Abstract
Background
Perioperative neurocognitive disorders (PND) are common complications in older adults associated with increased 1-year mortality and long-term cognitive decline. One risk factor for worsened long-term postoperative cognitive trajectory is the Alzheimer’s Disease (AD) genetic risk factor APOE4. APOE4 is thought to elevate AD risk partly by increasing neuroinflammation, which is also a theorized mechanism for PND. Yet, it is unclear whether modulating apoE4 protein signaling in older surgical patients would reduce PND risk or severity.
Objectives
MARBLE is a randomized, blinded, placebo-controlled phase II sequential dose escalation trial designed to evaluate perioperative administration of an apoE mimetic peptide drug, CN-105, in older adults (age ≥60 years). The primary aim is evaluating the safety of CN-105 administration, as measured by adverse event (AE) rates in CN-105 versus placebo-treated patients. Secondary aims include assessing perioperative CN-105 administration feasibility and its efficacy for reducing postoperative neuroinflammation and PND severity.
Methods
201 patients undergoing non-cardiac, non-neurological surgery will be randomized to control or CN-105 treatment groups and receive drug or placebo before and every six hours after surgery, for up to three days after surgery. Chart reviews, pre- and postoperative cognitive testing, delirium screening, and blood and CSF analyses will be performed to examine effects of CN-105 on perioperative adverse event rates, cognition, and neuro-inflammation. Trial results will be disseminated by presentations at conferences and peer-reviewed publications.
Conclusion
MARBLE is a transdisciplinary study designed to measure CN-105 safety and efficacy for preventing PND in older adults and to provide insight into the pathogenesis of these geriatric syndromes.
KEY WORDS (MESH DATABASE): Alzheimer’s Disease, Apolipoprotein E4, Apolipoprotein E, Neurocognitive Disorders, Delirium, Inflammation, Surgery
INTRODUCTION
Postoperative cognitive dysfunction (POCD; also known as Neurocognitive Disorder-Postoperative, or NCD) and delirium each occur in up to 40% of the ≥16 million adults age ≥60 who undergo surgery each year, and both are associated with decreased quality of life, increased one-year mortality, and a possible increased dementia risk [1–7]. Mild NCD refers to a 1 or 2 standard deviation cognitive decline (either from before to after surgery, or in comparison to population norms) that occurs between 1–12 months after surgery, coupled with either a subjective cognitive complaint (for mild NCD) or inability to care for oneself (NCD major) [8]. These perioperative neurocognitive disorders [8] (PND) pose a mounting public health concern as increasing numbers of older adults undergo surgery [1]. While there are several behavioral interventions such as the Hospital Elder Life Program (HELP) [9] and the ABCDEF bundle [10] that have demonstrated efficacy for reducing delirium incidence, there are no currently FDA-approved drugs for preventing delirium or other types of PND, likely due to our poor understanding of their pathogenesis.
Two mechanisms hypothesized to underlie PND are neuro-inflammation and exacerbation of pre-existing AD pathology. The role of neuro-inflammation in PND is supported by animal studies revealing postoperative increases in brain inflammatory cytokines [11] and microglial activation [12], and human studies demonstrating postoperative CSF inflammatory cytokine increases [13–15]. Further, some surgical patients display postoperative increases in blood and CSF tau and amyloid beta (Aβ) levels and the tau/amyloid beta (Aβ) ratio [14, 16], similar to alterations seen in patients with AD [17–19]. Low preoperative CSF and plasma Aβ levels and elevated preoperative CSF tau/Aβ have also been associated with increased POCD incidence [18, 20] and severity [21], respectively. Thus, AD neuropathology increases PND risk, and PND may be associated with postoperative worsening of AD pathology. Additionally, the relationship between AD pathology and neuro-inflammation is complex: neuro-inflammation plays both pathogenic and protective roles in AD [22–24], and may interact with AD pathology in PND pathogenesis, suggesting an ideal PND prevention drug may need to act on both of these processes.
One potential target implicated in both neuro-inflammation and AD pathology is the late-onset AD genetic risk factor APOE4 [25], whose protein product apoE4 increases glial activation and pro-inflammatory cytokine levels in animal models [26–29]. Human APOE4 carriers have elevated systemic IL-1β, IL-6, and TNFα levels and increased postoperative IL-1β levels [30, 31], increased Aβ and tau pathology [32, 33], and have worsened long term cognitive trajectories after surgery [34, 35]. In murine models, blocking apoE4 with the apoE mimetic peptide drug CN-105 reduced neuro-inflammation and improved cognitive, neurobehavioral, and motor outcomes in traumatic brain injury, ischemic stroke, and cerebral hemorrhage models [36–38]. Additionally, a phase I trial found that CN-105 was safe at doses up to 20 times greater than those given in preclinical studies, with no serious adverse events (SAEs) [39]. Based on these data, we hypothesize that CN-105 will be safe and effective for preventing PND in older adults. Thus, we have initiated MARBLE, a phase II randomized controlled trial (RCT) to determine the safety, feasibility and efficacy of perioperative CN-105 treatment in older surgical patients at risk for PND.
