Abstract
Simple Summary
Tribbles pseudokinases represent a sub-branch of the CAMK (Ca2+/calmodulin-dependent protein kinase) subfamily and are associated with disease-associated signaling pathways associated with various cancers, including melanoma, lung, liver, and acute leukemia. The ability of this class of molecules to regulate cell proliferation was first recognized in the model organism Drosophila and the fruit fly genetic model and continues to provide insight into the molecular mechanism by which this family of adapter molecules regulates both normal development and disease associated with corruption of their proper regulation and function.
Abstract
The Tribbles (Trib) family of pseudokinase proteins regulate cell growth, proliferation, and differentiation during normal development and in response to environmental stress. Mutations in human Trib isoforms (Trib1, 2, and 3) have been associated with metabolic disease and linked to leukemia and the formation of solid tumors, including melanomas, hepatomas, and lung cancers. Drosophila Tribbles (Trbl) was the first identified member of this sub-family of pseudokinases and shares a conserved structure and similar functions to bind and direct the degradation of key mediators of cell growth and proliferation. Common Trib targets include Akt kinase (also known as protein kinase B), C/EBP (CAAT/enhancer binding protein) transcription factors, and Cdc25 phosphatases, leading to the notion that Trib family members stand athwart multiple pathways modulating their growth-promoting activities. Recent work using the Drosophila model has provided important insights into novel facets of conserved Tribbles functions in stem cell quiescence, tissue regeneration, metabolism connected to insulin signaling, and tumor formation linked to the Hippo signaling pathway. Here we highlight some of these recent studies and discuss their implications for understanding the complex roles Tribs play in cancers and disease pathologies.
Keywords: Trib protein family, pseudokinase, growth, cancer
1. Overview
The tribbles (trbl) gene was first identified in Drosophila mutational screens for regulators of cell proliferation and migration [1,2,3,4]. Drosophila genes are often named after their mutant phenotypes, and trbl mutations result in the over-proliferation of invaginating mesodermal cells that superficially resemble the piles of small, furry, and fecund “Tribbles” animals that vexed the fictional crew of the Enterprise in the classic “Trouble with Tribbles” episode from the original Star Trek television series. The Drosophila Trbl gene sequence revealed a pioneer protein with homology to a database cDNA induced by mitogens in dog thyroid cells (C5FW, subsequently renamed mammalian Trib2) [5,6]. Subsequent genome sequencing efforts confirmed that all family members share a unique central kinase-like domain flanked by a variable N-terminus and a (largely) conserved C-terminal COP1 site predicted to bind ubiquitin ligase [7,8]. Over the last 20 years, cell biological, genetic, structural, and biochemical approaches have converged on the idea that Tribbles (Trib) family members function as molecular scaffolds, binding a specific set of target molecules to coordinate the activity of multiple signaling pathways regulating cell growth and differentiation.
Tribs 1, 2 and 3 have oncogenic and tumor suppressor effects, depending on the cellular context (Table 1 and [9]). Trib2 exemplifies this enigma; while reduced Trib2 levels accelerate NOTCH1-driven T-cell acute lymphoblastic leukemia (T-ALL) [10,11,12,13], increased Trib2 levels result in the degradation of the C/EBPα p42 isoform and increased phosphorylation of ERK leading to hematopoietic cell proliferation and leukemia pathologies [14,15,16,17]. Trib isoforms have distinct and overlapping functions [18] and exhibit a complex cross-regulation in various cancer cell types [19]; due to this, the association of any one isoform with a particular cancer may be attributed to a dysregulation among all isoforms. The notion that a common mechanism underlies their multifactorial roles in human disease and cancer underscores the importance of observations made in the model organism field. Recent reviews have focused on the role of Tribs in signaling, cancer, and metabolism (e.g., [9,20,21,22]), and here we survey recent studies from the Drosophila model that point to a deep conservation in Trib functions.
Table 1.
Association of Tribbles (Trib) family members with cancer subtypes, with recent references.
| TRIB | Cancer Type | Recent Reference |
|---|---|---|
| Trib1 | prostate | [23,24] |
| acute myeloid leukemia | [22,25] | |
| Trib2 | hepatocarcinogenesis | [26] |
| laryngeal squamous cell carcinoma | [27] | |
| human melanoma | [28] | |
| osteosarcoma | [29] | |
| acute myeloid leukemia | [30] | |
| Trib3 | nasopharyngeal cancer | [31] |
| lung cancer | [32,33] | |
| esophageal squamous cell carcinoma | [34] | |
| colorectal cancer | [35] | |
| ovarian cancer | [36] | |
| breast cancer | [37,38] | |
| endometrial cancer | [39,40] | |
| hepatocarcinogenesis | [41] | |
| glioblastoma | [42] | |
| lymphoma | [43] | |
| acute myeloid leukemia | [44,45] | |
| adenocarcinoma | [46] |
2. Tribbles Pseudokinases Are Conserved Adaptor Proteins
The primary structure of all Trib family members is marked by three features: (1) a central kinase-like domain that shares considerable homology to CAM-II kinases, (2) a divergent N-terminus that in some isoforms contains multiple PEST motifs (peptide sequence that is rich in proline [P], glutamic acid [E], serine [S], and threonine [T] that likely mediate protein turnover, and (3) a C-terminal tail that contains a conserved binding sites for MAPK (mitogen-activated protein kinase) proteins and COP1 E3 ubiquitin ligases. Since their discovery in 2000, a large amount of work has shown that Trib family members act as adaptor molecules that bind target proteins via the central pseudokinase core. The core has an aspartic acid-leucine-lysine (DLK) motif common to bona fide kinases, however the Trib kinase-like domain lacks both the ATP coordination site VAIK (valine–alanine–isoleucine–lysine in kinases) and the DFG motif (aspartic acid–phenylalanine–glycine in kinases) responsible for binding magnesium. The DFG motif characteristic of kinases is also absent and is replaced by serine/asparagine–leucine–glutamic acid (S/NLE) in almost all Tribs. Based on biochemical evaluations, it remains unclear whether Tribs serve solely in a non-catalytic role, or can in some instances phosphorylate targets via a novel mechanism, or even can act in both ways, depending on the isoform and cellular context [47].
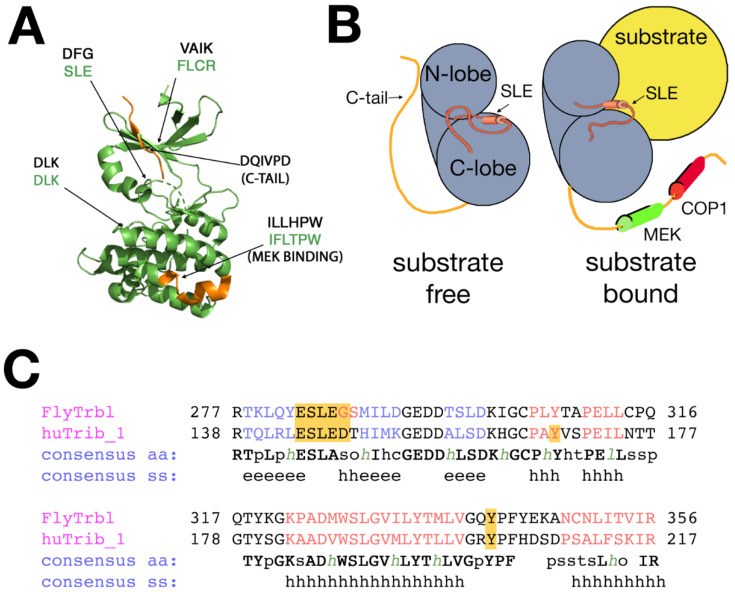
Solving the crystal structure of Trib1 unbound and bound to substrate has afforded substantial insight into the dynamic interactions among these three domains [48,49]. The inactive, substrate-free form of hTrib1 (Figure 1A) reveals a bi-lobed tertiary structure similar to kinases, with the C-terminal tail folded back against the pseudokinase spine, making several contacts with surface amino acids on a unique alpha-C helix in the N-lobe (Figure 1B, “substrate-free”). This intramolecular binding of the C-terminal tail to the N-lobe partially obscures the putative substrate-binding domain on the kinase-like domain, suggesting the C-terminal tail acts as a distinct pseudosubstrate to maintain an inactive conformation in the absence of bonafide substrate [48,50].
Figure 1.
The paradigm of Trib family function. (A) Proposed structure of the Drosophila Trbl kinase-like domain core, based on the structure of Trib1. Kinase/Trib homologies are indicated (aspartic acid–leucine–lysine (DLK)/DLK, aspartic acid–phenylalanine–glycine in kinases (DFG)/SLE and valine–alanine–isoleucine–lysine in kinases (VAIK) is phenylalanine-leucine-cysteine-arginine (FLCR). SLE motif resides in a flexible domain, similar to its mammalian counterpart. Location of the MEK (Mitogen-activated protein kinase kinase) binding site and C-terminal tail (orange) is indicated. (B) Simplified representation of the bi-lobed Trbl pseudokinase structure unbound (right) and bound (left) to substrate (yellow). The C-terminal tail is released upon degron binding and the SLE motif swings from an “in” to “out” configuration [56]. MEK1 and COP1 sites on the tail are indicated. (C) The primary structure of the flexible SLE domain is part of a highly conserved region in the kinase-like domain. The residues in red indicate helical structure, the residues in blue indicate beta sheet structure. SS = secondary structure, h = helical, and e = β-sheet.
