Abstract
Essential oils (EOs) are known to have varying degrees of antimicrobial properties that are mainly due to the presence of bioactive compounds. These include antiviral, nematicidal, antifungal, insecticidal and antioxidant properties. This review highlights the potential of EOs and their compounds for application as antifungal agents for the treatment of skin diseases via conventional and nonconventional approaches. A search was conducted using three databases (Scopus, Web of Science, Google Scholar), and all relevant articles from the period of 2010–2020 that are freely available in English were extracted. In our findings, EOs with a high percentage of monoterpenes showed strong ability as potential antifungal agents. Lavandula sp., Salvia sp., Thymus sp., Citrus sp., and Cymbopogon sp. were among the various species found to show excellent antifungal properties against various skin diseases. Some researchers developed advanced formulations such as gel, semi-solid, and ointment bases to further evaluate the effectiveness of EOs as antifungal agents. To date, most studies on the application of EOs as antifungal agents were performed using in vitro techniques, and only a limited number pursued in vivo and intervention-based research.
Keywords: skin fungus, essential oils, in vitro, in vivo, intervention
1. Introduction
Fungi are ubiquitous environmental microorganisms that may be categorized, according to their dimorphic morphology, as unicellular (yeast) or filamentous (molds). Almost one million mycotic species have been reported to exist in nature, with approximately 200 species identified as human pathogenic [1]. It has been found in recent years that fungal infections have contributed to increased mortality rates [2]. This phenomenon has been linked to certain age groups, especially premature neonates, infants and elderly people who are susceptible to underdeveloped or poor immune systems [3,4,5]. The most common species associated with deadly invasive and superficial infections are Candida sp., Aspergillus sp., and Cryptococcus sp. [6]. In addition, Fusarium sp. has been shown to cause opportunistic invasive fungal infections [5,7].
Aspergillus spp. is a filamentous and ubiquitous fungi with A. fumigatus as the major species associated with human disease, followed by A. flavus, A. niger and A. terreus [3,8,9]. In addition to the most common species, several other emerging species exist, including A. clavatus, A. nidulans, A. glaucus and A. ustus [6,9]. Fusarium spp. are other fungi that can cause human infections, and are the primary cause of fungal keratitis. This fungus is the second most common to infect severely immunocompromised patients and cause disseminated infection [10]. F. solani has been identified as the most frequent pathogen in fusarial keratitis incidence, while F. oxysporum leads to major incidences of onychomycosis [11,12,13]. Candida spp. are tiny, oval-shaped fungi with a thin cell wall that are capable of budding or fission. Among the identified species, five are the leading cause of invasive infections (C. albicans, C. glabrata, C. parapsilosis, C. tropicals and, C. krusei) [14]. Invasive candidiasis often occurs as a form of healthcare-associated infection, where affected patients are typically receiving broad-spectrum antibiotic treatment, immunosuppressants, or suffering from cancer [15]. Candidiasis infections typically exist on the epithelial surfaces of the mouth, gastrointestinal tract, vagina and skin surfaces. C. albicans remains the most common cause of skin, nail and mucous membrane infections in healthy individuals, in whom it may also induce more severe infections of the vital organs [16,17].
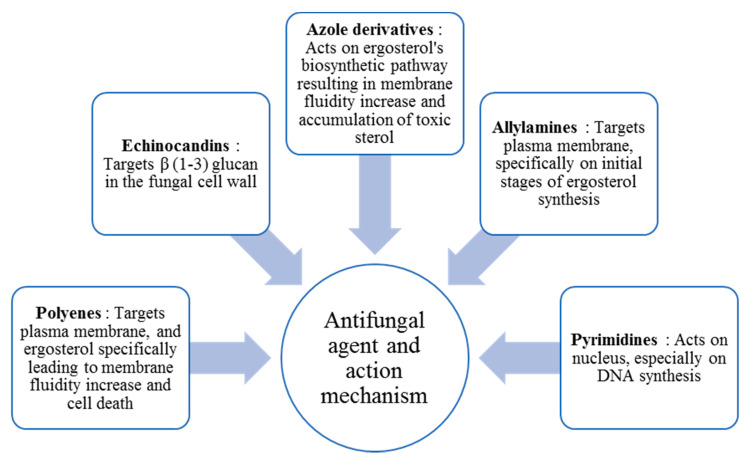
In general, fungal diseases are differentiated into four groups: dermatophytosis, subcutaneous mycoses, systemic mycoses and other mycoses [4]. Dermatophytosis is caused by dermatophytes that attack and grow on dead animal keratin. Epidermophyton, Microsporum and Trichophyton are the three main genera related to dermatophytes. Dermatophytes are known as a species of fungi that typically infect and invade a living host’s skin, hair and nails. Diseases caused by dermatophytes are typically classified according to the infection site, but are broadly referred to as tinea. Several forms of tinea are common such as Tinea capitis (scalp and hair), Tinea corporis (nonhairy skin), Tinea barbae (beard), Tinea cruris (groin), Tinea manuum (hand), Tinea pedis (feet) and Tinea unguium (nails, also called onchomyosis) [18]. According to current practice, five classes of conventional antifungal treatments are commonly applied. Figure 1 shows each antifungal agent and its mechanism of action.
Figure 1.
Conventional antifungal agents and their mechanisms of action (Adapted from [19]).
However, the treatment of fungal infections has encountered serious difficulties in the form of increased resistance due to the extensive use of antifungal agents. This situation has led to the insight that alternative, nonconventional approaches are required for effective antifungal treatment strategies. One of the possible directions proposed is the use of essential oils (EOs) as potential antifungal agents. EOs can be extracted from various plant parts and are volatile, aromatic, concentrated, hydrophobic oily liquids. Monoterpenes, sesquiterpenes and phenolic compounds are the key components of EOs. Phenolic compounds are chiefly responsible for the antimicrobial properties of Eos [20]. Numerous studies have proven the efficacy of EOs in antifungal treatments, although not all have addressed the underlying mechanisms of action [21]. The most widely used parameter in the antimicrobial assessment is minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC), i.e., the lowest antifungal agent concentration required to inhibit fungal growth or to kill mycetes, respectively [2,22]. At present, thyme (Thymus sp.), tea tree (Melaleuca alternifolia), peppermint (Mentha piperita) or clove oil (Syzygium aromaticum) are the most widely tested EOs in terms of their antifungal activities [23,24,25,26]. Nevertheless, examination of the potential of EOs has extended to other types of oils [27]. Several examples of the use of EOs in the treatment of fungal diseases are described below.
Thyme EO (Thymus sp.) is recognized as a promising antifungal agent due to the presence of thymol and carvacrol at high concentrations [28]. A study conducted with one of the most common thyme EO, T. vulgaris, indicated strong antifungal activity against A. flavus at a concentration of 350 ppm [29]. Rasooli and colleagues performed a comparative study of antifungal activity against A. niger using T. eriocalyx (Ronniger) Jalas, Rech.f. and T. x-porlock, showing superior activity of T. eriocalyx, with a MIC value of 125 ppm [30]. Another EO that has been widely explored is cinnamon oil (Cinnamomum sp.), with cinnamaldehyde as its major component. An investigation of the antifungal effect of C. zeylanicum exhibited a synergistic effect with Fluconazole against A. fumigatus [31,32,33]. Similarly, Eucalyptus globulus EO has been shown to possess potent antifungal activity; one study examined its effect on growth inhibition of A. flavus, A. niger, A. terreus and A. fumigatus [34]. S. aromaticum has also shown antifungal efficacy against different Aspergillus spp. (A. terreus, A. flavus and A. fumigatus) [29,35,36,37]. Additionally, Citrus sp., Mentha sp., and Cuminum sp. have been applied against Aspergillus spp. [36,38].
Fungal infections arising from Candida spp. are called candidiasis; the most commonly implicated species is C. albicans. Fluconazole and itraconazole are widely utilized for such infections, but these agents have caused azole resistance within Candida spp. [3,39,40]. The antifungal properties of EOs derived from Melaleuca sp., Origanum sp., Thymus sp., Mentha sp., Syzgium sp., Coriandrum sp., Cuminum sp. and several others against Candida spp. were noted [2]. Melaleuca sp. is the main genus to have been studied for its anticandida properties. In a study by Mondello and colleagues, it was observed that C. krusei and C. gabrata showed sensitivity towards M. alternifolia EO; additionally, all azole resistant C. albicans were killed within 30 and 60 min at 1% and 0.25% concentration, respectively [41]. Antifungal activity was also observed from T. pulegiosides EO, that inhibited C. albicans, C. glabrata, C. parapsilosis, C. krusei and C. guillermondii with a MIC value range of 0.32–0.64 μL/mL [28]. Peppermint EO is also highly capable as an antifungal agent. Studies have revealed its inhibition capacity against Candida spp., dermatophytes and Aspergillus spp. when tested within 40–7000, 800–3500 and 400–3500 μg/mL, respectively [42]. Lavandula angustifolia, or lavender EO has also exhibited excellent antifungal activity against Candida spp. [43,44]. Cumin EO effectiveness as an antifungal agent has also been proven with respect to C. albicans, resulting in MIC values of 3.90 and 11.71 μg/mL [45].
Despite the existence of numerous studies suggesting the suitability of EOs as an alternative treatment strategy for fungal infections, it should be noted that these properties are highly dependent on the composition of the EO itself. These compositions are prone to significant variability based upon the use of different plant parts or the harvesting season [27]. Terpenes and their metabolic derivatives constitute the major component of Eos; these include functional derivatives of alcohols (geraniol), ketones (menthone), esters (cedryl acetate) and phenols (thymol). In addition, nonterpene compounds derived from phenylpropane (eugenol) are present in smaller percentages [46]. Thus, the effects are subject to variations, due in part to combinations of ingredients. Curcuma longa L. EO effects have been demonstrated against A. flavus, in addition to aflatoxin inhibition. An interesting observation with exposure to this EO was reported via scanning electron microscopy (SEM) analysis, that signified hyphae membranes and conidiophores damage in A. flavus [47]. Likewise, EO of Matricaria chamomilla L. flower was investigated against A. niger, revealing apparent destruction of cytoplasmic membranes and intracellular organelles, plasma membrane detachment from the cell wall and complete disorientation of the hyphal compartment [48]. In addition to modification of the ultrastructure, a number of studies have examined the underlying mechanisms behind the proapoptotic effects of EOs. An example of this is Ocimum sanctum L. EO, which induced major cytotoxicity in C. albicans, where complete ergosterol depletion, membrane disintegration, DNA fragmentation, increased outsourcing of membrane phosphatidylserine and cytochrome c oxidase activity were observed [49].
However, it has also been suggested that the cytotoxic effects of EOs are mediated by induction of reactive oxygen species (ROS) over synthesis and oxidative stress. A study performed with Anethum graveolens L. seed EO revealed apoptosis in a C. albicans strain via ATPase activity decrease, chromatin condensation, DNA fragmentation, phosphatidylserine exposure, cytochrome c release and metacaspase activation. This study highlighted the role of L-cysteine in the prevention of apoptosis, which is a sign of ROS action [50]. Aflatoxins, i.e., harmful fungal toxins, have also attracted much interest relative to the potential effects of EOs [21]. Several studies have suggested positive inhibition outcomes for aflatoxin with the application of EOs, such as Chenopodium ambrosioides L., that suppressed the synthesis of aflatoxin B1 by the aflatoxigenic strain of A. flavus [51]. Similar results were also observed with Zataria multiflora Boiss EO, which decreased the growth of aflatoxin in A. parasiticus, indicating a link with gene inhibition of the biosynthesis pathways for aflatoxin [52].
To date, our knowledge of the mechanisms of action of EOs is still minimal, with emphasis on concentration-dependence. In general, some apparent effects of EOs include loss of membrane integrity, reduction of ergosterol levels, inhibition of wall formation, inhibition of gene expression, and suppression of membrane ATPases and cytokine interactions [53,54]. As such, the effectiveness of the antifungal characteristics is believed to be associated with the properties of the respective major component, but standardization of assessment methodologies and measurement units is important for further comparisons and investigations [27,55]. It is widely anticipated that EOs will eventually replace traditional agents. Considerable research has been performed and reported on the possible utilization of these nonconventional approaches, mostly in the form of ointment, gels and others [56,57].
In this review, we focus on the potential utilization of EOs as an alternative treatment of fungal infections in humans, along with the classification of these approaches as conventional and nonconventional techniques.
