Abstract
Background
Certain sequences of genomic mutations can lead to cancer formation and affect treatment outcomes and drug resistance. We constructed a cancer evolutionary tree using bulk-targeted deep sequencing to explore the impact of sequential and co-occurring somatic mutations on patients with stage III colorectal cancer (CRC).
Methods
A total of 108 stage III CRC patients from National Cheng Kung University Hospital (NCKUH) were recruited for this study between Jan. 2014 and Jan. 2019. Clinical information and tumor-targeted deep sequencing data were collected. Phylogenetic trees were reconstructed for evolutionary trajectories. We used a machine learning model for survival analysis.
Results
Six sequential somatic mutations stratified patients into seven subgroups based on survival. Patients carrying sequential germline followed by DNA damage response-related ATM or BRCA2 somatic mutations or non-TP53, APC somatic mutations had a better outcome than those without such mutations. The 4-year recurrence-free survival (RFS) probability was 88% in the low-risk group (G1) and 46% in the high-risk group (G2) (log-rank p-value 2e-05). The predictive efficacy by the area under the curve (AUC) was 0.73, 0.7, 0.797, and 0.88 at 2, 4, 6, and 8 years, respectively. The mutation status of mismatch repair (MMR) genes was not associated with RFS. Different genomic features were found between the groups. The orders of APC, KRAS and APC, BRCA2 sequential somatic mutations were associated with clinical outcomes. The occurrence of somatic mutations in BRCA2, such as TP53 somatic mutations, affected recurrence-free survival.
Conclusions
According to the evolution model, DNA damage response (DDR)-related ATM or BRCA2 somatic mutations are promising biomarkers for assessing the response of stage III CRC patients to oxaliplatin-based chemotherapy. The sequential order and co-occurring DDR somatic mutations are associated with recurrence-free survival.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-07926-1.
Keywords: Sequential somatic mutations, DNA damage response gene, Colorectal cancer, Tumor heterogeneity, Tumor evolution
Background
Patients with cancers that are associated with high intratumor heterogeneity might experience poor clinical outcomes [1]. As a result of this heterogeneity, the bulk tumor might include a diverse group of cells harboring distinct molecular signatures. Despite the success of targeted therapies for cancer, the development of resistance limits the ability to translate this method into a curative treatment. One possible resistance mechanism has traditionally been thought of as the intrinsic co-occurrence of acquired genetic mutations [2, 3].
Certain sequences of genomic mutations can lead to cancer development and affect treatment outcomes and drug resistance. Phylogenetic evolutionary trees with different genetic mutation orders may reveal information on cancer biology that can be used to plan therapeutic strategies [4, 5]. In a mouse model, we found that the temporal sequence of two mutations might impact tumorigenesis due to different sequential orders of RAS and TP53 somatic mutations and practical cooperation with other genetic mutations in cell line models [6, 7]. Clinically, EGFR-mutant/TP53-mutant lung adenocarcinoma should be regarded as a unique subgroup with a poor prognosis [8]. Studies have implied that the genetic mutation order or co-occurring genetic mutations are important in cancer biology.
In Pan-Cancer Atlas datasets, there are 141/528 cases of putative driver mutations in 29 genes associated with the DNA damage response (DDR) and DNA repair (including the mismatch repair and homologous recombination pathways) [9, 10]. For example, poly (ADP)-ribose polymerase inhibitors have shown promising results in preclinical studies on colorectal cancer (CRC) patients carrying BRCA1/BRCA2 mutations. Other DNA repair-targeting therapies, such as ATR and CHK1 inhibitors, which are most effective against cancers carrying ATM mutations, can be applied along with current genotoxic chemotherapy in CRC [11]. Targeting alternative DDR mechanisms may also improve the clinical outcomes of CRC patients.
In this study, we constructed a cancer evolutionary tree using bulk-targeted deep sequencing. We also explored the impact of DDR genes on stage III CRC patients and evaluated the effect of sequential and co-occurring somatic mutations.
Methods
Enrollment of patients
A total of 108 CRC patients from National Cheng Kung University Hospital (NCKUH) were recruited for this study between Jan. 2014 and Jan. 2019. All CRC cases were at pathological stage III and were treated with standard surgical resection followed by adjuvant chemotherapy with the mFOLFOX6 regimen (5-fluorouracil, leucovorin, and oxaliplatin). Clinical information was obtained from medical records. Tumor tissues and blood samples were collected at the time of enrollment. This study was approved by the Institutional Review Board of NCKUH (A-ER-103-395 and A-ER-104-153) and conducted according to the principles of the Declaration of Helsinki. All participants provided written informed consent.
Targeted tumor sequencing with a cancer panel
A total of 108 primary tumor samples were subjected to histological assessment, followed by nucleic acid extraction from formalin-fixed paraffin-embedded blocks at NCKUH. Pathologists reviewed the specimens and determined the percentage of viable tumor nuclei and the adequacy of the sample for mutational profile detection. Targeted deep sequencing of tumor samples was performed with Oncomine Comprehensive Assays (OCAs) (Thermo Fisher Scientific). All samples were analyzed using Torrent Suite Software 5.0.4. All reads were aligned to the hg19 reference genome, and variant calling was performed using the Torrent Variant Caller plugin (version 5.0.4.0).
Phylogenetic tree construction and evolutionary trajectory analysis
All somatic events (single-nucleotide variants (SNVs)/indels) were used to build a phylogenetic tree for each patient. Somatic SNVs with a minor allele frequency (MAF) less than 0.1% were filtered with ExAC EAS and in-house Taiwan Biobank WGS data. Driver genes were defined as nonsynonymous or indel variants; nondriver genes were defined as other intronic or synonymous variants. A clonal inference analysis of each patient was performed using the R package sciClone [12]. Phylogenetic trees and repeated evolutionary trajectories were produced with the R package Revolver [13]. Only driver genes were analyzed, and those that only occurred once were ignored.
