Abstract
Controlled perturbation of protein activity is essential to study protein function in cells and living organisms. Small molecules that hijack the cellular protein ubiquitination machinery to selectively degrade proteins of interest, so-called degraders, have recently emerged as alternatives to selective chemical inhibitors both as therapeutic modalities and as powerful research tools. These systems offer unprecedented temporal and spatial control over protein function. Here we review recent developments in this field with a particular focus on the use of degraders as research tools to interrogate complex biological problems.
In living cells, proteins are constantly synthesized and degraded. Two pathways control the bulk of protein degradation in mammals: the ubiquitin-proteasome system (UPS) and the autophagy-lysosome pathway1,2. The UPS is mainly responsible for degrading misfolded and damaged proteins, and, more importantly, short-lived regulatory proteins that control many critical cellular processes, including cell cycle progression, cell proliferation and differentiation, cell signaling and transcription3. The autophagy-lysosome pathway is primarily a stress response mechanism that degrades proteins, but also larger cellular structures, such as organelles, under conditions of starvation and other types of stress2.
Since the discovery that specific protein features, termed degrons, which often bind ubiquitin ligases4 or the proteasome directly5, render a protein susceptible to UPS-mediated degradation, researchers have sought ways to use degrons for controlled destabilization of proteins of interest (POI)6. Compared with genetic techniques, such as CRISPR-Cas9 or RNA interference methods, targeted protein degradation (TPD) has several advantages. First, TPD commonly depletes the target proteins fast, in minutes to hours, rather than the days or weeks required when manipulating mRNAs or genomic DNA. This prevents molecular compensation and cellular adaptation, which otherwise often convolutes phenotypic readouts. Second, while genetic manipulation generally affects all protein copies regardless of post-transcriptional RNA processing or posttranslational protein modifications, methods targeting proteins directly allow certain protein variants to be selectively degraded. Third, protein targeting methods are rapidly reversible and offer more fine-grained control. Incorporation of additional functional groups in TPD approaches allows protein degradation to be controlled by chemical or physical stimulation7,8. For example, photo-activable degradation tags enable control of the degradation of fusion proteins through irradiation9.
TPD approaches modify proteins with degrons or adapt existing scaffolding or enzymatic components of protein degradation machineries to redirect their activity10–17. More recently, powerful small molecule degrader systems have been developed that allow exquisite temporal control of protein degradation18. Small molecule-induced protein degradation has gained widespread attention not only because of its ability to precisely modulate target protein levels, but also because of the potential to develop new therapeutics that differ from classical inhibitors19–23. In this context, target proteins are often referred to as neo-substrates, because they are recruited to the protein degradation machinery only when the small molecule degrader is present. Induced degradation of therapeutic targets offers the advantage of activity at sub-stoichiometric doses24, and opens up therapeutic opportunities for classically “undruggable” targets, such as transcription factors and scaffold proteins25. Overall, three common TPD approaches have emerged: small molecule induced degradation, genetically encoded degradation tags and the use of protein conjugates, all of which will be discussed in detail below.
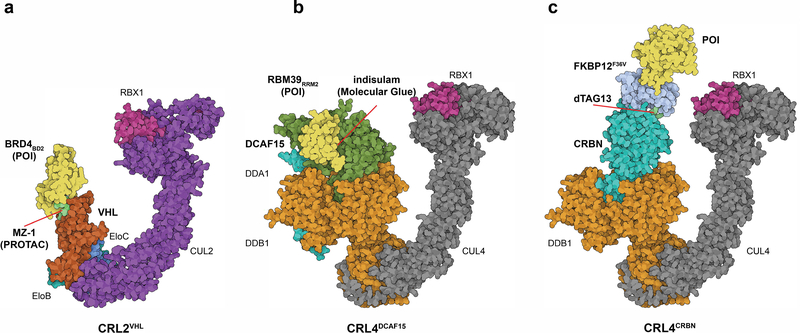
Over the last two decades, developments in the field of protein degradation have converged from two independent directions. Efforts in the early 2000’s established the concept of using two distinct binding moieties, one for the neo-substrate and one for the ubiquitin ligase, bridged by a linker (Fig. 1a). This approach is commonly referred to as PROteolysis TArgeting Chimeras (PROTACs) or bifunctional degraders17,25,26. Parallel studies uncovered that thalidomide and its analogs lenalidomide and pomalidomide (all FDA approved drugs for the treatment of hematologic malignancies) exert their therapeutic activity by induced degradation of neo-substrates, such as the zinc-finger transcription factors Ikaros (IKZF1) and Aiolos (IKZF3), the kinase Casein Kinase 1 alpha (CSNK1A1), and many more27–33. Strikingly, these molecules operate by a mechanism similar to the plant hormone auxin, which was coined a ‘molecular glue’ (Fig. 1b)34. This term commonly refers to molecules that display ligand affinity for only one of the two binding partners, either the neo-substrate or, more often, the ubiquitin ligase, and that enable engagement of the neosubstrate with the ubiquitin ligase35–40. These two lines of research converged and now thalidomide analogs are commonly used as ubiquitin ligase recruiters for bifunctional degraders41,42. Moreover, structural work (Fig. 1) has revealed that both bifunctional and molecular glue degraders can use extensive protein-protein contacts between ubiquitin ligase and neo-substrate, and hence the differences between the two classes may be more subtle than initially thought30,31,35–37,40,43–45. In this review, we therefore collectively refer to molecules that trigger protein degradation as ‘degraders’. Only if necessary do we further distinguish between bifunctional or molecular glue degraders. While the therapeutic potential of degraders is exciting and widely discussed19–22, we will focus here on recent advances in using TDP approaches as research tools.
Fig. 1.
Schematic representations of key concepts in protein degradation. a, Bifunctional degrader (PROTAC). Model of CRL2VHL E3 ligase in complex with MZ-1 (PROTAC) and BRD4BD2 (as a model POI) PDB: 5T35 and 5N4W (chain A and R). b, ‘Molecular glue’ degrader. Model of CRL4DCAF15 E3 ligase in complex with indisulam and RBM39 (POI). PDB: 6Q0R (chain B, C and D), PDB: 4A0C (chain C and D) and PDB: 4A0K (chain C). c, dTAG system as an example for a tagging strategy. Model of CRL4CRBN E3 ligase in complex with dTAG-13 recruiting FKBP12F36V-BRD4BD1 (POI). PDB models as in B, FKBP12 PDB: 1FKJ. Structure visualization created with the Illustrate program101
Small molecule degraders
Control of ubiquitin ligase activity by a low-molecular-weight ligand was first discovered in plants in the late 1990s18,34,46,47. The plant hormone auxin was found to exert its activity by recruiting auxin responsive transcriptional repressors, the AUX/IAA proteins, to the Cul1-Rbx1-Skp1-Tir1 (SCFTir1) ubiquitin ligase for degradation. The structural basis of auxin recognition by the SCFTir1 ligase revealed that auxin binds to the substrate receptor Tir1 and contributes a small additional hydrophobic patch between Tir1 and the AUX/IAA substrates that is sufficient for AUX/IAA recruitment to SCFTir1 34. As mentioned above, the molecular mechanism of auxin was described as a ‘molecular glue’, a term now widely used for small molecule degraders that primarily bind the ligase.
