Abstract
Climate change and an increasing world population means traditional farming methods may not be able to meet the anticipated growth in food demands. Therefore, alternative agricultural strategies should be considered. Here, plant cell and tissue cultures (PCTCs) may present a possible solution, as they allow for controlled, closed and sustainable manufacturing of extracts which have been or are still being used as colorants or health food ingredients today. In this review we would like to highlight developments and the latest trends concerning commercial PCTC extracts and their use as food ingredients or even as food. The commercialization of PCTC‐derived products, however, requires not only regulatory approval, but also outstanding product properties or/and a high product titer. If these challenges can be met, PCTCs will become increasingly important for the food sector in coming years.
Keywords: adventitious root culture, elicitation, food colorants, health food ingredients, suspension culture
Abbreviations
- 2.4‐D
2,4‐dichlorophenoxyacetic acid
- ABR
Active Botanicals Research
- CMC
Cambial Meristematic Cell
- D
Dark
- DDC
Dedifferentiated Cell
- EFSA
European Food Safety Authority
- EU
European Union
- FDA
Food and Drug Administration
- GRAS
Generally Recognized As Safe
- IRB
Instituto di Ricerche Biotecnologiche S.R.l.
- L
Light
- PCTC
Plant Cell and Tissue Culture
- VTT
Technical Research Center of Finland Ltd.
1. INTRODUCTION
There are currently more than 7 billion people on earth, with predictions by the United Nations estimating that this number is set to increase to 10 billion by 2050 [1]. While this has led to increased demands for food, the limited amount of arable land per capita available for food production has constantly been decreasing due to population growth and factors such as urbanization, erosion, soil salinization, and desertification. Furthermore, sustainability in food production [2, 3] and the threat of crop losses due to climate change and plant diseases are playing an increasingly important role and need to be taken into account. These factors could be countered using new and ethically more justifiable technologies such as cellular agriculture, which aims to produce agricultural products such as textiles, leather, meat, milk or egg proteins or fats without plants or animals in bioreactors, utilizing the cells of microorganisms, animals or plants as renewable factories. The products produced in this manner are either cellular or acellular in nature [1, 2] and while the commercially available acellular products are usually produced with genetically modified organisms, their cellular counterparts are still based on native organisms [3].
Commercial products based on plant cell and tissue cultures (i.e. aseptically isolated plant tissues and cells cultivated under defined chemical and physical conditions) have been on the market since the 1980s [4]. They represent ingredients for products of the pharmaceutical, cosmetics and food industries. These products, the majority of which are cosmetic products, can be produced in a controlled manner under closed conditions independent of climate, weather and soil conditions. Variations in product quantity and quality, which are typical for the use of collected or cultivated whole plants, can be avoided [5, 6]. In addition, the use of protected and endangered plant species becomes possible and the metabolism of the established plant cell and tissue culture (PCTC) can be specifically influenced. In other words, the formation of substances that are beneficial to the consumer can be supported and those that are harmful to the consumer can be reduced or even suppressed.
Starting with an overview of commercial PCTC‐based food ingredients, this review intends to shed light on select details, application principles, as well as technologies used for the manufacture of PCTC‐based food and food ingredients, including approaches to increasing process efficiency. In addition, the latest developments aimed at producing new PCTC extracts with nutritional potential and PCTC‐based flavour production is briefly discussed. Finally, the authors address regulatory issues regarding the application of PCTC‐based products in the food industry.
2. COMMERCIAL FOOD INGREDIENTS BASED ON PLANT CELL CULTURE TECHNOLOGY
2.1. Product overview
In Table 1 PCTC‐based food ingredients which have been commercialized at an industrial scale are summarized. The list of products is assigned to two product classes based on their application: (1) colorants and (2) compounds with health promoting effects. The colorants cover anthocyanins, betacyanins and shikonin. Production processes for these colorants run for a maximum of 23 day, with bioreactors using maximum working volumes of between 500 L [7] to 600 L [4, 8, 9] and, excepting the production of betacyanin [10, 11, 12, 13], delivering product yields up to the kilogram range. The commercial production of shikonin, the first authorized secondary metabolite worldwide, with Lithospermum erythrorhizon is particularly noteworthy, as the PCTC‐based process of Mitsui Petrochemical Industries Ltd. allows for the production of 5 kg of pure shikonin per bioreactor run. With comparable shikonin quality, productivity is about 800 times higher than with the traditional process using ginseng roots grown in a field [4, 14]. Another remarkable example of a PCTC‐based process is the scale‐up of echinacoside manufacturing to a production bioreactor size of 75 m3, which was achieved by the Diversa Gesellschaft für Bio‐ und Verfahrenstechnik in Germany in the 1990s [15]. This company, which came to be known as Phyton Biotech, ceased the production of echinacoside in favor of paclitaxel and has been successfully manufacturing this product in bioreactors for more than 20 years [16, 17].
TABLE 1.
Overview of commercialized food colorants and health food ingredients based on PCTCs (arranged in alphabetic order)
| Classification | Compound | Plant species, cell culture type | Manufacturer | Commercial availability | Reference |
|---|---|---|---|---|---|
| Colorant | Anthocyanins | Euphorbia milii, suspension culture e | Nippon Paint Co. Ltd. | Not clear | [18, 19] |
| Aralia cordata, suspension culture e | Tonen Co. Ltd. | Not clear | [7, 20, 21] | ||
| Betacyanins | Beta vulgaris L., suspension culturee | Nippon Shinyaku Co. Ltd. | Not clear | [10, 12, 13, 22, 23] | |
| Somar Corporation | Not clear | [11] | |||
| Shikonin | Lithospermum erythrorhizon, suspension culture e | Mitsui Petrochemical Industries a | Not clear g | [4, 8, 9, 14] | |
| Health food ingredient | Echinacosides |
Echinacea angustifolia, suspension culturee Echinacea purpurea, suspension culture e and adventitious root culture |
ABR | Product available, novel food (Europe) | [24, 25] |
| CBN Plantech Co. Ltd | Not clear | [26] | |||
| IRB | Product available, novel food (Europe) | [27, 28] | |||
| Diversa Gesellschaft für Bio‐ und Verfahrens‐technik mbH c | Commercial manufacture stopped in 1993 | [15, 16, 17] | |||
| Ginseng saponins | Panax ginseng, adventitious root culture | CBN Biotech | Product approved (Korean Food and Drug Association and FDA) | [29, 30, 31, 32, 33, 34] | |
| Nitto Denko Corporation | Not clear | [35, 36] | |||
| Wild ginseng, suspension culture f | Unhwa Corporation | Product approved as healthcare supplement | [37, 38] |
| Classification | Compound | Plant species, cell culture type | Manufacturer | Current status | Reference |
|---|---|---|---|---|---|
| Health food ingredients | Cocoa polyphenols | Theobroma cacao, suspension culture e | Diana Plant Sciences d | Commercial manufacture stopped in 2014 | [39, 40, 41] |
| Teupoloside | Ajuga reptans, suspension culture f | ABR, IRBb | Product available, novel food (Europe) | [24, 42, 43] | |
| Verbascoside | Lippia citriodora, suspension culture f | ABR | Product available, novel food (Europe) | [25, 44, 45] |
Now Mitsui Chemicals, Inc.
Now Part of Croda International.
Now Phyton Biotech.
Now part of Symrise AG.
Dedifferentiated cells (DDCs).
Cambial meristematic cells (CMCs).
