Significance
Mechanisms of anesthesia remain obscure. We developed a β-subunit-specific tethered photoswitch containing the widely used anesthetic, propofol, to study the anesthetic mechanisms through γ-aminobutyric acid type A (GABAA) receptors critical for this drug’s effects. Photoswitches are molecules that shift between two isomeric forms after irradiation with light at a specific wavelength, which would produce different biological outcomes on their targets. Tethered photoswitches possess reactive groups that can covalently bind to their target. We identified several residues in the receptor transmembrane domains that are suitable for tethering. Once bound and after photoirradiation, the propofol moiety of MAP20 swings into its binding site, allowing for light-controlled potentiation of the GABAA receptor expressed in Xenopus laevis oocytes.
Keywords: optopharmcology, anesthetic, molecular modeling, azobenzene, methanethiosulfonate
Abstract
Tethered photoswitches are molecules with two photo-dependent isomeric forms, each with different actions on their biological targets. They include reactive chemical groups capable of covalently binding to their target. Our aim was to develop a β-subunit-tethered propofol photoswitch (MAP20), as a tool to better study the mechanism of anesthesia through the GABAA α1β3γ2 receptor. We used short spacers between the tether (methanethiosulfonate), the photosensitive moiety (azobenzene), and the ligand (propofol), to allow a precise tethering adjacent to the putative propofol binding site at the β+α− interface of the receptor transmembrane helices (TMs). First, we used molecular modeling to identify possible tethering sites in β3TM3 and α1TM1, and then introduced cysteines in the candidate positions. Two mutant subunits [β3(M283C) and α1(V227C)] showed photomodulation of GABA responses after incubation with MAP20 and illumination with lights at specific wavelengths. The α1β3(M283C)γ2 receptor showed the greatest photomodulation, which decreased as GABA concentration increased. The location of the mutations that produced photomodulation confirmed that the propofol binding site is located in the β+α− interface close to the extracellular side of the transmembrane helices. Tethering the photoswitch to cysteines introduced in the positions homologous to β3M283 in two other subunits (α1W288 and γ2L298) also produced photomodulation, which was not entirely reversible, probably reflecting the different nature of each interface. The results are in agreement with a binding site in the β+α− interface for the anesthetic propofol.
While photoswitches have been a popular topic in numerous reviews (1), their application in research is still very rare. Photoswitches are freely diffusible molecules containing a photosensitive moiety which can alternate between two isomeric forms depending on light irradiation at specific wavelengths. These isomeric forms of the photoswitch possess different affinities or efficacies toward their biological target, such that their pharmacological activity can be turned on or off depending on the light wavelength used, thus providing temporal and spatial control. When photoswitches covalently tether to a native or engineered residue at the specific biological target, the resulting conformation would determine the corresponding pharmacological activity. One way the tethered photoswitch can be activated is by positioning the tether in such a way that the ligand moiety can reach its binding site in only one of the photoswitch conformations. Additional major advantages of the tethered photoswitches are high local concentration (the residue cannot diffuse away) and spatial restriction within the biological target. The ligand groups of photoswitches can possess diverse pharmacological activities, acting as agonists, inverse agonists, or antagonists at specific binding sites.
The GABAA receptor is formed by five subunits (usually two α, two β, and one γ or δ) arranged in pseudosymmetry around a central channel, in the following order (counterclockwise, viewed from the extracellular side): γ-β-α-β-α (2). Each interface is named after the subunits that form it, with “+” and “−” designated following the counterclockwise order (a model of the α1β3γ2 GABAA receptor can be found in SI Appendix, Fig. S1). Each subunit consists of an extracellular domain, attached to a sequence of four transmembrane helices (TMs), with a large intracellular loop inserted between TM3 and TM4. Multiple anesthetic drugs that act through this receptor possess relatively low binding affinities; therefore, precise identification and characterization of their binding sites require the use of techniques like mutagenesis, substituted cysteine modification protection (SCAMP), and photolabeling with photoreactive anesthetic analogs (3). Structures of the GABAA receptor with bound anesthetic drugs have recently been made available (4), but one unexplored way of obtaining valuable, functional information would be by using appropriately designed tethered photoswitches. In previous studies of GABAA receptors, the photoswitches used have included diffusible propofol photoswitches (5, 6) and a tethered propofol photoswitch with a very long spacer between the tethering cysteine (located in the extracellular domain) and the propofol moiety (6). Though all three positively modulated GABAA receptors, none could be used to define the propofol binding site.
Multiple studies point to propofol binding cavities being located in the TM interfaces between GABAA receptor subunits. The observation that specific mutations in TM2 or TM3 of GABAA β subunits could dramatically decrease propofol potentiation of GABA responses, and even direct activation of the receptor (7–9) led to the development of the β3(N265M) knockin mouse, which showed either a greatly decreased or absent hypnotic effect of propofol, depending on the test used (10). More recent studies have focused on a more complete characterization of binding sites for propofol as well as other intravenous anesthetics. A photoreactive propofol analog labeled three amino acids in the β+α− interface (β3M286, α1M236, and α1I239) and one in the α+β− interface (β3M227) in α1β3 receptors. Using etomidate and R-mTFD-MPAB [R-5-allyl-1-methyl-5-(m-trifluoromethyldiazirinylphenyl)barbituric acid] to inhibit photolabeling established that propofol also binds to the β+β− interface, suggesting that propofol shows little selectivity for either interface (11). Another study mutated photolabeled residues (α1M236, β3M227, and their homologs, all located in TM1) to tryptophan (causing steric occupancy of the pocket) and cysteine (to test propofol protection against cysteine-specific labeling) (12). SCAMP studies confirmed that propofol binds to the β+α− and α+β− interfaces, and also to the γ+β− interface; there was no evidence of binding to the α+γ− interface. A more recent study has expanded the analysis of residues at the β+α− interface that line a putative propofol binding site (13) (SI Appendix, Fig. S1). And cryoelectron microscopy (cryo-EM) structures of α1β2γ2 GABAA receptors bound to intravenous anesthetics and benzodiazepines have recently been published (4), consolidating the evidence toward a propofol binding site at the β+α− interfaces.
