Significance
In female mosquitoes, repeated blood feedings are essential for maintaining gonadotrophic cycles and serve as the basis for pathogen transmission. We demonstrate that the ecdysone-induced protein 93 (E93), a transcription factor implicated in insect metamorphic transitions, regulates reproductive switches in adult females of the major arboviral vector Aedes aegypti. E93 RNA interference before the first gonadotrophic cycle disrupts the second. E93 silencing disturbed the expression of Hormone Receptor 3 and autophagy, both essential for vitellogenesis termination. Transcriptomic analysis reveals a E93-mediated shutdown of gene expression during the end of a reproductive cycle, particularly genes linked to translation, yolk protein precursors, and metabolism. This study has advanced our understanding of the E93 role in controlling the gonadotrophic cyclicity in female mosquitoes.
Keywords: reproduction, ecdysone-induced protein 93, mosquito, juvenile hormone, autophagy
Abstract
Repeated blood feedings are required for adult female mosquitoes to maintain their gonadotrophic cycles, enabling them to be important pathogen carriers of human diseases. Elucidating the molecular mechanism underlying developmental switches between these mosquito gonadotrophic cycles will provide valuable insight into mosquito reproduction and could aid in the identification of targets to disrupt these cycles, thereby reducing disease transmission. We report here that the transcription factor ecdysone-induced protein 93 (E93), previously implicated in insect metamorphic transitions, plays a key role in determining the gonadotrophic cyclicity in adult females of the major arboviral vector Aedes aegypti. Expression of the E93 gene in mosquitoes is down-regulated by juvenile hormone (JH) and up-regulated by 20-hydroxyecdysone (20E). We find that E93 controls Hormone Receptor 3 (HR3), the transcription factor linked to the termination of reproductive cycles. Moreover, knockdown of E93 expression via RNAi impaired fat body autophagy, suggesting that E93 governs autophagy-induced termination of vitellogenesis. E93 RNAi silencing prior to the first gonadotrophic cycle affected normal progression of the second cycle. Finally, transcriptomic analysis showed a considerable E93-dependent decline in the expression of genes involved in translation and metabolism at the end of a reproductive cycle. In conclusion, our data demonstrate that E93 acts as a crucial factor in regulating reproductive cycle switches in adult female mosquitoes.
To reproduce successfully, hematophagous female mosquitoes need to feed on blood repeatedly, the feature which makes them crucial vectors of disease. The global incidence of devastating human diseases transmitted by mosquitoes has dramatically risen in recent decades. The yellow fever mosquito Aedes aegypti is the most important carrier of flaviviruses, causing yellow fever, dengue fever, Zika, and other viral diseases (1–4). Female mosquito reproduction is cyclic, with each gonadotrophic cycle activated by a separate blood feeding. Consequently, female mosquitoes are able to both acquire and transmit disease vectors in successive gonadotrophic cycles. Thorough investigations of the reproductive process in female mosquitoes could provide valuable insight into screening key targets for interrupting this gonadotrophic cyclicity.
In mosquitoes, each gonadotrophic cycle consists of two phases—previtellogenesis or posteclosion (PE) in the first cycle and vitellogenesis or postblood meal (PBM) development—which are regulated by two principal insect hormones, juvenile hormone (JH) and 20E-hydroxyecdysone (20E), respectively (5). During the mosquito PE reproductive phase, JH receptor Methoprene tolerant (Met) mediates the activating and repressive actions of JH (6). The downstream factors in the JH/Met signaling pathway, Krüppel homolog 1 (Kr-h1) and Hairy, are involved in the repression of JH-responsive genes (7–9). A high JH titer mediates the translation of the competence factor betaFTZ-F1 (fushi tarazu binding factor 1), enabling mosquitoes to acquire responsiveness to 20E after a blood meal (10). During the PBM phase, the ecdysone receptor (EcR) mediates 20E signaling. A suite of transcription factors including ecdysone-induced protein 74 (E74), ecdysone-induced protein 75 (E75), and Broad are implicated in the 20E/EcR early response, synergistically activating the expression of late genes, such as vitellogenin (Vg) (11–13). However, during the terminal phase of vitellogenesis (when 20E titer declines), Hormone Receptor 3 (HR3) is involved in a timely shutdown of Vg expression and betaFTZ-F1 activation, which is critical for terminating the first reproductive cycle and entering the second. HR3 is also involved in inhibiting target of rapamycin (TOR) signaling and activating programmed autophagy. The combined actions of HR3 are essential for maintaining the repeated cycles of egg maturation (11, 14). Our previous finding that ecdysone-induced protein 93 (E93, also known as Eip93F) is expressed in adult female A. aegypti mosquitoes (6, 15), has prompted us to investigate a potential role of this transcription factor in the regulation of the transition between reproductive cycles in this vector mosquito.
E93, a member of the helix–turn–helix transcription factors (including nuclear receptor interaction motif, corepressor C-terminal-binding protein interaction motif, and two DNA-binding HTH motifs), has been shown to serve as the developmental switch during metamorphosis to adult in hemimetabolous and holometabolous insects (16–18). It is expressed during the metamorphic transition of a pupa or a last instar nymph to an adult insect and functions as an important determinant of the responsiveness of downstream target genes in this process. Several studies have reported that E93 is a 20E-induced early gene that transduces the 20E signaling to activate autophagy and apoptosis in the fat body (FB), salivary glands, and midgut by promoting remodeling of these tissues (19, 20). Up-regulating E93 expression by 20E induces the onset of adult metamorphosis, whereas activation of the JH-responsive gene Kr-h1 prevents this process (18, 21–23). Moreover, research in several insects, including Drosophila melanogaster, Nilaparvata lugens, Tribolium castaneum, and Bombyx mori, has demonstrated that silencing of E93 could disrupt the transition of pupa to adult, leading to the failure of tissue remodeling (18–21, 24).
The role of E93 in reproduction is not well understood, although it would be particularly advantageous to elucidate its potential in controlling reproductive cyclicity in adult female mosquitoes. Here, we uncover an important regulatory role for E93 during female mosquito reproduction. Transcriptome analysis reveals that E93 promotes the end of vitellogenesis and progression into the next cycle by regulating large gene cohorts, including those involved in translation, metabolism, and yolk protein precursors (YPPs). In addition, HR3 and autophagy, both associated with the termination of reproductive cycles, are disrupted by E93 RNA interference (RNAi). E93 silencing before the first gonadotrophic cycle alters the normal progression of the second reproductive cycle. Thus, this study has uncovered an additional regulatory function of E93, which plays a crucial role in the regulation of the reproductive cyclicity in adult female mosquitoes.
Results
E93 Expression during Gonadotrophic Cycles in A. aegypti Adult Females Is Alternately Regulated by JH and 20E.
