Abstract
The GATA proteins, functioning as transcription factors (TFs), are involved in multiple plant physiological and biochemical processes. In this study, 28 GATA TFs of Brachypodium distachyon (BdGATA) were systematically characterized via whole-genome analysis. BdGATA genes unevenly distribute on five chromosomes of B. distachyon and undergo purifying selection during the evolution process. The putative cis-acting regulatory elements and gene interaction network of BdGATA were found to be associated with hormones and defense responses. Noticeably, the expression profiles measured by quantitative real-time PCR indicated that BdGATA genes were sensitive to methyl jasmonate (MeJA) and salicylic acid (SA) treatment, and 10 of them responded to invasion of the fungal pathogen Magnaporthe oryzae, which causes rice blast disease. Genome-wide characterization, evolution, and expression profile analysis of BdGATA genes can open new avenues for uncovering the functions of the GATA genes family in plants and further improve the knowledge of cellular signaling in plant defense.
Keywords: Brachypodium distachyon, GATA transcription factors, genome-wide characterization, phylogenetic analysis, profile analysis
1. Introduction
GATA transcription factors (TFs), as important regulatory proteins for gene expression and biological processes, are widely distributed in eukaryotes, including animals, plants, and fungi [1]. GATA family TFs are well characterized as specifically recognizing a conserved DNA motif (T/A)GATA(G/A), and their DNA binding domain has one or two typical Cys2/Cys2 type-IVb zinc finger motifs [2]. NtGATA1, homologous to the Neurospora crassa nit-2 gene, was the first identified and cloned member of the GATA TFs found in tobacco [3]. As a subsequent study, GATA TFs were determined and classified into four subfamilies (A to D) on the basis of the evolutionary relationship and structure domains [4]. There are distinctions among the zinc loop motifs found in the four GATA subfamilies: subfamily A contains a CX2CX18CX2C zinc loop; subfamily B possesses a similar zinc loop, except for the four amino acid motifs (XTNMMX) existing between the first and second Cys; subfamily C contains a CX2CX20CX2C and a TIFY motif related to biological clock and hormone signaling responses; and subfamily D possesses a CX2CX18CX2C zinc loop at the N-terminal end. In addition, the exon numbers of these GATA TFs subfamilies are distinct, with two exons in subfamily A, three in subfamily B, seven in subfamily C, and six in subfamily D [5].
GATA TFs are reported to be widely involved in regulating the processes of plant growth and secondary metabolism. PdGATA19 in Populus plays multiple crucial roles in secondary xylem differentiation and plant growth, predicted by cis-acting regulatory elements (CREs) [6]. Overexpression of PdGATA19 in Populus plants showed added biomass accumulation, increased stem height and photosynthetic rate, and 20% higher chlorophyll content; on the other hand, CRISPR/Cas9-mediated mutant pdgata19 exhibited severe growth retardation and a higher proportion of secondary xylem [6]. There is a similar situation with rice and Arabidopsis—GATA TFs are related to chlorophyll content and plant growth, and even yield [7,8]. For Arabidopsis GATA12 as a target of RGA-LIKE2 (RGL2, a pivotal transcriptional repressor of the gibberellic acid signaling pathway), its transcription level was negatively regulated by gibberellic acid (GA) and sharply reduced in specific broken dormancy environments of cold stratification and dry storage of seeds [9,10]. In addition, the seeds derived from GATA12 suppressed transgenic plants exhibiting a lower degree of dormancy, while seeds from overexpressed GATA12 lines showed a high degree of dormancy [10].
Plants frequently encounter various hormones and stresses in their ecosystems. Jasmonate ZIM-domain (JAZ) protein, a key regulator of the jasmonate acid signaling pathway, is capable of interacting with GATA TFs [11]. The GATA TF LESION SIMULATING DISEASE1 (LSD1) was originally discovered in Arabidopsis, which is related to the salicylic acid (SA) signaling pathway and negatively regulates programmed cell death; the expression level of LSD1 affects the expression of SA signaling-related genes [12]. It is reported that GATA TFs play a significant role in resistance to abiotic stress [13,14,15]. For example, the expression of the Arabidopsis GATA TF gene GATA nitrate-inducible, carbon metabolism-involved (AtGNC) in low-concentration nitrogen treatment is about 1.5 folds higher than that in high-concentration nitrogen treatment [16]. Similarly, overexpressed lines of the rice GATA TF gene CYTOKININ-RESPONSIVE GATA FACTOR1 (OsCga1) under a nitrogen-deficient environment are still able to sustain increased chlorophyll content, but not the oscga1 mutant [17]. Moreover, GATA genes play an important role in biotic stress. It is reported that the GATA proteins are involved in the rice–Magnaporthe oryzae interaction, validated by gene expression profiling [18]. An Arabidopsis GATA family protein, At4G20380, participates in the plant hypersensitive response (HR) to pathogen invasion [19]. Additionally, TF clique analysis indicated that GATA TFs co-occurred with WRKY and NAC TFs to regulate plant immunity [20].
Brachypodium distachyon is an ideal model monocot plant for studying interactions with fungi, which can be infected by multiple cereal pathogens, such as M. oryzae. In this study, we performed a genome-wide survey of GATA genes in B. distachyon and conducted an expression profiling analysis of all GATA genes with hormone treatments and M. oryzae inoculation. It was found that 10 GATA genes in B. distachyon responded to fungal infection. The findings obtained in this study elucidate the evolutionary process of GATA proteins in B. distachyon, which will provide evidence for further study of GATA gene functions.
2. Results
2.1. Identification and Phylogenetic Analysis of GATA Family Proteins in Brachypodium Distachyon
In this study, a total of 28 proteins with complete GATA domain were found in B. distachyon. The open reading frame (ORF) length of B. distachyon GATA TFs varied from 252 to 1635 bp, and the theoretical molecular weight of the putative GATA proteins ranged from 9 to 59 kDa (Supplementary Table S1).
