Significance
Opioid use disorder is on the rise, as are efforts to understand vulnerability to misuse. However, understanding resilience to drug abuse is also critical to the development of novel treatments. Here we demonstrate that a brief postnatal exposure to a low-resource environment—using the limited bedding and nesting (LBN) manipulation—promoted resilience to addiction-related phenotypes in adult male rats. Specifically, LBN reduced impulsivity, morphine self-administration, and nucleus accumbens (NAc) glutamate transmission in males, effects not seen in females. By comparing the sexes, we found that changes in NAc gene transcription unique to LBN males may contribute to resilience. Importantly, these molecular changes reveal new targets that could guide the development of pharmacotherapies for opioid use disorder.
Keywords: substance use disorder, sex difference, stress, nucleus accumbens, transcription
Abstract
Experiencing some early life adversity can have an “inoculating” effect that promotes resilience in adulthood. However, the mechanisms underlying stress inoculation are unknown, and animal models are lacking. Here we used the limited bedding and nesting (LBN) model of adversity to evaluate stress inoculation of addiction-related phenotypes. In LBN, pups from postnatal days 2 to 9 and their dams were exposed to a low-resource environment. In adulthood, they were tested for addiction-like phenotypes and compared to rats raised in standard housing conditions. High levels of impulsivity are associated with substance abuse, but in males, LBN reduced impulsive choice compared to controls. LBN males also self-administered less morphine and had a lower breakpoint on a progressive ratio reinforcement schedule than controls. These effects of LBN on addiction-related behaviors were not found in females. Because the nucleus accumbens (NAc) mediates these behaviors, we tested whether LBN altered NAc physiology in drug-naïve and morphine-exposed rats. LBN reduced the frequency of spontaneous excitatory postsynaptic currents in males, but a similar effect was not observed in females. Only in males did LBN prevent a morphine-induced increase in the AMPA/NMDA ratio. RNA sequencing was performed to delineate the molecular signature in the NAc associated with LBN-derived phenotypes. LBN produced sex-specific changes in transcription, including in genes related to glutamate transmission. Collectively, these studies reveal that LBN causes a male-specific stress inoculation effect against addiction-related phenotypes. Identifying factors that promote resilience to addiction may reveal novel treatment options for patients.
The opioid epidemic is a pervasive crisis, with negative impacts on individuals and society that are not abating, including a dramatic increase in opioid overdoses (1). Given this crisis, many efforts have focused on identifying mechanisms that underlie vulnerability to opioid abuse. However, it is also important to consider factors that promote resilience to substance use disorder (SUD). If the molecular mechanisms underlying resilience are identified, they may guide the development of novel therapies to prevent opioid abuse.
Exposure to severe stress early in life is associated with the later development of SUD (2, 3). However, most people exposed to early life adversity do not develop illnesses, and many are protected from later stressful experiences (4, 5). Some adversity early in life can actually improve coping skills to deal with later stressors (5). These data are consistent with early studies in monkeys revealing that intermittent stress early in life has an “inoculating” effect that promotes the development of resilience in adulthood (4). Since this early primate work, researchers have often turned to rodent models to identify mechanisms by which early life experience reprograms the brain. Some rodent studies support the stress inoculation hypothesis, while others find that early life adversity increases vulnerability (6–8). A recent synthesis of this literature found that such factors as the quality of maternal care, duration of the manipulation, developmental timing, and genetic background of the species contribute to these discrepancies (9).
We used a model of early life adversity, limited bedding and nesting (LBN) (10), to test whether a low-resource environment early in development promotes resilience to addiction-related phenotypes in adulthood. In our version of LBN, we breed rats in-house rather than ship pregnant dams, which can cause prenatal stress (11). Then rat dams and postnatal day (PND) 2 to 9 pups are placed in an impoverished environment. We previously found that LBN alters maternal care by increasing pup-directed behaviors, such as nursing and pup grooming, while reducing self-care, such as self-grooming and resting outside the nest (12).This shift in maternal behavior is consistent with a hypervigilant phenotype (13), and although it may be an attempt to compensate for the altered environment, offspring differ from controls. Specifically, progeny weigh less throughout development and exhibit endocrine changes that are particularly pronounced in male rats (12).
Here we extended our previous findings to determine how LBN affects addiction-related behaviors. We first assessed the effect of LBN on impulsive choice and morphine self-administration. Given the role of the nucleus accumbens (NAc) in these behaviors, we then tested the effect of LBN on NAc physiology. Finally, we evaluated LBN-induced changes in gene expression in the NAc. Given the shift in maternal care toward pups and the relatively mild nature of the model—altering only the postnatal environment for 7 d, versus prenatal and postnatal manipulations or postnatal manipulations that take several weeks (14)—we predicted that LBN would have an inoculating effect on addiction-related phenotypes.
Results
LBN Decreased Impulsive Choice in Male Rats.
We first investigated whether LBN exposure altered impulsive choice, given that impulsivity is associated with SUD (15). Long–Evans dams and pups were placed in standard housing (ample bedding, two nestlets/cage, one tunnel enrichment device/cage) or the LBN condition (no access to bedding materials, one paper towel for nesting, no enrichment) on PND2. Rats were transferred to normal housing on PND10 and tested as adults (PND 60 to 90) in all studies. As detailed in SI Appendix, Methods, we did not statistically compare the sexes in the behavior and physiology studies and so are limited in making direct conclusions regarding sex differences.
