Abstract
Medicinal plants and their derived compounds have drawn the attention of researchers due to their considerable impact on human health. Among medicinal plants, mint (Mentha species) exhibits multiple health beneficial properties, such as prevention from cancer development and anti-obesity, antimicrobial, anti-inflammatory, anti-diabetic, and cardioprotective effects, as a result of its antioxidant potential, combined with low toxicity and high efficacy. Mentha species are widely used in savory dishes, food, beverages, and confectionary products. Phytochemicals derived from mint also showed anticancer activity against different types of human cancers such as cervix, lung, breast and many others. Mint essential oils show a great cytotoxicity potential, by modulating MAPK and PI3k/Akt pathways; they also induce apoptosis, suppress invasion and migration potential of cancer cells lines along with cell cycle arrest, upregulation of Bax and p53 genes, modulation of TNF, IL-6, IFN-γ, IL-8, and induction of senescence phenotype. Essential oils from mint have also been found to exert antibacterial activities against Bacillus subtilis, Streptococcus aureus, Pseudomonas aeruginosa, and many others. The current review highlights the antimicrobial role of mint-derived compounds and essential oils with a special emphasis on anticancer activities, clinical data and adverse effects displayed by such versatile plants.
Keywords: mint; Mentha, phytochemicals; essential oils; anticancer; antimicrobial
1. Introduction
Medicinal plants and their derived compounds (phytochemicals) have been considered of pharmacological significance since ancient times. The use of plants in medicine dates back to 60,000 years ago, before the birth of civilization [1]. Today, more than 30% of all medicinal drugs (and their derivatives and analogs) derive from plants, and natural products will continue to possess considerable impact in human medicine. Most synthetic bioactive drugs are structurally similar to the phytochemicals of plants from which they were firstly isolated [2,3]. In many developing countries, plant materials have an important role in primary care or disease treatment. In addition, due to contraindications in the usage of chemical drugs, there is a growing interest in the utilization of the plant-derived medicinal products [4], since compared to synthetic drugs some of these natural products have lower toxicity and higher efficacy [5]. Anticancer drugs, antibiotics, anti-inflammatory drugs, immunomodulators are among the most important drugs having herbal origin [6,7,8]. Moreover, some of the aforementioned plant-derived compounds have pleasant taste and odor and can be used in kitchen as flavorings, spices and food [9]. Among the plants with global economic and culinary importance, mint is used worldwide for perfuming sweet and savoury dishes and flavouring tea, in addition to its pharmacological importance.
2. Mentha Genus
Mentha is a genus belonging to the family of Lamiaceae, whose plants are among the most aromatic and spread in diverse environments worldwide [10], having simple, characteristic leaves with pleasant scent. Mentha taxonomy is highly complicated and includes about 42 species and 15 hybrids, with hundreds of subspecies and cultivars [11]. Eleven naturally occurring hybrids have been produced from the species M. arvensis L., M. aquatica L., M. spicata L., M. longifolia L., and M. suaveolens Ehrh; most hybrids are infertile but can propagate due to their highly invasive rhizome [12]. Plants of this genus are perennial and are used for essential oil production, mainly in USA, India, China, and Iran [13]. Fresh and dried plant materials of Mentha species are widely used in industry as part of confectionaries, flavor enhancing agents, pharmaceuticals, cosmetics, etc. [14]. Some Mentha species have common names, as listed in Table 1 [15,16,17].
Table 1.
Scientific names and common names of Mentha species.
| Scientific Name | Common Name |
|---|---|
| M. aquatica L. | Water mint |
| M. piperita ‘Lavendula’ | Lavender mint |
| M. arvensis L. | Corn mint, field mint, ginger mint, wild mint |
| M. canadensis L. | American wild mint, Canada mint, Chinese mint, East Asian wild mint, Japanese mint, Sakhalin mint |
| M. longifolia L. | Himalayan silver mint, horsemint |
| M. piperita L. | Peppermint |
| M. piperita f. citrate | Bergamotmint, eau de cologne mint, orange mint |
| M. pulegium | Mosquito plant, pennyroyal mint, pennyrile, pudding grass, squaw mint |
| M. spicata L. | Ciudad del Este mint, common mint, garden mint, homegrown mint, lamb mint, mackerel mint, spearmint |
| M. suaveolens | Apple mint, pineapple mint, round-leafed mint, woolly mint |
| M. suaveolens ‘Variegata’ | Pineapple mint |
| M. x piperitaf. citrate ‘Chocolate’ | Chocolate mint |
| M. suaveolens× piperita | Grapefruit mint |
3. Phytochemical Composition of Mentha
Mentha species are rich in polyphenols [18] and, moreover, contain caffeic acid and its derivatives caftaric acid, cinnamic acid, ferulic acid, and oleanolic acid [19,20,21]. Flavonoids including luteolin and its derivatives apigenin, acacetin, diosmin, salvigenin and thymonin, have also been detected in these plants, accounting for some 10–70 compounds out of the total phenolics, and also flavanols such as catechin, epicatechin and coumarins, including esculetin and scopoletin [22,23,24,25]. With regard to mint compositions, the essential oils represent a major focus. They are colorless, pale yellow or greenish yellow [26] and alcohols, ketones, esters, ethers and oxides are their main components in Mentha species [13,27]. Menthol, menthone, isomenthone, menthyl acetate, linalool, linalyl acetate, lippione, pulegone, carvone, piperitenone oxide and cis-piperitone epoxide are the main constituents of essential oils prepared from different mint species [13,28,29,30]. Table 2 lists the main compounds isolated from different species of this genus.
Table 2.
Main chemical compounds isolated from different Mentha species.
| Species Name | Essential Oil Components |
Other Polyphenol Compounds |
References |
|---|---|---|---|
| M. aquatica L. | epi-bicyclosesquiphellandrene, 1,8-cineole, menthofuran, β-caryophyllene, limonene, p-menthone, β-pinene, germacrene D, α-pinene, α-humulene, δ-cadinene, caryophyllene oxide, viridiflorol, viridiflorol epoxide II, α-cadinol, β-bisabolenol, α-trans-bergamotene, p-cymene, borneol, sabinene, β-myrcene, terpinyl acetate, eucalyptol | Rosmarinic acid, lavandulifolioside, rutin-O-glc, eriodictyol-O-rut, quercetin-3-O-soph, verbascoside, caffeic acid | [31,32,33,34,35,36] |
| M. arvensis L. | 3-Octanol, fenchone, endo-fenchol, p-menthone, iso-menthone, neo-menthol, menthol, epi-bicyclosesquiphellandrene, isopulegone, 1-α-terpineol, pulegone, eugenol, cis-jasmone, β-bisabolene, cis-3-hexenyl phenyl acetate, β-eudesmol, oxygenated monoterpenes, 1,8-cineole, β-caryophyllene oxide, linalyl acetate, α-phellandrene, terpinolene, limonene, pulegone | Monogalactosyl diglycerides, digalactosyldiglycerides, decyl anhydride, 1-decanol | [17,35,36] |
| M. canadensis L. | Oxygenated monoterpenes, 1-menthol, isomenthone, 1-limonene, menthone, neomenthol, isopulegone, pulegone, linalyl acetate, piperitone | 3,4-Dihydro-3,6,7-trihydroxy-2(1H)-quinolinone, (E)-2-methoxy-2- oxethyl-3-(4-hydroxyphenyl) acrylate, syringic acid, p-coumaric acid, esculetin, methyl rosmarinate, nepetoidin B, syringaresinol, methyl ester of caffeoyl glycollic acid, 2″,3″-diacetyl- martynoside and bracteanolide A, cis-3-[2-[1-(3,4-dihydroxyphenyl)-1-hydroxymethyl]-1,3-benzodioxol-5-yl]-(E)-2-propenoic acid | [17,35,37] |
| M. longifolia L. | τ-Cadinol, γ-cadinene, γ-gurjunene, 1-limonene, piperitone oxide, piperitenone oxide, piperitenone, menthone, borneol, pulegone, verbenone, β-caryophyllene, linalool, 3-tripinolenone, dihydrocarvon, 1,8-cineol, germacrene D, citronellal | Prasterone acetate, sclareol |
[38,39] |
| M. mozaffarian L. | Piperitone, 1,8-cineol, linalool, α-terpineol | Piperitenone, pulegone, piperitenone oxide, menthone, cis-piperitone epoxide | [38,39,40] |
| M. piperita L. | Oxygenated monoterpenes, menthol, methyl petroselinate, menthyl acetate, isopulegol, pulegone, carvone, menthone, cineole, menthofuran, isomenthone, limonene, β-pinene, β-myrcene, α-pinene, α-thujene, linalool | Riboflavin, cis-carvone oxide, caffeic acid, p-cumaric acid, ferulic acid, rosmarinic acid, caftaric acid, chlorogenic acid, m-coumaric acid, o-coumaric acid, | [35,41,42,43,44,45] |
| M. pulegium L. | Piperitone, piperitenone, 4-terpineol, menthone, limonene, naringenin, pulegone, iso-methone | Rosmarinic acid, ellagic acid, caffeic acid, caftaric acid, chlorogenic acid, m-coumaric acid, o-coumaric acid, p-coumaric acid, cryptochlorogenic acid, isochlorogenic acid, neochlorogenic acid, protocatechuic acid | [35,46,47] |
| M. rotundifolia L. | Menthol, menthone, menthyl acetate, menthofuran, piperitone oxide, linalyl acetate, neomenthol, piperitone, isomenthone, 1,8-cineole, linalool, geraniol, myrcene, geranyl acetate, germacrene D, carveol, limonene, rotundifolone, p-menthane-1,2,3-triol, D-limonene, piperitol, diosphenol, β-caryophyllene,, germacrene D, calamenene, trans-piperitone epoxide, piperitenone oxide, cis-piperitone oxide, cyclohexanol, trans-sabinene hydrate | Hypericin, apigenin, quercetin, trans-cinamaldehyde acid, rosmarinic acid, quercetin3-O-galactoside, hydroxybenzoic acid, procyanidin B2 | [48,49,50,51,52] |
| M. spicata L. | Carvone, piperitenone oxide, pulegone, 1,8-cineole, limonene, cis-piperitone oxide, piperitone, piperitenone, menthofuran, caryophyllene | Rosmarinic acid, salvianolic acids, hydroxybenzoic acids, caffeoylquinic acids, hydroxycinnamic acids, flavanones, and flavones |
[53,54,55] |
| M. suaveolens Ehrh L. | Piperitenone oxide, pulegone, trans-caryophyllene, germacrene D, nepetalactone, piperitenone, cis-piperitone, limonene, menthone, terpinen-4-ol, p-cymen-8-ol, E-hydrate sabinene | 4-Hydroxybenzoic acid, vanillic acid, chlorogenic acid, syringic acid, o-coumaric acid, p-coumaric acid | [56,57,58] |
| M. viridis L. | Carvone, 1,8-cineole, 2-methyl- 5-(1-methylethenyl) limonene | Rosmarinic acid, caffeic acid, luteolin-7-O-rutinoside, rosmarinic acid and luteolin-7-O-glucoside, 3-O-caffeoylquinic acid, 3-acylchlorogenic acids | [59,60,61,62,63,64] |
4. Properties of the Mentha Genus
Mentha species are characterized by a great chemical diversity and were reported to contain a number of chemical compounds responsible for pharmacological properties (Table 3).
Table 3.
Chemical compounds in Mentha genus and their pharmacological properties.
| Pharmacological Properties | Chemical Compounds Responsible for Pharmacological Properties | References |
|---|---|---|
| Antioxidant | Ascorbic acid, rosmarinic acid, δ-terpinene, α-terpinene, p-cymene, 1,8-cineole, cis-carveol, carvone, rosmarinic acid, cynaroside, cryptochlorogenic acid, naringin | [59,65,66] |
| Antibacterial | Luteolin, rosmarinic acid, caffeic acid, gallocatechin, epigallocatechin gallate, catechins, menthone, isomenthone, hexadecanoic acid, cis-carveol, carvone, limonene | [4,65,66] |
| Antifungal and Antiyeast | Limonene, piperitenone oxide, menthol, menthone, carvone, cis-carveol and carvone, piperitone, citronellal, caffeic acid, naringin, cryptochlorogenic acid, rosmarinic acid | [4,65,67] |
| Antiviral | Menthol, eriocitrin, rosmarinic acid, luteolin 7-O-rutinoside, hesperidin, phytol | [4,68] |
| Anticancer | Eugenol, caryophyllene, t-cadinol, menthone, menthol crotonate, naringin, cryptochlorogenic acid, rosmarinic acid | [69,70,71] |
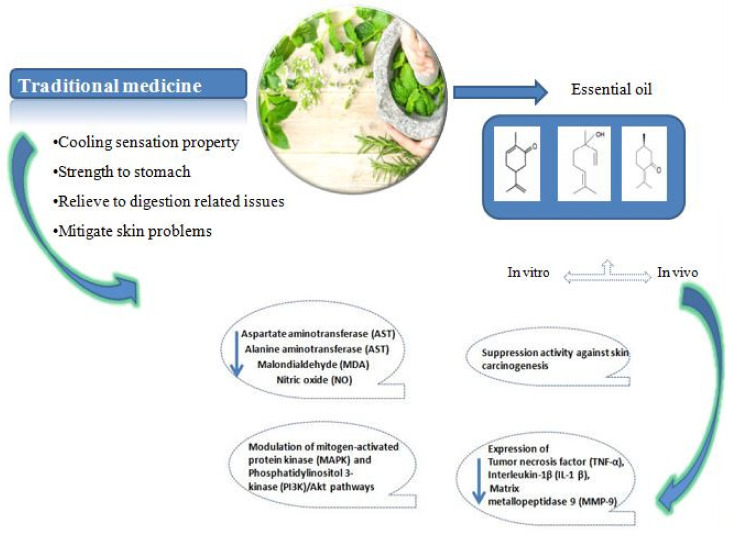
The use of essential oils has a long history being widely exploited in the food, beverage, confectionery and cosmetic industries. In traditional Iranian medicine, it is reported that Mentha species have cooling sensation properties, strengthen the stomach and are effective to relieve digestive symptoms, respiratory tract problems and hemorrhoids [72]. It has been shown that Mentha spicata essential oil can reduce pain caused by Caesarean section [73,74]. Recent investigations have suggested that the transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8) and transient receptor potential cation channel, subfamily A, member 1 (TRPA1) are implicated in pain relief (analgesia) and sensation of cooling [73,75]. In Ayurvedic medicine, some Mentha species were used to mitigate skin problems and headaches [76]. In vitro studies showed that peppermint essential oil and menthol acted as smooth muscle relaxants via blocking Ca2+ influx through L-type Ca2+ channels [77,78]. Also peppermint juice led to reduction in total cholesterol levels, triglycerides and could increase HDL levels in the blood of university students [79] (Figure 1).
