Abstract
The cannabis plant (Cannabis sativa L.) produces an estimated 545 chemical compounds of different biogenetic classes. In addition to economic value, many of these phytochemicals have medicinal and physiological activity. The plant is most popularly known for its two most-prominent and most-studied secondary metabolites—Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). Both Δ9-THC and CBD have a wide therapeutic window across many ailments and form part of a class of secondary metabolites called cannabinoids—of which approximately over 104 exist. This review will focus on non-cannabinoid metabolites of Cannabis sativa that also have therapeutic potential, some of which share medicinal properties similar to those of cannabinoids. The most notable of these non-cannabinoid phytochemicals are flavonoids and terpenes. We will also discuss future directions in cannabis research and development of cannabis-based pharmaceuticals. Caflanone, a flavonoid molecule with selective activity against the human viruses including the coronavirus OC43 (HCov-OC43) that is responsible for COVID-19, and certain cancers, is one of the most promising non-cannabinoid molecules that is being advanced into clinical trials. As validated by thousands of years of the use of cannabis for medicinal purposes, vast anecdotal evidence abounds on the medicinal benefits of the plant. These benefits are attributed to the many phytochemicals in this plant, including non-cannabinoids. The most promising non-cannabinoids with potential to alleviate global disease burdens are discussed.
Keywords: cannabinoids, Δ9-tetrahydrocannabinol, cannabidiol, non-cannabinoids, flavonoids, terpenes, secondary metabolites
1. Introduction
A central Asian site of domestication of the cannabis plant is often cited [1]. The plant’s medicinal value was first recorded in the Pen Ts’ao Ching, the Chinese pharmacopoeia—the world’s oldest, compiled in the Han dynasty, first or second century A.D. [1]. The cannabis plant, its resin, and some derivatives of its compounds have been used in traditional Eastern medicine. Uses include, for recreation, due to its hallucinogenic/hypnotic effects; for religious celebrations and meditative purposes; industrially for fibers, textiles and ropes; and medicinally as an analgesic anticonvulsant, antidiarrheal, sedative, relaxant, anxiolytic, antibacterial, and antioxidant, and as treatment for tetanus, epilepsy and delirium tremens.
The cannabis plant produces an estimated 545 [2] chemical compounds. The main biogenetic classes of compounds produced are shown in Table 1 below. These include primary metabolites, such as amino acids, fatty acids, vitamins, sugars, and proteins, and secondary metabolites produced in trichomes, the cell’s factory. Primary metabolites provide nutritional value. Secondary metabolites also provide nutritional value and include approximately 120 terpenoids (61 monoterpenes, 52 sesquiterpenoids, and 5 triterpenoids [2], over 26 flavonoids [2], lignans, stilbenes and derivatives such as dihydro-resveratrol (3,5,4’-trihydroxybibenzyl) [3], cannabinaceous alkaloids such as cannabisativine and anhydrocannabisativine [4], and 20 steroids [2]), dihydrophenanthrenes, glycoproteins, and dibenzyls [5,6]. In addition to sharing analgesic, antimicrobial anticancer, anti-inflammatory, and neuroprotective properties, a majority also share antioxidant capabilities [7].
Table 1.
The main biogenetic classes of compounds produced by Cannabis sativa L. [11].
During many pathological conditions and diseases processes such as age-related inflammatory and autoimmune diseases, asthma, atherosclerosis, cancer, chronic obstructive pulmonary disease, hypertension, ischemia/perfusion, diabetes, HIV and dementia [8], the cells of the body accumulate high levels of toxic reactive oxygen species (R.O.S.)/free radicals in comparison to antioxidants [9]. This imbalance can cause significant damage to cell structures [9]. Antioxidants mitigate excessively produced reactive oxygen species within the cells, and in doing so, reduce the oxidative stress in cells [10]. Antioxidants may therefore have therapeutic applicability against many human diseases including but not limited to cancer, viral infections, cardiovascular diseases and inflammatory diseases [10]. Natural antioxidants may also be revered due to their safer side effect profile compared to synthetic antioxidants used in preventative medicine and in the food industry [10].
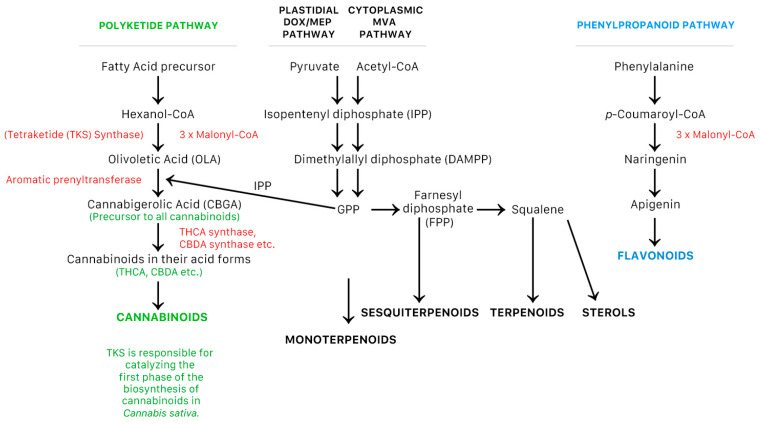
The biosynthesis of the non-cannabinoids in cannabis is driven by pyruvate/acetyl-CoA for the terpenes via the plastidial deoxyxylulose phosphate/methyl-erythritol phosphate (DOXP/MEP) and cytoplasmic MVA pathways [2], and p-coumaril-CoA via the phenylpropanoid pathway [2] for flavonoids [2]. The synthesis of cannabinoids is via the precursor molecule olivetolic acid (OLA) and the polyketide pathway, and another precursor molecule, olivetolic acid (GPP) and the plastidial deoxyxylulose phosphate/methyl-erythritol phosphate (DOXP/MEP) pathway [2]. These processes are simplified in Figure 1 below.
Figure 1.
Synthesis of cannabinoids and non-cannabinoids produced by Cannabis sativa L. [2].
In addition to having unique organoleptic properties [12], terpenes provide a wide range of therapeutic benefits to humans, secondary metabolites, particularly terpenes and flavonoids, play a primary role in a plant’s defenses against hostile environments, herbivores and phytophagous insects.
The identified non-cannabinoid metabolites of cannabis are discussed in more detail in the ensuing sections.
2. Non-Cannabinoid Compounds
Cannabinoid and non-cannabinoid phytochemicals possess bioactive and protective properties that are beneficial to human health. In addition to cannabinoids, the cannabis plant also produces hundreds of non-cannabinoid secondary metabolites including approximately 120 terpenoids (61 monoterpenes, 52 sesquiterpenoids, and 5 triterpenoids [2], essential oils, over 26 flavonoids [2] lignans, stilbenoid derivatives, alkaloids, amino acids, spiroindans, polyphenols, 20 steroids [2]), dihydrophenanthrenes, glycoproteins (such as galactose, glucose, mannose and xylose), and dibenzyls [5,6]. This list also includes a-cannabispiranol, chrysoeriol, 6-prenylapigenin, cannflavin A and b-acetyl cannabispiranol [13].
Flavonoids and terpenoids in particular have a wide therapeutic window, including but not limited to cancer, inflammation, excessive reactive oxygen species in cells, and enhancement of neuroprotection. In addition to the phytochemicals mentioned below, a 2008 study by Radwan and colleagues identified and isolated six new non-cannabinoid phytochemicals from a high potency Cannabis sativa L. strain. These were 5-acetoxy-6-geranyl-3-n-pentyl-1,4-benzoquinone, 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene, 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene, cannflavin C, cb-sitosteryl-3-O-b-D-glucopyranoside-20-O-palmitate, and 4-hydroxy-2,3,6,7-tetramethoxy-9,10-dihydrophenanthrene [13]. The review below focuses on non-cannabinoid phytochemicals with therapeutic potential.