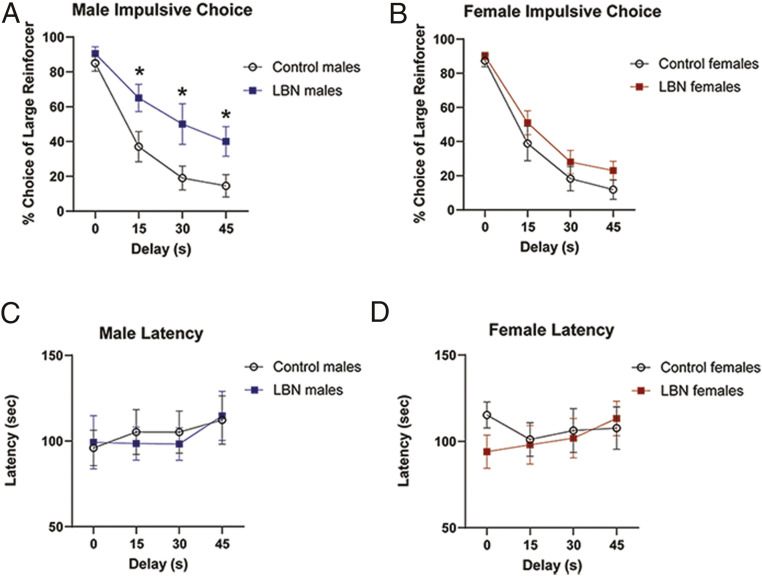
For the delayed discounting task, rats must choose between a small/immediate reward and a larger/delayed reward. During training, there were no differences in the time it took LBN (n = 8) vs. control males (n = 10) and LBN (n = 10) vs. control females (n = 9) to learn to lever press or to reach stable performance on delayed discounting (SI Appendix, Results). For delayed discounting, the percentage choice of the larger/delayed option relative to the smaller/no-delay option was calculated, such that a higher value indicated less impulsivity. There was a significant interaction for males (F3,48 = 4.59, P = 0.007). Sidak post hoc tests showed that LBN and control males performed similarly on the 0-s delay (P = 0.812), but that as delays increased, LBN males chose the larger/delayed reward more frequently (15 s, P = 0.0005; 30 s, P = 0.0002; 45 s, P = 0.0012), indicating reduced impulsive choice (Fig. 1A). Preference for larger/delayed reward was similar in LBN and control females (delay: F3,51 <1; LBN: F1,17 = 1.54, P = 0.231; interaction: F3,51 = 0.496, P = 0.687) (Fig. 1B). Neither response latencies nor omissions (not responding; SI Appendix, Fig. S1) differed between LBN and control males or females (Fig. 1 C and D) (SI Appendix, Results).
Fig. 1.
LBN reduces impulsive choice in male rats. (A) LBN exposure reduced impulsive choice in male rats, as evidenced by the increase in preference of the larger/delayed option at the 15 s, 30 s, and 45 s delays. *P < 0.05. (B) Discounting did not differ significantly between LBN and control females. LBN exposure did not affect male (C) or female (D) latency to respond.
LBN Reduces Self-Administration of a Low Dose of Morphine in Male Rats.
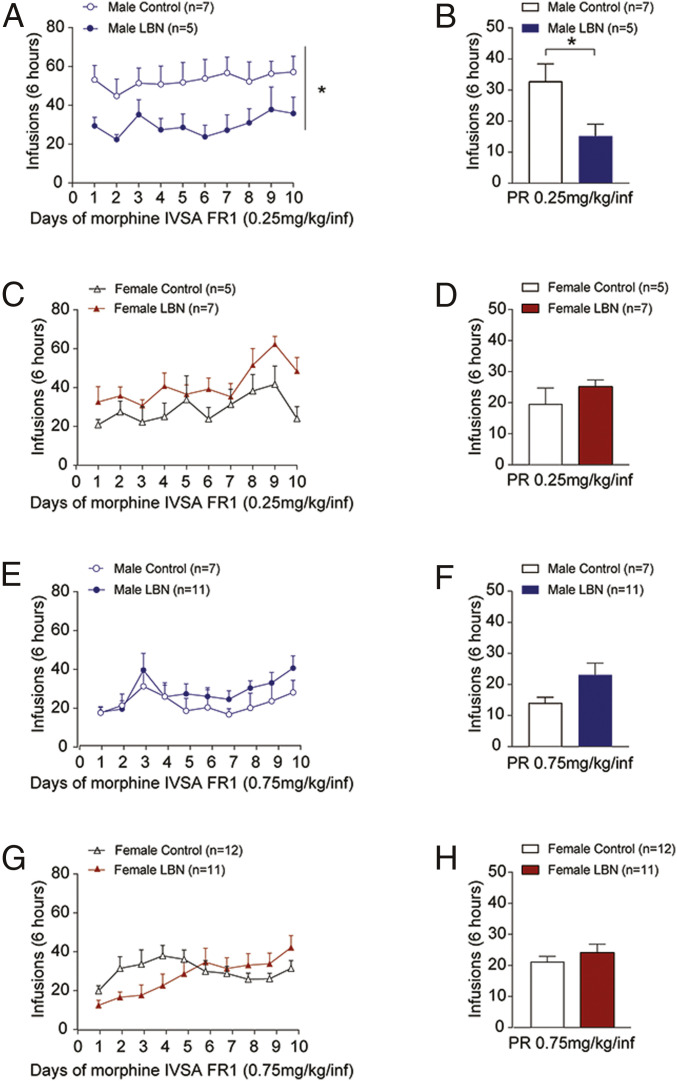
Lower impulsivity may reduce the risk for SUD, so we performed morphine self-administration studies using a low and high dose. For the low dose of morphine (0.25 mg/kg/infusion), LBN males (n = 5) earned a significantly fewer number of infusions within a 6-h period than their control counterparts (n = 7) on a fixed ratio (FR) 1 reinforcement schedule (LBN: F1,10 = 5.974, P = 0.03; days: F9,90 = 1.10, P = 0.39; interaction: F9,90 <1) (Fig. 2A). On a progressive ratio (PR) schedule, LBN males earned a fewer number of infusions compared to control males (t10 = 2.44, P = 0.03) (Fig. 2B). In females (control, n = 5; LBN, n = 7), there were no significant differences in the number of morphine infusions at the 0.25 mg/kg/infusion dose between LBN and control rats (LBN: F1,10 = 2.93, P = 0.12; days: F9,90 = 4.26, P < 0.0001; interaction: F9,90 = 1.00, P = 0.45) (Fig. 2C). LBN had no effect on PR for the low dose of morphine in females (t10 = 1.11, P = 0.29) (Fig. 2D).
