Abstract
N-3 polyunsaturated fatty acids (n-3 PUFAs), and especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are essential compounds for human health. They have been proven to act positively on a panel of diseases and have interesting anti-oxidative, anti-inflammatory or anti-cancer properties. For these reasons, they are receiving more and more attention in recent years, especially future food or feed development. EPA and DHA come mainly from marine sources like fish or seaweed. Unfortunately, due to global warming, these compounds are becoming scarce for humans because of overfishing and stock reduction. Although increasing in recent years, aquaculture appears insufficient to meet the increasing requirements of these healthy molecules for humans. One alternative resides in the cultivation of microalgae, the initial producers of EPA and DHA. They are also rich in biochemicals with interesting properties. After defining macro and microalgae, this review synthesizes the current knowledge on n-3 PUFAs regarding health benefits and the challenges surrounding their supply within the environmental context. Microalgae n-3 PUFA production is examined and its synthesis pathways are discussed. Finally, the use of EPA and DHA in food and feed is investigated. This work aims to define better the issues surrounding n-3 PUFA production and supply and the potential of microalgae as a sustainable source of compounds to enhance the food and feed of the future.
Keywords: microalgae, n-3 PUFA, EPA, DHA, food industry, feed industry
1. Algae, a Source of Bioactive Compounds
The term “algae” describes a diversity of micro and macro aquatic organisms able to survive and proliferate by photosynthesis [1]. Macroalgae (multicellular) and microalgae (unicellular) develop in both freshwater and marine environments [1]. Microalgae are planktonic or benthic algae floating in the water, while macroalgae are benthic and sedentary [2,3]. They include either eukaryotic species or prokaryotic cyanobacteria that are devoid of a membrane-bound nucleus and are classified between bacteria and plants [1]. The most abundant microalgal phyla are blue-green algae (Cyanophyceae), green algae (Chlorophyceae), Bacillariophyceae (including diatoms), and Chrysophyceae (including golden algae) [1,4].
Microalgae represent an exciting competitive source of biomass, especially because of their very efficient photosynthetic system. Indeed, thanks to their aqueous environment and submerged nature, microalgae have continuous access to water, CO2, and nutrients that allow them to be very effective in converting energy to biomass [3,5]. They are also easy to produce and can develop under various trophic regimes. Like terrestrial plants, most microalgae are autotrophic organisms efficiently using light to grow [6]. However, some species, for example, those belonging to the phylum Dinoflagellata, can use organic molecules to grow in addition to the inorganic ones and are called mixotrophic [7]. Heterotrophic microalgae, such as the Dinoflagellate Crypthecodinium cohnii, also exist, and exclusively use organic sources like glucose for carbon metabolism and energy [3]. This is also the case with marine protists from the Thraustochytrid family such as Schizochytrium. Even if they cannot be considered algae as they lack plastid and chlorophyll, these organisms are exclusively heterotrophic [8,9]. Microalgae are also very diverse, and more than 30,000 species have been described so far [10,11]. The ease with which they can be cultured explains in part why they have been receiving increasing interest in recent years. Microalgae chemical composition has been widely studied in the literature, with proportions varying widely between the different species and culture conditions used [1,12]. They have been recognized as a reliable source of bioactive compounds such as proteins, lipids, carotenoids, hydrocarbons and vitamins [3,6]. The levels of these compounds of interest in microalgae are similar or even higher than they are in plants and animals [5,6]. The diversity of the compounds associated with the critical number of species available makes it possible to select and choose specific strains and molecules for various applications. These molecules also exhibit very interesting properties such as antioxidative, anti-microbial, anti-inflammatory, coloring, texturing, stabilizing, or even emulsifying capacities, which explains why they are so widely valued in the food and feed industry, in the cosmetics and medical sectors, and for bioenergy, biodiesel or even aquaculture [3,6,12].
In recent years, particular attention has been given to microalgae lipids. Indeed, they can represent up to 74% of microalgae’s total biochemical content according to species [12]. These molecules are built with fatty acids with 12 to 24 atoms of carbon and include the polyunsaturated fatty acids of the n-3 or n-6 families (n-3 PUFAs and n-6 PUFAs, respectively) [1]. N-3 PUFAs, also called omega-3 fatty acids, are characterized by their health benefits for both animals and humans. Their properties are expected to be of central interest in developing novel ingredients or compounds for various sectors, including the feed and food industry, with high commercial value. So far, microalgae are still poorly explored and valued as a natural source for a healthy diet [13]. Among the critical number of species known to date, only a few are commercially produced due to strict food safety regulations, namely in Europe, and because their cultivation in industrial quantities is only a few decades old [10,11]. This translates into a high potential for the production and commercialization of these organisms in the coming years.
Thus, this review aims to focus on the future challenges of n-3 PUFA production by microalgae and its availability and role for humans and aquaculture in the context of stock reduction and global warming. For this purpose, the first part includes a review of the background situation concerning overfishing and environmental changes. Then, the production of n-3 PUFAs by microalgae is developed, and the current knowledge on their biosynthetic pathways is discussed. Finally, examples of the application of these healthy compounds in the food and feed industries are presented to help better foresee the future of this field in the years to come.
2. Polyunsaturated Fatty Acids and Human Health
Human consumption of seafood has been increasing for several years, mainly due to seafood’s richness in protein, n-3 PUFAs, vitamins and minerals, and its well-known health benefits [14]. In fact, marine products are one of the best food sources of n-3 PUFAs, also called omega 3s. At the bottom of the marine food chain, microalgae can produce EPA and DHA from smaller polyunsaturated fatty acids, linoleic acid (LA—18:2n-6) and α-linolenic acid (ALA—18:3n-3), due to a series of dedicated desaturases and elongases. However, unlike these organisms, humans do not possess these enzymes and are unable to produce EPA and DHA in sufficient quantities [15]. Both fatty acids are known for their therapeutic properties with regard to cardiovascular diseases, hypertension, and autoimmune disorders [14,16]. They are therefore of the most importance for humans and need to be included in their daily diet [17].
Microalgae are known to synthesize large quantities of PUFAs, a variable amount of monounsaturated fatty acids (MUFA) and low amounts of saturated fats (SFAs) [18]. Dietary intakes of n-3 PUFAs, particularly eicosapentaenoic acid (EPA—20:5n-3) and docosahexaenoic acid (DHA—22:6n-3) have long been known to reduce the risk of developing cardiovascular problems [19,20].
2.1. n-3 PUFAs and Cardiovascular Diseases
Numerous studies have been conducted in past years to understand how these nutrients can act on cardiovascular disorders. Research is ongoing and sometimes appears incoherent. Indeed, while early trials attested to beneficial cardiovascular outcomes, recent works are nuanced [21]. Difficulties in authenticating the impact of these two PUFAs stem from the heterogeneous nature of the population tested, the absence of standardized clinical trials to evaluate their actions, varying sample sizes, the omega-3 doses tested, and the EPA to DHA ratio [22]. For example, conflicting results on the impact of EPA, DHA, and a combination of both on myocardial infarction have been observed [23]. EPA and DHA used individually (5 g × day−1) seemed to diminish infarct size, while a combination of both to a level of 5 g × kg−1 did not [23]. Supplementation of EPA+DHA to a level of 1.8 g/day−1 for elderly patients with a recent acute myocardial infarction did not reduce clinical events either [24]. This might be due to n-3 PUFAs’ competition for the same receptors or biased signaling as observed with some protein receptors [23]. Generally, the available data seem to support the positive impact of dietary n-3 PUFAs on blood pressure [20], congestive heart failure [25], and supplementation of more than one g/day−1 of n-3 PUFAs seem effective in lowering the risk of fatal cardiac events [21]. Thus, further studies are warranted to better define these nutrients’ role and activity in cardiovascular disease development and prevention.
2.2. n-3 PUFAs Against Obesity and Diabetes
EPA and DHA can also prevent obesity by inhibiting certain enzymes responsible for lipid synthesis, affecting serum lipids and lipoproteins [14]. In animal models, omega-3 supplementation improved hepatic insulin sensitivity, the production of adipocytokines and direct and indirect anti-inflammatory effects [22]. For example, omega-3 supplementation can prevent diabetic complications such as diabetic nephropathy connected to renal phospholipid metabolism in rats, and n-3 PUFAs and MUFAs phospholipid synthesis is enhanced, and linked to anti-inflammatory mechanisms [26]. For humans, the mechanisms might vary as a function of genetics and/or lifestyle [22]. Insulin sensitivity seems to increase after supplementation of n-3 PUFAs in relation to non-esterified fatty acid reduction [27]. EPA and DHA prescription for Type 2 diabetes mellitus significantly reduces the levels of triglyceride known to be responsible for fat accumulation, thereby reducing the risk of developing hypertriglyceridemia [28]. However, the EPA-only prescription seems slightly better as it does not increase the concentration of low-density lipoprotein cholesterol, decreases total cholesterol levels, and results in fewer gastrointestinal effects than DHA-only prescription [28]. Interestingly, these n-3 PUFA-reinforced diets do not result in glucose metabolism impairment in diabetic patients, as shown in 14 clinical trials [29].
2.3. Anti-Inflammatory Properties of n-3 PUFAs in the Context of the COVID-19 Pandemic
EPA, 20:5n-3, is a precursor of eicosanoids and can limit inflammatory developments related to chronic diseases [16,30]. Both EPA and DHA can regulate the production of bioactive substances such as eicosanoids [15]. Increasing the ratio of n-3 to n-6 PUFAs decreases inflammation, as omega-6 is a precursor of pro-inflammatory molecules while eicosanoids issued from omega-3 have antagonist properties [14]. N-3 PUFAs can alter the decomposing enzymes and inflammatory factors or up-regulate the activities of some proteins or enzymes such as lipoprotein lipase fatty acid-binding protein [15]. More precisely, mechanisms underlying the anti-inflammatory actions of EPA and DHA involve inhibition of leukocyte chemotaxis, reduction of adhesion molecule expression and leukocyte-endothelial adhesive interactions, disruption of lipid rafts, inhibition of activation of NF-κB, activation of anti-inflammatory transcription factors, such as Peroxisome Proliferator-Activated Receptor Gamma (PPARγ), and binding to the G protein-coupled receptor (GPCR120) [30]. It is also important to note that pro-resolving mediators, such as resolvins, protectins, and maresins synthesized through the enzymatic oxidation of EPA and DHA, involve the COX and LOX pathways, and are inflammation-resolving, inhibiting transendothelial migration of neutrophils and cytokines (IL-1β and TNF-α), and chemokines production. N-3 PUFAs also increase phagocytotic capacity, decreasing the reactive oxidative species (ROS) of innate immune cells, including macrophages and neutrophils. Moreover, n-3 PUFAs and their metabolites also promote activation of NK cells and modulate T cell activation by altering the activation of antigen-presenting cells (APCs, such as macrophages or dendritic cells) and prevent the differentiation of CD4+ cells to Th1 cells. This anti-inflammatory effect appears all the more important in the current context of the global pandemic. Even if there is no known definitive treatment for SARS-CoV-2 induced COVID-19, antiviral and supportive treatments are essential for patients with COVID-19. As cytokine storm is a very common manifestation in severe patients and often leads to exacerbation, intervention with anti-inflammation therapy may help in preventing further injury [31]. Recently, Torrinhas et al. [32] reported that immune modulatory properties of EPA and DHA provided in emulsions might play a key role in changing clinical outcomes of SARS-CoV-2 infected patients. Even if the benefits expected based on the anti-inflammatory activity of EPA and DHA are anecdotal and need to be verified by rigorous clinical trials, they suggest that a prescription based on body weight (e.g., 0.2 g pure fish-oil lipid emulsions/kg body weight/day), combined with low oral aspirin intake to trigger resolvin synthesis from EPA and DHA, could be beneficial to patients [32].
2.4. EPA and DHA Roles in Gut Microbiota
Omega-3 supplementation has also been proven to impact the development and composition of gut microbiota positively. The intestinal microbiota is involved in immune response and prevention of pathogenic invasions [33]. They can also stimulate the resident macrophages, for example, via the production of tryptophan and indole-3-acetate [34]. Both can help in eliminating the pro-inflammatory molecules induced by lipopolysaccharides or palmitate. The composition of gut microbiota is influenced by phenotype, age, gender, exercise frequency, dietary habits, the use of antibiotics, drugs, probiotics, immune function, geographical location, environment, and host-specific in humans [35]. Any alteration of the gut microbiota or imbalance between microbial communities, called dysbiosis, might be responsible for metabolic or neurologic disease development [35]. Diet is one of the main factors pressuring microbial communities. The nature of dietary fats influences the types and number of gut microbes and the host’s intestinal health [36]. The communities present determine the level of resistance to infection and susceptibility to inflammatory diseases. Some communities’ changes have been recorded following the incorporation of alimentary fats including n-3 PUFAs [36]. For example, diets enriched with n-3 PUFAs for high-cardiometabolic-risk patients seem to be associated with modification of lipid metabolism with the promotion of Bifidobacteria that produces butyrate, an essential metabolite for the human colon [37]. N-3 PUFAs are also thought to have positive actions on gut microbiota. As an illustration, Bentley-Hewitt et al. [38] studied the interactions between Lactobacillus sp. and n-3 PUFAs. They showed that direct exposure of Lactobacilli, a prominent community of intestinal bacteria, to ALA, EPA, and especially DHA could enhance their probiotic potential, as these nutrients improved their abilities to adhere to the epithelial layer. Furthermore, the health benefits of EPA and DHA, such as their antimicrobial activities, can be triggered by intestinal microbiota’s enzymatic actions [36].
2.5. Daily Recommendation and Requirement of n-3 PUFAs
Accordingly, European authorities have established their recommendations based on epidemiological studies attesting to the beneficial effects of a Mediterranean-style diet [39]. This diet consists of high fat intake (40–50% of total daily calories) with less than 8% of saturated fats and 15 and 25% of monounsaturated fatty acids. A higher consumption of n-3 PUFAs than n-6 PUFAs is also advised in addition to a low ratio of n-6:n-3 comprised between 2:1 and 1:1 [39]. Because of the rising health concern for humans, there is an urgent need to target food and bioactive compounds that could meet the requirements of n-3 PUFAs for general health [40]. Health agencies’ recommendations on the daily consumption of these two fatty acids have been stated [41]. A worldwide consensus (World Health Organization, Europe, USA, Australia, New Zealand, United Kingdom, Netherlands, France, Canada and Japan) has been reached in recent years, recommending between 250 and 500 mg of EPA+DHA per day. More specifically for France (ANSES), the recommendation has remained unchanged since 2011 at 500 mg × day−1 EPA+DHA (250 mg.day−1 each) [42]. It is advised to consume at least two servings of fatty fish per week to ensure good health and reduce the risk of mortality from cardiovascular diseases [43].
2.6. Role of n-3 PUFAs on Brain Development, Tumors and Cancer
Polyunsaturated fatty acids also have a positive impact on brain development. DHA is one of the main structural components of nerve cells and helps transmit signaling messages to maintain the plasticity of the brain [44,45]. Arachidonic acid (ARA—20:4n-6) is also involved in brain functioning and eases neuronal transmission and long-term potentiation [44]. They are both preserving it against oxidative stress. DHA, as a component of photoreceptor cells, is also indispensable for vision [44]. Rodent and human models show that DHA supplementation could not only have a beneficial impact on brain functions, including learning and memory [46,47], depressive and aggressive behaviors [45,48] but also on audition [49,50], and olfaction [51,52].
N-3 PUFAs also participate in synapse formation, neuronal proliferation, and differentiation and extension of nerve fibers [53]. DHA had also been studied as a valuable compound to delay brain aging and protect against the onset of Alzheimer’s disease (AD) [54]. Among those with other brain disorders, patients with Schizophrenia might also present low levels of n-3 PUFAs and ARA in their body tissues [55] and research suggests that treatment including supplementation with EPA and/or DHA could decrease violence in schizophrenic patients [56].
EPA and DHA also have antitumoral and anticancer properties. Indeed, EPA and DHA can improve drug delivery by modifying the tumor vasculature with architectural remodeling [57], and present antiangiogenic activities [58,59]. They also induce apoptosis [60,61], block the cell cycle resulting in stopping tumor development [53], and participate in lipid peroxidation-mediated endoplasmic-reticulum-stress which triggers deterioration of cancer cells [62].
2.7. N-3 PUFAs and Retinopathies
PUFAs and their derivatives can also play a role in the pathogenesis of retinal diseases (retinopathies) like retinopathy of prematurity (ROP), age-related macular degeneration (AMD), or diabetic retinopathy (DR). They are essential nutrients for the visual system, and DHA is a major structural component of retinal photoreceptors [63]. Due to their anti-angiogenetic proprieties, they can reduce retinal stress in ROP [64], their anti-oxidant capacities they can protect eye photoreceptors from oxidative stress [65]. N-3 PUFAs can avert visual loss owing to AMD [63,66]. Lipid metabolism impairment in diabetic patients can also impact the retinal system [58]. Hyperglycemia triggers various pathological alterations such as oxidative stress, inflammation, angiogenesis, increase of apoptosis of endothelial cells and neurons, and injury to the retinal blood capillaries [67]. Thus, with the previously described effects of omega-3 fatty acids, including α-linolenic acid (anti-inflammatory, anti-oxidative or anti-angiogenic proprieties among others) on such disorders, they are of immense interest in preventing DR [68].