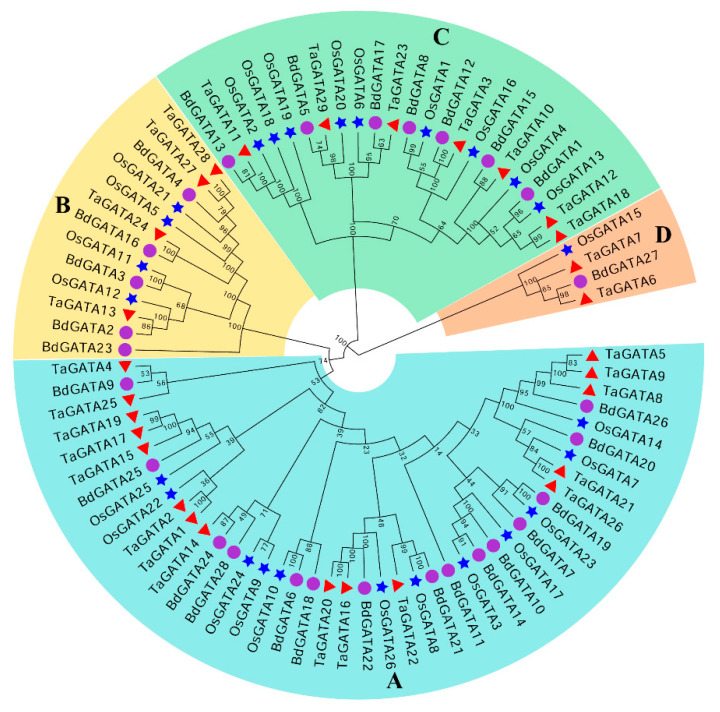
In order to analyze the evolutionary diversity of GATA genes, we constructed a comprehensive neighbor-joining phylogenetic tree for the three Gramineae species B. distachyon, rice (Oryza sativa L.), and wheat (Triticum aestivum L.), and the locus names of the GATA genes from these three are listed in Supplementary Table S2. The phylogenetic clustering analysis revealed that GATA genes were divided into four subgroups, named A to D according to their motifs (Figure 1). Among them, group A accounted for over half of the total (53.5%) and group D for only one member (BdGATA27) of B. distachyon. Group B contained the integrated conserved domain (GATA/CCT/TIFY); however, group D lacked some motifs. Consistently, the vast majority of GATA proteins within the same group had similar physicochemical characteristics, as shown in Table S1.
Figure 1.
Phylogenetic tree of GATA proteins from Brachypodium distachyon, rice, and wheat. Phylogenetic relationships of GATA proteins from Brachypodium distachyon (28), rice (26), and wheat (29) were performed with MEGA v5.0 using the NJ method with 1000 bootstrap replicates. The GATA proteins were clustered into four major groups—A, B, C, and D. Members of B. distachyon, rice, and wheat are represented by purple circles, blue stars, and red triangles, respectively. Gene IDs of GATAs from B. distachyon, rice and wheat were listed in Table S2.
2.2. Chromosome Location and Duplication Analysis of BdGATA Genes
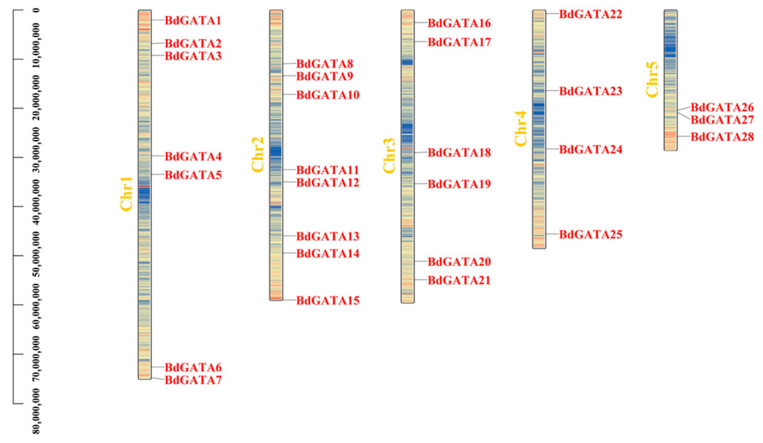
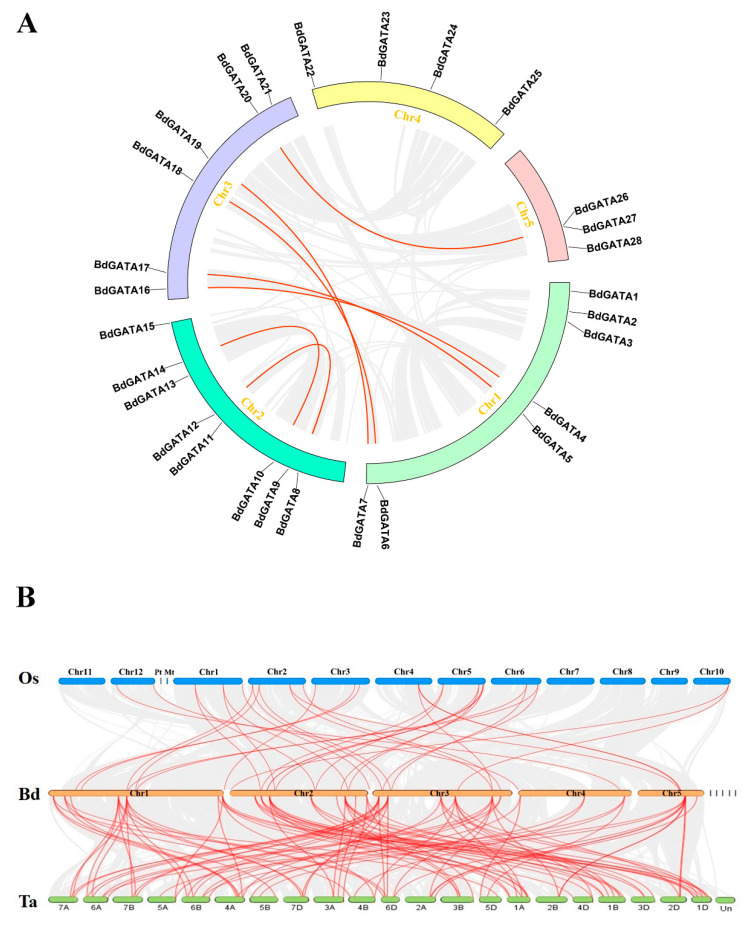
To further determine the chromosome position distribution of BdGATA genes, we utilized the tblastn program of the National Center for Biotechnology Information database to search for the nucleotide sequence of each BdGATA gene. The distribution of blast hits is shown in Figure 2. The GATA genes were non-uniformly distributed in the five chromosomes of B. distachyon, and there was no biological relevance between BdGATA gene number/density and the chromosome, the maximum of which is eight BdGATA genes existing on chromosome 2, with a minimum of three on chromosome 5 (Figure 2). For the vast majority of species, tandem and segmental duplication are the crucial evolutionary driving forces [21]. Therefore, we analyzed the tandem and segmental duplication characteristics of GATA genes and identified a BdGATA tandem duplication cluster (BdGATA26/BdGATA27). Moreover, seven BdGATA gene pairs were found as segmental duplication events (Figure 3A, Supplementary Table S3), which may partially contribute to GATA gene generation. The rate of nonsynonymous (Ka) to synonymous (Ks) substitution was used to evaluate the tendency and strength of natural selection action. The Ka/Ks values of all GATA gene pairs were less than 1.00 (0.28–0.90), which indicates that the GATA gene pairs in B. distachyon undergo intense purifying selection during the evolutionary process (Supplementary Table S3). Moreover, we also calculated the approximate date of duplication events. The duplication events of BdGATA genes occurred from 53.88 Mya (Ks = 0.700) to 89.75 Mya (Ks = 1.166), with a mean of 73.53 Mya (Ks = 0.955). To deduce the synteny of GATA genes, we conducted a collinearity analysis between B. distachyon and the other two cereal species (rice and wheat) using One Step MCScan. Consequently, 33 paired collinearity relationships between BdGATA (B. distachyon) and OsGATA (rice) genes were found, and there were 98 pairs between BdGATA and TaGATA (wheat) genes (Figure 3B). Detailed information of the synteny analysis, including gene pairs and chromosome locations, is shown in Supplementary Table S4.