Preferably used as colorant in the cosmetics industry (products such as lipsticks, lotions and soaps are no longer available).
Active Botanicals Research (ABR) and the Instituto di Ricerche Biotecnologiche S.R.l. (IRB) claim on their website that they produce “Echinan 4P” and “Echigena plus” from cell cultures of Echinacea angustifolia in production facilities up to m3 scale, however no detailed information on the production process has been published. CBN Plantech Co. Ltd. also works with Echinacea angustifolia based cell cultures at a scale of up to 500 L. Following one week of cultivation, 1.75 kg dry biomass containing 33.44 mg g–1 total caffeic acid can be harvested [26] of which an accumulation of 12.3 mg g–1 echinacoside has been reported.
Another breakthrough was the PCTC‐based production of ginseng saponins, as the traditional agricultural cultivation of ginseng is considered a very time‐consuming and labor‐intensive process, requiring up to 7 years. Nitto Denko Corporation scaled up the cultivation of Panax ginseng cell cultures to a 2 m3 scale, achieving 19 g L–1 dry cell biomass (700 mg L–1 d–1) in merely 4 weeks, and receiving permission for commercial use of the manufactured biomass as a food additive in Japan in the late 1980s [35]. Further attempts to produce ginseng saponins from PCTCs have also been made by CBN Biotech and Unhwa Corporation.
Through bioreactor and process optimization (see also Sections 2.2.2 and 2.2.3) CBN Biotech was able to achieve a total saponin content (5% of cell dry weight) more than twice as high as that of field‐grown ginseng in 0.5 and 1 m3 bioreactors [29]. The 10 m3 production bioreactor version provides an average production of 45 t of biomass fresh weight per year [30, 31]. Unhwa Corporation, on the other hand, succeeded in establishing a highly productive biotechnological production process by cultivating cambial meristematic cells (see Section 2.2.1) of wild ginseng for the first time [37, 38]. The product for the food sector, DDB20, has been on the market since 2014. In the same year, the first cocoa PCTC‐derived nutraceutical from the US company Diana Plant Sciences received the Global Frost & Sullivan Award for Visionary Innovation Leadership. It was named Cocovanol and was characterized by a higher flavanol content than basic cocoa, with only traces of caffeine and theobromine [46, 47]. However, since the acquisition of Diana Plant Sciences by Symrise 6 years ago, no information has been made available regarding this product. Finally, it should be mentioned that Active Botanicals Research and the Instituto di Ricerche Biotecnologiche S.R.I., in addition to the PCTC‐based echinacoside‐containing products ECHINAN 4P and ECHIGENA PluS, manufacture the teupoloside products TEUPOL 10P and 50P as well as TEOSIDE with the same technology [24, 42, 48, 49] and offer them as nutraceuticals alongside Active Botanicals Research PCTC‐based and verbacosid‐containing product ACETOS 10P, which has been approved as novel food (see also Section 4) in Europe [25, 44, 45].
2.2. Principles and technologies
2.2.1. Three main cell culture types
The majority of the products shown in Table 1 are based on plant suspension cells (dedifferentiated cells, DDCs) grown from callus cultures (Figure 1B). Callus is wound tissue induced through injury using a sterile scalpel, following a three‐stage surface sterilization [50, 51] of the mother plant segment containing the target substance(s) in the highest quality and quantity. For this purpose, the sterilized and injured parts of plants are incubated at temperatures between 23°C and 27°C in the dark or under light on agar plates with solid culture media [52, 53]. This is followed by mass propagation of the callus to obtain sufficient biomass for initiating a suspension culture using shaking flasks. Here it is important to work with a friable callus [54]. An efficient production process in the bioreactor requires a homogeneous, well‐growing and productive suspension culture, which sequentially requires a homogenization procedure [55] for the suspension culture, performed over several weeks. DDC‐based suspension cells have doubling times of between 0.6 and 5 days [52] and typically grow as aggregates [56]. Their secondary metabolites, usually formed intracellularly, are present in concentrations between 0.025 and 5 g L‐1 [57, 58] and may decrease with increasing passage number [59].
FIGURE 1.

Schematic representation of the procedure for the establishment of PCTCs: (A) CMC‐based suspension culture, (B) DDC‐based suspension culture and (C) Adventitious root culture. Created with Biorender.com
Cambium meristematic cell (CMC)‐derived suspension cells (Figure 1A), which represent true plant stem cells, grow as single cells [60] facilitating their long‐term storage. They are embedded in meristems located at the tips of shoots, as well as roots, or are contained inside the vascular system and can grow faster as well as reach higher product concentrations than DDC‐based suspension cells [61]. The technology for the generation of CMCs is still in its infancy and has been reported for Taxus cuspidata [38], Catharanthus roseus [62, 63], Ginko biloba and Solanum lycopersicum [64], Tripterygium wilfordii [65] and Ocimum basilicum (Mehring A, University of Kaiserslautern, personal communication, 2019). Unhwa Corporation having secured the world`s first patent technology in plant stem cell isolation is the leading company in this field. The first step in CMC establishment is surface sterilization of the cambium after removal of the pith and xylem. To induce CMC growth, the sterilized explants are transferred into an osmotic agent for 16 to 24 h. Subsequently, the explants are incubated for up to 30 days on a solid growth medium in Petri dishes [57, 66]. Afterwards, the propagated CMCs are sub‐cultivated in Petri dishes with fresh growth medium. If sufficient CMCs are available (100 g L–1 fresh weight), shake flask cultures may be prepared and cultivated under standard conditions. No homogenization procedure is necessary.
In order to generate an adventitious root culture (Figure 1C), the callus must first be induced and a maintenance culture in Petri dishes established, as with the DDC‐based suspension culture [67]. Afterwards, root induction is carried out with a solid culture medium that supports root formation. The roots are first propagated in Petri dishes and then in shaking flasks, in order to generate a maintenance culture, the latter also serving in the inoculum production for bioreactor cultivation.
As mentioned in the introduction, no genetically modified cultures have been used for the production of PCTC‐based commercial food products or ingredients. While the potential of genetically modified PCTCs in terms of product titer increases is undisputed, challenges primarily concerning the acceptance of such products in the European market persist. For an overview of methods commonly used to produce genetically modified PCTCs, the interested reader is referred to the reviews by Kowalczyk et al. (2020) and Nielsen et al. (2019) [68, 69].
2.2.2. Bioreactor cultivation
There are two dominant bioreactor types which have become established in commercial production processes for the products in Table 1, depending on the PCTC type used. While for suspension cultures stirred stainless steel bioreactors are preferred, root cultures are cultivated in modified bubble column bioreactors, with maximum working volumes in the m3 range [70]. Due to the morphology of the suspension cells, their limitation‐free cultivation on a large scale is easier than those of the adventitious root cultures. However, a possible challenge regarding the cultivation in stirred bioreactors is the strong increase in the viscosity of the culture broth when using plant suspension cultures which propagate very well (maximum biomass between 10 and 18 g dry weight L–1 or 200 and 350 g fresh weight L–1) [71, 72]. For such applications, it is recommended to equip the bioreactor with impellers close to bioreactor wall [73]. Typically, the vessel of a stirred production bioreactor for plant suspension cultures has a height‐to‐diameter ratio of 2:1 or 3:1, is equipped with one or more Rushton, marine or pitched blade impellers or combinations thereof, baffles and a dynamic seal [55]. Another critical point is foam formation due to polysaccharides or media ingredients secreted during cell growth [74]. This leads to the wall growth phenomenon, which is considerably more pronounced in bubble column bioreactors, due to their height‐to‐diameter ratio of 6:1 or 14:1.