2. Results
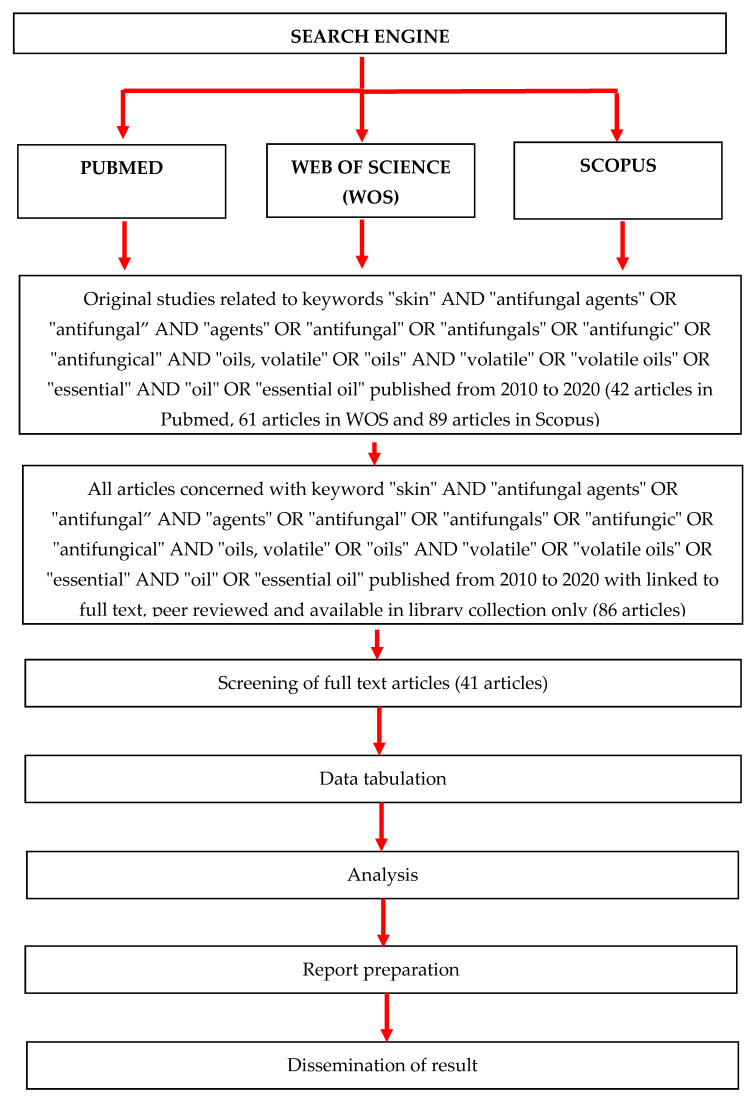
The search resulted in a total of 192 articles from the three search engines: Pubmed (42 articles), WoS (61 articles) and Scopus (89 articles). Nevertheless, 86 articles were identified with a refined search based on the availability of full text, peer reviewed articles and library collection access. Upon further assessment, only 41 full articles were found to be relevant and were included for final review (Figure 2). All of these articles were printed for further assessment. Upon conducting a thorough review, these 41 articles were classified into three categories: 32 articles were categorized as in vitro research, 7 as combination of in vitro and in vivo research and 2 articles as clinical interventions. Out of this, 5 in vitro studies and 3 combination studies were classified as descriptions of nonconventional technology (Table 1). Due to a lack of randomized controlled trials (RCT) in all but two articles, we could only include this topic in the form of a narrative review.
Figure 2.
PRISMA flow diagram of the identification of literature for inclusion in this review.
Table 1.
Potential of EOs as antifungal agents in human skin disease.
| No. | Techniques | Reference | Type of Skin Infections/ Fungi Species |
Essential Oils | Methods | Findings | Conclusion |
|---|---|---|---|---|---|---|---|
| 1. | In vitro—Conventional technology | [58] | Skin infections (C. albicans, C. parapsilosis) | Ten EOs: oregano, thyme, clove, arborvitae, cassia, lemongrass, melaleuca, eucalyptus, lavender, and clary sage |
|
|
|
| 2. | [59] | Dermatophytosis by dermatophytes (Trichophyton rubrum PTCC (Persian Type Culture Collection)5143, T. mentagrophytes PTCC5054, Microsporum canis PTCC5069, M. gypseum PTCC 5070, T. schoenleinii PTCC5221, T. verrucosum var. album PTCC 5056) | Artemisia sieberi oils |
|
|
|
|
| 3. | In vitro—Conventional technology | [60] | Various skin infection due to pathogens (C. albicans ATCC 10231, and dermatophyte T. rubrum SNB-TR) | Coridothymus capitatus L., |
|
|
|
| Lavandula stoechas L., | |||||||
| Lavandula angustifolia Mill., | |||||||
| Mentha spicata L. subsp. condensata, | |||||||
| Origanum syriacum L., | |||||||
| Rosmarinus officinalis, | |||||||
| Salvia fruticosa Miller., | |||||||
| Satureja cuneifolia Ten., | |||||||
| Satureja thymbra L., | |||||||
| Thymbra spicata L., and Vitex agnus-castus L. | |||||||
| 4. | [61] | Skin disease due to six fungal strains (C. tropicalis, C. albicans, Rhizomucor miehei, C. glabrata, A. niger and A. fumigates) | Myristica fragrans |
|
|
|
|
| 5. | [62] | Keratophilic fungi, a type of dermatophytes causing infection to hair, glabrous skin and nails (A. flavus, T. mentagrophytes, T. tonsurans, T. verrucosum, Epidermatophyton floccosum and Microsporum nanum) | Four different commercially available Itra (Volatile plant oils): Bella (Lonicera x bella zabel), Kewda (Pandanus odoratissimus), Rajnigandha (Polianthes tuberosa) and Mogra (Jasminum sambac) |
|
|
|
|
| 6. | In vitro—Conventional technology | [63] | Various skin diseases (C. krusei H9, C. guillermondii MAT23, C. albicans ATCC 10231, C. tropicalis ATCC 13803, C. parapsilosis ATCC 90018, Cryptococcus neoformans CECT 1078, T. mentagrophytes FF7, Microsporum canis FF, T. rubrum CECT 2794, M. gypseum CECT 2908, A. niger F01, A. fumigatus F05, A. fumigatus F07, A. fumigatus F17, A. flavus F44, A. niger ATCC 16404, A. fumigatus ATCC 46645 | Four type oils obtained from Thymus zygis subsp. Sylvestris (collected at four sites) |
|
|
|
| 7. | [64] | Dermatophytosis or ring worm infections (T. rubrum PTCC 5143, T. mentagrophytes PTCC 5054, M. canis PTCC 5069, M. gypseum PTCC 5070, T. schoenleinii PTCC 5221, T verrucosum var. album PTCC 5056) | Satureja khuzistanica |
|
|
|
|
| 8. | In vitro—Conventional technology | [65] | Management of dermatophytosis (Candida krusei H9, C. guillermondii MAT 23, C. albicans ATCC 10231, C. tropicalis ATCC 13803, C. parapsilosis ATCC 90018, C. neoformans CECT 1078; T. mentagrophytes FF7, M. canis FF, E. FF9, T. rubrum CECT 2794, M. gypseum CECT 2908, A. flavus F44, A. niger ATCC 16404, A. fumigatus ATCC 46645 | D. carota subsp. gummifer (Syme) Hook.f. |
|
|
|
| 9. | In vitro—Conventional technology | [66] | Various skin diseases (C. albicans ATCC 10231, C. parapsilosis ATCC 90018, C. tropicalis ATCC 13803, C. neoformans CECT 1078, C. guillermondii MAT2, C. krusei H9, A. niger ATCC 16404, A. fumigatus ATCC 46645, A. flavus F44, E. floccosum FF9, T. mentagrophytes FF7, M. canis FF1, T. rubrum CECT 2794, M. gypseum CECT 2908, T. mentagrophytes var. interdigitale CECT 2958, and T. verrucosum CECT 2992) |
Salvia officinalis L. |
|
|
|
| 10. | [67] | Various skin diseases (C. albicans ATCC 10231, T. rubrum SNB-TR1, T. mentagrophytes SNBTM1, T. soudanense SNB-TS1, T. violaceum SNB-TV1 and T. tonsurans SNB-TT1) | Abies cilicica, Cupressus sempervirens, Juniperus excelsa, Juniperus oxycedrus, Cedrus libani and Cupressus macrocarpa gold crest |
|
|
|
|
| 11. | In vitro—Conventional technology | [68] | Contact dermatitis and skin infections (C. krusei H9, C. guillermondii MAT23, C. albicans ATCC 10231, C. tropicalis ATCC 13803, C. parapsilosis ATCC 90018, C. neoformans CECT 1078, E. floccosum FF9, T. mentagrophytes FF7, M. canis FF1, T. rubrum CECT 2794, Microsporum gypseum CECT 2908, A. flavus F44, A. niger ATCC 16404, A. fumigatus ATCC 46645) |
Distichoselinum tenuifolium |
|
|
|
| 12. | In vitro—Conventional technology | [69] | Skin infections (T. rubrum and T. mentagrophytes) | Agathosma betulina and Coleonema album |
|
|
|
| 13. | [70] | Skin infections (T. rubrum and T. mentagrophytes) | C. album and C. pulchellum |
|
|
|
|
| 14. | In vitro—Conventional technology | [71] | Skin infections (Fusarium sporotrichioides, F. moniliforme, T.n mentagrophytes, A. niger, and Rhizoctonia lilacina) | Bursera morelensis |
|
|
|
| 15. | [72] | Skin infections (C. albicans, Candida stellatoidea and Candida torulopsis) |
Physalis angulata |
|
|
|
|
| 16. | In vitro—Conventional technology | [73] | Dermatophytes and fungi infections (Microsporum gypseum, M. canis, Arthroderma cajetani, T. mentagrophytes, T. tonsurans, E. floccosum, Botrytis cinerea, Pythium ultimum) |
Marrubium vulgare L. |
|
|
|
| 17. | [74] | Skin infections (C.a albicans 1, C. albicans 2, T. rubrum 1, T. rubrum 2, T. violaceum, and T. soudanensis) |
Eugenia caryophylla and Mentha sp cf piperita |
|
|
|
|
| 18. | In vitro—Conventional technology | [75] | Skin diseases (E. floccosum, M. canis, M. gypseum, T. mentagrophytes, T. mentagrophytes var. interdigitale, T. rubrum, T. verrucosum, C. albicans, C. guilliermondii, C. krusei, C. parapsilosis, C. tropicalis, C. neoformans, A. flavus, A. fumigatus, A. niger). |
Juniperus communis subsp. alpina (Suter) Celak |
|
|
|
| 19. | [76] | Skin infection sporotrichosis (Sporothrix schenckii fungus) | Turmeric (Curcuma longa) |
|
|
|
|
| 20. | [77] | Skin infections (T. rubrum and T. mentagrophytes) | Lavandula luisieri and Cymbopogon citratus |
|
|
|
|
| 21. | In vitro—Conventional technology | [78] | Skin diseases (Propionibacterium acnes, Malassezia spp., C. albicans and Trichophyton spp.) | Nine EOs (Cyperus scariosus R. Br., Syzgium aromaticum L. Merr et L.M. Perry, Carum carvi L., Coriandrum sativum L., Boswellia serrata Roxb. Ex Colebr., Syzgium cumini L. Skeels, Elettaria cardamom L. Maton, Occimum sanctum L. and Piper nigrum L.) |
|
|
|
| 22. | In vitro—Conventional technology | [79] | Skin infections by yeast (C. albicans 31, C. tropicalis 32, C. glabrata 33, C. glabrata 35 and C. glabrata 38) |
Six commercial lemon EOs (ETJA, Vera-Nord, Avicenna-Oil, Dufti by Gies, Art, and Croce Azzurra |
|
|
|
| 23. | [80] | Skin diseases (M. canis KCTC 6348, 6349, 6591, T. rubrum KCTC 6345, 6352, 6375, T. mentagrophytes KCTC 6077 and 6085) |
Lonicera japonica Thunb. |
|
|
|
|
| 24. | In vitro—Conventional technology | [81] | Skin diseases (Fusarium oxysporum, T. rubrum and T. mentagrophytes) |
Seventeen EOs |
|
|
|
| 25. | In vitro—Conventional technology | [82] | Pityriasis versicolor (Malassezia furfur) | Citrus lemon (lemon) and Citrus sinensis (orange) |
|
|
|
| 26. | [83] | Skin infections (C. albicans, C. dubliniensis, C. glabrata, C. guilliermondii, C. parapsilosis, C. tropicalis, C. neoformans, Sacharomyces cerevisiae, A. fumigatus,
A. flavus, A. niger, T. rubrum, M. canis, Fonsecaea pedrosoi, Pseudallescheria boydii, Fusarium solani, S. schenckii) |
Tropaeolum pentaphyllum Lam. tubers |
|
|
|
|
| 27. | In vitro—Conventional technology | [84] | Skin infections (T. rubrum and C. albicans) | Twenty-nine oils |
|
|
|
| 28. | In vitro—Nonconventional technology | [85] | Skin infections (Candida pseudotropicalis) |
Lippia multiflora (lippia oil) |
|
|
|
| 29. | In vitro—Nonconventional technology | [56] | Different skin infecting pathogens (C. albicans, A. niger) | Piper betle |
|
|
|
| 30. | [86] | Candidiasis (C. krusei ATCC 6528, C. parapsilosis ATCC 22019, C. krusei, C. parapsilosis, C. albicans, C. glabrata, C. tropicalis | Bidens tripartita |
|
|
|
|
| 31. | [87] | Candidiasis (C. albicans ATCC 10231, C. krusei ATCC 6258 and C. parapsilosis ATCC 90098) | Mediterranean EOs (Rosmarinus officinalis, Lavandula x intermedia “Sumian”, Origanum vulgare subsp. hirtum) |
|
|
|
|
| 32. | In vitro—Nonconventional technology | [88] | Skin infections due to pathogenic fungi(C. albicans, C. tropicalis and T. mentagrophytes) | Black pepper, Cardamom, Cumin, Boswellia and Patcholi |
|
|
|
| 33. | In vitro and In vivo—Conventional technology | [89] | Body aches and dermatitis (A. flavus, A. niger, F. solani, Mucor piriformis, Wickerhamomyces anomalus, Wickerhamomyces anomalus, Deboromyces hansenii, and C. albicans) | Parrotiopsis jacquemontiana |
|
|
|
| 34. | In vitro and In vivo—Conventional technology | [90] | Skin infections (Chrysosporium tropicum, Trichophyton simii, T. rubrum and Chrysosporium indicum) |
Trachyspermum ammi |
|
|
|
| 35. | [91] | Skin disease due to fungi strains(C. albicans, C. parapsilosis, C. tropicalis; A. niger, A. terreus, A. flavus, A. fumigatus, Penicillium sp. and Mucor sp. | Lemon grass (Cymbopogon citratus (DC.) Stapf) |
|
|
|
|
| 36. | In vitro and In vivo—Conventional technology | [92] | Skin infections (M. gypseum and T. mentagrophytes) |
Ageratum houstonianum Mill., |
|
|
|
| 37. | In vitro and In vivo—Nonconventional technology | [93] | Skin disease (C. albicans strain ATC 100231) | Lemongrass oil (LGO) is a volatile oil extracted from the leaves of C. citratus |
|
|
|
| 38. | In vitro and In vivo—Nonconventional technology | [94] | Skin infections (T. mentagrophytes, T. rubrum, T. verrucosum and M. canis) | Cymbopogon martini |
|
|
|
| 39. | In vitro and In vivo—Nonconventional technology | [95] | Dermatophytes and some filamentous fungi (A. flavus, A. niger, A. fumigatus, Microspoum audouni, M. nanum, T. mentagrophytes, T. verrucosm and T. violaceum) | C. martini and Chenopodium ambrosioides |
|
|
|
| 40. | Clinical Interventions | [96] | Ringworm (tenia corporis) (M. and T. mentagrophytes) | Curcuma longa L. |
|
|
|
| 41. | [97] | Pityriasis versicolor (Malassezia sp.) | Myrtus communis |
|
|
|
3. Discussion
In general, body aches are recognized and accepted as an initial symptom that could signify various underlying ailments. Dermatitis is normally acknowledged as a common condition of skin irritation that manifests in the form of dry skin or rashes. It usually instigates itchiness and may also cause blisters or skin flaking. Common conditions include atopic dermatitis (eczema), seborrheic dermatitis (dandruff) and contact dermatitis [98,99]. Acne is another inflammatory condition widely known to affect teenagers. Although this is usually considered a common ailment, certain severe forms can result in scarring, that may affect self-esteem and social interaction, along with causing psychological distress [100].