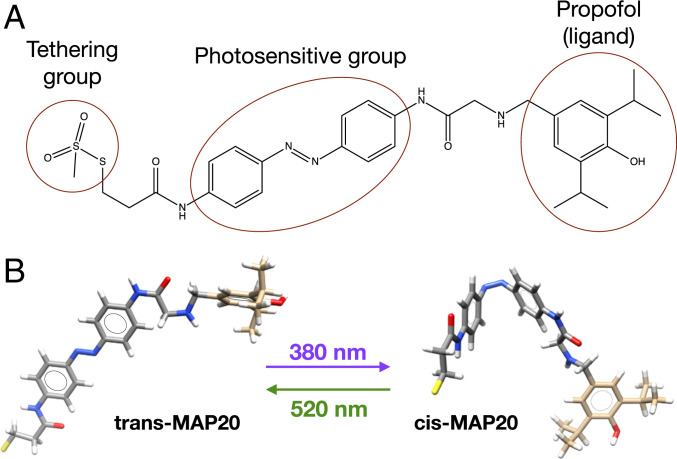
Our aim was to develop a β-subunit-tethered propofol photoswitch, as a tool to better study the mechanism of anesthesia through the GABAA α1β3ɣ2 receptor, merging both structural and functional approaches. This tethered photoswitch (Fig. 1A) consists of propofol as the ligand group, linked to an azobenzene group (which is photosensitive and can change between cis and trans isomeric forms, Fig. 1B), and finally a tethering group (methanethiosulfonate, that spontaneously forms a covalent bond with the thiolate group of cysteines located in water-filled cavities). This photoswitch was abbreviated as MAP20 (methanethiosulfonate azobenzene propofol 2020). These three basic components of this tethered photoswitch are connected by short spacers, decreasing the range between tethering and target residues. We used β3 subunits in our study because the immobilizing and hypnotic effects of propofol are mostly mediated by β3-containing receptors (10).
Fig. 1.
Tethered photoswitch. (A) Methanethiosulfonate azobenzene propofol 2020 (MAP20) consists of propofol (ligand group), an azobenzene moiety (photosensitive), and a tether (methanethiosulfonate). (B) The azobenzene moiety can change between cis and trans isomeric forms depending on the wavelength of the light irradiated.
Results
Before testing the photoswitch in relevant cysteine mutants, endogenous cysteines located in the TM and extracellular domains of the GABAA subunits were replaced by alanines, creating a Cys-to-Ala background into which tethering cysteines could be introduced without fear of endogenous cysteines interfering with the interpretation of the photomodulation results. The function of the resulting Cys-to-Ala α1β3γ2 GABAA receptor was compared with the wild-type receptor, and no differences were observed, except for a modest, albeit significant, increase in propofol potentiation (SI Appendix, Fig. S2 and Table S1).
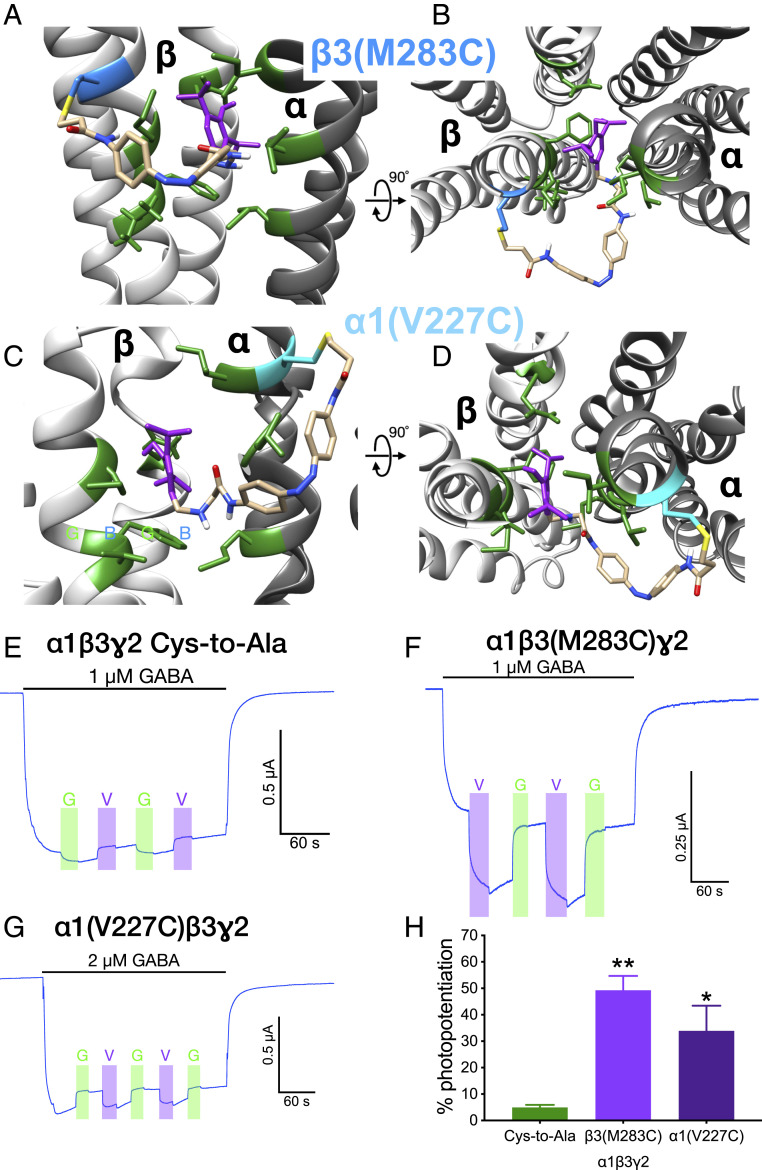
We used molecular modeling to position propofol in the putative binding site located in the β+α− interface in a GABAA receptor structure. This cavity is lined by several residues critical for propofol action: βN265 and βM286, and αL232 and αM236. Then we added the rest of the MAP20 structure (photosensitive and tethering groups with the corresponding spacers, Fig. 1A) and explored possible tethering sites among the residues within reach of the methanethiosulfonate group. We identified three residues in α1 TM1 as possible tethering points: α1V227, α1I235, and α1V243, and another three in β3 TM3 (β3M283, β3G287, and β3V290). Only the resulting models for MAP20 attached to β3(M283C) (Fig. 2 A and B) and α1(V227C) (Fig. 2 C and D) are shown.