Thus, there is increasing interest around EPA and DHA for their positive effects on health for both humans and animals. This correlates to a continually increasing demand for these two compounds in recent years. It is thus imperative to find sustainable ways of supplying polyunsaturated fatty acids to meet this demand.
3. Increasing Demand for n-3 Polyunsaturated Fatty Acids
A significant increase in fish and marine product consumption was registered between 1961 and 2016, with an average annual growth of 3.2% according to the FAO (2018) [69]. However, these trends are being challenged by supply issues [40]. Supply of n-3 PUFA comes mainly from the ocean and the vast majority (almost 90%) from capture fisheries [40]. Thus, access to fish and seafood determines to what extend the supply can meet PUFA demand. Global fisheries have reached a plateau following stock reduction (90 million tons per annum) [40,69]. Areas that were previously unexploited or underexploited in the 1950s are now undergoing an unprecedented expansion in fishing by industrial fleets [70]. Exploitation in the Southwestern Atlantic, the Indian Ocean, and the Western Central and Southwest Pacific has peaked [70]. These demanding industrial fisheries are responsible for removing top predators and thus the release of predatory pressure on lower trophic levels [70]. Pauly et al. (1998) [71] observed a decline in mean trophic levels. They proposed the concept of “fishing down marine food webs” which illustrates how the intensive capture pressure exercised on top predators leads to a change of target for lower trophic levels. Indeed, the diminution of top-down predatory pressure on smaller fish leads to a subsequent increase in their biomass, thus promoting new intensive captures by fisheries [70]. Overfishing is also responsible for stabilizing fish harvesting in the last few decades (33.1% of stocks were overfished in 2018) [69]. Most fisheries are at or beyond their sustainable levels of exploitation [40]. Technological improvement in fishing gear keeps increasing catching efficiency [72]. The fishing power of fleets and their range of action and versatility are dramatically affecting small pelagic fish [70].
However, these small trophic levels, including phytoplankton are also affected by climate change [73]. Global warming is suspected to impact polyunsaturated fatty acids production by phytoplankton, thus reducing the availability of these essential compounds for higher trophic levels [74]. Temperature is expected to have effects on the quantity and quality of fatty acids in phytoplankton [74]. At the bottom of marine food webs, phytoplankton can adapt to changing temperatures by modifying their membrane characteristics [75]. Indeed, lipids and especially fatty acids are critical elements of cell membranes and can be modulated in response to environmental changes to maintain the desired level of fluidity [73,76,77]. The unsaturation of PUFA enhances the fatty acid’s ability to fold and increase flexibility [74]. Generally, n-3 PUFAs decrease with increasing temperature, while n-6 PUFAs have antagonist dynamics with rising temperature [74]. The increase in water temperature may then induce major shifts in global PUFA production by phytoplankton and their first consumers, microzooplankton, which could then have reverberating effects on higher terrestrial and aquatic trophic levels [18,74].
Consequently, considering the constant growth of the global population and the resulting demand for these compounds, aquaculture now appears to be one of the solutions for meeting future seafood needs and filling the gap between supply and demand [40,78]. World aquaculture’s contribution to world fish production increased to 46% in 2016–2018 compared to only 26% in 2000 [79]. It is the first source of fish and ensures a continuing rise in fish supply for human consumption [69]. Global aquaculture production in 2018 consisted of 82.1 million tons of aquatic animals, 32.4 million tons of aquatic algae, and 26,000 tons of ornamental seashells and pearls [79]. Unfortunately, aquaculture remains mostly dependent on other fisheries’ products such as fish meal or fish oils (22 million tons in 2018) [78,79]. Consequently, finite and limited fisheries of small pelagic species like anchovy or sardine are more harvested, exacerbating pressure on fish stocks and significantly increasing sales prices [79]. In the longer term, aquaculture thus appears not to be economically sustainable, especially since climate change already impacts the natural production of these oils.
The new task is to find alternative n-3 PUFA sources and more sustainable fish oils and meal origins able to supplement aquaculture production and capture fisheries. One of such alternatives is the use of lower trophic level species like zooplankton, krill or copepods. This option is challenging in terms of harvesting and raises environmental questions on higher trophic levels. Top predators like whales and penguins are directly relying on these groups to survive. By collecting the prey this alternative might impact the predators, and then not be sustainable in the longer term [72]. Utilization of higher plants as a substitute for aquaculture feed is conceivable [72,80], however since they are not able to synthesize 20:5n-3 and 22:6n-3 due to the absence of dedicated enzymes [78,81], they would only supply precursors (like 18:2n-6 or 18:3n-3) and be difficult to promote for n-3 long-chain PUFAs (LC-PUFA) production by aquacultured fish. Consequently, the production of EPA and DHA by higher plants is currently attempted to implement genes of organisms naturally producing these compounds, i.e., microalgae, yeast and/or bacteria [78].
Developed in the mid-1970s for use on common crops, genetic engineering has been adapted in recent years, thanks to improvement in techniques, more specific DNA-markers and the use of genomics [82]. Genetic modifications have already introduced the n-3 PUFA biosynthesis trait to oleaginous plants. For example, this genetic engineering has been tested on Nicotiana tabacum [83], Arabidopsis thaliana [84], Brassica juncea [85], Cannabis sativa, and Camelina sativa [86,87]. At first, these transgenic plants could not match the level of EPA and DHA of microalgae or the requirement for farmed fish [78], especially in DHA [81]. However, recent studies have shown more promising results, proving, for example, the efficiency of Camelina crops to substitute both fish and vegetable oil without any negative effects on fish development or health [38,87,88]. They are real alternatives to less sustainable aquafeeds satisfying the demand for essential fatty acids as well as increasing 20:5n-3 and 22:6n-3 content for the human diet [40,88]. However, these genetically modified plants are often negatively regarded by consumers as artificial and unnatural and are perceived to carry risks for health and the environment [89]. Industries have to work on their transparency and communication strategies in order to increase the acceptance of such products on the market.
Another encouraging solution is the direct production of microalgae rich in these two PUFAs. Algal biomasses are currently used and formulated for farmed fish feed and human consumption and are commercialized by different industries [40]. These aspects will be developed later on in this review. However, even if the fatty acid composition of the different microalgae species is widely studied [76,90,91,92,93,94,95], more knowledge is needed to elucidate their synthesis pathways, as they will be particularly useful in developing new sustainable products for aquafeed as well as for human food.
4. Microalgae as n-3 Polyunsaturated Fatty Acids Producers
As already stated above, humans or top predators cannot synthesize de novo n-3 PUFAs or produce them from their precursors in large enough quantities. They thus have to obtain them from their diet [96,97]. It is well accepted that the main sources of LC-PUFAs are microalgae. Their average lipid content varies from between 1 and 40% according to the species and growth conditions [5]. Fatty acids that cannot be synthesized by all organisms are considered essential fatty acids [98]. This is the case for linoleic acid, α-linolenic acid or even longer PUFAs such as EPA, DHA or ARA [97].
The percentage of 20:5n-3 and 22:6n-3 varies with microalgae species and depends on the productivity rate and the growth conditions imposed [96]. Microalgae richer in EPA and DHA consequently present a higher nutritional value for consumers than microalgae with lower n-3 PUFA levels [96].
DHA-producers are known to be Dinophytes, Haptophytes, some Cryptophytes, Thraustochytrids, or Euglenoids [92,93,97,99,100]. Dinophytes can produce high amounts of 22:6n-3 with up to 40% of the total fatty acids in some taxa [99,101,102]. In Haptophytes, DHA production can reach 30% of total fatty acids [103]. Thraustochytrids remain some of the most essential 22:6n-3 producers and synthesize around 60% of total fatty acids in the form of triacylglycerides (TAG) in the genus Aurantiochytrium and Schizochytrium [100,104].
EPA-synthesizing taxa are Diatoms, such as Phaeodactylum tricornutum, Eustigmatophyte such as Nannochloropsis sp. and some Haptophytes [97,105,106,107,108,109,110,111]. Diatoms can synthesize around 20% of EPA and a low proportion of DHA [109]. The Eustigmatophyte, Nannochloropsis oculata, produces between 15 and 30% of 20:5n-3 [110].
For other taxa such as Cyanobacteria or Chlorophytes, long chain n-3 PUFA production can be assumed negligible [96], and only certain levels of PUFAs such as ALA and stearidonic acid (SDA—18:4n-3) can be found and play a subsiding role in consumer development, namely Chlorella vulgaris [97].
In addition to inter-species variability, LC-PUFA production can also be modulated by environmental parameters and varies with phytoplankton growth. Factors such as temperature, growth rate, irradiance, salinity, and nutrient availability can control phytoplankton PUFA production. Indeed, as stated earlier in this review, microalgae can modify their membrane fluidity in response to temperature variations, thus adapting their fatty acid composition [76,77]. Anterior works studied the impact of nitrogen and nutrient availability and showed that microalgae tended to accumulate neutral lipids when nutrients became scarce [112,113]. Microalgae sensitivity varies with taxa and can also have a tremendous impact on this lipid accumulation intensity following environmental stress [112]. In contrast, phytoplankton’s exponential growth phase associated with replete nutrient conditions would be more likely linked to higher production of polar lipids used to build cell membranes [113]. Taipale et al. [97] studied the influence of the growth stage on the production of n-3 and n-6 PUFAs in 16 species belonging to the six main groups of phytoplankton (Cryptophytes, Dinophytes, Chrysophytes, Diatoms, Chlorophytes and Cyanobacteria). They showed that PUFA content can vary greatly within phytoplanktonic groups to variation in cell size. Both increasing temperature and concentration of nitrogen were related to the production of SDA, EPA, DHA and docosapentaenoic acid (DPA-6—22:5n-6) in Cryptophytes, Chrysophytes, and Dinophytes, and the PUFA production of some taxa was more favored during the stationary phase (Cryptophytes and Chrysophytes), while others would be during exponential phase (such as fast-growing diatoms and dinophytes) [97].
Despite the extensive knowledge of the diverse species able to produce these two compounds, the synthesis pathways leading to the production of EPA and DHA are still under investigation. They would be of vital interest in improving microalgae cultures and enhancing the n-3 PUFA yields for industries, especially for healthy food and feed preparations.
5. Synthesis and Production of n-3 Polyunsaturated Fatty Acids
5.1. Nomenclature
Fatty acids are chains of hydrocarbons with a carboxyl group (COOH) and a methyl group (CH) on opposite sides of the molecule. These chains can vary in length, degree of unsaturation and ramification. Fatty acids without double bonds within the hydrocarbon chain are called saturated fatty acids. The mono-unsaturated fatty acids present only one double bond, while the polyunsaturated fatty acids possess several double bonds. These differences in length, location and numbers of unsaturation are used in the conventional nomenclature. The positioning of the first double bond relative to the methyl terminus carbon (“n”) is used to name fatty acids. SFAs are then noted X:0 with X the number of carbon atoms while MUFAs are annotated X:1n-Z and PUFAs X:Yn-Z with Y the number of double bonds and Z the position of the first double bond relative to the methyl terminus carbon, respectively [114]. Differences in the configuration are possible: a “cis” configuration indicates that carbon functional groups are on the same side of the carbon chain, while a “trans” configuration has functional groups at opposing sides of the carbon chain [114]. N-3 PUFAs, which include fatty acids such as EPA and DHA, are then polyunsaturated fatty acids presenting their first double bond on the third carbon from the methyl-end side of the carbon chain, while for n-6 PUFA (such ARA), the first double bond is located between the sixth and the seventh carbons.
5.2. Fatty Acid Synthase (FAS): Synthesis of Saturated Fatty Acids
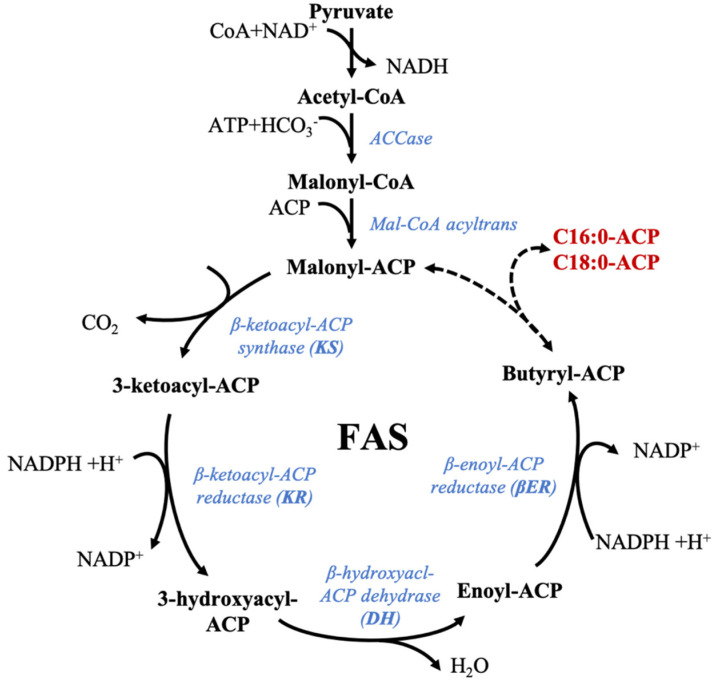
The biosynthesis of fatty acids is similar among plants and animals. It is initiated by de novo synthesis of acetyl-CoA via the concerted action of acetyl-CoA carboxylase (ACCase) and fatty acid synthase [115]. The metabolic pathway involved in lipid synthesis is initiated by glucose. Glucose is metabolized into pyruvate, then decarboxylated and oxidized by the pyruvate dehydrogenase to produce acetyl-CoA. In microalgae, fatty acid biosynthesis is assumed to be similar to that of higher plants: acetyl-CoA and de novo fatty acid synthesis seem to take place in the plastid (chloroplast) and require energy (ATP) and reducing power (NADPH) [116]. Differences in morphology and physiology of microalgae species, their cellular organization and carbon metabolism is expected to impact lipid trafficking and synthesis pathway [116]. Little is known about the routes responsible for acetyl-CoA synthesis in microalgae, but like in plants, acetyl-CoA is assumed to have two origins: it can be provided by direct supply from plastid by pyruvate dehydrogenase/decarboxylase or indirectly from mitochondrial pyruvate dehydrogenase where the mitochondrial acetyl-CoA has to be transferred to the plastid after hydrolysis to be regenerated by ACCase [117].
The first step of the fatty acid synthesis pathway is the reaction between acetyl-CoA and bicarbonate to form malonyl-CoA [116,117]. Malonyl-CoA has a central role in this synthesis as the initial precursor of the de novo fatty acids synthesis, but also by being useful in the later elongation steps occurring in the endoplasmic reticulum (ER). The conventional fatty acid synthesis pathway described in plants and microalgae is divided into three distinct pathways supported by three enzymatic systems: (i) first, the biosynthesis of palmitic acid (16:0) and other saturated fatty acids is completed from acetyl-CoA, following the Fatty Acid Synthase (FAS) pathway (Figure 1), (ii) then further chain elongations, and (iii) desaturations occur as part of the n-3 and n-6 pathways to produce more complex polyunsaturated fatty acids [116,117,118]. In microalgae, chain elongation and desaturation happen in the mitochondria and endoplasmic reticulum, desaturation steps in the endoplasmic reticulum, and de novo synthesis in the plastid [116]. Lipid chains are created from malonyl-CoA by successive additions of two-carbon units involving four enzymatic steps: condensation, reduction, dehydration, and another reduction and consuming NADPH while releasing CO2 [116]. In microalgae, fatty acid biosynthesis is performed by stromal fatty acid synthase (type II) which consists of a multisubunit composed of four monofunctional enzymes ensuring the following four enzymatic reactions [116]. The different reactions catalyzed by plant FAS and supposed to be identical for microalgae FAS as genes coding for these enzymes have been characterized for phytoplankton and were described earlier [116] (references therein). The condensation step is made by β-ketoacyl-ACP synthase (KS) and forms a simple carbon-carbon bond. Each type of KS is responsible for a different reaction: KSIII is in charge of the initial condensation between malonyl-CoA and acetyl-CoA, KSI is used during the six following iterative steps to produce 16:0-ACP, and the last elongation reaction of 16:0-ACP is conducted by KSII to form 18:0-ACP [117]. The first reduction reaction by β-ketoacyl-ACP reductase (KR) is NADPH-dependent. Next, the hydration step by β-hydroxyacyl-ACP participates in the synthesis of enoyl-ACP, which in turn is reduced by a second reductase (β-enoyl-ACP-reductase—βER) to form a saturated acyl-ACP [117]. Type I fatty acid synthase, present in mammal cytoplasms, consists of seven catalytic components linked together in a multifunctional megasynthase [116]. Its existence in microalgae cytoplasms remains unclear, but it could be possible, as putative FAS I enzymes have been identified in Nannochloropsis oceanica and Euglena gracilis [119,120]. The schematic representation of the supposed microalgae FAS pathway is available in Figure 1. Several iteration steps of the FAS pathways allow for the formation of palmitic acid (16:0).
