Abstract
Background
A growing body of research has documented the effects of prenatal risk factors on a wide spectrum of adverse offspring health outcomes. Childhood behavior problems, such as externalizing and internalizing problems, are no exception. This comprehensive literature review aims to summarize and synthesize current research about commonly experienced prenatal risk factors associated with internalizing and externalizing problems, with a focus on their impact during childhood and adolescence. Potential mechanisms as well as implications are also outlined.
Data sources
The EBSCO, Web of Science, PubMed, Google Scholar, and Scopus databases were searched for studies examining the association between prenatal risk factors and offspring internalizing/externalizing problems, using keywords “prenatal” or “perinatal” or “birth complications” in combination with “internalizing” or “externalizing”. Relevant articles, including experimental research, systematic reviews, meta-analyses, cross-sectional and longitudinal cohort studies, and theoretical literature, were reviewed and synthesized to form the basis of this integrative review.
Results
Prenatal risk factors that have been widely investigated with regards to offspring internalizing and externalizing problems encompass health-related risk factors, including maternal overweight/obesity, substance use/abuse, environmental toxicant exposure, maternal infection/inflammation, as well as psychosocial risk factors, including intimate partner violence, and anxiety/depression. Collectively, both epidemiological and experimental studies support the adverse associations between these prenatal factors and increased risk of emotional/behavioral problem development during childhood and beyond. Potential mechanisms of action underlying these associations include hormonal and immune system alterations. Implications include prenatal education, screening, and intervention strategies.
Conclusions
Prenatal risk factors are associated with a constellation of offspring internalizing and externalizing problems. Identifying these risk factors and understanding potential mechanisms will help to develop effective, evidence-based prevention, and intervention strategies.
Keywords: Externalizing, Internalizing, Obstetrical, Pregnancy, Prenatal risk factors
Introduction
Childhood behavioral and emotional problems are associated with increased risk for a wide spectrum of later adverse outcomes during adolescence and adulthood [1–6]. Indeed, the Global Burden of Disease study from 2010 estimated that behavioral disorders in childhood accounted for nearly 6 million disability-adjusted life years [7], rendering childhood behavioral problems an increasingly critical public health concern and major societal issue. While much literature has focused on early childhood risk factors for internalizing and externalizing behavior, fewer studies have explored the potential effects of prenatal risk factors on behavioral problems [8]. Understanding this phenomenon has the potential to facilitate recognition, prevention, and mitigation of prenatal risk factors-related childhood behavioral problems, as well as the associated long-lasting negative health consequences. The current literature review aims to examine empirical evidence on the association between common prenatal risk factors and childhood internalizing/externalizing behavior, as well as provide a synthesis of the selected studies. Given our comprehensive examination of seven prenatal risk factors, we chose to perform an integrative literature review to allow for the inclusion and simultaneous analysis of diverse research methodologies, both experimental and non-experimental in nature, to provide a broad, all-inclusive overview of available evidence. First, we will provide an overview of child behavioral problems, focusing on internalizing and externalizing behaviors, followed by an introduction of prenatal risk factors significantly associated with those behavioral problems. We will then discuss the link between those factors with internalizing and/or externalizing disorders across the lifespan, with an emphasis on childhood and adolescence. We will also discuss the potential mechanisms underlying those associations. Finally, we will discuss implications of current findings on prevention and intervention strategies.
Childhood behavioral problems
Internalizing and externalizing behaviors are well established and widely used behavioral classifications within the field of child and adolescent psychology [9]. Internalizing problems refer to inwardly focused negative behaviors such as anxiety, depression, and somatic symptoms [10], while externalizing problems refer to outwardly focused negative behaviors such as hyperactivity, aggression, disruptive conduct, and substance use [11, 12]. However, this distinction is not clearly delineated. In fact, there exist high rates of systematic comorbidity between internalizing and externalizing problems [13]. Despite the high degree of overlap, these groupings of behavioral, emotional, and social problems remain highly valuable in guiding research, clinical practices, and policy.
Demonstration of internalizing and/or externalizing behaviors during childhood are predictive of later negative adolescent and adult behavioral, emotional, cognitive, and physical health outcomes, including increased risk for aggression and violence [2, 14], substance abuse [5], depression [3], anxiety disorders [15], lowered academic competence [1], and increased long-term mortality risk in adulthood [16]. Given these profoundly enduring outcomes, research to investigate correlates and early risk factors for childhood internalizing and externalizing behavior is critically important, impacting prevention and intervention approaches for a wide array of negative health consequences.
Prenatal risk factors
For the purposes of this paper, prenatal risk factors will be operationally defined as any condition or exposure during pregnancy that increases fetal susceptibility for internalizing and externalizing behavior. The literature search revealed that two primary groups of prenatal risk factors were significantly associated with childhood internalizing and externalizing behavior: health-related risk factors and psychosocial risk factors. Health-related factors include maternal overweight/obesity, substance use/abuse, environmental toxicant exposure, and maternal infection/inflammation, while psychosocial factors include intimate partner violence and maternal depression and anxiety (Fig. 1).
Fig. 1.
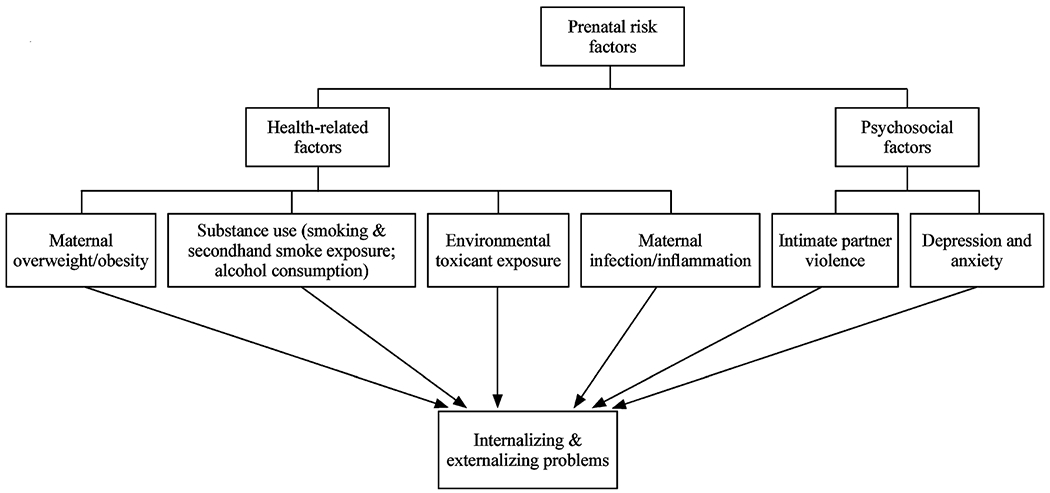
Prenatal risk factors for internalizing and externalizing problems
The link between prenatal risk factors and childhood internalizing/externalizing behavior
Health-related factors
Maternal overweight/obesity
Maternal overweight/obesity is one of the most common pregnancy complications in the developed world [17]. High maternal pre-pregnancy body mass index (BMI) poses significant risk to the development of offspring behavioral disorders, with considerable evidence for attention-deficit hyperactivity disorder (ADHD) in both human and animal studies. Research from human studies indicated the trend of a higher risk for ADHD in offspring whose mothers had a pre-pregnancy BMI of ≥ 25 kg/m2. Notably, Rodriguez et al. found a dose-dependent increase in ADHD symptomology as BMI increased from overweight to obese, and when compared to mothers who had normal weight, obese mothers had a twofold increased risk of having a child with ADHD [18]. A recent UK cohort replicated these findings, demonstrating that prenatal maternal very severe obesity is a strongly significant predictor of ADHD symptomology [19]. This association remained even after adjusting for all major confounders, including demographic factors, other prenatal risk factors, and maternal psychological wellbeing. However, when familial confounders, including genetics and shared environment, were taken into account, this association was lost [20]. Another study found that while children of white obese women had increased risk for ADHD symptoms, the same relationship did not hold true for offspring of black obese women [21]. Taken together, these results indicate the need for future research to elucidate the role of a broader range of potential confounders, including but not excluded to racial differences and familial factors.
Animal models of high maternal pre-pregnancy BMI, induced by high fat diet (HFD) consumption, provide support for human epidemiologic data linking maternal overweight/obesity with ADHD. Mouse models of obesity showed that maternal obesity during gestation alone was enough to increase hyperactivity in male offspring performing open-field tests, and decrease sociability (a primary characteristic of ADHD) in female offspring performing chamber social interaction tests [22]. These results are in accordance with those of a previous study, which found increased hyperactivity in adult offspring of obese mice evaluated in open-field tests, light/dark boxes, and novel home cage tests [23].
While there exists substantial evidence for the association between high maternal pre-pregnancy BMI and externalizing behaviors, the association with internalizing behaviors, on the other hand, exhibits mixed results. A large Swedish cohort (n = 1714) found that maternal pre-pregnancy overweight and obesity were associated with a teacher-reported twofold increased risk for difficulty with emotional regulation [24]. An Australian cohort (n = 2785) similarly found that increased maternal pre-pregnancy BMI was significantly associated with internalizing behavior [25]. The elevated risk for internalizing behavior first emerged at age 8 and increased through age 17. In contrast, a recent US longitudinal study (n = 2952) found no significant associations between maternal pre-pregnancy BMI and internalizing problems [26].