Fig. 2.
GABAA receptors containing β3(M283C) or α1(V227C) subunits. (A–D) Models of the β+α− interface (β in light gray, α in dark gray), with MAP20 attached to β3(M283C), depicted in blue (A and B) and α1(V227C), depicted in cyan (C and D). Propofol moiety from MAP20 in purple, residues lining the putative binding site of propofol at this interface in green. Side view of the interface as seen from the membrane in A and C. View of the interface from the extracellular side in B and D. The relative position of the models is indicated by the rotation axis and degree symbol. (E and H) Photopotentiation of submaximal GABA responses in α1β3(M283C)γ2 and α1(V227C)β3γ2 GABAA receptors. Representative tracings of photomodulation in (E) α1β3γ2 Cys-to-Ala, (F) α1β3(M283C)γ2, and (G) α1(V227C)β3γ2. Light wavelength: G (green), 520 nm; V (violet), 380 nm. (H) Quantitative summary of photomodulation in these receptors. Data are shown as mean ± SEM and were analyzed using one-way ANOVA, followed by Dunnett’s multiple comparisons test, *P < 0.05, **P < 0.01 versus Cys-to-Ala receptor (n = 4 to 5).
The candidates for tethering points were mutated to cysteine and their GABA sensitivity and modulation by flunitrazepam, propofol, and zinc were tested in pilot experiments. All the mutated receptors expressed on the surface of the oocyte, as shown by their activation by GABA. Their submaximal GABA responses were potentiated by 2 µM propofol and 0.1 µM flunitrazepam, and they showed small inhibition by 10 µM Zn++, indicating expression of the γ2 subunit. Results are shown only for α1β3(M283C)γ2 and α1(V227C)β3γ2 (SI Appendix, Fig. S3 and Table S1).
Structural characterization of MAP20 can be found in SI Appendix. The photoswitching behavior of MAP20 when irradiated with violet (380 nm) and green (520 nm) lights was reflected in the changes of its ultraviolet/visible (UV/Vis) absorption spectra (SI Appendix, Fig. S4). We tested the cysteine mutant receptors for photomodulation after incubation with MAP20. The α1β3γ2 Cys-to-Ala GABAA receptor (control) showed very small modulation upon irradiation with either violet or green light (Fig. 2E). The only mutated receptors to show photomodulation after incubation with 50 µM MAP20 were α1β3(M283C)γ2 (Fig. 2F) and α1(V227C)β3γ2 (Fig. 2G). A quantitative summary of photomodulation results for each receptor is shown in Fig. 2H (F = 9.348, P < 0.01). Violet light (that induces the cis isomer) produced positive modulation of the GABA response, while green light (that induces the trans isomer) reversed this effect. The other mutated receptors showed very small modulation upon irradiation, similarly to Cys-to-Ala receptors (representative tracings can be observed in SI Appendix, Fig. S5).
Two control experiments were carried out: first, a control photoswitch identical to MAP20 but lacking the propofol (methanethiosulfonate azobenzene 2020, MA20, SI Appendix, Fig. S6A) was synthesized and its structure characterized (described in SI Appendix). Oocytes expressing α1β3γ2 Cys-to-Ala and α1β3(M283C)γ2 GABAA receptors were incubated with 50 µM MA20, following the labeling protocol used with MAP20. After incubation with MA20, there were no significant changes in the GABA responses of Cys-to-Ala receptors, nor the β3(M283C)-containing receptors (SI Appendix, Fig. S6 B and C). The second control experiment was the acute application of MAP20 to α1β3γ2 Cys-to-Ala and α1β3(M283C)γ2 GABAA receptors (SI Appendix, Fig. S7). No significant changes were observed in the GABA responses of Cys-to-Ala GABAA receptors, while the β3(M283C)-containing receptors developed a sustained photomodulation of the GABA responses, as observed after incubation with MAP20 and subsequent electrophysiological recording. The absence of photomodulation due to the noncysteine residue 283 and lack of propofol moiety in the MA20 photoswitch clearly identify MAP20 as a tethered photoswitch. MAP20 is only capable of photomodulating the receptor when the tethering cysteine is present at a suitable location, with minimal effects on the GABA responses of the Cys-to-Ala receptor when coapplied, and virtually none after washout. Therefore, tether and ligand moieties in the photoswitch, and a precisely located tethering residue are the key elements for direct photomodulation of GABA by MAP20.