Fig. 2.
LBN reduces self-administration of a low dose of morphine in male rats. LBN exposure reduced self-administration on FR1 (A) and PR (B) of a low dose of morphine (0.25 mg/kg/infusion) in male rats. *P < 0.05. LBN did not significantly alter FR1 (C) or PR (D) for this low dose in females. FR1 and PR for the high dose of morphine (0.75 mg/kg/infusion) was not affected by LBN in male rats (E and F) or female rats (G and H).
We also assessed the effect of LBN on self-administration of a higher dose of morphine (0.75 mg/kg/infusion). There were no differences in the number of morphine infusions between control (n = 7) and LBN (n = 11) males on an FR1 schedule and on a PR schedule (Fig. 2 E and F) or between control (n = 12) and LBN (n = 11) females on an FR1 schedule and on a PR schedule (Fig. 2 G and H). A subset of the rats was tested for drug-seeking behavior in response to previously drug-paired cues at 7 d after the last self-administration session. LBN had no impact on drug-seeking behavior (SI Appendix, Fig. S2). Note that the 0.75 mg/kg/infusion morphine dose was used for both the cue test and the subsequent physiology studies because it did not result in any group differences in morphine intake (SI Appendix, Table S1), which could confound the interpretation of these measures.
LBN Leads to Alterations in Glutamate Transmission in the NAc Core of Male Rats.
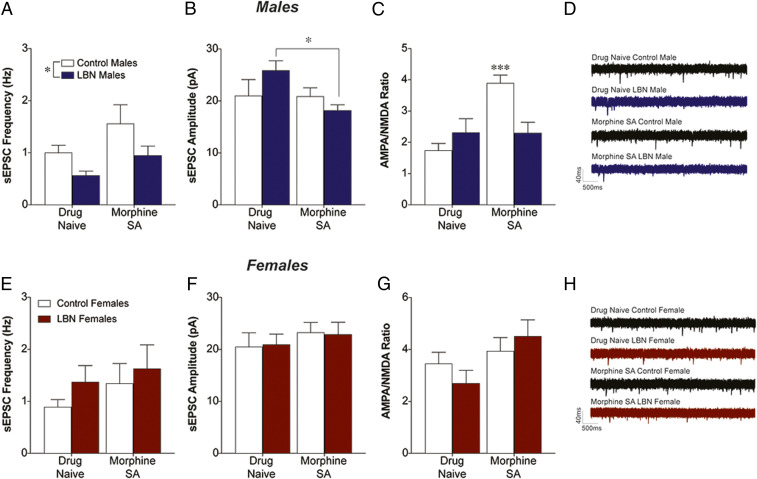
The NAc core (NAcC) mediates impulsive choice and self-administration (14), so we chose this region to explore physiological changes induced by LBN in males that could account for the behavioral findings. Spontaneous excitatory transmission in the NAcC of both naïve and morphine-experienced adult LBN rats was examined. LBN male rats had a decreased frequency of spontaneous excitatory postsynaptic currents (sEPSCs) compared to controls (8 to 12 cells/group from three rats/group) (LBN: F1,32 = 4.20, P = 0.049; morphine: F1,32 = 3.42, P = 0.074; interaction: F1,32 = 1) (Fig. 3A). While there were no effects of LBN on sEPSC amplitude in the males, there was a significant effect of morphine that may have been driven primarily by LBN males (LBN: F1,35 = 0.32, P = 0.58; morphine: F1,35 = 4.26, P = 0.047; interaction: F1,35 = 3.98, P = 0.05; Sidak’s post hoc drug naïve LBN vs. morphine SA LBN, P = 0.015) (Fig. 3B). We examined the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/NMDA (N-methyl-d-aspartate) ratio within the NAcC of drug naïve and morphine-experienced rats (eight to nine cells/group from three rats/group). The ratio between AMPA and NMDA receptors is a key factor governing plasticity of glutamatergic synapses and has been examined extensively in animal models of SUD (16–19). Morphine self-administration increased the AMPA/NMDA ratio in control males, but the LBN males did not show this drug-induced change (LBN: F1,37 = 2.15, P = 0.15; morphine: F1,37 = 9.45, P = 0.004; interaction: F1,37 = 9.68, P = 0.004; Sidak’s post hoc drug naïve control vs. morphine SA control, P < 0.001) (Fig. 3C).
Fig. 3.