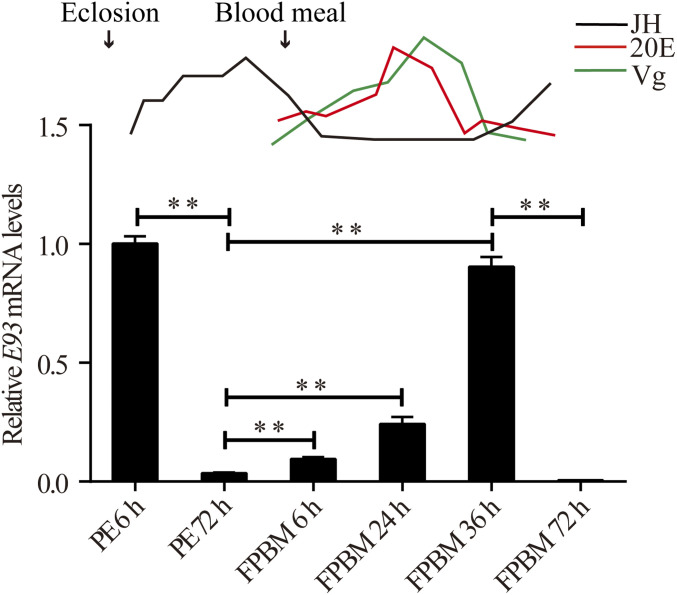
We first conducted a time-course transcription analysis of the E93 gene using qPCR. There are two E93 isoforms, differing only in their first exon region. Thus, the common region of these two isoforms was chosen for qPCR analysis. We analyzed E93 transcript abundance in the FB, the insect metabolic and reproductive tissue analogous to the vertebrate liver and adipose tissue. FB samples were collected from adult females at various stages during two successive gonadotrophic cycles: the PE phase at 6 h and 72 h; the first PBM (FPBM) cycle at 6 h, 24 h, 36 h, and 72 h; and the second PBM cycle (SPBM) at 6 h, 24 h, 36 h, and 72 h. The first blood meal was given at 72 h PE, while the second blood meal was given at 7 d FPBM, when mosquitoes completed egg deposition. The E93 transcript level was relatively higher at 6 h PE but significantly lower at 72 h PE (Fig. 1). During the FPBM cycle, E93 mRNA abundance increased dramatically, reaching a peak at 36 h FPBM. Subsequently, it decreased by 72 h FPBM to a low level similar to that at 72 h PE (Fig. 1). Likewise, E93 mRNA level was elevated once again after a second blood meal, exhibiting a trend resembling that of the FPBM (SI Appendix, Fig. S1). Together, these indicate a cyclic pattern of E93 expression.
Fig. 1.
The cyclic pattern of E93 expression across the first gonadotrophic cycles. Time course of E93 gene expression during the first cycle including the PE period (6 h and 72 h), FPBM period (6 h, 24 h, 36 h, and 72 h). The expression of E93 at PE 6 h was used as controls during the first cycle. The titers of JH, 20E, and the expression of Vg during the first reproductive cycle are schematically shown at Top. Data are shown as mean ± SEM **P < 0.01.
In mosquitoes, the hormones JH and 20E are involved in coordination of gonadotrophic cycles (5). Thus, in order to make clear the correlation of these two hormones with E93, a schematic representing the fluctuation of the titers of JH and 20E during the gonadotrophic cyclicity was shown in Fig. 1. Meanwhile, the expression of Vg, the important marker gene during vitellogenesis, was also presented along with JH and 20E (25–27). To further investigate potential roles of these hormones in controlling the E93 expression dynamics during gonadotrophic cycles, we used an in vitro FB culture assay in combination with the double-stranded RNA (dsRNA) depletion of hormone receptor genes. The E93 mRNA level in the 1 h PE FB tissue was notably lower than the control after 20 nM of the JH analog Methoprene was added to the medium for 8 h (SI Appendix, Fig. S2A). Moreover, the abundance of E93 mRNA was significantly boosted after Met RNAi at 3 d PE in comparison with the GFP (green fluorescent protein gene) RNAi control, indicating the repressive action of Met on E93 gene expression (SI Appendix, Fig. S2B). To investigate the signaling role of JH on E93 gene expression during the second gonadotrophic cycle, the FBs of the first blood-fed female mosquitoes were dissected at 3 h FPBM, before the 20E increase and after the JH decline. Treatment of these FBs with 20 nM Methoprene for 8 h led to a pronounced repression of the E93 transcript level in comparison with control (SI Appendix, Fig. S2C).
By contrast, when FBs at 96 h PE (when the E93 mRNA level is normally low) were incubated in 10 µM 20E for 10 h, the E93 mRNA level was greatly increased (SI Appendix, Fig. S2D). Additionally, E93 expression was significantly reduced at 24 h FPBM in FBs where the 20E receptor EcR was silenced via RNAi (SI Appendix, Fig. S2E). The function of 20E in regulating E93 expression during the second gonadotrophic cycles was also investigated. FB samples were collected from 7 d FPBM, when 20E levels are low. mRNA levels of E93 were significantly elevated when FBs were incubated in the medium containing 10 µM 20E for 10 h (SI Appendix, Fig. S2F).
Taken together, these results have clearly established the essential roles of JH, 20E, and their corresponding receptors in coordinating E93 gene cyclical expression throughout adult female mosquito gonadotrophic cycles. JH and Met suppressed E93 expression not only at the late PE phase, but also at the end of each PBM phase. In contrast, 20E and EcR induced its expression after each blood meal in successive gonadotrophic cycles.
Molecular Mechanism Controlling E93 Expression by the JH Pathway in Adult Female Mosquitoes.
Previously, two transcription factors, Hairy and Kr-h1, have been implicated in JH/Met-mediated gene repression in adult mosquitoes (8, 9). To investigate whether either of these intermediate factors is required for the JH-dependent repression of E93, in vitro FB culture experiments were carried out as described previously (9). FBs of female mosquitoes at 1 h PE were incubated in medium containing 20 nM Methoprene in the presence or absence of cycloheximide (CHX), a protein synthesis inhibitor, for 8 h. E93 transcript level was significantly lower in the presence of Methoprene than the medium containing solvent (ethanol). However, the addition of 20 µM CHX to the culture medium rendered E93 unresponsive to the repressive action of Methoprene (SI Appendix, Fig. S3A), suggesting that the action of JH is indirect. Transcript abundance of Kr-h1 and Hairy, on the other hand, still increased with the addition of Methoprene in the presence of CHX (SI Appendix, Fig. S3 B and C). Thus, these results indicate a possible role of intermediate factors downstream of Met in the JH-mediated repression of E93.