Substrate binding to the kinase-like domain elicits global rearrangements in conformation, in particular dramatic changes at the interface between the alpha-C helix and the conserved SLE motif on the activation loop (Figure 1B, “substrate-bound”) [48]. Molecular dynamic modeling and analysis of bound and unbound crystal structures suggests that the SLE motif lies at the center of a conserved activation loop (Figure 1C) that swings from an inactive, substrate-free “SLE-out” conformation to an active, substrate-bound “SLE-in” conformation. The gated infolding of the SLE loop (reminiscent of the “DFG-out/DFG-in” conformational change that activates bona fide kinases upon substrate binding) disrupts intramolecular binding between the N-lobe’s alpha-C helix and the C-terminal tail. The C-tail is freed and extends to bind MAP kinase kinase (MAPKK), via its MEK1 binding site [51,52,53,54], or the COP1 E3-ubiquitin ligase, via its distal COP1 binding site (Figure 1B) [55].
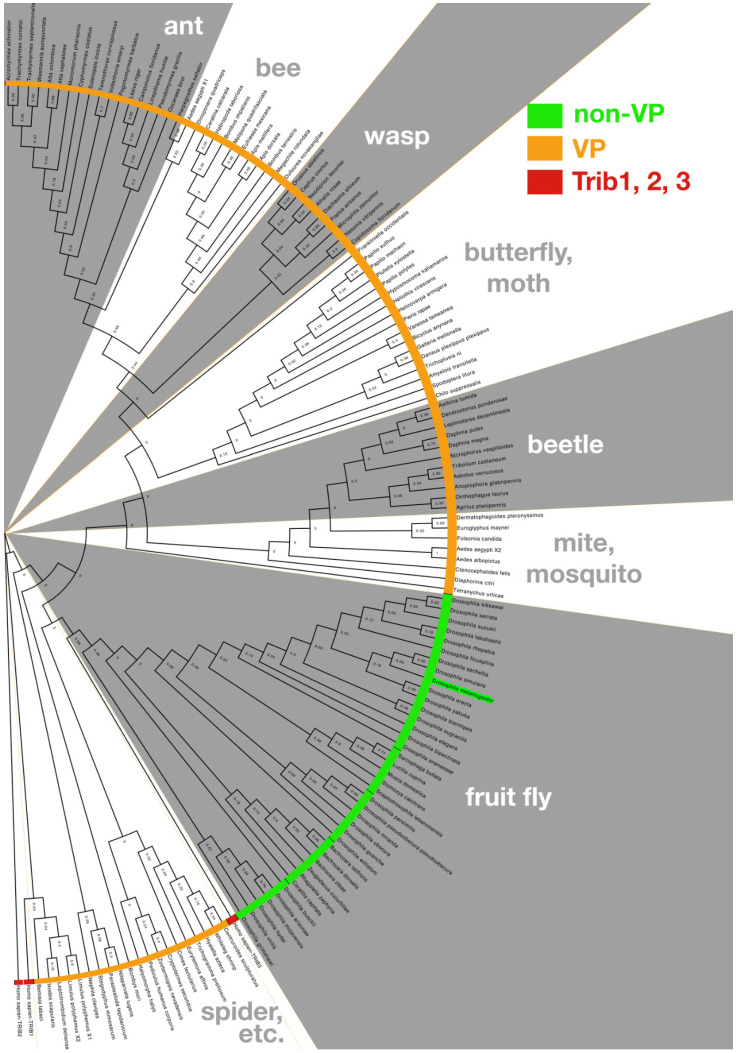
Based on structural analysis of Trib1, substrate binding triggers dynamic interactions among the SLE loop, alpha-C helix, and C-terminal tail leading to target degradation, and studies in Drosophila will be useful to understand better how these conserved motifs mediate the conformational changes that contribute to protein activity. In a quest to broaden our understanding of the evolutionary context of the C-terminal tail in Drosophila, we aligned Trib family member sequences from arthropods beginning with residues distal to the highly conserved MEK1 binding site, and rooted the sequences to the ancestral Trib isoform Trib2 [8]. From the tree of arthropod C-terminal tails displayed in Figure 2, two subgroups can be distinguished. First, a broad cluster of related C-terminal tails shares the consensus COP1 binding site (E/DQxVPE/D; highlighted in orange in Figure 2) identified by Uljon et al., who used alanine-scanning mutagenesis to show that the valine and proline (VP) residues are critical to potent COP1 binding. Structural analysis confirmed that this VP motif inserts into a conserved WD40 ß-propeller structure in COP1 E3-ubiquitin ligase [55]. A second broad subgroup of C-terminal tails lacks a clear homology to the COP1 binding site, and in particular does not display the critical VP motif. As shown in Figure 2, this cluster (highlighted in green) is limited to fruit flies, including Drosophilids. Consistent with the absence of a COP1 binding site motif in the C-terminal tail of Drosophila Trbl, it has been noted previously that the fly genome lacks a COP1 ubiquitin ligase homolog [57], thus it remains to be determined how Trbl interacts with the proteasome via its distinctive tail.
Figure 2.
The Drosophila Tribbles carboxy-terminal tail is divergent from other metazoan Tribbles. The C-terminal tails of Tribbles proteins from 136 taxa were used to build a phylogenic tree rooted on the human homolog, Trib2; distances are displayed at nodes of tree. Drosophila melanogaster Trbl is highlighted in green and clusters with Drosophilidae and related fruit flies sharing a C-terminal tail bearing a “non-VP” motif distinct from other arthropods bearing a COP1 binding site similar to other metazoan (“VP”) and the three human homologs (human Trib1, 2, and 3 are each red in tree). Methods: Drosophila Tribbles protein BLAST was run against the NCBI (National Center for Biotechnology Information) database of Arthropoda (taxid:6656) and Drosophilidae (taxid:7214). The non-redundant database was used with the following settings: Expect threshold of 10, word size of 6, Matrix-BLOSUM62, Gap Costs-Existence:11 extension:1, Computational adjustments-Conditional Score matrix adjustment. Sequences were then eliminated from the data set that did not retain important defining features of the Tribbles pseudokinase core. Each sequence was then trimmed to only represent the C-terminal tail as marked by the MEK1 binding site. The gap-spaced alignment of 137 sequences was analyzed for optimal tree model using MEGA X (all sites were used in a neighbor-joining tree using the maximum likelihood statistical method). MEGA X [58,59] was used to make a maximum likelihood tree and 50x bootstrap phylogeny test, using the Jones–Taylor–Thornton substitution model [60] with gamma (n = 4) distribution and nearest-neighbor interchange. The tree was then edited in FigTree v1.4.4.
3. Tribbles Targets Cdc25 Phosphatase to Block Cyclin-Dependent Mitosis
During Drosophila gastrulation, increased Tribbles levels in the ventral mesoderm temporarily block mitosis during invagination and promote migration of these cells between the overlying ectoderm and internal endoderm [1,2,3,4]. Subsequently, Tribbles expression decreases in these mesodermal precursors as they resume proliferation to form the muscle, heart, and peripheral nervous system. Recently, binding sites for the transcriptional repressor Tramtrack (Ttk) have been identified in a mesoderm-specific cis regulatory motif upstream of the trbl gene, which together with evidence that Ttk is expressed in the mesoderm upon migration, suggest a mechanism by which trbl gene expression is down-regulated after gastrulation [61,62].
Early work with flies showed that Trbl blocked cell division by binding and degrading Cdc25 phosphatase, a key activator of cell mitosis [1,3,4]. In all metazoans, Cdc25 phosphatase removes inhibitory phosphate residues from target cyclin-dependent kinases (Cdks) to control entry into and progression through various phases of the cell cycle, and mutations in Cdc25 have been associated with a variety of tumors [63]. Only relatively recently has the Trib–Cdc25 interaction been detected in a vertebrate model, when the Keeshan laboratory demonstrated that overexpression of human Trib2 in human cell culture led to reduced levels of CDC25C and CDC25B in a manner dependent on the proteasome [64]. Like Drosophila Trbl, Trib2 protein requires an intact kinase domain to poly-ubiquitinate CDC25 isoforms and, consistent with the notion that Trib2 controls the cell cycle, Trib2 levels oscillate to establish a G1/M gate in mitotically synchronized T-cell acute lymphoblastic leukemia (T-ALL) cells. In tissue culture cells, Trib2 binds and degrades CDC25C and CDC25B isoforms—but not the CDC25A isoform—specifically in the nuclear compartment, showing that Trib–Cdc25 interactions are selective and may be regulated by trafficking between subcellular compartments [64].
While human Trib2 discriminately binds CDC25C and CDC25B in preference to CDC25A, Drosophila Trbl targets both the Cdc25 paralogs encoded by string (Stg protein) during gastrulation as noted above, and twine (Twe protein), which is active earlier when cell cycle decelerates coincident with a switch from maternal to zygotic gene expression at the mid-blastoderm transition (MBT) [65,66,67]. Blastodermal injection of Twe RNAi does not delay MBT, suggesting down-regulation of Twe protein levels is post-transcriptional at this stage [68,69]. Based on observations that tribbles RNA levels are upregulated in cycle 13, workers in the O’Farrell lab injected an RNA encoding Trbl prior to this stage and observed effective destabilization of Twe protein leading to precocious MBT; conversely, injection of Trbl RNAi has the opposite effect to suppress Twe destruction and delay MBT [68].
