Significance
Disgust likely evolved to regulate exposure to pathogen-related stimuli and behaviors. One key prediction, that individuals with greater pathogen disgust sensitivity (PDS) will be exposed to fewer pathogens and thus suffer fewer infections, has never been tested directly. To function adaptively, PDS must respond to the local cost/benefit context of avoidance, but this too has been undertested. We provide a test of these predictions among an Indigenous population with notable variation in PDS, infection, and infrastructure. We document predicted negative associations between PDS and pathogen exposure, while illuminating complex, multidirectional relationships among disgust, infection, and environmental variation. Our findings support the hypothesis that disgust functions to regulate pathogen exposure, demonstrating the importance of evolved psychological mechanisms in disease avoidance.
Keywords: disgust, pathogen avoidance, behavioral immune system, market integration, Shuar
Abstract
Disgust is hypothesized to be an evolved emotion that functions to regulate the avoidance of pathogen-related stimuli and behaviors. Individuals with higher pathogen disgust sensitivity (PDS) are predicted to be exposed to and thus infected by fewer pathogens, though no studies have tested this directly. Furthermore, PDS is hypothesized to be locally calibrated to the types of pathogens normally encountered and the fitness-related costs and benefits of infection and avoidance. Market integration (the degree of production for and consumption from market-based economies) influences the relative costs/benefits of pathogen exposure and avoidance through sanitation, hygiene, and lifestyle changes, and is thus predicted to affect PDS. Here, we examine the function of PDS in disease avoidance, its environmental calibration, and its socioecological variation by examining associations among PDS, market-related lifestyle factors, and measures of bacterial, viral, and macroparasitic infection at the individual, household, and community levels. Data were collected among 75 participants (ages 5 to 59 y) from 28 households in three Ecuadorian Shuar communities characterized by subsistence-based lifestyles and high pathogen burden, but experiencing rapid market integration. As predicted, we found strong negative associations between PDS and biomarkers of immune response to viral/bacterial infection, and weaker associations between PDS and measures of macroparasite infection, apparently mediated by market integration-related differences. We provide support for the previously untested hypothesis that PDS is negatively associated with infection, and document variation in PDS indicative of calibration to local socioeconomic conditions. More broadly, findings highlight the importance of evolved psychological mechanisms in human health outcomes.
Darwin first recognized disgust as an evolved human emotion, hypothesizing that it aided in avoidance or expulsion of “tainted” food (1). Since then, many studies have supported the hypothesis that disgust is a universal human emotional response that evolved to motivate avoidance of certain kinds of fitness-reducing substances, activities, or individuals, particularly those associated with infection (2–9). What constitutes “fitness-reducing,” however, varies depending on individual and environmental circumstances (8–12). To function adaptively, how sensitive someone is to pathogen-related cues (i.e., pathogen disgust sensitivity [PDS]) should be context-specific, calibrated to the local costs and benefits associated with infection risk and avoidance behaviors, and influenced by endemic parasites, lifestyle, nutritional status, phenotypic qualities, and life-history parameters (8–13). Additionally, PDS to certain pathogen-related stimuli—and the resultant disease-avoidance behaviors—show cross-cultural variation. Disgust-related variation in food preferences and taboos (14, 15), disease-limiting behaviors (e.g., hand washing, wearing of surgical masks) (16), and even political ideology (17) exists cross-culturally, although the degree to which environmental triggers (18) and socially transmitted information (19) account for these differences remains unclear.
Despite general acceptance of the hypothesis that pathogen disgust functions to regulate pathogen exposure, evidence has been largely indirect. Research shows that pathogen disgust is activated in response to hypothesized cues of potential pathogen-harboring stimuli (2, 5, 7, 20, 21) and seems to function to provide answers to the adaptive problems of what to touch, what to eat, and with whom to interact (13). Higher PDS is predicted to motivate greater avoidance of cues recurrently associated with pathogens, and therefore be associated with lower prevalence and intensity of infections (8, 21). While some research suggests that frequent past infections may up-regulate PDS and fear of contamination (22), no studies have directly tested whether greater PDS is associated with fewer current infections.
In addition to environmental cues, individuals are expected to use social information and individual experience to identify local environment-specific sources of contagion or toxicity (8, 23). While prior studies have attempted to examine how disgust is calibrated to local environmental (e.g., hygiene practices, sanitation infrastructure, subsistence strategies) and individual phenotypic conditions (e.g., sex, age, reproductive and health status) (24–28), no studies have explored this among an Indigenous subsistence-based population living in high-pathogen environments, conditions more like the selective environments hypothesized to have shaped disgust psychology than those studied to date.
Evolutionarily Relevant Pathogens and Cues
Pathogens have imposed important selective pressures throughout evolutionary history, at least since the origin of eukaryotic organisms (29–33). In response, hosts evolved several physiological, psychological, and behavioral defense mechanisms (10, 34). Specifically, the disgust response likely evolved because it motivated strategic avoidance of cues associated with the presence of evolutionarily relevant pathogens (13), including viruses, bacteria, and macroparasites.
To adaptively regulate avoidance of pathogen-containing substances, PDS must result in selective avoidance of evolutionarily relevant cues associated with substances for which the costs of contact or ingestion outweigh the potential benefits (13). Selection is expected to have primarily targeted cues with high signal value, including clear cues of pathogen infection in other people (e.g., vomit, coughing, sputum, diarrhea, mucus/blood in feces), food spoilage (e.g., decay, rotten scent), substances that often harbor infectious agents (e.g., blood, feces), and animal vectors (e.g., maggots, flies, cockroaches, rats) (2, 7, 13, 23).
Calibration of PDS
Furthermore, to function adaptively, pathogen disgust is predicted to include regulatory mechanisms that calibrate PDS based on trade-offs among the local costs and benefits of avoidance, including the likelihood of encountering a pathogen, types of pathogens encountered, and probable fitness-reducing costs of both exposure and avoidance (8, 13, 35, 36). For example, in subsistence-based populations, potential pathogenic substances that must regularly be encountered to acquire food or shelter are relatively costly to avoid (e.g., direct exposure to soil, animal feces, dirt floors, possibly contaminated natural water sources). As disgust-motivated avoidance becomes relatively more costly, PDS is predicted to be down-regulated in response (7, 8, 13). When the costs of avoidance are lower (e.g., in areas with reliably clean water and cooking surfaces that are easily cleaned), PDS is predicted to be up-regulated (7, 8, 13). To date, however, these hypotheses have seldom been tested (9).
In addition, disgust in humans likely evolved to utilize information acquired through both individual experience and social transmission (13). Food aversion based on sickness following ingestion of a particular food is the clearest example of individual experience-induced avoidance, and is present across animal taxa (37, 38). However, since individual experience with a pathogenic contaminant can be costly (even deadly), socially acquired information can be used to reduce these costs when cues are absent, ambiguous, or evolutionarily novel. Thus, PDS is likely to be influenced by social transmission, including via observation of others’ sickness experience, instruction and observation of appropriate foods and food-preparation techniques, and hygiene and disease-avoidance measures. Additionally, within-family variation in PDS may highlight a heritable component (39). Thus, studies are needed to track social influences within households and communities, especially as cultural practices change with time, and partition these influences from environmental calibration and possible heritable variance.
