Significance
Cells sense and respond to nutrients liberated by the degradative activity of lysosomes. PQLC2 is a transporter that mediates the efflux of lysosomal cationic amino acids and also recruits a signaling complex containing WDR41, SMCR8, and C9orf72 proteins to the surface of lysosomes by interacting with WDR41. This interaction is regulated by the availability of the amino acids that PQLC2 transports. Here we define sites of contact between PQLC2 and WDR41 and relate them to conformational changes required for transport activity of PQLC2. In addition to directly advancing our understanding of the WDR41–PQLC2 interaction, this study provides a model for how a transporter can also serve as a sensor that recruits signaling proteins to the membrane where it resides.
Keywords: lysosome, transporter, transceptor, C9orf72, PQLC2
Abstract
PQLC2, a lysosomal cationic amino acid transporter, also serves as a sensor that responds to scarcity of its substrates by recruiting a protein complex composed of C9orf72, SMCR8, and WDR41 to the surface of lysosomes. This protein complex controls multiple aspects of lysosome function. Although it is known that this response to changes in cationic amino acid availability depends on an interaction between PQLC2 and WDR41, the underlying mechanism for the regulated interaction is not known. In this study, we present evidence that the WDR41–PQLC2 interaction is mediated by a short peptide motif in a flexible loop that extends from the WDR41 β-propeller and inserts into a cavity presented by the inward-facing conformation of PQLC2. The data support a transceptor model wherein conformational changes in PQLC2 related to substrate transport regulate the availability of the WDR41-binding site on PQLC2 and mediate recruitment of the WDR41-SMCR8-C9orf72 complex to the surface of lysosomes.
Lysosomes have long been known as sites where hydrolytic enzymes break down pathogens and cellular macromolecules into the basic building blocks of cells (amino acids, nucleic acids, sugars, lipids, and metals) so that diverse transporters can return these nutrients to the cytoplasm to support ongoing anabolic needs (1). More recently, it has been established that in addition to their function in clearing potentially toxic material and recycling nutrients, important signals are transduced from lysosomes. This prominently includes signaling via mTORC1, which is initiated on the cytoplasmic surface of lysosomes under the control of a highly regulated network of proteins that sense and respond to changes in nutrient and growth factor availability (1–3). Cellular responses to various pathogens are also initiated from lysosomes via several members of the Toll-like receptor (TLR) family (4). Both mTORC1 and TLR signaling from lysosomes depend on mechanisms to transduce signals from the lysosome lumen to the cytoplasm.
In addition to these long-established pathways for signaling from lysosomes, the lysosomal cationic amino acid transporter PQLC2/SLC66A1 was recently found to recruit a heterotrimeric complex composed of the C9orf72, SMCR8, and WDR41 proteins to the cytoplasmic surface of lysosomes (5). Knockout studies in multiple model organisms have established that this protein complex is critical for normal lysosome function and has impacts on both mTORC1 and TLR signaling (6–11). Considerable attention has been focused on the C9orf72 gene due to a hexanucleotide expansion in a noncoding region, which causes amyotrophic lateral sclerosis and frontotemporal dementia (12–14). Multiple mechanisms have been proposed to explain how the C9orf72 hexanucleotide expansion causes neurodegenerative diseases and even though the expansion occurs in a noncoding region, epigenetic silencing of the affected allele results in an overall decrease in C9orf72 expression levels in carriers of the repeat expansion and has been proposed to have disease relevance (12, 14–19). Understanding PQLC2-dependent recruitment of the C9orf72-SMCR8-WDR41 complex to lysosomes thus is broadly relevant for both lysosome cell biology and neurodegenerative diseases that potentially arise from C9orf72 deficiency.
Within the C9orf72-SMCR8-WDR41 protein complex, WDR41 forms a bridge between C9orf72-SMCR8 and PQLC2 and thus is essential for lysosome recruitment of the C9orf72-SMCR8-WDR41 complex (5, 20). This interaction between WDR41 and PQLC2 is negatively regulated by the availability of the cationic amino acids that are transported out of lysosomes by PQLC2 (5). As a result, the C9orf72-SMCR8-WDR41 complex is maximally recruited to lysosomes when cells are starved of cationic amino acids (5, 11, 20). Like members of the SWEET family of transporters, PQLC2 contains seven transmembrane-spanning segments that are critical for its transporter activity (21, 22). However, PQLC2 contains only minimal sequences that are exposed to the cytoplasm and has no folded cytoplasmic domains as candidates for mediating either amino acid sensing or WDR41 interactions. Therefore, although the PQLC2–WDR41 interaction is central to lysosomal nutrient sensing, it is unknown how PQLC2 acts as both an amino acid transporter and a platform for the regulated recruitment of the C9orf72 complex to the surface of lysosomes.
In this study, we have established the basis for interactions between WDR41 and PQLC2 through an iterative process of structural predictions and biochemical validation. Our results support a model wherein WDR41 recruitment to lysosomes is mediated by a short peptide motif within a flexible loop that extends from the WDR41 β-propeller and inserts into the large cavity exposed by the inward-facing conformation of PQLC2. Our results thus help explain how PQLC2 acts as both a cationic amino acid transporter and a WDR41 receptor to support the ability of cells to sense and respond to changing cationic amino acid availability.
Results and Discussion
Prediction of a Mechanism for the WDR41–PQLC2 Interaction.