Statistical analysis
The chi-square test or Fisher’s exact test was used to determine the association between CRC patients’ clinical characteristics and survival. Kaplan–Meier curves and Cox proportional hazards models were employed to evaluate recurrence-free survival (RFS), which was defined as the time between surgery and cancer recurrence. A p-value adjusted by a false discovery rate (FDR) or a Bonferroni (BF) critical value of < 0.05 was considered statistically significant. All statistical analyses were conducted in the R environment.
Machine learning model and analysis
CRC survival decision tree
The CRC survival decision tree was built with the package Recursive Partitioning and Regression Trees (rpart) [14] and visualized with partykit [15]. We set the parameter minsplit to 30, with each node containing at least ten patients. The 108 CRC patients were divided into seven subgroups.
Feature selection
We used the least absolute shrinkage and selection operator (LASSO) regression algorithm for feature selection. To obtain more specific features, we implemented the algorithm 100 times and used all 22 features.
Results
Clinical features and genomic phylogenetic mutations
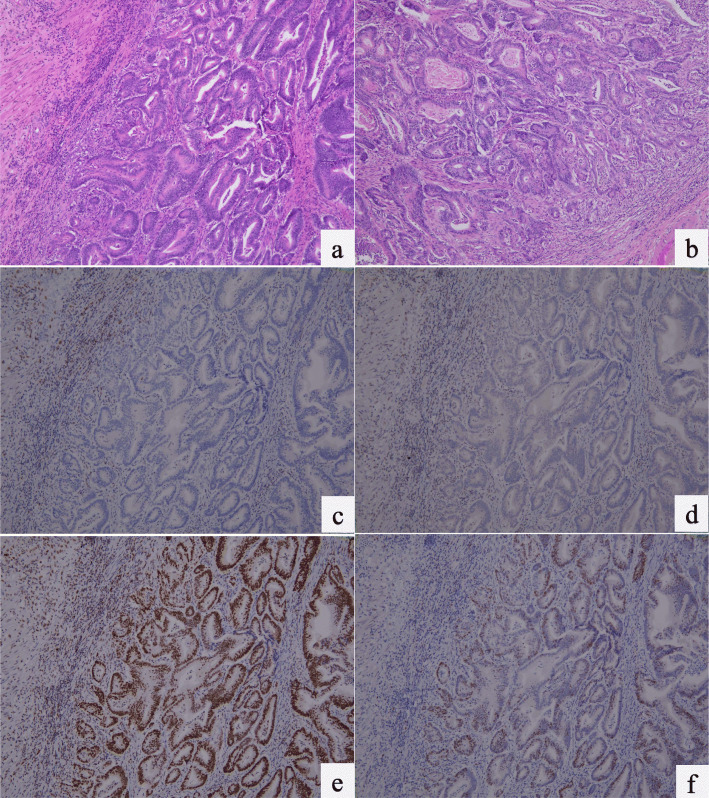
We enrolled 108 stage III colorectal cancer (CRC) patients in this study. For all subjects, we collected their primary tumor tissues for targeted deep sequencing. The baseline characteristics of the patients are provided in Supplementary Table S1. There was an equal sex distribution among the patients. The median patient age was 57.5 years (interquartile range (IQR), 49.75–65.0 years). Sex ratios were equal (50%, N = 54). The LENT primary tumor site was the left colon (75.9%, N = 82). Most of the cases were at a highly invasive tumor stage (T3/T4 stage) (87%, N = 94) but a low lymph node stage (N0/N1 stage) (71.3%, N = 77). Microsatellite instability (MSI) high, or DNA mismatch repair (MMR) protein loss by immunohistochemistry staining of tumors (mismatch repair deficiency (dMMR)) (5.6%, N = 6) was detected in only a few patients. Immunohistochemical (IHC) expression of MMR proteins was interpreted by the same pathologist in our institute. An example of MMR protein expression in colonic adenocarcinoma tissues is shown in Fig. 1, hematoxylin and eosin staining (H&E × 100) with moderately differentiated carcinoma in Fig. 1a and b, loss of MLH1 and PMS2 expression in Fig. 1c and d, and positive IHC expression of MSH2 and MSH6 in Fig. 1e and f. Positive MSI markers were reexamined to confirm the results.
Fig. 1.
An example of dMMR colorectal cancer stained with hematoxylin-eosin (a, b). The cancer cells revealed loss of nuclear staining of MLH1 (c) and PMS2 (d), and preserved nuclear staining of MSH2 (e) and MSH6 (f) (100x)
The mutational status of mismatch repair genes, including MLH1 and MSH2, in all patients was analyzed. Twenty-two of the 108 CRC patients showed MMR genetic variants. The association of group 1 (G1) and group 2 (G2) subjects with MMR genetic variants was tested by Fisher’s exact test (p-value = 0.99). (Supplementary Table S1). A heatmap of MMR and homologous recombination genetic variants is shown in Supplementary Fig. S1. The association of MLH1 and BRCA2 genetic variants was mutually exclusive, whereas others coexisted (Supplementary Table S2).
To understand each patient’s sequential somatic mutations, we constructed evolutionary trees and extracted sequential somatic mutations with the Revolver tool [13]. Overall, 302 sequential somatic mutations were detected. Each CRC patient showed an average of 18 sequential somatic mutations, and the distribution of sequential somatic mutations ranged from 2 to 107. The spectrum and frequency of sequential somatic mutations are depicted in Fig. 2. Germline followed by TP53 (Germline, TP53) was the most common sequential somatic mutation (70.4%). Other sequential somatic mutations were TP53 followed by APC (TP53, APC) (62%) and germline followed by APC (Germline, APC) (55.6%). A bar plot of the spectrum of sequential somatic mutations occurring at least 15 times was constructed.
Fig. 2.
