaimat A.N., Albiss B.A., Mehyar G., Ayyash M., Holley R. Antimicrobial activity of chitosan coating containing ZnO nanoparticles against E. coli O157:H7 on the surface of white brined cheese. Int. J. Food Microbiol. 2020;334:108838. doi: 10.1016/j.ijfoodmicro.2020.108838. [DOI] [PubMed] [Google Scholar]
- 83.Turlybekuly A., Pogrebnjak A.D., Sukhodub L.F., Sukhodub L.B., Kistaubayeva A.S., Savitskaya I.S., Shokatayeva D.H., Bondar O.V., Shaimardanov Z.K., Plotnikov S.V., et al. Synthesis, characterization, in vitro biocompatibility and antibacterial properties study of nanocomposite materials based on hydroxyapatite-biphasic ZnO micro- and nanoparticles embedded in Alginate matrix. Mater. Sci. Eng. C. 2019;104:109965. doi: 10.1016/j.msec.2019.109965. [DOI] [PubMed] [Google Scholar]
- 84.Kulshrestha S., Khan S., Meena R., Singh B.R., Khan A.U. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling. 2014;30:1281–1294. doi: 10.1080/08927014.2014.983093. [DOI] [PubMed] [Google Scholar]
- 85.Oves M., Rauf M.A., Ansari M.O., Aslam Parwaz Khan A., Qari H.A., Alajmi M.F., Sau S., Iyer A.K. Graphene Decorated Zinc Oxide and Curcumin to Disinfect the Methicillin-Resistant Staphylococcus aureus. Nanomaterials. 2020;10:1004. doi: 10.3390/nano10051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dharmalingam P., Jp P., Sundararajan V., Veluswamy P., Chelliah R., Samal D., Oh D.-H., Sahabudeen S.M., Venkatasubbu D. Mechanism of inhibition of graphene oxide/zinc oxide nanocomposite against wound infection causing pathogens. Appl. Nanosci. 2019;10 doi: 10.1007/s13204-019-01152-9. [DOI] [Google Scholar]
- 87.Wang Y.-W., Cao A., Jiang Y., Zhang X., Liu J.-H., Liu Y., Wang H. Superior Antibacterial Activity of Zinc Oxide/Graphene Oxide Composites Originating from High Zinc Concentration Localized around Bacteria. ACS Appl. Mater. Interfaces. 2014;6:2791–2798. doi: 10.1021/am4053317. [DOI] [PubMed] [Google Scholar]
- 88.Valenzuela L., Iglesias-Juez A., Bachiller-Baeza B., Faraldos M., Bahamonde A., Rosal R. Biocide mechanism of highly efficient and stable antimicrobial surfaces based on zinc oxide–reduced graphene oxide photocatalytic coatings. J. Mater. Chem. B. 2020;8:8294–8304. doi: 10.1039/D0TB01428A. [DOI] [PubMed] [Google Scholar]
- 89.Fakhroueian Z., Harsini F., Chalabian F., Katouzian F., Shafiekhani A., Esmaeilzadeh P. Influence of Modified ZnO Quantum Dots and Nanostructures as New Antibacterials. Adv. Nanopart. 2013;2:247–258. doi: 10.4236/anp.2013.23035. [DOI] [Google Scholar]
- 90.Singh A.K., Pal P., Gupta V., Yadav T.P., Gupta V., Singh S.P. Green synthesis, characterization and antimicrobial activity of zinc oxide quantum dots using Eclipta alba. Mater. Chem. Phys. 2018;203:40–48. doi: 10.1016/j.matchemphys.2017.09.049. [DOI] [Google Scholar]
- 91.Kumar S., Mudai A., Roy B., Basumatary I.B., Mukherjee A., Dutta J. Biodegradable Hybrid Nanocomposite of Chitosan/Gelatin and Green Synthesized Zinc Oxide Nanoparticles for Food Packaging. Foods. 2020;9:1143. doi: 10.3390/foods9091143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madhumitha G., Elango G., Roopan S.M. Biotechnological aspects of ZnO nanoparticles: Overview on synthesis and its applications. Appl. Microbiol. Biotechnol. 2016;100:571–581. doi: 10.1007/s00253-015-7108-x. [DOI] [PubMed] [Google Scholar]
- 93.Gold K., Slay B., Knackstedt M., Gaharwar A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018;1:1700033. doi: 10.1002/adtp.201700033. [DOI] [Google Scholar]
- 94.Melchionna M., Prato M., Fornasiero P. Mix and match metal oxides and nanocarbons for new photocatalytic frontiers. Catal. Today. 2016 doi: 10.1016/j.cattod.2016.04.024. [DOI] [Google Scholar]