Animal research of overweight and obesity shows more consistent results. Rodent models demonstrated increased anxiety in offspring of obese mice in open-field tests [22] and elevated plus mazes [27]. Strikingly, even grandmaternal exposure to HFD exacerbated anxiety- and depression-like phenotypes in the grand-progeny female adult offspring, suggesting the enduring, intergenerational effects of obesity [28]. Likewise, non-human primate models also demonstrated increased anxiety-like behavior in female offspring of obese mothers in response to threatening novel objects compared to controls [29]. The robust results exhibited by animal studies compared to the mixed results by human studies point to the possibility that significant confounders mediate the obesity-internalizing behavior relationship, as well to the importance of future studies to adjust for these confounders.
Substance use
Smoking and secondhand smoke exposure
Pregnancy is a critical period in which tobacco exposure may impact fetal development. Maternal smoking during pregnancy (MSDP) has been associated with offspring emotional and behavioral problems [6], with this link studied widely across the lifespan in numerous epidemiological studies. In childhood, an Australian longitudinal cohort study (n = 7555 families) utilizing multi-informant data from parents, teachers, and children found a robust relationship between MSDP and level of externalizing symptomology, including hyperactivity, difficulty with peers, and difficulty with behavior [30]. In adolescence, MSDP has been associated with rule-breaking and aggressive behavior [31], as well as hyperactive/impulsive ADHD [32], and increased risk for persisting and increasing externalizing symptom trajectory [33]. While powerful genetic and environmental factors contribute to disorders like ADHD, rendering it difficult to isolate MSDP as an independent risk factor for offspring ADHD, the consistency of results of several carefully controlled studies suggest that maternal smoking during the prenatal period acts as a strong predictor of attention deficits and hyperactive behavior. In fact, a study investigating a large, population-based sample of Dutch twin children (n = 15,228 pairs) observed a direct causal effect of MSDP on externalizing behavior in children at age 3, with mothers’ smoking cessation prior to conception associated with less overall externalizing problems, including overactive, aggressive, and oppositional behavior [34]. The effects of MSDP on externalizing behavior are strikingly enduring, persisting also into adulthood. A prospective longitudinal investigation of middle-aged adults (n = 3433) linked MSDP to elevated levels of antisocial behavior as well as violent and non-violent outcomes, with a pack-a-day increase in MSDP more than doubling the odds of an adult violent offense [35], underscoring the lasting adverse effects of smoking during pregnancy on offspring.
Evidence from animal models, primarily rodents, has similarly demonstrated the adverse consequences of prenatal nicotine, the major psychoactive ingredient in tobacco smoke, on offspring externalizing behavior, with most research conducted on ADHD. Focusing on hyperactivity as a measure of ADHD-like behavior, several studies show that prenatal nicotine exposure is associated with offspring impulsivity and elevated locomotor activity [36–38]. However, apart from ADHD, few other forms of externalizing behavior have been investigated with regards to prenatal nicotine exposure, thereby highlighting the need for future studies to employ animal models to understand a broader scope of externalizing behaviors.
While the literature demonstrates consistent results between MSDP and externalizing behavior, those examining the relationship between MSDP and internalizing behaviors show more mixed results, as well as weaker associations. For example, while the Norwegian Mother and Child Cohort Study reported that maternal smoking during early pregnancy shared a dose–response relationship with increased anxiety and depressive behaviors in children at 18 months and 3 years-of-age [39], The Netherlands Twin Register Study, failed to observe an association between MSDP and offspring anxiety or depression at 3 years-of-age [34]. These mixed results for MSDP-associated internalizing behavior extend into adolescence and adulthood as well [40–43]. In contrast to human population studies, experimental animal studies examining the association between MSDP and internalizing behavior, albeit limited, demonstrate a slightly stronger association with prenatal nicotine exposure inducing depression and anxiety-like behavior in offspring rats, with female rats demonstrating greater susceptibility [44–46]. Several possible explanations have been posited for the weaker association between MSDP and internalizing behavior than with externalizing behavior in human studies, including difficulty for adults to accurately perceive internalizing symptomology in young children [30] and the expression of internalizing behavior through “masked” symptoms like aggression in young children [47].
Compared with MSDP, evidence regarding the relationship between maternal passive smoking, or secondhand smoke exposure (SHS) during pregnancy is scarce; however, existing literature shows a robust relationship between prenatal SHS exposure and offspring externalizing behavior, with a high level of consistency demonstrated across countries. Epidemiological studies in China [48, 49], Japan [50], and the United States [36] have all reported a link between prenatal SHS exposure and hyperactivity. These results show that even indirect exposure through secondhand smoke may have adverse consequences on the development of offspring behavioral problems, further reinforcing the need for preventative efforts and continued education on the health hazards of tobacco use, especially with pregnancy as a period of vulnerability.
Alcohol consumption
During pregnancy, women are uniformly advised by physicians and other healthcare providers against alcohol consumption; however, the discussion on whether a “safe” level of alcohol may be consumed during pregnancy continues, and is especially relevant in light of recent Centers for Disease Control and Prevention’s statistics that one in ten pregnant women in the United States reported alcohol use [51]. Studies examining the effects of low-level alcohol consumption in relation to behavioral problems have found mixed results. A Japanese cohort study (n = 1933) found that even low prenatal alcohol exposure (PAE) exposure levels (1–4 times per month or less) were associated with children’s internalizing problems, as well as overall behavior problems [52]. Day et al. found that these results extend into young adulthood, with all levels of PAE exerting significant effects on internalizing and/or externalizing behavior at each trimester across pregnancy. In fact, the study reported that binge drinking was not a better predictor of adverse behavioral outcomes compared to low/moderate exposure levels [53]. These results remained consistent even after taking into account current offspring substance use, demonstrating the likely permanency of even low/moderate PAE. In contrast, a Danish cohort study observed no associations between low/moderate PAE (an accumulated 0–90 drinks throughout pregnancy) and externalizing and internalizing scores at 7 years-of-age [54]. Strikingly, an earlier Australian cohort (n = 2370) reported that children of mothers who were light to moderate drinkers during pregnancy (2–10 standard drinks per week) actually exhibited clinically meaningful reduced risk for internalizing, externalizing, and total behavioral problems compared to children of non-drinkers across 14-years-of-age [55].
While the effects of low-to-moderate PAE does not exhibit clear patterns, those of heavier alcohol consumption practices demonstrate more consistent results, especially with externalizing behavior. Using both parent and teacher reports, the Avon Longitudinal Study of Parents and Children found that even episodic binge drinking patterns (≥ 4 drinks in a day) are associated with higher levels of hyperactivity and inattention problems in 11-year-old children, an effect that was not contingent upon daily drinking during pregnancy [56]. Pagnin et al. similarly reported that binge drinking during pregnancy was associated with a fivefold increased risk for ADHD in offspring at 12-years-of-age [57]. Furthermore, a meta-analysis estimated the prevalence of “disturbance of activity and attention” in offspring with PAE to range from 23.6 to 78.4%, and the prevalence of conduct disorder to even higher percentages of 77.9–97.4% [58]. The detrimental effects of heavy alcohol usage extend well beyond childhood, exerting long-term effects on legal difficulties even in young adulthood [59]. These studies demonstrate the enduring, pervasive effects of heavy PAE, affecting offspring behavioral functioning throughout the life span.
However, several complexities continue to surround human studies of PAE, including stigma, potential underreporting of alcohol consumption during pregnancy [60, 61], varying thresholds of low, moderate, and heavy PAE from study to study [62], and pattern/timing of alcohol exposure during fetal development [63]. These complexities make the comprehensive assessment of the effects of PAE on adverse behavioral development challenging; however, substantial evidence from human studies has indicated that there is perhaps no safe level of maternal alcohol consumption during pregnancy.
Maternal infection/inflammation
Infections are common events during pregnancy, affecting up to nearly 60% of pregnant women [64]. Increasing evidence suggests that maternal exposure to infection during critical periods of prenatal development is associated with increased risk of offspring internalizing and externalizing problems, rendering the high prevalence of maternal infection highly concerning. Much epidemiological literature utilizing prospective, longitudinal cohort designs have linked prenatal maternal infection/immune activation to internalizing behavior in offspring, with a particular focus on depressive and anxious symptomology. This relationship has been well documented across the life course. In childhood, Giollabhui et al. found that maternal inflammation during pregnancy predicts more severe offspring internalizing symptoms, with especially marked effects noted in female offspring [65]. During adolescence, Murphy et al. found that maternal infection specifically during the second trimester was significantly associated with offspring depression at ages 15–17 [66]. Notably, a nationwide Danish cohort found that the risk of mental disorder in both childhood and adolescence increased in a dose–response relationship with the number of maternal infections [67]. The lasting effects of maternal infection endure all the way up to adulthood [68]. Strikingly, maternal infection and inflammation demonstrate strong transgenerational transmission effects, with the second-generation offspring showing enhanced depression-like behavior [69]. Similar behavioral alterations have been found in the animal literature, in which maternal infection is associated with elevated anxiety- and depression-like behaviors with increasing age in mice [70].
Compared to internalizing behavior, the relationship between maternal infection and externalizing behavior is less clear. While several human studies found that maternal infection is associated with lowered impulse control [71] and increased risk for ADHD in offspring [72], other studies found no adverse association between maternal infection during the prenatal period and increased ADHD occurrence in offspring, instead positing that any associations that are found to exist between the two derive largely from unmeasured familial confounding [73, 74]. With regards to experimental research, an animal study utilizing a rodent model found that prenatal inflammation due to Streptococcus exposure is significantly associated with hyperactivity in female rats [75]. However, animal studies with regards to externalizing behavior remain limited. These mixed results as well as limited research point to the importance of future research to examine a broader range of infection categories and identify specific time windows of pregnancy during which maternal infection renders offspring more susceptible to externalizing behavior.
