Abstract
Sleep disturbance, particularly insomnia, is common in children with autism spectrum disorders (ASD). Furthermore, disturbed sleep affects core symptoms and other related comorbidities. Understanding the causes and consequences of sleep disturbances in children with ASD is an important step toward mitigating these symptoms. To better understand the connection between sleep duration and ASD severity, we analyzed ASD-related symptoms using the Autism Diagnostic Interview-Revised (ADI-R), Autism Diagnostic Observation Schedule (ADOS), IQ scores, and parent reports of the average amount of time slept per night that were available in the medical histories of 2,714 children with ASD in the Simons Simplex Collection (SSC). The mean (SD) sleep duration was 555 minutes. Sleep duration and severity of core ASD symptoms were negatively correlated, and sleep duration and IQ scores were positively correlated. Regression results indicated that more severe social impairment, primarily a failure to develop peer relationships, is the core symptom most strongly associated with short sleep duration. Furthermore, increased severity for numerous maladaptive behaviors assessed on the Child Behavior Checklist, as well as reports of attention deficit disorder, depressive disorder, and obsessive compulsive disorder were associated with short sleep duration. Severity scores for social/communication impairment and restricted and repetitive behaviors (RRB) were increased, and IQ scores were decreased, for children reported to sleep ≤420 minutes per night (lower 5th percentile) compared to children sleeping ≥660 minutes (upper 95th percentile). Our results indicate that reduced amounts of sleep are related to more severe symptoms in children with ASD.
Keywords: comorbid conditions, sleep (disorders), subtypes (of ASD), social cognition, neurology
Introduction
Autism spectrum disorders (ASD) are heterogeneous neurodevelopmental disorders characterized by social and communication impairments and the presence of restricted and repetitive behavioral patterns (APA, 2013). Sleep disturbances, are commonly observed in children with ASD. The most prevalent sleep problem identified in children with ASD is insomnia, with estimates ranging from 50 to 80% (Couturier et al., 2005; Goldman et al., 2009;Krakowiak, Goodlin-Jones, Hertz-Picciotto, Croen, & Hansen, 2008; Sivertsen, Posserud, Gillberg, Lundervold, & Hysing, 2012; Souders et al., 2009). This high prevalence is in contrast to 20–30% in children of typical development (Maski & Owens, 2016; Owens, 2016). Insomnia is defined as difficulty falling asleep or staying asleep. Children with ASD often sleep for shorter total periods of time during the night compared to typically developing children and have long periods of awakenings throughout the night (Reynolds & Malow, 2011; Wiggs & Stores, 2004). A growing body of evidence indicates that sleep disturbances exacerbate impaired social interactions, communication problems, and presence of repetitive behaviors in children with ASD (Cohen, Conduit, Lockley, Rajaratnam, & Cornish, 2014;Gabriels, Cuccaro, Hill, Ivers, & Goldson, 2005; Malow et al., 2006). In particular, increased severity of sleep onset delay and short sleep duration were observed to predict of increased severity of core ASD symptoms, including social skill deficits and stereotypic behavior (Tudor, Hoffman, & Sweeney, 2012). Additionally, fewer hours of sleep per night were observed to predict difficulties with social interactions, stereotypic behavior, and overall diagnostic characteristics of autism that were measured via the Gilliam Autism Rating Scale (Schreck, Mulick, & Smith, 2004). Furthermore, children with ASD who sleep fewer hours at night, with waking during the night, have been observed to have more socialization and communication problems (Taylor, Schreck, & Mulick, 2012). Shorter sleep durations were also observed to relate to maladaptive behaviors and other serious comorbidities in ASD, including reduced overall adaptive functioning, daily living skills, motor development, and lower overall intelligence (Taylor et al., 2012). Increased severity of short sleep duration has also been found to correlate with increased physical aggression, hostility, inattention, and hyperactivity (Mazurek & Sohl, 2016).
In typically developing children, sleeping too little is associated with attention, behavior, and learning problems. Insufficient sleep is also associated with increased risk for depression, and in teenagers, increased risk of self-harm, suicidal thoughts, and suicide attempts (Paruthi et al., 2016). Notably, recent evidence suggests that sleep promotes normal brain development (Kayser, Yue, & Sehgal, 2014) and sleep disruption during development may affect adult brain connectivity (Billeh et al., 2016). This indicates that sufficient sleep is essential for normal neuronal development. Sleep deprivation has been observed to affect the neural circuitry underlying emotional regulation, including abnormal connectivity of the amygdala and prefrontal cortex (Maski & Kothare, 2013); this abnormal amygdala-prefrontal cortex connectivity has also been observed in ASD (Wiggins & Monk, 2013). In ASD, children may be even more vulnerable to the detrimental effects of sleep disruption on daytime behavior. There is potentially a critical window during neurodevelopment where the quality of sleep has a lasting impact on neurological function. As such, the need for effective treatments for sleep disturbances in children with ASD is profound.
It is possible that ASD symptoms drive disturbed sleep or that expression of ASD symptoms with comorbid sleep disturbances are modified by convergent genetic risk factors (Hu et al., 2009; Pagan et al., 2014; Veatch, Goldman, Adkins, & Malow, 2015a; Veatch, Maxwell-Horn, & Malow, 2015b; Yang et al., 2016). Defining the relationship of disturbed sleep to ASD symptom severity in large databases with extensive phenotypic and genetic data available is a necessary step toward generating hypotheses about how short sleep duration may affect ASD, and moving toward more effective approaches to mitigate these symptoms. The majority of sleep-related information available in large ASD databases is limited to data derived from parent report, primarily questions in medical history forms and questionnaires. To determine the accuracy of parent reports of sleep problems, our group previously compared parent report (i.e., the Children’s Sleep Habits Questionnaire (CSHQ)(Owens, Spirito, & McGuinn, 2000)) to actigraphy-measured data from 78 children with ASD (Veatch et al., 2016). We observed only one parent reported insomnia-related trait was significantly correlated with the comparable actigraphy-measure. This trait was the amount of time the child slept and actigraphy-measured nighttime sleep duration (ρ = 0.30, P = 8.0 × 10−3). Notably, parent reports of the severity of sleep onset delay were not correlated with actigraphy-measured sleep latency, nor were parent-reported severity of night wakings correlated with actigraphy-measured wake after sleep onset. Furthermore, there was no difference between actigraphy measurements for children whose parents did report a problem in a sleep-related behavior compared to those whose parent did not report a problem. Thus, sleep duration appears to be the most accurate parent-reported sleep trait.
The aim of the present study was to use data available in one of the largest collections of ASD samples, the Simons Simplex Collection (SSC), to examine the relationship between shorter sleep duration and ASD symptomatology (Fischbach & Lord, 2010). Furthermore, our goal was to pinpoint specific core symptoms and/or important comorbidities associated with short sleep duration in children with ASD. Our hypothesis was that shorter sleep duration would be associated with increased impairment of core symptoms, expression of comorbidities with evidence for a connection with sleep restriction, and more challenging daytime behaviors in ASD.
Methods
Dataset Demographics
The analysis dataset consisted of 2,714 children in the Simons Simplex Collection (SSC), which represents the largest cohort of autism simplex families currently collected (Fischbach & Lord, 2010). All children included in our analyses met DSM-5 criteria for ASD (APA, 2013) with confirmatory Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994) and Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1989). Verbal and nonverbal IQ scores were derived from a developmental hierarchy of cognitive measures, mainly the Differential Ability Scales, 2nd Edition (Elliott, 2006) and Mullen Scales of Early Learning (Mullen, 1995), and were available for all but one individual in the analysis dataset (n = 2,713). Deviation IQs (i.e., IQs derived from standard scores) were used to estimate verbal IQs for 85% of the dataset, while Ratio IQs (i.e., IQs derived by dividing verbal mental age by chronological age for children who were not able to obtain a Verbal Deviation IQ) were used for 15% of the dataset. The mean of the verbal IQ scores was 78.09+/−31.24, and scores ranged from 5 to 167. Deviation IQ scores were used to estimate nonverbal IQs for 81% of the dataset. The mean of the nonverbal IQ scores was 84.60+/−26.11, and ranged from 9 to 161.
The dataset was 86% male and children were between 4 and 18 years old, with a mean age of 9.04+/−3.58 years old, at the time of the ADOS and medical history collection. As sleep duration typically varies based on the individual’s age and environmental influences, it is notable that the proportion of children who were age 4–5 years old (preschool age) was ~17.7%, 6–12 years old (primary school-age) was −63.7%, and ≥13 years old (secondary-school age) was ~18.5%.
Phenotype Data Comparisons
All variables included in analyses were obtained from the SSC’s standard research battery and detailed medical histories. From the Autism Diagnostic Interview-Revised (ADI-R), we analyzed subscale scores evaluating specific symptoms related to social impairment, communication impairment, and restricted and repetitive behaviors (RRB), as well as overall severity scores in each domain. The ADI-R is an interview given by trained experts to caregivers of children suspected of having ASD (Lord et al., 1994). Communication severity scores are divided into verbal and nonverbal scores, and each individual receives a nonverbal score but not necessarily a verbal score. Of the 2,714 children in the total analysis dataset, 2,388 children (88%) had verbal communication impairment scores.
We evaluated calibrated severity scores (CSS) for core ASD symptoms obtained from the Autism Diagnostic Observation Schedule (ADOS). The ADOS is a semistructured observational assessment of children suspected of having ASD (Lord et al., 1989). Similar to the ADI-R, the ADOS provides overall severity scores related to social impairment, communication impairment and RRB. The ADOS has four modules used for assessment, based on the individual’s age and current developmental level. There were 508 children with ADOS scores from Module 1 (18.7%), 595 from Module 2 (21.9%), 1,540 from Module 3 (56.7%), and 71 from Module 4 (2.6%). ADOS CSS are generated in order to standardize ADOS scores allowing for comparisons of scores across Modules 1, 2, and 3. ADOS CSS are not calculated for individuals who are assessed using Module 4 (Gotham, Pickles, & Lord, 2009), therefore we analyzed CSS in 97% of the dataset (n = 2,643). Procedures for separately deriving the Social Affect (impairment in social and communication skills) and RRB CSS from the ADOS are detailed in Hus, Gotham, and Lord (2014).
Based on results from our previous study comparing parent-reported sleep traits to objective measures that were determined via actigraphy (Veatch et al., 2016), we expected that the most reliable sleep trait that could be derived from parent-report would be sleep duration. To approximate sleep duration, current answers to the question “On average, how many hours/night [does your child sleep]?” in the “Sleep Problems: Regularity and Duration” portion of the SSC medical history form was used. Subjective responses of “normal,” “less than normal,” “more than normal,” and “not sure” were coded as missing data. Of the 2,714 children with a numeric response to this question who also had available ADI-R, ADOS, there were 65 (2.4%) children for whom parents reported a range of hours. For these children, the median value in the range was used. For example, if a parent reported that the child sleeps 10–12 hours per night, a value of 11 hours was used in analyses. Prior to analyses, the reported number of hours per night were multiplied by 60 to determine the number of minutes, allowing for more interpretable effect estimates. Of the 2,714 children with a “current” average sleep duration reported, only 320 children (~12%) had an “ever” sleep duration reported. Therefore, we focused on current reports of sleep duration. All analyses were conducted using STATA, Version 13, College Station, TX (Statacorp, 2013).