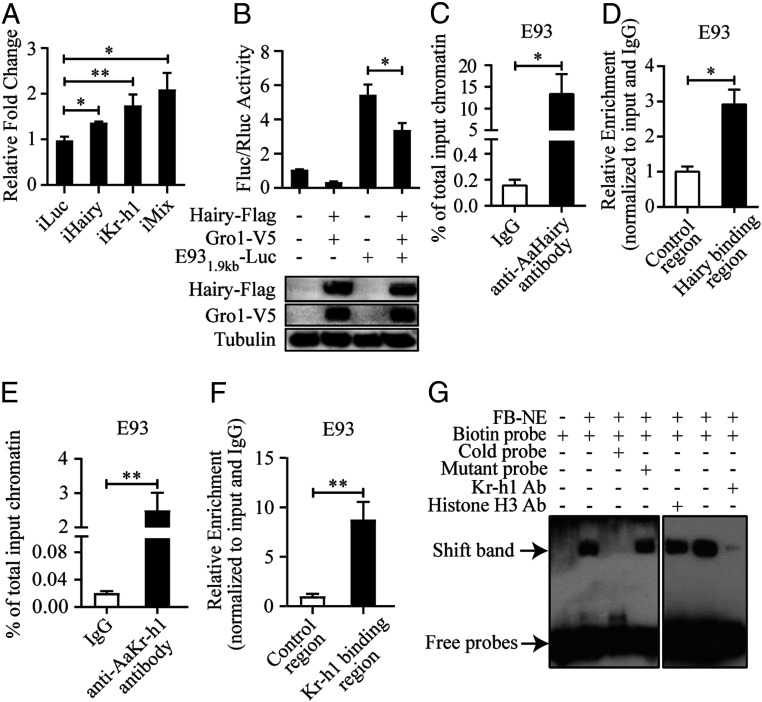
Next, Kr-h1 and Hairy dsRNAs were injected into separate groups of female mosquitoes within 24 h PE. For the simultaneous knockdown of Hairy and Kr-h1, equal amounts of both dsRNAs were used (iMix). FB samples were collected 4 d postinjection to perform qPCR analysis. In comparison with bacterial luciferase gene RNAi (iLuc) controls, E93 expression was increased in all tested samples: iKr-h1, iHairy, and iMix (Fig. 2A), suggesting E93 repression by both Kr-h1 and Hairy. The E93 activation in iMix was notably higher than that in either iKr-h1 or iHairy, indicating a synergistic action of these factors, as previously observed for other JH-regulated genes (8).
Fig. 2.
Hairy and Kr-h1 act as intermediate factors in JH/Met repression of the E93 gene. (A) qPCR analysis of E93 transcript abundance after RNAi knockdowns of Kr-h1, hairy, and a mixture of both (Kr-h1 and hairy). (B) The reporter plasmid (E931.9kb-Luc) and a control Renilla luciferase reporter vector pCopia were cotransfected into Drosophila S2 cells along with the Hairy-Flag and Gro1-V5 expression plasmids. Western blot results showing the expression of fusion protein Hairy-Flag and Gro1-V5. (C) The Hairy binding site occupancy on the E93 gene promoter region, as measured using ChIP-qPCR. Chromatin was immunoprecipitated with anti-Hairy and anti-IgG (control) antibodies from FBs at 72 h PE. Results are shown as % of total input chromatin compared with IgG control. (D) The E93 coding sequence was used as a control to compare with the enrichment of Hairy binding sites in the E93 promoter region. (E) The Kr-h1 binding site occupancy on the E93 gene promoter region, as measured using ChIP-qPCR. Chromatin was immunoprecipitated with anti-Kr-h1 and anti-IgG (control) antibodies from FBs at 72 h PE. Results are shown as % of total input chromatin compared with IgG control. (F) The E93 coding sequence was used as a control to compare with the enrichment of Kr-h1 binding sites in the E93 promoter region. (G) EMSA results confirming the binding of Kr-h1 to biotin-labeled E93 probe. The unlabeled E93 (cold) probe in 200× molar excess was used to test the binding specificity, while a mutant probe of the same concentration was a control. Histone H3 antibody served as a nonspecific antibody control. Data are shown as mean ± SEM *P < 0.05, **P < 0.01.
The genetic interaction of the E93 gene with these transcription factors was then investigated. We first looked to Hairy involvement in E93 repression using the dual luciferase reporter assay with a 1.9-kb region of the E93 promoter (see SI Appendix, Supplemental Results for details). These results indicated a repressive role of Hairy/Groucho1 in E93 regulation (Fig. 2B). Next, the chromatin immunoprecipitation (ChIP)-qPCR experiments were conducted to analyze the binding between Hairy and the E93 promoter. As shown in Fig. 2 C and D, the Hairy-binding region was significantly enriched when Hairy antibody was applied. At the same time, the binding in the promoter region was nearly 2.91-fold higher than that in the coding region. In order to elucidate further the Kr-h1 regulatory function on the E93 gene, we also performed ChIP-qPCR analysis (SI Appendix, Supplemental Results). A significantly higher enrichment of identified Kr-h1 DNA-binding sites was observed when ChIP was performed using the Kr-h1 antibody than that of the IgG (Fig. 2E). For the DNA enriched by the Kr-h1 antibody, we also compared the binding of Kr-h1 to the downstream coding region (control) of E93 with the regulatory region. There was an 8.76-fold stronger binding in the promoter region than in the control region (Fig. 2F). Electrophoretic mobility shift assay (EMSA) was also performed to further demonstrate a potential physical interaction between Kr-h1 and the E93 promoter using the FB nuclear extract (FB-NE). As shown in Fig. 2G, a band shift was detected when FB-NE was incubated with the biotin-labeled E93 probe. However, this band was abolished when it was preincubated with the unlabeled E93 probe, but not the unlabeled mutant probe. A diminished band was observed using the anti-Kr-h1 polyclonal antibody, suggesting the presence of Kr-h1 in FB-NE–probe complexes. When the histone H3 antibody was preincubated with FB-NE, the intensity of the band shift was similar to that in normal binding samples. These results demonstrate the specificity of genetic interaction between Kr-h1 and the E93 promoter and further confirm Kr-h1 as the intermediate factor mediating the Met repressive action on the E93 gene.
Molecular Mechanism Controlling E93 Expression by the 20E Pathway in Adult Female Mosquitoes.