Environmental toxicant exposure
There is growing epidemiological evidence demonstrating the associations between prenatal environmental toxicant exposure and offspring emotional/behavior problems. Here, we focus on two major categories of harmful chemicals that have received increasing attention with regards to child and adolescent behavioral problems in recent years. One such major class of environmental toxicants is heavy metals. Mercury is one particularly well-studied heavy metal toxicant, with birth cohorts in several countries including Korea, Canada, and the United States reporting that even low-level prenatal mercury exposure is linked with externalizing behavior [76], especially ADHD [77, 78]. Prenatal lead exposure has also long demonstrated similarly detrimental results on externalizing behavior, with longitudinal [79] and cross-sectional studies [80] finding associations between lead exposure and aggression, attention, antisocial behavior, and juvenile delinquency. Significant gender differences have also been found, with male offspring more susceptible to the effects of lead toxicity than females [79, 81]. While the neurotoxic impact of mercury and lead on behavioral development has been well established, recent studies have also recognized manganese as an emerging neurotoxicant. Longitudinal birth cohort studies examining prenatal manganese exposure in teeth dentine [82], maternal blood [83], and umbilical cord serum [84] have found adverse associations with hyperactivity, attention, neurobehavioral development, and emotional dysfunction.
Besides heavy metals, synthetic organic chemicals are another major class of neurotoxicants whose exposure has been found to increase externalizing problems in children. Prenatal phthalate exposure has been widely investigated by several birth cohorts, with studies utilizing maternal urine and blood serum samples for toxicant measurement largely suggesting that externalizing problem scores, specifically in the dimensions of aggression [85], conduct problems [86], inattention, and hyperactivity [87], are higher in children whose mothers have greater phthalate exposure [85, 86]. However, prenatal phthalate exposure research on neurodevelopment has primarily been examined in children 1–10 years of age, and the effects of phthalates on adolescent neurodevelopment and behavior are unclear. In one recent study following neurobehavioral development in a 15-year follow-up Taiwanese birth cohort, Huang et al. found that elevated maternal urinary phthalate levels are associated with attention problems, delinquent behavior, aggression, and other externalizing problems in children 8–14 years old [88]. Further research in this age group as well as in adolescents is necessary to confirm these associations. Polybrominated diphenyl ethers (PBDE) are yet another class of harmful chemicals that have raised concerns regarding their impact upon offspring development. Their exposure has been evaluated at various points of development, including prenatal exposure in maternal blood serum [89, 90] and postnatal exposure in breast milk [91], indicating the positive association between PBDE concentration and activity, impulsivity, and attention problems up to 12-years of age. Furthermore, bisphenol A (BPA) studies in both humans and animal models [92–98] have extensively documented the association of prenatal and early postnatal BPA exposure with increased aggression and hyperactivity. Given the ubiquity of these environmental toxins, not only is it important to independently assess safe levels of exposure for a wider spectrum of toxicants, but also to further elucidate the interplay of multiple neurotoxicant co-exposures on the development of behavioral problems. More research assessing the timing of exposure on the severity and prevalence of internalizing and externalizing problems should also be assessed to best inform best maternity leave policies that minimize workplace exposure.
Psychosocial factors
Intimate partner violence
The World Health Organization has declared intimate partner violence (IPV) a global public health concern, estimating that one in three women throughout the world will experience physical and/or sexual violence by a partner or sexual violence by a non-partner [99]. These statistics are especially alarming in lights of the considerable health risks that IPV poses to offspring. However, while much research has focused on IPV exposure during childhood, research on the role of prenatal IPV as a risk factor for behavioral and emotional problems after birth is scarce. Current studies have documented significant associations between prenatal IPV and both internalizing and externalizing behavior across infancy and childhood. Martinez-Torteya et al. found that in utero IPV exposure acts as a strong predictor of offspring externalizing behavior at 12 months of age. They also found associations with internalizing behavior, but this relationship was attenuated after taking into account stress hormone activity patterns [100]. At 24 months, McFarlane et al. reported that children residing with mothers exposed to IPV during pregnancy had greater problems with internalizing problems, specifically anxiety and depression [101]. At 42 months, Flach et al. found children exhibited externalizing problems, including conduct problems, if their mothers were exposed to antenatal IPV [102]. The long-term behavioral outcomes of prenatal IPV exposure have also been evaluated using prospective, longitudinal design. The study found that IPV exposure during pregnancy predicted child-reported internalizing and externalizing problems, as well as mother-rated externalizing problems, at age 10 [103]. A meta-analysis involving adolescents up to 16 years of age, however, did not find consistent associations between prenatal IPV exposure and adolescent internalizing and/or externalizing behavior [104], indicating that enduring influences may only be maintained up to childhood. Given the dearth of research in this area, future research should aim to rule out confounders that may mediate the associations, including genetic, familial, and environmental influences, as well as evaluate the frequencies, severity, and context of violence.
Maternal psychopathology—anxiety and depression
Pregnancy is a time of increased vulnerability for psychiatric disorders, with anxiety and depression being two of the most common psychopathologies experienced by expecting mothers [105]. Maternal anxiety and depression during pregnancy are known risk factors for internalizing and externalizing behavior in offspring, with several longitudinal cohort studies from multiple countries providing extensive evidence of their associations across the lifespan. During early childhood, a Canadian birth cohort study found that having a mother with either subclinical or high depressive symptoms trajectory across pregnancy is associated with an increased risk for hyperactivity, inattention, physical aggression, emotional/anxiety disorder, and separation anxiety symptoms in offspring at 3 years-of-age [106]. These associations shared a dose-response relationship, with the proportion of children with internalizing and/or externalizing behavior highest for those whose mothers presented with the most persistent and severe depressive symptoms. Similarly, data from an earlier Australian pregnancy cohort found consistent results that 4-year-old children whose mothers experienced either subclinical or high depressive symptoms had at least two-times elevated risk for emotional–behavioral difficulties compared to their unaffected counterparts [107]. In middle childhood, the results found by a French longitudinal cohort continued to be in agreement, with prenatal depression linked to heightened depressive and antisocial behavior symptoms [108]. These effects have also been observed in many adolescent longitudinal cohorts. Two Finish cohorts reported associations between prenatal depression and emotional/behavioral problems; however, while one determined that mother’s depressed mood in the first trimester best predicted children’s externalizing problems at age 12 [109], another found symptom pattern and trajectory, rather than timing of depressive symptoms, better explain higher levels of adolescent internalizing and externalizing behavior [110]. When participants were in adulthood, an Australian-based pre-birth cohort reported the long-term effects of prenatal psychopathology on behavioral problems at the 21-year follow-up, with comorbid depressive, anxious, and stress symptoms during pregnancy predicting mental health outcomes in adult offspring [111].
Results consistent with those found in human studies have also been documented in animal research. Prenatal exposure to selective serotonin reuptake inhibitors, a staple pharmacological treatment for women experiencing depression, elicits a variety of lasting emotional and behavioral abnormalities in rodents, including increased adult behavioral despair as indicated by immobility on the forced swim test [112, 113], anxiety-like behavior as shown by reduced exploratory behavior in the elevated plus maze [114] and open-field test [115, 116], and aggression in the social exploration [117] and territorial behavior [118] tests. Together, findings from human and experimental studies suggest offspring susceptibility to maternal emotional problems and early life antidepressant exposure. Given the detrimental consequences of fetal insult resulting from prenatal anxiety and depression, two highly prevalent risk factors during pregnancy, future research investigating this relationship should take into account more comprehensive measures, including symptom severity, recurrence and pattern of symptoms, symptom chronicity, and exposure to treatment/medication.
Potential mechanisms of action
While the specific mechanisms of action through which the health-related and psychosocial prenatal risk factors presented above act on offspring internalizing and externalizing problems are not well understood, several potential mechanisms have been posited. One widely investigated biological hypothesis involves hormonal mechanisms. In particular, the associations between several prenatal risk factors and offspring emotional/behavioral problems have been proposed to be mediated by glucocorticoids [119, 120], the central hormone of the hypothalamic–pituitary–adrenal (HPA) axis. Prenatal drug exposure, including tobacco and alcohol, has been found to attenuate cortisol reactivity in adolescents, an HPA axis disruption that manifested as risky health behaviors, aggression, and other externalizing behaviors [121]. Furthermore, the epigenetic modulation of the human glucocorticoid receptor gene, NR3C1, in offspring has been widely implicated in the development of internalizing and/or externalizing behaviors with regards to intimate partner violence [122], prenatal depression [123], and anxiety [124, 125], with higher methylation levels dysregulating stress responses and increasing vulnerability to emotional and behavioral problems. These modulations have been studied in several physiological contexts, including blood, placenta, and saliva. Apart from glucocorticoids, other hormones have also been found to mediate the relationship between health-related factors and offspring behavioral problems. For example, prenatal BPA exposure has been linked to disruptions in the fetal endocrine system, specifically the thyroid–adipokine axis. Previous results of several studies have suggested multiple mechanisms, including BPA-induced disruption of thyroid hormone receptors [126], interference of thyroid hormone functions by inhibiting synthesis [126], and alteration of thyrotropin-releasing hormone that inhibited iodide uptake by the sodium iodide symporter [127]. Further research is necessary to take into account potential social and environmental confounders given the length of time between prenatal exposure and the onset of behavioral problems [128], as well as examine the modifiability of these epigenetic mechanisms as a way of mitigating their adverse effects [123].