Figure 1.
Summary of the main effects of Mentha species.
4.1. Antioxidant Activities
There is increasing interest in the utilization of plant-based natural antioxidants due to their safer nature and medicinal benefits compared to synthetic formulations. In this regard, extracts and essential oils (EOs) from many medicinal and food herbs have been investigated as promising source of effective antioxidant agents [80] showing a well-known action against reactive oxygen species and free radicals. A range of in vitro antioxidant assays such as (2,2-diphenyl-1-picrylhydrazyl) (DPPH) radical scavenging [81], 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) inhibition of linoleic acid peroxidation [82], and reducing power [83] assays have been successfully employed by the researchers to examine the antioxidant effects of Mentha plants. Numerous medicinal plants, including the Mentha genus, contain high levels of antioxidants including phenolic compounds, ascorbic acid and carotenoids that can delay or inhibit the oxidation of different molecules [15]; for example, phenolic compounds act as free radical scavengers and inhibit lipid peroxidation [84]. Notably, the oxidation products such as hydroxyl radicals are extremely reactive oxygen species (ROS) that interact with all kinds of biological molecules within their vicinity and cause cellular damages potentially leading to acute or chronic diseases, including cancer [85,86]. Among the several studies carried out on the antioxidant potential of Mentha species, Park et al. reported that out of nine Mentha species M. longifolia was the most effective one, showing a 88.6% antioxidant activity compared to the 93.0% activity of ascorbic acid at a concentration of 100 µL/mL, whereas M. suaveolens ‘Variegata’ (pineapple mint) had no antioxidant activity [15]. In contrast, in another study [82] the order of DPPH scavenging activity level was as follows: M. piperita, M. pulegium, M. rotundifolia, M. spicata and M. longifolia. Numerous factors are implicated in these apparently conflicting results, for example cultivation methods vs wild harvest of plants, type of laboratory investigations or extraction methods, etc. Without a standardized approach it is not possible to define the consistency of comparisons among different works. It was reported that M. aquatica has the highest phenolic, flavonoid and tannin contents as well as the highest antioxidant activities [87]. In vitro studies showed that the n-butanol soluble fraction derived from a methanolic extract of M. spicata at 10 µg/mL exhibited a significant protective activity against DNA damage caused by free ·OH radicals [88]. Differently, Bahadori et al. found that M. longifolia showed the highest phenolic content and antioxidant activity [55].
Dzamic et al. showed that Mentha longifolia essential oil (MLEO) was an effective DPPH free radical scavenger and exhibited scavenging activity in a dose-dependent manner (IC50 = 0.66 mL/mL of solution) [89]. Similarly, MLEO reduced DPPH radicals to their neutral DPPH-H form (IC50 = 10.5 μg/mL) [90]. Moreover, it has been observed that extract of naturally dried M. longifolia had higher content of phenols (113.8 mg GAE/g) and flavonoids (106.7 mg RTE/g) than the laboratory oven-dried samples. Similarly, a higher antioxidant activity, in terms of ferric reducing power and DPPH scavenging, was also reported for the naturally dried extracts (2.76 mmol Fe2+/mg and EC50 = 0.02 mg/mL) compared with the laboratory oven-dried samples (1.13 ± 0.11 mmol Fe2+/mg of dry extract and EC50 = 0.03 mg/mL) [91]. This suggests that processing of Mentha plants under appropriate conditions is a key step to retain their maximum antioxidant value. In another study, the superoxide radical scavenging activity of different solvent fractions of M. spicata were investigated, showing that the ethyl acetate and aqueous fractions of ethanol extract of M. spicata had higher superoxide radical scavenging among others [81]. According to Anwar et al., MLEOs of different chemotypes, harvested in different regions of Saudi Arabia, exhibited a reasonably high extent of DPPH free radical scavenging that was mainly correlated to the variable polyphenols and carvone contents of the tested oils [4]. Saba and Anwar evaluated the effect of harvesting regions on physico-chemical and biological attributes of supercritical fluid-extracted spearmint (Mentha spicata L.) leaves EO [92]. The researchers noted that the oils tested effectively scavenged DPPH free radicals as well as inhibited linoleic acid peroxidation depending upon variable contents of total phenolics and flavonoids. In another study carried out by Ed-Dra et al., Mentha suaveolens EO showed a significant ferric reducing antioxidant potential and DPPH free radical scavenging activity [93]. Dhifi et al., reported that essential oil of Mentha spicata showed high activity against S. epidermidis and S. aureus, as well as Gram-negative cells of Salmonella spp. and E. coli [94]. Sokovi’c et al. also reported that menthol was more active than other compounds extracted from tested plants: linalyl acetate, limonene, β-pinene, α-pinene, camphor, linalool and 1,8-cineole [95]. Singh et al., reported the antioxidant activity of M. piperita measured by evaluating its antioxidant capacity, DPPH free radical scavenging activity and reducing power. A chloroform extract and peppermint oil showed antioxidant potency of about 90% and minimum activity was recorded for the aqueous extract. The IC50 (μg/mL) of peppermint oil by using DPPH scavenging method was found to be 15.2 ± 0.9 [96].
Though it is known that the extraction method, the solvent used and the extracted fractions analyzed have direct effect on the composition and ratio of compounds, the results of different studies showed that Mentha species, given their significant antioxidant activities, can be confidently used in pharmaceuticals, food and cosmetic productions when antioxidant effects are needed.
4.2. Antibacterial Activities
Infectious diseases are considered as one of the growing concerns in medical science worldwide [97] and, in the latter respect, the microorganisms such as pathogenic bacterial and fungal strains are major agents of infectious diseases. Importantly, most such microorganisms have the ability to survive under harsh environmental conditions and can develop multidrug resistance. Regardless of the availability and use of effective antibiotic drugs, a range of multidrug-resistant strains of microorganisms have been posing health threats [98]. Moreover, in the developing and underdeveloped countries, synthetic drugs are not only expensive and available in limited amount to treat infectious diseases, but they are often under the standard requirements and exhibit the lowest and/or side effects. Therefore, there is the need to search for novel and safer natural antimicrobial agents to control and fight against microbial infections [81,99]. Plant-based drugs and phytomedicines not only act as natural remedies to treat different diseases, but also serve as prototype to develop novel, safer, and effective modern medicines. The antimicrobial activities of Mentha EOs have mainly been attributed to volatile bioactives such as oxygenated monoterpenoids along with monoterpene hydrocarbons (MHs) and sesquiterne hydrocarbons [81]. Mentha EOs are found to exhibit antibacterial activities against pathogenic bacteria including both Gram- negative and Gram-positive, such as Pseudomonas aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aerogenosa, Serratia marcesens, and Streptococcus aureus [81,84]. Saba and collaborators reported the Mentha piperita broad spectrum antibacterial activity against bacterial strains, such as E. coli, Salmonella typhius, B. subtilius, S. aureus, P. aeruginosa, Staphylococcus epidermititis, and Klebsiella pneumonia [92]. According to another study, MLEO exhibited strong antibacterial effects especially against Gram-negative strains including P. aerginosa, E. coli, and S. enteric [100]. Mahady et al. found that methanol extract from peppermint was weakly active against 15 strains of Helicobacter pylori with minimum inhibitory concentration (MIC) in the range of 25–100 μg/mL [101]. However, the reported antibacterial effects of peppermint oil against different bacteria are random type, possibly due to difference of the plant varieties and bacterial strain used and/or testing conditions. In Anwar et al.’s investigation, MLEOs showed a natural antimicrobial effect against different strains of bacteria, but their activity varied with respect to the concentration of volatile chemical constituents [81]. The antibacterial potential of the oils tested was comparable with synthetic drug and its major chemical constituent, carvone. In another report, the EO isolated by supercritical fluid extraction (SCFE) from Mentha spicata leaves also revealed good antibacterial potential against selected strains of bacteria such as E. coli, S. aureus, B. aereus, B. pumilis, B. subtilis, P. aeruginosa, and S. poona [102]. The authors noted that the antibacterial activity of the tested oils varied with respect to the oil composition depending on harvesting regions; however, it was relatively comparable with positive control (synthetic drug). Generally, the oil extracted from drought stressed spearmint populations showed greater antimicrobial activity and those from colder/hilly region exhibited a greater antioxidant activity and total phenolics and flavonoids content [103]. Similarly, Ed-Dra et al. evaluated the antimicrobial effect of Mentha suaveolens EO against pathogenic bacteria, showing that the EO of this species has antibacterial effect against Gram-negative and Gram-positive bacteria and hence could be used as a food additive to enhance the shelf- life of food products [93].
Several studies showed that essential oils exerted their antibacterial effects via disrupting the structure of membranes, resulting in loss of integrity and increased cell permeabilization [103,104,105]. The hydroxyl group in phenol compounds was supposed to have a significant role in the antimicrobial activity of essential oils [106,107]; treatment of E. coli cells by phenolic compounds caused surface blebbing and inhibition of RNA and protein synthesis [108]. Over the last years, the antimicrobial, antifungal, antiyeast and antiparasitic activities of essential oils and extracts of Mentha species have been studied. Stanisavljević et al. prepared the essential oils from M. longifolia using three different methods including the natural way, in the laboratory oven (45 °C) and in the absorptional low-temperature condensational drier (35 °C). They showed that the essential oils obtained by the last method had the strongest antimicrobial and antifungal effects, while the essential oils prepared in natural way showed the strongest antioxidant activities [109]. Nikšić et al. reported that essential oils from M. longifolia exhibited significant antibacterial effects on gram-negative bacteria including E. coli, P. aeruginosa and S. enterica [90]. In another study, Gulluce et al. showed that the volatile oils of M. longfolia ssp. longfolia revealed strong antimicrobial activities against 15 bacteria, 14 fungi and four yeast species [110]. Samber et al. [111] showed that menthol, one of the main components of Mentha species essential oils, inhibited the H+-ATPase pump and intracellular acidification in C. albicans cells. They also reported that menthol inhibited ergosterol biosynthesis pathway and influenced the membrane fluidity and integrity, thus leading to leakage of the intracellular contents [111]. The volatile oil of M. spicata showed remarkable antibacterial activity on different Xanthomonas strains, and also showed mycelium growth inhibition effect on A. solani, R.solani, V. dahlia and FORL (F. oxysporum f. spradicis-lycopersici), in a dose-dependent manner [112].
It was reported that ethanol extract of M. arvensis induced the generation of ROS in A. baumannii cells in a dose-dependent manner, triggering cell membrane damage and protein leakage from the treated cells in a dose-dependent and time-dependent manner [113]. Moreover, structural equation modeling (SEM) visualizations indicated that increasing the extract concentration could provoke considerable cellular damages and morphological changes, consistent with ROS generations and protein leakage [113]. In two different studies, the antibacterial and antiadhesive activity of M. piperita ethanol extract on beverage spoilage bacteria was investigated on some acetic acid bacteria of Asaia genus, showing that the mint extract caused a reduction in cell adhesion of As. Bogorensis and As. Lannensis and biofilm formation through its antibacterial activity and its direct effects on extracellular substances [112,113]. Husain et al. reported that treatment of P. aeruginosa PAO1 by MPEO resulted in a decrease in the production of: LasB elastase (the major virulence factor); pyocyanin (a toxin produced and secreted by P. aeruginosa) up to 85%; exopolymeric substance (EPS); β-galactosidase activity up to 54.5%; acyl homoserine lactone (AHL) levels that regulate virulence factors and biofilm formation in P. aeruginosa and Aeromonas hydrophila [114].
In another study, M. piperita L. leaf extract showed stronger activity against Gram-positive Staphylococcus aureus, Bacillus subtilis than against Gram-negative Escherichia coli [115]. Another study conducted by Laggoune et al. found the E. coli and Proteus mirabilis strains were sensitive to Mentha spicata [116]. Antibacterial effects of peppermint water extract were also observed against Pseudomonas aeruginosa and Serratia marcescens [117]. Golestan et al. observed that M. spicata EO had the highest inhibition activity against S. aureus and Clostridium perfringens [118]. In another study, Zaidi and Dahiya [119] determined the antimicrobial activity of Mentha spicata and Mentha piperita EOs against 11 bacterial and four fungal clinical isolates. They reported maximum zone of inhibition of 21±0.09 mm against S. aureus, with Mentha spicata and 19.2±0.07 mm with Mentha piperita [119]. Singh et al. [96] assessed the antibacterial activity of M. piperita oil and different extracts (petroleum ether, chloroform, ethyl acetate, ethanol, aqueous) using the agar well diffusion method. Gram positive bacterial species (S. aureus and S. pyogenes) were tested sensitive to peppermint essential oil with the inhibition zone 17.2 and 13.1 mm, respectively. The inhibition zone for Gram negative bacteria ranges from 5.1 to 12.4 mm [96].
