To the Editor:
The prevalence of hiatus hernia in patients with idiopathic pulmonary fibrosis (IPF) is ~40% [1–3], greater than the prevalence of hiatus hernia in other chronic lung diseases. While the role of gastro-oesophageal reflux disease (GORD) and its treatment remain an area of controversy in IPF [4, 5], hiatus hernia is associated with reduced survival [2, 3] and more rapid lung function decline [3]. The cause for the increased prevalence of hiatus hernia in IPF remains unknown. It is possible that the increased rate of GORD associated with hiatus hernia leads to frequent chronic micro-aspiration events [1], which could contribute to the development of pulmonary fibrosis. Alternatively, it is plausible that as IPF progresses, the biomechanics of the fibrotic lung result in progressively more negative intrathoracic pressure causing cranial migration of the oesophagogastric junction (GOJ) and stomach into the thorax. The association between hiatus hernia and early stages of pulmonary fibrosis has not been assessed previously.
Interstitial lung abnormalities (ILA) are patterns of density seen on thoracic computed tomography (CT) scans identified incidentally in those without a pre-existing diagnosis of interstitial lung disease (ILD) [6]. These probably represent a broader range of disorders than IPF alone [7], and there is evidence that early developing stages of pulmonary fibrosis are present in some individuals identified with ILA [8–10]. To explore the role of hiatus hernia in the early development of pulmonary fibrosis, we sought to evaluate the association between hiatus hernia and ILA. Additionally, we sought to determine the associations between hiatus hernia and ILA progression and mortality.
Protocols for participant enrolment in the Age Gene/Environment Susceptibility (AGES)-Reykjavik study have been described previously [11]. Hiatus hernia status was characterised in 4885 (92%) of the 5320 participants recruited between 2002 and 2006, who had both chest CT and mortality data as of 2016. The methods for thoracic CT characterisation of ILA and ILA progression in the AGES-Reykjavik cohort have been described previously [10, 12, 13]. The presence of hiatus hernia was evaluated on thoracic CT scans by a single reader. Two radiologists additionally graded all cases of hiatus hernia using a previously described four-point scale [14]. Briefly, a grade 1 hiatus hernia is a “sliding” hernia with the GOJ above the level of the diaphragm; a grade 2 hiatus hernia is a “rolling” hernia with a portion