Abstract
Objective
To evaluate a novel liquid fiducial marker for intraoperative marking of the tumour resection surface in oral cancer patients to facilitate precise postoperative delineation of the interface between the tumour resection border and reconstructed tissue for intensity-modulated radiation therapy.
Methods
A total of 200 markers were created by injecting the volumes of 10 µl, 20 µl, 30 µl, 40 µl and 50 µl of a liquid marker composed of sucrose acetoisobutyrate (SAIB) and iodinated sucrose acetoisobutyrate (x-SAIB) into the soft tissue of porcine mandible segments. Visibility of the resulting markers was quantified by threshold-based segmentation of the marker volume in CT- and CBCT imaging and by a comparison of signal intensities in MRI.
Results
Even the lowest volume of SAIB-/x-SAIB investigated (10 µl) resulted in a higher visibility (CTSoft tissue: 88.18 ± 13.23 µl; CTBone: 49.55 ± 7.62 µl; CBCT: 54.65 ± 12.58 µl) than observed with the incorporation of titanium ligature clips (CTSoft tissue: 50.15 ± 7.50 mm3; CTBone: 23.90 ± 3.39 mm3; CBCT: 33.80 ± 9.20 mm3). Markers created by the injection of 10 µl and 20 µl could reliably be delineated from markers created by the injection of higher volumes.
Conclusion
SAIB/x-SAIB, which has recently become available as a Conformité Européenne (CE)-marked fiducial marker, provides an option for fast and reliable production of markers with excellent visibility in imaging modalities used in oral cancer radiation therapy (RT) planning routine.
Keywords: mouth neoplasms, radiotherapy, fiducial markers, feasibility studies
Introduction
Head and neck cancer was the seventh most common cancer worldwide in 2018 (890,000 new cases and 450,000 deaths).1,2 In Germany, there have been 17,524 (12,992 males; 4,532 females) newly diagnosed cases of head and neck cancer in 2013 and 19,800 new cases (14,300 males; 5,500 females) are anticipated in 2020.3 The therapy approach for these patients is multimodal, with primary surgical resection of the tumour, including tumour-free safety margins, being an important treatment component in the majority of oral cavity cancer patients.2 Adjuvant radiation therapy (RT) is indicated in case of advanced tumour, close or positive resection margins and cases of cervical lymph node, vascular and/or perineural involvement.4 In these patients, adjuvant RT has been demonstrated to significantly improve locoregional control, cause-specific survival and overall survival.5,6 However, it also has the potential to increase morbidity due to the irradiation of non-target tissue.6
Advances in radiotherapy, such as intensity-modulated radiation therapy (IMRT), can increase the accuracy of radiation and reduce the dose to the surrounding healthy tissue. This facilitates delivering a local tumour bed boost that can contribute to an increased local control rate, while at the same time reducing normal tissue toxicity.6–8
Traditionally, radiation treatment planning is based on combined information from (pre- and postoperative) radiological imaging, pathology reports and operative notes.9 In order to fully utilize the advantages of IMRT, precise orientation of the interface between the tumour resection border and native/reconstructed tissue is paramount. However, reconstruction of large resection defects using vascularized free flaps is often necessary in oral cancer patients. In these cases, similar contrast values between native tissue and graft tissue impair subsequent delineation of the tumour resection surface.10,11
In breast cancer surgery, intraoperative implantation of metal-based fiducial markers for tumour bed delineation is well known since the early 1990s and considered standard to define the former tumour cavity and RT target volume.12–14 In oral cancer surgery, this has been found to be a feasible approach for postoperative identification of the tumour resection surface as well.10,11
The key characteristics for fiducial markers have been defined by Habermehl et al as follows: (1) Importance of visibility, (2) absence of artefacts, (3) easy application and (4) sufficient immovability.15 In this context, injectable liquid fiducial markers might be an advantageous alternative to metal-based markers and promising characteristics have been reported for iodinated sucrose acetate isobutyrate (x-SAIB), which has recently become available as a CE-marked medical product (BioXmark®; Nanovi, Kgs. Lyngby, Denmark). This marker has been demonstrated to provide excellent visibility whilst only causing a low degree of (beam hardening) artefacts.16,17 Its application has been reported to be easy and fast with the option to create several markers in an uninterrupted procedure, the sizes of which can be adapted by altering the injected volume.18,19 Initially, the marker has a low viscosity. After injection into soft tissue, ethanol added as a solvent, diffuses out of the marker, causing an increase in viscosity. Eventually, this results in a semi-solid (gel-like) implant, which is positionally stable due to its stickiness.20,21 Previous investigations have demonstrated the marker to be safe and fully biocompatible.19
To date, BioXmark® has mainly been investigated in the context of IGRT for tumours of movable organs like lungs, oesophagus and pancreas, with relatively large volumes injected in most cases.18,21–23 In this study, BioXmark® was investigated applied in a high number of low injection-volumes for intraoperative marking of the tumour resection surface in surgically treated oral cancer. The aim of this preclinical investigation was to evaluate the technical feasibility of the marking procedure as well as the correlation between the injected volume and the visibility of the resulting markers in CT, CBCT and MRI.
Methods and materials
The liquid fiducial marker
BioXmark® (Nanovi, Kgs. Lyngby, Denmark) is a biocompatible, injectable soft tissue marker. It is composed of sucrose acetate isobutyrate (SAIB), iodinated SAIB (x-SAIB) and ethanol (EtOH), providing suitable viscosity for injection using thin needles (<25G). Upon injection into soft tissue, EtOH diffuses out of the marker, causing an increase of marker viscosity that results in the formation of a hydrophobic semi-solid, sticky and radio-opaque gel-like marker at the injection site.