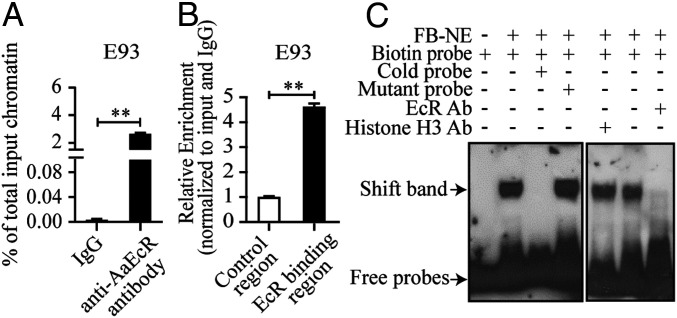
To examine whether an intermediate factor(s) is required for the 20E-dependent activation of E93, we again used in vitro FB cultures and CHX application (SI Appendix, Supplemental Results). CHX was ineffective in preventing the 20E activation of E93 gene expression, suggesting a possible direct control of the E93 gene by the 20E regulatory pathway (SI Appendix, Fig. S4A). The early genes E74 and E75, which are directly activated by EcR, were used as controls. The mRNA expression of E74 and E75 exhibited a similar responsive tendency to CHX as E93 (SI Appendix, Fig. S4 B and C). To investigate the genomic interaction of EcR and E93, we first analyzed the E93 upstream regulatory region (nucleotides [nt] −1900 to −1) using a luciferase cell transfection assay. The results also suggested a direct action of EcR/Ultraspiracle (USP) in activation of the E93 gene after 20E treatment (SI Appendix, Fig. S4D). Furthermore, ChIP-qPCR analysis demonstrated a direct genetic interaction between EcR and the E93 promoter (Fig. 3 A and B) (SI Appendix, Supplemental Results). We also performed EMSA to verify the physical interaction between EcR and the E93 promoter. As shown in Fig. 3C, a band shift was detected when FB-NE was incubated with the biotin-labeled E93 probe. However, the band was abolished when FB-NE was preincubated with excess of the unlabeled E93 probe, but not the unlabeled mutant probe. A diminished band was observed using anti-EcR polyclonal antibody, suggesting the existence of EcR in FB-NE–probe complexes. When the histone H3 antibody was preincubated with FB-NE, the intensity of the band shift was similar to that in normal binding samples. Thus, these results demonstrate a direct action of 20E/EcR on the E93 gene, as has been previously observed for D. melanogaster and B. mori (20, 28).
Fig. 3.
E93 is the 20E-regulated primary response gene. (A) The occupancy of EcR on the promoter region of the E93 gene as measured using the ChIP-qPCR assay. Chromatin from FBs at 36 h PBM was immunoprecipitated with anti-EcR or anti-IgG (control) antibodies. Results are shown as % of total input chromatin compared with IgG control. (B) The E93 coding sequence was used as a control in the ChIP-qPCR analysis to compare with the enrichment of the EcR-binding region in the E93 promoter. (C) EMSA results showing the binding of EcR to biotin-labeled E93 probe. The unlabeled E93 (cold) probe in 200× molar excess was used to test the binding specificity, while a mutant probe of the same concentration was a control. Histone H3 antibody served as nonspecific antibodies. Data are shown as mean ± SEM **P < 0.01.
E93 Is Essential for the Regulation of Gonadotrophic Cycles in Adult Female A. aegypti Mosquitoes.
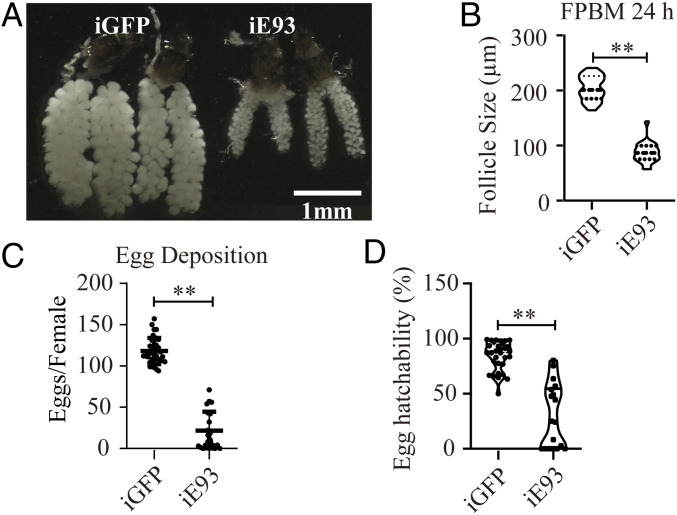
We investigated the potential role of E93 in controlling reproductive switches. We injected dsRNA targeting E93 (dsE93) into female mosquitoes within 24 h PE, fed them blood 3 d after injection, and examined the outcome during the FPBM period. The other batch of E93 RNAi mosquitoes was permitted to lay eggs, feed on blood, and was examined during the SPBM period. The E93 mRNA abundance was reduced to a low level throughout the FPBM and SPBM periods after a single dsE93 injection (SI Appendix, Fig. S5). During FPBM, ovarian development was severely retarded following E93 knockdown, with a similar defect in ovaries in the second cycle (Fig. 4A and SI Appendix, Fig. S6A). The average follicle length of iE93 mosquitoes was 57.46% lower (86.86 µm on average) in the FPBM and 55.96% lower (86.40 µm on average) in the SPBM than iGFP controls (204.20 µm and 196.20 µm, on average, respectively) (Fig. 4B and SI Appendix, Fig. S6B). Consistent with ovarian defects, egg deposition was dramatically lower in iE93 mosquitoes than iGFP (21.6 versus 118.1 eggs per female mosquito in the FPBM period; 47.10 versus 108.0 eggs per female mosquito in the SPBM period) (Fig. 4C and SI Appendix, Fig. S6C). E93 RNAi also had a significant impact on the hatchability of mosquito eggs. A single E93 RNAi depletion event severely reduced the egg hatchability by 65.08% in the first reproductive cycle and by 79.68% in the second reproductive cycle (Fig. 4D and SI Appendix, Fig. S6D).
Fig. 4.
E93 RNAi negatively affects the development of mosquito ovaries during the first gonadotrophic cycle. Female mosquitoes were injected with dsRNA of E93 or GFP within 24 h PE, and ovarian development was examined at 24 h FPBM. (A) The ovary phenotypes of iGFP and iE93 mosquitoes are shown. Images were captured under a 5-megapixel high-definition CMOS camera built into a Leica EZ4W stereoscopic microscope. (B) The follicle lengths (24 h FPBM) of iGFP and iE93 mosquitoes were measured in ImageJ software. Comparison of the egg deposition (C) and hatchability (D) between iE93 and iGFP mosquitoes. Data shown in B–D are represented as mean ± SEM **P < 0.01.
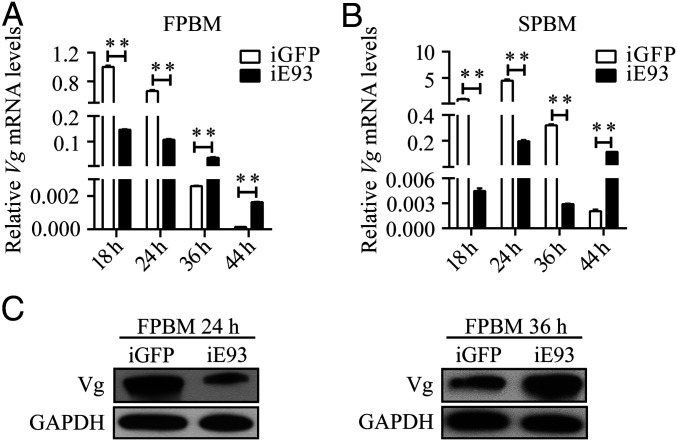
Next, we examined the effect of the E93 knockdown on Vg gene expression throughout both FPBM and SPBM periods. According to the previous report (27), Vg expression in mosquitoes normally peaks at 24 h PBM and then it rapidly declines by 36 h PBM. After E93 RNAi knockdown, Vg expression was significantly reduced at 18 h and 24 h PBM, but sharply roused at 36 h and 44 h FPBM in comparison with that in GFP knockdown mosquitoes (Fig. 5A). During the SPBM period, the expression tendency of the Vg gene after E93 knockdown was similar to the FPBM period, but the repressive role of E93 on Vg expression was delayed until 44 h (Fig. 5B). We also examined the protein levels of Vg at 24 h FPBM and 36 h FPBM using Western blotting analysis. After the E93 knockdown, the Vg protein level was nearly 50% lower at 24 h FPBM, while significantly higher at 36 h FPBM (Fig. 5C), which is similar to the pattern observed with mRNA expression in Fig. 5A. This suggests the E93 role in the regulation of the Vg gene switches at the time of vitellogenesis termination from being an activator to a repressor.
