Abstract
One hundred fifty years ago Darwin published The Descent of Man, and Selection in Relation to Sex, in which he presented his theory of sexual selection with its emphasis on sexual beauty. However, it was not until 50 y ago that there was a renewed interest in Darwin’s theory in general, and specifically the potency of mate choice. Darwin suggested that in many cases female preferences for elaborately ornamented males derived from a female’s taste for the beautiful, the notion that females were attracted to sexual beauty for its own sake. Initially, female mate choice attracted the interest of behavioral ecologists focusing on the fitness advantages accrued through mate choice. Subsequent studies focused on sensory ecology and signal design, often showing how sensory end organs influenced the types of traits females found attractive. Eventually, investigations of neural circuits, neurogenetics, and neurochemistry uncovered a more complete scaffolding underlying sexual attraction. More recently, research inspired by human studies in psychophysics, behavioral economics, and neuroaesthetics have provided some notion of its higher-order mechanisms. In this paper, I review progress in our understanding of Darwin’s conjecture of “a taste for the beautiful” by considering research from these diverse fields that have conspired to provide unparalleled insight into the chooser’s mate choices.
Keywords: sexual selection, mate choice, neuroscience
One hundred fifty years ago Charles Darwin published The Descent of Man, and Selection in Relation to Sex (1), in which he presented his theory of sexual selection with its emphasis on sexual beauty. However, it was not until 50 y ago that there was renewed interest in sexual selection, especially the potency of mate choice, in influencing the evolution of sexual beauty. Darwin suggested that in many cases mate preferences of females (more generally, choosers) for elaborately ornamented males (more generally, courters) derived from a female’s taste for the beautiful, the notion that females were attracted to sexual beauty for its own sake (2–4). In this paper, I review progress toward understanding animal mate attraction by considering research from a number of diverse fields that have conspired to provide unparalleled insights into the chooser’s brain and behavior.
The foundational narrative of Darwin’s formulation of sexual selection theory is well known to evolutionary biologists as well as to historians and philosophers of science (5, 6). A short recap here should suffice. Darwin and Wallace presented a theory of natural selection which explained the evolution of adaptations for survivorship and fecundity, a theory that was later fleshed out in Darwin’s On the Origin of Species. Later, Darwin penned a now-famous letter to the botanist Asa Gray and complained that “the sight of a feather in a peacock’s tail, whenever I gaze at it, makes me sick!” (7). That physical reaction was probably rooted in cognitive dissonance—how could his theory of natural selection explain the evolution of traits certainly maladaptive for survival? The conundrum was resolved by the theory of sexual selection, which focuses on another critical aspect of Darwinian fitness, traits that enhance an individual’s ability to acquire mates. This theory went a long way in explaining the evolution of many sexually dimorphic traits that appeared intricately involved in reproduction because they either gave the bearer the ability to compete with others for access to mates or they enhanced the sexual attractiveness of the bearer. Of course, both natural and sexual selection are critical in understanding organic evolution: You cannot reproduce if you do not survive; but if you survive and do not reproduce or help someone else reproduce you might as well be dead—at least from a Darwinian perspective.
Sexual selection became a target of criticisms from Alfred Wallace (8), among others. What stuck in their craw was Darwin’s assertions about mate choice. He had found that many species were sexually dimorphic, and often the more elaborately adorned sex was the male; these traits were quite varied in form and included the melodious songs of birds, brilliant colors of fish, and the dances of spiders. Darwin posited that these traits evolved to charm females during courtship. However, critics countered that there was scant evidence to suggest that females were the ones who executed the mating decision, and even if that were true Darwin did not propose a convincing theory as to why females should be attracted to more adorned males. Darwin, in fact, did provide an explanation: He suggested that females had “a taste for the beautiful” and that males evolved traits that appeal to the female’s perception of sexual beauty. His critics found such an explanation wanting (5, 6).
Sexual selection did not wither on the vine, as it attracted attention from some renowned thinkers such as Weismann (9), Fisher (10, 11), and Maynard Smith (12). Nevertheless, sexual selection was hardly a focal point, but that was soon to change. The “revenge of the ugly duckling” (13) commenced in 1972 with the publication of Campbell’s Sexual Selection and the Descent of Man (14), and especially Trivers’ chapter on parental investment and sexual selection (15). Female mate choice was now at the forefront, and a number of studies ensued that demonstrated variation in male mating success was correlated to variation in male display traits. These findings were followed by experimental studies that assuaged any further skepticism about the evolutionary potency of female mate choice (reviewed in ref. 2). Our understanding of the “hows” and “whys” of sexual selection by mate choice has since extended its reach into a number of parallel fields such as neuroscience, cognitive biology, and psychophysics. I will review some of the progress achieved, focus on recent studies of mate choice and the brain, and suggest some possibly interesting new avenues of research.
Species Recognition and Mate Choice
It is ironic that sexual selection by mate choice received only middling attention during the Modern Synthesis given that mate choice between species, under the rubric of species recognition, was one of its touchstones (16). Regardless of how one feels about Mayr’s biological species concept (reviewed in ref. 17), it is clear that gene flow among populations of sexually reproducing species is an important modulator of evolutionary divergence, and reproduction is what usually makes genes flow. A number of researchers documented female preferences for conspecific versus heterospecific mating signals. There has never been much consternation over why females would evolve such preferences, as the reproductive costs of mating with heterospecifics can be quite severe (reviewed in ref. 2).
Paralleling evolutionary studies of species recognition were those of neuroethologists exploring how animals extract and analyze biologically relevant information in the world around them. Lettvin et al.’s (18) study of “what the frog’s eye tells the frog’s brain” revealed the neural basis of finding a meal and helped launch the field of neuroethology. It also was an inspiration for studies by Capranica and coworkers asking what the frog’s ear tells the frog’s brain when it comes to finding a mate (19, 20). Other researchers provided similar insights into how species recognition occurred in crickets (21), fish (22), moths (23), fruit flies (24, 25), and birds (26), among other taxa.
It is not clear why studies of species recognition and sexual selection were siloed for so long. Theoreticians such as Fisher (11), West-Eberhard (27), and Lande (28) all saw the connections earlier on, and Ryan and Rand (29) argued that mate choice resulting in either species recognition or sexual selection is on a continuum: Selection for species recognition can generate sexual selection, and sexual selection can drive speciation. Our view of sexual selection was expanded when West-Eberhard (30) made the compelling argument that it should include the concept of social selection, which embodies all forms of competition among conspecifics for access to resources, including but not limited to mates. Social selection has many of the features of Darwin’s original notion of sexual selection and shares many of its differences with natural selection. It was against this background that behavioral ecologists resuscitated the field of sexual selection.