Alterations to the immune system are another biological mechanism that may mediate susceptibility to these conditions. With regards to maternal infection, infectious agents have been proposed to affect the developing fetus during the prenatal period through activation of the immune response [129]. With regards to pre-pregnancy weight, obesity has been found to systemically activate the maternal immune system, producing increased fetal brain inflammation via elevated cytokine expression within the offspring brain at birth. This thereby heightens susceptibility to mental health disorders [130, 131]. With regards to maternal infection, several studies utilizing animal models have found that maternal infection during pregnancy is associated with behavioral phenotypes in mice offspring that reflect depressive symptomology in humans [132, 133]. Specifically, maternal infection has been found to lead to the upregulation of pro- and anti-inflammatory cytokines, which are significantly implicated in the development of depression [134]. Many cytokines have been investigated to elucidate their specific roles within the pathway linking maternal infection with offspring emotional and behavioral aberrations, with several lines of experimental research indicating interleukin-6 as a key mediator [135, 136]. However, the exact mechanisms of cytokine augmentation as well as cytokine-specific effects are still not clearly understood. With regards to maternal anxiety and depression during pregnancy, elevated maternal inflammatory cytokines levels have been found to mediate the effects of psychological distress on infant internalizing behavior, particularly sadness [137]. However, while other studies to date have also observed the association between maternal psychopathology with altered cytokine levels during pregnancy [138–140], few have definitively found the link between inflammation levels and offspring behavioral outcomes, exposing the need for future research to elucidate this relationship. Inflammatory cytokines have also been implicated in prenatal tobacco and alcohol exposure [141, 142], environmental toxins [143], and intimate partner violence [144]; however, more research must be performed to establish causal pathways between these risk factors, inflammatory cytokines, and offspring outcomes.
Implications
Pregnancy is a time during which women re-assess their health, behavioral, and lifestyle choices, thus making it a unique window of opportunity. Understanding the broad spectrum of internalizing and externalizing outcomes that prenatal risk factors pose to offspring will not only aid clinicians, researchers, and policy-makers in integrating successful evidence-based prevention and intervention strategies into a regular part of obstetric care, but also better help expecting mothers manage health for both themselves and their offspring during a time when they are particularly receptive to care. The prenatal risk factors presented in this paper and their key clinical implications are summarized in Table 1.
Table 1.
Prenatal risk factors and their key clinical implications for healthcare providers
| Variables | Prenatal risk factors | Key clinical implications |
|---|---|---|
| Health-related factors | Maternal overweight/obesity | Promote prenatal care for expecting mothers to receive standardized metabolic tests and weight-management interventions [115] Emphasize guidelines for expecting mothers in dietary, physical activity, and behavioral management [116, 117] Refer overweight/obese expecting mothers to nutritional counseling [142] |
| Substance use/abuse (smoking and secondhand smoke exposure/alcohol consumption) | Educate parents on the negative consequences of substance use on offspring [122] Promote pre-pregnancy quitting through educational means in clinical and every-day settings [123] Screen prospective and expecting mothers for tobacco and alcohol use at initial prenatal visits, monitor status at each visit, and support feasible strategies for continued cessation, including behavioral and psychosocial interventions, smoke/alcohol cessation counseling, and pharmacotherapy [123, 124, 126, 143] |
|
| Environmental toxic exposure | Educate expecting mothers on risks of environmental health hazards (e.g., lead exposure) and appropriate prevention methods Collect environmental health exposure histories as routine part of obstetric care [129] Conduct screenings of ubiquitous environmental toxins and assess environmental toxicity risk in the home [129] Provide dietary advice of foods that may act as risk or protective factors for environmental toxin exposure [131, 132] Encourage expecting mothers to consult Teratology Information Services [133] and Pediatric Environmental Health Specialty Units [145] |
|
| Maternal infection/inflammation | Improve immunization rates for pregnant women and women of childbearing age Inform patients of increased complication and hospitalization risk due to infection during pregnancy Test pregnant women for common infectious agents as a part of standard prenatal care [146] In the case of infection, discuss the pros and cons of anti-viral drugs with patients Screen children for prenatal exposure to maternal infection |
|
| Psychosocial factors | Intimate partner violence (IPV) | Inquire about IPV exposure throughout multiple points throughout prenatal care to increase disclosure rates Offer ongoing support through counseling, home visits, and mentoring [96, 138] |
| Depression and anxiety | Screen expecting mothers for depression and anxiety throughout prenatal care Referral for those who exhibit symptoms of emotional problems for further evaluation Follow-up with treatment and therapy, including cognitive behavioral therapy and nutritional medicine [140, 144] |
Health-related implications
With regards to many health-related prenatal risk factors, pregnancy represents a window of opportunity for expecting women to re-evaluate their lifestyles and health behaviors. Thus, elucidating the associations between these risk factors and emotional/behavioral problems is critical in both clinical and public health settings for developing successful prevention and intervention methods during a unique time frame when women regularly interact with healthcare professionals.
Concerning maternal overweight and obesity, health care providers should promote prenatal care for expecting mothers to receive standardized metabolic tests and weight-management interventions [145–147]. Furthermore, obstetricians should regularly promote a combination of dietary [148], physical activity [149], and behavioral modifications specific for weight management during pregnancy. Apart from generalized guidelines, physicians should also refer expecting mothers who are overweight or obese to dieticians for nutritional counseling and other tailored prenatal weight-management methods that take into account factors such as socioeconomic status [150] and culture [151, 152]. However, while nutritional strategies have generally found to be useful in preventing maternal overweight/obesity, studies have also found insufficient evidence regarding the efficacy of lifestyle interventions on birth outcomes [153], therefore underscoring the need for further research to develop effective strategies.
Concerning substance use during pregnancy, including smoking and alcohol consumption, healthcare providers should educate parents on the negative consequences of substance use on offspring [154], as well as support prepregnancy quitting. Strategies for pre-pregnancy quitting include widely displaying media messages that emphasize the link between prenatal smoke/alcohol exposure and offspring emotional/behavioral health, and engaging clinicians, such as primary care physicians, to promote cessation prior to pregnancy [155]. They should furthermore universally screen for tobacco/alcohol use at initial prenatal visits, monitor usage status during each subsequent visit, and support continued cessation throughout the duration of the pregnancy to prevent relapse. Strategies for cessation during pregnancy must be cognizant of individuals who may be resistant to educational interventions and quitting. Interventions, including behavioral [156] and psychosocial [157, 158] cessation counseling sessions, as well as pharmacotherapy interventions [159, 160], have all shown to be effective in smoking cessation. Finally, arranging for follow-up visits to monitor progress is critical for continued cessation during the intervention process.
Concerning environmental toxicant exposure, education on proper avoidance or mitigation of effects of hazards during pregnancy is integral to both maternal and fetal health. Currently, healthcare providers are not routinely trained in environmental health, and environmental health screening is not incorporated as part of standard practice. Inclusion of such training and development of standardized screening tools is a necessity for risk reduction during pregnancy. Healthcare providers should include reproductive environmental health as a routine part of obstetric care, including the collection of patients’ environmental health exposure histories [161], as well as conduct screenings of ubiquitous environmental toxins and assess the risk of environmental toxin exposure in the home and workplace. Furthermore, obstetricians should provide dietary advice, including educating women that healthful nutrition may act as protective factors modulating the toxicity of environmental pollutants [145, 162]. They should furthermore advise women to avoid diets that may contain significant amounts of environmental toxins [163]. Apart from dietary advice, expecting mothers should also be encouraged to consult Teratology Information Services, a platform focused on advising women planning pregnancy, pregnant women, breast-feeding women, and their families on the reproductive safety or risk of prenatal exposures [146, 164], as well as the Pediatric Environmental Health Specialty Units, a nationwide network of specialists who provide consultation and training to health care providers and the general public [165].
Concerning maternal infection/inflammation, public health campaigns and community health efforts should focus on improving immunization rates for pregnant women and women of childbearing age. For example, although the Advisory Committee on Immunization Practices recommends the influenza vaccine for women who are pregnant during the influenza season, only 53.6% of pregnant women reported having received the vaccine before or during pregnancy during the 2016–17 influenza season [166]. To promote vaccination uptake, clinicians providing care for pregnant women may inform patients of the increased risk for complications and hospitalizations of infection during pregnancy, as well as the lasting implications upon offspring emotional and behavioral development. Furthermore, obstetricians may consider testing pregnant women for common infectious agents, including hepatitis B, human immunodeficiency virus, rubella, gonorrhea, and chlamydia as a standard part of prenatal care [167]. In the case of infection, the benefits of anti-viral drugs may be discussed with the patient, taking into consideration the type of drug, the timing of exposure, and the gestational period. Following birth, pediatricians should screen children for prenatal exposure to maternal infections for early identification of potential internalizing and externalizing behavior.
Prevention and intervention strategies for all previously discussed health-related factors should involve mobilization of not only the individual, but also community and governmental efforts. Policies and political action, such as improved maternal access to food in underserved communities [168], improved healthcare coverage benefits for smoking and alcohol cessation services during pregnancy [169], governmental regulation of workplace chemicals known to cause reproductive harm, and policies that limit permissible exposure in expecting women [161], should supplement clinical approaches for maximum effectiveness. These broad-scaled policy changes would complement individualized obstetric care by helping women generate their own capacity for change.
Psychosocial implications
The stigma surrounding IPV and mental health coupled with their deleterious effects warrant rigorous, active detection, and monitoring of psychosocial risk factors during pregnancy. With regards to IPV, inquiries should be made as a universal part of obstetric care (during the initial visit and at least once per trimester) [170] to increase the opportunity for disclosure. Indeed, routine and repeated screening for IPV during the prenatal period has been shown to increase disclosure rates among women exposed to IPV during pregnancy [101, 171]. When screening results indicate IPV, healthcare providers should offer ongoing support, review available prevention and intervention options including counseling, home visits, and mentoring support, and provide referrals to support services for abused women.