Paek et al. (2005) succeeded in foam reduction by modifying a bubble column bioreactor and designing the balloon‐type bubble bioreactor [29]. This bioreactor type has also been recommended for the mass propagation of tissue cultures [46, 75–77], including adventive root cultures, with the largest balloon type bubble bioreactors (1 and 10 m3) used by CBN Biotech for the commercial production of ginseng saponins [78].
The use of single‐use bioreactors [79, 80, 81] for the production of PCTC‐based food ingredients is only beneficial for research and development due to size limitations and the high prices of the pharma‐grade plastic vessels or bags. Due to their homogeneous energy dissipation, wave‐mixed bioreactors [82], such as the BIOSTAT RM (Sartorius), the Wave Bioreactor (Cytiva, formerly GE Healthcare), the HyPerforma Rocker Bioreactor (ThermoFisher) or the CELL‐tainer (Celltainer Biotech) are often used. Figure 2A depicts the CELL‐tainer CT20, in which suspension cells of Vitis vinifera were grown. The biomass generated within 38 days (Figure 2B) is comparable to that from a BIOSTAT RM 20/50 operated with a 20 L bag (unpublished data).
FIGURE 2.
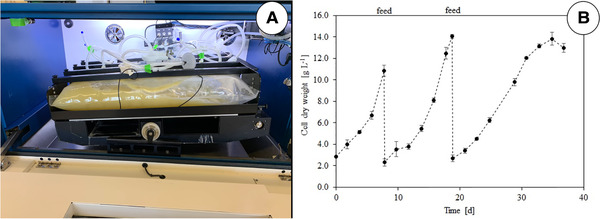
Propagation of V. vinifera DDCs in a CELL‐tainer bioreactor system (A) CELL‐tainer containing a 20 L bag. (B) Typical growth curve of the V. vinifera suspension cells
Specialists of the Technical Research Center of Finland Ltd. (VTT) developed the first prototype of a 3D printed bioreactor for the cultivation of PCTCs in a domestic kitchen. This aptly named “Home Bioreactor” represents a completely new approach based on a modified bubble column and can be compared to a Nespresso machine [83, 84]. The user inserts a capsule containing PCTC, adds water and turns on the bioreactor. During cultivation, the bioreactor maintains optimal growth conditions until the biomass is harvested and processed. Approximately 500 g of biomass (fresh weight) can be generated within one week and further processed (e.g. muesli) in this manner. The “Home Bioreactor”, however, remains to be made commercially available.
2.2.3. Elicitation for the product titer increase
Regardless of the bioreactor and PCTC type, elicitation approaches have proven to be most effective in the production processes shown in Table 1. For example, product concentrations of secondary metabolites in processes with elicited PCTCs could be increased 55‐fold [85, 86] and in some cases even product secretion [87] could be achieved. Elicitation is a non‐transgenic technique that stimulates secondary metabolite production through physical cues or by adding trace amounts of chemical compounds, called elicitors [88]. The elicitors can either be classified according their origin, as exogenous or endogenous, or on the basis of their nature, as biotic or abiotic [89, 90]. Exogenous elicitors are chemicals originating from outside the target cell, such as fatty acids, polysaccharides, peptides and enzymes, whereas endogenous elicitors include substances such as galacturonide or hepta‐β‐glucosides, which are synthesized inside the target cell by induction of intracellular biotic or abiotic signals [88]. Biotic elicitors are of biological origin, either derived from the plant itself or from a bacterial, fungal or viral pathogen source [88, 91]. Abiotic elicitors cover physical factors (e.g. high pH‐value, temperature shifts, osmolarity, oxidative stress and light) and chemical compounds, such as inorganic salts [91]. Physical elicitation is less investigated, more difficult to monitor and more seldom applied when compared to chemical elicitation.
Since the elicitation process is generally complex and the metabolic pathways not always fully understood, many factors and cultivation conditions may affect the impact of elicitors on the synthesis of secondary metabolites. A popular abiotic elicitor is methyl jasmonate [92] often used in concentrations between 0.5%–10%. By its addition growth and production phase of the PCTC are decoupled [93, 94]. With regard to efficient elicitation procedures, it is important to take into account the complex and time‐consuming preliminary tests required to select the suitable elicitor(s) and to determine the optimum addition time, dosage and exposure time of the elicitor [95].
If an ingredient for the food industry or food itself is to be produced with PCTCs, it is vital that not only the selected elicitor is food grade, but also the other ingredients of the culture medium. Synthetic phytohormones are commonly used as pesticides in agriculture, their usage is therefore regulated (see also Section 4) by the EU pesticide database in Europe and toxicological assessments of intracellular phytohormone accumulation are mandatory [96] if used in PCTC cell culture medium. A study by Häkkinen et al. (2020) evaluated the intracellular accumulation of such phytohormones in arctic bramble and birch cell suspension cultures (DDC‐derived). While they were able to detect free 2.4‐dichlorophenoxyacetic acid (2.4‐D) (0.33–0.83 μg g–1 dry weight), the values were below the reported median lethal dose levels measured in rats and mice (320–1000 μg g‐1). To circumvent these regulatory hurdles the replacement of synthetic phytohormones, such as 2.4‐D, kinetin and 1‐naphthalenacetic acid, with natural versions such as indole‐3‐acetic acid, casein, yeast extract, zeatin or coconut water [97] can be considered. A completely different approach is the use of hairy root cultures, as these can be cultivated without phytohormones [98]; however, these have yet to be used for the commercial production of food or food ingredients.
3. LATEST DEVELOPMENTS
3.1. Three new PCTC extracts with potential for human nutrition
Researchers at the Zurich University of Applied Sciences (ZHAW) have succeeded in producing a model chocolate using a DDC‐based Theobroma cacao suspension culture propagated in a wave‐mixed bioreactor. The main steps towards establishing mass propagation of the cell culture have already been described by Eibl et al (2018) [99]. The same applies to the production of cell culture chocolate. The sensory analysis by trained chocolate testers showed that the cell culture chocolate, with an intense fruity and sour aroma (comparable to citrus and red berry flavors), presented a promising sensory profile. Chemical analyses showed that the biomass contained both volatile and non‐volatile flavor compounds at a total polyphenol content of 6.69 g kg–1. However, the aroma profile of chocolate produced with biomass from the wave‐mixed bioreactor differed from those recently produced in stirred bioreactors [100], with an increase in bitterness observed for the “stirred bioreactor chocolate”. Biomass production in the stirred reactor and wave‐mixed bioreactor were comparable with the harvest of approximately 0.23 kg L‐1 biomass (fresh weight) after 16 days. Subsequent research work is now focusing on the possibility of transferring the process from a wave‐mixed bioreactor to a scalable stirred bioreactor and increasing process efficiency.