A Changing Focus in Studies of Sexual Selection and Mate Choice
In the last 40 y there has been a shift in the focus of mate-choice studies from the message that males might be sending to females to how females perceive the signals that complement their taste for the beautiful.
Most early studies in behavioral ecology have assumed that a male’s outer sexual beauty was indicative of his inner genetic quality, his “good genes” for survival (31–33). Studies rarely determined the effect of male traits on offspring survivorship or even proxies for survivorship (but see refs. 34 and 35) but based their arguments on the demonstration that male traits were costly (36). Currently, the relative importance of good genes selection does not seem overwhelming (reviewed in ref. 2).
Somewhat related to the “good genes” hypothesis is selection for complementary genes, which motivates preferences for conspecifics over heterospecifics and might also be important in mate choice within populations when it comes to certain genes such as the major histocompatibility complex (37). A favorite hypothesis of theoretical population geneticists is Fisherian runaway sexual selection, but its empirical support, usually based on demonstrating genetic correlations of traits and preferences, is somewhat sparse (38).
Eventually, studies began to ask how females make their choice of mates. These studies of mate choice were motivated not only by neurobiologists interested in neural circuitry but also by evolutionary biologist asking how the sexual brain drove the evolution of male traits, and vice versa. Much of this interest can be traced back to West-Eberhard’s notion of the sensory trap (30), whereby choosers’ responses in other contexts are elicited by courters’ traits. Examples include, but are not limited to, sand pillars built by male crabs that approximate refugia to females (39), fins of male fish that mimic food (40), and male moths that mimic bat echolocation calls (41). This is somewhat similar to Ryan’s notion of sensory exploitation (42) that suggests males evolve traits that exploit female preexisting biases. An example includes female platyfish showing a preference for their typically swordless males to whom artificial swords have been added (43); for additional examples see ref. 44.
Studies of direct (good fathers, good resources) and indirect (good genes, sexy sons) benefits of mate choice are often considered orthogonal to those of mechanisms underlying these choices. In many cases this is true. Identifying how the brain is biased to certain mating signals is an important contribution in its own right but does not necessarily provide insights into the adaptive significance of these mate preferences. In other examples, some of which are reviewed below, mechanistic studies reveal that the underpinnings of mate choice have often evolved in other domains (foraging or predator avoidance) or operate under some general neural (what you can see, hear, or smell) and cognitive (how you compare, how you make complex decisions) constraints. Thus, integrating proximate and ultimate studies is not only necessary for a complete understanding of mate choice but might also be necessary for a correct understanding. This is well-illustrated in studies of signal design.
Sensory Biology and Signal Design
Male courtship signals are designed to be detected, to be perceived, and to influence mate choice. This is at the crux of Endler’s theory of sensory drive which emphasizes the context of the ecological environment in which signals are transmitted as well as the neural environment in which they are processed (45, 46). Studies of bird song, for example, have demonstrated that acoustic design of signals are adapted to increase the signal’s active space in different habitats (47, 48) and also show that in urban settings some songbirds produce higher-frequency songs out of the range of anthropogenic noise (49). Similarly, Endler and Thery showed that birds selected display sites that maximized the animals’ visual contrast with the environment (50).
When studies started to probe the sexual senses of animals they usually focused on how peripheral sensory end organs are biased to properties of courtship signals. Archer et al. (51), for example, showed substantial photopigment variation in female guppies and suggested this might contribute to the diversity of courtship colors displayed by these fish. Cummings (52) showed that photopigment sensitivity in surfperch enhances contrast of their prey items against ambient background light and that males subsequently evolved colors and patterns to exploit these sensory biases. Seehausen et al. demonstrated in wonderful detail that changes in the ambient light spectrum with water depth in Lake Victoria correlated with cichlid diversity, color pattern, photopigment sensitivity, and mate choice preferences (53). In terrestrial taxa, Leal and Fleishman showed that visual sensitivity in two species of anolis lizards is adapted to the microhabitat’s light conditions of each species in both the female’s visual biases and the male’s signal design (54). In sum, these studies have shown that the retina can be both an important target of selection, as it evolves in response to the vagaries of the light environment, and an agent of selection, as it biases the response of animals to certain signal qualities.
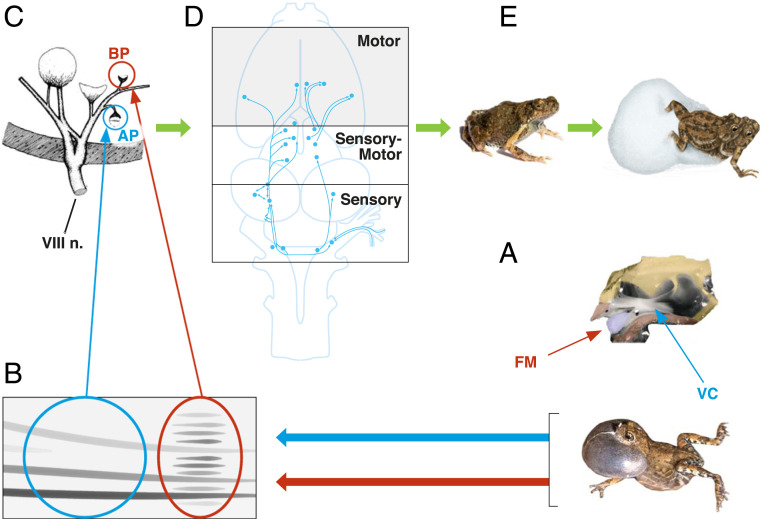
Analogous results have emerged from probing peripheral auditory systems. For example, although ultrasonic sensitivity in moths evolved prior to the evolution of bats (55), moth hearing organs were then coopted for detection of bat predators (56). Conner (57) argued that this sensory channel was then later exploited by male courtship sounds in some moths. In another example, studies of túngara frogs have been instructive in showing how auditory sensitivity influences acoustic-based mating preferences, the details of which are presented in Fig. 1. Males produce a long-distance mating call that consists of a whine, which can be followed by zero to seven chucks. The addition of chucks increases the attractiveness of the calls fivefold, but chucks are also attractive to the frog-eating bat (58). Thus, males vary the number of chucks as a function of competition with other males and predation risk from bats. The dominant frequency of the whine and the chuck each match one of the two inner ear organs of the frog, the amphibian papilla (AP) and the basilar papilla (BP), respectively. The tuning of the túngara frog’s BP and the BPs of its close relatives are strikingly similar, even though all but one of those species lack chucks and seem not to recruit the BP for communication (59). Thus, it appears that the chuck evolved to exploit a sensory channel that had not been used for communication in at least the last 15 My. This situation is somewhat similar to the male moths exploiting an ultrasonic channel that was not being used for sexual communication (59).