Market Integration and Disgust
Societies that traditionally relied on small-scale hunting, gathering, and horticulture are increasingly producing for and purchasing from the market economy, a process frequently referred to as market integration. Market integration affects many factors related to pathogen exposure and the costs of avoidance, and should consequently also shape PDS. For example, market integration is frequently accompanied by increased sanitation infrastructure (e.g., latrines, clean water sources, more hygienic cooking surfaces/practices) and greater access to Western medical care (40–42). It is therefore likely to affect not only disease barriers, but social expectations of cleanliness and hygiene, as well as education about biomedical ideas of disease transmission (42). Market integration is also associated with changes in food production, acquisition, and availability (42), thus likely altering the relative costs and benefits of exposure, expectations about contact with and ability to avoid disgust-eliciting substances, and norms about appropriate and taboo foods and practices. The context of market integration therefore presents a natural experiment for studying how disgust and infection vary with changing ecological and social circumstances.
Disgust and Market Integration among the Indigenous Shuar
In this study, we test: 1) If PDS is associated with lower infection prevalence and intensity; 2) if PDS is calibrated to the local environment; and 3) how relationships among PDS, infection, and environment vary at individual, household, and community levels among Ecuadorian Shuar. In so doing, we also examine the applicability of disgust domains originally defined in high-income populations.
The Shuar are a large Indigenous Amazonian population (>100,000 individuals, according to the Consejo de Desarrollo de las Nacionalidades y Pueblos del Ecuador, https://latinno.net/es/case/8080/#:∼:text=El%20Consejo%20de%20Desarrollo%20de,de%20Ecuador%20y%20el%20Estado, in 2012). To capture important lifestyle and infrastructural variation necessary to test relationships among PDS, market integration, and pathogen exposure, participants were recruited from three communities: One in the Upano Valley and two east of the Cordillera de Cutucú (i.e., “Cross Cutucú”). Shuar in both regions rely heavily on traditional cultigens, engage in subsistence horticulture, and interact regularly with domesticated animals (43–49). Cross Cutucú communities are relatively isolated, and subsistence continues to be based primarily on traditional horticulture, hunting, and fishing (43–48). However, in the Upano Valley, hunting and fishing have declined with increasing population size and economic development. Market access, agricultural sales, and wage labor are more pronounced in Upano Valley communities (46–49). At the time of this study, Upano Valley participants could access a regional market center and medical facility within 60 min by bus or truck. Cross Cutucú participants could only access this center after 7 to 12 h by motorized canoe then bus, although more limited services were available 1.5 to 3 h away by motorized canoe.
Among the Shuar, regional- and household-level market integration is linked to differences in both soil-transmitted helminth (STH) exposure and intestinal microbial diversity. Individuals living in the Cross Cutucú region tend to have higher prevalence and intensity of STH infection (50, 51). More market-integrated housing, particularly floor type (i.e., wood versus dirt) and water access (i.e., piped/well versus river/stream), are associated with reduced STH burden (52) and are more common in the Upano Valley. Additionally, more modern housing and reduced engagement in traditional subsistence activities are linked to less-diverse intestinal microbiota (53).
Data were collected as part of the Shuar Health and Life History Project (SHLHP, http://www.shuarproject.org/). To test relationships between PDS, socioecological factors, market integration, and infection, we administered a 19-item disgust questionnaire (SI Appendix, Table S1) modified for use with the Shuar from previous disgust scales (7, 54, 55) and a material style-of-life (SOL) interview used previously by the SHLHP (47), and collected stool and finger-prick dried blood spot samples from 75 Shuar participants (ages 5 to 59 y) from 28 households in three communities. This distribution allows us to examine clustering of PDS and infection at the individual, community, and household levels to identify patterns more closely related to environmental calibration. We used three markers of parasite infection: 1) Ascaris lumbricoides (large roundworm) and 2) Trichuris trichiura (whipworm) eggs per gram (EPG; indicative of infection presence and intensity) measured from stool samples, and 3) immunoglobulin E (IgE; a long-term marker of macroparasite infection) (56–58) measured from dried blood spot samples. We also analyzed two dried blood spot biomarkers of acute inflammatory response to immediate viral and bacterial infection (interleukin-6 [IL-6] and C-reactive protein [CRP]) (58–61). Material SOL variables were calculated to assess market-integrated SOL (MSOL; indicative of greater ownership of market-purchased items [e.g., propane stove, cellphone, refrigerator]), household SOL (HSOL; indicative of degree of market-integrated household construction and infrastructure [e.g., cinder block, lumber or palmwood walls, cement, wood or dirt floors, water from river, well or spring-fed water system, access to electricity]), and traditional SOL (TSOL; indicative of higher number of items owned that are associated with traditional subsistence and cultural activities [e.g., fishing nets, blowguns]) (47).
We predicted that among individual participants, higher PDS would be associated with lower levels of current infection indicators. Beyond the individual level, we expected to see associations between PDS and infection levels at both the family and community level. Because the costs of pathogen cue avoidance will be higher and the ability to successfully mitigate contamination will be lower among less market-integrated individuals, households, and communities, we predicted that these participants would have lower PDS and higher pathogen exposure.
Results
We used principal components analysis (PCA) to reduce the disgust questionnaire to a single factor (Disgust) (SI Appendix, Table S1). Infection and biomarker data were reduced to two factors using PCA (SI Appendix, Table S2). Factor 1 (Parasites) was associated with indicators of parasitic infection (e.g., IgE, species-specific EPG). Factor 2 (Inflammation) was associated with inflammatory indicators (CRP and IL-6), consistent with immune responses to other kinds of infection. We used Bayesian models to analyze associations, and report posterior parameter means, 95% credible intervals (CIs), and the proportion of the posterior greater than zero (P>0, values close to zero or one indicate that most of the posterior probability suggests a nonzero effect).
Disgust, infection measures, and SOL data clustered strongly at the household and community levels (Fig. 1 and SI Appendix, Figs. S1 and S2 and Tables S3 and S4). Specifically, one community had a high level of market integration (i.e., high MSOL/HSOL; low TSOL), and was characterized by high levels of Disgust and low levels of Inflammation and Parasites. A second community had intermediate levels for all variables. The third community had lower levels of market integration (i.e., low MSOL/HSOL; high TSOL), low levels of Disgust, and elevated levels of Inflammation and Parasites.
Fig. 1.
(A) The proportion of variance in each variable attributable to the community, household, and individual level. C1 to C3 are scores from the three components extracted from the disgust scale by PCA. See SI Appendix, Table S4. (B) Mean standardized values by community (yellow = high, blue = low). See SI Appendix, Table S3.
We analyzed variance components for each variable (Fig. 1A and Table S4) at the community, household, and individual levels, and found high degrees of variance attributable to community for Disgust (65%), Parasites (69%), and market-integration variables (HSOL 91%; MSOL: 60%). Additionally, household accounted for 13% of the variance in both Inflammation and Parasites, and 35% of the variance in MSOL and TSOL. Residual variance, attributable to the individual, was highest for Inflammation (62%) and Disgust (31%), with little individual level variation in the other variables.
We first adjusted for this clustering with random effects (SI Appendix, Table S5). However, to explicitly model the connections between household and community members, we calculated two variables for each individual: 1) The mean disgust and infection levels for all household members, excluding the individual in question, and 2) the mean disgust and infection values for all community members outside of the individuals’ household. Using these variables, we constructed path models, fit simultaneously as multivariate models (Fig. 2 and SI Appendix, Tables S6 and S7) and as partial models, to examine how household and community effects mediate associations between disgust and infection (see SI Appendix, Fig. S3 for partial model results).