With regards to anxiety and depression, screenings for the detection and treatment of maternal psychopathology should be a core part of women’s obstetric health visits. Recent evidence suggests that simply screening pregnant women, even without additional treatment-related support, increased rates of treatment seeking and reduced the prevalence of depression [172]. Those who screen positively should promptly be referred for consultation for mental health care, including further evaluation, a formal diagnosis, and treatment, including cognitive behavioral therapy [173] and nutritional medicine [174]. These services should be provided by collaborative, multidisciplinary care teams of clinicians, therapists, and social workers to best mitigate the deleterious effects of maternal psychopathology on offspring outcomes.
Conclusions
Mounting evidence shows that prenatal health and psychosocial risk factors predispose offspring to negative outcomes, including childhood internalizing/externalizing problems. These risk factors may modify the in utero environment, leading to stress and immune system alterations that shape offspring emotional/behavioral outcomes in childhood and beyond. Understanding these risk factors are significant given that the prenatal period is an important window of time for fetal neurobehavioral development, as well as childhood internalizing/externalizing problems are risk factors for later adverse physical and mental health outcomes. Thus, developing evidence-based prevention/intervention strategies for expecting mothers and clinicians is critical to reducing negative behavioral outcomes in future generations.
Acknowledgments
Funding This work was supported by the National Institutes of Environmental Health Sciences and the National Institutes of Health (R01-ES-018858, K02-ES-019878, K01-ES015877, and P30-ES-013508).
Footnotes
Ethical approval Not applicable.
Conflict of interest No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
References
- 1.Van der Ende J, Tiemeier H. The bidirectional pathways between internalizing and externalizing problems and academic performance from 6 to 18 years. Dev Psychopathol. 2016;28:855–67. [DOI] [PubMed] [Google Scholar]
- 2.Farrington DP. Early predictors of adolescent aggression and adult violence. Violence Vict. 1989;4:79–100. [PubMed] [Google Scholar]
- 3.Loth AK, Drabick DA, Leibenluft E, Hulvershorn LA. Do childhood externalizing disorders predict adult depression?A metaanalysis. J Abnorm Child Psychol. 2014;42:1103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathyssek CM, Olino TM, Verhulst FC, van Oort FV. Childhood internalizing and externalizing problems predict the onset of clinical panic attacks over adolescence: the TRAILS study. PLoS One. 2012;7:e51564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miettunen J, Murray GK, Jones PB, Mäki P, Ebeling H, Taanila A, et al. Longitudinal associations between childhood and adulthood externalizing and internalizing psychopathology and adolescent substance use. Psychol Med. 2014;44:1727–38. [DOI] [PubMed] [Google Scholar]
- 6.Liu J Early health risk factors for violence: conceptualization, evidence, and implications. Aggress Violent Behav. 2011;16:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease study 2010. The Lancet. 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Raine A, Wuerker A, Venables PH, Mednick S. The association of birth complications and externalizing behavior in early adolescents: direct and mediating effects. J Res Adolesc. 2009;9:93–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achenbach TM. The child behavior profile: i. Boys aged 6–11. J Consult Clin Psychol. 1978;46:478–88. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Chen X, Lewis G. Childhood internalizing behaviour: analysis and implications. J Psychiatr Ment Health Nurs. 2011;18:884–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achenbach TM, Ivanova MY, Rescorla LA, Turner LV, Althoff RR. Internalizing/externalizing problems: review and recommendations for clinical and research applications. J Am Acad Child Adolesc Psychiatry. 2016;55:647–56. [DOI] [PubMed] [Google Scholar]
- 12.Liu J Childhood externalizing behavior: theory and implications. J Child Adolesc Psychiatr Nurs. 2004;17:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willner CJ, Gatzke-Kopp LM, Bray BC. The dynamics of internalizing and externalizing comorbidity across the early school years. Dev Psychopathol. 2016;28:1033–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J Concept analysis: aggression. Issues Ment Health Nurs. 2009;25:693–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouquette A, Pingault J-B, Fried EI, Orri M, Falissard B, Kossakowski JJ, et al. Emotional and behavioral symptom network structure in elementary school girls and association with anxiety disorders and depression in adolescence and early adulthood: a network analysis. JAMA Psychiatry. 2018;75:1173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jokela M, Ferrie J, Kivimaki M. Childhood problem behaviors and death by midlife: the British National Child Development Study. J Am Acad Child Adolesc Psychiatry. 2009;48:19–24. [DOI] [PubMed] [Google Scholar]
- 17.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9:140–50. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Obel C, Taanila A, et al. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes (Lond). 2008;32:550–7. [DOI] [PubMed] [Google Scholar]
- 19.Mina TH, Lahti M, Drake AJ, Räikkönen K, Minnis H, Denison FC, et al. Prenatal exposure to very severe maternal obesity is associated with adverse neuropsychiatric outcomes in children. Psychol Med. 2017;47:353–62. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Sjölander A, Långström N, Rodriguez A, Serlachius E, D’Onofrio BM, et al. Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: a population-based cohort study using a sibling-comparison design. Int J Epidemiol. 2014;43:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanda R, Salsberry PJ. Racial differences in the association between maternal prepregnancy obesity and children’s behavior problems. J Dev Behav Pediatr. 2014;35:118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang SS, Kurti A, Fair DA, Fryer JD. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J Neuroinflammation. 2014;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes C, Grayton H, Poston L, Samuelsson A-M, Taylor PD, Collier DA, et al. Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Mol Psychiatry. 2012;17:1159–60. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez A Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. 2010;51:134–43. [DOI] [PubMed] [Google Scholar]
- 25.Van Lieshout RJ, Robinson M, Boyle MH. Maternal pre-pregnancy body mass index and internalizing and externalizing problems in offspring. Can J Psychiatry. 2013;58:151–9. [DOI] [PubMed] [Google Scholar]
- 26.Deardorff J, Smith LH, Petito L, Kim H, Abrams BF. Maternal prepregnancy weight and children’s behavioral and emotional outcomes. Am J Prev Med. 2017;53:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peleg-Raibstein D, Luca E, Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res. 2012;233:398–404. [DOI] [PubMed] [Google Scholar]
- 28.Winther G, Eskelund A, Bay-Richter C, Elfving B, Müller HK, Lund S, et al. Grandmaternal high-fat diet primed anxiety-like behaviour in the second-generation female offspring. Behav Brain Res. 2019;359:47–55. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30:3826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutin AR, Flynn HA, Terracciano A. Maternal cigarette smoking during pregnancy and the trajectory of externalizing and internalizing symptoms across childhood: similarities and differences across parent, teacher, and self reports. J Psychiatr Res. 2017;91:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Indredavik MS, Brubakk A-M, Romundstad P, Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr. 2007;96:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gard AM, Owens EB, Hinshaw SP. Prenatal smoke exposure predicts hyperactive/impulsive but not inattentive ADHD symptoms in adolescent and young adult girls. Infant Child Dev. 2016;25:339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nivard MG, Lubke GH, Dolan CV, Evans DM, St Pourcain B, Munafò MR, et al. Joint developmental trajectories of internalizing and externalizing disorders between childhood and adolescence. Dev Psychopathol. 2017;29:919–28. [DOI] [PubMed] [Google Scholar]
- 34.Dolan CV, Geels L, Vink JM, van Beijsterveldt CEM, Neale MC, Bartels M, et al. Testing causal effects of maternal smoking during pregnancy on offspring’s externalizing and internalizing behavior. Behav Genet. 2016;46:378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paradis AD, Shenassa ED, Papandonatos GD, Rogers ML, Buka SL. Maternal smoking during pregnancy and offspring antisocial behaviour: findings from a longitudinal investigation of discordant siblings. J Epidemiol Commun Health. 2017;71:889–96. [DOI] [PubMed] [Google Scholar]
- 36.Gatzke-Kopp LM, Beauchaine TP. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry Hum Dev. 2007;38:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacy RT, Brown RW, Morgan AJ, Mactutus CF, Harrod SB. Intravenous prenatal nicotine exposure Alters METH-induced hyperactivity, conditioned hyperactivity, and BDNF in adult rat offspring. DNE. 2016;38:171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alkam T, Mamiya T, Kimura N, Yoshida A, Kihara D, Tsunoda Y, et al. Prenatal nicotine exposure decreases the release of dopamine in the medial frontal cortex and induces a tomoxetine-responsive neurobehavioral deficits in mice. Psychopharmacology. 2017;234:1853–69. [DOI] [PubMed] [Google Scholar]
- 39.Moylan S, Gustavson K, Øverland S, Karevold EB, Jacka FN, Pasco JA, et al. The impact of maternal smoking during pregnancy on depressive and anxiety behaviors in children: the Norwegian Mother and Child Cohort Study. BMC Med. 2015;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashford J, van Lier PA, Timmermans M, Cuijpers P, Koot HM. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. J Am Acad Child Adolesc Psychiatry. 2008;47:779–87. [DOI] [PubMed] [Google Scholar]
- 41.Menezes AM, Murray J, László M, Wehrmeister FC, Hallal PC, Gonçalves H, et al. Happiness and depression in adolescence after maternal smoking during pregnancy: birth cohort study. PLoS One. 2013;8:e80370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monshouwer K, Huizink AC, Harakeh Z, Raaijmakers QA, Reijneveld SA, Oldehinkel AJ, et al. Prenatal smoking exposure and the risk of behavioral problems and substance use in adolescence: the TRAILS study. Eur Addict Res. 2011;17:342–50. [DOI] [PubMed] [Google Scholar]
- 43.Taylor AE, Carslake D, de Mola CL, Rydell M, Nilsen TIL, Bjørngaard JH, et al. Maternal smoking in pregnancy and offspring depression: a cross cohort and negative control study. Sci Rep. 2017;7:12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, Fan SJ, Sun AB, Liu ZZ, Liu L. Prenatal nicotine exposure induces depression-like behavior in adolescent female rats via modulating neurosteroid in the hippocampus. Mol Med Rep. 2019;19:4185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee H, Chung S, Noh J. Maternal nicotine exposure during late gestation and lactation increases anxiety-like and impulsive decision-making behavior in adolescent offspring of rat. Toxicol Res. 2016;32:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sobrian SK, Marr L, Ressman K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:501–18. [DOI] [PubMed] [Google Scholar]