Subsequent work has shown that Trbl lies at the center of a web of pathways that serve in a redundant fashion to reduce Twine/Cdc25 activity at MBT [67]. Components in this network include the cyclin:Cdk inhibitor encoded by the gene product Frühstart [70] and the phosphatase PpV (protein phosphatase V), homologous to the catalytic subunit of human PP6 [71]. Buoyed by novel methods to precisely measure the timing of MBT developed by Grosshans et al. [72], the MBT model system holds the promise to uncover a more detailed understanding of the conserved molecular mechanisms underlying how Trbl interacts with cell-cycle regulators during both normal and aberrant cell division [73].
4. Drosophila Genetic Screens Uncover a Tumor-Suppressor Role for Trbl
Loss of Trib function promotes undifferentiated tumor formation in mammals and this tumor suppressor effect is explained in part by the potent ability of Trib family members to block cell growth and proliferation as well as cell differentiation during normal development [74]. The effect of Trbl on cell differentiation was recognized in a misexpression genetic screen conducted in the year 2000 that showed increased Trbl levels degrade the C/EBP homolog Slbo (slow border cells) to block differentiation of the border cells in the ovary [2,75]. Subsequent work showed that mammalian Trib1 and 2 bind and degrade C/EBP to promote T cell maturation, revealing the deep conservation of the interaction between Tribs and C/EBPs [10,76]. While multiple genetic screens conducted over the past 20 years have linked fly Trbl to tissue differentiation in the central nervous system [77,78,79], peripheral nervous system [80], musculature [81], testes [82], ovary [3], and eye [83,84], the specific Trbl targets active during these tissue differentiation events remain unknown.
Mutations in human Trib2 are associated with increased levels of C/EBP in undifferentiated cancers like T-cell acute lymphoblastic leukemia (T-ALL) [10,14,85,86,87], hence it is unsurprising that trbl mutations have been uncovered in screens for regulators of tumor formation in Drosophila. Workers in the lab of Marcos Milan conducted screens for regulators of ionizing radiation-induced tumorigenesis in the developing wing disc and showed that Trbl is a potent tumor suppressor in this tissue [88]. While the molecular basis of Trbl anti-oncogenic function in this model is unclear, the ability of Trbl to block JNK (c-Jun N-terminal kinases) kinase activity and extend G2 may reduce tumorigenic genomic instabilities by allotting more time to facilitate DNA repair of double-stranded breaks that spur tumor formation [89].
In mouse and human models, both the progression of epithelial tumors and their successful colonization of target tissues rely on the nature of the tumor microenvironment (TME), which is thought to act as a niche to provide secreted signaling molecules and growth factors both to maintain tumor cells and recruit surrounding normal cells to the growing tumor. In work also conducted in Marco Milan’s lab, a Drosophila epithelial TME model was developed that exploited the availability of powerful genetic tools in the fly to generate tumors and track their effect on cell behavior both in the tumor and in surrounding normal tissue [90]. Using this system, they found that delaminating tumor cells acted upon adjacent normal epithelial cells to increase the expression of cytokines, such as the Wnt homolog Wg, leading to neoplastic transformation of these heretofore quiescent cells and further cell delamination, effectively growing the size of the tumor by the recruitment of outside cells. Trbl overexpression interfered with this dynamic feedback to reduce the number of Wg-expressing cells and block the effect of excessive tumor growth and delamination, leading to cell sheet disruption and tumor cell detachment. These data recall the complex roles reported for Trib2 in liver cancer cells as both a target of Wnt signaling to relieve C/EBPα-mediated inhibition of YAP/TEAD transcriptional activation [87] and to modulate the levels of Wnt signaling by targeting β-catenin/TCF4 for degradation [91].
While a molecular mechanism for the role of Trbl in cell delamination in flies is unclear, it may be noteworthy that a pair of genetic and proteomic screens in the whole animal combined with a separate cell-based RNA knockdown screen in tissue culture identified CLASP (cytoplasmic-linker-associated proteins) as a Trbl interactor. CLASP is an important microtubule regulatory protein acting in the Abl pathway [92,93]. The association of Trbl with a key component in the mitotic spindle recalls Xenopus Xtrb2 associated with the mitotic spindle [94] and may explain spindle orientation defects seen in Trbl-dependent tumors, and more broadly, Trbl–tubulin interactions may lie at the root of neural differentiation defects linked to behavior noted below.
5. Tribbles Integrates Developmental/Nutritional Inputs to Modulate Akt-Mediated Tissue Growth
Defects in the insulin/insulin-like growth factor signaling pathway (insulin/IGF signaling or IIS) manifest as a metabolic syndrome [95], a precursor to the onset of type 2 diabetes mellitus (T2DM) associated with an increased risk of some cancers. In the United States, 27% of adults and up to 50% of children have been diagnosed as severely obese and world-wide, TD2M diagnoses have grown to epidemic proportions [96,97]. Drosophila models of diet-induced obesity, insulin resistance, hyperglycemia, and hyperinsulinemia have been used to study the role of Trbl in modulating energy storage in response to dietary stress using two storage tissues analogous to the liver and adipose tissues of mammals: (1) the larval fat body, a repository of fats and proteins, the fat body sustains tissue rebuilding during the non-feeding stages of metamorphosis, and (2) the adult fat body, which develops independently to store nutrients required to fuel energy-expensive adult behaviors including flight and gametogenesis [98,99].
Our lab showed that like mammalian Trib3, Trbl binds Akt kinase to block its phosphorylation and inhibit insulin responses in the larval fat body [100]. Moreover, a Trbl mutant designed to mimic a Trib3 variant associated with type 2 diabetes in humans contributed to insulin-resistant phenotypes in the larval model [101]. Akt is a key transducer of insulin-regulated cell metabolism [102] and oncogenic activation of AKT is thought to augment the activity of nutrient transporters and metabolic enzymes to support the anabolic demands of rapidly growing tumors [103]. Like Trib3, fly Trbl blocks Akt phosphorylation at the conserved pThr308 residue necessary to recruit Akt to the cell membrane, however Trib3 does not reduce Akt levels, suggesting a mechanism for Akt repression distinct from the Trib-mediated proteosomal degradation of other targets [104,105].
In the adult, it has been shown that a high fat diet (HFD) alters glucose metabolism and induces high triglycerides (TG) leading to heart defects, similar to the association of HFD with diabetic cardiomyopathy in mammals. Hong and co-workers showed that HFD increased Trbl levels in the adult fat body and these increased Trbl levels correlated with decreased phospho-Akt levels, the hallmark of insulin resistance [106]. Combining the powerful tools available in the fly model with organ culture, Hong et al. showed that exogenous insulin potently increased phospho-Akt levels in fat body explants and this increase was effectively blocked by misexpressing Trbl using a fat-body-specific driver (transcriptional activator), GAL4. Misexpression of a UAS-regulated trbl RNAi transgene in the fat body is sufficient to relieve all the measurable metabolic effects of HFD, leading the authors to conclude that Trbl must be the central mediator of insulin resistance elicited by the HFD regimen in the adult fly [106].
Previous work has shown that mutations in the TGF-β ligand encoded by the gene glass bottom boat (Gbb) resulted in transparent larvae due to reduced fat body formation [107]. Hong et al. show that Trbl is an autocrine target of Gbb, which itself is upregulated in response to HFD. Demonstrating the deep conservation of this Gbb-Trbl signaling axis, Hong et al. and went on to show that TGF-β potently induced the expression of Trib3 in HepG2 cells. Mammalian TGF-β signaling through Smad3 plays a key role in adipogenesis related to the onset of diet-induced obesity and Trib3 is elevated both in patients with type 2 diabetes and animal models of this disease [108,109,110], leading Hong et al. to speculate that targeting Trib3 could offer new strategies for preventing type 2 diabetes associated with defects in TGF-β signaling [106].
6. Role for Trbl in Tissue Regeneration and Stem Cell Regulation
Quiescent stem cells can re-activate during both normal development and during wound healing, and powerful fly models have been developed to study both these processes [111]. Tissue regeneration studies in response to wound healing models rely on genetic manipulations that destroy defined sections of the tissue so that the genes required for compensatory tissue remodeling can be identified by various and sundry means. Microarrays were used to detect increased Trbl levels in the wing following genetic wounding, suggesting a role in restraining cell proliferation during the complex cell shape changes and rearrangements required for proper wing patterning during regrowth [112].