Another interesting approach to the use PCTC‐based products was described by the researchers of the already mentioned VVT (Section 2.2.2) and is focused on the use of berry suspension cells as food. Nordlund et al. (2017) studied the nutritional properties of DDC‐based plant cell suspension cultures of Rubus chamaemorus L., Vaccinium vitis‐idaea L. and Rubus saxatilis L. [101], also investigating the carbohydrate, lipid and protein composition, in vitro protein digestibility and sensory properties. The results confirm the potential use of the plant suspension cells as a source of food itself for the first time. A fresh odor, as well as flavor and a favorable composition (21%–37% dietary fiber, 0.3%–1% starch, 18%–33% sugars, satisfactory lipid quality, 14%–19% proteins, balanced amino acid profile) of the cell culture biomass were achieved. Furthermore, it was shown that it was possible to mass propagate Rubus chamaemorus L suspension cells up to pilot scale (300 L working volumes, stirred bioreactor, feeding) [102]. Interestingly, flavanols, to which beneficial health effects are attributed [103], are atypical for Rubus fruits cultivated in the field or collected in nature.
Finally, Bianconi et al. (2020) demonstrated the industrial potential of a red carrot cell line extract (R4G extract) for food application (colorant and health food ingredient). It is based on DDC‐derived suspension cells (dark culture) of Daucus carota, which were grown in Gamborg's B5 medium on shake flask and 50 L bioreactor (30 L working volume) scale [104]. The establishment of the production cell line was described by Ceoldo et al. (2005) and Ceoldo et al. (2009) [105, 106]. The R4G extract is characterized by large quantities of anthocyanins, which were higher and more stable than those found in natural red carrot extract, while the metabolic profiles of both extracts were comparable. Furthermore, a noticeable increase of anthocyanin content was achieved by increasing the sucrose level in the culture medium from 25 to 40 g L–1. The antioxidant and anti‐inflammatory activities of the R4G extract were confirmed in vivo using mice.
3.2. PCTC‐derived citrus oil ingredients
Citrus, one of the most important crops worldwide, is essential for both the beverage and flavor industry [107, 108, 109] amongst others. However, climate change, dwindling potable water supplies, soil salinity and plant diseases (e.g. citrus greening disease) has led to supply issues concerning citrus fruit and their products. As these threats are only expected to grow in the near future, callus and suspension cultures of numerous citrus varieties have been established and the PCTCs capabilities of producing typical citrus oil ingredients have been investigated (Figure 3).
FIGURE 3.

Citrus fruit derived PCTCs grown in the dark (D) or under light (L) regime (16 h/8 h): (A) Callus cell lines (B) Suspension cell lines (C) Suspension cell culture in a 20 L wave‐mixed bioreactor (D) Light microscopy image of citrus fruit vesicles in suspension culture
Analysis showed that even in the callus tissue various known citrus volatiles, in the range of several hundred mg/kg dry weight, could already be found. To further increase the production of citrus volatiles, a precursor [110, 111, 112] feeding approach was used. This concept is based on the idea that a natural derived, less expensive substance (intermediate) can be added, in order to induce and increase the production of the compound(s) of interest [18, 113].
To achieve this, a suspension culture (Figure 3B) originating from the flavedo of a citrus fruit was used. The doubling time of the suspension cells (batch culture, 26°C, 120 rpm) was 3.9 days. A two‐step process (10 days growth and 14 days production) was developed on a shaking flask scale (100 mL working volume). After adding the precursor (sesquiterpene), a balsamic fruity scent of orange flower accompanied by a strong citrus taste was produced. Following extraction of the biomass, 37 volatile compounds from various organic classes (aldehydes, alcohols, ethers, furans and other) were detected by gas chromatography‐mass spectrometry analysis of the headspace and solvent. To enable quantitative studies, the process was scaled up to a wave‐mixed BIOSTAT RM 20/50 (Figure 3C), with a working volume of 10 L. Upon completion of the cultivation, 2 kg of biomass fresh weight and 4.5 L of liquid were harvested. Both the biomass and culture supernatant carried the balsamic fruity orange flower like scent, while the taste of the liquid was described as that of white grapefruit by a flavorist of Givaudan. Approximately, 0.6% of the terpenic compound, responsible for this characteristic scent and taste, was detected in the biomass dry weight, twofold of that which can be found naturally occurring in the fruit. However, in order to improve the efficiency and commercial viability of the developed method, further process scale‐up to a stirred stainless‐steel system in the m3 range is necebssary.
4. REGULATORY ISSUES
The progress in the PCTC technology over the last two decades demonstrates that this technology has great potential as an alternative production method in the food sector. As already written, only a handful of PCTC based products have been commercialized. Besides the challenges on process efficiency, the complex regulatory landscape is another limiting factor for their commercialization. As countries have their own regulatory frameworks, the definitions as well as approval processes may vary, which may result in an increase in time, as well in costs to get a product to market.
Already, the definition of a novel food/food ingredient varies broadly. In Europe a food is considered as novel and will fall in the scope of the EU Regulation 2015/2283 on novel food, if it has not been used to a significant degree for food consumption in the EU before May 15, 1997. Food additives (e.g. colorants) and flavourings are not in the scope of this legislation and are covered by their own legislative framework with separate authorisation procedures. In the USA all substances added intentionally to food are considered as “food additives” and require pre‐market approval by the FDA, unless the substance is generally recognized as safe (GRAS) through scientific procedures, through safe history of use in food (dating to before 1958), or it meets one of the other exclusions from the food additive definition in section 201(s) of the Federal Food, Drug and Cosmetic Act.
Although the path to approval of different categories of food additives varies from jurisdiction to jurisdiction, there are many commonalities in terms of the data requirements and considerations for assessment regarding the safety of use of food additives, flavouring or novel food substances, including the use of positive lists of approved substances, pre‐market approval, as well as separation between science and policy decisions. All the different approaches do have a main purpose in common, which is to ensure the safety of the consumed food.
The safety of food regarding traditional use is usually accepted on the basis of its history of safe use. Within a safety assessment, traditional foods/food ingredients are used as reference points, whereas this approach is based on the concept of substantial equivalence of the traditional food/food ingredients versus the novel food/food ingredient under assessment. However, this approach has its limitation in the space of PCTCs, as many PCTCs do not necessarily deliver the same product profile as the whole plant part does. Furthermore, it is mostly not sufficient to take only the source materials and its composition into account, as all characteristics of the product as well as the production process needs to be assessed.
Even the EU Regulation 2015/2283 on novel food clearly defines that food consisting of, isolated from, or produced from a PCTC is considered as one of the novel food categories listed in the EU and requires pre‐market authorisation, which includes a safety assessment performed by the European Food Safety Authority (EFSA). For proper characterisation of the novel food, EFSA has provided guidance [114] in which the specific data requirements in relation to PCTCs are described.
Currently the PCTC extract from Ajuga reptans, Lippia citriodora and Echinacea augustifolia suspension cells (Table 1, Section 2.1) had been authorised as novel food for the use in food supplements under EU Regulation 2015/2283 and therefore imparted in the Union List of authorised novel foods (Commission Implementing Regulation EU 2017/2470). So far, no authorizations have been granted in Europe for food, like e.g. anthocyanins under the food additive legislation.
Another important factor for successful commercialization is the acceptance of the consumer. Consumers can sometimes be hesitant in accepting a novel food technology, even if it has already been perceived as safe by the experts. Most consumers viewed food manufactured with a minimum of processing with more positive attributes than highly processed foods or food manufactured using new technologies. As some authorisations might include specific labelling requirements as, for example in the case of Lippia citriodora cell culture extract, for which the designated labelling of the foodstuffs containing shall be ‘dried extract of Lippia citriodora from cell cultures HTN®Vb’, consumer acceptance can be a challenging factor.