Figure 1.
Fatty acid synthase (FAS). Fatty acid synthesis is initiated by malonyl-CoA. The different iteration of the cycle following the action of the four enzymes of the complex (KS, KR, DH, βER) add two atoms of carbon to produce saturated fatty acids such as 16:0 and 18:0. See the text for further details.
5.3. Elongation and Desaturation Steps of the n-3 and n-6 Pathways
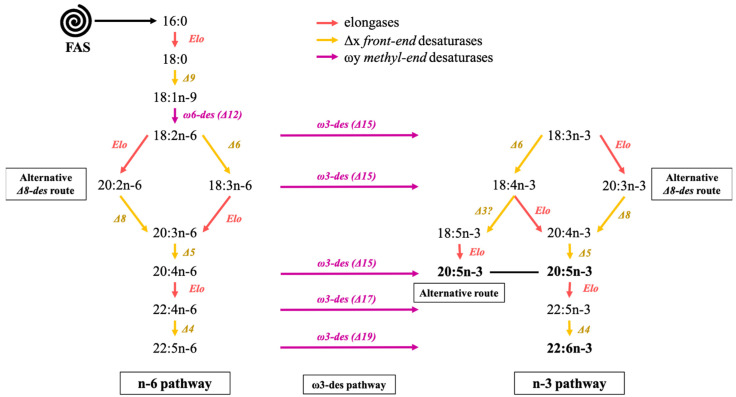
In microalgae and higher plants, at this point, palmitic acid can follow three different routes: (i) it can be used in the plastid to form chloroplast lipids such as the galactolipids, (ii), further elongated into 18:0 and then more complex fatty acids or (iii) converted into a free fatty-acid [121,122]. The next steps of the synthesis pathway allow the production of a panel of fatty acids varying in both chain length and degree of unsaturation. After being synthesized by the FAS pathway, 16:0 encompass one last condensation, reduction, dehydration and reduction step, respectively, and gives a saturated two-carbon longer fatty acid: stearic acid (18:0). 16:0 and 18:0 are the major products of the FAS pathway in microalgae [116]. An acyl-CoA desaturase is then involved in the following reaction step. A desaturase is a special type of oxygenase able to remove two hydrogens from a hydrocarbon chain, catalyzing the formation of a double bond in the substrate [121,123]. According to their regioselectivity, desaturases are noted Δx where x refers to the position from the carboxyl-end of the fatty acid where the double bond is added [123]. After FAS synthesis, a Δ9-desaturase (adding the double bond on the ninth carbon from the carboxyl-end) is responsible for the formation of the first double bond, leading to the formation of the first monounsaturated fatty acids, palmitoleic acid (16:1n-7—PAL) and oleic acid (18:1n-9—ODE) respectively, from 16:0 and 18:0 [116].
The fatty acids formed by FAS and from FAS-products following the action of desaturases have two possible fates in plants: (i) they can remain in the plastid and be involved in the synthesis of glycolipids especially galactolipids or (ii) they can be released to the cytosol and transfered to the endoplasmic reticulum to be used in extraplastidial lipid synthesis [116]. In microalgae, similar fatty acid trafficking could exist as studied in the diatom Chaetoceros muelleri with 13C-labelling [124].
After being synthesized in the plastid, saturated fatty acids are transferred to the cytosol to be elongated and/or desaturated. The elucidation of fatty acid synthesis pathways in microalgae is still ongoing. Even if a synthesis pathway of some species such as the diatoms are more extensively studied [107,125,126,127], for other species the synthetic routes remain to be identified. The following paragraph describes the polyunsaturated fatty acid synthesis pathway as it has been assumed to occur in plants and microalgae [116]. The conventional elongation and desaturation pathway present in numerous eukaryotes names, hereafter n-3 and n-6 pathways and the polyketide synthase pathway (PKS pathway), also exist to produce polyunsaturated fatty acids.
Both n-3 and n-6 pathways are reported to performed in the mitochondria and the endoplasmic reticulum (ER) and present a similar synthesis sequence as those of palmitate, and involve specific enzymes acyl-CoA derivatives (Figure 2). In the conventional oxygen-dependent pathway, 18:1n-9 is desaturated by Δ12 desaturase and further by a Δ15 desaturase to form linoleic acid and α-linolenic acid, respectively, the precursors of the n-6 and n-3 fatty acid families [128]. Exclusively in diatoms, 16:1n-7 can be transformed by Δ6 and Δ15 desaturations into more complex C16 PUFAs (16:2n-7, 16:2n-4, 16:3n-4 or 16:4n-1) [127]. It is called the “C16 PUFAs pathway”. The different reactions producing more complex fatty acids from 18:2n-6 and 18:3n-3 are available in Figure 2. In nature, several desaturases complexes have been identified. Front-end desaturases (noted Δx) add the double bond at position x from the carboxyl-end of the carbon chain, while methyl-end desaturase (noted ωy) introduces the double bond at position y from the methyl-end [121,129]. Metazoan cells, for example, contain front-end desaturases such as Δ5 and Δ6 desaturases but do not possess methyl-end desaturases like ω6 (Δ12) or ω3 (Δ15). Mammals can elongate and desaturase dietary 18:2n-6 and 18:3n-3 into C20-C22 PUFAs (20:4n-6, 20:5n-3, 22:6n-3) using their Δ4, Δ5 and Δ6 desaturase. Nevertheless, as previously stated, the conversion rate of 18:2n-6 and 18:3n-3 into essential C20-C22 PUFAs is generally insufficient and thus has to be supplied by the diet. On the contrary, microalgae have both methyl-end and front-end desaturases and thus play an important role in providing higher trophic levels with these essential compounds.
Figure 2.
Fatty acid synthase pathway (FAS pathway) assumed for microalgae. Enzymes involved in desaturation and elongation processes from the conventional pathway are colored: yellow for front-end desaturases, magenta for methyl-end desaturases and red for elongases. Enzyme names are written next to or above the corresponding arrow.
5.4. Polyketide Synthase Pathway (PKS)
Another oxygen-independent pathway exists to synthesize unsaturated fatty acids and is called the polyketide synthase pathway, or PKS pathway [128]. In contrast to n-3 and n-6, which are more widely represented among microalgae taxa, the PKS pathway is limited to only some families, namely bacteria but also Thraustochytrids or Dinophytes [130,131]. The PKS pathway and FAS pathway share functioning similarities and rely on the same four basic reactions, namely condensation, reduction, dehydration and reduction by the four same enzymes: β-ketoacyl-ACP synthase (KS), β-ketoacyl-ACP reductase (KR), β-hydroxyacyl-ACP dehydrase (DH), and β-enoyl-ACP reductase (βER). While FAS synthesis and elongation/desaturation pathways, involving numerous enzymatic activities and reactions are very energy-consuming [132], the PKS pathway, in constrat, has fewer reduction and dehydration steps and can produce PUFAs more efficiently [133]. Indeed, the metabolites used to synthesize the fatty acid remain unsaturated as it gets lengthened. The PKS pathway, even if fundamentally anaerobic, can take place in aerobic conditions [134]. The PKS pathway was first identified in the deep-sea bacteria, Shewanella [133]. Later on, the discovery of PKS encoding genes as well as the identification of PKS routes in Thraustochytrids [135,136], Dinophytes [137,138,139], Haptophytes [140,141], and Chlorophytes [140] supported the broader existence of this pathway among protists.
The polyketide synthases are subdivided into three types. Type I PKS are large multifunctional enzymes with several catalytic domains located on the same polypeptide. Each module is responsible for a set of distinct, non-iteratively acting activities responsible for the catalysis of one cycle of polyketide chain elongation [142]. Type II PKS are complexes composed of monofunctional enzymes, where each enzyme carries one catalytic domain and a single set of iteratively acting activities [142]. Finally, Type III PKS are homodimeric enzymes essentially involved in condensation reactions [142]. Type I and Type II need an acyl carrier protein (ACP) to activate the substrate responsible for the channeling of the growing polyketide intermediates. In contrast, Type III acts directly on the acetyl-CoA independently of ACP [142]. Type I PKS is mainstream of protist and has been identified in Haptophytes [141,143], Chlorophytes [140], Dinophytes [137,144], and Thraustochytrids [145]. In Dinophytes, the multifunctional activities characteristic of Type I PKS is not always verified because some species like Karenia brevis, Alexandrium ostenfeldii, and Heterocapsa triquetra possess an atypical architecture for Type I PKS with only one catalytic module [144,146]. Type II PKS is found in Haptophyte such as in the coccolithophorid Emiliania huxleyi, in Cryptophyte, and in Dinophyte [145]. Type III PKS is usually restricted to higher plants, fungi and bacteria [147,148].
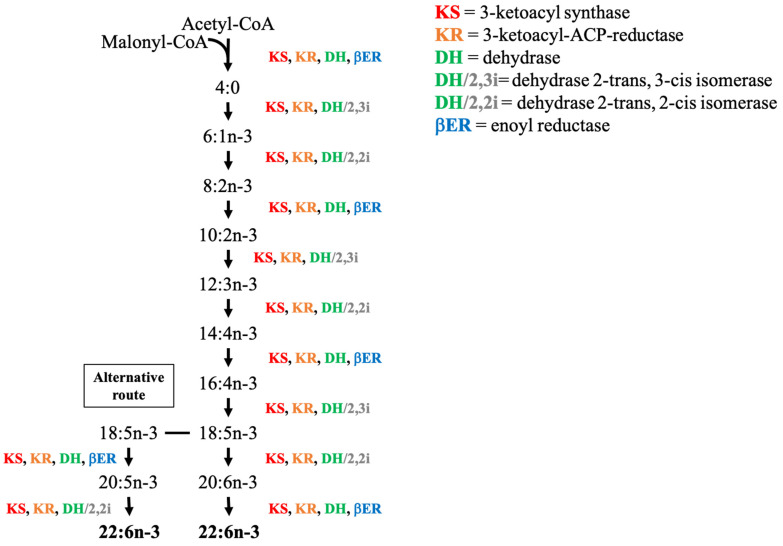
As mentioned earlier, the PKS pathway relies on the same four enzymes as the FAS pathway and can be completed with two isomerases (2.3 I or 2.2 I) (Figure 3). The precursors of the PKS pathway are identical to those of the FAS pathway i.e., acetyl-CoA and malonyl-CoA. The ketoacyl-synthase (KS) and ketoacyl-reductase (KR) are in charge of the addition of two carbon units while the dehydrogenase (DH) (or dehydrogenase associated with the isomerases) and enoyl-reductase (βER) are inserting the double bond. The difference between the functioning of PKS and elongation/desaturation pathways resides in this double bond insertion: the PKS pathway creates fatty acid by adding carbons and directly placing the unsaturation on a nascent acyl chain, while in n-3 and n-6 pathways, the double bond is inserted into an intact acyl chain [118]. Consequently, the PKS pathway requires less energy, as the ATP needed during the desaturation steps of the conventional pathway is not needed [118]. The different precursors and fatty acids assumed to be involved in the PKS pathway and proposed by Bell and Tocher (2009) [118] are presented in Figure 3. The large majority of the studies working on PKS have given a lot of attention to production of n-3 PUFAs, especially 22:6n-3 (DHA) and 20:5n-3 (EPA) [118,149]. However, more recent works show that PKS might not only be limited to n-3 PUFAs. Indeed, Zhang et al. (2017) [150] suggested the existence of a PKS-like pathway synthesizing the n-6 PUFA DPA-6, and Remize et al. (2020) [139] studied the fatty acid synthesis pathways of Alexandrium minutum and also proposed a n-6 PKS pathway to produce 22:5n-6. However, the difficulty in studying these pathways results from the fact that precursors used to form longer n-3 and n-6 PUFAs are generally absent, leading to further challenges in elucidating their synthesis routes [151].
Figure 3.
Polyketide synthase (PKS) pathway assumed for microalgae. Enzymes involved in the PKS pathway are the 3-ketoacyl synthase (KS—red), 3-ketoacyl-reductase (KR—orange), dehydrase with associated isomerases (DH—green, i—grey) and enoyl-reductase (βER—blue).
5.5. Elucidation of Microalgae Synthesis Pathways
To better understand the pathways used by microalgae to synthesize fatty acids, several studies have focused on the elucidation of synthesis pathways using different techniques. While certain taxa, such as diatoms like Phaeodactylum tricornutum, are studied more extensively, other groups remain to be understood. Preliminary work used radioactive label 14C-acetate to study P. tricornutum synthesis pathway [107,125]. Using 14C-labeled substrate they showed that 20:5n-3 in diatoms might use a combination of both n-3 and n-6 pathways and that C18 PUFAs of the n-6 family (18:2n-6 and 18:3n-6) can be used to form corresponding C18 PUFAs of the n-3 family 18:3n-3 and 18:4n-3, respectively, by a ω3-desaturase. More recent works have focused on understanding gene expression. Indeed, genes are interesting targets for enhancing fatty acid metabolism and improving the production of DHA and EPA [152,153]. The discovery of new genes or new enzymes involved in these pathways could also guide research and help find new objectives. Consequently, new genes involved in the n-3 and n-6 conventional pathways or PKS pathway have been discovered in P. tricornutum and Karenia mikimotoi (Dinophyte), respectively, and could be used in understanding the selected routes for EPA and DHA synthesis [138,154].
Comprehension of these pathways, however, remains incomplete. More recent studies have thus focused on characterizing the metabolic pathways quantitatively. To do so, 13C-labelling flux analyses appear to be a promising approach and have already been used on microalgae [124,139,155]. While relying on an isotopically labeled substrate, this technique aims to monitor the label’s progressive incorporation into organic molecules of interest. This will identify the metabolic intermediates and end products, giving insights into biosynthesis pathways. Thanks to the development of a new generation instruments such as gas chromatography with mass spectrometry (GC-MS) or gas chromatography coupled to isotopic ratio mass spectrometry (GC-c-IRMS), considerable improvements have been made, and it is now possible to resolve the isotopic composition of organic macromolecules including fatty acids [156,157].
In the future, further understanding of microalgae polyunsaturated fatty acid synthesis pathways is necessary. The genes and enzymes involved and the identification of metabolic intermediates will be of central interest to increase polyunsaturated fatty acid production and to ease the development of valuable ingredients for food and feed industries.
6. Use of n-3 Polyunsaturated Fatty Acids in Food and Feed Industries
6.1. Use and Proprieties of n-3 Polyunsaturated Fatty Acids in the Food Industry
Microalgae have been used in the human diet for centuries, especially in Asia and in Africa for species such as Spirulina sp. [4]. Thanks to their healthy and bioactive compounds such as carotenoids, phycobilins, fatty acids, polysaccharides, vitamins, and sterols, and also to their capability for production, they have been widely used in the health food market [4]. They have a range of relevant properties that can meet the increasing demand for sophisticated products [158]. The most popular way to consume microalgae is as a dietary supplement in tablet, capsule or powder form [159]. Indeed, the addition of the entire microalgal biomass is not always easy because of its color, fishy taste, smell and consistency [4]. An interesting approach in using microalgae as food is to employ the biomass as a source of specific biomolecules as it has already been tested in food and nutraceutical formulations [159]. Numerous studies have focused on extracting bioactive molecules and compounds such as fatty acids, proteins, pigments or antioxidants as additives for the food industry and as alternatives to the usual molecules [160,161,162,163].
The microalgal lipids can be divided into two major groups: (i) the neutral lipids (non-polar lipids) such as sterol, triacylglycerol (TAG), and free fatty acids are storage lipids and used as a stock of energy, and (ii) the polar lipids are structural lipid usually found in cell membranes. Short-chain saturated fatty acids (mainly neutral lipids) are valorized in the biofuel industry while, thanks to their health benefits, long-chain polyunsaturated fatty acids (esterified with glycerol in microalgae and generating TAG or polar lipids) are used in the nutraceutical industry or in active drug processing [164,165].
Microalgae biomass is predominantly utilized in the health food market and represents 75% of the annual microalgal biomass production [166]. Powders rich in n-3 PUFAs are manufactured into capsules or tablets and are expected to ensure a stable industry in the future [158]. This is the case for PureOneTM, a supplement capsule composed of EPA and DHA [165]. Allmicroalgae Natural Products (Portugal) developed health-benefits capsules and tablets with Chlorella vulgaris that are rich in omega-3, proteins, fiber, vitamins and iron under the brand Allma (known as Easy Capsules and Easy Tablets) [167]. Microalgae n-3 PUFAs are also formulated as oils with unique characteristics and different from plant or animal oils where oil is usually composed of a range of saturated and unsaturated fatty acids [130]. For example, Algae Omega 3 is formed with Schizochytrium sp. fatty acids and has been approved for human consumption [165]. OmegaTech (USA) exploits Schizochytrium to produce a low-cost oil known as DHA Gold [168,169]. This oil is currently supplemented as an adult dietary supplement in food (like cheese, yogurts, spreads and dressings) and beverages [168]. Nutrinova (Germany), and Martek (USA), respectively, cultivate the microalgae Ulkenia sp. (Thraustochytrid) and Crypthecodinium cohnii (Dinophyte) to produce similar oils rich in DHA [130].