Fig. 2.
Complete models showing relationships between disgust and infection at the individual, household, and community levels. Models show relationships between (A) Disgust and Inflammation (Inflam) and (B) Disgust and Parasites. Line thickness is proportional to the mean posterior effect size. Line type indicates the posterior certainty: Solid line = more than 95% of the posterior is on one side of zero; long dashes = <95% of the posterior is on one side of zero. Color indicates direction of effect: blue = negative, yellow = positive. Effects with less than 80% of the posterior on one side of zero are shaded gray. See SI Appendix, Table S6. (C) Parameter values for models with disgust components and Inflammation or Parasites (SI Appendix, Tables S8 and S9). Blue = individual disgust on individual infection, green = household disgust on individual infection, yellow = household disgust on household infection. Line thickness indicates 25%, 80%, and 95% highest posterior density interval.
The models revealed strong negative associations between Disgust and Inflammation, at both the individual (standardized β: −0.31, CI: −0.56, −0.06; P>0 < 0.01) and household levels (β: −0.34, CI: −0.73, 0.06; P>0 = 0.05). This relationship was also present in each of the three communities when examined individually (Fig. 3). The models also revealed a weaker effect with a wider credibility interval of other household members’ Disgust on an individual’s Inflammation (β: −0.21, CI: −0.58, 0.16; P>0 = 0.13).
Fig. 3.
The relationship between Disgust and infection variables across and within communities. Dashed line shows the linear fit line across all communities. Colored lines and points show the linear fit within each of the three communities in a model with random slopes (yellow circle = community 1; blue triangle = community 2; green square = community 3). Shading represents the 95% CI for each fit line. Points are marginal values corrected for age and sex.
When examined without accounting for village-level clustering, there was a negative association between Disgust and Parasites (Fig. 3 and SI Appendix, Fig. S3). However, once village-level clustering was accounted for, there was very little evidence for associations between Disgust and Parasites at the individual level (β: −0.06, CI: −0.26, 0.15; P>0 = 0.29), and within individual villages there was almost no relationship (Fig. 3). However, at the household level, there was evidence for an association, although weaker than that seen for Inflammation (β: −0.18, CI: −0.39, 0.02; P>0 = 0.04).
Community-level clustering in Disgust was reiterated in complete models as there were strong associations between community-level disgust and individual- and household-level disgust (Fig. 2 and SI Appendix, Tables S6 and S7). Since disgust and infection might be jointly influenced by individual and local circumstances, we added market-integration variables (MSOL, HSOL, and TSOL) to the models (Fig. 4 and SI Appendix, Fig. S4). The strongest evidence for their effect was a positive association between MSOL and Disgust, evident at both the individual and household levels (β: 0.26, CI: −0.01, 0.53; P>0 = 0.97; β: 0.15, CI: −0.09, 0.37; P>0 = 0.90) (Fig. 4 and SI Appendix, Fig. S4). HSOL was also positively associated with Disgust at the household level, albeit with a relatively broad posterior (β: 0.28, CI: −0.10, 0.61; P>0 = 0.94). Also notable was a strong negative association between HSOL and Parasites at the household level (β: −0.41; CI: −0.96, 0.11; P>0 = 0.06). Other associations with market-integration variables were relatively weak and uncertain.
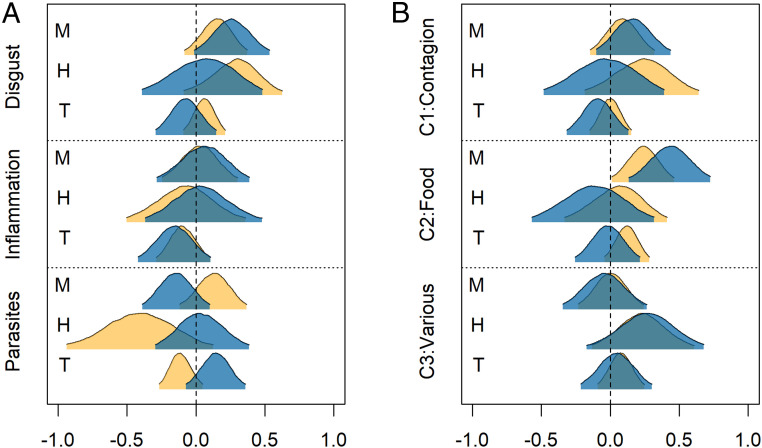
Fig. 4.
Market integration, disgust, and infection. (A) Posterior parameter distributions for effects of market integration on infection and disgust. (B) Effects of market integration on disgust components. Parameters are from full multivariate path models (SI Appendix, Fig. S4 and Tables S7–S9), with each plotted posterior distribution representing the combined distribution from the two models with that pathway. Yellow distributions are effects on households. Blue distributions represent effects at the individual level, controlling for levels of other household members. Shaded area is the 95% highest posterior density interval. The abbreviations M, H, and T represent market-integrated, household, and traditional SOL, respectively.
Due to the cultural inappropriateness of some items for use with the Shuar, we did not replicate the full disgust surveys used in past studies (7, 54, 55). We therefore used PCA to examine components of disgust based on the questions included in our questionnaire. Parallel analysis and scree plots suggested three factors (SI Appendix, Table S1). The first factor (C1: contagion) was most closely associated with direct contact with potential pathogens from other individuals (e.g., vomiting, coughing, sharing food with an ill individual), as well as bodily contamination (e.g., not bathing) or contamination of water and food (e.g., worms in food, dirty water). The second factor (C2: food) was closely associated with eating spoiled or raw food or touching a dead animal. In this context, touching a dead animal was interpreted to mean hunted meat and was thus perceived as food related. The third factor was comprised of disparate elements not easily classified, and so is referred to simply as C3. As with total Disgust, a high proportion of the variance in contagion and food components clustered at the community level (Fig. 1), whereas C3 was dominated by individual variance. The contagion component showed the strongest association with lower Inflammation (Fig. 2B and SI Appendix, Table S8) (β: −0.39; CI: −0.66, −0.12; P>0 = 0.003), followed by the food component (β: −0.29; CI: −0.57, 0.01; P>0 = 0.03). Food Disgust was associated with lower Parasites, but only at the household level (SI Appendix, Table S9) (β: −0.15; CI: −0.31, 0.01; P>0 = 0.03). Food Disgust also showed a strong positive association with MSOL (Fig. 4 and SI Appendix, Tables S8 and S9), whereas other components were not clearly associated with market-integration variables.
Discussion
The present paper is unique in directly testing hypothesized relationships between PDS and various types of pathogen infection among an Indigenous subsistence-based population living in a high-pathogen environment. Significant intracultural environmental and lifestyle variability in this population, due to rapid market integration, also allowed us to test predictions about the calibration of disgust psychology to local socioecological conditions. This research is important for several reasons. First, we provide evidence that higher PDS is associated with lower levels of pathogen infection, as predicted if it functions as a disease-avoidance mechanism. Second, we show that PDS varies at the household and community levels due to factors associated with market integration, consistent with theories of environmental and social calibration of disgust. Third, we provide some support for the applicability of disgust categories first documented in high-income nations to a sample from a subsistence-based population.
Pathogen Disgust Reduces Exposure to Relevant Pathogens.