2.1. Terpenes and Their Derivatives, Terpenoids
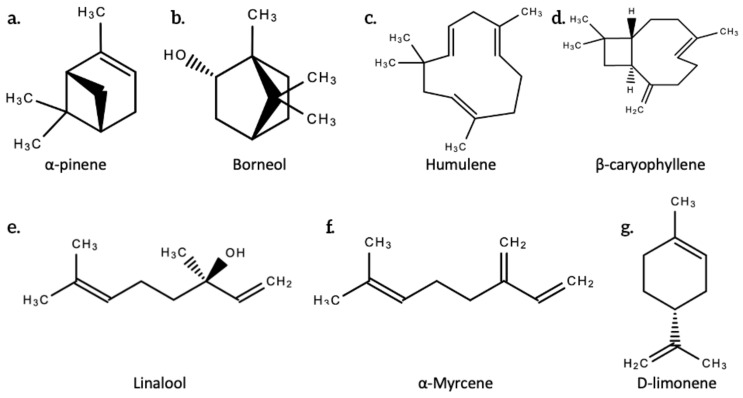
The word “terpene” was devised in 1866 by August Kekulé, a German organic chemist. Terpenes are hydrocarbons and are made up of isoprene units (5-carbon building blocks) [14], while terpenoids are an oxidized and denatured form of terpenes that differ in that they contain an additional functional group with oxygen [14]. They are not the same, despite being used synonymously. This oxidation occurs during the drying and curing processes when the plant is exposed to open air. Terpenes are typically classified by the number of isoprene units in the molecule. Isoterpene is the only hemiterpenes. Hemiterpenes and hemiterpenoids such as prenol and isovaleric acid only have a single isoprene unit. Monoterpenes and monoterpenoids such as pinene (most common terpene produced across plant species), limonene, myrcene, geraniol, and terpineol have two isoprene units. Sesquiterpenes and sesquiterpenoids such as humulene and farnesol have three isoprene units. Triterpenes, such as squalene—the precursors to all steroids [14]—have six isoprene units. Sesquiterpenes and tetraterpenes have seven and eight isoprene units, respectively. Polyterpenes and norisoprenoids have multiple isoprene units in their molecule. Figure 2 below shows the structures of some terpenoids.
Figure 2.
The chemical structures of some terpenoids produced by Cannabis sativa L. (a.) α-pinene; (b.) Borneol; (c.) Humulene; (d.) β-caryophyllene; (e.) Linalool; (f.) α-Myrcene; (g.) D-linonene.
Figure 1 above shows the biosynthetic pathway of terpenes and terpenoids, which are synthesized from an isoprenoid precursor Isopentenyl pyrophosphate (IPP). This is achieved via the plastidial deoxyxylulose phosphate/methyl-erythritol phosphate (DOXP/MEP) pathway (monoterpenoids), and the cytoplasmic mevalonate (MVA) pathway (sesquiterpenoids, triterpenoids, and sterols) [2]. Along with cannabinoids, terpenes are considered a main physiological marker of secondary metabolites [15,16].
Terpenes are a large and diverse class of aromatic compounds responsible for the unique flavors and scents of many herbs and plants and phenotypic variation across plant species. Over 20,000 terpenes exist across plant species [17], and over 150 alone in the cannabis plant [18]. This makes terpenes the largest classification of phytochemicals (Andre, Hausman, Guerriero, 2016). Their primary role in plants vary but, in some plants, may attract pollinators, and in others, deter herbivores and inhibit microbial growth.
The most common terpenes in cannabis include limonene, α-pinene, β-pinene, humulene, β-caryophyllene, linalool and myrcene [2]. Other common terpenes in cannabis include bisabolol, borneol, camphene, geraniol, ocimene, terpineol, and valencene. These fall into the monoterpenoids and sesquiterpenoids categories. Refer to Table 2 below for examples of mono- and sesquiterpenoids profiled in a 2020 study by Jin and colleagues that profiled secondary metabolites in cannabis inflorescences, leaves, stem bars, and roots for medicinal purposes [2].
Table 2.
Examples of mono- and sesquiterpenoids profiled in cannabis inflorescences, leaves, stem bars, and roots for medicinal purposes [2].
| Monoterpenoids | Sesquiterpenoids | |||
|---|---|---|---|---|
| α-Pinene | Eucalyptol | Borneol | (-)-β-Elemene | Viridiflorol |
| Camphene | Ocimene | Terpinen-4-ol | β-Caryophyllene | (-)-Guaiol |
| Sabinene | γ-Terpinene | -Terpineol | Aromadendrene | (+)-Cedrol |
| (-)-β-Pinene | Sabinene Hydrate | (+)-Dihydrocarvone | Trans-β-Farnesene | β-Eudesmol |
| β-Myrcene | Terpinolene | Nerol | α-Humulene | α-Bisabolol |
| α-Phellandrene | Frenchone | Pulegone | Valencene | |
| Δ 3-Carene | Linalool | Carvone | Ledene | |
| α-Terpinene | Frenchol | Geraniol | Trans-Nerolidol | |
| p-Cymene | (-)-Isopulegol | Geranyl Acetate | Caryophyllene Oxide | |
| Limonene | Camphor | Globulol |
Terpenes provide aromatherapeutic benefits to humans including stress, anxiety, and depression relief, decongestion, and a general pharmacologically synergistic effect in combination with cannabinoids and flavonoids. As a result, terpenes are frequently used in cosmeceuticals, perfumes and beverage flavoring.
Terpenes and terpenoids are highly concentrated in the essential oils extracted from the cannabis plant, and are also responsible for the therapeutic benefits that essential oils provide. A study by Piccaglia et al., 2016 explored the biological properties of some terpenes making up said essential oils extracted from the cannabis plant (Table 3) by distillation and characterized by gas chromatography–mass spectrometry (GC–MS). Table 3 also shows the biological properties of other common terpenes found in cannabis.
Table 3.
The biological properties of some common terpenes in cannabis.
| Terpene | Biological Property | References |
|---|---|---|
| Myrcene | Potent analgesic | [19] |
| Antioxidant; neuroprotective; anti-inflammatory | [20] | |
| Anticonvulsant | [21] | |
| 1,8-cineole | Increases cerebral blood flow and enhances cortical activity | [22] |
| Limonene | inhibits many species of bacteria and fungi—repellant | [23] |
| Anti-inflammatory; antioxidant; antiviral; antidiabetic; anticancer | [20] | |
| Antidepressant; anticonvulsant | [21] | |
| α-Pinene | Antimicrobial; repellant | [23] |
| Bronchodilator; anti-inflammatory, | ||
| Memory improvement/enhancement; acetylcholinesterase inhibitor | [21] | |
| Linalool (Lavender scent) |
Anxiolytic; anti-inflammatory; antimicrobial; anticancer; neuroprotective; antidepressant | [20] |
| Anti-influenza | [24] | |
| Sedative; induces apoptosis in cancer cells | [21] | |
| α-terpineol | Antimicrobial; repellant | [23] |
| Anti-inflammatory | [25] | |
| Analgesic | [26] | |
| Nociception inhibition | [26] | |
| Anticonvulsant | [27] | |
| Antimicrobial | [21] | |
| Gastroprotective | [28] | |
| Borneol | Antimicrobial; repellant | [23] |
| β- caryophyllene | Anti-inflammatory Analgesic |
[20] |
| Antispasmic in gut muscles | [21] | |
| Humulene | Antiallergy; anticancer | [20] |
| Ocimene | Antifungal; antibacterial; antioxidant; antiviral; anti-inflammatory | [29,30,31,32,33] |
The Medical Cannabis Network also reports that a current study is being undertaken by researchers at the Israel Institute of Technology investigating the therapeutic efficacy of a cannabis terpene inhalant formulation in suppressing the immune system response against COVID-19.