Immunomodulatory drugs (IMiDs).
Thalidomide and its close derivatives pomalidomide and lenalidomide, collectively known as immunomodulatory drugs, are clinically used in the treatment of multiple myeloma (MM), 5q-deletion associated myelodysplastic syndrome (del (5q)-MDS), and leprosy48–50. Thalidomide is infamous for its teratogenic effects when given to pregnant women for morning sickness in the 1950s, but second generation analogs such as lenalidomide and pomalidomide are now effective cancer treatments51. Re-approval of these drugs as anti-cancer agents was granted despite a lack of understanding of their mechanism of action. It was only subsequently discovered that IMiDs work by hijacking the ubiquitin ligase CRL4CRBN in a ‘molecular glue’ mechanism akin to auxin. IMiDs target the transcription factors IKZF1/3, ZFP91, ZNF692, SALL4, the kinase CSNK1A, and others, for degradation by CRL4CRBN 27,29,30,32,40,52,53 (Table 1). Importantly, this retrospective discovery of the mechanism of action provided clinical proof of concept for protein degradation therapeutics and has fostered widespread interest in this therapeutic modality20.
Table 1.
Neo-substrates, E3 ligases and ligands for the current molecular glue systems.
| Molecular glue | Known neo-substrates | Regulatory stage |
|---|---|---|
| IMiDs (CRL4CRBN) | ||
| Thalidomide32,40,52,102 | ZNF692, SALL4, RNF166, ZFP91, IKZF1, IKZF3 | FDA approved for leprosy, multiple myeloma |
| Lenalidomide27,28,32,40 | CSNK1A1 (CK1α), ZNF692, SALL4, RNF166, IKZF1, IKZF3, ZNF827 | FDA approved for multiple myeloma, del5q myelodysplastic syndrome |
| Pomalidomide32,40 | ZNF692, SALL4, RNF166, IKZF1, IKZF3, ZNF827, ZFP91, ZBTB39, GZF1, ZNF98, ZNF276, FAM83F, WIZ, RAB28, DTWD1, ZNF692, ZNF653 | FDA approved for multiple myeloma |
| Avadomide (CC-122)103 | IKZF1, IKZF3 | In clinical trials: Phase 1/2 |
| Iberdomide (CC-220)32,104 | IKZF1, IKZF3, ZFP91, RNF166, SALL4, RAB28, ZNF653, ZNF98 | In clinical trials: Phase 1 |
| CC-88531 | GSPT1, GSPT2, IKZF1, IKZF2, (possibly others as translational effects convolute proteomics) | Preclinical |
| FPFT-2216105 | CSNK1A1, IKZF1 | Preclinical |
| Aryl-sulfonamides (CRL4DCAF15) | ||
| Indisulam(E7070)38,57–59 | RBM39, RBM23, PRPF39 | In clinical trials: Phase 2 – terminated |
| E782038,57 | RBM39, RBM23 | In clinical trials: Phase 2 - completed |
| Tasisulam38,58 | RBM39 | In clinical trials: Phase 1/2 - terminated |
| Chloroquinoxaline sulfonamide (CQS)38,58 | RBM39 | In clinical trials: Phase 2 - completed |
While the therapeutic potential of CRL4CRBN-binding degraders has been widely discussed19,20, thalidomide analogs also serve as research tools. In contrast to most bifunctional degraders that are at the chemical porbe stage, the physicochemical properties and oral bioavailability of IMiDs make them great tools for in vivo studies54,55. Initially, the use of IMiDs in rodents was hindered by polymorphisms in CRBN that prevented target degradation. Specifically, murine Crbn can bind IMiDs, but Ile391, a residue at the putative Crbn–neo-substrate interface, causes steric hindrance that prevents recruitment of the neo-substrates56. However, a recently developed mouse model harboring a CRBNI391V humanizing mutation has emerged as a useful tool to assess the effects of IMiD-induced protein degradation in genetically engineered or xenografted mouse tumor models56.
Aryl-sulfonamides.
Reminiscent of IMiDs, aryl-sulfonamides, including indisulam, E7820, tasisulam and chloroquinoxaline sulfonamide (CQS), were shown to induce degradation of the splicing factor RBM39 via recruitment of the ubiquitin ligase CUL4-RBX1-DDB1-DCAF15 (CRL4DCAF15) (Table 1)38,57–59. We and others have recently established the molecular basis of aryl-sulfonamide activity35–37 and demonstrated that E7820 and its analogs bind to a relatively shallow pocket on DCAF15, facilitating the recruitment of RBM39 and RBM23 through the second RNA recognition motif (RRM) present in RBM39 or RBM23. Aryl-sulfonamides act as interface stabilizers that preferentially bind to the complex of the ligase and neo-substrate, in contrast to IMiDs that tightly interact with CRBN even in the absence of neo-substrates60. While the use of aryl-sulfonamides as effective research tools remains to be established, they expand our knowledge of how small molecules promote substrate recruitment and could encourage the development of novel molecular glue compounds as well as tagging strategies based on the RBM39 RRM domain.
Bifunctional degraders (PROTACs).
Fueled by discoveries of small-molecule ligands for ubiquitin E3 ligases, the field of bifunctional degraders (PROTACs)17 is rapidly evolving24,26. Combining high-affinity ligands for ubiquitin ligases such as CRL4CRBN, CUL2-RBX1-ElonginB/C-VHL (CRL2VHL)61, IAPs62, and MDM263 or covalent binders of the ubiquitin ligases DCAF1664, RNF14465 and RNF466 with ligands targeting a variety of POI has yielded degraders with exquisite efficacy and selectivity. The ever expanding space of proteins that have been successfully targeted with bifunctional degraders includes kinases67–70, nuclear receptors24,71, and epigenetic regulators72,73.