Traits included in these analyses represent different types of statistical variables including ordinal, finite variables (i.e., ADI-R, ADOS), and continuous, infinite variables (i.e., sleep duration, age at exam, verbal IQ, and nonverbal IQ). Therefore, nonparametric Spearman rank correlation coefficients (ρ) were calculated to determine the relationship between sleep duration and social, communication, and RRB scores from the ADI-R and the ADOS, nonverbal and verbal IQ scores, and age at the time of the ADOS.
Association tests evaluating the relationship of sleep duration with core ASD symptoms, IQ, and comorbidities.
To determine if sleep duration was associated with severity scores from the ADI-R and ADOS, IQ scores, or age at ADOS, linear regression was conducted with sleep duration included as a quantitative outcome variable. Each ASD-related trait was analyzed independently, as well as in a “full” model that included all of the traits associated with sleep duration in independent tests. The full regression model included: ADI-R Social Impairment, ADI-R Nonverbal Communication Impairment, ADI-R Verbal Communication Impairment, ADI-R RRB, ADOS Social Affect CSS, Verbal IQ Score, Nonverbal IQ Score, and Age at ADOS. To determine if impairment in any specific behaviors contributed more strongly to the association signal, subscale-level scores that are included in the calculation of total severity scores from the ADI-R that remained significantly associated with sleep duration in the full model regression analysis were further analyzed in a linear regression model that incorporated all subscale-level scores, IQs, and age. To ensure that collinearity was not a concern in multiple regression analyses, the variance inflation factor (VIF) was calculated. VIF estimates indicated that the severity of multicollinearity was low (Total Scores_Mean VIF = 2.57, Subscale Scores_Mean VIF = 1.56), suggesting there were no appreciable effects on the variance of the estimated coefficients (Kutner, Nachtsheim, & Neter, 2004).
To determine if sleep duration was associated with any comorbidities of interest in children with ASD, linear regression was conducted with sleep duration included as a quantitative outcome variable and parent reports of a comorbid diagnosis in the child with ASD as predictors. Comorbidities were analyzed if they were reported in the SSC medical histories to be present in at least 10 children and observed in previous studies to have a connection with sleep disturbances. These comorbidities included anxiety disorder (Neckelmann, Mykletun, & Dahl, 2007; Ohayon & Roth, 2003), attention deficit disorder (ADD) (Hysing, Lundervold, Posserud, & Sivertsen, 2016), bipolar disorder (Wulff, Gatti, Wettstein, & Foster, 2010), depressive disorder (Nechita, Pirlog, & ChiriTa, 2015; Nutt, Wilson, & Paterson, 2008; Stewart, Barnard, Pearson, Hasan, & O’brien, 2006), obsessive compulsive disorder (Raines et al., 2015; Timpano, Carbonella, Bernert, & Schmidt, 2014), epileptic seizures (Accardo & Malow, 2015), migraines (Luc, Gupta, Birnberg, Reddick, & Kohrman, 2006; Peres, 2005; Vogler, Rapoport, Tepper, Sheftell, & Bigal, 2006), and constipation and irritable bowel disorder (Aldinger, Lane, Veenstra-VanderWeele, & Levitt, 2015;Ferguson et al., 2016). For associated comorbidities, studen’s t-tests were conducted comparing the mean sleep duration for children with the reported comorbidity to those without. Reports of comorbidities in the SSC medical histories indicate presence of a comorbid diagnosis at any point in the child’s developmental history. To better understand the relationship of current sleep duration with current severity of comorbidity-related symptoms as well as maladaptive behaviors, we evaluated the relationship of sleep duration with age-standardized Child Behavior Checklist (CBCL) T scores (Achenbach, 1991) using linear regression. Of the 2,714 children included in the above analyses, there were 2,709 children with caregiver reported behavior problems available from the CBCL.
Evaluating the effects of age on the associations of sleep duration and ASD-related traits.
To evaluate the effects of age on the relationship of ASD-related symptom severity and sleep duration, variables from the ADI-R that remained significantly associated in full models, as well as comorbidities and CBCL scores that were independently associated with sleep duration were further assessed using interaction analyses as follows: Sleep Duration = β0+βASD-related Trait+βAge at ADOS+ (βASD-related Trait*βAge at ADOS). ASD-related traits and comorbidities where the effect of age on the association with sleep duration was significant (P < 0.05) were further analyzed in distinct age-based subgroups defined as: 4–5 years old (preschool age), 6–12 years old (primary school-age), and ≥13 years old (secondary-school age).
Evaluating the effects of neurological impairment and medication use on the associations of sleep duration and ASD-related traits.
To evaluate the potential influence of neurological conditions and medication use on the relationship between sleep duration and ASD severity, we conducted regression testing the association of sleep duration with reports of neurological issues and current medication usage. Children that were reported to be currently taking the medications that were associated with sleep duration in this dataset were excluded and the remaining 2,042 children were evaluated for relationships between sleep duration and ASD-related symptoms that were associated in the full dataset.
Comparison of ASD-related symptoms in subgroups representing extreme tails of the overall sleep duration distribution.
Correlations indicated that many of the evaluated ASD-related symptoms had a nonlinear relationship with sleep duration. To better understand these relationships, the distribution of sleep duration across the analysis dataset was determined to identify children with extremely short, extremely long, and nonextreme sleep duration. There are currently no accepted criteria regarding the amount of sleep that is considered ideal for children with ASD. In typically developing children, several studies have used a definition of short sleep that was based on sleep duration of less than the 10th percentile for reported norms by age (Nixon et al., 2008; Owens, Mehlenbeck, Lee, & King, 2008; Paavonen et al., 2009; Pesonen et al., 2010). As children included in these analyses were not typically developing it seemed appropriate to use a more stringent cutoff for defining extremes. Furthermore, given the large dataset and the broad age range of 4–18 years, we defined phenotypic extremes of sleep duration as the lower 5th and upper 95th percentiles of the observed distribution. Based on this cutoff, short sleep was defined as ≤7 hours, which is not sufficient sleep to promote optimal health in children, according to the American Academy of Sleep Medicine (Paruthi et al., 2016). Kruskal-Wallis tests were then conducted to determine if ADI-R, ADOS, IQ scores, or CBCL T scores were different across sleep duration subgroups representing children with sleep duration: (1) in the lower 5th percentile (i.e., extremely short), (2) between the 5th and 95th percentile (i.e., nonextreme), and (3) in the upper 95th percentile (i.e., extremely long) of the distribution. We were particularly interested in determining if ASD symptoms were more severe in children having extremely short sleep duration compared to those with longer sleep duration. ANCOVAs were conducted, while adjusting for age, to compare ADI-R, ADOS, IQ, and CBCL scores between children with short versus long sleep duration. Post-hoc comparisons of mean scores for ASD-related symptom severity between sleep duration subgroups were conducted via Tukey Kramer tests. Logistic regression was used to determine if any comorbidities of interest were associated with the short sleep duration subgroup.
Results
Associations of Core ASD Symptoms, IQ, and Relevant Comorbidities with Sleep Duration
Social/communication impairment and RRB severity scores from the ADI-R were negatively correlated with parent-reported sleep duration (Fig. 1A–D). Increased Social Affect scores from the ADOS were moderately correlated with shorter sleep duration; however, RRB scores from the ADOS were not correlated with sleep duration (Fig 1E, F). Verbal and nonverbal IQ scores were positively correlated with sleep duration (Fig. 1G, H).
Figure 1.
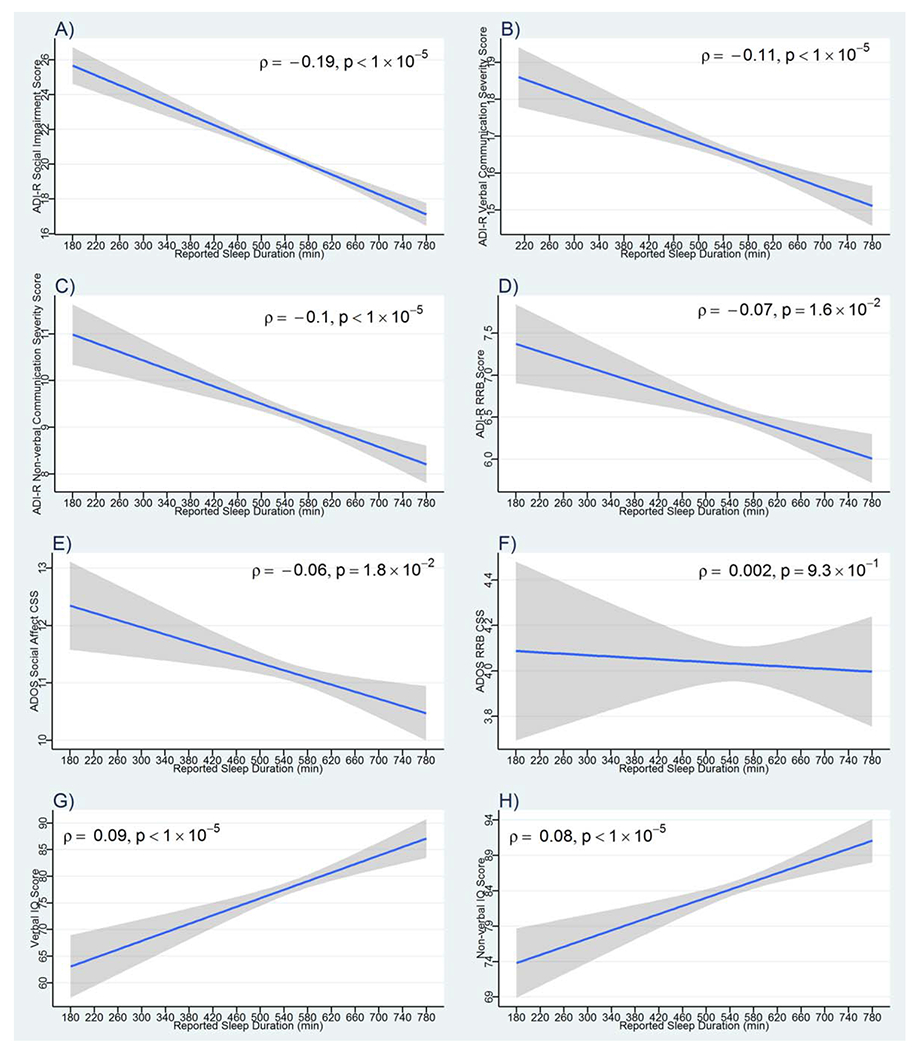
Correlation between ASD-related symptom severity and sleep duration. Plotted are the linear predictions for the relationships between sleep duration in minutes with: (A) ADI-R Social Impairment Scores, (B) ADI-R Verbal Communication Impairment Scores, (C) ADI-R Nonverbal Communication Impairment Scores, (D) ADI-R Restricted and Repetitive Behavior Scores, (E) ADOS Social Affect Calibrated Severity Scores, (F) ADOS Restricted and Repetitive Behavior Calibrated Severity Scores, (G) Verbal IQ scores, and (H) Nonverbal IQ scores. 95% confidence intervals around fitted lines are indicated in gray; Spearman’s rank correlation coefficients (ρ) and the corresponding P-values are provided. ADI-R, Autism Diagnostic Interview-Revised; ADOS, Autism Diagnostic Observation Schedule.