A more detailed understanding of the role of Trbl in tissue regeneration comes from the work of Leo Otsuki and Andrea Brand, who focused on the reactivation of quiescent neural stem cells (NSCs) that occurs at the larva-to-adult transition [113]. Using an elegant series of experiments that combined tissue-specific knockdowns with misexpression of activated molecules, they demonstrated that high levels of Trbl in a subset of NSCs in the larval brain actively blocks stem cell proliferation by promoting CDC25 turnover. Arrested at G2, these specialized NSCs can be distinguished from a second group of Trbl-independent NSCs arrested at G0. Following the first feeding that occurs after larval hatching, an insulin-dependent signal mediated by Akt downregulates Trbl, which in turn releases the G2 block on proliferation of this NSC subset. They demonstrated that the link between Trbl and Akt is a balancing act—prior to feeding, Trbl inhibits Akt while subsequently Akt inhibits Trbl transcription, in a nutrient-dependent fashion.
Their demonstration that Trbl plays a critical role in the activation of quiescent neural stem cells during early larval development is intriguing in light of previous work done by Zars et al. showing that trbl mutations result in adult behavior phenotypes, including sleep arrhythmias and short-term memory loss [78,79]. The Zars group showed that memory defects can be rescued by transgenic expression of Trbl in a subset of cells in the nervous system, making a compelling case that Trbl is critical for reorganization of neural circuitry necessary for subsequent adult behaviors.
7. Tribbles Balances Hippo-Pathway-Mediated Cell Division and Death
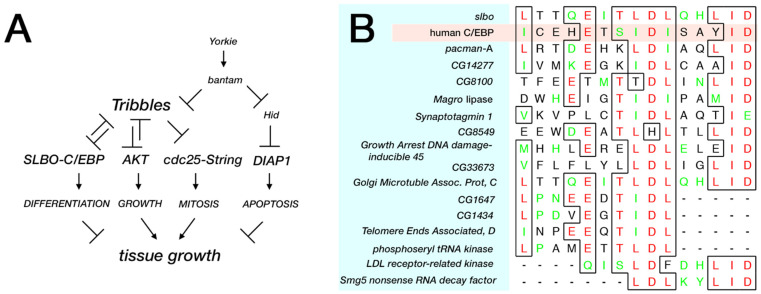
As noted above, the documented roles of Tribs in cancers are inconsonant; they can act as tumor suppressors or oncogenes, depending on the cellular context [8]. While CDC25, C/EBP, and Akt are conserved downstream targets (Figure 3A), how Trbl fits into a comprehensive set of growth regulatory pathways controlling cell proliferation and apoptosis remains unclear. Recent work from the Harrant lab focused on Trbl regulation by the 21 nucleotide microRNA (miRNA) encoded by the bantam gene [114]. The bantam locus of Drosophila was identified almost 20 years ago in a gain-of-function screen for genes that promote Hippo–Yorkie pathway-dependent growth [115], and significantly, in humans Hippo signaling defects are associated with mesotheliomas and cancers of the head and neck and gastrointestinal and gynecologic cancers [116].
Figure 3.
Trbl upstream regulation and candidate downstream targets. (A) Based on work from Gerlach et al., a comprehensive pathway of Tribbles function incorporates Hippo regulation by the bantam miRNA [108]. (B) Alignment of the Trib degron from human C/EBP proteins and the Drosophila C/EBP ortholog, Slbo, with candidate proteins from the Drosophila genome. Methods: A protein-protein BLAST search was conducted of non-redundant Drosophila sequences derived from the GenBank coding sequence (CDS) translations (including the PDB [Protein Data Bank], SwissProt, PIR [Protein Information Resource], and PRF [Protein Research Foundation]) with four sequences derived from the degron Slbo (ICEhEtSIDISAYIDPAA). The four sequences (JXXXEXTJDJXXXID, JXXXEXSJDJXXXID, LXXXEXTLDLXXXID, and IXXXEXTIDIXXXID) were chosen to exclude poorly conserved residues (X) and include where possible ambiguous amino acids (B, Z, and J) with the goal of expanding the binding sites obtained from the search. More functional data to evaluate important residues in the candidate binding sites was absent and top-ranked homologies overall are listed followed by strong homologies to subdomains of the motif.
In silico searches of the fly genome using microRNA target prediction algorithms identified a potential bantam miRNA binding site in the 3′ UTR of trbl RNA [114]. Tests of a trbl GFP sensor constructed by fusing the trbl RNA 3′ UTR to GFP and expressed ubiquitously in fly tissue revealed that bantam miRNA efficiently reduced GFP levels from the GFP-trbl3′UTR gene sensor; conversely a mutation in the bantam gene that fails to produce the miRNA led to increased GFP–trbl3′UTR gene sensor levels in a cell-autonomous fashion. The tumor-promoting effects of the bantam miRNA can be reversed by overexpression of Trbl, supporting the notion that Trbl is bantam miRNA’s major target to promote cell division and acts in parallel with bantam miRNA’s other target, the caspase 3 encoded by the hid (head involution defective) gene, which mediates bantam miRNA’s ability to block apoptosis simultaneously (Figure 3A). While the bantam miRNA has no identical vertebrate homolog [117], a growing body of evidence supports the idea that mammalian Trib isoforms are regulated by miRNAs [23,27,118,119,120], and evidence that Trib2 binds to the Hippo target YAP transcription factor to stabilize it indicates that Trbl may play a role in Hippo-mediated growth at several points in the pathway [26]. These observations suggest that the role of Trbl in the Hippo pathway depicted in Figure 3A will turn out to be greatly oversimplified.
8. Drosophila as a Platform to Understand Trib Family Members in Cancer
In various RNAi screens conducted in Drosophila cell culture, Trbl knockdown results in defects in cell adiposity, aberrant cell cycle regulation, and actin cytoskeletal changes in cell morphology [121], phenotypes consistent with trbl mutant phenotypes in vivo. Looking forward, it will become important to expand our understanding of the organ-specific functions of Trbl function in the context of the whole animal—its tissue-specific regulated expression, subcellular distribution, and interactions with targets that mediate regionalized effects, both during normal development and in response to environmental stress. The powerful genetic tools available in the fly system, including tissue- and stage-specific knockdown/misexpression, combined with the ability to collect large amounts of tissues and hemolymph from animals exposed to various environmental stresses and dietary regimens will allow us to attack these questions comprehensively.
While genetic approaches have been successful in identifying Trbl pathway components, biochemical searches for bona fide substrates have been hobbled by the instability of Trbl itself and rapid turnover of its targets in vivo when co-expressed. Better understanding of the conserved features of Trbl will allow the development of mutants that bind but do not degrade targets, potentially permitting the capture of tissue-specific binding partners from any tissue via high-throughput biochemical approaches. Other Trbl targets are suggested by observations in mammals and tests of their respective fly homologs may be fruitful. For example, the noted interaction between Smad3 and Trib3 to regulate expression of the three targets E-cadherin, Twist, and Snail in mammals [122] may explain in part the role of Trbl in mesoderm invagination, where the homologs of each of these genes are co-expressed.
In silico searches for Trbl interactors is facilitated by the simplicity of the fly genome, which is ca. one-fourth the size of the human genome depending on the yardstick used to compare the two. Sequence comparisons of the degron motif present in the N-terminal region of human C/EBP that strongly binds human Trib1 in vitro reveals strong homology to a motif in the Drosophila C/EBP Slbo (Figure 3B) [48], suggesting that the interaction interface between these proteins is highly conserved. We used this Trbl binding site (TBS) motif in Slbo (ICEhEtSIDISAYIDPAA) to conduct a BLAST screen [123] of the fly proteome to identify similar motifs, using reduced amino acid stringency as detailed in Figure 3. From this genome-wide search it is noteworthy that all other fly b-ZIP transcription factors lack a good match to the TBS, suggesting that Trbl targets Slbo selectively, a contention validated in one case by the demonstration that Trbl did not interact with the b-ZIP dFos in the Drosophila eye [1]. Proteins that did display a sequence similar to the TBS are listed in Figure 3B and include (1) Pacman, a 5′-3′ exoribonuclease involved in growth control [124,125,126,127], and (2) Synaptotagmin, a calcium-binding protein that functions in neurotransmitter release at synapses [128,129]. While the interactions between Trbl and these candidate TBS motifs is unexplored, available molecular and genetic tools make validating these interactions in vivo straightforward.
While the fly model simplifies interaction tests in some ways, it is worth noting that the single Trbl isoform in flies is tasked with a wide array functions that in vertebrates are split among three Trib isoforms, so that interpreting the mutant and overexpression Trbl phenotypes in the fly must be done carefully. For example, it might be expected that mutagenesis of the COP1 site in Trbl would disrupt the proteasome binding and degradation of Cdc25, resulting in a failure to block that pathway, however this same mutant may result in a stabilized version of Trbl that simultaneously is more effective at binding MEK kinase to regulate that signaling pathway. Alternatively, the inability of a COP1-mutated tail to interact with its intramolecular binding site the N-lobe could destabilize the protein, leading to its degradation. Thus, despite the attractive minimalism of model organisms, interpreting the results of manipulating a multi-faceted protein like Trbl, should be conducted with caution.