Fig. 1.
The role of vocal morphology, call morphology, the inner ear, and the brain in sexual communication in túngara frogs. (A) The túngara frog has an unusual larynx characterized by a large fibrous mass (FM) that protrudes from the vocal cords (VC). (B) Comparative studies (106), biomechanical models (107), and ablation of the fibrous mass (108) all show that the vocal cord vibration is primarily responsible for the whine (blue circle) and the vibration of the fibrous mass for the production of the chuck (red circle). (C) The dominant frequency of the whine, about 750 Hz, matches the average most sensitive frequency of the AP, while the dominant frequency of the chuck, about 2,500 Hz, is a close match to the average most sensitive frequency of the BP; the location of these two sensory end organs in the inner ear are indicated by the blue and red arrows and circles, respectively (109). (D) Information from the two inner ear organs enters the brain via the VIIIth cranial nerve. As the information ascends through the brain it is processed in the sensory, sensory-motor, and motor areas of the brain and results in movement of the female to the call, that is, phonotaxis (see details in ref. 71). Reprinted from ref. 71. Copyright (2010), with permission from Elsevier. (E) Stimulation of both inner ear organs by the whine–chuck results in a fivefold increase in the attractiveness of the call compared to the whine only (58). Females choose as mates those males making complex calls and lower-frequency chucks. They then construct a foam nest with ∼250 fertilized eggs (110).
Olfactory sexual signals in the form of volatile pheromones have been well documented in insects, and especially in fruit flies and moths. The antennae contain numerous olfactory sensory neurons (OSNs) which exhibit olfactory receptors (ORs) that bind to specific odorants. In the fruit fly, Drosophila melanogaster, the male-specific pheromone 11-cis-vaccenyl acetate (cVA) binds to the olfactory receptor OR67d to promote sexual behavior in females. If a mutant female lacks the OR67d gene she is less receptive to courting males. Quite amazingly, if OR67d is replaced with the moth receptor gene BmOr1, which codes for the pheromone bombykol, the female flies are attracted to the moth pheromone (60).
Sensory end organs are the portals to the brain, but sexual signals do not get a free pass. Only stimuli that meet their stringent criteria for generating sensory transduction proceed to the central nervous system for more detailed processing.
The Brain and the Hardware of Mate Choice
Although eyes, ears, and olfactory receptors, as well as other sensory end organs, are the portals to the brain, it is in the brain that perception and decision-making take place (61). A number of studies have shown how courtship stimuli are perceived by the brain and trigger mating decisions in comparing conspecifics with one another. Songbirds have been an important model system for both animal behavior and neuroscience. Although most of the mechanistic studies have been directed toward parsing the neural circuits involved in song learning and song production, other studies have provided insights into how courtship signals are evaluated in the brain. Margoliash (62), for example, identified song-selective neurons in the bird’s HVC, a nucleus that is central to song production and song learning. These neurons exhibited their greatest response to the bird’s own song, and the author suggested these neurons might serve as templates for comparison when evaluating songs of potential mates.
Studies of sexual signals and the brain have benefited from quantifying expression of immediate early genes (IEG) as a proxy for neural activation (63). It has long been known that some songbirds habituate to repeated song stimuli (64). Clayton and coworkers (65) showed that in zebra finches their behavior, action potentials, and IEG expression in auditory units all habituate to repeated stimulation of identical song notes. In addition, expression profiles of a large number of genes also change in response to habituation (66). These studies provide insights into how habituation in the bird’s brain might drive the evolution of larger song repertoire size in birds as more and different songs should better hold the attention of the receiver.
Courtship often produces signals and cues in different sensory modalities, which can introduce an additional layer of complexity to mate choice decisions. As do many other animals, cowbirds simultaneously evaluate multimodal signals in different sensory channels, in this case the auditory and visual (67). Females varied in their sensory acuity and this affected their feature weighting of different signal components. Females with greater temporal auditory resolution preferred shorter songs and those with better temporal visual resolution preferred less-intense visual displays. These differences in sensory efficacy of females might contribute to maintenance of variation in male courtship signals, which is consistent with the popular notion that mate choice can be a fickle business (4).
Studies of swordtail fish, also using IEG expression, have revealed significant correlations between levels of gene expression and mate choice behavior in areas of the brain that are part of the social decision-making network. One gene in particular, neuroserpin, a gene implicated in synaptic plasticity and learning, has emerged as a potential regulator of mate choice (68). Studies of guppies have taken a different approach and evaluated the relationship between overall brain size and mate-choice decisions. Researchers compared laboratory lines of guppies with small brains and large brains. In a dichotomous choice test, large-brained females showed strong preferences for more colorful males, while small-brained females showed no such preferences (69). As with the cowbirds, variation in the neural phenotype of female guppies might also contribute to maintaining variation in male courtship signals.
Bloch et al. (70) used these same lines of small- and large-brained guppies to address the link between sensory perception and social decision-making. They compared neurogenomic responses in these two lines when females were presented with either colorful or dull males. The choosy large-brained and the nonchoosy small-brained females exhibited a similar set of differentially expressed genes in the optic tectum, where integration of visual stimuli occurs. In the telencephalon, where social decisions are thought to be made (61), however, the choosy and the nonchoosy females differed in their gene expression. Thus, the difference in preferences between these two lines is not due to how females perceive the males but in the decisions they make based on these similar perceptions.
Frogs have provided fertile ground for examining sexual selection and the brain. Wilczynski and Ryan (71) reviewed a number of studies that have moved the understanding of neural substrates of mate choice from the peripheral auditory system deeper into the brain (Fig. 1). These studies have shown that biases toward mating calls that are first manifest in the tuning of the inner ear cascade throughout the brainstem, and feature detectors emerge when stimuli reach the midbrain. In the midbrain and the forebrain there is differential representation of conspecific and heterospecific signals as well as some variation in conspecific signals that involves both changes in overall activity levels across subnuclei and in the functional correlations among acoustically active areas. It appears that the frog’s auditory midbrain is a regulatory gateway for responses to sexual signals between the stimulus analysis of the brainstem and the behavioral and physiological control centers of the forebrain (Fig. 1).