Infections are typically defined as the acquisition of a microbe by a host, which results when the microorganism is not eradicated from the host upon direct contact, thus initiating disease [101,102]. It has been observed that fungal infections are becoming more prominent; this could be explained by the sharp growth of high-risk populations and the application of treatment modes that allow for improved survival rate [103]. Several variations of endemic fungal infections have been observed with specific geographical distribution due to climate change, human habitats expansion, ease of travel and population migrations [1]. There are four key prerequisite criteria for fungi to infect humans: its ability to colonize or penetrate surface barriers, its ability to absorb nutrients via lysis and absorption of human tissue, its ability to counteract innate and adaptive immunity associated pressure, and its ability to grow at typical human body temperature [104].
The current practice in the treatment of fungal infections is solely focused on the application of traditional antifungal agents. However, as new species of fungi emerge, different approaches are necessary, given the increased resistance toward commonly available antifungal medications. Based on extensive research performed on EOs, the potential of these oils for antifungal based therapies has gained huge attention in recent years. Our review highlights numerous studies that have evaluated the potential of various types of EOs against human pathogenic fungi that were examined via in vitro, in vivo as well as clinical research. The listed research articles were also defined and classified as conventional and nonconventional, where applicable.
3.1. EOs in Conventional Approaches
Among the in vitro studies, Candida spp. infections were among the most common. The hypothesis of the previous study was that the spread of candida infections could be avoided in the occurrence of a healthy immune system, except when scrapes or cuts are found on the body [105]. Numerous EOs were shown to be suitable to control candida infections. Commercially available lemon EOs have gained attention and also been shown to possess antifungal potential against three Candida species (C. albicans, C. tropicalis and C. glabrata), where the growth of C. albicans specifically was inhibited across the full spectrum of concentrations used [79]. In another study, anticandidal activities were examined against C. torulopsis, C. stellatoidea and C. albicans using Physalis angulata EO. P. angulata is an annual herbaceous plant in the Solanaceae family which is recognized for its medicinal value. P. angulata EO demonstrated excellent characteristics as an antifungal agent against all three candida strains that are usually resistant to antibiotics. As such, additional research on its anti-infective properties is warranted [72].
Cabral and colleagues evaluated the antifungal potential of Juniperus communis subsp. alpina (Suter) Celak needles EO [75]. J. communis L. is an evergreen shrub or tree with fleshy female cones, where the cone scales resemble berries; it occurs throughout Europe, Asia and North America [106]. The evaluation of juniper needle oil revealed excellent activity, in particular to M. canis and T. rubrum, with MIC and minimum lethal concentrations (MLC) of 0.32 μL/mL [75]. The oil was made up largely of monoterpene hydrocarbons (78.4%), with the main components being sabinene (26.2%), α-pinene (12.9%) and limonene (10.4%). Cell viability studies indicated the superiority of needle oil with no cytotoxic effects observed in HaCat keratinocytes when tested at 0.32 and 0.64 μL/mL, compared to a previous study on berry EO [75].
Another study investigated the effect of two EOs (E. caryophylla and M. sp cf piperita) on six human pathogenic fungi (four filamentous fungi (dermatophytes): T. rubrum (1 and 2), T. violaceum, and T. soudanense) and two pathogenic yeasts (C. albicans 1 and 2). Mentha sp cf piperita is a herbal plant belonging to the Lamiaceae family, native to temperate regions, with a fresh, sweet smell that is traditionally applied in the treatment of gastritis, muscular pains or toothache [107]. E. caryophylla (Myrtaceae) (clove) is a tree of 10–12 m in height that originates from Indonesia but which is also harvested in African countries. It is widely used as a spice, as well as for the treatment of toothache and in infection remedies [108]. The study showed that Mentha sp cf piperita EO presented weak activity, with MIC of 2.5 μL/mL against Tricophyton strains, while no activity was shown with C. albicans [74]. Additionally, E. caryophylla EO exhibited the highest activity, with MIC and MFC of 0.25 µL/mL and 0.125 µL/mL for filamentous fungi and MIC of 0.5 µL/mL for both yeast strains, while the MFC value was 1 µL/mL for one yeast strain and was not determined for the second [74].
In a study by Valente et al. [65], the potential of D. carota subsp. gummifer EO as an antifungal and anti-inflammatory was evaluated against multiple collections and clinical strains via macrodilution broth assays. Among the tested strains were two Candida strains isolated from recurrent cases of vulvovaginal candidosis (C. krusei H9 and C. guillermondii MAT 23), three type strains from the American Type Culture Collection (C. albicans ATCC 10231, C. tropicalis ATCC 13803, C. parapsilosis ATCC 90018), one type strain from the Colección Española de Cultivos Tipo (C. neoformans CECT 1078), three dermatophyte strains isolated from nails and skin (T. mentagrophytes FF7, M. canis FF1, E. floccosum FF9), two type strains from CECT (T. rubrum CECT 2794, M. gypseum CECT 2908), one Aspergillus strain isolated from bronchial secretions (A. flavus F44) and two type strains from ATCC (A. niger ATCC 16404 and A. fumigatus ATCC 46645). Chemical characterization of the oil revealed high amounts of monoterpenes with geranyl acetate and α-pinene as the major components. This EO action was shown to be active against dermatophytes and C. neoformans (MIC range: 0.32–0.64 μL/mL). The promising results shown by this EO led to the recommendation of future studies with a specific emphasis on peripheral and central nervous systems (CNS), implying the need for in vivo research focused in dermatophytosis and inflammatory disease management [65].
Two more genera of fungi are commonly related to skin diseases: Microsporum and Trichophyton. These opportunistic filamentous fungi belong to dermatophytes [109]. From our review, we found that many studies have been done to find methods to counter these two species. Artemisia is one of the most widely distributed species of the Asteraceae family, and is well-known for its pharmacological benefits. A. sieberi was tested on dermatophytes against several strains (T. rubrum, T. mentagrophytes, M. canis, M. gypseum, T. schoenleinii and T. verrucosum var. album), revealing higher sensitivity of M. gypseum, T. rubrum and M. canis and suggesting its suitability as a topical antifungal agent. However, this study indicated that collection site and harvest time affect the yield and chemical composition of the EO, although no significant differences were observed with respect to its antidermatophyte activities [59].
Rezgui and colleagues studied the effects of M. vulgare EO, extract and its active compound (marrubiin), and observed approximately 50% inhibition for T. mentagrophytes and E. floccosum with respect to marrubiin tested at 100 mg/mL. Antiphytopathogenic activity was also conducted, with only marrubiin being found to be active against B. cinerea at the highest dose (32.40%). The authors concluded that M. vulgare and marrubiin could be used as natural antifungal agents for skin dermatophyte infections [73]. Myristica fragrans is an edible plant that grows in the evergreen forests of West Africa. The seeds have both economic and medicinal value, especially for the local community. Inhibition was observed against several pathogenic fungi, e.g., C. tropicalis (1.3 cm), C. albicans (0.8 cm), R. miehei (0.6 cm) and C. glabrata (0.6 cm) with M. fragrans EO, although no inhibition was reported with A. niger and A. fumigates [61].
In Lebanon, Lamiaceae species such as C. capitatus L., L. stoechas L., L. angustifolia Mill., M. spicata L. subsp. condensata, O. syriacum L., R. officinalis, S. fruticosa Miller., S. cuneifolia Ten., S. thymbra L., T. spicata L., and V. agnus-castus L. are quite popular due to their use as a food, a condiment or as traditional medicine in the treatment gastrointestinal disorders and microbial infections. The EOs of Lamiaceae sp. was shown to be efficient, especially toward T. rubrum and C. albicans, that reported MIC values ranging between 64–128 µg/mL; this was attributed to the presence of large amounts of thymol and carvacrol components [60]. A recent review by Karpinski highlighted the antifungal properties of EOs from 72 Lamiaceae plants. Linalool, β-caryophyllene, limonene, β-pinene, 1,8-cineole, carvacrol, α-pinene, p-cymene, γ-terpinene, and thymol were identified as the major components among the tested plants; these compounds are known to possess antifungal characteristics. On the whole, the review concluded that more than half of these EOs presented MIC activity (<1000 μg/mL), with Clinopodium, Lavandula, Mentha, Thymbra and Thymus EOs displaying the best activities [110].
In addition to the species mentioned above, Lebanon is also well-known for EOs from conifer plants such as A. cilicica, C. sempervirens, J. excelsa, J. oxycedrus, C. libani and C. macrocarpa gold crest. The efficacy of such oils was evaluated against C. albicans and several isolates of Trichophyton spp. Based on the results, the highest sensitivity was observed with Trichophyton spp. to all tested EOs (MIC: 32–512 μg/mL). This study, however, highlighted interesting activity of a C. macrocarpa EO on dermatophytes (MIC: 32–64 μg/mL), whereby each major component, as well as an artificial EO, were tested on T. rubrum, suggesting a possible contribution of a minor component to the overall observed activity. The findings strengthen the potential of EOs of Lebanese conifers for use in antimicrobial preparations for the treatment of superficial infections [67].
In another test against T. rubrum and T. mentagrophytes, the antifungal activities of A. betulina and C. album EOs was evaluated using electron microscopy based on the mechanism of action. It was found that fungal growth was inhibited on all plates exposed to the EOs volatiles with different inhibition rates observed among the various fungal species, EOs and tested volumes. The highest inhibition was recorded on T. rubrum at 40 μL via the action of A. betulina EO, with a fungal growth index (FGI) of 2.3% indicating its capacity as a strong antifungicidal agent. The hyphae and spores of T. rubrum were also destroyed due to the volatile effects. The major components identified in A. betulina EO are limonene (29.8%), menthone (21.6%), and isomenthone (14.7%), while pinene (27.4%) and myrcene (14.5%) makes up the major part of C. album [69]. In the formulation of skincare products, the C. album (Thunb) Bart. & H. L. Wendl (Rutaceae) has been used by the Khoisan people by rubbing it on their skin. However, it is less popular in South Africa as a traditional medicine. The mechanism of action of A. betulina EO volatiles was shown to be through spore growth inhibition as well as production and morphology alteration via destruction of hyphae and spores. Overall, this research gave remarkable insights into the possible use of A. betulina as an antifungal agent [70].
Another study on the potential of EOs from L. luisieri and C. citratus against T. rubrum and T. mentagrophytes was conducted, whereby strong antifungal activity was observed with most clinical strains. Positive interaction between L. luisieri EO combined with terbinafine was observed against terbinafine-resistant strain (Tr ATCC MYA-4438). Significant reduction of the germination was observed above 100 µg/mL. Both oils were found to be safe to macrophage mammalian cells at the tested concentrations. This study describes the antifungal activity of L. luisieri and C. citratus EOs against dermatophytes, which could be useful in the design of new formulations of topical treatments [77].