Technical procedure
To evaluate the technical feasibility of the marking procedure and the visibility of SAIB/x-SAIB, markers were created by injecting different volumes into the soft tissue of porcine mandible segments. Porcine mandible segments were chosen as they were considered an adequate option to replicate the anatomical structures of the oral cavity (soft tissue, bone, teeth) in medical imaging.
Markers were created using the amounts of 10 µl, 20 µl, 30 µl, 40 µl and 50 µl with 40 injections performed per volume. To ensure reproducible injection of the different volumes, the marker was injected using unit dose injectors (MicroDose, Vlow Medical, Eindhoven, Netherlands). Considering previous reports on BioXmark® as well as its instruction for use, a 25G cannula was used for injection.
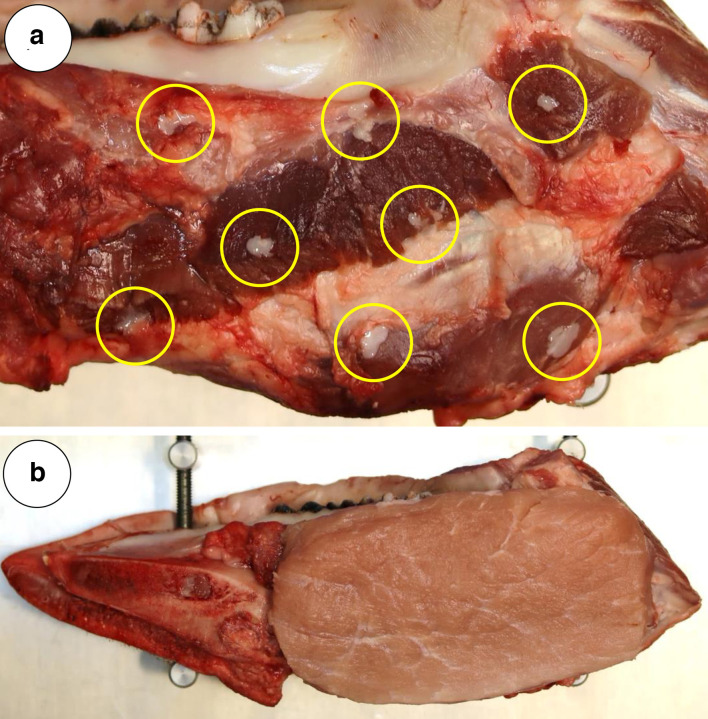
As the aim of this study was to investigate a procedure for marking of the soft tissue resection surface in oral cancer patients, the marker was injected superficially by advancing the needle tip 3–4 mm at a depth of 1–2 mm before injection. To replicate soft tissue reconstruction performed in oral cancer patients, the area marked with SAIB/x-SAIB was covered with muscle tissue subsequently to the injection of the desired number of markers (Figure 1).
Figure 1.
(A) Low amount of backflow of SAIB/x-SAIB as observed with the injection of 20 µl per marker. Tarnishing of the marker backflow (marked with circles) will help in visually controlling the marking procedure (B) Site of injection of SAIB/x-SAIB markers covered with muscle tissue to simulate resection defect soft tissue closure performed in oral cancer patients.
Evaluation of marker visibility
CT (soft tissue and bone kernel), CBCT and T1-weighted MRI scans (volume interpolated gradient echo sequence using the Dixon method for fat suppression) were acquired from all porcine mandible segments (n = 20). Imaging was acquired not earlier than 12 h after injection to allow for reliable efflux of ethanol, as this might impact the size of the marker visible in imaging.18 To allow for the transmission of the results of this study into the clinical setting, all imaging was acquired on clinical scanners, applying protocols used in clinical routine (Table 1).
Table 1.
Imaging protocols
| Imaging modality | In-plane resolution (mm2) | Slice thickness (mm) | TE (Dual Echo) | TR | BW (Hz/pixel) | FA (°) | GRAPPA Factor | X-ray exposure (mA) | X-ray voltage (kV) |
|---|---|---|---|---|---|---|---|---|---|
| CT (soft tissue and bone kernel)a | 0.64 × 0.64 | 2 | - | - | - | - | - | 120 | 120 |
| CBCTb | 0.25 × 0.25 | 0.5 | - | - | - | - | - | 87 | 90 |
| MRI – T1wc | 1.14 × 1.14 | 2 | 2.46/3.69 | 9.13 | 370 | 13 | 2 | - | - |
acquired on Siemens SOMATOM Definition Flash (Siemens Healthineers, Erlangen, Germany).
acquired on Morita 3D Accuitomo F17 (Morita, Osaka, Japan).
acquired on Siemens Magnetom Prisma Fit (Siemens Healthineers, Erlangen, Germany).
For objective quantitative analysis of the markers in CT and CBCT imaging, a two-step threshold-based segmentation procedure was performed, using the web-based medical imaging platform “Nora Imaging” (www.nora-imaging.com).24 In a first step, the window width was set such that all soft tissue surrounding the markers was assigned a grayscale value of 0. In a second step, the lower level of this window was applied as threshold to segment the voxels representing the marker volume, with a constant threshold value used for all images acquired with the same protocol/modality (CTsoft tissue, CTbone, CBCT).
In order to compare the visibility of SAIB/x-SAIB to the visibility of titanium clips, the segmentable volume of titanium ligature clips (Ligaclip Extra Titanium Medium; Ethicon Endo-Surgery, Cincinnati, Ohio, United States) was determined the same way.