Mentha component activity against multiple strains of bacteria mentioned above will have a pronounced impact in the future production of novel plant-derived drugs and in food storage/protection. However, the antimicrobial effect needs to be better elucidated considering that it might be due to the occurrence or cooccurrence of bioactives such as luteolin, rosmarinic acid, caffeic acid, gallocatechin, epigallocatechin gallate and catechins, menthone, isomenthone, and hexadecanoic acid in this species.
4.3. Antifungal and Antiyeast Activities
Fungal diseases are a severe health issue especially in subtropical and tropical regions of the world [120]. Due to microbial resistance against common antifungal drugs, there is an urgent need for discovery and development of novel plant-based natural antifungal agents [121]. Besides antibacterial activity, Mentha species have also been investigated as a potential source of antifungal agents to control pathogenic molds [92]. Antifungal activity of M. spicata was studied by Nosrati et al. and it was found that the EO significantly restricted the mycelia growth of Fusarium oxysporum sp. in a dose-dependent manner [122]. The antifungal potential of EO of four Mentha species, including M. arvensis, M. piperita, M. longifolia, and M. spicata, was evaluated by Hussain et al. [65]. The results of the latter research from the disc diffusion assay followed by MIC revealed that M. arvensis exhibited maximum antimicrobial activity with larger IZ (14–33 and 16–30 mm) and smallest MIC values (20.0–330.3 and 56.2–139.0 μg/mL) against selected strains of bacteria and fungi, respectively. Mentha piperita, M. longifolia, and M. spicata also exhibited a remarkable antimicrobial potential with IZ 15–20, 16–31, and 12–29 mm and 11–32, 19–30, and 16–29 mm against selected strains of bacteria and fungi, respectively. In another report, M. spicata EO was shown to be a good natural antifungal agent against pathogenic molds such as Mucor mucedo, Aspergillus niger, Fusarium solani, Botryodiplodiatheobromae, and Rhizopus solani [123]. Furthermore, recent report on the antifungal activity of chitosan in both its natural and nanoparticle forms revealed that incorporation of mint extract into chitosan nanoparticles resulted in increased antifungal effects against mycelium growth of A. niger [124]. The latter finding supports the potential uses of Mentha extracts and oils as antifungal agents in nanocapsulation of different food bioactives such as biopeptides. Moreover, the extract from M. longifolia (5 μL/mL) showed an effective fungicidal potential against Aspergillus, Fusarium, Penicillium funiculosum, and Trichoderma viride [89]. The most sensitive strains were Cladosporium fulvum, Penicillium ochrochloron, and Cladosporium cladosporioides where a concentration of as small as 2.5 μL/mL was found to be lethal [89]. Similarly, Moghtader evaluated the antifungal activity of MPEO against A. niger and reported that the oil possessed stronger antifungal activity than standard antibiotic, gentamycin [125]. The antifungal activity of M. piperita oil can be mainly ascribed to high content of oxygenated monoterpenoids, such as menthone and menthol. The broad-spectrum antifungal activity of Mentha EOs can be linked to the presence of major chemical constituents such as menthone, menthol, piperitenone oxide, and carvone [89].
The essential oil of M. longifolia showed antifungal activity against C. albicans at a concentration of 7120 mg/mL [126]. Mentha x piperita (Mentha of Pancalieri) EO exerted the most remarkable antifungal activity against Cryptococcus neoformans and showed non-negligible activity against Candida krusei and C. glabrata displaying the lowest minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values ranging from 0.06–0.125%, v/v [127].
Saccharomyces cerevisiae has been frequently used as a model organism to study the anti-yeast activities and investigate the mode of actions of different compounds [128,129]. It was shown that treatment of S. cerevisiae cells with MPEO caused the increase of intracellular ROS species, mtDNA damages, mitochondrial fragmentations, formation of petite mutants because of loss of respiratory chain function and chromatin condensation (with no effect on plasma membrane) [130]. In contrast, in another study, plasma membrane rupture, its depolarization and increased permeability was reported [131], thus confirming that different methods can lead to different results.
4.4. Antiviral Properties
Viral dependent infectious diseases are a dilemma in medical sciences. Many viruses are resistant to different therapies due to their adaptable lifestyle and, therefore, the development of long-term effective antiviral chemotherapeutic agents is a very challenging task. Natural products have always been deemed the best source for isolating chemically diverse new lead molecules and served as a platform for the future development of potent and safer antiviral agents. Preliminary evidence suggested that the main peppermint oil component, menthol, might have served as natural antiviral agent and protected against Herpes simplex [132]. As far as antiviral potential of Mentha plants is concerned, it is reported that Mentha spicata and Mentha spicata essential oil (MPEOs) contain some compounds acting as antiviral agents [133]. Appropriate studies showed that phenolic constituents such as rosmarinic acid, luteolin, and phytol present in the extracts of Mentha spicata are effective for their antimicrobial and antiviral actions [134]. Yamasaki et al. reported that water soluble extract (16 μg/mL) of M. piperita exhibited strong antihuman immunodeficiency virus-1 (HIV)-1 activity in MT-4 cells assay [135]. Similarly, methanolic and ethyl acetate extract of M. longlifolia showed significant inhibitory effects against human HIV-1, and ethyl acetate extract exerted its anti-HIV-1 effects via inhibiting the reverse transcriptase enzyme [136]. Water-soluble (polar substances) extract of M. piperita also exhibited inhibitory activity against HIV-reverse transcriptase. MPEO was also reported to have direct virucidal activity against Herpes simplex virus type 1 (HSV-1) and reduced plaque formation effectively [137].
4.5. Anticancer Activity
Cancer is a multistep disease that is characterized by out-of-control multiplication of cells and currently it is a challenging health problem worldwide [138]. According to the recent report of the World Health Organization (WHO), there are more than 18.1 million new cases and 9.6 million cancer deaths in 2018 [139]. It has been reported that 10–70% cancer mortality may be related to the diet, and about two-third of human cancers would be avoidable by choosing an appropriate lifestyle [140,141]. Cancer has been a continuing health problem in the medical sciences globally, with a lot of developments achieved in the treatments of this chronic disease via use of different preventive and curing therapies. The disease is characterized by the continuous multiplication of the human body cells, with the inability to be controlled or stopped, and the consequent formation of malignant cell tumors with the potential to be metastatic [142]. Current treatments include surgery, radiotherapy, and chemically derived drugs. Treatments such as chemotherapy can put patients under a lot of strain and further damage to their health, and therefore there is a focus on using alternative treatments and therapies against cancer [143]. Herbal medicines have been used for many years in developing countries as the primary source of medical treatment [144,145], and recently research is being directed to investigating the potential uses of terrestrial plant extracts as reducing and capping agents for the preparation of nanomaterial-oriented drugs for cancer control [146]. Many plant species have been screened by the researchers for their anticancer potential, and several of them are also employed in herbal medicine for cancer relief [147]. Plant potential in cancer prevention and treatment has received much attention, due to the presence of phytochemicals [141]. Although some plant-originating anticancer drugs including vinblastine, vincristine, vinorelbine, vindesine, estramustine, taxol and colchicine have been used in clinic [148], the ongoing clinical trials on the application of plant-based nutritional supplements and diets aimed to prevent cancers are still few [149].
The Labiatae family includes some of the most acknowledged and famous medicinal plants, since numerous studies have reported the cytotoxicity effect of Mentha species. For example, Rahimifard et al. [150] studied the cytotoxicity effects extracts and essential oils of five Mentha species on HeLa (human malignant cervix carcinoma), Hep2 (human laryngeal carcinoma), and Vero (green African monkey kidney) cell lines. They found that EOs showed greater cytotoxicity potential than the extracts and were more toxic in Hela cell line (IC50 ≤ 42.3 μg/mL), while the extracts were more toxic against Vero cell line (IC50 ≥ 94.3 μg/mL). Hep2 cells showed less sensitivity than the other two cell lines [150]. Differently, aqueous extract of M. spicata showed cytotoxicity effect on U937 leukemia cells (IC50 = 4.8 mg/mL) [151]. The methanolic extract of M. longifolia at a concentration >0.5 µg/µl demonstrated antiproliferative effects in adrenocortical tumor cell lines, with modulating activity on MAPK and PI3k/Akt pathways [152]. Ohara and Matsuhisa screened 120 medicinal plants for antitumor effects against the okadaic aid (OA) tumor inducer and noted that Mentha species were among the most effective in antiproliferative activity (86–100%) [153]. According to Hussain and co-workers, hydrodistilled EOs from four commonly cultivated Mentha species such as M. longifolia, M. spicata, M. arvensis, and M. piperita exhibited good cytotoxicity against the human breast cancer cell line MCF-7 [123]. Another work screened various species of Mentha plant extracts for antitumor activities and found that aqueous extract of M. pulegium showed the best antitumor activity (94%), whereas M. longifolia extracts exhibited reasonable antitumor activity [154]. Similarly, methanolic extract of M. longifolia at a dose of 500 μg/mL resulted in 25% cell death and this cytotoxic effect was dose-dependent, that is, further increase of the extract doses up to 1000, 1500, and 2000 μg/mL produced 25, 50, and 62.5% deaths, respectively [155]. In a recent study, Al-Ali et al. reported that water and methanolic extracts of M. longifolia had significant antimutagenic and anticancer activities as depicted by brine shrimp bioassay and Ames mutagenicity bioassay [156]. Furthermore, it was showed that the methanolic extract of M. pulegium had no cytotoxic effects, but its essential oil proved to be a potent cytotoxic agent on SKOV-3, HeLa and A549 cell lines [157]. Jain et al. showed that chloroform and ethyl acetate extracts of Mentha leaves have significant cytotoxicity in time- and dose-dependent manner on HeLa, MCF-7, Jurkat, T24, HT-29, MIAPaCa-2 cell lines; these extracts also induced G1 cell cycle arrest, apoptosis, upregulation of Bax and p53 genes, some cytokines including TNF, IL-6, IFN-γ, IL-8, and induction of senescence phenotype in treated cells [158]. Silver nanoparticles containing the aqueous extract of M. pulegium showed significant cytotoxicity on MCF-7 [159] and triggered caspase 9-dependent cell death in MCF-7 and MDA-MB-231 cells [160]. l-Menthol, one of the main components of Mentha species, has multiple applications in traditional medicine. Faridi et al. observed that it modulated tubulin polymerization, induced apoptosis and also suppresses the expression of HSP90 [161]. In their proposed model, the latter authors suggested that l-menthol provoked apoptosis directly via caspase 10 activation and also activated caspase 9 which indirectly triggered caspase 3 and mitochondrial cell death [161]. Perillyl alcohol is a monoterpene isolated from lavendin, peppermint, spearmint and some other plants [162], and several studies showed that this compound could inhibit tumor progression. Reddy et al. reported that perillyl alcohol at 1 g/kg level led to apoptosis induction and significantly inhibited the incidence and multiplicity of the adenocarcinoma of the colon in treated rats [163]. Further studies showed that perillyl alcohol induces G0/G1 cell cycle arrest and apoptosis in Bcr/Abl transformed myeloid cells through inhibition of Ras signaling pathway [164,165], or induction of Bak protein in pancreatic ductal adenocarcinoma cells involved in mitochondrial cell death pathway [166]. Perillyl alcohol also delayed and inhibited tumor formation in non-melanoma model of mouse skin carcinogenesis and inhibited UV-B-induced AP-1 transactivation in cultured human keratinocytes and transgenic mice that expressed a luciferase reporter regulated by AP-1 response element [167]. M. piperita extract showed a neuro-protective effect in gamma-irradiated mice [168], and the oral administration of its water extract in papilloma tumors mice (initiated by 7,12-dimethyl benz(a)anthracene, DMBA) reduced the incidence of tumors by 64% and increased the latency period of the appearance of papilloma [169]. 4-nitroquinoline-1-oxide (4-NQO) is a quinoline derivative that has tumorigenic potential through induction of DNA lesions and chromosome damages [170]. It was reported that the chloroform, the hexane, and the ethyl acetate fractions of M. spicata reduced the frequency of micro-nucleated polychromatic erythrocytes in mouse bone-marrow induced by 4-NQO, and moreover, treatment of mice with these fractions resulted in a significant decrease in apoptotic cells. Among the latter fractions, the ethyl acetate showed the highest effectiveness against 4-NQO [171]. Ifosfamide is a chemotherapy drug and an immunosuppressive agent that is used to treat many different types of cancers including testicular cancer, bladder cancer, small cell lung cancer, cervical cancer, ovarian cancer and osteosarcoma [59,172]. The exact mechanism of ifosfamide action has not been determined yet, but it reportedly binds to the N-7 position of guanine and results in inter- and intra-strand cross-links in the DNA and cell death [173]. M. spicata extract at dose 400 mg/kg showed strong anti-mutagenic effect against clastogenic action of ifosfamide in bone marrow cells and sperm abnormalities [174]. Ethanol extract of M. arvensis showed the highest anti-angiogenic effects in the chick chorioallantoic membrane (CAM) assay, followed by methanol and ethyl acetate extracts. Treatment of CAMs with 500 μg of the ethanol extract at 96 h resulted in completely suppression of angiogenesis [175,176]. M. aquatica essential oil revealed suppressor activity against skin carcinogenesis by suppression of keratin 14 and COX-2 overexpression in 7,12-dimethylbenz[a]anthracene/12-O-tetradecanoylphorbol-13-acetate (DMBA/TPA)- induced two-stage carcinogenesis mouse models [177]. Matrix metalloproteinases (MMPs) are a group of Ca2+- and Zn2+ -dependent endopeptidases that participate in extracellular matrix remodeling and degradation [178]. They have key roles in numerous basic and fundamental physiological processes including tissue remodeling, angiogenesis, wound healing, and migration and also have significant role in several pathological conditions including cancer progression and invasion [179]. Mentha species exhibited modulatory effects on the expression or activity of some MMPs, and Liu et al. reported that 10, 30, and 100 mg/kg of spearmint oil significantly decreased the expression of TNF-α, IL-1β, and MMP-9 in rat lung tissues [180]. Moreover, treatment of Wehi-164 fibrosarcoma cells with aqueous extract of spearmint led to significant reduction in MMP-2/MMP-9 activity in a dose-dependent manner, as there was no detectable activity of MMP-2/MMP-9 in Wehi-164 cells treated with 10 mg/mL of the extract after 24, 48, and 72 h [151]. A phytomics analysis (analysis of primary and secondary metabolites of plant extracts) showed that 500 µg/mL of M. piperita extract had significant inhibitory effect on the activity of MMP-1 (84.4%), MMP-8 (80.46%), MMP-13 (91.02%) [181]. Wound-closure assay demonstrated that treatment of HT-29 cells with concentration of 500 μg/mL of spearmint phenolic extract produced a blockade of invasion potential of HT-29 cells, though gelatin zymography experiments showed that the activity of MMP-2 and MMP-9 was not significantly inhibited. Also, spearmint extract led to reduced expression of iNOS (inducible nitric oxide synthase) in the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced chronic inflammation rat models [182]. Heat-induced expression of MMP-1, MMP-3, MMP-10, MMP-12, and MMP-13 in human dermal fibroblast was significantly inhibited by 100 μg/mL of apple mint leaves extract (ALE). Also, the activity of p-ERK, p-JNK, p-p38, and the expression of IL-8 were inhibited in a dose-dependent manner in HDF cells treated with ALE [183].