Many bifunctional degraders promote extensive protein-protein interactions between the neo-substrate and the ligase. These often provide an additional layer of substrate selectivity41–44 beyond or distinct from the target binding ligand alone. For example, achieving CDK4/6 homolog selectivity with active site inhibitors has been challenging due to the highly conserved CDK active site. By adapting the CDK4/6 dual inhibitor palbociclib as a degrader, Brand et al. were able to achieve excellent CDK6 selectivity and subsequently used the degrader to parse the consequences of acute CDK6 depletion on downstream signaling networks and transcriptional programs in AML74,75. Another application for bifunctional degraders is as probes for drug target identification (ID) and validation. For example, a bifunctional degrader was used to verify the transcription factor pirin as the target of a new chemical probe. The probe was discovered in an unbiased phenotypic screen designed to identify inhibitors of the HSF1 stress pathway76. Attaching a thalidomide moiety to this compound generated a degrader that specifically depleted pirin in cells, thus providing evidence for intracellular engagement of pirin. While this approach adds to the chemical biology toolbox commonly used for drug target ID, it is important to remember that false positive and false negative results are common for any target-ID method, including degraders.
Adding another layer of spatiotemporal control, optical-controlled degraders were recently described. Two independent groups reported photo-cleavable caging groups that can mask the degrader compounds. Upon radiation, such cages break down and release the active degrader, leading to degradation of neo-substrates 77–79. Photo-switchable bifunctional degraders were also designed and synthesized. Photo-PROTACs that contain an ortho-F4-azobenzene linker can switch between the cis- and trans-configurations upon visible light radiation, thus keeping the degrader in an active and inactive photostationary state, respectively. These probes allow very precise spatiotemporal control of protein degradation within the limits of intracellular diffusion of the activated degrader molecules7,8,80.
The discovery of ligands that covalently bind E3 ligases, such as DCAF16, RNF114 and RNF464–66 offer exciting new opportunities to access new protein target space. Proof of concept has been established by synthesizing bifunctional molecules using covalent E3 ligands and established neo-substrate ligands to degrade targets such as BRD4 and FKBP64,66. It remains to be shown, however, if covalent ligands will exhibit similar broad applicability as observed for CRBN and VHL ligands. Interestingly, covalent E3 binders can be discovered from fragment-based libraries64,66, but can also be found in the vast realm of natural products. One such example is nimbolide, which recruits the E3 ligase RNF11465.
One of the most exciting byproducts of the widespread therapeutic interest in degraders is the number of selective and well-characterized chemical probes that become available for biological research. While it is impressive how quickly the field has leveraged the technology, the development of bifunctional degraders will become increasingly difficult once the existing ligand space is exhausted and new ligands need to be discovered. In addition, while it is possible for some substrates to develop highly effective degraders in a few structure-activity relationship iterations, other potential substrates are refractory to degradation81. Substantial efforts are often required to obtain probes and ultimately therapeutics for some of the most promising therapeutic targets. Therefore, when available, target-specific degraders are ideal research tools, but one has to carefully consider whether development of a new degrader to answer a biological question is feasible or whether a degradation inducing fusion tag would provide a more adequate alternative.
Genetic fusion of degradation tags
While the degraders discussed above control endogenous protein levels, their development requires substantial synthetic efforts that are not guaranteed to succeed for every POI. Degradation tags offer an alternative and faster way to obtain precise control over protein abundance using established ligands (Fig. 1c). Here, we will cover a representative set of genetic tagging strategies as well as some general considerations, such as the size of the tag, selectivity and potential off-target effects. We will further discuss the feasibility of genome editing to achieve endogenous tagging and in vivo properties of ligands, which are relevant when choosing a particular approach for drug target validation and for studying biological pathways.
Auxin-inducible degron (AID).
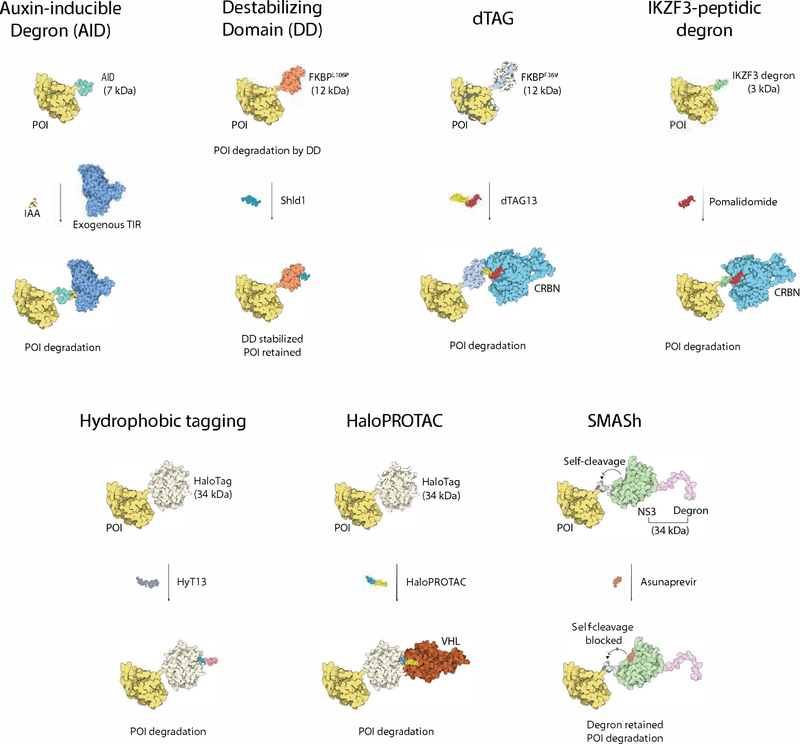
As described earlier, the plant hormone auxin can bind to the ubiquitin ligase SCFTIR1 and trigger the degradation of auxin responsive transcriptional repressors34. The degron sequence of these repressors (Tag size: 7 kDa) can be fused to a POI, and in the presence of exogenous plant E3 ligase SCFTIR1, the fused target protein is rapidly degraded upon auxin treatment13 (Fig. 2). An advantage of this system is that the plant hormone auxin has no known mammalian targets and is therefore assumed to have no off-target effects in mammalian cells. A major limitation of the system is the requirement for exogenous expression of the plant E3 ligase adapter TIR1, which complicates experimental design. In addition, TIR1 might have unknown activity in non-plant cells. Genetic knock-in of the AID sequence into a target locus to generate an endogenously tagged target protein has been reported, suggesting that this strategy is amenable to gene editing approaches82 (Table 2).
Fig. 2.
An overview of degradation tag fusion strategies for targeted protein degradation. Please refer to descriptions in the main text. POI based on BRD4 as an example, PDB: 6BOY; Auxin-inducible Degron based on TIR/IAA structure, PDB: 2P1Q (chain C), TIR structure, PDB: 2P1Q (chain B); Destabilzing Domain FKBP12 structure, PDB: 1FKJ; dTAG CRBN structure, PDB: 6BOY; IKZF3 tag based on structure of IKZF1 ZF2, PDB: 6H0F (chain C); HaloTag based apo HaloTag structure, PDB: 5UY1; VHL structure, PDB: 5T35 (chain D); NS3 protease structure in SMASh, PDB: 4WF8; NS4A degron based structure of NS4A peptide (shorter than the length of degron), PDB: 2OBO (chainB); Structure visualizations created with theIllustrate program101.