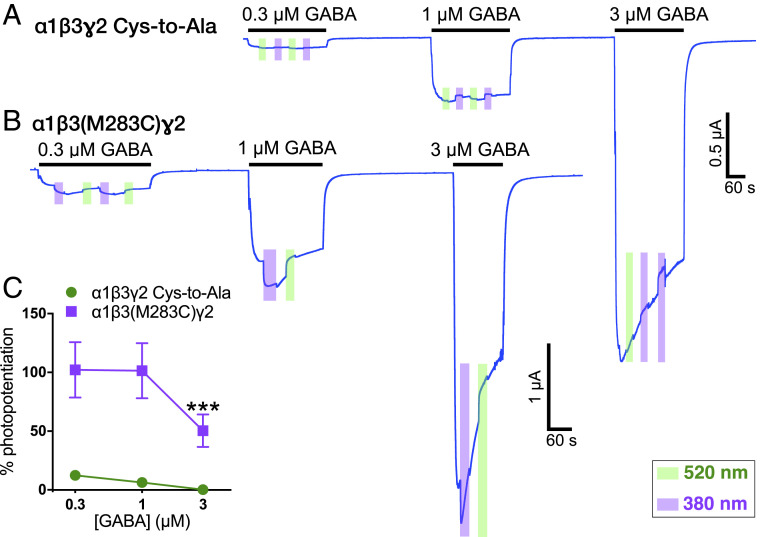
When α1β3(M283C)γ2 receptors were tested at different GABA concentrations after incubation with MAP20, the photomodulation decreased as the GABA concentration increased (F1,8 = 15.93, P < 0.01, effect of receptor; F2,14 = 12.40, P < 0.001, effect of concentration; F2,14 = 5.705, P < 0.05, receptor × concentration interaction), as expected for an allosteric modulator (Fig. 3).
Fig. 3.
Photomodulation at different GABA concentrations after incubation with 50 µM MAP20. Representative tracings of (A) α1β3γ2 Cys-to-Ala and (B) α1β3(M283C)γ2 GABAA receptors. (C) Quantitative summary of the photomodulation at different GABA concentrations. Data are shown as mean ± SEM, error bars not shown are smaller than symbols. Data were analyzed using two-way repeated measures ANOVA, followed by Dunnett’s multiple comparisons test, ***P < 0.01 versus 0.3 µM concentration (n = 4 to 5).
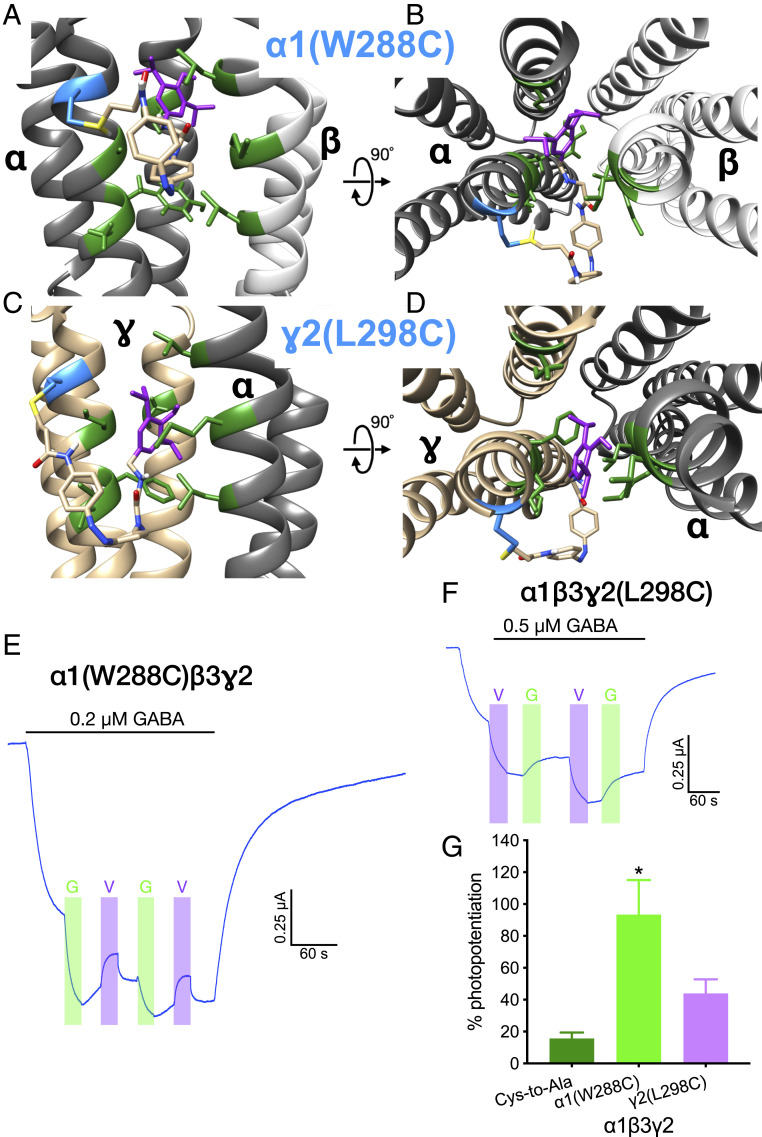
To test other TM interfaces, the residues homologous to β3M283 were replaced with cysteine in the other subunits and MAP20 modeled in the corresponding interfaces. Thus, when MAP20 was tethered to α1(W288C), its propofol group was predicted to bind to the α+β− interface in the model (Fig. 4 A and B). The binding of MAP20 to the α+γ− interface was not modeled, as there is evidence that it does not possess a propofol binding site. Similarly, when MAP20 was tethered to γ2(L298C), the propofol group was predicted to bind to the γ+β− interface in the model (Fig. 4 C and D).
Fig. 4.