Since ancient times, several Thymus species have been used in traditional medicine to combat pathogenic microorganisms. In a study by Goncalves et al. [63], chemical profiling and antifungal activity of four types of oils from Thymus zygis subsp. sylvestris were evaluated against yeasts, dermatophyte and Aspergillus strains. Carvacrol (25.0%), thymol (23.8%), geranyl acetate (20.8%), geraniol (19.8%) and linalool (30.0%) were the major identified components. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay revealed that the oil composed of high carvacrol concentrations presented stronger antifungal activities against dermatophytes, with no cytotoxic effect at 0.08–0.16 μL/mL, for up to 24 h. A lack of cytotoxicity was also observed with this oil in mouse skin dendritic cells at concentrations where significant antifungal activity was obtained (0.16 μL/mL). Based on these findings, it was proposed that T. zygis EO is highly capable as an antifungal agent with minimal detrimental effects, and that it should be explored in more detail [63].
In combatting ringworm infections, S. khuzistanica EO, ethanol and aqueous extract were tested for their antifungal activity. In the study, the antifungal activities of S. khuzistanica EO (MIC: 40–190 µg/mL) were found to be higher than its ethanol extract (MIC: 40–770 µg/mL), while S. khuzistanica aqueous extract had no antifungal activity against dermatophytes (MIC: 1550–3100 µg/mL). This study noted the potential of S. khuzistanica EO as a topical therapy in the treatment of skin fungal infections. It also noted that both S. khuzistanica EO and its ethanol extract could be further explored for their antifungal activities [64].
S. officinalis L. belongs to the Lamiaceae family; it was evaluated for its antifungal and anti-inflammatory activity. S. officinalis EO indicated antifungal activities against dermatophytes and significantly inhibited nitric oxide (NO) production stimulated by lipopolysaccharide (LPS) in macrophages without affecting cell viability in concentrations up to 0.64 μL/mL. This study claimed to be the first report on the in vitro anti-inflammatory potential of S. officinalis oil; the authors demonstrated that the bioactive concentrations of S. officinalis oils did not affect the mammalian macrophages and keratinocytes, making them suitable for use in skin care formulation for cosmetic and pharmaceuticals applications [66].
The antifungal activity of D. tenuifolium EO was evaluated against yeast, dermatophyte and Aspergillus strains. The oils revealed significant antifungal activity against C. neoformans and dermatophyte strains, and significantly inhibited NO production stimulated by LPS in macrophages, without affecting cell viability at concentrations ranging from 0.64–1.25 L/mL. A chemical analysis found that the oil was mostly composed of monoterpene hydrocarbons with myrcene as the major component. This research provided significant information about the pharmacological activities of D. tenuifolium EO which is relevant to its use as an antifungal and anti-inflammatory agent for treatment of contact dermatitis, skin infections as well as its role as an anti-inflammatory agent. Thus, it was proposed that its beneficial effects and usage for the prevention of diseases related to fungal and inflammation need to be further examined [68].
In a screening of C. lemon and C. sinensis oils by disc diffusion method in combatting M. furfur, the diameter of the inhibition zone was found to be 50 and 20 mm, i.e., greater than those of reference antibiotics, gentamycin and streptomycin, at 16.5 and 17 mm, respectively. MIC of both lemon and orange oil against M. furfur was found to be 0.8 and 2.2 µL/mL. These findings support the use of C. lemon and C. sinensis oil as a traditional herbal medicine for the treatment of P. versicolor infection of the skin [82]. The evaluation of Bursera morelensis EO indicated inhibition of all the filamentous fungi. F. monilifome (IC50: 2.27 mg/mL) was the most sensitive fungal strain. This work provided scientific evidence for the antimicrobial activity of the B. morelensis [71].
In addition to in vitro approaches, several investigations were also conducted with in vivo models combined with in vitro approaches. Among the most widely covered EOs in this review for combined approaches were P. jacquemontiana, C. martini, C. ambrosioides, C. citratus, T. ammi and A. houstonianum Mill. P. jacquemontiana is an aromatic plant of the Hamamelidaceae family, and is well-recognized for its medicinal value, especially in terms of treating skin infections or eruptions, relieving body aches and minimizing dermatitis, along with its anti-inflammatory, antioxidant hepatoprotective activities, as shown in experimental rats [111,112,113]. The study revealed excellent antifungal characteristics of P. jacquemontiana EO against seven fungi and one yeast strain. The maximum inhibition zone was obtained with Mucor piriformis ATCC 52554 (19.83 ± 1.04 mm), while F. solani ATCC 36031 resulted in a minimum inhibition zone of 13.13 ± 1.03 mm and a maximum MIC value of 512 μg/mL [89].
The properties of this oil with a wide spectrum of antifungal capacity were believed to be strongly influenced by its chemical composition, i.e., mostly phenolic compounds. Past research has suggested that alcohols, phenolic compounds or aldehydes are capable of preventing fungi and yeast growth [114]. An in vivo study to evaluate the wound healing capacity of this EO was performed with Sprague-Dawley rats. Outstanding wound contraction rates were observed. This phenomenon was attributed to the presence of phytochemical compounds (phenolic compounds, flavonoids, terpenoids, alkaloids) which were reported previously to assist in wound contraction and facilitate epithelialization. Wound healing encompasses a critical step of increased proliferation and migration over the wound via re-epithelialization, that further strengthens this hypothesis [115]. Although P. jacquemontiana oil was presented as an effective approach for wound healing, this group of researchers also proposed that extended studies with regards to the mechanism involved should be undertaken in the future [89].
In another study, the capacity of T. ammi fruits EO was demonstrated for antidermatophytic activity using Swiss Albino mice model. T. ammi L. is a member of the Apiaceae family, that was known as an aromatic herb and spice grown in Egypt, Persia, Bangladesh, Afghanistan, Ethiopia and India [116]. These fruits possess numerous medicinal properties, including antimicrobial, anti-inflammatory, antioxidant, antiviral and anticandidal effects [117,118,119,120,121,122]. Chemical profiling of the oil revealed thymol as the major component, at 58.88%, followed by p-cymene (24.02%) and γ-terpinene (13.77%). The MIC value of T. ammi oil was noted in the range of 0.025–0.5 μL/mL against tested fungi. Maximum zone of inhibition was observed against C. tropicum (63.83 ± 0.166 mm) followed by T. simii (57 ± 0.288 mm), T. rubrum (51.33 ± 0.333 mm) and C. indicum (45 ± 0.577 mm). From a total of five concentrations tested, no irritations were observed at low concentrations, i.e., up to 3%, while three mice presented symptoms of mild erythema and all five mice exhibited well-defined erythema at 5% and 7% concentrations, respectively. This finding concluded that toxic side effects were not demonstrated at low concentrations. Based on the excellent antidermatophytic activity of the oil, T. ammi EO could be potentially used in the treatment of tinea or ringworm infections [90].
An EO originating from A. houstonianum Mill leaves was shown to be capable of inhibiting the growth of both M. gypseum and T. mentagrophytes, with MIC of 80 μg/mL. A. houstonianum is an annual or biannual herb and a member of the Asteraceae family, that has been utilized as a traditional medicine in many countries. The MIC value of A. houstonianum Mill EO when tested with multiple serial dilutions was 20 μg/mL for both (8:2) while 8 and 10 μg/mL value were obtained for M. gypseum and T. mentagrophytes (10:1), respectively. An in vivo study of the acute dermal toxicity effects with guinea pig indicated no changes in treated skin and fur, appearance along with the absence of diarrhea. In addition, dosage increase resulted in a reduction of noise sensitivity degree, pinch reaction, locomotion effects, as well as reactivity. However, a slight darkening of the liver, kidneys and heart was noticed with dosage increase. The observed effects indicated the suitability of A. houstonianum EO as an antidermatophytic compound. This supported a previous hypothesis that this EO may have depressant or sedative effects on the CNS at high doses [92,123].
C. citratus (DC.) Stapf (Poaceae family), commonly known as lemon grass, is a perennial tropical grass with thin, long leaves. It is one of the main medicinal and aromatic plants cultivated in Algeria, as well as in the tropical and sub-tropical regions of Asia, South America, and Africa [124]. C. citratus EO (LGEO) exhibits promising antifungal effect against C. albicans, C. tropicalis and A. niger, with different inhibition zone diameters (IZD), i.e., 35–90 mm, that increase proportionately with the increase in the oil volume. A chemical characterization of LGEO indicated the presence of geranial and neral as the major components, representing about 42.2 and 31.5%, respectively; its antifungal activity was associated with the presence of these compounds. The diameter of the growth inhibition zone (DGIZ) of LGEO in the vapor phase was found to be superior to the liquid phase, which is believed to be more advantageous in terms of application ease, elimination of the need for direct contact with the oil, and the requirement of using less oil. In vivo evaluation with Swiss albino mice showed considerable anti-inflammatory activity, where the degree of edema inhibition was similar for 10 and 100 mg/kg LGEO 90 min after oral administration (82.75 and 86.2%, respectively). This level of edema inhibition was comparable to that observed using 50 mg/kg oral doses of the standard reference drug, diclofenac (86.2%). The analysis of the inhibition degree of croton oil topical application resulted in noticeable edema on the left ear. On the whole, LGEO, as an antifungal and anti-inflammatory agent, is believed to be effective, both in the prevention and treatment of acute inflammatory skin conditions. In line with this potential, this research introduced ideas for additional evaluations of active constituents, specific action mechanisms and drug discovery for alternative antifungal therapies [91].
Commonly used antifungal methods include the disc diffusion technique, macro and microbroth dilution assays, the food poisoning method, the volatile release plate method, as well as the use of electron microscopy. Certain methods like disc diffusion, well diffusion and agar dilution methods have been shown to produce unreliable and inconsistent results [125,126]; this is primarily due to difficulties related to stable oil dispersion in aqueous media, lipophilic substance distribution in the aqueous medium, and various methods which are used to determine the number of viable microbes that will remain following the addition of EOs. In addition, true representation of activity is not possible with the disc and well diffusion methods, as different components diffuse through the agar at varying rates, and only the more water-soluble components, such as terpine 4-ol, are capable of agar penetration [125]. This phenomenon also occurs in the agar dilution method, where the incorporation of EO into agar can result in the loss of certain active compounds (e.g. terpinen-4-ol, linalool and limonene), either through evaporation or absorption into the petri dish plastic [127]. These techniques yield unreproducible outcomes for certain EOs, and are thus unreliable for assessments of the true behaviour of EOs. Usually, Tween 80 (a commercial polyoxyethylene sorbitan monooleate) will be added to emulate the oils for the study of the antifungal activity of hydrophobic and viscous EOs [128]. This may be linked to the fact that surfactants decrease the degradation rates of EOs, thereby causing the continued release of antimicrobial agents [129].
3.2. EOs in Nonconventional Approaches
Nonconventional technology is defined as a combinatorial approach of an EO with an absorption base (e.g., ointments). This approach was applied in one study that examined the effect of L. multifora (Lippia oil) in combination with six types of ointment base and incorporation of Tween 80 at four different concentrations to another set. The findings of this study revealed that the most effective inhibitory action was obtained when lippia oil (10% w/w) was combined with Hydrous Wool Fat Ointment BP (British Pharmacopoeia method) or Simple Ointment BP, with added Tween 80 (6% w/w) upon testing of C. pseudotropicalis and C. albicans. The incorporation of Tween 80 further enhanced the efficacy of this formulation, suggesting its suitability as a nonconventional treatment strategy [85].
The potential of P. betle EO in combination with polymer gel as a suitable dermatological formulation against multiple skin pathogens was studied. The reported results showed the highest sensitivity of C. albicans and A. niger toward P. betle EO, with inhibition up to 24 mm and 20 mm when tested at 20 µL concentrations, respectively. The inhibition zones were found to be less affected by gel incorporation, as confirmed by the antimicrobial results from the use of the final formulation [56]. Another potential treatment of candidiasis was tested on B. tripartita. An EO of B. tripartita (BTEO) was obtained via hydrodistillation of the aerial parts of the plant, which were then transformed into a gel formulation. The authors of this study concluded that both active phytoconstituents of B. tripartita, as well as the vehicle properties of the gel, contributed to the observed therapeutic activity. The highest inhibition was recorded on C. tropicalis and C. krusei with 3.7 mm difference compared to hydrogel without BTEO. Through the comparison, the hydrogel-based formulation combined with BTEO appeared to be the preferred mixture, exhibiting superior antifungal activity against all tested Candida strains and revealing the oil as a promising anticandidal agent [86].
Another study screened the potential of nine EOs, where B. serrata was cited for its superior activity, with maximal activity against Trichophyton spp. B. serrata EO was reported to contain mostly of α-thujene, ρ-cymene and sabinene compounds. The synergistic activity demonstrated with azoles in azole-resistant C. albicans indicated the suitability of this oil for use as an antifungal agent. However, as the study was limited in terms of its examination of the mechanism of action, individual constituent analyses and clinical studies, further investigations were deemed necessary [78]. Similarly, the antifungal activity of several EOs (black pepper, cardamom, cumin, Boswellia and Patcholi) were examined in another study against fluconazole-resistant fungi. The results revealed the effectiveness of Boswellia and Cardamom oil against C. tropicalis and T. mentagrophytes, respectively. Furthermore, a synergistic effects of a combination of Boswellia EO and fluconazole was noted, revealing itself to be the most effective of the applied approaches against C. tropicalis, even at 1:10 dilution and 100 μg/mL, respectively. As indicated by Sadhasivam and colleagues [78], an earlier study also proposed that the active compound, action mechanism, toxicity and stability be the focus of future investigations [88].
