Abstract
Filamentous microorganisms are main producers of organic acids, enzymes, and pharmaceutical agents such as antibiotics and other active pharmaceutical ingredients. With their complex cell morphology, ranging from dispersed mycelia to dense pellets, the cultivation is challenging. In recent years, various techniques for tailor‐made cell morphologies of filamentous microorganisms have been developed to increase product formation and have been summarised under the term morphology engineering. These techniques, namely microparticle‐enhanced cultivation, macroparticle‐enhanced cultivation, and alteration of the osmolality of the culture medium by addition of inorganic salts, the salt‐enhanced cultivation, are presented and discussed in this review. These techniques have already proven to be useful and now await further proof‐of‐concept. Furthermore, the mechanical behaviour of individual pellets is of special interest for a general understanding of pellet mechanics and the productivity of biotechnological processes with filamentous microorganisms. Correlating them with substrate uptake and finally with productivity would be a breakthrough not to be underestimated for the comprehensive characterisation of filamentous systems. So far, this research field is under‐represented. First results on filamentous pellet mechanics are discussed and important future aspects, which the filamentous expert community should deal with, will be presented and critically discussed.
Keywords: filamentous microorganisms, macroparticle, mechanical induced stress, microparticle, morphology engineering, pellet, salt‐enhanced cultivation
Abbreviations
- CPO
chloroperoxidase
- DEM
discrete element method
- GFP
Green Fluorescent Protein
- MPEC
microparticle‐enhanced cultivation
- PBE
population balance equation
- VOF
volume of fluid
1. INTRODUCTION
Filamentous microorganisms are in the focus of many industrial biotechnological processes for the production of organic acids, enzymes, and pharmaceutical agents such as antibiotics, and are often cultivated as disperse mycelia or pellets in shaken and stirred tank bioreactors [1, 2, 3, 4, 5]. Beside filamentous growing eukaryotic fungi like from the family of Aspergilli, also bacterial filamentous microorganisms, actinomycetes, especially Streptomyces species, are of great pharmaceutical interest due to their production of various secondary metabolites [6, 7, 8].
PRACTICAL APPLICATION
In recent years, a variety of techniques for tailor‐made morphologies of filamentous microorganisms was developed to promote product formation. These morphology engineering techniques range from microparticle‐ and macroparticle‐enhanced cultivation, in which the particles can also have different surface modifications, to salt‐enhanced cultivation by adding inorganic salts (e.g. NaCl, KCl or (NH4)2SO4), to increase broth osmolality and change the cell morphology. The review dicusses how the cell morphology of filamentous microorganisms can lead to higher end product concentrations by these techniques and to what extent micromechanical, multiphase flows and fluid mechanical investigations can support biotechnology in implementing these processes in growth and product formation models, which allow a correlation to productivity. Finally, the remaining gaps and challenges in this complex research area are discussed.
A characteristic feature of filamentous microorganisms is that their growth and productivity are closely linked to particular morphologic phenotypes [9, 10, 11, 12, 13, 14]. The macro morphology determines the micro environment of hyphae through effects on mixing, mass transfer, and broth rheology, which in turn affect product formation [15]. For example, the density of the filament network determines mass transport within a pellet and can be crucial for the viability of filaments inside the pellet and possible substrate limitations. Controlling the morphology of filamentous organisms to increase the yield of a desired product is still a challenging task.
Besides the classical morphology influencing cultivation parameters, e.g. pH value, temperature, culture medium composition, dissolved oxygen, viability/vitality of the inoculum, and inoculum concentration, morphology engineering has been developed in recent years. It is also known that filamentous cell morphology is mainly influenced by mechanical stress. For this reason, the mechanical behaviour of individual pellets is of particular interest for a general understanding of pellet mechanics and the productivity of biotechnological processes with filamentous microorganisms.
This review deals with morphology influencing techniques for filamentous microorganisms and discusses results closely related to cell morphology and pellet mechanics. Therefore, the cell morphological parameters of filamentous microorganisms are presented and discussed in Section 2, followed by the description and evaluation of morphology engineering techniques, i.e. microparticle‐ and macroparticle‐enhanced cultivation and the progress of salt‐enhanced cultivation, which have become part of research in the last 15 years. In Section 3 the experimental investigations for the micromechanical characterisation of pellets are presented. Section 4 discusses the techniques and methods of fluid mechanical models for the description of pellet morphology and their influence in multiphase flows, as they prevail in stirred bioreactors, as well as in shaking reactors with free surface. Section 5 presents the challenges for future research for the understanding of cultivation processes with filamentous microorganisms. The results and findings as well as a look into the future for this highly complex biotechnological topic of filaments in bioprocesses are finally summarised in Section 6.
2. CELL MORPHOLOGY OF FILAMENTOUS MICROORGANISMS
One of the most influential properties of filamentous microorganisms is their complex cellular morphology [16, 17, 18, 19]. It is well‐known that there is a strong relationship between the cultivation process conditions, the cell morphology, and the productivity of filamentous microorganisms [15, 20–22]. Thus, the cell morphology is a key aspect in understanding filamentous microorganisms. In submerged cultivations, filamentous microorganisms can develop two different boundary forms of cell morphology depending on the cultivation conditions. In the beginning of filamentous cultivation the differentiation growth process involves different cell types leading from conidia over germ tubes to cylindrical fibers called hyphae, which grow and branch. Each individual hyphal filament is surrounded by the cultivation medium and can be described by the micro morphology. Segregation of cells after cell division is not accomplished [18]. Instead, biomass growth is strongly polarised and takes place only at the tip of the hyphae. Tip growth and branching eventually result in different macroscopic appearances (macro morphology) of the culture, ranging from single hyphal elements, so‐called dispersed mycelia, over connected networks of hyphae up to distinct spherical particles of biomass termed as (bio)pellets [18]. These spherical pellets resemble porous particles, consisting of an outwardly growing densely branched and partially intertwined filament network. In addition to these two boundary forms of cell morphologies, mixed forms can also occur in which the filamentous growing biomass termed as clumps. The development of the macro morphology is mainly driven by aggregation, fragmentation, and growth [23, 24]. Dense hyphal networks are known to limit the transport of substrates into filamentous pellets and thus influence their viability and productivity [25, 26, 27].
The availability of growth‐limited substrates (in most cases oxygen) inside a pellet is determined by the equilibrium of the hyphae‐specific oxygen uptake and the convective/diffusive oxygen transport over the pellet surface into the porous hyphal network. The transport processes inside the pellet are mainly influenced by the pellet porosity. The pellet porosity, defined by the density of the hyphal network, describes the free liquid phase between the hyphal structures, in which transport processes can occur. Therefore, the hyphal fraction is often used to characterise the pellet porosity by describing the ratio of the hyphal volume to total volume [25, 28–30]. Hille et al. [25] investigated the hyphal fraction in pellet slices and found a varying porosity over the radius. Further, the authors determined the effective diffusion of oxygen as a function of hyphal density along a radial pellet coordinate. Dense pellets with a large diameter often face oxygen and nutrient limitations in the pellet core [25, 31]. The diffusion of oxygen in the pellet core is hindered when a critical diameter is exceeded. This also influences the microorganisms metabolism and the product formation [32, 33]. However, in very porous or hairy pellets with small diameters the uptake of the growth‐limiting oxygen substrate is not limited in transport.
The formation of hyphae, which are intertwining to loose mycelia or dense pellets, leads to challenges in the cultivation process [15, 34] as is exemplarily shown in Figure 1.
FIGURE 1.
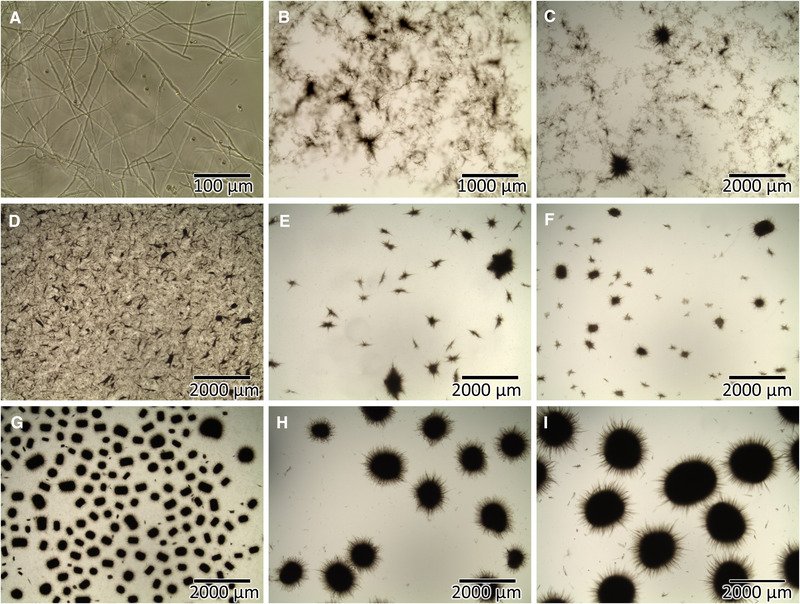
Morphological diversity of the filamentous actinomycete Lentzea aerocolonigenes in different cultivations ranging from single hyphae (A), over mostly mycelial structures (B‐D) up to dense and defined pellets in various sizes (E‐I)
These challenges are accepted because many filamentous bacteria and fungi produce substances that are interesting for food or pharmaceutical industry [34]. The bacterial filamentous Actinomycetes, including Actinomadura namibiensis and Lentzea aerocolonigenes, are of special interest, because of the production of mainly pharmaceutically relevant secondary metabolites such as labyrinthopeptin and rebeccamycin, respectively [6, 8, 35]. In 2000, 60% of the known biologically active secondary metabolites were isolated from Actinomycetes [35]. Although there are clear differences between filamentous bacteria and fungi, their morphology and its influence during a cultivation process are similar [6, 36].
In industrial cultivations, mycelium and pellet populations are characterised by different, often contradictory advantages and disadvantages [37]. In comparison to mycelial cultures, pellet cultivations have a lower apparent viscosity and often require a lower power input and lower operating costs for suspension and mixing [38]. Compared to mycelial cultures, pellet‐like growing microorganisms are easier to separate for processing [39, 40, 41].
The different morphological forms of filamentous microorganisms can affect the cultivation process by influencing different parameters such as the viscosity of the cultivation broth and therefore the mixing performance as well as the costs for downstream processing [15]. At high biomass concentrations mycelial structures or pellets with many freely exposed hyphae in their periphery can lead to a high viscosity with a non‐Newtonian flow behaviour [15, 42, 43]. In a highly viscous cultivation broth, the mass transfer and mixing performance are impeded. In order to ensure a sufficient nutrient and oxygen supply of the microorganism an increased power input is necessary [38]. A large power input in turn causes high mechanical stress and therefore leads to morphological changes [44] as well as variations in biomass growth and product titers [45].
For many filamentous microorganisms a correlation between cell morphology and productivity has been observed [46]. Depending on the strain of the microorganism and on the product, the morphological form (mycelium, clumps, pellets) varies, allowing the highest possible productivity in a bioreactor. So far, no strain‐, genus‐family‐, or product‐type related correlation exist to improve process understanding of filamentous production systems [34, 47].
