Abstract
Atmospheric fine particulate matter (PM2.5) transports polycyclic aromatic hydrocarbons (PAHs) regionally and globally, influencing the air quality of communities around the planet. Concentrations of 130 PAHs extracted from PM2.5, collected on a Native American Tribal Reservation in the Northern Puget Sound region of the American Pacific Northwest, were used to assess the air quality impacts of regional and local PAH sources, atmospheric transport, and human health implications. Wind coming from the southeast of the sampling locations increased the overall PAH concentration of the PM2.5, while winds from the southwest decreased the PAH concentration. Concentrations of PAH subclasses increased or decreased independently at the two sampling locations with different changes in wind patterns, changing the excess lifetime cancer risk significantly. No long-range transport was measured, but emissions from local and regional PAH sources were measured. Samples collected during regional wildfires showed increased PAH concentrations. Samples collected during predicted weather inversions resulted in the highest PAH concentrations, and up to a ten-fold increase in excess lifetime cancer risk over the normal days.
Keywords: Polycyclic Aromatic Hydrocarbons (PAHs), Atmospheric Fine Particulate Matter (PM2.5), Tribal reservation air quality
GRAPHICAL ABSTRACT
1. 0. INTRODUCTION
Atmospheric fine particulate matter (PM2.5) exposure is responsible for an array of respiratory and cardiovascular diseases. (Harrison and Yin (2000)). As of 2017, PM2.5 exposure has been directly linked to around 7 million deaths per year around the world. (Landrigan, et al. (2018)). The search to understand the mechanisms for PM2.5 toxicity have led to the identification of organic components in PM2.5 which are responsible for an array of adverse health effects. (Patel and Rastogi (2018), Tuet, et al. (2017)). Anthropogenic activity has increased the mass of organic compounds traversing the global atmosphere in PM2.5. (Landrigan, et al. (2018), Li, et al. (2017)).
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants found naturally in petrochemicals, and released as byproducts of incomplete combustion. Primbs, et al. (2008) Genualdi, et al. (2009) Jia, et al. (2011) Atmospheric emissions, mainly attributed to incomplete combustion, can undergo long-range atmospheric transport bound up in PM2.5. (Primbs, et al. (2008), Genualdi, et al. (2009), Jia, et al. (2011)). Some unsubstituted PAHs (UPAHs) have been well characterized, in terms of toxicity, which has led to the inclusion of 16 of these compounds on the US Environmental Protection Agency’s (US EPA) Priority Pollution List (PPL). PAHs exist in many subclasses other than UPAHs: heterocyclic (HPAHs) contain a non-carbon atom in the ring structure, −NO2 substituted (NPAHs), and carbonyl substituted (OPAHs), and high molecular weight (HMWs), characterized by having an atomic mass of more than 302 Da. These subclasses can all be directly emitted from various sources to the atmosphere along with UPAHs, and have been characterized in PM2.5. (Jariyasopit, et al. (2014), Zhao, et al. (2014), Wang, et al. (2011) (Genualdi, et al. (2009), Jariyasopit, et al. (2014)). Substitution reactions can occur through atmospheric reactions of UPAHs with an array of atmospheric reactants, specifically, nitrogen oxide compounds (NOx), ozone (O3), and hydroxyl (OH•) radicals. These reactions can lead to NPAHs, OPAHs, and hydroxy (OHPAHs) substitutions to a hydrogen on the UPAH rings. (Jariyasopit, et al. (2014), Kramer, et al. (2019)). While UPAH exposure has been linked to inflammation (Liu, et al. (2010)), cardiopulmonary and respiratory diseases (Hu, et al. (2018)), and cancer, the human health implications (Luo, et al. (2015), Geier, et al. (2018)) of the other subclasses of PAHs remains highly unclassified.
Native Americans have strong cultural, spiritual, and physical relationships with the natural environment they evolved to inhabit. (Indians (2015). Due to colonization and relocation by the US Federal government, Native American reservations comprise a fraction of the lands each Tribe previously inhabited. (Dippel (2014)). This constriction of land for Native American societies, results in increased sensitivity to changes to the environment. (Dippel (2014), Basnayake, et al. (2017)). Globally, indigenous peoples are disproportionately susceptible to and affected by PM2.5 adverse health outcomes. (Basnayake, et al. (2017)). One example is the Swinomish Indian Tribal Community whose reservation was recognized in 1855 by the Treaty of Point Elliot. In 1955 and 1958, two oil refineries were built on March Point, which is within the Treaty Reservation Boundary. These oil refineries have contributed to local PAH pollution in the marine environment Minick, et al. (2019)). Unplanned air toxic releases have also impacted the Swinomish reservation Rohlman, et al. (2019)).
When air masses move over emissions sources, PM2.5 is transported downwind of the source, sometimes resulting in PM2.5 transport thousands of miles from sources. (Primbs, et al. (2008), Genualdi, et al. (2009), Jia, et al. (2011), Jariyasopit, et al. (2014)). Events such as wildfires greatly increase the amount of PAH containing PM2.5 entering and transporting throughout the atmosphere. (Navarro, et al. (2016)). When atmospheric inversions occur, warm air masses move over cold air masses, trapping the cold air nearer the surface. Such atmospheric pressure systems result in lessened vertical air movement, which results in increased PM2.5, O3, and NOx concentrations during the inversion events. (Gillies, et al. (2010) Hou and Wu (2016)). Changes in weather patterns, wind directions, and atmospheric conditions can result in significantly different air quality concerns at any one location.
The objective of this study was to assess local and regional source contributions to PAH concentrations on a tribal reservation in the Pacific Northwest, and their impact on inhalation health risks. In partnership with the Swinomish Indian Tribal Community (SITC), located near the San Juan Islands of the upper Pacific Northwest (USA), an extensive air quality study measured UPAHs, as well as NPAHs, OPAHs, HPAHs, OHPAHs, and HMWs, on PM2.5 from two air samplers on the SITC Reservation. PM2.5 samples were collected at the same two locations as meteorological measurements for the assessment of the impact of local and/or regional emission sources and weather patterns on the air quality of the SITC Reservation. Well established diagnostic ratio analysis of PAHs, and positive matrix factorization was used to assess if sources of PAHs could be attributed.
2.0. MATERIALS AND METHODS
2.1. Materials.
Compound names, abbreviations, manufacturers, main emission sources, and estimated detection limits can be found on Table A1 of the appendix. Derivatization of hydroxylated PAHs (OHPAH) occurred using N-tertbutyldimethylsilyl-N-methyl-trifluoroacetamide (MTBSTFA). (Schummer, et al. (2009)). Quartz fiber filters (QFFs) were purchased from G. E. Whatman (Buckinghamshire, UK) in 8 × 11 inch sheets (PM2.5), and in 4 × 5.5 inch slotted sheets (larger PM). QFFs were prepared by placing individually into aluminum packets and baked at >350°C for 12 hours to remove organic contaminants and sealed in the aluminum packets, and then in plastic bags. After PM collection, all filters were resealed in their packets, and then stored and transported on ice to reduce vaporization losses during transport.
2.2. Sample collection.
Two Tisch Environmental (Village of Cleves Ohio, USA) High Volume Cascade Impact air samplers were installed 7 km apart from each other on the Swinomish Reservation on the Northwest coast of the US State of Washington (Figure 1). Sampling locations were chosen to coordinate with meteorological stations owned and operated by the Swinomish Indian Tribal Community. The first location was situated in a neighborhood on a hill, 48 meters (asl) above the main business district of the Swinomish Village (Town). (Town: 48.397789 (latitude), −122.504971 (longitude)). The second location was on the Reservation, situated near a Tribal owned and operated casino, and about 1 km off the fence-line (Fence) of two oil refineries that are located within the Reservation recognized by the 1855 Treaty (Fence: 48.459928 (latitude), −122.520388(longitude), 1.5 m asl). Tribal community employees were trained to operate and collect ambient PM2.5 samples. Paired samples were collected during the same 24-hour periods between April 2016 and September 2018. A total of 56 pairs of filters were analyzed for this study. Of the 56 pairs, 48 were used for wind pattern analysis, 5 pairs were collected during periods of National Weather Service (NWS) predicted impact from nearby wildfires (Fires), and 3 pairs were collected during weather inversion events (Inversions) predicted by weather forecasts. Days with more than 3 hours of ≥ 0.01 inch of precipitation were not used for this study. Data from Fires and Inversions were analyzed separately from the rest of the data (Normal).
Figure 1. Local and Regional map of the Swinomish Indian Tribal Community Reservation.