R. officinalis, Lavandula x intermedia “Sumian” and O. vulgare subsp. hirtum are among the most common medicinal plants in the Mediterranean region. They were widely used and have been synergistically tested for the treatment of candidiasis. Research on the HaCaT (normal cell line) and A431 (tumoral cell line) reported its safety for use as an antiproliferative agent. In vitro studies against C. albicans, C. krusei and C. parapsilosis showed an increase in the antifungal activity of clotrimazole-loaded nanoparticles (NLC). NLC containing Mediterranean EOs represents a promising strategy to improve drug effectiveness against topical candidiasis [87]. Infection of hair, globous skin and nails on humans is believed to be caused by keratinophilic fungi. Four types of an available commercial formulation called Itra-Bella (Lonicera x bella zabel), Kewda (P. odoratissimus), Rajnigandha (P. tuberosa) and Mogra (J. sambac) were tested against A. flavus, T. mentagrophytes, T. tonsurans, T. verrucosum, E. floccosum and M. nanum. The results showed better antifungal activity with EOs compared to synthetic drugs, i.e., terbinafine, itraconazole and fluconazole. T. tosurans was inhibited up to 47 mm by Itra-Bella, indicating that keratinophilic fungi could be successfully inhibited by Itra-Bella [62].
Several combination studies with in vitro and in vivo approaches also evaluated nonconventional technologies. One example is a study that investigated the potential of LGEO using nonconventional technology that incorporated the formulation of a topical hydrogel with lemongrass-loaded nano-sponges for antifungal action against C. albicans strain ATC 100231. Nano-sponges are a novel class of encapsulated nanoparticles that serves as an excellent delivery system in both the pharmaceutical and cosmeceutical industries [130]. This technology has received much attention in recent years, as it combines the benefits of both microsponges and nanosized-mediated delivery systems. Nanosponges with topical hydrogels have been shown to cause minimal irritation, fewer adverse effects as well as improved skin retention when compared with conventional topical delivery systems [57,131]. Similarly, this nonconventional approach was proven to be effective, i.e., the results confirmed the superior nonirritant and antifungal effects of the final selected hydrogel (F9) from among nine tested formulations. The MIC and MFC concentrations of LGEO using the broth macrodilution method was found to be 2 and 8 µL/mL, respectively. The results were promising, especially for the practical approach of using pharmaceutical formulations as a measure to minimize the risk of folk medicine usage in the crude form [93].
Another study by Gemeda and others conducted with EO from C. martini showcased broad-spectrum antimicrobial potency against all tested organisms, with MIC value ranging from 0.65 to 10 μg/mL. C. martini EO demonstrated absolute growth inhibitions of Trichophyton mentagrophytes and T. rubrum (>1% EO concentration), as well as M. canis and T. verrucosum (>4% EO concentration) [94]. This study incorporated a nonconventional approach, whereby various formulations were prepared and tested. A total of five base formulations (hydrophilic ointment, macrogol blend ointment, macrogol cream, simple ointment and white petrolatum base) were prepared with different concentrations of the EO by incorporating the oil into soft mass of different dermatological bases. The findings demonstrated that both hydrophilic and macrogol blend ointments with C. martini EO demonstrated superior activity upon tested organisms than relevant commercial drugs. Furthermore, a skin sensitization test with guinea pig showed no sign of irritation or sensitization when tested with 5% EO topical formulation. Hence, this product was believed to be a suitable alternative to established treatments, although extended studies are required to evaluate the safety, chronic toxicity and efficacy [94].
One more interesting study described the antifungal activity of C. martini and C. ambrosioides EO, along with their combinations, against several dermatophytes and filamentous fungi via in vitro as well as in vivo (guinea pig) tests. C. martini belongs to the Poaceae family, and is a known medicinal plant that possesses thermogenic, diuretic and insecticidal activity [132,133]. C. ambrosioides L. is a member of the Chenopodiaceae family and is widely used in Indian traditional medicines. Its antifungal and antiaflatoxigenic activities have been noted against certain storage fungi [51,132]. Its EO was prepared by mixing 1 mL of the oil with 100 g petroleum jelly. An in vivo study indicated significant efficacy of the EO against superficial mycosis, with a reduction in skin redness, lesion severity and dermatophyte occurrence upon ointment application, as confirmed via recurrence of hair growth at the infected site, compared to the control. The disease was successfully removed within 7–21 days via the application of EO ointments [95].
In addition, in vitro evaluation of both EO also inhibited the growth of T. rubrum and M. gypseum, with low MIC values that signified their potential role as alternatives to synthetic antifungal drugs. A chemical characterization indicated the presence of trans-geranoil (60.9%) and m-cymene (43.9%) as the major components of C. martini oil and C. ambrosioides EOs, respectively. One important element in this study was the more efficient synergistic effect obtained with a combination of both EOs in a 1:1 ratio than C. ambrosioides alone, indicating fungitoxicity alteration effects with the formation of combined chemical profiles. The MIC of EO and combination were shown to be around 150–500 ppm, which was comparatively lower than those of commercial drugs, which are usually higher, at around 1000–5500 ppm. In conclusion, this study highlighted the ability of both EOs, individually or in combination, to serve as a suitable strategy in the treatment of various superficial mycoses in humans, and recommended that clinical trials be performed [95].
3.3. EOs in Clinical Interventions
The effectiveness of EOs for the treatment of skin fungus has also been investigated through clinical interventions, in addition to in vitro and in vivo studies. In our review, we came across two interesting clinical research projects that had evaluated the antifungal activity of EOs originating from distinct plant species. In the first intervention, the antifungal activity of M. communis EO against Malassezia sp. isolated from the skin of patients with Pityriasis versicolor was examined. Malassezia sp. is a class of lipophilic yeasts that covers part of the skin microflora in humans and other warm-blooded animals [134]. However, the presence of this yeast is also known to lead to diseases such as inflammation, systemic infections or uninflamed lesions. Among these, uninflamed lesions in the presence of excess fungal load represent a disease known as Pityriasis versicolor (PV) [134,135]. PV is sometimes also known as tinea versicolor, and is characterized by the development of hypo or hyperpigmented scaly spots [134,136].
Malassezia furfur is recognized as one of the main causative agents of PV, from among the 14 recognized Malassezia species [134,135]. The EO applied in this study was isolated from Myrtus communis (Myrtaceae), which has been widely used in various medicinal remedies. To date, this myrtle EO is known to contain 1, 8-cineole and α-pinene. Its antifungal activity against several species (e.g. Rhizoctonia solani, F. solani, A. flavus, Colletotrichum lindemuthianum, F. culmorum, C. albicans) [137,138,139,140] has been documented. In this prospective case-series study, clinical isolates of PV were collected from 41 patients that had been deprived of treatment for at least two weeks. The clinical samples revealed the presence of 86 yeast colonies with seven identified species: M. furfur (42.5%), M. sympodialis (23.5%), M. sllooffiae (13.9%), M. globose (7.5%), M. obtusa (6%), M. japonica (4%) and M. restricta (2.5%). The antifungal inhibition revealed excellent activity of M. communis against Malassezia sp., especially M. furfur and M. sympodialis, at 96% and 83%, respectively. The findings of this study suggest the potential of M. communis EO as an alternative to conventional antifungal drugs, especially in the treatment of PV. Such an approach was considered cheaper, safe and nonhepatotoxic or nonnephrotoxic. Nevertheless, this study also addressed its limitations, and proposed the need for in vivo studies to confirm the effectiveness of this EO [97].
The second investigation focused on the antifungal activity of EO obtained from C. longa L. waste leaves. Curcuma longa L. is a member of the Zingiberaceae family that grows mostly in Sri Lanka, Indonesia and many parts of India. Curcuma longa L. is usually called turmeric, haldi or Indian saffron, with well-known usage as a spice, as well as a treatment against common skin diseases [141]. In a study by Pandey and colleagues, Curcuma longa L. EO exhibited positive effects against several human pathogenic fungi (Pandey et al. 2010). The selected patients in this study had been diagnosed with Tinea corporis. Tinea corporis is classified as a form of dermatophytic infection of glabrous skin. Trichophyton and Microsporum species were identified as the cause of this infection. Tinea corporis infection generally occurs within the stratum corneum of the epidermis, and may be transmitted via direct contact with infected individuals or animals [96,142,143].
Chemical characterization of the oil indicated the presence of terpinolene, α-phellendren, terpinen-4-ol and sabinyl acetate as the main components. It was also noted that the oil destroyed both M. gypseum and T. mentagrophytes within 5 s. In addition, antifungal activity of the oil was observed with other species such as E. floccosum, M. nanum, T. rubrum and T. violaceum. This study highlighted the significant potential of C. longa leaf EO based on in vitro and in vivo assays; additionally, strong fungicidal action, extended shelf-life, tolerability of higher inoculum density, thermal stability and a wider range of antidermatophytic activity without any adverse effects were noted. Intervention studies that involved the topical application of the oil formulation gave positive insights into its efficacy; complete healing was observed in 72% of cases after three weeks of treatment, without any relapse when patients were examined two months later. Hence, the study proposed the application of C. longa leaf EO as an inexpensive and effective formulation for commercial use [96].
3.4. EOs in Other Nonconventional Approaches and Their Advantages
The development of numerous nonconventional approaches in antifungal therapies indicates great potential. In addition to the reviewed articles, one prominent technique that has gained attention is the application of cyclodextrins via encapsulation. This technique involves the formation of inclusion complexes of selected oils with cyclodextrins (CDs). In a study performed by Torres-Alvarez and colleagues, concentrated orange oils were subjected to encapsulation with β-CD and evaluated for their antifungal activity. This study noted that the highest antifungal activity inhibition was achieved at 10 mg/mL, respective to 10× fraction orange oil in ratios of 12:88 and 16:84 (COEO: β-CD). Hence, the encapsulation technique proved to be beneficial toward inhibition, although the researchers proposed further studies to confirm the formation of the complex and the encapsulated compounds [144].
Pickering emulsions (PEs) are widely sought after at present, compared to conventional emulsions. PEs comprise particle-stabilized emulsions in which solid particles tend to adsorb instantly on the oil-water interface, resulting in a shell-like structure on the PE droplet surface. There are various advantages associated with the PE approach, e.g., improved stability in relation to the higher adsorption energy of solid particles, as well as appropriate emulsion droplet size, that could assist in longer retention of the antifungal drug at the treatment site. The approach of PE was demonstrated in one study that evaluated its effectiveness as an alternative therapy for onychomycosis topical treatment, also known as fungal nail infections. For this purpose, a nanotechnological approach was applied to formulate PEs in combination with azole derivative (tioconazole) and Melaleuca alternifolia EO tested against C. albicans and T. rubrum. The findings showed that PEs are an excellent choice for onychomycosis topical treatment compared to conventional emulsions or ethanolic solutions of reference drugs [145].
Nonconventional techniques are have gained broader recognition in recent years due to their excellent benefits. In this review, we have discussed various nonconventional approaches, and noted their superiority compared to the traditional methods. In general, we would like to highlight the fact that strategies involving nonconventional treatments are useful to reduce risks associated with the crude forms of folk medicines, along with lesser adverse effects and higher skin retention for better targeted drug delivery.
4. Materials and Methods
Search Strategy
Original articles were identified through searches of three databases (PubMed, WoS and Google Scholar) for the period of 2010 to 2020 using (“skin”[MeSH Terms] OR “skin”[All Fields]) AND (“antifungal agents”[Pharmacological Action] OR “antifungal agents”[MeSH Terms] OR (“antifungal”[All Fields] AND “agents”[All Fields]) OR “antifungal agents”[All Fields] OR “antifungal”[All Fields] OR “antifungals”[All Fields] OR “antifungic”[All Fields] OR “antifungical”[All Fields]) AND (“oils, volatile”[MeSH Terms] OR (“oils”[All Fields] AND “volatile”[All Fields]) OR “volatile oils”[All Fields] OR (“essential”[All Fields] AND “oil”[All Fields]) OR “essential oil”[All Fields]). Publications with abstracts were reviewed; the search was limited to studies published in the English and Malay languages. Papers on in vitro, in vivo, human and animal studies, and related to plant-based antifungal medication were included. Review articles and letters to the editor were excluded. Duplicate articles were eliminated.
5. Conclusions
In conclusion, our review underlines the fact that the importance of antifungal agents EOs is widely recognized. Their roles in reducing the severity of fungal infections vary according to species and origin. Based on our review, we strongly believe that EOs should be explored for commercial applications as alternatives to over-the-counter antifungal agents. In addition, commercial applications could be further enhanced with nonconventional strategies in combination with other components, such as fluconazole and Tween 80. Hence, it is vital that efforts continue for the development of EO-based skin antifungal therapies.
Acknowledgments
The authors thank the Director General of Health Malaysia and the Director of Institute for Medical Research (IMR), Malaysia, for giving the permission to publish this article. We also thank the staff of Nutrition, Metabolism and Cardiovascular Centre, Institute for Medical Research, NIH for their continuous support.