Table 2.
Genetic fusion strategies of degradation tags for targeted protein degradation.
| System | Tag | Tag size (kDa) | Drug | Molecular machinery for degradation | Note | Applications |
|---|---|---|---|---|---|---|
| AID | Auxin-inducible degron | 7 | Indole-3-acetic acid (IAA) | CRL1TIR1 | • CRISPR-Cas9 knock-In applicable • Exogenous expression of TIR1 is required |
13,82,106–108 |
| Destabilizing domain | FKBP12L106P mutant | 12 | Shld1 | Unidentified quality control pathway | • Continuous treatment of Shld1 ligand is required to keep the POI from degradation | 12,83–85 |
| dTAG | FKBP12F36V | 12 | dTAG13 | CRL4CRBN | • CRISPR-Cas9 knock-In applicable • Available in vivo |
15,86,87,109,110 |
| IKZF3-peptidic Degron | IKZF3 degron | 3 | Pomalidomide | CRL4CRBN | • CRISPR-Cas9 knock-In applicable • Available in vivo |
14 |
| HaloPROTACs | HaloTag | 34 | HaloPROTAC-3 | CRL2VHL/cIAP | • CRISPR-Cas9 knock-In applicable • Available in vivo |
16,91–93 |
| Hydrophobic | HaloTag | 34 | HyT13 | Unidentified quality control pathway | • Potential cellular perturbation from artificially unfolded protein | 88,89 |
| SMASh | NS3-NS4A | 34 | Asunaprevir | Unidentified quality control pathway | • No structural modification from tagging • Existing proteins cannot be degraded |
11,111–114 |
The Destabilizing domain.
This approach uses an engineered FKBP12L106P mutant protein as a fusion tag12 (Tag size: 12 kDa). The tag induces rapid and constitutive degradation of the fusion protein when expressed in mammalian cells, unless the cells are treated with a synthetic ligand (Shld1) that binds to the destabilizing domain and shields the fusion protein from degradation (Fig. 2). While this approach allows the reversible and tunable control of target protein stability, it requires continuous treatment with the Shld1 ligand to maintain the desired POI level. Unlike other degradation tag strategies, this system has the unique feature that it stabilizes the POI and turns on its downstream signaling upon ligand addition. This method has been used in many studies, including the investigation of lipophosphoglycan implicated in parasite survival83, P. falciparum protease falcipain-284, and PTEN85 (Table 2).
dTAG tag.
This tag (Tag size: 12 kDa)15 combines fusion of the targeted POI to mutant FKBP12F36V with the use of a degrader (dTAG13). The latter recruits the FKBP12F36V-fusion protein to CRL4CRBN and induces its degradation (Fig. 1c and Fig. 2). dTAG13 only binds the mutated FKBP12F36V and not the wild-type protein, thus minimizing off-target effects. Coupled with CRISPR-mediated knock-in, this system has been applied in multiple studies to deconvolute biological functions of POIs, as well as in drug target validation86,87. dTAG13 has been shown to have acceptable in vivo properties and in most cases triggers rapid protein degradation15. Empirically, dTAG appears to be a robust degradation tag, perhaps because the FKBP protein provides ubiquitination sites itself. Major limitations of this system are the rather large size for endogenous tagging (Table 2).
IKZF3-peptidic degron.
Another recent example of a degradation tag is the IKZF3-peptidic degron for IMiD-induced degradation14. Mapping of the boundaries in the zinc finger-2 domain of IKZF3 identified a 25 amino acid degron (Tag size: 3 kDa), which can be fused to a POI for IMiD-induced degradation (Fig. 2). Although it represents a single zinc-finger domain and not a linear peptide motif, this is still the smallest degron among all reported tags. The small size makes it less likely to interfere with the native function of target proteins and facilitates efficient knock-in. Another advantage is the good bioavailability of IMiDs, which cross the blood brain barrier and are active in the central nervous system (CNS), representing the only ligand-induced system suitable for modulation of proteins in the brain. An important consideration for this degron is the species-dependent sensitivity to IMiD-induced protein degradation discussed above. Many species, such as mice, harbor sequence variations in CRBN rendering them intrinsically resistant to IMiD-mediated recruitment of neo-substrates. The degron would thus be ineffective in wild-type animals, unless mouse CRBN is humanized to CRBNI391V as in reported mouse models56. To date, this degron has been reported to induce degradation for only ~ 50% of proteins that have been tagged, and further optimization may be required for it to be more widely applicable14 (Table 2). In addition, one has to take into account off-target effects: IMiDs induce the degradation of several transcription factors27,28, potentially confounding the interpretation of experimental results.
Halo Tag.
The HaloTag (Tag size: 34 kDa) is a modified haloalkane dehalogenase that can be specifically and covalently labeled with chloroalkane ligands. A HaloTag fused to a POI and labeled with a covalent bifunctional chemical probe consisting of such a chloroalkane ligand and a hydrophobic adamantyl group creates a hydrophobic degron (Fig. 2). This degron targets the fusion protein for degradation through the unfolded protein response in cell lines, zebrafish and mice88,89, but the precise mechanism of degradation triggered by the adamantyl group is not fully understood (Table 2). The HaloTag is widely used and the chemistry is thought to be bio-orthogonal (i.e. not exerting any off-target activity) in mammalian cells90. HaloTag fusion proteins can also be degraded using HaloPROTACs, covalent bifunctional molecules that react with the HaloTag and recruit VHL16 or cIAP91 ligases (Fig. 2). Combined with CRISPR/Cas9 genome editing technology, this method has been used to degrade endogenous HaloTag fusion proteins92 and target proteins in vivo93 (Table 2).
SMASh (Small Molecule Assisted Shutoff).
This system uses a destabilizing degron that is coupled to the NS3 protease from hepatitis C virus and a NS3 cleavage site. This cassette can be fused to a POI (Tag size: 34 kDa) (Fig. 2)11. After translation, the NS3 protease cleaves the recognition site between the fused cassette and the POI, generating an intact, native target protein. Administering the selective NS3 protease inhibitor, asunaprevir, blocks the cleavage event and leads to retention of the degron on the fusion protein and ultimately degradation. A main advantage of this method is that it produces a native protein without structural modifications. However, degradation is limited to newly synthesized proteins after asunaprevir addition, therefore the kinetics of degradation follow the rate of protein synthesis and are often slow. As a consequence, this tag may not be suitable for studying a biological process with fast dynamics (Table 2).