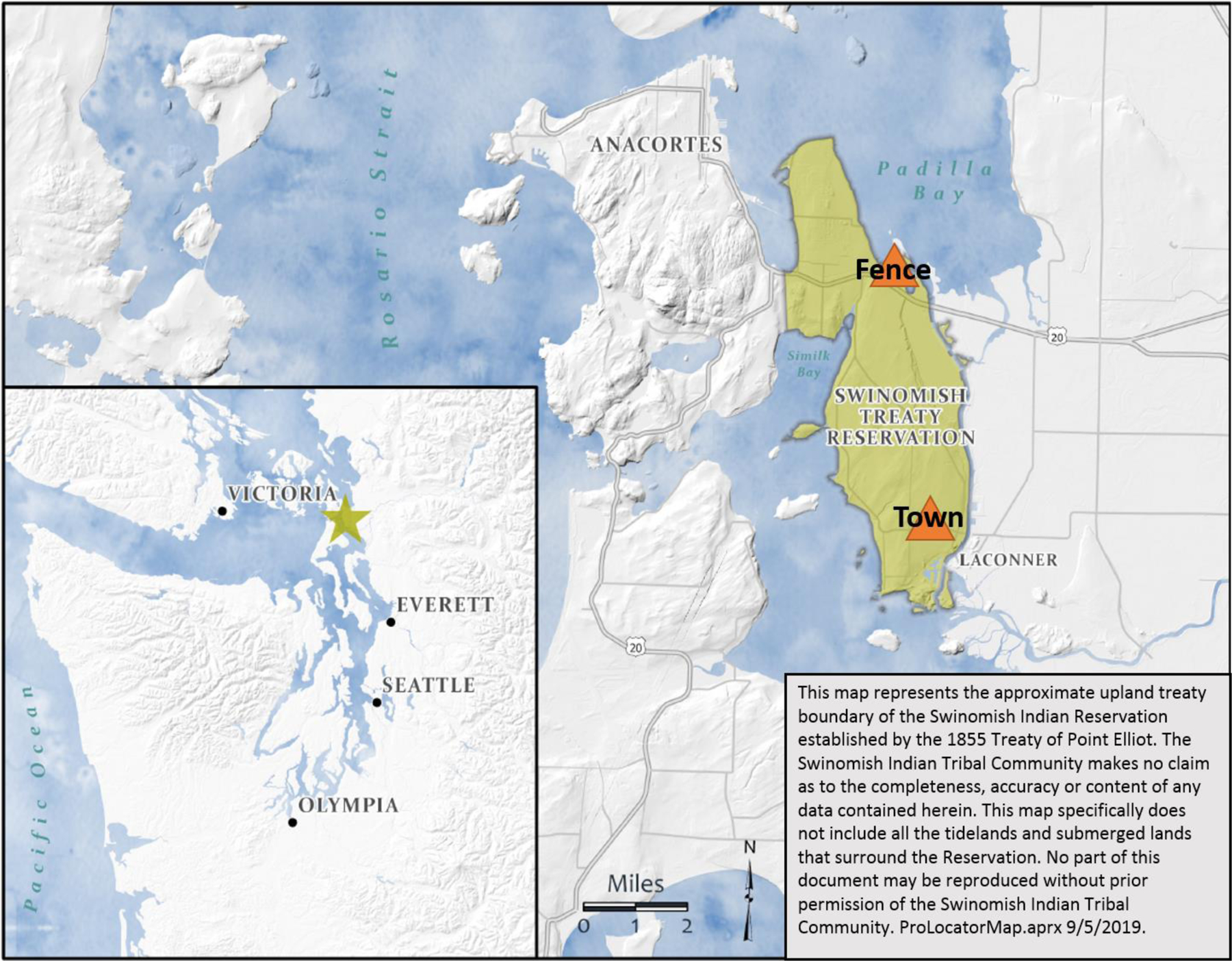
Map represents the 1855 Treaty of Point Elliot boundaries of the Swinomish Indian Tribal Reservation. Triangles mark the two sampling locations (Fence and Town).
2.3. PM2.5 extraction.
Collected sample filters were sealed in prebaked aluminum packets and sealed plastic bags, and stored at −20°C until extraction. Filters were extracted using ThermoFisher (USA) Dionex ASE 350 – Accelerated Solvent Extractor. Two cycles of dichloromethane and two cycles of a 2:1 mixture of ethyl acetate: acetone were used in 100% volume (66 mL ASE cells) with oven temperature set to 100°C, to extract both non-polar PAHs as well as more polar PAH transformation products. ASE extracts were cleaned using Agilent Technologies (Santa Clara California, USA) 500 mg silica solid phase extraction (SPE) cartridges, and concentrated to a final volume of 1000 µL in ethyl acetate. Filters were extracted without the additional isotope labeled surrogate compounds to ensure extracts could be used for future toxicological studies. Isotope labeled standards were added before GC/MS analysis for quantification purposes. Final extraction efficiency was evaluated by spiking analytes onto clean QFF and extracting along with filters. Extraction efficiency was measured at: UPAH = 81%, HMW = 86%, NPAH = 124%, OPAH = 127%, HPAH = 76%, and OHPAH 54%. To monitor extraction efficiency throughout extraction processes, standard reference material (NIST SRM 1648A) was weighed onto clean filters, and extracted alongside sample filters. SRM 1648A has certified concentrations for only some of the analytes measured in this study. For certified concentrations in the SRM, the relative standard deviation of the measured compounds ranged from 1% – 26%, suggesting the extraction was consistent throughout the study.
2.4. PAH characterization.
SPE extracts were characterized for UPAH, HPAH, OPAH, and OHPAH using Agilent 7890 Gas Chromatograph (GC), while HMWs were characterized on an Agilent 6890N GC, each partnered to a quadrupole mass spectrometer run in electron impact mode at 70 electron volt. NPAH characterization occurred using Agilent 6890 GC partnered to a quadrupole mass spectrometer run in chemical ionization mode. Identification and quantification for UPAH, NPAH, OPAH, OHPAH, and HPAH was performed using an Agilent J&W DB-50Mg 30 m × 0.25 mm i.d. (0.25μm film thickness) DB-5 capillary column, and HMW were analyzed using an Agilent J&W DB-17Mg 60 m × 0.25 mm i.d. Detailed GC-MS methods can be found in supplementary information (Appendix pages A10–A13). Extracts were analyzed using selected ion monitoring (SIM) mode, to look for and quantify PAHs and PAH-OPs with available analytical standards. Calibration curves, with linear ranges from 1–1000 pg/m3 (r2 > 0.995) were used for quantification of each class of PAH. To measure hydroxy substituted PAHs (OHPAHs), derivatization using MTBSTFA for was performed. This process is described in the Appendix (A14). Mass spectra of GC-MS fragmentation were interpreted using Mass Hunter (UPAH, OPAH, HPAH, OHPAH) or ChemStation (NPAH and HMW) software. To account for non-sample contamination, clean QFF were extracted alongside sample filters as lab blanks. Lab blank measured concentrations of all analytes were subtracted from their measured concentrations. Calibration on the High Volume motors, air samplers allowed for all measured concentrations to be calculated back to the mass of analyte per cubic meter of sampled air, and is presented as pg/m3 air.
2.5. Meteorological data.
Meteorological data such as wind speed, wind direction, relative humidity, precipitation, barometric pressure, etc., were collected hourly at the two sampling locations using R.M. Young Company model 05305 (Traverse City Michigan, USA). Using trigonometric sine and cosine functions, wind speed (mph) and direction (degrees) for each sample were transformed to (X,Y) coordinates for wind ratio analysis. Nitric oxide (NO), nitrogen dioxide (NO2) were collected, at the Fence location, using Teledyne API T200 (Caringbah New South Wales, Australia), and ozone (O3) readings were collected, at the Town location, using a Thermo 49i (Franklin Massachusetts, USA). To assess if local emissions were being transformed, versus transported from regional or global sources, NOx and O3 data were correlated with wind and compound measurements. Temperature and barometric pressure were monitored using Campbell Scientific Inc, (Logan Utah, USA) model Viasala HMP45C (temperature and relative humidity), and Vaisala PTB101B (barometric pressure). Meteorological and atmospheric instrumentation is owned and operated by the Swinomish Indian Tribal Community, and data was shared with us when available. Temperature and pressure were used to calibrate the High Volume air sample motors and calculate air mass sampled each day at each location.
To asses if air masses were influenced from trans-Pacific long-range atmospheric transport, HySplit back trajectories were calculated using the NOAA Air Resource Library (ARL) publically available on the www.noaa.gov website. Analysis of HySplit data for 7-day back trajectories for the sampling days did not indicate significant long-range atmospheric transport, indicating instead only local and regional transport.
2.6. Human Health Implications.
Benzo(a)pyrene (BaP) has long been used as the standard for assessing PAH toxicity in the environment. With well documented toxicity and carcinogenic properties, BaP concentrations are often used as a representation of all PAHs present in samples. Worldwide, there are exposure limits to BaP concentrations set under the context of expected lifetime cancer risk. In their 2010 report, The World Health Organization (WHO), documents that exposure to a BaP concentration of 1200 pg/m3 air, increases the risk of developing cancer to 1 in 10,000. (Hyunok Choi (2010)). Due to this, a recommended BaP concentration limit of 1000 pg/m3 air has been set in the European Union to diminish the increased risk of developing cancer due to BaP inhalation exposure. (Commission (2019)).