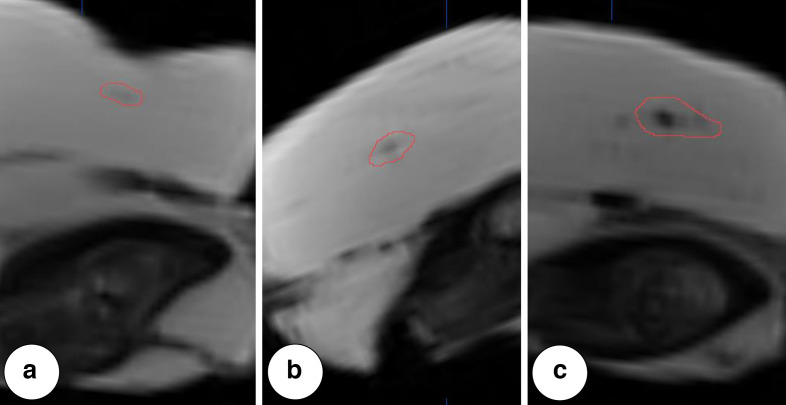
To analyse the visibility of the markers in MRI, the CTsoft tissue images were registered onto the MR images, applying a bone fusion registration based on mutual information using the iPlan CMF (Brainlab, Munich, Germany) planning software. This allowed for displaying the outline of the segmented marker volume from CT imaging into the MR images and thus reliable identification of the hypointensities representing the markers (Figure 2). For objective quantification of visibility, the signal intensity of these hypointensities was compared to the signal intensity of the surrounding tissue. Marker visibility was rated as poor, if the signal intensity at the centre of the marker was 95–80% the signal intensity of the surrounding tissue, rated as moderate if it was 80–60% the signal intensity of the surrounding tissue and rated as good, if it was <60% the signal intensity of the surrounding tissue. If the signal intensity at the centre of the marker was >95% the signal intensity of the surrounding tissue, the marker was rated as not visible.
Figure 2.
Examples of markers with visibility graded as (A) poor, (B) moderate and (C) good in T1W MRI. Outlined contour indicating marker volume as segmented in CT imaging.
Statistical analysis
For descriptive analysis, mean values, standard deviations and 95% CIs were calculated for the volumes segmented from CT and CBCT.
To objectively quantify the difference in size of markers created by the injection of a given volume, the norm intervals (mean ± 1.96*SD) for each injection volume and imaging modality were computed. Based on these intervals, the difference (∆) between the lower interval limit of the upper injection volumes compared to the upper interval limit of the lower injection volumes was calculated. Afterwards, the probability that an observation of the upper injection volume is greater than a border line (defined as interval limit of the upper injection volume - ∆/2) between lower and upper injection limit was computed.
Since the norm interval contains 97.5% of the observations, a reliable differentiation between the respective marker volumes is possible for values > 97.5% (Table 2).
Table 2.
Calculation of difference in segmentable volume between markers created by a given injection volume
| 10 µl | 20 µl | 30 µl | 40 µl | |
|---|---|---|---|---|
| 20 µl | ||||
| CT soft tissue | 95.70% | |||
| CT bone | 93.93% | |||
| CBCT | 90.65% | |||
| 30 µl | ||||
| CT soft tissue | 99.03% | 85.73% | ||
| CT bone | 99.11% | 83.73% | ||
| CBCT | 97.49% | 84.34% | ||
| 40 µl | ||||
| CT soft tissue | 99.74% | 94.77% | 73.87% | |
| CT bone | 99.47% | 92.33% | 73.42% | |
| CBCT | 99.22% | 94.52% | 76.75% | |
| 50 µl | ||||
| CT soft tissue | 99.73% | 97.49% | 89.67% | 79.12% |
| CT bone | 99.88% | 97.76% | 89.89% | 75.97% |
| CBCT | 99.28% | 96.84% | 89.00% | 75.69% |
Results for computation of the probability that an observation of the upper injection volume is greater than a border line between lower and upper injection limit.
With norm intervals containing 97.5% of observations, reliable differentiation between marker volumes is possible for values > 97.5%.
Results
Technical procedure
In our study, the technical feasibility of radiopaque and MRI-visible marking of oral soft-tissue resection surfaces using SAIB/x-SAIB could be demonstrated. This technique was found to allow for the simple creation of a high number of radiopaque and MRI-visible markers within a short period of time.
Marker visibility in CT and CBCT
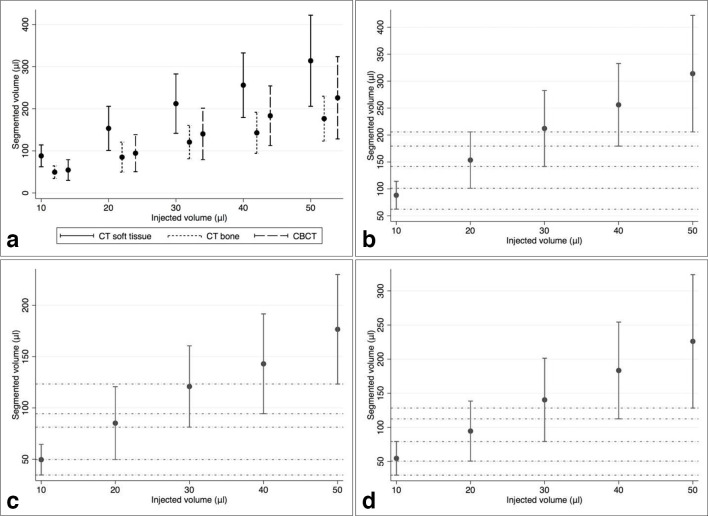
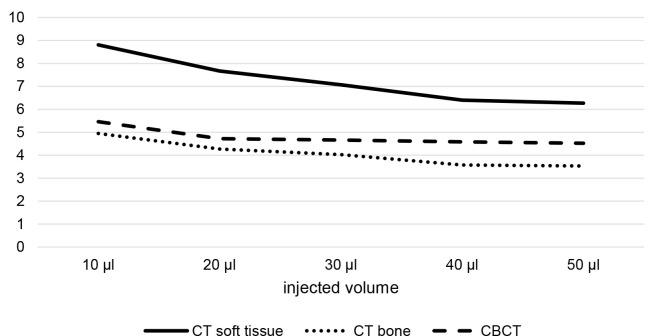
A total of 200 markers were placed in 20 porcine mandible segments, injecting volumes of 10–50 µl of BioXmark® (Nanovi, Kgs. Lyngby, Denmark). An overview of the segmented marker volume resulting from the different injection volumes in CT and CBCT is provided in Figure 3 and Table 3.