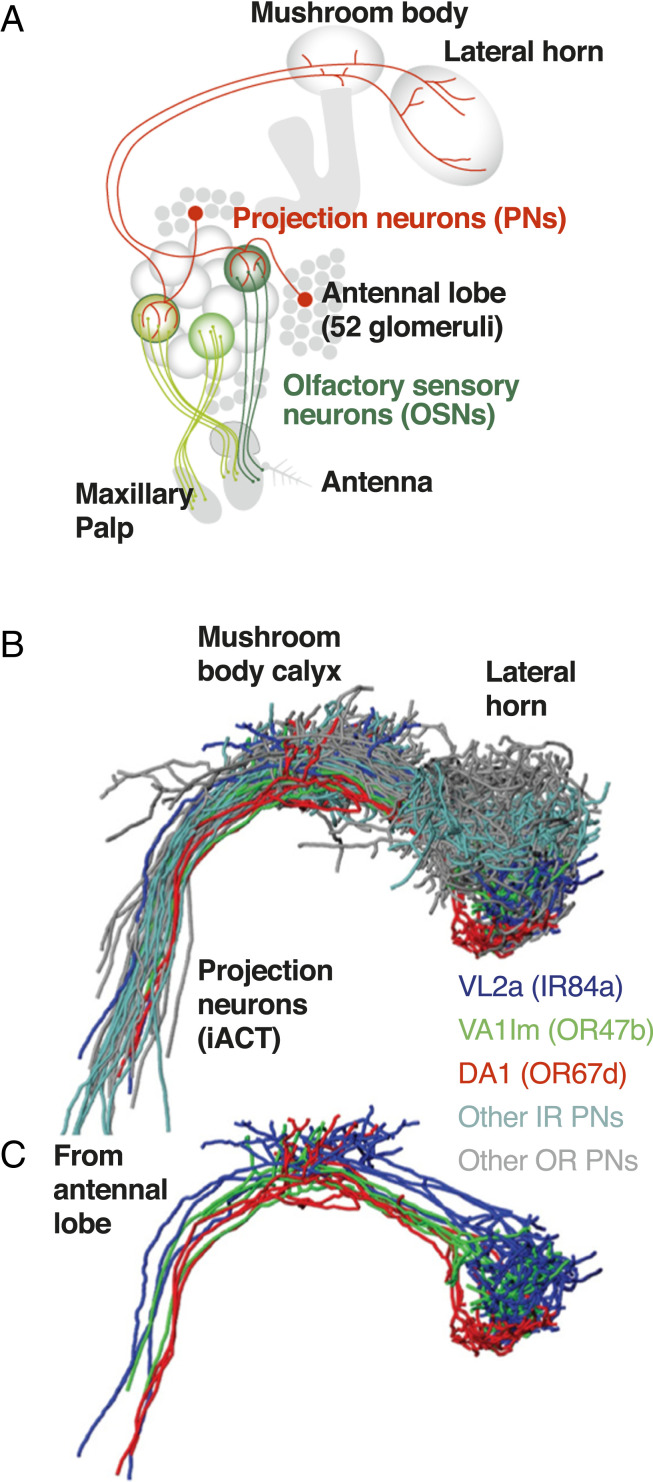
Studies of insect olfaction have also progressed from the sensory periphery to the brain, including the antennal lobe, the mushroom bodies, and the lateral horn (72, 73). The fly’s olfactory system functions in what is usually two separate domains, feeding and mating. Most of the olfactory sensory neurons involved in sensing food project from the antennal lobe to the mushroom body, while those involved in sex project to the lateral horn. One olfactory gene, IR84a, codes for ORs that are sensitive to volatiles associated with rotting fruit, which is where male D. melanogaster court females and where females deposit eggs. Unlike other receptors that detect food and eventually project to the mushroom body, this one terminates in the fly’s sexual brain, the lateral horn, along with projections of OSNs involved in courtship (Fig. 2). Even though these olfactory-sensitive neurons are detecting the presence of food they are critical for releasing male courtship behavior. In the absence of stimulation of IR84a receptors males do not court. The interpretation is that it is best to abstain from courtship if there is not a suitable site to deposit eggs (74).
Fig. 2.
The integration of projection neurons of the fruit-detecting olfactory receptor IR84a into the pheromone processing center in D. melanogaster. (A) OSNs in the antennae of the fruit fly project to glomeruli in the antennal lobe. Projection neurons (PNs) then project to the mushroom body and the lateral horn. Reprinted from ref. 73, which is licensed under CC BY 4.0. (B) Three-dimensional rendering of registered axonal projections of PNs receiving input from antennal glomeruli that are involved in fruit (VL2a glomerulus; IR84a-expressing PN; dark blue) or pheromone detection (VA1lm glomerulus; OR47b-expressing PN; green; DA1 glomerulus; OR67d-expressing PN; red). Other classes of PNs are shown in cyan and gray. Reprinted with permission from ref. 74 (Copyright 2011, Springer Nature: Nature). (C) When the other projection neurons are removed, the extensive comingling of fruit- and pheromone-detecting PNs in the lateral horn is evident. Reprinted with permission from ref. 74 (Copyright 2011, Springer Nature: Nature).
Studies of both the sensory periphery and the brain have provided a more sophisticated understanding of female preferences. Mate choice is a behavior that emerges from the interaction of the internal hormonal and neuronal milieu of the female with the external stimuli of male courtship and the environment where it is displayed. For female preferences to evolve there must be changes in the underlying hardware, and we now know what some of this hardware looks like. A number of these studies also suggest that females are leading the way in the receiver–signal coevolutionary dance. Also, the choreography of this dance is not dictated by sex alone. The brain is an important sex organ but it has other things on its mind, such as finding food and avoiding becoming food. The “sexual brain,” including its sensory end organs, does not act in isolation. The brain is a well-integrated unit that has evolved to acquire and analyze information from its surroundings in numerous domains, which, in turn, might have unanticipated effects on how females sense their sexual world. Studies of how this information is analyzed reveal some new perspectives on mate choice, as I review in the next section.
Cognition and Thinking about Mate Choice
A relatively new phase of mate choice studies falls into the realm of cognitive ecology. These studies do not imply consciousness or theory of mind but typically address the acquisition and analysis of external stimuli and their influence on decision-making (75). Studies of cognition and mate choice have been strongly influenced by studies of humans, especially in the fields of psychophysics, behavioral economics, and neuroaethetics. Furthermore, they have begun to challenge the assumption of “strict preferences” made in theoretical models of mate choice (76); that is, that all properties of a courtship display are reduced to a single preference value independent of other displays, physiological state, and social setting (77).