MATERIALS AND METHODS
Overview
MARBLE is a phase II escalating dose RCT that is registered with clinicaltrials.gov (NCT03802396). Since the MARBLE study drug (CN-105) was developed and patented by Duke University, MARBLE is overseen by an external IRB (Western Institutional Review Board) and monitored by an external Data and Safety Monitoring Board (DSMB+) comprised of five investigators external to Duke with no COI relative to CN-105 or AegisCN. In line with NIH recommendations, every attempt is being made to enroll a diverse group of study patients, in terms of race, ethnicity, as well as socioeconomic status in the MARBLE study, such that the results will be as broadly generalizable to the larger population of older adults as possible.
Eligibility
MARBLE has currently enrolled 40 of its 201 patient target. Inclusion criteria are: English-speaking, age ≥ 60 years old, and scheduled to undergo non-cardiac/non-neurologic surgery of ≥ 2 hours with a planned postoperative hospital admission at Duke University Hospital. Duke University Hospital is a tertiary care academic medical center. Eligible patients are informed about the study via brochures (distributed to the preoperative screening and surgical clinics), phone calls as well as electronic messages sent to their online medical charts in accord with Duke’s patient recruitment and engagement policy for clinical research [40]. Exclusion criteria include incarceration, planned systemic chemotherapy between the baseline and six week postoperative study visits, and inability to undergo lumbar punctures, e.g. due to anticoagulation [41]. There are no preoperative cognitive exclusion criteria; secondary analyses will be performed on patients stratified by preoperative cognitive function. Participants who undergo significant head trauma between the baseline and six week postoperative study visits will be withdrawn from the study due to the confounding effects of head trauma on cognition. Written informed consent is obtained from all patients or their legally-authorized representatives before participation.
Study Intervention
After enrollment, participants are randomized to receive CN-105 or placebo at a 3:1 ratio. There are three successive escalating dose groups of 67 patients; in each group, 50 patients receive CN-105 and 17 receive placebo. CN-105 dosage is 0.1 mg/kg, 0.5 mg/kg and 1 mg/kg in the three groups, respectively. After complete enrollment for each dose level, the DSMB+ reviews the data and makes a recommendation (based on safety data in that dose level) for whether the study should stopped or not. For each patient, CN-105 or placebo is given by intravenous infusion within one hour before the scheduled or actual surgery start and then every six hours ± 90 minutes after the start of surgery. Patients continue receiving drug (or placebo) until discharge orders are placed or until three days after surgery, whichever occurs first, for up to thirteen doses per patient. The participants, study team and hospital nurses are blinded to treatment randomization (i.e. to active drug vs placebo), thus the study is triple blinded. Randomization is tracked by the Duke hospital investigational drug service, and was designed electronically by an independent staff statistician.
Safety and Feasibility Assessments
Safety of perioperative CN-105 administration is assessed by AE rates in drug vs placebo-treated patients. Common terminology for classifying adverse events (CTCAE) criteria are used to assess and classify SAEs and AEs [42]. AEs are monitored by study staff that review participants’ clinical records, and by noting any symptoms reported directly by the participants to the study staff. All SAEs, even if unrelated to the intervention, are reported to the IRB in accordance with IRB and DSMB-designated reporting schedules. Since inflammation plays a role in both wound healing and postoperative infections, and CN-105 has been shown to modulate inflammation in animal studies [38], particular attention is payed to assessing for infection or delayed wound healing as AEs. As discussed above, our DSMB reviews the unblinded AE rate information after the enrollment of each 67 patient group (50 patients who receive drug, 17 who receive placebo), and then makes a determination whether the study should be stopped or not (see section below on stopping rules). The primary study team, patients and the PI remain blinded while the DSMB conducts these reviews of the un-blinded AE rate information.
The feasibility of perioperative CN-105 administration is assessed by tracking the percentage of doses given within the correct time window (i.e. within 1 hour prior to the scheduled or actual start time of the surgery, and within a +/− 90 minute time window for subsequent doses, which are administered every 6 hours after the start of surgery).
At study completion, and after un-blinding, we plan on assessing the characteristics of CN-105 treated patients who had AE’s, particularly if overall AE rates or specific types of AEs are more common in drug vs placebo treated patients in this study. This analysis could potentially identify predictors of drug-related AEs if they occur, such as potential drug-drug interactions between CN-105 and other medications administered to study participants. Identifying such drug-drug interactions, if they occur, would be important given overall concerns about the detrimental effects of polypharmacy in older adults [43].
Blood and CSF Sampling, Assays
Participants undergo baseline CSF and blood sampling within two months prior to surgery; repeat CSF and blood samples are obtained 24 ± two hours after the start of surgery, and six ± three weeks after surgery. Blood samples are centrifuged to separate plasma from the red cell pellet and buffy coat. CSF samples obtained using a 25 or 27 g pencil point needle, after topical benzocaine spray is sprayed on the patient’s back and allowed to soak in for 10 minutes. Then, up to 5 ml of 1–2% lidocaine are injected at the planned LP site, and two minutes is allowed to elapse before the LP needle itself is inserted, to allow the lidocaine to begin to work. We have recently shown that this protocol is effective for minimizing pain and adverse events after lumbar punctures[44]. CSF samples are then centrifuged to obtain cell pellets, which are cryopreserved and stored according to our recently published protocol [45] for future studies. Blood components and CSF supernatant aliquots are stored at −80 degrees C. CSF specimens are used to determine the effects of CN-105 treatments on levels of the cytokines IL-6, IL-8, G-CSF, and MCP-1 using Meso Scale Discovery multiplex assays [46]. Complete blood counts with differentials and serum chemistries are also obtained before and 24 hours and six weeks after surgery, to evaluate potential off-target effects of CN-105. Blood samples are used for APOE genotyping [16]. These genotyping results will be used to perform stratified exploratory analyses to examine whether CN-105 treatment efficacy varies by APOE genotype.