Figure 3.
(A) Overview of marker volumes segmented from CT and CBCT imaging and marker volumes segmented in (B) soft tissue kernel CT, (C) bone kernel CT and (D) CBCT. Dots are indicating mean values, vertical lines are indicating norm intervals and horizontal lines in B, C and D visualize difference in size between markers.
Table 3.
Overview of segmented marker volume
| Injected volume | 10 µl | 20 µl | 30 µl | 40 µl | 50 µl | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imaging modality | CTst | CTb | CBCT | CTst | CTb | CBCT | CTst | CTb | CBCT | CTst | CTb | CBCT | CTst | CTb | CBCT |
| Mean segmented volume (µl) | 88.18 | 49.55 | 54.65 | 153.47 | 85.15 | 94.55 | 212.12 | 120.86 | 140.32 | 256.03 | 142.94 | 183.38 | 313.93 | 176.61 | 226.03 |
| SD segmented volume | 13.23 | 7.62 | 12.58 | 26.68 | 18.14 | 22.43 | 35.98 | 20.22 | 31.17 | 39.08 | 24.80 | 36.16 | 55.20 | 27.21 | 49.83 |
| 95% CI segmented volume | 69.80– 113.40 |
37.80– 62.70 |
34.25– 73.30 |
111.80– 195.65 |
50.65– 113 |
57.80– 128.95 |
152.05– 262.95 |
88.05– 157.45 |
87.10– 177.65 |
186.75– 326.95 |
101.80– 192.75 |
134.45– 241 |
203.95– 406.3 |
124.3– 222.25 |
150.6– 314.05 |
| Ratio Segmented volume/ injected volume |
8.81 | 4.95 | 5.46 | 7.67 | 4.26 | 4.72 | 7.07 | 4.02 | 4.67 | 6.40 | 3.57 | 4.58 | 6.27 | 3.53 | 4.52 |
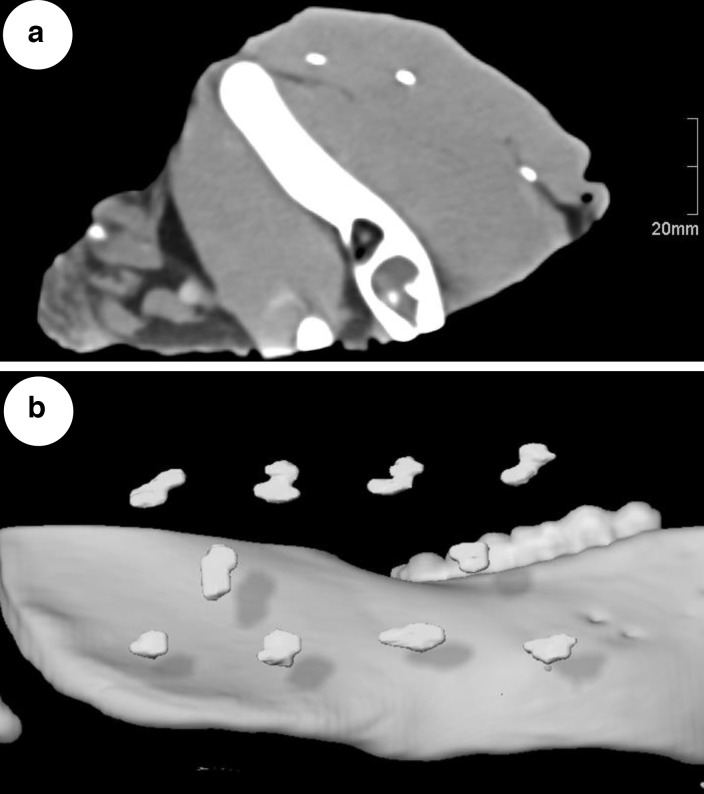
Even the lowest volume of SAIB/x-SAIB investigated (10 µl) resulted in a higher segmentable volume than observed with the incorporation of titanium clips (CTSoft tissue: 50.15 ± 7.50 mm3; CTBone: 23.90 ± 3.39 mm3; CBCT: 33.80 ± 9.20 mm3), thus providing excellent visibility (Figure 4).
Figure 4.
(A) Slice from soft tissue kernel CT, showing three markers resulting from injection of 10 µl of SAIB/x-SAIB each. (B) 3D-reconstruction of porcine mandible with segmented markers resulting from 10 µl injections of SAIB/x-SAIB.