LBN alters both baseline and morphine-induced glutamate transmission in male rats. (A) LBN exposure reduced sEPSC frequency in male rats regardless of morphine history. *P < 0.05. (B) sEPSC amplitude was lower following morphine exposure, and this effect was driven primarily by the LBN group. *P < 0.05, drug-naïve LBN males vs. morphine LBN males. (C) While no differences were seen in drug-naïve rats, control males exposed to morphine exhibited an increase in the AMPA/NMDA ratio that was not present in the LBN males. ***P < 0.001. (D) Example sEPSC traces from male rats. LBN exposure did not significantly affect sESPC frequency (E), sEPSC amplitude (F), or AMPA/NMDA ratio (G) in females. (H) Example sEPSC traces from female rats.
Females did not show any between-group differences in sEPSC frequency (Fig. 3E and SI Appendix, Results) or amplitude (Fig. 3F and SI Appendix, Results). While there was no effect of LBN on the AMPA/NMDA ratio in females, we did detect a trend toward an increase following morphine (7 to 12 cells/group from three rats/group) (LBN: F1,36 < 1; morphine: F1,36 = 4.11, P = 0.050: interaction: F1,36 = 1.36, P = 0.25) (Fig. 3G).
LBN Causes Sex-Specific Changes in Gene Transcription in the NAc.
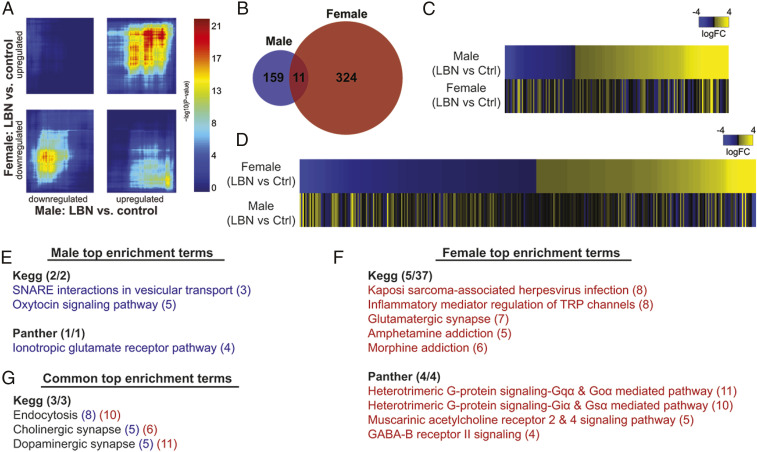
To delineate molecular signatures in the NAc that may drive the LBN phenotypes in males, we conducted RNA-sequencing in punches from whole NAc from adult behaviorally naïve rats (male control, n = 4; female control, n = 5; male LBN, n = 5; female LBN, n = 6). We first used rank-rank hypergeometric overlap (RRHO) analysis to compare overall gene expression patterns in males and females induced by LBN. This analysis included all genes that were differentially expressed, including gene changes not reaching the threshold of statistical significance, and thus facilitated the agnostic comparison of gene expression patterns after LBN in males and females. Although there was significant overlap between both up-regulated and down-regulated genes between males and females (Fig. 4A; hot spots in bottom left and top right quadrants), we also found that LBN induces a unique signature in males and females. For example, many genes were up-regulated by LBN in males but down-regulated by LBN in females (Fig. 4A; bottom right quadrant).
Fig. 4.
LBN produces sex-specific changes in gene expression in the NAc. (A) Threshold-free comparison of DEGs by rank-rank hypergeometric overlap. Hotspots represent the overlap between the impact of LBN on gene expression in females and males. Hotter colors indicate more overlap. The upper right quadrant includes co–up-regulated genes, while the lower left quadrant represents co–down-regulated genes. The top left and bottom right quadrants represent genes that are changed by LBN in opposite directions in males compared to females. (B) LBN induces 170 DEGs in males and 335 DEGs in females, with 11 overlapping DEGs between the sexes. (C) Heatmap sorted by fold change of DEGs in males (Top) compared to the expression of those genes in females (Bottom). LBN reduced the expression of 55 genes and increased the expression of 115 genes in males. (D) Heatmap sorted by fold change of DEGs in females (Top) compared to the expression of those genes in males (Bottom). LBN increased the expression of 162 genes and decreased the expression of 173 genes. (E) KEGG and Panther enrichment terms identified in males following LBN. The number of DEGs within each term is in parentheses to the right of the term. (F) KEGG and Panther enrichment terms identified in females following LBN. The number of DEGs within each term is in parentheses to the right of the term. (G) KEGG enrichment terms identified in both males and females following LBN. The number of DEGs within each term is in parentheses to the right of the term, with males in blue and females in red.
We next narrowed our analysis to genes showing a significant difference between control and LBN and found 170 differentially expressed genes (DEGs) in males and 335 DEGs in females. These gene expression changes were largely sex-specific, with only 11 common genes altered by LBN in both sexes (Fig. 4B and SI Appendix, Table S2). Heatmaps sorted by fold change of LBN DEGs revealed different patterns of up-regulated and down-regulated genes between males and females: in males, 67.6% of identified DEGs were up-regulated following LBN, while in females, only 48.4% of DEGs were up-regulated (Fig. 4 C and D). Thus, LBN induces unique patterns of gene expression in males and females, rather than oppositely regulating a common set of genes.