In addition to tagging substrate proteins, modifying or tagging the E3 ligases with a specific protein-recognizing entity, such as nanobodies, creates a degradation system that targets an endogenous POI. Nanobodies recognizing GFP or other target proteins were successfully fused to an F-box motif creating a functional Cul1-Rbx1-Skp1-nanobody ligase to degrade GFP fusion proteins10. In another study, nanobodies were fused to SPOP, an adaptor protein of the CUL3 E3 ubiquitin ligase, to degrade specific nuclear proteins in mammalian cells and zebrafish embryos94. Because of the general adaptability of nanobodies, this approach can potentially be used to target any endogenous protein, including specific post-translationally modified states or splice variants.
Macromolecular conjugates
Trim-away.
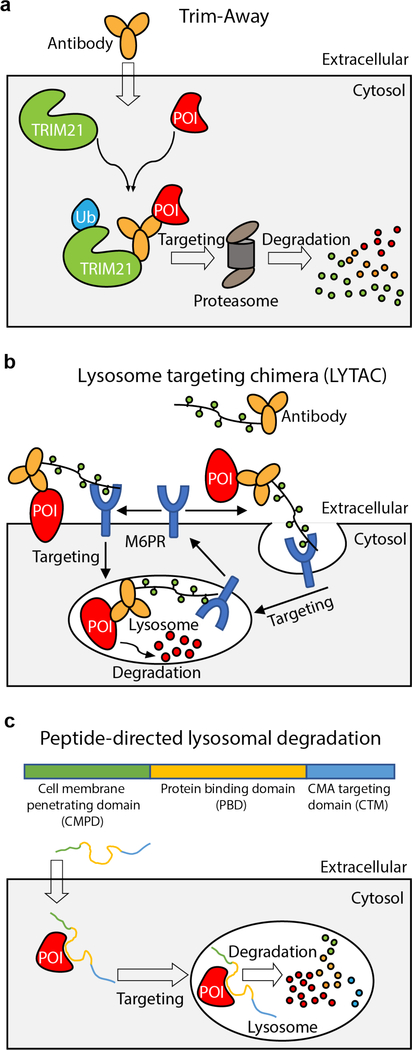
The Trim-away approach takes advantage of the fact that the ubiquitin ligase TRIM21 binds tightly to the Fc domain of antibodies and ubiquitinates itself, which results in degradation of the antibody-POI complex together with TRIM2195. The Trim-away method has been shown to degrade endogenous proteins for which specific antibodies are available (Fig. 3a). A major limitation is that antibodies are not cell penetrant. This issue can be overcome through microinjection of individual cells or electroporation of large cell populations with the antibody and the TRIM21 protein, resulting in rapid target protein degradation with protein half lives in the range of minutes to hours. In cell lines with high levels of TRIM21, injection or electroporation of the antibody alone is sufficient for target protein degradation95. An advantage of this method is its application in primary or non-dividing cells that are not amenable to genetic manipulation. Recently, this method was also used to degrade proteins in zebrafish embryos, acting faster than morpholinos96. Limitations of this method include the potential degradation of binding partners of the POI, observed for certain targeted proteins95. Further, as TRIM21 and antibodies are also degraded alongside the target protein, the method is prone to rapid recovery of POI levels95.
Fig. 3.
Schematic representations of targeted protein degradation strategies mediated by macromolecular conjugates. a, Trim-away. b, Lysosome targeting chimera. c, Peptide-directed lysosomal degradation.
Lysosome targeting chimeras (LYTACs).
While most TPD methods utilize the UPS to degrade a POI, a recently developed method allows degradation of secreted and membrane proteins through the lysosome pathway (Fig. 3b). LYTACs are macromolecular conjugates that are able to simultaneously bind a cell surface lysosome targeting receptor and the extracellular domain of a target protein. They consist of an antibody fused to agonist glycopeptide ligands for the cation-independent mannose-6-phosphate receptor (CI-M6PR)97. These LYTACs can induce degradation of proteins in a variety of cell lines. However, protein degradation using LYTACs generally requires days, compared to minutes or hours for methods directly engaging the UPS. Another potential limitation is the off-target degradation of interacting partners of the targeted membrane proteins.
Peptide-directed lysosomal degradation.
Another approach to degrade endogenous proteins uses a peptide fusion of a chaperone-mediated autophagy (CMA)-targeting motif (CTM) with a cell membrane-penetrating domain (CMPD) and a protein binding domain (PBD), to target a protein that is specifically recognized by the PBD98 (Fig. 3c). By simply adding the peptide into the cell culture medium, this method was effective in targeting a number of proteins in established cell lines as well as in primary neuronal cultures. Inherent limitations of this method include the inability to target proteins involved in the CMA pathway and lysosomal integrity98 and the limited stability of the peptide in cells and in animals. Recently, this method was used to target CDK5 to study the potential therapeutic effects of degrading CDK5 in prevention of strokes99.
Validation methods
The cellular activity of degraders relies on multiple factors, such as cellular uptake, efficient recruitment of the E3 ligase, ubiquitination of the target protein, and finally recruitment to the proteasome for degradation. As with any complex process, validation of expected results at each step is paramount to success. While not exhaustive, the methods outlined in Table 3 and Fig. 4 together with the use of established UPS inhibitors represent some of the available techniques for quality control at the various stages of the protein degradation pathway and are broadly applicable to all protein degradation modalities. In our experience the following assays are critical to troubleshoot protein degradation systems: 1) testing ligase or target protein engagement in cells, which also provides a qualitative readout of cell permeability, 2) in vitro ubiquitination assays, 3) target protein degradation in cells using a quantitative assay, such as ratiometric GFP/RFP40 or luminescence assays, and 4) assessment of the selectivity and efficacy for the targeted protein using mass spectrometry-based proteomics, which is a powerful profiling method for small molecule degraders.
Table 3.
Validation methods for targeted protein degradation.
| Steps | Purpose | Example Assays |
|---|---|---|
| Dimerization – in vitro | Ternary complex formation in vitro | TR-FRET40,43, SPR/BLI115 |
| Dimerization – cellular | Ternary complex formation in cells | NanoBit116 NanoBret116 |
| Cellular engagement | Cellular permeability and cellular engagement of the E3 ligase | Degradation based engagement assay43 NanoBret based Fluoro-ligand displacement117 |
| Ubiquitination – in vitro | Verify ubiquitination | In-vitro ubiquitination assay |
| Ubiquitination – cellular | Ubiquitination quantification and type | Western blot, NanoBret, TUBES118 |
| Identify ubiquitination sites | Proteomics | |
| Cellular degradation – targeted approach | Verify degradation in cells | Western blot, GFP-fusion, mCherry reporter lines119 Endogenous CRISPR fusions (HiBit Tag116, Split GFP120) |
| Cellular degradation – selectivity profiling by proteomics | Verify degradation selectivity in cells | Proteomics approaches32,52, library based screens40 |
| UPS inhibitors | Inhibitors for Cullin-RING family of ligases121,122 | MLN4924 - a specific inhibitor of the NAE1/UBA3 Nedd8 activating enzyme CSN5i-3 – inhibitor of COP9 signalosome |
| p97 inhibitor123 | CB-5083 | |
| Ubiquitin E1 (UBA1) inhibitor | MLN7243 | |
| Proteasome inhibitors124 | Bortezomib, Carfilzomib and MG132 |
Fig. 4.