With an increasing injection volume, an increase in the variance of the resulting segmentable volumes was observed (Figure 3). At the same time, increasing the injection volume resulted in a decrease of the ratio between segmented volume and injected volume (Figure 5).
Figure 5.
Ratio of segmented volume to injected volume in CT soft tissue kernel, CT bone kernel and CBCT.
Reliable differentiation is possible between markers created by the injection of 10 µl and markers created by the injection of ≥30 µl (Figure 3 and Table 2). Differentiation is less reliable between markers created by the injection of 20 µl and higher injection volumes and not possible between markers created by the injection of 30 µl and higher injection volumes.
Marker visibility in MRI
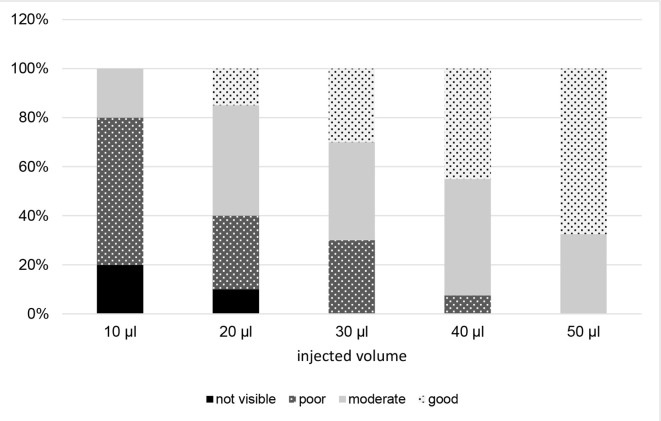
Figure 2 shows examples of markers with visibilities graded as poor, moderate and good in T1W MRI. In general, low injection volumes of SAIB/x-SAIB resulted in poorly or not visible structures in MRI. However, the ratio between the signal intensity of the marker and the signal intensity of the surrounding tissue showed a decrease with increasing injection volumes, resulting in 67.5% of the 50 µl injections providing good visibility in MRT1 (Figure 6).
Figure 6.
Visibility of markers in T1W MRI.
Discussion
Various studies have confirmed an improved accuracy of breast radiotherapy in patients with fiducial markers placed intraoperatively to facilitate postoperative delineation of the former tumour bed.7,25–30 Radio-opaque markers placed in the excision cavity can provide valuable additional localization information compared to (CT) imaging alone, significantly improving (inter-)observer consistency.26,31
It has been demonstrated, that implanting titanium surgical clips is a feasible approach for postoperative identification of the tumour resection surface in oral cancer patients as well: Once the results of intraoperative frozen section analysis have confirmed complete resection of the tumour, the surgeon can place the desired number of clips to mark the tumour bed. Subsequently to free flap reconstruction or full thickness closure of soft-tissue resection defects, this can help significantly in delineating the interface between the tumour resection border and native/reconstructed tissue in postoperative imaging.10,11
However, marking the resection surface with titanium clips goes along with shortcomings: If placed superficially in the oral cavity, there is a risk of detaching and subsequent aspiration of the clips.10,11 Moreover, migration of metallic clips may occur, potentially reducing the accuracy of the marking procedure and artefacts from metal-based markers might impair their identification on postoperative imaging.18,20,22,32,33 From a technical standpoint, placement of the desired number of clips can be impeded by the imperative to avoid intraoperative delay.10,11
The liquid, biocompatible marker BioXmark® (Nanovi, Kgs. Lyngby, Denmark) has been reported to provide a fast, safe and reliable alternative to create fiducial markers.18,22 When delineating the three-dimensional tumour resection surface in postoperative imaging, distances to be interpolated between fiducial markers should be low.11 Thus, placing a high number of markers helps to accurately identify the resection surface. Our preclinical investigation could demonstrate the feasibility of using a high number of low injection volumes of the liquid fiducial marker to mark oral soft tissue resection surfaces subsequently covered with muscle tissue to simulate defect closure. Injecting the marker superficially at a depth of 1–2 mm, a low amount of backflow of the liquid marker through the injection channel was observed, as reported by other investigators as well.22 However, the authors do not consider this a disadvantage; directly upon injecting the marker into the soft-tissue, viscosity of the marker will begin to increase due to the efflux of ethanol. This process is accompanied by tarnishing of the liquid (Figure 1A), helping in visually controlling the marking procedure.
In the literature, there is information available on SAIB/x-SAIB investigated in volumes as low as 10 µl and up to 300 µl per injection, with a relatively low number of injections performed in most cases.18,21,22 With the different organs the marker has been investigated in and its performance suspected to be influenced by the environment it is injected into, as well as the different imaging protocols used, it is difficult to directly compare the findings reported in the literature or to transfer them to other settings.18,21 In general however, good visibility is reported for all the volumes investigated in the literature in CT and CBCT sequences used clinically, with an increase in (hardening) artefacts reported with larger injection volumes (≥50 µl).18,19,21,22,34 The markers are reported to show good continuous radiopacity in CT and CBCT for up to 36 months without migration relative to the injection site.19,34 Reduction in marker size of approximately 35% has been found after 9 months due to degradation.19
Performing a high number of low-dose injections, the aim of this study was to investigate the minimum dose required to create markers with good visibility in imaging modalities used clinically, as a low injection volume per marker would allow for an increased total number of markers and thus more detailed delineation of the three-dimensional resection surface in postoperative imaging. Moreover, smaller volumes of the marker result in more homogeneous structures (Figure 4B), making the marking procedure more accurate and furthermore cause less hardening artefacts.