Kyoto Encyclopedia of Genes and Genomes (KEGG) and Panther pathway analysis were used to examine the biological processes altered by LBN. The top Gene Ontology (GO) terms are displayed in Fig. 4 E and F. Only three overlapping terms were identified between males and females (Fig. 4G). Within these GO terms identified in both sexes, largely different sets of DEGs were regulated within these networks (SI Appendix, Table S3), indicating unique transcriptional profiles following LBN in males and females. HOMER (Hypergeometric Optimization of Motif EnRichment) analysis of transcription factor binding sites was performed to identify potential master regulators affected by LBN that could account for the broad effects on transcription in the NAc. HOMER analysis found different transcription factor binding sites for the DEGs altered by LBN in males and females (SI Appendix, Fig. S3A). Importantly, none of the transcription factors predicted to influence LBN effects in males or females were represented in the transcription factors predicted to influence baseline sex differences in the NAc (comparing control males and control females; SI Appendix, Fig. S3B).
Discussion
Early life adversity can have an inoculating effect that promotes resilience in adulthood. Using the 7-d postnatal LBN manipulation, we revealed that LBN reduced impulsive choice and self-administration of a low dose of morphine in male rats. LBN also dampened NAcC glutamate transmission and prevented the morphine-induced increase in the AMPA/NMDA ratio, blocking this drug-induced plasticity in males. These physiological changes may underlie the resilience to addiction-related behaviors found in LBN males. Interestingly, LBN exposure in females did not significantly alter their behavior or NAcC physiology, so the effects of LBN on these phenotypes appear to be sex-specific. LBN also produced sex-specific changes in gene expression in the NAc. Overall, we show unique behavioral, electrophysiological, and molecular signatures associated with stress inoculation in LBN males.
LBN Reduces Impulsive Choice in Males.
We examined the effects of LBN on impulsivity using a delay discounting task, a well-established assay for impulsive choice. LBN reduced impulsive choice in males. A similar effect was not found in females. This result complements another report that a different type of early life deprivation—artificial rearing with low simulated grooming—reduced impulsive choice in adult male rats but not in adult female rats (20). Although in these two examples, early life manipulations had no effect on female impulsivity, a study that combined bedding restriction with exposure to a “substitute” mother found reduced impulsive choice in female rats (21). Males in that study were unaffected, suggesting that the social experience of having a substitute mother may have differential effects on impulsive choice in male vs. female animals later in adulthood. Collectively, these finding indicate that early life stress in rodents reduces impulsive choice, making their decision making more profitable. These changes would be beneficial in the long term, as such adaptations to an early stressful environment would prepare the organism to better cope with future stressors (4).
LBN did not affect the acquisition of delayed discounting, indicating that LBN does not alter performance factors, such as activity or instrumental learning. Other studies have indicated that LBN exposure can impair learning (22, 23); however, those previous reports examined behaviors mediated by the hippocampus, whereas instrumental learning is not dependent on the integrity of this region (24). Thus, LBN may affect learning in a circuit-specific manner. Another measure unaltered by LBN in either sex was response latency. This finding contrasts with the effect of an immediate acute stressor on delay discounting, which increases response latencies in adult male rats (25), and implicates proximity to the stressful event as critical for this measure.
LBN Decreases Self-Administration of a Low Dose of Morphine in Males.
High impulsivity is a risk factor for opioid misuse, whereas lower impulsivity may reduce the propensity to take drugs (15, 26). Consistent with this idea, LBN not only reduced impulsive choice in male rats, but also reduced self-administration for the lower dose of morphine on FR1 and PR schedules. The reduced responding under PR conditions indicates that LBN males may have diminished motivation and/or sensitivity to the reinforcing effects of this dose of morphine compared with controls. Another interpretation could be that LBN induces anhedonia in males; however, this interpretation is not supported by the delayed discounting data showing that LBN did not affect training for sucrose pellets and that LBN males were more likely to wait for the larger reward. In contrast to the low dose, self-administration of a higher dose of morphine was not affected by LBN exposure in males, suggesting that the changes in the reinforcing efficacy of morphine produced by LBN are dose-dependent. We chose two doses of morphine that are toward either the peak reinforcing efficacy (0.25 mg/kg/infusion) or the nadir (0.75 mg/kg/infusion) of the dose–response curve for motivation to earn morphine infusions (27).This experimental design permitted the detection of either enhancement or suppression of morphine dosing following LBN. The lack of effect at the higher dose may reflect the fact that the motivation to earn infusions at the higher dose is lower in control animals.
Unlike the stress-inoculating effects observed in males, LBN had no significant effect on either dose of morphine self-administration in females. The lack of LBN effect on impulsive choice and morphine self-administration in females could suggest that the way in which we implement this manipulation does not impact them. Another possibility is that females are affected in other ways that we did not assess. Levis et al. (28) examined the effects of LBN on heroin and remifentanil self-administration in female rats (males were not tested) and found no effect on the acquisition of self-administration but noted resistance to extinction and increased reinstatement. We found no difference between LBN and control males or females in cue-induced reinstatement after 1 wk of abstinence. None of the rats underwent extinction in our study, which could partly explain this discrepancy. However, in a behavioral economics task, LBN females had reduced demand elasticity for remifentanil, indicating they were willing to pay a higher price for the drug, an effect suggestive of higher motivation (28). We did not observe an effect of LBN on morphine PR in females for either dose, revealing no change in motivation for morphine. This result could be attributed to the different drugs used. However, another possibility is that differences in the implementation of the LBN model plays a role. As noted, in our manipulation, rats are bred in-house. In many other iterations of the model—including the one by Levis et al. (11)—dams are shipped while pregnant, which is a stressor. Therefore, effects on addiction-related behavior in females may require both a prenatal and postnatal hit (i.e., two hits) of stress.
LBN Dampens Glutamate Transmission in Male Rats, an Effect Not Found in Females.