Fig. 5.
Effect of E93 depletion on the Vg mRNA abundance during the first and second gonadotrophic cycles of female A. aegypti mosquitoes. (A) Female mosquitoes, injected with dsRNA of E93 or GFP within 24 h PE, were given the first blood meal 3 d postinjection. Vg transcript levels were examined at 18 h, 24 h, 36 h, and 44 h FPBM. (B) Another batch of mosquitoes was given a second blood meal after laying eggs, and Vg transcript levels were inspected at 18 h, 24 h, 36 h, and 44 h SPBM. Transcript levels of Vg were quantified using qPCR. Each sample was normalized to its internal control ribosomal protein 7 mRNA (rps7). All the data in iE93 mosquitoes were normalized to that in iGFP mosquitoes, which were represented as 1. (C) Western blotting showing Vg protein levels in iE93 and iGFP mosquitoes at 24 h and 36 h FPBM. Data are shown as mean ± SEM **P < 0.01.
In adult female A. aegypti mosquitoes, HR3 has been implicated in regulating reproductive cycles (11). Here, we show that the expression of this gene was affected by the E93 RNAi in FBs of adult female Aedes mosquitoes. We find that RNAi-mediated knockdown of E93 disrupts expression of the HR3 gene throughout both FPBM and SPBM periods, in a pattern very similar to that of Vg (SI Appendix, Fig. S7 A and B). This suggests that in HR3 gene expression, the E93 role also switches at the time of vitellogenesis termination from being an activator to a repressor. The molecular mechanism determining the E93 functional switch remains to be investigated. Collectively, the data indicate that E93 is required for the regulation of the ovarian development during vitellogenesis and plays a key role in determining the gonadotrophic cyclicity.
Effect of E93 RNAi Silencing on the FB Transcriptome at 24 h and 36 h FPBM in Adult Female Aedes Mosquitoes.
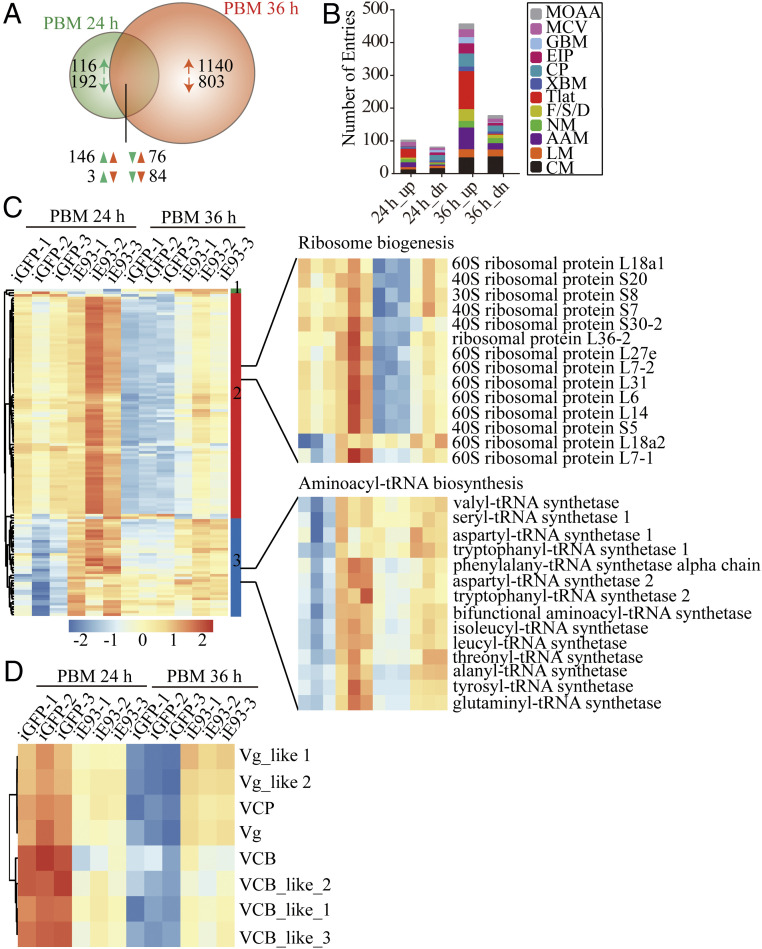
To uncover gene targets of E93 during reproduction, FBs of E93 dsRNA-injected mosquitoes were subjected to transcriptome analysis at 24 h and 36 h FPBM. Transcripts with a fold change (FC) of either >2 (up-regulated genes) or <0.05 (down-regulated genes) and a q value (adjusted P value) <0.05 were considered as differential expression genes (DEGs). Notably, a large number of gene cohorts (2,252) were significantly affected by E93 knockdown at 36 h FPBM, while only a small number at 24 h (617). DEGs detected at both time points are shown in SI Appendix, Table S1 and Fig. S8. Most genes were affected by silencing of E93 at 36 h FPBM. We find 309 genes commonly regulated at both time points (Fig. 6A), of which 146 were up-regulated and 84 were down-regulated in both samples. The remaining 79 genes displayed the opposite pattern of expression after E93 silencing. Additionally, 308 genes (116 up-regulated and 192 down-regulated) were uniquely regulated at 24 h FPBM and 1,943 genes were uniquely regulated at 36 h FPBM (1,140 up-regulated and 803 down-regulated) (Fig. 6A). This indicates that E93 plays an important role during adult female reproduction, especially at the end of the vitellogenesis.
Fig. 6.
Comparison of FB transcriptomes after E93 dsRNA injection between 24 h FPBM and 36 h FPBM. (A) The Venn diagram analysis of commonly and uniquely E93-regulated genes at 24 h and 36 h FPBM. (B) Distribution of gene functional groups within up- and down-regulated E93 RNAi-depleted transcriptome at 24 h and 36 h FPBM. Functional group abbreviations: MOAA, metabolism of other amino acids; MCV, metabolism of cofactors and vitamins; GBM, glycan biosynthesis and metabolism; EIP, environmental information processing; CP, cellular process; XBM, xenobiotics biodegradation and metabolism; Tlat, translation; F/S/D, folding, sorting, and degradation; NM, nucleotide metabolism; AAM, amino acid metabolism; LM, lipid metabolism; and CM, carbohydrate metabolism. Hierarchical clustering analysis of differentially expressed genes involved in translation (C) and vitellogenesis (D) at 24 h and 36 h FPBM between iE93 and iGFP control. Part of the genes associated with ribosome biogenesis and aminoacyl-tRNA biosynthesis are shown in C.