Cognitive Testing, NCD Assessment
Cognition is assessed with a standard test battery [47, 48] (Table 2), by staff trained by a board-certified neuropsychologist within two months before and again six weeks after surgery. Individual test scores will be combined by factor analysis into cognitive domain factors, as previously described [2]. Our prior experience is that this approach typically results in a four factor solution that accounts for the vast majority of the variance in the individual cognitive battery test scores [2]. The mean of these cognitive domain factor scores yields the Continuous Cognitive Index (CCI), a sensitive score used to quantify overall cognitive function which our group has used in multiple studies over the past ~20 years [2, 47–49]. CCI change from before to after surgery thus quantifies the degree of learning/cognitive improvement or cognitive decline. CCI change from before to after surgery will be compared between drug vs placebo treated patients as a secondary outcome, because this continuous measure (CCI change) provides significantly greater statistical power for determining differences between groups than comparing the between-groups difference in the incidence of a dichotomous outcome (such as the absence vs presence of NCD postoperative, mild or major). Further, examining the difference in CCI change between randomized groups (i.e. CN-105 vs placebo-treated) estimates the treatment effect of CN-105 after controlling for regression to the mean (since regression to the mean for cognitive test results over time should be similar in both groups), as described in [50]. Since the main purpose of this study is to simply compare outcomes (such as postoperative changes in cognition) between drug treated vs non-drug treated groups, this study does not contain a control group of non-surgical individuals.
Table 2.
The MARBLE Cognitive Testing Battery.
| Test | Cognitive Function Assessed |
|---|---|
| Brief Visuospatial Memory Test, Revised | Visuospatial learning and recall |
| Controlled Oral Word Association Test | Verbal Fluency and Information Retrieval |
| Hopkins Verbal Learning Test, Revised | Auditory learning and verbal recall |
| Lafayette Grooved Pegboard Test | Manual dexterity and motor speed |
| Montreal Cognitive Assessment | Mild cognitive impairment screening |
| Trail Making Test, Parts A & B | Complex executive functioning skills (eg, logical task switching) |
| Wechsler Adult Intelligence Scale, 3rd Revision Digit Span Subtest | Immediate auditory-verbal recall and complex attention |
| Wechsler Adult Intelligence Scale, 3rd Revision Digit Symbol Coding Subtest | Visual scanning and visuomotor production |
| Wechsler Test of Adult Reading | Premorbid intellectual function |
Since changes in specific cognitive domains have been observed from before to after surgery [51], we will also examine the change in each the individual cognitive domain factor scores from before to six weeks after surgery between drug vs placebo treated patients. This is the first human study to measure cognitive change between CN-105 vs placebo treated patients; thus, we have no a priori hypothesis about which particular cognitive domain(s) will be affected the most or the least by CN-105 treatment. Accordingly, we will examine differences between drug vs placebo treated patients in each of the cognitive domain score changes (from before to after surgery) as an exploratory outcome.
The incidence of NCD-postoperative, mild and/or major [8], between drug- vs placebo- treated patients will also be examined as an exploratory outcome. NCD postoperative (mild) will be defined as a 1–2 standard deviation (SD) decrease in score on any one of the four cognitive domain factors (which are used in calculating the Continuous Cognitive Index as described above) combined with a subjective cognitive complaint. Major NCD, postoperative will be defined as a ≥2 SD drop in any of these four cognitive domain factors. Here, a 1 or 2 standard deviation in any one of the four cognitive domain factors refers to the standard deviation in each of these factors in the entire population under study here at the baseline/preoperative timepoint. Subjective cognitive complaints will be assessed using the Cognitive Difficulties Scale [52], which our group has previously used to examine the association between subjective and objective cognitive deficits after surgery [53]. The Cognitive Difficulties Scale is administered to MARBLE study patients both before and six weeks after surgery.
NCD postoperative (major) will be defined as a 2 SD decrease in any one of the four cognitive domain factors, combined with a postoperative deficit in ability to perform one or more activities of daily living (ADLs). Patients’ ability to perform ADL’s will be assessed using the Duke Activity Status Index [54], which is administered to MARBLE study patients both before and six weeks after surgery.
Delirium Screenings
Delirium is assessed at the initial baseline study visit and twice daily during postoperative days one through day five using the 3D-CAM in non-intubated patients and the CAM-ICU in intubated patients [55, 56]. Delirium assessors are trained with materials from the Hebrew SeniorLife Program/Harvard Medical School SAGES study group [57], and begin conducting delirium assessments on study patients only once they demonstrate ≥90% agreement with standardized training assessments. To date, the accuracy of our delirium assessors measured using these standardized video-taped assessments [57] is 93%, and all assessors receive feedback to further improve their accuracy after this process. Delirium severity will be assessed by the 3D-CAM as well [58].