LBN decreased presynaptic glutamate transmission in males, as indexed by a reduction in sEPSC frequency. This effect was observed in both drug-naïve and morphine-experienced males, reflecting an LBN-induced general dampening of glutamate transmission in males. Females did not exhibit any significant changes in sEPSC frequency following LBN. This result provides electrophysiological evidence for early life adversity influencing glutamate transmission within the NAcC and complements previous work demonstrating that maternal separation stress leads to decreased GluA2 AMPA subunit expression in the NAc of males but not of females (29). Of note, many other studies have demonstrated stress-induced increases in glutamate transmission within the NAc, but those studies focused on recordings in the NAc shell and primarily examined the influence of stress exposure during adulthood (30–32).
LBN-induced decreases in glutamate transmission may be a key contributing factor to the behavioral effects of this manipulation. In support of this notion, reducing glutamate transmission with the NMDA receptor antagonist ifenprodil in male rats reduced impulsive choice during delay discounting (33). Similarly, dampening of accumbal glutamate transmission leads to decreased opioid-seeking (34) and decreased conditioned opioid reward (35). These results, juxtaposed with the behavioral and neurophysiological data reported here, suggest that reduced glutamate transmission in LBN males could contribute to the reduction in impulsive choice decrease in morphine self-administration.
Morphine Self-Administration Experience Enhances Glutamate Transmission in Male Rats, an Effect Blocked by LBN Exposure.
In this study examining the electrophysiological consequences of morphine self-administration with the NAcC, control male rats exhibited an increase in the AMPA/NMDA ratio within the NAcC following 13 d of morphine self-administration and then 7 d of forced abstinence. This change is consistent with an increased AMPA/NMDA ratio in the NAc shell of dopamine 1 (D1) receptor medium spiny neurons following experimenter-administered morphine in male mice (16). Thus, it is possible that the D1 receptors are driving the effects observed here, although this remains to be explored in future studies.
Unlike in males, we did not find an effect of morphine self-administration on the AMPA/NMDA ratio in females. Given that morphine self-administration did not change the AMPA/NMDA ratio in females, other types of plasticity must underlie female self-administration behavior. These findings may also extend to other drugs of abuse, as there is no published evidence that cocaine increases the AMPA/NMDA ratio in female animals.
Additionally, these studies demonstrate that LBN prevents the morphine-induced alteration in the AMPA/NMDA ratio in the NAcC of male rats. NAc AMPA receptors are necessary for the negative affective state induced by morphine withdrawal (36). As such, it is tempting to speculate that early life adversity decreases morphine-taking in males by preventing the plasticity that leads to the negative affect that drives drug-seeking, but this idea needs to be tested.
LBN Induces Sex-Specific Epigenetic and Transcriptional Changes in the NAc.
RNA-sequencing revealed sex-specific changes in gene expression induced by LBN in the NAc. Interestingly, these transcriptional changes were more pronounced in females, the sex that did not demonstrate any LBN-induced behavioral phenotypes. This finding indicates an active form of resilience in females and is consistent with previous animal models of stress. For example, while males are not susceptible to subchronic variable stress (SCVS), they demonstrate greater transcriptional changes within the NAc compared with females who are affected by SCVS (37).
Gene set enrichment (GSE) analysis revealed that LBN had differential effects on the NAc transcriptome in males and females. Only three overlapping pathways were identified between males and females: endocytosis, cholinergic synapse, and dopaminergic synapse. Within these pathways, the genes responsible for the enrichment were distinct between males and females. Two pathways that were uniquely overrepresented in male DEGs, “SNARE interactions in vesicular transport” and “ionotropic glutamate receptor,” are consistent with their LBN-induced changes in NAc glutamate transmission. Additionally, oxytocin signaling in the NAc is a well-known modulator of opiate addiction-like behaviors (38) and the “oxytocin signaling” pathway was represented only in male DEGs. In females, it was surprising that “glutamatergic synapse” and “morphine addiction” were in the KEGG enrichment terms, because LBN did not alter their glutamate transmission or morphine self-administration. These findings highlight the possibility that compensatory mechanisms reflected in the numerous female-specific pathways, such as “GABA-B receptor II signaling,” may result in little observable phenotypes in females.
Consistent with our observation that LBN was accompanied by unique transcriptional signatures in the male and female NAc, potential master regulators engaged by LBN were unique to each sex. In LBN males, HOMER motif analysis identified KLF1, a transcription factor that interacts with glucocorticoid receptors to regulate its target genes (39). Chronic restraint stress similarly increased KLF1 in erythroid progenitor cells of mice (39). Transcription factors important for autophagy (E2F1) and lysosomal biogenesis (TFE3) (40, 41)were likely regulators of DEGs in LBN males, raising the interesting possibility that baseline cellular clearance is altered (42). In females, thyroid hormone receptor beta (THRB) was a predicted master regulator of LBN-related DEGs in females. Thyroid hormone receptors can interact with repressive complexes, including histone deacetylases, in the absence of the hormone, while ligand binding results in conformational changes of the receptor and instead facilitates association with coactivators, including histone acetylases (43). Thus, THRB may facilitate changes in gene expression following LBN through changes to the epigenetic landscape of its target genes. Importantly, the predicted transcription factors that may orchestrate the broad changes in gene expression elicited by LBN in males and females are distinct from those predicted to coordinate baseline sex difference in the NAc. The only potential exception is Rfx1 in females, since RFX family transcription factors were identified by HOMER when comparing control males to control females (SI Appendix, Fig. S3B). Overall, these analyses highlight the potential master regulators in the NAc that warrant further investigation in the context of stress inoculation.