Mycelial structures are advantageous for the production of geldamycin in S. hygroscopicus and tylosin in S. fridae [48, 49]. The production of citric acid [17, 50], glucose oxidase [44], glucoamylase [29], and polygalacturonidase [51] is favoured in pellet growth by Aspergillus niger. Compared to mycelium cultures, pellet morphologies have also been used to achieve higher productivity in the production of nikkomycin by Streptomyces tendae [52], avermectin by Streptomyces avermitilis [53], rebeccamycin by Lentzea (formerly Lechevalieria) aerocolonigenes [7, 19, 54, 55], and the new peptide antibiotic labyrinthopeptin A1 by Actinomadura namibiensis [8]. For these reasons, a large number of studies focus exclusively on pellet‐like cultivations and the interaction between mechanically induced morphological changes and product formation. However, there are also microorganisms that exhibit nearly equal productivity in different morphological forms like S. clavuligerus and S. virginiae when producing clavulanic acid or virginiamycin [56, 57].
2.1. Control and design of the pellet morphology
Cellular morphology, productivity, and the initiation of mycelial or pellet‐like fungal and bacterial growth from an inoculum are influenced by various process conditions, e.g. strain, inoculum size and type, composition of the cultivation medium, pH value, dissolved oxygen content, temperature, presence of certain ions and, volumtric power input and therefore mechanical stress during cultivation [15, 17, 34, 38]. These cultivation parameters of a bioprocess, which influence cellular morphology and productivity, are also summarised under the holistic term environome [15, 58]. By adjusting the cell morphology according to the preferences of the microorganism, a targeted optimisation of the product concentration is aimed for. Consequently, various aspects have to be considered.
In pellet cultures, the mechanical interactions of the porous structures with the surrounding turbulent flow, the aeration, the stirrer, and the reactor wall play an important role, as they can cause permanent deformations and rearrangements or densifications of the outer filament network, cf. Figure 2.
FIGURE 2.
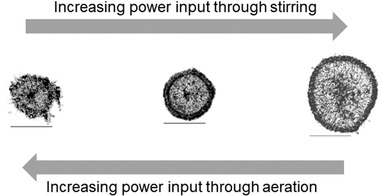
Microscopic images of equatorial pellet sections for the cultivation of Aspergillus niger AB1.13 after 62 h of cultivation in a 28 L stirred tank bioreactor with constant volumetric power input P/V of 143 W m−3 and non‐limiting oxygen supply at different combinations of the stirrer‐ and aeration‐related power input. Left: P/V 103 (aeration, aer) and 40 (stirring, stir) W m−3; middle: 78 (aer) and 65 (stir) W m−3; right: 52 (aer) and 91 (stir) W m−3; scale bar 500 μm). Adapted from Lin et al. [29]
Consequently, these morphological changes significantly affect the substrate transport inside the pellet and the filament productivity. For multiple filamentous microorganisms a high power input and high dissolved oxygen levels lead to pellet‐like growth, while a lower power input generates fluffier pellets and low dissolved oxygen levels promote mycelial growth [52, 53, 59, 60]. Besides mechanical stress, the pellet morphology can also be influenced by other cultivation parameters, e.g. substrate supply or cultivation time [52, 61, 62, 63].
2.1.1. Micro‐ and macroparticle‐enhanced cultivation
In recent years, various techniques for tailor‐made morphologies of filamentous microorganisms to promote product formation were developed and summarised under the term morphology engineering [12, 20, 54, 64, 65, 66, 67, 68, 69]. One approach to influence the cell morphology and productivity of filamentous microorganisms is the addition of microparticles, which is meanwhile a frequently used method by researchers [45, 70, 71]. This approach of morphology engineering is called microparticle‐enhanced cultivation (MPEC) [63]. The microparticles used in these cases usually have a diameter of <50 μm [54, 67, 72], in some cases even larger particles are treated as microparticles [73, 74].
The first application of microparticle addition to filamentous microorganisms was described by Kaup et al. [64]. The authors investigated the effects of aluminum oxide (Al2O3) and talc powder (3MgO∙4SiO2∙H2O) on growth and chloroperoxidase (CPO) formation of Caldariomyces fumago. Both types of microparticles had diameters ≤42 μm. The microparticle concentration was varied between 0.05 and 25 g L‐1. For an Al2O3 concentration of 15 g L‐1 a 4‐fold increase in accumulated CPO activity was observed after 12 days of cultivation. For 10 g L‐1 of talc powder even a 10‐fold increase of accumulated CPO activity was found. Moreover, the cell morphology of the fungus was significantly influenced by the addition of microparticle. Without particles C. fumago grew in pellets with a diameter of approximately 4 mm. With Al2O3 or talc powder the diameter was between 0.1 and 0.5 mm and many single hyphae between 60 and 600 μm were apparent. A similar effect on morphology was also observed for eight other filamentous fungi and the filamentous bacterium Streptomyces aurefaciens [64]. The addition of titanate microparticles to A. niger, however, resulted in pellets with a dense microparticle core [12].
Since then, many researchers worked with the procedure of MPEC. An overview of different studies applying MPEC is given in Table 1.
TABLE 1.
Overview of selected applications of MPEC with different microorganisms from the literature
| Microorganism | Microparticle type | Microparticle concentration [g L‐1] | Reference |
|---|---|---|---|
| Caldariomyces fumago | Aluminumoxide, talc | 0.05–25 | Kaup et al. [63] |
| Aspergillus niger | Aluminum oxide, talc, titanate | 0–30 | Driouch et al. [12, 72] |
| Aspergillus niger, Trichoderma atroviride | 17 particle types | 20 | Etschmann et al. [73] |
| Streptomyces sp. M‐Z18 | Talc | 0–20 | Ren et al. [75] |
| Aspergillus terreus | Talc | 1–15 | Gonciarz and Bizukojc [67], Gonciarz et al. [76] |
| Aspergillus sojae | Aluminumoxide, talc | 0–25 |
Karahalil et al. [71], Yatmaz et al. [77], Germec et al. [78] |
| Aspergillus niger | Talc | 12 | Kowalska et al. [79] |
| Lentzea aerocolonigenes | Talc, surface modified talc | 10 | Walisko et al. [54] |
| Trichoderma viride | Aluminum oxide | 0–30 | Dong et al. [80] |
| Grifola frondosa | Talc | 0–20 | Tao et al. [81] |
| Aspergillus terreus | Aluminum oxide | 6–12 | Boruta and Bizukojc [82] |
| Aspergillus terreus, Penicillium rubens, Chaetomium globosum, Mucor racemosus | Aluminum oxide | 6 | Kowalska et al. [83, 84, 85] |
| Aspergillus terreus | Talc | 0.48 | Saberi et al. [86] |
| Aspergillus nidulans | Talc | 20 | Niu et al. [74] |
As can be seen in Table 1, most MPEC approaches used filamentous fungi, while filamentous bacteria are under‐represented in this field of research. Furthermore, talc and aluminum oxide microparticles are used in almost all studies. Other microparticles were investigated by Etschmann et al. [73]. The effects of 17 microparticle types, differing in size (ø = 5‐250 μm) and chemical composition, were examined in cultivations of a 2‐phenylethanol producing A. niger and a 6‐pentyl‐α‐pyrone producing Trichoderma atroviride. However, the effects were highly strain‐ and particle‐specific and a general mechanistic description was not possible [73].
Etschmann et al. [73] proposed the design of tailor‐made particles with a defined surface, since the inhomogeneity regarding particle size and shape of different microparticle types might play a major role with respect to their effects on cultivations of filamentous microorganisms. Walisko et al. [54] applied a layer‐by‐layer surface modification technique on talc particles. Thus, talc particles with different surface modifications and thereby different surface properties (e.g. hydrophilic/hydrophobic) were created. These surface modified talc particles were then used in cultivations of L. aerocolonigenes. Ten different modifications together with unmodified talc were investigated. The production of rebeccamycin with some modified particles was higher than with unmodified talc, in other cases the product concentrations were lower. Rather hydrophilic or only slightly hydrophobic particles with a negative zeta potential showed the highest rebeccamycin concentrations. However, no clear trend of which properties are advantageous could be identified [54]. Further investigations to the type of surface modifications of particulate additives are required.
The mechanisms behind MPEC are widely discussed, but not yet fully understood. Driouch et al. [65] traced the effects of talc particles on morphology of A. niger back to the hindered spore aggregation before germination. Kowalska et al. [85] investigated the addition of aluminum oxide on four fungi with different agglomeration types and considered this aspect to be of major importance in MPEC cultivations. Driouch et al. [65] proposed the loosened mycelial structure, which enables an increased nutrient and oxygen supply to all areas as the reason for the increase in enzyme activity with talc or aluminum oxide. Although the addition of titanate led to a different cell morphology, a loosening of the pellet structure was nevertheless observed, which led to similar improvements in nutrient and oxygen supply throughout the pellet [12]. Talc and titanate microparticles were also added to a cultivation of the recombinant reporter strain A. niger ANip7‐MCS‐gfp2, producing a variant of Green Fluorescent Protein (GFP) which was coexpressed with the desired enzyme glucoamylase. While a pellet from an unsupplemented cultivation was only producing in a thin layer on the surface of the pellet, pellets grown with titanate produced GFP all over the pellet [12]. Mycelial structures created by the addition of talc also showed production in all areas [11].
Since the addition of microparticles proved to be beneficial for many filamentous microorganisms, some authors questioned whether larger particles might have similar effects. Kaup et al. [64] tested quarz (SiO2) particles with a diameter of approximately 350 μm and glass beads with a diameter of about 500 μm in cultivations of C. fumago. The quarz particles showed a qualitatively similar effect as the microparticles for biomass and CPO formation, but much less pronounced. The glass beads, however, did not affect biomass and CPO formation in the cultivation [64]. The addition of broken and porous SiO2 particles (ø = 120–200 μm) and glass beads (ø = 250–500 μm) to two different Streptomyces strains was investigated by Holtmann et al. [87]. 5 g L‐1 of SiO2 in a cultivation of Streptomyces coelicolor led to an increase in actinorhodin concentration of 85 up to 160% depending on the applied culture medium. The final streptavidin concentration after a cultivation of Streptomyces avidinii was not affected by the addition of 5 g L–1 glass beads. However, an accelerated production was observed [87].
Neither of the two authors mentioned above investigated the morphological changes caused by the addition of larger particles. Ochi [88] described the homogenisation of biomass, but did not give further details on the pellet size. Hotop et al. [89] added 4 mm glass beads to their pre‐cultures (50 in the first pre‐cultures, 400 in the second pre‐cultures) to reduce the pellet diameter of Penicillium chrysogenum. The pellet diameter in the pre‐cultures was reduced to <300 μm, leading to a diameter reduction from 1 to 0.6 mm in the main culture. The final product concentration of penicillin V was doubled [89]. The addition of glass beads was also investigated by Dobson et al. [48]. Beads with a diameter of 5 mm were used in a 250 mL shaking flask for the cultivation of Streptomyces hygroscopicus var. geldanus. Between 0 and 55 glass beads were added at the beginning and a spore suspension was used for inoculation. With increasing glass bead number, the average pellet diameter decreased. These changes in cell morphology were accompanied by an increased final geldamycin concentration with increasing glass bead number [48]. Sohoni et al. [90] applied glass beads between 0.75 and 4 mm in microtiter plate cultivations of S. coelicolor. Diameters of 0.75 ‐ 2 mm led to pellet‐like growth, while diameters of 3 and 4 mm produced dispersed mycelium. A glass bead diameter of 3 mm was considered optimal, as it led to reproducible and narrow defined morphology of S. coelicolor and, in addition, improved the product concentrations of actinorhodin and undecylprodigiosin [90]. The addition of glass beads to L. aerocolonigenes was described by Walisko et al. [54]. Beads, featuring diameters between 0.25 and 2.1 mm in a concentration of 80 g L‐1, were added to 250 mL baffled shaking flasks. In Walisko [91] even larger particles up to 5 mm were tested in cultivations. With increasing bead diameter, the pellet diameter decreased, resulting in a mycelial structure with glass beads of ≥3 mm glass beads. With 0.25‐0.5 mm beads a 19‐fold increase in rebeccamycin concentration compared to an unsupplemented control was observed [54, 91].