2.2. Phenolic Compounds in Cannabis sativa
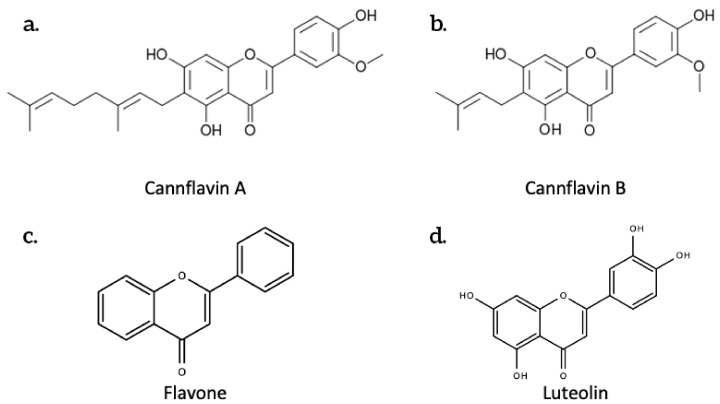
Polyphenols, also known as phenylpropanoids, are a class of over 10,000 chemical compounds that have phenyl groups/rings (C6H5). A phenyl ring consists of a 6-carbon structure formed on a hexagonal plane. Five of these carbon atoms are each individually bonded to hydrogen atoms (Figure 3a).
Figure 3.
The chemical structures of some flavonoids produced by Cannabis sativa L. (a.) Cannflavin A; (b.) Cannflavin B; (c.) Flavone; (d.) Luteolin.
A 2020 study by Izzo and colleagues analyzed some phenolic compounds in Cannabis sativa L. inflorescences using ultra-high-performance liquid chromatography-quadruple-orbitrap high-resolution mass spectrometry (UHPLC-Q-Orbitrap HRMS). Table 4 below lists some of the phenolic compounds investigated. Cannabisin A, B and C were the predominant lignanamides identified [34].
Table 4.
List of phenolic compounds in commercial Cannabis sativa L. [34].
| Flavonols | Phenolic Amides | Flavones | Phenolic Acids |
|---|---|---|---|
| Catechin | N-trans-caffeoyltyramine | Cannflavin A | Hydroxycinnamic acids |
| Epicatechin | Flavonoids | Cannflavin B | Chlorogenic acid |
| Flavanone | Flavonol | Luteolin-7-O-glucoside | Caffeic acid |
| Naringenin | Rutin | Apigenin-7-O-glucoside | p-Coumaric acid |
| Lignanamides | Quercetin-3-glucoside | Luteolin | Ferulic acid |
| Cannabisin A | Kaempferol-3-O-glucoside | Apigenin | |
| Cannabisin B | Quercetin | ||
| Cannabisin C | Kaempferol |
2.3. Flavonoids
Flavonoids are a large family of polyphenolic plant compounds that naturally occur in fruits, vegetables, chocolate, and beverages such as wine and tea.
There are six classes of flavonoids including anthocyanidins, flavan-3-ols, flavonols, flavanones, flavones, and isoflavones. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B), and a heterocyclic ring. This carbon structure can be abbreviated C6-C3-C6. Figure 3 below shows the structures of some flavonoids.
Flavonoids are found in a wide range of plants and may act as physiological regulators, cell-cycle inhibitors and/or chemical messengers. They are also responsible for plant pigmentation (flower coloration to attract pollinator animals/insects), UV filtration/protection, and symbiotic nitrogen fixation.
Flavonoids provide color, flavor and aroma. These class of compounds also have anticancer, antioxidant, antithrombogenic, antidiabetic and neuroprotective activities via modulation of a number of cell-signaling cascades.
A 2016 study by Bertoia and colleagues explored the relationship between dietary flavonoid intake (of flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, and flavonoid polymers) and weight change at 4-year intervals between 1986 and 2011 of 124,086 men and women. In conclusion of this study, intake of foods rich in flavonols, flavan-3-ols, anthocyanins, and flavonoid polymers, inversely related to weight gain. Anthocyanidins had the greatest negative correlation with weight maintenance [35]. See Table 5 and Table 6 below for some biological properties of some common classes of flavonoids.
Table 5.
The biological properties of some common classes of flavonoids.
| Flavonoid | Biological Property | |
|---|---|---|
| Flavonols (e.g., quercetin and kaempferol) |
Antioxidant; cardioprotective | [36] |
| Flavanones | Antioxidant; anticancer; anti-inflammatory | [36] |
| Isoflavonoids | Phytoestrogenic (mimic the hormone estrogen); hormone balance and metabolism | [36] |
| Anthocyanins (responsible for a plant’s unique colour) |
Antioxidant and anti-inflammatory | [36] |
Table 6.
More therapeutic benefits of flavonoids.
| Therapeutic Window/Benefits of Flavonoids | Patent Number |
|---|---|
| Flavonoid derivatives targeting kinases, sirtuins and oncogenic agents for the treatment of cancers. | [40,41] |
| Agent containing flavonoid derivatives for treating cancer and inflammation. | [42] |
| Therapeutic agents containing cannabis flavonoid derivative for ocular disorders. | [43] |
| Therapeutic agents containing cannabis flavonoid derivatives for the prevention and treatment of neurodegenerative disorders. | [44] |
| Pi 4-kinase inhibitor as a therapeutic for viral hepatitis, cancer, malaria. autoimmune disorders and inflammation, and a radiosensitizer and immunosuppressant. | [45] |
| Therapeutic antiviral agents containing cannabis cannabinoid derivatives. | [46] |
Over 20 different flavonoids have been identified in cannabis, most of which fall into the flavonol and flavone group [2]. One such flavonoid is cannflavin A, which is considered to be 30 times more effective than Aspirin at inhibiting prostaglandin E2, a significant modulator of inflammation [37]. Cannflavin B is also an inhibitor of prostaglandin E2 [38].
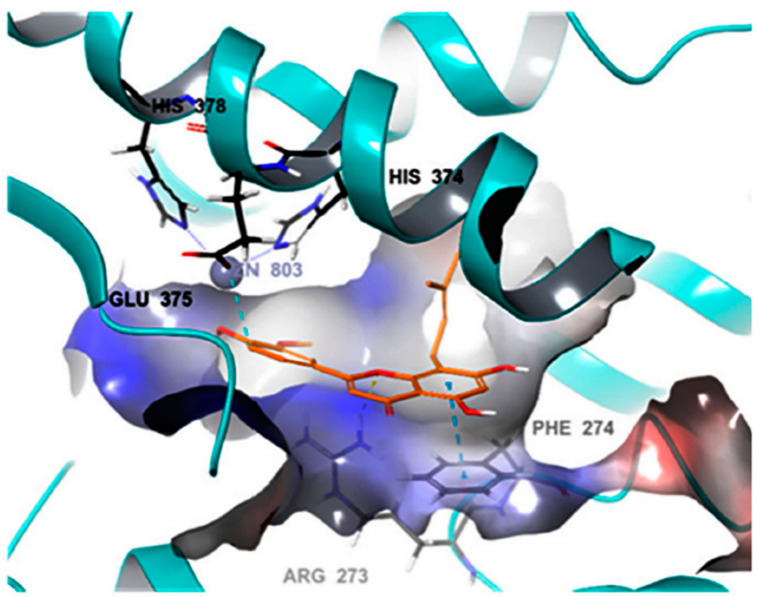
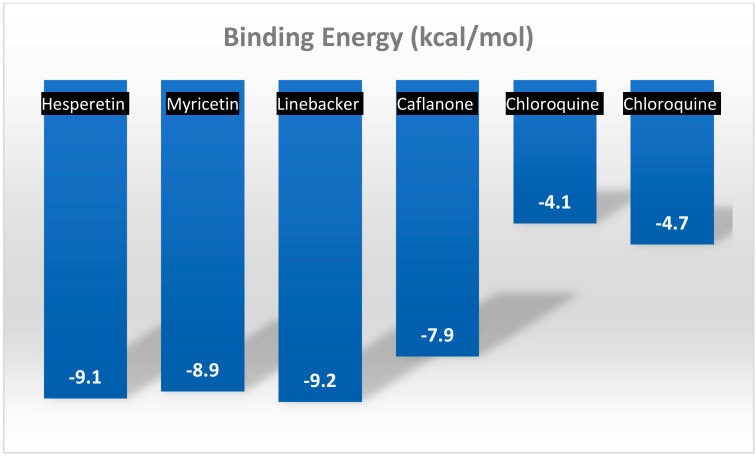
Another major flavonoid derived from cannabis is caflanone. In recent studies, caflanone demonstrated activity against the human coronavirus OC43 (HCoV-OC43) also known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). HCoV-OC43 belongs to clade b of the genus Betacoronavirus [39]. In vitro, caflanone inhibited HCoV-OC43 with an EC50 of 0.42 µM [39]. In silico studies showed that caflanone may act by inhibiting the angiotensin-converting enzyme 2 (ACE2) receptor found in the lung and respiratory tract, and used by the virus during cell entry and infection. Caflanone was also shown to have strong binding affinity to two of the proteases (PLpro and 3CLpro) are vital to the replication of SARS-CoV-2 in humans, thereby inhibiting viral entry to and/or replication within human cell [39]. Figure 4 shows the main interactions of caflanone with the proteins ACE2 (catalytic site/zinc metallopeptidase domain), Glu375, Glu402, ZN803, HIS374, Phe274 and Arg 273 [39]. The docking/binding studies results below show that the phytoantiviral flavonoids (Hesperetin, myricetin, linebacker, and caflanone) could bind equally or more effectively than chloroquine (CLQ), another investigated drug for SARS-CoV-2 [39] (Figure 5).