Epithelial-mesenchymal transition (EMT) is a feature of advanced carcinomas, essential for many developmental processes, wound healing, organ fibrosis and also is one of the causes of cancer cell invasion and metastasis [184]. EMT involves loss of epithelial markers including E-cadherin, weakening of cell-cell adhesion, and the expression of some fibroblast markers including vimentin, α-smooth muscle actin (α-SMA), and desmin [185,186]. Moreover, it is well known that adding TGF-β to epithelial cells in culture results in EMT induction in different epithelial cells [187]. Treatment of CCl4-induced liver fibrosis rats with MPEO produced a significant reduction in ALT, AST, MDA and NO (nitric oxide) that are triggered by CCl4 [188]. In addition, MPEO administration resulted in inhibition of the TGF-β1/SMAD signaling pathway, downregulation of desmin and α-SMA, which are mesenchymal markers, and p53 and upregulation of CYP2E1 in rats treated with CCl4. Nakamura et al. reported that piperitenone oxide, a component of the n-hexane fraction of the spearmint extract, induced duct formation in RCM-1 human colon cancer cells, a process that is a considered a differentiation marker, i.e., with potential anticarcinogenic activity [189]. In vitro anticancer potential of methanolic and aqueous extracts of Mentha arvensis, M. longifolia, M. spicata and M. viridis at concentration of 100 µg/mL was evaluated against eight human cancer cell lines—A-549, COLO-205, HCT-116, MCF-7, NCI-H322, PC-3, THP-1 and U-87MG from six different origins (breast, colon, glioblastoma, lung, leukemia and prostate) using sulphorhodamine blue (SRB) assay. Methanolic extracts of Mentha spp. displayed anti-proliferative effect in the range of 70–97% against four human cancer cell lines, namely COLO-205, MCF-7, NCI-H322 and THP-1; however, aqueous extracts were found to be active against HCT-116 and PC-3. However, essential oil from M. pulegium was found to be a cytotoxic agent against human ovary adenocarcinoma SK-OV-3, human malignant cervical adenocarcinoma HeLa and human lung carcinoma A-549 cell lines [157].
Mentha species contain numerous bioactive constituents, which have been shown to possess anticancer activity that in turn can act as lead molecule for discovery of new anticancer drugs with additional protective role against different pathogens, as underlined by their antimicrobial properties.
5. Clinical Trials
Limited data are available on clinical trials using Mentha species in humans [190], and indeed only two works reported the use of Mentha species in humans related to cancer. First a randomized, double-blind clinical trial was conducted in 200 patients to determine the efficacy of volatile oils of Mentha piperita or Mentha spicata in preventing chemotherapy-induced nausea and vomiting (CINV) in four groups, control, placebo, M. piperita, M. spicata. The results showed a significant reduction in the intensity and number of emetic events in the first 24 h with M. spicata and M. piperita in both treatment groups (p< 0.05) when compared with the control and no adverse effects were reported. The cost of treatment was also reduced when essential oils were used [191]. The second work is another randomized, double blind placebo clinical trial conducted in 60 patients to evaluate the effects of Mentha piperita (and Matricaria recutita) on oral mucositis (OM) in patients undergoing hematopoietic stem cell transplantation (HSCT) [192]. OM is one of the most common side effects of intensive chemotherapy in patients undergoing HSCT. Patients who received herbal mouthwash three times daily for 1 week before HSCT showed significant improvements in pain intensity (p = 0.009), dryness (p = 0.04) and dysphagia (p = 0.009), suggesting a therapeutic role for M. piperita in OM.
6. Adverse Effects of Mentha Species
Although medicinal plants such as Mentha species are commonly believed to be safe, they are not devoid of side effects that can be severe in some cases. Furthermore, allergic reactions can occur with any natural or synthetic compound in sensitive persons. No chronic toxicity studies in humans are available, therefore toxicity of Mentha species are scarcely reported. However, it seems that no adverse effects have been reported after consumption 0.24 mL of pure M. spicata essential oil daily for three continuous weeks in two different clinical studies [193,194]. Leaves of Mentha spicata are known for its contact allergy such as contact cheilitis caused by its essential oil use as toothpaste flavoring [195]. In addition, MPEO is also associated with adverse effects like vomiting, headaches, flushing, heartburn and nausea [196]. Mentha piperita and spearmint tea can deprive the human body of iron and cause anemia if consumed excessively, and carvone and limonene showed to be major allergens [195]. Gürbüz found that pulegone, contained in low concentrations in Mentha piperita oil extracts, is hepatotoxic, and Douros et al. also reported the likely liver injury caused by M. piperita [197,198]. Other research showed that menthol and pulegone could be toxic compounds; in particular, pulegone and its metabolite menthofuran have been suggested as the hepatotoxic compounds in Mentha pulegium and have been also found in smaller quantity in Mentha piperita [199]. Notably, inhalation of menthol can cause apnea and larygospasm in sensitive patients and indeed has been reported that mentholated preparation can be involved in nausea, anorexia, cardiac problems, ataxia and other CNS symptoms [200]. Mentha spicata extracts displayed toxicity to neuronal cells when applied at concentrations which are one order of magnitude higher than those effective for radical scavenging [201]. The positive correlation between the two aforementioned effects suggests that a higher desirable radical scavenging is associated with a higher undesirable toxicity.
Peppermint oil is contraindicated in obstruction of the bile ducts, gallbladder inflammation, and severe liver failure [199]. The American College of Gastroenterology has recommended reducing the peppermint intake as it is a risk factor for gastroesophageal reflux disease (GERD) and lifestyle changes [202,203]. Further, Zong et al. [204] reported that peppermint essential oil, not only stimulated bile fluid secretion, but might be involved in upregulating the bile acid synthesis-related gene, cholesterol 7α-hydroxylase (CYP7A1), and the nuclear bile acid receptor FXR (farnesoid X receptor) mRNA. In addition, peppermint oil could cause heartburn or perianal irritation, bradycardia and muscle tremor, a hypersensitivity reaction, contact dermatitis, abdominal pain and jaundice in newborn babies [205]. A study on a 58-year-old woman who smoked menthol cigarettes also established that she suffered from gastrointestinal upsets with occasional vomiting, hand tremor, mental confusion and depression which were all ascribed to menthol [206]. Similarly, in another case report, a 40-year-old woman with no history of asthma or any other forms of allergy has shown the symptoms of dyspnea, wheezing and nasal after using menthol containing candies and toothpaste, suggesting the development of classical symptoms of asthma [207]. Menthol administered for 28 days at a dose level (≤ 800 mg/kg) in rats caused hepatocellular changes and pulegone (≤ 160 mg/kg) has been reported as hepatotoxic and neurotoxic. Consequently, pulegone caused weight loss, atonia, decreased blood creatinine, histopathological changes in the liver and also in the white matter of the cerebellum [208]. Menthone (≤ 800 mg/kg orally) on the other hand, dose dependently decreased plasma creatinine, but increased alkaline phosphatase and bilirubin along with liver and spleen weight [209]. Menthone administered to rats at a high dose over 28 days did display some signs of hepatotoxicity and cerebellar histopathology [209]. In a later study examining peppermint constituents for their possible induction of encephalopathy, one-month treatment with limonene (≤ 1600 mg/kg) or 1,8-cineole (1000 mg/kg) produced an accumulation of protein droplets containing α-2µ-globulin in proximal tubular epithelial cells, but no encephalopathy in rats [210]. Peppermint and menthol have both been shown to possess Ca2+ channel blocking properties, which might underlie their mechanism of efficacy against irritable bowel syndrome in the clinic [200]. However, in some patients, the use of peppermint is accompanied by oral symptoms like burning mouth syndrome and oral ulceration [203]. Also in this context, direct application of peppermint oil to the chest or nasal area of infants is not recommended due to the risk of apnea, bronchial and/or laryngeal spasms [211].
7. Conclusions and Future Perspectives
Mentha species have been used in indigenous medicine for many centuries and this review attempts to provide an overview on Mentha species’ preventive and curative effects. The essential oils derived from Mentha species acts as a good expectorant and further have been used as a folk remedy for respiratory diseases such as bronchitis, sinusitis, tuberculosis and the common cold. Mentha species’ exploitation in pharmaceuticals formulations requires further research. Likewise, clinical trials are scarce and intense efforts should be made to confirm the claims of efficacy in humans. However, numerous preclinical works have been performed, underlining the antioxidant, antibacterial, antifungal, anti-yeast, antiviral, and anticancer activity. Indeed, Mentha species, and especially essential oils, are used to reduce microbial load, suggesting a strong bactericidal, virucidal, and fungicidal activity. Nevertheless, some adverse effects, such as allergic reactions, vomiting, headache, flushing, heartburn and nausea hepatotoxicity, apnea and larygospasm, neuronal cell damage, may arise due to the presence of some compounds (carvone, limonene, menthol, pulegone) also depending on the Mentha extract concentration applied. Moreover, the presence of harmful compounds in plant such as pulegone and menthone can be reduced by oven-drying or cooked before consumption in order to make it safer. In addition, care should be taken when this plant is consumed along with drugs which induce P450 enzymes.
The positive results described in the present review are a key-point of efficacy of Mentha species, to such an extent that they can be considered promising natural extracts with a clear application as preservatives, supplements, and antioxidants. This intended role of mint cannot be restricted to food preparation, elaboration, or storage, but should be also figured out as a new tool for pharmaceutical industries.