5. CONCLUDING REMARKS
The potential of PCTCs for the sustainable and controlled production of food ingredients is undisputed. Today, the number of PCTC‐based products in the food sector, representing colorants and substances stimulating the immune system or having health effects, is still limited. However, this could change in the near future through climate change, loss of arable land and potable water designated for food production, and plant diseases. Furthermore, smooth regulatory approval and consumer acceptance of new PCTC‐based products will play an important role in their success, with single‐use bioreactors, such as wave‐mixed systems, supporting and accelerating process development. In addition to elicitor and/or precursor feeding, experimental design may improve process efficiency by increasing biomass yield more than twofold and reducing the cost of goods by more than half, as demonstrated by Rasche et al. (2016) [113]. This could pave the way for increased use of PCTCs in the commercial production of food [96] and food ingredients.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
ACKNOWLEDGMENTS
We thank Berkan Ali Keke, Philipp Meier and Yannick Senn from our team, the analytical experts around Prof. Dr. Chahan Yeretzian (Institute of Chemistry and Biotechnology, ZHAW, Switzerland) and the team around Prof. Dr. Tilo Hühn (Institute for Food and Beverage Innovation, ZHAW, Switzerland) for their support in generating experimental data of the grape, citrus and cacao suspension cell cultivations, as well as in making the model chocolate. We also thank Silke Schubert (Regulatory Services, Givaudan) for fruitful discussions and Misha Teale (Institute of Chemistry and Biotechnology, ZHAW) for his linguistic correction of the manuscript.
Gubser G, Vollenweider S, Eibl D, Eibl R. Food ingredients and food made with plant cell and tissue cultures: State‐of‐the art and future trends. Eng Life Sci. 2021;21:87–98. 10.1002/elsc.202000077
This article is dedicated to Prof. Thomas Bley on the occasion of his 70th birthday.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Datar, I. , Kim, E. , d'Origny, G. , New harvest: building the cellular agriculture economy, in: Donaldson, B. (Ed.), The Future of Meat Without Animals, Rowman & Littlefield International, London: 2016, pp. 121–131. [Google Scholar]
- 2. Hoogenkamp, H. , Cellular agriculture shows future potential. FWI 2016, 3, 46–49. [Google Scholar]
- 3. Rischer, H. , Szilvay, G. R. , Oksman‐Caldentey, K‐ M. , Cellular agriculture — industrial biotechnology for food and materials. Curr. Opin. Biotechnol. 2020, 61, 128–134. [DOI] [PubMed] [Google Scholar]
- 4. Malik, S. , Bhushan, S. , Sharma, M. , Ahuja, P. S. , Biotechnological approaches to the production of shikonins: a critical review with recent updates. Crit Rev Biotechnol 2016, 36, 327–340. [DOI] [PubMed] [Google Scholar]
- 5. Stahl, U. , Donalies, U. E. B. , Nevoigt, E. (Eds.), Food Biotechnology. Berlin Heidelberg, Springer‐Verlag Berlin Heidelberg; 2008. [Google Scholar]
- 6. Alfermann, A. W. , Petersen, M. , Natural product formation by plant cell biotechnology: results and perspectives. Plant Cell Tiss Organ Cult 1995, 43, 199–205. [Google Scholar]
- 7. Kobayashi, Y. , Akita, M. , Sakamoto, K. , Liu, H. , et al. Large‐scale production of anthocyanin by Aralia cordata cell suspension cultures. Appl Microbiol Biotechnol 1993, 40, 215–218. [Google Scholar]
- 8. Yazaki, K. , Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnol. J. 2017, 34, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hara, Y. , Suga, C. , Method for producing secondary metabolites of plants., European Patent 0071999A2, 1983.
- 10. Gengatharan, A. , Dykes, G. A. , Choo, W. S. , Betalains: Natural plant pigments with potential application in functional foods. LWT‐Food Sci Technol 2015, 64, 645–649. [Google Scholar]
- 11. Murata, Y. , Otsuka, M. , Saimoto, H. , Kawashima, M. , Process for the production of betacyanin pigments, European Patent 0388143A1, 1990.
- 12. Akita, T. , Hina, Y. , Nishi, T. , Production of betacyanins by a cell suspension culture of table beet (Beta vulgaris L.). Biosci Biotechnol Biochem 2000, 64, 1807–1812. [DOI] [PubMed] [Google Scholar]
- 13. Hina, Y. , Akita, T. , Nishi, T. , Method of Producing Beet Red, Canadian Patent O91/03567, 1991.
- 14. Renneberg, R. , Berkling, V. , Loroch, V. (Eds.), Green Biotechnology, in: Biotechnology for Beginners, Elsevier, 2017, pp. 233–279. [Google Scholar]
- 15. Aitken‐Christie, J. , Kozai, T. , Smith, M. A. L. (Eds.), Automation and Environmental Control in Plant Tissue Culture, Springer, Dordrecht, Dordrecht: 1995. [Google Scholar]
- 16. Parsons, J. L. , Cameron, S. I. , Harris, C. S. , Smith, M. L. , Echinacea biotechnology: advances, commercialization and future considerations. Pharm Biol 2018, 56, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Georgiev, M. I. , Weber, J. , Maciuk, A. , Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biotechnol 2009, 83, 809–823. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto, Y. , Kinoshita, Y. , Yamada, Y. , Anthocyanin production in suspension cultures of high‐producing cells of Euphorbia millii. Agric. Biol. Chem. 1989, 53, 417–423. [Google Scholar]
- 19. Yamamoto, Y. , Kinoshita, Y. , Production of quercetin glucuronide, United States Patent 5212076, 1993.