To better encompass the breadth of PAHs present in PM2.5, relative potency factors (RPFs), established by the US Environmental Protection Agency, are used to calculate BaP equivalent (BaPEQ) concentrations of samples for risk assessment purposes. (Agency (2010)). These RPF values act to transfer a measured PAH concentration to a relative BaP concentration, using established carcinogenic potency factors, which can then be used to perform risk assessments. BaPEQ concentrations, (calculated using equation 1), derived from available RPF values were used in this study to perform inhalation risk assessment. Agency (2010)
| Equation 1: |
This assessment is based on known toxicity, assumes average inhalation rates, body mass, and exposure lengths over an adult lifetime (70 years). Together these assumed values are used to create unit risk factors (URBaP) which are set by various governmental and/or regulatory agencies. This risk assessment (equation 2) estimates the excess risk of developing cancer due to inhalation exposure of measured compounds.
| Equation 2: |
Due to the fact that many Native American Tribal lands encompass both sides of the US / Canadian border, the WHO inhalation URBaP (of 8.7 × 10−5 per ng/m3 of measured BaP) was used to calculate inhalation excess lifetime cancer risk for this study. Indians (2015) Hyunok Choi (2010) Jariyasopit, et al. (2014) Substituting the European Union regulatory limit of 1000 pg/m3 air BaP in this equation results in an extra 87 out of 1 million (excess cancer risk of 8.7 × 10−5) cases of cancer expected due to a lifetime of exposure to the measured concentration.
2.7. Statistical analysis.
PAH concentrations were censored for statistical modeling. Non-detect measurements were censored by using half of the method detection limits (MDL), and measurements that fell between the MDL and limit of quantification were assigned the MDL value for each compound Hites (2019), Claus P. Haslauer (2017)). MDLs, calculated using US EPA methods, for all compounds can be found in Appendix Table A1 US EPA (2010)). Pearson matrix correlations, and Student’s t-tests were performed using R statistical software, on the R-Studio user platform, on censored measurements for each day and location. Compounds measured in less than 60% of the samples were excluded from statistical analysis. Statistical significance is reported as significant if p-value ≤ 0.05. In reported data, the number of samples is indicated with ‘n = x’. Error bars on graphs, and (± x) values represent the standard error.
2.8. Source apportionment.
PAH Diagnostic ratios found in Yunker et al. 2002, were used to identify possible source contributions. To assess if crude petroleum from the nearby oil refineries was affecting the air quality over the Reservation, the ratio of PAHs with an atomic mass of 202, (FLN / FLN + PYR) was used. The ratio of PAHs with an atomic mass of 276, (IcdP / IcdP + BghiP), was used to distinguish between different types of combustion. Retene, which has been linked to biomass combustion, was analyzed along with UPAHs, and considered for evidence of biomass burning. Ramdahl (1983)
Positive matrix factorization (PMF) was performed using the EPA PMF 5.0 software package. Possible sources in the region include wood/biomass burning, crude oil, automobile emissions, ship emissions, and industrial emissions. PMF parameters included 6 factors, 20 runs, bootstrap of 100 times with a minimum correlation value of 0.7.
3.0. RESULTS
3.1. Analysis of wind speed and direction during normal atmospheric conditions.
To determine the effect of wind direction and wind speed on PAH concentrations at the two sampling locations, the plotted hourly polar coordinates of wind speed and wind direction, (for each 24-h sample and sampling location), were binned into one of four quadrants: Northwest (NW), Northeast (NE), Southeast (SE) and Southwest (SW). The number of hours the resulting coordinate was in one of the four quadrants was used to calculate the percentage of time the wind came from that wind direction. For comparative purposes, samples were grouped based on differences in wind direction during the sampling timeframe. A cutoff of < 20% of the wind coming from one wind direction was compared to when >80% of the wind was coming from same wind direction. For the NW wind direction, > 70% was used to keep the number of samples at or above three for statistical purposes. No days at the Fence location had winds from the NE that were more than 30% during the sampling period, while no days at the Town location had winds from the NE that were more than 50% during the sampling period. Correlations of individual compound concentrations with winds coming from both SE and SW directions indicated a significant air quality impact over the sampling locations (Table A3). Wind coming from the NW and NE had < 10 individual PAH concentrations correlated with the wind. Due to the low prevalence of winds from the NE, and the low number of PAH concentration correlations with NE winds, the NE wind direction was considered to not be influential on air quality during these sampling days, and is therefore not discussed in the detailed results.
Comparison of PAH concentrations in PM2.5 collected when there was <20% to >80% of SE winds during the sample collection, had significantly lower (p-value < 0.05) ΣOPAH concentrations at both sampling locations (Figure 2 C). At the Fence sampling location, comparison of <20% of the wind coming from the SE (n=23) to >80% of the wind coming from the SE (n=9) resulted in a significant decrease in the sum of ΣOHPAH concentrations. While the sum of the other classes of PAHs did not significantly change with the increase in SE wind during sampling, a number of individual quinone-, dione-, and hydroxy PAH concentrations decreased by more than 65% when there was more SE wind during the sample collection (Table A2).
Figure 2. ΣPAH class graphs for each location and wind direction.
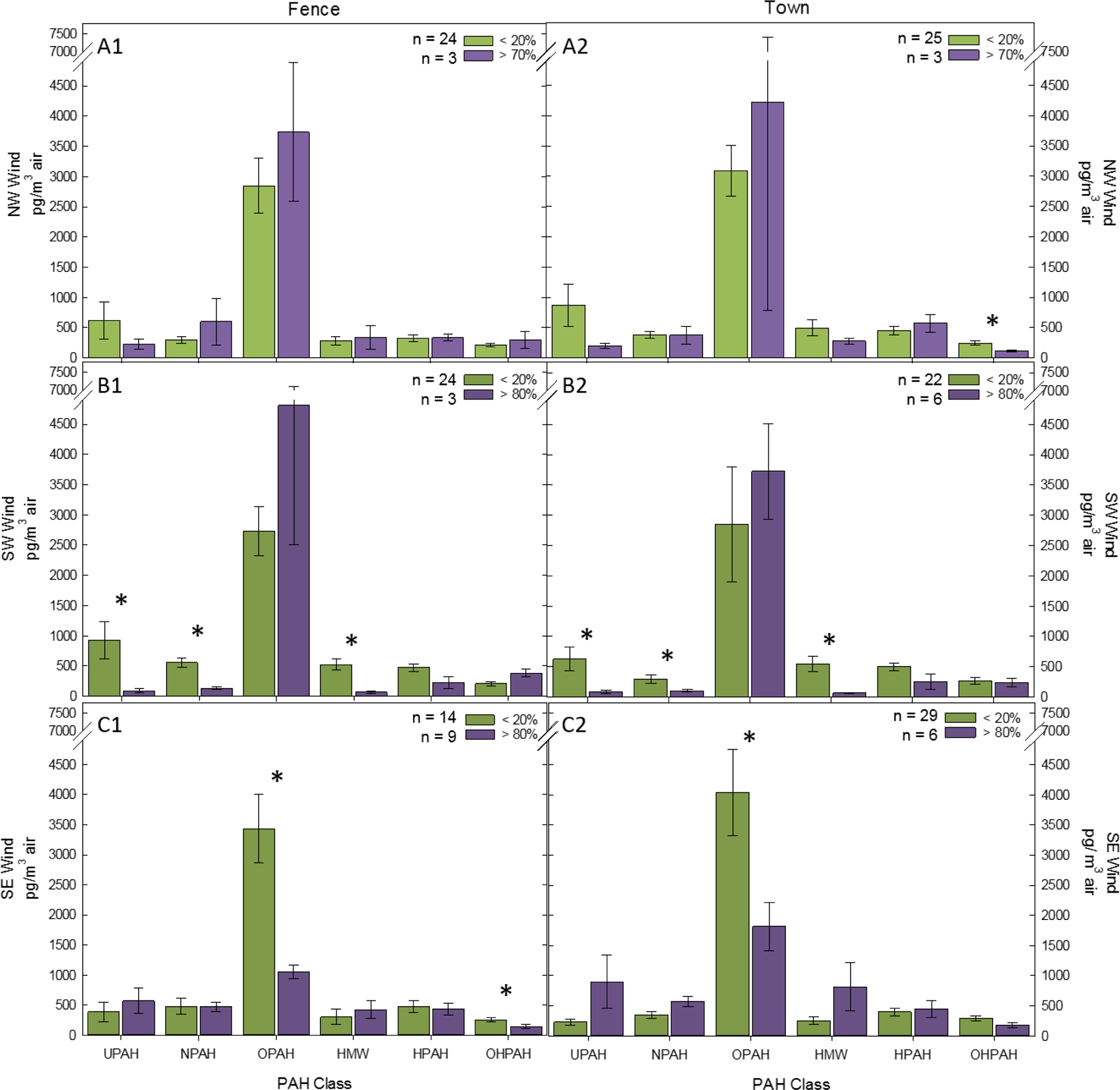
Figure 2 shows the average sum of concentrations for each PAH class (ǂ SE) for each wind direction (A = NW, B = SW, C = SE wind), for each location (1 & 2). In each panel, green bars represent data for days with < 20% wind coming from the indicated direction, and purple bars represent days with > 70% for NW, and > 80% for SW and SE wind coming from each direction. “n” indicates the number of samples that matched that particular wind direction criteria. * indicates a significant change (Student’s t-test p-value < 0.05) in the sum concentration of each class between low to high wind conditions.