Figure 4.
The main protein interactions of caflanone [39]. Reproduced with permission from Wilfred Ngwa, Potential of Flavonoid-Inspired Phytomedicines against COVID-19, published by Molecules Open-Access Journal, 11 June 2020 [39].
Figure 5.
A comparison of the docking/binding studies results between the phytoantiviral flavonoids hesperetin, myricetin, linebacker, and caflanone [39].
2.4. Fatty Acids of Cannabis Seeds
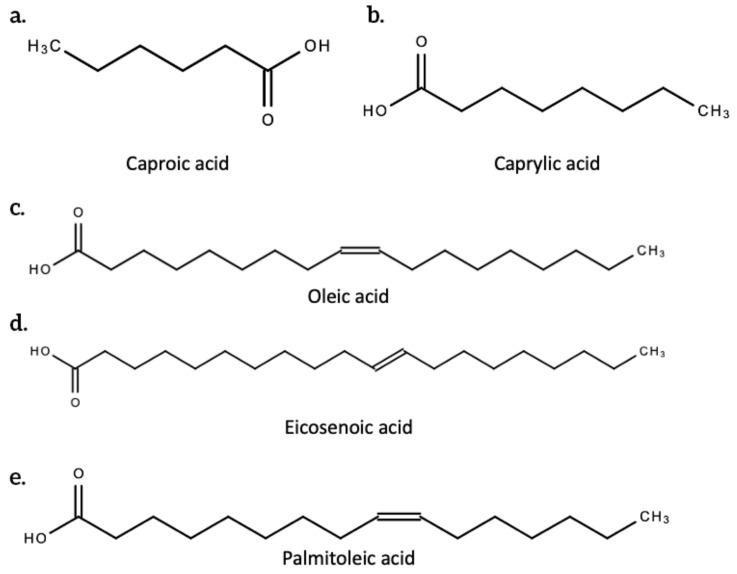
Fatty acids have high nutritional value. They are a class of molecules made up of a chain of carbon atoms bonded with hydrogen atoms, with a function carboxyl group (-COOH) attached to a terminal end. It is this functional group that participates in chemical reactions that allow fatty acids to perform their physiological roles. Figure 6 below shows the structure of some fatty acids produced by C. sativa L. These roles include providing insulation, storing and providing energy for cells in the absence of glucose, providing the precursor (cholesterol) for the production of hormones and intracellular membranes (estrogen, testosterone, vitamin D hormone, steroids, and prostaglandins), forming the building blocks of glycolipids and phospholipids which form the cell membrane and subcellular membrane, transporting fat soluble vitamins (A, D, E and K), and modifying proteins. Many of these fatty acids have nutritional and pharmaceutical potential [47].
Figure 6.
The structure of some fatty acids found in C. sativa L. [48]. (a.) Caproic acid; (b.) Caprylic acid; (c.) Oleic acid; (d.) Eicosenoic acid; (e.) Palmitoleic acid.
A 1996 study by Ross and colleagues explored the composition of fatty acids present in the lipid matter of [commercial] cannabis seeds from different countries. Some fatty acids in commercial Cannabis sativa (seeds) include caproic acid, caprylic acid, myristic acid, palmitoleic acid, palmitic acid, margaric acid, oleic acid, linolenic acid, isolinolenic acid, linoleic acid, stearic acid, eicosenoic acid, arachidic acid, isoarachidic acid, and behenic acid [48].
It should also be noted that C. sativa L. seeds, particularly those of the hemp variety, typically have nutritional value. The seed is made up of approximately 25% high-quality protein and 35% fat [49]. Table 7 below lists some vitamins, minerals and macronutrients found in C. sativa L. seeds. In addition to significant concentrations of fatty acids (particularly omega-6 fatty acids), hemp oil extracted from C. sativa L. seeds also contain vitamin C [50,51]; thiamine [50,51]; riboflavin [50,51]; vitamin E [50,51]; minerals such as calcium [50], magnesium [50,51], potassium [50,51], phosphorus [50,51], iron [50,51], zinc [50,51], sodium [50,51], and copper [51]; and macronutrients such as fat [50], carbohydrates [50], fiber [50], and protein [50].
Table 7.
Examples of vitamins, minerals and macronutrients produced by C. sativa L. seeds [50].
| Vitamins | Minerals | Macronutrients |
|---|---|---|
| C | Calcium | Fat |
| Thiamine | Iron | Carbohydrate |
| Riboflavin | Magnesium | Fiber |
| Niacin | Phosphorus | Protein |
| B-6 | Potassium | kJ:2313 |
| Folate | Sodium | |
| A | Zinc | |
| E |
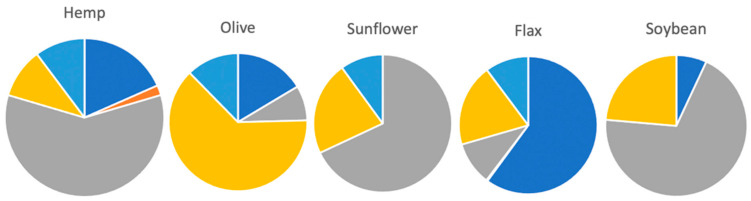
In comparison to other popular and nutritionally-rich edible oils such as canola, sunflower, pumpkin and soybean oils, only hemp oil provides the optimal ratio (3:1) of omega-6 fatty acid (linolic acid) and omega-3-fatty acids (alpha-linolenic acid), along with gamma-linolenic acid (GLA) and stearidonic acid (SDA) [49]. See Figure 7 for a comparison of the composition of edible plant oils.
Figure 7.
Comparison of the fatty acid composition of edible plant oils [49].
2.5. Alkaloids in Cannabis
In 1876, Preobrajensky claimed to find nicotine in cannabis resin (hashish) from Uzbekistan in 1876 [52]. This was later rejected on the basis that cannabis users from that part of the world tend to mix the resin with tobacco before smoking. This made it likely that the presence of nicotine was due to the tobacco. Later in 1881, at the British Pharmaceutical Conference, Siebold and Bradbury reported on the isolation of the alkaloid cannabinine [53]. Two years later, in 1883, Hay isolated tetanocannabin, another biologically active alkaloid. It was so called because it produced strychnine-like convulsions in frogs [54]. In 1986, even Merck (of Darmstadt) began marketing and advertising a “cannabine alkaloid” product [55].