Chlorella has been cultured in large-scale structures for the production and commercialization of fatty acids (including omega 3 and omega 6) because it has been recognized for its stimulation properties for the human immune system [167,170]. The green edible microalga, Dunaliella salina, a by-product of large-scale salt producing evaporative ponds, is also valorized as health food [171,172]. Babuskin et al. (2014) [173] used Nannochloropsis oculata for its richness in omega-3, EPA and DHA, to create functional cookies and pasta with health benefits. Pavlova lutheri was supplemented in the yogurt formula for its n-3 PUFA content having anti-inflammatory proprieties in vitro which can also be interesting for consumer health [174]. However, the algae-based yogurt was not pleasant enough for consumers and needed improvement [174]. Incorporating enriched n-3 PUFAs algae oils into yogurt seems like an interesting idea to consider for new food development, especially for vegetarians [175]. Ingestion of this enhanced-yogurt significantly increases the level of DHA in the plasma and blood erythrocytes [175]. Consequently, another advantage of using algae to synthesize these beneficial compounds for humans also resides in the fact that they seem an appropriate ingredient for vegetarians [175]. Species like Schizochytrium sp. presented before and recognized for their high DHA levels are more and more consumed for their DHA-rich oil and are also suitable for a vegetarian diet [169]. They are also particularly interesting as they are known to be safe, without detectable environmental pollutants or heavy metals [169].
Vegetarians who supplemented with DHA from Ulkenia sp. showed positive results on the accumulation of n-3 PUFAs of interest in red blood cells. This tested microalga oil also seemed to impact their appetite and regulation of food intake [176]. N-3 PUFAs (EPA, DHA, ALA) can be supplemented in health drinks, such as infant grade milk or in Chlorella-enriched beverages [158].
Microalgae lipids can be used for their antioxidant, anti-cancer and anti-inflammatory properties. For example, furan fatty acids are free radical scavengers and present an electron-rich furan ring within the carbon backbone [177]. They have been identified as powerful anti-inflammatory agents and are known so far in Isochrysis sp. and Phaeodactylum tricornutum [177,178,179]. Their development and use in the food industry are still in their infancy, as no clinical trials have been performed to evaluate their effect on cardiometabolic health because they are difficult to extract and purify [177]. The key link between n-3 PUFA and inflammation is eicosanoid metabolism [30]. Indeed, eicosanoids are mediators and regulators of inflammation and are generated from C20 PUFAs [30]. Nitrogen-containing compounds (lipopeptides) produced by cyanobacteria aim for tubulin or actin filaments in eukaryotic cells allowing their detection by anticancer agents [180]. Phytosterols containing food can also be interesting in reducing the concentration of blood cholesterol and preventing the onset of cardiovascular diseases, diabetes and hypertension [166].
However, microalgae PUFAs’ functionality in food products is not limited to their health aspects, as they can also have bioactive properties that can be used in food formulation, for example, in structuring processes [12]. In the following paragraph describes examples of the application of microalgae lipid fractions in the food industry.
Species rich in proteins such as Chlorella sp., Haematococcus pluvialis or Tetraselmis sp. were studied as promising microalgae for the production of surface-active ingredients [181,182]. Indeed, the complex structure of proteins makes them particularly suitable for production of stable viscoelastic film valuable in emulsion [183,184]. However, Law et al. [184] stated that proteins might not always be as good of a surfactant, i.e., reducing interfacial tension between oil and water phases despite being an excellent emulsifier as smaller molecular weight molecules especially in food processing. Indeed, small molecular-weight surfactants have a higher diffusivity and are more easily available to absorb and lower interfacial tension [183]. Consequently, small molecular-weight compounds produced by microalgae such as lipids, have been studied for their surface-active proprieties. This is the case of glycolipids (monoacylglycerol—MAG or diacylglycerol—DAG), phospholipids (phosphatidylcholine—PC also called lecithin) or fatty acids [185,186,187]. Marine phospholipids contain high levels of lecithin (phosphatidylcholine—PC) which have amphiphilic properties, and thus are particularly suitable for emulsion preparation [188]. Most of the current literature on the subject discusses the use of n-3 triacylglycerol fortified functional foods, whereas food fortification with phospholipids is less studied [188]. For example, n-3 PUFA phospholipid rich oils have been used to fortify surimi seafoods and confer them a better resistance towards lipid oxidation due to the presence of EPA and DHA [189]. Similar types of phospholipids have been used to create nutraceutically fortified egg products [190,191].
Microalgae biomass and lipids fraction are also used as food quality enhancers and preservatives. The development of algae-based lipid powders and flours is a trending topic and is more and more included in novel cuisine. The addition of the edible Dunaliella salina to pasta recipes increases its sensory characteristics especially due to the higher presence of polyunsaturated fatty acids, phytochemicals, and minerals [192]. They are being used as egg substitutes with really promising results, especially for vegetarians [193]. Lipid powders, especially those rich in 14:0, 16:0, 18:1n-9 and EPA from microalgae, are also interesting for their antimicrobial properties, which can play a significant role in product preservation [193]. Microalgal biomass is used to increase the nutritional properties of dairy food products such as cheese. Chlorella and Arthrospira have been used in cheese and reported to positively impact lactic acid bacteria viability and enhance quality and sensory properties [194]. Nannochloropsis oculata and Isochrysis galbana were used in novel cookies, pasta and biscuit formulas and significantly increased the concentration of n-3 PUFAs inducing a positive sensory evaluation by consumers [173,195]. Auxenochlorella protothecoides were used to create an algal flour (AlgaVia® Lipid-Rich Whole Algae by Corbion) rich in polyunsaturated fats be used in various types of foods [194]. Another important advantage in adding microalgae biomass rich in lipids, especially n-3 PUFAs, could be their higher resistance to thermal treatment [195,196]. This preserves the greater nutritional quality of the microalgae-enriched-products in comparison with conventional products.
6.2. Example of Microalgae Valuable in Food Industries
As previously mentioned, only a few microalgae species are intensively used for human consumption, among them Chlorella vulgaris, Spirulina (Arthrospira), and Tetraselmis chuii; nevertheless, Dunaliella, Haematococcus, Schizochytrium, Scenesdesmus, Aphanizomenon, Odontella, and Porphyridium are gaining acceptance in the food and health food market [197]. Currently, Chlorella and Arthrospira largely dominate the market [158]. Allmicroalgae, based in Portugal, is commercially supplying the species approved for human consumption in powder and frozen paste format to be added to soups, millets, juices, crackers, cookies, ice creams, smoothies or dietary supplements [198]. Indeed, Chlorella vulgaris and Arthrospira are relatively easy to grow. Arthrospira grows in an alkaline environment and under saline conditions that prevent the growth of competitive organisms or contaminants [199]. Cyanobacteria are mainly sold in Europe, North America, and Asia [168]. Spirulina (Arthrospira) has been used for years, especially by African and Mexican populations living around alkaline lakes where it develops naturally [200]. Although mainly used for its richness in polysaccharides and phycobiliproteins, Arthrospira is also known for its important production (3–35%) of the essential γ-linolenic acid (18:3n-6—GLA), which is pharmacologically significant as it is effective in lowering plasma cholesterol and in stimulating prostaglandins [198,200]. However, it can also synthesize DHA in lower proportions (9% of total fatty acids content) that is involved in regulation of inflammatory, immunological, and cardiovascular disorders [198,201]. Chlorella has been widely cultivated and commercialized in the health food market [199]. Chlorella is a spherical unicellular green alga that reproduces asexually [202]. One advantage of this species is its capacity to be cultivated in heterotrophic, autotrophic, and even mixotrophic conditions [199]. Its composition in polyunsaturated fatty acids (especially 18:2n-6 and 18:3n-6) has been shown to confer antioxidant, antitumor and anti-inflammatory proprieties valued in the food industry [201,203]. Chlorophytes generally contain low levels of EPA, with an exception made for the species Chlorella minutissima, that has been proven to produce a high content of this LC-PUFA in photoautotrophic conditions (31% of total fatty acids) or when incubated at low temperatures (45% w/w) [204,205]. Chlorella has been used for health food production in Germany, China, Japan, and other Asian countries and valorized as food supplements for humans [202]. Tetraselmis chuii (Chlorophyte) is another species valorized in food and health products. In Europe, T. chuii has been approved as a novel food to be used as a sauce, special salt, and condiment since 2004 [194], while the Thraustochytrid Ulkenia, rich in DHA, was approved in 2009 as a novel food ingredient in bakery products, cereal bars, and nonalcoholic beverages [206]. Other species such as Crypthecodinium, Nannochloropsis, Phaeodactylum, Monodus, Nitzschia or Isochrysis, are currently under investigation for their health supplement, especially their PUFA production [207]. In the United States, the Food and Drug Administration (FDA) ranked microalgae as generally recognized as safe (GRAS). This is the case for species such as Euglena gracilis for conventional food and beverage products such as baked goods, cereals, milk and dairy products, processed fruits, fruit juices, soft candy, and soups [194]. Other GRAS species are Arthrospira platensis, Auxenochlorella protothecoides, Dunaliella bardawil, Dunaliella salina, Schizochytrium sp., and Ulkenia sp. [194,208]. Microalgae species are authorized in Europe, France and the USA, and examples of applications are available in Table 1.
Table 1.
Microalgae used in the food industry and authorized in France, Europe, and the USA (GRAS: generally recognized as safe), and examples of application. DS: dietary supplement, E: extract, EP: Europe, F: Food, FR: France, USA: United States of America.
| Scientific Name | FR [209] | EP [209] | USA [194,208] | Example of Application |
|---|---|---|---|---|
| Aphanizomenon flos aquae | DS | F | Functional ingredient with antioxidant properties for cookies [210] | |
| Arthrospira sp. | F | F | DS, F | Fermenting agent for lactose-free beverages [211] |
| A. major | DS | |||
| A. maxima | DS | |||
| A. platensis | DS | F | DS, F | |
| Auxenochlorella protothecoides | DS, F | Algal flour for baked goods [194] | ||
| Chlamydomonas reinhardtii | DS, F | Omega-3 fatty acids valuable in healthy food [212] | ||
| Chlorella sp. | F | Tablets and powder made with whole biomass for human food [167] | ||
| C. luteoviridis | F | |||
| C. pyrenoidosa | F | |||
| C. vulgaris | DS | F | DS, F | |
| Dunaliella salina | DS | DS, F | Biomass used in pasta [192] | |
| D. badarwil | DS, F | |||
| Euglena gracilis | DS, F | Produce paramylon acting against fatigue [213] | ||
| Haematoccocus pluvialis | DS | DS, E | Astaxanthin-rich oleoresin with antioxidant capacity for healthy food [214] | |
| Nannochloropsis oculata | DS | Ingredient in functional cookies [173] | ||
| Odontella aurita | DS | DS | Produces fucoxanthin with antioxidant activities [215] | |
| Schizochytrium sp. | DS | E | E | Used for DHA-rich oil [165] |
| Tetraselmis chuii | DS, E | Ingredient for broccoli soup [216] | ||
| Ulkenia sp. | DS | E | E | Used for DHA-rich oil [130] |
6.3. Use of n-3 Polyunsaturated Fatty Acids in the Feed Industry and Aquaculture
Microalgae are also used for feed, especially in aquaculture, since they represent the conventional diet of marine and freshwater consumers in natural ecosystems [179,198]. Species like Dunaliella, Chlorella, Arthrospira, Nannochloropsis or Tetraselmis proved to have good application in aquaculture [1]. Indeed, species like Nannochloropsis sp. are characterized by high levels of PUFAs (37%) and are rich in EPA and DHA [217]. Chlorophytes such as Chlorella, generally have low nutritional values due to their low levels in essential long-chain polyunsaturated fatty acids, and are then often combined with other species when used as feed [218]. The Baccilariophyceae, including diatoms (such as Chaetoceros sp. or Phaeodactylum sp.), are more interesting as they produce important levels of EPA [111].
Microalgae are cultivated and concentrated in the form of dispersible pastes (which can be added directly in the water tank and dissolved in the liquid media) or as freeze-dried cubes [198]. When used alive, microalgae are a source of essential fatty acids [198]. To form the microalgae pastes, diatoms such as Chaetoceros sp. and Phaeodactylum sp. or the Eustigmatophyte, Nannochloropsis oculata are generally more used [198]. As for food industries, microalgae rich in PUFA can be used as functional feed. Microalgae biomass can enhance animal physiology by improving their immune response, disease resistance, antiviral and antibacterial protections, and better reproductive performance, feed conversion, and weight gain [219]. In the domain of aquaculture feeding, microalgae biomass appears really promising for a more sustainable animal production industry as they are more environment-friendly than classical land agriculture [219].
Mollusk species are the largest aquaculture group of animals that rely on microalgae as feed [179]. As an example, in 1999, 62% of the microalgae produced for aquaculture were used for mollusks, while 21% were used for crustaceans (mainly shrimps) and only 16% for fish [168]. Microalgae species such as Isochrysis affinis lutea (T-iso), Pavlova lutheri, and Chaetoceros sp. are commonly used in shellfish hatcheries for their richness in nutrients, especially n-3 PUFAs [199]. They are easily and efficiently filtered from the water where the shells grow [179]. Crustaceans also feed directly on microalgae, and their PUFAs are essential during the larval stage [168,220].
Microalgae can be used for their suspected role as probiotics. This remains to be proven, however, and concrete evidence of the action of living microalgae in aquaculture farms is still scarce [219]. For example, Chaetoceros sp., Pavlova sp., and/or Isochrysis sp. have been added to pearl oyster diets and have been shown to improve their resistance to bacterial pathogens [221].
Microalgae can also be utilized indirectly in aquaculture feed. Indeed, heterotrophic protists and small zooplankton (such as rotifers of genus Brachionus or the brine shrimp Artemia salina) play a major role in supplying and channeling energy and essential compounds from microalgae to higher consumers [222,223]. By feeding on microalgae, these organisms give access to nutrients, polyunsaturated fatty acids, amino-acids, and sterols to higher trophic levels that are originally unable to use them due to their small sizes [223,224]. For example, the heterotrophic dinoflagellate Crypthecodinium cohnii has been marketed for its DHA production and used as a substitute for fisheries-derived oils for seabream (Sparus aurata) microdiets [225]. The elevated DHA production of Crypthecodinium was particularly suitable for the high requirement for this essential PUFA of seabream larvae and resulted in similar performances as with classical fisheries-related diets [225]. Nannochloropsis sp. and Isochrysis galbana are also widely used for rotifer production and to enhance their richness in EPA and DHA [226,227,228]. Indeed, they increase their survival, productivity, efficiency of feed assimilation, and biochemical composition [227,228]. Schizochytrium can also be supplemented to zooplankton rotifers to increase their levels of DHA [229]. Special care has to be given to the microalgae’s culture condition because they can have a significant effect on their nutritional quality for predators, especially since rotifer fatty acid composition is closely related to that of their diet [228,229]. Rotifers enriched with high lipids levels can be used as the first food organisms in culture of fish and shrimp larvae [226,229]. They are in charge of supplying consumers with macro- and micronutrients, vitamins and even antibiotics [226]. An advantage of using lipids from microalgae for aquaculture is the possibility of adjusting and enhancing the nutritional composition of microalgae by monitoring environmental parameters [73].
Microalgae can also be incorporated into the feed of terrestrial animals and positively impact their survival, development, growth, and fertility [199]. Species like Spirulina, Chlorella, Tetraselmis, Nannochloropsis, Nitzschia, Navicula, Scenesdesmus, Crypthecodinium, and Chaetoceros are valorized and commercialized for pets, ruminants, pigs, poultry and other animals [230]. Arthrospira is also highly used in this domain and touches all types of animals, such as cats, dogs, aquarium fish, ornamental birds, horses, cows, and breeding bulls, because it affects their physiology to essential fatty acids among other [168]. Crypthecodinium cohnii and Schizochytrium sp. are used as poultry feed, especially for chickens to produce eggs rich in omega-3 [165]. As for humans, EPA and DHA have been credited with health benefits even when supplemented in small amounts, and can have positive effects on the improvement of the immune system, lipid metabolism, stress resistance, increase in appetite, enhancing reproductive performance, or even the reduction of cholesterol levels [231]. Nannochloropsis oceanica has been tested as a natural source of EPA for ruminants [232]. The algae’s thick cell walls protect the healthy compound from metabolization by rumen microbiota, inducing a full enrichment in the EPA for better ruminant development and health without inhibiting the growth and activity of rumen bacteria [232]. Providing n-3 PUFAs has a positively impact on atopic dermatitis, osteoarthritis, and the modulation of immunity indicators in dogs [233]. DHA in dog diets can improve their immune system, enhance the palatability of their food, increase digestibility, and enhance their cognitive responses [234,235]. The use of Algal Oil rich in EPA and DHA has been proven efficient in Beagle dogs during their reproduction, development, growth, and during the early life stages of their offspring [236]. In shrimp hatcheries, microalgae are necessary for their PUFA production that participates in the second stage of larval development (zoea) [218].