In many mating systems females compare males to one another, and hundreds of studies have shown that females prefer sexual traits of greater magnitude, be they song amplitude, tail length, or intensity of pheromones (78). Thus, how we, and other animals, compare stimuli should have an important influence on mate choice and the evolution of sexual beauty. Studies of human psychophysics dating back to the 19th century have shown there need not be a linear relationship between stimulus quantity and perceived sensation (79). One such phenomenon is known as Weber’s law, in which comparisons between stimuli that vary in magnitude are based on proportional differences rather than linear ones (80). A result is that as stimulus magnitude increases the difference between the stimuli in a pair must be greater to give rise to a just noticeable difference (JND).
Animals often follow Weber’s law when distinguishing numerous environmental stimuli (80). Biologists have also applied Weber’s law to the context of naturalistic tasks to determine just meaningful differences rather than JNDs (80). In túngara frogs, females prefer calls with more chucks over calls with fewer chucks. Are these comparisons based on linear or proportional differences? Would the strength of preference for a whine–six chucks versus a whine–five chucks, for example, be just as strong as a preference for whine–two chucks versus a whine–one chuck? Experiments show that a Weber-like function explains a larger amount of the variation in preferences for chuck number compared to a linear function (84% versus 12%). Is proportional comparison of chuck number an adaptive mechanism for mate choice in these frogs? That seems unlikely. Frog-eating bats also prefer túngara frog calls with more chucks. When the analogous experiments were conducted with the bats their preferences also followed a Weber-like function strikingly similar to that of the frogs (81). The interpretation is that Weber-like mate choice did not evolve as an adaptation in túngara frogs but occurs because this is how many animals compare quantities.
Economic and evolutionary analyses share some commonalities, an important one being the assumption that individuals should behave in a manner that maximizes some utility or payoff. In economics the payoff can be monetary or emotional, and in animals the payoff is assumed to be Darwinian fitness. When individuals behave in this manner in economics they are considered rational. The relatively new field of behavioral economics, and especially the works of Tversky and Kahneman, have revealed a number of human behaviors that do not fit the strict definition of rationality (82, 83). There are two assumptions to economic rationality. One is transitivity: If A is preferred to B, and B is preferred to C, then A is preferred to C. The second is regularity: The relative preference between A and B is independent of the presence or absence of a third inferior option C.
Although “rationality” has long been an implicit assumption of evolutionary models of mate choice, it has only recently been tested. Studies of mate preferences in cichlids (84) and fruit flies (85) show transitivity in mate choice. However, there are exceptions. Studies of swordtails used digital images of a courting male in which body size and courtship intensity could be varied. Female choice based only on variation in body size was intransitive, and older females were more likely to be irrational than younger ones. However, all the females exhibited transitivity when their choice was based on courtship intensity or courtship intensity plus body size (86). Studies of crickets also showed that violation of transitivity was dependent on which acoustic cues were being evaluated (87).
Violations of regularity, known as the decoy effect, are common in humans. For instance, if given a choice between two equally desirable options, a trip to Paris and a trip to Rome, both with a free breakfast included, people may not show a strong preference between the two. If a third option is added, a trip to Rome without a free breakfast, Rome with a free breakfast becomes the preferred option (88). This paradigm has been applied to foraging behavior in animals where the role of competitive decoys has been documented extensively (89).
The influence of competitive decoys on mate choice has only recently been studied. Lea and Ryan (90) tested female túngara frogs with natural calls that differed in their attractiveness based on the acoustic properties of the call; females also prefer calls with faster call rates. Three calls, A, B, and C, were broadcast with varying call rates; call C was the least attractive and was used as the competitive decoy. Binary choice phonotaxis tests were conducted between calls A and B and females did not exhibit a significant preference, although the majority of females responded to B. In a trinary experiment females were given a choice between A, B, and C, and this resulted in a statistically strong preference for A; thus, there is a clear competitive decoy effect.
There are far too few studies available to evaluate the general importance of competitive decoys in mate choice. Studies of peacock blennies showed that female preference was influenced both by the color and the size of the male. Some females weighted size more heavily and others weighted color more heavily in their choice. A competitive decoy effect only occurred if the less-preferred trait of the decoy was manipulated (91). Studies in another fish, the green swordtail, showed no evidence of a competitive decoy effect (92). Finally, a study of mosquito fish that was based on preference of group size, rather than characteristics of males per se, also failed to show such an effect (93).
As Rosenthal (94) pointed out, sensory and perceptual biases by themselves cannot explain a female’s taste for the beautiful. A splotch of red on the shoulder of a blackbird, for example, might induce sexual wanting in a female, but a splotch of the same red on a coral snake might induce fear and antipathy in the same female. A flip in the hedonic value of a signal can be brought about by changes in neural wiring in the brain as well as simple genetic changes with no change in the response properties of the sense organs (reviewed in ref. 94). In our own species, of course, this issue is more complex. However, the emergence of a relatively new field addressing this issue in humans, neuroaesthetics, combines empirical aesthetics and cognitive neuroscience to understand how we assign hedonic value to objects in our natural and cultural worlds (95).
The notion of perceptual fluency may inform our understanding of sensory and perceptual biases. Rebar et al. (96) argued that viewing stimuli that are easily processed, for example that involve sparse coding in the brain, can result in hedonic pleasure. They suggested that perceptual fluency is “hedonically marked.” Some evidence in support of this idea is presented by Renoult et al. (97). They estimated perceptual fluency related to processing images of women’s faces by examining sparse codes in neural models that previously had been trained on natural scenes. The attractiveness of these faces was then rated by men. The sparseness of the coding of the faces explained 17% of the variation in attractiveness. This relationship between perceptual fluency and attractiveness is probably not an adaptation for processing faces per se, the researchers suggested, but is a domain general effect that results from selection to decode the visual world around us. This is why, Changizi et al. (98) argued, forms of letters in most written languages show a strong match to patterns of visual scenes in our environments.
Renoult and Mendelson (99) introduced this idea of perceptual fluency to sexual selection and mate choice. They suggested that females may be motivated by hedonic pleasure when they search out males with attractive courtship traits. Data from Mendelson and coworkers (100) support this notion. Male darters are freshwater fish in which the male’s breeding coloration consists of spectacularly colored patterns that are attractive to females. The researchers assume that the visual system is adapted to process visual scenes in the natural world and thus images with spatial statistics of the local habitat should also be more fluently perceived. Spatial statistics of habitats vary among sites and the spatial statistics of the male’s visual courtship traits, but not pigment patterns of females, best match the local habitat statistics.
All of these studies addressing cognitive aspects of mate choice show how many of our assumptions about how females evaluate males might be wrong: Females do not always exhibit strict preferences, they need not adhere to rules of economic rationality, and they might be motivated by their own hedonic pleasure along with or instead of any utilitarian quality of the male.