- 20. Sakamoto, K. , Iida, K. , Sawamura, K. , Hajiro, K. , et al. Anthocyanin production in cultured cells of Aralia cordata Thunb. Plant Cell Tiss Organ Cult 1994, 36, 21–26. [Google Scholar]
- 21. Sakamoto, K. , Asada, Y. , Furuya, T. , Aralia cordata thunb: in vitro culture and the production of anthocyanins, in: Bajaj, Y.P.S. (Ed.), Medicinal and Aromatic Plants IX, Springer Berlin, Heidelberg: 1996. [Google Scholar]
- 22. Akita, T. , Hina, Y. , Nishi, T. , new medium composition for high betacyanin production by a cell suspension culture of table beet (Beta vulgaris L.). Biosci. Biotechnol. Biochem. 2002, 66, 902–905. [DOI] [PubMed] [Google Scholar]
- 23. Esatbeyoglu, T. , Wagner, A. E. , Schini‐Kerth, V. B. , Rimbach, G. , Betanin‐A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [DOI] [PubMed] [Google Scholar]
- 24. Next Generation Botanical Extracts: 3.0 Ecological Approach with an Added Value for Your Food Supplements, ABResearch, Brendola, Italy: 2017. [Google Scholar]
- 25. Fremont, M. , Cell culture: an innovative approach for production of plant actives. New Food Magazine 2017, 1, 1–7. [Google Scholar]
- 26. Cui, H‐ Y. , Abdullahil Baque, Md. , Lee, E‐ J. , Paek, K‐ Y. , Scale‐up of adventitious root cultures of Echinacea angustifolia in a pilot‐scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnol Rep 2013, 7, 297–308. [Google Scholar]
- 27. Dal Toso, R. , Melandri, F. , Echinacea angustifolia cell culture extract: added value for sport and fitness. Nutrafoods 2011, 10, 19–24. [Google Scholar]
- 28. King, C. (Ed.), Commercial Echinacea Production, Alberta Agriculture, Food, and Rural Development, Edmonton, Canada: 2005. [Google Scholar]
- 29. Paek, K. Y. , Chakrabarty, D. , Hahn, E. J. , Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tiss Organ Cult 2005, 81, 287–300. [Google Scholar]
- 30. Baque, Md.A. , Moh, S‐ H. , Lee, E‐ J. , Zhong, J‐ J. , et al. Production of biomass and useful compounds from adventitious roots of high‐value added medicinal plants using bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267. [DOI] [PubMed] [Google Scholar]
- 31. Niranjana Murthy, H. , Dandin, V. S. , Yoeup Paek, K. , Hepatoprotective activity of ginsenosides from Panax ginseng adventitious roots against carbon tetrachloride treated hepatic injury in rats. J Ethnopharmacol 2014, 158, 442–446. [DOI] [PubMed] [Google Scholar]
- 32. Sivakumar G., Yu K. W., Paek K. Y. Biosafe Ginseng: A Novel Source for Human Well‐Being. Engineering in Life Sciences. 2005, 5, 6:527–533. [Google Scholar]
- 33. Choi, S. M. , Ho Son, S. , Rho Yun, S. , Woung Kwon, O. , et al. Pilot‐scale culture of adventitious roots of ginseng in a bioreactor system. Plant Cell Tiss Org 2000, 62, 187–193. [Google Scholar]
- 34. Paek, K‐ Y. , Murthy, H. N. , Hahn, E‐ J. , Zhong, J‐ J. , Large Scale Culture of Ginseng Adventitious Roots for Production of Ginsenosides, in: Zhong, J.‐J. , Bai, F.‐W. , Zhang, W. (Eds.), Biotechnology in China I, Springer Berlin, Heidelberg: 2009, pp. 151–176. [DOI] [PubMed] [Google Scholar]
- 35. Hibino, K. , Ushiyama, K. , Commercial production of ginseng by plant tissue culture technology, in: Fu, T.‐J. , Singh, G. , Curtis, W.R. (Eds.), Plant Cell and Tissue Culture for the Production of Food Ingredients, Springer US, Boston: 1999, pp. 215–224. [Google Scholar]
- 36. Nitto Denko: Annual Report 2006, Nitto Denko: KPMG AZSA & Co., Osaka, Japan: 2006. [Google Scholar]
- 37. Wilson, S. A. , Roberts, S. C. , Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules: Development and commercialization of plant cell culture. Plant Biotechnol J. 2012, 10, 249–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee, E‐ K. , Jin, Y‐ W. , Park, J. H. , Yoo, Y. M. , et al. Cultured cambial meristematic cells as a source of plant natural products. Nat Biotechnol 2010, 28, 1213–1217. [DOI] [PubMed] [Google Scholar]
- 39. Zumbé, A. , Polyphenols in cocoa: are there health benefits? Nutr Bulletin 1998, 23, 94–102. [Google Scholar]
- 40. Barney, L. , DianaPlantSciences creates natural, plant‐based products: the innovative method uses green, sustainable and non‐genetically modified technology DianaPlantSciences. OBA 2019, 1, 1–3. [Google Scholar]
- 41. Georgiev, V. , Mass propagation of plant cells‐ an emerging technology platform for sustainable production of biopharmaceuticals. Biochem Pharmacol 2015, 4, 5. [Google Scholar]
- 42. Di Paola, R. , Esposito, E. , Mazzon, E. , Riccardi, L. , et al. Teupolioside, a phenylpropanoid glycosides of Ajuga reptans, biotechnologically produced by IRBN22 plant cell line, exerts beneficial effects on a rodent model of colitis. Biochem. Pharmacol. 2009, 77, 845–857. [DOI] [PubMed] [Google Scholar]
- 43. Corino, C. , Prost, M. , Pastorelli, G. , Chiapparini, S. , et al. Dietary biotechnological Ajuga reptans extract in post weaning piglets: effects on growth performance, oxidative status and immune parameters. Ann. Anim. Sci. 2019, 19, 793–806. [Google Scholar]
- 44. Kubica, P. , Szopa, A. , Ekiert, H. , Production of verbascoside and phenolic acids in biomass of Verbena officinalis L. (vervain) cultured under different in vitro conditions. Nat. Prod. Res. 2017, 31, 1663–1668. [DOI] [PubMed] [Google Scholar]
- 45. Boustani, A. , Omidi, M. , Torabi, S. , Zarekarizi, A. , Callus induction and plant regeneration in lemon verbena (Lippia Citrodora L.), an important medicinal plant. TJS 2016, 14, 30–38. [Google Scholar]
- 46. Georgiev, M. I. , Design of bioreactors for plant cell and organ cultures, in: Paek, K.‐Y. , Murthy, H.N. , Zhong, J.‐J. (Eds.), Production of Biomass and Bioactive Compounds Using Bioreactor Technology, Springer Netherlands, Dordrecht: 2014, pp. 3–15. [Google Scholar]
- 47. Lange, B. M. , Commercial‐scale tissue culture for the production of plant natural products: successes, failures and outlook, in: Schwab, W. , Lange, B.M. , Wüst, M. (Eds.), Biotechnology of Natural Products, Springer International Publishing, Cham: 2018, pp. 189–218. [Google Scholar]
- 48. Toso, R. D. , Melandri, F. , Sustainable sourcing of natural food ingredients by plant cell cultures. Agro Food Ind Hi Tech 2011, 22, 4. [Google Scholar]
- 49. Dal Monte, R. , Dal Toso, R. , Minghetti, A. , Crespi Perellino, N. , et al. Extracts from Ajuga reptans cell lines, their preparation and use, European Patent 1736166A2, 2006.