of the gastric fundus above the diaphragm with a normal position of the GOJ; a grade 3 hiatus hernia is a mix of the previous two with both an abnormal placement of the gastric fundus and the GOJ; and a grade 4 hiatus hernia includes additional elements of the abdominal viscera in the hernia sac (figure 1). Written informed consent was obtained from all participants, and the Icelandic Bioethics Committee (VSN: 00–063) and the institutional review board of the Brigham and Women’s Hospital approved this study.
FIGURE 1.
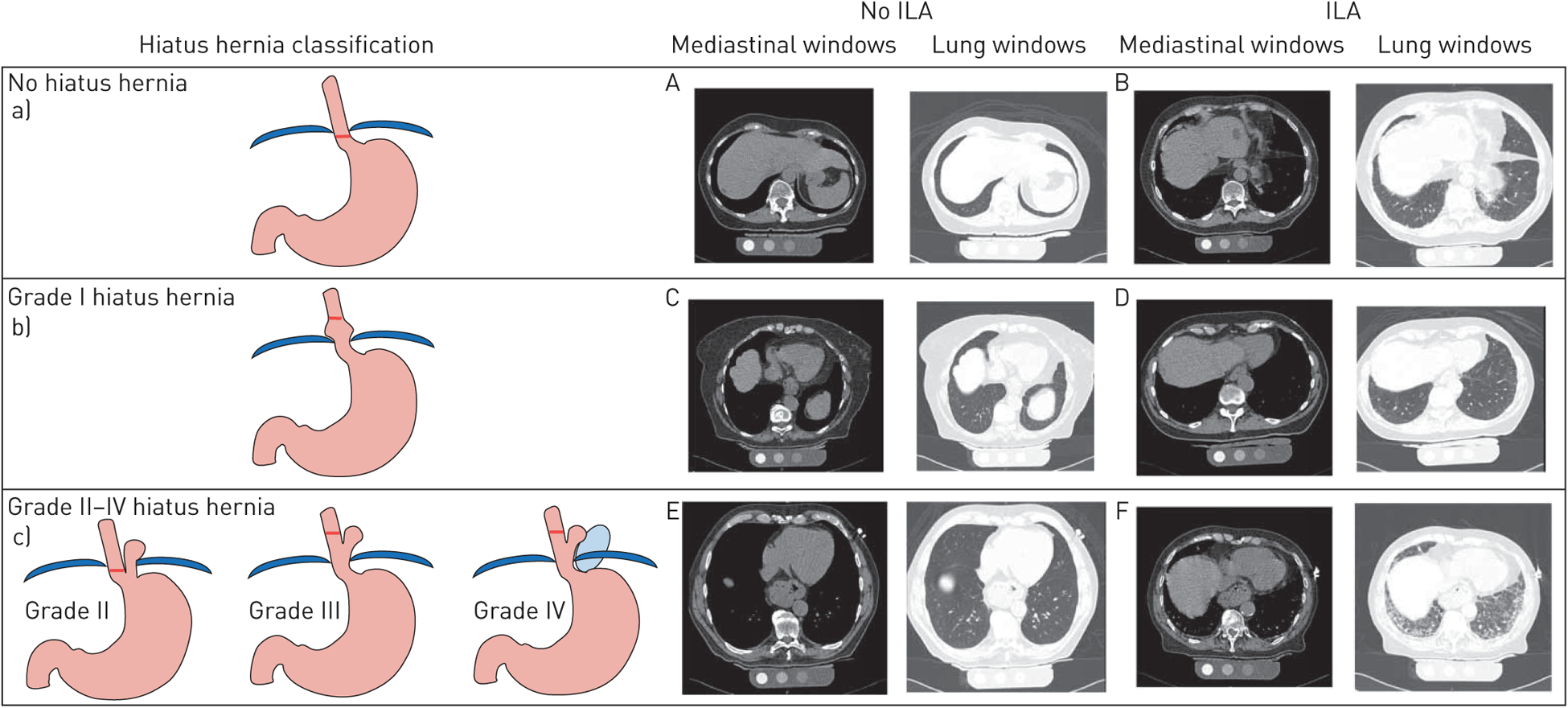
Hiatus hernia and interstitial lung abnormality (ILA) classification. Graphical representation of a) normal thoracoabdominal anatomy (no hiatus hernia), b) grade I hiatus hernia and c) grades II–IV hiatus hernia. Mediastinal and lung windows from axial chest computed tomography scans demonstrate hiatus hernia anatomy and parenchymal involvement respectively for participants with and without ILA (A–F). Participant A has no hiatus hernia and no ILA. Participant B has no hiatus hernia and subpleural ILA with fibrosis. Participant C has a grade I hiatus hernia and no ILA. Participant D has a grade I hiatus hernia and subpleural ILA without fibrosis. Participant E has a grade II–IV hiatus hernia and no ILA. Participant F has a grade II–IV hiatus hernia and subpleural ILA with fibrosis.
Analyses evaluating the association between hiatus hernia and ILA, ILA subtypes and ILA progression were performed using logistic regression. Multivariable analyses were adjusted for age, sex, body mass index, smoking pack-years and current smoking status. Cox models were used to evaluate associations between hiatus hernia and mortality. Reported p-values were two-sided, with <0.05 considered statistically significant.
The prevalence of hiatus hernia in research participants with ILA (21%) was similar to the prevalence of hiatus hernia in the entire cohort (23%). Regardless of size, the presence of hiatus hernia was not associated with ILA (OR, 1.01, 95% CI 0.76–1.35; p=0.94) when adjusting for covariates. Similarly, moderate to large hiatus hernia (grade II–IV) was not associated with the presence of ILA (OR 1.26, 95% CI 0.73–2.19; p=0.41), after adjusting for covariates. The presence of hiatus hernia, regardless of size, was not associated with an increased risk of ILA progression (OR 1.50, 95% CI 0.75–2.97; p=0.25). Furthermore, the presence of a hiatus hernia was not associated with development of an ILA when serial imaging was analysed (OR 1.05, 95% CI 0.81–1.37; p=0.71).