EEG Recording
Baseline 32 channel EEG measurements are obtained just before surgery and CN-105 administration, and during anesthesia/surgery, (i.e. after the initial CN-105 dose is given) as described [49]. These recordings will help identify potential intraoperative EEG markers of PND and/or neuroinflammation [59–61], and whether CN-105 treatment prevents these intraoperative EEG patterns.
Physical and Quality of Life Assessments
Physical function is assessed via Timed-Up-and-Go (TUG) [62] and Romberg tests, Duke Activity Status Index (DASI) [54], Elderly Falls Screening Test [63], Fall-Risk Screening Test, and the physical function subscale of the Short-Form-36 Health Survey (SF-36) [64]. QOL is assessed via the SF-36 [64]. These assessments occur within two months before and six ± three weeks after surgery.
Stopping Rules
MARBLE may be stopped if after any group of 67 patients the rate of grade III or higher SAEs (per 2018 CTCAE guidelines [42]) in drug-treated patients is >10% and more than three times the rate of such events in the placebo-treated group. Considering the wide range of surgical procedures and patient comorbidities that could contribute to AEs in this study, the DSMB+ has been advised to use both this quantitative cutoff and its clinical judgement in considering whether to recommend that the study be stopped or not.
Statistical Analysis
Based on prior studies, we expect ≥80% of patients to complete all aspects of the study (drug administration, blood and CSF sampling, neurocognitive testing, EEG recording, quality of life assessments, and delirium screening), yielding ≥117 CN-105-treated patients across the three dose groups and ≥39 placebo-treated patients. We anticipate an AE incidence of 5% among placebo-treated patients, based on prior AE rates in older Duke non-cardiac surgical patients. Based on these parameters, this sample size provides >80% power (with α=0.05) in a two- sample two-sided un-pooled variance chi-square test to detect an absolute difference of 14.8% between AE rates in placebo versus CN-105 treated patients. Missing data will be categorized by cause, and baseline characteristics of patients who do not complete the study will be compared to those who do, to evaluate for response bias. Multiple imputation will be pursued if found necessary for the analysis of efficacy endpoints. In the case that imputation is used, sensitivity analyses will also be performed using only actual data to ensure that the results are not biased by the imputation strategy.
The primary safety outcome will be compared between placebo and drug treated patients via chi-square tests and post-hoc logistic regression to investigate dose-response patterns or subgroup effects. The feasibility analysis will be performed via construction of confidence intervals for the rate of dose administration per protocol. Post-hoc analysis will be performed among drug treated patients and by dose level to determine if the drug itself or drug dose level affects feasibility. For our secondary efficacy endpoints, we will perform t-tests, Wilcoxon rank sum tests, or chi-square tests as appropriate to compare outcomes for drug and placebo treated patients. Subsequently we will use ANOVA or regression models to investigate potential dose-response and subgroup effects for each endpoint. Additional exploratory sensitivity analyses will be conducted for primary and secondary outcomes by stratifying patients based on baseline cognitive status. These additional exploratory analyses will be performed in case there are interaction effects between CN-105 effects (whether beneficial or harmful) and baseline/preoperative cognitive status.
Each study participant is assigned a unique study ID; all data and subsequent analyses are stored securely under this unique ID without patient identifiers in a redcap database. Data are to be analyzed using standard software packages including SAS and R.
DISCUSSION
MARBLE is a phase II clinical trial designed to evaluate the maximum safe dose of perioperative CN-105 administration in older non-cardiac, non-neurological surgery patients. It is secondarily intended to evaluate the feasibility and potential of CN-105 for preventing PND and reducing postoperative neuroinflammation.
We hypothesize that CN-105 administration at all dose levels will be well-tolerated by participants (i.e. with no significant increase in AE rates among drug vs placebo treated patients), based on CN-105’s phase I safety profile [39]. We expect CN-105 administration will mitigate postoperative neuroinflammation and AD pathology changes, as measured by CSF inflammatory cytokine and tau, p-tau, and Aß levels, respectively. We also hypothesize that CN-105 will be effective for decreasing the incidence and severity of POCD/NCD.
MARBLE’s design allows for the identification of possible CN-105 response predictors for preventing POCD/NCD, delirium, and neuroinflammation, which could then be formally evaluated in future studies. For example, since apoE mimetic peptides have demonstrated differential efficacy for reducing neuroinflammation in APOE3 and APOE4 transgenic animals, [65, 66], we will also perform stratified exploratory analyses to examine whether CN-105 treatment efficacy varies by APOE genotype.
In conclusion, MARBLE is the first clinical trial to examine the effect of modulating presence of APOE4 on PND risk, severity and underlying mechanisms in older surgical patients. Its findings should guide future PND studies and may also assist in identifying predictors of susceptibility and resilience to these disorders. Finally, regardless of whether CN-105 is safe or efficacious, the data collected in MARBLE should further elucidate the role of neuroinflammation and AD pathology in PND.
Figure 1.