The selected examples of recent Drosophila Tribbles research presented in this review show the continued relevance of the fruit fly model to understanding the conserved role of the Trib gene family in tumor formation and growth. The fly can now brag of models for colorectal, brain, and lung cancers and offers genetically enhanced approaches to developing personalized therapies and to drug discovery [130]. Recently, the fly system has served as an in vivo platform to test kinase inhibitors [131], pointing to the exciting possibility that this model can serve as an in vivo ‘guinea pig’ to test small molecules targeting Trbl functions. The ever-expanding genetic tool kit in Drosophila is boosted by advances in new technologies in molecular genetics including next-generation sequencing, in vivo imaging, CRISPR-Cas (clustered regularly interspaced short palindromic repeats) editing and metabolomics analyses. Going forward, the fly system will offer valuable insights into the poorly understood impacts of the Trib gene family on cancer formation and possibly contribute to the design and development of treatments.
Acknowledgments
We thank Flybase curators for the constant updating of the database flybase.org which has alerted us to interesting screens that have revealed novel functions for Tribbles. Due to the focus of this review, we could not cover all important work in a fast-moving field, so apologies to colleagues whose work was not included. We thank members of the Dobens lab and members of the BIO312 course for helpful comments on earlier versions of the manuscript [132].
Author Contributions
L.L.D., C.N., Z.F. and X.Y. contributed to the design of the figures, analysis of the literature and the writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding support came from the NSF (IOS-1456023), NIH (NIH R21 CA197317) to L.L.D.; and UMKC School of Graduate Studies (Graduate Student Research Award Program 2014–2017) to Z.F. and C.N.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grosshans J., Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell. 2000;101:523–531. doi: 10.1016/S0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- 2.Rorth P., Szabo K., Texido G. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol. Cell. 2000;6:23–30. doi: 10.1016/S1097-2765(05)00008-0. [DOI] [PubMed] [Google Scholar]
- 3.Mata J., Curado S., Ephrussi A., Rorth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell. 2000;101:511–522. doi: 10.1016/S0092-8674(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 4.Seher T.C., Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr. Biol. 2000;10:623–629. doi: 10.1016/S0960-9822(00)00502-9. [DOI] [PubMed] [Google Scholar]
- 5.Wilkin F., Savonet V., Radulescu A., Petermans J., Dumont J.E., Maenhaut C. Identification and characterization of novel genes modulated in the thyroid of dogs treated with methimazole and propylthiouracil. J. Biol. Chem. 1996;271:28451–28457. doi: 10.1074/jbc.271.45.28451. [DOI] [PubMed] [Google Scholar]
- 6.Wilkin F., Suarez-Huerta N., Robaye B., Peetermans J., Libert F., Dumont J.E., Maenhaut C. Characterization of a phosphoprotein whose mRNA is regulated by the mitogenic pathways in dog thyroid cells. Eur. J. Biochem. 1997;248:660–668. doi: 10.1111/j.1432-1033.1997.t01-1-00660.x. [DOI] [PubMed] [Google Scholar]
- 7.Dobens L.L., Bouyain S. Developmental roles of tribbles protein family members. Dev. Dyn. 2012;241:1239–1248. doi: 10.1002/dvdy.23822. [DOI] [PubMed] [Google Scholar]
- 8.Eyers P.A., Keeshan K., Kannan N. Tribbles in the 21st Century: The Evolving Roles of Tribbles Pseudokinases in Biology and Disease. Trends Cell Biol. 2017;27:284–298. doi: 10.1016/j.tcb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richmond L., Keeshan K. Pseudokinases: A tribble-edged sword. FEBS J. 2020;287:4170–4182. doi: 10.1111/febs.15096. [DOI] [PubMed] [Google Scholar]
- 10.Keeshan K., He Y., Wouters B.J., Shestova O., Xu L., Sai H., Rodriguez C.G., Maillard I., Tobias J.W., Valk P., et al. Tribbles homolog 2 inactivates C/EBPalpha and causes acute myelogenous leukemia. Cancer Cell. 2006;10:401–411. doi: 10.1016/j.ccr.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wouters B.J., Jordà M.A., Keeshan K., Louwers I., Erpelinck-Verschueren C.A., Tielemans D., Langerak A.W., He Y., Yashiro-Ohtani Y., Zhang P., et al. Distinct gene expression profiles of acute myeloid/T-lymphoid leukemia with silenced CEBPA and mutations in NOTCH1. Blood. 2007;110:3706–3714. doi: 10.1182/blood-2007-02-073486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannon M.M., Lohan F., Erbilgin Y., Sayitoglu M., O’Hagan K., Mills K., Ozbek U., Keeshan K. Elevated TRIB2 with NOTCH1 activation in paediatric/adult T-ALL. Br. J. Haematol. 2012;158:626–634. doi: 10.1111/j.1365-2141.2012.09222.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanda T., Lawton L.N., Barrasa M.I., Fan Z.P., Kohlhammer H., Gutierrez A., Ma W., Tatarek J., Ahn Y., Kelliher M.A., et al. Core transcriptional regulatory circuit controlled by the TAL1 complex in human T cell acute lymphoblastic leukemia. Cancer Cell. 2012;22:209–221. doi: 10.1016/j.ccr.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor C., Lohan F., Campos J., Ohlsson E., Salomè M., Forde C., Artschwager R., Liskamp R.M., Cahill M.R., Kiely P.A., et al. The presence of C/EBPα and its degradation are both required for TRIB2-mediated leukaemia. Oncogene. 2016;35:5272–5281. doi: 10.1038/onc.2016.66. [DOI] [PubMed] [Google Scholar]
- 15.King F.J., Lin H. Somatic signaling mediated by fs(1)Yb is essential for germline stem cell maintenance during Drosophila oogenesis. Development. 1999;126:1833–1844. doi: 10.1242/dev.126.9.1833. [DOI] [PubMed] [Google Scholar]
- 16.Zalokar M., Erk I., Santamaria P. Distribution of Ring-X Chromosomes in the Blastoderm of Gynandromorphic D. melanogaster. Cell. 1980;19:133–141. doi: 10.1016/0092-8674(80)90394-3. [DOI] [PubMed] [Google Scholar]
- 17.Liang K.L., Rishi L., Keeshan K. Tribbles in acute leukemia. Blood. 2013;121:4265–4270. doi: 10.1182/blood-2012-12-471300. [DOI] [PubMed] [Google Scholar]
- 18.Dedhia P.H., Keeshan K., Uljon S., Xu L., Vega M.E., Shestova O., Zaks-Zilberman M., Romany C., Blacklow S.C., Pear W.S. Differential ability of Tribbles family members to promote degradation of C/EBPalpha and induce acute myelogenous leukemia. Blood. 2010;116:1321–1328. doi: 10.1182/blood-2009-07-229450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomé M., Hopcroft L., Keeshan K. Inverse and correlative relationships between TRIBBLES genes indicate non-redundant functions during normal and malignant hemopoiesis. Exp. Hematol. 2018;66:63–78. doi: 10.1016/j.exphem.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Jadhav K.S., Bauer R.C. Trouble with Tribbles-1. Arterioscler. Thromb. Vasc. Biol. 2019;39:998–1005. doi: 10.1161/ATVBAHA.118.311573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiss-Toth E. Tribbles: ‘puzzling’ regulators of cell signalling. Biochem. Soc. Trans. 2011;39:684–687. doi: 10.1042/BST0390684. [DOI] [PubMed] [Google Scholar]
- 22.Stein S.J., Mack E.A., Rome K.S., Pear W.S. Tribbles in normal and malignant haematopoiesis. Biochem. Soc. Trans. 2015;43:1112–1115. doi: 10.1042/BST20150117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moya L., Lai J., Hoffman A., Srinivasan S., Panchadsaram J., Chambers S., Clements J.A., Batra J., Australian P.C.B. Association Analysis of a Microsatellite Repeat in the TRIB1 Gene with Prostate Cancer Risk, Aggressiveness and Survival. Front. Genet. 2018;9:428. doi: 10.3389/fgene.2018.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niespolo C., Johnston J.M., Deshmukh S.R., Satam S., Shologu Z., Villacanas O., Sudbery I.M., Wilson H.L., Kiss-Toth E. Tribbles-1 Expression and Its Function to Control Inflammatory Cytokines, Including Interleukin-8 Levels are Regulated by miRNAs in Macrophages and Prostate Cancer Cells. Front. Immunol. 2020;11:574046. doi: 10.3389/fimmu.2020.574046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugast E., Kiss-Toth E., Soulillou J.P., Brouard S., Ashton-Chess J. The Tribbles-1 protein in humans: Roles and functions in health and disease. Curr. Mol. Med. 2013;13:80–85. doi: 10.2174/156652413804486197. [DOI] [PubMed] [Google Scholar]
- 26.Xiang D., Zhu X., Zhang Y., Zou J., Li J., Kong L., Zhang H. Tribbles homolog 2 promotes hepatic fibrosis and hepatocarcinogenesis through phosphatase 1A-Mediated stabilization of yes-associated protein. Liver Int. 2021 doi: 10.1111/liv.14782. [DOI] [PubMed] [Google Scholar]