Author Contributions
Conceptualization: R.P. Data curation and collection; all authors. Writing—original draft; all authors. Writing—review & editing; all authors. Supervision: G.C., M.A., M.T., R.P. All authors have read and agreed to the published version of manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Solecki R.S. Shanidar IV, a Neanderthal Flower Burial in Northern Iraq. Sci. 1975;190:880–881. doi: 10.1126/science.190.4217.880. [DOI] [Google Scholar]
- 2.Nirmal S.A., Pal S.C., Otimenyin S.O., Aye T., Elachouri M., Kundu S.K., Thandavarayan R.A., Mandal S.C. Contribution of Herbal Products In Global Market. The pharma review. 2013:95–104. [Google Scholar]
- 3.Sevindik M. Pharmacological Properties of Mentha Species. J. Tradit. Med. Clin. Naturop. 2018;7:1–4. doi: 10.4172/2573-4555.1000259. [DOI] [Google Scholar]
- 4.Anwar F., Abbas A., Mehmood T., Gilani A.-H., Rehman N.-u. Mentha: A genus rich in vital nutra-pharmaceuticals—A review. Phytother. Res. 2019;33:2548–2570. doi: 10.1002/ptr.6423. [DOI] [PubMed] [Google Scholar]
- 5.Fabricant D.S., Fransworth N.R. The value of Plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rates S. Plants as source of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 7.Petrovska B.B. Historical review of medicinal plants’ usage. Phar. Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balasubramanian A., Ramalingam K., Krishnan S., Ajm C. Anti-inflammatory Activity of Morus indica Linn. Ira. J. of Phar. Thera. 2005;4:13–15. [Google Scholar]
- 9.Salehi B., Valussi M., Jugran A.K., Martorell M., Ramírez-Alarcón K., Stojanović-Radić Z.Z., Antolak H., Kręgiel D., Mileski K.S., Sharifi-Rad M., et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018;80:104–122. doi: 10.1016/j.tifs.2018.07.030. [DOI] [Google Scholar]
- 10.Šarić-Kundalić B., Fialová S., Dobeš C., Ölzant S., Tekeľová D., Grančai D., Reznicek G., Saukel J. Multivariate numerical taxonomy of Mentha species, hybrids, varieties and cultivars. Sci. Pharm. 2009;77:851–876. doi: 10.3797/scipharm.0905-10. [DOI] [Google Scholar]
- 11.Salehi B., Stojanovi´c-Radi´c Z., Mateji´c J., Sharopov F., Antolak H., Kr˛egiel D., Sen S., Sharifi-Rad M., Acharya K., Sharifi-Rad R., et al. Plants of Genus Mentha: From Farm to Food Factory. Plants. 2018;7:70. doi: 10.3390/plants7030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer A., Hamill J.D., Rhodes M.J. In Vitro biosynthesis of monoterpenes by Agrobacterium transformed shoot cultures of two Mentha species. Phytochem. 1993;32:911–919. doi: 10.1016/0031-9422(93)85228-J. [DOI] [Google Scholar]
- 13.Lawrence B.M. Mint. The Genus Mentha. CRC Press; Boca Raton, FL, USA: 2006. pp. 1–56. [Google Scholar]
- 14.Shaikh S., Bin Yaacob H., Rahim Z.H.A. Prospective Role In Treatment Of Major Illnesses And Potential Benefits As A Safe Insecticide And Natural Food Preservative of Mint (Mentha spp.): A Review. Asian J. Biomed. Pharm. Sci. 2014;4:1–12. doi: 10.15272/ajbps.v4i35.559. [DOI] [Google Scholar]
- 15.Park Y.J., Baek S.-A., Choi Y., Kim J.K., Park S.U. Metabolic Profiling of Nine Mentha Species and Prediction of Their An-tioxidant Properties Using Chemometrics. Molecules. 2019;24:258. doi: 10.3390/molecules24020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gracindo L.A.M.B., Grisi M.C.M., Silva D.B., Alvez R.B.N., Bizzo H.R., Vieira R.F. Chemical characterization of mint (Mentha spp.) germplasm at Federal District, Brazil. Rev. Bras. de Pla. Med. 2006;8:5–9. [Google Scholar]
- 17.Barros A.D.S., Morais S.M.d., Ferreira P.A.T., Vieira Í.G.P., Craveiro A.A., Fontenelle R.O.D.S., Menezes J.E.S.A.D., Silva F.W.F.D., Sousa H.A.D. Chemical composition and functional properties of essential oils from Mentha species. Ind. Cro. and Prod. 2015;76:557–564. doi: 10.1016/j.indcrop.2015.07.004. [DOI] [Google Scholar]
- 18.Pereira O.R., Cardoso S.M. Overview on Mentha and Thymus Polyphenols. Cur. Anal. Chem. 2013;9:382–396. doi: 10.2174/1573411011309030008. [DOI] [Google Scholar]
- 19.Benedec D., Vlase L., Oniga I., Mot A.C., Silaghi-Dumitrescu R., Hanganu D., Tiperciuc B., Crişan G. LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of mentha. Farmacia. 2013;61:262–267. [Google Scholar]
- 20.Taamalli A., Arráez-Román D., Abaza L., Iswaldi I., Fernandez-Gutierrez A., Zarrouk M., SeguraCarretero A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015;26:320–330. doi: 10.1002/pca.2566. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z., Tan B., Liu Y., Dunn J., Guerola P.M., Tortajada M., Cao Z., Ji P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules. 2019;24:2825. doi: 10.3390/molecules24152825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koşar M., Dorman H.J.D., Baser K.H.C., Hiltunen R. Screening of Free Radical Scavenging Compounds in Water Extracts ofMenthaSamples Using a Postcolumn Derivatization Method. J. Agric. Food Chem. 2004;52:5004–5010. doi: 10.1021/jf0496189. [DOI] [PubMed] [Google Scholar]
- 23.Bimakr M., Rahman R.A., Taip F.S., Ganjloo A., Salleh L.M., Selamat J., Hamid A., Zaidul I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011;89:67–72. doi: 10.1016/j.fbp.2010.03.002. [DOI] [Google Scholar]
- 24.Salin O., Törmäkangas L., Leinonen M., Saario E., Hagström M., Ketola R.A., Saikku P., Vuorela H., Vuorela P.M. Corn Mint (Mentha arvensis) Extract Diminishes Acute Chlamydia pneumoniae Infection in Vitro and in Vivo. J. Agric. Food Chem. 2011;59:12836–12842. doi: 10.1021/jf2032473. [DOI] [PubMed] [Google Scholar]
- 25.Fatiha B., Didier H., Naima G., Khodir M., Martin K., Léocadie K., Caroline S., Mohamed C., Pierre D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae) Ind. Crop. Prod. 2015;74:722–730. doi: 10.1016/j.indcrop.2015.04.038. [DOI] [Google Scholar]
- 26.Franz C., Novak J. Sources of Essential Oils. CRC Press/Taylor & Francis Group; Boca Raton, FL, USA: 2010. pp. 45–67. [Google Scholar]
- 27.Malingré T.M. Chemotaxonomic study of Mentha arvensis L. Pharm. week. 1971;106:165–171. [PubMed] [Google Scholar]
- 28.Maffei M., Codignola A. Photosynthesis, Photorespiration and Herbicide Effect on Terpene Production in Peppermint (Mentha piperitaL.) J. Essent. Oil Res. 1990;2:275–286. doi: 10.1080/10412905.1990.9697886. [DOI] [Google Scholar]
- 29.Sokovic M.D., Vukojevic J., Marin P.D., Brkic D.D., Vajs V., van Griensven L.J. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules. 2009;14:238–249. doi: 10.3390/molecules14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghaddam M., Pourbaige M., Tabar H.K., Farhadi N., Hosseini S.M.A. Composition and Antifungal Activity of Peppermint Mentha piperita Essential Oil from Iran. J. of Ess. Oil Bear. Pla. 2013;16:506–512. doi: 10.1080/0972060X.2013.813265. [DOI] [Google Scholar]
- 31.Andro A.-R., Boz I., Zamfirache M.-M., Burzo I. Chemical composition of essential oils from Mentha aquatica L. at different moments of the ontogenetic cycle. J. Med. Plant Res. 2013;7:470–473. [Google Scholar]
- 32.Dai D.N., Thang T.D., Emmanuel E.E., Abdulkabir O.O., Ogunwande I.A. Study on essential oil of Mentha aquatica L. from Vietnam. Am. J. Essent. Oil. 2015;2:12–16. [Google Scholar]
- 33.Getahun Z., Asres K., Mazumder A., Bucar F. Essential Oil Composition, Antibacterial and Antioxidant Activities of Mentha aquatica Growing in Ethiopia. Ethiop. Pharm. J. 2008;26:9–16. doi: 10.4314/epj.v26i1.35128. [DOI] [Google Scholar]
- 34.Morteza-Semnani K., Saeedi M., Akbarzadeh M. The Essential Oil Composition of Mentha aquatica L. J. Essent. Oil Bear. Plants. 2006;9:283–286. doi: 10.1080/0972060X.2006.10643505. [DOI] [Google Scholar]
- 35.Fancello F., Zara S., Petretto G.L., Chessa M., Addis R., Rourke J.P., Pintore G. Essential oils from three species of Mentha harvested in Sardinia: Chemical characterization and evaluation of their biological activity. Int. J. Food Prop. 2017;20:1–11. doi: 10.1080/10942912.2017.1354020. [DOI] [Google Scholar]
- 36.Pereira O.R., Macias R.I.R., Domingues M.R.M., Marin J.J.G., Cardoso S.M. Hepatoprotection of Mentha aquatica L., Lavandula dentata L. and Leonurus cardiaca L. Antioxidants. 2019;8:267. doi: 10.3390/antiox8080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M., Xu L., Li Z., Qian S., Qin M. Chemical constituents from Mentha canadensis. Biochem. Syst. Ecol. 2013;49:144–147. doi: 10.1016/j.bse.2013.03.021. [DOI] [Google Scholar]
- 38.Shelepova O.V., Voronkova T.V., Kondrat’eva V.V., Semenova M.V., Bidyukova G.F., Olehnovich L.S. Phenotypic and Phytochemical Differences between Mentha arvensis L. and Mentha canadiensis L. Biol. Bull. 2014;41:19–23. doi: 10.1134/S1062359014010099. [DOI] [PubMed] [Google Scholar]
- 39.Thawkar B.S., Jawarkar A.G., Kalamkar P.V., Pawar K.P., Kale M.K. Phytochemical and pharmacological review of Mentha arvensis. Int. J. Green Pharm. 2016;10:71–76. [Google Scholar]
- 40.Abdel-Hameed E.-S.S., Salman M.S., Fadl M.A., Elkhateeb A., Hassan M.M. Chemical Composition and Biological Activity of Mentha longifolia L. Essential Oil Growing in Taif, KSA Extracted by Hydrodistillation, Solvent Free Microwave and Microwave Hydrodistillation. J. Essent. Oil Bear. Plants. 2018;21:1–14. doi: 10.1080/0972060X.2018.1454343. [DOI] [Google Scholar]
- 41.Teymouri M., Alizadeh A. Chemical composition and antimicrobial activity of the essential oil of Mentha mozaffarianii Jamzad growing wild and cultivated in Iran. Nat. Prod. Res. 2017;32:1320–1323. doi: 10.1080/14786419.2017.1342082. [DOI] [PubMed] [Google Scholar]
- 42.Golparvar A.R., Hadipanah A., Gheisari M.M., Salehi S., Khaliliazar R., Ghasemi O. Comparative analysis of chemical composition of Mentha longifolia (L.) Huds. J Herb Med. 2017;7:235–241. [Google Scholar]
- 43.Daneshbakhsh D., Asgarpanah J., Najafizadeh P., Rastegar T., Mousavi Z. Safety Assessment of Mentha mozaffarianii Essential Oil: Acute and Repeated Toxicity Studies. Iran. J. Med. Sci. 2018;43:479–486. [PMC free article] [PubMed] [Google Scholar]
- 44.Tavakkoli-Khaledi S., Asgarpanah J. Essential Oil Chemical Composition of Mentha mozaffarianii Jamzad Seeds. J. Mex. Chem. Soc. 2017;60:19–22. doi: 10.29356/jmcs.v60i1.66. [DOI] [Google Scholar]
- 45.Alexa E., Danciu C., Radulov I., Obistioiu D., Sumalan R.M., Morar A., Dehelean C.A. Phytochemical Screening and Biological Activity of Mentha × piperita L. and Lavandula angustifolia Mill. Extracts. Anal. Cell. Pathol. 2018:2678924. doi: 10.1155/2018/2678924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goudjil M.B., Ladjel S., Ben S.E., Zighmi S., Hamada D. Chemical Composition, Antibacterial and Antioxidant Activities of the Essential Oil Extracted from the Mentha piperita of Southern Algeria. Res. J. Phytochem. 2015;9:79–87. doi: 10.3923/rjphyto.2015.79.87. [DOI] [Google Scholar]
- 47.Marwa C., Fikri-Benbrahim K., Ou-Yahia D., Farah A. African peppermint (Mentha piperita) from Morocco: Chemical composition and antimicrobial properties of essential oil. J. Adv. Pharm. Technol. Res. 2017;8:86–90. doi: 10.4103/japtr.JAPTR_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satmi F.R.S., Hossain M.A. In vitro antimicrobial potential of crude extracts and chemical compositions of essential oils of leaves of Mentha piperita L native to the Sultanate of Oman. Pac. Sci. Rev. A: Nat. Sci. Eng. 2016;18:103–106. doi: 10.1016/j.psra.2016.09.005. [DOI] [Google Scholar]
- 49.Tsai M., Wu C., Lin T., Lin W., Huang Y., Yang C. Chemical Composition and Biological Properties of Essential Oils of Two Mint Species. Trop. J. Pharm. Res. 2013;12:577–582. doi: 10.4314/tjpr.v12i4.20. [DOI] [Google Scholar]
- 50.Politeo O., Bektašević M., Carev I., Jurin M., Roje M. Cover Picture: Phytochemical Composition, Antioxidant Potential and Cholinesterase Inhibition Potential of Extracts from Mentha pulegium L. (C&B 12/2018) Chem. Biodivers. 2018;15:e1800624. doi: 10.1002/cbdv.201800624. [DOI] [PubMed] [Google Scholar]
- 51.Rahmani F., Rezaeian-Doloei R., Alimoradi L. Evaluation of Phytochemical Composition of Mentha pulegium L. Essential Oil and Its Antibacterial Activity against Several Pathogenic Bacteria. Iran. J. Med. Microbiol. 2018;11:167–177. [Google Scholar]
- 52.Siham F., Rachid B., Read A.-Z.M. Chemical Composition and Antioxidant Effect of Mentha rotundifolia Extracts. Pharmacogn. J. 2019;11:521–526. doi: 10.5530/pj.2019.11.83. [DOI] [Google Scholar]
- 53.Derwich E., Benziane Z., Boukir A., Benaabidate L. GC-MS Analysis of the Leaf Essential Oil of Mentha rotundifolia, a Traditional Herbal Medicine in Morocco. Chem. Bul. “Politehnica” Univ. Timisoara. 2009;54:85–88. [Google Scholar]