The mechanical stress induced by the glass beads is often considered to be responsible for the effect in cell morphology, since mechanical stress by aeration or agitation can also influence the pellet morphology [29], cf. Figure 2. For a precise estimation of the mechanical stress induced by macroparticles in shaking flask cultivations, simulations of the system are necessary (Section 4).
2.1.2. Salt‐enhanced cultivation
In addition to the techniques of microparticle‐ and macroparticle‐enhanced cultivation, the manipulation of osmolality by adding inorganic salts to the cultivation broth was found to be another method to adapt the cell morphology [66]. In analogy to MPEC this tool of morphology engineering was refered to as salt‐enhanced cultivation [8, 92]. Compared to an unsupplemented cultivation the increase in osmolality or osmolarity through the addition of salt leads to significantly higher productivity in some filamentous microorganisms such as A. niger for the production of fructofuranosidase or glucoamylase and Actinomadura namibiensis for the production of labyrinthopeptin [8, 66]. The pellet size is reduced while the pellet porosity is increased and a stronger mycelial growth occurred which caused this productivity increase. In all cases, the increased osmolality/osmolarity led to more mycelial and clump growth.
The terms osmolality/osmolarity refers to the substance quantity of osmotically active ions or particles of solute per kilogram/liter of solvent. The osmolality/osmolarity of cultivation broths mainly depends on the composition of the culture medium and changes during cultivation due to nutrient consumption, release of metabolic products and addition of an acid or base for pH control. Changes in the external osmolality/osmolarity trigger water fluxes along the osmotic gradient and require adaptive processes to counter either swelling or dehydration of the cells. Maintaining the hydrostatic pressure is important as a positive turgor is considered as driving force for cell expansion [93]. Microorganisms have developed mechanisms to adjust the intracellular pressure [94]. Because osmolality/osmolarity changes have the same physicochemical effects in all organisms, there are considerable similarities in their responses to osmotic shifts [95]. When medium osmolality/osmolarity is increased, specialised nontoxic osmolytes (compatible solutes) are taken up and/or synthesised in the cytoplasm. These molecules are highly soluble and do not carry a net charge on physiological pH. Exemplary osmoprotectants are glycine betaine, dimethylsulfoniopropionate, carnitine, proline, proline betaine, ectoine, trehalose, and gycosylglycerol [93]. Some halotolerant organisms also accumulate inorganic ions (mainly potassium) to balance the osmotic pressure [96].
Since osmoregulation is inseparable from metabolic regulation [95] and metabolic regulation affects morphology and productivity, the addition of salts to the cultivation medium can be used to manipulate the cell morphology. Studies on the impact of different inorganic salts on the morphology of filamentous microorganisms furthermore showed that different types of ions influence the cell morphology in distinct ways. Pellet‐like growth was enhanced by polycations and suppressed by polyanions, leading to the conclusion that ions also influence the agglomeration behavior of the cell walls [32, 48, 97].
Only a few studies are devoted to the effect of so‐called inert salts such as NaCl or KCl, which are supposed to have no effect on metabolism [98, 99, 100]. Bobowicz‐Lassociska and Grajek [99] increased the protein secretion of washed and filtered mycelia of A. niger by the addition of KCl [20]. Furthermore, Fiedurek [98] increased the activity of A. niger‐expressed glucose oxidase 2‐fold by adding NaCl to centrifuged mycelia, administering an osmotic shock to the fungus. Wucherpfennig et al. [66] investigated the salt‐enhanced cultivation with two A. niger strains. Increased NaCl concentrations led to stronger mycelial growth and an 18‐fold increased productivity of fructofuranosidase and a 4.5‐fold enhanced productivity of glucoamylase in A. niger SKAn1015 and AB1.13, respectively. However, the observed changes in productivity might not be due to the change in cell morphology alone. The parameter of osmolality might have influence the fungal physiology, which could in turn affect the cell morphology independently. The increase in the observed productivity was shown to correlate with the active pellet surface area [20, 66]. Table 2 shows an overview of selected applications of the manipulation of osmolality by the addition of inorganic salts to the cultivation broth.
TABLE 2.
Overview of selected applications of the manipulation of osmolality by the addition of inorganic salts to the cultivation broth–The salt‐enhanced cultivation with different microorganisms
| Microorganism | Inorganic salt | Salt concentration [mM]/Osmolality [mosmol/kg] | Reference |
|---|---|---|---|
| Dendryphiella salina |
NaCl NaCl, NaH2CO3, Na2SO4, CaCl2, RbCl, KCl, MgCl2 NaCl or KCl |
0–75/‐ 15/‐ 25/‐ |
Allaway and Jennings [98] |
| Aspergillus niger | KCl | 300–3110/‐ | Bobowicz‐Lassociska and Grajek [99] |
| Aspergillus niger | NaCl | 400–2800/‐ | Fiedurek [100] |
| Streptomyces azureus | Mg2+, Ca2+, Mn2+ | 0.2/‐ | Okba et al. [101] |
| Aspergillus niger SKAn1015 and AB1.13 | NaCl | ‐/350–2550 | Wucherpfennig et al., [66] |
| Streptomyces coelicolor | NaCl |
<500, >500/ <1070, >1070 |
Fuchino et al. [102] |
| Actinomadura namibiensis |
NaCl, NH4Cl, K2SO4 (NH4)2SO4 |
0, 25, 50, 75, 100/‐ 200 (only with (NH4)2SO4)/‐ |
Tesche et al. [8] |
Similar to A. niger, Okba et al. [101] investigated the impact on cell morphology of Streptomyces azureus (no information about the product) by the addition of Mg2+, Ca2+ and Mn2+. Here, the authors showed that the addition all induced pellet‐like growth. Ca2+ supplementation led to smaller pellets than Mg2+ supplementation and Mn2+ induced pellet formation at much lower levels than Mg2+ and Ca2+. Fuchino et al. [102] observed that osmotic upshifts of sucrose (1.33 osmol kg–1) and NaCl (0.97 osmol kg–1) both caused a significant change in the hyphal growth of Streptomyces coelicolor (no information about the product). After a 2 to 3 h growth arrest due to the osmotic shock, the developed hyphal tips did not grow again, but several new branches emerged from the lateral hyphal wall. The osmotic shock triggered a reprogramming of the cell polarity and a redistribution of the polar growth sites [102].
Similar observations were made by Tesche et al. [8] for the addition of ammonium sulfate to the filamentous actinomycete Actinomadura namibiensis. Here, a salt‐enhanced cultivation was used to increase the production of the pharmaceutically interesting peptide labyrinthopeptin A1 in shaking flask cultures. Among the inorganic salts added to a complex production medium, the addition of 50 mM (NH4)2SO4 resulted in the highest concentration of 325 mg L–1 Labyrinthopeptin A1 and increased up to sevenfold compared to the unsupplemented control after 10 days of cultivation. Additionally, the performance of other salts containing NH4 + and SO4 2‐ (e.g. NH4Cl, K2SO4) was significantly lower than the performance of (NH4)2SO4. The changes in cell morphology of A. namibiensis compared to the unsupplemented control were also quantified by image analysis. A. namibiensis always developed a heterogeneous cell morphology with pellets and loose mycelia. In contrast to the unsupplemented control, the cell morphology of the (NH4)2SO4‐supplemented cultures was characterised by smaller and more spherical pellets, which were more stable against pellet disintegration in the stationary production phase [8].
3. EXPERIMENTAL INVESTIGATIONS FOR THE MICROMECHANICAL CHARACTERISATION OF PELLETS
In the past, extensive experimental investigations for the rheological characterisation of pellet‐based cultivations were carried out [15, 42, 43, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112]. Although it is important to determine the viscous properties of a cultivation medium in order to estimate the power input of a process, it is not possible to conclude on the mechanical stress of an individual pellet or its influence on the pellet morphology without making major system assumptions [92, 113].
So far, the micromechanical behaviour of pellets has mainly been investigated by means of Atomic Force Microscopy (AFM), whereby different growth stages from single hyphae [113, 114, 115, 116] to spores and extracellular matrix adhesion [117, 118, 119, 120, 121, 122, 123, 124, 125] were analysed. Although the mechanical behaviour of individual pellets is of special interest for a general understanding of pellet mechanics, i.e. the understanding of load transfer mechanisms inside the pellet, as well as for biorector and stirrer development and optimisation, only one study exists which deals with the experimental sampling of pellets [92]. In this study, the authors investigate the influence of salt‐enhanced cultivation on the mechanical behaviour filamentous pellets (Aspergillus niger and Actinomadura namibiensis) by realising cyclic compression experiments. It is well‐known, that pellet growth strongly depends on the cultivation conditions, which was also shown in this study, see Figure 3.
FIGURE 3.
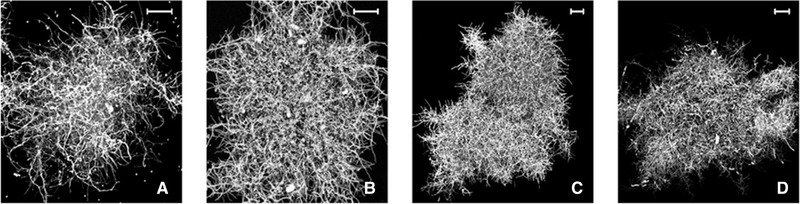
Morphological forms (maximum intensity projections) of pellets and hyphae in a salt‐enhanced cultivation of (A) A. niger and 0 mM NaCl, (B) A. niger and 500 mM NaCl, (C) A. namibiensis and 0 mM (NH4)2SO4, and (D) A. namibiensis and 50 mM (NH4)2SO4, recorded by confocal laser scanning microscopy (scale bar 25 μm) [92] (© Copyright 2019 Elsevier B.V.)
For the realisation of the compression tests, the authors used a custom‐made, micromechanical experimental apparatus, embedded in an inverted microscope. The used setup allowed the measurement of pellets of various sizes and fluffiness, cf. Figure 4. Generally, the pellets showed a nonlinear, dissipative material response with plastic deformations after cyclic loading histories. Further, the use of the strains A. niger and A. namibiensis under various cultivation conditions led to a similar qualitative, mechanical behaviour. However, clear differences became apparent in the maximum forces and standard deviations achieved for the measurements [92].
FIGURE 4.