Comparison of PAH concentrations in PM2.5 collected when there was <20% to >80% of SW winds during the sampling collection, had significantly lower ΣUPAH, ΣNPAH and ΣHMW concentrations at both sampling locations (Figure 2 B). While ΣOPAHs and ΣOHPAH concentrations did not significantly change, with the increase in SW wind during sampling, 1,4-anthraquinone, 9,10-phenanthrequinone, and 2-hydroxyfluorene concentrations significantly increased at the Town sampling location when the SW wind increased from <20% (n=22) to >80% (n=6) of the sample collection. Chromone concentrations significantly increased at the Fence sample location with the increase of SW wind from <20% (n=24) to >80% (n=3) during sample collection (Table A2). Many individual PAH concentrations decreased significantly with the increase of SW wind at both sampling locations (Table A2).
When the wind coming from the NW directional quadrant increased from < 20% (n=25) to > 70% (n=3), a significant decrease in the ΣOHPAH concentration was measured at the Town location (Figure 2, Table A2), while both 2,6-dihydroxynaphthalene and 3-hydroxybenz(a)anthracene concentrations both increased significantly (Table A2). No significant changes were measured in the sum of any of the PAH class concentrations at the Fence sampling location with the increase of wind coming from the NW (< 20% = 24 samples, > 70% = 3 samples). Several individual PAHs were measured in significantly lower concentrations in the Town sampling location collected when > 70% of the wind was coming from the NW during the sampling timeframe (Table A2).
3.2. Sampling Location Comparison.
The PAH concentrations at the two sampling locations were compared to identify local emissions sources unique to each sampling location. The PAH concentrations and profiles were not statistically significantly different between the Fence and Town sampling locations (Figure 2), indicating no definitive PAH sources impacting one or both of the sampling locations. While there were differences in which PAHs changed with increased winds from each direction, between the two sampling locations, no pattern was found in the data to provide evidence of specific sources or their contributions in each direction of the sampling locations.
Compound concentration correlations with wind ratios for each wind direction gave more information on local sources. For example, the concentration of chrysene & triphenylene (Cr/Tr) was positively correlated (p-value = 0.028) with the SE winds at the Town sampling location and negatively correlated (p-value = 0.047) with the SW wind at the Fence sampling location. This indicates that there is a source of Cr/Tr nearby the two sampling locations that gets enhanced at the Town sampling location with increasing SE winds, but also gets diluted at the Fence sampling location when the SW winds increase. A full list of correlations appears in the Appendix (Table A3). Correlation data provided clues, but no definitive local sources were identified based on compound correlation data.
3.3. Atmospheric reactant analysis.
To distinguish between local emissions and transformation products, atmospheric reactants responsible for PAH transformations were correlated with PAH concentrations. NO and NO2 concentrations (ppm) were both negatively correlated with SE wind at the Town sampling location (Table A3). O3 concentrations (ppm) were positively correlated with SE wind at the Fence sampling location, and negatively correlated with NW wind at both sampling locations. Due to the relatively short atmospheric lifetime of NOx species (2–8 hours), the presence of NOx, and any influence NOx species may have on the PM-bound PAHs, was thought to be distinguishable through correlations of NOx with PAHs or classes of PAHs. The O3 atmospheric lifetime is much longer than NOx (~22 days), and therefore has more time to react with PM-bound compounds during transport. High correlations of individual OPAH or OHPAH concentrations with O3 concentrations could indicate the transport of these compounds from sources far from the sampling locations.
Several individual PAH concentrations were correlated with individual atmospheric reactants, such as 1-hydroxy-9-fluorenone negatively correlated with O3 concentrations, and 12-hydroxybenzo(a)pyrene positively correlated with NO2 concentration at the Town sampling location, (Table A4). While, compound concentrations were positively correlated with NOx concentrations and negatively correlated with O3 concentrations, no clear pattern of PAH concentration correlation was observed at either location, resulting in no conclusion about the influence NOx and O3, on the PM2.5-bound PAHs in the Reservation airshed.
3.4. Fire and inversion events.
Data from PM2.5 samples collected during regional wildfires (n=5), and during a weather inversions (n=3), were interpreted separately from the rest of the samples. In general, both the fire and inversions days had higher PAH concentrations than the rest of the sampling days (normal days) (Figure 3). Data from samples collected during regional wildfires had significantly increased concentrations of ΣHMWs over data from Normal Days, at both sampling locations, suggesting that HMW PAHs may be emitted from wildfires. There was also a significant decrease in ΣOHPAH concentrations at the Town location during wildfire collections.
Figure 3. Atmospheric Condition Comparison.
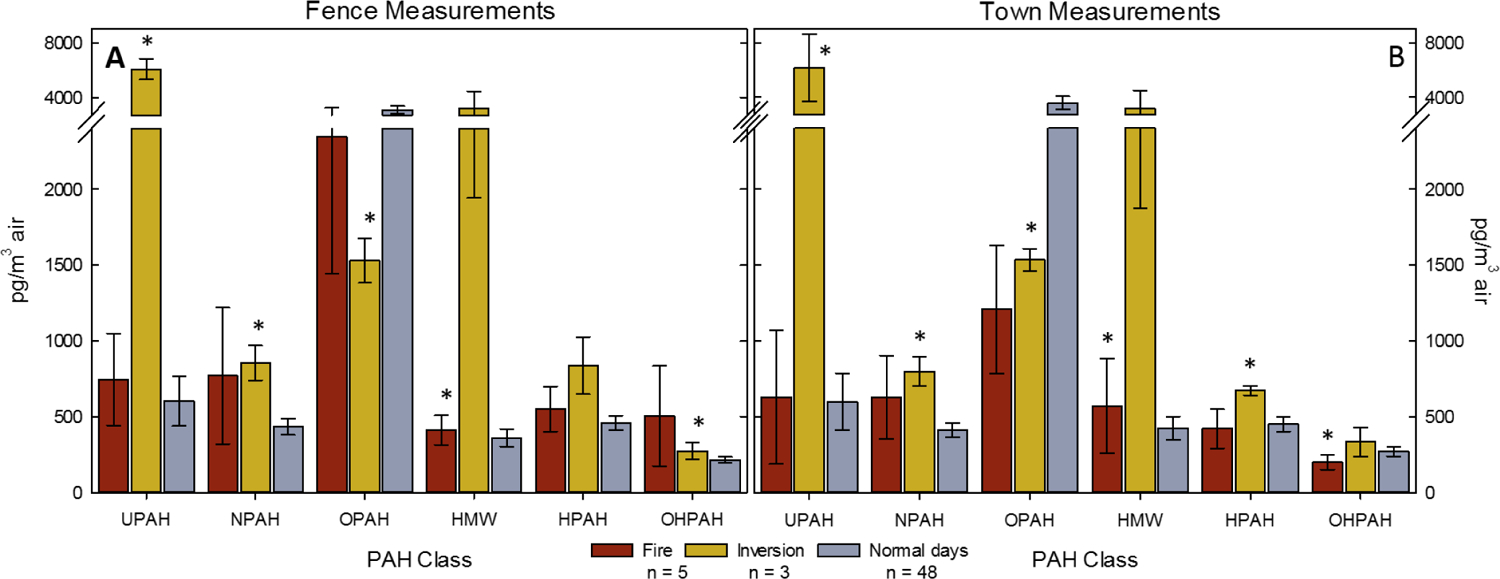
The average concentration of the sum of each PAH class during Normal Days (gray bars), compared to events of wildfires (red bars), or weather inversions (yellow bars) influencing the sampling area for both sampling locations; Fence (A) and Town (B) locations. * indicates a statistically significant (p-value < 0.05) difference from the Normal Days measurements. There were five sampling events during incidence of wildfire influence (n = 5) on the region, and three during inversions (n=3), and 48 days when neither of these atmospheric conditions were noted.
ΣUPAH and ΣNPAH concentrations were significantly higher during inversions at both sampling locations, while ΣOPAH concentrations were significantly lower during the inversions. ΣOHPAH concentrations were higher at the Fence location, and ΣHPAHs were higher at the Town location during inversion events.