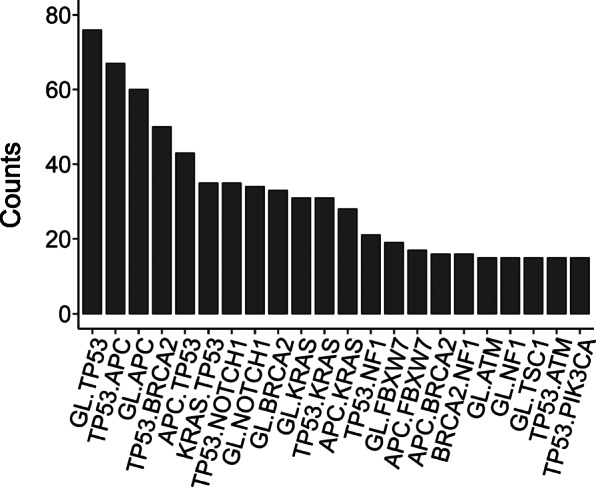
The spectrum and frequency of sequential somatic mutations in 108 CRC patients. The most common sequential somatic mutation was germline mutation. TP53 occurred 76 times in our cohort. TP53, APC occurred 67 times. Germline, APC occurred 60 times. Only sequential somatic mutations that occurred at least 15 times are shown in the plot
Survival stratification and predictive efficacy according to mutation of DNA damage genes
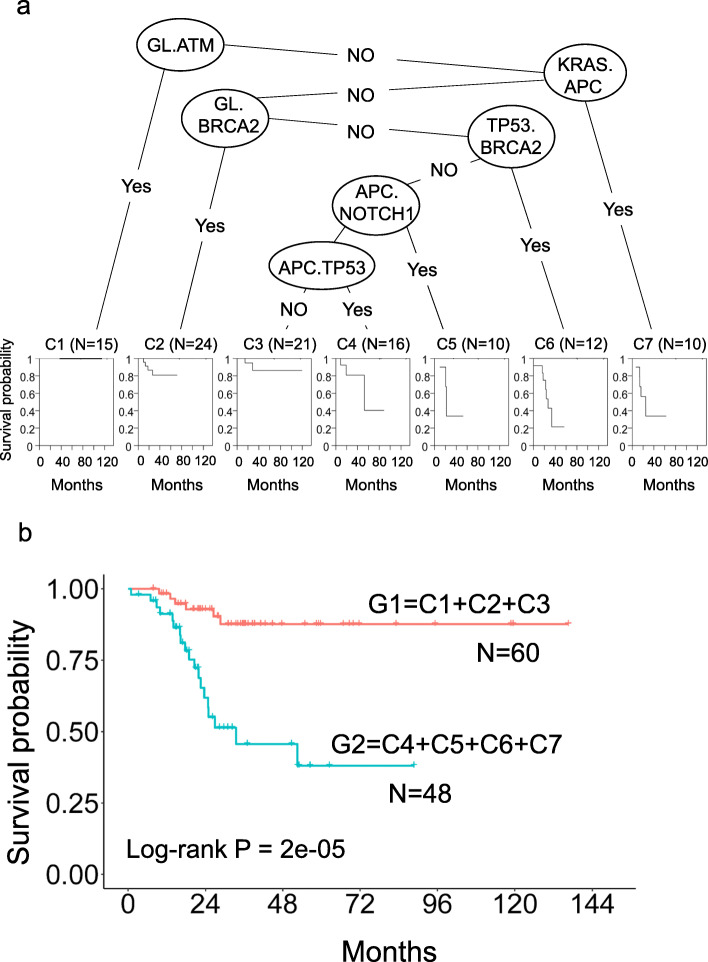
Recursive partitioning analysis (RPA) was implemented to construct a survival decision tree to further improve CRC risk stratification according to sequential somatic mutations. As a result, the survival decision tree was generated with six sequential somatic mutations that separated the patients into seven subgroups (C1–7). The patients in subgroups C1, C2, and C3 had a better survival rate than the patients in subgroups C4, C5, C6, and C7 (Fig. 3a). Patients carrying germline followed by ATM, BRCA2 or non-TP53 APC somatic mutations had a better outcome than those without such mutations (Fig. 3b). The ATM and BRCA2 somatic mutation sites are shown in Supplementary Table S3. DNA damage response (DDR) somatic mutations are promising biomarkers to assess the response of stage III CRC patients to oxaliplatin-based chemotherapy.
Fig. 3.
CRC survival decision tree analysis. a A decision tree was generated to optimize risk stratification in our cohort. The internal nodes of the tree represent sequential somatic mutations. The leaf nodes represent recurrence-free survival for each subgroup, and each node is representative of at least ten patients. b Kaplan–Meier plot of the decision subgroups. Patients in the G1 subgroup had a higher probability of 4-year recurrence-free survival (probability = 0.88, 95% CI = 0.79–0.98) than patients in the G2 subgroup (probability = 0.46, 95% CI = 0.30–0.69). The hazard ratio was 5.80 (95% CI = 2.30–14.61), and the log-rank p-value was 2e-05 (median recurrence-free survival, NA months [95% CI, NA-NA] vs 33.47 months [95% CI, 23.63-NA] (G1 = C1 + C2 + C3, G2 = C4 + C5 + C6 + C7)
We also classified patients in C1–3 as the low-risk group (G1) and patients in C4–7 as the high-risk group (G2). The clinical characteristics of the low-risk (G1) and the high-risk (G2) groups were not significantly different (Supplementary Table S1). The clinical characteristics of the DDR mutation group (C1–2), the no mutation group (C3), and the high-risk group (C4–7) also did not differ significantly (Supplementary Table S4). Kaplan–Meier curve analysis between the low-risk group (G1) and the high-risk group (G2) showed a significant difference (log-rank p-value 2e-05), and the 4-year recurrence-free survival probability was 88% in the G1 group and 46% in the G2 group (Fig. 3b). The median RFS was not reached in the low-risk group, and it was 33.5 months in the high-risk group. There was no statistically significant difference in survival between the DDR mutation subgroups and the no mutation subgroup (Supplementary Fig. S2). To assess predictive efficacy, time-dependent receiver operating characteristic (ROC) curves for 2, 5, 6, and 8 years before recurrence for the 2 groups were examined, and the area under the curves (AUCs) in the training set were 0.73, 0.7, 0.797, and 0.88, respectively. These results are shown in Supplementary Fig. S3.