Increases in severity for social/communication impairment and RRB scores from the ADI-R, and Social Affect scores from the ADOS were independently associated with shorter sleep duration. In addition, lower IQ scores were also associated with shorter sleep duration (Table 1). Following evaluation of these traits in a full regression model, which also corrected for age, more severe social impairment and RRB from the ADI-R remained associated with shorter sleep duration. Nonverbal communication scores from the ADI-R were also associated with shorter sleep duration in the full regression model; however, the direction of effect was different when correcting for all other independently associated variables (Table 1).
Table 1.
ASD-Related Traits Associated with Sleep Duration. Severity Scores for Each ASD-Related Trait were Independently Analyzed for Association with Sleep Duration. Threshold for Significance for Independent Tests was Set at a Bonferroni-Corrected P-Value < 5.0 × 10−3. Score Ranges are Included in Parentheses Next to the Variable Description. Significantly Associated Traits were Further Analyzed in a Full Model. Increased Social Impairment and RRB Scores from the ADI-R Remained Associated with Shorter Sleep Duration in the Full Model
| ASD-Related Trait (df = 2,713) | βa (95% CI) | P-value | R2 |
|---|---|---|---|
| Social Impairmentadi-R (8–30) | −2.54 (−3.04, −2.05) | <1.0 × 10−4 | 0.0363 |
| NV Communicationadi-R (0–14) | −2.28 (−3.11, −1.45) | <1.0 × 10−4 | 0.0106 |
| Verbal Communicationadi-R (6–26)* | −1.86 (−2.55, −1.16) | <1.0 × 10−4 | 0.0114 |
| RRBADI-R (0–12) | −2.13 (−3.27, −0.98) | <1.0 × 10−4 | 0.0048 |
| CSSADOS (4–10)** | −0.83 (−2.57, 0.90) | 3.5 × 10−1 | 0.0003 |
| Social Affect CSSADOS (2–20)** | −1.13 (−1.86, −0.41) | 2.0 × 10−3 | 0.0035 |
| RRB CSSADOS (0–8)** | −0.21 (−1.63, 1.21) | 7.7 × 10−1 | 0.000 |
| Verbal IQ score (5–167) | 0.24 (0.15, 0.33) | <1.0 × 10−4 | 0.0096 |
| Nonverbal IQ score (9–161) | 0.25 (0.14, 0.36) | <1.0 × 10−4 | 0.0071 |
| Age at ADOS (4–18 years) | −8.03 (−8.77, −7.89) | <1.0 × 10−4 | 0.1423 |
| Full regression model (df = 2,316) | |||
| Social ImpairmentADI-R | −1.20 (−1.89, −0.51) | 1.0 × 10−3 | 0.1803 |
| NV CommunicationADI-R | 1.95 (0.13, 3.78) | 3.6 × 10−2 | |
| Verbal CommunicationADI-R | −1.09 (−2.58, 0.39) | 1.5 × 10−1 | |
| RRBADI-R | −1.40 (−2.55, −0.25) | 1.7 × 10−2 | |
| Social Affect CSSADOS | −0.27 (−1.07, 0.54) | 5.3 × 10−1 | |
| Verbal IQ Score | 0.11 (−0.06, 0.29) | 2.0 × 10−1 | |
| Nonverbal IQ Score | −0.09 (−0.28, 0.10) | 3.7 × 10−1 | |
| Age at ADOS | −8.35 (−9.19, −7.51) | <1.0 × 10−4 |
For every one unit score increase in the ASD-related trait, or 1 year increase in age, the β-coefficient represents the number of minutes sleep duration either decreases or increases.
Indicates sample size reduction as a result of unavailable ADI-R Verbal Communication Impairment Scores, df = 2,387;
Indicates sample size reduction as a result of children having been evaluated on ADOS Module 4, df = 2,643.
ADI-R, Autism Diagnostic Interview-Revised; ADOS, Autism Diagnostic Observation Schedule; CSS, calibrated severity scores; RRB, restricted and repetitive behaviors; NV, nonverbal; df, degrees of freedom.
Evaluation of subscales for social impairment and RRB total severity scores from the ADI-R revealed that it was primarily a “failure to develop peer relationships” and presence of “apparently compulsive adherence to nonfunctional routines or rituals” that were associated with shorter sleep duration (Table 2). No subscales included in the total communication severity scores were associated with sleep duration (Table 2).
Table 2.
Association of Sleep Duration with Specific Behaviors Reflecting Impairments in Core ASD-Related Traits. Subscale Scores, from the Autism Diagnostic Interview-Revised, Representing Specific Behaviors That are Included in the Total Severity Scores That were Associated with Sleep Duration were Further Analyzed to Determine Which Specific Symptoms were Most Associated with Sleep. Increased Severity in Failure to Develop Peer Relationships and Compulsive Rituals were Associated with Shorter Sleep Duration. Score Ranges are Included in Parentheses Next to the Variable Description
| Autism-Related Trait | βa (95% CI) | P-value (df = 2,384) | R2 |
|---|---|---|---|
| Failure to use nonverbal behaviors (0–6) | −0.63 (−2.79, 1.52) | 5.6 × 10−1 | 0.1910 |
| Failure to develop peer relationships (0–8) | −4.17 (−6.13, −2.21) | <1.0 × 10−4 | |
| Lack of shared enjoyment (0–6) | −0.26 (−2.37, 1.84) | 8.1 × 10−1 | |
| Lack of socioemotional reciprocity (0–10) | −0.32 (−2.00, 1.37) | 7.1 × 10−1 | |
| Delay in language or use of gestures (0–8) | 0.38 (−0.92, 1.38) | 5.7 × 10−1 | |
| Failure to initiate conversation (0–4) | −0.54 (−4.65, 3.56) | 8.0 × 10−1 | |
| Stereotyped, repetitive or idiosyncratic speech (0–8) | −1.34 (−2.98, 0.30) | 1.1 × 10−1 | |
| Lack of make believe play (0–6) | 1.87 (−0.55, 4.30) | 1.3 × 10−1 | |
| Encompassing preoccupation (0–4) | −0.24 (−2.70, 2.23) | 8.5 × 10−1 | |
| Compulsive nonfunctional rituals (0–4) | −2.29 (−4.29, −0.29) | 2.5 × 10−2 | |
| Repetitive motor mannerisms (0–2) | −1.62 (−5.08, 1.84) | 3.6 × 10−1 | |
| Preoccupations with objects (0–2) | −0.38 (−5.19, 4.43) | 8.8 × 10−1 | |
| Verbal IQ Score (5–167) | 0.11 (−0.06, 0.28) | 1.9 × 10−1 | |
| Nonverbal IQ score (9–161) | −0.10 (−0.29, 0.08) | 2.8 × 10−1 | |
| Age at ADOS (4–18 years) | −8.42 (−9.22, −7.61) | <1.0 × 10−4 |
For every one unit score increase in the ASD-related trait, or 1 year increase in age, the β-coefficient represents the number of minutes sleep duration either decreases or increases.
ADOS, Autism Diagnostic Observation Schedule; df, degrees of freedom.
In addition to more severe core ASD-related symptoms and lower IQs, shorter sleep duration was associated with increased reports of ADD, depressive disorder, and obsessive compulsive disorder (Table 3). Shorter sleep duration was also associated with increased severity for numerous maladaptive behaviors that were measured via the CBCL (Table 4). These included having more severe pervasive developmental symptoms, social problems, sleep problems, attention deficit/attention deficit hyperactivity disorder (ADD/ADHD) behaviors, affective problems, anxiety, anxious/depressed symptoms, attention problems, emotional reactivity, internalizing problems, and thought problems.
Table 3.
Comorbidities Associated with Sleep Duration. (A) Reports in the Medical History of Attention Deficit Disorder, Depressive Disorder, and Obsessive Compulsive Disorder were All Independently Associated with Shorter Current Sleep Duration. The Threshold for Significance was Set at a Bonferroni-Corrected P-Value < 6.0 × 10−3. (B) for Associated Comorbidities, Mean Sleep Duration was Compared between Children with the Reported Comorbidity to Those without
| Comorbidity | βa (95% CI) | P-value | R2 | ||
|---|---|---|---|---|---|
| A | |||||
| Anxiety Disorder (n = 96/2,540) | −10.15 (−25.72, 5.42) | 2.0 × 10−1 | 0.0006 | ||
| Attention Deficit Disorder (n = 361/2,556) | −18.43 (−26.94, −9.92) | <1.0 × 10−4 | 0.0070 | ||
| Bipolar Disorder (n = 13/2,549) | −30.22 (−73.50, 13.05) | 1.7 × 10−1 | 0.0007 | ||
| Constipation (n = 727/2,702) | −3.57 (−10.06, 2.93) | 2.8 × 10−1 | 0.0004 | ||
| Depressive Disorder (n = 21/2,522) | −65.68 (−98.41, −32.94) | <1.0 × 10−4 | 0.0061 | ||
| Irritable Bowel Disorder (n = 20/2,709) | −5.62 (−39.23, 27.99) | 7.3 × 10−1 | 0.0000 | ||
| Migraines (n = 37/2,524) | −25.91 (−50.73, −1.08) | 4.1 × 10−2 | 0.0017 | ||
| Obsessive Compulsive Disorder (n = 85/2,552) | −25.76 (−42.27, −9.22) | 2.0 × 10−3 | 0.0036 | ||
| Seizures (n = 133/2,556) | −4.65 (−17.93, 8.64) | 4.9 × 10−1 | 0.0002 | ||
| Comorbidity | Mean SD w/Comorbidity |
Mean SD w/o Comorbidity |
t (df) | d (95% CI) | P-value |
| B | |||||
| Attention Deficit Disorder | 538.84+/−76.87 | 557.27+/−76.35 | 4.23 (2554) | 0.86 (0.43, 1.29) | <1.0 × 10−5 |
| Depressive Disorder | 490.00+/−97.37 | 555.68+/−75.99 | 3.08 (20.23W) | 0.24 (0.13, 0.35) | 2.9 × 10−3 |
| Obsessive Compulsive Disorder | 529.83+/−86.15 | 555.69+/−76.05 | 2.72 (88.68W) | 0.34 (0.12, 0.55) | 3.9 × 10−2 |
n = the number of children reported to have the comorbidity/the total number of children with these data available.
β-coefficients represent the number of minutes sleep duration decreases given that the parent reported presence of the comorbidity in the proband; WIndicates the use of Welch’s approximation as the variance of sleep duration was unequal between the comparison groups.