3.5. Excess Lifetime Cancer Risk assessment.
RPF values currently only exist for 19 PAHs (Table A5), 13 UPAHs and 6 HMWs. BaPEQ concentrations were calculated for samples in this study for both UPAHs and HMWs with these RPF values (Figure 4 & Table A7). The data shows decreased ΣUPAH and ΣHMW concentrations when SW winds increase from < 20% to > 80%, and have a significant effect on the BaPEQ concentrations at both sampling locations. There was also a significant change to the BaPEQ concentration when adding HMWs BaPEQ to the UPAH BaPEQ at the Town sampling location during times of <20% SE wind (Figure 4). UPAHs represented 68% or 63% of the overall BaPEQ concentration at the Fence and Town sampling locations, respectively.
Figure 4. Benzo(a)pyrene Equivalent Concentrations.
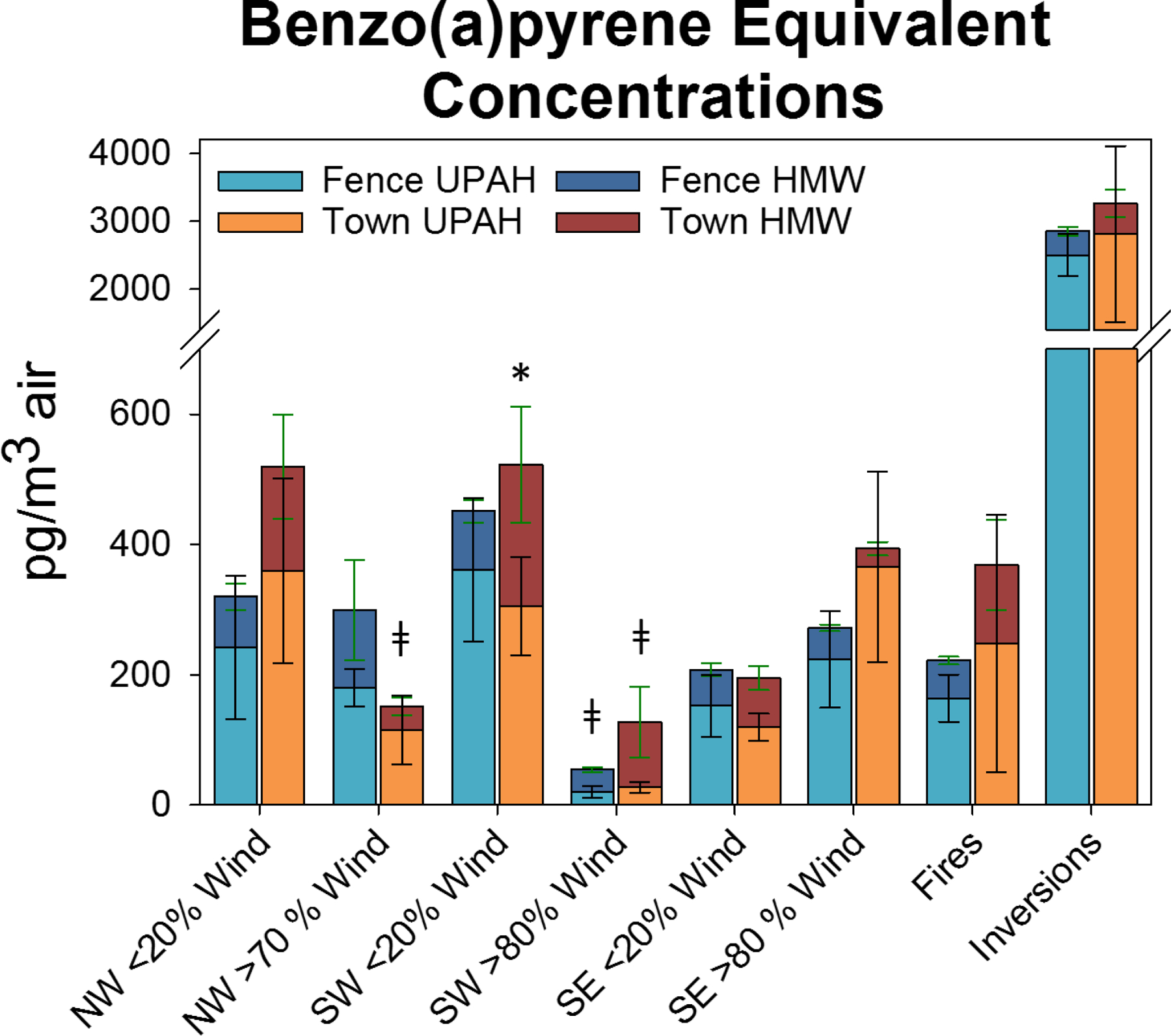
Calculated benzo(a)pyrene (BaP) equivalent concentrations. * indicates a significant (p-value < 0.05) difference between BaPEQ concentrations using RPFs for UPAHs (13 compounds) and all available (19 total compounds) (available on www.epa.gov) at the Town sample location. ǂ indicates a significant decrease in BaPEQ when winds increase from <20% to > 70 or 80% (as indicated). Shades of blue/teal are Fence concentrations and orange/red colors are Town concentrations for indicated wind direction or atmospheric condition (x-axis).
While no BaP concentrations were measured above the 1000 pg/m3 air EU regulatory limit at either sampling location (Figure A3), during Inversion events BaPEQ concentrations exceeded the EU BaP guideline by a factor of 3 (Figure 4). There currently is no regulatory limit on BaPEQ, which we are aware of, but the significant changes in BaPEQ with wind directions and inversion events demonstrate a need for updated regulatory information. During Inversion events, the BaPEQ concentration averaged 2855 (± 376) pg/m3 air and 3267 (± 1479) pg/m3 air at the Fence and Town sampling locations respectively (Figure 4). The median BaPEQ concentration for normal days was ~200 pg/m3 air at both sampling locations.
3.6. Source apportionment.
Diagnostic ratios of PAHs, with a molecular weight of 202 AMU, have been shown to indicate the presence of crude petroleum contamination. Specific ratios of PAHs, with a mass of 276, can give clues about specific combustion sources Yunker, et al. (2002)). These diagnostic ratios were applied to data from this study to assess if local PAH sources could be identified. While figure 5 indicates that the PM2.5 in the samples, heavily influenced by SE winds, primarily contained PAHs produced during liquid fuel and biomass combustion processes, there was no significant correlation with specific source PAH emissions. (Yunker, et al. (2002)). The same diagnostic ratios indicate that mainly liquid fuel combustion (~ 66%) was the source of the PAHs measured in the PM2.5 samples when > 70% of the wind came from the NW and SW (Figure 5). This indicates that gasoline, liquid natural gas, and diesel combustion is the dominant source of PAHs in the SITC Reservation air shed. The diagnostic ratios from the inversion days suggest that both diesel and biomass combustion are important local sources of PAHs in PM2.5 on the reservation.
Figure 5. Diagnostic Ratio Analysis.