Based on mutations in DDR genes (Fig. 3), we found co-occurrence of BRCA2 and TP53 somatic mutations to be associated with a poor prognosis. The median survival duration was 26.9 months. These results suggest the importance of the occurrence of somatic mutations.
Multivariate analysis of the clinical and prognostic significance of genomic groups
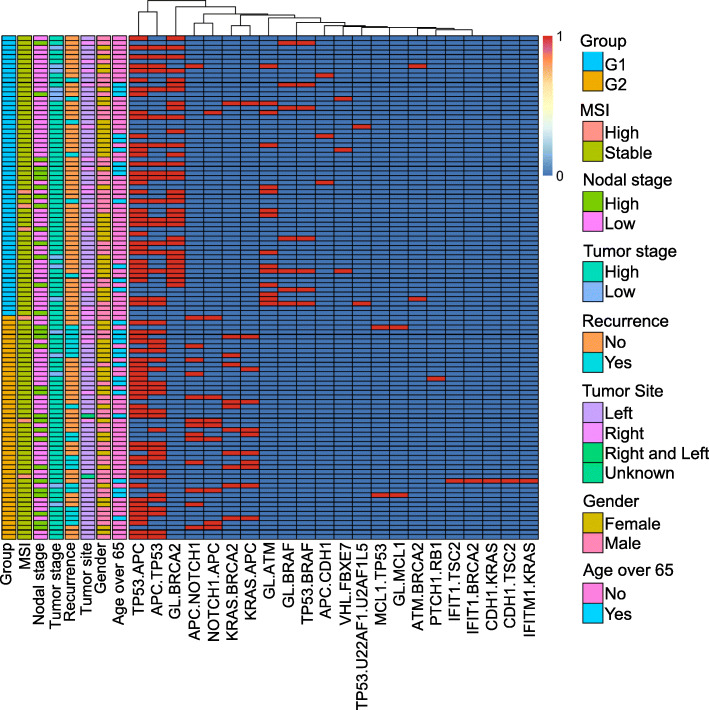
The LASSO regression model was implemented to distinguish sequential somatic mutations between the low-risk group (G1) and the high-risk group (G2). We selected 22 sequential somatic mutations with the bootstrap method. A heatmap of the hierarchical clustering of the sequential somatic mutations and their clinical characteristics is provided in Fig. 4. We also used principal component analysis (PCA) to evaluate the tendency of the critical features. As a result, germline mutations followed by BRCA2, ATM and BRAF somatic mutations were unique to the low-risk group (G1); APC, TP53 and APC, NOTCH1 somatic mutations were unique to the high-risk group (G2) (Fig. 4 and Supplementary Fig. S4). A heatmap of sequential somatic mutations occurring at least ten times is shown in Supplementary Fig. S5.
Fig. 4.
Heatmap of CRC sequential somatic mutations and clinical characteristics. We divided the patients into two subgroups (G1 and G2). Patients with a better prognosis in the previous survival decision subgroup (C1, C2, and C3) formed subgroup G1; the patients with a worse prognosis (C4, C5, C6, and C7) formed subgroup G2. We show only the 22 sequential somatic mutations that distinguished the two subgroups based on LASSO regression
Next, we performed univariate and multivariate analyses of recurrence-free survival. However, univariate and multivariate Cox regression models revealed no association between patient age, sex, primary tumor location site, tumor invasion, lymph node status, mismatch repair status, or MMR genetic variants and recurrence-free survival (RFS) (Table 1 and Supplementary Fig. 1). Nevertheless, a significant difference in RFS between the low-risk group (G1) and the high-risk group (G2) was observed in both models (adjusted hazard ratio (HR) = 7.46, 95% confidence interval (CI) = 2.48–18.56, p-value = 0.000192) (Table 1).
Table 1.
Univariate and multivariate analysis
| Characteristic | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | ||
| Age | < 65 vs > =65 | 1.48 (0.64–3.45) | 0.361 | 1.3 (0.55–3.07) | 0.56 |
| Gender | Female vs Male | 0.78 (0.35–1.71) | 0.534 | 0.72 (0.31–1.66) | 0.44 |
| Site | Left vs Right | 0.85 (0.29–2.49) | 0.769 | 1.81 (0.53–6.16) | 0.34 |
| T | T1/T2 vs T3/T4 | 1.08 (0.37–3.16) | 0.887 | 1.11 (0.36–3.45) | 0.853 |
| N | N0/N1 vs N2 | 0.68 (0.30–1.53) | 0.347 | 0.82 (0.35–1.92) | 0.649 |
| Mismatch Repair Status | Proficient vs Deficient | – | 0.997 | – | – |
| MMR genetic variants | Mutation vs Wild | 1.03 (0.39–2.7) | 0.954 | 0.94 (0.34–2.61) | 0.914 |
| Risk group | G1 vs G2 | 5.80 (2.30–14.61) | 0.000187 | 7.46 (2.48–18.56) | 0.000192 |
Abbreviations: T tumor invasion, N lymph node, MSI microsatellite instability, G1 low-risk group, G2 high-risk group, HR hazard ratio, MMR mismatch repair
The order of sequential somatic mutations is associated with clinical outcomes
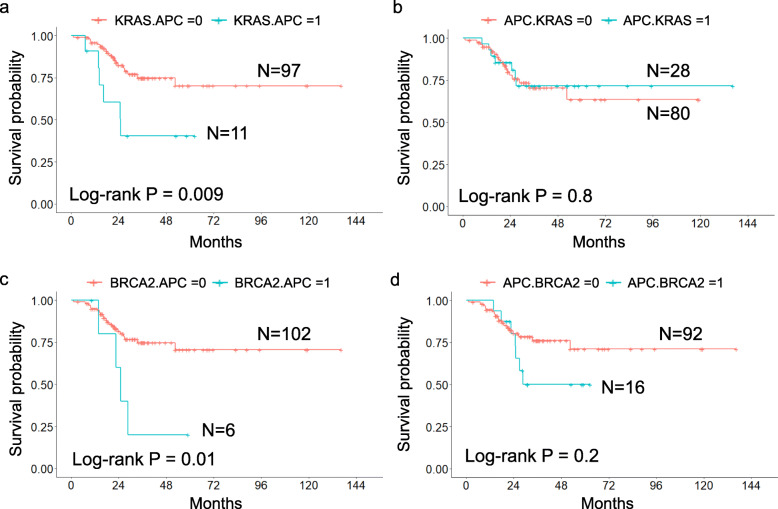
To determine whether the order of somatic mutations impacts recurrence-free survival, we examined sequential somatic mutations in two genetic orders: reverse and inverse. As a result, we found that the frequency of sequential KRAS followed by APC somatic mutations (KRAS, APC) was 10.2% and that of sequential BRCA2, APC somatic mutations was 5.6%; both were associated with a poor prognosis (hazard ratio (HR) = 3.2, 95% confidence interval (CI) = 1.3–7.99, p-value = 0.01, and HR =3.5, 95% CI = 1.2–10.31, p-value = 0.02). In contrast, there was no statistically significant difference in survival in the inverse order of sequential APC, KRAS somatic mutations (25.9%) and APC, BRCA2 mutations (14.8%) (HR = 0.89, 95% CI = 0.37–2.13, p-value = 0.8 and HR = 1.9, 95% CI = 0.78–4.47, p-value = 0.2). The results are illustrated as a scatterplot in Fig. 5.