To examine the functional classification of the DEGs at these two time points, the up- and down-regulated genes were subjected to the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. The functional distribution of DEGs at 24 h and 36 h FPBM exhibited similar tendencies (Fig. 6B). However, the gene number in most cohorts exhibited a remarkable increase at 36 h FPBM in comparison with 24 h FPBM. These included genes related to carbohydrate metabolism (CM), lipid metabolism (LM), nucleotide metabolism (NM), and amino acid metabolism (AM). The functional groups that showed significant overrepresentation in iE93-down-regulated clusters at 24 h FPBM and iE93-up-regulated clusters at 36 h FPBM were environmental information processing (EIP), glycan biosynthesis and metabolism (GBM), and cellular processes (CPs) (Fig. 7B). This indicates that E93 knockdown changed the basal metabolism and energy homeostasis in adult female mosquitoes. Importantly, the gene cohorts that include translation (Tlat) were significantly enriched in iE93-up-regulated gene sets, particularly at 36 h FPBM (Fig. 7B). We then performed the hierarchical clustering analysis of the differentially expressional transcripts involved in translation between 24 h and 36 h FPBM (Fig. 6C). Obviously, the expressional pattern of these genes could be divided into three groups. Group 1 included two genes referring to RNA transport. Transcripts in group 2 were involved in ribosome biogenesis. Except for a small number of genes associated with RNA transport and ribosome biogenesis, the majority of genes involved in aminoacyl-tRNA biosynthesis were in group 3. This analysis has confirmed the extensive E93-mediated shutdown of translation during vitellogenesis, especially at 36 h FPBM.
Fig. 7.
Autophagy of mosquitoes is affected by E93. (A) Hierarchical clustering analysis of autophagy-related genes at 36 h FPBM. (B) qPCR analysis of the mRNA levels of autophagy-related genes in E93 RNAi mosquitoes. (C) Immunofluorescence assays showing the ATG8 distribution in FBs of iE93 mosquitoes compared with iGFP control. Anti-ATG8 antibodies were used to detect the ATG8 protein (red). Alexa Fluor 594 was used as the secondary antibody. Nuclei stained with Hoechst 488 was blue. (D) The dual luciferase reporter assay showing the effect of E93 on the ATG8 gene. Cells transfected with the empty vector pAc5.1b or no transfection served as the control. The Renilla luciferase vector pGL4.73 was used as an expression control in the luciferase assays. The Western blot showed the protein levels of E93-V5 fusion proteins after transfection in S2 cells for 48 h using anti-V5 monoclonal antibody. GAPDH antibody was used as a loading control. Data are shown as mean ± SEM **P < 0.01.
Consistent with the qPCR results shown in Fig. 5A, the expression level of Vg and Vg-like genes in the iE93 transcriptome was down-regulated at 24 h and up-regulated at 36 h FPBM (Fig. 6D). Notably, expression of these genes in iGFP transcriptomic data exhibited higher expression at 24 h than that at 36 h, which was consistent with the observation reported previously (27). In fact, in addition to Vg, the female mosquito FB produces several other genes involved in vitellogenesis. For example, vitellogenic carboxypeptidase (VCP) is activated during embryogenesis, and cathepsin B-like protease (VCB) is involved in embryonic degradation of vitellin (29, 30). E93 knockdown negatively affected these genes at 24 h FPBM, suggesting an activating role of this factor at the beginning of vitellogenesis. However, transcripts of these genes were significantly increased at 36 h FPBM in iE93 mosquitoes, indicating that E93 acts as a repressor at the terminal phase of vitellogenesis (Fig. 6D). A timely shutdown of the expression of vitellogenesis-related genes is essential for mosquitoes to enter into the second reproductive cycle. These data further demonstrate the role of E93 in gonadotrophic cyclicity.
E93 Regulates Autophagy in Female Aedes Mosquitoes.
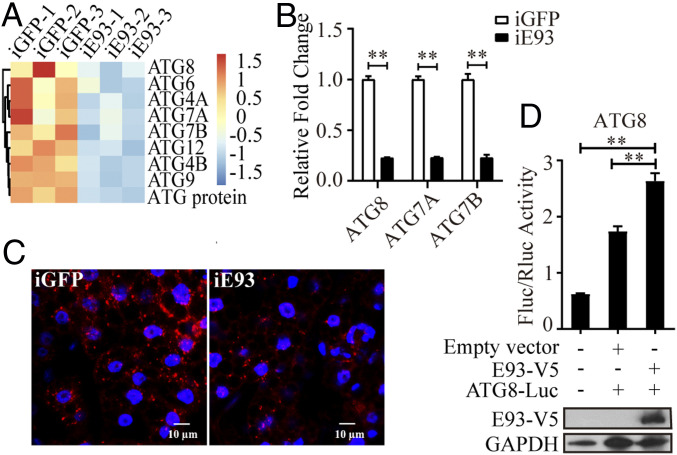
Autophagy is involved in tissue remodeling and has been linked with the regulation of reproductive cyclicity in A. aegypti female mosquitoes (14). We investigated whether E93 is involved in controlling autophagy activity. As expected, several autophagy-related genes (ATGs), including ATG8, ATG4B, and ATG2, were among DEGs and showed a significant reduction after E93 knockdown. It is worth mentioning that ATG8 was an important autophagy marker gene. A further transcriptome data mining (FC < 0.67) revealed that ATG18A also showed a reduction in mRNA expression after E93 knockdown at 24 h FPBM, except for ATG8, ATG4B, and ATG2 (SI Appendix, Fig. S9A). However, a considerably larger number of ATGs showed reduced expression levels at 36 h FPBM in E93-depleted mosquitoes (Fig. 7A). Furthermore, the mRNA levels of ATG8 and ATG4B were reduced at both time points, indicating a positive effect of E93 on the regulation of autophagy genes and thus the autophagy process during vitellogenesis. The larger amount of ATGs at 36 h FPBM than at 24 h FPBM suggests that the regulation of autophagy by E93 occurs mainly at 36 h FPBM.