Our results indicate a robust relationship between Disgust and Inflammation, with higher Disgust associated with lower levels of Inflammation. Here, CRP and IL-6 were used as continuous variables to indicate acute immune response to pathogens (59–61). In healthy individuals, CRP and IL-6 levels are usually negligible, but during infections they increase up to 1,000-fold (60). Past research among the Shuar shows no evidence of chronic low-grade inflammation (62), so elevation in these markers is likely capturing acute inflammatory responses associated with infection at the time of study. Specifically, Shuar with high inflammation scores are experiencing varying degrees of inflammatory immune responses to pathogens at the time of study, while individuals with lower Inflammation scores are likely not experiencing an acute infection (60). Intermediate values indicate a recent infection and thus serve as a graded signal of the likelihood of recent infection. Our results (SI Appendix, Figs. S6–S9) with simulated biomarkers suggest that measures of acute inflammation are likely to capture associations between disgust and infection.
Disgust predicted lower Inflammation, consistent with the hypothesis that disgust functions to lower pathogen exposure. This relationship was robust when analyzed within individual communities, and varied little between communities (Fig. 3). Negative relationships between Disgust and Inflammation were also observed at the household level. Furthermore, models provided evidence that the Disgust of household members can protect others in the household; when other household members had higher Disgust scores, individuals in those households also benefited by having lower Inflammation scores. These effects were partially mediated through shared PDS in the household (SI Appendix, Fig. S3), but suggest that household members can provide both direct and indirect protection from infection, likely through hygiene/sanitation-related pathogen avoidance practices and overall reduced pathogen exposure via household transmission.
The lack of strong relationships between Disgust and Parasites at the individual level, though not originally predicted, is not surprising. The parasitic infections identified in the Shuar and used in these analyses (i.e., A. lumbricoides, T. trichiura) are generally less virulent, but can incur major fitness-reducing costs including diarrhea, cognitive deficiencies, stunted growth, and altered fertility (29, 63–66). These types of parasite infections are not directly foodborne and instead occur through contact with or consumption of fecal-contaminated soil containing embryonated eggs that may become infectious after obvious signs of fecal contamination have faded. Therefore, the variables included in our Disgust questionnaire are not primary pathways of STH transmission. Analyses of larger datasets collected after this study also show that parasite load varies by region and community, likely due to variation in market integration-related hygiene/sanitation, infrastructure, and environmental factors, like water source (50–52). The present study supports this finding, with Parasites clustering strongly at the community level.
In fact, we might expect PDS to function differently with respect to STH infection prevention. Unlike viruses and bacteria, STHs cannot replicate in the host and frequency of exposure determines infectious load (64). Exposure to only a few eggs or larvae leads to less-intense infections, while exposure to many eggs or larvae leads to more-intense infections. Immunological pathways keep infection intensities in check while reducing the costs associated with damage to host tissue that would occur from a more aggressive inflammatory response (67). Thus exposure-level and immune response drive infection intensity. With STHs, there is relatively little cost to light initial exposures, thus PDS may be less sensitive to STH-specific vectors (e.g., soil and soil-contaminated foods). These vectors may be extremely difficult to avoid in contexts where exposure to soil is needed for subsistence (e.g., agriculture, hunting, foraging) or where infrastructure does not allow efficient avoidance (e.g., dirt floors, lack of running water/sewage systems), all factors associated with STH infection among the Shuar (52), and characteristic of the evolutionary environment that shaped the disgust response. In these cases, heightened disgust sensitivity related to these vectors may be maladaptive.
Since parasite load is dependent on repeated exposure, simulation results suggest that if PDS were protective against STHs, the effect should be easily detected with the current approach (SI Appendix, Figs. S6–S9). The fact that we find little effect likely indicates that PDS, as measured here, is negligibly protective against these kinds of parasites. On the other hand, microparasites, like viruses and bacteria, replicate in the host and can cause intense infections even with light initial exposure to limited numbers of bacteria or viral elements. For this reason, it makes sense for the disgust response system to treat vectors or cues of microparasites as contaminants (i.e., substances to be avoided even in small amounts and which are capable of spreading serious disease with even limited contact) (68–70) and a robust response to microparasite-related vectors, which are relatively more easily avoided in these settings, would be more adaptive.
While we have assumed that PDS influences infection, infection might also be capable of influencing PDS. Sickness behavior, for example, is characterized by loss of appetite (71–75) and as changes in depression and anxiety (75–77), and could therefore relate to PDS, though we know of no evidence for this. Indeed, over longer time periods, disgust is expected to be calibrated by environmental cues and thus to be influenced by infection. Based on our results, however, reversed causality for infection and PDS (SI Appendix, Fig. S5) seems less logically plausible, since we would expect Inflammation to increase Disgust, given greater vulnerability to other infections during illness (75).
The finding that infection, Disgust, and SOL variables clustered differently at the community, household, and individual level (Fig. 1) is also important. Most of the variance in Inflammation was at the individual level, demonstrating the importance of individual variation in exposure to viral and bacterial pathogens, and immune system development and function. Despite PDS being moderately heritable genetically (39), in our study Disgust varied considerably at the individual level with little clustering by household, suggesting that PDS is calibrated to individual experience, perhaps based on factors such as life-history stage, immune system development, and overall health.
Environmental and Social Calibration of Disgust.
Beyond direct relationships between individual PDS and infection, community explained a large portion of the variance in most variables (Fig. 1A and SI Appendix, Table S4). Consistent with expectations, Disgust was highest in the most market-integrated community, where access to sanitation and clean water is greater and the costs of disgust-motivated avoidance likely lowest (Fig. 1B and SI Appendix, Fig. S2). Conversely, Disgust was lowest in the least market-integrated community, where there is greater engagement in traditional subsistence activities of hunting and fishing, coupled with living conditions that make avoidance more costly (i.e., dirt floors, cooking fires on floors, no plumbing/latrines, lack of piped spring water, possibly contaminated water sources). In these conditions, PDS may be down-regulated in response to the relatively high costs of pathogen-avoidance behaviors. In more market-integrated communities, sanitation and improved water infrastructure may reduce costs of avoiding disgust-eliciting stimuli and effective preventative behaviors (e.g., more effective hand and dish washing). Due to greater ease of avoidance, PDS may be up-regulated to further motivate greater avoidance behaviors.
The patterns described here are perhaps contrary to some hypotheses that predict disease-avoidance behaviors should be highest in high pathogen areas (36). However, these hypotheses are largely based on cross-country comparisons, rather than individual-level data, and generally overlook the relative costs of avoidance under different circumstances (9). When we look at individual-level data, high PDS is associated with lower levels of infection and with more market-integrated conditions, which likely lower the relative costs of pathogen avoidance. This suggests a “Simpson’s paradox” (78): When we compare individuals within the same culture, we see that individuals with higher PDS have lower pathogen loads; but when whole regions or countries are compared, countries with higher parasite loads have higher PDS. It may be that the highest disgust sensitivity is found in individuals in high pathogen countries who can afford to avoid pathogens. Studies biased toward sampling more affluent individuals in countries with high parasite loads might therefore suggest a positive association between pathogens and pathogen avoidance, even though at an individual level the opposite might be observed.
Cross-Cultural Evidence for Pathogen Disgust Domains.