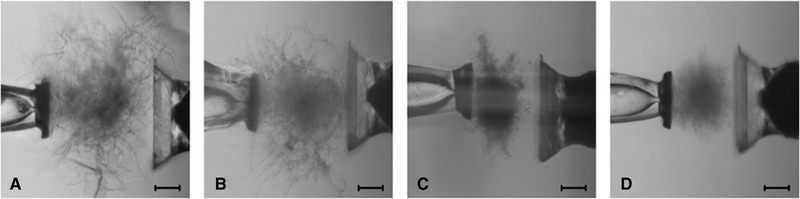
Pellets analysed within Dittmann et al. [92]: (A) A. niger and 0 mM NaCl, (B) A. niger and 500 mM NaCl, (C) A. namibiensis and 0 mM (NH4)2SO4, and (D) A. namibiensis and 50 mM (NH4)2SO4 (scale bar: 100 μm) (© Copyright 2019 Elsevier B.V.)
These investigations are particularly relevant for the quantification of forces that occur when the pellets collide with the stirrer, the reactor walls or suspended macroparticles and thereby affect the pellet morphology. Consequently, to achieve the highest possible productivity from a process engineering point of view, the mechanical properties of the pellets must lie within the correct process window of mechanical stress. On the one hand, the pellets have to be stable against mechanical loading and on the other hand, they must be open‐pored enough to be fully supplied with oxygen (in case of aerobic cultivation) so that no material restrictions occur. Additional experimental investigations and predictive modelling strategies are necessary in this area.
However, experimental studies at the pellet level are of outstanding importance as they provide key information for a deeper mechanical understanding from a process‐engineering point of view.
4. FLUIDMECHANICAL MODELS TO DESCRIBE THE PELLET MORPHOLOGY AND ITS CHANGES
Although the interaction between pellet morphology and mechanical stress is a key essential for the productivity of filamentous biological systems, even after intensive literature research no pellet‐specific models could be found that take into account stress‐induced morphological changes. Many of the existing pellet‐specific models focus on the formation of pellets from single vegetative hyphae or spores and aim to predict the development of inocula in mycelium‐ or pellet‐like cultures [126, 127, 128, 129, 130, 131, 132, 133]. In approaches to predict biomass concentration, substrate uptake, and product formation in ideally mixed bioreactors, pellets are often idealised as spherical, porous particles, growing due to metabolic processes [15, 37]. To this end, a constant density and a radius that changes over time describe the morphology of a pellet. In case substrate limitations occur, the change in radius over time is first approximated by the thickness of the outer active pellet layer in which the nutrient substrates are consumed [6], leading to the so‐called cubic root law, where exponential biomass growth takes place exclusively in the active, substrate‐supplied layer. Cui et al. [134] enhanced the description of a pellet as a porous sphere with a substrate‐limited core by a pellet density that varies linearly with the cultivation time.
However, in practice, pellets have an almost spherical geometry and a spherically symmetrical density distribution. Morphological investigations on equatorial sections have also shown that the filament distribution and the mass density within a pellet can vary considerably radially [25, 92, 135]. In addition, recent microscopic measurements confirm that the pellet density can vary significantly depending on the radius, see Figure 5. These observations contradict the assumption of a constant pellet density and suggest that spatially inhomogeneous diffusion coefficients and substrate uptake rates need also to be taken into account. Such inhomogeneities influence the pellet internal substrate transport and have a major impact on local substrate limitations and hyphal vitality.
FIGURE 5.
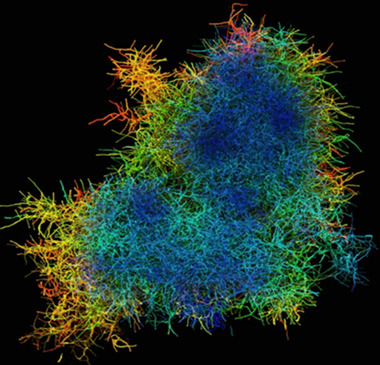
Depth‐coded representation of a filamentous biopellet of Actinomadura namibiensis. Note, the colour coding provides information about the position of the hyphae; blue hyphae are close to the microscope objective, red ones are further away
Summarising, new modelling approaches are necessary to resolve these morphological inhomogeneities and their temporal changes and thus to achieve an improved understanding of mechanically induced changes in pellet morphology. These approaches would have to consider the external loads acting on the individual pellet in order to be able to use them independently of a specific reactor configuration or given operating cultivation conditions for the analysis of the pellet morphology distributions and the prediction of product formation.
4.1. Multiphase flows in aerobic cultivation
Nutrient media in which pellets are aerobically cultivated are dispersed multiphase systems characterised by intensive coupling at the mechanical and biochemical level. In order to achieve an almost homogeneous dispersion of the pellets and to compensate for gradients of substrate and metabolic products, the cultivation broth is stirred under oxygen supply in industrial stirred tank bioreactors, whereas in bubble column and airlift reactors the mixing takes place exclusively through the pneumatic energy input via the gas phase.
The larger the bioreactor and the more local the energy input for homogenisation of the cultivation broth, the more difficult homogeneous mixing becomes [136]. With a focus on scaling bioreactors from the laboratory scale to industrial sizes, most of the previous research on spatially resolved models has focused on predicting biomass distribution in the bioreactor and productivity for a nominal metabolic kinetics. Mostly planktonic bacterial systems that follow the path lines of the flow and do not collide with each other have been considered [137, 138, 139].
In the case of filamentous pellet cultures, two challenges arise that have not yet been addressed in the literature. On the one hand, pellets grow to diameters larger than 500 μm [92], so that their trajectories deviate from the flow path lines and inertia effects influence the momentum exchange between the pellets and the turbulent flow. On the other hand, the mechanical forces exerted on the pellets by the turbulent flow can cause morphological changes and thus change the productivity of the pellets [17]. The mechanical forces reflect the intermittency of the velocity field to which the pellets are exposed.
The culture medium in stirred or shaken cultures is called continuous phase and can be described by the continuity equation and Euler's balance equations for momentum, energy, and mass transport. Depending on the description of the disperse phase, a distinction is made between Euler‐Euler and Euler‐Lagrange approaches. In Euler‐Euler models, the continuous and particulate phases are regarded as interpenetrating continua, each characterised by a volume fraction [140, 141, 142]. Continuity and momentum equations are also formulated for the particulate phase, which can be supplemented by transport equations for the mean properties of the particles, such as mean size. These equations represent the zero and first order moments of a population balance equation (PBE) for the property distribution of the particles at a given position and at a fixed time. The Euler‐Euler method is also known as the two‐fluid method because the continuous and particulate phases behave similarly to two coexisting fluids [143]. Generalis and Cartland Glover [144] used an Euler‐Euler formulation to study the interactions between the gas phase and monodisperse pellets of A. niger in a turbulent bubble column reactor for citric acid production. The k‐ε model was used to represent the momentum interactions between the disperse phases and the continuous phase of the liquid culture medium. Elqotbi et al. [145] analysed a laminar stirred reactor with bubble aeration in which A. niger produced gluconic acid in mycelium morphology. The mycelium fraction was modelled as a chemical component of the culture medium which causes an increase in viscosity.
A disadvantage of Euler‐Euler formulations is that the basic equations contain mathematical and physical approximations for whose validity and effects our understanding is still limited [146]. Furthermore, Euler‐Euler methods aim at predicting quantities that are averaged over the particle population at a given location. An alternative are Euler‐Lagrange methods in which a separate equation of motion is solved for each particle and the kinetic rates at which the properties of the particles change contribute to changes in the continuous phase. These methods are very well suited for polydisperse particles, whose inertia is variable and whose trajectories can deviate from the path lines of the flow and cross each other [147]. The change in the characteristic particle properties reflects the temporal intermittency of, e.g. resistivity or medium composition. For monodisperse yeast cultures in a stirred tank reactor an Euler‐Lagrange formulation of Lapin et al. [137] was presented and coupled with a detailed metabolic mechanism for glycolysis. In a follow‐up article [138] the authors adapted this formulation to culture broths of Escherichia coli, whose intracellular metabolism is controlled by glucose uptake.
A challenge in Euler‐Lagrange approaches for turbulent flows is to reconstruct the instantaneous flow velocity at the position of a particle from the Reynolds‐averaged velocity. For this purpose, the mean flow velocity is extended by a fluctuating component derived from the vortex viscosity [148]. The extended equations of motion represent a stochastic process, the realisations of which correspond to samples from the averaged property distribution of the particulate phase at a certain position. The property distribution can be described by an averaged PBE and the stochastic equations of motion of the particles correspond to a statistically equivalent reformulation of the PBE [149].
Even after intensive literature research, it has been shown that in the existing models for predicting the productivity of pellet cultures, the pellet morphology is assumed to be constant and the influence of morphological changes and the associated metabolic and mechanical changes are excluded.
4.2. Modelling of shaking reactors—influence of free surfaces
In shaking flask reactors, oxygen is usually introduced exclusively via the free surface. The shape of the free surface changes over time and influences the shape and position of the flow area in which the pellets are dispersed. Free surfaces exert a dissipative influence on turbulent flows, which dampens the fluctuations in the velocity field [150]. Previous studies on flow conditions and flow properties in shaking flasks used a Volume Of Fluid (VOF) formulation combined with a strategy to reconstruct the free surface between gas and liquid [151, 152, 153]. The VOF method is based on the idea to extend the area for physical modelling to the whole shaking flask and to indicate the phase at a certain location by an indicator field, which varies between 0 (liquid) and 1 (gas) and is transported with the current velocity field. At the interface between the two phases, the indicator field assumes intermediate values which can be used to approximately reconstruct the shape of the interface. Zhang et al. [151] used a VOF formulation to investigate the oxygen transport from the gas phase into the culture liquid of a shaking flask without flow baffles and to quantify the specific power input. This approach was later supplemented by the RNG k‐ε‐turbulence model [150] and applied in a recently published paper [154]. Liu et al. [153] derived a threshold value for the fluid mechanical shear stresses by means of VOF simulation, above which the specific growth rate of plant cells in a shaking flask decreases. In experimental investigations on pellet cultures of filamentous bacteria (L. aerocolonigenes) it was observed that the addition of macroscopic glass spheres in shaking flasks can increase product synthesis many times over [54]. One possible explanation is that the pellets are structurally altered in collisions with glass spheres or under the influence of the velocity field altered by the glass spheres, thereby increasing their porosity. This in turn facilitates the transport of oxygen within the pellets and leads to an intensification of the metabolism inside the pellets. To support this hypothesis, Schrader et al. [55] used a VOF‐DEM model to analyse the mean kinetic energy that can be transmitted by dispersed glass spheres in collisions and derived a measure for the stress energy in the dispersion. In the case of fixed mass occupancy, the stress energy increases with the size of the glass sphere. Even though the investigations here show that an optimal stress energy exists for which the pellets are porous without fragmenting, and pellet productivity is highest, the exact mechanism of pellet loosening remains unexplained, except for conjecture.
However, common feature of the above mentioned VOF‐based models is that the dispersed pellets were not represented and the mean energy dissipation rate in the flow [153] or the total stress energy [53] was interpreted as a direct measure of the pellet loads. On the one hand, the intermittency of the loads was not considered. On the other hand, the forces exerted by the flow on the pellets change very much with the size and morphology of the pellets.