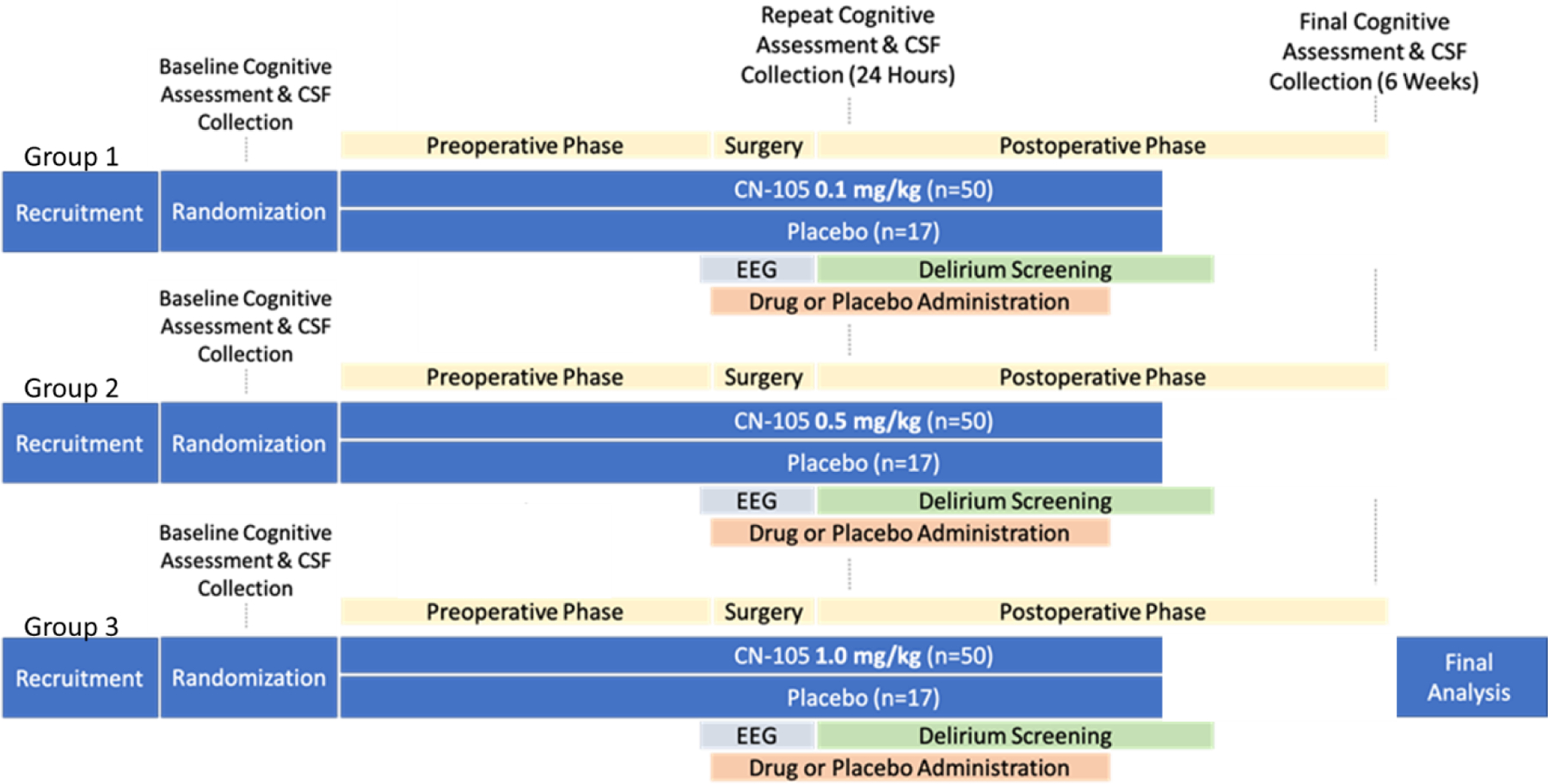
An overview of the MARBLE trial design. Precise time periods for each intervention or measurement are given in Table 1.
Table 1.
Activities and Timeline for MARBLE Study Participants. Abbreviations: CSF, cerebrospinal fluid; EEG, electroencephalogram.
| Baseline Study Visit | Day of Surgery | Postoperative day 1 | Postoperative days 2–3 | Postoperative days 4–5 | 6 weeks after surgery | |
|---|---|---|---|---|---|---|
| Neurocognitive testing, Quality of Life Assessments | X | X | ||||
| CSF, Blood Sample Collection | X | X | X | |||
| Drug administration | X | X | X | |||
| EEG | X | |||||
| Delirium screening | X | X | X | X | X | X |
ACKNOWLEDGEMENTS
We would like to thank Tanya Bolton, Diane Edwards, Galen Murphy and the rest of the perioperative nursing staff at Duke University Hospital for their enthusiastic support of our study and excellent care for its participants.