Caveats and Future Directions
There are a number of other interesting aspects of sexual selection besides the relationship of the brain to mate choice, and their omission in this paper is not meant to diminish their importance. Studies of good gene benefits and Fisherian runaway sexual selection are certain to continue unabated. The importance of combat in males and the developmental biology underlying these armaments also provide fertile grounds for research (101), as are studies examining the relationship between sexual selection and speciation (102). Finally, studies of eavesdropping on sexual signals are revealing a diverse set of counterselection forces that constrain the evolution of traits favored by sexual selection (103).
In this paper I have focused on female mate choice because Darwin suggested sexual aesthetics was primarily a female trait, albeit less so in our own species. We do know that both male mate choice and mutual mate choice are common in the animal kingdom, including our own species. Comparing the neural bases of male and female mate choice could be enlightening.
There is growing interest in same-sex sexual behavior throughout the animal kingdom (104). How would the sexual aesthetics of homosexual and heterosexual individuals differ or resemble one another? Although there are scant data in other animals, there are some intriguing hints in humans. Homosexual and heterosexual men and women rated the attractiveness of faces similarly, and functional MRI results also showed similar amounts of activation in areas of the brain involved in face recognition among the four groups. However, when reward areas of the brain were measured there was variation among subjects and sexual orientation; heterosexual women and homosexual men both showed enhanced activation of the reward circuit when they viewed men’s faces, while homosexual women and heterosexual men showed enhanced activation of the same circuit when they viewed women’s faces (105).
I would guess that 150 y after Darwin suggested his idea of “a taste for the beautiful” he would hardly recognize the research that this suggestion spawned. However, I would also guess that he would come to understand it quickly and, I would hope, do so approvingly.
Acknowledgments
I acknowledge my late friend and colleague Walt Wilczynski for his insights into the anuran brain. I thank the anonymous reviewers for their extremely helpful and constructive comments.
Footnotes
The author declares no competing interest.
This article is a PNAS Direct Submission.
Data Availability
There are no data underlying this work.
References
- 1.Darwin C., The Descent of Man, and Selection in Relation to Sex (Murray, London, 1871). [Google Scholar]
- 2.Rosenthal G. G., Mate Choice: The Evolution of Sexual Decision Making from Microbes to Humans (Princeton University Press, Princeton, 2017). [Google Scholar]
- 3.Prum R. O., The Evolution of Beauty: How Darwin’s Forgotten Theory of Mate Choice Shapes the Animal World–and Us (Anchor, New York, 2017). [Google Scholar]
- 4.Ryan M. J., A Taste for the Beautiful: The Evolution of Attraction (Princeton University Press, Princeton, 2018). [Google Scholar]
- 5.Cronin H., The Ant and the Peacock: Altruism and Sexual Selection from Darwin to Today (Cambridge University Press, Cambridge, UK, 1991). [Google Scholar]
- 6.Richards E., Darwin and the Making of Sexual Selection (University of Chicago Press, Chicago, 2017). [Google Scholar]
- 7.Darwin C., “Letter 2743 of Darwin correspondence project” (Cambridge University, 1860). https://www.darwinproject.ac.uk/ entry 2743.
- 8.Wallace A. R., G. J. Mivart’s “Lessons from Nature, as Manifested in Mind and Matter”. The Academy, 562 (1876). [Google Scholar]
- 9.Weismann A., The Evolutionary Theory (Arnold Co., London, 1904), vol. E. [Google Scholar]
- 10.Fisher R. A., The evolution of sexual preference. Eugen. Rev. 7, 184–192 (1915). [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher R. A., The Genetical Theory of Natural Selection (Oxford University Press, Oxford, 1930). [Google Scholar]
- 12.Maynard Smith J., Fertility, mating behaviour and sexual selection in Drosophila subobscura. J. Genet. 54, 261–279 (1956). [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick M., Revenge of the ugly duckling. Evolution 38, 704–706 (1984). [DOI] [PubMed] [Google Scholar]
- 14.Campbell B. G., Sexual Selection and the Descent of Man, 1871-1971 (Aldine Publishing Co., Chicago, 1972). [Google Scholar]
- 15.Trivers R. L., “Parental investment and sexual selection” in Sexual Selection and the Descent of Man, Campbell B., Ed. (Aldine Publishing Co., Chicago, 1972), pp. 136–179. [Google Scholar]
- 16.Mayr E., The bearing of the new systematics on genetical problems; the nature of species. Adv. Genet. 3b, 205–237 (1948). [DOI] [PubMed] [Google Scholar]
- 17.Coyne J., Orr H., Speciation (Sinauer, Sunderland, MA, 2004). [Google Scholar]
- 18.Lettvin J. Y., Maturana H. R., McCulloch W. S., Pitts W. H., “What the frog’s eye tells the frog’s brain” in Proceedings of the IRE (1959), vol. 47, pp. 1940–1951. [Google Scholar]
- 19.Frishkopf L. S., Capranica R. R., Goldstein M. H. Jr, Neural coding in the bullfrog’s auditory system, a teleological approach. Proc. IEEE 56, 969–980 (1968). [Google Scholar]
- 20.Capranica R. R., Why auditory neurophysiologists should be more inteeested in animal sound communication. Physiologist 15, 55–60 (1972). [PubMed] [Google Scholar]
- 21.Esch H., Huber F., Wohlers D. W., Primary auditory neurons in crickets: Physiology and central projections. J. Comp. Physiol. 137, 27–38 (1980). [Google Scholar]
- 22.Hopkins C. D., Bass A. H., Temporal coding of species recognition signals in an electric fish. Science 212, 85–87 (1981). [DOI] [PubMed] [Google Scholar]
- 23.Christensen T. A., Hildebrand J. G., Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 160, 553–569 (1987). [DOI] [PubMed] [Google Scholar]
- 24.Hoy R. R., Hoikkala A., Kaneshiro K., Hawaiian courtship songs: Evolutionary innovation in communication signals of Drosophila. Science 240, 217–219 (1988). [DOI] [PubMed] [Google Scholar]
- 25.van der Goes van Naters W., Carlson J. R., Receptors and neurons for fly odors in Drosophila. Curr. Biol. 17, 606–612 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margoliash D., Konishi M., Auditory representation of autogenous song in the song system of white-crowned sparrows. Proc. Natl. Acad. Sci. U.S.A. 82, 5997–6000 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West-Eberhard M. J., Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 (1983). [Google Scholar]
- 28.Lande R., Models of speciation by sexual selection on polygenic traits. Proc. Natl. Acad. Sci. U.S.A. 78, 3721–3725 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan M. J., Rand A. S., Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47, 647–657 (1993). [DOI] [PubMed] [Google Scholar]