Besides of the minimum amount of SAIB/x-SAIB required to produce markers reliably detectable in imaging, our study was aiming at investigating the variance of marker volumes in imaging resulting from a defined injection volume. While titanium ligature clips can define borders of excised tissue, they cannot provide any information on the distance between the tumour and the excision border in postoperative imaging.10,11 Information on the difference in injection volume required to produce markers that can reliably be differentiated by their visible size in imaging would facilitate incorporating pathohistological information on high-risk regions obtained from frozen section analysis (e.g., close margin or R1-resection) by marking these specific regions with a volume different from the volume used to mark the tumour resection surface in general. This way, high-risk regions can subsequently be identified in postoperative imaging during RT-planning, providing the opportunity of planning the delivery of a radiation “boost” to improve postoperative tumor control.
Visibility of markers created by the injection of SAIB/x-SAIB has been evaluated in the literature by determining their diameter, volume or applying a grading scale.18,19,22,35 Due to the non-spherical shape of the markers and their evaluation in three-dimensional imaging, the authors consider the segmentable marker volume to be the most appropriate parameter. Our findings on the ratio of segmented volume to injected volume are comparable to the findings of Schneider et al, who applied a similar segmentation approach.18 Comparing a high number of markers, this study could demonstrate injection volumes as low as 10 µl to provide excellent visibility in CT and CBCT imaging. Even considering the decrease in visible marker size of 35% reported within a period of 9 months due to degradation,19 an injected volume of 10 µl would still provide a visibility comparable to titanium ligature clips. The best visibility of the marker was found in soft tissue kernel CT, which is the modality used routinely for RT treatment planning. Markers created by the injection of 10 µl can be reliably distinguished from markers created by the injection of ≥30 µl, thus providing the option to use this difference to incorporate pathohistological information into the patients imaging.
Besides of the visibility in radiographical imaging, the aim of this study was to investigate the visibility of SAIB/x-SAIB in MRI, as this is an imaging modality gaining increasing attention in head and neck cancer RT treatment planning and setup and MR-based RT (MR-Linac) represents a promising new option in radiation therapy.36–38 Due to its composition, the marker appears as hypointense structure (signal void) in MRI, making identification more difficult in this modality. However, with the application of higher injection volumes, visibility gradually increased, resulting in in good visibility of 67.5% of the markers created by the injection of 50 µl. These findings on MRI visibility are in accordance with the literature: Detectability of the individual marker deposits on T1W and T2W imaging has been described as being challenging without information from the corresponding CT-images due to signal voids from tissue heterogeneity or air cavities.18,34 Increased MRI visibility is reported with higher marker volumes (>50 µl) and lower slice thickness (<3 mm).18,22
Coating (”painting”) the tumour cavity with SAIB/x-SAIB has been reported as an alternative to injecting the marker.16 Our main concern with this approach is the fact that degradation of the marker during the postoperative course will result in structures not clearly identifiable as the marker in CT and CBCT, potentially leading to misinterpretation as local pathological processes. Furthermore, while identifying the marker on MRI can be challenging in case of (low dose) injection, it would most likely not be possible at all with a “painting” approach. Moreover, the “painting” approach excludes applying different marker volumes to incorporate pathohistological information (e.g., location of high risk regions) obtained during surgery.
The authors thus consider injection of a high number of low doses of the marker to be the most reliable approach to identify the tumour resection surface in postoperative imaging, especially on the long term.
Conclusion
It can be concluded from this investigation that SAIB/x-SAIB provides a fast and reliable way to intraoperatively mark the tumour resection surface in oral cancer patients. Injection volumes as low as 10 µl result in markers with a visibility comparable to the visibility of titanium ligature clips in CT and CBCT. As CT imaging represents the standard in oral cancer RT planning, this is the modality with the highest relevance for excellent delineation of the tumour resection surface in daily clinical routine. MRI can provide valuable additional information, however, it is not used routinely in oral cancer RT planning. If visibility of SAIB/x-SAIB is desired in this modality, higher volumes should be injected.
In the past, promising results have been obtained at the authors’ department using titanium clips for the marking of the tumour resection surface.10,11 It is the authors’ belief that the improvements in the marking procedure made possible by the results obtained in this investigation of the novel liquid marker will help considerably in the adjuvant radiation treatment of oral cancer patients.
Fusing different CT images acquired during the follow-up course, the markers might also help to identify local structural changes of the tumour resection surface, potentially suggestive for recurrent cancer. Moreover, the liquid fiducial marker might be used to preoperatively mark the intraorally visible tumour extent for visualization in imaging to facilitate targeted neoadjuvant RT and planning of image-guided surgical resection.
Footnotes
Acknowledgements: The authors would like to thank Nanovi, Kgs. Lyngby, Denmark, for providing the amount of BioXmark® required to perform this investigation.
Competing interests: The authors declare that they have no competing interests.
Funding: This research was financially supported by the University of Freiburg Faculty of Medicine Research Committee.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 2018: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Chow LQM. Head and neck cancer. N Engl J Med 2020; 382: 60–72. doi: 10.1056/NEJMra1715715 [DOI] [PubMed] [Google Scholar]
- 3.Robert Koch Institut Bericht zum Krebsgeschehen in Deutschland 2016 [Internet]. 2016. Available from: https://edoc.rki.de/handle/176904/3264 [2020 Feb 23].
- 4.AWMF S3-Leitlinie Mundhöhlenkarzinom, Diagnostik und Therapie. [Internet]. 2012. Available from: https://www.awmf.org/leitlinien/detail/ll/ 007-100OL.html [2019 Oct 12].