Figure 2.
Chromosomal locations of BdGATA with gene density. The size of a chromosome is estimated from its relative length. The chromosome numbers are shown on the left of each chromosome. The gene density is indicated by the color bar (blue-to-red scale indicates gene density from low to high).
Figure 3.
Duplication pairs and synteny analysis of GATA genes between Brachypodium distachyon and rice/wheat. (A) Each chromosome is represented with a different color. Gray curves denote the details of syntenic regions in Brachypodium distachyon genome and red curves denote BdGATA gene pairs with segmental duplication. (B) Red lines indicate homologous genes between Brachypodium distachyon and rice/wheat chromosomes.
2.3. Structures and Conserved Motifs of BdGATA Genes
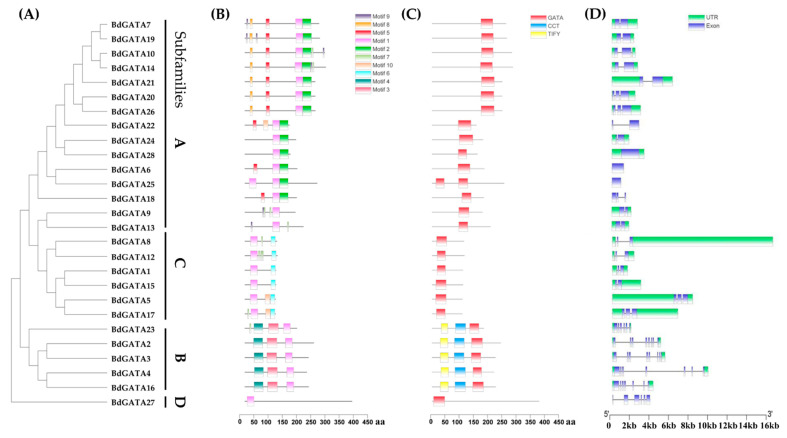
To illustrate the potential correlation between gene structure–function and the evolution process of BdGATA genes, we analyzed gene structure (exons/introns) and conserved domains/motifs. As shown in Figure 4, we found that the structures of GATA genes in the same subcluster were similar, which can also serve as additional evidence to support the classification of BdGATA genes. In the BdGATA proteins, 10 motifs were identified and defined as motifs 1 to 10 (Figure 4B and Supplementary Figure S1). In addition, besides the common GATA domain, there were two additional domains, CCT and TIFY, exclusively present in subfamily B (Figure 4C). In detail, BdGATA2/3/4/16/23 contains similar structures and multiple exons, usually six to seven, whereas BdGATA24/25/28 contains only one exon (Figure 4D). This, again, reveals the occurrence of duplication events. On the other hand, significant differences in the number of exons indicate gain or loss of DNA fragments throughout the evolutionary process.
Figure 4.
Putative conserved motifs, domains, and gene structures of the BdGATA family. (A) Phylogenetic tree. Multiple sequence alignment of BdGATA proteins was performed using ClustalX. The neighbor-joining tree was constructed using MEGA v5.0 with 1000 bootstrap replicates. (B) Conserved motifs. MEME Suite version 5.1.1 revealed the conserved motifs of the GATA proteins. (C) The distribution of conserved domains of BdGATA family. aa: amino acids. (D) Gene structure. The blue square, green square, and black lines represent the exon, untranslated region (UTR), and intron, respectively.
2.4. Prediction of CRE-Involved Pathways, BdGATA Targets of miRNA, and BdGATA Interaction Network
To illuminate the mechanisms of GATA genes in transcriptional regulation, we identified the CREs within a region 2 kb upstream from the start codon (ATG) of GATA genes using the PlantCARE website search tool. The predictions showed that the CREs of BdGATA genes are mainly involved in 11 biological pathways and that each GATA gene contains several types of CREs (Supplementary Figure S2, Supplementary Table S5) that participate in responses to stress.