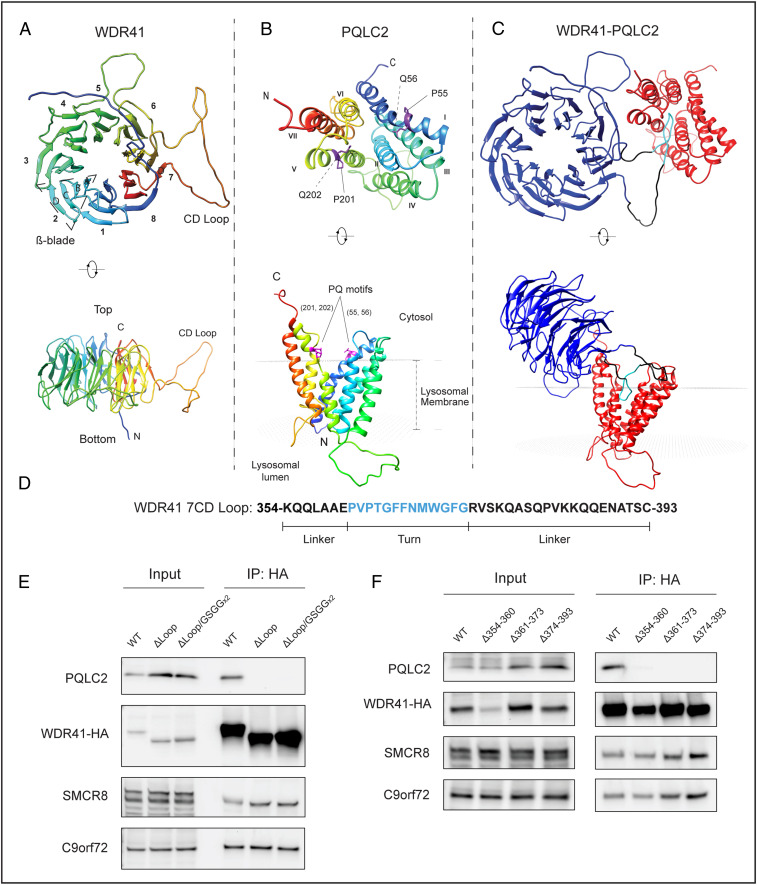
At endogenous expression levels, the interactions between WDR41 and PQLC2 are tightly regulated by cationic amino acid availability (5). Thus, defining the basis for this PQLC2–WDR41 interaction, which controls WDR41-SMCR8-C9orf72 complex recruitment to the surface of lysosomes, is important for understanding lysosome-based nutrient sensing and signaling. To address this problem, we first performed homology modeling using HHpred (MPI Bioinformatics Toolkit) to predict a WDR41 structure. The human ODA16 β-propeller protein (Protein Data Bank [PDB] ID code 5NNZ) displayed the highest predicted structural homology with WDR41 (SI Appendix, Fig. S1A) and was selected as the template for further WDR41 modeling using MODELER (23). This led to a model of WDR41 as an eight-bladed β-propeller with a prominent loop (or unstructured region) formed at the bottom of blade 7, between β-strands C and D (7CD loop; Fig. 1A). The secondary structure predicted by WDSP, a database for WD40 proteins, also contained a similar large loop extending from the WDR41 β-propeller (24). Although not available at the start of our study, two recently reported cryo-EM structures for the WDR41-C9orf72-SMCR8 complex did not resolve this loop within WDR41 (likely due to its predicted flexibility), but they nonetheless provide evidence consistent with the presence of the 7CD loop (25, 26). They further show that the interaction interface between WDR41 and SMCR8-C9orf72 is distinct from the 7CD loop and instead involves blade 8 and the C terminus of WDR41 (25, 26).
Fig. 1.
Generation and validation of a structural model predicting that the WDR41 7CD loop is necessary for interaction with PQLC2. (A) Ribbon diagram of the predicted WDR41 structure that forms an eight-blade β-propeller with a large loop (CD loop) formed between the C and D β-strands in blade 7. (B) Predicted structure of PQLC2 inward-facing conformation. PQ motifs in transmembrane helices 1 and 5 are highlighted. The position of PQLC2 in the lysosomal membrane was predicted via the PPM 2.0 server (30). (C) Model derived from the docking of predicted WDR41 and PQLC2 structures (model with the highest score shown) wherein the 7CD loop of WDR41 is inserted within the central cavity exposed by the inward-facing conformation of PQLC2. (D) WDR41 7CD loop amino acid sequence. (E) Anti-HA immunoprecipitation from cells expressing PQLC2-FLAG and HA-tagged wild-type or indicated mutant versions of WDR41-HA, followed by detection of the indicated proteins by immunoblotting. (F) Anti-HA immunoprecipitation followed by immunoblot detection of HeLa cells expressing PQLC2-FLAG along with either WT WDR41-HA or WDR41-HA mutants with the indicated deletions.
In parallel, we predicted a structure for PQLC2 based on homology to SWEET13 from Arabidopsis thaliana (another member of the PQ-loop transporter family; SI Appendix, Fig. S1B), whose structure was previously solved in the inward-facing conformation (PDB ID code 5XPD) (27). The resulting model for PQLC2 matches expectations for members of this family in that it contains an N-terminal three helix bundle (THB) made up by transmembrane domains (TMDs) 1 to 3 linked to the C-terminal THB composed of TMDs 5 to 7 via the TMD 4 linker (Fig. 1B) (27–29). This conformation contains a central cavity oriented toward the cytoplasm. In this model, the PQ motifs in TMDs 1 and 5 are located at the membrane-cytosol interface (Fig. 1B, side view; inferred with the assistance of PPM web server; ref. 30).