- 30.West-Eberhard M. J., Sexual selection, social competition, and evolution. Proc. Am. Philos. Soc. 123, 222–234 (1979). [Google Scholar]
- 31.Zahavi A., Mate selection-a selection for a handicap. J. Theor. Biol. 53, 205–214 (1975). [DOI] [PubMed] [Google Scholar]
- 32.Zahavi A., Zahavi A., The Handicap Principle: A Missing Piece of Darwin’s Puzzle (Oxford University Press, Oxford, 1997). [Google Scholar]
- 33.Hamilton W. D., Zuk M., Heritable true fitness and bright birds: A role for parasites? Science 218, 384–387 (1982). [DOI] [PubMed] [Google Scholar]
- 34.Petrie M., Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371, 598–599 (1994). [Google Scholar]
- 35.Welch A. M., Semlitsch R. D., Gerhardt H. C., Call duration as an indicator of genetic quality in male gray tree frogs. Science 280, 1928–1930 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Achorn A. M., Rosenthal G. G., It’s not about him: Mismeasuring ‘good genes’ in sexual selection. Trends Ecol. Evol. 35, 206–219 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Rymešová D., et al., Mate choice for major histocompatibility complex complementarity in a strictly monogamous bird, the grey partridge (Perdix perdix). Front. Zool. 14, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson G. S., Kahler H., Baker R. H., Evolution of female mating preferences in stalk-eyed flies. Behav. Ecol. 9, 525–533 (1998). [Google Scholar]
- 39.Christy J. H., Salmon M., Comparative studies of reproductive behavior in mantis shrimps and fiddler crabs. Am. Zool. 31, 329–337 (1991). [Google Scholar]
- 40.Kolm N., Amcoff M., Mann R. P., Arnqvist G., Diversification of a food-mimicking male ornament via sensory drive. Curr. Biol. 22, 1440–1443 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Nakano R., Takanashi T., Skals N., Surlykke A., Ishikawa Y., To females of a noctuid moth, male courtship songs are nothing more than bat echolocation calls. Biol. Lett. 6, 582–584 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan M. J., Sensory systems, sexual selection, and sensory exploitation. Oxf. Surv. Evol. Biol. 7, 157–195 (1990). [Google Scholar]
- 43.Basolo A. L., Female preference predates the evolution of the sword in swordtail fish. Science 250, 808–810 (1990). [DOI] [PubMed] [Google Scholar]
- 44.Ryan M. J., Cummings M. E., Perceptual biases and mate choice. Annu. Rev. Ecol. Evol. Syst. 44, 437–459 (2013). [Google Scholar]
- 45.Endler J. A., Signals, signal conditions and the direction of evolution. Am. Nat. 139, S125–S153 (1992). [Google Scholar]
- 46.Cummings M. E., Endler J. A.; Handling editor: Rebecca C. Fuller , 25 Years of sensory drive: The evidence and its watery bias. Curr. Zool. 64, 471–484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morton E. S., Ecological sources of selection on avian sounds. Am. Nat. 109, 17–34 (1975). [Google Scholar]
- 48.Hunter M. L., Krebs J. R., Geographical variation in the song of the great tit (Parus major) in relation to ecological factors. J. Anim. Ecol. 48, 759–785 (1979). [Google Scholar]
- 49.Slabbekoorn H., Peet M., Ecology: Birds sing at a higher pitch in urban noise. Nature 424, 267 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Endler J. A., Thery M., Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am. Nat. 148, 421–452 (1996). [Google Scholar]
- 51.Archer S. N., Endler J. A., Lythgoe J. N., Partridge J. C., Visual pigment polymorphism in the guppy Poecilia reticulata. Vision Res. 27, 1243–1252 (1987). [DOI] [PubMed] [Google Scholar]
- 52.Cummings M. E., Sensory trade-offs predict signal divergence in Surfperch. Evolution 61, 530–545 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Seehausen O., et al., Speciation through sensory drive in cichlid fish. Nature 455, 620–626 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Leal M., Fleishman L. J., Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species. Proc. Biol. Sci. 269, 351–359 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawahara A. Y., et al., Phylogenomics reveals the evolutionary timing and pattern of butterflies and moths. Proc. Natl. Acad. Sci. U.S.A. 116, 22657–22663 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roeder K. D., Treat A. E., Ultrasonic reception by the tympanic organ of noctuid moths. J. Exp. Zool. 134, 127–157 (1957). [DOI] [PubMed] [Google Scholar]
- 57.Conner W., Ultrasound: Its role in the courtship of the arctiid moth, Cycnia tenera. Experientia 43, 1029–1031 (1987). [Google Scholar]
- 58.Ryan M. J., et al., Nineteen years of consistently positive and strong female mate preferences despite individual variation. Am. Nat. 194, 125–134 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Wilczynski W., Rand A. S., Ryan M. J., Evolution of calls and auditory tuning in the Physalaemus pustulosus species group. Brain Behav. Evol. 58, 137–151 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Kurtovic A., Widmer A., Dickson B. J., A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546 (2007). [DOI] [PubMed] [Google Scholar]
- 61.DeAngelis R. S., Hofmann H. A., Neural and molecular mechanisms underlying female mate choice decisions in vertebrates. J. Exp. Biol. 223, jeb207324 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Margoliash D., Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J. Neurosci. 6, 1643–1661 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clayton D. F., The genomic action potential. Neurobiol. Learn. Mem. 74, 185–216 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Hartshorne C., The monotony-threshold in singing birds. Auk 73, 176–192 (1956). [Google Scholar]
- 65.Stripling R., Volman S. F., Clayton D. F., Response modulation in the zebra finch neostriatum: Relationship to nuclear gene regulation. J. Neurosci. 17, 3883–3893 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong S., Clayton D. F., Habituation in songbirds. Neurobiol. Learn. Mem. 92, 183–188 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronald K. L., Fernández-Juricic E., Lucas J. R., Mate choice in the eye and ear of the beholder? Female multimodal sensory configuration influences her preferences. Proc. Biol. Sci. 285, 20180713 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong R. Y., Ramsey M. E., Cummings M. E., Localizing brain regions associated with female mate preference behavior in a swordtail. PLoS One 7, e50355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corral-López A., et al., Female brain size affects the assessment of male attractiveness during mate choice. Sci. Adv. 3, e1601990 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bloch N. I., et al., Early neurogenomic response associated with variation in guppy female mate preference. Nat. Ecol. Evol. 2, 1772–1781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilczynski W., Ryan M. J., The behavioral neuroscience of anuran social signal processing. Curr. Opin. Neurobiol. 20, 754–763 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fishilevich E., Vosshall L. B., Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548–1553 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Seki Y., et al., Olfactory coding from the periphery to higher brain centers in the Drosophila brain. BMC Biol. 15, 56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grosjean Y., et al., An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478, 236–240 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Shettleworth S., Cognition, Evolution, and Behavior (Oxford University Press, Oxford, UK, 1998). [Google Scholar]