Fig. 5.
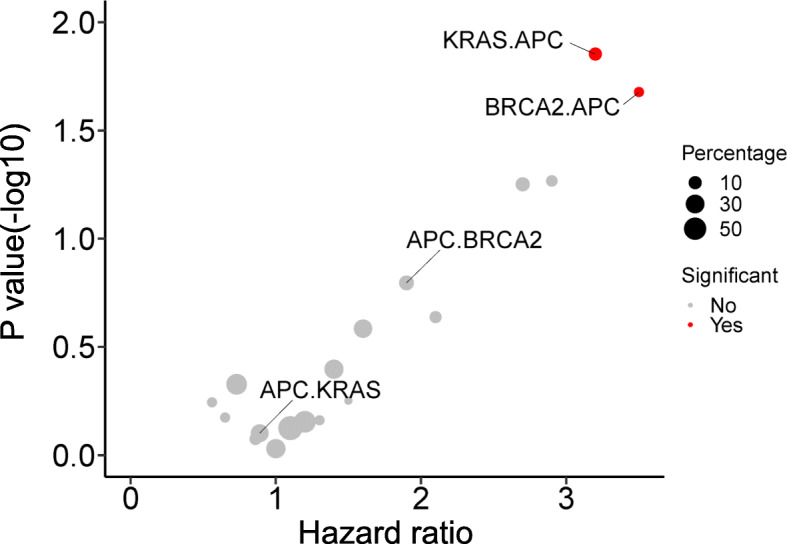
Scatter plot of sequential somatic mutations associated with clinical outcomes. The sequential genetic order of KRAS, APC with APC, KRAS and BRCA2, APC with APC, BRCA2 has a different impact on recurrence-free survival. KRAS, APC and BRCA2, APC were associated with RFS; APC, KRAS and APC, BRCA2 were not. The p-value was calculated using the hazard ratio. Red dots represent the statistical significance of the hazard ratio, and the size of the circle represents the percentage of patients with sequential somatic mutations
Kaplan–Meier analysis of patients (Fig. 6 a and c) with sequential KRAS, APC or BRCA2, APC somatic mutations also revealed a significantly poor survival outcome, with log-rank p-values of 0.009 and 0.01, respectively. When sequential somatic mutations were reversed (APC, KRAS somatic mutation and APC, BRCA2 somatic mutation), there were no significant differences in the Kaplan–Meier curves, with p-values of 0.8 and 0.2, respectively (Fig. 6 b and d). The 4-year recurrence-free survival probabilities for patients with KRAS, APC and BRCA2, APC somatic mutations were 40 and 20%, respectively, which were significantly lower than those for patients with APC, KRAS and APC, BRCA2 somatic mutations (72 and 50%, respectively) (Fig. 6). The median RFS duration in patients with sequential KRAS, APC somatic mutations was not reached, whereas it was reached for patients with APC, KRAS somatic mutations, at 24.9 months. Median RFS durations for patients with sequential BRCA2, APC and APC, BRCA2 somatic mutations were 24.9 and 28.6 months, respectively.
Fig. 6.
Kaplan–Meier plot of sequential genetic mutations with different orders. a Kaplan–Meier analysis of patients with sequential KRAS, APC somatic mutations revealed a significantly poor survival outcome, with log-rank p-values of 0.009. b There were no significant differences in patients with sequential genetic APC, KRAS somatic mutations (p-values of 0.8). c BRCA2, APC somatic mutations also led to a significantly poor survival outcome, with log-rank p-values of 0.01. d There were no significant differences in patients with APC, BRCA2 somatic mutations (p-values of 0.2). CRC patients with the sequential KRAS, APC or BRCA2, APC somatic mutations had a worse prognosis than patients without sequential somatic mutations. Sequential APC, KRAS and APC, BRCA2 somatic mutations were not associated with recurrence-free survival. The 4-year probability of RFS according to KRAS, APC somatic mutations (probability = 0.40, 95% CI = 0.19–0.86) were less than APC, KRAS somatic mutations (probability = 0.72, 95% CI = 0.55–0.92). The 4-year probability of RFS according to BRCA2, APC somatic mutations (probability = 0.20, 95% CI = 0.03–1) were less than APC, BRCA2 somatic mutations (probability = 0.5, 95% CI = 0.29–0.85)
Discussion
With advances in NGS technology, tumor-targeted sequencing is becoming an affordable tool for patients with cancer. Indeed, these advances enhanced our ability to identify the impact of tumor heterogeneity and evolution when developing drug response genetic markers in our previous study [16]. To address these issues, we performed a cancer genomics evolutionary trajectory analysis. Our results are highlighted as follows: (i) mutations in DNA damage genes, such as ATM or BRCA2 somatic mutations, can impact the clinical outcomes of stage III CRC patients; (ii) we divided patients into low-risk (G1) and high-risk (G2) groups to predict survival based on sequential somatic mutations; and (iii) the order and occurrence of sequential somatic mutations are associated with clinical outcomes.