List of Abbreviations
| ATCC | American Type Culture Collection |
| BTEO | EO of B. tripartita |
| BP | British Pharmacopoeia method |
| CECT | Colección Española de Cultivos Tipo |
| CLZ-NLC | Clotrimazole- nanostructured lipid carriers |
| CNS | Central nervous systems |
| CD | Cyclodextrin |
| DGIZ | Diameter of the growth inhibition zone |
| EO | Essential oil |
| EUCAST | European committee on antimicrobial susceptibility testing |
| FGI | Fungal growth index |
| FT-IR | Fourier Transformed- Infrared spectroscopy |
| GC-FID | Gas chromatography-flame ionisation detector |
| GC-MS | Gas chromatography-mass spectrometry |
| IZD | Different inhibition zone diameter |
| KOH | Potassium hydroxide |
| LGEO | Cymbopogon citratus EO |
| LPS | Lipopolysaccharide |
| MFC | Minimum fungicidal concentrations |
| MIC | Minimum inhibitory concentrations |
| MLC | Minimum lethal concentrations |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NLC | Clotrimazole-loaded nanoparticles |
| NO | Nitric oxide |
| PE | Pickering emulsion |
| PTCC | Persian Type Culture Collection |
| PV | Pityriasis versicolor |
| RCT | Randomised controlled trial |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| ZOI | Zone of inhibition test |
Author Contributions
Conceptualization: all authors; literature review: all authors; tables: all authors, writing and review all authors; editing: all authors; revisions and final editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guarner J., Brandt M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 2011;24:247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natu K.N., Tatke P.A. Essential oils—Prospective candidates for antifungal treatment? J. Essent. Oil Res. 2019;31:347–360. doi: 10.1080/10412905.2019.1604437. [DOI] [Google Scholar]
- 3.Warnock D.W. Trends in the epidemiology of invasive fungal infections. Nihon Ishinkin Gakkai Zasshi Jpn. J. Med. Mycol. 2007;48:1–12. doi: 10.3314/jjmm.48.1. [DOI] [PubMed] [Google Scholar]
- 4.Yoon H.J., Choi H.Y., Kim Y.K., Song Y.J., Ki M. Prevalence of fungal infections using National Health Insurance data from 2009–2013, South Korea. Epidemiol. Health. 2014;36:1–10. doi: 10.4178/epih/e2014017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiskopf D., Weinberger B., Grubeck-Loebenstein B. The aging of the immune system. Transpl. Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 6.Singh N. Trends in the epidemiology of opportunistic fungal infections: Predisposing factors and the impact of antimicrobial use practices. Clin. Infect. Dis. 2001;33:1692–1696. doi: 10.1086/323895. [DOI] [PubMed] [Google Scholar]
- 7.Nucci M., Marr K.A. Emerging fungal diseases. Clin. Infect. Dis. 2005;41:521–526. doi: 10.1086/432060. [DOI] [PubMed] [Google Scholar]
- 8.Riscili B.P., Wood K.L. Noninvasive pulmonary Aspergillus infections. Clin. Chest Med. 2009;30:315–335, vii. doi: 10.1016/j.ccm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Garbino D.J. Aspergillosis. In: Lew D., editor. Orphanet Encyclopedia. Orphanet; Paris, France: 2004. pp. 1–7. [Google Scholar]
- 10.Dignani M.C., Anaissie E. Human fusariosis. Clin. Microbiol. Infect. 2004;10(Suppl. 1):67–75. doi: 10.1111/j.1470-9465.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 11.Godoy P., Nunes E., Silva V., Tomimori-Yamashita J., Zaror L., Fischman O. Onychomycosis caused by Fusarium solani and Fusarium oxysporum in São Paulo, Brazil. Mycopathologia. 2004;157:287–290. doi: 10.1023/B:MYCO.0000024186.32367.d4. [DOI] [PubMed] [Google Scholar]
- 12.Brilhante R., Cordeiro R., Medrano D., Rocha M., Monteiro A., Cavalcante C., Meireles T., Sidrim J. Onychomycosis in Ceará (Northeast Brazil): Epidemiological and laboratory aspects. Mem. Inst. Oswaldo Cruz. 2005;100:131–135. doi: 10.1590/S0074-02762005000200005. [DOI] [PubMed] [Google Scholar]
- 13.Ninet B., Jan I., Bontems O., Léchenne B., Jousson O., Lew D., Schrenzel J., Panizzon R.G., Monod M. Molecular identification of Fusarium species in onychomycoses. Dermatology. 2005;210:21–25. doi: 10.1159/000081478. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez J.A., Sobel J.D. Candidiasis. In: Kauffman C.A., Pappas P.G., Sobel J.D., Dismukes W.E., editors. Essentials of Clinical Mycology. Springer; New York, NY, USA: 2011. pp. 167–206. [Google Scholar]
- 15.Darouiche R.O. Candida in the ICU. Clin. Chest Med. 2009;30:287–293. doi: 10.1016/j.ccm.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Bommanavar S., Gugwad S., Malik N. Phenotypic switch: The enigmatic white-gray-opaque transition system of Candida albicans. J. Oral Maxillofac. Surg. Med. Pathol. 2017;21:82–86. doi: 10.4103/0973-029X.203781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakrabarti A., Sood P., Rudramurthy S.M., Chen S., Kaur H., Capoor M., Chhina D., Rao R., Eshwara V.K., Xess I., et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensiv. Care Med. 2015;41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 18.Noble S.L., Forbes R.C., Stamm P.L. Diagnosis and management of common tinea infections. Am. Fam. Physician. 1998;58:163–174, 177–178. [PubMed] [Google Scholar]
- 19.Aguilar C.V.J., Alanio A.S.B., Frange P., Lanternier F., Lortholary O. Antifungals. [(accessed on 24 December 2020)]; Available online: https://www.em-consulte.com/article/946740/antifongiques.
- 20.Chanthaphon S., Chanthachum S., Hongpattarakere T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. against food-related microorganisms. Songklanakarin J. Sci. Technol. 2008;30:125–131. [Google Scholar]
- 21.Da Cruz Cabral L., Fernández Pinto V., Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013;166:1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Sharifi-Rad J., Sureda A., Tenore G.C., Daglia M., Sharifi-Rad M., Valussi M., Tundis R., Sharifi-Rad M., Loizzo M.R., Ademiluyi A.O., et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules. 2017;22:70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajkowska K., Otlewska A., Kunicka-Styczyńska A., Krajewska A. Candida albicans Impairments Induced by Peppermint and Clove Oils at Sub-Inhibitory Concentrations. Int. J. Mol. Sci. 2017;18:1307. doi: 10.3390/ijms18061307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tullio V., Roana J., Scalas D., Mandras N. Evaluation of the Antifungal Activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles. Molecules. 2019;24:3148. doi: 10.3390/molecules24173148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tullio V., Roana J., Scalas D., Mandras N. Enhanced Killing of Candida krusei by Polymorphonuclear Leucocytes in the Presence of Subinhibitory Concentrations of Melaleuca alternifolia and “Mentha of Pancalieri” Essential Oils. Molecules. 2019;24:3824. doi: 10.3390/molecules24213824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scalas D., Mandras N., Roana J., Tardugno R., Cuffini A.M., Ghisetti V., Benvenuti S., Tullio V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement. Altern. Med. 2018;18:143. doi: 10.1186/s12906-018-2219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Agostino M., Tesse N., Frippiat J.P., Machouart M., Debourgogne A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules. 2019;24:3713. doi: 10.3390/molecules24203713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto E., Pina-Vaz C., Salgueiro L., Gonçalves M.J., Costa-de-Oliveira S., Cavaleiro C., Palmeira A., Rodrigues A., Martinez-de-Oliveira J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006;55:1367–1373. doi: 10.1099/jmm.0.46443-0. [DOI] [PubMed] [Google Scholar]
- 29.Omidbeygi M., Barzegar M., Hamidi Z., Naghdibadi H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control. 2007;18:1518–1523. doi: 10.1016/j.foodcont.2006.12.003. [DOI] [Google Scholar]
- 30.Rasooli I., Rezaei M.B., Allameh A. Growth inhibition and morphological alterations of Aspergillus niger by essential oils from Thymus eriocalyx and Thymus x-porlock. Food Control. 2006;17:359–364. doi: 10.1016/j.foodcont.2004.12.002. [DOI] [Google Scholar]
- 31.Khan M.S., Ahmad I. In vitro antifungal, anti-elastase and anti-keratinase activity of essential oils of Cinnamomum-, Syzygium- and Cymbopogon-species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine. 2011;19:48–55. doi: 10.1016/j.phymed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Khan M.S., Ahmad I. Antifungal activity of essential oils and their synergy with fluconazole against drug-resistant strains of Aspergillus fumigatus and Trichophyton rubrum. Appl. Microbiol. Biotechnol. 2011;90:1083–1094. doi: 10.1007/s00253-011-3152-3. [DOI] [PubMed] [Google Scholar]
- 33.Angelini P., Pagiotti R., Menghini A., Vianello B. Antimicrobial activities of various essential oils against foodborne pathogenic or spoilage moulds. Ann. Microbiol. 2006;56:65–69. doi: 10.1007/BF03174972. [DOI] [Google Scholar]
- 34.Vilela G.R., de Almeida G.S., D’Arce M.A.B.R., Moraes M.H.D., Brito J.O., da Silva M.F.d.G., Silva S.C., de Stefano Piedade S.M., Calori-Domingues M.A., da Gloria E.M. Activity of essential oil and its major compound, 1, 8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J. Stored Prod. Res. 2009;45:108–111. doi: 10.1016/j.jspr.2008.10.006. [DOI] [Google Scholar]
- 35.Uniyal V., Bhatt R., Saxena S., Talwar A. Antifungal activity of essential oils and their volatile constituents against respiratory tract pathogens causing Aspergilloma and Aspergillosis by gaseous contact. J. Appl. Nat. Sci. 2012;4:65–70. doi: 10.31018/jans.v4i1.224. [DOI] [Google Scholar]
- 36.Verma R.K., Chaurasia L., Kumar M. Antifungal activity of essential oils against selected building fungi. Indian J. Nat. Prod. Resour. 2011;2:448–451. [Google Scholar]
- 37.Pinto E., Vale-Silva L., Cavaleiro C., Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009;58:1454–1462. doi: 10.1099/jmm.0.010538-0. [DOI] [PubMed] [Google Scholar]
- 38.Naeini A., Shokri H. Chemical composition and in vitro antifungal activity of the essential oil from Cuminum cyminum against various Aspergillus strains. J. Med. Plants Res. 2012;6:1702–1706. doi: 10.5897/JMPR11.1495. [DOI] [Google Scholar]
- 39.Kanafani Z.A., Perfect J.R. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008;46:120–128. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 40.Low C.Y., Rotstein C. Emerging fungal infections in immunocompromised patients. F1000 Med. Rep. 2011;3:14. doi: 10.3410/M3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mondello F., De Bernardis F., Girolamo A., Salvatore G., Cassone A. In vitro and in vivo activity of tea tree oil against azole-susceptible and -resistant human pathogenic yeasts. J. Antimicrob. Chemother. 2003;51:1223–1229. doi: 10.1093/jac/dkg202. [DOI] [PubMed] [Google Scholar]
- 42.Stringaro A., Colone M., Angiolella L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha spp. Essential Oils. Medicines. 2018;5:112. doi: 10.3390/medicines5040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Auria F.D., Tecca M., Strippoli V., Salvatore G., Battinelli L., Mazzanti G. Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med. Mycol. 2005;43:391–396. doi: 10.1080/13693780400004810. [DOI] [PubMed] [Google Scholar]