SD, sleep duration; df, degrees of freedom.
Table 4.
Association of Sleep Duration with Numerous Maladaptive Behaviors. Shorter Sleep Duration was Associated with Increased Severity for Numerous Maladaptive Behaviors Measured with the Child Behavior Checklist (CBCL). The Threshold for Significance for Independent Tests was Set at a Bonferroni-Corrected P-Value < 2.9 × 10−3. Score Ranges are Included in Parentheses Next to the Variable Description
| Child Behavior Checklist T scores | βa (95% CI) | P-value (df) | R2 |
|---|---|---|---|
| Activities (0–65)* | 0.19 (−0.13, 0.52) | 2.4 × 10−1 (2,042) | 0.0007 |
| ADD/ADHD (50–80) | −0.65 (−0.99, −0.31) | <1.0 × 10−4 (2,708) | 0.0052 |
| Affective Problems (50–95) | −1.70 (−2.03, −1.38) | <1.0 × 10−4 (2,703) | 0.0373 |
| Aggressive Behavior (50–100) | −0.33 (−0.64, −0.02) | 3.5 × 10−2 (2,706) | 0.0016 |
| Anxiety Problems (50–95) | −0.94 (−1.25, −0.63) | <1.0 × 10−4 (2,704) | 0.0127 |
| Anxious/Depressed (50–98) | −0.93 (−1.25, −0.61) | <1.0 × 10−4 (2,705) | 0.0119 |
| Attention Problems (50–100) | −0.57 (−0.85, −0.28) | <1.0 × 10−4 (2,706) | 0.0054 |
| Conduct Problems (50–93)* | −0.07 (−0.50, 0.36) | 7.5 × 10−1 (2,056) | 0.0001 |
| Emotionally Reactive (50–93)** | −1.05 (−1.66, −0.44) | 1.0 × 10−3 (647) | 0.0173 |
| Externalizing Problems (32–97) | −0.08 (−0.35, 0.19) | 5.5 × 10−1 (2,706) | 0.0001 |
| Internalizing Problems (33–90) | −0.67 (−0.97, −0.37) | <1.0 × 10−4 (2,706) | 0.0070 |
| Oppositional Defiant (50–80) | −0.31 (−0.66, 0.03) | 7.4 × 10−2 (2,704) | 0.0012 |
| Pervasive Developmental (50–95)** | −1.36 (−2.01, −0.71) | <1.0 × 10−4 (647) | 0.0257 |
| Rule Breaking (50–84)* | 0.08 (−0.44, 0.60) | 7.6 × 10−1 (2,058) | 0.0000 |
| School Problems (0–99)* | −0.19 (−0.60, 0.22) | 3.5 × 10−1 (1,894) | 0.0005 |
| Sleep Problems (50–100)** | −2.14 (−2.72, −1.57) | <1.0 × 10−4 (644) | 0.0764 |
| Social Problems (50–91)* | −0.64 (−1.03, −0.24) | 2.0 × 10−3 (2,057) | 0.0048 |
| Somatic Complaints (50–94) | −0.41 (−0.79, −0.04) | 3.2 × 10−2 (2,706) | 0.0017 |
| Somatic Problems (50–93)* | −0.33 (−0.75, 0.09) | 1.2 × 10−1 (2,055) | 0.0012 |
| Thought Problems (50–91)* | −1.46 (−1.84, −1.09) | <1.0 × 10−4 (2,057) | 0.0277 |
| Withdrawn (50–100) | −0.29 (−0.60, −0.01) | 6.0 × 10−2 (2,706) | 0.0013 |
| Total Competence (0–66)* | 0.26 (−0.10, 0.63) | 1.6 × 10−1 (1,861) | 0.0011 |
| Total Problems (27–92) | −0.93 (−1.25, −0.62) | <1.0 × 10−4 (2,706) | 0.0123 |
For every one unit score increase in the CBCL T score, the β-coefficient represents the number of minutes sleep duration either decreases or increases.
Indicates the variable was only available for children evaluated using the CBCL for ages 6–18;
Indicates the variable was only available for children evaluated using the CBCL for ages 2–5.
df, degrees of freedom.
Interaction analyses indicated that age had a significant effect on the associations of social and nonverbal communication impairment scores from the ADI-R with sleep duration, but not RRB severity scores from the ADI-R (Supporting Information Table S1). Age also had an impact on the relationship of sleep duration with failure to develop peer relationships, but not compulsive rituals (Supporting Information Table S1). When analyses were restricted to children in age-defined subgroups, it was observed that children who were ages 4–12 years old were primarily driving the associations between shorter sleep duration and increased severity of core symptoms (Table 5).
Table 5.
Effects of Age on Associations of ASD-Related Traits with Sleep Duration. ASD-Related Traits for Which Interaction Terms in Regression Indicated Significant Effects of Age on the Association with Sleep Duration were Further Analyzed in Age-Related Subgroups Defined by Children Being Preschool (4–5 Years Old), Primary School (6–12 Years Old), or Secondary School (13–18 Years Old) Age at the Time of Sleep-Related Data Collection (i.e., Age at ADOS). The Majority of the Evaluated Traits were Associated with Sleep Duration in Children Who were Preschool and Primary School Age, but Not Secondary School Age. Age Was Associated with Sleep Duration for Children Who were Primary and Secondary School, but Not for Children Who were Preschool Age. Score Ranges are Included in Parentheses under the Variable Description
| Age group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4–5 years (n = 481) |
6–12 years (n = 1,730) |
13–18 years (n = 503) |
|||||||
| ASD-Related Trait | βa (95% CI) | P-value (df) | R2 | βa (95% CI) | P-value (df) | R2 | βa (95% CI) | P-value (df) | R2 |
| Social ImpairmentADI-R (8–30) | −3.07 (−4.26, −1.89) | <1.0 × 10−4 (480) | 0.0515 | −2.05 (−2.65, −1.45) | <1.0 × 10−4 (1,729) | 0.0255 | 0.60 (−0.50, 1.71) | 2.8 × 10−1 (502) | 0.0023 |
| Failure to Develop Peer RelationshipsADI-R (0–8) | −7.81 (−11.50, −4.12) | 1.0 × 10−4 (480) | 0.0348 | −5.86 (−7.86, −3.87) | <1.0 × 10−4 (1,728) | 0.0189 | −1.36 (−4.90, 2.19) | 4.5 × 10−1 (501) | 0.0011 |
| NV CommunicationADI-R (0–14) | −3.08 (−5.10, −1.07) | 3.0 × 10−3 (480) | 0.0186 | −1.96 (−2.94, −0.98) | <1.0 × 10−4 (1,729) | 0.0088 | 1.13 (−0.62, 2.89) | 2.1 × 10−1 (502) | 0.0032 |
| Affective ProblemsCBCL (50–95) | −2.42 (−3.17, −1.68) | <1.0 × 10−4 (478) | 0.0789 | −1.74 (−2.13, −1.35) | <1.0 × 10−4 (1,725) | 0.0428 | −0.52 (−0.87, 0.71) | 8.4 × 10−1 (498) | 0.0001 |
| Anxiety ProblemsCBCL (50–95) | −1.45 (−2,17, −0.73) | <1.0 × 10−4 (478) | 0.0320 | −0.44 (−0.82, −0.06) | 2.3 × 10−2 (1,726) | 0.0030 | 0.53 (−0.16, 1.22) | 1.3 × 10−1 (498) | 0.0046 |
| Anxious/DepressedCBCL (50–98) | −1.72 (−2.74, −0.71) | 1.0 × 10−3 (478) | 0.0227 | −0.39 (−0.76, −0.01) | 4.5 × 10−2 (1,727) | 0.0023 | 0.12 (−0.50, 0.75) | 7.0 × 10−1 (498) | 0.0003 |
| Internalizing ProblemsCBCL (33–90) | −1.49 (−2.26, −0.72) | <1.0 × 10−4 (478) | 0.0295 | −0.65 (−1.00, −0.31) | <1.0 × 10−4 (1,727) | 0.0079 | 0.08 (−0.55, 0.71) | 8.0 × 10−1 (499) | 0.0001 |
| Total ProblemsCBCL (27–92) | −1.58 (−2.23, −0.94) | <1.0 × 10−4 (478) | 0.0464 | −0.91 (−1.29, −0.53) | <1.0 × 10−4 (1,727) | 0.0128 | 0.004 (−0.72, 0.73) | 9.9 × 10−1 (499) | 0.0000 |
| Age at ADOS_years | −10.59 (−24.94, 3.72) | 1.5 × 10−1 (480) | 0.0044 | −8.23 (−9.96, −6.50) | <1.0 × 10−4 (1,729) | 0.0479 | −4.68 (−8.44, −0.92) | 1.5 × 10−2 (502) | 0.0118 |
For every one unit score increase in the ASD-related trait, or 1 year increase in age, the β-coefficient represents the number of minutes sleep duration either decreases or increases.
ADI-R, Autism Diagnostic Interview-Revised; RRB, restricted and repetitive behaviors; NV, nonverbal; ADOS, Autism Diagnostic Observation Schedule; CBCL, Child Behavior Checklist; df, degrees of freedom.
Age did not have significant effects on the associations between sleep duration and reports in the medical histories of comorbid ADD, depressive disorder, nor obsessive compulsive disorder (Supporting Information Table S1). However, age did influence the relationship of sleep duration with some T scores from the CBCL. In particular, age had a significant effect on the association of short sleep duration with more severe scores for affective problems, anxiety problems, anxious/depressed symptoms, internalizing problems, and total problems (Supporting Information Table S1). Similar to results in age-defined subgroups for core symptom severity, children who were ages 4–12 years old were primarily driving the associations between shorter sleep duration and increased CBCL T scores (Table 5).
Of the 2,714 children included in the analysis dataset, 948 children (~35%) were reported to have at least one comorbid neurological condition (Supporting Information Table S2). Sleep duration was not associated with having a neurological condition (P = 8.5 × 10−1), or with any specific neurological condition (P ≥ 1.6 × 10−1). Sleep duration was associated with current use of ADD medications, mood stabilizers, sedatives, and thalidomide (Supporting Information Table S3). In the subset of children who were reported to not be currently taking these medications, sleep duration remained associated with ADI-R Social Impairment scores, failure to develop peer relationships, and compulsive nonfunctional rituals, but not ADI-R RRB total severity scores, or ADI-R Nonverbal Communication Impairment scores (Supporting Information Table S4). Increased severity for all of the previously associated CBCL T scores remained associated with shorter sleep duration in these children (Supporting Information Table S5). Similar results were also observed for reports of depressive disorder; however, sleep duration was no longer associated with reports of ADD, or obsessive compulsive disorder (Supporting Information Table S5).