- 95.Agelidis A., Koujah L., Suryawanshi R., Yadavalli T., Mishra Y.K., Adelung R., Shukla D. An Intra-Vaginal Zinc Oxide Tetrapod Nanoparticles (ZOTEN) and Genital Herpesvirus Cocktail Can Provide a Novel Platform for Live Virus Vaccine. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kadiyala U., Turali-Emre E.S., Bahng J.H., Kotov N.A., VanEpps J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA) Nanoscale. 2018;10:4927–4939. doi: 10.1039/C7NR08499D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rago I., Chandraiahgari C.R., Bracciale M.P., De Bellis G., Zanni E., Cestelli Guidi M., Sali D., Broggi A., Palleschi C., Sarto M.S., et al. Zinc oxide microrods and nanorods: Different antibacterial activity and their mode of action against Gram-positive bacteria. RSC Adv. 2014;4:56031–56040. doi: 10.1039/C4RA08462D. [DOI] [Google Scholar]
- 98.Yi G.-C., Wang C., Park W. ZnO Nanorods: Synthesis, Characterization and Applications. Semicond. Sci. Technol. 2005;20:22–34. doi: 10.1088/0268-1242/20/4/003. [DOI] [Google Scholar]
- 99.Reddy L.S., Nisha M.M., Joice M., Shilpa P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014;52:1388–1397. doi: 10.3109/13880209.2014.893001. [DOI] [PubMed] [Google Scholar]
- 100.Taghizadeh S.-M., Lal N., Ebrahiminezhad A., Moeini F., Seifan M., Ghasemi Y., Berenjian A. Green and Economic Fabrication of Zinc Oxide (ZnO) Nanorods as a Broadband UV Blocker and Antimicrobial Agent. Nanomaterials. 2020;10:530. doi: 10.3390/nano10030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma S., Naik D., Agarwala V. Synthesis, Characterization and Antibacterial Activity of ZnO Nanoparticles of Different Morphology. Adv. Mater. Res. 2012;585:154–158. doi: 10.4028/www.scientific.net/AMR.585.154. [DOI] [Google Scholar]
- 102.Ali S.G., Ansari M.A., Alzohairy M.A., Alomary M.N., Jalal M., AlYahya S., Asiri S.M.M., Khan H.M. Effect of Biosynthesized ZnO Nanoparticles on Multi-Drug Resistant Pseudomonas Aeruginosa. Antibiotics. 2020;9:260. doi: 10.3390/antibiotics9050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mishra Y.K., Modi G., Cretu V., Postica V., Lupan O., Reimer T., Paulowicz I., Hrkac V., Benecke W., Kienle L., et al. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces. 2015;7:14303–14316. doi: 10.1021/acsami.5b02816. [DOI] [PubMed] [Google Scholar]
- 104.Jeyabharathi S., Mahalakshmi R., Chandramohan S., Naveenkumar S., Sundar K., Muthukumaran A. Self-assembled hollow ZnO nano and micro donut shape by starch and its antimicrobial potentials. Mater. Lett. 2020;275:128128. doi: 10.1016/j.matlet.2020.128128. [DOI] [Google Scholar]
- 105.Ashajyothi C., Harish K.H., Dubey N., Chandrakanth R.K. Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: A nanoscale approach. J. Nanostruct. Chem. 2016;6:329–341. doi: 10.1007/s40097-016-0205-2. [DOI] [Google Scholar]
- 106.Sharma N., Singh V., Pandey A.K., Mishra B.N., Kulsoom M., Dasgupta N., Khan S., El-Enshasy H.A., Haque S. Preparation and Evaluation of the ZnO NP–Ampicillin/Sulbactam Nanoantibiotic: Optimization of Formulation Variables Using RSM Coupled GA Method and Antibacterial Activities. Biomolecules. 2019;9:764. doi: 10.3390/biom9120764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eftekhar B., Moghimipour E., Jahandideh P.P., Jalali S., Mahmoudian M. Analgesic effect of odontopaste and a compound intracanal medicament between root canal therapy appointments. Jundishapur J. Nat. Pharm. Prod. 2013;8:169–174. doi: 10.17795/jjnpp-12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koch T., Peutzfeldt A., Malinovskii V., Flury S., Häner R., Lussi A. Temporary zinc oxide-eugenol cement: Eugenol quantity in dentin and bond strength of resin composite. Eur. J. Oral Sci. 2013;121:363–369. doi: 10.1111/eos.12053. [DOI] [PubMed] [Google Scholar]