5. CHALLENGES FOR FUTURE RESEARCH WITH FILAMENTOUS MICROORGANISMS
The huge efforts in quantitative characterisation and tailoring of filamentous cell morphology have shown an evident interrelationship between the operating environment of the bioprocess, productivity, and metabolic properties of the individual bio‐agglomerate (mycelium, clump, and pellet). Nevertheless, the reproducibilities in product titers or cell morphologies to be optimally adjusted via morphology engineering techniques are still very unsatisfactory when cultivating filamentous microorganisms. Closing the gap between the intregral metabolic parameters, small‐scale processes at the pellet level, and the interconnections and transformation across the scales up to the process level remains the most interesting aspect for future research work.
Many existing modelling approaches for describing and predicting the viability and productivity of filamentous pellet cultures are linked to a specific bioreactor system and defined operating and cultivation conditions. Thus, in practice, reaction kinetics are almost without exception averaged over the entire heterogeneous pellet population, even if the individual pellets have very different cell morphologies and thus contribute differently to overall productivity. Therefore, the aim of future research activities in this field should be to develop a transferable, strain‐, genus‐family‐, or product‐type related models to describe the productivity of disperse pellet cultures and to predict process‐relevant properties [30, 43]. For this purpose, integral observation variables such as the mean oxygen‐, biomass‐, or product‐concentration can be traced back to physical processes taking place at the pellet level. The focus on small‐scale processes will make it possible to predict pellet growth, morphological characteristics, and production behaviour for any reactor geometry and operating conditions. In order to bridge the scales between pellet and the bioreactor, a turbulent flow model can be combined with a point particle‐method. The properties of the pellets change due to mechanical and chemical interactions with the surrounding culture medium. By means of special experimental investigations, kinetic rate expressions for these interactions can then be derived, calibrated, and validated.
Still this field is far from understanding the underlying metabolic and regulatory mechanisms. Experimental and computational technologies in systems biotechnology; however, will help to step towards a better understanding of this complex link between the biological and engineering aspects of filamentous microorganisms.
6. CONCLUSION AND FUTURE PERSPECTIVES
Due to their ability to produce a wide variety of bioactive substances, many eukaryotic and prokaryotic filamentous microorganisms (e.g. fungi and actinomycetes) are of great interest to the biotech‐related industry. The cell morphology of filamentous microorganisms plays a major role regarding their productivity and can be influenced in different ways. Beside parameters such as pH value, temperature, cultivation medium composition, dissolved oxygen, viability/vitality of the inoculum, inoculum concentration, power input, and mechanical stress, the most important proven technology of morphology engineering is the addition of micro‐ and macroparticles, the microparticle‐ and macroparticle‐enhanced cultivation, and the change of osmolality of the cultivation medium by adding inorganics salts, the salt‐enhanced cultivation. These techniques have already proven useful and now await further proof‐of‐concept ‐ a promising starting point for the next level of filaments in bioprocesses technology.
The mechanical behaviour of individual pellets is of special interest for a general understanding of pellet mechanics. Correlating it with the substrate uptake and finally to the productivity of the filamentous system would be a breakthrough for the comprehensive characterisation of filamentous systems which should not be underestimated. Up to now, this research field is under‐represented. Such investigations are particularly relevant for the quantification of forces that occur when the pellets interact with reactor internals (e.g. stirrer, baffles, reactor wall) or suspended particles and thereby affect the pellet morphology. Additionally, experimental studies at the pellet level are of high importance as they provide key information for the productivity of filamentous biological systems and a deeper mechanical understanding from a process‐engineering point of view.
Summarising, new experimental and modelling approaches are necessary to resolve morphological inhomogeneities and their temporal changes and thus to achieve an improved understanding of mechanically induced changes in pellet morphology. Such approaches would have to consider the external loads acting on the individual pellet in order to be able to use them for the analysis of the pellet morphology distributions and the prediction of product formation independently of a specific reactor configuration or given operating cultivation conditions. In addition it has been shown that in the existing models for predicting the productivity of pellet cultures, the pellet morphology is assumed to be constant and the influence of morphological changes and the associated metabolic and mechanical changes are excluded. Here, additional experimental investigations and predictive modelling strategies are necessary.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
ACKNOWLEDGMENTS
RK and KS acknowledge the financial support provided by the German Research Foundation (DFG) in the Priority Programme 1934 DiSPBiotech – Dispersity, structural and phase modifications of proteins and biological agglomerates in biotechnological processes (SPP 1934 DiSPBiotech, project number 315457657). Additionally, RK and ST acknowledge financial support from the Lower Saxony Ministry for Science and Culture for a PhD scholarship in the joint research project Processing of poorly soluble drugs at small scale (μ‐PROPS) within the Center of Pharmaceutical Engineering (PVZ) at the Technische Universität Braunschweig, Germany.
Open access funding enabled and organized by Projekt DEAL.
Böl M, Schrinner K, Tesche S, Krull R. Challenges of influencing cellular morphology by morphology engineering techniques and mechanical induced stress on filamentous pellet systems—A critical review. Eng Life Sci. 2021;21:51–67. 10.1002/elsc.202000060
This article is dedicated to Prof. Thomas Bley on the occasion of his 70th birthday.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Ward, M. , Lin, C. , Victoria, D. C. , Fox, B.P. et al., Characterization of humanized antibodies secreted by Aspergillus niger . Appl. Env. Microbiol. 2004, 70, 2567–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papagianni, M. , Advances in citric acid fermentation by Aspergillus niger: biochemical aspects, membrane transport and modeling. Biotechnol. Adv. 2007, 25, 244–263. [DOI] [PubMed] [Google Scholar]
- 3. Sauer, M. , Porro, D. , Mattanovich, D. , Branduardi, P. , Microbial production of organic acids: expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [DOI] [PubMed] [Google Scholar]
- 4. Meyer, V. , Genetic engineering of filamentous fungi — Progress, obstacles and future trends. Biotechnol. Adv. 2008, 26, 177–185. [DOI] [PubMed] [Google Scholar]
- 5. Lubertozzi, D. , Keasling, J. D. , Developing Aspergillus as a host for heterologous expression. Biotechnol. Adv. 2009, 27, 53–75. [DOI] [PubMed] [Google Scholar]
- 6. Nielsen, J. , Modelling the morphology of filamentous microorganisms. Trends Biotechnol. 1996, 14, 438–443. [Google Scholar]
- 7. Pommerehne, K. , Walisko, J. , Ebersbach, A. , Krull, R. , The antitumor antibiotic rebeccamycin ‐ Challenges and advanced approaches in production processes. Appl. Microbiol. Biotechnol. 2019, 103, 3627–3636. [DOI] [PubMed] [Google Scholar]
- 8. Tesche, S. , Rösemeier‐Scheumann, R. , Lohr, J. , Hanke, R. et al., Salt‐enhanced cultivation as morphology engineering tool for filamentous actinomycetes: Increased production of labyrinthopeptin A1 in Actinomadura namibiensis . Eng. Life Sci. 2019, 19, 781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papagianni, M. , Mattey, M. , Morphological development ofAspergillus niger in submerged citric acid fermentation as a function of the spore inoculum level. Application of neural network and cluster analysis for characterization of mycelial morphology. Microb. Cell Fact. 2006, 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahamed, A. , Vermette, P. , Effect of culture medium composition on Trichoderma reesei's morphology and cellulase production. Bioresour. Technol. 2009, 100, 5979–5987. [DOI] [PubMed] [Google Scholar]
- 11. Driouch, H. , Roth, A. , Dersch, P. , Wittmann, C. , Optimized bioprocess for production of fructofuranosidase by recombinant Aspergillus niger . Appl. Microbiol. Biotechnol. 2010, 87, 2011–2024. [DOI] [PubMed] [Google Scholar]
- 12. Driouch, H. , Hänsch, R. , Wucherpfennig, T. , Krull, R. et al., Improved enzyme production by bio‐pellets of Aspergillus niger: Targeted morphology engineering using titanate microparticles. Biotechnol. Bioeng. 2012, 109, 462–471. [DOI] [PubMed] [Google Scholar]
- 13. Babič, J. , Pavko, A. , Enhanced enzyme production with the pelleted form of D. squalens in laboratory bioreactors using added natural lignin inducer. J. Ind. Microbiol. Biotechnol. 2012, 39, 449–457. [DOI] [PubMed] [Google Scholar]
- 14. Wucherpfennig, T. , Lakowitz, A. , Driouch, H. , Krull, R. et al., Customization of Aspergillus niger morphology through addition of talc micro particles. J. Vis. Exp. 2012, 61, e4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wucherpfennig, T. , Kiep, K. A. , Driouch, H. , Wittmann, C. et al., Morphology and rheology in filamentous cultivations. Adv. Appl. Microbiol. 2010, 72, 89–136. [DOI] [PubMed] [Google Scholar]
- 16. Metz, B. , Kossen, N. W. F. , The growth of molds in the form of pellets – a literature review. Biotechnol. Bioeng. 1977, 19, 781–799. [Google Scholar]
- 17. Papagianni, M. , Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–295. [DOI] [PubMed] [Google Scholar]