Differences in ASD-Related Symptoms in Subgroups Representing Extreme Tails of the Overall Sleep Duration Distribution
The mean sleep duration reported for 2,714 children with ASD in the SSC was 555 ± 76 minutes (9.25 ± 1.30 hours). There were 179 children with extremely short sleep duration (≤420 minutes or ≤7 hours) and 302 children with extremely long sleep duration (≥660 minutes or ≥11 hours) (Fig. 2). Social/communication impairment and RRB severity scores from the ADI-R, IQ scores and ages were different when comparing children with extremely short, nonextreme, and extremely long sleep duration (Table 6A). In addition, severity of social/communication impairment measured with both the ADI-R and ADOS were increased in children with extremely short sleep duration compared to children with extremely long sleep duration. Furthermore, children with extremely short sleep duration had more severe RRB, measured with the ADI-R, and lower IQ scores (Table 6B) compared to extremely long sleep duration.
Figure 2.
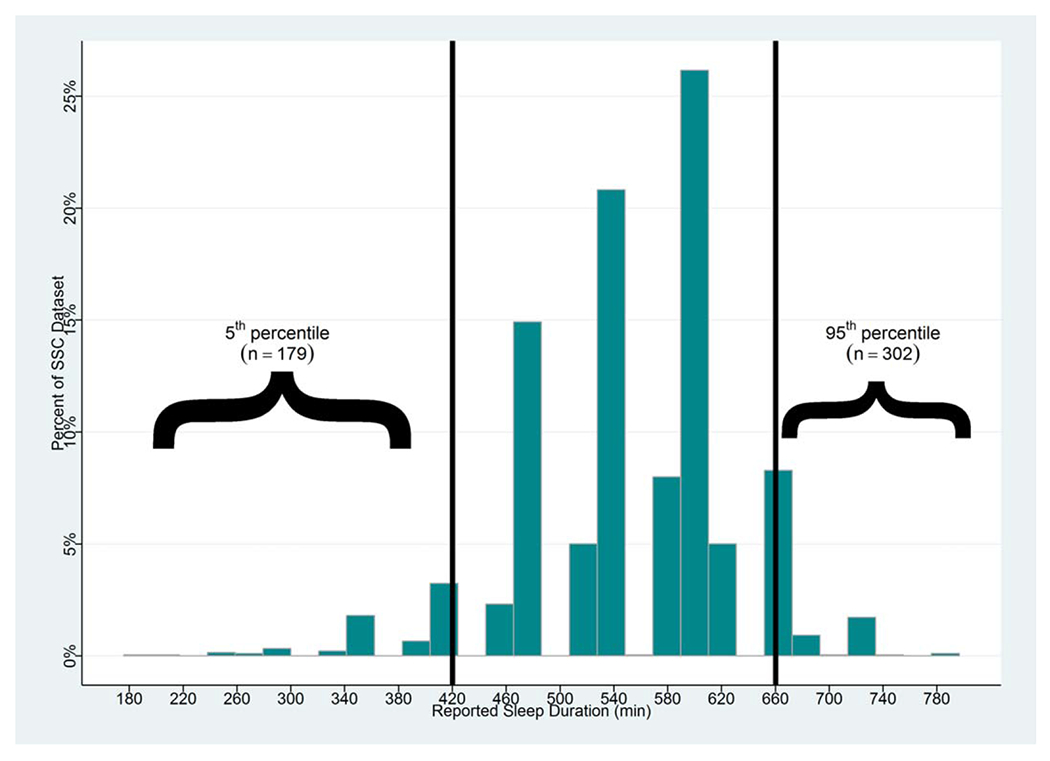
Distribution of parent-reported sleep duration. Plotted is the distribution of sleep durations that were reported in the Simons Simplex Collection medical histories for children with ASD (n = 2,714). The mean reported sleep duration was 555 +/−76 minutes. The frequency of sleep duration in the lower 5th (≤420 minutes) and upper 95th (≥660 minutes) percentile of the distribution are indicated with brackets.
Table 6.
Differences in ASD Severity Based on Sleep Duration Subgroups. (A) Results Comparing Scores for ASD-Related Traits across Children with Sleep Duration Reported in the (1) Lower 5th Percentile (N = 179), (2) between the 5th and 95th Percentile (N = 2,233), and (3) Upper 95th Percentile (N = 302) of the Distribution. (B) Analysis of Covariance and Age-Adjusted Mean Scores for ASD-Related Traits between Children with Sleep Duration Reported in the (1) Lower 5th Percentile, and (2) Upper 95th Percentile of the Distribution. Score Ranges Are Included in Parentheses Next to the Variable Description
| ASD-Related Trait | Chi2 (df = 2) | P-value | eta2 | ||
|---|---|---|---|---|---|
| A | |||||
| Social ImpairmentADI-R (8–30) | 57.92 | <1.0 × 10−4 | 0.0213 | ||
| NV CommunicationADI-R (0–14) | 21.55 | <1.0 × 10−4 | 0.0079 | ||
| Verbal CommunicationADI-R (6–26)* | 18.48 | <1.0 × 10−4 | 0.0068 | ||
| RRBADI-R (0–12) | 14.65 | 7.0 × 10−4 | 0.0054 | ||
| CSSADOS (4–10)** | 3.42 | 1.8 × 10−1 | 0.0013 | ||
| Social Affect CSSADOS (2–20)** | 3.32 | 1.9 × 10−1 | 0.0012 | ||
| RRB CSSADOS (0–8)** | 0.93 | 6.3 × 10−1 | 0.0003 | ||
| Verbal IQ Score (5–167) | 6.70 | 3.6 × 10−2 | 0.0025 | ||
| Nonverbal IQ Score (9–161) | 6.66 | 3.6 × 10−2 | 0.0025 | ||
| Age at ADOS (4–18 years) | 200.83 | <1.0 × 10−4 | 0.0740 | ||
| ASD-Related Trait | F (df) | P-value | eta2 | Mean 5th %ile | Mean 95th %ile |
| B | |||||
| Social ImpairmentADI-R (8–30) | 6.68 (480) | <1.0 × 10−5 | 0.61 | 21.37 | 18.32 |
| NV CommunicationADI-R (0–14) | 2.28 (480) | 4.1 × 10−3 | 0.07 | 9.42 | 8.52 |
| Verbal CommunicationADI-R (6–26) | 2.80 (403) | 4.0 × 10−4 | 0.10 | 16.64 | 15.43 |
| RRBADI-R (0–12) | 2.18 (480) | 6.4 × 10−3 | 0.07 | 6.87 | 5.96 |
| CSSADOS (4–10) | 1.39 (467) | 1.5 × 10−1 | 0.04 | 7.43 | 7.26 |
| Social Affect CSSADOS (2–20) | 7.81 (467) | 5.4 × 10−3 | 0.05 | 11.59 | 10.34 |
| RRB CSSADOS (0–8) | 1.32 (467) | 1.8 × 10−1 | 0.04 | 4.11 | 3.47 |
| Verbal IQ Score (5–167) | 2.83 (479) | 3.0 × 10−4 | 0.08 | 74.11 | 87.31 |
| Nonverbal IQ Score (9–161) | 2.99 (479) | 1.0 × 10−4 | 0.09 | 81.58 | 91.38 |
| Age at ADOS (4–18 years) | 182.09 (480) | <1.0 × 10−5 | 0.28 | 10.71 | 6.68 |
Indicates sample size reduction as a result of unavailable ADI-R Verbal Communication Impairment Scores (n1 = 143, n2 = 1,984, n3 = 261).
Indicates sample size reduction as a result of children having been evaluated on ADOS Module 4 (n1 = 170, n2 = 2,175, n3 = 298).
ADI-R, Autism Diagnostic Interview-Revised; ADOS, Autism Diagnostic Observation Schedule; CSS, calibrated severity score; RRB, restricted and repetitive behaviors; df, degrees of freedom.
The majority of maladaptive behaviors reported on the CBCL were also different when comparing sleep duration subgroups (Table 7A). Children with sleep duration reported in the lower 5th percentile had more severe CBCL T scores for social problems, pervasive developmental symptoms, ADD/ADHD symptoms, affective problems, anxious/depressed symptoms, and numerous other maladaptive behaviors (Table 7B). Increased reports of ADD (OR = 2.90, 95% CI = 1.66, 5.06, P < 1.0 × 10−4), depressive disorder (OR = 10.87, 95% CI = 1.30, 91.07, P = 2.8 × 10−2), and obsessive compulsive disorder (OR = 6.02, 95% CI = 1.63, 22.19, P = 7.0 × 10−3) were associated with the short sleep duration subgroup.
Table 7.