- 5.Lundahl RE, Foote RL, Bonner JA, Suman VJ, Lewis JE, Kasperbauer JL, et al. Combined neck dissection and postoperative radiation therapy in the management of the high-risk neck: a matched-pair analysis. Int J Radiat Oncol Biol Phys 1998; 40: 529–34. doi: 10.1016/S0360-3016(97)00817-1 [DOI] [PubMed] [Google Scholar]
- 6.Lavaf A, Genden EM, Cesaretti JA, Packer S, Kao J. Adjuvant radiotherapy improves overall survival for patients with lymph node-positive head and neck squamous cell carcinoma. Cancer 2008; 112: 535–43. doi: 10.1002/cncr.23206 [DOI] [PubMed] [Google Scholar]
- 7.Harrington KJ, Harrison M, Bayle P, Evans K, Dunn PA, Lambert HE, et al. Surgical clips in planning the electron boost in breast cancer: a qualitative and quantitative evaluation. Int J Radiat Oncol Biol Phys 1996; 34: 579–84. doi: 10.1016/0360-3016(95)02090-X [DOI] [PubMed] [Google Scholar]
- 8.Geretschläger A, Bojaxhiu B, Crowe S, Arnold A, Manser P, Hallermann W, et al. Outcome and patterns of failure after postoperative intensity modulated radiotherapy for locally advanced or high-risk oral cavity squamous cell carcinoma. Radiat Oncol 2012; 7: 175. doi: 10.1186/1748-717X-7-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bittermann G, Voss P, Duttenhoefer F, Zimmerer R, Vach K, Metzger MC. The validity of surgical clips as radiographic markers for the tumour resection cavity in head and neck cancer treatment. J Craniomaxillofac Surg 2015; 43: 758–62. doi: 10.1016/j.jcms.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 10.Bittermann G, Wiedenmann N, Bunea A, Schwarz SJ, Grosu A-L, Schmelzeisen R, et al. Clipping of tumour resection margins allows accurate target volume delineation in head and neck cancer adjuvant radiation therapy. Radiother Oncol 2015; 116: 82–6. doi: 10.1016/j.radonc.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 11.Bittermann G, Wiedenmann N, Voss P, Zimmerer R, Duttenhoefer F, Metzger MC. Marking of tumor resection borders for improved radiation planning facilitates reduction of radiation dose to free flap reconstruction in head and neck cancer surgery. J Craniomaxillofac Surg 2015; 43: 567–73. doi: 10.1016/j.jcms.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 12.Kirova YM, Fournier-Bidoz N, Servois V, Laki F, Pollet GA, Salmon R, et al. How to boost the breast tumor bed? A multidisciplinary approach in eight steps. Int J Radiat Oncol Biol Phys 2008; 72: 494–500. doi: 10.1016/j.ijrobp.2007.12.059 [DOI] [PubMed] [Google Scholar]
- 13.Furet E, Peurien D, Fournier-Bidoz N, Servois V, Reyal F, Fourquet A, et al. Plastic surgery for breast conservation therapy: how to define the volume of the tumor bed for the boost? Eur J Surg Oncol 2014; 40: 830–4. doi: 10.1016/j.ejso.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 14.Bedwinek J. Breast conserving surgery and irradiation: the importance of demarcating the excision cavity with surgical clips. Int J Radiat Oncol Biol Phys 1993; 26: 675–9. doi: 10.1016/0360-3016(93)90287-6 [DOI] [PubMed] [Google Scholar]
- 15.Habermehl D, Henkner K, Ecker S, Jäkel O, Debus J, Combs SE. Evaluation of different fiducial markers for image-guided radiotherapy and particle therapy. J Radiat Res 2013; 54 Suppl 1(Suppl 1): i61–8. doi: 10.1093/jrr/rrt071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciernik IF, Greiss AM. Visualization of the tumor cavity after lumpectomy of breast cancer for postoperative radiotherapy. Clin Transl Radiat Oncol 2019; 14: 47–50. doi: 10.1016/j.ctro.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherman Rydhög J, Irming Jølck R, Andresen TL. Munck AF Rosenschöld P. quantification and comparison of visibility and image artifacts of a new liquid fiducial marker in a lung phantom for image-guided radiation therapy: a new liquid fiducial marker for IGRT. Med Phys 2015; 42: 2818–26. [DOI] [PubMed] [Google Scholar]
- 18.Schneider S, Aust DE, Brückner S, Welsch T, Hampe J, Troost EGC, et al. Detectability and structural stability of a liquid fiducial marker in fresh ex vivo pancreas tumour resection specimens on CT and 3T MRI. Strahlenther Onkol 2019; 195: 756–63. doi: 10.1007/s00066-019-01474-1 [DOI] [PubMed] [Google Scholar]
- 19.de Blanck SR, Rydhög JS, Larsen KR, Clementsen PF, Josipovic M, Aznar MC, et al. Long term safety and visibility of a novel liquid fiducial marker for use in image guided radiotherapy of non-small cell lung cancer. Clin Transl Radiat Oncol 2018; 13: 24–8. doi: 10.1016/j.ctro.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobiasch S, Kampfer S, Burkhardt R, Schilling D, Schmid TE, Wilkens JJ, et al. BioXmark for high-precision radiotherapy in an orthotopic pancreatic tumor mouse model : Experiences with a liquid fiducial marker. Strahlenther Onkol 2017; 193: 1039–47. doi: 10.1007/s00066-017-1193-y [DOI] [PubMed] [Google Scholar]
- 21.Rydhög JS, Mortensen SR, Larsen KR, Clementsen P, Jølck RI, Josipovic M, et al. Liquid fiducial marker performance during radiotherapy of locally advanced non small cell lung cancer. Radiother Oncol 2016; 121: 64–9. doi: 10.1016/j.radonc.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 22.Machiels M, Voncken FEM, Jin P, van Dieren JM, Bartels-Rutten A, Alderliesten T, et al. A novel liquid Fiducial marker in esophageal cancer image guided radiation therapy: technical feasibility and visibility on imaging. Pract Radiat Oncol 2019; 9: e506–15. doi: 10.1016/j.prro.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 23.Scherman Rydhög J, Riisgaard de Blanck S, Josipovic M, Irming Jølck R, Larsen KR, Clementsen P, et al. Target position uncertainty during visually guided deep-inspiration breath-hold radiotherapy in locally advanced lung cancer. Radiotherapy and Oncology 2017; 123: 78–84. doi: 10.1016/j.radonc.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 24.Anastasopoulos C, Reisert M, Kellner E. “Nora Imaging”: A Web-Based Platform for Medical Imaging. Neuropediatrics [Internet]. 2017. Available from: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0037-1602977 [2020 Mar 3].