- 18. Grimm, L. H. , Kelly, S. , Krull, R. , Hempel, D.C. , Morphology and productivity of filamentous fungi. Appl. Microbiol. Biotechnol. 2005, 49, 375–384, [DOI] [PubMed] [Google Scholar]
- 19. Schrinner, K. , Veiter, L. , Schmideder, S. , Doppler, P. et al., Morphological and physiological characterization of filamentousLentzea aerocolonigenes: Comparison of biopellets by microscopy and flow cytometry. PLoS One 2020, 15, e0234125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krull, R. , Wucherpfennig, T. , Esfandabadi, M.E. , Walisko, R. et al., Characterization and control of fungal morphology for improved production performance in biotechnology. J. Biotechnol. 2013, 163, 112–123. [DOI] [PubMed] [Google Scholar]
- 21. Veiter, L. , Rajamanickam, V. , Herwig, C. , The filamentous fungal pellet‐relationship between morphology and productivity. Appl. Microbiol. Biot. 2018, 102, 2997–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zacchetti, B. , Wösten, H. A. B. , Claessen, D. , Multiscale heterogeneity in filamentous microbes. Biotechnol. Adv. 2018, 36, 2138–2149. [DOI] [PubMed] [Google Scholar]
- 23. Lin, P. J. , Grimm, L. H. , Wulkow, M. , Hempel, D.C. , et al., Population balance modeling of the conidial agglomeration of Aspergillus niger . Biotechnol. Bioeng. 2008, 99, 341–350. [DOI] [PubMed] [Google Scholar]
- 24. Zacchetti, B. , Smits, P. , Claessen, D. , Dynamics of pellet fragmentation and aggregation in liquid‐grown cultures of Streptomyces lividans . Front. Microbiol. 2018, 9, 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hille, A. , Neu, T.R. , Hempel, D.C. , Horn, H. , Oxygen profiles and biomass distribution in biopellets of Aspergillus niger . Biotechnol. Bioeng. 2005, 92, 614–623. [DOI] [PubMed] [Google Scholar]
- 26. Nieminen, L. , Webb, S. , Smith, M. C. M. , Hoskisson, P. A. , A flexible mathematical model platform for studying branching networks: experimentally validated using the model actinomycete, Streptomyces coelicolor . PLoS One 2013, 8, e54316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Veiter, L. , Herwig, C. , The filamentous fungus Penicillium chrysogenum analysed via flow cytometry ‐ a fast and statistically sound insight into morphology and viability. Appl. Microbiol. Biot. 2019, 103, 6725–6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hille, A. , Neu, T.R. , Hempel, D.C. , Horn, H. , Effective diffusivities and mass fluxes in fungal biopellets. Biotechnol. Bioeng. 2009, 103, 1202–1213. [DOI] [PubMed] [Google Scholar]
- 29. Lin, P. J. , Scholz, A. , Krull, R. , Effect of volumetric power input by aeration and agitation on pellet morphology and product formation of Aspergillus niger. Biochem. Eng. J. 2010, 49, 213–220. [Google Scholar]
- 30. Schmideder, S. , Barthel, L. , Müller, H. , Meyer, V. , et al., From three‐dimensional morphology to effective diffusivity in filamentous fungal pellets. Bioeng. Biotechnol. 2019, 116, 3360–3371. [DOI] [PubMed] [Google Scholar]
- 31. Bizukojc, M. , Gonciarz, J. , Influence of oxygen on lovastatin biosynthesis by Aspergillus terreus ATCC 20542 quantitatively studied on the level of individual pellets. Bioproc. Biosyst. Eng. 2015, 38, 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phillips, D. H. , Oxygen transfer into mycelial pellets. Biotechnol. Bioeng. 1966, 8, 456–460. [DOI] [PubMed] [Google Scholar]
- 33. Elmayergi, H. , Scharer, J. M. , Moo‐Young, M. , Effects of polymer additives on fermentation parameters in a culture of A. niger . Biotechnol. Bioeng. 1973, 15, 845–859. [Google Scholar]
- 34. Walisko, R. , Moench‐Tegeder, J. , Blotenberg, J. , Wucherpfennig, T. et al., The taming of the shrew – controlling the morphology of filamentous eukaryotic and prokaryotic microorganisms. Adv. Biochem. Eng./Biotechnol. 2015, 149, 1–27. [DOI] [PubMed] [Google Scholar]
- 35. Kieser, T. , Bibb, M. J. , Buttner, M. J. , Chater, K. F. et al., Practical Streptomyces Genetics. John Innes Foundation, Norwich, 2000. [Google Scholar]
- 36. Olmos, E. , Mehmood, N. , Haj Husein, L. , Goergen, J. L. et al., Effects of bioreactor hydrodynamics on the physiology of Streptomyces. Bioproc. Biosyst. Eng. 2013, 36, 259–272. [DOI] [PubMed] [Google Scholar]
- 37. Krull, R. , Hempel, D. C. , Wucherpfennig, T. , Bioverfahrenstechnik, in: Grote, K. H. , Bender, B. , Göhlich, D. (Eds.), Dubbel – Taschenbuch für den Maschinenbau, 25. Auflage, Springer Vieweg, Berlin: 2018, pp. N36–N58. [Google Scholar]
- 38. Oncu, S. , Tari, C. , Unluturk, S. , Effect of various process parameters on morphology, rheology, and polygalacturonase production by Aspergillus sojae in a batch bioreactor. Biotechnol. Prog. 2007, 23, 836–845. [DOI] [PubMed] [Google Scholar]
- 39. Reichl, U. , King, R. , Gilles, E. D. , Characterization of pellet morphology during submerged growth of Streptomyces tendae by image analysis. Biotechnol. Bioeng. 1992, 39, 164–170. [DOI] [PubMed] [Google Scholar]
- 40. Zhou, Y. , Du, J. , Tsao, G. , Mycelial pellet formation by Rhizopus oryzae ATCC 20344. Appl. Biochem. Biotechnol. 2000, 84–86, 779–789. [DOI] [PubMed] [Google Scholar]
- 41. Atkinson, B. , Daoud, I. , Microbial flocs and flocculation in fermentation process engineering. Adv. Biochem. Eng./ Biotechnol. 1976, 4, 41–124. [Google Scholar]
- 42. Wucherpfennig, T. , Lakowitz, A. , Krull, R. , Comprehension of viscous morphology ‐ Evaluation of fractal and conventional parameters for rheological characterization of Aspergillus niger culture broth. J. Biotechnol. 2013, 163, 124–132. [DOI] [PubMed] [Google Scholar]
- 43. Bliatsiou, C. , Schrinner, K. , Waldherr, P. , Tesche, S. et al., Rheological characteristics of filamentous cultivation broths and suitable model fluids. Biochem. Eng. J. 2020, 163, 107746. [Google Scholar]
- 44. El‐Enshasy, A. H. , Farid, M. A. , El‐Sayed, A. , Influence of inoculums type and cultivation conditions on natamycin production by Streptomyces natalensis . J. Basic Microbiol. 2000, 40, 333–342. [PubMed] [Google Scholar]
- 45. Rosa, J. C. , Baptista Neto, A. , Hokka, C. O. , Badino, A. C. , Influence of dissolved oxygen and shear conditions on clavulanic acid production by Streptomyces clavuligerus . Bioproc. Biosyst. Eng. 2005, 27, 99–104. [DOI] [PubMed] [Google Scholar]
- 46. Antecka, A. , Bizukojc, M. , Ledakowicz, S. , Modern morphological engineering techniques for improving productivity of filamentous fungi in submerged cultures. World J. Microbiol. Biotechnol. 2016, 32, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krijgsheld, P. , Bleichrodt, R. , van Veluw, G. J. , Wang, F. et al., Development in Aspergillus. Stud. Mycol. 2013, 74, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dobson, L. F. , O'Cleirigh, C. C. , O'Shea, D. G. , The influence of morphology on geldanamycin production in submerged fermentations of Streptomyces hygroscopicus var. geldanus. Appl. Microbiol. Biotechnol. 2008, 79, 859–866. [DOI] [PubMed] [Google Scholar]
- 49. Tamura, S. , Park, Y. , Toriyama, M. , Okabe, M. , Change of mycelial morphology in tylosin production by batch culture of Streptomyces fradiae under various shear conditions. J. Ferment. Bioeng. 1997, 83, 523–528. [Google Scholar]
- 50. Gomez, R. , Schnabel, I. , Garrido, J. , Pellet growth and citric acid yield of Aspergillus niger 110. Enzyme Microb. Technol. 1988, 10, 188–191. [Google Scholar]
- 51. Hermersdörfer, H. , Leuchtenberger, A. , Wardsack, C. , Ruttloff, H. , Influence of culture conditions on mycelial structure and polygalacturonase synthesis of Aspergillus niger . J. Basic Microbiol. 1987, 27, 309–315. [DOI] [PubMed] [Google Scholar]
- 52. Vecht‐Lifshitz, S. E. , Sasson, Y. , Braun, S. , Nikkomycin production in pellets of Streptomyces tendae . J. Appl. Bacteriol. 1992, 72, 195–200. [DOI] [PubMed] [Google Scholar]
- 53. Yin, P. , Wang, Y. H. , Zhang, S.L. , Chu, J. et al., Effect of mycelial morphology on bioreactor performance and avermectin production of Streptomyces avermitilis in submerged cultivations. J. Chin. Inst. Chem. Eng. 2008, 39, 609–615. [Google Scholar]
- 54. Walisko, J. , Vernen, F. , Pommerehne, K. , Richter, G. et al., Particle‐based production of antibiotic rebeccamycin with Lechevalieria aerocolonigenes . Process Biochem. 2017, 53, 1–9. [Google Scholar]
- 55. Schrader, M. , Pommerehne, K. , Wolf, S. , Finke, B. et al., Design of a CFD‐DEM‐based method for mechanical stress calculation and its application to glass bead‐enhanced cultivations of filamentous Lentzea aerocolonigenes . Biochem. Eng. J. 2019, 148, 116–130. [Google Scholar]
- 56. Belmar‐Beiny, M. T. , Thomas, C. R. , Morphology and clavulanic acid production ofStreptomyces clavuligerus: effect of stirrer speed in batch fermentations. Biotechnol. Bioeng. 1991, 37, 456–462. [DOI] [PubMed] [Google Scholar]
- 57. Yang, Y. K. , Morikawa, M. , Shimizu, H. , Shioya, S. et al., Image analysis of mycelial morphology in virginiamycin production by batch culture of Streptomyces virginiae . J. Ferment. Bioeng. 1996, 81, 7–12. [Google Scholar]
- 58. Krull, R. , Cordes, C. , Horn, H. , Kampen, I. et al., Morphology of filamentous fungi ‐ Linking cellular biology to process engineering using Aspergillus niger . Adv. Biochem. Eng./ Biotechnol. 2010, 121, 1–21. [DOI] [PubMed] [Google Scholar]
- 59. Tough, A. J. , Prosser, J. I. , Experimental verification of a mathematical model for pelleted growth of Streptomyces coelicolor A3(2) in submerged batch culture. Microbiol. 1996, 142 (Pt 3), 639–648. [DOI] [PubMed] [Google Scholar]
- 60. Bellgardt, K. H. , Process models for production of β‐lactam antibiotics, in: Schügerl, K. (Ed.), Relation Between Morphology and Process Performances, Springer, Berlin, Heidelberg, 1998, pp. 153–194. [Google Scholar]
- 61. Clark, D. S. , Lentz, C. P. , Submerged citric acid fermentation of beet molasses in tank‐type fermenters. Biotech. Bioeng. 1963, 5, 193–199. [Google Scholar]
- 62. Ayazi Shamlou, P. , Makagiansar, H. Y. , Ison, A. P. , Lilliy, D. et al., Turbulent breakage of filamentous micro‐organisms in submerged culture in mechanically stirred bioreactors. Chem. Eng. Sci. 1994, 49, 2621–2631. [Google Scholar]