FINANCIAL DISCLOSURE
MARBLE is supported by a Program to Accelerate Clinical Trials (PACT) grant from the Alzheimer's Drug Discovery Foundation (to Dr. Berger). Dr. Berger also acknowledges additional support from National Institutes of Health (NIH) grants 1K76AG057022 (to Dr. Miles Berger), R01DA043241 (to Dr. David Murdoch), the Physical Resilience Indicators and Mechanisms in the Elderly (PRIME) Collaborative UH2AG056925 (to Drs. Heather Whitson and Cathleen Colon-Emeric), and additional support from the Duke Claude D. Pepper Older American Independence Center (P30AG028716), a William L. Young neuroscience research award from the Society for Neuroscience in Anesthesiology and Critical Care (SNACC), and the Duke Anesthesiology Department. Dr. Devinney acknowledges support from a research fellowship grant from the Foundation for Anesthesia Education and Research. Dr. Laskowitz has received funding from CURE-AD for preclinical efficacy of CN-105 in murine AD models
Footnotes
CONFLICT OF INTEREST
Dr. Laskowitz is an officer of Aegis-CN, which provides study drug for MARBLE but maintains no editorial control over study design, execution, or the writing of this manuscript. Dr. Laskowitz is a co-inventor of the Duke-held patent for CN-105. Dr. Berger acknowledges private consulting income from two legal cases related to postoperative cognition, and material support from Masimo Inc. for another prior study.
REFERENCES
- [1].Berger M, Nadler JW, Browndyke J, Terrando N, Ponnusamy V, Cohen HJ, Whitson HE, Mathew JP (2015) Postoperative Cognitive Dysfunction: Minding the Gaps in Our Knowledge of a Common Postoperative Complication in the Elderly. Anesthesiol Clin 33, 517–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McDonagh DL, Mathew JP, White WD, Phillips-Bute B, Laskowitz DT, Podgoreanu MV, Newman MF, Neurologic Outcome Research G (2010) Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology 112, 852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Inouye SK, Westendorp RGJ, Saczynski JS (2014) Delirium in elderly people. Lancet (London, England) 383, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Raats JW, van Eijsden WA, Crolla RMPH, Steyerberg EW, van der Laan L (2015) Risk Factors and Outcomes for Postoperative Delirium after Major Surgery in Elderly Patients. PloS one 10, e0136071–e0136071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS (2009) Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110, 548–555. [DOI] [PubMed] [Google Scholar]
- [6].Cullen KA, Hall MJ, Golosinskiy A (2009) Ambulatory surgery in the United States, 2006. Natl Health Stat Report, 1–25. [PubMed]
- [7].Evered LA, Silbert BS, Scott DA, Maruff P, Ames D (2016) Prevalence of Dementia 7.5 Years after Coronary Artery Bypass Graft Surgery. Anesthesiology 125, 62–71. [DOI] [PubMed] [Google Scholar]
- [8].Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG (2018) Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 121, 1005–1012. [DOI] [PubMed] [Google Scholar]
- [9].Bradley EH, Schlesinger M, Webster TR, Baker D, Inouye SK (2004) Translating research into clinical practice: making change happen. J Am Geriatr Soc 52, 1875–1882. [DOI] [PubMed] [Google Scholar]
- [10].Ely EW (2017) The ABCDEF Bundle: Science and Philosophy of How ICU Liberation Serves Patients and Families. Crit Care Med 45, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M (2010) Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 107, 20518–20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M (2011) Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 70, 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hirsch J, Vacas S, Terrando N, Yuan M, Sands LP, Kramer J, Bozic K, Maze MM, Leung JM (2016) Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation 13, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG (2011) Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology 115, 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Berger M, Ponnusamy V, Greene N, Cooter M, Nadler JW, Friedman A, McDonagh DL, Laskowitz DT, Newman MF, Shaw LM, Warner DS, Mathew JP, James ML, FtM-PI (2017) The Effect of Propofol vs. Isoflurane Anesthesia on Postoperative Changes in Cerebrospinal Fluid Cytokine Levels: Results from a Randomized Trial. Frontiers in Immunology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Berger M, Nadler JW, Friedman A, McDonagh DL, Bennett ER, Cooter M, Qi W, Laskowitz DT, Ponnusamy V, Newman MF, Shaw LM, Warner DS, Mathew JP, James ML, team M-Pt (2016) The Effect of Propofol Versus Isoflurane Anesthesia on Human Cerebrospinal Fluid Markers of Alzheimer’s Disease: Results of a Randomized Trial. J Alzheimers Dis 52, 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Evered L, Silbert B, Scott DA, Zetterberg H, Blennow K (2018) Association of Changes in Plasma Neurofilament Light and Tau Levels With Anesthesia and Surgery: Results From the CAPACITY and ARCADIAN Studies. JAMA Neurol 75, 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Evered L, Silbert B, Scott DA, Ames D, Maruff P, Blennow K (2016) Cerebrospinal Fluid Biomarker for Alzheimer Disease Predicts Postoperative Cognitive Dysfunction. Anesthesiology 124, 353–361. [DOI] [PubMed] [Google Scholar]
- [19].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Evered LA, Silbert BS, Scott DA, Maruff P, Laughton KM, Volitakis I, Cowie T, Cherny RA, Masters CL, Li QX (2009) Plasma amyloid beta42 and amyloid beta40 levels are associated with early cognitive dysfunction after cardiac surgery. Ann Thorac Surg 88, 1426–1432. [DOI] [PubMed] [Google Scholar]