- 44.Behmanesh F., Pasha H., Sefidgar A.A., Taghizadeh M., Moghadamnia A.A., Adib Rad H., Shirkhani L. Antifungal Effect of Lavender Essential Oil (Lavandula angustifolia) and Clotrimazole on Candida albicans: An In Vitro Study. Scientifica (Cairo) 2015;2015:1–5. doi: 10.1155/2015/261397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minooeianhaghighi M.H., Sepehrian L., Shokri H. Antifungal effects of Lavandula binaludensis and Cuminum cyminum essential oils against Candida albicans strains isolated from patients with recurrent vulvovaginal candidiasis. J. Mycol. Med. 2017;27:65–71. doi: 10.1016/j.mycmed.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dias Ferreira F., Mossini S.A., Dias Ferreira F.M., Arrotéia C.C., da Costa C.L., Nakamura C.V., Machinski M., Jr. The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus link growth and morphology. Sci. World J. 2013;2013:1–6. doi: 10.1155/2013/343804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tolouee M., Alinezhad S., Saberi R., Eslamifar A., Zad S.J., Jaimand K., Taeb J., Rezaee M.B., Kawachi M., Shams-Ghahfarokhi M., et al. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. Int. J. Food Microbiol. 2010;139:127–133. doi: 10.1016/j.ijfoodmicro.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Khan A., Ahmad A., Akhtar F., Yousuf S., Xess I., Khan L.A., Manzoor N. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res. Microbiol. 2010;161:816–823. doi: 10.1016/j.resmic.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y., Zeng H., Tian J., Ban X., Ma B., Wang Y. Dill (Anethum graveolens L.) seed essential oil induces Candida albicans apoptosis in a metacaspase-dependent manner. Fungal Biol. 2014;118:394–401. doi: 10.1016/j.funbio.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Kumar R., Mishra A.K., Dubey N.K., Tripathi Y.B. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int. J. Food Microbiol. 2007;115:159–164. doi: 10.1016/j.ijfoodmicro.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Yahyaraeyat R., Khosravi A., Shahbazzadeh D., Khalaj V. The potential effects of Zataria multiflora Boiss essential oil on growth, aflatoxin production and transcription of aflatoxin biosynthesis pathway genes of toxigenic Aspergillus parasiticus. Braz. J. Microbiol. 2013;44:649–655. doi: 10.1590/S1517-83822013000200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Oliveira Lima M.I., Araújo de Medeiros A.C., Souza Silva K.V., Cardoso G.N., de Oliveira Lima E., de Oliveira Pereira F. Investigation of the antifungal potential of linalool against clinical isolates of fluconazole resistant Trichophyton rubrum. J. Mycol. Med. 2017;27:195–202. doi: 10.1016/j.mycmed.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Shreaz S., Shiekh R.A., Raja V., Wani W.A., Behbehani J.M. Impaired ergosterol biosynthesis mediated fungicidal activity of Co(II) complex with ligand derived from cinnamaldehyde. Chem. Biol. Interact. 2016;247:64–74. doi: 10.1016/j.cbi.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Kurita N., Koike S. Synergistic Antimicrobial Effect of Ethanol, Sodium Chloride, Acetic Acid and Essential Oil Components. Agric. Biol. Chem. 1983;47:67–75. doi: 10.1080/00021369.1983.10865580. [DOI] [Google Scholar]
- 56.Satpathy B., Sahoo M., Sahoo P., Patra S. Formulation and evaluation of herbal gel containing essential oils of piper betle against skin infecting pathogens. Int. J. Res. Pharm. Sci. 2011;2:373–378. [Google Scholar]
- 57.Sharma R., Pathak K. Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm. Dev. Technol. 2011;16:367–376. doi: 10.3109/10837451003739289. [DOI] [PubMed] [Google Scholar]
- 58.Kozics K., Bučková M., Puškárová A., Kalászová V., Cabicarová T., Pangallo D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules. 2019;24:4570. doi: 10.3390/molecules24244570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahboubi M., Kazempour N. The antifungal activity of Artemisia sieberi essential oil from different localities of Iran against dermatophyte fungi. J. Mycol. Med. 2015;25:e65–e71. doi: 10.1016/j.mycmed.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 60.Khoury M., Stien D., Eparvier V., Ouaini N., El Beyrouthy M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. Based Complement. Altern. Med. 2016;2016:1–17. doi: 10.1155/2016/2547169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mary H., Tina A.V., Jeeja K.J., Abiramy M. Phytochemical analysis and anticancer activity of essential oil from Myristica fragrans. Int. J. Curr. Pharm. Res. 2012;2:188–198. [Google Scholar]
- 62.Singh P., Bundiwale R., Dwivedi L. In-Vitro study of antifungal activity of various commercially available itra (Volatile plant oil) against the keratinophilic fungi isolated from soil. Int. J. Pharma Bio Sci. 2011;2:178–184. [Google Scholar]
- 63.Gonçalves M., Cruz M., Cavaleiro C., Lopes M., Salgueiro L. Chemical, antifungal and cytotoxic evaluation of the essential oil of Thymus zygis subsp. sylvestris. Ind. Crop. Prod. 2010;32:70–75. doi: 10.1016/j.indcrop.2010.03.005. [DOI] [Google Scholar]
- 64.Mahboubi M., Kazempour N. Comparison of antifungal activity of different extracts from Satureja khuzistanica Jamzad against dermatophytes. Biharean Biol. 2015;9:105–107. [Google Scholar]
- 65.Valente J., Zuzarte M., Resende R., Gonçalves M., Cavaleiro C., Pereira C., Cruz M., Salgueiro L. Daucus carota subsp. gummifer essential oil as a natural source of antifungal and anti-inflammatory drugs. Ind. Crop. Prod. 2015;65:361–366. doi: 10.1016/j.indcrop.2014.11.014. [DOI] [Google Scholar]
- 66.Abu-Darwish M., Cabral C., Ferreira I., Gonçalves M., Cavaleiro C., Cruz M., Al-Bdour T., Salgueiro L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. BioMed Res. Int. 2013;2013:1–9. doi: 10.1155/2013/538940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fahed L., Khoury M., Stien D., Ouaini N., Eparvier V., El Beyrouthy M. Essential oils composition and antimicrobial activity of six conifers harvested in Lebanon. Chem. Biodivers. 2017;14:e1600235. doi: 10.1002/cbdv.201600235. [DOI] [PubMed] [Google Scholar]
- 68.Tavares A.C., Gonçalves M.J., Cruz M.T., Cavaleiro C., Lopes M.C., Canhoto J., Salgueiro L.R. Essential oils from Distichoselinum tenuifolium: Chemical composition, cytotoxicity, antifungal and anti-inflammatory properties. J. Ethnopharmacol. 2010;130:593–598. doi: 10.1016/j.jep.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 69.Fajinmi O., Kulkarni M., Benická S., Zeljković S.Ć., Doležal K., Tarkowski P., Finnie J., Van Staden J. Antifungal activity of the volatiles of Agathosma betulina and Coleonema album commercial essential oil and their effect on the morphology of fungal strains Trichophyton rubrum and T. mentagrophytes. S. Afr. J. Bot. 2019;122:492–497. doi: 10.1016/j.sajb.2018.03.003. [DOI] [Google Scholar]
- 70.Fajinmi O.O., Grúz J., Tarkowski P., Kulkarni M.G., Finnie J.F., Van Staden J. Antifungal and antioxidant activities of Coleonema album and C. pulchellum against skin diseases. Pharm. Biol. 2017;55:1249–1255. doi: 10.1080/13880209.2017.1296470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canales-Martinez M., Rivera-Yañez C., Salas-Oropeza J., Lopez H., Jimenez-Estrada M., Rosas-Lopez R., Duran D., Flores C., Hernandez L., Rodriguez-Monroy M. Antimicrobial activity of Bursera morelensis ramírez essential oil. Afr. J. Tradit. Complement. Altern. Med. 2017;14:74–82. doi: 10.21010/ajtcam.v14i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osho A., Adetunji T., Fayemi S.O., Moronkola D.O. Antimicrobial activity of essential oils of Physalis angulata. L. Afr. J. Tradit. Complement. Altern. Med. 2010;7:303–306. doi: 10.4314/ajtcam.v7i4.56696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rezgui M., Majdoub N., Mabrouk B., Baldisserotto A., Bino A., Kaab L.B., Manfredini S. Antioxidant and antifungal activities of marrubiin, extracts and essential oil from Marrubium vulgare L. against pathogenic dermatophyte strains. J. Mycol. Med. 2020:100927. doi: 10.1016/j.mycmed.2020.100927. [DOI] [PubMed] [Google Scholar]
- 74.Nyegue M.A., Ndoyé-Foe F.-C., Essama S.R., Hockmeni T., Etoa F.-X., Menut C. Chemical composition of essential oils of Eugenia caryophylla and Mentha sp cf piperita and their in vitro antifungal activities on six human pathogenic fungi. Afr. J. Tradit. Complement. Altern. Med. 2014;11:40–46. doi: 10.4314/ajtcam.v11i6.3. [DOI] [Google Scholar]
- 75.Cabral C., Francisco V., Cavaleiro C., Gonçalves M.J., Cruz M.T., Sales F., Batista M.T., Salgueiro L. Essential Oil of Juniperus communis subsp. alpina (Suter) Čelak Needles: Chemical Composition, Antifungal Activity and Cytotoxicity. Phytother. Res. 2012;26:1352–1357. doi: 10.1002/ptr.3730. [DOI] [PubMed] [Google Scholar]
- 76.Prakash R., Agarwal S., Srivastava A., Nigam V. GC-MS Analysis of essential oil of Turmeric rhizome and its activity against Sporothrix schenckii fungus. Orient. J. Chem. 2015;31:2213–2214. doi: 10.13005/ojc/310445. [DOI] [Google Scholar]
- 77.Dias N., Dias M., Cavaleiro C., Sousa M., Lima N., Machado M. Oxygenated monoterpenes-rich volatile oils as potential antifungal agents for dermatophytes. Nat. Prod. Res. 2017;31:460–464. doi: 10.1080/14786419.2016.1195379. [DOI] [PubMed] [Google Scholar]
- 78.Sadhasivam S., Palanivel S., Ghosh S. Synergistic antimicrobial activity of Boswellia serrata Roxb. ex Colebr. (Burseraceae) essential oil with various azoles against pathogens associated with skin, scalp and nail infections. Lett. Appl. Microbiol. 2016;63:495–501. doi: 10.1111/lam.12683. [DOI] [PubMed] [Google Scholar]
- 79.Białoń M., Krzyśko-Łupicka T., Koszałkowska M., Wieczorek P.P. The influence of chemical composition of commercial lemon essential oils on the growth of Candida strains. Mycopathologia. 2014;177:29–39. doi: 10.1007/s11046-013-9723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahman A., Al-Reza S.M., Siddiqui S.A., Chang T., Kang S.C. Antifungal potential of essential oil and ethanol extracts of Lonicera japonica Thunb. against dermatophytes. EXCLI J. 2014;13:427–436. [PMC free article] [PubMed] [Google Scholar]
- 81.Tangarife Castaño V., Roa Linares V., Betancur L., Durán D., Stashenko E., Mesa A. Antifungal activity of Verbenaceae and Labiatae families essential oils. Pharmacologyonline. 2012;1:133–145. [Google Scholar]
- 82.Sharma R., Sharma G., Sharma M. Anti-Malassezia furfur activity of essential oils against causal agent of Pityriasis versicolor disease. Afr. J. Pharm. Pharmacol. 2012;6:979–983. doi: 10.5897/AJPP12.097. [DOI] [Google Scholar]
- 83.da Cruz R.C., Denardi L.B., Mossmann N.J., Piana M., Alves S.H., de Campos M.M. Antimicrobial Activity and Chromatographic Analysis of Extracts from Tropaeolum pentaphyllum Lam. Tubers. Molecules. 2016;21:566. doi: 10.3390/molecules21050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tabassum N.M.V. Antimicrobial activity of medicinal oil plants against human pathogens from Hyderabad Karnataka Region. Int. J. Pharm. Sci. Rev. Res. 2014;26:182–188. [Google Scholar]
- 85.Oladimeji F.A., Akinkunmi E.O., Raheem A.I., Abiodun G.O., Bankole V.O. Evaluation of topical antimicrobial ointment formulations of essential oil of Lippia multiflora moldenke. Afr. J. Tradit. Complement. Altern. Med. 2015;12:135–144. doi: 10.4314/ajtcam.v12i5.18. [DOI] [Google Scholar]
- 86.Tomczykowa M., Wróblewska M., Winnicka K., Wieczorek P., Majewski P., Celińska-Janowicz K., Sawczuk R., Miltyk W., Tryniszewska E., Tomczyk M. Novel gel formulations as topical carriers for the essential oil of Bidens tripartita for the treatment of candidiasis. Molecules. 2018;23:2517. doi: 10.3390/molecules23102517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carbone C., Teixeira M.d.C., Sousa M.d.C., Martins-Gomes C., Silva A.M., Souto E.M.B., Musumeci T. Clotrimazole-Loaded mediterranean essential oils NLC: A synergic treatment of Candida skin infections. Pharmaceutics. 2019;11:231. doi: 10.3390/pharmaceutics11050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rabadia A.G., Kamat S., Kamat D. Antifungal activity of essential oils against fluconazole resistant fungi. Int. J. Phytomed. 2011;3:506. [Google Scholar]
- 89.Ali S., Khan M.R., Batool R., Maryam S., Majid M. Wound healing potential of oil extracted from Parrotiopsis jacquemontiana (Decne) Rehder. J. Ethnopharmacol. 2019;236:354–365. doi: 10.1016/j.jep.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 90.Jain N., Sharma M., Joshi S., Kaushik U. Chemical composition, toxicity and antidermatophytic activity of essential oil of Trachyspermum ammi. Indian J. Pharm. Sci. 2018;80:135–142. doi: 10.4172/pharmaceutical-sciences.1000338. [DOI] [Google Scholar]
- 91.Boukhatem M.N., Ferhat M.A., Kameli A., Saidi F., Kebir H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med. 2014;9:1–10. doi: 10.3402/ljm.v9.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Njateng G., Kuiate J., Gatsing D., Tamokou J., Mouokeu R., Kuete V. Antidermatophytic activity and dermal toxicity of essential oil from the leaves of Ageratum houstonianum (Asteraceae) J. Biol. Sci. 2010;10:448–454. doi: 10.3923/jbs.2010.448.454. [DOI] [Google Scholar]