Validation methods for targeted protein degradation at different stages of the ubiquitin-proteasome pathway. P97 PDB: 5FTJ; model of CRL4CRBN E3 ligase apo structure and structure with BRD4 created with PDB: 4A0C (chain C and D), PDB: 4A0K (chain C) and 6BOY (chain B and C); Human 26S proteasome PDB: 5GJR;Ubiquitin PDB: 1UBQ;POI BRD4 PDB: 6BOY (chain C). Structure visualizations created with the Illustrate program101.
Concluding remarks
Protein homeostasis is an important field of study for understanding human physiology and disease, as evidenced by an increasing number of small molecule therapeutics targeting this process. Key contributions to our mechanistic understanding of protein modification and degradation make it possible to engineer these systems to control the turnover of a POI. Most current TPD methods use the UPS, but several approaches using large biomolecular conjugates target the autophagy/lysosome pathway. R ecently reported small molecule degraders based on the autophagy pathway (autophagy-targeting chimeras or AUTACs)100 offer a glimpse into future possibilities. With this review focusing on the impact of TPD methods as research tools, we hope to encourage and inspire more efforts in creating new TPD methods and to provide readers with a resource to help navigate this expanding toolbox.
Acknowledgements
We thank all members of the Fischer lab for discussions and insights. This work was supported by NIH grant NCI R01CA214608 and R01CA218278 (grant to E.S.F.), a Mark Foundation Emerging Leader Award (grant to E.S.F.). E.S.F. is a Damon Runyon a Damon Runyon-Rachleff Innovator supported in part by the Damon Runyon Cancer Research Foundation (DRR-50-18). H.Y. is supported by a Chleck Foundation fellowship.
Footnotes
Competing Interests statement E.S.F. is a founder, scientific advisory board (SAB) member and equity holder of Civetta Therapeutics. E.S.F. is a SAB member and equity holder of C4 Therapeutics. E.S.F. is or has consulted for to Novartis, AbbVie, Astellas, Deerfield, EcoR1 and Pfizer. The Fischer lab receives or has received research funding from Novartis, Deerfield and Astellas.
References
- 1.Ciechanover A Intracellular protein degradation: from a vague idea, through the lysosome and the ubiquitin-proteasome system, and onto human diseases and drug targeting (Nobel lecture). Angew Chem Int Ed Engl 44, 5944–67 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Dikic I Proteasomal and Autophagic Degradation Systems. Annu Rev Biochem 86, 193–224 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Zheng N & Shabek N Ubiquitin Ligases: Structure, Function, and Regulation. Annu Rev Biochem 86, 129–157 (2017). [DOI] [PubMed] [Google Scholar]
- 4.King RW, Glotzer M & Kirschner MW Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol Biol Cell 7, 1343–57 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi J, Chen H, Hoyt MA & Coffino P Structural elements of the ubiquitin-independent proteasome degron of ornithine decarboxylase. Biochem J 410, 401–7 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Park EC, Finley D & Szostak JW A strategy for the generation of conditional mutations by protein destabilization. Proc Natl Acad Sci U S A 89, 1249–52 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaff P, Samarasinghe KTG, Crews CM & Carreira EM Reversible Spatiotemporal Control of Induced Protein Degradation by Bistable PhotoPROTACs. ACS Central Science 5, 1682–1690 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin R et al. PHOTACs Enable Optical Control of Protein Degradation, (2019).
- 9.Renicke C, Schuster D, Usherenko S, Essen LO & Taxis C A LOV2 domain-based optogenetic tool to control protein degradation and cellular function. Chem Biol 20, 619–26 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Caussinus E, Kanca 0 & Affolter M. Fluorescent fusion protein knockout mediated by anti-GFP nanobody. Nat Struct Mol Biol 19, 117–21 (2011).This paper describes engineering of a nanobody based CRL substrate receptor for the degradation of GFP-fusion proteins.
- 11.Chung HK et al. Tunable and reversible drug control of protein production via a self-excising degron. Nat Chem Biol 11, 713–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG & Wandless TJ A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995–1004 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura K, Fukagawa T, Takisawa H, Kakimoto T & Kanemaki M An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods 6, 917–22 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Koduri V et al. Peptidic degron for IMiD-induced degradation of heterologous proteins. Proc Natl Acad Sci USA 116, 2539–2544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabet B et al. The dTAG system for immediate and target-specific protein degradation. Nat Chem Biol 14, 431–441 (2018).This work describes the dTAG systems and demonstrates its utility in target validation by degrading KRASG12V.
- 16.Buckley DL et al. HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins. ACS Chem Biol 10, 1831–7 (2 015).This paper describes the development of a HaloPROTAC for the degradation of Halo-tagged proteins.
- 17.Sakamoto KM et al. Protacs: chimeric molecules that target proteins to the Skpl-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA 98, 8554–9 (2001).This paper shows the first example of using chimeric molecules to redirect the specificity of an ubiquitin ligase toward a target protein of interest.
- 18.Gray WM, Kepinski S, Rouse D, Leyser O & Estelle M Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414, 271–6 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Pettersson M & Crews CM PROteolysis TArgeting Chimeras (PROTACs) Past, present and future. Drug Discov Today Technol 31, 15–27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churcher I Protac-Induced Protein Degradation in Drug Discovery: Breaking the Rules or Just Making New Ones? J Med Chem 61, 444–452 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Watt GF, Scott-Stevens P & Gaohua L Targeted protein degradation in vivo with Proteolysis Targeting Chimeras: Current status and future considerations. Drug Discov Today Technol 31, 69–80 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Burslem GM & Crews CM Small-Molecule Modulation of Protein Homeostasis. Chem Rev 117, 11269–11301 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Salami J & Crews CM Waste disposal-An attractive strategy for cancer therapy. Science 355, 1163–1167 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Bondeson DP et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol 11, 611–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J et al. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chemistry & Biology 22, 755–763 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter GE et al. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kronke J et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–5 (2014).This study and the concurrent study by Lu et al., demonstrate how thalidomide promotes degradation of IKZF1/3 transcription factors.
- 28.Lu G et al. The Myeloma Drug Lenalidomide Promotes the Cereblon-Dependent Destruction of Ikaros Proteins. Science 343, 305 (2014).This study and the concurrent study by Kronke et al., demonstrate how thalidomide promotes degradation of IKZF1/3 transcription factors.