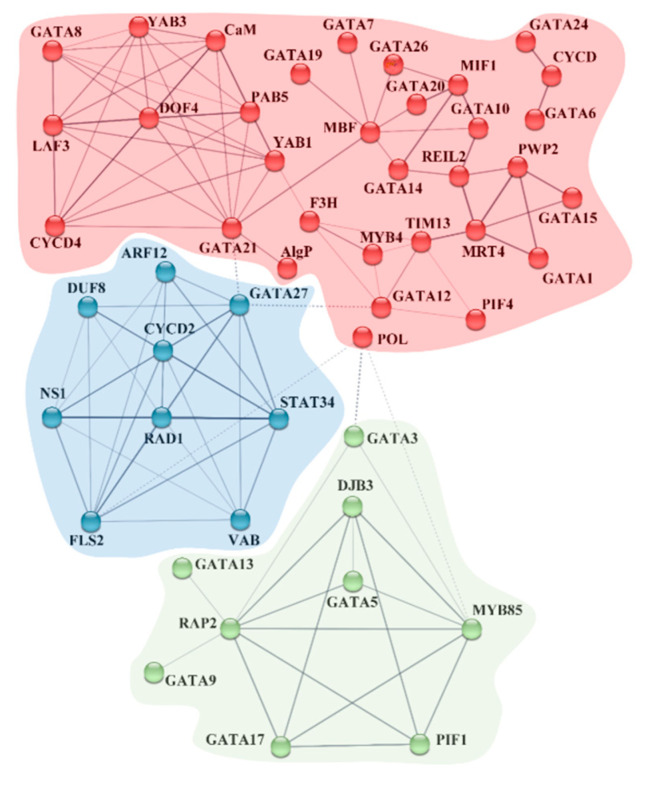
To further reveal the potential interactions between BdGATA proteins and other proteins, we drafted the BdGATA protein interaction network on the basis of the String database (Figure 5, Supplementary Table S6). The network is clustered into three sets, which were divided according to K-means. Several BdGATA proteins directly or indirectly interact with each other (e.g., BdGATA12–BdGATA21–BdGATA27). Coincidently, both related to AP2-like ethylene-responsive transcription factor 2 (RAP2) and multiprotein bridging factor (MBF) were predicted to interact with five BdGATA proteins in varying degrees.
Figure 5.
Interaction network of GATA and related genes. Line thickness indicates the strength of data support. The associations were inferred from the evidence from the STRING database—known interactions, predicted interactions, and others.
Numerous microRNAs (miRNAs) have a strong tendency to target genes involved in defense and development, especially TF genes [22]. A previous report demonstrated that some GATA genes were potential regulatory targets of miRNAs [23]. To rapidly predict GATA genes that are possible targets of miRNA, we performed a web-based plant small RNA target analysis (Supplementary Table S7). The results revealed that 16 BdGATA genes were predicted targets of 13 miRNAs. It should be noted that potato miR2673 targets seven GATA genes, and miR2673-GATA (PGSC id: DMT400024205) in potato was predicted to regulate resistance against late blight disease [24]. Furthermore, miR2673 was reported to implicate plant oxidative burst and HR via proline dehydrogenase [25].
2.5. Expression Profile of BdGATA Genes in Response to Magnaporthe Oryzae and Hormone Treatments
To detect the BdGATA gene expression profile in specific B. distachyon organs (roots, stems, leaves, and seeds), we performed a reverse-transcription polymerase chain reaction (RT-PCR) with the BdUBC gene as the internal control. As shown in Figure 6, BdGATA genes had different expressions in leaves, roots, stems, and seeds. For example, BdGATA1/6/21 showed similar expression patterns, which were at a high level in roots, stems, and seeds, but a low level in leaves. BdGATA2/25/27 were ubiquitously expressed in all tested organs, with varying transcript levels.
Figure 6.
RT-PCR analysis of BdGATA expression in different tissues of Brachypodium distachyon.
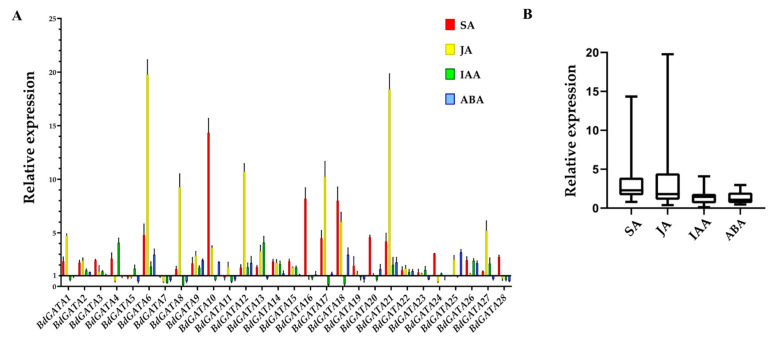
The expression of BdGATA genes was detected after treatment with different phytohormones. Compared to the other three hormone treatments, BdGATA10/16/18/20/24/28 showed more significant expression change after SA treatment. In addition, BdGATA6/8/12/17/21/27 showed significant expression change upon JA treatment (Figure 7A). To make the results more intuitive, we have shown the results of BdGATA gene expression after phytohormone treatments as a box–whisker plot containing a median (line) and interquartile range (box ends). According to Figure 7B, one can see clearly that BdGATA genes were strongly induced by JA and SA, but not abscisic acid (ABA) and indoleacetic acid (IAA).
Figure 7.
Expression profiles of BdGATA genes with phytohormone treatments. (A) Fold change in expression of BdGATA genes after hormone treatments. Fold change in BdGATA gene expression after hormone treatments assessed by qRT-PCR of cDNA isolated from hormone treatment plants were compared with mock treatment plants. Fold change of 1 means no difference in gene expression between hormone treatment plants and mock treatment plants. This assay was repeated three times with similar results. (B) Box–whisker plot of BdGATA gene expression after phytohormone treatments. The y-axis represents relative gene expression level. Whiskers represent maximum/minimum values, boxes indicate the first quartile and third quartile, and the horizontal line represents the median.