- 54.Riahi L., Elferchichi M., Ghazghazi H., Jebali J., Ziadi S., Aouadhi C., Chograni H., Zaouali Y., Zoghlami N., Mliki A. Phytochemistry, antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L. in Tunisia. Ind. Crop. Prod. 2013;49:883–889. doi: 10.1016/j.indcrop.2013.06.032. [DOI] [Google Scholar]
- 55.Bahadori M.B., Zengin G., Bahadori S., Dinparast L., Movahhedin N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.) Int. J. Food Prop. 2018;21:183–193. doi: 10.1080/10942912.2018.1440238. [DOI] [Google Scholar]
- 56.Brada M., Bezzina M., Marlier M., Lognay G.C. Chemical Composition of the Leaf Oil of Mentha rotundifolia (L.) from Algeria. J. Essent. Oil Res. 2006;18:663–665. doi: 10.1080/10412905.2006.9699198. [DOI] [Google Scholar]
- 57.Yahia I.B.H., Jaouadi R., Trimech R., Boussaid M., Zaouali Y. Variation of chemical composition and antioxidant activity of essential oils of Mentha x rotundifolia (L.) Huds. (Lamiaceae) collected from different bioclimatic areas of Tunisia. Biochem. Syst. Ecol. 2019;84:8–16. doi: 10.1016/j.bse.2019.03.001. [DOI] [Google Scholar]
- 58.Sytar O., Hemmerich I., Zivcak M., Rauh C., Brestic M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018;25:631–641. doi: 10.1016/j.sjbs.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bardaweel S.K., Bakchiche B., Alsalamat H.A., Rezzoug M., Gherib A., Flamini G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement. Altern. Med. 2018;18:1–7. doi: 10.1186/s12906-018-2274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bayan Y., Küsek M., Ahi Evran University Kahramanmaras Sutcu Imam University Chemical Composition and Antifungal and Antibacterial Activity of Mentha spicata L. Volatile Oil. Cienc. Investig. Agrar. 2018;45:64–69. doi: 10.7764/rcia.v45i1.1897. [DOI] [Google Scholar]
- 61.Sevindik E., Yamaner Ç., Kurtoğlu C., Tin B. Chemical Composition of Mentha spicata L. subsp. tomentosa and M. pulegium L., and their Antimicrobial Activity on Strong Pathogen Microorganisms. Not. Sci. Biol. 2017;9:73–76. doi: 10.15835/nsb919923. [DOI] [Google Scholar]
- 62.Bouyahya A., Belmehdi O., Abrini J., Dakka N., Bakri Y. Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities. Asian Pac. J. Trop. Med. 2019;12 doi: 10.4103/1995-7645.254937. [DOI] [Google Scholar]
- 63.Božović M., Pirolli A., Ragno R. Mentha suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry. Molecules. 2015;20:8605–8633. doi: 10.3390/molecules20058605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rita I., Pereira C., Barros L., Santos-Buelga C., Ferreira I.C.F.R. Mentha spicata L. infusions as sources of antioxidant phenolic compounds: Emerging reserve lots with special harvest requirements. Food Funct. 2016;7:4188–4192. doi: 10.1039/C6FO00841K. [DOI] [PubMed] [Google Scholar]
- 65.Hussain A.I., Anwar F., Shahid M., Ashraf M., Przybylski R. Chemical Composition, and Antioxidant and Antimicrobial Activities of Essential Oil of Spearmint (Mentha spicata L.) From Pakistan. J. Essent. Oil Res. 2010;22:78–84. doi: 10.1080/10412905.2010.9700269. [DOI] [Google Scholar]
- 66.Al-Okbi S.Y., Fadel H.H., Mohamed D.A. Phytochemical constituents, antioxidant and anticancer activity of Mentha citrata and Mentha longifolia. Res. J. Pharm. Biol. Chem. 2015;6:739–751. [Google Scholar]
- 67.Shahbazi Y. Chemical Composition and In Vitro Antibacterial Activity of Mentha spicata Essential Oil against Common Food-Borne Pathogenic Bacteria. J. Pathog. 2015;2015:1–5. doi: 10.1155/2015/916305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barros A.D.S., De Morais S.M., Ferreira P.A.T., Vieira Í.G.P., Craveiro A.A., Fontenelle R.O.D.S., De Menezes J.E.S.A., Da Silva F.W.F., De Sousa H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crop. Prod. 2015;76:557–564. doi: 10.1016/j.indcrop.2015.07.004. [DOI] [Google Scholar]
- 69.Li Y., Liu Y., Ma A., Bao Y., Wang M., Sun Z. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017;26:1675–1683. doi: 10.1007/s10068-017-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elansary H.O., Szopa A., Kubica P., Ekiert H., Klimek-Szczykutowicz M., El-Ansary D.O., Mahmoud E.A. Polyphenol Profile and Antimicrobial and Cytotoxic Activities of Natural Mentha × piperita and Mentha longifolia Populations in Northern Saudi Arabia. Processes. 2020;8:479. doi: 10.3390/pr8040479. [DOI] [Google Scholar]
- 71.Yassin M.T., Mostafa A.A., Al-Askar A.A. Anticandidal and anti-carcinogenic activities of Mentha longifolia (Wild Mint) extracts in vitro. J. King Saud Univ.-Sci. 2020;32:2046–2052. doi: 10.1016/j.jksus.2020.02.008. [DOI] [Google Scholar]
- 72.Kee L.A., Shori A.B., Baba A.S. Bioactivity and health effects of Mentha spicata. Integr. Food, Nutr. Metab. 2017;5:1–2. doi: 10.15761/ifnm.1000203. [DOI] [Google Scholar]
- 73.Liu B., Fan L., Balakrishna S., Sui A., Morris J.B., Jordt S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154:2169–2177. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uritu C.M., Mihai C.T., Stanciu G.-D., Dodi G., Alexa-Stratulat T., Luca A., Leon-Constantin M.-M., Stefanescu R., Bild V., Melnic S., et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018;2018:1–44. doi: 10.1155/2018/7801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karashima Y., Damann N., Prenen J., Talavera K., Segal A., Voets T., Nilius B. Bimodal Action of Menthol on the Transient Receptor Potential Channel TRPA1. J. Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahboubi M. Mentha spicata L. essential oil, phytochemistry and its effectiveness in flatulence. J. Tradit. Complement. Med. 2018:1–7. doi: 10.1016/j.jtcme.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amato A., Liotta R., Mulè F. Effects of menthol on circular smooth muscle of human colon: Analysis of the mechanism of action. Eur. J. Pharmacol. 2014;740:295–301. doi: 10.1016/j.ejphar.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 78.Grigoleit H.-G., Grigoleit P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine. 2005;12:612–616. doi: 10.1016/j.phymed.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Brabalho S.M., Machado F.M.V.F., Oshiiwa M., Abreu M., Guiger E.L., Tomazela P., Goulart R.A. Investiga-tion of the effects of peppermint (Mentha piperita) on the biochemical and anthropometric profile of university students. Ciência Tecnol. Alime. 2011;31:584–588. doi: 10.1590/S0101-20612011000300006. [DOI] [Google Scholar]
- 80.Ceylan R., Zengin G., Uysal S., Ilhan V., Aktumsek A., Kandemir A., Anwar F. GC-MS analysis and in vitro antioxidant and enzyme inhibitory activities of essential oil from aerial parts of endemic Thymus spathulifolius Hausskn. et Velen. J. Enzym. Inhib. Med. Chem. 2015;31:983–990. doi: 10.3109/14756366.2015.1077822. [DOI] [PubMed] [Google Scholar]
- 81.Anwar F., Alkharfy K.M., Najeeb-ur-Rehman, Adam E.H.K., Gilani A.-U.-H. Chemo-geographical variations in the com-position of volatiles and the biological attributes of Mentha longifolia (L.) essential oils from Saudi Arabia. Int. J. Pharmacol. 2017;13:408–424. doi: 10.3923/ijp.2017.408.424. [DOI] [Google Scholar]
- 82.Nickavar B., Alinaghi A., Kamalinejad M. Evaluation of the Antioxidant Properties of Five Mentha Species. Iran. J. Pharm. Sci. 2008;7:203–209. [Google Scholar]
- 83.Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 84.Rice-Evans C.A., Miller N.J., Paganga G. Antioxidant properties of phenolic compounas. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- 85.Becker E.M., Nissen L.R., Skibsted L.H. Antioxidant evaluation protocols: Food quality or health effects. Eur. Food Res. Technol. 2004;219:561–571. doi: 10.1007/s00217-004-1012-4. [DOI] [Google Scholar]
- 86.Dorman H.J.D., Koşar M., Kahlos K., Holm A.Y., Hiltunen R. Antioxidant Properties and Composition of Aqueous Extracts fromMenthaSpecies, Hybrids, Varieties, and Cultivars. J. Agric. Food Chem. 2003;51:4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- 87.Benabdallah A., Rahmoune C., Boumendjel M., Aissi O., Messaoud C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. Biomed. 2016;6:760–766. doi: 10.1016/j.apjtb.2016.06.016. [DOI] [Google Scholar]
- 88.Kumar A., Chattopadhyay S. DNA damage protecting activity and antioxidant potential of pudina extract. Food Chem. 2007;100:1377–1384. doi: 10.1016/j.foodchem.2005.12.015. [DOI] [Google Scholar]
- 89.Džamić A.M., Soković M.D., Ristić M.S., Novaković M., Grujić-Jovanović S., Tešević V., Marin P.D. Antifungal and antioxidant activity of Mentha longifolia (L.) Hudson (Lamiaceae) essential oil. Botanica serbica. 2010;34:57–61. [Google Scholar]
- 90.Niksic H., Bešović E.K., Makarević E., Duric K. Chemical composition, antimicrobial and antioxidant properties of Mentha longifolia (L.) Huds. essential oil. J. Heal. Sci. 2012;2:192–200. doi: 10.17532/jhsci.2012.38. [DOI] [Google Scholar]
- 91.Stanisavljevic D., Stojicevic S., Djordjevic S., Zlatkovic B., Velickovic D., Karabegovic I., Lazic M. Antioxidant activity, the content of total phenols and flavonoids in the ethanol extracts of Mentha longifolia (L.) Hudson dried by the use of different techniques. Chem. Ind. Chem. Eng. Q. 2012;18:411–420. doi: 10.2298/CICEQ110919017S. [DOI] [Google Scholar]
- 92.Saba I., Anwar F. Effect of Harvesting Regions on Physico-chemical and Biological Attributes of Supercritical Fluid-Extracted Spearmint (Mentha spicata L.) Leaves Essential Oil. J. Essent. Oil Bear. Plants. 2018;21:400–419. doi: 10.1080/0972060X.2018.1458658. [DOI] [Google Scholar]
- 93.Ed-Dra A., Filai F.R., Bou-Idra M., Zekkori B., Bouymajane A., Moukrad N., Benhallam F., Bentayeb A. Application of mentha suaveolens essential oil as an antimicrobial agent in fresh turkey sausages. J. Appl Biol & Biotechnol. 2018;6:7–12. [Google Scholar]
- 94.Dhifi W., Jelali N., Mnif W., Litaiem M., Hamdi N. Chemical composition of the essential oil of Mentha spicata L. from Tunisia and its biological activities. J. Food Biochem. 2013;37:362–368. doi: 10.1111/j.1745-4514.2012.00656.x. [DOI] [Google Scholar]
- 95.Sokovi´c M.D., Glamoˇclija J., Marin P.D., Brki´c D., van Griensven L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh R., Shushni M.A., Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015;8:322–328. doi: 10.1016/j.arabjc.2011.01.019. [DOI] [Google Scholar]
- 97.Nathan C. Antibiotics at the crossroads. Nat. Cell Biol. 2004;431:899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 98.Ahameethunisa A.R., Hopper W. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopath-ogenic bacteria. BMC Complement. Altern Med. 2010;10:6. doi: 10.1186/1472-6882-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muhammad G., Hussain M.A., Anwar F., Ashraf M., Gilani A.H. Alhagi: A plant genus rich in bioactives for pharma-ceuticals. Phytother. Res. 2015;29:1–13. doi: 10.1002/ptr.5222. [DOI] [PubMed] [Google Scholar]
- 100.Irshad S., Butt M., Younus H. In-vitro antibacterial activity of two medicinal plants neem (Azadirachta indica) and pep-permint. Int Res. J. Pharma. 2011;1:9–14. [Google Scholar]
- 101.Mahady G.B., Pendland S.L., Stoia A., Hamill F.A., Fabricant D., Dietz B.M., Chadwick L.R. In Vitro susceptibility ofHelicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytotherapy Res. 2005;19:988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- 102.Dixit P. A comparative screening of antibacterial activity of Anisomeles indica and Mentha piperita against Human patho-genic micro-organisms. Ind J. Fund. Appl. Life Sci. 2013;3:85–88. [Google Scholar]
- 103.Dorman H.J.D., Deans S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 104.O’Bryan C.A., Pendleton S.J., Crandall P.G., Ricke S.C. Potential of Plant Essential Oils and Their Components in Animal Agriculture–in vitro Studies on Antibacterial Mode of Action. Front. Veter- Sci. 2015;2 doi: 10.3389/fvets.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang X.-N., Khan I., Kang S.C. Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub. Asian Pac. J. Trop. Med. 2015;8:694–700. doi: 10.1016/j.apjtm.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 106.Alves M.J., Ferreira I.C.F.R., Froufe H.J.C., Abreu R.M.V., Martins A., Pintado M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013;115:346–357. doi: 10.1111/jam.12196. [DOI] [PubMed] [Google Scholar]