- 109.Panzarini S.R., Trevisan C.L., Brandini D.A., Poi W.R., Sonoda C.K., Luvizuto E.R., Dos Santos C.L. Intracanal dressing and root canal filling materials in tooth replantation: A literature review. Dent. Traumatol. 2012;28:42–48. doi: 10.1111/j.1600-9657.2011.01023.x. [DOI] [PubMed] [Google Scholar]
- 110.Ghorbani M., Nezhad-Mokhtari P., Ramazani S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020;153:921–930. doi: 10.1016/j.ijbiomac.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 111.Hamdan S., Pastar I., Drakulich S., Dikici E., Tomic-Canic M., Deo S., Daunert S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017;3:163–175. doi: 10.1021/acscentsci.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramya R., Venkatesan J., Kim S., Sudha P.N. Biomedical Applications of Chitosan: An Overview. J. Biomater. Tissue Eng. 2012;2:100–111. doi: 10.1166/jbt.2012.1030. [DOI] [Google Scholar]
- 113.Sahariah P., Másson M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules. 2017;18:3846–3868. doi: 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- 114.Zhang J., Xia W., Liu P., Cheng Q., Tahirou T., Gu W., Li B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Mar. Drugs. 2010;8:1962–1987. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Donnadio A., Cardinali G., Latterini L., Roscini L., Ambrogi V. Nanostructured zinc oxide on silica surface: Preparation, physicochemical characterization and antimicrobial activity. Mater. Sci. Eng. C. 2019;104:109977. doi: 10.1016/j.msec.2019.109977. [DOI] [PubMed] [Google Scholar]
- 116.Mousavi S.A., Ghotaslou R., Khorramdel A., Akbarzadeh A., Aeinfar A. Antibacterial and antifungal impacts of combined silver, zinc oxide, and chitosan nanoparticles within tissue conditioners of complete dentures in vitro. Ir. J. Med Sci. 2020 doi: 10.1007/s11845-020-02243-1. [DOI] [PubMed] [Google Scholar]
- 117.Qiu S., Zhou H., Shen Z., Hao L., Chen H., Zhou X. Synthesis, characterization, and comparison of antibacterial effects and elucidating the mechanism of ZnO, CuO and CuZnO nanoparticles supported on mesoporous silica SBA-3. RSC Adv. 2020;10:2767–2785. doi: 10.1039/C9RA09829A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sophee S., Prasad V., Srinivas J., Aparna R., Phani A. Antibacterial Activity of TiO2 and ZnO Microparticles Combination on Water Polluting Bacteria. J. Green Sci. Technol. 2013;1 doi: 10.1166/jgst.2013.1008. [DOI] [Google Scholar]
- 119.Predoi D., Iconaru S.L., Predoi M.V., Stan G.E., Buton N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials. 2019;9:1295. doi: 10.3390/nano9091295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jose A., Sunaja Devi K.R., Pinheiro D., Lakshmi Narayana S. Electrochemical synthesis, photodegradation and antibacterial properties of PEG capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2018;187:25–34. doi: 10.1016/j.jphotobiol.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 121.Mocanu A., Isopencu G., Busuioc C., Popa O.-M., Dietrich P., Socaciu-Siebert L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 2019;9:17687. doi: 10.1038/s41598-019-54118-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao S.-W., Guo C.-R., Hu Y.-Z., Guo Y.-R., Pan Q.-J. The preparation and antibacterial activity of cellulose/ZnO composite: A review. Open Chem. 2018;16:9. doi: 10.1515/chem-2018-0006. [DOI] [Google Scholar]