Differences in Maladaptive Behavior Severity Based on Sleep Duration Subgroups. (A) Results Comparing Scores for Child Behavior Checklist (CBCL) T Scores across Children with Sleep Duration Reported in the (1) Lower 5th Percentile, (2) between the 5th and 95th Percentile, and (3) Upper 95th Percentile of the Distribution. (B) Analysis of Covariance and Age-Adjusted Mean CBCL T Scores between Children with Sleep Duration Reported in the (1) Lower 5th Percentile, and (2) Upper 95th Percentile of the Distribution. Score Ranges are Included in Parentheses Next to the Variable Description
| Child Behavior Checklist T scores | n1 | n2 | n3 | Chi2 (df = 2) | P-value | eta2 |
|---|---|---|---|---|---|---|
| A | ||||||
| Activities (0–65)* | 149 | 1,744 | 150 | 6.79 | 3.4 × 10−2 | 0.0033 |
| ADD/ADHD (50–80) | 179 | 2,224 | 301 | 20.46 | <1.0 × 10−4 | 0.0076 |
| Affective Problems (50–95) | 179 | 2,224 | 301 | 71.10 | <1.0 × 10−4 | 0.0263 |
| Aggressive Behavior (50–100) | 179 | 2,224 | 301 | 9.30 | 9.6 × 10−3 | 0.0034 |
| Anxiety Problems (50–95) | 179 | 2,225 | 301 | 27.30 | <1.0 × 10−4 | 0.0101 |
| Anxious/Depressed (50–98) | 179 | 2,226 | 301 | 25.35 | <1.0 × 10−4 | 0.0094 |
| Attention Problems (50–100) | 179 | 2,227 | 301 | 18.68 | <1.0 × 10−4 | 0.0069 |
| Conduct Problems (50–93)* | 150 | 1,754 | 153 | 14.85 | 6.0 × 10−4 | 0.0072 |
| Emotionally Reactive (50–93)** | 29 | 471 | 148 | 3.92 | 1.4 × 10−1 | 0.0061 |
| Externalizing Problems (32–97) | 179 | 2,227 | 301 | 10.43 | 5.4 × 10−3 | 0.0039 |
| Internalizing Problems (33–90) | 179 | 2,227 | 301 | 11.70 | 2.9 × 10−3 | 0.0043 |
| Oppositional Defiant (50–80) | 179 | 2,225 | 301 | 6.62 | 3.7 × 10−2 | 0.0024 |
| Pervasive Developmental (50–95)** | 29 | 471 | 148 | 9.72 | 7.8 × 10−3 | 0.0150 |
| Rule Breaking (50–84)* | 150 | 1,756 | 153 | 8.44 | 1.5 × 10−2 | 0.0041 |
| School Problems (0–99)* | 133 | 1,635 | 137 | 2.23 | 3.3 × 10−1 | 0.0012 |
| Sleep Problems (50–100)** | 29 | 468 | 148 | 35.89 | <1.0 × 10−4 | 0.0557 |
| Social Problems (50–91)* | 150 | 1,755 | 153 | 8.48 | 1.4 × 10−2 | 0.0041 |
| Somatic Complaints (50–94) | 179 | 2,227 | 301 | 4.48 | 1.1 × 10−1 | 0.0017 |
| Somatic Problems (50–93)* | 150 | 1,753 | 153 | 7.09 | 2.9 × 10−2 | 0.0035 |
| Thought Problems (50–91)* | 150 | 1,756 | 152 | 58.16 | <1.0 × 10−4 | 0.0283 |
| Withdrawn (50–100) | 179 | 2,227 | 301 | 10.56 | 5.1 × 10−3 | 0.0039 |
| Total Competence (0–66)* | 129 | 1,602 | 131 | 2.95 | 2.3 × 10−1 | 0.0016 |
| Total Problems (27–92) | 179 | 2,227 | 301 | 30.55 | <1.0 × 10−4 | 0.0113 |
| Child Behavior Checklist T scores | F (df) | P-value | eta2 | Mean 5th %ile | Mean 95th %ile | |
| B | ||||||
| Activities (0–65)* | 158 (298) | 9.1 × 10−1 | 0.07 | 38.11 | 40.28 | |
| ADD/ADHD (50–80) | 2.59 (479) | 1.0 × 10−3 | 0.08 | 63.82 | 60.98 | |
| Affective Problems (50–95) | 5.58 × (479) | <1.0 × 10−5 | 0.15 | 66.82 | 59.59 | |
| Aggressive Behavior (50–100) | 1.49 (479) | 1.1 × 10−1 | 0.05 | 61.66 | 58.64 | |
| Anxiety Problems (50–95) | 2.62 (479) | 8.0 × 10−4 | 0.08 | 62.65 | 59.60 | |
| Anxious/Depressed (50–98) | 2.93 (479) | 2.0 × 10−4 | 0.09 | 60.31 | 57.69 | |
| Attention Problems (50–100) | 4.32 (479) | <1.0 × 10−5 | 0.12 | 68.78 | 65.01 | |
| Conduct Problems (50–93)* | 1.22 (302) | 2.6 × 10−1 | 0.05 | 59.29 | 56.66 | |
| Emotionally Reactive (50–93)** | 2.46 (176) | 6.4 × 10−2 | 0.04 | 64.84 | 59.67 | |
| Externalizing Problems (32–97) | 1.52 (479) | 9.2 × 10−2 | 0.05 | 59.11 | 55.54 | |
| Internalizing Problems (33–90) | 1.73 (479) | 4.2 × 10−2 | 0.05 | 62.73 | 59.21 | |
| Oppositional Defiant (50–80) | 1.33 (479) | 1.8 × 10−1 | 0.04 | 60.22 | 58.06 | |
| Pervasive Developmental (50–95)** | 4.91 (176) | 2.7 × 10−3 | 0.08 | 76.56 | 70.18 | |
| Rule Breaking (50–84)* | 0.74 (302) | 7.3 × 10−1 | 0.03 | 56.70 | 55.12 | |
| School Problems (0–99)* | 2.32 (269) | 6.2 × 10−3 | 0.11 | 34.31 | 36.06 | |
| Sleep Problems (50–100)** | 13.53 (176) | <1.0 × 10−5 | 0.19 | 66.13 | 54.72 | |
| Social Problems (50–91)* | 1.46 (302) | 1.3 × 10−1 | 0.06 | 65.16 | 63.49 | |
| Somatic Complaints (50–94) | 0.84 (479) | 6.4 × 10−1 | 0.03 | 58.31 | 56.76 | |
| Somatic Problems (50–93)* | 0.68 (302) | 7.8 × 10−1 | 0.03 | 56.92 | 55.16 | |
| Thought Problems (50–91)* | 5.55 (301) | <1.0 × 10−5 | 0.20 | 72.11 | 65.67 | |
| Withdrawn (50–100) | 4.98 (479) | <1.0 × 10−5 | 0.14 | 67.12 | 62.29 | |
| Total Competence (0–66)* | 0.74 (259) | 7.3 × 10−1 | 0.04 | 30.92 | 33.16 | |
| Total Problems (27–92) | 2.91 (479) | 2.0 × 10−4 | 0.09 | 65.91 | 61.06 | |
Indicates the variable was only available for children evaluated using the CBCL for ages 6-18.
Indicates the variable was only available for children evaluated using the CBCL for ages 2-5.
df, degrees of freedom.
Discussion
This study represents one of the largest analyses conducted evaluating the effects of sleep duration on ASD severity. Across the entire SSC dataset, we observed that increased severity of core ASD symptoms were correlated with decreased sleep duration. Furthermore, more severe social impairment and RRB from the ADI-R were associated with shorter sleep duration, when correcting for age and IQ. The specific symptoms associated with shorter sleep were a failure to develop peer relationships within the social impairment domain, and an adherence to nonfunctional routines or rituals in the RRB domain. In addition, shorter sleep duration was associated with more maladaptive behaviors measured via the CBCL and increased reports of ADD, depressive disorder, and obsessive compulsive disorder.
When comparing subgroups representing children with extremely short and extremely long sleep duration, we found that children with ASD and extremely short sleep duration had increased severity of core ASD symptoms (i.e., social/communication impairment, and RRB), lower IQ scores, more severe behavior problems (e.g., ADD/ADHD symptoms, affective problems, anxiety problems, anxious/depressed symptoms, attention problems, internalizing problems, thought problems, problems at school, and sleep problems), and increased reports of ADD, depressive disorder and obsessive compulsive disorder. While we have yet to establish whether short sleep duration is a cause or consequence of more severe symptoms in children with ASD (or that both sleep duration and ASD symptoms are the consequence of a third unknown factor), our results demonstrate the importance of identifying and treating sleep problems in this patient population.
Increased Severity of ASD-Related Symptoms are Associated with Shorter Sleep Duration
It is notable that estimates of sleep duration based on parent responses to questionnaires are subject to some level of bias leading to potential inaccuracies. For example, if the child is not alerting the parents, many may be unaware of the exact amount of time it takes for the child to fall asleep or the number awakenings occurring throughout the night. As such, sleep duration measured via parent-report is an imperfect phenotype. However, we were able to corroborate findings from previous studies in smaller ASD datasets, where sleep data was obtained using well-defined clinical diagnostic criteria and/or objective measures (Goldman et al., 2009; Malow et al., 2012; Schreck et al., 2004), observing that the severity of social/communication impairment and RRB is increased in children with comorbid short sleep duration. This indicates that using subjective reports of sleep duration in large ASD datasets is useful for understanding the relationship of sleep disturbances to symptom severity in children with ASD.
Furthermore, we observed that a failure to develop peer relationships was the strongest symptom associated with shorter sleep duration in children with ASD. Specifically, for every 4-minute decrease in sleep duration, there was a one unit increase in this ADI-R subscale. This is particularly important as early problems with establishing peer relationships negatively impact the eventual social and emotional development of the child, including increased psychological adjustment problems (Ladd & Troop-Gordon, 2003), and increased risk of educational under-achievement and unemployment (Woodward & Fergusson, 2000). It has been shown in typically developing individuals that a significant impact of daytime sleepiness relates to issues with establishing interpersonal relationships (Mulgrew et al., 2007). While we did not directly assess daytime sleepiness, increased reports of problems with the child being unusually sleepy during the day were associated with shorter sleep duration (β = −19.92, P < 1.0 × 10−4), and daytime sleepiness has been observed to be associated with short sleep duration in other studies (Perez-Lloret et al., 2013). Therefore, it can be inferred that in a child with ASD, who is already prone to social impairment, shorter sleep duration that results in daytime sleepiness could further impede the establishment of personal relationships. The net result may impact the effectiveness of therapies and negatively alter the developmental trajectory of the individual.
Notably, while communication scores were independently associated with sleep duration, verbal communication scores from the ADI-R were not associated in the full regression model. This suggests that sleep duration is not associated with verbal ability. These findings are similar to observations in typically developing children (Paavonen et al., 2010). In addition, while nonverbal communication scores from the ADI-R remained associated, the direction of effect changed when correcting for all other independently associated variables. This would indicate that decreased severity of nonverbal communication impairment is associated with shorter sleep duration. The connection between sleep duration and the individual’s ability to communicate nonverbally is unclear; however, one study previously observed that children with less severe impairment in nonverbal communication had significantly greater impairment in social functioning (Joseph, Tager-Flusberg, & Lord, 2002). It is possible that our results reflect a discrepancy between the relationships of sleep duration and social impairment, compared to nonverbal communication impairment.
It is interesting that the effects of sleep duration on severity of core symptoms were primarily observed when symptoms were evaluated with the ADI-R compared to the ADOS. One explanation is that the specific symptoms that had the strongest associations with sleep duration included issues with peer relationships and adherence to routines or rituals which may not be as extensively evaluated with the ADOS. For example, direct evaluation of developing peer relationships is not assessed with the ADOS and RRBs observed on the ADOS are more likely to be simple repetitive behaviors, easily observed in a brief interaction (Hus et al., 2014). In contrast, the ADI-R captures a broader array of RRBs and provides information for more complex repetitive behaviors. While comparable scores between the ADI-R and ADOS were significantly correlated with each other, the correlation coefficients indicated some level of discrepancy across the two diagnostic methods in this dataset (ρSocial = 0.35, ρRRB = 0.12, P < 1.0 × 10−5). It should be noted that scores from the ADI-R diagnostic version are based on summing the most abnormal manifestation of the evaluated behavior between the ages of 4 and 5 years old (Lord et al., 1994). Therefore, it is possible that given the age range of the analysis dataset, reports of core symptom severity from the diagnostic version of the ADI-R are less likely than ADOS scores to accurately reflect a relationship with current reports for sleep duration. However, when separate analyses were conducted in age-based subgroups, we observed that sleep duration remained associated with social impairment, failure to develop peer relationships, nonverbal communication, and RRB scores in children who were age 4–5 years old. This suggests that shorter sleep duration is related to increased severity of ASD in the subgroup of children where scores on the ADI-R are more likely to reflect the current state of this relationship.
Decreases in Sleep Duration are Associated with Lower IQs, Maladaptive Behaviors, and Reports of ADD, Depressive Disorder, and Obsessive Compulsive Disorder in ASD
Intellectual capability is an important distinguishing factor, affecting the functional level of children with ASD (Matson & Shoemaker, 2009; Rommelse et al., 2015). We observed that decreases in IQ scores were independently associated with decreases in sleep duration. While IQ scores were no longer associated in the full regression model, IQ scores for children with ASD were lower in the short sleep duration subgroup compared to children in the long sleep duration subgroup. These findings are similar to those reported for typically developing school-age children, indicating that sleep duration is differentially related to cognitive functioning (Gruber et al., 2010). Specifically, longer sleep duration has been associated with increased overall IQ scores in typically developing children. It is possible that short sleep duration in children with ASD negatively impacts the level of intellectual functioning.