- 107.Puupponen-Pimia R., Nohynek L., Meier C., Ka¨hko¨nen M., Heinonen M., Hopia A., Oksman-Caldentey K.-M. Anti-microbial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001;90:494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 108.Lucchini J., Corre J., Cremieux A. Antibacterial Activity of Phenolic Compound and Aromatic Alcohols. Res. Microbiol. 1990;141:499–510. doi: 10.1016/0923-2508(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 109.Stanisavljević D., Đorđević S., Milenković M., Lazić M., Veličković D., Ranđelović N., Zlatković B. Antimicrobial and Antioxidant Activity of the Essential Oils Obtained from Mentha longifolia L. Hudson, Dried by Three Different Techniques. Rec. Nat. Prod. 2014;8:61–65. [Google Scholar]
- 110.Gulluce M., Sahin F., Sokmen M., Ozer H., Daferera D., Sokmen A., Polissiou M., Adiguzel A., Ozkan H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007;103:1449–1456. doi: 10.1016/j.foodchem.2006.10.061. [DOI] [Google Scholar]
- 111.Samber N., Khan A., Varma A., Manzoor N. Synergistic anti-candidal activity and mode of action of Mentha piperita essential oil and its major components. Pharm. Biol. 2015;53:1496–1504. doi: 10.3109/13880209.2014.989623. [DOI] [PubMed] [Google Scholar]
- 112.Zhang L., Xu S.-G., Liang W., Mei J., Di Y.-Y., Lan H.-H., Yang Y., Wang W.-W., Luo Y.-Y. Antibacterial Activity and Mode of Action of Mentha arvensis Ethanol Extract against Multidrug-Resistant Acinetobacter baumannii. Trop. J. Pharm. Res. 2015;14:2099. doi: 10.4314/tjpr.v14i11.21. [DOI] [Google Scholar]
- 113.Antolak H., Czyżowska A., Kręgiel D. Activity of Mentha piperita L. Ethanol Extract against Acetic Acid Bacteria Asaia spp. Foods. 2018;7 doi: 10.3390/foods7100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Husain F.M., Ahmad I., Khan M.S., Ahmad E., Tahseen Q., Khan M.S., Alshabib N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015;6:420. doi: 10.3389/fmicb.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sujana P., Sridhar T.M., Josthna P., Naidu C.V. Antibacterial Activity and Phytochemical Analysis of Mentha piperita L. (Peppermint)—An Important Multipurpose Medicinal Plant. Am. J. Plant. Sci. 2013;4:77–83. doi: 10.4236/ajps.2013.41012. [DOI] [Google Scholar]
- 116.Laggoune S., Öztürk M., Erol E., Duru M.E., Abaza I., Kabouche A., Kabouche Z. Chemical composition, antioxidant and antibacterial activities of the essential oil of Mentha spicata L. from Algeria. J. Mater. Environ. Sci. 2016;7:4205–4213. [Google Scholar]
- 117.Bupesh G., Amutha C., Nandagopal S., Ganeshkumar A., Sureshkumar P., Murali K.S. Antibacterial activity of Mentha piperita L. (peppermint) from leaf extracts-A medicinal plant. Acta Agric. Slov. 2007;89:73–79. doi: 10.2478/v10014-007-0009-7. [DOI] [Google Scholar]
- 118.Golestan L., Seyedyousefi L., Kaboosi H., Safari H. Effect ofMentha spicataL. andMentha aquaticaL. essential oils on the microbiological properties of fermented dairy product, kashk. Int. J. Food Sci. Technol. 2016;51:581–587. doi: 10.1111/ijfs.13014. [DOI] [Google Scholar]
- 119.Zaidi S., Dahiya P. In vitro antimicrobial activity, phytochemical analysis and total phenolic content of essential oil from Mentha spicata and Mentha piperita. Int. Food Res. J. 2015;22:2440–2445. [Google Scholar]
- 120.Portillo A., Vila R., Freixa B., Adzet T., Cañigueral S. Antifungal activity of Paraguayan plants used in traditional medicine. J. Ethnopharmacol. 2001;76:93–98. doi: 10.1016/S0378-8741(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 121.Fortes T.O., Alviano D.S., Tupinambá G., Padrón T.S., Antoniolli Â.R., Alviano C.S., Seldin L. Production of an antimi-crobial substance against Cryptococcus neoformans by Paenibacillus brasilensis Sa3 isolated from the rhizosphere of Kalanchoe brasiliensis. Microbiol. Res. 2008;163:200–207. doi: 10.1016/j.micres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 122.Nosrati S., Esmailzadeh-Hosseini S., Sarpeleh A., Soflaei Shahrbabak M., Soflaei Shahrbabak Y. Antifungal activity of spearmint (Mentha spicata L.) essential oil on Fusarium oxysporum f. sp. radices cucumerinum the causal agent of stem and crown rot of greenhouse cucumber in Yazd, Iran; Proceedings of the International Conference on Environmental and Agricultural Engineering; Chengdu, China. 5 May 2011; pp. 52–56. [Google Scholar]
- 123.Hussain A.I., Anwar F., Nigam P.S., Ashraf M., Gilani A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010;90:1827–1836. doi: 10.1002/jsfa.4021. [DOI] [PubMed] [Google Scholar]
- 124.Mohamed A.E.-A.A.R., Rashed A.-O.M., Mahmoud M., Shehata S.M., Abdelazim N.S. Chitosan Nanoparticles as a Carrier for Mentha longifolia Extract: Synthesis, Characterization and Antifungal Activity. Curr. Sci. 2018;114:2116–2122. doi: 10.18520/cs/v114/i10/2116-2122. [DOI] [Google Scholar]
- 125.Moghtader M. In vitro antifungal effects of the essential oil of Mentha piperita L. and its comparison with synthetic menthol on Aspergillus niger. Afr. J. Plant Sci. 2013;7:521–527. doi: 10.5897/AJPS2013.1027. [DOI] [Google Scholar]
- 126.Mimica-Dukić N., Božin B., Soković M., Mihajlović B., Matavulj M. Antimicrobial and Antioxidant Activities of ThreeMenthaSpecies Essential Oils. Planta Medica. 2003;69:413–419. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 127.Tullio V., Roana J., Scalas D., Mandras N. Evaluation of the Antifungal Activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles. Molecules. 2019;24 doi: 10.3390/molecules24173148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ling H., Chen B., Kang A., Lee J.-M., Chang M.W. Transcriptome response to alkane biofuels in Saccharomyces cerevisiae: Identification of efflux pumps involved in alkane tolerance. Biotechnol. Biofuels. 2013;6 doi: 10.1186/1754-6834-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schenk M., Raffellini S., Guerrero S., Blanco G.A., Alzamora S.M. Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae by UV-C light: Study of cell injury by flow cytometry. LWT. 2011;44:191–198. doi: 10.1016/j.lwt.2010.05.012. [DOI] [Google Scholar]
- 130.Ferreira P., Cardoso T., Ferreira F., Fernandes-Ferreira M., Piper P., Sousa M.J. Mentha piperita essential oil induces apoptosis in yeast associated with both cytosolic and mitochondrial ROS-mediated damage. FEMS Yeast Res. 2014;14:1006–1014. doi: 10.1111/1567-1364.12189. [DOI] [PubMed] [Google Scholar]
- 131.Almeida E.T.D.C., De Souza G.T., Guedes J.P.D.S., Barbosa I.M., De Sousa C.P., Castellano L.R.C., Magnani M., De Souza E.L. Mentha piperita L. essential oil inactivates spoilage yeasts in fruit juices through the perturbation of different physiological functions in yeast cells. Food Microbiol. 2019;82:20–29. doi: 10.1016/j.fm.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 132.Melzer J., Rösch W., Reichling J., Brignoli R., Saller R. Meta-analysis: Phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast) Aliment. Pharmacol. Ther. 2004;20:1279–1287. doi: 10.1111/j.1365-2036.2004.02275.x. [DOI] [PubMed] [Google Scholar]
- 133.Orhan İ.E., ÖZÇELİK B., Kartal M., Kan Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012;36:239–246. [Google Scholar]
- 134.McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother. Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 135.Yamasaki K., Nakano M., Kawahata T., Mori H., Otake T., Ueda N., Oishi I., Inami R., Yamane M., Nakamura M., et al. Anti-HIV-1 Activity of Herbs in Labiatae. Biol. Pharm. Bull. 1998;21:829–833. doi: 10.1248/bpb.21.829. [DOI] [PubMed] [Google Scholar]
- 136.Amzazi S., Ghoulami S., Bakri Y., Idrissi A.I., Fkih-Tétouani S., Benjouad A. Human immunodeficiency virus type 1 inhibitory activity of Mentha longifolia. Therapie. 2003;58:531–534. doi: 10.2515/therapie:2003086. [DOI] [PubMed] [Google Scholar]
- 137.Schuhmacher A., Reichling J., Schnitzler P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine. 2003;10:504–510. doi: 10.1078/094471103322331467. [DOI] [PubMed] [Google Scholar]
- 138.Clurman B.E., Roberts J.M. Cell Cycle and Cancer. J. Natl. Cancer Inst. 1995;87:1499–1501. doi: 10.1093/jnci/87.20.1499. [DOI] [PubMed] [Google Scholar]
- 139.World Health Organization Cancer report. [(accessed on 12 December 2020)];Dec 12; Available online: https://www.who.int/health-topics/cancer.
- 140.Doll R., Peto R. The Quantitative of Cancer Causes of Cancer: Estimates of Avoidable Risks in the United States Today. J. Natl. Cancer Inst. 1981;66:1191–1308. doi: 10.1093/jnci/66.6.1192. [DOI] [PubMed] [Google Scholar]
- 141.Surh Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 142.Ochwang’I D.O., Kimwele C.N., Oduma J.A., Gathumbi P.K., Mbaria J.M., Kiama S.G. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014;151:1040–1055. doi: 10.1016/j.jep.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 143.Greenwell M., Rahman P. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015;6:4103–4112. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Anwar F., Muhammad G., Hussain M.A., Zengin G., Alkharfy K.M., Ashraf M., Gilani A.-H. Capparis spinosa L.: A Plant with High Potential for Development of Functional Foods and Nutraceuticals/Pharmaceuticals. Int. J. Pharmacol. 2016;12:201–219. doi: 10.3923/ijp.2016.201.219. [DOI] [Google Scholar]
- 145.Gull T., Anwar F., Sultana B., Alcayde M.A.C., Nouman W. Capparis species: A potential source of bioactives and high-value components: A review. Ind. Crop. Prod. 2015;67:81–96. doi: 10.1016/j.indcrop.2014.12.059. [DOI] [Google Scholar]
- 146.Sivaraj R., Rahman P.K.S.M., Rajiv P., Narendhran S., Venckatesh R. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014;129:255–258. doi: 10.1016/j.saa.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 147.Fouche G., Cragg G., Pillay P., Kolesnikova N., Maharaj V., Senabe J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008;119:455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 148.Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manson M.M. Cancer prevention–the potential for diet to modulate molecular signalling. Trends Mol. Med. 2003;9:11–18. doi: 10.1016/S1471-4914(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 150.Rahimifard N., Hajimehdipoor H., Hedayati M., Bagheri O., Pishehvar H., Ajani Y. Cytotoxic Effects of Essential Oils and Extracts of some Mentha species on Vero, Hela and Hep2 Cell Lines. J. Med. Plants. 2010;9:88–92. [Google Scholar]
- 151.Fatemeh H., Vida H., Hajighasemi F., Hashemi V. Down regulation of matrix metalloproteinases by spearmint extract in Wehi-164 cells. J. Med. Plants Res. 2012;6:5222–5227. doi: 10.5897/JMPR12.031. [DOI] [Google Scholar]
- 152.Patti F., Palmioli A., Vitalini S., Bertazza L., Redaelli M., Zorzan M., Rubin B., Mian C., Bertolini C., Iacobone M., et al. Anticancer Effects of Wild Mountain Mentha longifolia Extract in Adrenocortical Tumor Cell Models. Front. Pharmacol. 2020;10:1647. doi: 10.3389/fphar.2019.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ohara A., Matsuhisa T. Anti-Tumor Promoting Activities of Edible Plants against Okadaic Acid. Food Sci. Technol. Res. 2002;8:158–161. doi: 10.3136/fstr.8.158. [DOI] [Google Scholar]
- 154.Karakas F.P., Yildirim A., Turker A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities. Turkish J. Biol. 2012;36:641–652. [Google Scholar]
- 155.Khan R.A., Khan N.A., Khan F.U., Ahmed M., Shah A.S., Khan M.R., Shah M.S. Phytochemical, antioxidant and cytotoxic activities of Periploca aphyla and Mentha longifolia, selected medicinal plants of District Bannu, Pakistan. Afr. J. Pharm. Pharmacol. 2012;6:3130–3135. doi: 10.5897/AJPP12.445. [DOI] [Google Scholar]
- 156.Al-Ali K., Abdelrazik M., Alghaithy A., Diab A., El-Beshbishy H., Baghdadi H. Antimutagenic and Anticancer Activity of Al Madinah Alhasawy Mint (Mentha longifolia) Leaves Extract. Pak. J. Biol. Sci. 2014;17:1231–1236. doi: 10.3923/pjbs.2014.1231.1236. [DOI] [PubMed] [Google Scholar]
- 157.Shirazi F.H., Ahmadi N., Kamalinejad M. Evaluation of northern Iran Mentha pulegium L. cytotoxicity. DARU J. Pharm. Sci. 2004;212:106–110. [Google Scholar]
- 158.Jain D., Pathak N., Khan S., Raghuram G.V., Bhargava A., Samarth R., Mishra P.K. Evaluation of Cytotoxicity and An-ticarcinogenic Potential of Mentha Leaf Extracts. Int. J. Toxicol. 2011;30:225–236. doi: 10.1177/1091581810390527. [DOI] [PubMed] [Google Scholar]