- 27.Liu C., Lu Z., Liu H., Zhuang S., Guo P. LncRNA XIST promotes the progression of laryngeal squamous cell carcinoma via sponging miR-125b-5p to modulate TRIB2. Biosci. Rep. 2020;40 doi: 10.1042/BSR20193172. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Chen Z., Chen J., Wa Q., He M., Wang X., Zhou J., Cen Y. Knockdown of circ_0084043 suppresses the development of human melanoma cells through miR-429/tribbles homolog 2 axis and Wnt/β-catenin pathway. Life Sci. 2020;243:117323. doi: 10.1016/j.lfs.2020.117323. [DOI] [PubMed] [Google Scholar]
- 29.Guo J., Wu Q., Peng X., Yu B. miR-509-5p Inhibits the Proliferation and Invasion of Osteosarcoma by Targeting TRIB2. BioMed Res. Int. 2019;2019:2523032. doi: 10.1155/2019/2523032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Salomé M., Magee A., Yalla K., Chaudhury S., Sarrou E., Carmody R.J., Keeshan K.A. Trib2-p38 axis controls myeloid leukaemia cell cycle and stress response signalling. Cell Death Dis. 2018;9:443. doi: 10.1038/s41419-018-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Y., Peng H., Lu X.Q., Liang C.Q., Fan J.P., Liu H.H. Role of lncRNA-ENST00000412010 in regulating nasopharyngeal cancer cell survival. Rhinology. 2020;58:588–596. doi: 10.4193/Rhin19.341. [DOI] [PubMed] [Google Scholar]
- 32.Yu J.J., Zhou D.D., Yang X.X., Cui B., Tan F.W., Wang J., Li K., Shang S., Zhang C., Lv X.X., et al. TRIB3-EGFR interaction promotes lung cancer progression and defines a therapeutic target. Nat. Commun. 2020;11:3660. doi: 10.1038/s41467-020-17385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao X., Fang X., Malik W.S., He Y., Li X., Xie M., Sun W., Xu Y., Liu X. TRB3 interacts with ERK and JNK and contributes to the proliferation, apoptosis, and migration of lung adenocarcinoma cells. J. Cell Physiol. 2020;235:538–547. doi: 10.1002/jcp.28993. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S., Liu S., Lin C., Li Y., Ye L., Wu X., Jian Y., Dai Y., Ouyang Y., Zhao L., et al. TRIB3 confers radiotherapy resistance in esophageal squamous cell carcinoma by stabilizing TAZ. Oncogene. 2020;39:3710–3725. doi: 10.1038/s41388-020-1245-0. [DOI] [PubMed] [Google Scholar]
- 35.Makino S., Takahashi H., Okuzaki D., Miyoshi N., Haraguchi N., Hata T., Matsuda C., Yamamoto H., Mizushima T., Mori M., et al. DCLK1 integrates induction of TRIB3, EMT, drug resistance and poor prognosis in colorectal cancer. Carcinogenesis. 2020;41:394–396. doi: 10.1093/carcin/bgaa016. [DOI] [PubMed] [Google Scholar]
- 36.Wang S., Wang C., Li X., Hu Y., Gou R., Guo Q., Nie X., Liu J., Zhu L., Lin B. Down-regulation of TRIB3 inhibits the progression of ovarian cancer via MEK/ERK signaling pathway. Cancer Cell Int. 2020;20:418. doi: 10.1186/s12935-020-01509-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J.M., Sun W., Wang Z.H., Liang X., Hua F., Li K., Lv X.X., Zhang X.W., Liu Y.Y., Yu J.J., et al. TRIB3 supports breast cancer stemness by suppressing FOXO1 degradation and enhancing SOX2 transcription. Nat. Commun. 2019;10:5720. doi: 10.1038/s41467-019-13700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y.C., Wang W.L., Chang W.C., Huang Y.H., Hong G.C., Wang H.L., Chou Y.H., Tseng H.C., Lee H.T., Li S.T., et al. Tribbles Homolog 3 Involved in Radiation Response of Triple Negative Breast Cancer Cells by Regulating Notch1 Activation. Cancers. 2019;11:127. doi: 10.3390/cancers11020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devis-Jauregui L., Eritja N., Davis M.L., Matias-Guiu X., Llobet-Navàs D. Autophagy in the physiological endometrium and cancer. Autophagy. 2020:1–19. doi: 10.1080/15548627.2020.1752548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qu J., Liu B., Li B., Du G., Li Y., Wang J., He L., Wan X. TRIB3 suppresses proliferation and invasion and promotes apoptosis of endometrial cancer cells by regulating the AKT signaling pathway. Onco-Targets Ther. 2019;12:2235–2245. doi: 10.2147/OTT.S189001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X.J., Li F.F., Zhang Y.J., Jiang M., Ren W.H. TRIB3 promotes hepatocellular carcinoma growth and predicts poor prognosis. Cancer Biomark. 2020;29:307–315. doi: 10.3233/CBM-201577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Z., Chen H., Zhong D., Wei W., Liu L., Duan Q., Han B., Li G. TRIB3 facilitates glioblastoma progression via restraining autophagy. Aging (Albany NY) 2020;12:25020–25034. doi: 10.18632/aging.103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li K., Wang F., Yang Z.N., Zhang T.T., Yuan Y.F., Zhao C.X., Yeerjiang Z., Cui B., Hua F., Lv X.X., et al. TRIB3 promotes MYC-associated lymphoma development through suppression of UBE3B-mediated MYC degradation. Nat. Commun. 2020;11:6316. doi: 10.1038/s41467-020-20107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li K., Wang F., Yang Z.N., Cui B., Li P.P., Li Z.Y., Hu Z.W., Zhu H.H. PML-RARα interaction with TRIB3 impedes PPARγ/RXR function and triggers dyslipidemia in acute promyelocytic leukemia. Theranostics. 2020;10:10326–10340. doi: 10.7150/thno.45924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo X., Zhong L., Yu L., Xiong L., Dan W., Li J., Ye J., Chu X., Liu C., Liu B. TRIB3 destabilizes tumor suppressor PPARα expression through ubiquitin-mediated proteasome degradation in acute myeloid leukemia. Life Sci. 2020;257:118021. doi: 10.1016/j.lfs.2020.118021. [DOI] [PubMed] [Google Scholar]
- 46.Xing Y., Luo P., Hu R., Wang D., Zhou G., Jiang J. TRIB3 Promotes Lung Adenocarcinoma Progression via an Enhanced Warburg Effect. Cancer Manag. Res. 2020;12:13195–13206. doi: 10.2147/CMAR.S287956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey F.P., Byrne D.P., Oruganty K., Eyers C.E., Novotny C., Shokat K.M., Kannan N., Eyers P.A. The Tribbles 2 (TRB2) pseudokinase binds to ATP and autophosphorylates in a metal-independent manner. Biochem. J. 2015;467:47–62. doi: 10.1042/BJ20141441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jamieson S.A., Ruan Z., Burgess A.E., Curry J.R., McMillan H.D., Brewster J.L., Dunbier A.K., Axtman A.D., Kannan N., Mace P.D. Substrate binding allosterically relieves autoinhibition of the pseudokinase TRIB1. Sci. Signal. 2018;11:549. doi: 10.1126/scisignal.aau0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy J.M., Nakatani Y., Jamieson S.A., Dai W., Lucet I.S., Mace P.D. Molecular Mechanism of CCAAT-Enhancer Binding Protein Recruitment by the TRIB1 Pseudokinase. Structure. 2015;23:2111–2121. doi: 10.1016/j.str.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Kung J.E., Jura N. The pseudokinase TRIB1 toggles an intramolecular switch to regulate COP1 nuclear export. EMBO J. 2019;38:e99708. doi: 10.15252/embj.201899708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiss-Toth E., Bagstaff S.M., Sung H.Y., Jozsa V., Dempsey C., Caunt J.C., Oxley K.M., Wyllie D.H., Polgar T., Harte M., et al. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J. Biol. Chem. 2004;279:42703–42708. doi: 10.1074/jbc.M407732200. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama T., Kanno Y., Yamazaki Y., Takahara T., Miyata S., Nakamura T. Trib1 links the MEK1/ERK pathway in myeloid leukemogenesis. Blood. 2010;116:2768–2775. doi: 10.1182/blood-2009-10-246264. [DOI] [PubMed] [Google Scholar]
- 53.Jin G., Yamazaki Y., Takuwa M., Takahara T., Kaneko K., Kuwata T., Miyata S., Nakamura T. Trib1 and Evi1 cooperate with Hoxa and Meis1 in myeloid leukemogenesis. Blood. 2007;109:3998–4005. doi: 10.1182/blood-2006-08-041202. [DOI] [PubMed] [Google Scholar]
- 54.Guan H., Shuaib A., Leon D.D., Angyal A., Salazar M., Velasco G., Holcombe M., Dower S.K., Kiss-Toth E. Competition between members of the tribbles pseudokinase protein family shapes their interactions with mitogen activated protein kinase pathways. Sci. Rep. 2016;6:32667. doi: 10.1038/srep32667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uljon S., Xu X., Durzynska I., Stein S., Adelmant G., Marto J.A., Pear W.S., Blacklow S.C. Structural Basis for Substrate Selectivity of the E3 Ligase COP1. Structure. 2016;24:687–696. doi: 10.1016/j.str.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huse M., Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. doi: 10.1016/S0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 57.Yi C., Deng X.W. COP1-from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–625. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stecher G., Tamura K., Kumar S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020;37:1237–1239. doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones D.T., Taylor W.R., Thornton J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 61.Kumar S., Konikoff C., Van Emden B., Busick C., Davis K.T., Ji S., Wu L.W., Ramos H., Brody T., Panchanathan S., et al. FlyExpress: Visual mining of spatiotemporal patterns for genes and publications in Drosophila embryogenesis. Bioinformatics. 