Conclusions.
Here we have identified a stress inoculating effect of early life adversity on addiction-related phenotypes in males. These finding lay the groundwork for further examination of the specific mechanisms underlying behavioral phenotypes of LBN in males and females at the behavioral, electrophysiological, and molecular levels. Importantly, understanding the mechanisms that promote resilience to SUD can guide the development of novel treatments to reduce opioid abuse.
Materials and Methods
Subjects and LBN.
Long–Evans rats bred in-house from Charles River stock were assigned at random to be reared in LBN or control housing condition on PND2, as detailed in SI Appendix, Materials and Methods (12). For all experiments, no more than four rats (two males, two females) were used from each litter. Dependent measures were assessed in adult animals. All studies were conducted in accordance with NIH guidelines and were approved by Temple University’s Institutional Animal Use and Care Committee.
Delay Discounting.
Delay discounting was adapted from previous work (44); details are provided in SI Appendix, Materials and Methods. One lever, designated the small reward lever, delivered one pellet, while the other large reward lever delivered four pellets. The delay to the large reward increased across four blocks of trials per session (0 s block 1, 15 s block 2, 30 s block 3, and 45 s block 4). Once rats displayed stable choice behavior, data from the last 3 d of training were analyzed.
Morphine Self-Administration.
Rats were implanted with intravenous catheters (SI Appendix, Materials and Methods). After recovery (7 d), rats were trained to self-administer morphine for 6 h/d for 10 d on an FR1 reinforcement schedule; one active lever press resulted in one morphine infusion. An inactive lever with no programmed consequences was also present. After 10 d on FR1, rats were switched to a PR schedule, in which the number of active lever presses required to obtain an infusion increased steadily throughout the session (SI Appendix, Materials and Methods). A subset of rats received a cue test, in which each lever press was reinforced by a previously drug-paired cue but morphine was not available, on day 7 of forced abstinence following 13 d of morphine self-administration (0.75 mg/kg/infusion).
Whole-Cell Recordings in the NAcC.
Rats exposed to the cue test were euthanized 24 h later and compared with drug-naïve controls, which were taken from their home cage. Slices (300 µm) of NAc tissue were prepared for recording (SI Appendix, Materials and Methods). Mean sEPSC frequencies and amplitudes were analyzed, and AMPA/NMDA current ratios were computed, as detailed in SI Appendix, Materials and Methods. For all measures, cells from at least three animals within each group were used. Recordings were taken from cells within the NAcC.
RNA Sequencing.
RNA was extracted from bilateral NAc punches. Samples with an RNA integrity number >8 were used for library preparation (performed by BGI Americas) and were sequenced on an Illumina HiSeq 4000. Fastqc version 0.11.8 was used to evaluate the quality of reads (45) with adaptors and nonpaired reads removed using Trimmomatic version 0.39 (46). RNA-seq analysis was performed, and DEGs were identified using an adjusted P value of <0.1 and a 50% change in the expression (|log2 fold change|>0.58) as cutoffs to determine significance. The RRHO version 2 test (47) evaluated the degree of overlap in gene signatures between sexes. HOMER identified putative transcription factors of the DEGs and their motifs (48). GSE analysis was conducted using DEGs from the male and female groups separately. KEGG pathways with an adjusted P value of <0.1 were selected. More details are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Alessandro Jean-Louis and Neil Sekhawat for their technical assistance. This research includes calculations carried out on High-Performance Computing resources supported in part by the NSF (Major Research Instrumentation Grant 1625061) and by the US Army Research Laboratory (Contract W911NF-16-2-0189). This work was supported by NIH Grant R01 DA049837 (to D.A.B.), NSF CAREER Grant IOS1552416 (to D.A.B.), NSF Grant IOS-1929829 (to D.A.B.), NIH–National Institute on Drug Abuse (NIDA) Grant P30 DA13429-21 (Ellen Unterwald, PI of the P30; pilot project support to D.A.B.), NIH-NIDA Grant DP1 DA046537 (to M.E.W.), NIH-NIDA Grant T32 DA007237 (Ellen Unterwald, PI of the T32, supporting C.C.B.), NIH Grant R01 DA047265 (to L.A.B.), and Deutsche Forschungsgemeinschaft Grant DE 2828/1-1 (to A.U.D.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2020173118/-/DCSupplemental.
Data Availability
Data for Figs. 1–3 have been deposited in Figshare (https://doi.org/10.6084/m9.figshare.13303397; https://doi.org/10.6084/m9.figshare.13308743; https://doi.org/10.6084/m9.figshare.13315214). RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE160495).