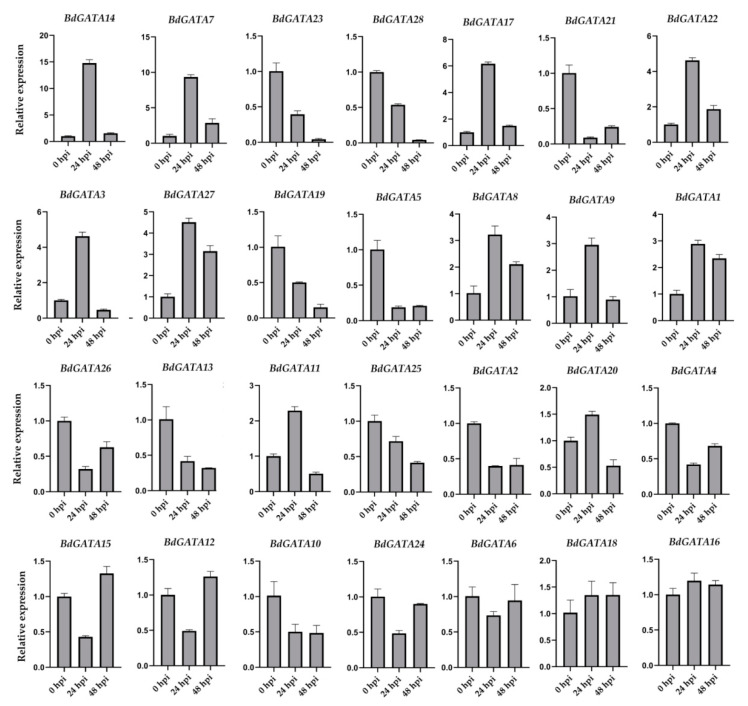
In addition, the transcription levels of BdGATA genes were detected in B. distachyon inoculated with M. oryzae isolate RO1-1 at 0, 24, and 48 h post inoculation (hpi). The relative expression of some BdGATA genes displayed significant induction at 24 and/or 48 hpi, such as BdGATA1/7/8/9/11/14/17/22/27; however, several GATA genes were induced at 24 but not 48 hpi (Figure 8). On the contrary, some BdGATA genes were suppressed after inoculation, for instance, BdGATA2/4/5/10/13/19/21/25/26/28 (Figure 8). On the whole, most of the BdGATA proteins might participate in B. distachyon disease resistance responses.
Figure 8.
Expression of BdGATA genes inoculated with Magnaporthe oryzae. The relative expression levels of BdGATA genes were normalized with BdUBC. The experiments in all panels were repeated three times with similar results.
3. Discussion
The GATA TFs participate in the regulation of various vital physiological and biochemical processes in plants [26]. However, the functions and mechanisms of GATA TFs in regulating hormone pathways and plant immunity are still largely unknown. In this study, we conducted a genome-wide analysis of the GATA gene family to explore their potential functions and expression diversity upon hormone and pathogen infection in B. distachyon. A total of 28 GATA genes were presumed throughout the whole genome of B. distachyon, which is close to the 31 GATA proteins identified in Malus domestica [27] and 28 GATA proteins identified in rice [28]. According to phylogenetic analysis, we classified GATA genes identified from B. distachyon into four subfamilies, which is consistent with classification in other plant species, such as the Gossypium genus and M. domestica [29]. BdGATA genes are widely expressed in different organs of B. distachyon, which suggests the important roles of BdGATA in plants. BdGATA11 is a pseudogene, considering it has large deletions and no detectable expression in any organs.
To explore the evolutionary pattern of GATA, we calculated selective pressure. The values of the Ka/Ks ratio for all BdGATA duplicated pairs were less than 1, indicating that purifying selection played a significant role in the evolution of BdGATA genes. In terms of multiple sequences alignment, subfamily B of BdGATA genes contain two additional domains, CCT and TIFY, in addition to the fully conserved GATA domain. This is consistent with the conclusion previously reported for dicot plant Brassica napus and monocot plant Zea mays [30,31].
Hormones are essential signaling molecules that regulate plant immune responses and development [32]. In this study, BdGATA genes showed diverse expression patterns after SA, JA, ABA, and IAA treatment. We suggest that an analysis of GATA genes expression profile under hormone treatment could provide an effective reference for the following screening genes related to rice blast resistance.
B. distachyon is host to a range of cereal pathogens. For example, the Brachypodium–M. oryzae interaction closely models the rice–M. oryzae interaction [33,34]. In the prediction of BdGATA interacting proteins, RAP2 and MBF proteins exhibited strong associations with GATA proteins. MBF functions as a key regulator of resistance to Pseudomonas syringae pv. tomato DC3000 and Puccinia striiformis f. sp. tritici by activating pathogenesis-related genes, SA, and ethylene pathways [35,36]. Likewise, RAP2 was reported to be an active transcriptional repressor of elevated Botrytis cinerea resistance and responses to ethylene [37]. All of these data suggest that BdGATA is likely to regulate disease resistance together with the interacting genes. In this study, on the basis of expression patterns in response to hormone treatment and M. oryzae inoculation, we found that several B. distachyon GATA genes were upregulated upon both treatments, including BdGATA1/8/14/19, which suggests that these upregulated GATA genes could contribute to regulating plant disease resistance against M. oryzae.
In addition, the transcription levels of duplicated BdGATA gene pairs exhibited similar expression patterns, indicating that their role could be redundant. The functional redundancy of duplicated genes may be associated with the analogous CREs, gene models, and conserved motifs [38]. For instance, BdGATA7/19 duplicated pairs showed similar tertiary structures and expression patterns in response to M. oryzae inoculation, and both are predicted to interact with MBF proteins. At any rate, further study of the functions and molecular mechanisms of BdGATA on plant disease resistance and hormone signaling transduction would be valuable.
4. Materials and Methods
4.1. Plant Materials, Hormone Treatments, and Rice Blast Inoculation
B. distachyon Bd21-3 genotype seeds were germinated on wet filter paper in a culture dish for 3 days and planted into a float tray containing vermiculite-based soil in a 22 °C greenhouse [39]. Seedlings were supplied with nutrient solution weekly for 2 months until processing.
For hormone treatment, 30-day-old B. distachyon plants were sprayed with 100 µM jasmonic acid (JA), 100 µM salicylic acid (SA), 100 µM indoleacetic acid (IAA), 100 µM abscisic acid (ABA), or distilled H2O as mock treatment, and then the leaves were sampled 2 h after treatment [40].