- 50. Endress, R. , Plant Cell Biotechnology, Springer Berlin, Heidelberg: 1994. [Google Scholar]
- 51. Bhojwani, S. S. , Razdan, M. K. , Plant Tissue Culture: Theory and Practice, Elsevier Science, Amsterdam: 1996. [Google Scholar]
- 52. Heß, D. , Biotechnologie der Pflanzen: Eine Einführung, UTB, Stuttgart, Stuttgart: 1992. [Google Scholar]
- 53. Murashige, T. , Skoog, F. , A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 1962, 15, 473–497. [Google Scholar]
- 54. Evans, D. E. , Coleman, J. O. D. , Kearns, A. , Plant Cell Culture, BIOS Scientific Publishers, London: 2003. [Google Scholar]
- 55. Eibl, R. , Eibl, D. , Plant cell‐based bioprocessing, in: Cell and Tissue Reaction Engineering: Principles and Practice, Springer‐Verlag Berlin, Heidelberg: 2009, pp. 315–356. [Google Scholar]
- 56. Hall, R. D. , Yeoman, M. M. , Intercellular and intercultural heterogeneity in secondary metabolite accumulation in cultures of Catharanthus roseus following cell line selection. J Exp Bot 1987, 38, 1391–1398. [Google Scholar]
- 57. Imseng, N. , Schillberg, S. , Schürch, C. , Schmid, D. , et al. Suspension Culture of Plant Cells Under Heterotrophic Conditions, in: Meyer, H.‐P. , Schmidhalter, D.R. (Eds.), Industrial Scale Suspension Culture of Living Cells, Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim, Germany: 2014, pp. 224–258. [Google Scholar]
- 58. Jeandet, P. , Clément, C. , Tisserant, L‐ P. , Crouzet, J. , et al. Use of grapevine cell cultures for the production of phytostilbenes of cosmetic interest. C. R. Chimie 2016, 19, 1062–1070. [Google Scholar]
- 59. Deus‐Neumann, B. , Zenk, M. , Instability of indole alkaloid production in Catharanthus roseus cell suspension cultures. Planta Med 1984, 50, 427–431. [DOI] [PubMed] [Google Scholar]
- 60. Yun, B‐ W. , Yan, Z. , Amir, R. , Hong, S. , et al. Plant natural products: history, limitations and the potential of cambial meristematic cells. Biotechnol Genet Eng Rev 2012, 28, 47–60. [DOI] [PubMed] [Google Scholar]
- 61. Ochoa‐Villarreal, M. , Howat, S. , Jang, M. O. , Kim, I. S. , et al. Cambial meristematic cells: a platform for the production of plant natural products. N Biotechnol 2015, 32, 581–587. [DOI] [PubMed] [Google Scholar]
- 62. Moon, S. H. , Venkatesh, J. , Yu, J‐ W. , Park, S. W. , Differential induction of meristematic stem cells of Catharanthus roseus and their characterization. C R Biol 2015, 338, 745–756. [DOI] [PubMed] [Google Scholar]
- 63. Zhu, J. , He, S. , Zhou, P. , Zi, J. , et al. eliciting effect of catharanthine on the biosynthesis of vallesiachotamine and isovallesiachotamine in catharanthus roseus Cambial Meristematic Cells. NPC 2018, 13, 543–546. [Google Scholar]
- 64. Loake, V. I. P. , Ochoa‐Villarreal, M. , Cambial Meristematic Cells: A Sustainable Platform for the Production of Plant‐Derived Anticancer Drugs, in: Malik, S. (Ed.), Biotechnology and Production of Anti‐Cancer Compounds, Springer International Publishing, Cham: 2017, pp. 143–156. [Google Scholar]
- 65. Song, Y. , Chen, S. , Wang, X. , Zhang, R. , et al. A novel strategy to enhance terpenoids production using cambial meristematic cells of Tripterygium wilfordii Hook. f. Plant Methods 2019, 15, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jang, M. O. , Lee, E‐ K. , Jin, Y‐ W. , Plant stem cell line derived from cambium of herbaceous plant with storage root and method for isolating the same, International Patent 2009/038417A2, 2009.
- 67. Rahmat, E. , Kang, Y. , Adventitious root culture for secondary metabolite production in medicinal plants: a review. J Plant Biotechnol 2019, 46, 143–157. [Google Scholar]
- 68. Kowalczyk, T. , Wieczfinska, J. , Skała, E. , Śliwiński, T. , et al. Transgenesis as a tool for the efficient production of selected secondary metabolites from plant in vitro cultures. Plants 2020, 9, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nielsen, E. , Temporiti, M. E. E. , Cella, R. , Improvement of phytochemical production by plant cells and organ culture and by genetic engineering. Plant Cell Rep 2019, 38, 1199–1215. [DOI] [PubMed] [Google Scholar]
- 70. Pavlov, A. , Georgiev, M. , Bley, T. , batch and fed‐batch production of betalains by red beet (beta vulgaris) hairy roots in a bubble column reactor. Zeitschrift Für Naturforschung C 2007, 62, 439–446. [DOI] [PubMed] [Google Scholar]
- 71. James, E. , Lee, J. M. , The production of foreign proteins from genetically modified plant cells, in: Zhong, J.‐J. , Byun, S.Y. , Cho, G.H. , Choi, J.W. , et al. (Eds.), Plant Cells, Springer Berlin, Heidelberg: 2001, pp. 127–156. [DOI] [PubMed] [Google Scholar]
- 72. Eibl, R. , Werner, S. , Eibl, D. , Disposable bioreactors for plant liquid cultures at Litre‐scale. Eng. Life Sci. 2009, 9, 156–164. [Google Scholar]
- 73. Eibl, R. , Brändli, J. , Eibl, D. , Plant cell bioreactors, in: Doelle, H.W. , Rokem, S. , Berovic, M. (Eds.), Biotechnology, Eolss Publishers, Oxford, UK: 2012. [Google Scholar]
- 74. Wongsamuth, R. , Doran, P. M. , Foaming and cell flotation in suspended plant cell cultures and the effect of chemical antifoams. Biotechnol. Bioeng. 1994, 44, 481–488. [DOI] [PubMed] [Google Scholar]
- 75. Faizah, H. , Tanjung, M. , Purnobasuk, H. , Sri Wulan, Y. , Biomass and Flavonoid Production of Gynura procumbens (L.) . Merr Adventitious Root Culture in Baloon‐type Bubble‐bioreactor Influenced by Elicitation. Asian J. of Plant Sciences 2018, 17, 107–119. [Google Scholar]
- 76. Wu, S. Q. , Lian, M. L. , Gao, R. , Park, S. Y. , et al. Bioreactor application on adventitious root culture of Astragalus membranaceus. In Vitro Cell.Dev.Biol.‐Plant 2011, 47, 719–724. [Google Scholar]
- 77. Murthy, H. N. , Paek, K‐ Y. , Park, S‐ Y. , Micropropagation of orchids by using bioreactor technology, in: Lee, Y.‐I. , Yeung, E.C.‐T. (Eds.), Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols, Springer New York, New York, NY: 2018, pp. 195–208. [Google Scholar]
- 78. Murthy, H. N. , Kim, Y‐ S. , Jeong, C‐ S. , Kim, S‐ J. , et al. Production of ginsenosides from adventitious root cultures of panax ginseng, in: Paek, K.‐Y. , Murthy, H.N. , Zhong, J.‐J. (Eds.), Production of Biomass And Bioactive Compounds Using Bioreactor Technology, Springer Netherlands, Dordrecht: 2014, pp. 625–651. [Google Scholar]
- 79. Lehmann, N. , Dittler, I. , Lämsä, M. , Ritala, A. , et al. Disposable bioreactors for cultivation of plant cell cultures, in: Paek, K.‐Y. , Murthy, H.N. , Zhong, J.‐J. (Eds.), Production of Biomass and Bioactive Compounds Using Bioreactor Technology, Springer Netherlands, Dordrecht: 2014, pp. 17–46. [Google Scholar]
- 80. Jossen, V. , Eibl, R. , Eibl, D. , Single‐use bioreactors – an overview, in: Eibl, R. , Eibl, D. (Eds.), Single‐Use Technology in Biopharmaceutical Manufacture, 1st ed., Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim, Germany: 2019, pp. 37–52. [Google Scholar]
- 81. Werner, S. , Greulich, J. , Geipel, K. , Steingroewer, J. , et al. Mass propagation of Helianthus annuus suspension cells in orbitally shaken bioreactors: improved growth rate in single‐use bag bioreactors. Eng. Life Sci. 2014, 14, 676–684. [Google Scholar]
- 82. Eibl, R. , Werner, S. , Eibl, D. , Bag bioreactor based on wave‐induced motion: characteristics and applications, in: Eibl, R. , Eibl, D. (Eds.), Disposable Bioreactors, Springer Berlin, Heidelberg: 2009, pp. 55–87. [DOI] [PubMed] [Google Scholar]
- 83. Räty, N. , Home bioreactor –Local food from plant cell cultures, Master thesis, Aalto University School of Arts, Design and Architecture, 2017.