2011;27:3319–3320. doi: 10.1093/bioinformatics/btr567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ciglar L., Girardot C., Wilczyński B., Braun M., Furlong E.E. Coordinated repression and activation of two transcriptional programs stabilizes cell fate during myogenesis. Development. 2014;141:2633–2643. doi: 10.1242/dev.101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen T., Huang S. The role of Cdc25A in the regulation of cell proliferation and apoptosis. Anticancer Agents Med. Chem. 2012;12:631–639. doi: 10.2174/187152012800617678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang K.L., Paredes R., Carmody R., Eyers P.A., Meyer S., McCarthy T.V., Keeshan K. Human TRIB2 Oscillates during the Cell Cycle and Promotes Ubiquitination and Degradation of CDC25C. Int. J. Mol. Sci. 2016;17:1378. doi: 10.3390/ijms17091378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalle Nogare D.E., Pauerstein P.T., Lane M.E. G2 acquisition by transcription-independent mechanism at the zebrafish midblastula transition. Dev. Biol. 2009;326:131–142. doi: 10.1016/j.ydbio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. Characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- 67.Farrell J.A., O’Farrell P.H. From egg to gastrula: How the cell cycle is remodeled during the Drosophila mid-blastula transition. Annu. Rev. Genet. 2014;48:269–294. doi: 10.1146/annurev-genet-111212-133531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farrell J.A., O’Farrell P.H. Mechanism and regulation of cdc25/twine protein destruction in embryonic cell-cycle remodeling. Curr. Biol. 2013;23:118–126. doi: 10.1016/j.cub.2012.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sung H.W., Spangenberg S., Vogt N., Großhans J. Number of nuclear divisions in the Drosophila blastoderm controlled by onset of zygotic transcription. Curr. Biol. 2013;23:133–138. doi: 10.1016/j.cub.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 70.Gawliński P., Nikolay R., Goursot C., Lawo S., Chaurasia B., Herz H.M., Kussler-Schneider Y., Ruppert T., Mayer M., Grosshans J. The Drosophila mitotic inhibitor Frühstart specifically binds to the hydrophobic patch of cyclins. EMBO Rep. 2007;8:490–496. doi: 10.1038/sj.embor.7400948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu B., Sung H.W., Großhans J. Multiple Functions of the Essential Gene PpV in Drosophila Early Development. G3 Genes Genomes Genet. 2019;9:3583–3593. doi: 10.1534/g3.119.400662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu B., Gregor I., Müller H.A., Großhans J. Fluorescence fluctuation analysis reveals PpV dependent Cdc25 protein dynamics in living embryos. PLoS Genet. 2020;16:e1008735. doi: 10.1371/journal.pgen.1008735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu B., Grosshans J. Link of Zygotic Genome Activation and Cell Cycle Control. Methods Mol. Biol. 2017;1605:11–30. doi: 10.1007/978-1-4939-6988-3_2. [DOI] [PubMed] [Google Scholar]
- 74.Sakai S., Miyajima C., Uchida C., Itoh Y., Hayashi H., Inoue Y. Tribbles-Related Protein Family Members as Regulators or Substrates of the Ubiquitin-Proteasome System in Cancer Development. Curr. Cancer Drug Targets. 2016;16:147–156. doi: 10.2174/1568009616666151112122645. [DOI] [PubMed] [Google Scholar]
- 75.Masoner V., Das R., Pence L., Anand G., Laferriere H., Zars T., Bouyain S., Dobens L.L. The kinase domain of Drosophila Tribbles is required for turnover of fly C/EBP during cell migration. Dev. Biol. 2013;375:33–44. doi: 10.1016/j.ydbio.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 76.Myrdal S.E., Twardzik D.R., Auersperg N. Cell-mediated co-action of transforming growth factors: Incubation of type beta with normal kidney cells produces a soluble activity that prolongs the ruffling response to type alpha. J. Cell. Biol. 1986;102:1230–1234. doi: 10.1083/jcb.102.4.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu C.H., Bollepalli M.K., Long S.V., Asteriti S., Tan J., Brill J.A., Hardie R.C. Genetic dissection of the phosphoinositide cycle in Drosophila photoreceptors. J. Cell Sci. 2018;131:jcs214478. doi: 10.1242/jcs.214478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.LaFerriere H., Guarnieri D.J., Sitaraman D., Diegelmann S., Heberlein U., Zars T. Genetic dissociation of ethanol sensitivity and memory formation in Drosophila melanogaster. Genetics. 2008;178:1895–1902. doi: 10.1534/genetics.107.084582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.LaFerriere H., Zars T. The Drosophila melanogaster tribbles pseudokinase is necessary for proper memory formation. Neurobiol. Learn. Mem. 2017;144:68–76. doi: 10.1016/j.nlm.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdelilah-Seyfried S., Chan Y.M., Zeng C., Justice N.J., Younger-Shepherd S., Sharp L.E., Barbel S., Meadows S.A., Jan L.Y., Jan Y.N. A gain-of-function screen for genes that affect the development of the Drosophila adult external sensory organ. Genetics. 2000;155:733–752. doi: 10.1093/genetics/155.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schnorrer F., Schönbauer C., Langer C.C., Dietzl G., Novatchkova M., Schernhuber K., Fellner M., Azaryan A., Radolf M., Stark A., et al. Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature. 2010;464:287–291. doi: 10.1038/nature08799. [DOI] [PubMed] [Google Scholar]
- 82.Schulz C., Kiger A.A., Tazuke S.I., Yamashita Y.M., Pantalena-Filho L.C., Jones D.L., Wood C.G., Fuller M.T. A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics. 2004;167:707–723. doi: 10.1534/genetics.103.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukherjee T., Schäfer U., Zeidler M.P. Identification of Drosophila genes modulating janus kinase/signal transducer and activator of transcription signal transduction. Genetics. 2006;172:1683–1697. doi: 10.1534/genetics.105.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muller D., Kugler S.J., Preiss A., Maier D., Nagel A.C. Genetic modifier screens on Hairless gain-of-function phenotypes reveal genes involved in cell differentiation, cell growth and apoptosis in Drosophila melanogaster. Genetics. 2005;171:1137–1152. doi: 10.1534/genetics.105.044453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keeshan K., Bailis W., Dedhia P.H., Vega M.E., Shestova O., Xu L., Toscano K., Uljon S.N., Blacklow S.C., Pear W.S. Transformation by Tribbles homolog 2 (Trib2) requires both the Trib2 kinase domain and COP1 binding. Blood. 2010;116:4948–4957. doi: 10.1182/blood-2009-10-247361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang D.E., Zhang P., Wang N.D., Hetherington C.J., Darlington G.J., Tenen D.G. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J., Park J.S., Wei Y., Rajurkar M., Cotton J.L., Fan Q., Lewis B.C., Ji H., Mao J. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPα function. Mol. Cell. 2013;51:211–225. doi: 10.1016/j.molcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dekanty A., Barrio L., Milán M. Contributions of DNA repair, cell cycle checkpoints and cell death to suppressing the DNA damage-induced tumorigenic behavior of Drosophila epithelial cells. Oncogene. 2015;34:978–985. doi: 10.1038/onc.2014.42. [DOI] [PubMed] [Google Scholar]
- 89.Cosolo A., Jaiswal J., Csordás G., Grass I., Uhlirova M., Classen A.K. JNK-dependent cell cycle stalling in G2 promotes survival and senescence-like phenotypes in tissue stress. eLife. 2019;8:41036. doi: 10.7554/eLife.41036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muzzopappa M., Murcia L., Milán M. Feedback amplification loop drives malignant growth in epithelial tissues. Proc. Natl. Acad. Sci. USA. 2017;114:E7291–E7300. doi: 10.1073/pnas.1701791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu S., Tong M., Huang J., Zhang Y., Qiao Y., Weng W., Liu W., Wang J., Sun F. TRIB2 inhibits Wnt/β-Catenin/TCF4 signaling through its associated ubiquitin E3 ligases, β-TrCP, COP1 and Smurf1, in liver cancer cells. FEBS Lett. 2014;588:4334–4341. doi: 10.1016/j.febslet.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 92.Long J.B., Bagonis M., Lowery L.A., Lee H., Danuser G., Van Vactor D. Multiparametric analysis of CLASP-interacting protein functions during interphase microtubule dynamics. Mol. Cell Biol. 2013;33:1528–1545. doi: 10.1128/MCB.01442-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lowery L.A., Lee H., Lu C., Murphy R., Obar R.A., Zhai B., Schedl M., Van Vactor D., Zhan Y. Parallel genetic and proteomic screens identify Msps as a CLASP-Abl pathway interactor in Drosophila. Genetics. 2010;185:1311–1325. doi: 10.1534/genetics.110.115626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saka Y., Smith J.C. A Xenopus tribbles orthologue is required for the progression of mitosis and for development of the nervous system. Dev. Biol. 2004;273:210–225. doi: 10.1016/j.ydbio.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 95.Baker K.D., Thummel C.S. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ford E.S., Li C., Zhao G., Pearson W.S., Mokdad A.H. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31:587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 97.Li C., Ford E.S., Zhao G., Mokdad A.H. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care. 2009;32:342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Das R., Dobens L.L. Conservation of gene and tissue networks regulating insulin signalling in flies and vertebrates. Biochem. Soc. Trans. 2015;43:1057–1062. doi: 10.1042/BST20150078. [DOI] [PubMed] [Google Scholar]