The predicted structures of WDR41 and PQLC2 were used to perform ab initio asymmetric protein-protein docking via GalaxyTongDock (31). In the highest-ranked model emerging from these predictions, the interaction was mediated by insertion of the 7CD loop of WDR41 into the central cavity of PQLC2 (Fig. 1C). In this model, the tip of the WDR41 7CD loop occupies a space within the central cavity close to where a substrate analog was found to reside in the SWEET13 inward-facing structure (27).
Mutagenesis Experiments Validate Structural Predictions.
We next generated WDR41 mutants with either a complete deletion of the region encompassing the 7CD loop (residues 354 to 393; Fig. 1D) or its replacement by a flexible linker sequence (GGSGGGSG) and used the strong constitutive interaction that occurs between overexpressed WDR41 and PQLC2 (5) to test our prediction about the WDR41–PQLC2 interaction mechanism. Immunoprecipitation experiments revealed that neither of the WDR41 loop deletion mutants bound to PQLC2; however, both of these mutants were expressed at wild-type levels and retained their ability to interact with C9orf72 and SMCR8, suggesting that folding of their β-propeller remains intact (Fig. 1E). To identify determinants within the WDR41 loop for PQLC2 interaction, we defined three subregions corresponding to the turn region, which was predicted to insert most deeply into the PQLC2 inward-facing cavity, as well as the two flanking linker sequences (Fig. 1D), and tested the impact of deleting each of these subregions. Although none of these WDR41 mutants interacted with PQLC2, they all still interacted with C9orf72 and SMCR8 (Fig. 1F).
A Short Motif within the WDR41 7CD Loop Is Sufficient for PQLC2 Binding.
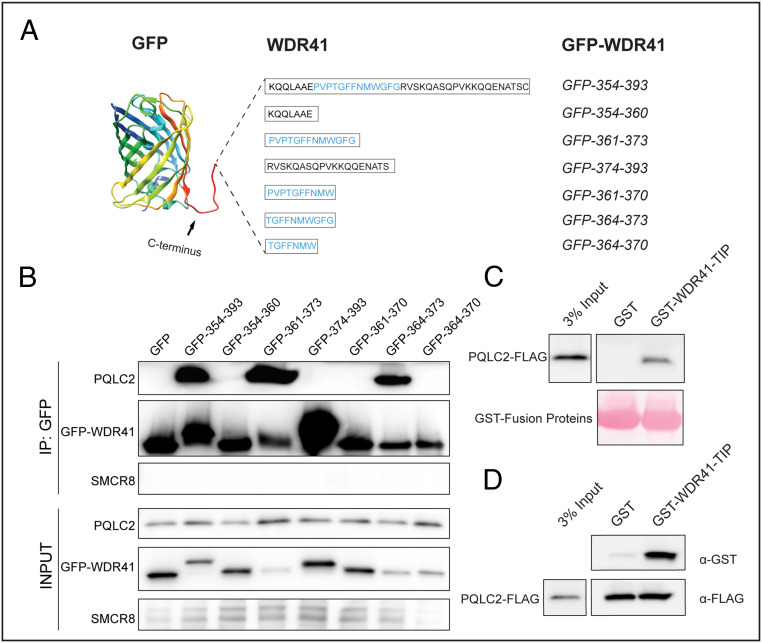
Based on the predicted model for WDR41–PQLC2 interactions (Fig. 1C), the negative impact of deletions to multiple parts of the WDR41 loop could reflect a need for specific sequences within the WDR41 turn region at the proposed site of contact with PQLC2, as well as flanking linker regions that extend the tip of this loop away from the β-propeller surface. To test this hypothesis, we fused the entire WDR41 loop sequence (354 to 393) or three small portions—354 to 360 (linker), 361 to 373 (turn), and 374 to 393 (linker)—to the C terminus of EGFP (Fig. 2A). Anti-GFP immunoprecipitations revealed that the entire WDR41 7CD loop sequence (39 residues) and the GFP fusion protein containing the WDR41 turn region (361-PVPTGFFNMWGFG-373), but not either of the flanking sequences, interacted with PQLC2 (Fig. 2B). Further deletions to the turn region established that the 10 amino acids defined by WDR41 amino acids 364 to 373 (TGFFNMWGFG) represents the minimal linear peptide sequence able to interact with PQLC2, which we henceforth refer to as the transporter-interacting peptide (TIP; Fig. 2B). As a negative control, no interactions were observed between any of these GFP fusions and SMCR8. Consistent with the critical role for the TIP in mediating the interaction with PQLC2, multiple sequence alignments of the WDR41 7CD loop from diverse species revealed that the TIP region is more highly conserved than its flanking sequences (SI Appendix, Fig. S2).
Fig. 2.