- 93.Aldawsari H.M., Badr-Eldin S.M., Labib G.S., El-Kamel A.H. Design and formulation of a topical hydrogel integrating lemongrass-loaded nanosponges with an enhanced antifungal effect: In vitro/in vivo evaluation. Int. J. Nanomed. 2015;10:893–902. doi: 10.2147/IJN.S74771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gemeda N., Tadele A., Lemma H., Girma B., Addis G., Tesfaye B., Abebe A., Gemechu W., Yirsaw K., Teka F. Development, characterization, and evaluation of novel broad-spectrum antimicrobial topical formulations from Cymbopogon martini (Roxb.) W. Watson essential oil. Evid. Based Complement. Altern. Med. 2018;2018:1–16. doi: 10.1155/2018/9812093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prasad C.S., Shukla R., Kumar A., Dubey N.K. In vitro and in vivo antifungal activity of essential oils of Cymbopogon martini and Chenopodium ambrosioides and their synergism against dermatophytes. Mycoses. 2010;53:123–129. doi: 10.1111/j.1439-0507.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 96.Pandey K.P., Mishra R.K., Kamran A., Mishra P., Bajaj A.K., Dikshit A. Studies on antidermatophytic activity of waste leaves of Curcuma longa L. Physiol. Mol. Biol. Plants. 2010;16:177–185. doi: 10.1007/s12298-010-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barac A., Donadu M., Usai D., Spiric V.T., Mazzarello V., Zanetti S., Aleksic E., Stevanovic G., Nikolic N., Rubino S. Antifungal activity of Myrtus communis against Malassezia sp. isolated from the skin of patients with pityriasis versicolor. Infection. 2018;46:253–257. doi: 10.1007/s15010-017-1102-4. [DOI] [PubMed] [Google Scholar]
- 98.Borda L.J., Wikramanayake T.C. Seborrheic Dermatitis and Dandruff: A Comprehensive Review. J. Clin. Investig. Dermatol. 2015;3:1–22. doi: 10.13188/2373-1044.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pugliarello S., Cozzi A., Gisondi P., Girolomoni G. Phenotypes of atopic dermatitis. JDDG J. Dtsch. Dermatol. Ges. 2011;9:12–20. doi: 10.1111/j.1610-0387.2010.07508.x. [DOI] [PubMed] [Google Scholar]
- 100.Gallitano S.M., Berson D.S. How Acne Bumps Cause the Blues: The Influence of Acne Vulgaris on Self-Esteem. Int. J. Womens Dermatol. 2017;4:12–17. doi: 10.1016/j.ijwd.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Casadevall A., Pirofski L.A. Host-Pathogen interactions: Redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999;67:3703–3713. doi: 10.1128/IAI.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Casadevall A., Pirofski L.A. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naggie S., Perfect J.R. Molds: Hyalohyphomycosis, phaeohyphomycosis, and zygomycosis. Clin. Chest Med. 2009;30:337–353, vii–viii. doi: 10.1016/j.ccm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 104.Kohler J., Hube B., Puccia R., Casadevall A., Perfect J. Fungi that infect humans. Microbiol. Spectr. 2017;5:1–29. doi: 10.1128/microbiolspec.FUNK-0014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guruprasad B., Famna Roohi N., Gowda D. A Review on Candidiasis resistance current drug development process in its prevention and treatment. Int. J. Res. Pharm. Sci. 2020;11:1073–1079. doi: 10.26452/ijrps.v11i1.1938. [DOI] [Google Scholar]
- 106.Farjon A., Juniperus L. In: Flora of Turkey and the East Aegean Islands. Davis P.H., Cullen J., Coode M.J.E., editors. Volume 11. Ediburgh University Press; Ediburgh, UK: 2000. pp. 8–10. [Google Scholar]
- 107.Shah P.P., Mello P. A review of medicinal uses and pharmacological effects of Mentha piperita. Nat. Prod. Radiance. 2004;3:214–221. [Google Scholar]
- 108.Nurdjannah N., Bermawie N. Handbook of Herbs and Spices. Elsevier; Amsterdam, The Netherlands: 2001. Clove; pp. 154–163. [Google Scholar]
- 109.Weedon D. 25—Mycoses and algal infections. In: Weedon D., editor. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; Edinburgh, UK: 2010. pp. 581–606.e24. [Google Scholar]
- 110.Karpiński T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules. 2020;10:103. doi: 10.3390/biom10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malik A., Siddique M., Sofi P., Butola J. Ethnomedicinal practices and conservation status of medicinal plants of North Kashmir Himalayas. Res. J. Med. Plant. 2011;5:515–530. [Google Scholar]
- 112.Kumar K., Sharma Y.P., Manhas R., Bhatia H. Ethnomedicinal plants of Shankaracharya Hill, Srinagar, J&K, India. J. Ethnopharmacol. 2015;170:255–274. doi: 10.1016/j.jep.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 113.Ali S., Khan M.R., Sajid M. Protective potential of Parrotiopsis jacquemontiana (Decne) Rehder on carbon tetrachloride induced hepatotoxicity in experimental rats. Biomed. Pharmacother. 2017;95:1853–1867. doi: 10.1016/j.biopha.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 114.Bruni R., Medici A., Andreotti E., Fantin C., Muzzoli M., Dehesa M., Romagnoli C., Sacchetti G. Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm.(Lauraceae) flower calices. Food Chem. 2004;85:415–421. doi: 10.1016/j.foodchem.2003.07.019. [DOI] [Google Scholar]
- 115.Fox L.T., Mazumder A., Dwivedi A., Gerber M., du Plessis J., Hamman J.H. In vitro wound healing and cytotoxic activity of the gel and whole-leaf materials from selected aloe species. J. Ethnopharmacol. 2017;200:1–7. doi: 10.1016/j.jep.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 116.India G.O. Ayurvedic Pharmacopoeia of India, Part 11999. Volume 1. Ministry of Health and Family Welfare, Department of Ayush; New Delhi, India: 2011. p. 170. [Google Scholar]
- 117.Ishwar S., Singh V. Antifungal properties of aqueous and organic solution extracts of seed plants against Aspergillus flavus and A. niger. Phytomorphology. 2000;50:151–157. [Google Scholar]
- 118.Singh V., Ali M., Saxena R., Shekhar C., Anand D. Volatile constituents and antimicrobial and antifungal activities of immature green seeds of Trachyspermum ammi Linn. J. Essent. Oil Bear. Plants. 2008;11:120–123. doi: 10.1080/0972060X.2008.10643607. [DOI] [Google Scholar]
- 119.Paul S., Dubey R., Maheswari D., Kang S.C. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control. 2011;22:725–731. doi: 10.1016/j.foodcont.2010.11.003. [DOI] [Google Scholar]
- 120.Umar S., Asif M., Sajad M., Ansari M.M., Hussain U., Ahmad W., Siddiqui S.A., Ahmad S., Khan H.A. Anti-Inflammatory and antioxidant activity of Trachyspermum ammi seeds in collagen induced arthritis in rats. Int. J. Drug Dev. Res. 2012;4:210–219. [Google Scholar]
- 121.Roy S., Chaurvedi P., Chowdhary A. Evaluation of antiviral activity of essential oil of Trachyspermum Ammi against Japanese encephalitis virus. Pharmacogn. Res. 2015;7:263–267. doi: 10.4103/0974-8490.157977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pozzatti P., Scheid L.A., Spader T.B., Atayde M.L., Santurio J.M., Alves S.H. In vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can. J. Microbiol. 2008;54:950–956. doi: 10.1139/W08-097. [DOI] [PubMed] [Google Scholar]
- 123.Gatsing D., Tchakoute V., Ngamga D., Kuiate J., Tamokou J.d.D., Nji-Nkah B., Tchouanguep F., Fodouop S. In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci. 2009;34:126–136. [Google Scholar]
- 124.Akhila A. Essential Oil-Bearing Grasses: The Genus Cymbopogon. CRC Press; New York, NY, USA: 2009. [Google Scholar]
- 125.Hood J.R., Wilkinson J.M., Cavanagh H.M.A. Evaluation of Common Antibacterial Screening Methods Utilized in Essential Oil Research. J. Essent. Oil Res. 2003;15:428–433. doi: 10.1080/10412905.2003.9698631. [DOI] [Google Scholar]
- 126.Ezadi F., Ardebili A., Mirnejad R. Antimicrobial Susceptibility Testing for Polymyxins: Challenges, Issues, and Recommendations. J. Clin. Microbiol. 2019;57:1–20. doi: 10.1128/JCM.01390-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Griffin S.G., Markham J.L., Leach D.N. An Agar Dilution Method for the Determination of the Minimum Inhibitory Concentration of Essential Oils. J. Essent. Oil Res. 2000;12:249–255. doi: 10.1080/10412905.2000.9699509. [DOI] [Google Scholar]
- 128.Chouhan S., Sharma K., Guleria S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chu Y., Xu T., Gao C., Liu X., Zhang N., Feng X., Liu X., Shen X., Tang X. Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int. J. Biol. Macromol. 2019;122:388–394. doi: 10.1016/j.ijbiomac.2018.10.194. [DOI] [PubMed] [Google Scholar]
- 130.Selvamuthukumar S., Anandam S., Krishnamoorthy K., Rajappan M. Nanosponges: A novel class of drug delivery system-review. J. Pharm. Pharm. Sci. 2012;15:103–111. doi: 10.18433/j3k308. [DOI] [PubMed] [Google Scholar]
- 131.Minelli R., Cavalli R., Ellis L., Pettazzoni P., Trotta F., Ciamporcero E., Barrera G., Fantozzi R., Dianzani C., Pili R. Nanosponge-Encapsulated camptothecin exerts anti-tumor activity in human prostate cancer cells. Eur. J. Pharm. Sci. 2012;47:686–694. doi: 10.1016/j.ejps.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 132.Prajapati N.D., Purohit S., Sharma A.K., Kumar T. Handbook of Medicinal Plants: A Complete Source Book. Agrobios Publishers; Jodhpur, India: 2003. [Google Scholar]
- 133.Kumar R., Srivastava M., Dubey N.K. Evaluation of Cymbopogon martinii oil extract for control of postharvest insect deterioration in cereals and legumes. J. Food Prot. 2007;70:172–178. doi: 10.4315/0362-028X-70.1.172. [DOI] [PubMed] [Google Scholar]
- 134.Gaitanis G., Velegraki A., Mayser P., Bassukas I.D. Skin diseases associated with Malassezia yeasts: Facts and controversies. Clin. Dermatol. 2013;31:455–463. doi: 10.1016/j.clindermatol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 135.Velegraki A., Cafarchia C., Gaitanis G., Iatta R., Boekhout T. Malassezia infections in humans and animals: Pathophysiology, detection, and treatment. PLoS Pathog. 2015;11:e1004523. doi: 10.1371/journal.ppat.1004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharma A., Rabha D., Ahmed G. In vitro antifungal susceptibility of Malassezia isolates from pityriasis versicolor lesions. Indian J. Dermatol. Venereol. Leprol. 2017;83:249–251. doi: 10.4103/0378-6323.193617. [DOI] [PubMed] [Google Scholar]
- 137.Kordali S., Usanmaz A., Cakir A., Komaki A., Ercisli S. Antifungal and Herbicidal Effects of Fruit Essential Oils of Four Myrtus communis Genotypes. Chem. Biodivers. 2016;13:77–84. doi: 10.1002/cbdv.201500018. [DOI] [PubMed] [Google Scholar]
- 138.Sara C., Paola M., Melania R., Marina C., Maurizio S., Bruno M., Stefania Z. Antimycotic activity of Myrtus communis L. towards Candida spp. from clinical isolates. J. Infect. Dev. Ctries. 2013;7 doi: 10.3855/jidc.2799. [DOI] [PubMed] [Google Scholar]
- 139.Deriu A., Branca G., Molicotti P., Pintore G., Chessa M., Tirillini B., Paglietti B., Mura A., Sechi L.A., Fadda G., et al. In vitro activity of essential oil of Myrtus communis L. against Helicobacter pylori. Int. J. Antimicrob. Agents. 2007;30:562–563. doi: 10.1016/j.ijantimicag.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 140.Aleksic V., Knezevic P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014;169:240–254. doi: 10.1016/j.micres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 141.Jain S., Shrivastava S., Nayak S., Sumbhate S. PHCOG MAG: Plant review recent trends in Curcuma Longa Linn. Pharmacogn. Rev. 2007;1:119–128. [Google Scholar]
- 142.Mochizuki T., Watanabe S., Kawasaki M., Tanabe H., Ishizaki H. A Japanese case of tinea corporis caused by Arthroderma benhamiae. J. Dermatol. 2002;29:221–225. doi: 10.1111/j.1346-8138.2002.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 143.Roxburgh A.C., Borrie P. Roxburgh’s Common Skin Diseases. 12th ed. The English Language Book Society and H.K. Lewis and Co. Ltd.; London, UK: 1973. [Google Scholar]
- 144.Torres-Alvarez C., Castillo S., Sánchez-García E., Aguilera González C., Galindo-Rodríguez S.A., Gabaldón-Hernández J.A., Báez-González J.G. Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations. Molecules. 2020;25:5109. doi: 10.3390/molecules25215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vörös-Horváth B., Das S., Salem A., Nagy S., Böszörményi A., Kőszegi T., Pál S., Széchenyi A. Formulation of Tioconazole and Melaleuca alternifolia Essential Oil Pickering Emulsions for Onychomycosis Topical Treatment. Molecules. 2020;25:5544. doi: 10.3390/molecules25235544. [DOI] [PMC free article] [PubMed] [Google Scholar]