4.2. Rice Blast Inoculation
M. oryzae isolate RO1-1 was grown on oat medium and incubated at 26 °C for about 15 days until spores germinated. Subsequently, 30-day-old B. distachyon plants were inoculated with 1 × 106 spores/mL using a spraying method as previously described [41]. Samples were harvested at 0, 24, and 48 h post-inoculation and kept at −80 °C until subsequent RNA extraction.
4.3. Identification of Brachypodium Distachyon GATA Gene Family and Phylogenetic Analysis
Information and sequences of B. distachyon GATA family genes were obtained from the Plant Transcription Factor Database v5.0 (http://planttfdb.cbi.pku.edu.cn/family). Annotation files in general feature format (.gff) and gene transfer format (.gtf) were retrieved from EnsemblPlants (https://plants.ensembl.org/info/website/ftp/index.html). The genome sequence was accessed from https://www.ncbi.nlm.nih.gov/genome.
Multiple alignments of amino acid sequences were conducted using the ClustalX program (version 2.08) with default parameters. A phylogenetic tree was generated with MEGA v5.0 software by the neighbor-joining (NJ) method, including pairwise deletion, Poisson distribution, and 1000 bootstrap replicates.
4.4. Gene Structure, Synteny Analysis, and Bioinformatics Resources
The theoretical isoelectric point (pI) and molecular weight (MW) of the deduced protein were calculated with the ExPASy web tool (https://web.expasy.org/compute_pi/). Motifs were identified by web-based MEME Suite v5.1.0 software (https://meme-suite.org/meme/) with standard parameters. Protein structural domain data were detected from the online conserved domain (CD) search tool (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi), with an expected value of ≤0.01.
The chromosomal locations of the TF genes were learned from TBtools v0.6683 (a program for annotated gene locations from GFF3/GTF files). The multiple alignments were performed using the procedure of the One Step MCScanX with genome sequence file (.fa) and genome structure annotation file (.gff); subsequently, the collinearity results were depicted by the dual synteny plotter for MCScanX program, and two steps above were realized by Toolkit for Biologists (github.com/CJ-Chen) [42].
For cis-acting regulatory element analysis, we extracted 2000 bp DNA sequences upstream from the translation initiation site and then analyzed these regions in PlantCare (http://bioinformatics.psb.ugent.be/plantcare/html) [43]. We performed miRNA target analysis using the psRNATarget web tool (http://plantgrn.noble.org/psRNATarget) [44]. For retrieval of interacting proteins, protein sequences were analyzed using the STRING web portal (http://string-db.org) [45].
4.5. RNA Extraction and qRT-PCR Analysis
Total RNA of B. distachyon was extracted using TRIzol Reagent (Ambion, Waltham, MA, USA), and purified with an EasyPure Plant RNA Kit (TianGen, Beijing, China). Subsequently, cDNA first-strand synthesis was conducted by reverse transcription using a NovoScript Plus All-in-one SuperMix kit (Novoprotein, Shanghai, China). Specific primers for RT-PCR and qRT-PCR are listed in Supplementary Table S8. The roots, stems, leaves, and seeds were sampled at the same time intervals. To investigate the expression of BdGATA genes in different tissues/organs, we carried out the method for tissue sample collection as previously described [46]. RT-PCR was conducted using a MonScript RTase II kit (Monad, Suzhou, China). PCR was performed with 10–15 pmol of each primer, DNA templates (60 ng), double-distilled H2O, and Hieff PCR Master Mix (Yeasen, Shanghai, China) in a total volume of 20 μL. qRT-PCR was performed using a TransStart Tip Green qPCR SuperMix kit (TransGen, Beijing, China) in a CFX 96 qPCR instrument (BioRad, Hercules, CA, USA). BdUBC (Bradi4g00660) was amplified as the normalization control.
5. Conclusions
Genome-wide characterization of the GATA gene family in B. distachyon was performed, with special emphasis on the response to rice blast infection. A total of 28 B. distachyon GATA genes were identified and grouped into four subfamilies. The chromosomal location, gene duplication, structural features, conserved domain and motif, CREs, miRNA target, and expression profiles (organ-specific, response to M. oryzae and hormone treatment) of the GATA gene were analyzed. In conclusion, the 28 predicted BdGATA genes were classified into four subfamilies in this study, on the basis of their motifs. The BdGATA genes, widely expressed in different organs, were responsive to SA and JA hormone signaling pathways, and 10 of them responded to invasion of the fungal pathogen M. oryzae, which indicates that they are involved in plant immunity. In addition, the purifying selection played a significant role in the evolution of BdGATA genes. Overall, these investigations and findings provide a foundation for further study on the disease resistance functions of the GATA family and present new ideas for the breeding of disease-resistant plants.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/4/2026/s1.