Consequently, industrial companies are progressively developing feed for pets, poultry and cattle based on microalgae biomass or extracts to improve their health or a better nutritional quality. This is the case, for example, with Allvitae by Allmicroalgae Natural Products, which propose various ranges of dog, cat, swine, bird, and poultry feeds rich in omega-3, especially DHA from species such as Chlorella vulgaris, Tetraselmis chui, Nannochloropsis oceanica, Scenesdesmus obliquus [237]. These feeds enhance their vital functions, heart conditions, cognitive development, or stimulate their intestinal immunity [237]. Veramaris proposes a feed (Veramaris® Pets) rich in EPA and DHA via the use of algal oil and used for aquafeeds. Schizochytrium limacinum is used to made All-G-RichTM (Alltech), a dehydrated whole-cell meal for chicken composed of 16% of DHA [238]. Hens supplemented with All-G-Rich can produce eggs richer in DHA without modifying their quality [238]. AlgaPrimeTM from Corbion is also marketed for animal feed and consists of a DHA source from Schizochytrium sp. [239].
7. Conclusions
EPA and DHA are receiving increasing interest for their positive impact on human health thanks to their antioxidant, anti-inflammatory and anti-cancer properties. Originating mainly from marine sources such as fish, these two compounds face supply challenges due to current stock reduction and overfishing. Because of environmental stress and increased pressure on the natural stock, aquaculture is not sufficient for filling the gap between supply and demand. New techniques, such as genetic engineering to increase the production of these molecules for humans, are in development.
As primary producers of EPA and DHA, microalgae have been used in recent years to develop the future ingredients and foods. Currently, only a small proportion of known microalgae species are used in the food and feed industry, as authorization proving they are safe for consumption is difficult to obtain. A rising number of companies are using n-3 PUFA as a functional ingredient for health food, food, supplementary food, or even for their physicochemical properties. The nutritional quality can enhance the final product for humans and both marine and terrestrial animals.
In the future, increasing the application of these healthy compounds is expected to and must be associated with increased knowledge of microalgae synthesis pathways to improve their production sustainably. Authorities should also allow more microalgae to be used in this sector to expand the range of possibilities.
Acknowledgments
We thank the help of Aswani Volety, Elon University (North Carolina).
Author Contributions
Conceptualization, M.R., E.F. and J.L.S.; validation: all authors; resources: J.-Y.B. and Y.B.; writing—original draft preparation, M.R.; writing—review and editing: all authors; visualization, M.R.; supervision, E.F., Y.B. and J.-Y.B.; project administration, Y.B. and J.-Y.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patras D., Moraru C.V., Socaciu C. Bioactive Ingredients from Microalgae: Food and Feed Applications. BUASVMCN-FST. 2019;76:1–9. doi: 10.15835/buasvmcn-fst:2018.0018. [DOI] [Google Scholar]
- 2.Levring T., Hoppe H.A., Schmid O.J. Marine Algae: A Survey of Research and Utilization. Walter de Gruyter GmbH & Co KG; Hamburg, Germany: 2019. [Google Scholar]
- 3.Pinckney J.L. A Mini-Review of the Contribution of Benthic Microalgae to the Ecology of the Continental Shelf in the South Atlantic Bight. Chesap. Sci. 2018;41:2070–2078. doi: 10.1007/s12237-018-0401-z. [DOI] [Google Scholar]
- 4.García J.L., De Vicente M., Galán B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017;10:1017–1024. doi: 10.1111/1751-7915.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chacón-Lee T., González-Mariño G. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010;9:655–675. doi: 10.1111/j.1541-4337.2010.00132.x. [DOI] [PubMed] [Google Scholar]
- 6.Barka A., Blecker C. Microalgae as a Potential Source of Single-Cell Proteins. A Review. Biotechnol. Agron. Société Environ. 2016;10 doi: 10.25518/1780-4507.13132. [DOI] [Google Scholar]
- 7.Spier M.R., Peron-Schlosser B., Paludo L.C., Gallo-García L.A., Zanette C.M. Handbook of Microalgae-Based Processes and Products. Elsevier; Amsterdam, The Netherlands: 2020. Microalgae as enzymes biofactories; pp. 687–706. [Google Scholar]
- 8.Raghukumar S. Thraustochytrid Marine Protists: Production of PUFAs and Other Emerging Technologies. Mar. Biotechnol. 2008;10:631–640. doi: 10.1007/s10126-008-9135-4. [DOI] [PubMed] [Google Scholar]
- 9.Leyland B., Leu S., Boussiba S. Are Thraustochytrids algae? Fungal Biol. 2017;121:835–840. doi: 10.1016/j.funbio.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Gouveia L., Batista A.P., Sousa I., Raymundo A., Bandarra N. Microalgae in novel food products. In: Papadopoulos K.N., editor. Food Chemistry Research Developments. Nova Science Publishers; Hauppauge, NY, USA: 2008. pp. 2–37. [Google Scholar]
- 11.Nova P., Martins A.P., Teixeira C., Abreu H., Silva J.G., Silva A.M., Freitas A.C., Gomes A.M. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. Environ. Boil. Fishes. 2020;32:1789–1802. doi: 10.1007/s10811-020-02129-w. [DOI] [Google Scholar]
- 12.Bernaerts T.M., Gheysen L., Foubert I., Hendrickx M.E., Van Loey A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019;37:107419. doi: 10.1016/j.biotechadv.2019.107419. [DOI] [PubMed] [Google Scholar]
- 13.Sathasivam R., Radhakrishnan R., Hashem A., Allah E.F.A. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019;26:709–722. doi: 10.1016/j.sjbs.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamed I., Özogul F., Özogul Y., Regenstein J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015;14:446–465. doi: 10.1111/1541-4337.12136. [DOI] [Google Scholar]
- 15.Li D., Wahlqvist M.L., Sinclair A.J. Advances in n-3 polyunsaturated fatty acid nutrition. Asia Pac. J. Clin. Nutr. 2019;28:1–5. doi: 10.6133/apjcn.201903_28(1).0001. [DOI] [PubMed] [Google Scholar]
- 16.Dyall S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozaffarian D., Wu J.H.Y. (n-3) Fatty Acids and Cardiovascular Health: Are Effects of EPA and DHA Shared or Complementary? J. Nutr. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladyshev M.I., Sushchik N.N., Makhutova O.N. Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat. 2013;107:117–126. doi: 10.1016/j.prostaglandins.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Rimm E.B., Appel L.J., Chiuve S.E., Djoussé L., Engler M.B., Kris-Etherton P.M., Mozaffarian D., Siscovick D.S., Lichtenstein A.H. A Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation. 2018;138:e35–e47. doi: 10.1161/CIR.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colussi G., Catena C., Novello M., Bertin N., Sechi L.A. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017;27:191–200. doi: 10.1016/j.numecd.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Casula M., Olmastroni E., Gazzotti M., Galimberti F., Zambon A., Catapano A.L. Omega-3 polyunsaturated fatty acids supplementation and cardiovascular outcomes: Do formulation, dosage, and baseline cardiovascular risk matter? An updated meta-analysis of randomized controlled trials. Pharmacol. Res. 2020;160:105060. doi: 10.1016/j.phrs.2020.105060. [DOI] [PubMed] [Google Scholar]
- 22.Itsiopoulos C., Marx W., Mayr H., Tatucu-Babet O., Dash S., George E., Trakman G., Kelly J., Thomas C., Brazionis L. The role of omega-3 polyunsaturated fatty acid supplementation in the management of type 2 diabetes mellitus: A narrative review. J. Nutr. Intermed. Metab. 2018;14:42–51. doi: 10.1016/j.jnim.2018.02.002. [DOI] [Google Scholar]
- 23.Madingou N., Gilbert K., Tomaro L., Touchette C.P., Trudeau F., Fortin S., Rousseau G. Comparison of the effects of EPA and DHA alone or in combination in a murine model of myocardial infarction. Prostaglandins Leukot. Essent. Fat. Acids. 2016;111:11–16. doi: 10.1016/j.plefa.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Kalstad A.A., Myhre P.L., Laake K., Tveit S.H., Schmidt E.B., Smith P., Nilsen D.W.T., Tveit A., Fagerland M.W., Solheim S., et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients after Myocardial Infarction: A Randomized Controlled Trial. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.052209. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto A., Saotome M., Iguchi K., Maekawa Y. Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Heart Failure: Current Understanding for Basic to Clinical Relevance. Int. J. Mol. Sci. 2019;20:4025. doi: 10.3390/ijms20164025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uljević M.V., Starčević K., Mašek T., Bočina I., Restović I., Kević N., Racetin A., Kretzschmar G., Grobe M., Vukojević K., et al. Dietary DHA/EPA Supplementation Ameliorates Diabetic Nephropathy by Protecting from Distal Tubular Cell Damage. Cell Tissue Res. 2019;378:301–317. doi: 10.1007/s00441-019-03058-y. [DOI] [PubMed] [Google Scholar]
- 27.Farsi P.F., Djazayery A., Eshraghian M.R., Koohdani F., Akbar A., Derakhshanian H., Zarei M., Javanbakht M.H., Djalali M. Effects of Supplementation with Omega-3 on Insulin Sensitivity and Non-Esterified Free Fatty Acid (NEFA) in Type 2 Di-abetic Patients. Arq. Bras. Endocrinol. Metabol. 2014;58:1–6. doi: 10.1590/0004-2730000002861. [DOI] [PubMed] [Google Scholar]
- 28.Avallone L., Shaikh A., Hassan A., Tajuddin N. Prescription omega-3 fatty acid products: Considerations for patients with diabetes mellitus. Diabetes Metab. Syndr. Obesity Targets Ther. 2016;9:109–118. doi: 10.2147/DMSO.S97036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis C.E.G., Landim K.C., Nunes A.C.S., Dullius J. Safety in the hypertriglyceridemia treatment with N-3 polyunsaturated fatty acids on glucose metabolism in subjects with type 2 diabetes mellitus. Nutr. Hosp. 2014;31:570–576. doi: 10.3305/nh.2015.31.2.7845. [DOI] [PubMed] [Google Scholar]
- 30.Calder P.C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 31.Chang J.P.-C., Pariante C.M., Su K.-P. Omega-3 fatty acids in the psychological and physiological resilience against COVID-Prostaglandins Leukot. Essent. Fat. Acids. 2020;161:102177. doi: 10.1016/j.plefa.2020.102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrinhas R.S., Calder P.C., Lemos G.O., Waitzberg D.L. Parenteral fish oil: An adjuvant pharmacotherapy for coronavirus disease 2019? Nutrition. 2021;81:110900. doi: 10.1016/j.nut.2020.110900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan S., Ding Y., Saedi N., Choi M., Sridharan G.V., Sherr D.H., Yarmush M.L., Alaniz R.C., Jayaraman A., Lee K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018;23:1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganism. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costantini L., Molinari R., Farinon B., Merendino N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017;18:2645. doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vetrani C., Maukonen J., Bozzetto L., Della Pepa G., Vitale M., Costabile G., Riccardi G., Rivellese A.A., Saarela M., Annuzzi G. Diets Naturally Rich in Polyphenols and/or Long-Chain n-3 Polyunsaturated Fatty Acids Differently Affect Mi-crobiota Composition in High-Cardiometabolic-Risk Individuals. Acta Diabetol. 2020;8:853–860. doi: 10.1007/s00592-020-01494-9. [DOI] [PubMed] [Google Scholar]
- 38.Bentley-Hewitt K.L., Narbad A., Majsak-Newman G., Philo M.R., Lund E.K. Lactobacilli survival and adhesion to colonic epithelial cell lines is dependent on long chain fatty acid exposure. Eur. J. Lipid Sci. Technol. 2017;119:1700062. doi: 10.1002/ejlt.201700062. [DOI] [Google Scholar]
- 39.Duru M. Trends in agri-food choices for health since the 1960s: The case of fatty acids. Oilseeds fats Crop. Lipids. 2019;26:44. doi: 10.1051/ocl/2019038. [DOI] [Google Scholar]
- 40.Tocher D.R., Betancor M.B., Sprague M., Olsen R.E., Napier J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients. 2019;11:89. doi: 10.3390/nu11010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richter C., Skulas-Ray A., Kris-Etherton P. Fish and Fish Oil in Health and Disease Prevention. Elsevier; London, UK: 2016. Recommended intake of fish and fish oils worldwide; pp. 27–48. [Google Scholar]
- 42.ANSES . Actualisation des Repères du PNNS: Révision des Repères de Consommation Alimentaires. Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’environnement et du Travail; CEDEX 94701, Maison-Alfort, France: 2016. p. 280. [Google Scholar]
- 43.Bowen K.J., Harris W.S., Kris-Etherton P.M. Omega-3 Fatty Acids and Cardiovascular Disease: Are There Benefits? Curr. Treat. Options Cardiovasc. Med. 2016;18:1–16. doi: 10.1007/s11936-016-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg P., Pejaver R.K., Sukhija M., Ahuja A. Role of DHA, ARA, & phospholipids in brain development: An Indian perspective. Clin. Epidemiol. Glob. Heal. 2017;5:155–162. doi: 10.1016/j.cegh.2017.09.003. [DOI] [Google Scholar]
- 45.DiNicolantonio J.J., O’Keefe J.H. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients. 2020;12:2333. doi: 10.3390/nu12082333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugasini D., Thomas R., Yalagala P.C.R., Tai L.M., Subbaiah P.V. Dietary Docosahexaenoic Acid (DHA) as Lysophospha-tidylcholine, but Not as Free Acid, Enriches Brain DHA and Improves Memory in Adult Mice. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-11766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J.-H., Wang Q., You Q.-L., Li Z.-L., Hu N.-Y., Wang Y., Jin Z.-L., Li S.-J., Li X.-W., Yang J.-M., et al. Acute EPA-induced learning and memory impairment in mice is prevented by DHA. Nat. Commun. 2020;11:1–15. doi: 10.1038/s41467-020-19255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNamara R.K., Jandacek R., Tso P., Blom T.J., Welge J.A., Strawn J.R., Adler C.M., Strakowski S.M., DelBello M.P. Adolescents with or at ultra-high risk for bipolar disorder exhibit erythrocyte docosahexaenoic acid and eicosapentaenoic acid deficits: A candidate prodromal risk biomarker. Early Interv. Psychiatry. 2015;10:203–211. doi: 10.1111/eip.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unay B., Sarıcı S.Ü., Ulaş Ü.H., Akın R., Alpay F., Gökçay E. Nutritional effects on auditory brainstem maturation in healthy term infants. Arch. Dis. Child. Fetal Neonatal Ed. 2004;89:F177–F179. doi: 10.1136/adc.2002.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haubner L.Y., Stockard J.E., Saste M.D., Benford V.J., Phelps C.P., Chen L.T., Barness L., Wiener D., Carver J.D. Maternal dietary docosahexanoic acid content affects the rat pup auditory system. Brain Res. Bull. 2002;58:1–5. doi: 10.1016/S0361-9230(01)00764-X. [DOI] [PubMed] [Google Scholar]
- 51.Le Belle J., Sperry J., Ludwig K., Harris N., Caldwell M., Kornblum H. Docosahexaenoic Acid Has Stem Cell-Specific Effects in the SVZ and Restores Olfactory Neurogenesis and Function in the Aging Brain. Neuroscience. 2020 doi: 10.1101/2020.02.10.942870. [DOI] [Google Scholar]