Early research grouped disgust-eliciting stimuli into five categories, the first three of which are specific to pathogen disgust: 1) Bodily excretions and body parts, 2) decayed and spoiled food, and 3) particular types of living creatures (e.g., rats, worms, maggots, cockroaches, lice) (2). More recent research suggests that disgust-regulating cues are linked to the specific evolutionary problems of what to touch, what to eat, and with whom to interact or have sex (13). Until now, these categorizations have not been validated among populations living in high-pathogen environments. We provide some preliminary support for these categorizations.
The two disgust components (i.e., contagion disgust and food disgust) identified by factor analysis in this study appear to address the evolutionary problems of “what to touch” and “what to eat,” respectively, and the variables included in each appear to factor into similar categories to bodily excretions and body parts, as well as decayed and spoiled food. Furthermore, the third factor (C3) did include exposure to animals like rats and spiders, which demonstrates some overlap with the third category (SI Appendix, Table S1). Although our PCA is somewhat informal in terms of validating domains, these findings provide some limited support for these basic psychological disgust categories among an Indigenous subsistence-based population. It would be useful for future studies to assess whether pathogen disgust stimuli fall into even more specific domains and the role of each in disease avoidance (2, 6, 13). Additional disgust domains (i.e., sexual and moral disgust) (7, 79) are also important; we did not assess them here for reasons of cultural appropriateness, but future studies may find ways to investigate these in a broader cross-cultural context.
These more specific pathogen-related disgust domains may be important because they each respond to different stimuli, are shaped by distinct environmental factors, likely protect against different pathogens, and thus entail different context-sensitive costs and benefits of avoidance. For example, while higher contagion and food disgust scores were both associated with lower levels of Inflammation, contagion disgust had a stronger relationship. Further work to understand the kinds of pathogens contributing to elevated inflammation could shed light on nuances in these variables. It is possible, for example, that contagion disgust protects from viral and bacterial pathogens that spread via person-to-person contact through bodily fluids (i.e., what to touch), while food disgust protects from foodborne pathogens and toxins and is an important component in deciding what to eat (13).
In fact, food disgust, but not contagion disgust, was associated with one of our indicators of market integration, specifically MSOL (Fig. 3 and SI Appendix, Fig. S4). Generally, market integration affects subsistence strategies, including how foods are obtained, processed, and stored. While traditional cultigens formed the subsistence base in all study communities, the more market-integrated community engaged in less hunting and fishing. Furthermore, income from market-based activities and proximity to market centers allowed greater purchase of market foods, some of which are preprocessed or prepackaged (e.g., noodles, sardines, rice). Tellingly, MSOL included specific items directly related to food preparation (e.g., how food is cooked) and storage (e.g., whether refrigeration is available). To take one example, cooking on a propane stove—compared to an open dirt-floor fire—enhances food preparation hygiene with relatively little individual effort. Similarly, refrigeration facilitates easy and safe food storage, allowing both fresh and cooked foods to be kept longer without spoilage. Without refrigeration, Shuar food storage entails either leaving root crops growing in the ground, processing manioc into a fermented beverage (niahamanche in Shuar, chicha in Spanish), smoking excess game or fish over the fire, or simply leaving leftover food at ambient temperature until the next meal. However, even smoked meat spoils rapidly in tropical heat and humidity. This may increase the relative value of consuming marginally spoiled foods—rather than letting them go to waste—under these conditions, which likely lowers food disgust sensitivity. Based on these findings, it may be useful to further explore how particular features of different disgust domains relate to specific forms of pathogen avoidance and environmental calibration.
Limitations
Although simulations suggest that the sample size in this study is sufficient to credibly detect the associations tested here with a low probability of a false positive (SI Appendix, Figs. S6–S9), the sample of 75 individuals is not sufficient to test additional individual phenotypic factors that might influence PDS. Although we did not detect appreciable effects of age or sex (SI Appendix, Tables S6–S9), many other factors (e.g., reproductive status) might interact with these variables.
This study is also relatively limited in the scope of market integration sampled. All participants were Shuar horticulturalists living in rural communities. Approximately half the sampled individuals resided in an Upano Valley community, while the other half lived in Cross Cutucú communities. While the sampling strategy was intentionally designed to capture variation in market integration, these communities are still much less market integrated than individuals living in the regional market center, a group not included in this study.
Furthermore, this study adapted preexisting disgust scales (7, 54, 55) created in high-income countries for use with an Indigenous population living in a high-pathogen environment whose diet remains primarily based on subsistence horticulture. The scale may not have been effective at measuring stimuli relevant to transmission of STHs and other macroparasites, which are less common than microparasites in high-income regions. Future research should incorporate disgust elicitors associated with contaminated soil and oral-fecal contamination (e.g., consumption of soil or soil-covered produce, eating with soil-covered hands) to test relationships between PDS and macroparasite exposure more directly.
Besides STH infection intensity, measured using EPG of feces, other measures of infection were indirect and based on immune biomarkers. The biomarkers CRP and IL-6 are related to increased inflammatory immune activity associated with acute infection with microparasites (59–61), but they cannot tell us about the type, intensity, or duration of the infection, or about individual variation in immune response. Furthermore, IgE is associated with long-term immune response to macroparasites over several years (56). While IgE increases based on infection intensity (24) and does eventually diminish after an infection clears (56, 80, 81), it provides only a snapshot of the immune response to macroparasites and cannot be used to deduce how long individuals have been infected, when they were first exposed, and how regularly they are being reexposed. We also cannot dissect individual variation in IgE based on genetics, immune-priming, and age. If PDS is calibrated to current conditions, its measure at time of study may be temporally out of sync with the IgE measure of macroparasitic infection.
The study is also limited in its ability to parse environmental calibration and evoked culture (18) from factors related to cultural transmission. While these are difficult to fully assess among human samples, future studies could incorporate longitudinal data or data that look explicitly at individual behavior, knowledge, and beliefs to better separate these effects. Longitudinal data would also allow for more direct tests of causality between environmental calibration and PDS as it occurs throughout an individual’s lifetime. It is worth noting, however, that market integration among the Shuar has increased so rapidly that adults in the most market-integrated community grew up in conditions similar to or even less market-integrated than the Cross Cutucú communities sampled at the time of this study, suggesting that environmental calibration continues throughout life.
Conclusions
This study provides support for the hypothesis that disgust is an evolved human emotion that functions to limit infection by regulating pathogen exposure in response to the local costs and benefits of avoidance and infection. It is unique in its findings that higher PDS is associated with lower levels of pathogen infection among an Indigenous subsistence-based population living in a high pathogen environment, conditions that are, in important ways, more similar to those experienced throughout human evolutionary history than those tested to date. Furthermore, it shows that market integration is associated with higher PDS, as is predicted if disgust sensitivity is calibrated to the local costs/benefit structure of avoidance and infection. Finally, although we did not test the idea that pathogen disgust evolved to solve the adaptive problem of with whom to interact, PCA suggests that two domains of pathogen disgust exhibited by the Shuar may be consistent with those first described in high income, industrialized populations, providing cross-cultural support for the hypothesis that pathogen disgust functions to solve adaptive problems related to what to touch and what to eat.
Materials and Methods
Participants and Sampling.
Cross-sectional data were collected over two field seasons (August to September 2011, August to September 2012) in one Upano Valley community (population ∼350 individuals) and two Cross Cutucú communities (combined population ∼360 individuals). Seventy-five participants (Upano Valley n = 30; Cross Cutucú n = 45; ages 5 to 59 y) completed the disgust questionnaire, SOL interview, and provided finger-prick blood and stool samples. The study was approved by the Institutional Review Board at the University of Oregon and all participants provided informed consent.