Author Contributions
W.P. and W.L.: conceptualization, data curation, writing—original draft preparation. N.S. and Z.T.: data curation. J.L., Y.W., and S.P.: resources, reviewing, and editing. L.D. and B.W.: writing—reviewing, editing, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (31801721; 31672017); National Key Research and Development Project (2016YFD0200800; 2016YFD0300707); the Natural Science Foundation of Hunan Province, China (2020JJ5240); the Scientific Research Fund of Hunan Provincial Education Department (19B247); and the Youth Fund Project of Hunan Agricultural University (19QN31).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reyes J.C., Muro-Pastor M.I., Florencio F.J. The GATA Family of Transcription Factors in Arabidopsis and Rice. Plant Physiol. 2004;134:1718–1732. doi: 10.1104/pp.103.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y., Jia Z., Yi Q., Song X., Liu Y., Jia Y., Wang L., Song L. A novel GATA-like zinc finger transcription factor involving in hematopoiesis of Eriocheir sinensis. Fish Shellfish Immunol. 2018;74:363–371. doi: 10.1016/j.fsi.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Daniel-Vedele F., Caboche M. A tobacco cDNA clone encoding a GATA-1 zinc finger protein homologous to regulators of nitrogen metabolism in fungi. Mol. Gen. Genet. MGG. 1993;240:365–373. doi: 10.1007/BF00280388. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C., Hou Y., Hao Q., Chen H., Chen L., Yuan S., Shan Z., Zhang X., Yang Z., Qiu D., et al. Genome-Wide Survey of the Soybean GATA Transcription Factor Gene Family and Expression Analysis under Low Nitrogen Stress. PLoS ONE. 2015;10:e0125174. doi: 10.1371/journal.pone.0125174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranftl Q.L., Bastakis E., Klermund C., Schwechheimer C. LLM-Domain Containing B-GATA Factors Control Different Aspects of Cytokinin-Regulated Development in Arabidopsis thaliana. Plant Physiol. 2016;170:2295–2311. doi: 10.1104/pp.15.01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An Y., Zhou Y., Han X., Shen C., Wang S., Liu C., Yin W., Xia X. The GATA transcription factor GNC plays an important role in photosynthesis and growth in poplar. J. Exp. Bot. 2020;71:1969–1984. doi: 10.1093/jxb/erz564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu G., Casaretto J.A., Ying S., Mahmood K., Liu F., Bi Y.-M., Rothstein S.J. Overexpression of OsGATA12 regulates chlorophyll content, delays plant senescence and improves rice yield under high density planting. Plant Mol. Biol. 2017;94:215–227. doi: 10.1007/s11103-017-0604-x. [DOI] [PubMed] [Google Scholar]
- 8.Bastakis E., Hedtke B., Klermund C., Grimm B., Schwechheimer C. LLM-Domain B-GATA Transcription Factors Play Multifaceted Roles in Controlling Greening in Arabidopsis. Plant Cell. 2018;30:582–599. doi: 10.1105/tpc.17.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamm P., Ravindran P., Mohanty B., Tan E., Yu H., Kumar P.P. Insights into the molecular mechanism of RGL2-mediated inhibition of seed germination in Arabidopsis thaliana. BMC Plant Biol. 2012;12:179. doi: 10.1186/1471-2229-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravindran P., Verma V., Stamm P., Kumar P.P. A Novel RGL2–DOF6 Complex Contributes to Primary Seed Dormancy in Arabidopsis thaliana by Regulating a GATA Transcription Factor. Mol. Plant. 2017;10:1307–1320. doi: 10.1016/j.molp.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Sen S., Kundu S., Dutta S.K. Proteomic analysis of JAZ interacting proteins under methyl jasmonate treatment in finger millet. Plant Physiol. Biochem. 2016;108:79–89. doi: 10.1016/j.plaphy.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Pant S.R., Krishnavajhala A., McNeece B.T., Lawrence G.W., Klink V.P. The syntaxin 31-induced gene, LESION SIMULATING DISEASE1 (LSD1), functions in Glycine max defense to the root parasite Heterodera glycines. Plant Signal. Behav. 2015;10:e977737. doi: 10.4161/15592324.2014.977737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhardwaj A.R., Joshi G., Kukreja B., Malik V., Arora P., Pandey R., Shukla R.N., Bankar K.G., Katiyar-Agarwal S., Goel S., et al. Global insights into high temperature and drought stress regulated genes by RNA-Seq in economically important oilseed crop Brassica juncea. BMC Plant Biol. 2015;15:9. doi: 10.1186/s12870-014-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta P., Nutan K.K., Singla-Pareek S.L., Pareek A. Abiotic Stresses Cause Differential Regulation of Alternative Splice Forms of GATA Transcription Factor in Rice. Front. Plant Sci. 2017;8:1944. doi: 10.3389/fpls.2017.01944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nutan K.K., Singla-Pareek S.L., Pareek A. The Saltol QTL-localized transcription factor OsGATA8 plays an important role in stress tolerance and seed development in Arabidopsis and rice. J. Exp. Bot. 2019:erz368. doi: 10.1093/jxb/erz368. [DOI] [PubMed] [Google Scholar]
- 16.Bi Y.-M., Zhang Y., Signorelli T., Zhao R., Zhu T., Rothstein S. Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity: A GATA gene for chlorophyll and glucose sensitivity. Plant J. 2005;44:680–692. doi: 10.1111/j.1365-313X.2005.02568.x. [DOI] [PubMed] [Google Scholar]
- 17.Hudson D., Guevara D.R., Hand A.J., Xu Z., Hao L., Chen X., Zhu T., Bi Y.-M., Rothstein S.J. Rice Cytokinin GATA Transcription Factor1 Regulates Chloroplast Development and Plant Architecture. Plant Physiol. 2013;162:132–144. doi: 10.1104/pp.113.217265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W.-T., Chen W.-L., Yang C., Wang J., Yang L., He M., Wang J.-C., Qin P., Wang Y.-P., Ma B.-T., et al. Identification and network construction of zinc finger protein (ZFP) genes involved in the rice-Magnaporthe oryzae interaction. Plant Omics. 2014;7:540–548. [Google Scholar]