- 84. Lempert, P. , Ten food trends that will shape 2017. Forbes 2016, 1, 1–7. [Google Scholar]
- 85. Sabater‐Jara, A‐ B. , Onrubia, M. , Moyano, E. , Bonfill, M. , et al. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus x media cell cultures. Plant Biotechnol J 2014, 12, 1075–1084. [DOI] [PubMed] [Google Scholar]
- 86. Jensen, M. , Keller, J. , Elicitierung‐ ein Ansatz zur Erhöhung der Sekundärstoffproduktion bei Produktionsprozessen mit pflanzlichen Zellkulturen, Bachelor Thesis, Zurich University of Applied Sciences, 2020. [Google Scholar]
- 87. Sevòn, N. , Tropan Alkaloids in Hairy Roots and Regenerated Plants of Hyoscyamus Muticus, Doctoral Thesis, University of Helsinki, 1997. [Google Scholar]
- 88. Namdeo, A. G. , Plant cell elicitation for production of secondary metabolites: a review. Phcog Rev. 2007, 1, 69–79. [Google Scholar]
- 89. Ramirez‐Estrada, K. , Vidal‐Limon, H. , Hidalgo, D. , Moyano, E. , et al. Elicitation, an effective strategy for the biotechnological production of bioactive high‐added value compounds in plant cell factories. Molecules 2016, 21, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shakya, P. , Marslin, G. , Siram, K. , Beerhues, L. , et al. Elicitation as a tool to improve the profiles of high‐value secondary metabolites and pharmacological properties of Hypericum perforatum. J Pharm Pharmacol 2019, 71, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Patel, H. , Krishnamurthy, R. , Journal of Pharmacognosy and Phytochemistry. J Pharmacogn Phytochem 2013, 2, 60–65. [Google Scholar]
- 92. Singh, A. , Dwivedi, P. , Methyl‐jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: a review. J Pharmacogn Phytochem 2018, 7, 750–757. [Google Scholar]
- 93. Cusido, R. M. , Palazon, J. , Bonfill, M. , Navia‐Osorio, A. , et al. Improved Paclitaxel and Baccatin III production in suspension cultures of Taxus media. Biotechnol. Prog. 2002, 18, 418–423. [DOI] [PubMed] [Google Scholar]
- 94. Chen, W‐ H. , Xu, C‐ M. , Zeng, J‐ L. , Zhao, B. , et al. Improvement of echinacoside and acteoside production by two‐stage elicitation in cell suspension culture of Cistanche deserticola. World J Microbiol Biotechnol 2007, 23, 1451–1458. [Google Scholar]
- 95. Naik, P. M. , Al‐Khayri, J. M. , Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants, in: Shanker, A.K. , Shanker, C. (Eds.), Abiotic and Biotic Stress in Plants‐ Recent Advances and Future Perspectives, InTech, 2016, pp. 247–277. [Google Scholar]
- 96. Häkkinen, S. T. , Nygren, H. , Nohynek, L. , Puupponen‐Pimiä, R. , et al. Plant cell cultures as food—aspects of sustainability and safety. Plant Cell Rep 2020, 1655–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Murthy, H. N. , Georgiev, M. I. , Park, S‐ Y. , Dandin, V. S. , et al. The safety assessment of food ingredients derived from plant cell, tissue and organ cultures: a review. Food Chem. 2015, 176, 426–432. [DOI] [PubMed] [Google Scholar]
- 98. Gutierrez‐Valdes, N. , Häkkinen, S. T. , Lemasson, C. , Guillet, M. , et al. Hairy root cultures—a versatile tool with multiple applications. Front. Plant Sci. 2020, 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Eibl, R. , Meier, P. , Stutz, I. , Schildberger, D. , et al. Plant cell culture technology in the cosmetics and food industries: current state and future trends. Appl Microbiol Biotechnol 2018, 102, 8661–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gnehm, R. , Scale‐up of Growth and Production of a Theobroma cacao Suspension Culture, Bachelor Thesis, Zurich University of Applied Sciences, 2018. [Google Scholar]
- 101. Nordlund, E. , Lille, M. , Silventoinen, P. , Nygren, H. , et al. Plant cells as food – a concept taking shape. Food Res. Int. 2018, 107, 297–305. [DOI] [PubMed] [Google Scholar]
- 102. Nohynek, L. , Bailey, M. , Tähtiharju, J. , Seppänen‐Laakso, T. , et al. Cloudberry (Rubus chamaemorus) cell culture with bioactive substances: establishment and mass propagation for industrial use. Eng. Life Sci. 2014, 14, 667–675. [Google Scholar]
- 103. Serrano, J. , Puupponen‐Pimiä, R. , Dauer, A. , Aura, A‐ M. , et al. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [DOI] [PubMed] [Google Scholar]
- 104. Bianconi, M. , Ceriotti, L. , Cuzzocrea, S. , Esposito, E. , et al. Red carrot cells cultured in vitro are effective, stable, and safe ingredients for skin care, nutraceutical, and food applications. Front. Bioeng. Biotechnol. 2020, 8, 575079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ceoldo, S. , Levi, M. , Marconi, A. M. , Baldan, G. , et al. Image analysis and in vivo imaging as tools for investigation of productivity dynamics in anthocyanin‐producing cell cultures of Daucus carota. New Phytol. 2005, 166, 339–352. [DOI] [PubMed] [Google Scholar]
- 106. Ceoldo, S. , Toffali, K. , Mantovani, S. , Baldan, G. , et al. Metabolomics of Daucus carota cultured cell lines under stressing conditions reveals interactions between phenolic compounds. Plant Sci. J. 2009, 176, 553–565. [DOI] [PubMed] [Google Scholar]
- 107. Ohloff, G. , Pickenhagen, W. , Kraft, P. , Scent and chemistry: the molecular world of odors, New and rev. ed., Verlag Helvetica Chimica Acta ; Wiley‐VCH, Zürich, Weinheim: 2012. [Google Scholar]
- 108. Iglesias, D. J. , Cercós, M. , Colmenero‐Flores, J. M. , Naranjo, M. A. , et al. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar]
- 109. Ladaniya, M. S. , Citrus fruit production and prospects, in: Citrus Fruit: Biology, Technology and Evaluation, Elsevier, 2008, pp. 1–11. [Google Scholar]
- 110. Tůmová, L. , Gallová, K. , Rimáková, J. , Silybum marianum in vitro. Ceska Slov Farm. 2004, 53, 135–140. [PubMed] [Google Scholar]
- 111. Namdeo, A. G. , Jadhav, T. S. , Rai, P. K. , Gavali, S. , et al. Precursor feeding for enhanced production of secondary metabolites: a review. Phcog Rev. 2007, 1, 227–231. [Google Scholar]
- 112. Smetanska, I. , Production of Secondary Metabolites Using Plant Cell Cultures, in: Stahl, U. , Donalies, U.E.B. , Nevoigt, E. (Eds.), Food Biotechnol., Springer Berlin, Heidelberg: 2008, pp. 187–228. [DOI] [PubMed] [Google Scholar]
- 113. Rasche, S. , Herwartz, D. , Schuster, F. , Jablonka, N. , et al. More for less: Improving the biomass yield of a pear cell suspension culture by design of experiments. Sci Rep 2016, 6, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Turck, D. , Bresson, J. , Burlingame, B. , Dean, T. , et al. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J 2016, 14, 1–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