A 10-aa peptide from the WDR41 7CD loop is sufficient for interaction with PQLC2. (A) Schematic representation of GFP chimeric proteins containing fragments of the WDR41 7CD loop. The indicated amino acid sequence of WDR41 regions of interest were fused to an unstructured C-terminal extension from GFP. (B) Analysis of PQLC2-FLAG binding to GFP-WDR41 chimeras by immunoprecipitation and immunoblotting. Proteins were immunoprecipitated with GFP-Trap beads, and samples corresponding to the lysates (Input) and immunoprecipitated proteins (IP: GFP) were immunoblotted with FLAG, GFP, and SMCR8 antibodies. (C) GST pull-down assay testing for an interaction between recombinant GST and GST-WDR41 TIP and PQLC2-FLAG from HeLa cell lysates. (D) PQLC2-FLAG was isolated by immunoprecipitation and used as bait to test for interaction with recombinant GST and GST-WDR41 TIP, respectively.
WDR41 Interacts Directly with PQLC2 via the TIP Motif.
To test for a direct interaction between the WDR41 TIP and PQLC2, we performed pull-down assays with a recombinant protein consisting of GST fused to the 10-aa TIP sequence. When immobilized on beads, this fusion protein interacted with PQLC2-FLAG from cell lysates (Fig. 2C). In reciprocal interaction experiments, in which PQLC2-FLAG was first purified by immunoprecipitation and then used as bait, it selectively interacted with the recombinant GST-TIP fusion protein but not with GST alone (Fig. 2D). These results corroborate the previous interaction assays using GFP fusions expressed in cells and support a direct interaction between the WDR41 TIP and PQLC2.
Specific Residues within the WDR41 TIP Are Required for PQLC2 Binding.
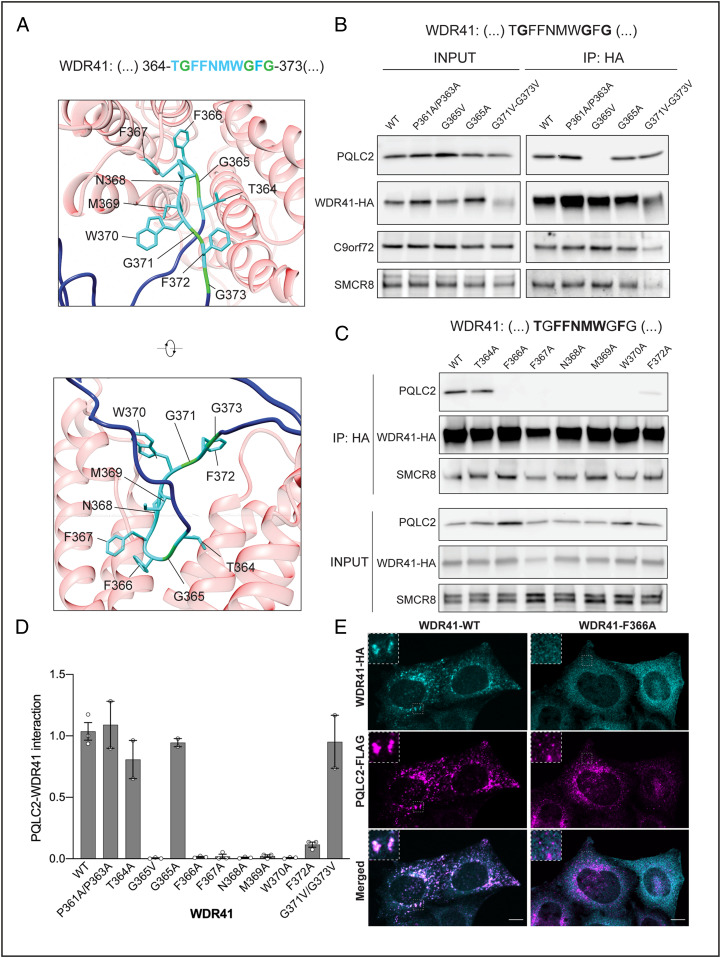
We next carried out a series of site-directed mutagenesis experiments to define key residues within the WDR41 TIP that are required for PQLC2 binding (Fig. 3A). Consistent with our previous experiments that defined the minimal TIP as amino acids 364 to 373, mutating prolines 361 and 363 to alanine had no impact on the interaction between full-length WDR41 and PQLC2. Although the WDR41-G365A mutant still interacted with PQLC2, the bulkier G365V substitution at this site abolished the interaction. However, glycine-to-valine substitutions at 371 and 373 were both tolerated (Fig. 3B).
Fig. 3.
Site-directed mutagenesis defines WDR41-specific residues in the 7CD loop that are required for PQLC2 binding. (A) Structural representation of the predicted interaction between WDR41-PQLC2 (front view and side view), showing the side chains of the WDR41 TIP residues. (B) Impact of site-directed mutagenesis of selected glycines and prolines within and adjacent to the WDR41-HA TIP on PQLC2–FLAG interactions. (C) Alanine-scanning mutagenesis of the remaining residues within the WDR41 TIP (T364A, F366A, F367A, N368A, M369A, W370A, or F372A). HeLa cells transiently cotransfected with plasmids expressing PQLC2-FLAG and the indicated WDR41-HA mutants were lysed and immunoprecipitated with anti-HA affinity matrix and then immunoblotted with FLAG, HA, C9orf72, and SMCR8 antibodies. (D) Quantification of PQLC2 interactions with the indicated WDR41 mutants (normalized to wild-type WDR41). Data are mean ± SEM plotted with data points from independent experiments as open circles. Quantification of this experiment is shown in SI Appendix, Fig. S2A. (E) Spinning-disk confocal immunofluorescence microscopy of transiently cotransfected HeLa WDR41 KO cells expressing PQLC2-FLAG and WDR41-HA-WT vs. the WDR41-HA-F366A mutant. (Insets) 3× magnifications of the selected regions denoted by dashed lines. (Scale bars: 10 µm.) Quantification of this experiment is shown in SI Appendix, Fig. S2B.