The prevalence of hiatus hernia was 37% (seven out of 19) in those with a usual interstitial pneumonia (UIP) pattern of ILA. Although there was a greater than two-fold increase in the odds of a hiatus hernia in those with a UIP pattern compared to those without ILA, after adjusting for covariates, this finding was not statistically significant (OR 2.34, 95% CI 0.89–6.17; p=0.09).
In the overall cohort, while the presence of hiatus hernia was not associated with mortality (hazard ratio (HR) 0.92, 95% CI 0.77–1.10; p=0.35), after adjusting for covariates there was an increased risk of death in participants with a moderate to large (grade II–IV) hiatus hernia (HR 1.65, 95% CI 1.24–2.18; p=0.0005). The median follow-up time was 5.4 years. By the 2-year follow-up time point, 14 (6.3%) out of 222 of those with a moderate to large (grade II–IV) hiatus hernia had died compared with 80 (1.7%) out of 4663 of those without a hiatus hernia or with a small (grade 0–I) hiatus hernia. By the 4-year follow-up time point, 37 (17%) out of 222 of those with a grade II–IV hiatus hernia had died compared with 458 (9.8%) out of 4663 of those with a grade 0–I hiatus hernia.
Among those with ILA, the presence of hiatus hernia of any size, and specifically a moderate to large hiatus hernia was not associated with mortality (HR 1.36, 95% CI 0.85–2.16; p=0.20), and (HR 1.71, 95% CI 0.68–4.31; p=0.26), respectively. During the follow-up period, 63% (12 out of 19) of those with a UIP pattern of ILA had died; 58% (seven out of 12) of those with UIP and no hiatus hernia died; and 71% (five out of seven) of those with UIP and a hiatus hernia died (of these, both participants with a moderate to severe hiatus hernia died).
There was a positive association between prescription of antacid therapy (both proton pump inhibitors and H2-blockers) and the presence of hiatus hernia (OR 1.88, 95% CI 1.59–2.23; p<0.0001). There was no evidence for an association between antacid therapy and ILA (OR 1.25, 95% CI 0.94–1.66; p=0.13) or risk of ILA progression (OR 1.48, 95% CI 0.73–2.99; p=0.27).
This is the first study to utilise thoracic CT scans to systematically evaluate the prevalence of hiatus hernia in a general population sample and to explore the potential impact of hiatus hernia on the development of early stages of ILD. We report that the prevalence of hiatus hernia in ILA is similar to that of the general population and that the presence of hiatus hernia is not associated with an increased risk of ILA or ILA progression. These data do not support the view that GORD is a major contributor to the early stages of ILD, although it is conceivable that GORD and micro-aspiration become more relevant in susceptible individuals as disease progresses and the lungs become primed for fibrogenesis.
We find that the prevalence of hiatus hernia in those with a UIP pattern of ILA is twice that of the general population at ~40%, a rate consistent with that previously observed in prior studies of IPF patients [1–3]. Furthermore, our data demonstrate that in those with UIP, the presence of a hiatus hernia is associated with an increased risk of death, although this analysis was limited by a small sample size. The increased prevalence of hiatus hernia in UIP and observed mortality signal could be the consequence of accelerated progression of fibrotic lung disease mediated by repeated micro-aspiration events among those with UIP and a hiatus hernia. Alternatively, it is possible that a hiatus hernia may serve as a marker of a group of patients with more advanced fibrotic lung disease whose progressive negative intrathoracic pressure results in a retraction of the stomach into the thoracic cavity. Definitively addressing this question will require longitudinal gastrointestinal physiology studies in patients with pulmonary fibrosis.
This analysis has a number of limitations. Lung function data were not available, which limits our ability to determine if the extent of restrictive deficits could contribute to the prevalence of hiatus hernia among those with ILA. The analysis of participants with UIP was limited by small numbers, and so it is not possible to establish whether the presence of hiatus hernia in those with UIP was associated with ILA progression or development of clinically relevant ILD. Thoracic CT is not the gold standard test for the diagnosis of hiatus hernia and is likely to underestimate its prevalence. However, the positive association between hiatus hernia and prescription of antacid therapy in this study suggests that participants with a hiatus hernia were sufficiently symptomatic of GORD to require pharmacological therapy and that thoracic CT is a pragmatic and effective tool for identification of clinically relevant hiatus hernia.