- 63. Fujita, M. , Iwahori, K. S. T. , Yanakawa, K. , Analysis of pellet formation of Aspergillus niger based on shear‐stress. J. Fermet. Bioeng. 1994, 78, 368–373. [Google Scholar]
- 64. Kaup, B. A. , Ehrich, K. , Pescheck, M. , Schrader, J. , Microparticle‐enhanced cultivation of filamentous microorganisms. Increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol. Bioeng. 2008, 99, 491–498. [DOI] [PubMed] [Google Scholar]
- 65. Driouch, H. , Sommer, B. , Wittmann, C. , Morphology engineering of Aspergillus niger for improved enzyme production. Biotech. Bioeng. 2010, 105, 1058–1068. [DOI] [PubMed] [Google Scholar]
- 66. Wucherpfennig, T. , Hestler, T. , Krull, R. , Morphology engineering ‐ osmolality and its effect on Aspergillus niger morphology and productivity. Microb. Cell Fact. 2011, 11, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gonciarz, J. , Bizukojc, M. , Adding talc microparticles to Aspergillus terreus ATCC 20542 preculture decreases fungal pellet size and improves lovastatin production. Eng. Life Sci. 2014, 14, 190–200. [Google Scholar]
- 68. Krull, R. , Bley, T. (Vol. Eds.), Filaments in Bioprocesses, Adv. Biochem. Eng./ Biotechnol., Vol. 149, Scheper, T. (Series Ed.), Springer Cham Heidelberg New York Dordrecht London, Springer International Publishing Switzerland, 2015. [Google Scholar]
- 69. Antecka, A. , Blatkiewicz, M. , Bizukojć, M. , Ledakowicz, S. , Morphology engineering of basidiomycetes for improved laccase biosynthesis. Biotechnol. Lett. 2016, 38, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Walisko, R. , Krull, R. , Schrader, J. , Wittmann, C. , Microparticle based morphology engineering of filamentous microorganisms for industrial bio‐production. Biotechnol. Lett. 2012, 34, 1975–1982. [DOI] [PubMed] [Google Scholar]
- 71. Karahalil, E. , Demirel, F. , Evcan, E. , Germeç, M. et al., Microparticle‐enhanced polygalacturonase production by wild type Aspergillus sojae . 3 Biotech 2017, 7, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Driouch, H. , Roth, A. , Dersch, P. , Wittmann, C. , Filamentous fungi in good shape: microparticles for tailor‐made fungal morphology and enhanced enzyme production. Bioengineered bugs 2011, 2, 100–104. [DOI] [PubMed] [Google Scholar]
- 73. Etschmann, M. M. W. , Huth, I. , Walisko, R. , Schuster, J. et al., Improving 2‐phenylethanol and 6‐pentyl‐α‐pyrone production with fungi by microparticle‐enhanced cultivation (MPEC). Yeast 2015, 32, 145–157. [DOI] [PubMed] [Google Scholar]
- 74. Niu, K. , Wu, X. P. , Hu, X. L. , Zou, S. P. et al., Effects of methyl oleate and microparticle‐enhanced cultivation on echinocandin B fermentation titer. Bioproc. Biosyst Eng. 2020, 43, 2009–2015. [DOI] [PubMed] [Google Scholar]
- 75. Ren, X. D. , Xu, Y. J. , Zeng, X. , Chen, X. S. et al., Microparticle‐enhanced production of ε‐poly‐ l ‐lysine in fed‐batch fermentation. RSC Adv. 2015, 5, 82138–82143. [Google Scholar]
- 76. Gonciarz, J. , Kowalska, A. , Bizukojc, M. , Application of microparticle‐enhanced cultivation to increase the access of oxygen to Aspergillus terreus ATCC 20542 mycelium and intensify lovastatin biosynthesis in batch and continuous fed‐batch stirred tank bioreactors. Biochem. Eng. J. 2016, 109, 178–188. [Google Scholar]
- 77. Yatmaz, E. , Karahalil, E. , Germec, M. , Ilgin, M. et al., Controlling filamentous fungi morphology with microparticles to enhanced β‐mannanase production. Bioproc. Biosyst. Eng. 2016, 39, 1391–1399. [DOI] [PubMed] [Google Scholar]
- 78. Germec, M. , Yatmaz, E. , Karahalil, E. , Turhan, İ. , Effect of different fermentation strategies on β‐mannanase production in fed‐batch bioreactor system. 3 Biotech 2017, 7, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kowalska, A. , Antecka, A. , Owczarz, P. , Bizukojć, M. , Inulinolytic activity of broths of Aspergillus niger ATCC 204447 cultivated in shake flasks and stirred tank bioreactor. Eng. Life Sci. 2017, 17, 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dong, M. , Wang, S. , Xu, F. , Li, Q. et al., Addition of aluminum oxide microparticles toTrichoderma viride My preculture enhances cellulase production and influences fungal morphology. Eng. Life Sci. 2018, 18, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tao, T. L. , Cui, F. J. , Chen, X. X. , Sun, W. J. et al., Improved mycelia and polysaccharide production of Grifola frondosa by controlling morphology with microparticle Talc. Microb. Cell Fact. 2018, 17, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Boruta, T. , Bizukojc, M. , Application of aluminum oxide nanoparticles inAspergillus terreus cultivations: Evaluating the effects on lovastatin production and fungal morphology. BioMed. Res. Int. 2019, 5832496, 10.1155/2019/5832496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kowalska, A. , Boruta, T. , Bizukojć, M. , Morphological evolution of various fungal species in the presence and absence of aluminum oxide microparticles. Comparative and quantitative insights into microparticle‐enhanced cultivation (MPEC). MicrobiologyOpen 2018, 6, e00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kowalska, A. , Boruta, T. , Bizukojc, M. , Kinetic model to describe the morphological evolution of filamentous fungi during their early stages of growth in the standard submerged and microparticle‐enhanced cultivations. Eng. Life Sci. 2019, 19, 557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kowalska, A. , Boruta, T. , Bizukojć, M. , Performance of fungal microparticle‐enhanced cultivations in stirred tank bioreactors depends on species and number of process stages. Biochem. Eng. J. 2020, 161, 107696. [Google Scholar]
- 86. Saberi, A. , Jalili, H. , Nikfarjam, A. , Koohsorkhi, J. et al., Monitoring of Aspergillus terreus morphology for the lovastatin production in submerge culture by impedimetry. Biochem. Eng. J. 2020, 159, 107615. [Google Scholar]
- 87. Holtmann, D. , Vernen, F. , Müller, J. M. , Kaden, D. et al., Effects of particle addition to Streptomyces cultivations to optimize the production of actinorhodin and streptavidin. Sustain. Chem. Pharm. 2017, 5, 67–71. [Google Scholar]
- 88. Ochi, K. , Occurrence of the stringent response in Streptomyces sp. and its significance for the initiation of morphological and physiological differentiation. J. Gen. Microbiol. 1986, 132, 2621–2631. [DOI] [PubMed] [Google Scholar]
- 89. Hotop, S. , Möller, J. , Niehoff, J. , Schügerl, K. , Influence of the preculture conditions on the pellet size distribution of Penicillium chrysogenum cultivations. Process. Biochem. 1993, 28, 99–104. [Google Scholar]
- 90. Sohoni, S. V. , Bapat, P. M ,, Lantz, A. E. , Robust, small‐scale cultivation platform for Streptomyces coelicolor . Microb. Cell Fact. 2012, 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Walisko, J. , Morphologiebeeinflussung von Lechevalieria aerocolonigenes und heterologe Produktion von Rebeccamycin, in: Krull, R. (Ed.), ibvt‐Schriftenreihe, Vol. 78, Cuvillier‐Verlag, Göttingen: 2017, also PhD thesis, Technische Universität Braunschweig, Germany. [Google Scholar]
- 92. Dittmann, J. , Tesche, S. , Krull, R. , Böl, M. , The influence of salt‐enhanced cultivation on the micromechanical behaviour of filamentous pellets. Biochem. Eng. J. 2019, 148, 65–76. [Google Scholar]
- 93. Kempf, B. , Bremer, E. , Uptake and synthesis of compatible solutes as microbial stress responses to high‐osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [DOI] [PubMed] [Google Scholar]
- 94. Kültz, D. , Osmotic regulation of DNA activity and the cell cycle, in: Cell Mol. Response to stress. Amsterdam, The Netherlands: Elsevier, 1994, 1, pp. 157–179. [Google Scholar]
- 95. Csonka, L. N. , Hanson, A. D. , Prokaryotic osmoregulation: Genetics and physiology. Annu. Rev. Microbiol. 1991, 45, 569–606. [DOI] [PubMed] [Google Scholar]
- 96. Stumpe, S. , Schlösser, A. , Schleyer, M. , Bakker, E.P. , K+ circulation across the prokaryotic cell membrane: K+‐uptake systems. Handb. Biol. Phys. 1996, 2, 473–499. [Google Scholar]
- 97. Braun, S. , Vecht‐Lifshitz, S. E. , Mycelial morphology and metabolite production. Trends Biotechnol. 1991, 9, 63–68. [Google Scholar]
- 98. Allaway, A. E. , Jennings, D. H. , The effect of cations on glucose utilization by, and on the growth of, the fungus Dendryphiella salina . New Phytol. 1971, 70, 511–518. [Google Scholar]
- 99. Bobowicz‐Lassociska, T. , Grajek, W. , Changes in protein secretion of Aspergillus niger caused by the reduction of the water activity by potassium chloride. Acta Biotechnol. 1995, 15, 277–287. [Google Scholar]
- 100. Fiedurek, J. , Effect of osmotic stress on glucose oxidase production and secretion by Aspergillus niger . J. Basic Microbiol. 1998, 38, 107–112. [PubMed] [Google Scholar]
- 101. Okba, A. K. , Ogata, T. , Matsubara, H. , Matsuo, S. et al., Effects of bacitracin and excess Mg2+ on submerged mycelial growth of Streptomyces azureus. J. Ferment. Bioeng. 1998, 86, 28–33. [Google Scholar]
- 102. Fuchino, K. , Flärdh, K. , Dyson, P. , Ausmees, N. , Cell‐biological studies of osmotic shock response in Streptomyces spp. J. Bacteriol. 2017, 199, e00465‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kim, J. H. , Lebeault, J. M. , Reuss, M. , Comparative study on rheological properties of mycelial broth in filamentous and pelleted forms. European Appl. Microbiol. Biotechnol. 1983, 18, 11–16. [Google Scholar]
- 104. Wittler, R. , Matthes, R. , Schügerl, K. , Rheology of Penicillium chrysogenum pellet suspensions. European J. Appl. Microbiol. Biotechnol. 1983, 18, 17–23. [Google Scholar]
- 105. Mitard, A. , Riba, J. P. , Rheological properties of Aspergillus niger pellet suspensions. European J. Appl. Microbiol. Biotechnol. 1986, 25, 245–249. [Google Scholar]
- 106. Olsvik, E. , Kristiansen, B. , Rheology of filamentous fermentations. Biotechnol. Adv. 1994, 12, 1–39. [DOI] [PubMed] [Google Scholar]
- 107. Ruohang, W. , Webb, C. , Effect of cell concentration on the rheology of glucoamylase fermentation broth. Biotechnol. Techniques 1995, 9, 55–58. [Google Scholar]