Alkaloids are a class of heterocyclical organic compounds that contain one or more nitrogen atoms. They may also contain an oxygen, sulfur, chlorine, bromine, or phosphorus atom attached to the molecule. They are most associated with plants, but are also produced by microorganisms and animals [56]. In plants, alkaloids are a form of chemical defense against herbivores. Many alkaloids are pharmacologically active. In fact, alkaloids make up about 60% of plant-derived drugs [57]. On the same tangent, dndogenous indole alkaloids have been confirmed in hemp [58].
Alkaloids have a variety of therapeutic applications as analgesics, antibacterial, anticancer, antiarrhythmic, antiasthma agents, antimalarials, anticholinergics, bronchodilatory, laxative, miotic, oxytocic, vasodilatory, psychotropic, and stimulating agents. This class of compounds include morphine, cocaine, nicotine, caffeine, quinine, ephedrine, among others.
Cannabinaceous alkaloids are alkaloids produced by Cannabis sativa. These include, but are not limited to, cannabisativine and anhydrocannabisativine [59].
A 1971 study by Klein and colleagues investigated the constituents of alkaloids mixtures extracted from cannabis plants and reported the isolated of four alkaloids, namely cannabimines A–D [60]. It was also noted that both cannabimine A and anhydrocannabisativine (isolated in 1976) share the same molecular formular (C21 H37 N3 O2) [59].
Cannabisativine was the first cannabinaceous alkaloid to be fully identified. It was isolated in 1975 in Mississippi from the roots of a Mexican variant of Cannabis sativa [61]. See Figure 8 below. These alkaloids demonstrated antiparasitic, antipyretic, antiemetic, antitumor, diuretic and analgesic properties [62]. In a 2004 study, Kuethe and Comins were able to achieve total asymmetric synthesis of cannabisativine with a high degree of stereo control [63].
Figure 8.
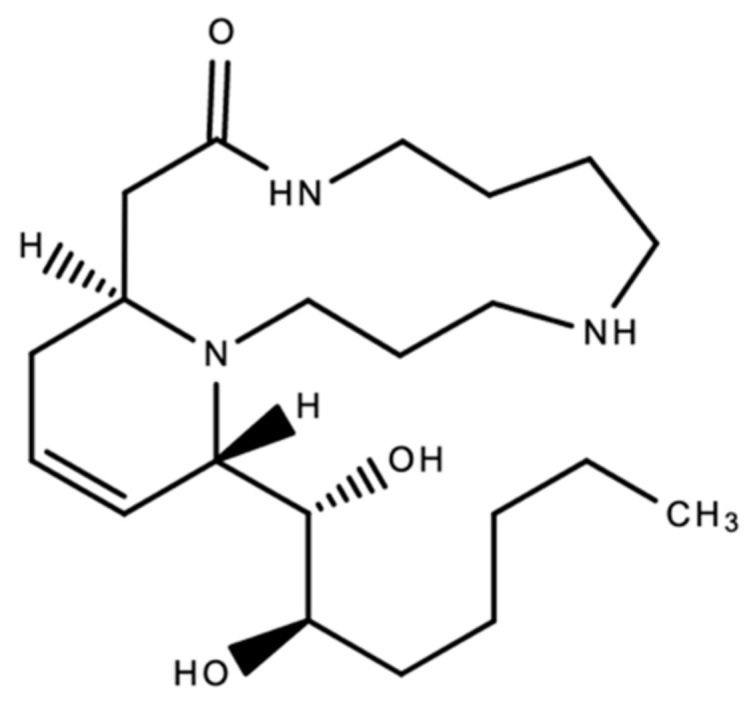
The chemical structure of cannabisativine, the first cannabinaceous alkaloid to be fully identified.
2.6. Lignanamides and Phenolic Acids
Lignanamides are another example of naturally occurring, non-cannabinoid secondary metabolites with bioactivity. This class of phytochemicals produced in cannabis include Cannabisin A–G, and Grossamide [64].
These phytochemicals are powerful anticancer, antitumor, antiviral, antioxidant, antidiabetic, cardiovascular, cytotoxic, antineoplastic, anti-inflammatory, anti-obesity, analgesic, antihyperlipidemic and antihyperlipidemic agents [64], and they also inhibit acetylcholinesterase [65]. In this 2015 study by Yan and colleagues, four new lignanamides were also isolated from the hemp seed. These were Cannabisin M, Cannabisin N, Cannabisin O, and 3,3′-demethyl-heliotropamide [65]. Cannabisins are unique to the cannabis plant. It should also be noted that phenolic amides share these bioactive properties [65].
2.7. Amino Acids
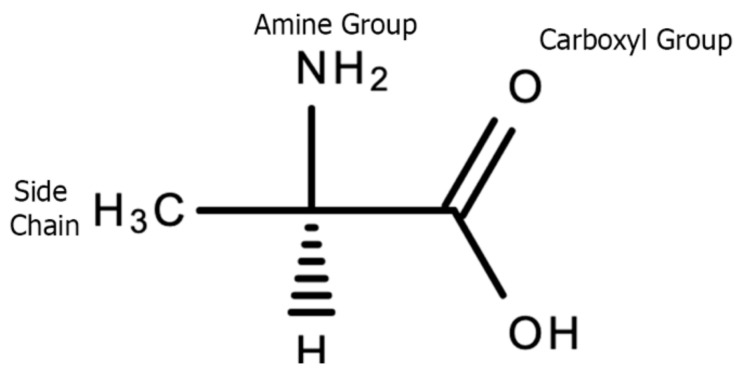
Amino acids are the building blocks of proteins which are involved in multiple biological functions in the body including energy production, fat metabolism, muscle metabolism, immune system function, response and maintenance, the synthesis of enzymes, hormones and neurotransmitters, tissue growth, nutrient absorption, sleep–wake cycles, regulation of blood sugar levels, hemoglobin production, digestion, and sexual function [66]. The amino acid molecule consists of a central carbon atom, an amino group (-NH2), a function R group side chain that defines the chemical properties of that specific amino acid, and a carboxyl group (COOH). Refer to Figure 9 below for the chemical structure of an amino acid.
Figure 9.
The basic structure of alanine, an amino acid.
Cannabis sativa (hemp) seeds contain nine essential amino acids, namely include histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and valine [67]. Essential amino acids are defined as those that cannot be synthesized in our bodies, but instead have to be obtained through food. In this same 2014 study by Audu, and colleagues, it is suggested that amino acids might be most concentrated within the leaves of the Cannabis sativa plant [67]. This is understandable because trichomes (the cell’s factory) are most concentrated in the leaves of plant. In one study aspartic acid, glutamic acid and arginine were the most prominent amino acids produced in hempseed [68]. In another study, hemp (C. sativa L.) isolates produced significantly higher levels of essential amino acid to total produced amino acids (with the exception of lysine), in comparison to a soy protein isolate [69].
Hempseed protein is unique within the plant kingdom because it contains the highest composition (65%) of the globulin edistin which make up many enzymes, antibodies, and hormones in the body [70].
2.8. Stilbenes and Stilbenoids
Stilbenes (and their derivatives) are naturally occurring polyphenolic phytochemicals that, aside from being a form of herbivore and disease resistance mechanisms for many lower and higher plants in the plant kingdom [71], have a wide range of biological activity and medicinal value to humans [72]. Stilbenoids are stilbene derivates that have been hydroxylated [73].
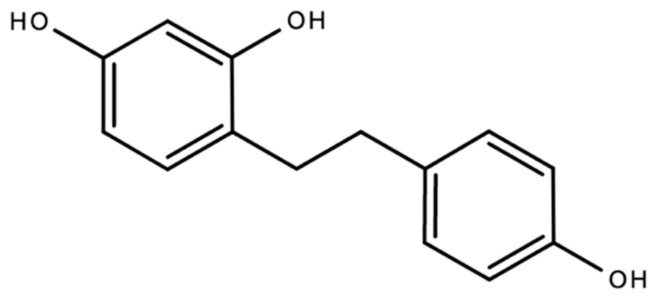
A 2004 study by El-Feraly isolated, characterized and synthesized dihydro-resveratrol (3,5,4′-trihydroxybibenzyl), a metabolite derivative of the antioxidant, resveratrol, a dihydrostilbenoid from Cannabis sativa (Figure 10 above) [74]. A 2019 study by Giménez-Bastida and colleagues reported that dihydro-resveratrol and other metabolite derivatives of resveratrol induced senescence in breast cancer cells [75]. Studies on the antioxidant activity of dihydro-resveratrol are limited. A 2016 study by Tsang and colleagues report that dihydro-resveratrol demonstrated the ability to attenuate pancreatic oxidative damage and could possibly have therapeutic potential in the management of acute pancreatitis [3].