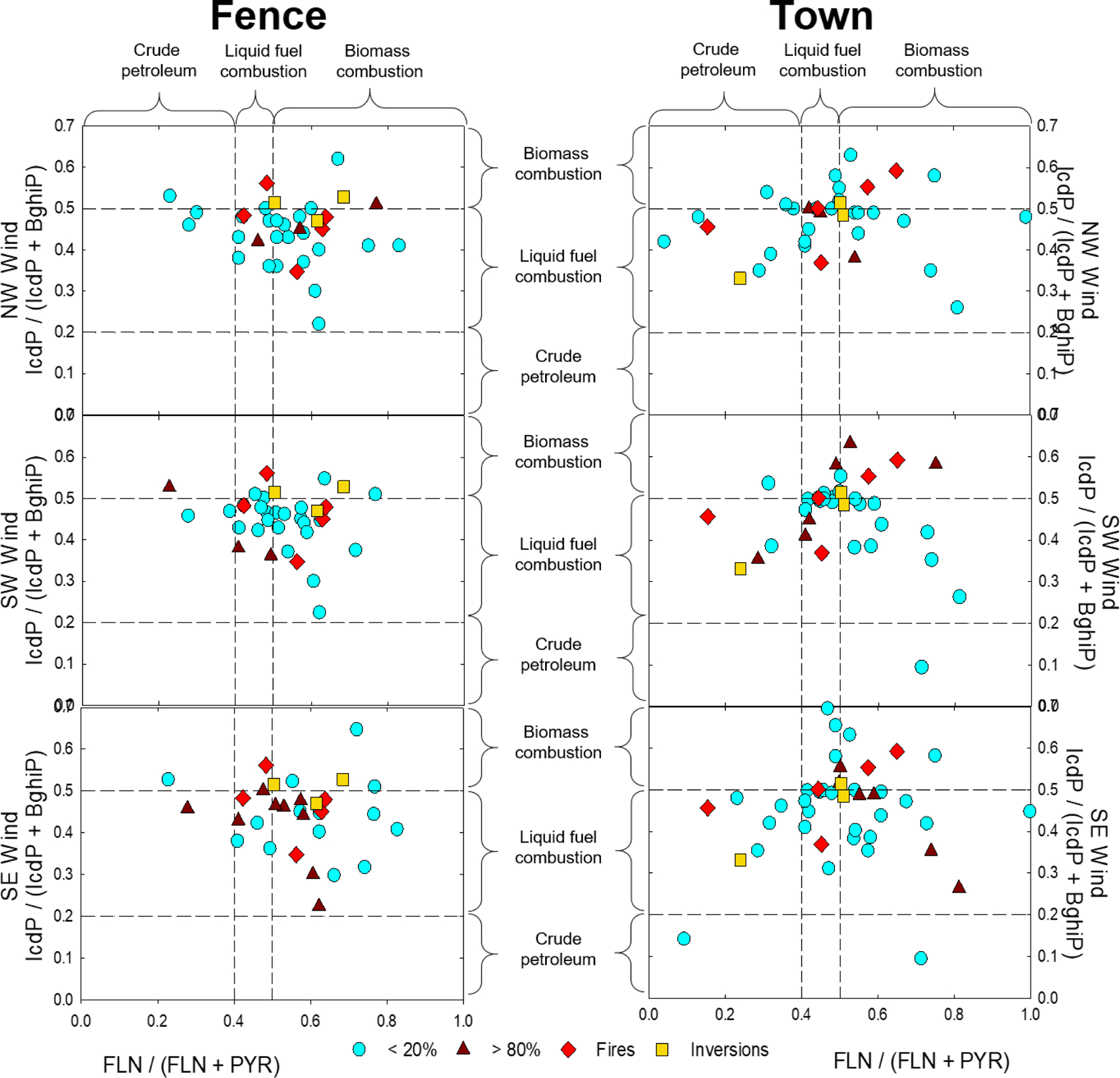
Scatter plots of data for diagnostic ratios of PAHs. PAH202 (FLN/FLN+PYR), used to assess if crude petroleum is a contributing source, and PAH 276 (IcdP/IcdP+BghiP), used to distinguish between types of combustion (Yunker 2002). Circles (teal) indicate low wind in quadrant direction, while triangles (burgundy) indicate high winds coming from specified direction at the two locations for the three impactful wind directions. Diamonds (red) show ratios for samples collected during regional wildfires, and squares (yellow) are ratios during weather inversions.
PMF results indicated no clear pattern of PAH profiles that matched specific source profiles available when there was no prevalent wind direction, nor when specific wind directions prevailed during the sampling period at either sampling location.
4.0. DISCUSSION
4.1. Source regions in wind directions.
At the Fence and Town sampling locations, the SW and SE winds resulted in the greatest change in PAH concentrations (Figure 2). At both sampling locations, when SW winds were >80%, the concentrations of multiple PAH classes and individual PAHs significantly decreased, relative to when SW winds were <20% (Table A2). This suggests that higher winds from the SW diluted PAH concentrations in air at both sampling locations, which is reinforced by correlation data (Table A3). In addition, at both sampling locations, SE winds >80% resulted in increased PAH concentrations, relative to when SE winds were <20%. The Seattle/Tacoma metropolitan area is located 100 km SE of the reservation and contains many industrial centers, transportation media, and energy sectors that may be contributing to the PAHs concentrations in the PM2.5 at the sampling locations when winds are from the SE.
In contrast, NW winds did not significantly impact the PAH concentrations at the sampling locations (Figure 2), even though there are a number of shipping lanes for major Canadian ports, including the city of Vancouver, BC, and minor US ports, as well as a number of oil refineries NW of the sampling locations. While shipping lanes are an important source of PM, and many ocean-going vessels use diesel fuel, leading to complex PM chemistry around shipping lanes (Yau, et al. (2013)), there was no evidence in our data to confirm shipping lane influence on the PAH concentrations.
4.2. Local sources of PAHs.
Increased PAH concentrations, at both sampling locations, during inversion events indicated that there are a number of local PAH sources, including traffic, home heating, local industries, and marinas. There is a lack in the current data to suggest which, if any, of these sources are contributing specific portions of the measured PAH concentrations to the PM2.5. The data from this study, added to previous studies, which have shown that weather inversions during winter months increase the amount of PM2.5 measured in different locations, provides information about air quality during inversion events (Gillies, et al. (2010)).
While diagnostic ratios give clues to possible sources, the variation in individual source PAH profiles, along with the number of possible PAH sources within the local/regional air-shed of the sampling locations makes their use alone ambiguous (Galarneau (2008). Retene was analyzed for changes with wind direction influence, as well as during predicted wildfire influence, and weather inversions. While no statistical difference was measured between the two locations, or between normal days and Fire events, retene concentrations were statistically higher at both locations during inversions (Figure A1). Retene was measured in 98% of all samples, further suggesting there is some biomass burning in the local and/or regional air-shed during all of our sampling periods. Retene inclusion in the PMF modeling did not resolve source fingerprints.
The SITC Reservation is surrounded on the East side by a large number of agricultural fields. These fields may result in PAH emissions from farming equipment and resuspension of dust particles during tilling and harvest that may contribute to increased PAH concentrations during SE winds. Interstate (I5) is located 19 km east of the Reservation. There are railroads, local wood mills, and local marinas located on Reservation lands. The northern peninsula of the Reservation, March Point, contains several industries including oil refineries. Diesel trains transporting materials to and from the oil refineries cross the reservation daily and have over 100 train cars.
The oil refining process was thought to be a significant source of PAHs in the PM2.5 over the Reservation, but our analysis shows mixed emission sources nearby and subsequently was unable to prove or disprove this theory. The number of different crude oils that may be coming into the two independent refineries, as well as the number of possible refined products that are leaving the area, make distinguishing a specific molecular marker for the refineries difficult. Our efforts to identify a PAH fingerprint coming from the refineries was unsuccessful, in part due to a lack of replicate sampling events when emissions of these sources would be directly affecting the sampling locations.
4.3. UPAH Transformation Products.
At both sampling locations, during inversion events the ΣOPAH concentrations decreased, while the ΣOHPAH and ΣNPAH concentrations increased, relative to non-inversion days (Figure 3). This suggests that during inversion events, atmospheric conditions favor the transformation of UPAHs to either NPAHs or OHPAHs on PM2.5. Laboratory experiments using O3 to form secondary organic aerosol (SOA) particles with UPAHs present have shown that OHPAHs are the most prevalent transformation products formed during the process of SOA formation Kramer, et al. (2019)). Studies have shown that PM-bound UPAHs can react with NOx to form NPAHs Jariyasopit, et al. (2014)). During inversions, the suppressed vertical movement of air traps atmospheric reactants along with volatile emissions and PM Esen, et al. (2005)). An increase in atmospheric reactants during inversion events explains an increase in both ΣNPAHs and ΣOHPAHs during these conditions. The decrease in ΣOPAHs however does not fit, unless transformations into OPAHs are occurring through a different mechanism. This discrepancy illustrates a greater need to study the reaction pathways of UPAHs in PM2.5.
4.4. Human Health Implications.
The Inhalation Cancer Risk Assessment over the reservation indicated that, when NW winds increase (from < 20% to > 70% of the sampling time) the cancer risk at the Town sampling location significantly decreases, while at the Fence sampling location it remains unchanged (Figure 6). Increasing SW winds significantly decrease the cancer risk at both sampling locations, and SE winds do not significantly change the risk.
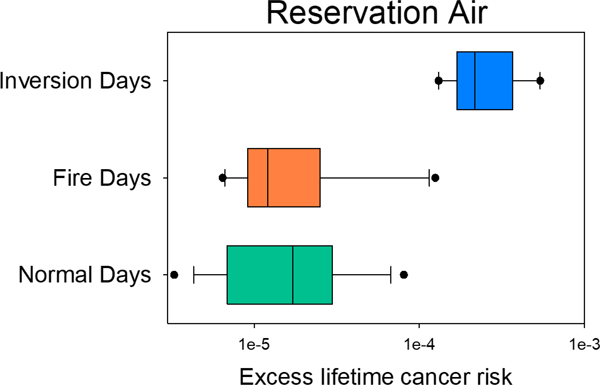
Figure 6.