GABAA receptors containing α1(W288C) or γ2(L298C) subunits. (A and B) Model of the α+β− interface (α in dark gray, β in light gray), with MAP20 attached to α1(W288C) (in cyan). (C and D) Model of the γ+α− interface (γ in gold, α in dark gray), with MAP20 attached to γ2(L298C) (in cyan). Propofol moiety from MAP20 in purple, residues lining the putative binding site of propofol at this interface in green. Side view of the interface as seen from the membrane in A and C. View of the interface from the extracellular side in B and D. The relative position of the models is indicated by the rotation axis and degree symbol. (E–G) Photopotentiation of submaximal GABA responses in α1(W288C)β3γ2 and α1β3γ2(L298C) GABAA receptors. Representative tracings of photomodulation in (E) α1(W288C)β3γ2 and (F) α1β3γ2(L298C). Light wavelength: G (green), 520 nm; V (violet), 380 nm. (G) Quantitative summary of photomodulation in these mutants. Data are shown as mean ± SEM and analyzed using one-way ANOVA, followed by Dunnett’s multiple comparisons test, *P < 0.05 versus Cys-to-Ala, (n = 4 to 8).
The replacement of these residues with cysteine did not greatly modify the functionality of the receptor when expressed in oocytes; the GABA sensitivity was not altered (SI Appendix, Fig. S8A and Table S1), and there was only a small increase in the propofol potentiation in α1β3γ2(L298C) (SI Appendix, Fig. S8B).
Photomodulation of MAP20 tethered to these cysteines resulted in potentiation in both receptors (Fig. 4 E–G). However, some differences from the previous results were apparent. Photopotentiation of α1(W288C)β3γ2 was highly variable, it was induced by the wavelength producing the trans isomer but could be induced in some cases by either wavelength. The photopotentiation in α1β3γ2(L298C) was induced by the cis isomer [as in β3(M283C)], but its magnitude was not different from the Cys-to-Ala receptor (Fig. 4G). In both mutants, after inducing photopotentiation with one wavelength, irradiation with the other did not totally reverse it, suggesting that for these interfaces, reversing the isomerization after the propofol was bound was not entirely possible. Fig. 4G shows the magnitude of the photopotentiation for the first light irradiation during submaximal GABA application [580 nm for α1(W288C)β3γ2, 380 nm for α1β3γ2(L298C)] (F2,14 = 4.994, P < 0.05). A list of all the mutants tested and results obtained with each can be found in SI Appendix, Table S2.
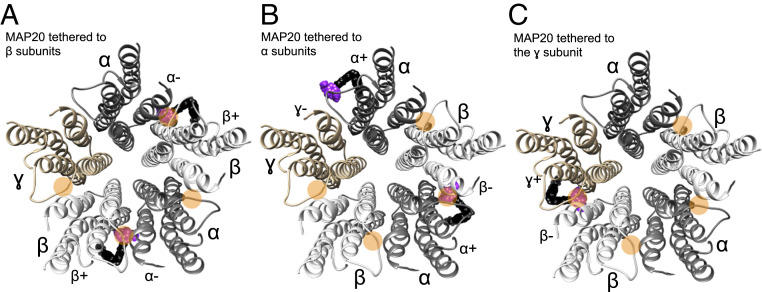
Fig. 5 shows a summary diagram with MAP20 at each of the interfaces after tethering to each subunit. Since not all transmembrane interfaces possess propofol binding cavities, the relative position of the tethering residue determines how many sites the photoswitch propofol can reach. When MAP20 is tethered to both β subunits (Fig. 5A), the propofol moieties of MAP20 can occupy two binding sites (β+α− interfaces). But even when MAP20 is tethered to both α subunits (Fig. 5B), only one propofol moiety of the tethered MAP20 reaches a binding site (α+β− interface, no propofol binding site in the α+γ− interface). When MAP20 is tethered to the γ subunit (Fig. 5C), there is only one propofol binding site that the propofol moiety of MAP20 can occupy (γ+β− interface). Therefore, depending on the tethering subunit, the propofol moiety of MAP20 can reach one or two binding sites.
Fig. 5.
Diagram of the GABAA receptor transmembrane domains viewed from the extracellular side. MAP20 tethered to (A) β subunits, (B) α subunits, and (C) γ subunit. Orange circles mark the interfaces where putative binding sites for propofol exist (four binding sites, the α+γ− interface is apparently lacking a propofol binding site). MAP20 is shown as a black and purple sphere-shaped molecule, with purple representing the propofol moiety.
Of all the tethering positions tested, β3(M283C) was the one that offered the best photopotentiation with MAP20. We introduced that cysteine in a β3 wild-type background (i.e., a subunit with all its endogenous cysteines), and tested its GABA sensitivity (SI Appendix, Fig. S9A), modulation by GABAergic drugs (SI Appendix, Fig. S9B), and its photopotentiation (SI Appendix, Fig. S9 C–E). The presence of β3(M283C) in addition to the endogenous cysteines did not modify the receptor functionality, and after incubation with MAP20, it showed a robust photopotentiation.
Discussion
Tethered photoswitches have distinct advantages over photoaffinity labeling and SCAMP in the study of anesthetics. Photoaffinity labeling requires a modified anesthetic to come close to an attachment point to allow the covalent reaction in the presence of light. High ligand occupancy at a specific location is therefore reinforced or detected by the formation of the covalent bond, but only in the presence of an activating light source once the ligand positioned at the target site. Furthermore, the modification of the ligand to include a photosensitive tethering group could result in substantial changes in the ligand chemical structure. SCAMP is similar in that “a side-chain hypothesized to be in or near an anesthetic binding site is mutated to cysteine, providing a free sulfhydryl. These cysteine substituted receptors are then exposed to sulfhydryl-reactive chemical probes, and after washout of these reagents, electrophysiology is used to detect whether covalent bond formation produced an irreversible functional change” (3). In the case of tethered photoswitches, a cysteine mutation in the region close to anesthetic binding is introduced like in SCAMP, and a photoswitch is covalently linked to the engineered cysteine without the need for photoactivation. Then the light is used to switch the conformation to produce a controllable on/off transition which is detected electrophysiologically. This is distinct from the SCAMP method because SCAMP does not involve the photo-dependent “switching” capabilities. The covalent bond created in SCAMP can be reduced, but not recreated unless more reagent is applied. In contrast, once the photoswitch is tethered, it can be turned on-off using light in a temporal and spatially controlled manner. Most importantly, photoswitches can be activated/deactivated multiple times in functional studies of live cells or animals.