- 47.Luby JL, Heffelfinger AK, Mrakotsky C, Brown KM, Hessler MJ, Wallis JM, et al. The clinical picture of depression in preschool children. J Am Acad Child Adolesc Psychiatry. 2003;42:340–8. [DOI] [PubMed] [Google Scholar]
- 48.Lin Q, Hou XY, Yin XN, Wen GM, Sun D, Xian DX, et al. Prenatal exposure to environmental tobacco smoke and hyperactivity behavior in Chinese young children. Int J Environ Res Public Health. 2017;14:E1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Leung PW, McCauley L, Ai Y, Pinto-Martin J. Mother’s environmental tobacco smoke exposure during pregnancy and externalizing behavior problems in children. NeuroToxicology. 2013;34:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka K, Miyake Y, Furukawa S, Arakawa M. Perinatal smoking exposure and behavioral problems in Japanese children aged 5 years: the Kyushu Okinawa Maternal and Child Health Study. Environ Res. 2016;151:383–8. [DOI] [PubMed] [Google Scholar]
- 51.CDC. CDC Press Releases. CDC. 2016. https://www.cdc.gov/media/releases/2015/p0924-pregnant-alcohol.html. Accessed 28 Dec 2018. [Google Scholar]
- 52.Ichikawa K, Fujiwara T, Kawachi I. Prenatal alcohol exposure and child psychosocial behavior: a sibling fixed-effects analysis. Front Psychiatry. 2018. https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00570/full#h5. Accessed 26 Nov 2018. [DOI] [PMC free article] [PubMed]
- 53.Day NL, Helsel A, Sonon K, Goldschmidt L. The association between prenatal alcohol exposure and behavior at 22 years of age. Alcohol Clin Exp Res. 2013;37:1171–8. [DOI] [PubMed] [Google Scholar]
- 54.Niclasen J, Nybo Andersen AM, Teasdale TW, Strandberg-Larsen K. Prenatal exposure to alcohol, and gender differences on child mental health at age seven years. J Epidemiol Commun Health. 2014;68:224–32. [DOI] [PubMed] [Google Scholar]
- 55.Robinson M, Oddy WH, McLean NJ, Jacoby P, Pennell CE, de Klerk NH, et al. Low–moderate prenatal alcohol exposure and risk to child behavioural development: a prospective cohort study. BJOG. 2010;117:1139–50. [DOI] [PubMed] [Google Scholar]
- 56.Sayal K, Heron J, Draper E, Alati R, Lewis SJ, Fraser R, et al. Prenatal exposure to binge pattern of alcohol consumption: mental health and learning outcomes at age 11. Eur Child Adolesc Psychiatry. 2014;23:891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pagnin D, Zamboni Grecco ML, Furtado EF. Prenatal alcohol use as a risk for attention-deficit/hyperactivity disorder. 2018. 10.1007/s00406-018-0946-7. Accessed 5 Jan 2019. [DOI] [PubMed]
- 58.Popova S, Lange S, Shield K, Mihic A, Chudley AE, Mukherjee RAS, et al. Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta-analysis. Lancet. 2016;38:978–87. [DOI] [PubMed] [Google Scholar]
- 59.Lynch ME, Kable JA, Coles CD. Effects of prenatal alcohol exposure in a prospective sample of young adults: mental health, substance use, and difficulties with the legal system. Neurotoxicol Teratol. 2017;64:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S. Underreporting of alcohol use in pregnancy. Alcohol Clin Exp Res. 1988;12:506–11. [DOI] [PubMed] [Google Scholar]
- 61.Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundström Poromaa I. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT—a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol. 2008;198:e1–5. [DOI] [PubMed] [Google Scholar]
- 62.Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a metaanalysis. Alcohol Clin Exp Res. 2014;38:214–26. [DOI] [PubMed] [Google Scholar]
- 63.O’Leary CM, Nassar N, Zubrick SR, Kurinczuk JJ, Stanley F, Bower C. Evidence of a complex association between dose, pattern and timing of prenatal alcohol exposure and child behaviour problems. Addiction. 2010;105:74–86. [DOI] [PubMed] [Google Scholar]
- 64.Collier SA, Rasmussen SA, Feldkamp ML, Honein MA, National Birth Defects Prevention Study. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth Defects Res Part A Clin Mol Teratol. 2009;85:193–201. [DOI] [PubMed] [Google Scholar]
- 65.Mac Giollabhui N, Breen EC, Murphy SK, Maxwell SD, Cohn BA, Krigbaum NY, et al. Maternal inflammation during pregnancy and offspring psychiatric symptoms in childhood: timing and sex matter. J Psychiatr Res. 2019;111:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy SK, Fineberg AM, Maxwell SD, Alloy LB, Zimmermann L, Krigbaum NY, et al. Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Res. 2017;257:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lydholm CN, Köhler-Forsberg O, Nordentoft M, Yolken RH, Mortensen PB, Petersen L, et al. Parental infections before, during, and after pregnancy as risk factors for mental disorders in childhood and adolescence: a nationwide Danish study. Biol Psychiat. 2019;85:317–25. [DOI] [PubMed] [Google Scholar]
- 68.Depino AM. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 2015;299:56–65. [DOI] [PubMed] [Google Scholar]
- 69.Ronovsky M, Berger S, Zambon A, Reisinger SN, Horvath O, Pollak A, et al. Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav Immun. 2017;63:127–36. [DOI] [PubMed] [Google Scholar]
- 70.Enayati M, Solati J, Hosseini MH, Shahi HR, Saki G, Salari AA. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res Bull. 2012;87:295–302. [DOI] [PubMed] [Google Scholar]
- 71.Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, et al. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiat. 2018;83:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Instanes JT, Halmøy A, Engeland A, Haavik J, Furu K, Klungsøyr K. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiat. 2017;81:452–9. [DOI] [PubMed] [Google Scholar]
- 73.Werenberg Dreier J, Nybo Andersen AM, Hvolby A, Garne E, Kragh Andersen P, Berg-Beckhoff G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. J Child Psychol Psychiatry. 2016;57:540–8. [DOI] [PubMed] [Google Scholar]
- 74.Ginsberg Y, D’Onofrio BM, Rickert ME, Class QA, Rosenqvist MA, Almqvist C, et al. Maternal infection requiring hospitalization during pregnancy and attention-deficit hyperactivity disorder in offspring: a quasi-experimental family-based study. J Child Psychol Psychiatry. 2019;60:160–8. [DOI] [PubMed] [Google Scholar]
- 75.Allard M-J, Brochu M-E, Bergeron JD, Sebire G. Hyperactive behavior in female rats in utero-exposed to group B Streptococcus-induced inflammation. Int J Dev Neurosci. 2018;69:17–22. [DOI] [PubMed] [Google Scholar]
- 76.Kim S, Eom S, Kim HJ, Lee JJ, Choi G, Choi S, et al. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2 years of age- CHECK cohort study. Sci Total Environ. 2018;624:377–84. [DOI] [PubMed] [Google Scholar]
- 77.Boucher O, Jacobson SW, Plusquellec P, Dewailly É, Ayotte P, Forget-Dubois N, et al. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Quebec. Environ Health Perspect. 2012;120:1456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sagiv SK, Thurston SW, Bellinger DC, Amarasiriwardena C, Korrick SA. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch Pediatr Adolesc Med. 2012;166:1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joo H, Choi JH, Burm E, Park H, Hong Y-C, Kim Y, et al. Gender difference in the effects of lead exposure at different time windows on neurobehavioral development in 5-year-old children. Sci Total Environ. 2018;615:1086–92. [DOI] [PubMed] [Google Scholar]
- 80.Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–8. [DOI] [PubMed] [Google Scholar]
- 81.Burns JM, Baghurst PA, Sawyer MG, McMichael AJ, Tong S. Lifetime low-level exposure to environmental lead and children’s emotional and behavioral development at ages 11–13 years: the Port Pirie Cohort study. Am J Epidemiol. 1999;149:740–9. [DOI] [PubMed] [Google Scholar]
- 82.Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernández-Bonilla D, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMA-COS cohort. Environ Int. 2015;84:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung SE, Cheong HK, Ha EH, Kim BN, Ha M, Kim Y, et al. Maternal blood manganese and early neurodevelopment: the Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2015;123:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu XD, Zhang J, Yan CH, Shen XM. Prenatal exposure to manganese at environment relevant level and neonatal neurobehavioral development. Environ Res. 2014;133:232–8. [DOI] [PubMed] [Google Scholar]
- 85.Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, et al. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort study. Environ Health Perspect. 2015;123:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minatoya M, Itoh S, Yamazaki K, Araki A, Miyashita C, Tamura N, et al. Prenatal exposure to bisphenol A and phthalates and behavioral problems in children at preschool age: the Hokkaido Study on Environment and Children’s Health. Environ Health Prev Med. 2018;23:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu D, Wang YX, Chen WJ, Zhang Y, Li HH, Xiong L, et al. Associations of phthalates exposure with attention deficits hyperactivity disorder: a case-control study among Chinese children. Environ Pollut. 2017;229:375–85. [DOI] [PubMed] [Google Scholar]
- 88.Huang HB, Kuo PH, Su PH, Sun CW, Chen WJ, Wang SL. Prenatal and childhood exposure to phthalate diesters and neurobe-havioral development in a 15-year follow-up birth cohort study. Environ Res. 2019;172:569–77. [DOI] [PubMed] [Google Scholar]
- 89.Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, et al. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. NeuroToxicology. 2017;62:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sagiv SK, Kogut K, Gaspar F, Gunier R, Harley K, Parra K, et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicol Teratol. 2015;52:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffman K, Adgent M, Goldman BD, Sjödin A, Daniels JL. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ Health Perspect. 2012;120:1438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casas M, Forns J, Martínez D, Avella-García C, Valvi D, Ballesteros-Gómez A, et al. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ Res. 2015;142:671–9. [DOI] [PubMed] [Google Scholar]
- 93.Evans SF, Kobrosly RW, Barrett ES, Thurston SW, Calafat AM, Weiss B, et al. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology. 2014;45:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Findlay LC, Kohen DE. Bisphenol A and child and youth behaviour: Canadian Health Measures Survey 2007 to 2011. Health Rep. 2015;26:3–9. [PubMed] [Google Scholar]
- 95.Roen EL, Wang Y, Calafat AM, Wang S, Margolis A, Herbstman J, et al. Bisphenol A exposure and behavioral problems among inner city children at 7-9 years of age. Environ Res. 2015;142:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chatsantiprapa K, Sophon T, Sattayasai J. Effects of continuous exposure to bisphenol A on male and female mice from prenatally to adulthood. 2016. http://www.tjps.pharm.chula.ac.th/ojs/index.php/tjps/article/view/98. Accessed 27 Jun 2016.