Having observed the variable importance of specific amino acids within this WDR41 TIP region, we next performed an alanine scanning mutagenesis of this whole region, which revealed that replacement of F366, F367, N368, M369, W370, or F372 with alanine abolished PQLC2 interactions (Fig. 3 C and D). Meanwhile, the T364A mutation had minimal effects (Fig. 3 C and D). Collectively, the selective importance of multiple amino acids within the WDR41 TIP sequence strengthens our conclusion that this peptide motif within the 7CD loop of WDR41 mediates the interaction with PQLC2. As all WDR41 mutants retained their interaction with SMCR8 (Fig. 3C and SI Appendix, Fig. S3A), these changes within the 7CD loop did not grossly disrupt the β-propeller architecture of WDR41.
WDR41 TIP Is Required for PQLC2-Dependent Recruitment of WDR41 to Lysosomes.
To test our model wherein the WDR41 loop mediates PQLC2 interactions and WDR41 recruitment to lysosomes in cells, we analyzed the subcellular localization of the WDR41-F366A mutant as a representative example of a TIP motif point mutant that cannot interact with PQLC2. Whereas the wild-type WDR41 was enriched on PQLC2-positive lysosomes, the WDR41-F366A mutant was not recruited to lysosomes and was instead dispersed throughout the cytoplasm (Fig. 3E and SI Appendix, Fig. S3B).
Residues within the Predicted PQLC2 Internal Cavity Are Critical for WDR41 Binding.
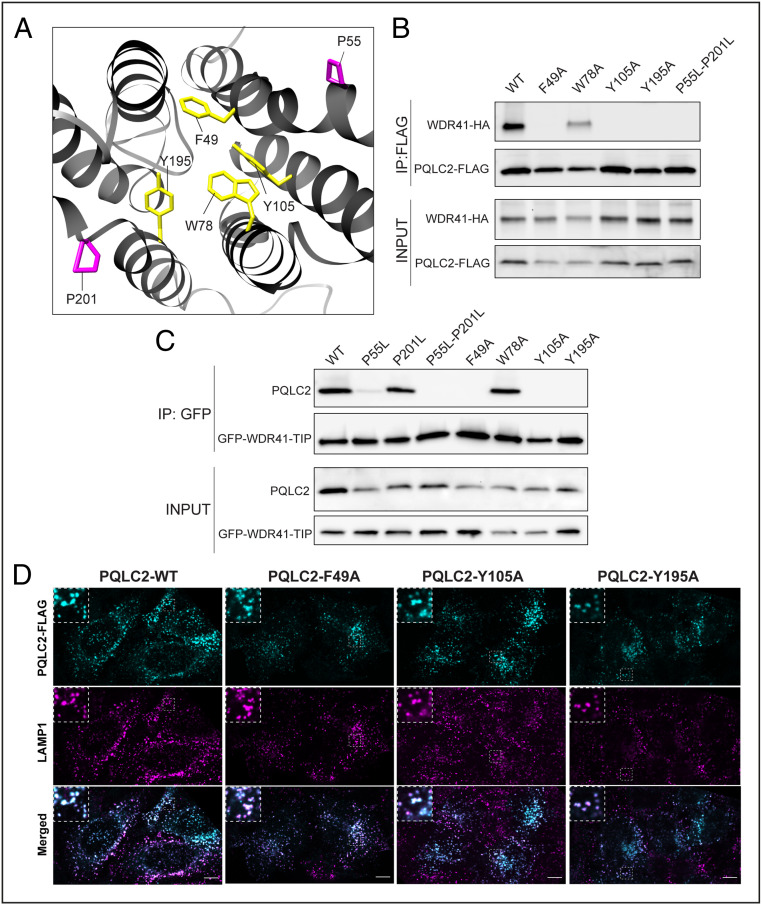
To further test the model of WDR41 TIP insertion into the cavity exposed by the inward-facing conformation of PQLC2, we performed site-directed mutagenesis on a set of highly conserved aromatic residues that are predicted to line this cavity: F49, W78, Y105, and Y195A (Fig. 4A). Immunoprecipitation of the resulting mutants revealed that F49, Y105, and Y195A are essential for the interaction between PQLC2 and WDR41 (full-length protein; Fig. 4B) and the isolated TIP sequence (Fig. 4C). Importantly, these mutations did not have any effect on the PQLC2 protein levels or on the trafficking of PQLC2 to lysosomes (Fig. 4D). In contrast, W78, another highly conserved aromatic amino acid within the predicted cytosol-facing cavity, was not required for the interaction between PQLC2 and the WDR41 TIP (Fig. 4 B and C).
Fig. 4.
Proline hinges and specific aromatic residues predicted to face the inward-facing cavity of PQLC2 are required for WDR41 binding. (A) Structural representation of PQLC2 transporter (front view), showing the side chains of prolines 55 and 201 (magenta) and aromatic residues predicted to line the inward-facing cavity (F49, W78, Y105, and Y195; yellow). (B) HeLa cells transiently cotransfected with WDR41-HA and the indicated PQLC2-FLAG mutants were lysed and immunoprecipitated (anti-FLAG M2 affinity gel) and immunoblotted with HA and FLAG antibodies. (C) HeLa cells transiently cotransfected with plasmids expressing GFP-TIP and the indicated PQLC2 mutants were lysed and immunoprecipitated (IP: GFP) and immunoblotted with FLAG antibody. Experiments in B and C were independently replicated at least three times. (D) Spinning-disk confocal immunofluorescence microscopy of transiently transfected HeLa cells expressing PQLC2-FLAG with the indicated mutations along with detection of endogenous LAMP1 (late endosomes and lysosomes). (Insets) 3× magnifications of the selected regions denoted by dashed lines. (Scale bars: 10 µm.)