- 52.Khoury S., Soubeyre V., Cabaret S., Merle L., Grégoire S., Deprêtre N., Jarriault D., Grosmaitre X., Bretillon L., Berdeaux O., et al. Perinatal exposure to diets with different n-6:n-3 fatty acid ratios affects olfactory tissue fatty acid composition. Sci. Rep. 2020;10:1–15. doi: 10.1038/s41598-020-67725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang T.-T., Xu J., Wang Y.-M., Xue C.-H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019;75:100997. doi: 10.1016/j.plipres.2019.100997. [DOI] [PubMed] [Google Scholar]
- 54.Mohajeri M.H., Troesch B., Weber P. Inadequate supply of vitamins and DHA in the elderly: Implications for brain aging and Alzheimer-type dementia. Nutrition. 2015;31:261–275. doi: 10.1016/j.nut.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Sun G.Y., Simonyi A., Fritsche K.L., Chuang D.Y., Hannink M., Gu Z., Greenlief C.M., Yao J.K., Lee J.C.-M., Beversdorf D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids. 2018;136:3–13. doi: 10.1016/j.plefa.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao Y., Mei Y., Han H., Liu F., Yang X.M., Shao Y., Xie B., Long B. Effects of Omega-3 in the treatment of violent schizophrenia patients. Schizophr. Res. 2018;195:283–285. doi: 10.1016/j.schres.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 57.Goupille C., Vibet S., Frank P.G., Mahéo K. EPA and DHA Fatty Acids Induce a Remodeling of Tumor Vasculature and Potentiate Docetaxel Activity. Int. J. Mol. Sci. 2020;21:4965. doi: 10.3390/ijms21144965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elmasry K., Ibrahim A.S., Abdulmoneim S., Al-Shabrawey M. Bioactive lipids and pathological retinal angiogenesis. Br. J. Pharmacol. 2019;176:93–109. doi: 10.1111/bph.14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y., Wang J., Li Q., Cao B. The Effect of Omega-3 Polyunsaturated Fatty Acid Supplementations on anti-Tumor Drugs in Triple Negative Breast Cancer. Nutr. Cancer. 2020:1–10. doi: 10.1080/01635581.2020.1743873. [DOI] [PubMed] [Google Scholar]
- 60.Chen H., Sun S., Li J. Docosahexaenoic acid (DHA) induces apoptosis in human hepatocellular carcinoma cells. HPB. 2016;18:e166. doi: 10.1016/j.hpb.2016.02.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue M., Ge Y., Yu C., Zheng Z., He X., Zhao J. Apoptosis is induced by docosahexaenoic acid in breast cancer cells via death receptor and mitochondria-mediated pathways. Mol. Med. Rep. 2017;16:978–982. doi: 10.3892/mmr.2017.6678. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Yang Y., Wang F., Yang W., Zou Z. MAG-DHA Induces Apoptosis and Autophagy in Breast Cancer Cells via Lipid Peroxidation-Mediated Endoplasmic Reticulum Stress. Preprint. 2020 doi: 10.21203/rs.3.rs-35529/v1. [DOI] [PubMed] [Google Scholar]
- 63.Wu J., Cho E., Giovannucci E.L., Rosner B.A., Sastry S.M., Willett W.C., Schaumberg D.A. Dietary Intakes of Eicosapentaenoic Acid and Docosahexaenoic Acid and Risk of Age-Related Macular Degeneration. Ophthalmology. 2017;124:634–643. doi: 10.1016/j.ophtha.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu Z., Lofqvist C.A., Shao Z., Sun Y., Joyal J.-S., Hurst C.G., Cui R.Z., Evans L.P., Tian K., SanGiovanni J.P., et al. Dietary ω-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose–endoplasmic reticulum stress reduction to increase adiponectin. Am. J. Clin. Nutr. 2015;101:879–888. doi: 10.3945/ajcn.114.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simón M.V., Agnolazza D.L., German O.L., Garelli A., Politi L.E., Agbaga M., Anderson R.E., Rotstein N.P. Synthesis of docosahexaenoic acid from eicosapentaenoic acid in retina neurons protects photoreceptors from oxidative stress. J. Neurochem. 2015;136:931–946. doi: 10.1111/jnc.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prokopiou E., Kolovos P., Kalogerou M., Neokleous A., Papagregoriou G., Deltas C., Malas S., Georgiou T. Therapeutic potential of omega-3 fatty acids supplementation in a mouse model of dry macular degeneration. BMJ Open Ophthalmol. 2017;1:e000056. doi: 10.1136/bmjophth-2016-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Behl T., Kotwani A. Omega-3 fatty acids in prevention of diabetic retinopathy. J. Pharm. Pharmacol. 2017;69:946–954. doi: 10.1111/jphp.12744. [DOI] [PubMed] [Google Scholar]
- 68.Suzumura A., Terao R., Kaneko H. Protective Effects and Molecular Signaling of n-3 Fatty Acids on Oxidative Stress and Inflammation in Retinal Diseases. Antioxidants. 2020;9:920. doi: 10.3390/antiox9100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.FAO . Meeting the Sustainable Development Goals. The State of World Fisheries and Aquaculture; FAO; Rome, Italy: 2018. [Google Scholar]
- 70.Caddy J.F., Garibaldi L. Apparent changes in the trophic composition of world marine harvests: The perspective from the FAO capture database. Ocean Coast. Manag. 2000;43:615–655. doi: 10.1016/S0964-5691(00)00052-1. [DOI] [Google Scholar]
- 71.Pauly D., Christensen V., Dalsgaard J., Froese R., Torres F. Fishing Down Marine Food Webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- 72.Tocher D. Issues surrounding fish as a source of omega-3 long-chain polyunsaturated fatty acids. Lipid Technol. 2009;21:13–16. doi: 10.1002/lite.200800079. [DOI] [Google Scholar]
- 73.Guschina I.A., Harwood J.L. Algal lipids and effect of the environment on their biochemistry. In: Kainz M., Brett M.T., Arts M.T., editors. Lipids in Aquatic Ecosystems. Springer; New York, NY, USA: 2009. pp. 1–24. [Google Scholar]
- 74.Hixson S.M., Arts M.T. Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton. Glob. Chang. Biol. 2016;22:2744–2755. doi: 10.1111/gcb.13295. [DOI] [PubMed] [Google Scholar]
- 75.Winter R., Dzwolak W. Exploring the temperature-pressure configurational landscape of biomolecules: From lipid membranes to proteins. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004;363:537–563. doi: 10.1098/rsta.2004.1507. [DOI] [PubMed] [Google Scholar]
- 76.Renaud S.M., Thinh L.-V., Lambrinidis G., Parry D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture. 2002;211:195–214. doi: 10.1016/S0044-8486(01)00875-4. [DOI] [Google Scholar]
- 77.Rousch J.M., Bingham S.E., Sommerfeld M.R. Changes in fatty acid profiles of thermo-intolerant and thermo-tolerant marine diatoms during temperature stress. J. Exp. Mar. Biol. Ecol. 2003;295:145–156. doi: 10.1016/S0022-0981(03)00293-4. [DOI] [Google Scholar]
- 78.Adarme-Vega T.C., Thomas-Hall S.R., Schenk P.M. Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 2014;26:14–18. doi: 10.1016/j.copbio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 79.FAO . The State of World Fisheries and Aquaculture 2020: Sustainability in Action. The State of World Fisheries and Aquaculture (SOFIA); FAO; Rome, Italy: 2020. [Google Scholar]
- 80.Domergue F., Abbadi A., Heinz E. Relief for fish stocks: Oceanic fatty acids in transgenic oilseeds. Trends Plant Sci. 2005;10:112–116. doi: 10.1016/j.tplants.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Robert S.S. Production of Eicosapentaenoic and Docosahexaenoic Acid-Containing Oils in Transgenic Land Plants for Human and Aquaculture Nutrition. Mar. Biotechnol. 2006;8:103–109. doi: 10.1007/s10126-005-5142-x. [DOI] [PubMed] [Google Scholar]
- 82.Khachatourians G.C., Hui Y.H., Scorza R., Nip W.-K. Transgenic Plants and Crops. CRC Press; Boca Raton, FL, USA: 2002. [Google Scholar]
- 83.Abbadi A., Domergue F., Bauer J., Napier J.A., Welti R., Zähringer U., Cirpus P., Heinza E. Biosynthesis of Very-Long-Chain Polyunsaturated Fatty Acids in Transgenic Oilseeds: Constraints on Their Accumulation. Plant Cell. 2004;16:2734–2748. doi: 10.1105/tpc.104.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qi B., Fraser T., Mugford S.T., Dobson G., Sayanova O., Butler J., Napier J.A., Stobart A.K., Lazarus C.M. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol. 2004;22:739–745. doi: 10.1038/nbt972. [DOI] [PubMed] [Google Scholar]
- 85.Wu G., Truksa M., Datla N., Vrinten P., Bauer J., Zank T., Cirpus P., Heinz E., Qiu X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005;23:1013–1017. doi: 10.1038/nbt1107. [DOI] [PubMed] [Google Scholar]
- 86.Ruiz-Lopez N., Haslam R.P., Napier J.A., Sayanova O. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J. 2014;77:198–208. doi: 10.1111/tpj.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Betancor M., Sprague M., Usher S., Sayanova O., Campbell P., Napier J.A., Tocher D.R. A nutritionally-enhanced oil from transgenic Camelina sativa effectively replaces fish oil as a source of eicosapentaenoic acid for fish. Sci. Rep. 2015;5:8104. doi: 10.1038/srep08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Napier J.A., Olsen R., Tocher D.R. Update on GM canola crops as novel sources of omega-3 fish oils. Plant Biotechnol. J. 2019;17:703–705. doi: 10.1111/pbi.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ribeiro T.G., Barone B., Behrens J.H. Genetically modified foods and their social representation. Food Res. Int. 2016;84:120–127. doi: 10.1016/j.foodres.2016.03.029. [DOI] [Google Scholar]
- 90.Volkman J., Jeffrey S., Nichols P., Rogers G., Garland C. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1989;128:219–240. doi: 10.1016/0022-0981(89)90029-4. [DOI] [Google Scholar]
- 91.Ötleş S., Pire R. Fatty Acid Composition of Chlorella and Spirulina Microalgae Species. J. Assoc. Off. Agric. Chem. Int. 2001;84:1708–1714. doi: 10.1093/jaoac/84.6.1708. [DOI] [PubMed] [Google Scholar]
- 92.Leblond J.D., Evans T.J., Chapman P.J. The biochemistry of dinoflagellate lipids, with particular reference to the fatty acid and sterol composition of a Karenia brevis bloom. Phycologia. 2003;42:324–331. doi: 10.2216/i0031-8884-42-4-324.1. [DOI] [Google Scholar]
- 93.Pernet F., Tremblay R., Demers E., Roussy M. Variation of lipid class and fatty acid composition of Chaetoceros muelleri and Isochrysis sp. grown in a semicontinuous system. Aquaculture. 2003;221:393–406. doi: 10.1016/S0044-8486(03)00030-9. [DOI] [Google Scholar]
- 94.Patil V., Källqvist T., Olsen E., Vogt G., Gislerød H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2006;15:1–9. doi: 10.1007/s10499-006-9060-3. [DOI] [Google Scholar]
- 95.Niccolai A., Zittelli G.C., Rodolfi L., Biondi N., Tredici M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019;42:101617. doi: 10.1016/j.algal.2019.101617. [DOI] [Google Scholar]
- 96.Gladyshev M.I., Sushchik N.N. Long-chain Omega-3 Polyunsaturated Fatty Acids in Natural Ecosystems and the Human Diet: Assumptions and Challenges. Biomolecules. 2019;9:485. doi: 10.3390/biom9090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taipale S., Peltomaa E., Salmi P. Variation in ω-3 and ω-6 Polyunsaturated Fatty Acids Produced by Different Phytoplankton Taxa at Early and Late Growth Phase. Biomolecules. 2020;10:559. doi: 10.3390/biom10040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arts M.T., Ackman R.G., Holub B.J. “Essential fatty acids” in aquatic ecosystems: A crucial link between diet and human health and evolution. Can. J. Fish. Aquat. Sci. 2001;58:122–137. doi: 10.1139/f00-224. [DOI] [Google Scholar]
- 99.Mendes A., Reis A., Vasconcelos R., Guerra P., Da Silva T.L. Crypthecodinium cohnii with emphasis on DHA production: A review. Environ. Boil. Fishes. 2009;21:199–214. doi: 10.1007/s10811-008-9351-3. [DOI] [Google Scholar]
- 100.Fan K.-W., Jiang Y., Faan Y.-W., Chen F. Lipid Characterization of Mangrove Thraustochytrid—Schizochytrium mangrovei. J. Agric. Food Chem. 2007;55:2906–2910. doi: 10.1021/jf070058y. [DOI] [PubMed] [Google Scholar]
- 101.Leblond J.D., Chapman P.J. Lipid class distribution of highly unsaturated long chain fatty acids in marine dinoflagellates. J. Phycol. 2000;36:1103–1108. doi: 10.1046/j.1529-8817.2000.00018.x. [DOI] [Google Scholar]
- 102.Usup G., Hamid S.Z., Chiet P.K., Wah C.K., Ahmad A. Marked Differences in Fatty Acid Profiles of Some Planktonic and Benthic Marine Dinoflagellates from Malaysian Waters. Phycologia. 2008;47:105–111. doi: 10.2216/07-55.1. [DOI] [Google Scholar]
- 103.Da Costa F., Le Grand F., Quéré C., Bougaran G., Cadoret J.P., Robert R., Soudant P. Effects of growth phase and nitrogen limitation on biochemical composition of two strains of Tisochrysis lutea. Algal Res. 2017;27:177–189. doi: 10.1016/j.algal.2017.09.003. [DOI] [Google Scholar]
- 104.Chang K.J.L., Nichols C.M., Blackburn S.I., Dunstan G.A., Koutoulis A., Nichols P.D. Comparison of Thraustochytrids Aurantiochytrium sp., Schizochytrium sp., Thraustochytrium sp., and Ulkenia sp. for Production of Biodiesel, Long-Chain Omega-3 Oils, and Exopolysaccharide. Mar. Biotechnol. 2014;16:396–411. doi: 10.1007/s10126-014-9560-5. [DOI] [PubMed] [Google Scholar]
- 105.Moreno V.J., De Moreno J.E.A., Brenner R.R. Biosynthesis of unsaturated fatty acids in the Diatom Phaeodactylum tricornutum. Lipids. 1979;14:15–19. doi: 10.1007/BF02533560. [DOI] [Google Scholar]
- 106.Gillan F., McFadden G., Wetherbee R., Johns R. Sterols and fatty acids of an antarctic sea ice diatom, Stauroneis amphioxys. Phytochemistry. 1981;20:1935–1937. doi: 10.1016/0031-9422(81)84038-1. [DOI] [Google Scholar]
- 107.Arao T., Sakaki T., Yamada M. Biosynthesis of polyunsaturated lipids in the diatom, Phaeodactylum tricornutum. Phytochemistry. 1994;36:629–635. doi: 10.1016/S0031-9422(00)89787-3. [DOI] [Google Scholar]
- 108.Tonon T., Harvey D., Larson T.R., Graham I.A. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry. 2002;61:15–24. doi: 10.1016/S0031-9422(02)00201-7. [DOI] [PubMed] [Google Scholar]
- 109.Liang Y., Maeda Y., Yoshino T., Matsumoto M., Tanaka T. Profiling of fatty acid methyl esters from the oleaginous diatom Fistulifera sp. strain JPCC DA0580 under nutrition-sufficient and -deficient conditions. Environ. Boil. Fishes. 2014;26:2295–2302. doi: 10.1007/s10811-014-0265-y. [DOI] [Google Scholar]
- 110.Yao L., Gerde J.A., Lee S.-L., Wang T., Harrata K.A. Microalgae Lipid Characterization. J. Agric. Food Chem. 2015;63:1773–1787. doi: 10.1021/jf5050603. [DOI] [PubMed] [Google Scholar]
- 111.Zulu N.N., Zienkiewicz K., Vollheyde K., Feussner I. Current trends to comprehend lipid metabolism in diatoms. Prog. Lipid Res. 2018;70:1–16. doi: 10.1016/j.plipres.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 112.Adams C., Godfrey V., Wahlen B., Seefeldt L., Bugbee B. Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Bioresour. Technol. 2013;131:188–194. doi: 10.1016/j.biortech.2012.12.143. [DOI] [PubMed] [Google Scholar]
- 113.Saha S.K., McHugh E., Hayes J., Moane S., Walsh D., Murray P. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 2013;128:118–124. doi: 10.1016/j.biortech.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 114.Parrish C.C. Lipids in Marine Ecosystems. Int. Sch. Res. Not. Oceanogr. 2013;2013:604045. doi: 10.5402/2013/604045. [DOI] [Google Scholar]
- 115.Watkins S.M., German B.J. Food Lipid: Chemistry, Nutrition, and Biotechnology. CRC Press/Taylor & Francis Group; Boca Raton, CA, USA: 2008. Unsaturated fatty acids; pp. 513–537. Food Science and Technology. [Google Scholar]
- 116.Khozin-Goldberg I. Lipid metabolism in microalgae. In: Borowitzka M.A., Beardall J., Raven J.A., editors. The Physiology of Microalgae. Springer International Publishing; Cham, Switzerland: 2016. pp. 413–484. Developments in Applied Phycology. [Google Scholar]