Mutations in DNA damage repair (DDR) genes, such as those involved in mismatch repair (MMR), nucleotide excision repair (NER), homologous recombination (HR), Fanconi anemia (FA), and checkpoints, play an important role in DNA damage repair, and defects in these genes may predict the response to platinum-based chemotherapy [11, 17]. Currently, colorectal cancer patients receive an adjuvant chemotherapy mFOLFOX6 regimen with 5-fluorouracil, leucovorin, and oxaliplatin. 5-Fluorouracil (5-FU) is an antimetabolite drug that inhibits thymidylate synthase and is thought to inhibit DNA replication [11, 18]. 5-FU is also incorporated into DNA, causing DNA mismatches to be recognized and repaired by the mismatch repair (MMR) pathway [19]. Oxaliplatin is a platinum-based compound that induces cell death through several mechanisms [20]. Oxaliplatin causes DNA interstrand cross-link damage that is repaired by nucleotide excision repair proteins. This type of damage is repaired during G1 and by the Fanconi anemia and HR proteins during S phase [18, 21].
Mismatch repair (MMR) status can be used for clinical decision-making for patients with stage II colon cancer receiving 5-FU chemotherapy regimen. However, colon cancer patients in stage III with MMR-proficient tumors have clinical outcomes similar to those of MMR-deficient patients [22]. As MMR status was not associated with the prognosis of stage III colon cancer patients, we used other DNA damage response (DDR) somatic mutations in our study. Precision treatment strategies also target tumor-specific DDR somatic mutations [23]. In a previous study, ATM defects were detected in 15% of CRC patients and were associated with improved overall survival [24]. BRCA mutations can help personalize patient treatment by potentially adding platinum/DNA-damaging agents or PARP inhibitors [25]. Our study is notable because a significant survival advantage was observed in stage III CRC patients with sequential DDR gene mutations (39/108 (36.1%) patients in our cohort). The germline mutation followed by ATM and BRCA2 somatic mutations was found in 15/108 (13.8%) and 24/108 (22.2%) patients, respectively. Although the median RFS duration for the entire cohort was not reached, the occurrence of mutations in BRCA2, such as TP53 somatic mutation, resulted in different survival rates, as did different sites of the primary tumor (patients with a tumor on the left side of the colon and those with DDR gene mutations; p-value = 0.017). Our results show that DDR somatic mutations and co-occurring somatic mutations play an important role in clinical outcomes. We used cBioPortal, a web platform for gene-based data exploration, to demonstrate proof of concept. Based on CRC genomic data, we found that ATM mutations were associated with better survival. The presence of BRCA1 and TP53 somatic mutations resulted in a poorer prognosis than BRCA1 somatic mutations only in CRC patients. The results are shown in Supplementary Fig. S6.
Oxaliplatin-containing combination chemotherapy, including the FOLFOX regimen, is a well-established standard of care for stage III CRC patients receiving adjuvant therapy. Nonetheless, side effects such as neuropathy can negatively influence the quality of life. Interestingly, we built a survival prediction model for recurrence according to six sequential somatic mutations. Multivariate analysis revealed that patients in the high-risk group (G2) had a higher recurrence rate. These results suggest that we should consider another therapeutic strategy for patients at a high risk of recurrence.
Cancers result from the accumulation of somatic mutations, and their properties are thought to reflect the sum of these mutations. The order in which somatic mutations are acquired determines how individual cancers behave [4, 5], and the order in which JAK2 and TET2 are mutated influences clinical features and response to targeted therapy in patients with myeloproliferative neoplasms [26]. Compared with patients in whom TET2 mutation was acquired first, patients in whom the JAK2 mutation was acquired first had an increased risk of thrombosis and a poor prognosis. Overall, the tumor phenotype is directly influenced by different sequential orders of RAS and TP53 somatic mutations [6]. Patients harboring EGFR and comutation of tumor suppressor genes have poor recurrence-free survival [7, 8]. These studies indicate that sequential or co-occurring somatic mutations are important in cancer biology. In our study, sequential KRAS and APC somatic mutations as well as sequential BRCA2 and APC somatic mutations affected RFS. These results indicate that sequential somatic mutations have a key role in drug resistance.
Several clinical and pathological factors, as shown in Table 1, were considered when analyzing the prognostic impact of risk groups stratified by sequential somatic mutations. The small sample size is a limitation of our study. However, our model showed a strong statistical significance for RFS (adjusted hazard ratio (HR) = 7.46, p-value = 0.000192). There are also some limitations in our methods. We did not consider the effect of copy number variants (CNVs), which will influence variant allele frequency (VAF). The number of SNVs and the distribution of VAFs in targeted deep sequencing may not be enough to represent our evolution model, and in terms of the case numbers, more data may be needed to make a strong conclusion.
Conclusions
In conclusion, mutations in DNA damage response (DDR) genes represent promising biomarkers to assess the response of stage III CRC patients to oxaliplatin-based chemotherapy. The sequential order of and co-occurring DDR somatic mutations are associated with recurrence-free survival. We should consider the impact of tumor evolution when developing therapeutic strategies.