PQLC2 Conformational Changes Link Its Transporter and Receptor-Like Functions.
The PQ motifs in transmembrane helices 1 and 5 of PQLC2 are thought to function as hinges that support the conformational changes required for substrate transport, and proline-to-leucine mutations at these sites block transporter activity of PQLC2 (22, 32, 33). In particular, this hinge is critical for the inward-facing conformation of this family of transporters (34). Consistent with the predicted importance of the inward-facing conformation for the WDR41 TIP interactions, PQLC2 PQ motif proline-to-leucine mutants reduced (when made singly) or abolished (double P55L + P201L mutation) interactions with the WDR41 TIP (Fig. 4D). This result is further supported by our previous observation that although this PQLC2 mutant still localizes to lysosomes, it cannot recruit the WDR41-SMCR8-C9orf72 complex (5).
Transporters that also have receptor-like signaling activities have been referred to as “transceptors” (35–37). Although the transceptor concept is supported by multiple observations, mechanisms that relate the distinct transporter and receptor activities of transceptors have been elusive. Thus, our results support a model wherein a specific conformational state within the alternating access transporter model mediates WDR41 recruitment to the cytoplasmic surface of lysosomes and thus provides a potential mechanism for the transceptor properties of PQLC2.
We also sought to test whether the subcellular localization of PQLC2 affects its ability to interact with WDR41. However, in contrast to previous studies in which mutation of a single dileucine motif within the cytoplasmic C terminus resulted in accumulation of rat PQLC2 on the plasma membrane rather than on lysosomes (22, 38), we found that human PQLC2 contains two distinct dileucine motifs, both of which must be mutated to alanine to yield a plasma membrane rather than lysosome localization (SI Appendix, Fig. S4A). This plasma membrane-localized PQLC2 mutant exhibited reduced interaction with the WDR41 TIP GFP fusion protein (SI Appendix, Fig. S4B). This may reflect a requirement for an acidic luminal pH and/or unique aspects of the lysosomal membrane for promoting the inward-facing PQLC2 conformation that we predict to be a prerequisite for WDR41 interactions.
A Foundation for Understanding Signaling from Lysosomes in Health and Disease.
We have provided a model for the regulated, PQLC2-mediated recruitment of the C9orf72 complex to the cytoplasmic surface of lysosomes based on the insertion of a short peptide motif from the WDR41 7CD loop region (WDR41 TIP) into the large cavity that is predicted to be formed by the inward-facing conformation of PQLC2. In addition to the fundamental biological importance of understanding how this interaction allows cells to maintain lysosome homeostasis and adapt to changes in cationic amino acid availability (12, 15–19, 39), this discovery raises the possibility that drugs that stabilize a conformation of PQLC2 that supports WDR41 interactions could have therapeutic value by enhancing C9orf72-SMCR8-WDR41 signaling from lysosomes.
Methods
Structural Predictions.
Database searches and secondary structure prediction were performed with HHpred (https://toolkit.tuebingen.mpg.de/tools/hhpred), a sensitive protein homology detection and structure prediction by hidden Markov model (HMM)-HMM comparison (40), the core of the MPI Bioinformatics Toolkit (41). The modeling of WDR41 and PQLC2 was achieved with MODELER, a program for comparative protein structure modeling (42) as part of the MPI Bioinformatics Toolkit (41). WDR41 secondary structure prediction was performed with the WD40-repeat protein Structures Predictor (WDSP) (43). Protein-protein docking analysis was carried out by GalaxyTongDock in an ab initio asymmetric fashion (44). Templates and models were analyzed and selected based on HHpred and GalaxyTongDock criteria and scores. UCSF Chimera (www.rbvi.ucsf.edu/chimera) was used for visualization and three-dimensional analysis (45).
Cell Culture and Transfection.
HeLa cells (provided by Pietro De Camilli, Yale University) were grown in Dulbecco’s Modified Eagle Medium (DMEM) plus 4.5 g/L d-glucose, l-glutamine (Gibco), 10% fetal bovine serum, and 1% penicillin/streptomycin supplement (Mediatech). Transfections and cotransfections were performed using FuGENE 6 transfection reagent (Promega). Cells were analyzed at 2 d posttransfection.
Plasmids.
PQLC2-FLAG plasmids have been described previously (5). Single substitutions of the PQLC2 gene were incorporated into this PQLC2-FLAG plasmid. The WDR41-HA plasmid (pCMV6-WDR41-HA) was a gift from Nicolas Charlet-Berguerand (Addgene plasmid 74159). Deletions and single/double substitutions of the WDR41 gene were incorporated into this plasmid. GFP-WDR41 fusions were constructed in the pEGFP-N1 plasmid (Clontech), and the GST-WDR41 fusion was done in pGEX-6T-1 (GE Life Sciences). These new plasmids were generated by Gibson assembly or Q5 site-directed mutagenesis according to the manufacturer’s instructions (New England BioLabs). Primers for making these plasmids and various mutants are listed in SI Appendix, Table S1. Plasmids were confirmed by restriction enzyme digestion and sequencing (Yale University Keck DNA Sequencing Facility).