Disgust Sensitivity Measures.
Commonly used disgust scales (7, 54, 55) were adapted for relevance to Shuar culture and translated to Spanish. Most Shuar speak Spanish fluently, but a bilingual (Spanish/Shuar) assistant translated as necessary. The questionnaire measured PDS to 19 items (SI Appendix, Table S1 provides English translation for all questionnaire items) using a 5-point Likert scale, with higher values indicating greater disgust (1, “not disgusting” to 5, “very disgusting”).
Lifestyle Measures.
Structured interviews were administered in Spanish to collect basic demographic and lifestyle information from adult household members. Participants were asked questions from a material SOL index (82, 83) modified for use with the Shuar (47, 49). Three SOL variables were calculated: HSOL, TSOL, MSOL. See SI Appendix for more details. Participants could be high on all scales, low on all, or any combination, so these variables were not further combined.
Dried Blood Spot Collection and Analyses.
Capillary whole blood samples were collected via finger prick and preserved on protein saver cards (Whatman #903, GE Healthcare) following standard methods (84). Samples were dried, stored at −20 °C, and shipped frozen to the Global Health Biomarker Laboratory at the University of Oregon. They were analyzed using commercially available ELISA for IgE and IL-6, and commercially available antibodies for CRP, based on previously established dried blood spot protocols (56, 57, 65, 85–87). See SI Appendix for assay details.
Stool Collection and Analysis.
Fresh stool samples were collected and processed based on methods previously described (50–52). For each specimen, a single Kato-Katz thick smear (Vestergaard Frandsen) (88) was examined microscopically by a trained observer (T.J.C.-R.). Species-specific EPG of feces were calculated by multiplying the total number of eggs of each STH species on a single slide by 24 (89). Higher EPG represent higher-intensity infections (89).
Statistical Analyses.
All analyses were conducted in R 4.0.3 (https://cran.r-project.org/). We first reduced the disgust questionnaire to a single principal component and extracted scores via regression. In secondary analyses, we extracted three components as suggested by parallel analysis and scrutiny of scree plots (90) (SI Appendix, Table S1). Infection variables (CRP, IL-6, IgE, A. lumbricoides and T. trichiura EPG counts) were log-transformed and standardized prior to analysis. Missing values for IgE, CRP, and IL-6 (n = 15, 11, and 19, respectively) were imputed with multivariate imputation by chained equations [mice (91)] and a PCA of infection variables was performed on the combined 10 imputed datasets, yielding two oblimin-rotated components as suggested by parallel analysis and scrutiny of scree plots (SI Appendix, Table S2). Individual scores were extracted by regression and labeled Parasites and Inflammation.
For each individual, we calculated the mean disgust, infection, and market-integration value for all household members excluding the target individual, and for all community members excluding the household. Modeling using these variables explicitly modeled the contribution of other household and community members to the variance in the dependent variable. Some models included random effects to control for lack of independence in repeat measures, as appropriate. Models with imputed values were fit using brm_multiple in the brms package (92). Components of multivariate path models were fit simultaneously in the same model. Reported values are the mean posterior estimate and 95% CI. Further details on statistical methods and model specifications are given in SI Appendix.
Supplementary Material
Acknowledgments
We thank our colleagues, research assistants, and friends who helped make this research possible (Dr. Richard Bribiescas, Luzmila Jempeket, Estela Jempeket, Oswaldo Mankash, Marilu Utitiaj); the Federación Interprovincial de Centros Shuar; John Tooby and Leda Cosmides of the Center for Evolutionary Psychology; and our study participants. Funding for this study came from the Wenner-Gren Foundation for Anthropological Research (Grants 8476, 7970); National Science Foundation (NSF) Doctoral Dissertation Improvement Grants (BCS-1341165, BCS-0824602, BCS-0925910); NSF Graduate Research Fellowship 2011109300; the Ryoichi Sasakawa Young Leaders Fellowship Fund; NIH contract Grant 5DP1O000516-04; the University of Oregon’s Institute of Cognitive and Decision Sciences, Department of Anthropology, Faculty Excellence Award, and Faculty Research Award; and the Boettcher Foundation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018552118/-/DCSupplemental.
Data Availability
The deidentified dataset for this article is available upon request on the SHLHP website (https://www.shuarproject.org/data-sharing) in accordance with data use agreements to ensure compliance with relevant Institutional Review Board, participant expectations, and authorship conditions. Code for all analyses is available at http://doi.org/10.5281/zenodo.4487336 (93).
References
- 1.Darwin C., “Disdain, contempt, disgust, pride, etc., Helplessness, patience, affirmation and negation” in The Expression of the Emotions in Man and Animals (D. Appleton & Company, New York, 1872), pp. 253–277. [Google Scholar]
- 2.Curtis V., Biran A., Dirt, disgust, and disease. Is hygiene in our genes? Perspect. Biol. Med. 44, 17–31 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Ekman P., Are there basic emotions? Psychol. Rev. 99, 550–553 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Lieberman D., Tooby J., Cosmides L., Does morality have a biological basis? An empirical test of the factors governing moral sentiments relating to incest. Proc. Biol. Sci. 270, 819–826 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oaten M., Stevenson R. J., Case T. I., Disgust as a disease-avoidance mechanism. Psychol. Bull. 135, 303–321 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Rozin P., Haidt J., McCauley R., “Disgust” in Handbook of Emotions, Lewis M., Haviland-Jones J. M., Barrett L. F., Eds. (Guilford Press, New York, NY, ed. 3, 2008), pp. 757–776. [Google Scholar]
- 7.Tybur J. M., Lieberman D., Griskevicius V., Microbes, mating, and morality: Individual differences in three functional domains of disgust. J. Pers. Soc. Psychol. 97, 103–122 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Tybur J. M., Lieberman D., Human pathogen avoidance adaptations. Curr. Opin. Psychol. 7, 6–11 (2016). [Google Scholar]
- 9.Tybur J. M., Çınar Ç., Karinen A. K., Perone P., Why do people vary in disgust? Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackerman J. M., Hill S. E., Murray D. R., The behavioral immune system: Current concerns and future directions. Soc. Personal. Psychol. Compass 12, e12371 (2018). [Google Scholar]
- 11.Fessler D. M. T., Eng S. J., Navarrete C. D., Elevated disgust sensitivity in the first trimester of pregnancy: Evidence supporting the compensatory prophylaxis hypothesis. Evol. Hum. Behav. 26, 344–351 (2005). [Google Scholar]
- 12.Fessler D. M. T., Navarrete C. D., Domain-specific variation in disgust sensitivity across the menstrual cycle. Evol. Hum. Behav. 24, 406–417 (2003). [Google Scholar]
- 13.Lieberman D., Billingsley J., Patrick C., Consumption, contact and copulation: How pathogens have shaped human psychological adaptations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallon A. E., Rozin P., Pliner P., The child’s conception of food: The development of food rejections with special reference to disgust and contamination sensitivity. Child Dev. 55, 566–575 (1984). [PubMed] [Google Scholar]
- 15.Hartmann C., Shi J., Giusto A., Siegrist M., The psychology of eating insects: A cross-cultural comparison between Germany and China. Food Qual. Prefer. 44, 148–156 (2015). [Google Scholar]