A recent publication described cryo-EM structure of the α1β3γ2 GABAA receptor bound to propofol (4). The cryo-EM-derived structure is highly similar to our model, including the position of the propofol binding site in the interface (SI Appendix, Fig. S10). The minor differences could be attributed to our model being based on an open channel template (used because anesthetics are known to stabilize the open state of the GABAA receptor), while the cryo-EM structure is most likely that of a desensitized state. While the propofol molecules in the cryo-EM structure were only detected in the two β+α− interfaces, there is evidence suggesting that propofol binds to two other interfaces (SI Appendix, Fig. S1C).
Several photoswitches have been developed to target the GABAA receptor, and most are competitive antagonists that tethered near the GABA binding site at the β+α− interface in the extracellular domains (14, 15). Three are modulators, with propofol as the ligand group: two diffusible (5, 6) and the other (MPC100) tethers to the extracellular domain of the γ subunit (6). Most of the experiments were carried out with the diffusible photoswitches. To our knowledge, the tethered photoswitch used in this study (MAP20) is the first for any target that is tethered to a residue in a transmembrane domain whose location on the side of the receptor is only accessible from within the membrane itself.
We identified possible tethering sites using a α1β3γ2 GABAA receptor model based on the crystal structure of GluCl bound to ivermectin (Protein Data Bank [PDB]: 3RIF). Our decision to use this as the basis of the model was based on the fact that kinetic studies show that anesthetics bind most favorably to and stabilize activated receptors (open channels) (16, 17), and GluCl is a pentameric ligand-gated ion channel that shows the proper characteristics of an activated receptor with an open channel bound to two separate activating ligands, glutamate and ivermectin (18).
Most of the tethering positions at the β+α− interface that we identified via molecular modeling, either in the β or α subunit, did not result in photomodulation after preincubation with MAP20 (SI Appendix, Fig. S10). Only receptors containing either β3(M283C) or α1(V227C) showed photomodulation. MAP20, once tethered, potentiated GABA-mediated currents in the cis isomeric form, while the trans isomer produced no potentiation. This agrees with the molecular models, that show propofol in an interface cavity only when MAP20 is in the cis isomeric form. This binding cavity is in good agreement with the putative binding site supported by evidence provided by mutagenesis, photolabeling, SCAMP (13), and cryo-EM structures (4). The magnitude of propofol modulation is dependent on the GABA activation: at low GABA concentrations, propofol modulation is larger than that at the higher GABA concentrations (19), and MAP20 behaved in a similar way, providing further evidence for them sharing the same mechanism of action on the GABAA receptor. Even though the irradiation of MAP20 with green light did not fully reverse the isomerization to the trans isoform in the spectroscopy experiments, the photopotentiation of GABA currents in response to violet light was fully reversed upon green light illumination.
The absence of photomodulation when the residue 283 is not a cysteine, along with the development of photomodulation independently of the application method only in the β3(M283C)-containing receptors, indicate that MAP20 is only capable of photomodulating the receptor when the tethering cysteine is present at a suitable location. Similarly, the absence of photomodulation when the propofol moiety is lacking from the compound (MA20), indicate that the photomodulation is a specific effect due to the propofol moiety, and not due to the tethering of an azobenzene derivative to a residue critical for receptor function. Therefore, these results clearly identify MAP20 as a tethered photoswitch, that lacks a direct effect on receptors lacking a suitable cysteine.
The actions of MAP20 both agree and contrast with those of MPC100, the other tethered propofol photoswitch (6). As with MPC100, MAP20 produced light-sensitive potentiation of submaximal GABA currents (mediated by GABAA receptors expressed in oocytes), after incubation with the photoswitch and subsequent perfusion to remove the untethered compound. This photopotentiation was not observed in the control GABAA receptors, indicating that both photoswitches covalently reacted with the cysteine introduced in the mutated receptors. The main two differences are the active isomer and the position of the tethering cysteine in each case. MPC100 potentiation was induced after irradiation with high-intensity visible light, which produces the trans isomer, and inactivated with UV light, which produces the cis isomer (6). In contrast, MAP20 produced photopotentiation after UV light, whereas green (visible) light eliminated it. The tethering residue for MPC100 was in the extracellular domain of the γ2 subunit; the most effective tethering place for MAP20 was in TM3 of the β3 subunit. These differences are due to the chemical structure of the photoswitches. MPC100 has a long spacer between the tethering group and the photosensitive group (separated by a chain formed by 84 C/O/N atoms), while MAP20 has a much shorter spacer (4 C/N atoms). The best way for the propofol in MPC100 to reach the binding cavity is for the photoswitch to be in the trans isomeric form, while the reverse is true for MAP20. MAP20’s short spacers between the structural components (tethering, photosensitive, and ligand groups) require the tethering residue to be near to the propofol binding cavity for photoswitching to occur.