- 97.Komada M, Itoh S, Kawachi K, Kagawa N, Ikeda Y, Nagao T. Newborn mice exposed prenatally to bisphenol A show hyperactivity and defective neocortical development. Toxicology. 2014;323:51–60. [DOI] [PubMed] [Google Scholar]
- 98.Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav. 2008;53:580–8. [DOI] [PubMed] [Google Scholar]
- 99.WHO. Global and regional estimates of violence against women. https://www.who.int/reproductivehealth/publications/violence/9789241564625/en/. Accessed 7 Dec 2018.
- 100.Martinez-Torteya C, Bogat GA, Lonstein JS, Granger DA, Levendosky AA. Exposure to intimate partner violence in utero and infant internalizing behaviors: moderation by salivary cortisol-alpha amylase asymmetry. Early Hum Dev. 2017;113:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McFarlane J, Maddoux J, Cesario S, Koci A, Liu F, Gilroy H, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123:839–47. [DOI] [PubMed] [Google Scholar]
- 102.Flach C, Leese M, Heron J, Evans J, Feder G, Sharp D, et al. Antenatal domestic violence, maternal mental health and subsequent child behaviour: a cohort study. BJOG. 2011;118:1383–91. [DOI] [PubMed] [Google Scholar]
- 103.Martinez-Torteya C, Bogat GA, Levendosky AA, von Eye A. The influence of prenatal intimate partner violence exposure on hypothalamic-pituitary-adrenal axis reactivity and childhood internalizing and externalizing symptoms. Dev Psychopathol. 2016;28:55–72. [DOI] [PubMed] [Google Scholar]
- 104.Bianchi AL, McFarlane J, Cesario S, Symes L, Maddoux J. Continued intimate partner violence during pregnancy and after birth and its effect on child functioning. J Obstet Gynecol Neonatal Nurs. 2016;45:601–9. [DOI] [PubMed] [Google Scholar]
- 105.Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kingston D, Kehler H, Austin M-P, Mughal MK, Wajid A, Vermeyden L, et al. Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PLoS One. 2018;13:e0195365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giallo R, Woolhouse H, Gartland D, Hiscock H, Brown S. The emotional-behavioural functioning of children exposed to maternal depressive symptoms across pregnancy and early childhood: a prospective Australian pregnancy cohort study. Eur Child Adolesc Psychiatry. 2015;24:1233–44. [DOI] [PubMed] [Google Scholar]
- 108.Eichler A, Walz L, Grunitz J, Grimm J, Van Doren J, Raabe E, et al. Children of prenatally depressed mothers: externalizing and internalizing symptoms are accompanied by reductions in specific social-emotional competencies. J Child Fam Stud. 2017;26:3135–44. [Google Scholar]
- 109.Pihlakoski L, Sourander A, Aromaa M, Rönning J, Rautava P, Helenius H, et al. Do antenatal and postnatal parental psychological distress, and recognized need of help predict preadolescent’s psychiatric symptoms? The Finnish Family Competence Cohort study. Child Psychiatry Hum Dev. 2013;44:305–19. [DOI] [PubMed] [Google Scholar]
- 110.Korhonen M, Luoma I, Salmelin R, Tamminen T. Maternal depressive symptoms: associations with adolescents’ internalizing and externalizing problems and social competence. Nord J Psychiatry. 2014;68:323–32. [DOI] [PubMed] [Google Scholar]
- 111.Betts KS, Williams GM, Najman JM, Alati R. The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depress Anxiety. 2015;32:82–90. [DOI] [PubMed] [Google Scholar]
- 112.Boulle F, Pawluski JL, Homberg JR, Machiels B, Kroeze Y, Kumar N, et al. Developmental fluoxetine exposure increases behavioral despair and alters epigenetic regulation of the hippocampal BDNF gene in adult female offspring. Horm Behav. 2016:80:47–57. [DOI] [PubMed] [Google Scholar]
- 113.Glover ME, Pugh PC, Jackson NL, Cohen JL, Fant AD, Akil H, et al. Early-life exposure to the SSRI paroxetine exacerbates depression-like behavior in anxiety/depression-prone rats. Neuroscience. 2015;284:775–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zohar I, Shoham S, Weinstock M. Perinatal citalopram does not prevent the effect of prenatal stress on anxiety, depressive-like behaviour and serotonergic transmission in adult rat offspring. Eur J Neurosci. 2016;43:590–600. [DOI] [PubMed] [Google Scholar]
- 115.Sprowles JL, Hufgard JR, Gutierrez A, Bailey RA, Jablonski SA, Williams MT, et al. Perinatal exposure to the selective serotonin reuptake inhibitor citalopram alters spatial learning and memory, anxiety, depression, and startle in Sprague-Dawley rats. Int J Dev Neurosci. 2016;54:39–52. [DOI] [PubMed] [Google Scholar]
- 116.Zahra A, Jiang J, Chen Y, Long C, Yang L. Memantine rescues prenatal citalopram exposure-induced striatal and social abnormalities in mice. Exp Neurol. 2018;307:145–54. [DOI] [PubMed] [Google Scholar]
- 117.Svirsky N, Levy S, Avitsur R. Prenatal exposure to selective serotonin reuptake inhibitors (SSRI) increases aggression and modulates maternal behavior in offspring mice. Dev Psychobiol. 2016;58:71–82. [DOI] [PubMed] [Google Scholar]
- 118.Kiryanova V, Meunier SJ, Vecchiarelli HA, Hill MN, Dyck RH. Effects of maternal stress and perinatal fluoxetine exposure on behavioral outcomes of adult male offspring. Neuroscience. 2016;320:281–96. [DOI] [PubMed] [Google Scholar]
- 119.Cicchetti D, Handley ED. Methylation of the glucocorticoid receptor gene, nuclear receptor subfamily 3, group C, member 1 (NR3C1), in maltreated and nonmaltreated children: associations with behavioral undercontrol, emotional lability/negativity, and externalizing and internalizing symptoms. Dev Psychopathol. 2017:29:1795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dadds MR, Moul C, Hawes DJ, Mendoza Diaz A, Brennan J. Individual differences in childhood behavior disorders associated with epigenetic modulation of the cortisol receptor gene. Child Dev. 2015:86:1311—20. [DOI] [PubMed] [Google Scholar]
- 121.Buckingham-Howes S, Mazza D, Wang Y, Granger DA, Black MM. Prenatal drug exposure and adolescent cortisol reactivity: association with behavioral concerns. J Dev Behav Pediatr. 2016;37:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry. 2011;l:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Räikkønen K, Pesonen AK, O’Reilly JR, Tuovinen S, Lahti M, Kajantie E, et al. Maternal depressive symptoms during pregnancy, placental expression of genes regulating glucocorticoid and serotonin function and infant regulatory behaviors. Psychological Medicine. 2015. https://www./core/journals/psychological-medicine/article/maternal-depressive-symptoms-during-pregnancy-placental-expression-of-genes-regulating-glucocorticoid-and-serotonin-function-and-infant-regulatory-behaviors/E06FA206B0A8ED9668F84139675072C2. Accessed. 24 Jan 2019. [DOI] [PubMed]
- 124.Hompes T, Izzi B, Gellens E, Morreels M, Fieuws S, Pexsters A, et al. Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. J Psychiatr Res. 2013;47:880–91. [DOI] [PubMed] [Google Scholar]
- 125.Parade SH, Ridout KK, Seifer R, Armstrong DA, Marsit CJ, McWilliams MA, et al. Methylation of the glucocorticoid receptor gene promoter in preschoolers: links with internalizing behavior problems. Child Dev. 2016;87:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.In vitro molecular mechanisms of bisphenol A action. https://www.sciencedirect.com/science/article/pii/S0890623807001864. Accessed 19 May 2019.