- 117.Harwood J.L. Plant Lipids: Biology, Utilisation and Manipulation. John Wiley & Sons; Cardiff, Wales, UK: 2009. Fatty acid biosynthesis; pp. 27–66. [Google Scholar]
- 118.Bell M.V., Tocher D.R. Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: General pathways and new direc-tions. In: Kainz M., Brett M.T., Arts M.T., editors. Lipids in Aquatic Ecosystems. Springer; New York, NY, USA: 2009. pp. 211–236. [Google Scholar]
- 119.Vieler A., Wu G., Tsai C.-H., Bullard B., Cornish A.J., Harvey C., Reca I.-B., Thornburg C., Achawanantakun R., Buehl C.J., et al. Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga Nannochloropsis oceanica CCMP1779. PLoS Genet. 2012;8:e1003064. doi: 10.1371/journal.pgen.1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoffmeister M., Piotrowski M., Nowitzki U., Martin W. Mitochondrial trans-2-Enoyl-CoA Reductase of Wax Ester Fermentation from Euglena gracilis Defines a New Family of Enzymes Involved in Lipid Synthesis. J. Biol. Chem. 2005;280:4329–4338. doi: 10.1074/jbc.M411010200. [DOI] [PubMed] [Google Scholar]
- 121.Los D.A., Murata N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1998;1394:3–15. doi: 10.1016/S0005-2760(98)00091-5. [DOI] [PubMed] [Google Scholar]
- 122.Li-Beisson Y., Beisson F., Riekhof W. Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J. 2015;82:504–522. doi: 10.1111/tpj.12787. [DOI] [PubMed] [Google Scholar]
- 123.Meesapyodsuk D., Qiu X. The Front-end Desaturase: Structure, Function, Evolution and Biotechnological Use. Lipids. 2012;47:227–237. doi: 10.1007/s11745-011-3617-2. [DOI] [PubMed] [Google Scholar]
- 124.Remize M., Planchon F., Loh A.N., Le Grand F., Bideau A., Le Goic N., Fleury E., Miner P., Corvaisier R., Volety A., et al. Study of Synthesis Pathways of the Essential Polyunsaturated Fatty Acid 20:5n-3 in the Diatom Chaetoceros Muelleri Using 13C-Isotope Labeling. Biomolecules. 2020;10:797. doi: 10.3390/biom10050797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arao T., Yamada M. Biosynthesis of polyunsaturated fatty acids in the marine diatom, Phaeodactylum tricornutum. Phytochemistry. 1994;35:1177–1181. doi: 10.1016/S0031-9422(00)94817-9. [DOI] [Google Scholar]
- 126.Domergue F., Lerchl J., Zähringer U., Heinz E. Cloning and functional characterization of Phaeodactylum tricornutum front-end desaturases involved in eicosapentaenoic acid biosynthesis. Eur. J. Biochem. 2002;269:4105–4113. doi: 10.1046/j.1432-1033.2002.03104.x. [DOI] [PubMed] [Google Scholar]
- 127.Domergue F., Spiekermann P., Lerchl J., Beckmann C., Kilian O., Kroth P.G., Boland W., Zähringer U., Heinz E. New Insight into Phaeodactylum tricornutum Fatty Acid Metabolism. Cloning and Functional Characterization of Plastidial and Microsomal Δ12-Fatty Acid Desaturases. Plant Physiol. 2003;131:1648–1660. doi: 10.1104/pp.102.018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gurr M.I., Harwood J.L. Fatty acid structure and metabolism. In: Gurr M.I., Harwood J.L., editors. Lipid Biochemistry: An Introduction. Springer; Boston, MA, USA: 1991. pp. 23–118. [Google Scholar]
- 129.Buist P.H. Fatty acid desaturases: Selecting the dehydrogenation channel. Nat. Prod. Rep. 2004;21:249–262. doi: 10.1039/b302094k. [DOI] [PubMed] [Google Scholar]
- 130.Ratledge C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie. 2004;86:807–815. doi: 10.1016/j.biochi.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 131.Armenta R.E., Valentine M.C. Single-Cell Oils as a Source of Omega-3 Fatty Acids: An Overview of Recent Advances. J. Am. Oil Chem. Soc. 2012;90:167–182. doi: 10.1007/s11746-012-2154-3. [DOI] [Google Scholar]
- 132.Wallis J.G., Watts J.L., Browse J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci. 2002;27:467–473. doi: 10.1016/S0968-0004(02)02168-0. [DOI] [PubMed] [Google Scholar]
- 133.Metz J.G., Roessler P., Facciotti D., Levering C., Dittrich F., Lassner M., Valentine R., Lardizabal K., Domergue F., Yamada A., et al. Production of Polyunsaturated Fatty Acids by Polyketide Synthases in Both Prokaryotes and Eukaryotes. Science. 2001;293:290–293. doi: 10.1126/science.1059593. [DOI] [PubMed] [Google Scholar]
- 134.Qiu X. Biosynthesis of docosahexaenoic acid (DHA, 22:6-4, 7,10,13,16,19): Two distinct pathways. Prostaglandins Leukot. Essent. Fat. Acids. 2003;68:181–186. doi: 10.1016/S0952-3278(02)00268-5. [DOI] [PubMed] [Google Scholar]
- 135.Hauvermale A., Kuner J., Rosenzweig B., Guerra D., Diltz S., Metz J.G. Fatty acid production in Schizochytrium sp.: Involvement of a polyunsaturated fatty acid synthase and a type I fatty acid synthase. Lipids. 2006;41:739–747. doi: 10.1007/s11745-006-5025-6. [DOI] [PubMed] [Google Scholar]
- 136.Uttaro A.D. Biosynthesis of polyunsaturated fatty acids in lower eukaryotes. Int. Union Biochem. Mol. Biol. Life. 2006;58:563–571. doi: 10.1080/15216540600920899. [DOI] [PubMed] [Google Scholar]
- 137.Murray S.A., Garby T., Hoppenrath M., Neilan B.A. Genetic Diversity, Morphological Uniformity and Polyketide Production in Dinoflagellates (Amphidinium, Dinoflagellata) PLoS ONE. 2012;7:e38253. doi: 10.1371/journal.pone.0038253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kimura K., Okuda S., Nakayama K., Shikata T., Takahashi F., Yamaguchi H., Skamoto S., Yamaguchi M., Tomaru Y. RNA Sequencing Revealed Numerous Polyketide Synthase Genes in the Harmful Dinoflagellate Karenia mikimotoi. PLoS ONE. 2015;10:e0142731. doi: 10.1371/journal.pone.0142731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Remize M., Planchon F., Loh A.N., Le Grand F., Lambert C., Bideau A., Bidault A., Corvaisier R., Volety A., Soudant P. Identification of Polyunsaturated Fatty Acids Synthesis Pathways in the Toxic Dinophyte Alexandrium minutum Using 13C-Labelling. Biomolecules. 2020;10:1428. doi: 10.3390/biom10101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.John U., Beszteri B., Derelle E., Van De Peer Y., Read B., Moreau H., Cembella A. Novel Insights into Evolution of Protistan Polyketide Synthases through Phylogenomic Analysis. Protist. 2008;159:21–30. doi: 10.1016/j.protis.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 141.John U., Beszteri S., Glöckner G., Singh R., Medlin L., Cembella A.D. Genomic characterisation of the ichthyotoxic prymnesiophyte Chrysochromulina polylepis,and the expression of polyketide synthase genes in synchronized cultures. Eur. J. Phycol. 2010;45:215–229. doi: 10.1080/09670261003746193. [DOI] [Google Scholar]
- 142.Shen B. Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr. Opin. Chem. Biol. 2003;7:285–295. doi: 10.1016/S1367-5931(03)00020-6. [DOI] [PubMed] [Google Scholar]
- 143.Freitag M., Beszteri S., Vogel H., John U. Effects of physiological shock treatments on toxicity and polyketide synthase gene expression in Prymnesium parvum (Prymnesiophyceae) Eur. J. Phycol. 2011;46:193–201. doi: 10.1080/09670262.2011.591438. [DOI] [Google Scholar]
- 144.Eichholz K., Beszteri B., John U. Putative Monofunctional Type I Polyketide Synthase Units: A Dinoflagellate-Specific Feature? PLoS ONE. 2012;7:e48624. doi: 10.1371/journal.pone.0048624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kohli G.S., John U., Van Dolah F.M., Murray S.A. Evolutionary distinctiveness of fatty acid and polyketide synthesis in eukaryotes. ISME J. 2016;10:1877–1890. doi: 10.1038/ismej.2015.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Monroe E.A., van Dolah F.M. The Toxic Dinoflagellate Karenia brevis Encodes Novel Type I-like Polyketide Synthases Con-taining Discrete Catalytic Domains. Protist. 2008;159:471–482. doi: 10.1016/j.protis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 147.Jenke-Kodama H., Sandmann A., Müller R., Dittmann E. Evolutionary Implications of Bacterial Polyketide Synthases. Mol. Biol. Evol. 2005;22:2027–2039. doi: 10.1093/molbev/msi193. [DOI] [PubMed] [Google Scholar]
- 148.Cock J.M., Sterck L., Rouzé P., Scornet D., Allen A.E., Amoutzias G.D., Anthouard V., Artiguenave F., Aury J.-M., Badger J.H., et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nat. Cell Biol. 2010;465:617–621. doi: 10.1038/nature09016. [DOI] [PubMed] [Google Scholar]
- 149.Ye C., Qiao W., Yu X., Ji X., Huang H., Collier J.L., Liu L. Reconstruction and analysis of the genome-scale metabolic model of schizochytrium limacinum SR21 for docosahexaenoic acid production. BMC Genom. 2015;16:799. doi: 10.1186/s12864-015-2042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang K., Li H., Chen W., Zhao M., Cui H., Min Q., Wang H., Chen S., Li D. Regulation of the Docosapentaenoic Acid/Docosahexaenoic Acid Ratio (DPA/DHA Ratio) in Schizochytrium limacinum B4D1. Appl. Biochem. Biotechnol. 2017;182:67–81. doi: 10.1007/s12010-016-2311-5. [DOI] [PubMed] [Google Scholar]
- 151.Leblond J.D., Lasiter A.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. II.Lepidodinium chlorophorum, Karenia brevis, and Kryptoperidinium foliaceum, three dinoflagellates with aberrant plastids. Eur. J. Phycol. 2009;44:199–205. doi: 10.1080/09670260802524611. [DOI] [Google Scholar]
- 152.Courchesne N.M.D., Parisien A., Wang B., Lan C.Q. Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J. Biotechnol. 2009;141:31–41. doi: 10.1016/j.jbiotec.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 153.Gong Y., Wan X., Jiang M., Hu C., Hu H., Huang F. Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Prog. Lipid Res. 2014;56:19–35. doi: 10.1016/j.plipres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 154.Mühlroth A., Li K., Røkke G., Winge P., Olsen Y., Hohmann-Marriott M.F., Vadstein O., Bones A.M. Pathways of Lipid Metabolism in Marine Algae, Co-Expression Network, Bottlenecks and Candidate Genes for Enhanced Production of EPA and DHA in Species of Chromista. Mar. Drugs. 2013;11:4662–4697. doi: 10.3390/md11114662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cui J., Diao J., Sun T., Shi M., Liu L., Wang F., Chen L., Zhang W. 13C Metabolic Flux Analysis of Enhanced Lipid Accumulation Modulated by Ethanolamine in Crypthecodinium cohnii. Front. Microbiol. 2018;9:956. doi: 10.3389/fmicb.2018.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Menzel R., Ngosong C., Ruess L. Isotopologue profiling enables insights into dietary routing and metabolism of trophic biomarker fatty acids. Chemoecology. 2017;27:101–114. doi: 10.1007/s00049-017-0236-2. [DOI] [Google Scholar]
- 157.Wei X., Shi B., Koo I., Yin X., Lorkiewicz P., Suhail H., Rattan R., Giri S., McClain C.J., Zhang X. Analysis of stable isotope assisted metabolomics data acquired by GC-MS. Anal. Chim. Acta. 2017;980:25–32. doi: 10.1016/j.aca.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 159.Levasseur W., Perré P., Pozzobon V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020;41:107545. doi: 10.1016/j.biotechadv.2020.107545. [DOI] [PubMed] [Google Scholar]
- 160.Pan-Utai W., Iamtham S. Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process. Biochem. 2019;82:189–198. doi: 10.1016/j.procbio.2019.04.014. [DOI] [Google Scholar]
- 161.Kulkarni S., Nikolov Z. Process for selective extraction of pigments and functional proteins from Chlorella vulgaris. Algal Res. 2018;35:185–193. doi: 10.1016/j.algal.2018.08.024. [DOI] [Google Scholar]
- 162.Castejón N., Señoráns F.J. Simultaneous extraction and fractionation of omega-3 acylglycerols and glycolipids from wet microalgal biomass of Nannochloropsis gaditana using pressurized liquids. Algal Res. 2019;37:74–82. doi: 10.1016/j.algal.2018.11.003. [DOI] [Google Scholar]
- 163.Lu K., Zhao X., Ho S.-H., Ma R., Xie Y., Chen J. Biorefining and the Functional Properties of Proteins from Lipid and Pigment Extract Residue of Chlorella pyrenoidosa. Mar. Drugs. 2019;17:454. doi: 10.3390/md17080454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bhattacharya M., Goswami S. Microalgae—A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020;25:101580. doi: 10.1016/j.bcab.2020.101580. [DOI] [Google Scholar]
- 165.Tang D.Y.Y., Yew G.Y., Koyande A.K., Chew K.W., Vo D.-V.N., Show P.L. Green technology for the industrial production of biofuels and bioproducts from microalgae: A review. Environ. Chem. Lett. 2020;18:1967–1985. doi: 10.1007/s10311-020-01052-3. [DOI] [Google Scholar]
- 166.Galasso C., Gentile A., Orefice I., Ianora A., Bruno A., Noonan D.M., Sansone C., Albini A., Brunet C. Microalgal Derivatives as Potential Nutraceutical and Food Supplements for Human Health: A Focus on Cancer Prevention and Interception. Nutrients. 2019;11:1226. doi: 10.3390/nu11061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Allmicroalgae Natural Products Allma by Allmicroalgae. [(accessed on 7 December 2020)]; Available online: https://www.allmicroalgae.com/en/food/
- 168.Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- 169.Krupanidhi S., Sanjeevi C.B. Omega-3 Fatty Acids for Nutrition and Medicine: Considering Microalgae Oil as a Vegetarian Source of EPA and DHA. Curr. Diabetes Rev. 2007;3:198–203. doi: 10.2174/157339907781368968. [DOI] [PubMed] [Google Scholar]
- 170.Borowitzka M.A. Algal physiology and large-scale outdoor cultures of microalgae. In: Boro-witzka M.A., Beardall J., Raven J.A., editors. The Physiology of Microalgae. Springer International Publishing; Cham, Switzerland: 2016. pp. 601–652. [Google Scholar]
- 171.Oren A. A hundred years of Dunaliella research: 1905–2005. Saline Syst. 2005;1:2. doi: 10.1186/1746-1448-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beuzenberg V., Smith K., Packer M. Isolation and characterisation of halo-tolerant Dunaliella strains from Lake Grassmere/Kapara Te Hau, New Zealand. N. Zealand J. Bot. 2014;52:136–152. doi: 10.1080/0028825X.2013.859627. [DOI] [Google Scholar]
- 173.Babuskin S., Krishnan K.R., Saravana P.A., Sivarajan M., Sukumar M. Functional Foods Enriched with Marine Microalga Nannochloropsis oculata as a Source of W-3 Fatty Acids. Food Technol. Biotechnol. 2014;52:292–299. [Google Scholar]
- 174.Robertson R.C., Mateo M.R.G., O’Grady M.N., Guihéneuf F., Stengel D.B., Ross R.P., Fitzgerald G.F., Kerry J.P., Stanton C. An assessment of the techno-functional and sensory properties of yoghurt fortified with a lipid extract from the microalga Pavlova lutheri. Innov. Food Sci. Emerg. Technol. 2016;37:237–246. doi: 10.1016/j.ifset.2016.03.017. [DOI] [Google Scholar]
- 175.Lane K.E., Li W., Smith C.A., Derbyshire E. The bioavailability of an omega-3-rich algal oil is improved by nanoemulsion technology using yogurt as a food vehicle. Int. J. Food Sci. Technol. 2014;49:1264–1271. doi: 10.1111/ijfs.12455. [DOI] [Google Scholar]
- 176.Geppert J., Kraft V., Demmelmair H., Koletzko B. Docosahexaenoic acid supplementation in vegetarians effectively increases omega-3 index: A randomized trial. Lipids. 2005;40:807–814. doi: 10.1007/s11745-005-1442-9. [DOI] [PubMed] [Google Scholar]