- 16.Burgess A., Horii M., Risk, ritual and health responsibilisation: Japan’s ‘safety blanket’ of surgical face mask-wearing. Sociol. Health Illn. 34, 1184–1198 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Tybur J. M., et al., Parasite stress and pathogen avoidance relate to distinct dimensions of political ideology across 30 nations. Proc. Natl. Acad. Sci. U.S.A. 113, 12408–12413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tooby J., Cosmides L., “The psychological foundations of culture” in The Adapted Mind: Evolutionary Psychology and the Generation of Culture, Barkow J. H., Tooby J., Cosmides L., Eds. (Oxford University Press, 1992), pp, 19–136. [Google Scholar]
- 19.Boyd R., Richerson P. J., Henrich J., The cultural niche: Why social learning is essential for human adaptation. Proc. Natl. Acad. Sci. U.S.A. 108 (suppl. 2), 10918–10925 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apicella C. L., Rozin P., Busch J. T. A., Watson-Jones R. E., Legare C. H., Evidence from hunter-gatherer and subsistence agricultural populations for the universality of contagion sensitivity. Evol. Hum. Behav. 39, 355–363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis V., Aunger R., Rabie T., Evidence that disgust evolved to protect from risk of disease. Proc. Biol. Sci. 271, S131–S133 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson R. J., Case T. I., Oaten M. J., Frequency and recency of infection and their relationship with disgust and contamination sensitivity. Evol. Hum. Behav. 30, 363–368 (2009). [Google Scholar]
- 23.Kavaliers M., Ossenkopp K.-P., Choleris E., Social neuroscience of disgust. Genes Brain Behav. 18, e12508 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Batres C., Perrett D. I., Pathogen disgust sensitivity changes according to the perceived harshness of the environment. Cogn. Emotion 34, 377–383 (2020). [DOI] [PubMed] [Google Scholar]
- 25.de Barra M., Islam M. S., Curtis V., Disgust sensitivity is not associated with health in a rural Bangladeshi sample. PLoS One 9, e100444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gassen J., et al., Behavioral immune system activity predicts downregulation of chronic basal inflammation. PLoS One 13, e0203961 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones B. C., et al., Hormonal correlates of pathogen disgust: Testing the compensatory prophylaxis hypothesis. Evol. Hum. Behav. 39, 166–169 (2018). [Google Scholar]
- 28.Oaten M. J., Stevenson R. J., Case T. I., Compensatory up-regulation of behavioral disease avoidance in immune-compromised people with rheumatoid arthritis. Evol. Hum. Behav. 38, 350–356 (2017). [Google Scholar]
- 29.Hurtado A. M., Frey M., Hill K., Hurtado I., Baker J., “The role of helminths in human evolution: Implications for global health in the 21st century” in Medicine and Evolution: Current Applications, Future Prospects, Elton S., O’Higgins P., Eds. (Taylor and Francis, New York, 2008) pp. 151–178. [Google Scholar]
- 30.Jackson J. A., Friberg I. M., Little S., Bradley J. E., Review series on helminths, immune modulation and the hygiene hypothesis: Immunity against helminths and immunological phenomena in modern human populations: Coevolutionary legacies? Immunol. 126, 18–27 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell P. D., The origins of human parasites: Exploring the evidence for endoparasitism throughout human evolution. Int. J. Paleopathol. 3, 191–198 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Wolfe N. D., Dunavan C. P., Diamond J., Origins of major human infectious diseases. Nature 447, 279–283 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tooby J., Pathogens, polymorphism, and the evolution of sex. J. Theor. Biol. 97, 557–576 (1982). [DOI] [PubMed] [Google Scholar]
- 34.Schaller M., “The behavioral immune system” in The Handbook of Evolutionary Psychology, Buss D. M., Ed., (Wiley, ed. 2, 2015), vol. 1, pp. 206–224. [Google Scholar]
- 35.Curtis V., de Barra M., Aunger R., Disgust as an adaptive system for disease avoidance behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 389–401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornhill R., Fincher C. L., Murray D. R., Schaller M., Zoonotic and non-zoonotic diseases in relation to human personality and societal values: Support for the parasite-stress model. Evol. Psychol. 8, 151–169 (2010). [PubMed] [Google Scholar]
- 37.Bernstein I. L., Taste aversion learning: A contemporary perspective. Nutrition 15, 229–234 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Moore R. S., Kaletsky R., Murphy C. T., Piwi/PRG-1 argonaute and TGF-β mediate transgenerational learned pathogenic avoidance. Cell 177, 1827–1841.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tybur J. M., Wesseldijk L. W., Jern P., Genetic and environmental influences on disgust proneness, contamination sensitivity, and their covariance. Clin. Psychol. Sci. 8, 1054–1061 (2020). [Google Scholar]
- 40.Campbell S. J., et al., Water, sanitation, and hygiene (WASH): A critical component for sustainable soil-transmitted helminth and schistosomiasis control. PLoS Negl. Trop. Dis. 8, e2651 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman M. C., Clasen T., Brooker S. J., Akoko D. O., Rheingans R., The impact of a school-based hygiene, water quality and sanitation intervention on soil-transmitted helminth reinfection: A cluster-randomized trial. Am. J. Trop. Med. Hyg. 89, 875–883 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godoy R., Reyes-García V., Byron E., Leonard W. R., Vadez V., The effect of market economies on the well-being of Indigenous peoples and on their use of renewable natural resources. Annu. Rev. Anthropol. 34, 121–138 (2005). [Google Scholar]
- 43.Harner M. J., The Jı’varo, People of the Sacred Waterfalls (University of California Press, Berkley, 1984). [Google Scholar]
- 44.Karsten R., The Head-Hunters of Western Amazonas: The Life and Culture of the Jibaro Indians of Eastern Ecuador and Peru (Societas Scientarium, Helsinki, Finland, 1935). [Google Scholar]
- 45.Stirling M., Historical and Ethnographical Material on the Jivaro Indians (Government Printing Office, Washington, DC, 1938). [Google Scholar]
- 46.Blackwell A. D., Pryor G. 3rd, Pozo J., Tiwia W., Sugiyama L. S., Growth and market integration in Amazonia: A comparison of growth indicators between Shuar, Shiwiar, and nonindigenous school children. Am. J. Hum. Biol. 21, 161–171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liebert M. A., et al., Implications of market integration for cardiovascular and metabolic health among an indigenous Amazonian Ecuadorian population. Ann. Hum. Biol. 40, 228–242 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Madimenos F. C., Snodgrass J. J., Blackwell A. D., Liebert M. A., Sugiyama L. S., Physical activity in an indigenous Ecuadorian forager-horticulturalist population as measured using accelerometry. Am. J. Hum. Biol. 23, 488–497 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urlacher S. S., et al., Heterogeneous effects of market integration on sub-adult body size and nutritional status among the Shuar of Amazonian Ecuador. Ann. Hum. Biol. 43, 316–329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cepon-Robins T. J., et al., Soil-transmitted helminth prevalence and infection intensity among geographically and economically distinct Shuar communities in the Ecuadorian Amazon. J. Parasitol. 100, 598–607 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Gildner T. E., et al., Regional variation in Ascaris lumbricoides and Trichuris trichiura infections by age cohort and sex: Effects of market integration among the indigenous Shuar of Amazonian Ecuador. J. Physiol. Anthropol. 35, 28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gildner T. E., et al., Market integration and soil-transmitted helminth infection among the Shuar of Amazonian Ecuador. PLoS One 15, e0236924 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stagaman K., et al., Market integration predicts human gut microbiome attributes across a gradient of economic development. mSystems 3, e00122–e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haidt J., McCauley C., Rozin P., Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Pers. Individ. Dif. 16, 701–713 (1994). [Google Scholar]