- 76.Kirkpatrick M., Ryan M. J., The paradox of the lek and the evolution of mating preferences. Nature 350, 33–38 (1991). [Google Scholar]
- 77.Kirkpatrick M., Rand A. S., Ryan M. J., Mate choice rules in animals. Anim. Behav. 71, 1215–1225 (2006). [Google Scholar]
- 78.Ryan M. J., Keddy-Hector A., Directional patterns of female mate choice and the role of sensory biases. Am. Nat. 139, S4–S35 (1992). [Google Scholar]
- 79.Stevens S., Psychophysics: Introduction to Its Perceptual, Neural, and Social Prospects (Transaction, New Brunswick, NJ, 1975). [Google Scholar]
- 80.Akre K. L., Johnsen S., Psychophysics and the evolution of behavior. Trends Ecol. Evol. 29, 291–300 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Akre K. L., Farris H. E., Lea A. M., Page R. A., Ryan M. J., Signal perception in frogs and bats and the evolution of mating signals. Science 333, 751–752 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tversky A., Kahneman D., “Rational choice and the framing of decisions” in Multiple Criteria Decision Making and Risk Analysis Using Microcomputers, Zionts S., Karpak P., Eds. (Springer, Berlin, 1989), pp. 81–126. [Google Scholar]
- 83.Kahneman D., Thinking, Fast and Slow (Macmillan, New York, 2011). [Google Scholar]
- 84.Dechaume-Moncharmont F.-X., Freychet M., Motreuil S., Cézilly F., Female mate choice in convict cichlids is transitive and consistent with a self-referent directional preference. Front. Zool. 10, 69 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arbuthnott D., Fedina T. Y., Pletcher S. D., Promislow D. E., Mate choice in fruit flies is rational and adaptive. Nat. Commun. 8, 13953 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reding L., Cummings M. E., Rational mate choice decisions vary with female age and multidimensional male signals in swordtails. Ethology 124, 641–649 (2018). [Google Scholar]
- 87.Gabel E., Hennig R. M., Evidence for comparative decision making in female crickets. Behav. Ecol. 27, 1216–1222 (2016). [Google Scholar]
- 88.Ariely D., Predictably Irrational, Revised and Expanded Edition (HarperCollins, London, 2009). [Google Scholar]
- 89.Bateson M., Healy S. D., Comparative evaluation and its implications for mate choice. Trends Ecol. Evol. 20, 659–664 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Lea A. M., Ryan M. J., Irrationality in mate choice revealed by túngara frogs. Science 349, 964–966 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Locatello L., Poli F., Rasotto M. B., Context-dependent evaluation of prospective mates in a fish. Behav. Ecol. Sociobiol. 69, 1119–1126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Royle N. J., Lindström J., Metcalfe N. B., Context-dependent mate choice in relation to social composition in green swordtails Xiphophorus helleri. Behav. Ecol. 19, 998–1005 (2008). [Google Scholar]
- 93.Reding L., Cummings M. E., Rational choice of social group size in mosquitofish. Biol. Lett. 15, 20180693 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenthal G. G.; Handling editor: Becky Fuller , Evaluation and hedonic value in mate choice. Curr. Zool. 64, 485–492 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pearce M. T., et al., Neuroaesthetics: The cognitive neuroscience of aesthetic experience. Perspect. Psychol. Sci. 11, 265–279 (2016). [DOI] [PubMed] [Google Scholar]
- 96.Reber R., Schwarz N., Winkielman P., Processing fluency and aesthetic pleasure: Is beauty in the perceiver’s processing experience? Pers. Soc. Psychol. Rev. 8, 364–382 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Renoult J. P., Bovet J., Raymond M., Beauty is in the efficient coding of the beholder. R. Soc. Open Sci. 3, 160027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Changizi M. A., Zhang Q., Ye H., Shimojo S., The structures of letters and symbols throughout human history are selected to match those found in objects in natural scenes. Am. Nat. 167, E117–E139 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Renoult J. P., Mendelson T. C., Processing bias: Extending sensory drive to include efficacy and efficiency in information processing. Proc. Biol. Sci. 286, 20190165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hulse S. V., Renoult J. P., Mendelson T. C., Sexual signaling pattern correlates with habitat pattern in visually ornamented fishes. Nat. Commun. 11, 2561 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Brien D. M., et al., On the evolution of extreme structures: Static scaling and the function of sexually selected signals. Anim. Behav. 144, 95–108 (2018). [Google Scholar]
- 102.Cooney C. R., et al., Sexual selection predicts the rate and direction of colour divergence in a large avian radiation. Nat. Commun. 10, 1773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Page R. A., Bernal X. E., The challenge of detecting prey: Private and social information use in predatory bats. Funct. Ecol. 34, 344–363 (2020). [Google Scholar]
- 104.Monk J. D., Giglio E., Kamath A., Lambert M. R., McDonough C. E., An alternative hypothesis for the evolution of same-sex sexual behaviour in animals. Nat. Ecol. Evol. 3, 1622–1631 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Kranz F., Ishai A., Face perception is modulated by sexual preference. Curr. Biol. 16, 63–68 (2006). [DOI] [PubMed] [Google Scholar]
- 106.Ryan M. J., Drewes R. C., Vocal morphology of the Physalaemus pustulosus species group (Leptodactylidae): Morphological response to sexual selection for complex calls. Biol. J. Linn. Soc. Lond. 40, 37–52 (1990). [Google Scholar]
- 107.Kime N. M., Ryan M. J., Wilson P. S., Modelling the production of complex calls in the túngara frog (Physalaemus pustulosus). Bioacoustics 28, 345–363 (2019). [Google Scholar]
- 108.Griddi-Papp M., Rand A. S., Ryan M. J., Complex call production in túngara frogs. Nature 441, 38 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Ryan M. J., Fox J. H., Wilczynski W., Rand A. S., Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature 343, 66–67 (1990). [DOI] [PubMed] [Google Scholar]
- 110.Ryan M. J., The Túngara Frog: A Study in Sexual Selection and Communication (University of Chicago Press, Chicago, 1985). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.