In summary, our study does not demonstrate an association between hiatus hernia and ILA and in those with an ILA, the prevalence of hiatus hernia is similar to that of the general population at ~20%, suggesting that GORD is not a driver for the early stages of ILD. We report that those with a UIP pattern of ILA have a two-fold increase in the prevalence of hiatus hernia, a rate similar to the 40% reported in IPF cohorts. In participants with UIP and a moderate to large hiatus hernia there was a suggested increased risk of mortality and although this analysis was limited by small numbers, this group of individuals should undergo close surveillance and may be at higher risk of worse outcomes. The finding that across the whole cohort, the presence of a moderate to large hiatus hernia was associated with an increased risk of death is potentially important and requires further study.
Footnotes
Conflict of interest: P.M. George reports personal fees from Roche Pharmaceuticals and Teva, grants and personal fees from Boehringer Ingelheim, outside the submitted work. T. Hida has nothing to disclose. R.K. Putman reports grants from NIH, during the conduct of the study. T. Hino has nothing to disclose. S.R. Desai has nothing to disclose. A. Devaraj reports personal fees from Boehringer Ingelheim, Galapagos, Galecto Biotech and GSK, outside the submitted work. S. Kumar has nothing to disclose. J.A. Mackintosh reports personal fees for lectures from Roche, outside the submitted work. V. Gudnason has nothing to disclose. H. Hatabu reports grants from Canon Medical System Inc. and Konica-Minolta Inc., personal fees for consultancy from Mitsubishi Chemical Inc., and has been member of an advisory board for Canon Medical System Inc., outside the submitted work. G. Gudmundsson has nothing to disclose. G.M. Hunninghake reports personal fees from Boehringer Ingelheim, Mitsubishi Chemical and Gerson Lehrman Group, outside the submitted work.
References
- 1.Noth I, Zangan SM, Soares RV, et al. Prevalence of hiatal hernia by blinded multidetector CT in patients with idiopathic pulmonary fibrosis. Eur Respir J 2012; 39: 344–351. [DOI] [PubMed] [Google Scholar]
- 2.Tossier C, Dupin C, Plantier L, et al. Hiatal hernia on thoracic computed tomography in pulmonary fibrosis. Eur Respir J 2016; 48: 833–842. [DOI] [PubMed] [Google Scholar]
- 3.Mackintosh JA, Desai SR, Adamali H, et al. In patients with idiopathic pulmonary fibrosis the presence of hiatus hernia is associated with disease progression and mortality. Eur Respir J 2019; 53: 1802412. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Collard HR, Anstrom KJ, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med 2013; 1: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreuter M, Wuyts W, Renzoni E, et al. Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis. Lancet Respir Med 2016; 4: 381–389. [DOI] [PubMed] [Google Scholar]
- 6.Washko GR, Lynch DA, Matsuoka S, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol 2010; 17: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobbs BD, Putman RK, Araki T, et al. Overlap of genetic risk between interstitial lung abnormalities and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2019; 200: 1402–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller ER, Putman RK, Vivero M, et al. Histopathology of interstitial lung abnormalities in the context of lung nodule resections. Am J Respir Crit Care Med 2018; 197: 955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araki T, Putman RK, Hatabu H, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med 2016; 194: 1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putman RK, Gudmundsson G, Axelsson GT, et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am J Respir Crit Care Med 2019; 200: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007; 165: 1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putman RK, Gudmundsson G, Araki T, et al. The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes. Eur Respir J 2017; 50: 1700537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putman RK, Hatabu H, Araki T, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA 2016; 315: 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skinner D. Hernias (hiatal, traumatic, and congenital). In: Berk JE, ed. Gastroenterology. 4th Edn. Philadelphia, WB Saunders, 1985; pp. 705–716. [Google Scholar]