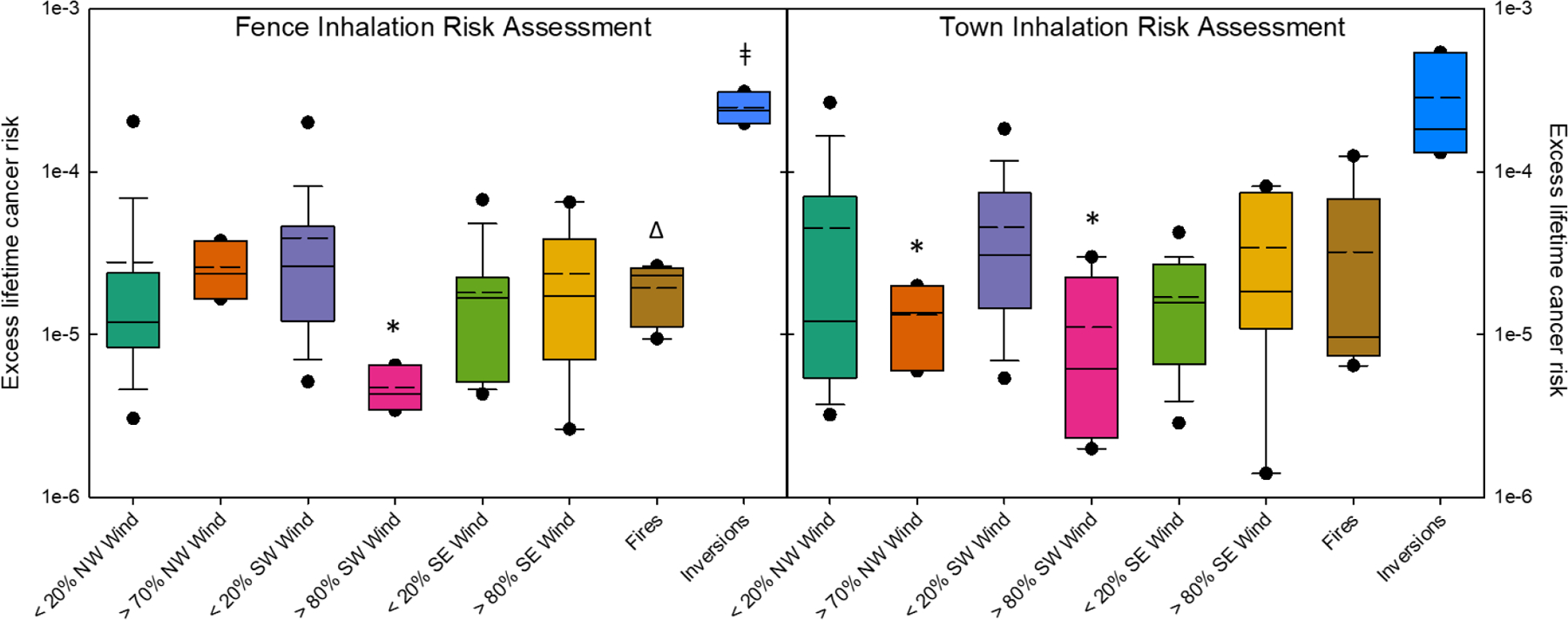
Estimated Lifetime Cancer risk assessment using World Health Organization (WHO) unit risk (8.7 × 10−5 per ng/m3 BaP) for BaP (URBaP) as a proxy for the mixture of PAHs measured in PM2.5. Boxes represent the top and bottom 25% of estimates for each weather condition (x-axis) with the solid lines representing the average value for excess cancer risk. Whiskers represent Standard Deviation, the 5th and 95th percentiles are represented by the dots, and dashed lines represent the geometric mean estimate. * indicate a significant difference between low (<20% wind) and high (> 70 or 80% wind) from one direction, ǂ indicates a significant difference in inversion Lifetime Cancer Risk over all other data in this study, Δ indicates significantly higher excess lifetime cancer risk during fire events than during periods of >80% SW winds at the Fence site location.
During normal atmospheric conditions, both sampling locations had similar excess lifetime cancer risk of 23 (± 5) people in a million at the Fence sampling location and 28 (± 6) people in a million at the Town sampling location. During Fire events, the Town sampling location had a higher Inhalation cancer risk (~32 people in a million) than the Fence sampling location (~19 people in a million). During Inversion events, both sampling locations had a significant increase in the excess lifetime cancer risk, with 248 (± 33) people and 284 (± 129) people per million at the Fence and Town locations, respectively.
As demonstrated by the BaPEQ concentrations of this study, the RPF values available for 19 PAHs, are helpful in distinguishing possible toxicity of PM2.5 samples, but a broader list of RPFs is needed to better understand the complexity of PM2.5 toxicity. The HMW BaPEQ concentrations represented between 10–40% of the overall BaPEQ concentrations, suggesting that studies using only the UPAHs on the PPL could result in the under-prediction of risk.
The order of magnitude increase in inhalation cancer risk during predicted periods of weather inversions, observed in this study, illustrate the gaps in the available data surrounding such events and the best way to protect the health of people during them. The differences in transformation products measured during the inversion events illustrates a gap in available knowledge about the reaction pathways for PAH transformation products. While other studies have shown increases in atmospheric reactants and PM during inversions, more toxic compound analysis is needed to fully understand the health risks of such events (Esen, et al. (2005), Gillies, et al. (2010)).
5.0. CONCLUSIONS
The number of PAHs measured in PM2.5 in this study, illustrates the need to expand upon the US EPAs PPL. The 16 PAHs included on the PPL, are all UPAHs and are commonly measured in PM2.5 around the globe. This study shows the relevance of the other subclasses of PAHs. While the toxicity of many UPAHs have been well studied, the human health effects of NPAH, OPAH, HPAH, HMW and OHPAHs in PM2.5 remains highly unknown. PM2.5 exposure has been linked to over 7 million deaths per year. (Landrigan, et al. (2018)). However, the components of the PM2.5 responsible for these deaths are still not known. Expanding the current screening lists to include the subclasses of PAHs measured in PM2.5 in this study will help global scientists better understand the nature of PM2.5 exposure. Toxicological studies of the impact of the compounds measured here is needed to fully understand the contribution to PM2.5 toxicity linked to PAHs.
Data presented here illustrates the negative impact of regional PAH sources on the air quality of the SITC Reservation. When winds increased from the SE, the reservation air quality was impacted by increased PAH concentration in the PM2.5 over the reservation. While SW winds diluted PAHs in the PM2.5 from local sources, inversion events trapped local emissions and greatly increased the harmful PM2.5 components in the Reservation air.
Indigenous communities are less equipped to measure and address environmental health concerns. (Basnayake, et al. (2017)). The SITC sought scientific help in understanding the environment in which they live. The air quality over the SITC Reservation is directly impacted by different anthropogenic sources of PAH contaminated PM2.5 from nearby local and regional sources. Data from this study demonstrates the need to be vigilant of atmospheric conditions in efforts to protect the health of the SITC peoples. The highest risk of adverse health effects occurred during Inversion events, which coincide with winter months in the US. Pacific Northwest. Mote and Salathé (2010) During these months, communities are heating their homes and businesses, often using wood burning stoves and fireplaces. The data in this study suggest there are different air quality concerns on different parts of the Reservation at any given time. General steps to reduce exposure may not have the same efficacy across all parts of the Reservation. To more fully understand the local PAH sources on the SITC Reservation, more detailed studies are needed.
As global climate change continues to change weather patterns across the planet, the Pacific Northwest will experience increases land and sea temperatures, which will change the local weather patterns. (Mote and Salathé (2010), IPCC (2014)). Communities, such as SITC, need to be take measures to ensure the safety of their people, from the local, regional, and global sources of air pollution moving into their air-shed. Increased wildfires and changing inversion patterns will continue to raise PM2.5 concentrations across impacted areas, increasing the concentration of toxic compounds, such as PAHs. (Gillies, et al. (2010), Navarro, et al. (2017), Navarro, et al. (2016)).
Supplementary Material
HIGHLIGHTS.
Paired PM2.5 samples were collected over a Native American Reservation
Extracted PM2.5 samples were analyzed using GC-MS for 131 PAHs
PAH concentrations were compared to local weather patterns and analyzed for correlations
Weather inversions and regional wildfires increased PAH concentrations on the Reservation
ACKNOWLEDGMENTS
Funding. This publication was made possible in part by Grants AGS-1411214 from the National Science Foundation (NSF), and P42-ES016465 and P30-ES00210 from National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH). Its contents are the sole responsibility of the authors and do not represent the official view of the NIEHS or NIH.
Footnotes
Supplementary data
Supplementary data to this article can be in Appendix A
REFERENCES
- Agency USEP. 2010. Development of a Relative Potency Factor (Rpf) Approach for Polycyclic Aromatic Hydrocarbon (PAH) MIxtures (External Review Draft). Washington DC: U.S. Environmental Protection Agency. [Google Scholar]
- Basnayake TL, Morgan LC, Chang AB. 2017. The global burden of respiratory infections in indigenous children and adults: A review. Respirology 22(8):1518–1528. [DOI] [PubMed] [Google Scholar]
- Claus P Haslauer JRM, Andras Bardossy, and Parker Beth L. 2017. Estimating a Representative Value and Proportion of True Zeros for Censored Analytical Data with Application to Contaminated Site Assessment. Environmental Science & Technology 51(13):7502–7510. [DOI] [PubMed] [Google Scholar]
- Commission TE. 2019. Air Quality Standards. 07/18/2019 ed.