References
- 1.Rudd R. A., Aleshire N., Zibbell J. E., Gladden R. M., Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb. Mortal. Wkly. Rep. 64, 1378–1382 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Anda R. F., et al., The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 256, 174–186 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn K., et al., The relationships of childhood trauma and adulthood prescription pain reliever misuse and injection drug use. Drug Alcohol Depend. 169, 190–198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons D. M., Parker K. J., Schatzberg A. F., Animal models of early life stress: Implications for understanding resilience. Dev. Psychobiol. 52, 616–624 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masten A. S., Ordinary magic. Resilience processes in development. Am. Psychol. 56, 227–238 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Santarelli S., et al., An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology 78, 213–221 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Biggio F., et al., Maternal separation attenuates the effect of adolescent social isolation on HPA axis responsiveness in adult rats. Eur. Neuropsychopharmacol. 24, 1152–1161 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Hsiao Y.-M., et al., Early life stress dampens stress responsiveness in adolescence: Evaluation of neuroendocrine reactivity and coping behavior. Psychoneuroendocrinology 67, 86–99 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Murthy S., Gould E., Early life stress in rodents: Animal models of illness or resilience? Front. Behav. Neurosci. 12, 157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivy A. S., Brunson K. L., Sandman C., Baram T. Z., Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience 154, 1132–1142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachs B. D., Lumia A. R., Is stress due to shipment of animals a confounding variable in developmental research? Dev. Psychobiol. 14, 169–171 (1981). [DOI] [PubMed] [Google Scholar]
- 12.Eck S. R., et al., The effects of early life adversity on growth, maturation, and steroid hormones in male and female rats. Eur. J. Neurosci., 10.1111/ejn.14609 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallo M., et al., Limited bedding and nesting induces maternal behavior resembling both hypervigilance and abuse. Front. Behav. Neurosci. 13, 167 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banqueri M., Méndez M., Arias J. L., Behavioral effects in adolescence and early adulthood in two length models of maternal separation in male rats. Behav. Brain Res. 324, 77–86 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Marino E. N., et al., Impulsivity but not sensation-seeking is associated with opioid analgesic misuse risk in patients with chronic pain. Addict. Behav. 38, 2154–2157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hearing M. C., et al., Reversal of morphine-induced cell type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc. Natl. Acad. Sci. U.S.A. 113, 757–762 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lüscher C., Malenka R. C., Drug-evoked synaptic plasticity in addiction: From molecular changes to circuit remodeling. Neuron 69, 650–663 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mameli M., et al., Cocaine-evoked synaptic plasticity: Persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 12, 1036–1041 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Moussawi K., et al., Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc. Natl. Acad. Sci. U.S.A. 108, 385–390 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovic V., Keen D., Fletcher P. J., Fleming A. S., Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav. Neurosci. 125, 481–491 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Fuentes S., et al., Sex-dependent effects of an early life treatment in rats that increases maternal care: Vulnerability or resilience? Front. Behav. Neurosci. 8, 56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunson K. L., et al., Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 25, 9328–9338 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice C. J., Sandman C. A., Lenjavi M. R., Baram T. Z., A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149, 4892–4900 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbit L. H., Balleine B. W., The role of the hippocampus in instrumental conditioning. J. Neurosci. 20, 4233–4239 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafiei N., Gray M., Viau V., Floresco S. B., Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology 37, 2194–2209 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vest N., Reynolds C. J., Tragesser S. L., Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addict. Behav. 60, 184–190 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Swain Y., Muelken P., LeSage M. G., Gewirtz J. C., Harris A. C., Locomotor activity does not predict individual differences in morphine self-administration in rats. Pharmacol. Biochem. Behav. 166, 48–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levis S. C., et al., On the early life origins of vulnerability to opioid addiction. Mol. Psychiatry, 10.1038/s41380-019-0628-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganguly P., Honeycutt J. A., Rowe J. R., Demaestri C., Brenhouse H. C., Effects of early life stress on cocaine conditioning and AMPA receptor composition are sex-specific and driven by TNF. Brain Behav. Immun. 78, 41–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khibnik L. A., et al., Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol. Psychiatry 79, 898–905 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muir J., et al., Ventral hippocampal afferents to nucleus accumbens encode both latent vulnerability and stress-induced susceptibility. Biol. Psychiatry 88, 843–854 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Williams E. S., et al., Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol. Psychiatry 87, 492–501 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yates J. R., Bardo M. T., Effects of intra-accumbal administration of dopamine and ionotropic glutamate receptor drugs on delay discounting performance in rats. Behav. Neurosci. 131, 392–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bossert J. M., Gray S. M., Lu L., Shaham Y., Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology 31, 2197–2209 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baharlouei N., Sarihi A., Moradi M., Zarrabian S., Haghparast A., Microinjection of the mGluR2/3 agonist, LY379268, into the nucleus accumbens attenuates extinction latencies and the reinstatement of morphine-induced conditioned place preference in rats. Behav. Pharmacol. 29, 385–392 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Russell S. E., et al., Nucleus accumbens AMPA receptors are necessary for morphine-withdrawal-induced negative-affective states in rats. J. Neurosci. 36, 5748–5762 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodes G. E., et al., Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 35, 16362–16376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leong K. C., Cox S., King C., Becker H., Reichel C. M., Oxytocin and rodent models of addiction. Int. Rev. Neurobiol. 140, 201–247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voorhees J. L., et al., Chronic restraint stress upregulates erythropoiesis through glucocorticoid stimulation. PLoS One 8, e77935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu J., et al., Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion. Biochem. Pharmacol. 150, 202–213 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Martina J. A., et al., The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal. 7, ra9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raben N., Puertollano R., TFEB and TFE3: Linking lysosomes to cellular adaptation to stress. Annu. Rev. Cell Dev. Biol. 32, 255–278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernal J., Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 3, 249–259 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Stopper C. M., Green E. B., Floresco S. B., Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cereb. Cortex 24, 154–162 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Andrews S., FastQC: A quality control tool for high-throughput sequence data (2015), www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed 27 January 2021.
- 46.Bolger A. M., Lohse M., Usadel B., Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahill K. M., Huo Z., Tseng G. C., Logan R. W., Seney M. L., Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci. Rep. 8, 9588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinz S., et al., Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for Figs. 1–3 have been deposited in Figshare (https://doi.org/10.6084/m9.figshare.13303397; https://doi.org/10.6084/m9.figshare.13308743; https://doi.org/10.6084/m9.figshare.13315214). RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE160495).