- 123.Hewlings S.J., Kalman D.S. Curcumin: A Review of Its Effects on Human Health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perera W.P.T.D., Dissanayake R.K., Ranatunga U.I., Hettiarachchi N.M., Perera K.D.C., Unagolla J.M., De Silva R.T., Pahalagedara L.R. Curcumin loaded zinc oxide nanoparticles for activity-enhanced antibacterial and anticancer applications. RSC Adv. 2020;10:30785–30795. doi: 10.1039/D0RA05755J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Teh S.J., Yeoh S.L., Lee K.M., Lai C.W., Abdul Hamid S.B., Thong K.L. Effect of reduced graphene oxide-hybridized ZnO thin films on the photoinactivation of Staphylococcus aureus and Salmonella enterica serovar Typhi. J. Photochem. Photobiol. B Biol. 2016;161:25–33. doi: 10.1016/j.jphotobiol.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 126.Bajpai S.K., Thomas V., Bajpai M. Novel Strategy for Synthesis of ZnO Microparticles Loaded Cotton Fabrics and Investigation of their Antibacterial Properties. J. Eng. Fibers Fabr. 2011;6:155892501100600310. doi: 10.1177/155892501100600310. [DOI] [Google Scholar]
- 127.Noman M.T., Petrů M. Functional Properties of Sonochemically Synthesized Zinc Oxide Nanoparticles and Cotton Composites. Nanomaterials. 2020;10:1661. doi: 10.3390/nano10091661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salat M., Petkova P., Hoyo J., Perelshtein I., Gedanken A., Tzanov T. Durable antimicrobial cotton textiles coated sonochemically with ZnO nanoparticles embedded in an in-situ enzymatically generated bioadhesive. Carbohydr. Polym. 2018;189:198–203. doi: 10.1016/j.carbpol.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 129.Subash A.A., Chandramouli K.V., Ramachandran T., Rajendran R., Muthusamy M. Preparation, characterization, and functional analysis of zinc oxide nanoparticle-coated cotton fabric for antibacterial efficacy. J. Text. Inst. 2012;103:298–303. doi: 10.1080/00405000.2011.570046. [DOI] [Google Scholar]
- 130.Yilmaz Atay H. Antibacterial Activity of Chitosan-Based Systems. Funct. Chitosan. 2020 doi: 10.1007/978-981-15-0263-7_15. [DOI] [Google Scholar]
- 131.Jaros J., Wilson C., Shi V.Y. Fabric Selection in Atopic Dermatitis: An Evidence-Based Review. Am. J. Clin. Dermatol. 2020;21:467–482. doi: 10.1007/s40257-020-00516-0. [DOI] [PubMed] [Google Scholar]
- 132.Perinelli D.R., Fagioli L., Campana R., Lam J.K.W., Baffone W., Palmieri G.F., Casettari L., Bonacucina G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018;117:8–20. doi: 10.1016/j.ejps.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 133.Tanpichai S., Witayakran S., Wootthikanokkhan J., Srimarut Y., Woraprayote W., Malila Y. Mechanical and antibacterial properties of the chitosan coated cellulose paper for packaging applications: Effects of molecular weight types and concentrations of chitosan. Int. J. Biol. Macromol. 2020;155:1510–1519. doi: 10.1016/j.ijbiomac.2019.11.128. [DOI] [PubMed] [Google Scholar]
- 134.Wang H., Qian J., Ding F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018;66:395–413. doi: 10.1021/acs.jafc.7b04528. [DOI] [PubMed] [Google Scholar]
- 135.Marpu S.B., Benton E.N. Shining Light on Chitosan: A Review on the Usage of Chitosan for Photonics and Nanomaterials Research. Int. J. Mol. Sci. 2018;19:1795. doi: 10.3390/ijms19061795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karahaliloglu Z., Kilicay E., Denkbas E.B. Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Artif. Cells Nanomed. Biotechnol. 2017;45:1172–1185. doi: 10.1080/21691401.2016.1203796. [DOI] [PubMed] [Google Scholar]
- 137.Javed R., Rais F., Fatima H., Haq I.U., Kaleem M., Naz S.S., Ao Q. Chitosan encapsulated ZnO nanocomposites: Fabrication, characterization, and functionalization of bio-dental approaches. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;116:111184. doi: 10.1016/j.msec.2020.111184. [DOI] [PubMed] [Google Scholar]