Figure 10.
The chemical structure of dihydroresveratrol, a hydroxylated stilbene derivative.
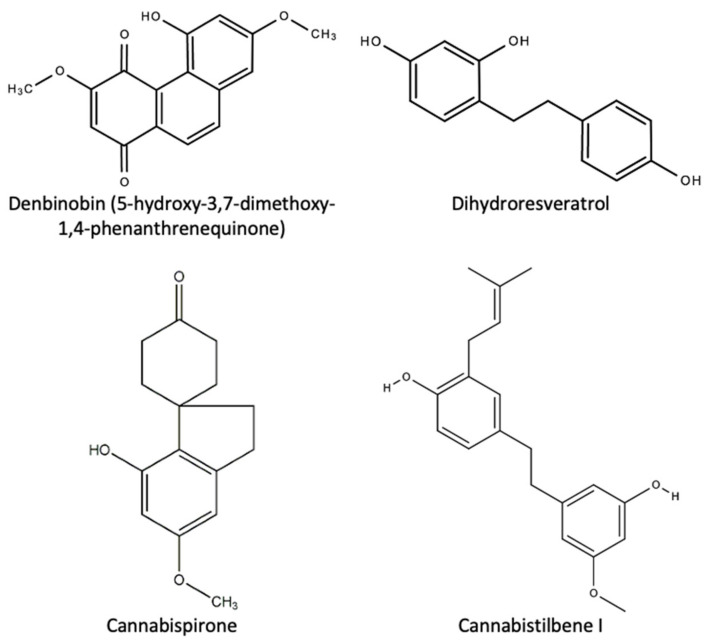
Nineteen stilbenoids unique to the cannabis plant were identified in a 1995 study by Ross and ElSohly [5]. These can be divided into three main structural classes: eleven spiroindans, eight dihydrostilbenes/bibenzyls, five phenanthrenes and prenylated, geranylated and glycosylated derivates [76]. Table 8 below is a list of these compounds.
Table 8.
Some stilbenes and their derivatives found in C. sativa L.
| Spiroindans | Eight Dihydrostilbenes/Bibenzyls | Phenanthrenes and Derivates |
|---|---|---|
|
|
|
Denbinobin, first isolated in a 2008 study by Sànchez-Duffhues, is reported to have beneficial bioactive properties to human health as an anti-HIV, antioxidant, antitumor, and inhibitor of platelet aggregation [77]. The structure of denbinobin is shown in Figure 11 below.
Figure 11.
The chemical structures of some stilbenes and their derivates found in C. sativa L.
3. Conclusions and Future Prospects
The cannabis plant produces an estimated 545 cannabinoid and non-cannabinoid phytochemicals of great economic and medicinal value [1]. These include some 104 cannabinoids (including CBD, Δ9-THC, and CBG), 120 terpenoids, over 26 flavonoids [1], lignans, phenolic amides, amino acids, nitrogenous compounds, steroids, fatty acids, alkaloids, stilbenes, vitamins, minerals and other phytochemicals. These phytochemicals are produced in virtually all areas of the plant (root, leaves, and stem). The aforementioned cannabinoids and non-cannabinoid secondary metabolites are produced primarily in the trichomes of the cannabis plant [78].
Cannabinoid and non-cannabinoid secondary metabolites have wide therapeutic application across many ailments including different types of cancers, diabetes, cardiovascular disorders, neurodegenerative disorders, inflammation-related diseases, and viral infections. Unlike THC, one of the most-studied cannabinoids, the majority of these phytochemicals are non-psychotropic. This means that they will not produce the psychoactive effects produced by THC, but will still have therapeutic benefit.
A majority of these phytochemicals require further in-depth characterization for their therapeutic efficacy and safety. There is also the need for a more rigorous standardization of cannabis cultivation practices so as to ensure consistent reproducibility of the profiles of secondary metabolites such as terpenes. The ability to reconstruct synthetic pathways, elucidate regulatory mechanism involved in the production of secondary metabolites, and establish genomic, metabolomic, transcriptomic maps/“fingerprints” of said secondary metabolites will allow drug manufacturers to produce more targeted drugs [79].
There is a need for more systematic botanical, physicochemical and chemical analyses of the cannabis plant. Further research is required to determine the efficacy, dosage standards, optimum extraction methods/solvents, cytotoxicity/hepatoxicity, pharmacokinetics, molecular mechanisms of action, phytoantiviral screening methods, and drug interactions for many phytochemicals in the cannabis plant.
Author Contributions
Conceptualization, H.L. and B.S.; writing, review and editing, H.L., B.S., J.B., N.T. and W.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This study did not involve humans or animals.
Informed Consent Statement
Not applicable. This study did not involve humans or animals.
Data Availability Statement
Data sharing is not applicable to this article. No new data were created or analyzed in this study.
Conflicts of Interest
Authors H.L. and N.T. are employees of Vilotos and Flavocure and both companies have commercial interest in caflanone. The other authors declare no conflicts of interest. Neither Vilotos nor Flavocure took part in the study or experience.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li H. An archaeological and historical account of cannabis in China. Econ. Bot. 1973;28:437–448. doi: 10.1007/BF02862859. [DOI] [Google Scholar]
- 2.Jin D., Dai K., Xie Z., Chen J. Secondary metabolites profiled in Cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-60172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang S.W., Guan Y., Wang J., Bian Z., Zhang H. Inhibition of pancreatic oxidative damage by stilbene derivative dihydro-resveratrol: Implication for treatment of acute pancreatitis. Sci. Rep. 2016;6 doi: 10.1038/srep22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur R., Matta T., Kaur H. Plant derived alkaloids. Haya Saudi J. Life Sci. 2017;2:158–189. [Google Scholar]
- 5.Ross S.A., ElSohly M.A. Constituents of Cannabis sativa L. XXVIII a review of the natural constituents: 1980–1994. Zagazig J. Pharm. Sci. 1995;4:1–10. [Google Scholar]
- 6.Turner C.E., ElSohly M.A., Boeren E.G. Constituents of Cannabis sativa L. XVII: A review of the natural constituents. J. Nat. Prod. 1980;43:169–243. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- 7.Hampson A.J., Grimaldi M., Axelrod J., Wink D. Cannabidiol and (-) 9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA. 1988;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampson A.J., Grimaldi M., Lolic M., Wink D., Rosenthal R., Axelrod J. Neuroprotective antioxidants from marijuana. Ann. N. Y. Acad. Sci. 2000;899:274–282. doi: 10.1111/j.1749-6632.2000.tb06193.x. [DOI] [PubMed] [Google Scholar]
- 9.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 11.Grotenhermen F., Russo E. Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential. 1st ed. Routledge; Oxfordshire, UK: 2002. [Google Scholar]
- 12.Booth J.K., Page J.E., Bohlmann J. Terpene synthases from Cannabis sativa. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0173911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radwan M.M., Elsohly M.A., Slade D., Ahmed S.A., Wilson L., El-Alfy A.T., Khan I.A., Ross S.A. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry. 2008;69:2627–2633. doi: 10.1016/j.phytochem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Taweel A., Perveen S. Terpenes and Terpenoids. IntechOpen; London, UK: 2018. Chapter 1. [Google Scholar]
- 15.Fischedick J.T., Hazekamp A., Erkelens T., Choi Y.H., Verpoorte R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 2010;71 doi: 10.1016/j.phytochem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Elzinga S., Fischedick J., Podkolinski R., Raber J.C. Cannabinoids and terpenes as chemotaxonomic markers in cannabis. Nat. Prod. Chem. Res. 2015;3:81. doi: 10.4172/2329-6836.1000181. [DOI] [Google Scholar]
- 17.Jacobs M. The Difference between Cannabinoids and Terpenes (Analytical Cannabis). 27 July 2020. [(accessed on 28 July 2020)]; Available online: https://www.analyticalcannabis.com/articles/the-difference-between-cannabinoids-and-terpenes-311502.