- 159.Kelkawi A.H.A., Kajani A.A., Bordbar A.-K. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnology. 2017;11:370–376. doi: 10.1049/iet-nbt.2016.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Banerjee P.P., Bandyopadhyay A., Harsha S.N., Policegoudra R.S., Bhattacharya S., Karak N., Chattopadhyay A. Mentha arvensis (Linn.)-mediated green silver nanoparticles trigger caspase 9-dependent cell death in MCF7 and MDA-MB-231 cells. Breast Cancer: Targets Ther. 2017;9:265–278. doi: 10.2147/BCTT.S130952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Faridi U., Dhawan S.S., Pal S., Gupta S., Shukla A.K., Darokar M.P., Sharma A., Shasany A.K. Repurposing L-Menthol for Systems Medicine and Cancer Therapeutics? L-Menthol Induces Apoptosis through Caspase 10 and by Suppressing HSP90. OMICS: A J. Integr. Biol. 2016;20:53–64. doi: 10.1089/omi.2015.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Belanger J.T. Perillyl alcohol: Applications in oncology. Altern. Med. Rev.: A J. Clin. Ther. 1998;3:448–457. [PubMed] [Google Scholar]
- 163.Reddy B.S., Wang C.X., Samaha H., Lubet R., E Steele V., Kelloff G.J., Rao C.V. Chemoprevention of colon carcinogenesis by dietary perillyl alcohol. Cancer Res. 1997;57:420–425. [PubMed] [Google Scholar]
- 164.Clark S.S., Zhong L., Filiault D., Perman S., Ren Z., Gould M., Yang X. Anti-leukemia effect of perillyl alcohol in Bcr/Abl-transformed cells indirectly inhibits signaling through Mek in a Ras- and Raf-independent fashion. Clin. Cancer Res. 2003;9:4494–4504. [PubMed] [Google Scholar]
- 165.Sahin M., Perman S., Jenkins G., Clark S. Perillyl alcohol selectively induces G0/G1 arrest and apoptosis in Bcr/Abltransformed myeloid cell lines. Leukemia. 1999;13:1581–1591. doi: 10.1038/sj.leu.2401536. [DOI] [PubMed] [Google Scholar]
- 166.Stayrook K.R., McKinzie J.H., Burke Y.D., Crowell P.L. Induction of the apoptosis-promoting protein Bak by perillyl alcohol in pancreatic ductal adenocarcinoma relative to untransformed ductal epithelial cells. Carcinog. 1997;18:1655–1658. doi: 10.1093/carcin/18.8.1655. [DOI] [PubMed] [Google Scholar]
- 167.Barthelman M., Chen W., Gensler H.L., Huang C., Dong Z., Bowden G.T. Inhibitory effects of perillyl alcohol on UVB-induced murine skin cancer and AP-1 transactivation. Cancer Res. 1998;58:711–716. [PubMed] [Google Scholar]
- 168.Hassan H.A., Hafez H.S., Goda M.S. Mentha piperita as a pivotal neuro-protective agent against gamma irradiation induced DNA fragmentation and apoptosis. Cytotechnology. 2012;65:145–156. doi: 10.1007/s10616-012-9470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kumar A., Samarth R.M., Yasmeen S., Sharma A., Sugahara T., Terado T., Kimura H. Anticancer and radioprotective potentials of Mentha piperita. BioFactors. 2004;22:87–91. doi: 10.1002/biof.5520220117. [DOI] [PubMed] [Google Scholar]
- 170.Arima Y., Nishigori C., Takeuchi T., Oka S., Morimoto K., Utani A., Miyachi Y. 4-Nitroquinoline 1-Oxide Forms 8-Hydroxydeoxyguanosine in Human Fibroblasts through Reactive Oxygen Species. Toxicol. Sci. 2006;91:382–392. doi: 10.1093/toxsci/kfj161. [DOI] [PubMed] [Google Scholar]
- 171.Arumugam P., Ramesh A. Antigenotoxic and antioxidant potential of aqueous fraction of ethanol extract of Mentha spicata (L.) against 4-nitroquinoline-1-oxide–induced chromosome damage in mice. Drug Chem. Toxicol. 2009;32:411–416. doi: 10.1080/01480540903127316. [DOI] [PubMed] [Google Scholar]
- 172.Willits I., Price L., Parry A., Tilby M.J., Ford D., Cholerton S., Pearson A.D.J., Boddy A.V. Pharmacokinetics and metabolism of ifosfamide in relation to DNA damage assessed by the COMET assay in children with cancer. Br. J. Cancer. 2005;92:1626–1635. doi: 10.1038/sj.bjc.6602554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Furlanut M., Franceschi L. Pharmacology of Ifosfamide. Oncol. 2003;65:2–6. doi: 10.1159/000073350. [DOI] [PubMed] [Google Scholar]
- 174.Saleem M.A., Al-Attar M.S.M. Protective Effects Of Mentha Spicata Aqueous Extract Against Ifosfamide Induced Chromosomal Aberrations And Sperm Abnormalities In Male Albino Mice. Trends Biotechnol. Res. 2013;2:17–23. [Google Scholar]
- 175.Dhanasekaran S., Sangavi J., Ramya R., Nithya K. Mint Leaves Inhibits Tumour Angiogenesis via Suppression of Sonic Hedgehog Pathway by In vitro Analysis for Colorectal Cancer. Int. J. Eng. Techn. Res. 2014;2:131–134. [Google Scholar]
- 176.Sonawane H., Shinde A., Jadhav J. Evaluation of anti-angiogenic potential of Mentha arvensis Linn. Leaf extracts using chorioallantoic membrane assay. World J. Pharm. Res. 2016;5:677–689. [Google Scholar]
- 177.Chang C.-T., Soo W.-N., Chen Y.-H., Shyur L.-F. Essential Oil of Mentha aquatica var. Kenting Water Mint Suppresses Two-Stage Skin Carcinogenesis Accelerated by BRAF Inhibitor Vemurafenib. Molecules. 2019;24:2344. doi: 10.3390/molecules24122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 179.Lo¨ffek S., Schilling O., Franzke C.-W. Biological role of matrix metalloproteinases: A critical balance. Eur Res-Piratory J. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 180.Liu X., Zheng J., Zhou H. TLRs as pharmacological targets for plant-derived compounds in infectious and inflammatory diseases. Int. Immunopharmacol. 2011;11:1451–1456. doi: 10.1016/j.intimp.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 181.Gonulalan E.-M., Nemutlu E., Demirezer L.-O. A new perspective on evaluation of medicinal plant biological activities: The correlation between phytomics and matrix metalloproteinases activities of some medicinal plants. Saudi Pharm. J. 2019;27:446–452. doi: 10.1016/j.jsps.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Direito R., Rocha J., Lima A., Gonçalves M.M., Duarte M.P., Mateus V., Sousa C., Fernandes A., Pinto R., Ferreira R.B., et al. Reduction of Inflammation and Colon Injury by a Spearmint Phenolic Extract in Experimental Bowel Disease in Mice. Medicine. 2019;6:65. doi: 10.3390/medicines6020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Son D., Kim M., Woo H., Park D., Jung E. Anti-Thermal Skin Aging Activity of Aqueous Extracts Derived from Apple Mint (Mentha suaveolens Ehrh.) in Human Dermal Fibroblasts. Evidence-Based Compl. Altern. Med. 2018;1:1–7. doi: 10.1155/2018/4595982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nisticò P., Bissell M.J., Radisky D.C. Epithelial-Mesenchymal Transition: General Principles and Pathological Relevance with Special Emphasis on the Role of Matrix Metalloproteinases. Cold Spring Harbor Persp. Biol. 2012;4:a011908. doi: 10.1101/cshperspect.a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rygiel K.A., Robertson H., Marshall H.L., Pekalski M., Zhao L., Booth T.A., Jones D.E., Burt A.D., Kirby J.A. Epithelial–mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab. Invest. 2008;88:112–123. doi: 10.1038/labinvest.3700704. [DOI] [PubMed] [Google Scholar]
- 186.Scanlon C.S., Tubergen E.A.V., Inglehart R.C., D’Silva N.J. Biomarkers of Epithelial Mesenchymal Transition in Squamous Cell Carcinoma. J. Dental Res. 2012;92:114–121. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xu J., Lamouille S., Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ogaly H.A., Eltablawy N.A., Abd-Elsalam R.M. Antifibrogenic Influence of Mentha piperita L. Essential Oil against CCl4-Induced Liver Fibrosis in Rats. Oxid. Med. Cell. Long. 2018;2018:4039753. doi: 10.1155/2018/4039753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nakamura Y., Hasegawa Y., Shirota K., Suetome N., Nakamura T., Chomnawang M.T., Thirapanmethee K., Khuntayaporn P., Boonyaritthongchai P., Wongs-Aree C., et al. Differentiation-inducing effect of piperitenone oxide, a fragrant ingredient of spearmint (Mentha spicata), but not carvone and menthol, against human colon cancer cells. J. Funct. Foods. 2014;8C:62–67. doi: 10.1016/j.jff.2014.03.005. [DOI] [Google Scholar]
- 190.ClinicalTrials.gov Clinical Trials. [(accessed on 3 January 2020)]; Available online: https://clinicaltrials.gov.
- 191.Tayarani-Najaran Z., Talasaz-Firoozi E., Nasiri R., Jalali N., Hassanzadeh M. Antiemetic activity of volatile oil from Mentha spicata and Mentha × piperita in chemotherapy-induced nausea and vomiting. Canc. Med. Sci. 2013;7:290. doi: 10.3332/ecancer.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tavakoli Ardakani M., Ghassemi S., Mehdizadeh M., Mojab F., Salamzadeh J., Ghassemi S., Hajifathali A. Evaluating the effect of Matricaria recutita and Mentha piperita herbal mouthwash on management of oral mucositis in patients undergoing hematopoietic stem cell transplantation: A randomized, double blind, placebo controlled clinical trial. Compl. Ther. Med. 2016;29:29–34. doi: 10.1016/j.ctim.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 193.Amui Roknabad M., Sarafraz N. Comparison between the Effect of Supermint and Ibuprofen on Primary Dysmenorrheal: A Randomized Clinical Trial. Qom Univ Med. Sci J. 2011;5:37–41. [Google Scholar]
- 194.Nasiri A., Pakmehr M., Shahdadi H., Balouchi A., Sepehri Z., Ghalenov A.R. A Comparative Study of Dimethicone and Supermint Anti-flatulence Effects on Reducing Flatulence in Patients with Irritable Bowel Syndrome. Der. Pharm. Lett. 2015;7:432–436. [Google Scholar]
- 195.Akdoğan M., Tamer M.N., Cüre E., Cüre M.C., Köroğlu B.K., Delibaş N. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother. Res. 2007;21:444–447. doi: 10.1002/ptr.2074. [DOI] [PubMed] [Google Scholar]
- 196.Peixoto I.T.A., Furletti V.F., Anibal P.C., Duarte M.C.T., Höfling J.F. Potential pharmacological and toxicological basis of the essential oil from Mentha spp. Rev. Ciênc. Farm. Básica Apl. 2010;30:235–239. [Google Scholar]
- 197.Gürbüz P. An Overview about Adverse Hepatic Effects of the Plants Used in Turkey. Cerrahpaşa Med J. 2020;44:115–124. [Google Scholar]
- 198.Douros A., Bronder E., Andersohn F., Klimpel A., Kreutz R., Garbe E., Bolbrinker J. Herb-induced liver injury in the Berlin case-control surveillance study. Int J. Mol. Sci. 2016;17:114. doi: 10.3390/ijms17010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Balakrishnan A. Therapeutic uses of peppermint –A review. J. Pharm. Sci. Res. 2015;7:474–476. [Google Scholar]
- 200.Hawthorn M., Ferrante J., Luchowski E., Rutledge A., Wei X.Y., Triggle D.J. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Alim. Pharm. Ther. 1988;2:101–118. doi: 10.1111/j.1365-2036.1988.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 201.Amat-ur-Rasool H., Symes F., Tooth D., Schaffert L.-N., Elmorsy E., Ahmed M., Hasnain S., Carter W.G. Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants. Biomolecules. 2020;10:1556. doi: 10.3390/biom10111556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jarosz M., Taraszewska A. Risk factors for gastroesophageal reflux disease: The role of diet. Prz Gastroenterol. 2014;9:297. doi: 10.5114/pg.2014.46166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.DeVault K.R., Castell D.O. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am. J. Gastroenter. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 204.Zong L., Qu Y., Luo D.X., Zhu Z.Y., Zhang S., Su Z., Shan J.C., Gao X.P., Lu L.G. Preliminary experimental research on the mechanism of liver bile secretion stimulated by peppermint oil. J. Dig. Dis. 2011;12:295–301. doi: 10.1111/j.1751-2980.2011.00513.x. [DOI] [PubMed] [Google Scholar]
- 205.Sharma V., Hussain S., Gupta M., Saxena A.K. In vitro anticancer activity of extracts of Mentha spp. against human cancer cells. Indian J. Biochem. Biophys. 2014;51:416–419. [PubMed] [Google Scholar]
- 206.Alankar S. A review on peppermint oil. Asian J. Pharm. Clin. Res. 2009;2:27–33. [Google Scholar]
- 207.Dos Santos M., CE S.G. Menthol-induced asthma: A case report. J. Invest. Allerg. Clin. Immun. 2001;11:56–58. [PubMed] [Google Scholar]
- 208.Thorup I., Würtzen G., Carstensen J., Olsen P. Short term toxicity study in rats dosed with pulegone and menthol. Toxicol. Let. 1983;19:207–210. doi: 10.1016/0378-4274(83)90120-0. [DOI] [PubMed] [Google Scholar]
- 209.Madsen C., Würtzen G., Carstensen J. Short-term toxicity study in rats dosed with menthone. Toxicol. Let. 1986;32:147–152. doi: 10.1016/0378-4274(86)90061-5. [DOI] [PubMed] [Google Scholar]
- 210.Kristiansen E., Madsen C. Induction of protein droplet (α2μ-globulin) nephropathy in male rats after short-term dosage with 1, 8-cineole and l-limonene. Toxicol. Let. 1995;80:147–152. doi: 10.1016/0378-4274(95)03390-7. [DOI] [PubMed] [Google Scholar]
- 211.Shah P.P., Mello P. A review of medicinal uses and pharmacological effects of Mentha piperita. Nat. Prod. Rad. 2004;3:214–221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.