- 108. Sinha, J. , 0, J. , Pil Park, J. , Hyun Song, C. , et al., Effect of substrate concentration on broth rheology and fungal morphology during exo‐biopolymer production by Paecilomyces japonica in a batch bioreactor. Enzyme Microbial Technol. 2001, 29, 392–399. [Google Scholar]
- 109. Cho, Y. J. , Hwang, H. J. , Kim, S. W. , Song, C. H. et al., Effect of carbon source and aeration rate on broth rheology and fungal morphology during red pigment production by Paecilomyces sinclairii in a batch bioreactor. J. Biotechnol. 2002, 95, 13–23. [DOI] [PubMed] [Google Scholar]
- 110. Casas López, J. L. , Sánchez Pérez, J. A. , Fernández Sevilla, J. M. , Rodríguez Porcel, E. M. et al., Pellet morphology, culture rheology and lovastatin production in cultures of Aspergillus terreus . J. Biotechnol. 2005, 116, 61–77. [DOI] [PubMed] [Google Scholar]
- 111. Rodríguez Porcel, E. M. , Casas López, J. L. , Sánchez Pérez, J. A. , Fernández Sevilla, J. M. , et al. Effects of pellet morphology on broth rheology in fermentations of Aspergillus terreus . Biochem. Eng. J. 2005, 26, 139–144. [Google Scholar]
- 112. Sainz Herrán, N. , Casas López, J. L. , Sánchez Pérez, J. A. , Chisti, Y. , Influence of ultrasound amplitude and duty cycle on fungal morphology and broth rheology of Aspergillus terreus . World J. Microbiol. Biotechnol. 2010, 26, 1409–1418. [Google Scholar]
- 113. Li, Z. J. , Shukla, V. , Wenger, K. , Fordyce, A. et al., Estimation of hyphal tensile strength in production‐scale Aspergillus oryzae fungal fermentations. Biotechnol. Bioeng. 2002, 77, 601–613. [DOI] [PubMed] [Google Scholar]
- 114. Stocks, S. M. , Thomas, C. R. , Viability, strength, and fragmentation of Saccharopolyspora erythraea in submerged fermentation. Biotechnol. Bioeng. 2001, 75, 702–709. [DOI] [PubMed] [Google Scholar]
- 115. Stocks, S. M. , Thomas, C. R. , Strength of mid‐logarithmic and stationary phase Saccharopolyspora erythraea hyphae during a batch fermentation in defined nitrate‐limited medium. Biotechnol. Bioeng. 2001, 73, 370–378. [DOI] [PubMed] [Google Scholar]
- 116. Zhao, L. , Schaefer, D. , Xu, H. , Modi, S. et al. Elastic properties of Aspergillus nidulans studied with atomic force microscopy. Biotechnol. Prog. 2005, 21, 292–299. [DOI] [PubMed] [Google Scholar]
- 117. Zhao, L. , Schaefer, D. , Marten, M. R. , Assessment of elasticity and topography ofAspergillus nidulans spores via atomic force microscopy. Appl. Environ. Microbiol. 2005, 71, 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dague, E. , Alsteens, D. , Latgé, J. P. , Verbelen, C. et al., Chemical force microscopy of single live cells. Nano Lett. 2007, 7, 3026–3030. [DOI] [PubMed] [Google Scholar]
- 119. Dague, E. , Alsteens, D. , Latgé, J. P. , Dufrêne, Y. F. , High‐resolution cell surface dynamics of germinating Aspergillus fumigatus conidia. Biophys. J. 2008, 94, 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dufrêne, Y. F. , Towards nanomicrobiology using atomic force microscopy. Nature Rev. Microbiol. 2008, 6, 674–680. [DOI] [PubMed] [Google Scholar]
- 121. Dufrêne, Y. F. , Atomic force microscopy of fungal cell walls: an update. Yeast 2010, 27, 465–471. [DOI] [PubMed] [Google Scholar]
- 122. Wargenau, A. , Kwade, A. , Determination of adhesion between single Aspergillus niger spores in aqueous solutions using an atomic force microscope. Langmuir 2010, 26, 11071–11076. [DOI] [PubMed] [Google Scholar]
- 123. Fang, T. H. , Kang, S. H. , Hong, Z. H. , Wu, C. D. , Elasticity and nanomechanical response of Aspergillus niger spores using atomic force microscopy. Micron 2012, 43, 407–411. [DOI] [PubMed] [Google Scholar]
- 124. Papi, M. , Maiorana, A. , Bugli, F. , Torelli, R. et al., Detection of biofilm‐grown Aspergillus fumigatus by means of atomic force spectroscopy: Ultrastructural effects of alginate lyase. Microsc. Microanal. 2012, 18, 1088–1094. [DOI] [PubMed] [Google Scholar]
- 125. Maiorana, A. , Papi, M. , Bugli, F. , Torelli, R. et al., A fast and quantitative evaluation of the Aspergillus fumigatus biofilm adhesion properties by means of digital pulsed force mode. Appl. Surface Sci. 2013, 279, 409–415. [Google Scholar]
- 126. Yang, H. , King, R. , Reichl, U. , Gilles, E. D. , Mathematical model for apical growth, septation, and branching of mycelial microorganisms. Biotechnol. Bioeng. 1992, 39, 49–58. [DOI] [PubMed] [Google Scholar]
- 127. Yang, H. , Reichl, U. , King, R. , Gilles, E. D. , Measurement and simulation of the morphological development of filamentous microorganisms. Biotechnol. Bioeng. 1992, 39, 44–48. [DOI] [PubMed] [Google Scholar]
- 128. Meyerhoff, J. , Bellgardt, K. H. , Two mathematical models for the development of a single microbial pellet. Bioproc. Eng. 1995, 12, 315–322. [Google Scholar]
- 129. Lejeune, R. , Baron, G. V. , Simulation of growth of a filamentous fungus in 3 dimensions. Biotechnol. Bioeng. 1997, 53, 139–150. [DOI] [PubMed] [Google Scholar]
- 130. Knudsen, G. R. , Stack, J. P. , Schuhmann, S. O. , Orr, K. et al., Individual‐based approach to modeling hyphal growth of a biocontrol fungus in soil. Phytopathology 2006, 96, 1108–1115. [DOI] [PubMed] [Google Scholar]
- 131. Grimm, L. H. , Kelly, S. , Hengstler, J. , Göbel, A. et al., Kinetic studies on the aggregation of Aspergillus niger conidia. Biotechnol. Bioeng. 2004, 87, 213–218. [DOI] [PubMed] [Google Scholar]
- 132. Grimm, L. H. , Kelly, S. , Völkerding, I. I. , Krull, R. et al., Influence of mechanical stress and surface interaction on the aggregation of Aspergillus niger conidia. Biotechnol. Bioeng. 2005, 92, 879–888. [DOI] [PubMed] [Google Scholar]
- 133. Blackledge, J. M. , Barry, D. J. , Morphological analysis from images of hyphal growth using a fractional dynamic model, in Carr, H. , Grimstead, I. (Eds.), EG UK Theory and Practice of Computer Graphics. Warwick, United Kingdom: Warwick University, 2011, pp. 17–24. 10.21427/3ssf-3335. [DOI] [Google Scholar]
- 134. Cui, Y. Q. , Okkerse, W. J. , van der Lans, R. G. J. M. , Luyben, K. C. A. M. , Modeling and measurements of fungal growth and morphology in submerged fermentations. Biotechnol. Bioeng. 1998, 60, 216–229. [DOI] [PubMed] [Google Scholar]
- 135. Hille, A. , Neu, T. R. , Hempel, D. C. , Horn, H. , Einfluss der Morphologie auf Stofftransport und‐umsatz in Aspergillus niger‐Pellets. Chem. Ing. Tech. 2006, 78, 627–632. [Google Scholar]
- 136. Lencastre Fernandes, R. , Nierychlo, M. , Lundin, L. , Pedersen, A. E. , et al., Experimental methods and modeling techniques for description of cell population heterogeneity. Biotechnol. Adv. 2011, 29, 575–599. [DOI] [PubMed] [Google Scholar]
- 137. Lapin, A. , Müller, D. , Reuss, M. , Dynamic behavior of microbial populations in stirred bioreactors simulated with Euler−Lagrange methods: Traveling along the lifelines of single cells. Ind. Eng. Chem. Res. 2004, 43, 4647–4656. [Google Scholar]
- 138. Lapin, A. , Schmid, J. , Reuss, M. , Modeling the dynamics of E. coli populations in the three‐dimensional turbulent field of a stirred‐tank bioreactor ‐ A structured‐segregated approach. Chem. Eng. Sci. 2006, 61, 4783–4797. [Google Scholar]
- 139. Lapin, A. , Klann, M. , Reuss, M. , Multi‐scale spatio‐temporal modeling: Lifelines of microorganisms in bioreactors and tracking molecules in cells. Adv. Biochem. Eng./ Biotechnol. 2010, 121, 23–43. [DOI] [PubMed] [Google Scholar]
- 140. Anderson, T. B. , Jackson, R. , Fluid mechanical description of fluidized beds. Equations of motion. Ind. Eng. Chem. Fundamen. 1967, 6, 527–539. [Google Scholar]
- 141. van Wachem, B. G. M. , Almstedt, A. E. , Methods for multiphase computational fluid dynamics. Chem. Eng. J. 2003, 96, 81–98. [Google Scholar]
- 142. Eslahpazir, M. , Krull, R. , Krühne, U. , Computational Fluid Dynamics, in: Moo‐Young, M. (ed.), Comprehensive Biotechnology, Third Edition, Vol. 2, Elsevier: Pergamon, 2019, pp. 95–107. [Google Scholar]
- 143. Schmalzriedt, S. , Jenne, M. , Mauch, K. , Reuss, M. , Integration of physiology and fluid dynamics. Adv. Bochem Eng./ Biotechnol. 2003, 80, 19–68. [DOI] [PubMed] [Google Scholar]
- 144. Generalis, S. C. ; Cartland Glover, G. M. , Modelling a biochemical reaction with computational fluid dynamics. International J. Chem. Reactor Eng. 2005, 3, A50. 10.2202/1542-6580.1288. [DOI] [Google Scholar]
- 145. Elqotbi, M. , Montastruc, L. , Vlaev, S. D. , Nikov, I. , CFD stimulation of gluconic acid production in a stirred gas‐liquid fermenter, in: Mixing 2006, 12th European Conference on Mixing Bologna, 2006, Bologna, Italy. [Google Scholar]
- 146. Fox, R. O. , Large‐eddy‐simulafion tools for multiphase Flows. Ann. Rev. Fluid Mechanics 2012, 44, 47–76. [Google Scholar]
- 147. Eaton, J. K. , Two‐way coupled turbulence simulations of gas‐particle flows using point‐particle tracking. International J. Multiphase Flow 2009, 35, 792–800. [Google Scholar]
- 148. Gosman, A. D. , loannides, E. , Aspects of computer simulation of liquid‐fueled combustors. Journal of Energy 1983, 7, 482–490. [Google Scholar]
- 149. Minier, J. P. , On Lagrangian stochastic methods for turbulent polydisperse two‐phase reactive flows. Prog. Energy Combust. Sci. 2015, 50, 1–62. [Google Scholar]
- 150. Berry, J. D. , Liovic, P. , Šutalo, I. D. , Stewart, R. L. et al., Characterisation of stresses on microcarriers in a stirred bioreactor. Applied Mathematical Modelling 2016, 40, 6787–6804. [Google Scholar]
- 151. Zhang, H. , Williams‐Dalson, W. , Keshavarz‐Moore, E. , Ayazi Shamlou, P. A. , Computational‐fluid‐dynamics (CFD) analysis of mixing and gas–liquid mass transfer in shake flasks. Biotechnol. Appl. Biochem. 2005, 41, 1–8. [DOI] [PubMed] [Google Scholar]
- 152. Li, Z. , Song, P. , Adaptive mesh refinement techniques for the immersed interface method applied to flow problems. Computers & Structures 2013, 122, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Liu, Y. , Wang, Z. J. , Zhang, J. W. , Xia, J. Y. et al, Quantitative evaluation of the shear threshold on Carthamus tinctorius L. cell growth with computational fluid dynamics in shaken flask bioreactors. Biochem. Eng. J. 2016, 113, 66–76. [Google Scholar]
- 154. Zhu, L. , Zhang, X. , Cheng, K. , Lv, Z. et al., Characterizing the fluid dynamics of the inverted frustoconical shaking bioreactor. Biotechnol. Prog. 2018, 34, 478–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