- 18.Booth J.K., Bohlmann J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019;284:67–72. doi: 10.1016/j.plantsci.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Rao V.S., Menezes A.M., Viana G.S. Effect of myrcene on nociception in mice. J. Pharm. Pharmacol. 1990;42:877–878. doi: 10.1111/j.2042-7158.1990.tb07046.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson J., Theisen E. What Are Terpenes? 6 March 2020. [(accessed on 28 July 2020)]; Available online: https://www.medicalnewstoday.com/articles/what-are-terpenes.
- 21.McPartland J.M., Russo E.B. Non-Phytocannabinoid Constituents of Cannabis and Herbal Synergy. In: Pertwee R.G., editor. Handbook of Cannabis. Oxford University Press; Oxford, UK: 2014. [Google Scholar]
- 22.Ambrosch S., Duliban C., Heger H., Moser E., Laistler E., Windischberger C., Heuberger E. Effects of 1,8-Cineole and (-)-Linalool on functional brain activation in a working memory task. Flavour Fragr. J. 2018;33:235–244. doi: 10.1002/ffj.3436. [DOI] [Google Scholar]
- 23.Vieira-Brock P.L., Vaughan B.M., Vollmer D.L. Comparison of antimicrobial activities of natural essential oils and synthetic fragrances against selected environmental pathogens. Biochim. Open. 2017;5:8–13. doi: 10.1016/j.biopen.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi H. Chemical constituents of essential oils possessing anti-influenza A/WS/33 virus activity. Osong Publ. Health Res. Perspect. 2018;9:348–353. doi: 10.24171/j.phrp.2018.9.6.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Held S., Schieberle P., Somoza V. Characterization of α-Terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J. Agric. Food Chem. 2007;55:8040–8046. doi: 10.1021/jf071691m. [DOI] [PubMed] [Google Scholar]
- 26.Quintans-Júnior L.J., Oliveira M.G., Santana M.F., Santana M.T., Guimarães A.G., Siqueira J.S., Almeida R.N. α-Terpineol reduces nociceptive behavior in mice. Pharm. Biol. 2011;49:583–586. doi: 10.3109/13880209.2010.529616. [DOI] [PubMed] [Google Scholar]
- 27.Sousa D.P., Quintans L., Almeida R.N. Evolution of the anticonvulsant activity of α-terpineol. Pharm. Biol. 2007;45:69–70. doi: 10.1080/13880200601028388. [DOI] [Google Scholar]
- 28.Souza R., Cardoso M., Menezes C., Silva J., De Sousa D., Batista J. Gastroprotective activity of α-terpineol in two experimental models of gastric ulcer in rats. DARU J. Fac. Pharm. Tehran. Univ. Med. Sci. 2011;19:277–281. [PMC free article] [PubMed] [Google Scholar]
- 29.Valente J., Zuzarte M., Gonçalves M.J., Lopes M.C., Cavaleiro C., Salgueiro L., Cruz M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxic. Intern. J. Publ. Br. Indus. Biol. Res. Assoc. 2013;62:349–354. doi: 10.1016/j.fct.2013.08.083. [DOI] [PubMed] [Google Scholar]
- 30.Cavaleiro C., Salgueiro L., Gonçalves M.J., Hrimpeng K., Pinto J., Pinto E. Antifungal activity of the essential oil of Angelica major against Candida, Cryptococcus, Aspergillus and dermatophyte species. J. Nat. Med. 2015;69:241–248. doi: 10.1007/s11418-014-0884-2. [DOI] [PubMed] [Google Scholar]
- 31.Golfakhrabadi F., Khanavi M., Ostad S.N., Saeidnia S., Vatandoost H., Abai M.R., Hafizi M., Yousefbeyk F., Rad Y.R., Baghenegadian A., et al. Biological activities and composition of ferulago carduchorum essential Oil. J. Arthropod. Borne Dis. 2014;9:104–115. [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M.J., Yang K.W., Kim S.S., Park S.M., Park K.J., Kim K.S., Choi Y.H., Cho K.K., Hyun C.G. Chemical composition and anti-inflammation activity of essential oils from Citrus unshiu flower. Nat. Prod. Comm. 2014;9:727–730. doi: 10.1177/1934578X1400900538. [DOI] [PubMed] [Google Scholar]
- 33.Loizzo M.R., Saab A.M., Tundis R., Statti G.A., Menichini F., Lampronti I., Gambari R., Cinatl J., Doerr H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodiver. 2008;5:461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izzo L., Castaldo L., Narváez A., Graziani G., Gaspari A., Rodríguez-Carrasco Y., Ritieni A. Analysis of phenolic compounds in commercial Cannabis sativa L. Inflorescences using UHPLC-Q-Orbitrap HRMS. Molecules. 2020;25:631. doi: 10.3390/molecules25030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertoia M.L., Rimm E.B., Mukamal K.J., Hu F.B., Willett W.C., Cassidy A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ. 2016;352:1–53. doi: 10.1136/bmj.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter N. What are Flavonoids? (Comprehensive Guide). 10 March 2020. [(accessed on 28 July 2020)]; Available online: https://wayofleaf.com/education/what-are-flavonoids.
- 37.Barrett M.L., Gordon D., Evans F.J. Isolation from Cannabis sativa L. Of cannflavin—A novel inhibitor of prostaglandin production. Biochem. Pharm. 1985;34:2019–2024. doi: 10.1016/0006-2952(85)90325-9. [DOI] [PubMed] [Google Scholar]
- 38.Werz O., Seegers J., Schaible A.M., Weinigel C., Barz D., Koeberle A., Appendino G. Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. Pharma Nutr. 2014;2:53–60. doi: 10.1016/j.phanu.2014.05.001. [DOI] [Google Scholar]
- 39.Ngwa W., Kumar R., Thompson D., Lyerly W., Moore R., Reid T., Toyang N. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules. 2020;25:2707. doi: 10.3390/molecules25112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowe H.I., Toyang N.J. Washington, DC: U.S. Patent and Trademark Office. No. US20180098961A1. U.S. Patent. 2019 Sep 3;
- 41.Lowe H.I., Toyang N.J. Washington, DC: U.S. Patent and Trademark Office. No. WO2017091837A2. U.S. Patent. 2017 Jun 1;
- 42.Lowe H.I., Toyang N.J., Bryant J. Washington, DC: U.S. Patent and Trademark Office. No. US20170360744A1. U.S. Patent. 2020 Sep 8;
- 43.Lowe H.I., Toyang N.J. Washington, DC: U.S. Patent and Trademark Office. No. US10278950B2. U.S. Patent. 2019 May 7;
- 44.Lowe H.I., Toyang N.J. Washington, DC: U.S. Patent and Trademark Office. No. US20190083452A1. U.S. Patent. 2020 Aug 25;
- 45.Lowe H.I., Toyang N.J. Washington, DC: U.S. Patent and Trademark Office. No. WO2018022868A1. U.S. Patent. 2018 Feb 1;
- 46.Lowe H.I., Toyang N.J. Washington, DC: U.S. Patent and Trademark Office. No. US20180214389A1. U.S. Patent. 2018 Aug 2;
- 47.Mölleken H., Theimer R.R. Survey of minor fatty acids in Cannabis sativa L. fruits of various origins. [(accessed on 15 November 2020)];J. Int. Hemp Assoc. 1997 4:13–17. Available online: http://www.internationalhempassociation.org/jiha/jiha4107.html. [Google Scholar]
- 48.Ross S.A., ElSohly H.N., ElKashoury E.A., ElSohly M.A. Fatty acids of Cannabis seeds. Phytochem. Anal. 1996;7:279–283. doi: 10.1002/(SICI)1099-1565(199611)7:6<279::AID-PCA322>3.0.CO;2-P. [DOI] [Google Scholar]
- 49.Hazekamp A., Fischedick J., Llano-Diez M., Lubbe A., Ruhaak L. Comprehensive Natural Products Chemistry II. 2nd ed. Elsevier Ltd.; Oxford, UK: 2010. Chemistry of Cannabis. [DOI] [Google Scholar]
- 50.Cooke J. Does Cannabis Have Nutritional Benefits? [(accessed on 28 July 2020)]; Available online: https://thesunlightexperiment.com/blog/cannabis-nutritional-benefits.