- 29.Kronke J, Hurst SN & Ebert BL Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology 3, e941742 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petzold G, Fischer ES & Thoma NH Structural basis of lenalidomide-induced CK1alpha degradation by the CRL4(CRBN) ubiquitin ligase. Nature 532, 127–30 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Matyskiela ME et al. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature 535, 252–7 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Donovan KA et al. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. Elife 7(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito T et al. Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–50 (2010).This study identified Cereblon as target of thalidomide.
- 34.Tan X et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–5 (2007).This paper provides the structural basis for auxin perception, the first proof-of-principle of a ‘molecular glue’ regulatory mechanism.
- 35.Faust TB et al. Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15. Nat Chem Biol 16, 7–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bussiere DE et al. Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex. Nat Chem Biol 16, 15–23 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Du X et al. Structural Basis and Kinetic Pathway of RBM39 Recruitment to DCAF15 by a Sulfonamide Molecular Glue E7820. Structure 27, 1625–1633.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Ting TC et al. Aryl Sulfonamides Degrade RBM39 and RBM23 by Recruitment to CRL4-DCAF15. Cell Reports 29, 1499–1510.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheard LB et al. Jasmonate perception by inositol-phosphatepotentiated C0I1-JAZ co-receptor. Nature 468, 400–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sievers QL et al. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 362(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang HT et al. A Chemoproteomic Approach to Query the Degradable Kinome Using a Multi-kinase Degrader. Cell Chem Biol 25, 88–99.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bondeson DP et al. Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead. Cell Chem Biol 25, 78–87.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowak RP et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat Chem Biol 14, 706–714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gadd MS et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol 13, 514–521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farnaby W et al. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat Chem Biol 15, 672–680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pozo JC, Timpte C, Tan S, Callis J & Estelle M The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science 280, 1760–3 (1998). [DOI] [PubMed] [Google Scholar]
- 47.Schwechheimer C et al. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIRI in mediating auxin response. Science 292, 1379–82 (2001). [DOI] [PubMed] [Google Scholar]
- 48.D'Amato RJ, Loughnan MS, Flynn E & Folkman J Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 91, 4082–5 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan B & Lentzsch S The application and biology of immunomodulatory drugs (IMiDs) in cancer. Pharmacol Ther 136, 56–68 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Teo S et al. Thalidomide in the treatment of leprosy. Microbes Infect 4, 1193–202 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Thomas DA & Kantarjian HM Current role of thalidomide in cancer treatment. Curr Opin Oncol 12, 564–73 (2000). [DOI] [PubMed] [Google Scholar]
- 52.An J et al. pSILAC mass spectrometry reveals ZFP91 as IMiD-dependent substrate of the CRL4(CRBN) ubiquitin ligase. Nat Commun 8, 15398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi AK et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol 164, 811–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y et al. In Vivo Assessment of the Effect of CYP1A2 Inhibition and Induction on Pomalidomide Pharmacokinetics in Healthy Subjects. The Journal of Clinical Pharmacology 58, 1295–1304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen N, Zhou S & Palmisano M Clinical Pharmacokinetics and Pharmacodynamics of Lenalidomide. Clinical Pharmacokinetics 56, 139–152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fink EC et al. Crbn (I391V) is sufficient to confer in vivo sensitivity to thalidomide and its derivatives in mice. Blood 132, 1535–1544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uehara T et al. Selective degradation of splicing factor CAPERalpha by anticancer sulfonamides. Nat Chem Biol 13, 675–680 (2017).This paper and the concurrent study by Han et al., demonstrate how aryl-sulfonamides promote degradation of RBM39.
- 58.Han T et al. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 356(2017).This paper and the concurrent study by Uehara et al., demonstrate how aryl-sulfonamides promote degradation of RBM39.
- 59.Jia X et al. pSILAC method coupled with two complementary digestion approaches reveals PRPF39 as a new E7070-dependent DCAF15 substrate. Journal of Proteomics 210, 103545 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Fischer ES et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512, 49–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckley DL et al. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1alpha interaction. J Am Chem Soc 134, 4465–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itoh Y, Ishikawa M, Naito M & Hashimoto Y Protein knockdown using methyl bestatin-ligand hybrid molecules: design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins. J Am Chem Soc 132, 5820–6 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Hines J, Lartigue S, Dong H, Qian Y & Crews CM MDM2-Recruiting PROTAC Offers Superior, Synergistic Antiproliferative Activity via Simultaneous Degradation of BRD4 and Stabilization of p53. Cancer Res 79, 251–262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Crowley VM, Wucherpfennig TG, Dix MM & Cravatt BF Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16. Nat Chem Biol 15, 737–746 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spradlin JN et al. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat Chem Biol 15, 747–755 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ward CC et al. Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications. ACS Chem Biol 14, 2430–2440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burslem GM, Song J, Chen X, Hines J & Crews CM Enhancing Antiproliferative Activity and Selectivity of a FLT-3 Inhibitor by Proteolysis Targeting Chimera Conversion. Journal of the American Chemical Society 140, 16428–16432 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Burslem GM et al. The Advantages of Targeted Protein Degradation Over Inhibition: An RTK Case Study. Cell Chem Biol 25, 67–77.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li W et al. Phthalimide conjugations for the degradation of oncogenic PI3K. Eur J Med Chem 151, 237–247 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Tinworth CP et al. PROTAC-Mediated Degradation of Bruton's Tyrosine Kinase Is Inhibited by Covalent Binding. ACS Chem Biol 14, 342–347 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Salami J et al. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol 1, 100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang K et al. Development of the first small molecule histone deacetylase 6 (HDAC6) degraders. Bioorg Med Chem Lett 28, 2493–2497 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Bassi ZI et al. Modulating PCAF/GCN5 Immune Cell Function through a PROTAC Approach. ACS Chem Biol 13, 2862–2867 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Brand M et al. Homolog-Selective Degradation as a Strategy to Probe the Function of CDK6 in AML. Cell Chem Biol 26, 300–306 e9(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang B et al. Development of Dual and Selective Degraders of Cyclin-Dependent Kinases 4 and 6. Angew Chem Int Ed Engl 58, 6321–6326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chessum NEA et al. Demonstrating In-Cell Target Engagement Using a Pirin Protein Degradation Probe (CCT367766). J Med Chem 61, 918–933 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J et al. Light-induced control of protein destruction by opto-PROTAC. Sci Adv 6, eaay5154 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xue G, Wang K, Zhou D, Zhong H & Pan Z Light-Induced Protein Degradation with Photocaged PROTACs. J Am Chem Soc 141, 18370–18374 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Naro Y, Darrah K & Deiters A Optical Control of Small Molecule-Induced Protein Degradation. J Am Chem Soc 142, 2193–2197 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin Y et al. Azo-PROTAC: novel light-controlled small-molecule tool for protein knockdown. J Med Chem (2020). [DOI] [PubMed] [Google Scholar]
