Li et al. (1) recently report the discovery of 16 Food and Drug Administration–approved drugs as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease (Mpro) inhibitors. They were identified from a computational virtual screening approach using the Mpro as the drug target, and their enzymatic inhibition against SARS-CoV-2 Mpro was validated in the fluorescence resonance energy transfer (FRET)-based enzymatic assay (inhibitory constant Ki = 0.04 to 3.27 µM).
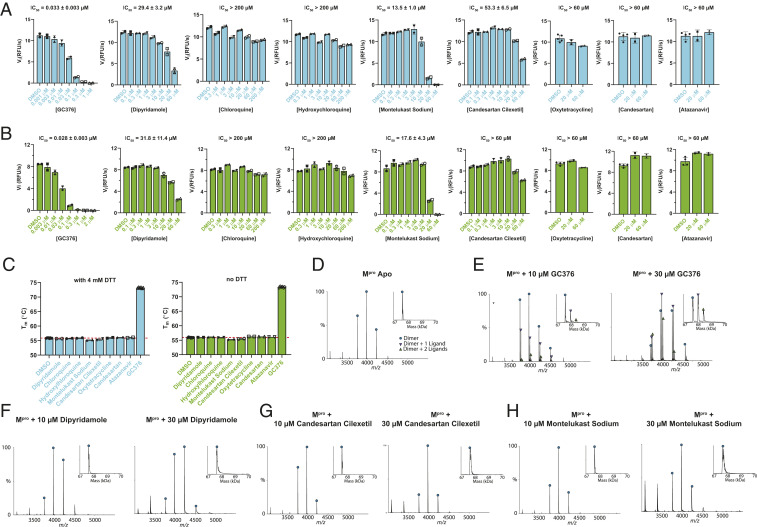
Among the list of 16 discovered hits, disulfiram was recently proved by us as a nonspecific promiscuous cysteine protease inhibitor that not only inhibits SARS-CoV-2 Mpro but also five other cysteine proteases, and the inhibition was only observed in the absence of reducing reagent, 1,4-dithiothreitol (DTT) (2). As these claimed hits do not share structural similarities with existing Mpro inhibitors (3), we therefore chose the eight most potent compounds, including dipyridamole, candesartan cilexetil, hydroxychloroquine, chloroquine, montelukast sodium, atazanavir, candesartan, and oxytetracycline, for the hit validation. GC376 was included as a positive control (4, 5). To rule out false positives, we tested all eight compounds in the FRET-based enzymatic assay with and without DTT, thermal shift binding assay, and native mass spectrometry (MS) binding assay. Collectively, our results have shown that, first, the most potent compound claimed by Li et al. (1), dipyridamole (Ki = 0.04 µM, half-maximum inhibitory concentration [IC50] = 0.6 µM), is a weak inhibitor of SARS-CoV-2 Mpro with an IC50 value of 29.4 ± 3.2 µM (Fig. 1A). However, dipyridamole did not show binding to Mpro in either the thermal shift assay (Fig. 1C) or the native MS assay (Fig. 1F), suggesting dipyridamole is not a potent inhibitor of Mpro. Second, chloroquine and hydroxychloroquine did not shown inhibition against Mpro in the enzymatic assay either with or without DTT (IC50 >200 µM) (Fig. 1 A and B). They also did not show binding to Mpro in the thermal shift binding assay (Fig. 1C). Third, montelukast sodium inhibited Mpro with an IC50 value of 13.5 ± 1.0 µM in the presence of DTT (Fig. 1A). However, it did not show binding to Mpro in either the thermal shift assay (Fig. 1C) or native MS assay (Fig. 1H). This suggests that the apparent enzymatic inhibition might be a false positive. Fourth, candesartan cilexetil, candesartan, oxytetracycline, and atazanavir did not inhibit Mpro (IC50 >50 µM) (Fig. 1 A and B), nor did they bind to Mpro as shown by the thermal shift binding assay (Fig. 1C). Candesartan cilexetil also did not show binding in the native MS assay (Fig. 1G).
Fig. 1.
Hit validation/invalidation of dipyridamole, chloroquine, hydroxychloroquine, montelukast sodium, candesartan, candesartan cilexetil, oxytetracycline, and atazanavir as SARS-CoV-2 Mpro inhibitors. The most active 8 compounds out 16 identified in ref. 1 were evaluated in a FRET-based enzymatic assay in the presence (A) or absence (B) of 4 mM DTT, thermal shift binding assay (C), and native MS binding assay (D–H). The FRET-based enzymatic assay was carried out with 100 nM SARS-CoV-2 Mpro protein with 10 µM FRET substrate Dabcyl-KTSAVLQ/ SGFRKME-Edans (2, 4–6), the thermal shift binding assay was carried out with 3 µM Mpro protein and 40 µM testing compounds (2, 4–6), and the native MS binding assay was carried out with 4 µM Mpro protein and 10 to 30 µM testing compounds (2, 4, 5).
Overall, our data suggest that there might be a significant flaw with the enzymatic assay inhibition results presented in this study. None of the identified hits was confirmed to inhibit or bind to SARS-Co-2 Mpro. A GST-tagged Mpro was used in their enzymatic assay; however, it is known that Mpro requires a native N terminus to form the enzymatic active dimer (4). DTT was not added in the enzymatic assay, but the nonspecific reactivity did not explain the results presented in the paper, as we did not observe significant enzymatic inhibition even in the absence of DTT for six of the tested compounds (Fig. 1B).
Acknowledgments
This research was supported by NIH grants AI147325 and AI157046 and Arizona Biomedical Research Centre Young Investigator grant ADHS18-198859 to J.W. We thank Julia Alma Townsend and Michael Marty at the University of Arizona Department of Chemistry and Biochemistry for the native MS data.
Footnotes
The authors declare no competing interest.
References
- 1.Li Z., et al., Identify potent SARS-CoV-2 main protease inhibitors via accelerated free energy perturbation-based virtual screening of existing drugs. Proc. Natl. Acad. Sci. U.S.A. 117, 27381–27387 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma C., et al., Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are nonspecific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharmacol. Transl. Sci. 3, 1265–1277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullrich S., Nitsche C., The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 30, 127377 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma C., et al., Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 30, 678–692 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacco M. D., et al., Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Sci. Adv., 10.1126/sciadv.abe0751 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y., et al., Boceprevir, calpain inhibitors II and XII, and GC-376 have broad-spectrum antiviral activity against coronaviruses in cell culture. bioRxiv [Preprint] (2020). https://www.biorxiv.org/content/10.1101/2020.10.30.362335v1 (Accessed 3 February 2021). [DOI] [PMC free article] [PubMed]