- 55.Olatunji B. O., et al., The Disgust Scale: Item analysis, factor structure, and suggestions for refinement. Psychol. Assess. 19, 281–297 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Iancovici Kidon M., et al., Serum immunoglobulin E levels in Israeli-Ethiopian children: Environment and genetics. Isr. Med. Assoc. J. 7, 799–802 (2005). [PubMed] [Google Scholar]
- 57.Blackwell A. D., et al., Evidence for a peak shift in a humoral response to helminths: Age profiles of IgE in the Shuar of Ecuador, the Tsimane of Bolivia, and the U.S. NHANES. PLoS Negl. Trop. Dis. 5, e1218 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urlacher S. S., et al., Tradeoffs between immune function and childhood growth among Amazonian forager-horticulturalists. Proc. Natl. Acad. Sci. U.S.A. 115, E3914–E3921 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez L., Acute phase protein response to viral infection and vaccination. Arch. Biochem. Biophys. 671, 196–202 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slaats J., Ten Oever J., van de Veerdonk F. L., Netea M. G., IL-1β/IL-6/CRP and IL-18/ferritin: Distinct inflammatory programs in infections. PLoS Pathog. 12, e1005973 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rose-John S., Winthrop K., Calabrese L., The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat. Rev. Rheumatol. 13, 399–409 (2017). [DOI] [PubMed] [Google Scholar]
- 62.McDade T. W., et al., Analysis of variability of high sensitivity C-reactive protein in lowland Ecuador reveals no evidence of chronic low-grade inflammation. Am. J. Hum. Biol. 24, 675–681 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Ahmed A., Al-Mekhlafi H. M., Surin J., Epidemiology of soil-transmitted helminthiases in Malaysia. Southeast Asian J. Trop. Med. Public Health 42, 527–538 (2011). [PubMed] [Google Scholar]
- 64.Bethony J., et al., Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 367, 1521–1532 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Blackwell A. D., Snodgrass J. J., Madimenos F. C., Sugiyama L. S., Life history, immune function, and intestinal helminths: Trade-offs among immunoglobulin E, C-reactive protein, and growth in an Amazonian population. Am. J. Hum. Biol. 22, 836–848 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blackwell A. D., et al., Helminth infection, fecundity, and age of first pregnancy in women. Science 350, 970–972 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McSorley H. J., Maizels R. M., Helminth infections and host immune regulation. Clin. Microbiol. Rev. 25, 585–608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cisler J. M., Reardon J. M., Williams N. L., Lohr J. M., Anxiety sensitivity and disgust sensitivity interact to predict contamination fears. Pers. Individ. Dif. 42, 935–946 (2007). [Google Scholar]
- 69.Brown S. D., Harris G., Disliked food acting as a contaminant during infancy. A disgust based motivation for rejection. Appetite 58, 535–538 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Olatunji B. O., Sawchuk C. N., Lohr J. M., de Jong P. J., Disgust domains in the prediction of contamination fear. Behav. Res. Ther. 42, 93–104 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Hart B. L., Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137 (1988). [DOI] [PubMed] [Google Scholar]
- 72.Kent S., Bret-Dibat J. L., Kelley K. W., Dantzer R., Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci. Biobehav. Rev. 20, 171–175 (1996). [DOI] [PubMed] [Google Scholar]
- 73.Dantzer R., Cytokine-induced sickness behavior: Where do we stand? Brain Behav. Immun. 15, 7–24 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Shattuck E. C., Muehlenbein M. P., Human sickness behavior: Ultimate and proximate explanations. Am. J. Phys. Anthropol. 157, 1–18 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Schrock J. M., Snodgrass J. J., Sugiyama L. S., Lassitude: The emotion of being sick. Evol. Hum. Behav. 41, 44–57 (2020). [Google Scholar]
- 76.Raison C. L., Miller A. H., The evolutionary significance of depression in pathogen host defense (PATHOS-D). Mol. Psychiatry 18, 15–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stieglitz J., et al., Depression as sickness behavior? A test of the host defense hypothesis in a high pathogen population. Brain Behav. Immun. 49, 130–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blyth C. R., On Simpson’s paradox and the sure-thing principle. J. Am. Stat. Assoc. 67, 364–366 (1972). [Google Scholar]
- 79.Olatunji B. O., et al., The three domains of disgust scale: Factor structure, psychometric properties, and conceptual limitations. Assessment 19, 205–225 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Cooper P. J., et al., Environmental determinants of total IgE among school children living in the rural tropics: Importance of geohelminth infections and effect of anthelmintic treatment. BMC Immunol. 9, 33 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hagel I., et al., Ascaris reinfection of slum children: Relation with the IgE response. Clin. Exp. Immunol. 94, 80–83 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bindon J. R., Knight A., Dressler W. W., Crews D. E., Social context and psychosocial influences on blood pressure among American Samoans. Am. J. Phys. Anthropol. 103, 7–18 (1997). [DOI] [PubMed] [Google Scholar]
- 83.Leonard W. R., Galloway V. A., Ivakine E., Osipova L., Kazakovtseva M., “Ecology, health, and lifestyle change among the Evenki herders of Siberia” in Human Biology of Pastoral Populations, Leonard W. R., Crawford M. H., Eds. (Cambridge University Press, Cambridge, 2002), pp. 206–235. [Google Scholar]
- 84.McDade T. W., Williams S., Snodgrass J. J., What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 44, 899–925 (2007). [DOI] [PubMed] [Google Scholar]
- 85.McDade T. W., Burhop J., Dohnal J., High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin. Chem. 50, 652–654 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Miller E. M., McDade T. W., A highly sensitive immunoassay for interleukin-6 in dried blood spots. Am. J. Hum. Biol. 24, 863–865 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanner S., McDade T. W., Enzyme immunoassay for total immunoglobulin E in dried blood spots. Am. J. Hum. Biol. 19, 440–442 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Katz N., Chaves A., Pellegrino J., A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo 14, 397–400 (1972). [PubMed] [Google Scholar]
- 89.Montresor A., Crompton D. W. T., Hall A., Bundy D. A. P., Savioli L., Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level: A Guide for Managers of Control Programmes (World Health Organization, Geneva, Switzerland, 1998). [Google Scholar]
- 90.Revelle W. R., psych: Procedures for personality and psychological research (2020). https://cran.r-project.org/web/packages/psych/index.html. Accessed 13 July 2020.
- 91.Van Buuren S., Groothuis-Oudshoorn K., Buuren S., Groothuis-Oudshoorn K., MICE: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011). [Google Scholar]
- 92.Bürkner P.-C., brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 28781 (2017). [Google Scholar]
- 93.Blackwell A. D., adblackwell/shuardisgust: “Pathogen disgust sensitivity protects against infection in a high pathogen environment” publication release (Version 1.0). Zenodo . 10.5281/zenodo.4487336. Deposited 1 February 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified dataset for this article is available upon request on the SHLHP website (https://www.shuarproject.org/data-sharing) in accordance with data use agreements to ensure compliance with relevant Institutional Review Board, participant expectations, and authorship conditions. Code for all analyses is available at http://doi.org/10.5281/zenodo.4487336 (93).