Immunoprecipitation and Immunoblotting.
Cells were washed with cold phosphate-buffered saline (PBS) and then lysed in 50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, plus protease and phosphatase inhibitor mixture tablets (cOmplete mini, EDTA-free; Roche Diagnostics and PhosSTOP; Sigma-Aldrich). Insoluble cell material was removed by centrifugation. Immunoprecipitation was performed on the resulting lysates. Anti-HA affinity matrix (Roche Diagnostics), GFP-Trap (ChromoTek), and anti-FLAG M2 affinity gel (Sigma-Aldrich) were used for anti-HA, anti-GFP, and anti-FLAG immunoprecipitation, respectively. Samples were resolved in 4 to 15% gradient Mini-PROTEAN TGX precast polyacrylamide gels (Bio-Rad) and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% nonfat dry milk for 1 h and then incubated overnight at 4 °C with primary antibodies in 5% nonfat dry milk or bovine serum albumin in Tris-buffered saline (TBS) with 0.05% Tween 20. The antibodies used for Western blot analysis were HA (3F10; Roche, 1:2,000), GFP-HRP (Rockland, 1:2,000), FLAG (Cell Signaling Technology, 1:1,000), C9orf72 (GT1553; Genetech, 1:2,000), SMCR8 (Bethyl Laboratories, 1:4,000), and GST (kindly provided by P. De Camilli, 1:2,000). Chemiluminescence detection (SuperSignal West Pico or Femto; Thermo Fisher Scientific) of horseradish peroxidase signals from primary or secondary antibodies was acquired with a Bio-Rad Versa-Doc imaging station. Fiji (46) was used to quantify band intensities.
GST Fusion Protein Purification and Pull-Down Assays.
Plasmids for expression of GST fusion proteins were transformed in BL21 Escherichia coli. The GST-tagged proteins were expressed and purified using glutathione Sepharose 4B GST-tagged protein purification resin (GE Healthcare) (47). Dialysis was performed via 3.5 k MWCO Slide-A-Lyzer-G2 dialysis cassettes (Thermo Fisher Scientific). Pull-down assays were performed by adding GST-tagged proteins to the lysates from HeLa cells, transiently expressing PQLC2-FLAG plasmids, or HEK293FT C9orf72-HA PQLC2 KO cells (5), stably expressing PQLC2-FLAG (pLVX-puro vector; Clontech). Proteins bound to the glutathione resin were then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted with anti-FLAG antibodies while the GST fusion proteins were visualized with Ponceau S staining (Apacor).
Immunofluorescence and Microscopy.
Control or WDR41 KO HeLa cells (20) were grown on 12-mm coverslips (Carolina Biological Supply) were transiently transfected with the indicated plasmids using Fugene 6 transfection reagent (Promega). Cells were subsequently fixed by a 1:1 addition of 8% paraformaldehyde in 0.1 M sodium phosphate to their growth media. Cells were permeabilized by immersing coverslips in ice-cold methanol for 3 s. After rinsing with PBS, they were blocked in TBS with 5% normal donkey serum, and antibody incubations were performed in the same buffer (5, 46). The following antibodies were used: HA (1:200, Cell Signaling Technology), FLAG (1:200, Cell Signaling Technology), LAMP1 (clone H4A3; 1:300, Developmental Studies Hybridoma Bank), anti-mouse Alexa Fluor 594 (1:300, Thermo Fisher Scientific), and anti-rabbit Alexa Fluor 488 (1:300, Thermo Fisher Scientific). A Nikon Eclipse TI-E inverted microscope equipped with 60× CFI PlanApo VC, NA 1.4, oil immersion and 40× CFI Plan Apo, NA 1.0, oil immersion objectives, a spinning disk confocal scan head (CSU-X1; Yokogawa), and Volocity (PerkinElmer) software were used for spinning-disk confocal microscopy. Mander’s coefficients (with threshold corrections, M1: fraction of WDR41-HA or mutant overlapping PQLC2-FLAG) were calculated with the JACoP colocalization plugin for Fiji (45, 47).
Supplementary Material
Acknowledgments
We thank the members of the S.M.F. laboratory for helpful advice and thoughtful discussions. This research was supported by NIH Grant GM105718 (to S.M.F.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014941118/-/DCSupplemental.
Data Availability
All study data are included in the main text and SI Appendix.