- 127.Effect of triclosan, triclocarban, 2,2′,4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis, https://www.sciencedirect.com/science/article/pii/S0887233316300145. Accessed 19 May 2019. [DOI] [PubMed]
- 128.Lin B, Ostlund BD, Conradt E, Lagasse LL, Lester BM. Testing the programming of temperament and psychopathology in two independent samples of children with prenatal substance exposure. Dev Psychopathol. 2018;30:1023–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009:33:1061–79. [DOI] [PubMed] [Google Scholar]
- 130.Bolton JL, Bilbo SD. Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms. Dialog Clin Neurosci. 2014;16:307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daraki V, Roumeliotaki T, Koutra K, Georgiou V, Kampouri M, Kyriklaki A, et al. Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: the Rhea mother–child cohort, Crete, Greece. Eur Child Adolesc Psychiatry. 2017;26:703–14. [DOI] [PubMed] [Google Scholar]
- 132.The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. https://www.sciencedirect.com/science/article/pii/S0163725815000029. Accessed 18 May 2019. [DOI] [PubMed]
- 133.Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. https://www.sciencedirect.com/science/article/pii/S0006322313006380. Accessed 18 May 2019. [DOI] [PubMed]
- 134.Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. https://www.sciencedirect.com/science/article/abs/pii/S030645221300393X. Accessed 18 May 2019. [DOI] [PMC free article] [PubMed]
- 135.Babri S, Doosti M-H, Salari A-A. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain Behav Immun. 2014;37:164–76. [DOI] [PubMed] [Google Scholar]
- 136.Boksa P Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–97. [DOI] [PubMed] [Google Scholar]
- 137.Gustafsson HC, Sullivan EL, Nousen EK, Sullivan CA, Huang E, Rincon M, et al. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain Behav Immun. 2018;73:470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang JP, Lin CY, Lin PY, Shih YH, Chiu TH, Ho M, et al. Polyunsaturated fatty acids and inflammatory markers in major depressive episodes during pregnancy. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:273–8. [DOI] [PubMed] [Google Scholar]
- 139.Karlsson L, Nousiainen N, Scheinin NM, Maksimow M, Salmi M, Lehto SM, et al. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy-the FinnBrain Birth Cohort study. Arch Womens Ment Health. 2017:20:39^-8. [DOI] [PubMed] [Google Scholar]
- 140.Walsh K, Basu A, Werner E, Lee S, Feng T, Osborne LM, et al. Associations among child abuse, depression, and interleukin-6 in pregnant adolescents: paradoxical findings. Psychosom Med. 2016;78:920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chahal N, McLain AC, Ghassabian A, Michels KA, Bell EM, Lawrence DA, et al. Maternal smoking and newborn cytokine and immunoglobulin levels. Nicotine Tob Res. 2017;19:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sowell KD, Uriu-Adams JY, Van de Water J, Coles CD, Kable JA, et al. Implications of altered maternal cytokine concentrations on infant outcomes in children with prenatal alcohol exposure. Alcohol. 2018;68:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection-maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol. 2018:299:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Robertson Blackmore E, Mittal M, Cai X, Moynihan JA, Matthieu MM, O’Connor TG. Lifetime exposure to intimate partner violence and proinflammatory cytokine levels across the perinatal period. J Women’s Health. 2016;25:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Screening for and management of obesity in prenatal patients, https://www.ahrq.gov/professionals/prevention-chronic-care/healthier-pregnancy/preventive/prenatalobesity.html. Accessed 30 Jan 2019.
- 146.Flynn AC, Dalrymple K, Barr S, Poston L, Goff LM, Rogozińska E, et al. Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev. 2016;74:312–28. [DOI] [PubMed] [Google Scholar]
- 147.Evenson KR, Barakat R, Brown WJ, Dargent-Molina P, Haruna M, Mikkelsen EM, et al. Guidelines for physical activity during pregnancy: comparisons from around the world. Am J Lifestyle Med. 2014;8:102–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cahill AG, Haire-Joshu D, Cade WT, Stein RI, Woolfolk CL, Moley K, et al. Weight control program and gestational weight gain in disadvantaged women with overweight or obesity: a randomized clinical trial. Obesity. 2018;26:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lindberg NM, Stevens VJ, Vega-López S, Kauffman T, Calderón MR, Cervantes MA. A weight-loss intervention program designed for Mexican-American women: cultural adaptations and results. J Immigr Minor Health. 2012;14:1030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sampson MR. Effectiveness of a culturally tailored weight loss intervention for overweight and obese postpartum African American women. 2013. https://open.bu.edu/handle/2144/12209. Accessed 16 Jan 2019.
- 151.Muktabhant B, Lumbiganon P, Ngamjarus C, Dowswell T. Interventions for preventing excessive weight gain during pregnancy. Cochrane Database Syst Rev. 2012:4:7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lander L, Howsare J, Byrne M. The impact of substance use disorders on families and children: from theory to practice. Soc Work Public Health. 2013;28:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.WHO Gender, women, and the tobacco epidemic. https://www.who.int/tobacco/publications/gender/women_tob_epidemic/en/. Accessed 16 Jan 2019.
- 154.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. 2017. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001292.pub3/abstract. Accessed 19 Jan 2019.
- 155.Brandon AR. Psychosocial interventions for substance use during pregnancy. J Perinat Neonatal Nurs. 2014;28:169–77. [DOI] [PubMed] [Google Scholar]
- 156.Chamberlain C, O’Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. 2017. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001055.pub5/abstract. Accessed 19 Jan 2019. [DOI] [PMC free article] [PubMed]
- 157.Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. 2015. http://minerva-access.ummelb.edu.au/handle/11343/57346. Accessed 19 Jan 2019. [DOI] [PubMed]
- 158.Rolland B, Paille F, Gillet C, Rigaud A, Moirand R, Dano C, et al. Pharmacotherapy for alcohol dependence: the 2015 recommendations of the French Alcohol Society, issued in partnership with the European Federation of Addiction Societies. CNS Neurosci Ther. 2016;22:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stotland NE, Sutton P, Trowbridge J, Atchley DS, Conry J, Trasande L, et al. Counseling patients on preventing prenatal environmental exposures-a mixed-methods study of obstetricians. PLoS One. 2014;9:e98771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hennig B, Ormsbee L, McClain CJ, Watkins BA, Blumberg B, Bachas LG, et al. Nutrition can modulate the toxicity of environmental pollutants: implications in risk assessment and human health. Environ Health Perspect. 2012;120:771–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoffman JB, Hennig B. Protective influence of healthful nutrition on mechanisms of environmental pollutant toxicity and disease risks. Ann NY Acad Sci. 2017;1398:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kirk LE, Jørgensen JS, Nielsen F, Grandjean P. Public health benefits of hair-mercury analysis and dietary advice in lowering methylmercury exposure in pregnant women. Scand J Public Health. 2017:45:444–51. [DOI] [PubMed] [Google Scholar]
- 163.Campbell SC, Kast TT, Kamyar M, Robertson J, Sherwin CM. Calls to a teratogen information service regarding potential exposures in pregnancy and breastfeeding. BMC Pharmacol Toxicol. 2016:17:33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hancock RL, Koren G, Einarson A, Ungar WJ. The effectiveness of teratology information services (TIS). Reprod Toxicol. 2007;23:125–32. [DOI] [PubMed] [Google Scholar]
- 165.Woolf AD, Sibrizzi C, Kirkland K. Pediatric environmental health specialty units: an analysis of operations. Acad Pediatr. 2016;16:25–33. [DOI] [PubMed] [Google Scholar]
- 166.Ding H, Black CL, Ball S, Fink RV, Williams WW, Fiebelkorn AP, et al. Influenza vaccination coverage among pregnant women—United States, 2016–17 influenza season. MMWR Morb Mortal Wkly Rep. 2017;66:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.AAP. Guidelines for perinatal care, 8th edition. https://ebooks.aappublications.org/content/guidelines-for-perinatal-care-8th-edition. Accessed 17 May 2019. [Google Scholar]
- 168.Hanson M, Barker M, Dodd JM, Kumanyika S, Norris S, Steegers E, et al. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017;5:65–76. [DOI] [PubMed] [Google Scholar]
- 169.Brown QL, Hasin DS, Keyes KM, Fink DS, Ravenell O, Martins SS. Health insurance, alcohol and tobacco use among pregnant and non-pregnant women of reproductive age. Drug Alcohol Depend. 2016:166:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.ACOG Committee Opinion No. 518. Intimate partner violence. Obstet Gynecol. 2012;119:412–7. [DOI] [PubMed] [Google Scholar]
- 171.Hunter T, Botfield JR, Estoesta J, Markham P, Robertson S, McGeechan K. Experience of domestic violence routine screening in Family Planning NSW clinics. Sex Health. 2017;14:155–63. [DOI] [PubMed] [Google Scholar]
- 172.O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US preventive services task force. JAMA. 2016;315:388–406. [DOI] [PubMed] [Google Scholar]
- 173.Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord. 2015;177:7–21. [DOI] [PubMed] [Google Scholar]
- 174.Lin PY, Chang CH, Chong MF, Chen H, Su KP. Polyunsaturated fatty acids in perinatal depression: a systematic review and meta-analysis. Biol Psychiat. 2017;82:560–9. [DOI] [PubMed] [Google Scholar]