Mutagenesis, photolabeling, and SCAMP studies suggest that propofol binds to at least four cavities at interfaces formed between bundles of transmembrane alpha helices, with only the α+γ− interface lacking a propofol binding site (12). We tested photomodulation of GABAA receptors containing cysteine mutations in either the α or γ subunits, in the homologous positions to β3M283. Tethering MAP20 to either α1(W288C) or γ2(L298C) did not produce as clean a photomodulation as β3(M283C) has shown. The photopotentiation observed after tethering MAP20 to γ2(L298C) and irradiating with violet light (which induces the cis isomer) was not fully reversible with green light (which induces the trans isomer). In addition, the photopotentiation was smaller than that observed for β3(M283C), which is likely due to MAP20 occupying only one binding site, instead of two as when it binds to the β subunit. The case of α1(W288C) is more complex; photopotentiation was variable and in some cases observed after either wavelength, which means that either the cis or the trans isomer can photopotentiate the GABA-mediated currents. We did however notice that when MAP20 was tethered to α1(W288C) and in the trans configuration, its propofol group was near β3H267, which has been previously labeled with the photoreactive ortho-propofol diazirine (20). This putative binding site located near the extracellular side of the β-interfaces (between TM1 and TM2 of β subunits and TM2 of the neighboring subunit) is closer to the pore than to the lipid membrane. It is possible that in a fraction of receptors, the propofol group of MAP20 reaches this putative propofol binding site when it is in the trans isomeric form, therefore producing potentiation in both isomeric configurations. Unfortunately, we were not able to identify an additional suitable residue to which we could tether MAP20 in order to directly test this binding site for photomodulation.
The exact number of propofol binding sites in the GABAA receptor remains undetermined. The goodness of fit of functional data from α1β2γ2 receptors (propofol concentration-response curves) does not vary when the number of sites is set at values between 2 and 5 (21). As mentioned above, there is considerable evidence supporting a propofol binding site present at four of the interfaces, near the extracellular side. Previous studies have suggested that each of these four propofol binding sites are equal and independent when it comes to energetic contributions to propofol effects on a concatemeric version of the trimeric receptor, with mutations located in either the β+ or β− interfaces (22). However, the differences in the residues present in each kind of interface are sufficient to produce significant pharmacological differences. This has been demonstrated by the differential affinity of photoreactive analogs of etomidate for β+ and mephobarbital for β− interfaces (23, 24), even though the photoreactive analog of propofol did not discriminate among these four interfacial binding sites (11). A more in-depth study using competition photolabeling concluded that propofol has 5.6-fold higher affinity for the β+α− interfaces than for the β− interfaces (25). This is in agreement with the recently published cryo-EM structures of α1β2γ2 receptors in combination with propofol (4). However, as mentioned before, there are limitations to these data.
We propose that our MAP20 photoswitch could be a valuable tool to identify brain regions that are essential to each pharmacological effect of propofol. MAP20 could be infused into a specific brain region of a knockin mouse with a cysteine in the appropriate location of the GABAA receptor. MAP20 application does not modify GABA responses in the wild-type GABAA receptor, indicating that the endogenous cysteines present in that receptor do not provide tethering points with a functional consequence. The labeling of β3(M283C) in the wild-type background with MAP20 produced the same kind of photomodulation as in the Cys-to-Ala background, indicating that it is not necessary to replace the endogenous cysteines with alanines in the knockin mice (SI Appendix, Fig. S7). This engineered mouse could then be subjected to appropriate behavioral tests, and MAP20 activated and deactivated using light delivered to the same brain region via an optic fiber during testing. Thus, the precise spatial-temporal control of the drug activity could reveal if that brain region participates in the drug effect. Furthermore, as the photoswitch would only bind to β3-containing receptors, it possesses a β-subunit selectivity (β3 over β1/2) that is not possible to achieve with currently available drugs. The high homology among β subunits, and the fact that propofol does not differentiate among them also suggests that it would be possible to replicate these experiments by introducing a cysteine in the homologous position of β1 and β2, thus obtaining a way to test the relevance of each subunit in different behavioral tests when each one is positively modulated in turn in specific brain regions.
In summary, we designed and tested a tethered photoswitch that can be covalently bound to an engineered cysteine in the TM helices of the GABAA receptor, and then controlled via lights at specific wavelengths to potentiate GABA-mediated currents in a propofol-like manner. Even though the results obtained in the β+α− interface did not translate directly to the other interfaces where propofol is predicted to bind, the location of the tethering cysteines that led to photomodulation confirmed that propofol binds in the previously proposed cavity present between βTM3 and αTM1. It is worth noting that these experiments were carried out before the structures of propofol bound to a GABAA receptor were published, proving that other experimental approaches can be combined with molecular modeling to correctly identify a binding site and predict suitable tethering points for photoswitches.
Materials and Methods
Molecular Modeling.
The α1β3γ2 GABAA receptor model was based on the crystal structure of GluCl bound to ivermectin and glutamate in its open form (PDB: 3RIF) (SI Appendix, Fig. S1). The optimized pose and conformation of a native propofol molecule docked to the GABAA receptor were obtained from our previous work (26, 27). The MAP20 compound was separately created in vacuo and optimized using molecular mechanics with the optically active double bond being restrained in the cis conformation. The propofol moiety end of MAP20 was manually overlapped with the aforementioned optimally docked propofol in the GABAA receptor and the native propofol was removed. We identified GABAA receptor residues for possible mutation to Cys for induced disulfide linkage to the sulfhydryl end of MAP20. This was done by fixing the propofol end of MAP20 in the binding site and rotating the torsion of the bond which links the propofol moiety to the rest of MAP20 through 360°. Residues in reasonable proximity to the sulfhydryl end of the MAP20 for such linkage are noted in capital letters: β3 TM3 sequence containing dMylmGcfV and α1 TM1 sequence containing ViqtyLpcIM. All the molecular graphics shown were created using the University of California San Francisco Chimera package version 1.14 (28).
Oocyte Isolation and Injection.
Xenopus laevis frog surgery was performed in accordance with a protocol approved by The University of Texas at Austin’s institutional animal care and use committee and the NIH Guide for the Care and Use of Laboratory Animals. Manually isolated X. laevis oocytes were injected with capped complementary RNAs encoding wild-type or mutant subunits. The injected oocytes were incubated for 3 to 5 d before recording. Complete methods are described in SI Appendix, Experimental Procedures.