- 138.Morais D.S., Guedes R.M., Lopes M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials. 2016;9:498. doi: 10.3390/ma9060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rajendiran K., Zhao Z., Pei D.-S., Fu A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers. 2019;11:1670. doi: 10.3390/polym11101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Z.-Y., Xiong H.-M. Photoluminescent ZnO Nanoparticles and Their Biological Applications. Materials. 2015;8:3101–3127. doi: 10.3390/ma8063101. [DOI] [Google Scholar]
- 141.Chen H., Zhang M., Li B., Chen D., Dong X., Wang Y., Gu Y. Versatile antimicrobial peptide-based ZnO quantum dots for in vivo bacteria diagnosis and treatment with high specificity. Biomaterials. 2015;53:532–544. doi: 10.1016/j.biomaterials.2015.02.105. [DOI] [PubMed] [Google Scholar]
- 142.Jin T., Sun D., Su J.Y., Zhang H., Sue H.J. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli O157:H7. J. Food Sci. 2009;74:M46–M52. doi: 10.1111/j.1750-3841.2008.01013.x. [DOI] [PubMed] [Google Scholar]
- 143.Gabros S., Nessel T.A., Zito P.M. Sunscreens and Photoprotection. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2020. [PubMed] [Google Scholar]
- 144.Gao A.L., Cole J.G., Woolsey Z.T., Stoecker W.V. Structured zinc oxide dressing for secondary intention wounds. J. Wound Care. 2017;26:S30–S36. doi: 10.12968/jowc.2017.26.Sup10.S30. [DOI] [PubMed] [Google Scholar]
- 145.Gao Y., Han Y., Cui M., Tey H.L., Wang L., Xu C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J. Mater. Chem. B. 2017;5:4535–4541. doi: 10.1039/C7TB00664K. [DOI] [PubMed] [Google Scholar]
- 146.Memarzadeh K., Sharili A.S., Huang J., Rawlinson S.C., Allaker R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. Part A. 2015;103:981–989. doi: 10.1002/jbm.a.35241. [DOI] [PubMed] [Google Scholar]
- 147.Suozzi K., Turban J., Girardi M. Cutaneous Photoprotection: A Review of the Current Status and Evolving Strategies. Yale J. Biol. Med. 2020;93:55–67. [PMC free article] [PubMed] [Google Scholar]
- 148.Thompson C.B., Wiemken T.L., Brown T.S. Effect of Postoperative Dressing on Excisions Performed on the Leg: A Comparison Between Zinc Oxide Compression Dressings Versus Standard Wound Care. Dermatol. Surg. 2017;43:1379–1384. doi: 10.1097/DSS.0000000000001209. [DOI] [PubMed] [Google Scholar]
- 149.Bae J.Y., Park S.N. Evaluation of anti-microbial activities of ZnO, citric acid and a mixture of both against Propionibacterium acnes. Int. J. Cosmet. Sci. 2016;38:550–557. doi: 10.1111/ics.12318. [DOI] [PubMed] [Google Scholar]
- 150.Cervantes J., Eber A.E., Perper M., Nascimento V.M., Nouri K., Keri J.E. The role of zinc in the treatment of acne: A review of the literature. Dermatol. Ther. 2018;31 doi: 10.1111/dth.12576. [DOI] [PubMed] [Google Scholar]
- 151.Chuang H.-C., Yang Y.-T., Chen H.-C., Hwang Y.-H., Wu K.-Y., Chen T.-F., Chen C.-L., Jhan M.-K., Cheng T.-J. Acute Effects of Pulmonary Exposure to Zinc Oxide Nanoparticles on the Brain in vivo. Aerosol Air Qual. Res. 2020 doi: 10.4209/aaqr.2019.10.0523. [DOI] [Google Scholar]
- 152.Hadrup N., Rahmani F., Jacobsen N.R., Saber A.T., Jackson P., Bengtson S., Williams A., Wallin H., Halappanavar S., Vogel U. Acute phase response and inflammation following pulmonary exposure to low doses of zinc oxide nanoparticles in mice. Nanotoxicology. 2019;13:1275–1292. doi: 10.1080/17435390.2019.1654004. [DOI] [PubMed] [Google Scholar]
- 153.Rasmussen K., Rauscher H., Mech A., Riego Sintes J., Gilliland D., González M., Kearns P., Moss K., Visser M., Groenewold M., et al. Physico-chemical properties of manufactured nanomaterials—Characterisation and relevant methods. An outlook based on the OECD Testing Programme. Regul. Toxicol. Pharmacol. 2018;92:8–28. doi: 10.1016/j.yrtph.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]