- [21].Wu Z, Zhang M, Zhang Z, Dong W, Wang Q, Ren J (2018) Ratio of β-amyloid protein (Aβ) and Tau predicts the postoperative cognitive dysfunction on patients undergoing total hip/knee replacement surgery. Experimental and therapeutic medicine 15, 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 4, 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP (2015) Neuroinflammation in Alzheimer’s disease. The Lancet. Neurology 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Regen F, Hellmann-Regen J, Costantini E, Reale M (2017) Neuroinflammation and Alzheimer’s Disease: Implications for Microglial Activation. Curr Alzheimer Res 14, 1140–1148. [DOI] [PubMed] [Google Scholar]
- [25].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- [26].Zhu Y, Nwabuisi-Heath E, Dumanis SB, Tai LM, Yu C, Rebeck GW, LaDu MJ (2012) APOE genotype alters glial activation and loss of synaptic markers in mice. Glia 60, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vitek MP, Brown CM, Colton CA (2009) APOE genotype-specific differences in the innate immune response. Neurobiol Aging 30, 1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM (2005) Apolipoprotein E4 enhances brain inflammation by modulation of the NF-κB signaling cascade. Neurobiology of Disease 20, 709–718. [DOI] [PubMed] [Google Scholar]
- [29].Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT (2003) APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem 278, 48529–48533. [DOI] [PubMed] [Google Scholar]
- [30].Fan YY, Cai QL, Gao ZY, Lin X, Huang Q, Tang W, Liu JH (2017) APOE epsilon4 allele elevates the expressions of inflammatory factors and promotes Alzheimer’s disease progression: A comparative study based on Han and She populations in the Wenzhou area. Brain Res Bull 132, 39–43. [DOI] [PubMed] [Google Scholar]
- [31].Grocott HP, Newman MF, El-Moalem H, Bainbridge D, Butler A, Laskowitz DT (2001) Apolipoprotein E genotype differentially influences the proinflammatory and anti-inflammatory response to cardiopulmonary bypass. J Thorac Cardiovasc Surg 122, 622–623. [DOI] [PubMed] [Google Scholar]
- [32].Huynh TV, Davis AA, Ulrich JD, Holtzman DM (2017) Apolipoprotein E and Alzheimer’s disease: the influence of apolipoprotein E on amyloid-beta and other amyloidogenic proteins. J Lipid Res 58, 824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhao N, Liu CC, Qiao W, Bu G (2018) Apolipoprotein E, Receptors, and Modulation of Alzheimer’s Disease. Biol Psychiatry 83, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bartels K, Li YJ, Li YW, White WD, Laskowitz DT, Kertai MD, Stafford-Smith M, Podgoreanu MV, Newman MF, Mathew JP (2015) Apolipoprotein epsilon 4 genotype is associated with less improvement in cognitive function five years after cardiac surgery: a retrospective cohort study. Can J Anaesth 62, 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schenning KJ, Murchison CF, Mattek NC, Silbert LC, Kaye JA, Quinn JF (2016) Surgery is associated with ventricular enlargement as well as cognitive and functional decline. Alzheimers Dement 12, 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lei B, James ML, Liu J, Zhou G, Venkatraman TN, Lascola CD, Acheson SK, Dubois LG, Laskowitz DT, Wang H (2016) Neuroprotective pentapeptide CN-105 improves functional and histological outcomes in a murine model of intracerebral hemorrhage. Scientific Reports 6, 34834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tu TM, Kolls BJ, Soderblom EJ, Cantillana V, Ferrell PD, Moseley MA, Wang H, Dawson HN, Laskowitz DT (2017) Apolipoprotein E mimetic peptide, CN-105, improves outcomes in ischemic stroke. Ann Clin Transl Neurol 4, 246–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Laskowitz DT, Wang H, Chen T, Lubkin DT, Cantillana V, Tu TM, Kernagis D, Zhou G, Macy G, Kolls BJ, Dawson HN (2017) Neuroprotective pentapeptide CN-105 is associated with reduced sterile inflammation and improved functional outcomes in a traumatic brain injury murine model. Scientific reports 7, 46461–46461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Guptill JT, Raja SM, Boakye-Agyeman F, Noveck R, Ramey S, Tu TM, Laskowitz DT (2017) Phase 1 Randomized, Double-Blind, Placebo-Controlled Study to Determine the Safety, Tolerability, and Pharmacokinetics of a Single Escalating Dose and Repeated Doses of CN-105 in Healthy Adult Subjects. J Clin Pharmacol 57, 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Research DOoC, Recruitment and Engagement Policy for Clinical Research https://medschool.duke.edu/research/clinical-and-translational-research/duke-office-clinical-research/docr-services-and-initiativesprojects/recruitment-and-engagement, Accessed 2/4/2020.
- [41].Berger M, Oyeyemi D, Olurinde MO, Whitson HE, Weinhold KJ, Woldorff MG, Lipsitz LA, Moretti E, Giattino CM, Roberts KC, Zhou J, Bunning T, Ferrandino M, Scheri RP, Cooter M, Chan C, Cabeza R, Browndyke JN, Murdoch DM, Devinney MJ, Shaw LM, Cohen HJ, Mathew JP, Investigators I (2019) The INTUIT Study: Investigating Neuroinflammation Underlying Postoperative Cognitive Dysfunction. J Am Geriatr Soc 67, 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].National Cancer Institute NIoH, US Department of Health and Human Services, Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0/, Accessed September 3rd.