- 19.He S., Tan G., Liu Q., Huang K., Ren J., Zhang X., Yu X., Huang P., An C. The LSD1-Interacting Protein GILP Is a LITAF Domain Protein That Negatively Regulates Hypersensitive Cell Death in Arabidopsis. PLoS ONE. 2011;6:e18750. doi: 10.1371/annotation/64462bfc-a7c6-48e2-9710-8ecea202e4a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deb A., Kundu S. Deciphering Cis-Regulatory Element Mediated Combinatorial Regulation in Rice under Blast Infected Condition. PLoS ONE. 2015;21 doi: 10.1371/journal.pone.0137295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leister D. Tandem and segmental gene duplication and recombination in the evolution of plant disease resistance genes. Trends Genet. 2004;20:116–122. doi: 10.1016/j.tig.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Guo Z., Maki M., Ding R., Yang Y. Genome-wide survey of tissue-specific microRNA and transcription factor regulatory networks in 12 tissues. Sci. Rep. 2009;10:729–731. doi: 10.1038/srep05150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Zhang Y.-Z. Computational detection of microRNAs targeting transcription factor genes in Arabidopsis thaliana. Comput. Biol. Chem. 2005;29:360–367. doi: 10.1016/j.compbiolchem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Singh R., Tiwari J.K., Rawat S., Sharma V., Singh B.P. In silico identification of candidate microRNAs and their targets in potato somatic hybrid Solanum tuberosum (+) S. pinnatisectum for late blight resistance. Plant Omics. 2016;9:159–164. doi: 10.21475/poj.160902.p7734x. [DOI] [Google Scholar]
- 25.Yang J., Zhang N., Ma C., Qu Y., Si H., Wang D. Prediction and verification of microRNAs related to proline accumulation under drought stress in potato. Comput. Biol. Chem. 2013;46:48–54. doi: 10.1016/j.compbiolchem.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Yuan Q., Zhang C.-L., Zhao T.-T., Xu, X.Y. Research advances of GATA transcription factor in plant. Mol. Plant Breed. 2017;15:1702–1707. doi: 10.13271/j.mpb.015.001702. [DOI] [Google Scholar]
- 27.Chen H., Shao H., Li K., Zhang D., Fan S., Li Y., Han M. Genome-wide identification, evolution, and expression analysis of GATA transcription factors in apple (Malus × domestica Borkh.) Gene. 2017;627:460–472. doi: 10.1016/j.gene.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 28.Luo Y., Wang Y.-C., Wang W.-P., Zhang C. Bioinformatics Analysis of GATA Gene Family in Rice. Mol. Plant Breed. 2018;16:5514–5522. doi: 10.13271/j.mpb.016.005514. [DOI] [Google Scholar]
- 29.Zhang Z., Zou X., Huang Z., Fan S., Qun G., Liu A., Gong J., Li J., Gong W., Shi Y., et al. Genome-wide identification and analysis of the evolution and expression patterns of the GATA transcription factors in three species of Gossypium genus. Gene. 2019;680:72–83. doi: 10.1016/j.gene.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Zhu W., Guo Y., Chen Y., Wu D., Jiang L. Genome-Wide Identification, Phylogenetic and Expression Pattern Analysis of GATA Family Genes in Brassica Napus. BMC Plant Biol. 2020;20:543. doi: 10.1186/s12870-020-02752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang L., Yu X., Chen D., Feng H., Li J. Identification, Phylogenetic Evolution and Expression Analysis of GATA Transcription Factor Family in Maize (Zea Mays) Int. J. Agric. Bio. 2020;23:7. doi: 10.17957/IJAB/15.1334. [DOI] [Google Scholar]
- 32.Jin J.-H., Zhang H.-X., Ali M., Wei A.-M., Luo D.-X., Gong Z.-H. The CaAP2/ERF064 Regulates Dual Functions in Pepper: Plant Cell Death and Resistance to Phytophthora capsici. Genes. 2019;10:541. doi: 10.3390/genes10070541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker D., Beckmann M., Zubair H., Enot D.P., Caracuel-Rios Z., Overy D.P., Snowdon S., Talbot N.J., Draper J. Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J. 2009;59:723–737. doi: 10.1111/j.1365-313X.2009.03912.x. [DOI] [PubMed] [Google Scholar]
- 34.Routledge A.P.M., Shelley G., Smith J.V., Talbot N.J., Draper J., Mur L.A.J. Magnaporthe grisea interactions with the model grass Brachypodium distachyon closely resemble those with rice (Oryza sativa) Mol. Plant Pathol. 2004;5:253–265. doi: 10.1111/j.1364-3703.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- 35.Ping H.-T., Guo P.-C., Yu P., Zhang Y.-J., Zhang B., Hu Z.-L. Cloning and Analysis of Pathogen Resistance of Multiprotein Bridging Factor Gene LeMBF1 in Tomato. Life Sci. Res. 2012;16:138–143. [Google Scholar]
- 36.Zhang Y., Zhang G., Dong Y.-L., Guo J., Huang L.-L., Kang Z.-S. Cloning and Characterization of a MBF1 Transcriptional Coactivator Factor in Wheat Induced by Stripe Rust Pathogen: Cloning and Characterization of a MBF1 Transcriptional Coactivator Factor in Wheat Induced by Stripe Rust Pathogen. ACTA Agron. Sin. 2009;35:11–17. doi: 10.3724/SP.J.1006.2009.00011. [DOI] [Google Scholar]
- 37.Zhao Y., Wei T., Yin K.-Q., Chen Z., Gu H., Qu L.-J., Qin G. Arabidopsis RAP2.2 plays an important role in plant resistance to Botrytis cinerea and ethylene responses. New Phytol. 2012;195:450–460. doi: 10.1111/j.1469-8137.2012.04160.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z., Fu M., Li H., Chen Y., Wang L., Liu R. Systematic analysis of NAC transcription factors in Gossypium barbadense uncovers their roles in response to Verticillium wilt. PeerJ. 2019;7:e7995. doi: 10.7717/peerj.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu X., Chen S., Li J., Liu Y., Ji W., Li H. Genome-wide identification of GRAS genes in Brachypodium distachyon and functional characterization of BdSLR1 and BdSLRL1. BMC Genom. 2019;20:635. doi: 10.1186/s12864-019-5985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebel C., BenFeki A., Hanin M., Solano R., Chini A. Characterization of wheat (Triticum aestivum) TIFY family and role of Triticum Durum TdTIFY11a in salt stress tolerance. PLoS ONE. 2018;13:e0200566. doi: 10.1371/journal.pone.0200566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang C., Yu Y., Huang J., Meng F., Pang J., Zhao Q., Islam M.A., Xu N., Tian Y., Liu J. Binding of the Magnaporthe oryzae Chitinase MoChia1 by a Rice Tetratricopeptide Repeat Protein Allows Free Chitin to Trigger Immune Responses. Plant Cell. 2019;31:172–188. doi: 10.1105/tpc.18.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai X., Zhuang Z., Zhao P.X. psRNATarget: A plant small RNA target analysis server (2017 release) Nucleic Acids Res. 2018;46:W49–W54. doi: 10.1093/nar/gky316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Feng L., Zhu Y., Li Y., Yan H., Xiang Y. Comparative genomic analysis of the WRKY III gene family in populus, grape, arabidopsis and rice. Biol. Direct. 2015;10:48. doi: 10.1186/s13062-015-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or supplementary material.