Furthermore, the observations that more severe CBCL T scores for ADD/ADHD symptoms, affective problems, anxious/depressed symptoms, and increased reports of ADD, depressive disorder, and obsessive compulsive disorder in the medical histories were associated with decreases in sleep duration, and were more common in the extremely short sleep duration subgroup, are especially intriguing as numerous genetic mechanisms implicated in these comorbidities overlap with risk factors for ASD and those involved in sleep regulation (Gehrman, Keenan, Byrne, & Pack, 2015;Herken et al., 2007;Mattheisen et al., 2015; Neale et al., 2010; Parsons et al., 2013; Wu, Xiao, Sun, Zou, & Zhu, 2012). While it is possible that presence of these comorbidities contribute to sleep disturbances in children with ASD, it is also likely that overlapping genetic risk factors have pleiotropic effects that influence risk for multiple clinically distinct conditions in the same individual. In addition, a potential relationship may exist where underlying biology modifies the negative consequences of sleep problems in children with ASD. Specifically, genetic risk factors for ASD that function in regulating sleep may increase the severity of symptoms observed in children with ASD and shorter sleep duration. Identifying pleiotropic effects related to underlying genetic architecture may be particularly useful to informing more effective treatment options for important comorbidities in individuals with ASD.
It is notable that we did not observe a significant relationship between sleep duration and irritable bowel syndrome or constipation. A previous study evaluating comorbidities reported in the SSC medical histories observed that there was increased risk for reports of sleep problems to co-occur with gastrointestinal disturbances (Aldinger et al., 2015). The differences we observed when compared to this previous study are likely due to the specific variables that were evaluated. For example, the previous study combined all reports of sleep problems into a single variable (Aldinger et al., 2015). We did not focus on evaluating parent reports of sleep problems, as we expect based on our previous work (Veatch et al., 2016), that these are less accurate when compared to the average amount of sleep reported for the child. Furthermore, sleep problems that were queried in the SSC medical history encompass a breadth of distinct sleep disorders, including questions related to insomnia, parasomnias, and sleep disordered breathing. We observed that, in addition to the child being unusually sleepy during the day as noted above, specific parent-reported sleep problems that were associated with decreases in sleep duration included the child having difficulty going to bed (β = −39.82, P < 1.0 × 10−4), difficulty falling asleep (β = −42.70, P < 1.0 × 10−4), difficulty waking in the morning (β = −28.61, P < 1.0 × 10−4), taking long or frequent naps (β = −35.32, P < 1.0 × 10−4), parents having to lay down with the child to get him/her to fall asleep (β = −15.44, P < 1.0 × 10−4), having frequent awakenings during the night (β = −32.77, P < 1.0 × 10−4), not having a regular bedtime (β = −62.18, P < 1.0 × 10−4), and the parent feeling the child did not get enough sleep (β = −61.79, P < 1.0 × 10−4). Sleep duration was not associated with reports of sleepwalking or frequent nightmares, wetting the bed, snoring, or difficulty breathing at night. In addition, the previous study combined all reports of gastrointestinal problems into one variable (Aldinger et al., 2015), while we separated out reports of constipation and irritable bowel syndrome due to the connection of these particular symptoms with sleep problems.
Study Limitations
Defining sleep status, particularly insomnia, can be challenging in large datasets where sleep-related data is largely derived from potentially biased self- or parent-report. Unfortunately, the majority of sleep-related information available in the large ASD databases is limited to these types of data. The use of parent-reported sleep duration in this study is a potentially biased source of information. However, by focusing these analyses on evaluating the parent-reported sleep trait that was previously observed to be the most accurate insomnia-related trait that could be obtained from parent reports of sleep in children with ASD (Veatch et al., 2016), we expect that this minimized potential biases. Furthermore, the large size of the evaluated dataset should also help account for inaccuracies in parent reports of sleep duration that could otherwise reduce the power of statistical analyses.
It is possible that age had an impact on our comparisons of ASD symptoms and sleep duration, as it is well-established that sleep duration decreases with age; however, as age was included in the full model regression analyses, we expect that there was minimal effect of age on the associations of shorter sleep duration with increased severity of social impairment, and RRB. Furthermore, shorter sleep duration remained associated with more severe social impairment, RRB, affective problems, anxiety problems, anxious/depressed symptoms, and internalizing problems when evaluated exclusively in children who were both preschool and primary school age (Table 5). Additionally, including interaction terms in regression analyses indicated that age did not have a significant effect on the association of sleep duration with reports of comorbidities, or the majority of CBCL T scores (Supporting Information Table S1).
Onset of puberty is also a potential confounder in sleep duration studies, and it should be noted that 51% of the SSC dataset were indicated to have “not yet started” puberty at the time of the medical history intake. For 33% of the dataset, puberty was indicated to be “underway” and 1% of the dataset were indicated to have completed puberty. Information regarding developmental stage related to puberty was missing for 15% of the dataset. As onset of puberty was missing for a portion of the dataset, is a more subjective variable than age, and was strongly correlated with age (ρ = 0.74, P < 1.0 × 10−5), we chose to report results correcting for age as opposed to both age and puberty. However, to ensure this did not overly influence results, we also conducted regression analyses including puberty status and observed similar results to those reported. The notable exception was that RRB scores were no longer significantly associated with sleep duration when correcting for both age and puberty status although the result was still trending toward significance (P = 7.5 × 10−2).
Another limitation is the potential impact of current medication use, presence of medical, neurological and psychiatric comorbidities, and the environment on sleep behavior. As such, we cannot ensure that the reported short sleep duration was not due to a medication, medical, neurological or psychiatric condition, or poor sleep habits. However, the lack of association between the neurological conditions that were reported in 948 of the children that were included in these analyses suggests that presence of these neurological conditions did not influence the effect of sleep duration on ASD symptom severity. Sleep duration was also not associated with the majority of medications that children were reported to be currently taking; indicating medication use had minimal influence on our results. Furthermore, the majority of our results did not change following removal of the children reported to be on medications that were associated with sleep duration from analyses. Notably, reports of obsessive compulsive disorder and ADD were no longer associated with sleep duration in children not taking these medications, although increased CBCL T scores for ADD/ADHD and attention problems remained associated. This suggests a potential influence of medications on the relationship between short sleep duration and more reports of ADD, but not more severe ADD symptoms. Regardless, the main goal of this study was to determine if the severity of ASD was increased in children with shorter sleep duration, and to identify specific symptoms associated with shorter sleep, despite the underlying reason for the sleep disturbance.
These results reflect cross-sectional analyses of the relationship of sleep duration to the severity of ASD-related symptoms and are therefore incapable of establishing causality. Additionally, it is unclear whether the current sleep duration has persisted, or fluctuated, throughout the individual’s development. Unfortunately, “ever” responses to the sleep duration question in the medical history were only available for 12% of the analysis dataset (n = 320/2,714 total). As such, we were unable to adequately evaluate the relationship between current reports to ever reports; however, in these 320 children, we observed that “current” sleep duration was strongly correlated with “ever” sleep duration (r = 0.64, P < 1.0 × 10−5). Additionally, children with responses for both “current” and “ever” sleep duration were older than children who only had reports for “current” sleep duration (μCurrent + Ever = 9.73+/−3.44, μCurrentOnly = 8.95+/−3.59, P = 1.0 × 10−4). It is possible that this indicates current reports of sleep duration may reflect sleep duration over time although to ultimately understand how the quality of sleep throughout development may fluctuate and impact symptom severity in children with ASD, future studies focused on evaluating longitudinal data will be necessary.
Our results support a relationship between increased severity of core symptoms, expression of comorbidities, more severe expression of maladaptive behaviors and short sleep duration in children with ASD. These findings not only indicate the importance of sleep to daytime behaviors, but support the hypothesis that sleep quality may impact neurological function.
Supplementary Material
Acknowledgments
Special thanks to the families and individuals who participated in the studies from which this work was made possible. We also acknowledge financial support received from the following grants: Grant sponsor The Simons Foundation (Drs. Sutcliffe and Warren);Grant award Simons Simplex Collection Support Award; Grant sponsor The Burry Family Endowment (Dr. Malow); and Grant award T32 HL07713 (Drs. Veatch and Pack).