Supplementary Information
Additional file 1: Supplementary Fig. S1. The association between mismatch repair and homologous recombination gene mutations. aHeatmap of CRC with MMR and homologous recombination genetic variants and clinical characteristics. bKaplan–Meier plot of MMR mutations in CRC patients, with no significant difference. Supplementary Fig. S2. Kaplan-Meier plot of decision subgroups T1, T2 and T3. Patients in T1 (N = 39) and T2 (N = 21) had longer recurrence-free survival than patients in T3 (N = 48). T1 vs T2 (HR = 1.05, 95% CI = 0.19–5.71, P = 1) T1 vs T3 (HR = 5.89, 95% CI = 1.99–17.4, P = 0.001) T2 vs T3 (HR = 5.75, 95% CI = 1.34–24.73, P = 0.02) (median recurrence-free survival, NA months [95% CI, NA-NA] vs 33.47 months [95% CI, 23.63-NA]. Supplementary Fig. S3. Time-dependent receiver operating characteristic (ROC) curves for 2, 5, 6, and 8 years before recurrence for the 2 groups assessed at follow-up visits. The area under the curve (AUC) of this model at 2, 4, 6, and 8 years in the training set was 0.73, 0.7, 0.797, and 0.88, respectively. Supplementary Fig. S4. PCA plot of subgroups G1 and G2 by sequential genetic mutations. There was a significant difference between G1 and G2 subgroups. Sequential germline (GL) genetic mutations followed by BRCA2, ATM and BRAF somatic mutations tend to be important features in G1; APC, TP53 and APC, NOTCH1 somatic mutations belong to G2. Red circles represent the G1 group and blue triangles the G2 group. Supplementary Fig. S5 Heatmap ofCRCsequential genetic mutations andclinical characteristics occurringin at least 10 patients. Supplementary Fig. S6. Kaplan-Meier plot of ATM and TP53 with BRCA1 mutations of CRC patients in cBioPortal. aCRC patients with ATM mutations tend to have better survival than patients with ATM mutations. bThe occurrence of BRCA1 and TP53 somatic mutations led to a poorer prognosis than BRCA1 somatic mutations only in CRC patients. Supplementary Table S1. Patients characteristics in low and high risk groups. Supplementary Table S2.The association of MMR genes and HRD. Supplementary Table S3. ATM and BRCA2 mutations in subgroup C1 and C2. Supplementary Table S4. Patients characteristics in three groups.
Acknowledgments
The authors gratefully acknowledge the significant contribution of KimForest LTD., Taiwan, for bioinformatics support.
Abbreviations
- BF
Bonferroni
- CNV
Copy number variant
- CRC
Colorectal cancer
- DDR
DNA damage response
- FA
Fanconi anemia
- FDR
False discovery rate
- HR
Hazard ratio
- HR
Homologous recombination
- IQR
Interquartile range
- LASSO
Least absolute shrinkage and selection operator
- MAF
Minor allele frequency
- MMR
Mismatch repair
- dMMR
Mismatch repair deficiency
- MSI-H
Microsatellite instability-high
- NCKUH
National Cheng Kung University Hospital
- NER
Nucleotide excision repair
- OCAs
Oncomine Comprehensive Assays
- RPA
Recursive partitioning analysis
- RFS
Recurrence-free survival
- SNVs
Single-nucleotide variants
Authors’ contributions
Conception and study design: PC Lin, YM Yeh, RH Chan, BW Lin, PC Chen and MR Shen. Development of methodology: PC Lin, YM Yeh, CC Pan, and MR Shen. Acquisition of data: PC Lin, YM Yeh, RH Chan, BW Lin, PC Chen. Statistical and computational analysis: PC Lin, YM Yeh, RH Chan, BW Lin, CC Pan, and MR Shen. Writing, review, and revision of the manuscript: PC Lin, YM Yeh, RH Chan, BW Lin, PC Chen and MR Shen. Study supervision: MR Shen. All authors have read and approved the manuscript. All authors agree to the publication of this paper.
Funding
This work was supported in part by the Ministry of Science and Technology (MOST), Taiwan (research grants MOST 110-2634-F-006-014 and MOST 109–2634-F-006-023), the Ministry of Health and Welfare (MOHW110-TDU-B-211-144018) and the Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University (NCKU).
Availability of data and materials
All data generated and analyzed during this study are presented in the Supplementary Information files of this manuscript. All sequencing datasets presented in this study can be found in online repositories. The names of the repositories/repositories and accession number(s) are as follows: NCBI BioProject (Accessions: PRJNA681983/ https://dataview.ncbi.nlm.nih.gov/object/PRJNA681983?reviewer=vocgf3viamqi24ehjp86kqlhvg).
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-ER-103-395 and A-ER-104-153) and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 2.Haber DA, Gray NS, Baselga J. The evolving war on Cancer. Cell. 2011;145:19–24. doi: 10.1016/j.cell.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 4.Levine AJ, Jenkins NA, Copeland NG. The roles of initiating Truncal mutations in human cancers: the order of mutations and tumor cell type matters. Cancer Cell. 2019;35:10–15. doi: 10.1016/j.ccell.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kent DG, Green AR. Order matters: the order of somatic mutations influences cancer evolution. Cold Spring Harb Perspect Med. 2017;7:a027060. doi: 10.1101/cshperspect.a027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbet M, Salomon A, Feige JJ, Thomas M. Acquisition order of Ras and p53 gene alterations defines distinct adrenocortical tumor phenotypes. PLoS Genet. 2012;8:e1002700. doi: 10.1371/journal.pgen.1002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Pan Y, Cheng C, Zheng D, Zhang Y, Gao Z, et al. EGFR-mutant lung adenocarcinoma harboring co-mutational tumor suppressor genes predicts poor prognosis. J Cancer Res Clin Oncol. 2020;146:1781–1789. doi: 10.1007/s00432-020-03237-3. [DOI] [PubMed] [Google Scholar]