References
- 1.Ballabio A., Bonifacino J. S., Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 21, 101–118 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Liu G. Y., Sabatini D. M., mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin H. R., Zoncu R., The lysosome at the intersection of cellular growth and destruction. Dev. Cell 54, 226–238 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinz L. X., et al., TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7-9. Nature 581, 316–322 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amick J., Tharkeshwar A. K., Talaia G., Ferguson S. M., PQLC2 recruits the C9orf72 complex to lysosomes in response to cationic amino acid starvation. J. Cell Biol. 219, e201906076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Rourke J. G., et al., C9orf72 is required for proper macrophage and microglial function in mice. Science 351, 1324–1329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan P. M., et al., The ALS/FTLD associated protein C9orf72 associates with SMCR8 and WDR41 to regulate the autophagy-lysosome pathway. Acta Neuropathol. Commun. 4, 51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., et al., The C9orf72-interacting protein Smcr8 is a negative regulator of autoimmunity and lysosomal exocytosis. Genes Dev. 32, 929–943 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAlpine W., et al., Excessive endosomal TLR signaling causes inflammatory disease in mice with defective SMCR8-WDR41-C9ORF72 complex function. Proc. Natl. Acad. Sci. U.S.A. 115, E11523–E11531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrionero A., Horvitz H. R., A C9orf72 ALS/FTD ortholog acts in endolysosomal degradation and lysosomal homeostasis. Curr. Biol. 28, 1522–1535.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Amick J., Roczniak-Ferguson A., Ferguson S. M., C9orf72 binds SMCR8, localizes to lysosomes, and regulates mTORC1 signaling. Mol. Biol. Cell 27, 3040–3051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeJesus-Hernandez M., et al., Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renton A. E.et al.; ITALSGEN Consortium , A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gijselinck I., et al., A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: A gene identification study. Lancet Neurol. 11, 54–65 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Belzil V. V., et al., Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol. 126, 895–905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi Z., et al., Hypermethylation of the CpG island near the G4C2 repeat in ALS with a C9orf72 expansion. Am. J. Hum. Genet. 92, 981–989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waite A. J., et al., Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol. Aging 35, 1779.e5–1779.e13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viodé A.et al.; NeuroCEB Brain Bank , New antibody-free mass spectrometry-based quantification reveals that C9ORF72 long protein isoform is reduced in the frontal cortex of hexanucleotide-repeat expansion carriers. Front. Neurosci. 12, 589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y., et al., Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 24, 313–325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amick J., Tharkeshwar A. K., Amaya C., Ferguson S. M., WDR41 supports lysosomal response to changes in amino acid availability. Mol. Biol. Cell 29, 2213–2227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng L., Frommer W. B., Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 40, 480–486 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Jézégou A., et al., Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc. Natl. Acad. Sci. U.S.A. 109, E3434–E3443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb B., Sali A., Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics 86, 5.6.1–5.6.37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J., et al., WDSPdb: An updated resource for WD40 proteins. Bioinformatics 35, 4824–4826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su M. Y., Fromm S. A., Zoncu R., Hurley J. H., Structure of the C9orf72 ARF GAP complex that is haploinsufficient in ALS and FTD. Nature 585, 251–255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang D., et al., Cryo-EM structure of C9ORF72-SMCR8-WDR41 reveals the role as a GAP for Rab8a and Rab11a. Proc. Natl. Acad. Sci. U.S.A. 117, 9876–9883 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han L., et al., Molecular mechanism of substrate recognition and transport by the AtSWEET13 sugar transporter. Proc. Natl. Acad. Sci. U.S.A. 114, 10089–10094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bräuer P., et al., Structural basis for pH-dependent retrieval of ER proteins from the Golgi by the KDEL receptor. Science 363, 1103–1107 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Tao Y., et al., Structure of a eukaryotic SWEET transporter in a homotrimeric complex. Nature 527, 259–263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomize M. A., Pogozheva I. D., Joo H., Mosberg H. I., Lomize A. L., OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park T., Baek M., Lee H., Seok C., GalaxyTongDock: Symmetric and asymmetric ab initio protein-protein docking web server with improved energy parameters. J. Comput. Chem. 40, 2413–2417 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Lee Y., Nishizawa T., Yamashita K., Ishitani R., Nureki O., Structural basis for the facilitative diffusion mechanism by SemiSWEET transporter. Nat. Commun. 6, 6112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B., Du H., Rutkowski R., Gartner A., Wang X., LAAT-1 is the lysosomal lysine/arginine transporter that maintains amino acid homeostasis. Science 337, 351–354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latorraca N. R., et al., Mechanism of substrate translocation in an alternating access transporter. Cell 169, 96–107.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donaton M. C., et al., The Gap1 general amino acid permease acts as an amino acid sensor for activation of protein kinase A targets in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 50, 911–929 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Popova Y., Thayumanavan P., Lonati E., Agrochão M., Thevelein J. M., Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc. Natl. Acad. Sci. U.S.A. 107, 2890–2895 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scalise M., et al., Insights into the transport side of the human SLC38A9 transceptor. Biochim. Biophys. Acta Biomembr. 1861, 1558–1567 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Llinares E., Barry A. O., André B., The AP-3 adaptor complex mediates sorting of yeast and mammalian PQ-loop-family basic amino acid transporters to the vacuolar/lysosomal membrane. Sci. Rep. 5, 16665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gijselinck I., et al., The C9orf72 repeat size correlates with onset age of disease, DNA methylation and transcriptional downregulation of the promoter. Mol. Psychiatry 21, 1112–1124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Söding J., Protein homology detection by HMM-HMM comparison. Bioinformatics 21, 951–960 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann L., et al., A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Sali A., Potterton L., Yuan F., van Vlijmen H., Karplus M., Evaluation of comparative protein modeling by MODELLER. Proteins 23, 318–326 (1995). [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Jiang F., Zhuo Z., Wu X. H., Wu Y. D., A method for WD40 repeat detection and secondary structure prediction. PLoS One 8, e65705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettersen E. F., et al., UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Schindelin J., et al., Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petit C. S., Roczniak-Ferguson A., Ferguson S. M., Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J. Cell Biol. 202, 1107–1122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolte S., Cordelières F. P., A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.