- 99.Texada M.J., Koyama T., Rewitz K. Regulation of Body Size and Growth Control. Genetics. 2020;216:269–313. doi: 10.1534/genetics.120.303095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Das R., Sebo Z., Pence L., Dobens L.L. Drosophila tribbles antagonizes insulin signaling-mediated growth and metabolism via interactions with Akt kinase. PLoS ONE. 2014;9:e109530. doi: 10.1371/journal.pone.0109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fischer Z., Das R., Shipman A., Fan J.Y., Pence L., Bouyain S., Dobens L.L. A Drosophila model of insulin resistance associated with the human Trib3 Q/R polymorphism. Dis. Model. Mech. 2017;10:1453–1464. doi: 10.1242/dmm.030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hanada M., Feng J., Hemmings B.A. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim. Biophys. Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Hoxhaj G., Manning B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He L., Simmen F.A., Mehendale H.M., Ronis M.J., Badger T.M. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with the cell membrane. Role of TRB3 in inhibition of Akt/protein kinase B activation. J. Biol. Chem. 2006;281:11126–11134. doi: 10.1074/jbc.M510724200. [DOI] [PubMed] [Google Scholar]
- 105.Cheng K.K., Iglesias M.A., Lam K.S., Wang Y., Sweeney G., Zhu W., Vanhoutte P.M., Kraegen E.W., Xu A. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9:417–427. doi: 10.1016/j.cmet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 106.Hong S.H., Kang M., Lee K.S., Yu K. High fat diet-induced TGF-β/Gbb signaling provokes insulin resistance through the tribbles expression. Sci. Rep. 2016;6:30265. doi: 10.1038/srep30265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doctor J.S., Jackson P.D., Rashka K.E., Visalli M., Hoffmann F.M. Sequence, biochemical characterization, and developmental expression of a new member of the TGF-beta superfamily in Drosophila melanogaster. Dev. Biol. 1992;151:491–505. doi: 10.1016/0012-1606(92)90188-M. [DOI] [PubMed] [Google Scholar]
- 108.Ti Y., Xie G.L., Wang Z.H., Bi X.L., Ding W.Y., Wang J., Jiang G.H., Bu P.L., Zhang Y., Zhong M., et al. TRB3 gene silencing alleviates diabetic cardiomyopathy in a type 2 diabetic rat model. Diabetes. 2011;60:2963–2974. doi: 10.2337/db11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Z.H., Shang Y.Y., Zhang S., Zhong M., Wang X.P., Deng J.T., Pan J., Zhang Y., Zhang W. Silence of TRIB3 suppresses atherosclerosis and stabilizes plaques in diabetic ApoE−/−/LDL receptor−/− mice. Diabetes. 2012;61:463–473. doi: 10.2337/db11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang W., Wu M., Kim T., Jariwala R.H., Garvey W.J., Luo N., Kang M., Ma E., Tian L., Steverson D., et al. Skeletal Muscle TRIB3 Mediates Glucose Toxicity in Diabetes and High-Fat Diet-Induced Insulin Resistance. Diabetes. 2016;65:2380–2391. doi: 10.2337/db16-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Otsuki L., Brand A.H. Quiescent Neural Stem Cells for Brain Repair and Regeneration: Lessons from Model Systems. Trends Neurosci. 2020;43:213–226. doi: 10.1016/j.tins.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 112.Blanco E., Ruiz-Romero M., Beltran S., Bosch M., Punset A., Serras F., Corominas M. Gene expression following induction of regeneration in Drosophila wing imaginal discs. Expression profile of regenerating wing discs. BMC Dev. Biol. 2010;10:94. doi: 10.1186/1471-213X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Otsuki L., Brand A.H. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science. 2018;360:99–102. doi: 10.1126/science.aan8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gerlach S.U., Sander M., Song S., Herranz H. The miRNA bantam regulates growth and tumorigenesis by repressing the cell cycle regulator tribbles. Life Sci. Alliance. 2019;2:e201900381. doi: 10.26508/lsa.201900381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hipfner D.R., Weigmann K., Cohen S.M. The bantam gene regulates Drosophila growth. Genetics. 2002;161:1527–1537. doi: 10.1093/genetics/161.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calses P.C., Crawford J.J., Lill J.R., Dey A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends. Cancer. 2019;5:297–307. doi: 10.1016/j.trecan.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 117.Brennecke J., Hipfner D.R., Stark A., Russell R.B., Cohen S.M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/S0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 118.Liu X., Zhao J., Liu Q., Xiong X., Zhang Z., Jiao Y., Li X., Liu B., Li Y., Lu Y. MicroRNA-124 promotes hepatic triglyceride accumulation through targeting tribbles homolog 3. Sci. Rep. 2016;6:37170. doi: 10.1038/srep37170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ilyas Z., Angyal A., Szili D., Johnston J., Kiss-Toth E. 223 MIRNA202 is a Novel Regulator of Tribbles-1 Expression. Heart. 2015;101:A121. doi: 10.1136/heartjnl-2015-308066.223. [DOI] [Google Scholar]
- 120.Ye Y., Wang G., Wang G., Zhuang J., He S., Song Y., Ni J., Xia W., Wang J. The Oncogenic Role of Tribbles 1 in Hepatocellular Carcinoma Is Mediated by a Feedback Loop Involving microRNA-23a and p53. Front. Physiol. 2017;8:789. doi: 10.3389/fphys.2017.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bjorklund M., Taipale M., Varjosalo M., Saharinen J., Lahdenpera J., Taipale J. Identification of pathways regulating cell size and cell-cycle progression by RNAi. Nature. 2006;439:1009–1013. doi: 10.1038/nature04469. [DOI] [PubMed] [Google Scholar]
- 122.Hua F., Mu R., Liu J., Xue J., Wang Z., Lin H., Yang H., Chen X., Hu Z. TRB3 interacts with SMAD3 promoting tumor cell migration and invasion. J. Cell Sci. 2011;124:3235–3246. doi: 10.1242/jcs.082875. [DOI] [PubMed] [Google Scholar]
- 123.NCBI R.C. Database resources of the National Center for Biotechnology Information. Nucleic Acids. Res. 2018;46:D8–D13. doi: 10.1093/nar/gkx1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chernukhin I.V., Seago J.E., Newbury S.F. Drosophila 5′-->3′-exoribonuclease Pacman. Methods Enzymol. 2001;342:293–302. doi: 10.1016/s0076-6879(01)42553-5. [DOI] [PubMed] [Google Scholar]
- 125.Grima D.P., Sullivan M., Zabolotskaya M.V., Browne C., Seago J., Wan K.C., Okada Y., Newbury S.F. The 5′-3′ exoribonuclease pacman is required for epithelial sheet sealing in Drosophila and genetically interacts with the phosphatase puckered. Biol. Cell. 2008;100:687–701. doi: 10.1042/BC20080049. [DOI] [PubMed] [Google Scholar]
- 126.Waldron J.A., Jones C.I., Towler B.P., Pashler A.L., Grima D.P., Hebbes S., Crossman S.H., Zabolotskaya M.V., Newbury S.F. Xrn1/Pacman affects apoptosis and regulates expression of hid and reaper. Biol. Open. 2015;4:649–660. doi: 10.1242/bio.201410199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zabolotskaya M.V., Grima D.P., Lin M.D., Chou T.B., Newbury S.F. The 5′-3′ exoribonuclease Pacman is required for normal male fertility and is dynamically localized in cytoplasmic particles in Drosophila testis cells. Biochem. J. 2008;416:327–335. doi: 10.1042/BJ20071720. [DOI] [PubMed] [Google Scholar]
- 128.Littleton J.T., Stern M., Schulze K., Perin M., Bellen H.J. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 129.Littleton J.T., Bellen H.J. Synaptotagmin controls and modulates synaptic-vesicle fusion in a Ca(2+)-dependent manner. Trends. Neurosci. 1995;18:177–183. doi: 10.1016/0166-2236(95)93898-8. [DOI] [PubMed] [Google Scholar]
- 130.Yamamura R., Ooshio T., Sonoshita M. Tiny Drosophila makes giant strides in cancer research. Cancer Sci. 2020 doi: 10.1111/cas.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sonoshita M., Scopton A.P., Ung P.M.U., Murray M.A., Silber L., Maldonado A.Y., Real A., Schlessinger A., Cagan R.L., Dar A.C. A whole-animal platform to advance a clinical kinase inhibitor into new disease space. Nat. Chem. Biol. 2018;14:291–298. doi: 10.1038/nchembio.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gramates L.S., Marygold S.J., Santos G.D., Urbano J.M., Antonazzo G., Matthews B.B., Rey A.J., Tabone C.J., Crosby M.A., Emmert D.B., et al. The FlyBase Consortium FlyBase at 25: Looking to the future. Nucleic Acids Res. 2017;45:D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.