References
- Accardo JA, & Malow BA (2015). Sleep, epilepsy, and autism. Epilepsy & Behavior, 47, 202–206. [DOI] [PubMed] [Google Scholar]
- Achenbach TM (1991). Manual for the Child Behavior Checklist/4-18 and 1991 profile. VT: Department of Psychiatry, University of Vermont Burlington. [Google Scholar]
- Aldinger KA, Lane CJ, Veenstra-VanderWeele J, & Levitt P (2015). Patterns of risk for multiple co-occurring medical conditions replicate across distinct cohorts of children with autism spectrum disorder. Autism Research, 8, 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Billeh YN, Rodriguez AV, Bellesi M, Bernard A, de Vivo L, Funk CM, … Tononi G (2016). Effects of chronic sleep restriction during early adolescence on the adult pattern of connectivity of mouse secondary motor cortex. eNeuro, 3. pii: ENEURO.0053-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Conduit R, Lockley SW, Rajaratnam SM, & Cornish KM (2014). The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. Journal of Neurodevelopmental Disorders, 6, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier JL, Speechley KN, Steele M, Norman R, Stringer B, & Nicolson R (2005). Parental perception of sleep problems in children of normal intelligence with pervasive developmental disorders: Prevalence, severity, and pattern. Journal of the American Academy of Child and Adolescent Psychiatry, 44, 815–822. [DOI] [PubMed] [Google Scholar]
- Elliott CD (2006). Differential ability scales-second edition (DAS-II). Sant Antonio, TX: The Psychological Corporation. [Google Scholar]
- Ferguson BJ, Marler S, Altstein LL, Lee EB, Akers J, Sohl K, … Beversdorf DQ (2016). Psychophysiological associations with gastrointestinal symptomatology in autism spectrum disorder. Autism Research. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, & Lord C (2010). The Simons Simplex Collection: A resource for identification of autism genetic risk factors. Neuron, 68, 192–195. [DOI] [PubMed] [Google Scholar]
- Gabriels RL, Cuccaro ML, Hill DE, Ivers BJ, & Goldson E (2005). Repetitive behaviors in autism: Relationships with associated clinical features. Research in Developmental Disabilities, 26, 169–181. [DOI] [PubMed] [Google Scholar]
- Gehrman PR, Keenan BT, Byrne EM, & Pack AI (2015). Genetics of sleep disorders. The Psychiatric Clinics of North America, 38, 667–681. [DOI] [PubMed] [Google Scholar]
- Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, & Malow BA (2009). Defining the sleep phenotype in children with autism. Developmental Neuropsychology, 34, 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Laviolette R, Deluca P, Monson E, Cornish K, & Carrier J (2010). Short sleep duration is associated with poor performance on IQ measures in healthy school-age children. Sleep Medicine, 11, 289–294. [DOI] [PubMed] [Google Scholar]
- Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, … Akyol O (2007). Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: Impact of antidepressant treatment. Archives of Medical Research, 38, 247–252. [DOI] [PubMed] [Google Scholar]
- Hu VW, Sarachana T, Kim KS, Nguyen A, Kulkarni S, Steinberg ME, … Lee NH (2009). Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: Evidence for circadian rhythm dysfunction in severe autism. Autism Research, 2, 78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, & Lord C (2014). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44, 2400–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysing M, Lundervold AJ, Posserud MB, & Sivertsen B (2016). Association between sleep problems and symptoms of attention deficit hyperactivity disorder in adolescence: Results from a large population-based study. Behavioral Sleep Medicine, 14, 550–564. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H, & Lord C (2002). Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 43, 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, Yue Z, & Sehgal A (2014). A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science, 344, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, & Hansen RL (2008). Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. Journal of Sleep Research, 17, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner MH, Nachtsheim CJ, Neter J (2004). Applied Linear Regression Models (4th ed.). McGraw-Hill Irwin. [Google Scholar]
- Ladd GW, & Troop-Gordon W (2003). The role of chronic peer difficulties in the development of children’s psychological adjustment problems. Child Development, 74, 1344–1367. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, & Schopler E (1989). Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders, 19, 185–212. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Luc ME, Gupta A, Birnberg JM, Reddick D, & Kohrman MH (2006). Characterization of symptoms of sleep disorders in children with headache. Pediatric Neurology, 34, 7–12. [DOI] [PubMed] [Google Scholar]
- Malow B, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, & Burnette C (2012). Melatonin for sleep in children with autism: A controlled trial examining dose, tolerability, and outcomes. Journal of Autism and Developmental Disorders, 42, 1729–1737; author reply 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, & Stone WL (2006). Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep, 29, 1563–1571. [DOI] [PubMed] [Google Scholar]
- Maski K, & Owens JA (2016). Insomnia, parasomnias, and narcolepsy in children: Clinical features, diagnosis, and management. The Lancet Neurology, 15, 1170–1181. [DOI] [PubMed] [Google Scholar]
- Maski KP, & Kothare SV (2013). Sleep deprivation and neurobehavioral functioning in children. International Journal of Psychophysiology, 89, 259–264. [DOI] [PubMed] [Google Scholar]
- Matson JL, & Shoemaker M (2009). Intellectual disability and its relationship to autism spectrum disorders. Research in Developmental Disabilities, 30, 1107–1114. [DOI] [PubMed] [Google Scholar]
- Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, … Nestadt G (2015). Genome-wide association study in obsessive-compulsive disorder: Results from the OCGAS. Molecular Psychiatry, 20, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, & Sohl K (2016). Sleep and behavioral problems in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46, 1906–1915. [DOI] [PubMed] [Google Scholar]
- Mulgrew AT, Ryan CF, Fleetham JA, Cheema R, Fox N, Koehoorn M, … Ayas NT (2007). The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Medicine, 9, 42–53. [DOI] [PubMed] [Google Scholar]
- Mullen E (1995). The Mullen scales of early learning. Circle Pines, MN: American Guidance Service, Inc. [Google Scholar]
- Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, … Nelson S (2010). Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 884–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechita F, Pirlog MC, & ChiriTa AL (2015). Circadian malfunctions in depression - neurobiological and psychosocial approaches. Romanian Journal of Morphology and Embryology, 56, 949–955. [PubMed] [Google Scholar]
- Neckelmann D, Mykletun A, & Dahl AA (2007). Chronic insomnia as a risk factor for developing anxiety and depression. Sleep, 30, 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon GM, Thompson JM, Han DY, Becroft DM, Clark PM, Robinson E, … Mitchell EA (2008). Short sleep duration in middle childhood: Risk factors and consequences. Sleep, 31, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Wilson S, & Paterson L (2008). Sleep disorders as core symptoms of depression. Dialogues in Clinical Neuroscience, 10, 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, & Roth T (2003). Place of chronic insomnia in the course of depressive and anxiety disorders. Journal of Psychiatric Research, 37, 9–15. [DOI] [PubMed] [Google Scholar]
- Owens JA (2016). Behavioral sleep problems in children. In Chervin RD & Hoppin AG (Eds.), UptoDate, Accessed 6 Dec 2016 ed. MA: Waltham. [Google Scholar]
- Owens JA, Mehlenbeck R, Lee J, & King MM (2008). Effect of weight, sleep duration, and comorbid sleep disorders on behavioral outcomes in children with sleep-disordered breathing. Archives of Pediatrics & Adolescent Medicine, 162, 313–321. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, & McGuinn M (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23, 1043–1051. [PubMed] [Google Scholar]
- Paavonen EJ, Raikkonen K, Lahti J, Komsi N, Heinonen K, Pesonen AK, … Porkka-Heiskanen T (2009). Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics, 123, e857–e864. [DOI] [PubMed] [Google Scholar]
- Paavonen EJ, Raikkonen K, Pesonen AK, Lahti J, Komsi N, Heinonen K, … Porkka-Heiskanen T (2010). Sleep quality and cognitive performance in 8-year-old children. Sleep Medicine, 11, 386–392. [DOI] [PubMed] [Google Scholar]
- Pagan C, Delorme R, Callebert J, Goubran-Botros H, Amsellem F, Drouot X, … Launay JM (2014). The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Translational Psychiatry, 4, e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Lester KJ, Barclay NL, Nolan PM, Eley TC, & Gregory AM (2013). Replication of Genome-Wide Association Studies (GWAS) loci for sleep in the British G1219 cohort. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162B, 431–438. [DOI] [PubMed] [Google Scholar]
- Paruthi S, Brooks LJ, D’ambrosio C, Hall WA, Kotagal S, Lloyd RM, … Wise MS (2016). Consensus statement of the American Academy of Sleep Medicine on the recommended amount of sleep for healthy children: Methodology and discussion. Journal of Clinical Sleep Medicine, 12, 1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres MF (2005). Melatonin, the pineal gland and their implications for headache disorders. Cephalalgia, 25, 403–411. [DOI] [PubMed] [Google Scholar]
- Perez-Lloret S, Videla AJ, Richaudeau A, Vigo D, Rossi M, Cardinali DP, & Perez-Chada D (2013). A multi-step pathway connecting short sleep duration to daytime somnolence, reduced attention, and poor academic performance: An exploratory cross-sectional study in teenagers. Journal of Clinical Sleep Medicine, 9, 469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Paavonen EJ, Heinonen K, Komsi N, Lahti J, … Strandberg T (2010). Sleep duration and regularity are associated with behavioral problems in 8-year-old children. International Journal of Behavioral Medicine, 17, 298–305. [DOI] [PubMed] [Google Scholar]
- Raines AM, Short NA, Sutton CA, Oglesby ME, Allan NP, & Schmidt NB (2015). Obsessive-compulsive symptom dimensions and insomnia: The mediating role of anxiety sensitivity cognitive concerns. Psychiatry Research, 228, 368–372. [DOI] [PubMed] [Google Scholar]
- Reynolds AM, & Malow BA (2011). Sleep and autism spectrum disorders. Pediatric Clinics of North America, 58, 685–698. [DOI] [PubMed] [Google Scholar]
- Rommelse N, Langerak I, van der Meer J, de Bruijn Y, Staal W, Oerlemans A, & Buitelaar J (2015). Intelligence may moderate the cognitive profile of patients with ASD. PloS One, 10, e0138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck KA, Mulick JA, & Smith AF (2004). Sleep problems as possible predictors of intensified symptoms of autism. Research in Developmental Disabilities, 25, 57–66. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Posserud MB, Gillberg C, Lundervold AJ, & Hysing M (2012). Sleep problems in children with autism spectrum problems: A longitudinal population-based study. Autism, 16, 139–150. [DOI] [PubMed] [Google Scholar]
- Souders MC, Mason TB, Valladares O, Bucan M, Levy SE, Mandell DS, … Pinto-Martin J (2009). Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep, 32, 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statacorp. (2013). Stata Statistical Software (13th ed.). College Station, TX: Statacorp LP. [Google Scholar]
- Stewart ME, Barnard L, Pearson J, Hasan R, & O’brien G (2006). Presentation of depression in autism and Asperger syndrome: A review. Autism, 10, 103–116. [DOI] [PubMed] [Google Scholar]
- Taylor MA, Schreck KA, & Mulick JA (2012). Sleep disruption as a correlate to cognitive and adaptive behavior problems in autism spectrum disorders. Research in Developmental Disabilities, 33, 1408–1417. [DOI] [PubMed] [Google Scholar]
- Timpano KR, Carbonella JY, Bernert RA, & Schmidt NB (2014). Obsessive compulsive symptoms and sleep difficulties: Exploring the unique relationship between insomnia and obsessions. Journal of Psychiatric Research, 57, 101–107. [DOI] [PubMed] [Google Scholar]
- Tudor ME, Hoffman CD, Sweeney DP (2012). Children with autism: sleep problems and symptom severity. Focus on Autism and Other Developmental Disabilities, 27, 254–262. [Google Scholar]
- Veatch OJ, Goldman SE, Adkins KW, & Malow BA (2015a). Melatonin in children with autism spectrum disorders: How does the evidence fit together? Journal of Nature and Science, 1, e125. [PMC free article] [PubMed] [Google Scholar]
- Veatch OJ, Maxwell-Horn AC, & Malow BA (2015b). Sleep in autism spectrum disorders. Current Sleep Medicine Reports, 1, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch OJ, Reynolds A, Katz T, Weiss SK, Loh A, Wang L, & Malow BA (2016). Sleep in children with autism spectrum disorders: How are measures of parent report and actigraphy related and affected by sleep education? Behavioral Sleep Medicine, 14, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler B, Rapoport AM, Tepper SJ, Sheftell F, & Bigal ME (2006). Role of melatonin in the pathophysiology of migraine: Implications for treatment. CNS Drugs, 20, 343–350. [DOI] [PubMed] [Google Scholar]
- Wiggins JL, & Monk CS (2013). A translational neuroscience framework for the development of socioemotional functioning in health and psychopathology. Development and Psychopathology, 25, 1293–1309. [DOI] [PubMed] [Google Scholar]
- Wiggs L, & Stores G (2004). Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Developmental Medicine and Child Neurology, 46, 372–380. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, & Fergusson DM (2000). Childhood peer relationship problems and later risks of educational under-achievement and unemployment. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 41, 191–201. [PubMed] [Google Scholar]
- Wu J, Xiao H, Sun H, Zou L, & Zhu LQ (2012). Role of dopamine receptors in ADHD: A systematic meta-analysis. Molecular Neurobiology, 45, 605–620. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, & Foster RG (2010). Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nature Reviews Neuroscience, 11, 589–599. [DOI] [PubMed] [Google Scholar]
- Yang Z, Matsumoto A, Nakayama K, Jimbo EF, Kojima K, Nagata K, … Yamagata T (2016). Circadian-relevant genes are highly polymorphic in autism spectrum disorder patients. Brain & Development, 38, 91–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