Subsequently, several ATGs have been analyzed using qPCR after E93 knockdown. In line with the transcriptome data, E93 RNAi led to an obvious reduction in transcript abundance of ATG8, ATG2, and ATG18A at 24 h FPBM (SI Appendix, Fig. S9B) and ATG8, ATG7A, and ATG7B at 36 h FPBM (Fig. 7B). We next determined the ATG8 protein level of iE93 and iGFP female mosquitoes using Western blotting. As shown in SI Appendix, Fig. S9C, the band of lipidated ATG8-PE (ATG8-phosphatidylethanolamine) was remarkably lower in iE93 mosquitoes than iGFP controls, indicating that the delivery of ATG8 to the autophagosome double membrane was blocked (31). Immunofluorescence analysis was conducted to monitor the intracellular distribution of the ATG8 protein in FBs at 36 h FPBM. At that time, there is a high level of lysosomal activity in FB cells (14). iE93 mosquitoes exhibited less fluorescent signal than control mosquitoes (iGFP), again indicating the E93-activating role in regulating autophagy (Fig. 7C). Next, we examined the role of E93 effect on the ATG8 promoter using the dual luciferase reporter assay. Luciferase activity was significantly increased after overexpressing the E93-V5 fusion protein and the ATG8 luciferase reporter plasmid in S2 cells compared with control groups (Fig. 7D) (SI Appendix, Supplemental Results). These findings highlight a critical role for E93 as an ATG8-positive regulator that interacts with the ATG8 promoter region. Taken together, the present findings suggest that autophagy in E93-depleted mosquitoes is impaired and the disruption of developmental transitions in iE93 mosquitoes during the gonadotrophic cycle might be caused, at least partially, by the incompetent autophagy at the terminal phase of vitellogenesis.
Discussion
A unique aspect of female mosquitoes is the cyclicity of their egg development. Importantly, each cycle is closely coupled with a separate blood meal and successive gonadotrophic cycles aid the transmission of mosquito-borne pathogens. Thus, deciphering the molecular mechanism governing mosquito reproductive cyclicity is vital for the development of innovative strategies for vector control. Our work described here has revealed that E93 has a remarkable impact on the reproduction of adult female A. aegypti mosquitoes by playing a critical role in the regulation of reproductive cyclicity.
Previously, E93 has been defined as the adult specifier determining metamorphic shifts from a pupa or a last instar nymph to an adult in both holo- and hemimetabolous insect species (16). During successive gonadotrophic cycles of an adult female mosquito, E93 gene expression is sequentially suppressed by JH and induced by 20E. Similar responses to JH and 20E on the E93 gene have been reported during metamorphic transitions in D. melanogaster and B. mori (17, 20, 28). We show that the repression of E93 expression during the PE phase of mosquito reproduction is indirect and mediated by a synergistic action of Kr-h1 and Hairy. This mode of JH-mediated gene repression has been characterized for other mosquito genes (8). The timely activating of E93 by 20E after a blood meal is required for inducing expression of Vg and other YPP genes essential for egg maturation. E93 RNAi-depleted mosquitoes displayed a retardation of ovary development in both the first and second reproductive cycles, once again indicating the importance of E93 in female mosquito reproduction. Similarly, studies in the brown planthopper N. lugens and red flour beetle T. castaneum showed that E93 knockdown delayed ovary development and reduced the number of egg depositions (32, 33). Thus, the E93 role in regulating reproduction is likely widespread among insects.
Our transcriptome-based analysis of the adult female mosquito FB has implicated E93 in mediating many gene cohorts, particularly those genes encoding translation, YPP, and metabolism. Of importance, this analysis has shown the massive E93-dependent shutdown of these genes’ expression at reproductive cycle termination. Intriguingly, we observed a switch in E93 activity on YPP and HR3 from being a transcriptional activator at the peak of vitellogenesis (24 h PBM) to a transcriptional repressor at the time of termination (36 h PBM). At that time period, the 20E titer is dropping from its 24-h peak to a low level at 36 h and JH titer begins to increase (25, 26). We hypothesize that this switch of the E93 action might be the cross-talk of the JH and 20E and involved in the recruitment of functionally different cofactors. E93 acts as an activator at 24 h PBM, when 20E exhibits its peak titer. However, the E93 role switches to transcriptional repression when the 20E titer is dramatically reduced and JH begins to increase at 36 h PBM, initiating the termination event. Indeed, these two opposing transcriptional outputs controlled by E93 have been indicated in D. melanogaster during wing development. E93 regulated its target gene expression by controlling chromatin accessibility, which was involved in chromatin opening and closing. Importantly, the regulation of chromatin accessibility in developmental timing was hormone dependent (34, 35). In addition, the multiple functional domains in E93 protein might contribute to either the activation or repression dependent on the developmental context. LCoR, the homolog of E93 in mammals, has been reported to function differentially through binding with various transcriptional regulator proteins (36). In A. aegypti, the elucidation of the molecular mechanism underlying this E93 functional switch over time awaits a future investigation.
Autophagy is a classic degradation pathway, which is mediated by lysosomes. It is involved in a variety of cellular processes, including immunity, tissue remodeling, metabolism, and reproduction (14, 37–39). In A. aegypti, FB remodeling caused by a programmed autophagy during oogenesis is important for a timely termination of vitellogenesis and maintenance of an unobstructed reproductive cycle. After a blood meal, activation of TOR signaling pathway, Vg production, and autophagy are successive events. At the early phase of vitellogenesis, amino acids and insulin-mediated TOR pathway activation are required for up-regulating Vg expression and inhibiting autophagy (11). However, during the terminal phase of vitellogenesis, programmed autophagy inhibits TOR signaling and Vg production. Autophagy-deficient mosquitoes show the continuously active TOR signaling and extended vitellogenesis (14). In D. melanogaster, autophagy protein ATG1 has also been shown to be a negative regulator of TOR (40–42). In addition, impairment of autophagy was unable to activate the expression of competence factor betaFTZ-F1 and hindered the second cycle of egg development (14). In the current study, we found that several ATGs were induced by E93 during the termination of vitellogenesis. Autophagic flux in the FB of E93 RNAi-silenced mosquitoes dramatically diminished at the late phase of vitellogenesis. Moreover, we have demonstrated an increased luciferase activity when cotransfected E93 plasmids with ATG8 promoter in S2 cells. Similarly, E93 has been confirmed to trigger autophagy by binding to the promoter region of ATG1 during the B. mori pupal–adult transition (20).
In summary, our study has identified the critical role of E93—previously implicated in metamorphic transitions—in controlling reproductive switches in the adult female A. aegypti mosquitoes. It has shed further light on the regulatory complexity of gonadotrophic cyclicity in this important vector of human disease. It has also expanded our understanding of the role of E93 in insect reproduction.
Materials and Methods
A detailed description of the materials and methods is given in SI Appendix, Materials and Methods. In brief, the culture of A. aegypti mosquitoes, qPCR, Western blot, EMSA, and dual luciferase report assay were performed according to the protocols described previously (43). ChIP assays were performed using Magna ChIP G Tissue Kit (Sigma-Aldrich) following the manufacturer’s instructions. FBs, stained with ATG8 antibody, were visualized under a Zeiss LSM 710 confocal microscope to collect the immunofluorescence data. Total mRNA extracted from FBs were sequenced using the Illumina platform. Primers used in this study are shown in SI Appendix, Table S2.
Supplementary Material
Acknowledgments
This work was supported by National Key Plan for Scientific Research and Development of China Grant 2019YFC1200504 (to Z.Z.), National Science Foundation of China Grant 31802013 (to X.W.), CAS Grant ZDBS-LY-SM027 (to Z.Z.), and NIH Grant R01 AI036959 (to A.S.R.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021910118/-/DCSupplemental.
Data Availability
All study data are included in the main text and supporting information.