- 51.Pontassuglia L., Dongo D. Hemp, the Italian Superfood–Hemp. [(accessed on 28 July 2020)]; Available online: https://www.greatitalianfoodtrade.it/en/markets/hemp-the-italian-superfood.
- 52.Kennedy G.W. Proceedings of the American Pharmaceutical Association at the Annual Meeting. Volume 34. The Association; Philadelphia, PA, USA: 1886. The Asserted Presence of Nicotine in Cannabis; pp. 119–121. [Google Scholar]
- 53.Siebold L., Bradbury T. Note on the alleged presence of Nicotine in Indian hemp. Pharm. J. Trans. 1881;12:326–327. [Google Scholar]
- 54.Hay M. A new alkaloid in Cannabis indica. Pharm. J. Trans. 1883;13:996–997. [Google Scholar]
- 55.Merck E. Merck's 1896 Index: An Encyclopedia for the Physician and the Pharmacist: Stating the Names and Synonyms, Source of Origin, Chemical Nature and Formulas, Physical Form, Appearance, and Properties, Melting and Boiling Points, Solubilities, Gravities and Percentage Strengths, Physiological Effects, Therapeutic Uses, Modes of Administration and Application, Regular and Maximum Dosage, Incompatibles, Antidotes: Special Cautions, Hints on Keeping and Handling, Methods of Testing, Market Values, etc., etc. of the Chemicals and Drugs Used in Medicine, in Chemistry, and in the Arts. Merck & Co.; New York, NY, USA: 1895. [Google Scholar]
- 56.Roberts M.F. In: Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Wink M., editor. Springer; Boston, MA, USA: 1998. OCLC 851770197. [Google Scholar]
- 57.Gowda L., Venkategowda A. Isolation & characterization of antimicrobial alklaoids from Plumeria albaflowers against food borne pathogens. Am. J. Life Sci. 2014;2:1–6. [Google Scholar]
- 58.Yan X., Zhou Y., Tang J., Ji M., Lou H., Fan P. Diketopiperazine indole alkaloids from hemp seed. Phytochem. Lett. 2016;18:77–82. doi: 10.1016/j.phytol.2016.09.001. [DOI] [Google Scholar]
- 59.Mechoulam R., Hanuš L. A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids. 2000;108:1–13. doi: 10.1016/S0009-3084(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 60.Klein F.K., Rapoport H., Elliott H.W. Cannabis alkaloids. Nature. 1971;232:258–259. doi: 10.1038/232258a0. [DOI] [PubMed] [Google Scholar]
- 61.Lotter H.L., Abraham D.J., Turner C.E., Knapp J.E., Schiff P.L., Slatkin D.J., Jr. Cannabisativine. A new alkaloid from Cannabis sati6a L. Root. Tetrahedron Lett. 1975;33:2815–2818. doi: 10.1016/S0040-4039(00)75003-9. [DOI] [Google Scholar]
- 62.Wink M. Potential of DNA intercalating alkaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity. 2020;12:175. doi: 10.3390/d12050175. [DOI] [Google Scholar]
- 63.Kuethe J.T., Comins D.L. Asymmetric total synthesis of (+)-cannabisativine (I) Chem. Inform. 2004;35 doi: 10.1021/jo049724. [DOI] [PubMed] [Google Scholar]
- 64.Flores-Sánchez I.J., Verpoorte R. Secondary metabolism in cannabis. Phytochem. Rev. 2008;7:615–639. doi: 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- 65.Yan X., Tang J., Passos C., Nurisso A., Simões-Pires C., Ji M., Lou H., Fan P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015;63 doi: 10.1021/acs.jafc.5b05282. [DOI] [PubMed] [Google Scholar]
- 66.Kubala J. Essential Amino Acids: Definition, Benefits and Food Sources. 12 June 2018. [(accessed on 28 July 2020)]; Available online: https://www.healthline.com/nutrition/essential-amino-acids.
- 67.Audu B.S., Ofojekwu P.C., Ujah A., Ajima M.N.O. Phytochemical, proximate composition, amino acid profile and characterization of Marijuana (Cannabis sativa L.) [(accessed on 26 July 2020)];J. Phytopharm. 2014 3:35–43. Scientific Figure on ResearchGate. Available online: https://www.researchgate.net/figure/Amino-acid-composition-of-the-leaf-stem-and-seeds-of-Cannabis-sativa-obtained-from_tbl3_273692476. [Google Scholar]
- 68.Jang H., Park S., Nam J. The Effects of Heat Treatment on the Nutritional Composition and Antioxidant Properties of Hempseed (Cannabis sativa L.) J. Korean Soc. Food Sci. Nutr. 2018;47:885–894. doi: 10.3746/jkfn.2018.47.9.885. [DOI] [Google Scholar]
- 69.Wang X., Tang C., Yang X., Gao W. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008;107:11–18. doi: 10.1016/j.foodchem.2007.06.064. [DOI] [Google Scholar]
- 70.Osburn L. Hemp seed: The most nutritionally complete food source in the world. Hemp. Line J. 1992;1:14–15. [Google Scholar]
- 71.Orsini F., Verotta L. Stilbenes and bibenzyls with potential anticancer or chemopreventive activity. In: Zappia V., Della Ragione F., Barbarisi A., Russo G.L., Iacovo R.D., editors. Advances in Nutrition and Cancer 2. Advances in Experimental Medicine and Biology. Volume 472. Springer; Boston, MA, USA: 1999. [DOI] [PubMed] [Google Scholar]
- 72.Baur J., Sinclair D. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 73.Montané X., Kowalczyk O., Reig-Vano B., Bajek A., Roszkowski K., Tomczyk R., Tylkowski B. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules. 2020;25:3342. doi: 10.3390/molecules25153342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Feraly F. Isolation, characterization, and synthesis of 3,5,4’–Trihydroxybibenzyl from cannabis sativa. J. Nat. Prod. 2004;47 doi: 10.1021/np50031a011. [DOI] [Google Scholar]
- 75.Giménez-Bastida J.A., Ávila-Gálvez M.Á., Espín J.C., González-Sarrías A. Conjugated physiological resveratrol metabolites induce senescence in breast cancer cells: Role of p53/p21 and p16/Rb pathways, and ABC transporters. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201900629. [DOI] [PubMed] [Google Scholar]
- 76.Pollastro F., Minassi A., Fresu L. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018;24 doi: 10.2174/0929867324666170810164636. [DOI] [PubMed] [Google Scholar]
- 77.Sànchez-Duffhues G., Calzado M.A., de Vinuesa A.G., Caballero F.J., Ech-Chahad A., Appendino G., Krohn K., Fiebich B.L., Muñoz E. Denbinobin, a naturally occurring 1,4-phenanthrenequinone, inhibits HIV-1 replication through an NF-kB-dependent pathway. Biochem. Pharmacol. 2008;76:1240–1250. doi: 10.1016/j.bcp.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Gonçalves J., Rosado T., Soares S., Simão A., Caramelo D., Luís Â., Duarte A. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines. 2019;6:31. doi: 10.3390/medicines6010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andre C., Hausman J.F., Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016:7. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article. No new data were created or analyzed in this study.