- 81.Zeng M et al. Exploring Targeted Degradation Strategy for Oncogenic KRAS(G12C). Cell Chem Biol 27, 19–31 e6 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Li S, Prasanna X, Salo VT, Vattulainen I & Ikonen E An efficient auxin-inducible degron system with low basal degradation in human cells. Nat Methods 16, 866–869 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Madeira da Silva L, Owens KL, Murta SM & Beverley SM Regulated expression of the Leishmania major surface virulence factor lipophosphoglycan using conditionally destabilized fusion proteins. Proc Natl Acad Sci U S A 106, 7583–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Armstrong CM & Goldberg DE An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat Methods 4, 1007–9 (2007). [DOI] [PubMed] [Google Scholar]
- 85.An W et al. Engineering FKBP-Based Destabilizing Domains to Build Sophisticated Protein Regulation Systems. PLoS One 10, e0145783 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang HT et al. MELK is not necessary for the proliferation of basal-like breast cancer cells. Elife 6(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Erb MA et al. Transcription control by the ENL YEATS domain in acute leukaemia. Nature 543, 270–274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neklesa TK et al. Small-molecule hydrophobic tagging-induced degradation of HaloTag fusion proteins. Nat Chem Biol 7, 538–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raina K et al. Targeted protein destabilization reveals an estrogen-mediated ER stress response. Nat Chem Biol 10, 957–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Los GV et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3, 373–82 (2008). [DOI] [PubMed] [Google Scholar]
- 91.Tomoshige S, Naito M, Hashimoto Y & Ishikawa M Degradation of HaloTag-fused nuclear proteins using bestatin-HaloTag ligand hybrid molecules. Org Biomol Chem 13, 9746–50 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Tovell H et al. Rapid and Reversible Knockdown of Endogenously Tagged Endosomal Proteins via an Optimized HaloPROTAC Degrader. ACS Chem Biol 14, 882–892 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.BasuRay S, Wang Y, Smagris E, Cohen JC & Hobbs HH Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc Natl Acad Sci U S A 116, 9521–9526 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shin YJ et al. Nanobody-targeted E3-ubiquitin ligase complex degrades nuclear proteins. Sci Rep 5, 14269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clift D et al. A Method for the Acute and Rapid Degradation of Endogenous Proteins. Cell 171, 1692–1706 e18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen X et al. Degradation of endogenous proteins and generation of a null-like phenotype in zebrafish using Trim-Away technology. Genome Biol 20, 19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steven B, Kayvon P, Simon W, Nicholas R & Carolyn B Lysosome Targeting Chimeras (LYTACs) for the Degradation of Secreted and Membrane Proteins, (2019).This paper shows the method of using chimeric macromolecular conjugates to target proteins for degradation by the lysosomal pathway.
- 98.Fan X, Jin WY, Lu J, Wang J & Wang YT Rapid and reversible knockdown of endogenous proteins by peptide-directed lysosomal degradation. Nat Neurosci 17, 471–80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li W et al. Chaperone-mediated autophagy: Advances from bench to bedside. Neurobiology of Disease 122, 41–48 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Takahashi D et al. AUTACs: Cargo-Specific Degraders Using Selective Autophagy. Mol Cell 76, 797–810.e10 (2019).This paper shows the first example of small molecule degraders that target proteins to the lysosomal pathway for degradation.
- 101.Goodsell DS, Autin L & Olson AJ Illustrate: Software for Biomolecular Illustration. Structure 27, 1716–1720.e1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matyskiela ME et al. SALL4 mediates teratogenicity as a thalidomide-dependent cereblon substrate. Nat Chem Biol 14, 981–987 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Hagner PR et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood 126, 779–789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matyskiela ME et al. A Cereblon Modulator (CC-220) with Improved Degradation of Ikaros and Aiolos. J Med Chem 61, 535–542 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Gemechu Y et al. Humanized cereblon mice revealed two distinct therapeutic pathways of immunomodulatory drugs. Proceedings of the National Academy of Sciences 115, 11802 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakazawa N, Arakawa O & Yanagida M Condensin locates at transcriptional termination sites in mitosis, possibly releasing mitotic transcripts. Open Biol 9, 190125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshiba S et al. HsSAS-6-dependent cartwheel assembly ensures stabilization of centriole intermediates. J Cell Sci 132(2019). [DOI] [PubMed] [Google Scholar]
- 108.Goto H et al. Chk1-mediated Cdc25A degradation as a critical mechanism for normal cell cycle progression. J Cell Sci 132(2019). [DOI] [PubMed] [Google Scholar]
- 109.Boija A et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 175, 1842–1855.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brunetti L et al. Mutant NPM1 Maintains the Leukemic State through HOX Expression. Cancer Cell 34, 499–512.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fay EJ et al. Engineered Small-Molecule Control of Influenza A Virus Replication. J Virol 93(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rago F et al. Degron mediated BRM/SMARCA2 depletion uncovers novel combination partners for treatment of BRG1/SMARCA4-mutant cancers. Biochem Biophys Res Commun 508, 109–116 (2019). [DOI] [PubMed] [Google Scholar]
- 113.Zhu W et al. Precisely controlling endogenous protein dosage in hPSCs and derivatives to model FOXG1 syndrome. Nat Commun 10, 928 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu Y, Yang L, Chang T, Kandeel F & Yee JK A Small Molecule-Controlled Cas9 Repressible System. Mol Ther Nucleic Acids 19, 922–932 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roy MJ et al. SPR-Measured Dissociation Kinetics of PROTAC Ternary Complexes Influence Target Degradation Rate. ACS Chem Biol 14, 361–368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Riching KM et al. Quantitative Live-Cell Kinetic Degradation and Mechanistic Profiling of PROTAC Mode of Action. ACS Chem Biol 13, 2758–2770 (2018). [DOI] [PubMed] [Google Scholar]
- 117.Robers MB et al. Quantitative, Real-Time Measurements of Intracellular Target Engagement Using Energy Transfer. Methods Mol Biol 1888, 45–71 (2019). [DOI] [PubMed] [Google Scholar]
- 118.Hjerpe R et al. Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Rep 10, 1250–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Emanuele MJ et al. Global identification of modular cullin-RING ligase substrates. Cell 147, 459–74 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feng S et al. Improved split fluorescent proteins for endogenous protein labeling. Nat Commun 8, 370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soucy TA et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–6 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Schlierf A et al. Targeted inhibition of the COP9 signalosome for treatment of cancer. Nat Commun 7, 13166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anderson Daniel J. et al. Targeting the AAA ATPase p97 as an Approach to Treat Cancer through Disruption of Protein Homeostasis. Cancer Cell 28, 653–665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hyer ML et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat Med 24, 186–193 (2018). [DOI] [PubMed] [Google Scholar]