References
- 1.McNeil C. J., Shetty A. K., Zika virus: A serious global health threat. J. Trop. Pediatr. 63, 242–248 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Tsetsarkin K. A., Chen R., Weaver S. C., Interspecies transmission and chikungunya virus emergence. Curr. Opin. Virol. 16, 143–150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S., et al., The global distribution and burden of dengue. Nature 496, 504–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett A. D., Higgs S., Yellow fever: A disease that has yet to be conquered. Annu. Rev. Entomol. 52, 209–229 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Roy S., Saha T. T., Zou Z., Raikhel A. S., Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 63, 489–511 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Zou Z., et al., Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc. Natl. Acad. Sci. U.S.A. 110, E2173–E2181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ojani R., Fu X., Ahmed T., Liu P., Zhu J., Krüppel homologue 1 acts as a repressor and an activator in the transcriptional response to juvenile hormone in adult mosquitoes. Insect Mol. Biol. 27, 268–278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saha T. T., et al., Synergistic action of the transcription factors Krüppel homolog 1 and Hairy in juvenile hormone/Methoprene-tolerant-mediated gene-repression in the mosquito Aedes aegypti. PLoS Genet. 15, e1008443 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha T. T., et al., Hairy and Groucho mediate the action of juvenile hormone receptor Methoprene-tolerant in gene repression. Proc. Natl. Acad. Sci. U.S.A. 113, E735–E743 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J., Chen L., Raikhel A. S., Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 100, 13338–13343 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mane-Padros D., Cruz J., Cheng A., Raikhel A. S., A critical role of the nuclear receptor HR3 in regulation of gonadotrophic cycles of the mosquito Aedes aegypti. PLoS One 7, e45019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun G., Zhu J., Raikhel A. S., The early gene E74B isoform is a transcriptional activator of the ecdysteroid regulatory hierarchy in mosquito vitellogenesis. Mol. Cell. Endocrinol. 218, 95–105 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Pierceall W. E., et al., E75 expression in mosquito ovary and fat body suggests reiterative use of ecdysone-regulated hierarchies in development and reproduction. Mol. Cell. Endocrinol. 150, 73–89 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Bryant B., Raikhel A. S., Programmed autophagy in the fat body of Aedes aegypti is required to maintain egg maturation cycles. PLoS One 6, e25502 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy S., et al., Regulation of gene expression patterns in mosquito reproduction. PLoS Genet. 11, e1005450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ureña E., Manjón C., Franch-Marro X., Martín D., Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc. Natl. Acad. Sci. U.S.A. 111, 7024–7029 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belles X., Santos C. G., The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem. Mol. Biol. 52, 60–68 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Belles X., Krüppel homolog 1 and E93: The doorkeeper and the key to insect metamorphosis. Arch. Insect Biochem. Physiol. 103, e21609 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Liu H., Wang J., Li S., E93 predominantly transduces 20-hydroxyecdysone signaling to induce autophagy and caspase activity in Drosophila fat body. Insect Biochem. Mol. Biol. 45, 30–39 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Liu X., et al., 20-Hydroxyecdysone (20E) primary response gene E93 modulates 20E signaling to promote Bombyx larval-pupal metamorphosis. J. Biol. Chem. 290, 27370–27383 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K. L., et al., The roles of E93 and Kr-h1 in metamorphosis of Nilaparvata lugens. Front. Physiol. 9, 1677 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayukawa T., Jouraku A., Ito Y., Shinoda T., Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 114, 1057–1062 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gujar H., Palli S. R., Krüppel homolog 1 and E93 mediate Juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci. Rep. 6, 26092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chafino S., et al., Upregulation of E93 gene expression acts as the trigger for metamorphosis independently of the threshold size in the beetle Tribolium castaneum. Cell Rep. 27, 1039–1049.e2 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Hagedorn H. H., et al., The ovary as a source of alpha-ecdysone in an adult mosquito. Proc. Natl. Acad. Sci. U.S.A. 72, 3255–3259 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao B., et al., Determination of juvenile hormone titers by means of LC-MS/MS/MS and a juvenile hormone-responsive Gal4/UAS system in Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 77, 69–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho W. L., Raikhel A. S., Cloning of cDNA for mosquito lysosomal aspartic protease. Sequence analysis of an insect lysosomal enzyme similar to cathepsins D and E. J. Biol. Chem. 267, 21823–21829 (1992). [PubMed] [Google Scholar]
- 28.Baehrecke E. H., Thummel C. S., The Drosophila E93 gene from the 93F early puff displays stage- and tissue-specific regulation by 20-hydroxyecdysone. Dev. Biol. 171, 85–97 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Cho W. L., Deitsch K. W., Raikhel A. S., An extraovarian protein accumulated in mosquito oocytes is a carboxypeptidase activated in embryos. Proc. Natl. Acad. Sci. U.S.A. 88, 10821–10824 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho W. L., et al., Mosquito cathepsin B-like protease involved in embryonic degradation of vitellin is produced as a latent extraovarian precursor. J. Biol. Chem. 274, 13311–13321 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Klionsky D. J., et al., Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 12, 1–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao Y., Li Y., Gao H., Lin X., The direct interaction between E93 and Kr-h1 mediated their antagonistic effect on ovary development of the Brown Planthopper. Int. J. Mol. Sci. 20, 2431 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eid D. M., Chereddy S. C. R. R., Palli S. R., The effect of E93 knockdown on female reproduction in the red flour beetle, Tribolium castaneum. Arch. Insect Biochem. Physiol. 104, e21688 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nystrom S. L., Niederhuber M. J., McKay D. J., Expression of E93 provides an instructive cue to control dynamic enhancer activity and chromatin accessibility during development. Development 147, dev181909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uyehara C. M., et al., Hormone-dependent control of developmental timing through regulation of chromatin accessibility. Genes Dev. 31, 862–875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palijan A., et al., Ligand-dependent corepressor LCoR is an attenuator of progesterone-regulated gene expression. J. Biol. Chem. 284, 30275–30287 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo C. J., Hansen M., Troemel E., Autophagy and innate immunity: Insights from invertebrate model organisms. Autophagy 14, 233–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K., Czaja M. J., Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 20, 3–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mpakou V. E., Nezis I. P., Stravopodis D. J., Margaritis L. H., Papassideri I. S., Different modes of programmed cell death during oogenesis of the silkmoth Bombyx mori. Autophagy 4, 97–100 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Lee S. B., et al., ATG1, an autophagy regulator, inhibits cell growth by negatively regulating S6 kinase. EMBO Rep. 8, 360–365 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neufeld T. P., TOR-dependent control of autophagy: Biting the hand that feeds. Curr. Opin. Cell Biol. 22, 157–168 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott R. C., Schuldiner O., Neufeld T. P., Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 7, 167–178 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Wang X., et al., Hormone and receptor interplay in the regulation of mosquito lipid metabolism. Proc. Natl. Acad. Sci. U.S.A. 114, E2709–E2718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and supporting information.