- [43].Inouye SK (2000) Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med 32, 257–263. [DOI] [PubMed] [Google Scholar]
- [44].Nobuhara CK, Bullock WM, Bunning T, Colin B, Cooter M, Devinney MJ, Ferrandino MN, Gadsden J, Garrigues G, Habib AS, Moretti E, Moul J, Ohlendorf B, Sandler A, Scheri R, Sharma B, Thomas JP, Young C, Mathew JP, Berger M, Madco PC, Teams II (2020) A protocol to reduce self-reported pain scores and adverse events following lumbar punctures in older adults. J Neurol ePub ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Berger M, Murdoch DM, Staats JS, Chan C, Thomas JP, Garrigues GE, Browndyke JN, Cooter M, Quinones QJ, Mathew JP, Weinhold KJ, Team M-PS (2019) Flow Cytometry Characterization of Cerebrospinal Fluid Monocytes in Patients With Postoperative Cognitive Dysfunction: A Pilot Study. Anesth Analg 129, e150–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dabitao D, Margolick JB, Lopez J, Bream JH (2011) Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. J Immunol Methods 372, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA, Neurological Outcome Research G, the Cardiothoracic Anesthesiology Research Endeavors I (2001) Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 344, 395–402. [DOI] [PubMed] [Google Scholar]
- [48].Mathew JP, White WD, Schinderle DB, Podgoreanu MV, Berger M, Milano CA, Laskowitz DT, Stafford-Smith M, Blumenthal JA, Newman MF, Neurologic Outcome Research Group of The Duke Heart C (2013) Intraoperative magnesium administration does not improve neurocognitive function after cardiac surgery. Stroke 44, 3407–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Giattino CM, Gardner JE, Sbahi FM, Roberts KC, Cooter M, Moretti E, Browndyke JN, Mathew JP, Woldorff MG, Berger M, Investigators M-P (2017) Intraoperative Frontal Alpha-Band Power Correlates with Preoperative Neurocognitive Function in Older Adults. Front Syst Neurosci 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Barnett AG, van der Pols JC, Dobson AJ (2005) Regression to the mean: what it is and how to deal with it. Int J Epidemiol 34, 215–220. [DOI] [PubMed] [Google Scholar]
- [51].Price CC, Tanner JJ, Schmalfuss I, Garvan CW, Gearen P, Dickey D, Heilman K, McDonagh DL, Libon DJ, Leonard C, Bowers D, Monk TG (2014) A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology 120, 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McNair DM, Kahn RJ, Eds: Crook T, Ferris S, Bartus R (1983) Assessment in geriatric psychopharmacology (Chapter: Self-Assessment of Cognitive Deficits; The Cognitive Difficulties Scale), Mark Powley and Associates, New Canaan, CN. [Google Scholar]
- [53].Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, Mark DB, Newman MF (2006) Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med 68, 369–375. [DOI] [PubMed] [Google Scholar]
- [54].Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB (1989) A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 64, 651–654. [DOI] [PubMed] [Google Scholar]
- [55].Kuczmarska A, Ngo LH, Guess J, O’Connor MA, Branford-White L, Palihnich K, Gallagher J, Marcantonio ER (2016) Detection of Delirium in Hospitalized Older General Medicine Patients: A Comparison of the 3D-CAM and CAM-ICU. J Gen Intern Med 31, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Marcantonio ER, Ngo LH, O’Connor M, Jones RN, Crane PK, Metzger ED, Inouye SK (2014) 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 161, 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO Jr., Fong TG, Metzger E, Inouye SK, Group SS (2012) Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc 13, 818 e811–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vasunilashorn SM, Guess J, Ngo L, Fick D, Jones RN, Schmitt EM, Kosar CM, Saczynski JS, Travison TG, Inouye SK, Marcantonio ER (2016) Derivation and Validation of a Severity Scoring Method for the 3-Minute Diagnostic Interview for Confusion Assessment Method--Defined Delirium. J Am Geriatr Soc 64, 1684–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Albrecht MA, Vaughn CN, Erickson MA, Clark SM, Tonelli LH (2018) Time and frequency dependent changes in resting state EEG functional connectivity following lipopolysaccharide challenge in rats. PLoS One 13, e0206985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gutierrez R, Egana JI, Saez I, Reyes F, Briceno C, Venegas M, Lavado I, Penna A (2019) Intraoperative Low Alpha Power in the Electroencephalogram Is Associated With Postoperative Subsyndromal Delirium. Front Syst Neurosci 13, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U (2015) Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol 15, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39, 142–148. [DOI] [PubMed] [Google Scholar]
- [63].Cwikel JG, Fried AV, Biderman A, Galinsky D (1998) Validation of a fall-risk screening test, the Elderly Fall Screening Test (EFST), for community-dwelling elderly. Disabil Rehabil 20, 161–167. [DOI] [PubMed] [Google Scholar]
- [64].Ware JE Jr., Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30, 473–483. [PubMed] [Google Scholar]
- [65].Laskowitz DT, Song P, Wang H, Mace B, Sullivan PM, Vitek MP, Dawson HN (2010) Traumatic brain injury exacerbates neurodegenerative pathology: improvement with an apolipoprotein E-based therapeutic. J Neurotrauma 27, 1983–1995. [DOI] [PubMed] [Google Scholar]
- [66].James ML, Sullivan PM, Lascola CD, Vitek MP, Laskowitz DT (2009) Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke 40, 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]