- 177.Li K., Sinclair A.J., Zhao F., Li D. Uncommon Fatty Acids and Cardiometabolic Health. Nutrients. 2018;10:1559. doi: 10.3390/nu10101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wakimoto T., Kondo H., Nii H., Kimura K., Egami Y., Oka Y., Yoshida M., Kida E., Ye Y., Akahoshi S., et al. Furan fatty acid as an anti-inflammatory component from the green-lipped mussel Perna canaliculus. Proc. Natl. Acad. Sci. USA. 2011;108:17533–17537. doi: 10.1073/pnas.1110577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Packer M.A., Harris G.C., Adams S.L. Food and feed applications of algae. In: Bux F., Chisti Y., editors. Algae Biotechnology. Springer International Publishing; Cham, Switzerland: 2016. pp. 217–247. Green Energy and Technology. [Google Scholar]
- 180.Jordan M.A., Wilson L. Microtubules as a Target for Anticancer Drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 181.Ba F., Ursu A.V., Laroche C., Djelveh G. Haematococcus pluvialis soluble proteins: Extraction, characterization, concentration/fractionation and emulsifying properties. Bioresour. Technol. 2016;200:147–152. doi: 10.1016/j.biortech.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 182.Caporgno M.P., Haberkorn I., Böcker L., Mathys A. Cultivation of Chlorella protothecoides under different growth modes and its utilisation in oil/water emulsions. Bioresour. Technol. 2019;288:121476. doi: 10.1016/j.biortech.2019.121476. [DOI] [PubMed] [Google Scholar]
- 183.Damodaran S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2006;70:R54–R66. doi: 10.1111/j.1365-2621.2005.tb07150.x. [DOI] [Google Scholar]
- 184.Law S.Q., Mettu S., AshokKumar M., Scales P.J., Martin G.J. Emulsifying properties of ruptured microalgae cells: Barriers to lipid extraction or promising biosurfactants? Colloids Surfaces B Biointerfaces. 2018;170:438–446. doi: 10.1016/j.colsurfb.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 185.Cabra V., Arreguín R. Emulsifying Properties of Proteins. Boletín Soc. Química México. 2008;2:80–89. [Google Scholar]
- 186.Hasenhuettl G.L. Analysis of food emulsifiers. In: Hasenhuettl G.L., Hartel R.W., editors. Food Emulsifiers and Their Applications. Springer; New York, NY, USA: 2008. pp. 39–62. [Google Scholar]
- 187.Mnif I., Ghribi D. High Molecular Weight Bioemulsifiers, Main Properties and Potential Environmental and Biomedical Applications. World J. Microbiol. Biotechnol. 2015;16:691–706. doi: 10.1007/s11274-015-1830-5. [DOI] [PubMed] [Google Scholar]
- 188.Lu F.S.H., Nielsen N.S., Baron C.P., Jacobsen C. Marine phospholipids: The current understanding of their oxidation mechanisms and potential uses for food fortification. Crit. Rev. Food Sci. Nutr. 2017;57:2057–2070. doi: 10.1080/10408398.2014.925422. [DOI] [PubMed] [Google Scholar]
- 189.Pietrowski B.N., Tahergorabi R., Matak K.E., Tou J.C., Jaczynski J. Chemical properties of surimi seafood nutrified with ω-3 rich oils. Food Chem. 2011;129:912–919. doi: 10.1016/j.foodchem.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 190.Sedoski H.D. Master’s Thesis. West Virginia University Libraries; Morgantown, WV, USA: 2011. Sensory Evaluation and Quality Indicators of Nutritionally Enhanced Egg Product with Omega-3 Rich Oils. [Google Scholar]
- 191.Kassis N.M., Gigliotti J.C., Beamer S.K., Tou J.C., Jaczynski J. Characterization of lipids and antioxidant capacity of novel nutraceutical egg products developed with omega-3-rich oils. J. Sci. Food Agric. 2011;92:66–73. doi: 10.1002/jsfa.4542. [DOI] [PubMed] [Google Scholar]
- 192.El-Baz F.K., Abdo S.M., Hussein A.M.S. Microalgae Dunaliella salina for Use as Food Supplement to Improve Pasta Quality. Int. J. Pharm. Sci. Rev. Res. 2017;46:45–51. [Google Scholar]
- 193.Pina-Pérez M.C., Rivas A., Martínez A., Rodrigo D. Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem. 2017;235:34–44. doi: 10.1016/j.foodchem.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sidari R., Tofalo R. A Comprehensive Overview on Microalgal-Fortified/Based Food and Beverages. Food Rev. Int. 2019;35:778–805. doi: 10.1080/87559129.2019.1608557. [DOI] [Google Scholar]
- 195.Gouveia L., Coutinho C., Mendonça E., Batista A.P., Sousa I., Bandarra N.M., Raymundo A. Functional biscuits with PUFA-ω3 from Isochrysis galbana. J. Sci. Food Agric. 2008;88:891–896. doi: 10.1002/jsfa.3166. [DOI] [Google Scholar]
- 196.Gouveia L., Batista A.P., Raymundo A., Bandarra N. Spirulina maxima and Diacronema vlkianummicroalgae in vegetable gelled desserts. Nutr. Food Sci. 2008;38:492–501. doi: 10.1108/00346650810907010. [DOI] [Google Scholar]
- 197.Kaur P. Microalgae as nutraceutical for achieving sustainable food solution in future. In: Singh J., Vyas A., Wang S., Prasad R., editors. Microbial Biotechnology: Basic Research and Applications. Springer; Singapore: 2020. pp. 91–125. Environmental and Microbial Biotechnology. [Google Scholar]
- 198.Raja R., Coelho A., Hemaiswarya S., Kumar P., Carvalho I.S., Alagarsamy A. Applications of microalgal paste and powder as food and feed: An update using text mining tool. Beni Suef Univ. J. Basic Appl. Sci. 2018;7:740–747. doi: 10.1016/j.bjbas.2018.10.004. [DOI] [Google Scholar]
- 199.Griffiths M., Harrison S.T.L., Smit M., Maharajh D. Major commercial products from micro and macroalgae. In: Bux F., Chisti Y., editors. Algae Biotechnology. Springer International Publishing; Cham, Switzerland: 2016. pp. 269–300. Green Energy and Technology. [Google Scholar]
- 200.Reboleira J., Freitas R., Pinteus S., Silva J., Alves C., Pedrosa R., Bernardino S. Nonvitamin and Nonmineral Nutritional Supplements. Elsevier; Amsterdam, The Netherlands: 2019. Spirulina; pp. 409–413. [Google Scholar]
- 201.Matos J., Cardoso C., Bandarra N.M., Afonso C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017;8:2672–2685. doi: 10.1039/C7FO00409E. [DOI] [PubMed] [Google Scholar]
- 202.Silva J., Alves C., Pinteus S., Reboleira J., Pedrosa R., Bernardino S. Nonvitamin and Nonmineral Nutritional Supplements. Elsevier; Amsterdam, The Netherlands: 2019. Chlorella; pp. 187–193. [Google Scholar]
- 203.Rodriguez-Garcia I., Guil-Guerrero J.L. Evaluation of the antioxidant activity of three microalgal species for use as dietary supplements and in the preservation of foods. Food Chem. 2008;108:1023–1026. doi: 10.1016/j.foodchem.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 204.Vazhappilly R., Chen F. Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. J. Am. Oil Chem. Soc. 1998;75:393–397. doi: 10.1007/s11746-998-0057-0. [DOI] [Google Scholar]
- 205.Seto A., Wang H.L., Hesseltine C.W. Culture conditions affect eicosapentaenoic acid content of Chlorella minutissima. J. Am. Oil Chem. Soc. 1984;61:892–894. doi: 10.1007/BF02542159. [DOI] [Google Scholar]
- 206.European Parliament European Council Commission Decision of 21 October 2009 Concerning the Extension of Uses of Algal Oil from the Microalgae Ulkenia Sp. as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (Notified under Document C(2009) 7932) Off. J. Eur. Union. 2009 Oct 21;L 278/54:2. [Google Scholar]
- 207.Fu W., Nelson D., Yi Z., Xu M., Khraiwesh B., Jijakli K., Chaiboonchoe A., Alzahmi A., Al-Khairy D., Brynjolfsson S., et al. Bioactive compounds from microalgae: Current development and prospects. In: Rahman A., editor. Studies in Natural Products Chemistry. Volume 54. Elsevier; Amsterdam, The Netherlands: 2017. pp. 199–225. [Google Scholar]
- 208.Torres-Tiji Y., Fields F.J., Mayfield S.P. Microalgae as a future food source. Biotechnol. Adv. 2020;41:107536. doi: 10.1016/j.biotechadv.2020.107536. [DOI] [PubMed] [Google Scholar]
- 209.CEVA . Edible Seaweed and Microalgae—Regulatory Status in France and Europe—2019 Update. Centre d’Etude et de Valorisation des Algues; Saint Brieuc, France: 2020. p. 15. [Google Scholar]
- 210.Nuzzo D., Contardi M., Kossyvaki D., Picone P., Cristaldi L., Galizzi G., Bosco G., Scoglio S., Athanassiou A., Carlo M.D. Heat-Resistant Aphanizomenon Flos-Aquae (AFA) Extract (Klamin®) as a Functional Ingredient in Food Strategy for Preven-tion of Oxidative Stress. Oxidative Med. and Cell. Longev. 2019:9481390. doi: 10.1155/2019/9481390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Niccolai A., Bažec K., Rodolfi L., Biondi N., Zlatić E., Jamnik P., Tredici M.R. Lactic Acid Fermentation of Arthrospira platensis (Spirulina) in a Vegetal Soybean Drink for Developing New Functional Lactose-Free Beverages. Front. Microbiol. 2020;11:560684. doi: 10.3389/fmicb.2020.560684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Darwish R., Gedi M.A., Akepach P., Assaye H., Zaky A.S., Gray D.A. Chlamydomonas reinhardtii Is a Potential Food Supplement with the Capacity to Outperform Chlorella and Spirulina. Appl. Sci. 2020;17:6736. doi: 10.3390/app10196736. [DOI] [Google Scholar]
- 213.Kawano T., Naito J., Nishioka M., Nishida N., Takahashi M., Kashiwagi S., Sugino T., Watanabe Y. Effect of Food Con-taining Paramylon Derived from Euglena Gracilis EOD-1 on Fatigue in Healthy Adults: A Randomized, Double-Blind, Pla-cebo-Controlled, Parallel-Group Trial. Nutrients. 2020;15:3098. doi: 10.3390/nu12103098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ruiz-Domínguez M.C., Espinosa C., Paredes A., Palma J., Jaime C., Vílchez C., Cerezal P. Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants. Molecules. 2019;24:4073. doi: 10.3390/molecules24224073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xia S., Wang K., Wan L., Li A., Hu Q., Zhang C. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella aurita. Mar. Drugs. 2013;11:2667–2681. doi: 10.3390/md11072667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lafarga T., Acién-Fernández F.G., Castellari M., Villaró S., Bobo G., Aguiló-Aguayo I. Effect of microalgae incorporation on the physicochemical, nutritional, and sensorial properties of an innovative broccoli soup. Food Sci. Technol. 2019;111:167–174. doi: 10.1016/j.lwt.2019.05.037. [DOI] [Google Scholar]
- 217.Matos Â.P., Feller R., Moecke E.H.S., De Oliveira J.V., Junior A.F., Derner R.B., Sant’Anna E.S. Chemical Characterization of Six Microalgae with Potential Utility for Food Application. J. Am. Oil Chem. Soc. 2016;93:963–972. doi: 10.1007/s11746-016-2849-y. [DOI] [Google Scholar]
- 218.Hemaiswarya S., Raja R., Kumar R.R., Ganesan V., Anbazhagan C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2010;27:1737–1746. doi: 10.1007/s11274-010-0632-z. [DOI] [Google Scholar]
- 219.Camacho F., Macedo A., Malcata F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs. 2019;17:312. doi: 10.3390/md17060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shah M.R., Lutzu G.A., Alam A., Sarker P., Chowdhury M.A.K., Parsaeimehr A., Liang Y., Daroch M. Microalgae in aquafeeds for a sustainable aquaculture industry. Environ. Boil. Fishes. 2018;30:197–213. doi: 10.1007/s10811-017-1234-z. [DOI] [Google Scholar]
- 221.Martínez-Fernández E., Southgate P.C. Use of tropical microalgae as food for larvae of the black-lip pearl oyster Pinctada margaritifera. Aquaculture. 2007;263:220–226. doi: 10.1016/j.aquaculture.2006.09.040. [DOI] [Google Scholar]
- 222.Breteler W.C.M.K., Schogt N., Baas M., Schouten S., Kraay G.W. Trophic upgrading of food quality by protozoans enhancing copepod growth: Role of essential lipids. Mar. Biol. 1999;135:191–198. doi: 10.1007/s002270050616. [DOI] [Google Scholar]
- 223.Bec A., Martin-Creuzburg D., Von Elert E. Trophic upgrading of autotrophic picoplankton by the heterotrophic nanoflagellate Paraphysomonas sp. Limnol. Oceanogr. 2006;51:1699–1707. doi: 10.4319/lo.2006.51.4.1699. [DOI] [Google Scholar]
- 224.Chu F., Lund E., Podbesek J. Quantitative significance of n-3 essential fatty acid contribution by heterotrophic protists in marine pelagic food webs. Mar. Ecol. Prog. Ser. 2008;354:85–95. doi: 10.3354/meps07215. [DOI] [Google Scholar]
- 225.Ganuza E., Benítez-Santana T., Atalah E., Vega-Orellana O., Ganga R., Izquierdo M. Crypthecodinium cohnii and Schizochytrium sp. as potential substitutes to fisheries-derived oils from seabream (Sparus aurata) microdiets. Aquaculture. 2008;277:109–116. doi: 10.1016/j.aquaculture.2008.02.005. [DOI] [Google Scholar]
- 226.Lubzens E. Raising rotifers for use in aquaculture. Hydrobiologia. 1987;147:245–255. doi: 10.1007/BF00025750. [DOI] [Google Scholar]
- 227.Ferreira M., Maseda A., Fabregas J., Otero A. Enriching Rotifers with “Premium” Microalgae Isochrysis aff. galbana Clone T-ISO. Aquaculture. 2008;279:126–130. doi: 10.1016/j.aquaculture.2008.03.044. [DOI] [Google Scholar]
- 228.Ferreira M., Coutinho P., Seixas P., Fábregas J., Otero A. Enriching Rotifers with “Premium” Microalgae. Nannochloropsis gaditana. Mar. Biotechnol. 2009;11:585–595. doi: 10.1007/s10126-008-9174-x. [DOI] [PubMed] [Google Scholar]
- 229.Castillo C.E.-D., Gapasin R.S., Leaño E.M. Enrichment potential of HUFA-rich thraustochytrid Schizochytrium mangrovei for the rotifer Brachionus plicatilis. Aquaculture. 2009;293:57–61. doi: 10.1016/j.aquaculture.2009.04.008. [DOI] [Google Scholar]
- 230.Koyande A.K., Chew K.W., Rambabu K., Tao Y., Chu D.-T., Show P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness. 2019;8:16–24. doi: 10.1016/j.fshw.2019.03.001. [DOI] [Google Scholar]
- 231.Kovač D.J., Simeunović J.B., Babić O.B., Mišan A.Č., Milovanović I.L. Algae in Food and Feed. Food Feed Res. 2013;11:21–32. [Google Scholar]
- 232.Alves S.P., Mendonça S.H., Silva J.L., Bessa R.J.B. Nannochloropsis oceanica, a novel natural source of rumen-protected eicosapentaenoic acid (EPA) for ruminants. Sci. Rep. 2018;8:10269. doi: 10.1038/s41598-018-28576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Beynen A.C. Microalgae in Petfood. Creat. Companion. 2019;40:7. [Google Scholar]
- 234.Souza C.M.M., De Lima D.C., Bastos T.S., De Oliveira S.G., Beirão B.C.B., Félix A.P. Microalgae Schizochytrium sp. as a source of docosahexaenoic acid (DHA): Effects on diet digestibility, oxidation and palatability and on immunity and inflammatory indices in dogs. Anim. Sci. J. 2019;90:1567–1574. doi: 10.1111/asj.13294. [DOI] [PubMed] [Google Scholar]
- 235.Hadley K., Bauer J., Milgram N. The oil-rich alga Schizochytrium sp. as a dietary source of docosahexaenoic acid improves shape discrimination learning associated with visual processing in a canine model of senescence. Prostaglandins Leukot. Essent. Fat. Acids. 2017;118:10–18. doi: 10.1016/j.plefa.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 236.Dahms I., Bailey-Hall E., Sylvester E., Parenteau A., Yu S., Karagiannis A., Roos F., Wilson J. Safety of a novel feed ingredient, Algal Oil containing EPA and DHA, in a gestation-lactation-growth feeding study in Beagle dogs. PLoS ONE. 2019;14:e0217794. doi: 10.1371/journal.pone.0217794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Allmicroalgae Natural Products Allvitae by Allmicroalgae. [(accessed on 7 December 2020)]; Available online: https://www.allmicroalgae.com/en/feed-2/
- 238.Ao T., Macalintal L.M., Paul M.A., Pescatore A.J., Cantor A.H., Ford M.J., Timmons B., Dawson K.A. Effects of supplementing microalgae in laying hen diets on productive performance, fatty-acid profile, and oxidative stability of eggs. J. Appl. Poult. Res. 2015;24:394–400. doi: 10.3382/japr/pfv042. [DOI] [Google Scholar]
- 239.Corbion Inc AlgaPrimeTM DHA Is the World’s Leading Source of Algae Omega-3 Feed Ingredient for Aquaculture, Production Animals and Companion Animals. [(accessed on 7 December 2020)]; Available online: https://algaprime.com.