- 25.Coles CE, Wilson CB, Cumming J, Benson JR, Forouhi P, Wilkinson JS, et al. Titanium clip placement to allow accurate tumour bed localisation following breast conserving surgery: audit on behalf of the import trial management group. Eur J Surg Oncol 2009; 35: 578–82. doi: 10.1016/j.ejso.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Yang TJ, Tao R, Elkhuizen PHM, van Vliet-Vroegindeweij C, Li G, Powell SN. Tumor bed delineation for external beam accelerated partial breast irradiation: a systematic review. Radiother Oncol 2013; 108: 181–9. doi: 10.1016/j.radonc.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 27.Donovan EM, Brooks C, Mitchell RA, Mukesh M, Coles CE, Evans PM, et al. The effect of image guidance on dose distributions in breast boost radiotherapy. Clin Oncol 2014; 26: 671–6. doi: 10.1016/j.clon.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 28.Krawczyk JJ, Engel B. The importance of surgical clips for adequate tangential beam planning in breast conserving surgery and irradiation. Int J Radiat Oncol Biol Phys 1999; 43: 347–50. doi: 10.1016/S0360-3016(98)00402-7 [DOI] [PubMed] [Google Scholar]
- 29.Coles CE, Harris EJ, Donovan EM, Bliss P, Evans PM, Fairfoul J, et al. Evaluation of implanted gold seeds for breast radiotherapy planning and on treatment verification: a feasibility study on behalf of the import Trialists. Radiother Oncol 2011; 100: 276–81. doi: 10.1016/j.radonc.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 30.Struik GM, Hoekstra N, Klem TM, Ghandi A, Verduijn GM, Swaak-Kragten AT, et al. Injection of radiopaque hydrogel at time of lumpectomy improves the target definition for adjuvant radiotherapy. Radiotherapy and Oncology 2019; 131: 8–13. doi: 10.1016/j.radonc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 31.van Mourik AM, Elkhuizen PHM, Minkema D, Duppen JC, van Vliet-Vroegindeweij C.Dutch Young Boost Study Group . Multiinstitutional study on target volume delineation variation in breast radiotherapy in the presence of guidelines. Radiother Oncol 2010; 94: 286–91. doi: 10.1016/j.radonc.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 32.Rosen EL, Vo TT. Metallic clip deployment during stereotactic breast biopsy: retrospective analysis. Radiology 2001; 218: 510–6. doi: 10.1148/radiology.218.2.r01fe39510 [DOI] [PubMed] [Google Scholar]
- 33.Kass R, Kumar G, Klimberg VS, Kass L, Henry-Tillman R, Johnson A, et al. Clip migration in stereotactic biopsy. Am J Surg 2002; 184: 325–31. doi: 10.1016/S0002-9610(02)00952-2 [DOI] [PubMed] [Google Scholar]
- 34.de Blanck SR, Scherman-Rydhög J, Siemsen M, Christensen M, Baeksgaard L, Irming Jølck R, et al. Feasibility of a novel liquid fiducial marker for use in image guided radiotherapy of oesophageal cancer. Br J Radiol 2018; 91: 20180236. doi: 10.1259/bjr.20180236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherman Rydhög J, Perrin R, Jølck RI, Gagnon-Moisan F, Larsen KR, Clementsen P, et al. Liquid fiducial marker applicability in proton therapy of locally advanced lung cancer. Radiother Oncol 2017; 122: 393–9. doi: 10.1016/j.radonc.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 36.Largent A, Marage L, Gicquiau I, Nunes J-C, Reynaert N, Castelli J, et al. Head-And-Neck MRI-only radiotherapy treatment planning: from acquisition in treatment position to pseudo-CT generation. Cancer Radiother 2020; 24: 288–97. doi: 10.1016/j.canrad.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 37.Chen AM, Hsu S, Lamb J, Yang Y, Agazaryan N, Steinberg ML, et al. Mri-Guided radiotherapy for head and neck cancer: initial clinical experience. Clin Transl Oncol 2018; 20: 160–8. doi: 10.1007/s12094-017-1704-4 [DOI] [PubMed] [Google Scholar]
- 38.Alongi F, Rigo M, Figlia V, Cuccia F, Giaj-Levra N, Nicosia L, et al. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat Oncol 2020; 15: 69. doi: 10.1186/s13014-020-01510-w [DOI] [PMC free article] [PubMed] [Google Scholar]