- Dippel C 2014. FORCED COEXISTENCE AND ECONOMIC DEVELOPMENT: EVIDENCE FROM NATIVE AMERICAN RESERVATIONS. Econometrica 82(6):2131–2165. [Google Scholar]
- Esen F, Tasdemir Y, Cindoruk SS. 2005. Evaluation of NOx and O3 Concentrations in the Atmosphere of Bursa, Turkey. Environmental Forensics 6(3):311–317. [Google Scholar]
- Galarneau E 2008. Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment. Atmospheric Environment 42(35):8139–8149. [Google Scholar]
- Geier MC, Chlebowski AC, Truong L, Massey Simonich SL, Anderson KA, Tanguay RL. 2018. Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Archives of toxicology 92(2):571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genualdi SA, Killin RK, Woods J, Wilson G, Schmedding D, Simonich SLM. 2009. Trans-Pacific and Regional Atmospheric Transport of Polycyclic Aromatic Hydrocarbons and Pesticides in Biomass Burning Emissions to Western North America. Environmental Science & Technology 43(4):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies RR, Wang S-Y, Booth MR. 2010. Atmospheric Scale Interaction on Wintertime Intermountain West Low-Level Inversions. Weather and Forecasting 25(4):1196–1210. [Google Scholar]
- Harrison RM, Yin J. 2000. Particulate matter in the atmosphere: which particle properties are important for its effects on health? Science of The Total Environment 249(1):85–101. [DOI] [PubMed] [Google Scholar]
- Hites RA. 2019. Correcting for Censored Environmental Measurements. Environmental Science & Technology. [DOI] [PubMed]
- Hou P, Wu S. 2016. Long-term Changes in Extreme Air Pollution Meteorology and the Implications for Air Quality. Scientific Reports 6(1):23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Hou J, Zhou Y, Sun H, Yin W, Zhang Y, Wang X, Wang G, Chen W, Yuan J. 2018. Association of polycyclic aromatic hydrocarbons exposure with atherosclerotic cardiovascular disease risk: A role of mean platelet volume or club cell secretory protein. Environmental Pollution 233:45–53. [DOI] [PubMed] [Google Scholar]
- Hyunok Choi RH, Hannu Komulainen, Delgado Saborit Juana M. 2010. WHO Guidelines for Indoor Air Quality: Selected Pollutants: World Health Organization, Regional Office for Europe. 454 p. [PubMed] [Google Scholar]
- Indians NCoA. 2015. Tribal Nations and the United States: An Introduction. Embassy of Tribal Nations. [Google Scholar]
- IPCC. 2014. Climate Chane 2014: Synthesis Report. Contributions of Working Groups I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Core writing team, Pachauri RK, Meyer LA). IPCC. [Google Scholar]
- Jariyasopit N, McIntosh M, Zimmermann K, Arey J, Atkinson R, Cheong PH-Y, Carter RG, Yu T-W, Dashwood RH, Massey Simonich SL. 2014. Novel Nitro-PAH Formation from Heterogeneous Reactions of PAHs with NO2, NO3/N2O5, and OH Radicals: Prediction, Laboratory Studies, and Mutagenicity. Environmental Science & Technology 48(1):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Stone D, Wang W, Schrlau J, Tao S, Massey Simonich Staci L. 2011. Estimated Reduction in Cancer Risk due to PAH Exposures If Source Control Measures during the 2008 Beijing Olympics Were Sustained. Environmental Health Perspectives 119(6):815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AL, Suski KJ, Bell DM, Zelenyuk A, Massey Simonich SL. 2019. Formation of Polycyclic Aromatic Hydrocarbon Oxidation Products in α-Pinene Secondary Organic Aerosol Particles Formed through Ozonolysis. Environmental Science & Technology 53(12):6669–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N, Baldé AB, Bertollini R, Bose-O’Reilly S, Boufford JI and others. 2018. The Lancet Commission on pollution and health. The Lancet 391(10119):462–512. [DOI] [PubMed] [Google Scholar]
- Li C, Martin RV, van Donkelaar A, Boys B, Hammer M, Xu J-W, Marais EA, Reff A, Strum M, Ridley D and others. 2017. Trends in chemical composition of global and regional population-weighted fine particulate matter estimated for 25 years. Environmental Science & Technology. [DOI] [PubMed]
- Liu H-H, Lin M-H, Chan C-I, Chen H-L. 2010. Oxidative damage in foundry workers occupationally co-exposed to PAHs and metals. International Journal of Hygiene and Environmental Health 213(2):93–98. [DOI] [PubMed] [Google Scholar]
- Luo P, Bao L-J, Li S-M, Zeng EY. 2015. Size-dependent distribution and inhalation cancer risk of particle-bound polycyclic aromatic hydrocarbons at a typical e-waste recycling and an urban site. Environmental Pollution 200:10–15. [DOI] [PubMed] [Google Scholar]
- Minick DJ, Paulik LB, Smith BW, Scott RP, Kile ML, Rohlman D, Anderson KA. 2019. A passive sampling model to predict PAHs in butter clams (Saxidomus giganteus), a traditional food source for Native American tribes of the Salish Sea Region. Marine Pollution Bulletin 145:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote PW, Salathé EP. 2010. Future climate in the Pacific Northwest. Climatic Change 102(1):29–50. [Google Scholar]
- Navarro KM, Cisneros R, Noth EM, Balmes JR, Hammond SK. 2017. Occupational Exposure to Polycyclic Aromatic Hydrocarbon of Wildland Firefighters at Prescribed and Wildland Fires. Environmental Science & Technology 51(11):6461–6469. [DOI] [PubMed] [Google Scholar]
- Navarro KM, Cisneros R, O’Neill SM, Schweizer D, Larkin NK, Balmes JR. 2016. Air-Quality Impacts and Intake Fraction of PM2.5 during the 2013 Rim Megafire. Environmental Science & Technology 50(21):11965–11973. [DOI] [PubMed] [Google Scholar]
- Patel A, Rastogi N. 2018. Oxidative potential of ambient fine aerosol over a semi-urban site in the Indo-Gangetic Plain. Atmospheric Environment 175:127–134. [Google Scholar]
- Primbs T, Piekarz A, Wilson G, Schmedding D, Higginbotham C, Field J, Simonich SM. 2008. Influence of Asian and Western United States Urban Areas and Fires on the Atmospheric Transport of Polycyclic Aromatic Hydrocarbons, Polychlorinated Biphenyls, and Fluorotelomer Alcohols in the Western United States. Environmental Science & Technology 42(17):6385–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdahl T 1983. Retene—a molecular marker of wood combustion in ambient air. Nature 306(5943):580–582. [Google Scholar]
- Rohlman D, Donatuto J, Heidt M, Barton M, Campbell L, Anderson AK, Kile LM. 2019. A Case Study Describing a Community-Engaged Approach for Evaluating Polycyclic Aromatic Hydrocarbon Exposure in a Native American Community. International Journal of Environmental Research and Public Health 16(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummer C, Delhomme O, Appenzeller BMR, Wennig R, Millet M. 2009. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 77(4):1473–1482. [DOI] [PubMed] [Google Scholar]
- Tuet WY, Chen Y, Fok S, Champion JA, Ng NL. 2017. Inflammatory responses to secondary organic aerosols (SOA) generated from biogenic and anthropogenic precursors. Atmos. Chem. Phys. 17(18):11423–11440. [Google Scholar]
- US EPA CMT. 2010. Definition and Procedure for the Determination of the Method Detection Limit, Revision 2. In: (4303T) EaASBE, editor. www.epa.gov: US Environmental Protection Agency. [Google Scholar]
- Wang W, Jariyasopit N, Schrlau J, Jia Y, Tao S, Yu T-W, Dashwood RH, Zhang W, Wang X, Simonich SLM. 2011. Concentration and Photochemistry of PAHs, NPAHs, and OPAHs and Toxicity of PM2.5 during the Beijing Olympic Games. Environmental Science & Technology 45(16):6887–6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau PS, Lee SC, Cheng Y, Huang Y, Lai SC, Xu XH. 2013. Contribution of ship emissions to the fine particulate in the community near an international port in Hong Kong. Atmospheric Research 124:61–72. [Google Scholar]
- Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S. 2002. PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry 33(4):489–515. [Google Scholar]
- Zhao Y, Hong B, Fan Y, Wen M, Han X. 2014. Accurate analysis of polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs homologs in crude oil for improving the gas chromatography/mass spectrometry performance. Ecotoxicology and Environmental Safety 100(Supplement C):242–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