- 8.Arbour KC, Jordan E, Kim HR, Dienstag J, Yu HA, Sanchez-Vega F, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24:334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knijnenburg TA, Wang L, Zimmermann MT, Chambwe N, Gao GF, Cherniack AD, et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018;23:239–254.e6. doi: 10.1016/j.celrep.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin PC, Yeh YM, Wu PY, Hsu KF, Chang JY, Shen MR. Germline susceptibility variants impact clinical outcome and therapeutic strategies for stage III colorectal cancer. Sci Rep. 2019;9:3931. doi: 10.1038/s41598-019-40571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reilly NM, Novara L, DiNicolantonio F, Bardelli A. Exploiting DNA repair defects in colorectal cancer. Mol Oncol. 2019;13:681–700. doi: 10.1002/1878-0261.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller CA, White BS, Dees ND, Griffith M, Welch JS, Griffith OL, et al. SciClone: inferring clonal architecture and tracking the spatial and temporal patterns of tumor evolution. PLoS Comput Biol. 2014;10:e1003665. doi: 10.1371/journal.pcbi.1003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caravagna G, Giarratano Y, Ramazzotti D, Tomlinson I, Graham TA, Sanguinetti G, et al. Detecting repeated cancer evolution from multi-region tumor sequencing data. Nat Methods. 2018;15:707–714. doi: 10.1038/s41592-018-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson EJ, Therneau TM. An introduction to recursive partitioning using the RPART routines. Mayo Clin Sect Biostat Tech Rep. 2000;61:33. [Google Scholar]
- 15.Hothorn T, Zeileis A. Partykit: a modular toolkit for recursive partytioning in R. J Mach Learn Res. 2015;16:3905–3909. [Google Scholar]
- 16.Lin PC, Yeh YM, Lin BW, Lin SC, Chen PC, Shen MR. Intratumor heterogeneity of MYO18A and FBXW7 variants impact the clinical outcome of stage III colorectal cancer. Front Oncol. 2020;10:2348. doi: 10.3389/fonc.2020.588557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger E, Gout J, Arnold F, Beutel AK, Müller M, Abaei A, Barth TFE, Rasche V, Seufferlein T, Perkhofer L, Kleger A. Maintenance therapy for ATM-deficient pancreatic Cancer by multiple DNA damage response interferences after platinum-based chemotherapy. Cells. 2020;9:2110. doi: 10.3390/cells9092110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 19.Iwaizumi M, Stephanie TR, Carethers JM. DNA mismatch repair proficiency executing 5-fluorouracil cytotoxicity in colorectal cancer cells. Cancer Biol Ther. 2011;12:756–764. doi: 10.4161/cbt.12.8.17169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ, et al. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med. 2017;23:461–471. doi: 10.1038/nm.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceccaldi R, Sarangi P, D’Andrea AD. The Fanconi anaemia pathway: new players and new functions. Nat Rev Mol Cell Biol. 2016;17:337–349. doi: 10.1038/nrm.2016.48. [DOI] [PubMed] [Google Scholar]
- 22.Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148:88–99. doi: 10.1053/j.gastro.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randon G, Fucà G, Rossini D, Raimondi A, Pagani F, Perrone F, et al. Prognostic impact of ATM mutations in patients with metastatic colorectal cancer. Sci Rep. 2019;9:2858. doi: 10.1038/s41598-019-39525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arena S, Corti G, Durinikova E, Montone M, Reilly NM, Russo M, et al. A subset of colorectal cancers with cross-sensitivity to olaparib and oxaliplatin. Clin Cancer Res. 2020;26:1372–1384. doi: 10.1158/1078-0432.CCR-19-2409. [DOI] [PubMed] [Google Scholar]
- 26.Ortmann CA, Kent DG, Nangalia J, Silber Y, Wedge DC, Grinfeld J, et al. Effect of mutation order on Myeloproliferative neoplasms. N Engl J Med. 2015;372:601–612. doi: 10.1056/NEJMoa1412098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Fig. S1. The association between mismatch repair and homologous recombination gene mutations. aHeatmap of CRC with MMR and homologous recombination genetic variants and clinical characteristics. bKaplan–Meier plot of MMR mutations in CRC patients, with no significant difference. Supplementary Fig. S2. Kaplan-Meier plot of decision subgroups T1, T2 and T3. Patients in T1 (N = 39) and T2 (N = 21) had longer recurrence-free survival than patients in T3 (N = 48). T1 vs T2 (HR = 1.05, 95% CI = 0.19–5.71, P = 1) T1 vs T3 (HR = 5.89, 95% CI = 1.99–17.4, P = 0.001) T2 vs T3 (HR = 5.75, 95% CI = 1.34–24.73, P = 0.02) (median recurrence-free survival, NA months [95% CI, NA-NA] vs 33.47 months [95% CI, 23.63-NA]. Supplementary Fig. S3. Time-dependent receiver operating characteristic (ROC) curves for 2, 5, 6, and 8 years before recurrence for the 2 groups assessed at follow-up visits. The area under the curve (AUC) of this model at 2, 4, 6, and 8 years in the training set was 0.73, 0.7, 0.797, and 0.88, respectively. Supplementary Fig. S4. PCA plot of subgroups G1 and G2 by sequential genetic mutations. There was a significant difference between G1 and G2 subgroups. Sequential germline (GL) genetic mutations followed by BRCA2, ATM and BRAF somatic mutations tend to be important features in G1; APC, TP53 and APC, NOTCH1 somatic mutations belong to G2. Red circles represent the G1 group and blue triangles the G2 group. Supplementary Fig. S5 Heatmap ofCRCsequential genetic mutations andclinical characteristics occurringin at least 10 patients. Supplementary Fig. S6. Kaplan-Meier plot of ATM and TP53 with BRCA1 mutations of CRC patients in cBioPortal. aCRC patients with ATM mutations tend to have better survival than patients with ATM mutations. bThe occurrence of BRCA1 and TP53 somatic mutations led to a poorer prognosis than BRCA1 somatic mutations only in CRC patients. Supplementary Table S1. Patients characteristics in low and high risk groups. Supplementary Table S2.The association of MMR genes and HRD. Supplementary Table S3. ATM and BRCA2 mutations in subgroup C1 and C2. Supplementary Table S4. Patients characteristics in three groups.
Data Availability Statement
All data generated and analyzed during this study are presented in the Supplementary Information files of this manuscript. All sequencing datasets presented in this study can be found in online repositories. The names of the repositories/repositories and accession number(s) are as follows: NCBI BioProject (Accessions: PRJNA681983/ https://dataview.ncbi.nlm.nih.gov/object/PRJNA681983?reviewer=vocgf3viamqi24ehjp86kqlhvg).