Recording Protocols.
The responses of GABAA receptors expressed in oocytes were studied using a two-electrode voltage clamp. Complete methods are described in SI Appendix, Experimental Procedures.
Photoswitch Tethering and Irradiation.
Oocytes expressing GABAA receptors were incubated in 50 µM MAP20 (Fig. 1, Toronto Research Chemicals) for 20 to 120 min. Following the MAP20 incubation, the oocyte was placed in a two-electrode voltage clamp rig, surrounded by a Faraday cage and blackout curtains. An optical fiber placed directly above the oocyte in the recording chamber provided light generated by high power light-emitting diode (LED) sources connected to a beam combiner (Prizmatix) allowing the illumination of the oocyte with either 520 nm (green) or 380 nm (violet) lights. Identical procedures were followed for labeling with MA20. Complete methods are described in SI Appendix, Experimental Procedures.
Statistical Analysis.
Complete information is described in SI Appendix, Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. Raluca Gearba-Dolocan and Olga Ponomareva for assistance with the ligand spectrophotometric analyses and Dr. Alam Jahangir for helpful consultation and medicinal chemistry expertise. This study was supported by the Waggoner Center for Alcohol and Addiction Research and by NIH Grants AA006399 (R.A.H.) and R01AA020980 (J.R.T.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2008178118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Hüll K., Morstein J., Trauner D., In vivo photopharmacology. Chem. Rev. 118, 10710–10747 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Scott S., Aricescu A. R., A structural perspective on GABAA receptor pharmacology. Curr. Opin. Struct. Biol. 54, 189–197 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Forman S. A., Miller K. W., Mapping general anesthetic sites in heteromeric γ-aminobutyric acid type A receptors reveals a potential for targeting receptor subtypes. Anesth. Analg. 123, 1263–1273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J. J., et al., Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 585, 303–308 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein M., et al., Azo-propofols: Photochromic potentiators of GABA(A) receptors. Angew. Chem. Int. Ed. Engl. 51, 10500–10504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue L., et al., Robust photoregulation of GABA(A) receptors by allosteric modulation with a propofol analogue. Nat. Commun. 3, 1095 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegwart R., Jurd R., Rudolph U., Molecular determinants for the action of general anesthetics at recombinant alpha(2)beta(3)gamma(2)gamma-aminobutyric acid(A) receptors. J. Neurochem. 80, 140–148 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Krasowski M. D., et al., Propofol and other intravenous anesthetics have sites of action on the gamma-aminobutyric acid type A receptor distinct from that for isoflurane. Mol. Pharmacol. 53, 530–538 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Pistis M., Belelli D., McGurk K., Peters J. A., Lambert J. J., Complementary regulation of anaesthetic activation of human (alpha6beta3gamma2L) and Drosophila (RDL) GABA receptors by a single amino acid residue. J. Physiol. 515, 3–18 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurd R., et al., General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 17, 250–252 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Jayakar S. S., et al., Multiple propofol-binding sites in a γ-aminobutyric acid type A receptor (GABAAR) identified using a photoreactive propofol analog. J. Biol. Chem. 289, 27456–27468 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nourmahnad A., et al., Tryptophan and cysteine mutations in M1 helices of α1β3γ2L γ-aminobutyric acid type a receptors indicate distinct intersubunit sites for four intravenous anesthetics and one orphan site. Anesthesiology 125, 1144–1158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziemba A. M., et al., Alphaxalone binds in inner transmembrane beta+-alpha- interfaces of alpha1beta3gamma2 gamma-aminobutyric acid type a receptors. Anesthesiology 128, 338–351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin W. C., et al., A comprehensive optogenetic pharmacology toolkit for in vivo control of GABA(A) receptors and synaptic inhibition. Neuron 88, 879–891 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin W. C., et al., Engineering a light-regulated GABAA receptor for optical control of neural inhibition. ACS Chem. Biol. 9, 1414–1419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbach J. H., Akk G., Modulation of GABA(A) receptor channel gating by pentobarbital. J. Physiol. 537, 715–733 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman S. A., Miller K. W., Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels. Can. J. Anaesth. 58, 191–205 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hibbs R. E., Gouaux E., Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanna E., et al., Actions of the general anesthetic propofol on recombinant human GABAA receptors: Influence of receptor subunits. J. Pharmacol. Exp. Ther. 274, 353–360 (1995). [PubMed] [Google Scholar]
- 20.Yip G. M., et al., A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat. Chem. Biol. 9, 715–720 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruesch D., Neumann E., Wulf H., Forman S. A., An allosteric coagonist model for propofol effects on α1β2γ2L γ-aminobutyric acid type A receptors. Anesthesiology 116, 47–55 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin D. J., et al., Propofol is an allosteric agonist with multiple binding sites on concatemeric ternary GABAA receptors. Mol. Pharmacol. 93, 178–189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiara D. C., et al., Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor. J. Biol. Chem. 288, 19343–19357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G. D., Chiara D. C., Cohen J. B., Olsen R. W., Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J. Biol. Chem. 285, 8615–8620 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayakar S. S., et al., Identifying drugs that bind selectively to intersubunit general anesthetic sites in the α1β3γ2 GABAAR transmembrane domain. Mol. Pharmacol. 95, 615–628 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertaccini E. J., Yoluk O., Lindahl E. R., Trudell J. R., Assessment of homology templates and an anesthetic binding site within the γ-aminobutyric acid receptor. Anesthesiology 119, 1087–1095 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cayla N. S., et al., A newly developed anesthetic based on a unique chemical core. Proc. Natl. Acad. Sci. U.S.A. 116, 15706–15715 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersen E. F., et al., UCSF Chimera–A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.